Chapter 39 Intrahepatic stones
Overview
Although hepatolithiasis is a rare disease prevalent only in East Asia—including China, Hong Kong, Korea, and Japan—it has increasingly been encountered in Western countries (Herman et al, 2005; Vetrone et al, 2006; Catena et al, 2006; Nuzzo et al, 2008; Al-Sukhni et al, 2008). Patients with intrahepatic stones follow a specific clinical course characterized by recurrent cholangitis with recurrent stones or liver abscess, which may cause pyogenic sepsis or liver dysfunction and result in biliary cirrhosis. Advances in hepatobiliary diagnostic modalities have allowed more precise delineation of the biliary anatomy and extent of disease, and the application of modern treatment modalities has resulted in a longer stone-free period over the past 2 decades. Intrahepatic stones include brown pigment stones (calcium bilirubinate stones), cholesterol stones, and other rare types. The majority of intrahepatic stones are brown pigment stones. Some centers, not only in Asia but also in Western countries, have reported increasing numbers of patients with primary cholesterol stones originating in the liver.
Definition and Surgical Anatomy
Intrahepatic stones are defined as concretions existing in the intrahepatic bile ducts. Although the confluence of the hepatic ducts is situated outside of the parenchyma of the liver, for convenience an intrahepatic bile duct is defined as any bile duct proximal to the confluence of the right and left hepatic ducts. Established by the Japanese Ministry of Health, Labor, and Welfare, the Japan Research Group for the Study of Hepatolithiasis proposed a classification of patients with hepatolithiasis into two groups: type I patients have stones only in the intrahepatic bile duct, and type IE patients have stones in the intrahepatic and extrahepatic bile ducts. Patients are classified further by location of stones within the liver: right side (type R), left side (type L), right and left sides (type LR), and caudate lobe (type C) (Nimura et al, 2005).
In Japan, the proportion of type I intrahepatic stones was 20.6% from 1975 through 1979, increasing to 45.5% from 1989 through 1992 (Tanimura et al, 1994). In Hong Kong, type I constituted 51% of intrahepatic stones from 1991 to 1996 (Liu et al, 1998). In Kaohsiung, Taiwan, the percentage of type I stones was 18.3% from 1980 through 1986 (Yuan et al, 1990). In addition to the findings in East Asian countries, type I intrahepatic stones were diagnosed in 77.3% of patients between 1986 and 2003 in Bologna, Italy (Vetrone et al, 2006).
Many patients with intrahepatic stones are reported to have no stones in the gallbladder. Yuan and colleagues (1990) reported that only 62 (30.7%) of 202 patients with intrahepatic stones had stones in the gallbladder. Regarding distribution of stones in the liver, type L is reported to be dominant. In Japan, the rate was 45.5% for type L, 26.1% for type R, and 26.3% for type LR for 1989 through 1992. The distribution was 52.1% (type L), 11.5% (type R), and 31.3% (type LR) in Hong Kong and 55% (type L), 18.3% (type R), and 26.7% (type LR) for 1989 through 1992 and 71.5% (type L), 16.2% (type R), and 12.2% (type LR) from 2000 through 2005 in Taiwan (Lee et al, 2007). Similarly, it was 59.0% (type L), 18.3% (type R), and 22.7% (type LR) for 1986 through 2003 in Italy (Vetrone et al, 2006). The incidence of stones in the caudate lobe (type C) was reported to be 6.6% (Nimura et al, 1998).
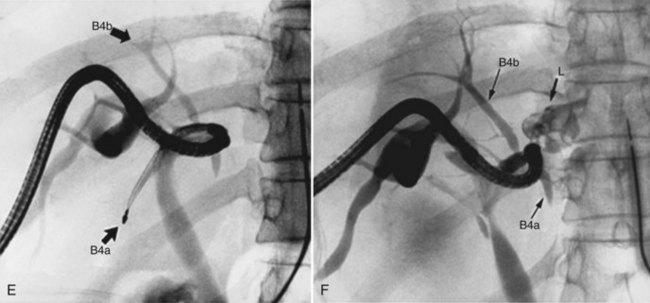
Hepatobiliary radiologists, endoscopists, and surgeons should have a thorough knowledge of the segmental and subsegmental anatomy of the intrahepatic bile ducts for preoperative diagnosis of intrahepatic stones, for an understanding of the variations of the intrahepatic biliary tree, and for decision making about therapeutic approaches to difficult biliary stenosis (see Chapter 1A, Chapter 1B ). The subsegmental anatomy of the intrahepatic bile ducts (Fig. 39.1) was established on the basis of clinical cholangiograms (Nimura, 1997). This cholangiographic anatomy is related to the radiologic study of the intrahepatic portal branches (Takayasu et al, 1985). The left lateral segmental ducts (B2, lateral posterior branch; B3, lateral anterior branch) join just behind the umbilical portion of the left portal vein. The left medial segmental duct (B4) enters this common trunk on the right of the umbilical portion of the left portal vein and forms the left hepatic duct. Segmental ducts B3 and B4 may make a common trunk that B2 joins to form the left hepatic duct. The right anterior segmental branches are superimposed over the right posterior segmental branches and are difficult to distinguish from each other on clinical cholangiograms taken with the patient in a supine position; they are divided clearly by the right hepatic vein on cholangiograms taken with the patient in the right lateral position, the right anterior in the left cranial area, and the right posterior in the right caudal area. The right posterior segmental duct joins the left hepatic duct in 12.6% of Taiwanese patients and 16.8% of Japanese patients with intrahepatic stones. This is an important anatomic variation when considering the distribution of intrahepatic stones (Kitagawa et al, 2003).
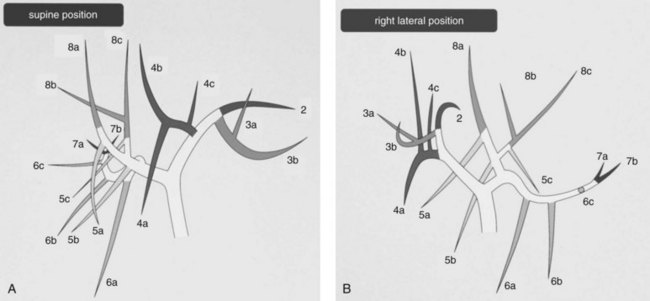
FIGURE 39.1 Cholangiographic anatomy of the intrahepatic segmental ducts according to the patient’s position. Numerals refer to Couinaud’s segments. 3a, Superior branch; 3b, inferior branch; 4a, inferior branch; 4b, superior branch; 4c, dorsal branch; 5a, ventral branch; 5b, dorsal branch; 5c, lateral branch; 6a, ventral branch; 6b, dorsal branch; 6c, lateral branch; 7a, ventral branch; 7b, dorsal branch; 8a, ventral branch; 8b, lateral branch; 8c, dorsal branch (Nimura et al, 1997).
Pathology
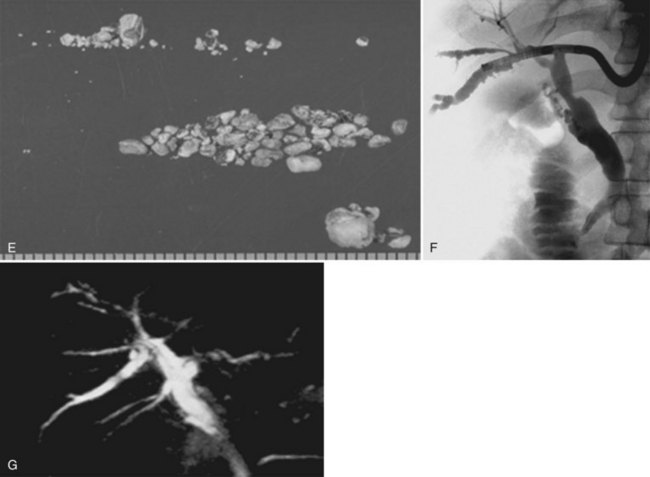
Intrahepatic calcium bilirubinate stones are composed mainly of bilirubin, cholesterol, fatty acids, and calcium. They are dark brown, soft, and friable. On a cut surface, a lamellar structure can be seen (Fig. 39.2). Stones are frequently located in the large bile ducts, such as the common, right, or left hepatic ducts. Diffuse dilation of the biliary tree, chiefly of the stone-containing ducts and their merging peripheral branches, and stenosis-like ductal lesions distal to the stones are commonly noted.
The histologic changes of the bile ducts with calcium bilirubinate stones are classified as either 1) chronic proliferative cholangitis, 2) suppurative cholangitis, or 3) chronic granulomatous cholangitis (Nakanuma et al, 1981). Chronic proliferative cholangitis consists of extensive proliferation of fibrous connective tissue in the ductal wall, moderate to severe infiltration of the inflammatory cells (primarily lymphocytes), and proliferation of mucin-producing glandular elements in the ductal wall. Suppurative cholangitis is characterized by ulceration and suppurative inflammation of the ductal wall. Chronic granulomatous cholangitis is characterized histologically by prominent granulomatous changes in the ductal wall and periductal tissues. In chronic proliferative cholangitis, histologic observation shows that most proliferative glands in the ducts have mucin-producing activity. These glands are observed to secrete a large amount of sialylated and sulfated acid mucins into the lumen (Maki et al, 1971; Sasaki et al, 1996). The affected part of the liver frequently shows atrophic changes. When the whole liver is involved, patients with intrahepatic stones may develop biliary cirrhosis and portal hypertension.
Intrahepatic cholesterol stones are composed mainly of cholesterol. They are yellow, small, and hard (Fig. 39.3). On a cut surface, a radial or crystalloid structure can been seen. Bile ducts containing cholesterol stones generally show a milder degree of fibrosis and glandular hyperplasia than ducts with calcium bilirubinate stones. Foamy cell aggregates and multinucleated giant cells are characteristic findings in patients with intrahepatic cholesterol stones.
Long-standing chronic cholangitis with biliary epithelial regeneration may be causally related to its malignant transformation, and several studies have demonstrated cholangiocarcinogenesis related to hepatolithiasis. Intraductal papillary growth of neoplastic epithelia in the liver (IPNL) is associated with hepatolithiasis and often displays variable gastroenteric metaplasia and significant intraductal spread. A continuous histologic spectrum may be observed, from papillary growths with low-grade and high-grade dysplasia to carcinoma in situ and finally to invasive carcinoma, particularly mucinous carcinoma (Chen et al, 2001). Mucin-secreting tumors of the intrahepatic biliary tract show frequent expression of MUC2 and nuclear CDX2. MUC2 expression in intestinal differentiation is closely related to the expression of CDX2 in IPNL, and mucinous intrahepatic cholangiocarcinoma (ICC) is associated with hepatolithiasis in the same manner as intestinal metaplasia in gastric mucosa and Barrett esophagus. This suggests that a common pathway of carcinogenesis may be involved in ICC with hepatolithiasis, gastric carcinoma, and adenocarcinoma in Barrett esophagus, in which CDX2-dependent intestinal metaplasia develops (Ishikawa et al, 2004). Further studies revealed that two types of neoplastic lesions preceding invasive ICC were identified: a flat-type neoplastic lesion called biliary intraepithelial neoplasia (BilIN) and intraductal papillary neoplasm of the bile duct (IPNB) or IPNL. Multistep carcinogenesis has been suggested in both lesions, and immunophenotypes of MUCs and cytokeratins might characterize three cholangiocarcinogenetic pathways in hepatolithiasis. IPNB and increased expression of MUC1 in BilIN are associated with tubular adenocarcinoma, and colloid carcinoma in IPNB is characterized by MUC1-negativity and less advanced pathologic stages (Zen et al, 2006).
Further immunohistochemical studies reveal that decreased membranous expression of β-catenin and E-cadherin is an early event in the tumorigenesis of both BilIN and IPNB lineages. However, different expression of cancer-related molecules was suggested in the progression of the BilIN and IPNB lineages in cholangiocarcinogenesis related to hepatolithiasis. The expression of matrix metalloproteinase (MMP) 7 and membrane type (MT) 1 MMT was closely associated with invasive growth of the BilIN lineage, and the Wnt signaling pathway may play an important role in the tumorigenesis of the IPNB lineage (Itatsu et al, 2007). The reported incidence of ICC detected at operation varies from 1.4% to 13% in Asian countries (Jeng et al, 1996; Liu et al, 1998), and it was 8.6% (three of 35 cases) in an Italian hospital (Nuzzo et al, 2008). A report from Hong Kong that assessed the long-term efficacy of hepatic resection for stone clearance in 103 patients noted cholangiocarcinoma in 10 patients (four known preoperatively) at the time of operation and in another three patients during follow-up (Chen et al, 2004). A similar report from Taiwan showed that three (2.4%) of 123 patients with intrahepatic stones had associated cholangiocarcinoma at the time of hepatectomy, and two (1.6%) had cholangiocarcinoma develop during a median follow-up of 40 months (range, 5 to 58 months) (Lee et al, 2007). Patients with retained stones or recurrent hepatolithiasis after percutaneous transhepatic cholangioscopic lithotomy have a high incidence of cholangiocarcinoma; four (17%) of 23 cases during a median follow-up of 11 years (range, 1 to 23 years; Chen et al, 2005). Intrahepatic stones associated with ICC are calcium bilirubinate stones in 89.1% of reported cases (57 of 64 cases; Sato et al, 1998). An autopsy case of intrahepatic cholesterol stones with peripheral ICC was reported, in which the causal relationship between the stones and the carcinoma remained speculative (Terada et al, 1989).
Etiology
Most intrahepatic stones appear as brown pigment (calcium bilirubinate) stones on macroscopic inspection and infrared analysis. The chemical composition of intrahepatic calcium bilirubinate stones is not identical to that of brown pigment stones in the extrahepatic bile ducts; the former contain more cholesterol and less bilirubin and bile acid and include lesser amounts of bile acids modified by bacterial metabolism (Shoda et al, 1991, 2003; Yamashita et al, 1988). The high incidence of bactibilia in patients with intrahepatic calcium bilirubinate stones suggests a relationship between bacteria and formation of stones (Maki, 1966). Among the bacteria in bile, Escherichia coli, Clostridium spp., and Bacteroides spp. show β-glucuronidase activity and are thought to be responsible for the hydrolysis of bilirubin glucuronide, the water-soluble form of bilirubin in bile, to free unconjugated bilirubin, which is water insoluble and combines with ionized calcium in bile to form calcium bilirubinate; this leads to stone formation. Bacterial infection alone cannot explain why intrahepatic brown pigment stones contain a higher amount of cholesterol than stones in the extrahepatic ducts. Upregulated cholesterogenesis and downregulated bile acid synthesis in the liver may be one of the causative factors responsible for the formation of hepatic bile substantially supersaturated with cholesterol, which leads to subsequent formation of cholesterol-rich stones (Shoda et al, 1995, 2003).
It is suggested that the risk factors of intrahepatic stone formation after surgery for choledochal cyst are anastomotic stricture, residual debris in the intrahepatic ducts, and dilated intrahepatic bile ducts. A Japanese surgical study reported secondary hepatolithiasis that developed after choledochal cyst excision with hepaticoenterostomy (CEHE). Although no stone formation was seen in the 145 children who had CEHE at the age of 5 years or younger, three (5.5%) of 55 children older than 5 years who underwent CEHE had intrahepatic stones develop. On the other hand, intrahepatic stones developed in five (12.5%) of the 40 adult patients with CEHE. Two of the five developed anastomotic stricture of the end-to-side hepaticojejunostomy distally to the stone at the porta hepatis with or without left intrahepatic duct dilation. The incidence of post-CEHE hepatolithiasis in children aged 5 years or younger was significantly lower than in other children and adults. In addition to the hepatolithiasis, one adult developed ICC at age 54 years, 6 years after CEHE converted from cholecystectomy performed 16 years previously (Yamataka et al, 1997). On the other hand, intrahepatic stones developed in patients with biliary atresia who had undergone hepatic portoenterostomy, and almost 100% calcium bilirubinate stones were found in bile lake in the resected liver (Tainaka et al, 2006).
A study from Taiwan investigated the possible association between biliary helminthic infestation (ascariasis or clonorchiasis) and hepatolithiasis (Huang et al, 2005). By using immunologic techniques, the authors showed a higher prevalence of ascariasis and clonorchiasis in patients with intrahepatic stones than in control subjects (33.6% vs. 17.4%; P = .005). However, analysis of patients with hepatolithiasis with and without evidence of helminthic infestation showed no differences in the rates of biliary stricture formation, stone recurrence, secondary biliary cirrhosis, or cholangiocarcinoma. An epidemiologic study suggested that lower socioeconomic and hygienic status may be involved in stone formation in Taiwan (Momiyama et al, 2008). In the Kami-Goto Islands of Japan, where incidence of this disease is high, there were similar hygienic and social problems from the 1960s to the 1980s. However, there has been no new case of hepatolithiasis in the generation born after the 1970s, after improvements in the environment and economic status (Yasaka, 2008).
The most important factors for the formation of intrahepatic calcium bilirubinate stones are biliary stasis, bacterial infection, and biliary mucin produced from the covering and proliferated mucous gland epithelium of the intrahepatic bile ducts. Calcium bilirubinate and fatty acid–calcium soap occur through hydrolysis of bilirubin conjugates and lecithin by bacterial β-glucuronidase and phospholipase in bile. These salts can precipitate and form microcalculi, particularly if a nidus is present, such as desquamated epithelium or biliary mucin (Forstner & Forstner, 1975). These microcalculi coalesce to form large stones. Chronic proliferative inflammation seems to precede bile duct damage: the development of intrahepatic stones leads to biliary strictures, which promote further propagation of stones through alterations of bile flow (Nakanuma et al, 1988).
A study on the biliary dynamics of patients with intrahepatic stones revealed that the time-activity curves of technetium-99m-ethylenediaminetetraacetic acid (EDTA) were prolonged significantly in affected and apparently unaffected intrahepatic bile ducts compared with the curves in normal subjects (Takahashi et al, 1986). This result suggests a global impairment of bile flow, even when only one side of the liver is affected by stones. The curves in the intrahepatic bile ducts of the left lobe were more prolonged than those of the right lobe, even in normal liver; this prolongation could explain why involvement of the left lobe is more common.
Sphincter of Oddi motility has been analyzed in patients with intrahepatic and common bile duct (CBD) stones by percutaneous transhepatic manometry (Yuasa et al, 1994). This study disclosed no significant manometric differences among three groups—an intrahepatic stone group, a cholecystectomy group with CBD stones, and a noncholecystectomy group with CBD stones—with the exception of the higher frequencies and greater duration of burst contractions during duodenal phase III of the migrating motor complex in the cholecystectomy group with CBD stones, although this abnormality probably plays little role in the etiology of intrahepatic stones.
Indirect evidence suggests that metabolic factors play a more important etiologic role in intrahepatic cholesterol stones. Cholesterol supersaturation and microscopic cholesterol crystallization, which represent major stages in the formation of cholesterol gallbladder stones, have been observed in the hepatic bile of patients with intrahepatic cholesterol stones (Schillio et al, 1992: Strichartz et al, 1991). Activity of apolipoprotein A1, which is one of the inhibiting factors of cholesterol nucleation in bile, is reported to be decreased in the hepatic segments containing cholesterol stones (Ohta et al, 1993).
Signs and Symptoms
Abdominal pain, either in the right upper quadrant or in the upper abdomen, is the most frequent initial symptom observed in 70% of hepatolithiasis patients (Tanimura et al, 1994). Jaundice and fever follow and are present in 10% to 30% of cases. Other abdominal complaints, including abdominal discomfort and vomiting, are also observed. These symptoms are not pathognomonic for intrahepatic stone disease. Asymptomatic cases have increased in number to 16.1% because of advances in diagnostic imaging modalities and more frequent medical checkups. Previous biliary operations for calculous disease had been performed in 42.1% of patients with intrahepatic stones, and 22.2% had had two or more operations, indicating an intractable course of disease.
Diagnosis
With the introduction of various new imaging modalities, intrahepatic stones are now diagnosed more accurately before surgery. Ultrasonography (US) is a noninvasive, reliable, and inexpensive procedure. It may reveal features of biliary obstruction, stones in the biliary system, pneumobilia, or liver abscesses. The major findings of stones on US examination are hyperechoic lesions with acoustic shadows or ductal dilation (Choen et al, 1991; Itai et al, 1980; Lim et al, 1990). Interpretation may be difficult, however, owing to the presence of pneumobilia as a result of a previous papillotomy or biliary-enteric anastomosis. In addition, intrahepatic stones that do not cast a sonic shadow may not be well visualized on US.
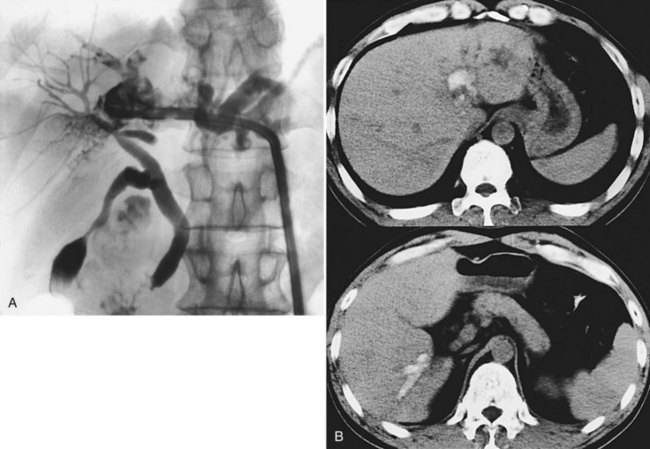
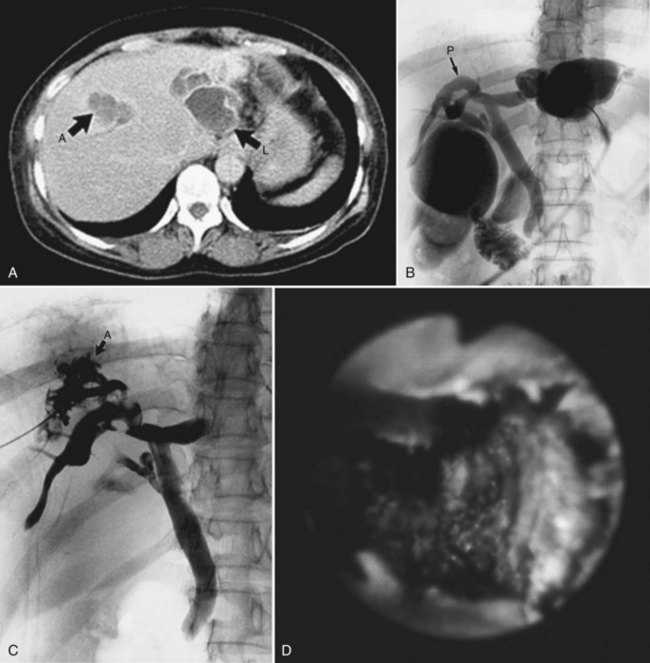
Computed tomography (CT) provides information regarding the location and composition of stones and generally offers more anatomic detail, which is necessary for decisions regarding definitive treatment. Round or oval high-density shadows in the dilated ducts are a sign of intrahepatic calcium bilirubinate stones (Fig. 39.4); the false-positive rate is quite low (Itai et al, 1980; Menu et al, 1985). Cholesterol stones or pigmented stones containing less calcium are hard to detect, so it is important to examine noncontrast films first (Fig. 39.5), because intrahepatic stones may be less conspicuous after parenchymal enhancement. Pneumobilia does not hinder the CT evaluation of the biliary system. In addition, CT determines the degree of atrophy of the affected part of the liver and detects the segmental or subsegmental duct involvement when cholangiography fails to visualize these ducts; CT also provides an accurate localization of liver abscesess, raises the suspicion of cholangiocarcinoma, and detects the presence of portal hypertension as a result of biliary cirrhosis. Accurate diagnosis of cholangiocarcinoma is difficult, however, because small tumors often appear as abscesses or escape detection altogether, even on high-quality helical CT scans. Park and colleagues (2006) reported several factors that were significantly associated with cholangiocarcinoma in patients with hepatolithiasis: 1) presence of periductal soft-tissue density, 2) higher enhancement of the duct than adjacent bile duct on portal venous phase images, 3) ductal thickening, 4) portal vein obliteration, and 5) lymph node enlargement.
Magnetic resonance imaging (MRI), especially MR cholangiopancreatography (MRCP), is useful for the defining stone location, obstructed intrahepatic segmental ducts, and bile duct anatomy (Kim et al, 2002; Park et al, 2004). In some cases, the bile duct diameter can be calculated for evaluation of bile duct dilation and stricture (Nimura et al, 2005).
If these modalities show intrahepatic stones only in the left lobe, no more imaging investigations for deciding the surgical intervention may be necessary. If stones are located in the right liver or are bilateral, however, percutaneous transhepatic biliary drainage (PTBD) followed by percutaneous transhepatic cholangioscopy (PTCS) should be done to determine the optimal treatment (Nimura et al, 1981, 1988). US, CT, and MRCP provide less precise information about segmental and subsegmental anatomy of the intrahepatic bile ducts and strictures than percutaneous transhepatic cholangiography (PTC), tube cholangiograms via PTBD, or selective cholangiography by PTCS. Endoscopic retrograde cholangiopancreatography (ERCP) frequently misdiagnoses intrahepatic stones, because intrahepatic bile ducts peripheral to stones and strictures are often poorly visualized. ERCP has the other associated disadvantage of potentially causing cholangitis in patients with multiple intrahepatic stones.
Making the diagnosis of associated cholangiocarcinoma is difficult, because intrahepatic stones and strictures hinder clear visualization of the intrahepatic ducts in their entirety on cholangiography. As diagnostic clues, the importance of persistent filling defects and obliteration of the involved intrahepatic ducts on cholangiography, mucobilia and mucosal changes on cholangioscopy, and gross appearance are emphasized (Chen et al, 1993).
Treatment
For patients with acute obstructive suppurative cholangitis, emergency PTBD is indicated and is preferable to operative and endoscopic transpapillary approaches, and local anesthesia for PTBD can alleviate much of the risk to such critically ill patients. Emergency exploration does not allow a definitive treatment and may not provide adequate biliary decompression (Cheung et al, 2005). An endoscopic transpapillary approach is an effective and safer treatment for distal bile duct stones (Cotton et al, 1998), but further studies are required to clarify the therapeutic benefit of this approach.
With the refinement of fiberoptic cholangioscopes and the development of percutaneous transhepatic approaches to the bile ducts, PTCS has allowed nonsurgical removal of intrahepatic stones (Nimura et al, 1981, 1988). The size of extractable stones is limited, however, by the caliber of the PTBD sinus tract. The presence of a biliary stricture in association with intrahepatic stones often makes stone extraction more difficult. To overcome this, laser cholangioscopy has been applied for intrahepatic stones with some success (Hayakawa et al, 1981), and electrohydraulic lithotripsy during cholangioscopy has facilitated extraction of larger stones (Matsumoto et al, 1987). Electrohydraulic lithotripsy is a unique method to disintegrate stones by means of shock waves generated by an electric discharge in a liquid medium. Under cholangioscopic control, the lithotriptor probe can be kept in contact with stones during application of discharge sparks to avoid any damage to the bile duct wall.
Hepatic resection may remove not only the stones and the strictures that led to their formation but also the consequences of repeated injury, such as cholangiocarcinoma. In general, hepatectomy is indicated when a sector or a lobe of the liver is grossly atrophic (Fan et al, 1993; Otani et al, 1999). In addition to atrophic liver, indications for hepatectomy include multiple intrahepatic strictures and cystic dilation of the peripheral intrahepatic bile duct that cannot be managed by endoscopic or radiologic intervention, particularly if the left lobe or the left liver is involved. The surgical mortality rate associated with hepatic resection in this setting is low, but morbidity rates are substantial. When PTCS shows biliary strictures involving the right or left hepatic duct or the biliary confluence, and the liver parenchyma is relatively normal, an end-to-side hepaticojejunostomy (Roux-en-Y) is performed based on the location and level of stricture. Hepatic resection with hepaticojejunostomy may be indicated when PTCS reveals a biliary stricture at the hepatic confluence in a patient with bilateral intrahepatic stones. If the function of the sphincter of Oddi deteriorates, hepaticojejunostomy is mandated.
For patients with difficult bilateral intrahepatic stones with bilateral biliary strictures, a stepwise approach is advocated (Nimura, 1984):
1 PTBD through B3 or the left hepatic duct is performed via a direct anterior approach, which has several advantages in the management of right intrahepatic stones. The most advantageous point is easy accessibility of a cholangioscope to the right intrahepatic ducts, especially to the right posterior sectoral ducts, which are much more difficult to access via intraoperative cholangioscopy or postoperative cholangioscopy through a T-tube sinus tract or jejunal loop.
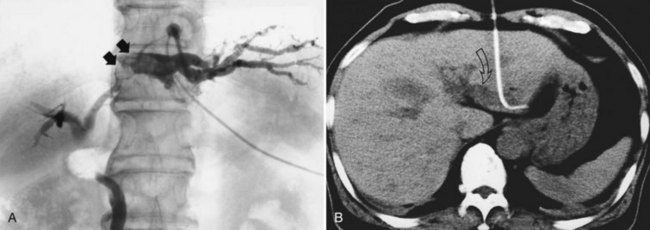
2 The PTBD sinus tract is dilated step by step. One week after the PTBD, the 6-Fr drainage catheter is replaced by a 10- or 11-Fr (PTCS) catheter (Fig. 39.6). The tract is dilated by exchanging the catheter for a larger one a few times a week, until a 16-Fr catheter can be introduced approximately 2 weeks after the initial PTBD. During this period, CT and US reveal pathologic changes of the liver. A hepatectomy is indicated in cases of unilateral atrophy of the liver as discussed earlier.
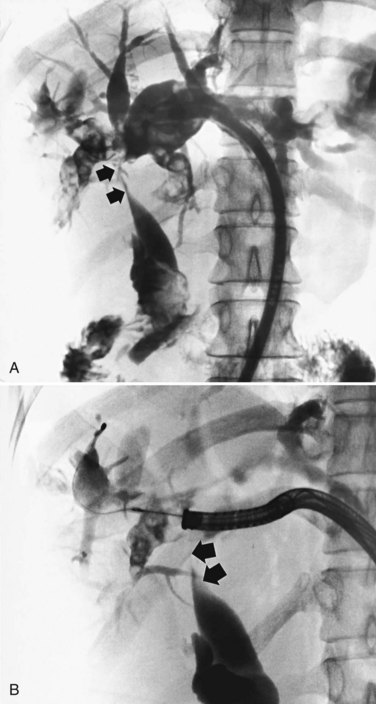
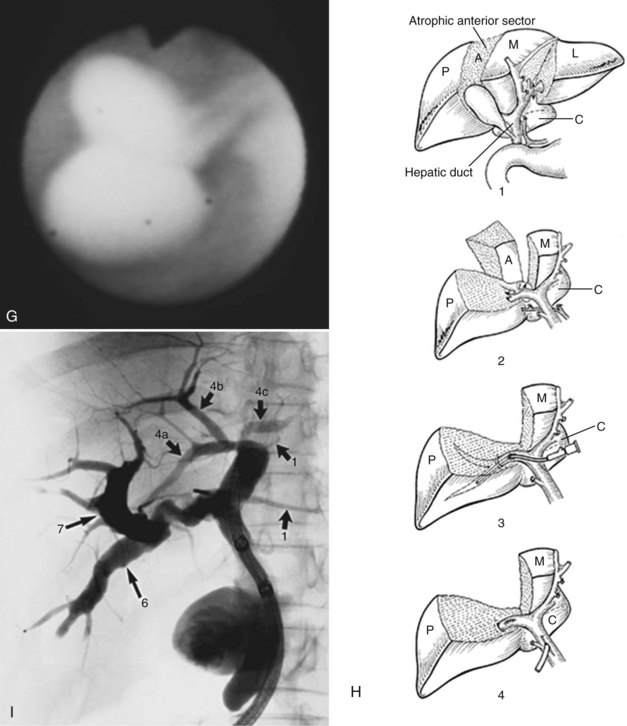
3 Cholangioscopic lithotomy is performed mainly in the hepatic sectors or lobes that will be preserved. If intrahepatic bile duct stenosis hinders the cholangioscopic lithotomy, the ducts should be dilated by introducing a 12- or 15-Fr PTCS catheter. Advances in lithotripsy technology make it possible to remove impacted stones without difficulty, and lithotripsy is warranted if the affected sectors or lobes are not atrophic. PTCS allows definition of biliary stenosis before surgical treatment. In the case of biliary stenosis at the hepatic hilus, hilar bile duct resection with hepaticojejunostomy is indicated (Fig. 39.7).
4 If neither atrophy nor strictures are seen after cholangioscopic lithotomy, surgery is not recommended. Cholecystectomy may be indicated in patients with documented cholelithiasis. In cases of hepatic sectoral or lobar atrophy without biliary stricture at the hepatic hilus, cholecystectomy, hepatectomy, choledochotomy, intraoperative cholangioscopy, and T-tube placement without biliary-enteric anastomosis are recommended (Fig. 39.8). For a patient with some element of atrophy and a stricture at the hepatic duct confluence, hilar bile duct resection with hepaticojejunostomy should be performed in addition to cholecystectomy, hepatectomy, and intraoperative cholangioscopy. If a biliary stricture is defined only at the hepatic duct confluence after endoscopic lithotomy by PTCS, cholecystectomy, hilar bile duct resection, intraoperative cholangioscopy, and hepaticojejunostomy are indicated.
5 In patients subjected to surgical intervention, postoperative cholangioscopy should be performed to assess for and possibly treat residual stones and to evaluate the postoperative changes of the intrahepatic biliary strictures. If the treatment effect is insufficient, biliary stenting with a silicone catheter should be applied.
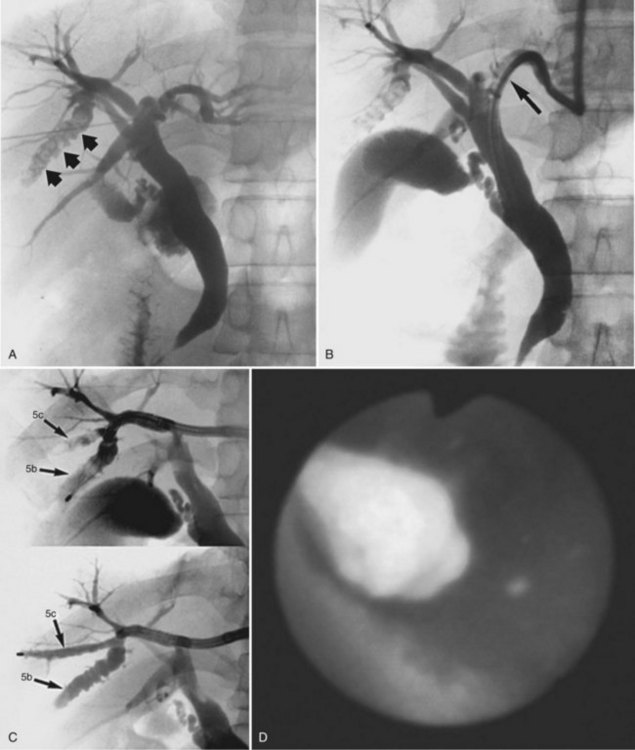
Regarding treatment of intrahepatic cholesterol stones, it is recommended to select a nonsurgical approach as the first treatment of choice (Fig. 39.9; Kondo et al, 1995). On the contrary, a high incidence (16 of 35 cases) of intrahepatic cholesterol stones was reported from a European institution, and partial hepatectomy is recommended as a safe and effective procedure (Nuzzo et al, 2008). An ERCP approach does not seem feasible because of the generally inaccessible location of the intrahepatic stones. Extracorporeal shock-wave lithotripsy may be effective, especially in combination with dissolution therapy (Okugawa et al, 2002; Staritz et al, 1990). Improvement in bile composition has been reported after oral dissolution therapy in patients with intrahepatic cholesterol stones, suggesting that this therapy is probably useful as a preventive adjunct (Schillio et al, 1992; Strichartz et al, 1991).
The indications for intervention in asymptomatic patients with intrahepatic stones are controversial. In a patient who has mostly cholesterol stones, treatment may not be indicated. On the contrary, if associated ICC is suspected, further evaluation and consideration for hepatectomy are mandatory. In Kami-Goto Islands of western Japan, a screening survey for intrahepatic stones was performed by US because of the high incidence of ICC in this population. This survey identified 122 asymptomatic patients with intrahepatic stones who had been followed for an average of 10 years (Furukawa et al, 1998). In this group, only 14 (11.5%) of the 122 patients had symptoms develop, and 13 of these (92.9%) had liver atrophy, whereas the incidence of liver atrophy was significantly lower (13%) in patients with no symptoms. Based on these data and other cancer-related investigations (Mori, 2008b; Tsuyuguchi, 2008), no treatment is necessary or recommended for asymptomatic patients without sectoral or lobar atrophy.
Prognosis
Intrahepatic stone disease carries a high rate of recurrence. Of all cases treated in Japan in the years 1989 through 1992, the overall recurrence rate was 6.6% (119 of 1815 cases; Tanimura et al, 1994). The recurrence rate according to treatment was as follows: 5.3% (38 of 719) for hepatic resection, 8.3% (21 of 254) for choledochojejunostomy, 6.4% (40 of 624) for choledochotomy followed by cholangioscopic lithotomy, and 9.6% (20 of 208) for PTCS. The lowest reported recurrence rate is 3.1% (3 of 96) at a mean follow-up period of 26 months in patients treated in Hong Kong (Liu et al, 1998). The highest reported recurrence rate is 32.6% (43 of 132) in patients undergoing lithotomy by PTCS for a mean follow-up period of 5 years in a report from Taiwan (Yeh et al, 1995). In 2008, a nationwide survey was performed in Japan. Despite recent progress in diagnosis and treatment for hepatolithiasis, the recurrence rate was still 18.6%, and cholangiocarcinoma with hepatolithiasis was found in 5.9% of the cases (Mori, 2008a). Another report from Korea showed that 11 (5%) of 225 patients with intrahepatic stones developed cholangiocarcinoma at 10.7 years (range, 6.6 to 19.7 years) after initial treatment of stones. The 11 cholangiocarcinoma patients consisted of six (4%) of 159 patients with complete stone removal and five (8%) of 66 patients with residual stones after treatment (Cheon et al, 2009).
The survival rate after resection of ICC associated with hepatolithiasis is quite low, primarily because most patients have advanced disease at the time of diagnosis and operation, and postoperative survival rates in 55 Taiwanese patients at 1, 2, and 4 years were 30%, 12.7%, and 3.6%, respectively (Chen et al, 1993). In Japan, 48% of patients with cholangiocarcinoma associated with hepatolithiasis died within one year of operation (Tanimura et al, 1994).
Al-Sukhni W, et al. Recurrent pyogenic cholangitis with hepatolithiasis: the role of surgical therapy in North America. J Gastrointest Surg. 2008;12:496-503.
Catena M, et al. Treatment of non-endemic hepatolithiasis in a Western country: the role of hepatic resection. Ann R Coll Surg Engl. 2006;88:383-389.
Chen CH, et al. Reappraisal of percutaneous transhepatic cholangioscopic lithotomy for primary hepatolithiasis. Surg Endosc. 2005;19:505-509.
Chen DW, et al. Immediate and long-term outcomes of hepatectomy for hepatolithiasis. Surgery. 2004;135:386-393.
Chen MF, et al. A reappraisal of cholangiocarcinoma in patients with hepatolithiasis. Cancer. 1993;71:2461-2465.
Chen TC, et al. Intraductal papillary neoplasia of the liver associated with hepatolithiasis. Hepatology. 2001;34:651-658.
Cheon YK, et al. Evaluation of long-term results and recurrent factors after operative and nonoperative treatment for hepatolithiasis. Surgery. 2009;146:843-853.
Cheung MT, et al. Liver resection for intrahepatic stones. Arch Surg. 2005;140:993-997.
Cohen SM, et al. Biliary sonography. Radiol Clin North Am. 1991;29:1171-1198.
Cotton PB, et al. Endoscopic sphincterotomy for stones by experts is safe, even in younger patients with normal ducts. Ann Surg. 1998;227:201-243.
Fan ST, et al. Hepatic resection for hepatolithiasis. Arch Surg. 1993;128:1070-1074.
Forstner JF, Forstner GG. Calcium binding to intestinal goblet cell mucin. Biochem Biophys Acta. 1975;386:283-292.
Furukawa M, et al. Asymptomatic hepatolithiasis: a follow-up study. In: Annual Reports of the Japanese Ministry of Health and Welfare [in Japanese]. Tokyo: Japanese Government; 1998:12-14.
Hayakawa N, et al. Studies on laser cholangioscopy application for lithotripsy of gallstones. In: Atsumi, K, editor. Laser Tokyo 81. Tokyo: Inter Group; 1981:2316-2318.
Herman P, et al. Non-Oriental primary intrahepatic lithiasis: experience with 48 cases. World J Surg. 2005;29:858-864.
Huang MH, et al. Relation of hepatolithiasis to helminthic infestation. J Gastroenterol Hepatol. 2005;20:141-146.
Ishikawa A, et al. Aberrant expression of CDX2 is closely related to the intestinal metaplasia and MUC2 expression in intraductal papillary neoplasm of the liver in hepatolithiasis. Lab Invest. 2004;84:629-638.
Itai Y, et al. Computed tomography and ultrasound in the diagnosis of intrahepatic calculi. Radiology. 1980;136:399-405.
Itatsu K, et al. Immunohistochemical analysis of the progression of flat and papillary preneoplastic lesions in intrahepatic cholangiocarcinogenesis in hepatolithiasis. Liver Int. 2007;27:1174-1184.
Jeng KS, et al. Reappraisal of the systemic management of complicated hepatolithiasis with bilateral intrahepatic biliary strictures. Arch Surg. 1996;131:141-147.
Kim TK, et al. Diagnosis of intrahepatic stones: superiority of MR cholangiopancreatography over endoscopic retrograde cholangiopancreatography. AJR Am J Roentgenol. 2002;179:429-434.
Kitagawa Y, et al. Intrahepatic segmental bile duct patterns in hepatolithiasis: a comparative cholangiographic study between Taiwan and Japan. J Hepatobiliary Pancreat Surg. 2003;10:377-381.
Kondo S, et al. A clinicopathologic study of primary cholesterol hepatolithiasis. Hepatogastroenterology. 1995;42:478-486.
Lee TY, et al. Outcomes of hepatectomy for hepatolithiasis. World J Surg. 2007;31:479-482.
Lim JH, et al. Oriental cholangiohepatitis: sonographic findings in 48 cases. AJR Am J Roentgenol. 1990;155:511-514.
Liu CL, et al. Primary biliary stones: diagnosis and management. World J Surg. 1998;22:1162-1166.
Maki T. Pathogenesis of calcium bilirubinate gallstone: role of E. coli, β-glucuronidase and coagulation by inorganic ions, polyelectrolytes and agitation. Ann Surg. 1966;164:90-100.
Maki T, et al. Role of sulfated glycoprotein in gallstone formation. Surg Gynecol Obstet. 1971;132:846-854.
Matsumoto S, et al. Electrohydraulic lithotripsy of intrahepatic stones during choledochoscopy. Surgery. 1987;102:852-856.
Menu Y, et al. Sonographic and computed tomographic evaluation of intrahepatic calculi. AJR Am J Roentgenol. 1985;145:579-583.
Momiyama M, et al. Lifestyle risk factors for intrahepatic stones: findings from a case-control study in an endemic area, Taiwan. J Gastroenterol Hepatol. 2008;23:1075-1081.
Mori T, et al. Etiology of hepatolithiasis: risk factor of cholangiocarcinoma. In: Annual Reports of the Japanese Ministry of Health and Welfare [in Japanese]. Tokyo: Japanese Government; 2008:123-125.
Mori T, et al. Report from Working Group for Etiology and Prognosis of Intrahepatic Stones. In: Annual Reports of the Japanese Ministry of Health and Welfare [in Japanese]. Tokyo: Japanese Government; 2008:21-23.
Nakanuma Y, et al. Histological studies on the livers with intrahepatic gallstones. Jpn J Gastroenterol. 1981;78:874-882.
Nakanuma Y, et al. Japanese Hepatolithiasis Study Group, 1988: Pathologic features of hepatolithiasis in Japan. Hum Pathol. 1988;19:1181-1186.
Nimura Y. Percutaneous transhepatic cholangioscopy (PTCS) in the treatment of intrahepatic stones. In: Sheen, PC, Ker, CG. Gallstone and Choledochoscope. Kaoshiung: Mei Yuh; 1984:71-85.
1997 Nimura Y. Surgical anatomy of the biliary ducts. In: Medical Radiology: Biliary Tract Radiology. Berlin: Springer-Verlag; 1997:21-30.
Nimura Y, et al. Report from Working Group for Classification of Intrahepatic Stones. In: Annual Reports of the Japanese Ministry of Health and Welfare [in Japanese]. Tokyo: Japanese Government; 2005:10-13.
Nimura Y, et al. Percutaneous transhepatic cholangioscopy (PTCS). Stomach Intestine [in Japanese with English abstract]. 1981;16:681-689.
Nimura Y, et al. Value of percutaneous transhepatic cholangioscopy (PTCS). Surg Endosc. 1988;2:213-219.
Nimura Y, et al. Intrahepatic stone in the caudate lobe. In: Annual Reports of the Japanese Ministry of Health and Welfare [in Japanese]. Tokyo: Japanese Government; 1998:45-49.
Nuzzo G, et al. Liver resection for primary intrahepatic stones: a single-center experience. Arch Surg. 2008;143:570-573.
Ohta T, et al. Histological evaluation of the intrahepatic biliary tree in intrahepatic cholesterol stones, including immunohistochemical staining against apolipoprotein A-1. Hepatology. 1993;17:531-537.
Okugawa T, et al. Peroral cholangioscopic treatment of hepatolithiasis: long-term results. Gastrointest Endosc. 2002;56:366-371.
Otani K, et al. Comparison of treatments for hepatolithiasis: hepatic resection versus cholangioscopic lithotomy. J Am Coll Surg. 1999;189:177-182.
Park DH, et al. Accuracy of magnetic resonance cholangiopancreatography for locating hepatolithiasis and detecting accompanying biliary stricture. Endoscopy. 2004;36:987-992.
Park HS, et al. CT Differentiation of cholangiocarcinoma from periductal fibrosis in patients with hepatolithiasis. Am J Roentgenol. 2006;187:445-453.
Sasaki M, et al. Carcinoembryonic antigen and blood group-related carbohydrate antigens in glycoproteins in human bile in hepatolithiasis. Hepatology. 1996;23:258-263.
Sato M, et al. Intrahepatic cholangiocarcinoma associated with hepatolithiasis. Hepatogastroenterology. 1998;45:137-144.
Schillio Y, et al. Primary intrahepatic cholesterol stones: report of one case and treatment with ursodeoxycholic acid. Dig Dis Sci. 1992;37:1460-1463.
Shoda J, et al. Microanalysis of bile acid composition in intrahepatic calculi and its etiological significance. Gastroenterology. 1991;101:821-830.
Shoda J, et al. Primary dual defect of cholesterol and bile acid metabolism in liver of patients with intrahepatic calculi. Gastroenterology. 1995;108:1534-1546.
Shoda J, et al. Hepatolithiasis: epidemiology and pathogenesis update. Front Biosci. 2003;8:e398-e409.
Staritz M, et al. Electromagnetically generated extracorporeal shockwaves for fragmentation of extra- and intrahepatic bile duct stones: indications, success and problems during a 15 month clinical experience. Gut. 1990;31:222-225.
Strichartz SD, et al. Intrahepatic cholesterol stones: a rationale for dissolution therapy. Gastroenterology. 1991;100:228-232.
Tainaka T, et al. Hepatolithiasis after hepatic portoenterostomy for biliary atresia. J Pediatr Surg. 2006;44:808-811.
Takahashi K, et al. Biliary dynamics in patients with intrahepatic gallstones. J Bil Tract Pancreas [in Japanese]. 1986;10:1317-1322.
Takayasu K, et al. Intrahepatic portal vein branches studied by percutaneous transhepatic portography. Radiology. 1985;154:31-36.
Tanimura H, et al. Epidemiology of hepatolithiasis in Japan. In: Annual Reports of the Japanese Ministry of Health and Welfare [in Japanese]. Tokyo: Japanese Government; 1994:17-27.
Terada T, et al. Intrahepatic cholesterol stones associated with peripheral cholangiocellular carcinoma: an autopsy case. Am J Gastroenterol. 1989;84:1434-1436.
Tsuyuguchi T. Long-term prognosis of hepatolithiasis: a study for risk factors. In: Annual Reports of the Japanese Ministry of Health and Welfare [in Japanese]. Tokyo: Japanese Government; 2008:135-137.
Vetrone G, et al. Surgical therapy for hepatolithiasis: a Western experience. J Am Coll Surg. 2006;202:306-312.
Yamashita N, et al. Composition of intrahepatic calculi: etiological significance. Gastroenterology. 1988;101:821-830.
Yamataka A, et al. Complications after cyst excision with hepaticoenterostomy for choledochal cysts and their surgical management in children versus adults. J Pediatr Surg. 1997;32:1097-1102.
Yasaka T. Investigation for hepatolithiasis distribution in the Kami-Goto area. In: Annual Reports of the Japanese Ministry of Health and Welfare [in Japanese]. Tokyo: Japanese Government; 2008:126-127.
Yeh YH, et al. Percutaneous trans-hepatic cholangioscopy and lithotripsy in the treatment of intrahepatic stones: a study with 5 year follow-up. Gastrointest Endosc. 1995;42:13-18.
Yuan CY, et al. A reevaluation of 202 patients after surgical removal of intrahepatic stones: with special emphasis on intrahepatic biliary stricture. J Formos Med Assoc. 1990;89:373-377.
Yuasa N, et al. Sphincter of Oddi motility in patients with bile duct stones: a comparative study using percutaneous transhepatic manometry. Dig Dis Sci. 1994;39:257-267.
Zen Y, et al. Different expression patterns of mucin core proteins and cytokeratins during intrahepatic cholangiocarcinogenesis from biliary intraepithelial neoplasia and intraductal papillary neoplasm of the bile duct: an immunohistochemical study of 110 cases of hepatolithiasis. J Hepatol. 2006;44:350-358.