Chapter 10 Intraventricular Conduction Abnormalities
General Considerations
A narrow QRS complex requires highly synchronous electrical activation of the ventricular myocardium, which can be achieved only through the rapidly conducting His-Purkinje system (HPS). The term intraventricular conduction disturbances (IVCDs) refers to abnormalities in the intraventricular propagation of supraventricular impulses resulting in changes in the morphology or duration, or both, of the QRS complex. These changes in intraventricular conduction can be fixed and present at all heart rates, or they can be intermittent (transient) and tachycardia- or bradycardia-dependent. They can be caused by structural abnormalities in the HPS or ventricular myocardium, functional refractoriness in a portion of the conduction system (i.e., aberrant ventricular conduction), or ventricular preexcitation over a bypass tract.1
Transient Bundle Branch Block
The term aberration is used to describe transient bundle branch block (BBB) and does not include QRS abnormalities caused by preexisting BBB, preexcitation, or the effect of drugs. Transient BBB can have several mechanisms, including phase 3 block, phase 4 block, and concealed conduction. These mechanisms of aberration can occur anywhere in the HPS, and, unlike in chronic BBB, the site of block during aberration can shift. Right BBB (RBBB) is the most common pattern of aberration, occurring in 80% of patients with aberration and in up to 100% of cases of aberration in normal hearts.2–7
Phase 3 Block
Conduction velocity depends, in part, on the rate of rise of phase 0 of the action potential (dV/dt) and the height to which it rises (Vmax). These factors, in turn, depend on the membrane potential at the time of stimulation. The more negative the membrane potential is, the more sodium (Na+) channels are available for activation, the greater the influx of Na+ into the cell during phase 0, and the greater the conduction velocity. Therefore, when stimulation occurs during phase 3 of the action potential, before full recovery and at less negative potentials of the cell membrane, a portion of Na+ channels remains refractory and unavailable for activation. Consequently, the Na+ current and phase 0 of the next action potential are reduced, and conduction is then slower.6,8–10
Phase 3 block, also called tachycardia-dependent block, occurs when an impulse arrives at tissues that are still refractory caused by incomplete repolarization. Manifestations of phase 3 block include BBB and fascicular block, as well as complete atrioventricular (AV) block.3,11
Functional or physiological phase 3 aberration can occur in normal fibers if the impulse is sufficiently premature to encroach on the physiological refractory period of the preceding beat, when the membrane potential is still reduced. This is commonly seen with very early premature atrial complexes (PACs) that conduct aberrantly. Phase 3 aberration can also occur pathologically if electrical systole and the refractory period are abnormally prolonged (with refractoriness extending beyond the action potential duration or the QT interval) and the involved fascicle is stimulated at a relatively rapid rate.3,11 Transient left BBB (LBBB) is less common than RBBB (only 25% of phase 3 aberration is of the LBBB type). The block usually occurs in the very proximal portion of the bundle branch.6,9,10
Aberration Caused by Premature Excitation
Premature excitation can cause aberration (BBB) by encroaching on the refractory period of the bundle branch prior to full recovery of the action potential, namely during so-called voltage-dependent refractoriness (see Fig. 4-29).11 In normal hearts, this type of aberration is almost always in the form of RBBB (Fig. 10-1), whereas such aberration in the abnormal heart can be that of RBBB or LBBB.
At normal heart rates, the effective refractory period (ERP) of the right bundle branch (RB) exceeds the ERP of the AV node (AVN), HB, and left bundle branch (LB). At faster heart rates, the ERP of both bundle branches shortens. However, RB ERP shortens to a greater degree than LB ERP, so that the duration of the refractory periods of the two bundles crosses over, and LB ERP becomes longer than that of the RB. This explains the tendency of aberration to be in the form of RBBB when premature excitation occurs during normal heart rates and in the form of LBBB when it occurs during fast heart rates.2,3,6,8–10,12
Ashman Phenomenon
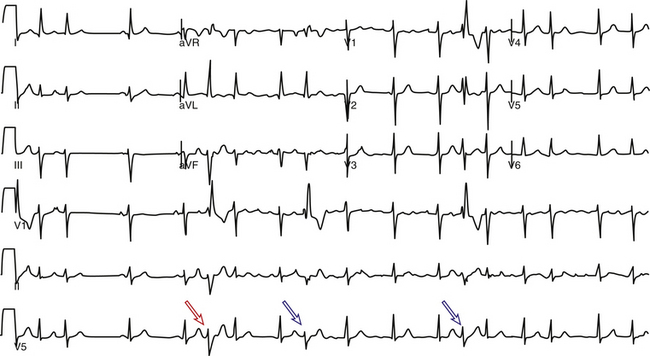
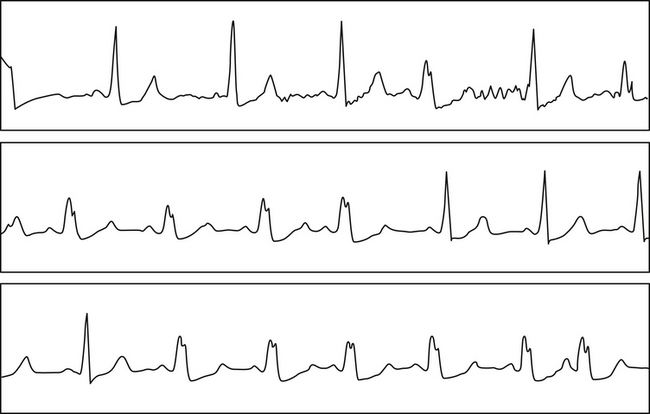
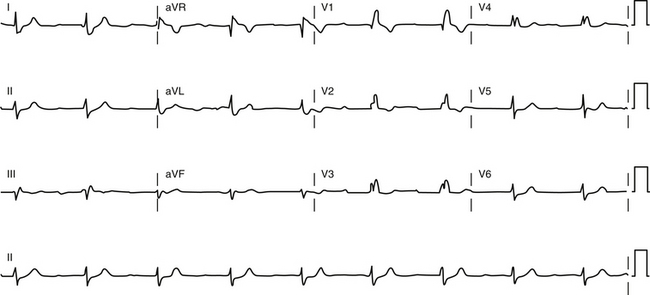
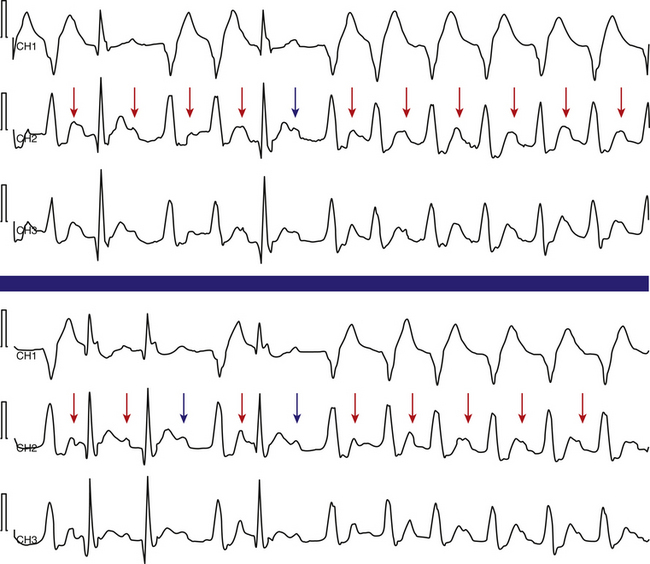
The Ashman phenomenon refers to aberration occurring when a short cycle follows a long one (long-short cycle sequence) (see Fig. 10-1).11,13 Aberrancy is caused by the physiological changes of the conduction system refractory periods associated with the R-R interval. Normally, the refractory period of the HPS lengthens as the heart rate slows and shortens as the heart rate increases, even when heart rate changes are abrupt. Thus, aberrant conduction can result when a short cycle follows a long R-R interval. In this scenario, the QRS complex that ends the long pause is conducted normally but creates a prolonged ERP of the bundle branches. If the next QRS complex occurs after a short coupling interval, it may be conducted aberrantly because one of the bundles is still refractory as a result of a lengthening of the refractory period (phase 3 block; Fig. 10-2).6,7
RBBB aberration is more common than LBBB in this setting because the RB has a longer ERP than the LB. The Ashman phenomenon can occur during second-degree AV block (see Fig. 9-9), but it is most common during atrial fibrillation (AF), whereby the irregularity of the ventricular response results in frequently occurring long-short cycle sequences.
The aberrancy can be present for one beat and have a morphology resembling a premature ventricular complex (PVC), or it can involve several sequential complexes, a finding suggesting ventricular tachycardia (VT). In the setting of aberrancy during AF, the long-short cycle sequence characteristic of the Ashman phenomenon may not be helpful in differentiating aberration from ventricular ectopy. Although a long cycle (pause) sets the stage for the Ashman phenomenon, it also tends to precipitate ventricular ectopy. Furthermore, concealed conduction occurs frequently during AF, and, therefore, it is never possible to know from the surface ECG exactly when a bundle branch is activated. Thus, if an aberrant beat does end a long-short cycle sequence during AF, it can be because of refractoriness of a bundle branch secondary to concealed conduction into it, rather than because of changes in the length of the ventricular cycle.8–1012
Nevertheless, there are several features of ventricular ectopy that can help distinguish a PVC from an aberrantly conducted or Ashman beat during AF. PVCs are usually followed by a longer R-R cycle, indicating the occurrence of a compensatory pause, the result of retrograde conduction into the AVN and anterograde block of the impulse originating in the atrium. A ventricular origin is also likely when there is a fixed coupling cycle between the normal and aberrant QRS complexes. The presence of long and identical R-R cycles after the aberrated beats and the absence of a long-short cycle sequence associated with the wide or aberrant QRS complex also suggest ventricular ectopy. Additionally, the absence of aberrancy, despite the presence of R-R cycle length (CL) combinations that are longer and shorter than those associated with the wide QRS complex, suggests ventricular ectopy. QRS morphology inconsistent with LBBB or RBBB aberrancy argues against aberration (Fig. 10-3).6,8–10,12
Aberration Caused by Heart Rate Acceleration
As the heart rate accelerates, the HPS refractory period shortens; normal conduction tends to be preserved because of this response. However, refractoriness of the HPS eventually reaches a critical value beyond which an increase in heart rate no longer abbreviates it; at this point, AV block may occur. Conversely, the refractory period lengthens as the heart rate slows. Acceleration-dependent BBB is a result of failure of the action potential of the bundle branches to shorten, or, paradoxically, the action potential lengthens in response to acceleration of the heart rate (Fig. 10-4).3,11 As noted, the ERP of the RB normally shortens at faster heart rates to a greater degree than that of the LB; this finding explains the more frequent RBBB aberration at longer CLs and LBBB aberration at shorter CLs.4,8,10
This form of aberration is a marker of some type of cardiac abnormality when it appears at relatively slow heart rates (less than 70 beats/min), displays LBBB (Fig. 10-5), appears after several cycles of accelerated but regular rate, or appears with gradual rather than abrupt acceleration of the heart rate and at CL shortening by less than 5 milliseconds. Because of the small changes in the duration of the CL that may initiate aberration (critical cycle), recognition of acceleration-dependent aberration may require a long record to document the gradual, and at times minimal, shortening of the R-R interval.8–10
With increasing heart rate or persistence of fast heart rate, acceleration-dependent aberration can occasionally disappear. The normalization of a previously aberrant QRS complex can be explained by a greater shortening of the ERP of the bundle branches than that of the AVN or by a time-dependent gradual shortening of the refractory period of the affected bundle branch (a phenomenon occasionally referred to as restitution).6
During slowing of the heart rate, intraventricular conduction often fails to normalize at the critical CL, and aberration persists at cycles longer than the critical cycle that initiated the aberration. Once acceleration-dependent BBB is established, the actual cycle for the blocked bundle does not begin until approximately halfway through the QRS complex because of concealed transseptal conduction (see later); thus, it is necessary for the heart rate to slow down more than would be expected to reestablish normal conduction.8–10
Phase 4 Block
Phase 4 block occurs when conduction of an impulse is blocked in tissues well after their normal refractory periods have ended.3,11 Phase 4 block is governed by the same physiological principles as those for phase 3 block. Membrane responsiveness is determined by the relationship of the membrane potential at excitation with the maximum height of phase 0. The availability of the Na+ channels is reduced at less negative membrane potentials, and activation at a reduced membrane potential is likely to cause aberration or block.6
The cause of membrane depolarization (i.e., reduction of membrane potential) in the setting of phase 4 block, however, is different from that in phase 3 block. Enhanced phase 4 depolarization within the bundle branches can be caused by enhanced automaticity or partial depolarization of injured myocardial tissue, or both. In this setting, the maximum diastolic potential immediately follows repolarization, from which point the membrane potential is steadily reduced (by the pacemaker current). This reduction, in turn, results in inactivation of some Na+ channels. Thus, an action potential initiated early in the cycle (immediately after repolarization) would have a steeper and higher phase 0 and consequently better conduction than would an action potential initiated later in the cycle when the membrane potential at the time of the stimulus is reduced, with resulting reductions in the velocity and height of phase 0 and slower conduction.8,10,12
Phase 4 aberration is one explanation for the development of aberration at the end of a long cycle. As a result of a gradual spontaneous depolarization made possible by a prolonged cycle, the cell is activated from a less negative potential, and the result is impaired conduction. This type of aberration is sometimes referred to as bradycardia-dependent BBB (Fig. 10-6).6 Importantly, the membrane potential has to depolarize significantly before conduction becomes impaired. Mild depolarization can actually improve conduction because the voltage is closer to threshold.
Phase 4 aberration would be expected in the setting of bradycardia or enhanced normal automaticity. However, despite the fact that bradycardia is common and cells with phase 4 depolarization are abundant, phase 4 block is not commonly seen; most reported cases are associated with structural heart disease. One explanation for this phenomenon is that in normal fibers, conduction is well maintained at membrane potentials more negative than −70 to −75 mV. Significant conduction disturbances are first manifested when the membrane potential is less negative than −70 mV at the time of stimulation; local block appears at −65 to −60 mV. Because the threshold potential for normal His-Purkinje fibers is −70 mV, spontaneous firing occurs before the membrane can actually be reduced to the potential necessary for conduction impairment or block. Phase 4 block is therefore pathological when it does occur, and it requires one or more of the following: (1) the presence of slow diastolic depolarization, which needs to be enhanced; (2) a decrease in excitability (a shift in threshold potential toward zero) so that, in the presence of significant bradycardia, sufficient time elapses before the impulse arrives, thus enabling the bundle branch fibers to reach a potential at which conduction is impaired; and (3) a deterioration in membrane responsiveness so that significant conduction impairment develops at −75 mV instead of −65 mV; this occurrence would also negate the necessity for such a long cycle before conduction fails.8,10,12
Bradycardia-dependent or phase 4 block almost always manifests an LBBB pattern, likely because the left ventricular (LV) conduction system is more susceptible to ischemic damage and has a higher rate of spontaneous phase 4 depolarization than the right ventricle (RV).12
Both tachycardia-dependent and bradycardia-dependent BBB can be seen in the same patient with an intermediate range of CLs associated with normal conduction. The prognosis of rate-dependent BBB largely depends on the presence and severity of the underlying heart disease. Its clinical implications are not clear, and it usually occurs in diseased tissue and in the setting of myocardial infarction (MI), especially inferior wall MI.6
Aberration Caused by Concealed Transseptal Conduction
Concealed transseptal conduction is the underlying mechanism of aberration occurring in several situations, including perpetuation of aberrant conduction during tachyarrhythmias, unexpected persistence of acceleration-dependent aberration, and alternation of aberration during atrial bigeminal rhythm.8–10,12,14,15
Perpetuation of Aberrant Conduction during Tachyarrhythmias
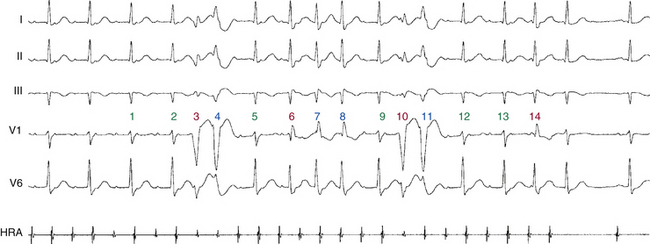
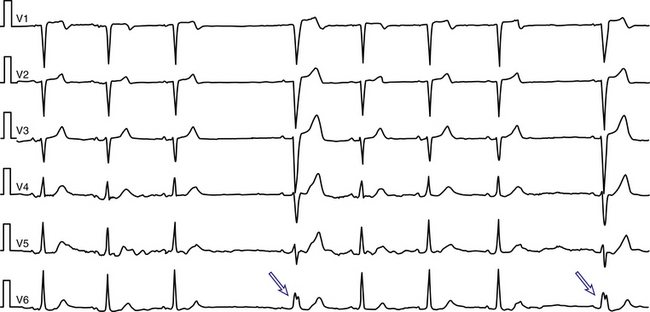
During a supraventricular tachycardia (SVT) with normal ventricular activation, a PVC originating from the RV can retrogradely activate the RB early, whereas retrograde activation of the LB occurs later, following transseptal conduction of the PVC. Consequently, although the RB ERP expires in time for the next SVT impulse, the LB remains refractory because its actual cycle began later than the RB. Therefore, the next SVT impulse traveling down the His bundle (HB) encounters an excitable RB and a refractory LB; thus, it propagates to the RV over the RB (with an LBBB pattern, phase 3 aberration). Conduction subsequently propagates from the RV across the septum to the LV. By this time, the distal LB has recovered, allowing for retrograde penetration of the LB by the SVT impulse propagating transseptally, thereby rendering the LB refractory to each subsequent SVT impulse (Fig. 10-7). This process is repeated, and the LBBB pattern continues until another well-timed PVC preexcites the LB (and either peels back or shortens its refractoriness), so that the next impulse from above finds the LB fully recovered (see Fig. 10-4).4,14,15
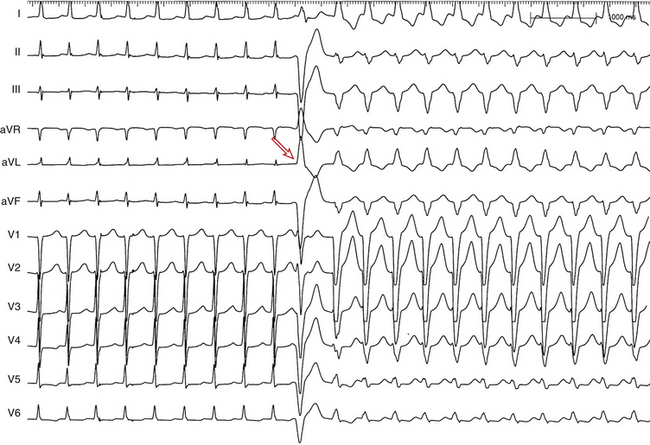
Unexpected Persistence of Acceleration-Dependent Aberration
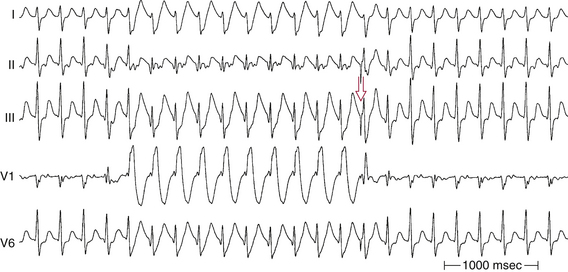
Acceleration-dependent BBB develops at a critical rate faster than the rate at which it disappears (Fig. 10-8). This paradox is most commonly ascribed to concealed conduction from the contralateral conducting bundle branch across the septum with delayed activation of the blocked bundle. Such concealed transseptal activation results in a bundle branch–to–bundle branch (RB-RB or LB-LB) interval shorter than the manifest R-R cycle. The reason is that the actual cycle for the blocked bundle does not begin until approximately halfway through the QRS complex, because it takes 60 to 100 milliseconds for the impulse to propagate down the RB and transseptally reach the blocked LB. Consequently, for normal conduction to resume, the cycle (R-R interval) during deceleration must be longer than the critical cycle during acceleration by at least 60 to 100 milliseconds.8–10,12,14,15
However, unexpected delay of normalization of conduction cannot always be explained by concealed conduction. Conduction sometimes normalizes with slowing of the heart rate, only to recur at cycles that are still longer than the critical cycle. Such a sequence excludes transseptal concealment as the mechanism of recurrence of the aberration. Similarly, when the discrepancy between the critical cycle and the cycle at which normalization finally occurs is longer than the expected transseptal activation time (approximately 60 milliseconds in the normal heart and 100 milliseconds in the diseased states), transseptal concealment alone cannot explain the delay (see Fig. 10-8). Fatigue and overdrive suppression have been suggested as possible mechanisms of the delayed normalization of conduction.8–10
Alternation of Aberration during Atrial Bigeminal Rhythm
A bigeminal rhythm can be caused by atrial bigeminy, 3:2 AV block, or atrial flutter with alternating 2:1 and 4:1 AV conduction. The alternation can be between a normal QRS complex and BBB or between RBBB and LBBB.
When alternation occurs between a normal QRS complex and RBBB during atrial bigeminy, the ERP of both RB and LB starts simultaneously following the normally conducted PAC, and the ERP of both branches is relatively short because of the preceding short cycle. After the pause, the sinus beat conducts normally, and the ERP of both bundle branches starts simultaneously but is relatively long because of the preceding long cycle. However, because the RB ERP is relatively longer than that of the LB, the next PAC encroaches on the RB refractoriness and conducts with an RBBB pattern (phase 3 block). Subsequently, that PAC is conducted down the LB and across the septum. The PAC activates the RB retrogradely after some delay (concealed transseptal conduction), so that the RB-RB interval (during the following pause) and the RB ERP become shorter. As a result, by the time the next PAC reaches the RB, the RB is fully recovered because of its abbreviated ERP (reflecting the shorter preceding RB-RB interval, which is shorter than the manifest R-R interval during the preceding pause), and normal conduction occurs (see Fig. 10-2).14,15
The same phenomenon (concealed transseptal conduction) explains alternating RBBB and LBBB during bigeminal rhythms. In the presence of RBBB, transseptal concealed conduction from the LB to the RB shortens the RB-RB interval relative to the now longer LB-LB interval. As a result, the ERP of the LB is longer and conduction in the LB fails. In the presence of a refractory LB, conduction propagates through the RB. The delayed transseptal activation of the LB shortens the LB-LB interval. The ERP of the RB is now relatively longer, because RB conduction is blocked.9,10,12
Chronic Bundle Branch Block
Anatomical Considerations
Normal ventricular activation requires the synchronized participation of the distal components of the specialized conduction system (i.e., the main bundle branches and their ramifications). An IVCD is the result of abnormal activation of the ventricles caused by conduction delay or block in one or more parts of the specialized conduction system. Abnormalities of local myocardial activation can further alter the specific pattern of venticular activation.1,6
Traditionally, three major fascicles are considered to be operative in normal persons: the RB, the left anterior fascicle (LAF), and the left posterior fascicle (LPF). An estimated 65% of individuals have a third fascicle of the LB, the left median fascicle (LMF). The HB divides at the junction of the fibrous and muscular boundaries of the intraventricular septum into the RB and LB. The RB is an anatomically compact unit that travels as the extension of the HB after the origin of the LB. The LB and its divisions are, unlike the RB, diffuse structures that fan out just beyond their origin. The LAF represents the superior (anterior) division of the LB, the LPF represents the inferior (posterior) division of the LB, and the LMF represents the septal (median) division of the LB.6
The bundle branches and fascicles consist of bundles of Purkinje cells covered by a dense sheath of connective tissue. The terminal Purkinje fibers connect the ends of the bundle branches to the ventricular myocardium. Purkinje fibers form interweaving networks on the endocardial surface of both ventricles and penetrate only the inner third of the endocardium, and they tend to be less concentrated at the base of the ventricle and at the papillary muscle tips. Purkinje cells are specialized to conduct rapidly, at 1 to 3 m/sec, because phase 0 of the action potential is dependent on the rapid inward Na+ current (INa). This characteristic results in almost simultaneous depolarization of the terminal HPS and propagation of the cardiac impulse to the entire RV and LV endocardium.6
Characteristics of the Right Bundle
The RB is vulnerable to stretch and trauma for two thirds of its course when it travels subendocardially. Chronic RBBB pattern can result from three levels of conduction delay in the RV: proximal, distal, or terminal.3 Proximal RBBB is the most common site of conduction delay. Distal RBBB occurs at the level of the moderator band, and it is an unusual site of conduction delay unless there has been transection of the moderator band during surgery. Terminal RBBB involves the distal conduction system of the RB or, more likely, the ventricular muscle itself, and it can be produced by ventriculotomy or transatrial resection of parietal bands in repair of tetralogy of Fallot. In addition, RBBB can be induced by events in the HB, because certain fibers of the HB are organized longitudinally and predestined to activate only one fascicle or bundle branch. Disease in these areas can result in activation that is asynchronous from that in the rest of the infranodal conducting system, possibly with resulting bundle branch or fascicular block.
Characteristics of the Left Bundle and Its Fascicles
The predivisional portion of the LB penetrates the membranous portion of the interventricular septum under the aortic ring and then divides into three discrete branches: the LAF, the LPF, and the LMF.3 The LAF crosses the anterobasal LV region toward the anterior papillary muscle and terminates in the Purkinje system of the anterolateral wall of the LV. The LPF appears as an extension of the main LB and is large in its initial course. It then fans out extensively toward the posterior papillary muscle and terminates in the Purkinje system of the posteroinferior wall of the LV. The LMF runs to the interventricular septum; it arises in most cases from the LPF, less frequently from the LAF, or from both, and in a few cases it has an independent origin from the central part of the main LB at the site of its bifurcation.16
The LAF can be injured by diseases that involve primarily the LV basal septum, the anterior half of the ventricular septum, and the anterolateral LV wall. In fact, isolated LAF block is the most common type of IVCD seen in acute anterior MI. Other disorders and situations that can also cause LAF block include hypertension, cardiomyopathies, aortic valve disease, Lev and Lenègre diseases, spontaneous and surgical closure of a ventricular septal defect, and other surgical procedures.16–18
In contrast, the LPF is the least vulnerable segment of the whole system because it is short and wide and is located in the inflow tract of the LV, which is a less turbulent region than the outflow tract. Additionally, the LPF has a dual blood supply, from the anterior and posterior descending coronary arteries, and it is not related to structures that are so potentially dangerous.16 LPF block is a rare finding, and rather nonspecific for cardiac disease. LPF block is almost always associated with RBBB.
Site of Block
Despite ECG anatomical correlates, the site of block producing BBB patterns is not certain in all cases. Data suggest that fibers to the RV and LV are already predestined within the HB and that lesions in the HB can produce characteristic BBB patterns. Longitudinal dissociation with asynchronous conduction in the HB can give rise to abnormal patterns of ventricular activation; hence, the conduction problem may not necessarily lie in the individual bundle branch. Moreover, it is not uncommon for intra-Hisian disease to be accompanied by BBB (especially LBBB).3 Pacing distal to the site of block can normalize the QRS. Furthermore, the problem may not be actual block, because conduction delay within the bundle in the range of 10 milliseconds can give rise to an ECG pattern of BBB.
Clinical Relevance
Bifascicular block (especially RBBB and LAF block) is the most common ECG pattern preceding complete heart block in adults. Other forms of IVCD precede the bulk of the remaining cases of complete intra-Hisian and infra-Hisian AV block. The incidence of progression to complete AV block is approximately 2% in asymptomatic patients with an IVCD and approximately 6% in patients with an IVCD and neurological symptoms (e.g., syncope).19,20
Patients with BBB have an unusually high incidence of cardiac disease and sudden cardiac death. The highest incidence of sudden cardiac death is among patients with LBBB and cardiac disease.19–22 However, most sudden cardiac deaths are caused by VT or ventricular fibrillation, do not seem to be related to AV block, and are not prevented by pacemakers (although pacing can potentially relieve symptoms such as syncope). Complete electrophysiological (EP) testing and ventricular stimulation are therefore necessary in patients with syncope and BBB because VT can be found in up to 30% to 50%. Although the poor prognosis associated with BBB is related to myocardial dysfunction, heart failure, and ventricular fibrillation rather than heart block, symptoms such as syncope are often related to heart block.23–25
RBBB is a common finding in the general population. In patients without evidence of structural heart disease, RBBB has no prognostic significance. However, new-onset RBBB does predict a higher rate of coronary artery disease, heart failure, and cardiovascular mortality. When cardiac disease is present, the coexistence of RBBB suggests advanced disease. RBBB is an independent predictor of all-cause mortality in patients with known or suspected coronary heart disease, and in the setting of an acute MI, RBBB is associated with a significant increase in mortality.26
The prevalence of LBBB in the general population has been reported to vary considerably according to population size and sampling criteria; it ranges from 0.1% to 0.8%. The presence of isolated LBBB has no adverse prognostic significance. In patients with LBBB associated with ischemic heart disease, hypertension, or cardiomyopathy, the prognosis depends on the severity of the underlying heart disease. Nevertheless, among patients with acute MI, cardiomyopathy, and heart failure, the presence of LBBB is associated with a worse prognosis. Because the presence of LBBB can represent the clinical onset of LV structural disease such as dilated cardiomyopathy or an infective, hypertensive, or valvular heart disease, the finding of LBBB on resting ECG requires further evaluation with echocardiogram and Holter monitoring for assessment of LV function, as well as identifying both advanced degrees of AV block and heart disease-related tachyarrhythmias. Evaluation for ischemic heart disease can be necessary based on the presence of risk factors and typical symptoms.26
The prevalence of LAF block in the general population ranges from 0.9% to 6.2%. Isolated LAF block does not itself imply a risk factor for cardiac mortality or morbidity, and in a healthy population it should be regarded as an incidental ECG finding.16 The prognosis of LAF block is primarily related to the underlying heart disease. LAF block in the setting of acute MI is probably associated with increased mortality.
As noted, LPF block is a rare finding and rather nonspecific for cardiac disease. LPF block is almost always associated with RBBB, in that LPF block and RBBB share cause, pathogenesis, and prognosis. The combination of LPF block and RBBB in acute MI is associated with a high mortality rate (80% to 87%) during the first weeks after the coronary event. Similarly, the risk of progression toward complete AV block (a form of trifascicular block) is also considerable (42%), and approximately 75% of these patients die of pump failure.16
Electrocardiographic Features
Bundle Branch Block
The ECG criteria for different types of fascicular blocks and BBB are listed in Table 10-1. The ECG pattern of BBB can represent complete block or conduction delay (relative to the other fascicles) that produces asynchronous ventricular activation without necessarily implying complete failure of conduction in the diseased fascicle. Therefore, an ECG pattern of complete BBB can have varying degrees or alternate with contralateral complete BBB pattern, phenomena explained by delay, rather than complete block, as the underlying pathophysiologic feature of the ECG pattern.3,20,27
TABLE 10-1 ECG Criteria for Fascicular and Bundle Branch Block
• QRS duration ≥120 msec in adults
• Broad notched or slurred R wave in leads I, aVL, V5, and V6 and an occasional RS pattern in V5 and V6 attributed to displaced transition of QRS complex
• Absent q waves in leads I, V5, and V6, but in the lead aVL, a narrow q wave may be present in the absence of myocardial disease
• R wave peak time >60 msec in leads V5 and V6 but normal in leads V1, V2, and V3, when small initial r waves can be discerned in the above leads
• ST and T waves usually opposite in direction to QRS
• Positive T wave in leads with upright QRS may be normal (positive concordance)
• Depressed ST segment and/or negative T wave in leads with negative QRS (negative concordance) are abnormal
• Appearance of LBBB may change the mean QRS axis in the frontal plane to the right, to the left, or superiorly, in some cases in a rate-dependent manner
BBB leads to prolongation of the QRS duration and sometimes to alterations in the QRS vector. The degree of prolongation of the QRS duration depends on the severity of the impairment. With complete BBB, the QRS is 120 milliseconds or longer in duration; with incomplete BBB, the QRS duration is 100 to 120 milliseconds. The QRS vector in BBB is generally oriented in the direction of the myocardial region in which depolarization is delayed.1
Right Bundle Branch Block
Development of RBBB alters the activation sequence of the RV but not the LV. Because the LB is not affected, the initial septal activation (the initial 30 milliseconds of the QRS complex), which depends on the LB, remains normal, occurring from left to right, and results in septal q waves in leads I, aVL, and V6 and r waves in leads V1, V2, and aVR.26 Thus, the Q wave of a prior MI remains unchanged. Septal activation is followed by LV activation (within the subsequent 40 to 60 milliseconds), occurring over the LB in a leftward and posterior vector and resulting in R waves in the leftward leads (I, aVL, and V6), as well as s (or S) waves in the anterior precordial leads (V1 and V2). This appearance is usually similar to that in normal subjects.
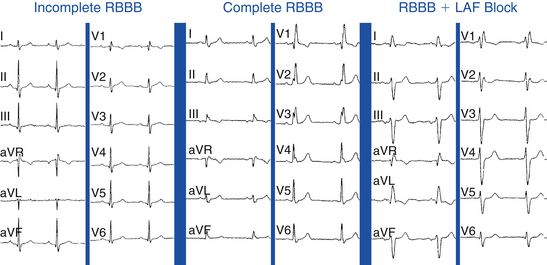
The asynchronous depolarization caused by RBBB is primarily manifested in the later portion of the QRS, at 80 milliseconds and beyond. During this time, RV activation spreads slowly by conduction through working muscle fibers rather than the specialized Purkinje system, and it occurs predominantly after activation of the LV has completed. The forces generated by the late, unopposed RV free wall activation result in a terminal rightward and anterior positivity, manifesting as a second positive deflection that can be small (r′) or large (R′) in the anterior precordial leads (V1 and V2) and S waves in the leftward leads (I, aVL, and V6; Fig. 10-9).3,26 The QRS axis is unaffected by RBBB; left or right axis deviation can indicate concurrent LAF or LPF block, respectively (see Fig. 10-9).
The time interval necessary for full depolarization of the ventricular free wall (from the endocardium to the epicardium) beneath any given ECG electrode corresponds to the interval from the beginning of the QRS complex to the time of initial downstroke of the R wave after it has peaked (or to the time of initial upstroke of the S wave after it has reached its nadir). This interval is termed R wave peak time (in preference to the term intrinsicoid deflection). In the right precordial leads, the upper limit of normal for R wave peak time is 35 milliseconds, whereas in the left precordial leads, it is 45 milliseconds. In RBBB, the R wave peak time is delayed in the right precordial leads (more than 50 milliseconds).1
Incomplete Right Bundle Branch Block
An incomplete RBBB can result from lesser degrees of conduction delay in the RB. The ECG pattern of incomplete RBBB is similar to that of complete RBBB, except that the QRS duration is between 110 and 120 milliseconds (see Fig. 10-9). An RBBB pattern with a QRS duration shorter than 100 milliseconds can be a normal variant, presumably reflecting a slight delay in the terminal posterobasal forces in some individuals.1
The ECG pattern of incomplete or complete RBBB in association with a distinct ST segment elevation in the right precordial leads can be observed in the Brugada syndrome. The Brugada ECG pattern, however, is characterized by the absence of a wide terminal S wave in the left lateral leads (I, aVL, V5, V6) and no broad terminal R wave in aVR, findings indicating that true RBBB is not present (see Fig. 31-5). Additionally, the ECG manifestations of the Brugada syndrome are often dynamic or concealed and can be unmasked or modulated primarily by sodium channel blockers but also during a febrile state or with vagotonic agents.28
Left Bundle Branch Block
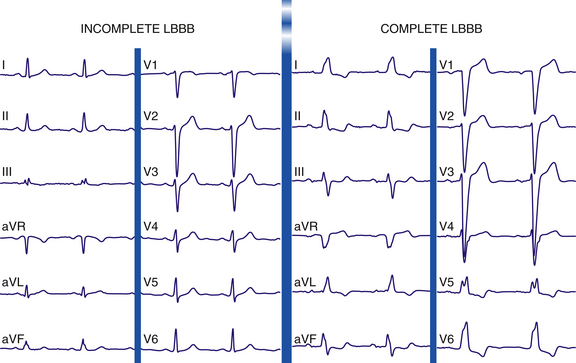
The normal sequence of ventricular activation is altered dramatically in LBBB. Complete LBBB results in delayed and abnormal activation and diffuse slowing of conduction throughout the LV. Activation of the LV originates from the RB in a right to left direction, in contrast to the normal situation in which the first part of the LV myocardium to be activated is the septum in a left to right direction via a small septal branch of the LB. Thus, LBBB results in reversal of the direction of the initial septal activation sequence (within the initial 30 milliseconds of the QRS complex), with the activation traveling from right to left and from apex to base and to the RV apex and free wall. RV activation is typically completed within the first 45 milliseconds of the onset of the QRS, before the onset of LV activation. However, because the septum is a larger structure than the RV free wall, septal activation predominates, eliciting a leftward and usually anterior vector and resulting in loss of the normal small q waves and initiation of a wide, slurred R wave in leads I, aVL, and V6 and an rS or QS pattern in lead V1 (Fig. 10-10). Thus, Q waves of a prior MI may disappear, and new Q waves can emerge.
Following septal activation, LV activation (starting as late as 44 to 58 milliseconds into the QRS) spreads slowly by conduction through working muscle fibers rather than the specialized conduction system, with spatial vectors oriented to the left and posteriorly because the LV is a leftward and posterior structure. As a consequence, the delayed LV activation (unopposed by the now completed RV activation) produces large, broad, and notched or slurred R waves (without q or s waves) in the leftward leads (I, aVL, and V6), with delayed R wave peak time in the left precordial leads (more than 60 milliseconds). The slowing and notching of the mid-QRS portion are caused by slow transseptal conduction. The terminal activation vector results from depolarization of the anterolateral LV wall that produces a small vector that is also directed to the left and posteriorly.1,3,25
Incomplete Left Bundle Branch Block
Incomplete LBBB can result from lesser degrees of conduction delay in the LB. Although LV activation begins abnormally on the right side of the septum (as in complete LBBB), much of the subsequent LV activation occurs via the normal conduction system. Incomplete LBBB is characterized by the following: (1) QRS duration of 110 to 119 milliseconds; (2) presence of an LV hypertrophy pattern; (3) R wave peak time longer than 60 milliseconds in leads V4, V5, and V6; and (4) absence of q wave in leads I, V5, and V6 and frequent replacement by a slurred initial upstroke (pseudo-delta wave) (see Fig. 10-10). This entity can bear an ECG resemblance to a Wolff-Parkinson-White ECG pattern secondary to the delayed upstroke of the R wave, although the PR interval is usually short in Wolff-Parkinson-White syndrome, whereas it should be normal in cases of incomplete LBBB.1
Fascicular Block
Hemiblocks in the LB system affect the LAF, LPF, or LMF. Fascicular block generally does not substantially prolong QRS duration, but alters only the sequence of LV activation. The primary ECG change is a shift in the frontal plane QRS axis because the conduction disturbance primarily involves the early phases of activation. The QRS duration is usually less than 100 milliseconds (unless complicated by BBB or hypertrophy), although some investigators allow a QRS duration of up to 120 milliseconds, or 20 milliseconds higher than the previous baseline.16–18
Left Anterior Fascicular Block
The LAF normally initiates activation in the upper part of the septum, the anterolateral LV free wall, and the left anterior papillary muscle. Delayed activation of these regions secondary to damage to the LAF causes unopposed activation wavefronts by the LPF and LMF early during the QRS complex and unopposed anterosuperior forces late during ventricular activation. All these changes occur with a QRS that widens no more than 20 milliseconds in pure and uncomplicated LAF block.16
As a consequence, the initial (first 20 milliseconds) QRS vector is normal in time, but it has an abnormal direction. Rather than proceeding superiorly and to the left, the QRS vector depicts an inferior and rightward shift in the frontal plane (frontal plane axis more than +120 degrees) that produces a small, sharp r wave in the inferior leads (II, III, and aVF) and a small q wave in leads I, aVL, V5, and V6. Additionally, the inferior and rightward shift in the initial QRS forces can occasionally result in a small, sharp q wave in leads V2 and V3 (mimicking old anteroseptal infarction patterns) when the electrodes are placed at the normal level and, in almost all cases, when they are placed in a higher position.16
During the midportion of the QRS, the main forces of LV activation are oriented superiorly and to the left (frontal plane axis more than –45 or –60 degrees), with a wide-open counterclockwise-rotated loop in the frontal plane caused by the delayed activation of the high lateral wall, which is normally activated by the LAF. This results in deep S waves in leads II, III, and aVF (S wave deeper in lead III than in lead II), and R waves in aVR and aVL. The net effect is an rS pattern in leads II, III, and aVF and a qR or R pattern in leads I, aVL, V5, and V6. Additionally, as a result of the superiorly directed forces, deeper S waves are recorded in leads V5 and V6; these waves tend to disappear when the electrodes are placed above the normal level and are deeper when the electrodes are placed below the normal level.1,16–18
Left Posterior Fascicular Block
The early unopposed activation of the anterolateral wall of the LV by the normally conducting LAF and LMF causes the initial forces to be oriented superiorly and to the left, producing initial small r waves in leads I, aVL, V1, and V6 and small q waves in leads II, III, and aVF. However, the main and terminal forces of the QRS are directed posteriorly, inferiorly, and to the right with a wide-open clockwise-rotated loop, because of the late unopposed activation of the areas normally activated by the LPF (the inferoposterior LV free wall). This is responsible for the characteristic rightward frontal plane axis of +120 to +180 degrees. As a result, there is a qR morphology in leads II, III, and aVF and an rS morphology in leads I and aVL. In fact, the ECG pattern of LPF block is the exact mirror image of LAF block in the standard and unipolar leads. LPF block is almost always associated with RBBB. Isolated LPF block is extremely rare, and a firm diagnosis requires that other causes of right axis deviation have been excluded.1,16
Other Types
Bifascicular Blocks
Bifascicular block refers to different combinations of fascicular block and BBB. Examples of bifascicular block include RBBB with LAF block (most common; see Fig. 10-9), RBBB with LPF block, and LAF block plus LPF block (which manifests as LBBB).
Trifascicular Blocks
The combination of bifascicular block (RBBB plus LAF block, RBBB plus LPF block, or LBBB) with first-degree AV block on the surface ECG (Fig. 10-11) cannot be considered as trifascicular block because the site of AV block can be in the AVN or HPS, and such a pattern can reflect slow conduction in the AVN with concomitant bifascicular block. In such circumstances, the PR interval on the ECG does not appear to be helpful in selecting those individuals with a prolonged His bundle–ventricular (HV) interval because a normal PR interval can easily conceal a significantly prolonged HV interval and a prolonged PR interval can be caused by a prolonged atrial–His bundle (AH) interval. However, two criteria can be of value: (1) a short PR interval (less than 160 milliseconds) makes a markedly prolonged HV interval (i.e., more than 100 milliseconds) unlikely, and (2) a markedly prolonged PR interval (more than 300 milliseconds) almost always indicates that at least some of the abnormality, if not all, is caused by AVN conduction.
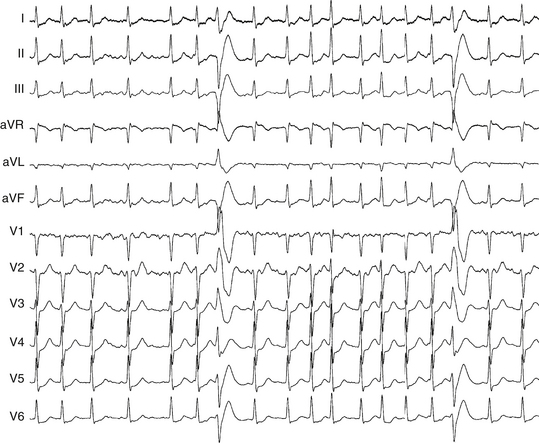
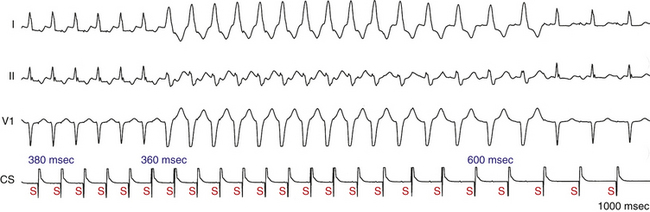
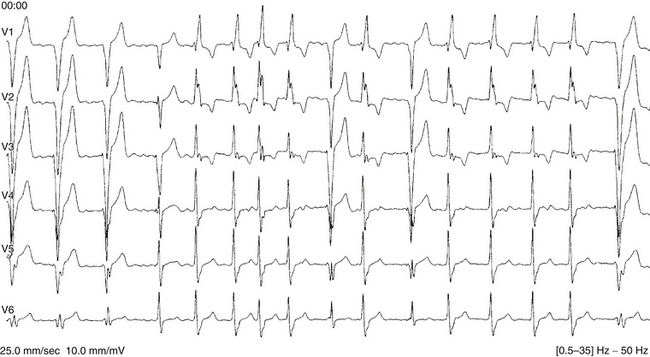
Alternating Bundle Branch Block
Alternating RBBB and LBBB is manifested by QRS complexes with LBBB morphology coexisting with complexes with RBBB morphology (Fig. 10-12). Often, every other complex is an RBBB or LBBB. Spontaneous alternating BBB, especially when associated with a change in the PR interval, represents the most common ominous sign for progression to AV block (Fig. 10-13). Beat-to-beat alternation is the most concerning, whereas a change in BBB noted on different days is less ominous.
This phenomenon implies instability of the HPS and a disease process involving the HB or bundle branches. In most patients with diffuse HPS disease, delay or block in one of the bundle branches consistently predominates, and alternating BBB is uncommon. The HV interval in alternating BBB is almost universally prolonged and typically varies with the change in BBB. This group has the highest incidence of HV interval exceeding 100 milliseconds.3
Intermittent Bundle Branch Block
Intermittent BBB, either right or left, is diagnosed on the surface ECG when there are occasional QRS complexes with RBBB or LBBB morphology, interspersed with QRS complexes that have a normal morphology. Most often, the intermittent BBB is rate-related; thus, the R-R intervals of the QRS complexes manifesting the BBB are shorter when compared with the intervals of the normal QRS complexes. In other cases, there is no rate-related change in the QRS intervals, but the occurrence of the BBB is a random or sporadic event.
Electrophysiological Testing
Baseline Intervals
His Bundle–Ventricular Interval
The use of multipolar catheters to record distal, middle, and proximal HB potentials can help localize the site of conduction delay or block within the HB. The value of prolonged HV interval in predicting the risk of AV block is controversial. Studies have shown that an HV interval longer than 70 milliseconds predicts a higher risk of AV block, especially in symptomatic patients. The risk of AV block, however, even in the high-risk group, approaches at most only 6% per year. An HV interval exceeding 100 milliseconds identifies a group of patients at a very high risk of AV block (25% over 22 months).3
In the presence of RBBB with or without additional fascicular block, the HV interval should be normal as long as conduction is unimpaired in the remaining fascicle (Fig. 10-14). However, 50% of patients with RBBB plus LAF block and 75% of those with LBBB have a prolonged HV interval; thus, a prolonged HV interval itself is nonspecific as a predictor of AV block.
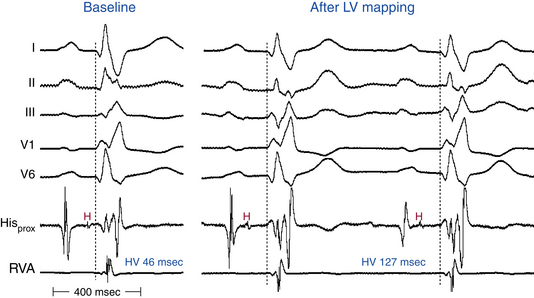
In the presence of LBBB, and in the absence of a change in the HB-RB and HB-RV intervals, the HV interval may be slightly prolonged because the earliest site of depolarization on the left side of the septum via the LB precedes activation of the RB by 5 to 15 milliseconds. Therefore, an HV interval of up to 60 milliseconds in the presence of LBBB should be considered normal and does not by itself indicate associated RB or HB disease (Fig. 10-15).3
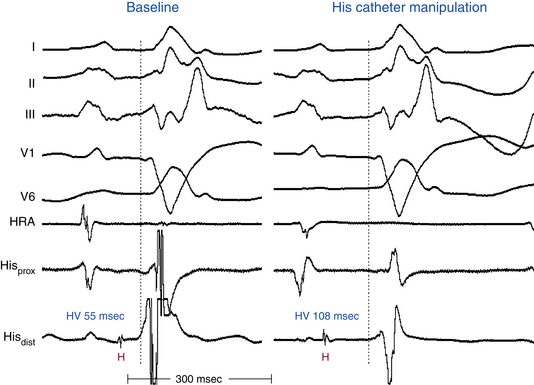
Catheter manipulation in the LV or RV can inadvertently produce prolongation of the HV interval and varying degrees of AV block or BBB, or both, usually temporarily (see Figs. 10-14 and 10-15). Complete heart block can occur during right-sided heart catheterization in a patient with preexisting LBBB or during LV catheterization (LV angiography or ablation procedures) in a patient with preexisting RBBB. Among patients with chronic LBBB, the presence of an r wave of more than 1 mm in lead V1 appears to identify a subgroup at lower risk for complete AV block in response to catheter trauma to the RB. This ECG sign likely suggests intact conduction over the septal fibers of the LBB.29
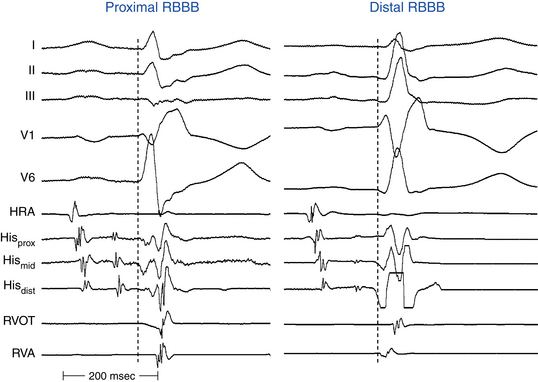
Localization of the Site of Block in Right Bundle Branch Block
Measurement of activation times at different areas of the RV has been used to assess conduction properties of the RB indirectly. In the presence of RBBB, measuring the HV interval, HB–proximal RB interval, ventricular-RV (V-RV) apex interval (from onset of the surface QRS to RV apical local activation), and V-RVOT interval can help localize the site of block (Fig. 10-16). In the presence of RBBB, a normal HV interval and a V-RV apex interval of less than 30 milliseconds suggest terminal RBBB. On the other hand, a normal HV interval and a V-RV apex interval of less than 30 milliseconds, with delayed activation in the anterior wall (prolonged V-RVOT interval), suggest distal RBBB. With proximal RBBB, the V-RV apex interval is longer than 30 milliseconds.
Localization of the Site of Block in Left Bundle Branch Block
A common observation in patients with LBBB is a delay in transseptal activation. The pattern in which the LV is activated initially (i.e., the site of breakthrough), as well as the remainder of the LV endocardium and transmural activation, depends critically on the nature of the underlying heart disease; the bizarreness of the QRS width and morphology is more a reflection of the underlying LV disease than of the primary conduction disturbance.3 Patients with normal hearts and those with cardiomyopathy appear to have an intact distal conducting system and, hence, early engagement and rapid spread throughout the rest of the intramural myocardium. In patients with large infarcts, the bulk of their distal specialized conducting system has been destroyed; consequently, endocardial activation occurs via muscle-to-muscle conduction and thus is much slower.
Diagnostic Maneuvers
Atrial Pacing
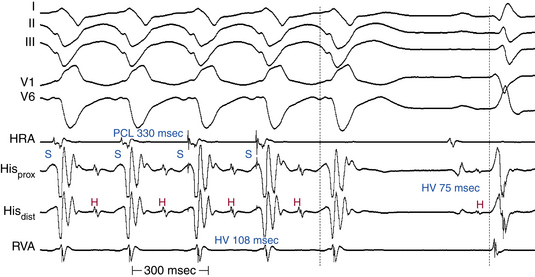
During incremental-rate atrial pacing, normal shortening of the HPS refractoriness at decreased pacing CLs facilitates 1:1 AV conduction. The development of second- or third-degree AV block within the HPS (in the absence of changing AH intervals) at pacing CLs longer than 400 milliseconds is abnormal and suggests a high risk (50%) for progression to high-grade AV block (Fig. 10-17).
His Bundle Pacing
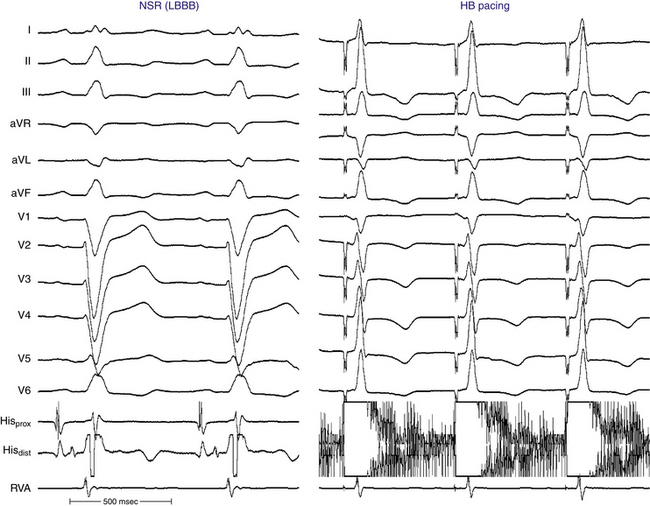
Normalization of the QRS during HB pacing is suggestive of HB disease proximal to the pacing site (Fig. 10-18).
Ventricular Pacing
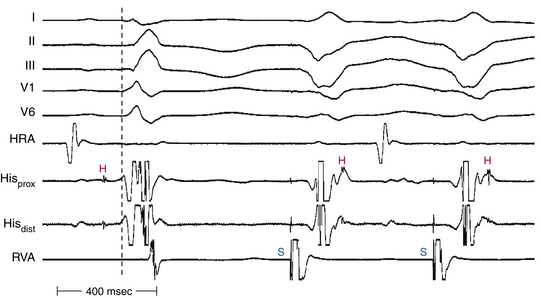
Assessment of retrograde VH (or VA) conduction is not useful as an indicator of anterograde HPS reserve. Anterograde RBBB is usually proximal, whereas during RV stimulation, block usually occurs at the gate, which is at the distal HPS–myocardial junction. Bidirectional RBBB is characterized by His activation following local ventricular activation in the HB recording, caused by propagation from the RV pacing site across the interventricular septum to the LB and then to the HB, rather than the more direct retrograde route up the RB (Fig. 10-19).
Role of Electrophysiological Testing
The guidelines for pacing in chronic BBB are listed in Table 10-2. EP testing is used to obtain information that could predict which patients are at risk for syncope, AV block, or sudden cardiac death (Table 10-3).3,18 Complete EP testing with programmed atrial and ventricular stimulation is necessary in patients with syncope and BBB because VT can be induced in 30% to 50% of such patients. Pacemaker therapy clearly can help prevent syncope in those patients in whom that event most likely was caused by transient bradyarrhythmia, but it has not been shown to prevent sudden cardiac death or reduce cardiac mortality.3,18,30
TABLE 10-2 Guidelines for Permanent Pacing in Chronic Bifascicular and Trifascicular Block
| Class I |
AV = atrioventricular; EP = electrophysiological; HV = His bundle–ventricular.
TABLE 10-3 Role of Electrophysiological Testing in Patients with Bundle Branch Block
| EP Indicators of HPS Disease |
| EP Indicators of High Risk for AV Block in Patients with IVCD |
| Recommendations for Pacing in Patients with IVCD |
|
• LBBB, RBBB, IVCD, RBBB + LAF block, or RBBB + LPF block associated with the following: HV interval >100 msec or HV interval = 60-99 msec in the presence of unexplained syncope or presyncope • Infra-Hisian block at atrial pacing CL ≥400 msec (regardless of HV interval or presence of symptoms) • Alternating BBB (regardless of HV interval or presence of symptoms) |
AV = atrioventricular; BBB = bundle branch block; CL = cycle length; EP = electrophysiological; ERP = effective refractory period; HPS = His-Purkinje system; HV = His bundle–ventricular; IVCD = intraventricular conduction defect; LAF = left anterior fascicle; LBBB = left bundle branch block; LPF = left posterior fascicle; RBBB = right bundle branch block.
1. Surawicz B., Childers R., Deal B.J., et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram. Part III. Intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53:976-981.
2. Chilson D.A., Zipes D.P., Heger J.J., et al. Functional bundle branch block: discordant response of right and left bundle branches to changes in heart rate. Am J Cardiol. 1984;54:313-316.
3. Josephson M.E. Intraventricular conduction disturbances. In: Josephson M.E., editor. Clinical cardiac electrophysiology. ed 4. Philadelphia: Lippincott Williams & Wilkins; 2008:114-144.
4. Eckardt L., Breithardt G., Kirchhof P. Approach to wide complex tachycardias in patients without structural heart disease. Heart. 2006;92:704-711.
5. Pavri B.B., Kocovic D.Z., Hanna M. Long-short RR intervals and the right bundle branch. J Cardiovasc Electrophysiol. 1999;10:121-123.
6. Rubart M., Zipes D.P. Genesis of cardiac arrhythmias: electrophysiological considerations. In: Zipes D.P., Libby P., Bonow R., Braunwald E., editors. Braunwald’s heart disease: a textbook of cardiovascular medicine. ed 7. Philadelphia: Saunders; 2004:653-688.
7. Suyama A.C., Sunagawa K., Sugimachi M., et al. Differentiation between aberrant ventricular conduction and ventricular ectopy in atrial fibrillation using RR interval scattergram. Circulation. 1993;88:2307-2314.
8. Josephson M.E. Miscellaneous phenomena related to atrioventricular conduction. In: Josephson M.E., editor. Clinical cardiac electrophysiology. ed 4. Philadelphia: Lippincott Williams & Wilkins; 2008:145-159.
9. Kilborn M.F. Electrocardiographic manifestations of supernormal conduction, concealed conduction, and exit block. In: Zipes D.P., Jalife J., editors. Cardiac electrophysiology: from cell to bedside. ed 4. Philadelphia: Saunders; 2004:733-738.
10. Wellens H.J., Boss D.L., Farre J., Brugada P. Functional bundle branch block during supraventricular tachycardia in man: observations on mechanisms and their incidence. In: Zipes D.P., Jalife J., editors. Cardiac electrophysiology and arrhythmias. New York: Grune & Stratton; 1995:435-441.
11. Fisch C., Knoebel S. Wolff-Parkinson-White syndrome. In: Fisch C., Knoebel S., editors. Electrocardiography of clinical arrhythmias. Armonk, NY: Futura; 2000:293-314.
12. Fisch C., Knoebel S. Atrioventricular and ventriculoatrial conduction and blocks, gap, and overdrive suppression. In: Fisch C., Knoebel S., editors. Electrocardiography of clinical arrhythmias. Armonk, NY: Futura; 2000:315-344.
13. Gouaux J.L., Ashman R. Auricular fibrillation with aberration simulating ventricular paroxysmal tachycardia. Am Heart J. 1947;34:366-373.
14. Fisch C., Zipes D.P., McHenry P.L. Electrocardiographic manifestations of concealed junctional ectopic impulses. Circulation. 1976;53:217-223.
15. Fisch C., Knoebel S.B. Concealed conduction. In: Fisch C., Knoebel S., editors. Electrocardiography of clinical arrhythmias. Armonk, NY: Futura; 2000:153-172.
16. Elizari M.V., Acunzo R.S., Ferreiro M. Hemiblocks revisited. Circulation. 2007;115:1154-1163.
17. Biagini E., Elhendy A., Schinkel A.F., et al. Prognostic significance of left anterior hemiblock in patients with suspected coronary artery disease. J Am Coll Cardiol. 2005;46:858-863.
18. MacAlpin R.N. In search of left septal fascicular block. Am Heart J. 2002;144:948-956.
19. Imanishi R., Seto S., Ichimaru S., et al. Prognostic significance of incident complete left bundle branch block observed over a 40-year period. Am J Cardiol. 2006;98:644-648.
20. McCullough P.A., Hassan S.A., Pallekonda V., et al. Bundle branch block patterns, age, renal dysfunction, and heart failure mortality. Int J Cardiol. 2005;102:303-308.
21. Baldasseroni S., Opasich C., Gorini M., et al. Left bundle-branch block is associated with increased 1-year sudden and total mortality rate in 5517 outpatients with congestive heart failure: a report from the Italian network on congestive heart failure. Am Heart J. 2002;143:398-405.
22. Auricchio A., Fantoni C., Regoli F., et al. Characterization of left ventricular activation in patients with heart failure and left bundle-branch block. Circulation. 2004;109:1133-1139.
23. Stenestrand U., Tabrizi F., Lindback J., et al. Comorbidity and myocardial dysfunction are the main explanations for the higher 1-year mortality in acute myocardial infarction with left bundle-branch block. Circulation. 2004;110:1896-1902.
24. Wong C.K., Stewart R.A., Gao W., et al. Prognostic differences between different types of bundle branch block during the early phase of acute myocardial infarction: insights from the Hirulog and Early Reperfusion or Occlusion (HERO)-2 trial. Eur Heart J. 2006;27:21-28.
25. Francia P., Balla C., Paneni F., Volpe M. Left bundle-branch block: pathophysiology, prognosis, and clinical management. Clin Cardiol. 2007;30:110-115.
26. Agarwal A.K., Venugopalan P. Right bundle branch block: varying electrocardiographic patterns. Aetiological correlation, mechanisms and electrophysiology. Int J Cardiol. 1999;71:33-39.
27. Rogers R.L., Mitarai M., Mattu A. Intraventricular conduction abnormalities. Emerg Med Clin North Am. 2006;24:41-51. vi
28. Kamakura S., Ohe T., Nakazawa K., et al. Long-term prognosis of probands with Brugada-pattern ST-elevation in leads V1-V3. Circ Arrhythm Electrophysiol. 2009;2:495-503.
29. Padanilam B.J., Morris K.E., Olson J.A., et al. The surface electrocardiogram predicts risk of heart block during right heart catheterization in patients with preexisting left bundle branch block: implications for the definition of complete left bundle branch block. J Cardiovasc Electrophysiol. 2010;21:781-785.
30. Epstein A.E., DiMarco J.P., Ellenbogen K.A., et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: executive summary. Heart Rhythm. 2008;5:934-955.