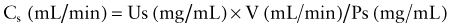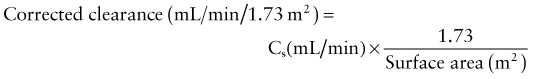Chapter 502 Introduction to Glomerular Diseases
502.1 Anatomy of the Glomerulus
The kidneys lie in the retroperitoneal space slightly above the level of the umbilicus. They range in length and weight, respectively, from approximately 6 cm and 24 g in a full-term newborn to ≥12 cm and 150 g in an adult. The kidney (see ![]() Fig. 502-1 on the Nelson Textbook of Pediatrics website at www.expertconsult.com) has an outer layer, the cortex, which contains the glomeruli, proximal and distal convoluted tubules, and collecting ducts; and an inner layer, the medulla, that contains the straight portions of the tubules, the loops of Henle, the vasa recta, and the terminal collecting ducts (see Fig. 502-2 on the Nelson Textbook of Pediatrics website at www.expertconsult.com).
Fig. 502-1 on the Nelson Textbook of Pediatrics website at www.expertconsult.com) has an outer layer, the cortex, which contains the glomeruli, proximal and distal convoluted tubules, and collecting ducts; and an inner layer, the medulla, that contains the straight portions of the tubules, the loops of Henle, the vasa recta, and the terminal collecting ducts (see Fig. 502-2 on the Nelson Textbook of Pediatrics website at www.expertconsult.com).
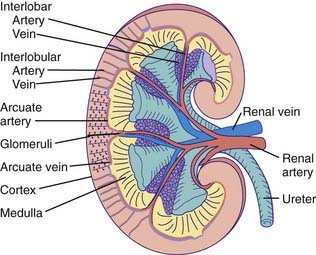
Figure 502-1 Gross morphology of the renal circulation.
(From Pitts RF: Physiology of the kidney and body fluids, ed 3, Chicago, 1974, Year Book Medical Publishers.)
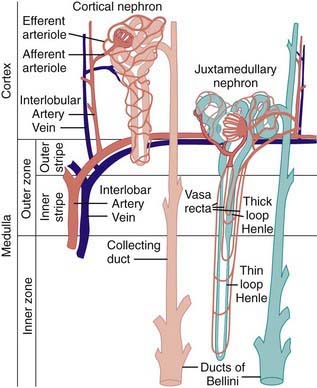
Figure 502-2 Comparison of the blood supplies of cortical and juxtamedullary nephrons.
(From Pitts RF: Physiology of the kidney and body fluids, ed 3, Chicago, 1974, Year Book Medical Publishers.)
The blood supply to each kidney usually consists of a main renal artery that arises from the aorta; multiple renal arteries can occur. The main artery divides into segmental branches within the medulla, becoming the interlobar arteries that pass through the medulla to the corticomedullary junction. At this point, the interlobar arteries branch to form the arcuate arteries, which run parallel to the surface of the kidney. Interlobular arteries originate from the arcuate arteries and give rise to the afferent arterioles of the glomeruli. Specialized muscle cells in the wall of the afferent arteriole and specialized distal tubular cells adjacent to the glomerulus (macula densa) form the juxtaglomerular apparatus that controls the secretion of renin. The afferent arteriole divides into the glomerular capillary network, which then recombines into the efferent arteriole (Fig. 502-2). The juxtamedullary efferent arterioles are larger than those in the outer cortex and provide the blood supply, as the vasa recta, to the tubules and medulla.
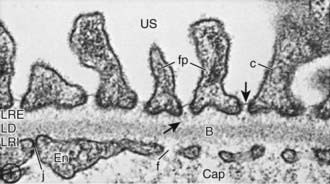
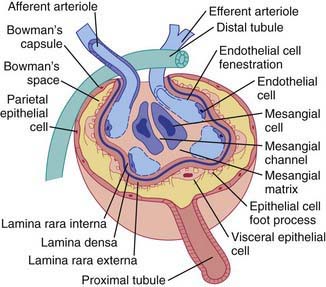
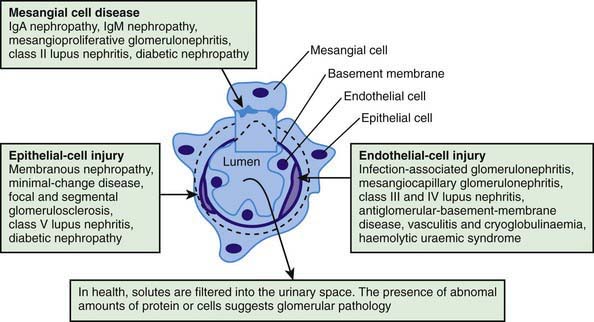
The glomerular network of specialized capillaries serves as the filtering mechanism of the kidney. The glomerular capillaries are lined by endothelial cells (Fig. 502-3) and have very thin cytoplasm that contains many holes (fenestrations). The glomerular basement membrane (GBM) forms a continuous layer between the endothelial and mesangial cells on one side and the epithelial cells on the other. The membrane has 3 layers: (1) a central electron-dense lamina densa; (2) the lamina rara interna, which lies between the lamina densa and the endothelial cells; and (3) the lamina rara externa, which lies between the lamina densa and the epithelial cells. The visceral epithelial cells cover the capillary and project cytoplasmic foot processes, which attach to the lamina rara externa. Between the foot processes are spaces or filtration slits. The mesangium (mesangial cells and matrix) lies between the glomerular capillaries on the endothelial cell side of the GBM and forms the medial part of the capillary wall. The mesangium may serve as a supporting structure for the glomerular capillaries and probably has a role in the regulation of glomerular blood flow, filtration, and the removal of macromolecules (such as immune complexes) from the glomerulus. Bowman’s capsule, which surrounds the glomerulus, is composed of a basement membrane, which is continuous with the basement membranes of the glomerular capillaries and the proximal tubules, and the parietal epithelial cells, which are contiguous with the visceral epithelium (Fig. 502-4).
Fogo AB. Renal pathology. In: Avner ED, Harmon WE, Niaudet P, et al, editors. Pediatric nephrology. ed 6. Heidelberg, Germany: Springer-Verlag; 2009:565-598.
Hunley TE, Kon V, Ichikawa I. Glomerular circulation and function. In: Avner ED, Harmon WE, Niaudet P, et al, editors. Pediatric nephrology. ed 6. Heidelberg, Germany: Springer-Verlag; 2009:31-64.
502.2 Glomerular Filtration
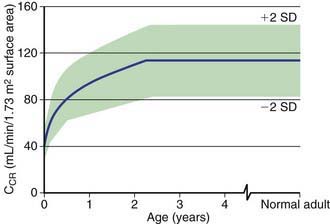
Although glomerular filtration begins at approximately the 6th wk of fetal life, kidney function is not necessary for normal intrauterine homeostasis because the placenta serves as the major fetal excretory organ. After birth, the glomerular filtration rate (GFR) increases until renal growth ceases (by age ~18-20 years in most people). To compare GFRs of children and adults, the GFR is standardized to the body surface area (1.73 m2) of an “ideal” 70-kg adult. Even after correction for surface area, the GFR of a child does not approximate adult values until the 3rd yr of life (Fig. 502-5).
The GFR may be estimated by measurement of the serum creatinine level (Fig. 502-6). Creatinine is derived from muscle metabolism. Its production is relatively constant, and its excretion is primarily through glomerular filtration, although tubular secretion can become important in renal insufficiency. In contrast to the concentration of blood urea nitrogen, which is affected by state of hydration and nitrogen balance, the serum creatinine level is primarily influenced by the level of glomerular function. The serum creatinine is of value only in estimating the GFR in the steady state. A patient can have a normal creatinine level without effective renal function very shortly after the onset of acute renal failure with anuria. In this clinical setting, serum creatinine may be an insensitive measure of decreased renal function because its level does not rise above normal until the GFR falls by 30-40%.
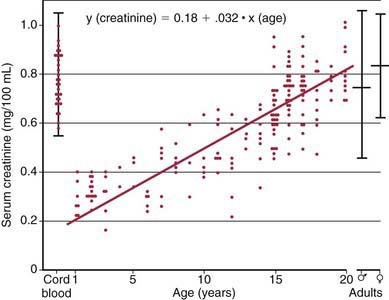
Figure 502-6 The serum creatinine in relation to age.
(From McCrory W: Developmental nephrology, Cambridge, MA, 1972, Harvard University Press.)
where Cs equals the clearance of substance s, Us reflects the urinary concentration of s, V represents the urinary flow rate, and Ps equals the plasma concentration of s. To correct the clearance for body surface area, the formula is
Arant BSJr. Postnatal development of renal function during the first year of life. Pediatr Nephrol. 1987;1:308-313.
Finney H, Newman DJ, Thakkar H, et al. Reference ranges for plasma cystatin C and creatinine measurements in premature infants, neonates, and older children. Arch Dis Child. 2000;82:71-75.
Hunley TE, Kon V, Ichikawa I. Glomerular circulation and function. In: Avner ED, Harmon WE, Niaudet P, Yoshikawa N, editors. Pediatric Nephrology. ed 6. Heidelberg, Germany: Springer-Verlag; 2009:31-64.
Schwartz GJ, Muñoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629-637.
502.3 Glomerular Diseases
Pathogenesis
Glomerular injury may be a result of genetic, immunologic, perfusion, or coagulation disorders. Genetic disorders of the glomerulus result from mutations in the exons of DNA encoding proteins located within the glomerulus, interstitium, or tubular epithelium; mutations in the regulatory genes controlling DNA transcription; abnormal post-transcriptional modification of RNA transcripts; or abnormal post-translational modification of proteins. Immunologic injury to the glomerulus results in glomerulonephritis, which is a generic term for several diseases and a histopathologic term signifying inflammation of the glomerular capillaries. Evidence that glomerulonephritis is caused by immunologic injury includes morphologic and immunopathologic similarities to experimental immune-mediated glomerulonephritis; the demonstration of immune reactants (immunoglobulin, complement) in glomeruli; abnormalities in serum complement; and the finding of autoantibodies (anti-GBM) in some of these diseases (see ![]() Fig. 502-7 on the Nelson Textbook of Pediatrics website at www.expertconsult.com). There appear to be 2 major mechanisms of immunologic injury: glomerular deposition of circulating antigen-antibody immune complexes and interaction of antibody with local antigen in situ. In the latter circumstance, the antigen may be a normal component of the glomerulus (the noncollagenous domain [NC-1] of type IV collagen, a putative antigen in human anti-GBM nephritis) or an antigen that has been deposited in the glomerulus.
Fig. 502-7 on the Nelson Textbook of Pediatrics website at www.expertconsult.com). There appear to be 2 major mechanisms of immunologic injury: glomerular deposition of circulating antigen-antibody immune complexes and interaction of antibody with local antigen in situ. In the latter circumstance, the antigen may be a normal component of the glomerulus (the noncollagenous domain [NC-1] of type IV collagen, a putative antigen in human anti-GBM nephritis) or an antigen that has been deposited in the glomerulus.
In immune complex–mediated diseases, antibody is produced against and combines with a circulating antigen that is usually unrelated to the kidney (see Fig. 502-7). The immune complexes accumulate in GBMs and activate the complement system, leading to immune injury. Acute serum sickness in rabbits is produced by a single intravenous injection of bovine albumin. Within 1 wk after injection, a rabbit produces antibody against bovine albumin, and the antigen remains in the blood in high concentration. As antibody enters the circulation, it forms immune complexes with antigen. Although the amount of antigen in the circulation exceeds that of antibody (antigen excess), the complexes formed are small, remain soluble in the circulation, and are deposited in glomeruli. The processes involved in glomerular localization are not well understood but include characteristics of the complex (concentration, charge, size), and/or the glomerulus (mesangial trapping, negatively charged capillary wall); hydrodynamic forces, and the influence of various chemical mediators (angiotensin II, prostaglandins).
The inflammatory reaction that follows immunologic injury results from activation of 1 or more mediator pathways. The most important of these is the complement system, which has 2 initiating sequences: the classic pathway, which is activated by antigen-antibody immune complexes, and the alternative or properdin pathway, which is activated by polysaccharides and endotoxin. These pathways converge at C3; from that point on, the same sequence leads to lysis of cell membranes (Chapter 127). The major noxious products of complement activation are produced after activation of C3 and include anaphylatoxin (which stimulates contractile proteins within vascular walls and increases vascular permeability) and chemotactic factors (C5a) that recruit neutrophils and perhaps macrophages to the site of complement activation, leading to consequent damage to vascular cells and basement membranes.
Pathology
Proliferation of glomerular cells occurs in most forms of glomerulonephritis and may be generalized, involving all glomeruli, or focal, involving only some glomeruli and sparing others. Within a single glomerulus, proliferation may be diffuse, involving all parts of the glomerulus, or segmental, involving only 1 or more tufts, but not others. Proliferation commonly involves the endothelial and mesangial cells and is often associated with an increase in the mesangial matrix (see Fig. 502-7). Mesangial proliferation can result from deposition of immune complex within the mesangium. The resultant increase in cell size and number, and production of mesangial matrix, can increase glomerular size and narrow the lumens of glomerular capillaries, leading to renal insufficiency.