40 Intradiscal and Peridiscal Therapies for Discogenic and Radicular Pain
Intradiscal therapies are those that involve the placement under imaging guidance of a needle, probe, or similar device into an intervertebral disc with the goal to reduce the patient’s presenting symptoms. Chemonucleolysis, a term coined by Smith and Garvin in 1963 when they injected chymopapain into a disc for the treatment of sciatica,1 was the first intradiscal therapy; it was designed as a minimally invasive treatment for radicular pain caused by a disc prolapse. Chymopapain was eventually shown to be an extremely effective treatment, but ironically ceased to exist mainly for political reasons. However, the main thrust on epidemiologic grounds has been the search for a minimally invasive panacea for spinal origin pain, particularly nonspecific low back pain (NSLBP). As a consequence, numerous device companies have developed experimental intradiscal devices, some of which have achieved modest success in the small cohort of patients who present with NSLBP and are given the questionable diagnosis of discogenic pain (DP).
The diagnosis established by provocation discography (PD) is internal disc disruption (IDD). In IDD, the disc is shown to be internally disrupted and the probable source of pain, and it is thought that the radial fissures are the prime source of pain. The credibility of the diagnosis IDD is dependent on the validity and reliability of discography. The diagnostic confidence of IDD can be manipulated by altering the criteria for calling a PD test as positive. Indeed, over the years these criteria have changed and it may be that they will change in the future. Initially discography was deemed positive when internal disruption was seen, but when morphologic change alone was shown to be nonspecific, pain provocation was added. With the recognition that pain provocation was itself nonspecific, the nature of pain provocation was redefined so that pain had to (1) reach a minimum intensity (currently about 7/10 on a VAS); (2) be exactly like the clinical pain; and (3) not be produced at adjacent discs The Diagnostic Criteria for IDD from the International Spine Intervention Society (ISIS) are presented in Table 40-1.2 It may be that further refinements of discography will increase the confidence that IDD is a credible diagnosis. If it is, then diagnosis should lead to a predictable outcome for this cohort, be it with conservative or interventional management.
Table 40-1 Diagnostic Criteria for IDD from ISIS (2004)∗
| 1. Reproduction of the patient’s pain by stimulation of the affected disc |
| 2. This pain must have an intensity of at least 7 on a 10-point scale |
| 3. This pain must be reproduced at a low pressure of stimulation: 15 psi (1 kg cm2) |
| 4. Stimulation of adjacent disc(s) must not reproduce pain |
| 5. Postdiscography CT demonstrates a grade III or IV fissure |
IDD, internal disc disruption; ISIS, International Spine Intervention Society.
∗ All categories must be satisfied. These criteria are being modified.
(From International Spinal Intervention Society. Lumbar disc stimulation. In Bogduk N (ed): Practice Guidelines for Spinal Diagnostic and Treatment Procedures. International Spinal Intervention Society, San Francisco, 2004, pp 20-46.)
Another problem in this area of medicine is proving treatment efficacy. The future development of treatments for IDD is likely to be constrained at least because of the costs of running multiple randomized controlled double-blind trials (RCDBTs). The estimated cost of a previous intradiscal electrothermal study was estimated to be more than $1,000,000.3 An alternative is to use well designed, properly conducted observational studies as the benchmarks that establish whether a particular procedure is backed by a suitable level of evidence of efficacy.3 Such studies are particularly good in at least refuting ineffective procedures and practices.3 The recent publication of the first RCDBT on MB suggests that it is an extremely effective treatment for IDD.4 Other studies are underway to further assess this treatment.
Intradiscal Thermal Therapies for Discogenic Pain
Intradiscal therapies for DP, and more specifically for IDD, are not standard treatments. They are in development. Bogduk has enunciated the status of intradiscal procedures elegantly as follows:5
Types and Methods
Discogenic and radicular pain can be treated with thermal intradiscal procedures (TIPs) that typically use radiofrequency (RF) energy delivered into the disc by a locally placed probe or similar device aimed at altering disc structure, removing disc tissue, or denervating nerves. Other patented energy sources that can be used include thermal, electric current, microwave emission, ultrasound emission, radioactive emission, and laser. Although RF is the most commonly used energy source, ultrasound is at least as good.6 RF technology has been used since at least 1891 when D’Arsonval used alternating current to prevent any unwanted effect of neuromuscular stimulation during surgery. Subsequently, Harvey Gushing developed the technique of high-frequency current for electrocoagulation of blood vessels during surgery.7 It was subsequently shown that high frequency lesions in the RF range were reproducible and well defined.8 Since this time, the technology has advanced, and RF current is used in multiple arenas, including neurosurgery, dermatology, chronic pain, and in the treatment of cardiac arrhythmias. The three minimally invasive TIPs for the treatment of IDD are intradiscal electrothermal therapy (IDET), percutaneous intradiscal radiofrequency thermocoagulation (PIRFT), and intradiscal biacuplasty.
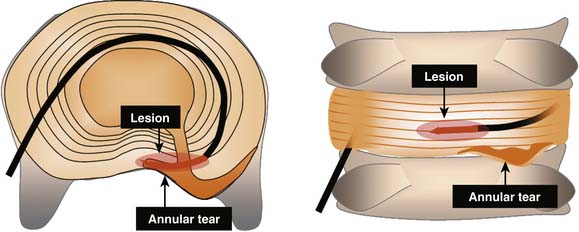
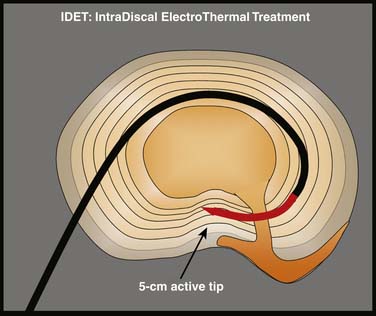
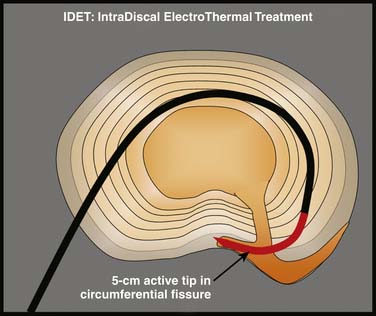
Intradiscal electrothermal annuloplasty (IDET) uses the Smith and Nephew Endoscopy (Andover, MA) SpineCath System in which a navigable catheter with an embedded thermal resistive coil is inserted posterolaterally into the AF. The catheter is then advanced through the disc circumferentially to return posteriorly. With the use of indirect radiofrequency energy, electrothermal heat is then generated with the thermal resistive coil; the disc material is heated for up to 20 minutes to 90° C (Fig. 40-1).
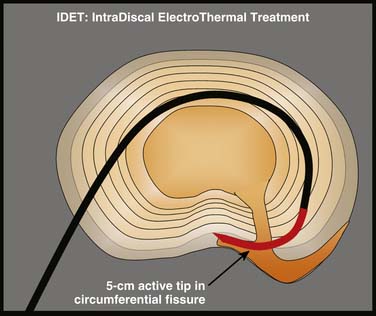
Figure 40-1 Schematic representation of IDET with the lesion seemingly applied across the radial tear.
Intradiscal biacuplasty (Baylis Medical, Montreal, Canada), uses RF energy directly to heat the AF while circulating water is used to cool the tissue that is adjacent to the needle. Two probes are placed on the posterolateral sides of the AF through introducer needles and the disc is heated by RF waves. The electrode surfaces are cooled by circulating water in the electrodes, thereby heating the disc tissue to 45 to 55° C—a temperature range that is considered to be neurotoxic.9–11 In fact, this technique can produce temperatures in the 46° to 67° C range in the inner two thirds of the AF, more than 45° C in the outer third and less than 42° C in the cauda equina,12 thus supporting other evidence that at least the procedure is safe13 (Fig. 40-2).
Heat Lesions: Extent and Effect
IDET and other thermal treatments were developed to produce intradiscal temperatures sufficient to at least ablate AF nerve fibers and also to denature AF collagen. RF lesions on peripheral nerves in dogs showed that at up to 6 weeks, there was a total loss of unmyelinated and a near total loss of myelinated fibers with lesions at temperatures as low as 45° C.14 This figure has not been verified in humans. It was considered that for denervation to occur, intradiscal lesions had to be above 45° C, but it may be that a significantly higher temperature is required. Safety was ensured by keeping temperatures in adjacent tissues below certain tolerance levels. Spinal cord and nerve root ischemia can be prevented by observing that the maximum tolerable temperatures in the CNS are 42.0 to 42.5° C for 40 to60 minutes and 43° C for 10 to 30 minutes.15 It had also been observed that collagen denaturation, from a triple helix to a random coil with variable crosslinking,16 occurred in other tissues at 60° -65° C.17,18 It was noted that this effect produced stiffening in shoulder capsule and tendons, leading to stabilization, as well as subsequent beneficial biologically mediated effects including increased strength; the process was accompanied by histologic changes, such as degradation and replacement of collagen fibrils, and signs of healing including increased cellularity, reactive fibroblasts, and increased vascularization.19 Thus, it was considered that thermal lesions in the disc could achieve collagen denaturation and denervation by producing intradiscal temperatures up to 65° C providing that surrounding structures were not heated beyond tolerance levels.
Subsequent cadaver experiments indicated that the temperatures developed by IDET could reach 60° C or more up to 2 mm from the catheter, 60° C between 2 mm and 4 mm away; and 45° C 9 mm to 14 mm away.20 It is notable that the region of increased temperature around IDET is small.16,20It was later reported that the temperatures reached in the AF by IDET were independent of the degree of DD,21 always reached 45° C, but did not always reach 60° C.21
Experimental Methods and Effects
Some of the cadaveric studies on thermal intradiscal thermal lesions have obvious limitations, not the least being their inability to model short- and long-term tissue responses to trauma.22–25 Live animal models also suffer because they address morphologic changes and not pain responses.
The most significant finding from these studies is that, perhaps consistent with morphologic differences between disc and shoulder capsule, the same process of stiffening does not occur in the in vivo animal disc;26–28 heat does not cause acute or chronic stiffening of the healthy disc.19,29 The first change that occurs after the introduction of any device within the disc is the development of fibrosis.30 There is no healing response.19 For example, at 12 weeks after disc incision, there is proteoglycan death, vascular granulation tissue consistent with an attempt at a healing response extending to one third of the AF depth, with grade 3 or 4 DD (Gries classification),31 and sparse neoinnervation in the outer AF. As an aside, it is notable that these changes do not necessarily occur with AF stab incision needles with a 19 gauge needle.21
The higher the temperature, the greater the AF destruction.21,26,32,33 Bass and colleagues performed an in vivo study, with animal sacrifice at 7, 45, or 180 days, on ovine cervical discs, demonstrating that high temperature treatment (70° C for 10 minutes) causes AF tissue degradation, but lower temperature lesions (52° to 54° C) have minimal adverse biomechanical effects compared to sham treatment.19 The most outstanding feature of intradiscal heat is it causes progressive deterioration of disc morphology without any healing response.19,21 Thus, the process is very different to that seen in joint capsule and tendon.
It is also unlikely that IDET lesions have an effect by denervation. It is theoretically possible that reinnervation of the NP is a significant mechanism for DP. Freemont and coworkers demonstrated a propensity for patients with IDD having fusion for LBP to develop reinnervation into the inner third of the AF (46%) and NP (22%), but also noted that these changes occurred but to a moderately lesser degree in nonpainful disc samples,34 a feature also noted in severely degenerated discs in any case.35 But do these thermal lesions actually denervate the disc? The heat required to produce denervation in peripheral nerves is at least 45° C.36 However, when IDET at 90° C was performed in sheep with experimentally induced posterolateral AF lesions with temperatures in the adjacent AF tissue raised to a mean of 63.6° C and in the NP of 67° C, there was not only a lack of tissue repair, but also a lack of denervation.21
Efficacy of Intradiscal Electrothermal Therapy
Intradiscal electrothermal therapy (IDET) is, by far, the most studied intradiscal therapy. Studies on IDET have been confined to the treatment of IDD. IDET has not been tested on other cohorts, such as patients with NSLBP who, for example, have DP diagnosed with nerve blocks or on patients with MRI-detected morphologic changes. It has also not been studied to any significant extent on other areas of the spine. As IDET targets fissures in the peripheral AF, it is logical that it should only be performed if the cause of pain is in the peripheral AF. The detection of annular fissures is one of a number of criteria for IDD.37
IDET is a technically demanding procedure. It requires precision: the lesion must be placed in the outer zone of the AF or across a radial fissure. IDET creates a small lesion (a few mm); even if the lesion seems well placed in axial views, it may be that in the horizontal dimension the lesion is applied above or below the painful fissure (Fig. 40-3).23 Additionally, if IDET works by neurotomy, the IDET lesion must be large enough to coagulate a critical number of nerve endings to produce pain relief:23 perhaps such widespread denervation is not achievable.
IDET has been found to, at best, benefit only a small cohort of patients with IDD and at worst to have no efficacy. The more important studies on IDET are on patients with IDD as determined by the strict ISIS criteria. In these studies, IDET has been performed optimally, with electrodes placed as far posteriorly in the AF as is feasible.23 Small lesions directed at poor targets should be no better than placebo!
The evidence about IDET derives primarily from two controlled trials on IDET using sham procedures as the control,38,39 and one prospective cohort study using patients denied interventional treatment as a comparison group.40,41 Although the Bogduk40,41and Pauza39 studies showed a number needed to treat (NNT) of five for excellent pain relief with the use of IDET, the Freeman42 study showed no benefit over placebo. Secondary information has been obtained from numerous observational case series.43–64 All of these studies, with the exception of one that did not record the device,46 used the SpineCATH. From these studies six systematic reviews have arisen.65–70
Group data from these observational studies show, in general, modest but definite improvements in pain and function,43,46,50,51,56,58,61,79, and these results seem to be much the same as might be expected from other interventional treatments including spinal fusion for the management of IDD. Reported outcomes have generally been the same for compensation versus noncompensation cases.40,41,47,80,81
There have been numerous systematic reviews that contain comments on IDET.65,66,70,84, Derby and associates consider that IDET provides a cheaper, less invasive, and safer alternative than does spinal surgery for the treatment of IDD—especially in patients with less functional impairment, relatively well maintained disc heights, and DP caused by annular tears or protrusions less that 3 to 4 mm.82 Derby further commented that of six IDET studies with sufficient reported data,39,40,46,52,85 between 38% and 94% (average 71%) of patients reported greater than 50% of pain relief 6 to 24 months after IDET.82
Overall, the success rates for IDET are modest. This may reflect the fact that IDET is a technically demanding procedure. It requires precision: the lesion must be placed in the outer zone of the AF or across a radial fissure, and even if on an optimal position, it creates only a small lesion (a few mm). Even if the lesion seems well placed in axial views, it may be that in the horizontal dimension the lesion is applied above or below the painful fissure.23 Additionally, if IDET works by neurotomy, the IDET lesion must be large enough to coagulate a critical number of nerve endings to produce pain relief:23 perhaps such widespread denervation is not achievable.
Randomized Controlled Studies on IDET
Pauza found 1360 eligible patients after a telephone interview, and, after performing discography on 260 of them, found 64 who met the criteria for IDD using pressure-controlled PD.39 Patients were treated with IDET or sham IDET, with the catheters in excellent position, and were followed for 6 months. The mean pretreatment VAS was 6.6 in the IDET group and 6.5 in the control group, and at 6 months, the improvement in VAS for IDET was 2.4 versus 1.1 in the control group. There was also a significant improvement of physical function in disabled patients and a trend to improvement in SF-36 Bodily Pain subscale. Approximately 40% of patients experienced greater than or equal to 50% pain relief, and approximately one out of five experienced greater than or equal to 75% pain relief.
Freeman performed a prospective, randomized, double-blind, placebo-controlled trial of IDET comparing this treatment group with a sham procedure for the treatment of chronic discogenic low back pain. Inclusion criteria included the presence of one- or two-level symptomatic disc degeneration with posterior or posterolateral annular tears as determined by provocative computed tomography (CT) discography.87 Of the 57 patients, 38 had IDET and 19 had sham IDET. The IDET catheter was positioned to cover at least 75% of the annular tear as defined by the CT discography. Follow-up was for 6 months. This study demonstrated no significant benefit over a 6-month posttreatment period for IDET. Interestingly, neither the sham nor the treatment group made any improvement over this period; there was no placebo effect.
Prospective Cohort Study on IDET
Bogduk and Karasek performed a prospective cohort study with 2-year follow-up in a group of 53 patients with similar pain scores evaluating the use of IDET after establishing the diagnosis of IDD according to ISIS protocol and then performing IDET on 36 patients and comparing them to another group of 17 patients who were refused treatment by the insurers.40 The comparison group can be considered representative of the natural history of LBP treated by conventional and conservative treatment regimens, although there may be a degree of negative effect relating to the intransigence of the insurers; perhaps this group then reflects the normal situation in real medicine because the insurers often are a significant barrier to patient management. This study found that the comparison group stayed the same in regard to pain over the 2 years, with only one individual reporting partial improvement, the rest no significant improvement, and none being completely relieved at either 12 or 24 months. This contrasted substantially with those treated with IDET; at 24 months, 54% of these patients had achieved at least 50% relief of their pain, were no longer using opioids, and were at work. Even more significantly, seven patients (20%) were totally free of pain and at work at 24 months. Thus, this data meant that for IDET, the number needed to NNT for a complete resolution of pain was five, and the NNT for 50% reduction in pain was three. Furthermore, 54% of IDET patients experienced a 50% or better reduction in pain and return to work and no need for opioids compared to 10% of the group undergoing typical treatment including rehabilitation.23
Efficacy of Percutaneous Intradiscal Radiofrequency Thermocoagulation (PIRFT)
Efficacy of Intranuclear PIRFT
In intranuclear PIRFT, a needle is inserted into the disc through which an electrode or flexible catheter is placed into the center of the NP of the nucleus and is slowly heated to 50 to 80° C for 90 to 360 seconds. The initial pilot study by Van Kleef and colleagues reported some success in a case series study of 39 consecutive patients with a minimum of 12 months LBP undergoing PIRFT with a 90-second 70° C lesion protocol. At 8 weeks 21/39 patients (54%), 7 of whom had had previous operation and 14 who had no operation, reported an adequate reduction in pain; at 16 months 16/39 patients (41%) reported reduction in pain.88
Barendse and coworkers performed an RCT on a cohort of NSLBP patients (n = 28) selected on the basis of positive (more than 50% relief) AD performed at L4-5 and L5-S1.89 Patients were randomized and after the RF probe was inserted underwent PIRFT with 90 second 70° C lesions (n = 11) or sham (n = 13). At follow-up there was no difference between the two groups. Either the selection process was inadequate (AD alone at two levels does not comply with the diagnostic protocol for IDD) and/or the treatment method itself was inadequate.
Ercelen and associates sought to determine whether the selection method or technique parameters made any difference to the RCT outcome.90 After 60 patients were diagnosed with IDD with PD, 39 patients were randomized into lesions over 120 seconds and 360 seconds both at 80° C. There was no difference between the groups at any time in the 6 months of evaluation. Pain scores were similar at 6 months to inception; the only difference was a significant decrease in pain in the first month seen in both groups. It could be concluded that PIRFT has no utility in the management of IDD or NSLBP diagnosed on the grounds of 50% relief with analgesic discography.
Azulay and coworkers used a different system to perform intranuclear RF thermal lesions on 17 patients with persistent IDD, based on at least one level positive PD, for at least 6 months (mean duration was 50.6 months).91 The criteria for diagnosing DP was abnormal T2-weighted images and if no radicular pain was present, disc bulging, or herniation. Treatment was not performed if the disc height was more than 50% reduced. The technique was different from previous descriptions; 0.9% saline solution was injected into the NP, impedance levels were monitored, and each disc took about 40 minutes to treat. It was considered that this treatment should deliver safe, persistent, comprehensive heat to the AF at a sufficient degree to cause neural ablation. Using 50% pain relief as a marker of success, 15 patients were responders at 1 month (88%), 9 at 3 months (53%), and 12 at 6 months (70.6%).
Efficacy of Annular PIRFT (Radiofrequency Annuloplasty with discTRODE)
There is one RCT study on RFA. As with intranuclear PIRFT, the RCT showed no benefit over placebo treatment.92 RFA has also been compared to IDET in trial with 42 patients matched into each treatment, with follow-up at 2 weeks and then at 2, 3, 6, 9, and 12 months; the IDET group had significantly better results from the 3-month period.62 In the IDET group VAS decreased from 7.4 ± 1.9 before IDET to 1.4 ±1.9 at 1 year follow-up, compared to a change from 6.6 ± 2.0 before to 4.4 ± 2.4 at 1 year after RFA.
Finch93 performed a case-controlled series on 46 patients with at least 6 months of LBP diagnosed as IDD at a single level. Thirty-one patients underwent RFA and the remaining group who could not get funding for the procedure persisted with usual care. After 12 months, the VAS decreased significantly by 37% in the RFA group, and did not change in the other group. Disability, as measured by the Oswestry Disability Index (ODI) also improved.
Subsequently, Kvarstein performed an RCT using RFA or sham RFA with the discTRODE probe with 10 patients in each group and followed the patients at 6 and 12 months.92 After selection according to strict criteria including positive PD with concordant pain at more than 7/10, patients underwent heating following the 10-minute protocol established by Finch,94 which is incremental heating starting at 50° C increasing by 5° C every second minute ending with a 4-minute interval at 65° C. There was no difference between the groups.
Efficacy of Disc Biacuplasty
Disc biacuplasty is in its infancy. Its safety has been studied by Kapural and colleagues,95 who found that it provided a more generalized heat throughout the posterior AF than did IDET, while not heating the CNS to excess. After a reporting successful outcomes on two patients,96 one of whom had previously had a surgical discectomy,97 Kapural and coworkers reported on a 6-month pilot trial on 15 patients with IDD diagnosed on PD—all with disc heights of more than 50% of controls.98 Reassessment occurred at 6 and 12 months.98,99 At 6 months, 13 patients were reassessed. The median VAS had reduced from 7 (±1) to 4 (±1) at 1 month, and stayed much the same at the 6-month and 12-month assessments. Disability and physical function scores also improved but the differences were insignificant. Opioid use continued to decrease after the 6-month follow-up.
Comments on Pulsed RF
Pulsed RF does not cause neural damage. A study on rat sciatic nerve compared the results of PRF lesions to that of RF lesions at 42° C and 70° C for 120 seconds. When the sciatic nerve was studied histologically at 21 days, it was recorded that most axonal damage occurred with the RF 70° C group.100 PRF does not cause significant damage to neural tissue,100 and any changes seem to be reversible.101 In egg white, PRF starts to produce minimal thermocoagulation at 60° C; above this temperature, PRF produces coagulation similar to, but smaller than, that of RF at the same temperature.102
PRF has been compared to RF treatment in the management of ZJ pain and been found to be substantially inferior. When 2 Hz PRF was compared to RF denervation at 80° C and to injection of local anesthetic for the treatment ZJ pain, with needles placed adjacent to the relevant medial branches or dorsal rami, there was a decrease in the VAS and disability scores at 6 and 12 months in the RF group but not the other two groups: PRF had a better short term outcome than did the control group but the improvement was not maintained at 6 or 12 months.103
Various studies have pilot-tested PRF. Intradiscal PRF has been pilot tested in a group of patients with IDD. It is theorized that high-voltage, long-duration intradiscal pulsed radiofrequency with the electrode in the center of the NP might work not from thermal effects but by exposure to electric fields.104 One pilot used PRF for 20 minutes at a setting of 2 × 20 ms/sec and 60 V on eight patients: it reported a reduction of pain score of 4/10 at 3 months and seven of eight patients were pain free at an average follow-up of 12.8 months.104 Pulsed RF has never been tested by an RCT and it seems that these results can be suitably ignored until an RCT on the efficacy is reported for any pain situation and then for the management for the management of DP or IDD.105
Neural RF Thermocoagulation for Discogenic Pain
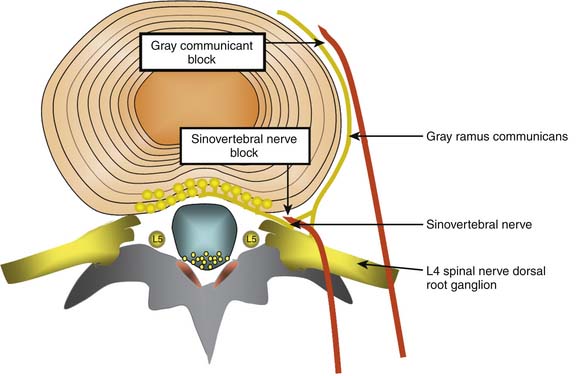
Conceptually, the possible minimally invasive targets for the treatment of a painful disc include the collagen and neural tissue within the disc, as well as the direct nerve supply of the disc (the sinuvertebral nerves, and the gray rami communicantes). As discussed earlier, it is difficult and perhaps impossible to selectively block the sinuvertebral nerve. The rami communicantes nerves are more accessible. However, both nerves innervate tissue other than the disc, so selective denervation of the disc is probably not possible in any case (Fig. 40-4).
Gray Rami communicantes
The efficacy of treatment to these nerves has not been established at any stage. Although RF of the gray rami communicantes was described in 1988 as being well tolerated and at times seemingly successful, it was noted that there was a discrepancy between clinical outcomes and the information gleaned by diagnostic blocks.106 Simopoulos and coworkers used L2 ramus communicans blocks as a basis for performing L2 ramus communicans RF lesions at 80° C for 60 seconds, and in a pilot study of five patients reported “consistent pain relief after a minimum of two radiofrequency lesioning treatments approximately 4 months apart.”107 However, there has been an RCT of RF treatment of these nerves that has established possible efficacy. Oh and Shim performed an RCT on a group of patients who had been diagnosed with IDD according to strict protocol and subsequently had failed treatment with IDET.108 Patients were then excluded if they had subsequent positive medial branch blocks for ZJ pain, and were included after they reported a 50% or greater improvement in pain following a diagnostic block of the appropriate ramus communicans with 2 mL 2% lidocaine and 1 mL contrast medium. The treatment group (n = 26) received RF thermocoagulation (Stryker system) of the ramus communicans nerve at 65° Cfor 60 seconds as well as local steroid injection; the control group (n = 23) received an injection of lidocaine without RF. Patients were followed only for an average of 4 months, and VAS scores were significantly lower in the treatment group at that time. In the treatment group VAS changed from 7.1 to 3.8 (± 1.1) and SF 36 subscales for bodily pain and physical functioning also improved significantly. There was no statistical change in the control group. This is an interesting study that needs to be replicated.
These nerves have also been injected in patients with refractory pain in association with osteoporotic vertebral compression fracture. In one retrospective study, gray rami communicantes blocks with local anesthetic and steroid at the fracture level produced or coincided with a modest improvement of pain over a 9-month period, with decreased analgesic requirements in 42% and medium-to-high patient satisfaction in 75%.109
Sinuvertebral Nerve Treatments
There is some reported evidence that the L2 nerve root might innervate the lower lumbar discs. If so, it might be possible to perform RF lesions to these nerves. However, it should be noted at the outset that bilateral L1 and L2 dorsal root ganglion (DRG) blocks with injectate including 4 mL bupivacaine 0.5% did not give any pain relief in 12 patients with IDD diagnosed with PD.110 As noted earlier, these nerves supply structures other than the disc, and any efficacy from sinuvertebral nerve treatment might have nothing to do with DP.
Murata and associates performed an RCT of L2 nerve root block on patients with NSLBP and/or radicular pain, reporting that the treatment group who had L2 spinal nerve root block with 1% lidocaine and 3.3 mg dexamethasone had a reduction of pain from 69/100 to 14/100 at 5 minutes and the control block group with the same medication at a depth of 2.5 cm had insignificant pain reduction from 68/100 before the injection to 62/100 5 minutes later; incidentally, they also reported a modest treatment effect at 1 and 10 weeks postinjection.111 They found that almost all patients with LBP or radicular pain had significant reduction of pain following the L2 nerve root injection, although it was not clear whether the leg pain was true radicular pain. Another study on the L2 nerve root was that of Nakamura and colleagues, who injected the L2 nerve root in 33 patients with NSLBP, some of whom had sciatica, and reported that needle insertion produced pain radiating to the low back in 23/33 injections, that LBP decreased substantially or totally, and that sciatica was not changed at all in the nine patients with sciatica.112
Furthermore, as sensory nerves from the L2 and L5 vertebral bodies may enter the paravertebral sympathetic trunks and reach the L2 DRG, Ohtori and colleagues performed an RCT on patients with LBP after acute L3 or L4 osteoporotic lumbar vertebral fracture. The treatment group had an L2 spinal nerve root block with 1.5 mL 1% lidocaine and the control group had a subcutaneous injection. Both groups had pain relief that was greater in the treatment group at 1 hour, 1 week, and 2 weeks after treatment, but there was no relief after this time in either group. It was concluded that the L2 DRG might innervate the L3 and L4 vertebral bodies in humans.113 In fact, this study randomized these patients into a treatment and a control group. The treatment group had injection of 1.5 mL 1% lidocaine directed onto the L2 nerve root with fluoroscopic control with contrast and the control group had the same injectate given subcutaneously. In the treatment group, VAS preblock was 70 ± 25 and VAS at 1 hour was 35 ± 23; in the control group, VAS preblock was 72 ± 22, and VAS at 1 hour was 56 ± 23. That is, although there was a possible trend for those patients with nerve root injection to have a decrease in pain, the confidence limits overlapped. It was not clear if any patient had 100% reduction in pain, a figure that might attract more interest if the L3 and L4 vertebral bodies are substantially innervated by the L2 DRG. Furthermore, Mendez and coworkers assessed the ability of anesthetic infiltration of the L2 spinal nerve to predict the results of PD. In 40 cases, L2 spinal nerve blocks were performed at least 2 weeks before PD. It was found that L2 nerve root block predicted the PD outcome in 46.5% of cases only, and the likelihood ratio of close to 1.0 determined that L2 nerve root block is not a surrogate for PD.114
Even if sinuvertebral nerve blocks eradicated pain and it was decided to treat with RF, the proximity of the sinuvertebral nerve to other structures—not the least being the spinal nerve root—makes such heating untenable. Sluijter has proposed the use of pulsed RF (PRF) as a nondestructive method of exposing tissue to RF electric fields.115 However, there is limited evidence that supports the use of low temperature lesions in any area of pain medicine. In a case series report, Tsou and associates performed PRF on the L2 DRG for patients with NSLBP without leg pain and added L3-S1 DRG for those with leg pain. The greatest improvement in pain occurred at 3 months, and at 3 and 12 months approximately 45% of patients had pain relief of 50% or more.116
Intradiscal Chemical Therapies for Discogenic Pain
Proliferants
In one study, 30 patients with chronic NSLBP for an average duration of 8.5 years were diagnosed with IDD on PD and treated with an intradiscal injection of a solution containing glucosamine and chondroitin sulfate combined with hypertonic dextrose and dimethyl sulfoxide (DMSO), which is an industrial solvent also used to treat interstitial cystitis. At an average posttreatment time of 12 months, Roland-Morris scores were 6.4±1.0 from 12.0±0.9 and VAS 3.0±0.4 from 6.1±. Overall, 17/30 (57%) did very well with average of 72% of disability scores and 76% of VAS, whereas the other 13/30 (43%) showed little or no improvement.117
The effect of intradiscal injection of a solution consisting of 1.5 mL 50% dextrose in water and 1.5 mL 0.25% bupivacaine was measured on patients with moderate-to-severe DD without herniation and with positive PD. Patients underwent biweekly injections (average number 3.5). At 18 months, 43% of patients had sustained improvement with an average reduction in VAS of 71%.118
In a pilot study, Derby and colleagues tested intradiscal injection of glucosamine and chondroitin sulfate combined with hypertonic dextrose and DMSO (N = 35) against IDET (N = 74) in patients with IDD diagnosed with PD.119 At 6 to 18 months the VAS improvement was 2.2 for the injection group and 1.3 for the IDET group, with 66% of the injection group and 47% of the IDET reporting feeling better.
Steroids
Intradiscal steroids have been reported for the treatment of disc problems including putative DP.120–125 They have been shown to be no more effective than placebo at 2 weeks and 1 year in patients with a positive PD.120,121 They might be effective in the short term for some patients with vertebral end-plate changes. The rationale for their use is that they interrupt the inflammatory pathway in their action as a phospholipase A2 inhibitor.
The first reports were by Feffer, who subsequently reported on 244 patients injected with intradiscal steroids finding that at follow-up between 4 and 10 years, 47% had recovered and the patients with predominantly LBP were more likely to recover.123,124
Graham compared chemonucleolysis with chymopapain to intradiscal hydrocortisone, with 20 patients in each group, in a double-blind study of patients with “discogenic back pain and sciatica.”126 The 2-year post-injection survey by an independent observer revealed that there was no statistical significance between the results, although the trend suggested superiority of CN, which had nine good, three fair, and eight unimproved results in 20 patients, contrasting with hydrocortisone, which produced three good, eight fair, and eight unimproved results in 20 patients—one patient dying from unrelated causes.
Then Wilkinson and Schuman performed 42 intradiscal injections on a series of 29 patients with NSLBP, and 18 injections on 13 patients with NSNP, none of whom had classic symptoms or signs of true radicular pain, although some were reported as likely to have radicular pain.127 All of the patients underwent analgesic and/or contrast discography, which was reported as showing morphologic changes consistent with degeneration in 27 of the 29 patients: pain provocation was not reported on. Each disc was injected with between 40 mg and 80 mg suspension of methyl prednisolone. There was a fair degree of pain relief early on, and a few patients had relief lasting 6 months to several years. No conclusions could be necessarily drawn from this, except that these injections prevented only 11 of the 29 of the lumbar group from having surgery, and most of these did poorly with surgery anyway.
Simmons reported on patients who had positive PD at 2 weeks, finding that there was no difference between those injected with steroid and those injected with bupivacaine.121 Khot reported on a similar cohort of 120 patients and found no difference at 1 year between injection of saline and steroid.120 However, Fayad investigated steroids in patients with NSLBP who had not recovered with 3 months of conservative treatment.122 This early intervention was found to be more successful at 1 month in those who had Modic type 1 changes or Modic type 2 changes with predominantly edematous changes. At 3 months, the outcomes were similar in all patients with any Modic type 1 or 2 changes.
Methylene Blue
Methylene blue (MB) is attracting increasing interest in the management of IDD. It was first reported as having a possible role in medicine in 1885 by Erlich and by 1928, it had attracted significant interest from its use as an indicator to its possible role as a therapeutic agent.128 Its neurotropic actions and its nerve blocking or neurolytic action129,130 have been used for a variety of applications.131 MB has been used, for example, in the treatment of methemoglobinemia, cyanide poisoning, carbon monoxide poisoning, pruritus ani, and Alzheimer disease. Sheng and coworkers introduced it as a possible treatment for pain by successfully injecting MB with procaine into pelvic fracture sites for the management of fracture pain, with each injection giving pain relief for about 3 weeks.132
MB, by inhibiting the enzyme guanylate cyclase, blocks the accumulation of cyclic guanosine monophosphate (cGMP), thereby reducing the responsiveness of vessels to cGMP-dependent vasodilators like nitric oxide. Nitric oxide is a final common mediator of cartilage degradation.133 As a consequence, it has been tested as a potential intradiscal treatment for DP. In a novel study, Peng and associates injected patients who were otherwise scheduled for lumbar interbody fusion for back pain with an intradiscal injection of one mL of 1% MB followed by 1 mL of 2% lignocaine.131 The criterion for selection was failure of other nonsurgical treatment for at least 6 months, and finding a painful abnormally morphologic disc on PD (no comment was made about adjacent controls). At a mean follow-up period of 18.2 months, 87% (21/24) reported a total or substantial relief of low back pain and improvement in physical function. VAS pain scores pretreatment, and at 3, 6, and more than 12 months were 7.52±1.31, 2.50±2.09, 2.37±2.06, and 2.18±1.79. Oswestry disability scores (ODI) over the same period were 48.71±5.28, 17.42±14.76, 16.79±15.02, and 15.38±13.63. That is, VAS and ODI dropped significantly early on and the improvement was maintained.
Peng and coworkers then ran an RCT on MB treatment for minimum 6 months” DP, with 36 patients in each group. At the 24-month follow-up, both the groups differed substantially with respect to the primary outcomes. The patients in MB injection group showed a mean reduction in pain measured by NRS of 52.50, a mean reduction in Oswestry disability scores of 35.58, and satisfaction rates of 91.6%, compared with 6.91, 1.68, and 14.3%, respectively, in placebo treatment group. Regular medication was still used by 42.9% of the placebo group 24 months after randomization, but in only 8.3% of the MB injection group. No adverse effects or complications were found in the group of patients treated with intradiscal MB injection. Thus, MB appears to be a safe, effective, and minimally invasive method for the treatment of IDD.4 As Peng and coworkers pointed out,4 these outcomes are similar to those reported for fusion or artificial disc replacement,134,140 and MB treatment has a significant advantage of now having been tested with a RCDBT.
Regenerative Therapies for Discogenic Pain
Disc homeostasis is mediated by various substances including cytokines, growth factors, enzymes, and enzyme inhibitors. Proteoglycans play a central role in the function of the disc, and restoration of normal proteoglycan production is a key component of minimizing DD.141 Whether this translates to less LBP is unknown, however. Proteoglycan production can be elevated by up-regulating their production or down-regulating proinflammatory cytokine-mediated catabolism.142
The cytokine TNF-α is found in increased quantities in DD and contributes to matrix degradation by increasing MMP-2 (also called gelatinase) activity.143 Gelatinase cleaves the fragments of collagen that have been degraded by collagenase (MMP-1), which itself has a more direct action as it cleaves type II collagen.144 TNF-α also has a possible role in DP through its role in neuronogenesis.145 Etanercept, a TNF-α blocker, has been injected intradiscally in a double-blind, placebo-controlled pilot study and found to be a safe but ineffective treatment for chronic radicular pain and IDD.146
Biologic substances and drugs can be delivered to the disc directly and also via gene therapy using a gene vector.141 Intradiscal delivery is favored over intravenous delivery because IV solutes do not penetrate the central disc and concentrations dissipate rapidly.147 However, because it is likely that molecules required to affect homeostasis will require a sustained interface, genes encoding the recombinant or natural proteins may turn out to be the optimal method of delivery.148
An example of a regenerative agent under study is osteogenic protein (OP-1). It is from the bone morphogenetic protein (BMP) family, and is unique in that it is both proanabolic and anticatabolic.149 OP-1 is safe when injected into rat NP and epidural space.150 In experimental studies, growth differentiation factor 5 (GDF-5) and osteogenic protein 1 (OP-1) stimulate proteoglycan and collagen synthesis in the disc, with corresponding in vivo reversal of DD features including restoration of disc height, improvement in MRI scores, and histologic grading.151,152 It has therefore been proposed that OP-1 can be used in combination with chemonucleolytic agents such as collagenase to prevent the inevitable degeneration related to chemonucleolysis. In rabbit models, the combined use reversed the decrease seen in disc height of those injected with collagenase alone.153 Perhaps it could also be used in combination with intradiscal thermal therapies, as they, as noted earlier, have been shown to stymie tissue regeneration. However, it may be that the effect of these agents may be insufficient to alter the molecular structure of the disc to a significant extent to ameliorate pain.
Stem cell therapies have also been investigated. It appears that, at least in rabbits, mesenchymal stem cells transplanted into disc tissue survive for many weeks and have the potential to promote disc healing.154 However, when 10 patients with a positive PD underwent intradiscal injection with hematopoietic precursor stem cells (HSCs) and a subsequent 2-week course of hyperbaric oxygen therapy and followed up at 6- and 12- month intervals there was no improvement.155
Intradiscal Chemical Therapies for Radicular Pain
Chemonucleolysis, Chymopapain, Collagenase, Chondroitinase, and Ethanol
After chymopapain (CP) was injected into the NP for the treatment of sciatica in 1963 in a process coined chemonucleolysis (CNL),156 various high-quality studies determined that it was a preeminent treatment for radicular pain because of disc prolapse. It was used in the United States for about 20 years until its demise, which was caused by a variety of political factors causing less utilization and withdrawal by its manufacturers.157 Subsequent to CP, other materials, such as collagenase,158 ethanol,159 and chondroitinase have been developed as potential surrogates.160 CP was definitively proved to be an effective and safe treatment for the treatment of radicular pain owing to disc prolapse but not disc sequestration.161 Its withdrawal indeed defies logic. Even though microsurgery for this condition is relatively safe and effective, it may be that these chemical therapies or other surrogates will be developed. Collagenase certainly showed promise. Ethanol has also been shown to be effective,159 but is difficult to handle. Subsequently, radiopaque gelified ethanol (RGE) has been developed and assessed.162,163 In cervical disc prolapse, the results were rated as satisfactory in 89.5% of patients with no adverse events recorded during the procedure or after.163 In another trial, 276 consecutive patients were divided into two groups, the first containing patients with simple disc prolapse and the second those with disc prolapse in association with more severe symptoms, disc sequestration, or foraminal/canal narrowing. The first group (A) was treated with RGE and intraarticular steroids only. The second group (B) was subdivided into two, the first being treated with RGE and automated percutaneous discectomy, and the third (C) with RGE and radiofrequency nucleoplasty. At follow-up, very good or good results were obtained in 202/221 (91%) patients in group A, in 37/44 (84%) in group B, and in 9/11 (82%) of group C. There was no allergic complication in any of our patients.164
Any chemical therapy should aim to be as effective as CP was. After various studies had shown good results and the optimal dosage,165,166 a high-quality and extensive study on CP for the treatment of disc prolapse was performed by Gogan and Fraser that proved CP was significantly superior to placebo.167 They compared 30 patients injected with CP with 30 patients injected with saline. At 10 years, 80% of the CP patients, who were still blinded to the original treatment, regarded the injection to be successful, compared with 34% for the saline group (P =.0006). Laminectomy had been required in 20% of the CP patients, compared with 47% of the saline patients (P = .028). Seventy-seven percent of the CP group was assessed by a blinded independent observer to be at least moderately improved, compared with 38% for the saline group (P = .004). Over these 10 years, the results for benefit of CP over placebo were maintained: for example, at 6 weeks posttreatment, 73% of CP patients and 37% of placebo patients were considered to have a successful outcome; at 6 months success was 80% and 57%;168 at 2 years, 77% and 47% and at that 2-year review 57% treated with CP were free of pain, compared to 23% in the saline group.169
A systematic review/meta-analysis of 22 eligible trials concerning CP found that chemonucleolysis with CP was superior to placebo and was as effective as collagenase in the treatment of lumbar disc prolapse, with a summary risk ratio estimate for pain relief as outcome of 1.51 (95% CI: 1.27 to 1.80) for the comparison between chemonucleolysis versus placebo, and 1.07 (95% CI: 0.95 to 1.20) for the comparison between chymopapain and collagenase.170 In a 5-year clinical follow-up assessment of a prospective DBRCT of chemonucleolysis using CP or collagenase for the treatment of 6 weeks or more radicular pain caused by disc prolapse short of extrusion revealed good and excellent results in 72% of the CP group and 52% of the collagenase group when progression to surgery was considered a failure. After injection, 18% of the CP group and 28% of the collagenase group underwent microdiscectomy at the injected level. Of the reexamined patients who did not have microdiscectomy, 100% of the CP group and 93% of the collagenase group had good and excellent results. Thus, in the doses used, CP (4000 IU) and collagenase (400 ABC units), collagenase was still effective but not as effective as CP. Both were considered to be safe when injected under controlled conditions.171
However, a review of all treatments on disc prolapse indicates that overall discectomy produces the best results. Gibson and Waddell analyzed all randomized controlled trials published up to January 1, 2007 on the treatment of disc prolapse, reporting that discectomy produced better clinical outcomes than chemonucleolysis, which itself was better than placebo.172 As an example, Van Alphen and colleagues performed a randomized trial comparing open discectomy with chemonucleolysis in 151 patients suffering from a disc herniation at L4-5 or L5-S1. In the immediate postoperative period there was an increase in radicular pain in 16 patients (22%) in the chemonucleolysis group compared to none in the discectomy group.173 Within a follow-up period of 1 year, 18 patients (25%) required open discectomy following failed chemonucleolysis; two patients (3%) in the discectomy group needed a second operation. Open discectomy following previous chemonucleolysis was successful in only 44% of cases. Comparison of the final results of the two modes of treatment 12 months after the last intervention (including second treatment) did not reveal any significant differences.
Ozone
Ozone, injected as an ozone/oxygen mixture, is considered to have a redox action on NP proteoglycan,174 and possibly an antiinflammatory action. A meta-analysis of the use of ozone for the treatment of disc prolapse presented at the Society of Interventional Radiology meeting in 2009 by Murphy, who disclosed a financial interest in the manufacturing of the device used, reported that there were 24 related articles of which 13 (with 8000 patients) met inclusion criteria.174 There was a 79.9% reported improvement in the Modified MacNab scale with this treatment. However, there is virtually no reasonable data on which to reach a conclusion that this treatment has significant benefit.
Most reports relate to the treatment of radicular pain for disc prolapse.174–178 One literature review found five controlled studies, all considered to have methodologic limitations, supporting the efficacy of ozone over microdiscectomy.179 However, a controlled trial by Wu and coworkers in which two groups of 108 patients presenting with lumbar disc prolapse were treated using surgery or the injection of oxygen-ozone combined with collagenase into the lumbar disc or the epidural space. The difference in the success rates of 86.11% and 88.89% in the minimally invasive group at 3 and 12 months, respectively, and 92.59% and 95.37% in surgical group were not statistically significant.178 There have also been reports of possible serious side-effects from this therapy.180,181
Saline
Intradiscal saline, which when injected under high pressure ruptures the thinned posterior longitudinal ligament, has been used and studied for the management of disc prolapse. Tearing of the ligament is confirmed by a sudden loss of resistance to injection and leakage of contrast medium from the disc to the epidural space. It was reported to be successful in patients with large disc herniations.182 However, there is insufficient data on its use to draw any conclusion.
Percutaneous Disc Decompression for Radicular Pain
Nucleoplasty
Gerszten anc colleagues performed a prospective audit of 67 patients (mean age 41 years) with primarily radicular pain owing to a contained disc herniation who underwent nucleoplasty with the Perc-DLE SpineWand and, although they reported improvements in disability at 3 and 6 months, the mean improvement of VAS at 3 months was only 1.3/10.183
A systematic review of the literature relating to mechanical lumbar disc decompression with nucleoplasty reported that there is level II to III evidence for mechanical lumbar percutaneous disc decompression with nucleoplasty in treatment of leg pain, but no evidence available in managing axial low back pain.184
Cervical spine nucleoplasty is at a very early stage of development and research. Percutaneous nucleoplasty and percutaneous cervical discectomy seem to be low-risk procedures that both lead to about 80% significant clinical improvement for the treatment of contained cervical disc herniation.185–187 It should be noted, however, that when cervical discectomy, physiotherapy, and no management was compared at 12 months in the management of cervical radicular pain owing to disc prolapse, the outcomes were identical.188
Bhagia and coworkers studied the complications and side effects of nuceloplasty in a prospective study in 53 patients; they found at 24 hours needle point entry soreness (76%), new numbness and tingling (26%), increased intensity of preprocedure back pain (15%), and new areas of back pain (15%). At 2 weeks, no patient had soreness at the needle insertion site or new areas of back pain; however, new numbness and tingling was present in 15% of patients and two patients (4%) had increased intensity of preprocedure back pain.189 However, there were statistically significant reductions in back and leg pain.
Laser
Lasers have been used in medicine since the 1960s. Percutaneous laser disc decompression has been used as a treatment for radicular pain secondary to contained disc herniation. A systematic review of the literature found only Level II-2 evidence for this use.190
However, laser has also been studied for the treatment of IDD. Percutaneous endoscopic laser annuloplasty (PELA) targets the AF alone. It uses a small endoscope enabling the use of Ho:YAG laser, irrigation and light. This tool used with an extreme posterolateral approach into the posterior AF allows entry into the disc with minimal damage. It was studied in 30 patients with chronic disabling back pain of at least 2 years over a 10-month follow-up period who also had (1) DD and a HIZ on MRI and (2) positive PD at only one level. The discography had been performed just prior to the procedure, and thus the cannula could be placed very close to any annular tear. The mean VAS score improved from 8.0 to 2.4 and the modified Macnab’s criteria also showed a good outcome, with a success rate of 90.0%.191 PELA needs to be studied further.
Automated Percutaneous Lumbar Discectomy
Automated percutaneous lumbar discectomy (APLD) is performed with a pneumatically driven, section-cutting probe that removes disc material about 1 cm anterior to the herniation. There have been two RCTs comparing chemonucleolysis (CN) favorably to APLD.192,193 However, two RCTs compared APLD with microdiscectomy.194,195 In the Chatterjee study, 71 patients with contained disc herniations and radicular pain were treated with either procedure and satisfactory results were seen in 80% of the microdiscectomy group and only 29% of the ALPD group.194 In the other study, only 27 patients were ultimately followed up from 5735 screened patients, of whom 95 were found to be eligible and 36 were enrolled; the success rate in each group was 40%.196
Technical Performance of Intradiscal Procedures
The Procedure
The process for discogram is also described in Chapter 38. Any intradiscal procedure requiring injection into the NP is performed using this method. After intravenous access has been obtained, the patient is prepped and draped, and, if required, adequately sedated. The entry site and the proposed needle track can be injected with local anesthetic with a 3.5-inch spinal needle.
Standard Entry to a Lumbar Disc
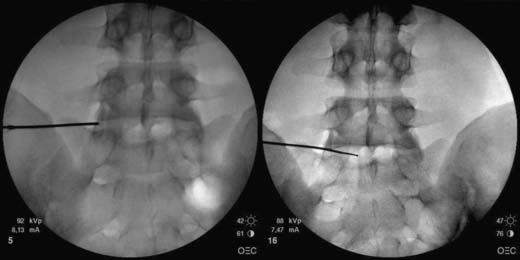
The operator must be aware of the position of the needle or device tip at all times; this requires repetitive C-arm repositioning into PA, oblique, and lateral views. Due to the natural lordotic curve of the lumbar spine, the C-arm is moved in a cephalocaudad direction to achieve a direct visualization into the index disc; the vertebral body should be as close to squared off as possible. The process of getting the end plate square or crisp is commonly referred to as “crisping” the disc. The vertebral end plates above and below the disc are seen to move from an elliptoid image to a crisp line as the angulation approaches ideal view to that disc (Fig. 40-5). The C-arm is then moved in an oblique way to the side of entry. The superior articular process of the ZJ is seen to move across the disc space as the obliquity increases and ideally is positioned at the midpoint of the disc. The skin entry site for direct disc access in the lumbar spine is found as the C-arm is rotated axially from a direct PA position to an oblique position when the superior articular process of the subjacent vertebra bisects the target disc space (Fig. 40-6).
Figure 40-5 Crisping the disc: the superior end plate of L5 is crisp; the inferior end plate of L4 is ellipsoid.
Figure 40-6 An oblique view of the L4-5 disc showing the needle aimed at the midpoint of the L4-5 disc.
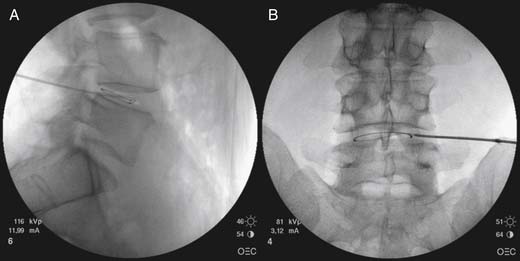
The entry point is just lateral to the superior articular process at the midpoint of the crisp disc. A 14-gauge intravenous cannula is ideal to breach the skin in a down-the-beam view toward the disc. When the stylet is removed from the intravenous cannula, the needle can be placed through the cannula aiming at the disc. It is common to gently brush the lateral aspect of the superior articular pillar and, at this point, the patient may have minor discomfort. When using a curved needle, it is best to allow the natural curve to bounce off the superior articular process when there is an immediate sense of release or loss of resistance; then the curve is rotated 180 degrees, such that the concave side of the curve is now medial and the approach angle into the disc is improved. Lateral and PA images may then be checked under fluoroscopy prior to entering the disc. Contact with the outer AF is associated with increased pressure. Lateral and PA views are required to determine final needle position (Fig. 40-7). Clearly, for discography or other intranuclear injection, the needle is placed centrally within the NP, whereas variations on this are more ideal for nucleoplasty and IDET.
Nucleoplasty
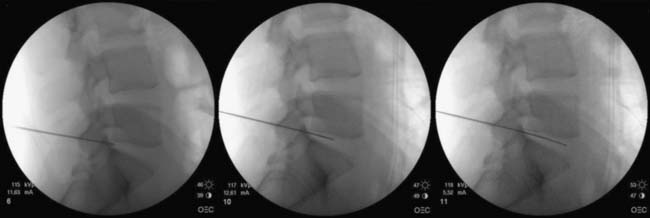
The nucleoplasty procedure is performed using a standard approach into the lumbar disc, as described earlier (Fig. 40-8). In the example shown, the L5-S1 disc has degraded and there is a small, central contained disc prolapse established as contained by discography (Fig. 40-9). In this demonstrated procedure, the needle entry is somewhat lateral to the central point described earlier (Figs. 40-10 and 40-11). When the 14-gauge cannula is accurately placed in the line of the C-arm beam aiming at the disc, then the 17-gauge Crawford needle supplied with the nucleoplasty kit is placed through the cannula toward the disc (Figs. 40-12 and 40-13). In the nucleoplasty procedure, it is ideal to be aiming at the center of the disc, although the Crawford needle is advanced only to the junction between the NP and the AF. When the Crawford needle has breached the AF, PA and lateral views are required to determine this position.
In the lateral view, the spine wand is then introduced through the Crawford needle. It is carefully advanced until the tip is at the end of the Crawford needle and it is then manipulated across the NP until a firm resistance is felt on the anterior AF wall. Regular fluoroscopic monitoring is required to ensure that the wand has not advanced beyond the anterior vertebral margin (Fig. 40-14). A PA view ensures that the wand lies across the central aspect of the disc (Fig. 40-15).
The cable is connected between the power source and the wand and power level 2 is selected (Fig. 40-16). The small spring depth lock is slipped down the spine wand while it is still in the fully deployed position; this helps to protect the wand from being inadvertently advanced beyond this point during the procedure.
IDET
The approach is similar to that described for nucleoplasty, however less obliquity is required and the superior articular process does not need to be at the midvertebral body. The introducing needle is ideally placed approximately one third of the way across the disc in the PA view, but central on the lateral view, thereby lying within the AF (Fig. 40-17).
Figure 40-17 IDET cannula being introduced toward the L4-5 disc. IDET, IntraDiscal ElectroThermal treatment.
The stylet is removed and the IDET Spine-cath is deployed into the disc. It is best to have the concavity of the catheter tip facing medially to facilitate navigation around the AF. Gentle constant pressure is applied to the proximal hub of the catheter as it is observed under fluoroscopy to pass across the anterior AF and then to track posteriorly to the posterolateral AF. AP and lateral views are used to check catheter position, and in particular, to check that the catheter has not inadvertently deployed through a fissure into the epidural space (Fig. 40-18).
At times, the catheter may not deploy to the correct position; it can then be withdrawn into the needle and redeployed with a different catheter orientation. If such manipulation fails to achieve satisfactory positioning, the catheter should be completely withdrawn from the body and the introducer needle adjusted, usually further anteriorly or to the anterolateral quadrant on the opposite side, followed by catheter placement (Figs. 40-19 and 40-20).
Complications
These can be minimized by strict adherence to preprocedural screening and operative technique. In a meta-analysis of IDET, Appleby coworkers197 found the overall incidence of complications for IDET to be 0.8%; of the 17 studies analyzed, 6 reported no complications in 240 patients,39,47,50,198–200 and 23 complications were reported in another 6 studies from procedures on 246 patients.51,201–205 Of these, most resolved, but eight went on to fusion. The complications included increased radicular pain, neuropathic sensations in legs, disc herniation at the level, L5 nerve root injury, dural puncture, discitis, anterolisthesis, increased pain in back, cauda equina lesion, referred leg pain, and headache. The neurologic complications of neuropathic leg sensations, cauda equina lesion, and L5 nerve root injury all resolved.
Should Antibiotics Be Used?
There is no consensus regarding the use of antibiotics in intradiscal procedures. If the risk of infection from discogram is similar to that of an intradiscal procedure, then the risk of infection is extremely small. There are no randomized or controlled trials on the efficacy of prophylactic antibiotics in discography.206 Based on animal experiments,207 the incidence of postdiscography discitis is lowered with the use of cephazolin given either intravenously or in the intradiscal suspension injection. In high concentrations, contrast medium can itself inhibit bacterial growth at least to some degree, and it does not reduce the inhibitory effect of antiobiotics.208 A follow-up of 127 consecutive patients having lumbar discography with contrast containing cephazolin 1 mg per mL revealed no cases of discitis. 207 However, Willems and colleagues had no cases of discitis when they performed two-needle technique discography without preemptive antibiotics on 200 patients and followed them all for a minimum period of 3 months.209 Willems and colleagues also reviewed the literature concerning discitis following discography. They reported on 10 papers spanning a period of 40 years from 1962 to 1990, one of which used prophylactic antibiotics and reported no cases of discitis,207 and the other 9,210–218 which when combined with the Willems study, showed that the overall rate of discitis without prophylactic antibiotics was 0.24% of all patients (12 cases in 5091 patients) or 0.091% of all discograms (12 cases in 13, 205 discs).209 Other possible complications with any of these procedures includes wound infection, superficial abscess, epidural abscess,180 discitis,180 osteomyelitis, and septic arthritis.
Can Disc Puncture Itself Cause Harm?
Animal models that study DD have used stab incisions into the AF because these induce generalized DD within the entire disc.26,219 In one experiment, looking for the best way to model for DD, a 21-gauge, three-puncture technique was superior to an 18-gauge, one-puncture in producing disc degeneration over a shorter period of time.219 This introduces a question about the relationship between number of penetrations versus size; it may be that a smoother technique with one introduction of a thin needle has a minimal chance of producing a detrimental effect. Two studies are worth assessing in this regard.
In a long-term follow-up of patients undergoing disc puncture, Carragee followed two matched cohorts of 75 each without serious LBP “illness.”220 Each subject was assessed with MRI and one group underwent L3-4, L4-5, and L5-S1 discography examination in 1997. There were 134 needle injections with a 25-gauge needle and 21 injections with a 22-gauge needle. Between 7 and 10 years after enrollment, each subject underwent a further MRI: 50 discography subjects and 52 control subjects were reassessed. The results were as follows: (1) in all graded or measured parameters, discs that had been exposed to puncture and injection had greater progression of degenerative findings compared to control (noninjected) discs—this was represented by: progression of disc degeneration, 54 discs (35%) in the discography group compared to 21 (14%) in the control group; ] 55 new disc herniations in the discography group compared to 22 in the control group; (2) new disc herniations were disproportionately found on the side of the AF puncture; (3) the quantitative measures of disc height and disc signal also showed significantly greater loss of disc height and signal intensity in the discography disc compared to the control disc; and (4) there was no statistical differences in the progression of degeneration findings between the smaller and larger gauge needles, although there was a trend to greater degeneration in the discs punctured with the larger 22-gauge needle. There was no comment made regarding the number of penetrations made per discogram.
In contrast, Freeman and colleagues performed a study on IDET in live sheep.21 As part of the study, normal discs were incised with a 19-gauge needle, and again, no comment was made about the number of disc penetrations. At 18 months, there was no alteration of disc morphology compared to controls.
If true, the consequences of disc puncture in discography are germane for any intradiscal procedure. It may be that the number of needle penetrations is as important as the gauge of the penetration instrument. However, if these reported effects are generalizable, they may or may not translate into symptoms and, if they do, it would seem that by now there should have been an epidemic of symptomatic disc prolapses in subjects who had undergone discography. Such an epidemic has not been reported.
1. Smith L., Garvin P.J., Gesler R.M., Jennings R.B. Enzyme dissolution of the nucleus pulposus. Nature. 198, 1963. 1311-1312
2. International Spinal Intervention Society. Lumbar disc stimulation. In Bogduk N ed: Practice Guidelines for Spinal Diagnostic and Treatment Procedures. San Francisco, 2004:20–46.
3. Bogduk N., Fraifeld E.M. Proof or consequences: Who shall pay for the evidence in medicine? Pain Med. 2010;11:1-2.
4. Peng B., Pang X., Wu Y., et al. A randomized placebo-controlled trial of intradiscal methylene blue injection for the treatment of chronic discogenic low back pain. Pain. 2010;149:124-129.
5. Bogduk N: Why I pursue discogenic pain. Annual Scientific Meeting of the German Pain Society. 2005.
6. Nau W.H., Diederich C.J. Evaluation of temperature distributions in cadaveric lumbar spine during nucleoplasty. Phys Med Biol. 2004;49:1583-1594.
7. McLean A. Bovie Electrosurgical Current Generator. Arch Surg. 1929;18:1863-1870.
8. Sweet W.H., Mark V.H. Unipolar anodal electrolyte lesions in the brain of man and cat: Report of five human cases with electrically produced bulbar or mesencephalic tractotomies. Arch Neurol Psychiatry. 1953;70:224-234.
9. Smith H.P., McWhorter J.M., Challa V.R. Radiofrequency neurolysis in a clinical model. Neuropathological correlation. J Neurosurg. 1981;55:246-253.
10. Bono C.M., Iki K., Jalota A., et al. Temperatures within the lumbar disc and endplates during intradiscal electrothermal therapy: formulation of a predictive temperature map in relation to distance from the catheter. Spine. 2004;29:1124-1129.
11. Shah R.V., Lutz G.E., Lee J., et al. Intradiskal electrothermal therapy: a preliminary histologic study. Arch Phys Med Rehabil. 2001;82:1230-1237.
12. Pauza K. Cadaveric intervertebral disc temperature mapping during disc biacuplasty. Pain Physician. 2008;11:669-676.
13. Petersohn J.D., Conquergood L.R., Leung M. Acute histologic effects and thermal distribution profile of disc biacuplasty using a novel water-cooled bipolar electrode system in an in vivo porcine model. Pain Med. 2008;9:26-32.
14. Smith H.P., McWhorter J.M., Challa V.R. Radiofrequency neurolysis in a clinical model. Neuropathological correlation. J Neurosurg. 1981;55:246-253.
15. Haveman J., Sminia P., Wondergem J., et al. Effects of hyperthermia on the central nervous system: what was learnt from animal studies? Int J Hyperthermia. 2005;21:473-487.
16. Kleinstueck F.S., Diederich C.J., Nau W.H., et al. Acute biomechanical and histological effects of intradiscal electrothermal therapy on human lumbar discs. Spine. 2001;26:2198-2207.
17. Hecht P., Hayashi K., Cooley A.J., et al. The thermal effect of monopolar radiofrequency energy on the properties of joint capsule. An in vivo histologic study using a sheep model. Am J Sports Med. 1998;26:808-814.
18. Lee J.M., Pereira C.A., Abdulla D., et al. A multi-sample denaturation temperature tester for collagenous biomaterials. Med Eng Phys. 1995;17:115-121.
19. Bass E.C., Nau W.H., Diederich C.J., et al. Intradiscal thermal therapy does not stimulate biologic remodeling in an in vivo sheep model. Spine. 2006;31:139-145.
20. Kleinstueck F.S., Diederich C.J., Nau W.H., et al. Temperature and thermal dose distributions during intradiscal electrothermal therapy in the cadaveric lumbar spine. Spine. 2003;28:1700-1708.
21. Freeman B.J., Walters R.M., Moore R.J., Fraser R.D. Does intradiscal electrothermal therapy denervate and repair experimentally induced posterolateral annular tears in an animal model? Spine. 2003;28:2602-2608.
22. Karasek M., Bogduk N. Intradiscal electrothermal annuloplasty: percutaneous treatment of chronic discogenic low back pain. Techniques in Regional Anesthesia and Pain Management. 2001;5:130-135.
23. Bogduk N., Lau P., Govind J., et al. Intradiscal electrothermal therapy. Tech Reg Anesth Pain Manag. 2005;9:25-34.
24. Skrzynski S., Sionkowska A., Marciniak A. DSC Study of Collagen in Disc Disease. J Biophys. 2009:819635.
25. Hecht P., Hayashi K., Cooley A.J., et al. The thermal effect of monopolar radiofrequency energy on the properties of joint capsule. An in vivo histologic study using a sheep model. Am J Sports Med. 1998;26:808-814.
26. Osti O.L., Vernon-Roberts B., Fraser R.D. 1990 Volvo Award in experimental studies. Anulus tears and intervertebral disc degeneration. An experimental study using an animal model. Spine. 1990;15:762-767.
27. Lipson S.J., Muir H. 1980 Volvo award in basic science. Proteoglycans in experimental intervertebral disc degeneration. Spine. 1981;6:194-210.
28. Niinimaki J., Ruohonen J., Silfverhuth M., et al. Quantitative magnetic resonance imaging of experimentally injured porcine intervertebral disc. Acta Radiol. 2007;48:643-649.
29. Bass E.C., Wistrom E.V., Diederich C.J., et al. Heat-induced changes in porcine annulus fibrosus biomechanics. J Biomech. 2004;37:233-240.
30. Osti O.L., Vernon-Roberts B., Moore R., Fraser R.D. Annular tears and disc degeneration in the lumbar spine. A post-mortem study of 135 discs. J Bone Joint Surg Br. 1992;74:678-682.
31. Gries N.C., Berlemann U., Moore R.J., Vernon-Roberts B. Early histologic changes in lower lumbar discs and facet joints and their correlation. Eur Spine J. 2000;9:23-29.
32. Carragee E.J., Don A.S., Hurwitz E.L., et al. Does discography cause accelerated progression of degeneration changes in the lumbar disc: a ten-year matched cohort study. Spine. 2009;34:2338-2345.
33. Shah R.V., Lutz G.E., Lee J., et al. Intradiskal electrothermal therapy: A preliminary histologic study. Arch Phys Med Rehabil. 2001;82:1230-1237.
34. Freemont A.J., Peacock T.E., Goupille P., et al. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178-181.
35. Coppes M.H., Marani E., Thomeer R.T., et al. Innervation of “painful” lumbar discs. Spine. 1997;22:2342-2350.
36. Smith H.P., McWhorter J.M., Challa V.R. Radiofrequency neurolysis in a clinical model. Neuropathological correlation. J Neurosurg. 1981;55:246-253.
37. Bogduk N. The lumbar disc and low back pain. Neurosurg Clin N Am. 1991;2:791-806.
38. Freeman B.J., Fraser R.D., Cain C.M., et al. A randomized, double-blind, controlled trial: intradiscal electrothermal therapy versus placebo for the treatment of chronic discogenic low back pain. Spine. 2005;30:2369-2378.
39. Pauza K.J., Howell S., Dreyfuss P., et al. A randomized, placebo-controlled trial of intradiscal electrothermal therapy for the treatment of discogenic low back pain. Spine J. 2004;4:27-35.
40. Bogduk N., Karasek M. Two-year follow-up of a controlled trial of intradiscal electrothermal anuloplasty for chronic low back pain resulting from internal disc disruption. Spine J. 2002;2:343-350.
41. Karasek M., Bogduk N. Twelve-month follow-up of a controlled trial of intradiscal thermal anuloplasty for back pain due to internal disc disruption. Spine. 2000;25:2601-2607.
42. Freeman B.J., Fraser R.D., Cain C.M., et al. A randomized, double-blind, controlled trial: Intradiscal electrothermal therapy versus placebo for the treatment of chronic discogenic low back pain. Spine. 2005;30:2369-2378.
43. Derby R., Lee S.H., Seo K.S., et al. Efficacy of IDET for relief of leg pain associated with discogenic low back pain. Pain Pract. 2004;4:281-285.
44. Gerszten P.C., Welch W.C., McGrath P.M., et al. A prospective outcomes study of patients undergoing intradiscal electrothermy (IDET) for chronic low back pain. Pain Physician. 2002;5:360-364.
45. Lee M.S., Cooper G., Lutz G.E., et al. Intradiscal electrothermal therapy (IDET) for treatment of chronic lumbar discogenic pain: a minimum 2-year clinical outcome study. Pain Physician. 2003;6:443-448.
46. Maurer P., Block J.E., Squillante D. Intradiscal electrothermal therapy (IDET) provides effective symptom relief in patients with discogenic low back pain. J Spinal Disord Tech. 2008;21:55-62.
47. Endres S.M., Fiedler G.A., Larson K.L. Effectiveness of intradiscal electrothermal therapy in increasing function and reducing chronic low back pain in selected patients. WMJ. 2002;101:31-34.
48. Saal J.A., Saal J.S. Intradiscal electrothermal treatment for chronic discogenic low back pain: A prospective outcome study with minimum 1-year follow-up. Spine. 2000;25:2622-2627.
49. Saal J.A., Saal J.S. Intradiscal electrothermal treatment for chronic discogenic low back pain: Prospective outcome study with a minimum 2-year follow-up. Spine. 2002;27:966-974.
50. Singh V. Intradiscal electrothermal therapy: a preliminary report. Pain Physician. 2000;3:367-373.
51. Derby R., Eek B., Chen Y. Intradiscal electrothermal annuloplasty (IDET): A novel approach for treating chronic discogenic back pain. Neuromodulation. 2000;3:82-88.
52. Lutz C., Lutz G.E., Cooke P.M. Treatment of chronic lumbar diskogenic pain with intradiskal electrothermal therapy: a prospective outcome study. Arch Phys Med Rehabil. 2003;84:23-28.
53. Cohen S.P., Larkin T., Abdi S., et al. Risk factors for failure and complications of intradiscal electrothermal therapy: a pilot study. Spine. 2003;28:1142-1147.
54. Freedman B.A., Cohen S.P., Kuklo T.R., et al. Intradiscal electrothermal therapy (IDET) for chronic low back pain in active-duty soldiers: 2-year follow-up. Spine J. 2003;3:502-509.
55. Spruit M., Jacobs W.C. Pain and function after intradiscal electrothermal treatment (IDET) for symptomatic lumbar disc degeneration. Eur Spine J. 2002;11:589-593.
56. Ergun R., Sekerci Z., Bulut H., et al. Intradiscal electrothermal treatment for chronic discogenic low back pain: a prospective outcome study of 39 patients with the Oswestry disability index at 18 month follow-up. Neurol Res. 2008;30:411-416.
57. Chen W., Tong G.H., Wu C.X., et al. [Intradiscal electrothermal treatment combined with epidural steroid injection for discogenic lumbago-leg pain]. Zhonghua Yi.Xue.Za Zhi. 2007;87:1742-1745.
58. Park S.Y., Moon S.H., Park M.S., et al. Intradiscal electrothermal treatment for chronic lower back pain patients with internal disc disruption. Yonsei Med J. 2005;46:539-545.
59. Bryce D.A., Nelson J., Glurich I., et al. Intradiscal electrothermal annuloplasty therapy: a case series study leading to new considerations. WMJ. 2005;104:39-46.
60. Glavinovic M.I., Law Min J.C., Kapural L., et al. Speed of action of various muscle relaxants at the neuromuscular junction binding vs. buffering hypothesis. J Pharmacol Exp Ther. 1993;265:1181-1186.
61. Mekhail N., Kapural L. Intradiscal thermal annuloplasty for discogenic pain: a outcome study. Pain Pract. 2004;4:84-90.
62. Kapural L., Hayek S., Malak O., et al. Intradiscal thermal annuloplasty versus intradiscal radiofrequency ablation for the treatment of discogenic pain: a prospective matched control trial. Pain Med. 2005;6:425-431.
63. Kapural L., Mekhail N., Korunda Z., et al. Intradiscal thermal annuloplasty for the treatment of lumbar discogenic pain in patients with multilevel degenerative disc disease. Anesth Analg. 2004;99:472-476.
64. Saal J.S., Saal J.A. Management of chronic discogenic low back pain with a thermal intradiscal catheter. A preliminary report. Spine. 2000;25:382-388.
65. Gibson J.N., Waddell G. Surgery for degenerative lumbar spondylosis: updated Cochrane Review. Spine. 2005;30:2312-2320.
66. Appleby D., Andersson G., Totta M. Meta-analysis of the efficacy and safety of intradiscal electrothermal therapy (IDET). Pain Med. 2006;7:308-316.
67. Andersson G.B., Mekhail N.A., Block J.E. Treatment of intractable discogenic low back pain. A systematic review of spinal fusion and intradiscal electrothermal therapy (IDET). Pain Physician. 2006;9:237-248.
68. Freeman B.J. IDET: a critical appraisal of the evidence. Eur Spine J. 2006;15(Suppl 3):S448-S457.
69. Helm S., Hayek S.M., Benyamin R.M., Manchikanti L., et al. Systematic review of the effectiveness of thermal annular procedures in treating discogenic low back pain. Pain Physician. 2009;12:207-232.
70. Urrutia G., Kovacs F., Nishishinya M.B., Olabe J. Percutaneous thermocoagulation intradiscal techniques for discogenic low back pain. Spine. 2007;32:1146-1154.
71. Bryce D.A., Nelson J., Glurich I., et al. Intradiscal electrothermal annuloplasty therapy: A case series study leading to new considerations. WMJ. 2005;104:39-46.
72. Chen W., Tong G.H., Wu C.X., et al. [Intradiscal electrothermal treatment combined with epidural steroid injection for discogenic lumbago-leg pain]. Zhonghua Yi.Xue.Za Zhi. 2007;87:1742-1745.
73. Lee M.S., Cooper G., Lutz G.E., et al. Intradiscal electrothermal therapy (IDET) for treatment of chronic lumbar discogenic pain: A minimum 2-year clinical outcome study. Pain Physician. 2003;6:443-448.
74. Lutz C., Lutz G.E., Cooke P.M. Treatment of chronic lumbar diskogenic pain with intradiskal electrothermal therapy: A prospective outcome study. Arch Phys Med Rehabil. 2003;84:23-28.
75. Spruit M., Jacobs W.C. Pain and function after intradiscal electrothermal treatment (IDET) for symptomatic lumbar disc degeneration. Eur Spine J. 2002;11:589-593.
76. Gerszten P.C., Welch W.C., McGrath P.M., et al. A prospective outcomes study of patients undergoing intradiscal electrothermy (IDET) for chronic low back pain. Pain Physician. 2002;5:360-364.
77. Saal J.A., Saal J.S. Intradiscal electrothermal treatment for chronic discogenic low back pain: Prospective outcome study with a minimum 2-year follow-up. Spine. 2002;27:966-973.
78. Saal J.A., Saal J.S. Intradiscal electrothermal treatment for chronic discogenic low back pain: A prospective outcome study with minimum 1-year follow-up. Spine. 2000;25:2622-2627.
79. Assietti R., Morosi M., Block J.E. Intradiscal electrothermal therapy for symptomatic internal disc disruption: 24-month results and predictors of clinical success. J Neurosurg Spine. 2010;12:320-326.
80. Lee M.S., Cooper G., Lutz G.E., et al. Intradiscal electrothermal therapy (IDET) for treatment of chronic lumbar discogenic pain: A minimum 2-year clinical outcome study. Pain Physician. 2003;6:443-448.
81. Lutz C., Lutz G.E., Cooke P.M. Treatment of chronic lumbar diskogenic pain with intradiskal electrothermal therapy: A prospective outcome study. Arch Phys Med Rehabil. 2003;84:23-28.
82. Derby R., Baker R.M., Lee C.H., Anderson P.A. Evidence-informed management of chronic low back pain with intradiscal electrothermal therapy. Spine J. 2008;8:80-95.
83. Boswell M.V., Trescot A.M., Datta S., et al. Interventional techniques: evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician. 2007;10:7-111.
84. Helm S., Hayek S.M., Benyamin R.M., et al. Systematic review of the effectiveness of thermal annular procedures in treating discogenic low back pain. Pain Physician. 2009;12:207-232.
85. Saal J.A., Saal J.S. Intradiscal electrothermal treatment for chronic discogenic low back pain: prospective outcome study with a minimum 2-year follow-up. Spine. 2002;27:966-973.
86. Lee M.S., Cooper G., Lutz G.E., et al. Intradiscal electrothermal therapy (IDET) for treatment of chronic lumbar discogenic pain: A minimum 2-year clinical outcome study. Pain Physician. 2003;6:443-448.
87. Freeman B.J., Fraser R.D., Cain C.M., et al. A randomized, double-blind, controlled trial: Intradiscal electrothermal therapy versus placebo for the treatment of chronic discogenic low back pain. Spine. 2005;30:2369-2377.
88. van Kleef M., Barendse G., Wilmink J.T., et al. Percutaneous intradiscal radio-frequency thermocoagulation in chronic non-specific low back pain. Pain Clinic. 1996;9:259-268.
89. Barendse G.A., van Den Berg S.G., Kessels A.H., et al. Randomized controlled trial of percutaneous intradiscal radiofrequency thermocoagulation for chronic discogenic back pain: lack of effect from a 90-second 70° C lesion. Spine. 2001;26:287-292.
90. Ercelen O., Bulutcu E., Oktenoglu T., et al. Radiofrequency lesioning using two different time modalities for the treatment of lumbar discogenic pain: a randomized trial. Spine. 2003;28:1922-1927.
91. Azulay N., Forgerit M., Alava E.G., et al. A novel radiofrequency thermocoagulation method for treatment of lower back pain: thermal conduction after instillation of saline solution into the nucleus pulposus—preliminary results. Acta Radiol. 2008;49:934-939.
92. Kvarstein G., Mawe L., Indahl A., et al. A randomized double-blind controlled trial of intra-annular radiofrequency thermal disc therapy—a 12-month follow-up. Pain. 2009;145:279-286.
93. Finch P.M., Price L.M., Drummond P.D. Radiofrequency heating of painful annular disruptions: one-year outcomes. J Spinal Disord Tech. 2005;18:6-13.
94. Finch P.M. The use of radiofrequency heat lesions in the treatment of lumbar discogenic pain. Pain Pract. 2002;2:235-240.
95. Kapural L., Mekhail N., Hicks D., et al. Histological changes and temperature distribution studies of a novel bipolar radiofrequency heating system in degenerated and nondegenerated human cadaver lumbar discs. Pain Med. 2008;9:68-75.
96. Kapural L., Mekhail N. Novel intradiscal biacuplasty (IDB) for the treatment of lumbar discogenic pain. Pain Pract. 2007;7:130-134.
97. Kapural L., Cata J.P., Narouze S. Successful treatment of lumbar discogenic pain using intradiscal biacuplasty in previously discectomized disc. Pain Pract. 2009;9:130-134.
98. Kapural L., Ng A., Dalton J., et al. Intervertebral disc biacuplasty for the treatment of lumbar discogenic pain: results of a six-month follow-up. Pain Med. 2008;9:60-67.
99. Kapural L. Intervertebral disk cooled bipolar radiofrequency (intradiskal biacuplasty) for the treatment of lumbar diskogenic pain: a 12-month follow-up of the pilot study. Pain Med. 2008;9:407-408.
100. Tun K., Cemil B., Gurcay A.G., et al. Ultrastructural evaluation of Pulsed Radiofrequency and Conventional Radiofrequency lesions in rat sciatic nerve. Surg Neurol. 2009;72:496-501.
101. Tun K., Savas A., Sargon M.F., et al. The histopathological and electron-microscopic examination of the stereotactic pulsed radiofrequency and conventional radiofrequency thermocoagulation lesions in rat brain. Neurol Res. 2006;28:841-844.
102. Heavner J.E., Boswell M.V., Racz G.B. A comparison of pulsed radiofrequency and continuous radiofrequency on thermocoagulation of egg white in vitro. Pain Physician. 2006;9:135-137.
103. Tekin I., Mirzai H., Ok G., et al. A comparison of conventional and pulsed radiofrequency denervation in the treatment of chronic facet joint pain. Clin J Pain. 2007;23:524-529.
104. Teixeira A., Sluijter M.E. Intradiscal high-voltage, long-duration pulsed radiofrequency for discogenic pain: a preliminary report. Pain Med. 2006;7:424-428.
105. van Boxem K., van Eerd M., Brinkhuize T., et al. Radiofrequency and pulsed radiofrequency treatment of chronic pain syndromes: the available evidence. Pain Pract. 2008;8:385-393.
106. Sluijter M.E. The use of radiofrequency lesions for pain relief in failed back patients. Int Disabil Stud. 1988;10:37-43.
107. Simopoulos T.T., Malik A.B., Sial K.A., et al. Radiofrequency lesioning of the L2 ramus communicans in managing discogenic low back pain. Pain Physician. 2005;8:61-65.
108. Oh W.S., Shim J.C. A randomized controlled trial of radiofrequency denervation of the ramus communicans nerve for chronic discogenic low back pain. Clin J Pain. 2004;20:55-60.
109. Chandler G., Dalley G., Hemmer J.Jr., Seely T. Gray ramus communicans nerve block: novel treatment approach for painful osteoporotic vertebral compression fracture. South Med J. 2001;94:387-393.
110. Richardson J., Collinghan N., Scally A.J., Gupta S. Bilateral L1 and L2 dorsal root ganglion blocks for discogenic low-back pain. Br J Anaesth. 2009;103:416-419.
111. Murata Y., Kato Y., Miyamoto K., Takahashi K. Clinical study of low back pain and radicular pain pathways by using l2 spinal nerve root infiltration: a randomized, controlled, clinical trial. Spine. 2009;34:2008-2013.
112. Nakamura S.I., Takahashi K., Takahashi Y., et al. The afferent pathways of discogenic low-back pain. Evaluation of L2 spinal nerve infiltration. J Bone Joint Surg Br. 1996;78:606-612.
113. Ohtori S., Yamashita M., Inoue G., et al. L2 spinal nerve-block effects on acute low back pain from osteoporotic vertebral fracture. J Pain. 2009;10:870-875.
114. Mendez R., Bailey S., Paine G., et al. Evaluation of the L2 spinal nerve root infiltration as a diagnostic tool for discogenic low back pain. Pain Physician. 2005;8:55-59.
115. Sluijter M.E. The role of radiofrequency in failed back surgery patients. Curr Rev Pain. 2000;4:49-53.
116. Tsou H.K., Chao S.C., Wang C.J., et al. Percutaneous pulsed radiofrequency applied to the L-2 dorsal root ganglion for treatment of chronic low-back pain: 3-year experience. J Neurosurg Spine. 2010;12:190-196.
117. Klein R.G., Eek B.C., O’Neill C.W., et al. Biochemical injection treatment for discogenic low back pain: a pilot study. Spine J. 2003;3:220-226.
118. Miller M.R., Mathews R.S., Reeves K.D. Treatment of painful advanced internal lumbar disc derangement with intradiscal injection of hypertonic dextrose. Pain Physician. 2006;9:115-121.
119. Derby R., Eek B., Lee S.H., et al. Comparison of intradiscal restorative injections and intradiscal electrothermal treatment (IDET) in the treatment of low back pain. Pain Physician. 2004;7:63-66.
120. Khot A., Bowditch M., Powell J., Sharp D. The use of intradiscal steroid therapy for lumbar spinal discogenic pain: a randomized controlled trial. Spine. 2004;29:833-837.
121. Simmons J.W., McMillin J.N., Emery S.F., et al. Intradiscal steroids. A prospective double-blind clinical trial. Spine. 1992;17(suppl 6):S172-S175.
122. Fayad F., Lefevre-Colau M.M., Rannou F., et al. Relation of inflammatory modic changes to intradiscal steroid injection outcome in chronic low back pain. Eur Spine J. 2007;16:925-931.
123. Feffer H.L. Therapeutic intradiscal hydrocortisone. A long-term study. Clin Orthop Relat Res. 1969;67:100-104.
124. Feffer H.L. Treatment of low-back and sciatic pain by the injection of hydrocortisone into degenerated intervertebral discs. J Bone Joint Surg Am. 1956;38-A:585-592.
125. Wilkinson H.A., Schuman N. Intradiscal corticosteroids in the treatment of lumbar and cervical disc problems. Spine. 1980;5:385-389.
126. Graham C.E. Chemonucleolysis: a double blind study comparing chemonucleolysis with intradiscal hydrocortisone: in the treatment of backache and sciatica. Clin Orthop Relat Res. 1976:179-192.
127. Wilkinson H.A., Schuman N. Intradiscal corticosteroids in the treatment of lumbar and cervical disc problems. Spine (Phila Pa 1976). 1980;5:385-389.
128. Guzman Barron E.S. Studies on blood cells. J Biological Chemistry. 1928.
129. Eusebio E.B., Graham J., Mody N. Treatment of intractable pruritus ani. Dis Colon Rectum. 1990;33:770-772.
130. Farouk R., Lee P.W. Intradermal methylene blue injection for the treatment of intractable idiopathic pruritus ani. Br J Surg. 1997;84:670.
131. Peng B., Zhang Y., Hou S., et al. Intradiscal methylene blue injection for the treatment of chronic discogenic low back pain. Eur Spine J. 2007;16:33-38.
132. Sheng Z.Y. Medical support in the Tangshan earthquake: a review of the management of mass casualties and certain major injuries. J Trauma. 1987;27:1130-1135.
133. Cohen N., Robinson D., Ben-Ezzer J., et al. Reduced NO accumulation in arthrotic cartilage by exposure to methylene blue. Acta Orthop Scand. 2000;71:630-636.
134. Fritzell P., Hagg O., Wessberg P., et al. Chronic low back pain and fusion: a comparison of three surgical techniques: a prospective multicenter randomized study from the Swedish lumbar spine study group. Spine. 2002;27:1131-1141.
135. Fritzell P., Hagg O., Wessberg P., et al. 2001 Volvo Award Winner in Clinical Studies: Lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine. 2001;26:2521-2534.
136. Moore K.R., Pinto M.R., Butler L.M. Degenerative disc disease treated with combined anterior and posterior arthrodesis and posterior instrumentation. Spine. 2002;27:1680-1686.
137. Lee C.K., Vessa P., Lee J.K. Chronic disabling low back pain syndrome caused by internal disc derangements. The results of disc excision and posterior lumbar interbody fusion. Spine. 1995;20:356-361.
138. Bertagnoli R., Yue J.J., Shah R.V., et al. The treatment of disabling single-level lumbar discogenic low back pain with total disc arthroplasty utilizing the Prodisc prosthesis: a prospective study with 2-year minimum follow-up. Spine. 2005;30:2230-2236.
139. Bertagnoli R., Yue J.J., Shah R.V., et al. The treatment of disabling multi-level lumbar discogenic low back pain with total disc arthroplasty utilizing the ProDisc prosthesis: a prospective study with 2-year minimum follow-up. Spine. 2005;30:2192-2199.
140. Hanley E.N.Jr., David S.M. Lumbar arthrodesis for the treatment of back pain. J Bone Joint Surg Am. 1999;81:716-730.
141. Tow B.P., Hsu W.K., Wang J.C. Disc regeneration: a glimpse of the future. Clin Neurosurg. 2007;54:122-128.
142. Masuda K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur Spine J. 2008;17(Suppl 4):441-451.
143. Seguin C.A., Pilliar R.M., Madri J.A., et al. TNF-alpha induces MMP2 gelatinase activity and MT1-MMP expression in an in vitro model of nucleus pulposus tissue degeneration. Spine. 2008;33:356-365.
144. Bogduk N. The inter-body joint and the intervertebral discs. In: Clinical Anatomy of the Lumbar Spine and Sacrum. Edinburgh: Churchill Livingstone; 1997:13-31.
145. Hayashi S., Taira A., Inoue G., et al. TNF-alpha in nucleus pulposus induces sensory nerve growth: a study of the mechanism of discogenic low back pain using TNF-alpha-deficient mice. Spine. 2008;33:1542-1546.
146. Cohen S.P., Wenzell D., Hurley R.W., et al. A double-blind, placebo-controlled, dose-response pilot study evaluating intradiscal etanercept in patients with chronic discogenic low back pain or lumbosacral radiculopathy. Anesthesiology. 2007;107:99-105.
147. Das D.B., Welling A., Urban J.P., et al. Solute transport in intervertebral disc: experiments and finite element modeling. Ann N Y Acad Sci. 2009;1161:44-61.
148. Evans C. Potential biologic therapies for the intervertebral disc. J Bone Joint Surg Am. 2006;88(Suppl 2):95-98.
149. Chubinskaya S., Hurtig M., Rueger D.C. OP-1/BMP-7 in cartilage repair. Int Orthop. 2007;31:773-781.
150. Kawakami M., Hashizume H., Matsumoto T., et al. Safety of epidural administration of Osteogenic Protein-1 (OP-1/BMP-7): behavioral and macroscopic observation. Spine. 2007;32:1388-1393.
151. Lin Z., Liu H., Wang W. [Study progress of growth differentiation factor 5 or osteogenic protein 1 injection into a degenerated disc]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2008;22:435-438.
152. Miyamoto K., Masuda K., Kim J.G., et al. Intradiscal injections of osteogenic protein-1 restore the viscoelastic properties of degenerated intervertebral discs. Spine J. 2006;6:692-703.
153. Imai Y., Okuma M., An H.S., et al. Restoration of disc height loss by recombinant human osteogenic protein-1 injection into intervertebral discs undergoing degeneration induced by an intradiscal injection of chondroitinase ABC. Spine. 2007;32:1197-1205.
154. Sobajima S., Vadala G., Shimer A., et al. Feasibility of a stem cell therapy for intervertebral disc degeneration. Spine J. 2008;8:888-896.
155. Haufe S.M., Mork A.R. Intradiscal injection of hematopoietic stem cells in an attempt to rejuvenate the intervertebral discs. Stem Cells Dev. 2006;15:136-137.
156. Smith L., Garvin P.J., Gesler R.M., et al. Enzyme dissolution of the nucleus pulposus. Nature. 1963;198:1311-1312.
157. Simmons J.W.Jr., Fraser R.D. The rise and fall of chemonucleolysis. In: Parviz K., editor. Arthroscopic and Endoscopic Spinal Surgery: Text and Atlas. Totowa, NJ: Humana; 2005:351-358.
158. Wittenberg R.H., Oppel S., Rubenthaler F.A., Steffen R., et al. Five-year results from chemonucleolysis with chymopapain or collagenase: a prospective randomized study. Spine. 2001;26:1835-1841.
159. Riquelme C., Musacchio M., Mont’Alverne F., et al. Chemonucleolysis of lumbar disc herniation with ethanol. J Neuroradiol. 2001;28:219-229.
160. Brown M.D. Update on chemonucleolysis. Spine. 1996;21(suppl 24):62S-68S.
161. Gogan W.J., Fraser R.D. Chymopapain. A 10-year, double-blind study. Spine. 1992;17:388-394.
162. Theron J., Guimaraens L., Casasco A., et al. Percutaneous treatment of lumbar intervertebral disk hernias with radiopaque gelified ethanol: A preliminary study. J Spinal Disord Tech. 2007;20:526-532.
163. Theron J., Cuellar H., Sola T., et al. Percutaneous treatment of cervical disk hernias using gelified ethanol. AJNR Am J Neuroradiol. 2010;31:1454-1456.
164. Theron J., Guimaraens L., Casasco A., et al. Percutaneous treatment of lumbar intervertebral disk hernias with radiopaque gelified ethanol: A preliminary study. J Spinal Disord Tech. 2007;20:526-532.
165. Benoist M., Bonneville J.F., Lassale B., et al. A randomized, double-blind study to compare low-dose with standard-dose chymopapain in the treatment of herniated lumbar intervertebral discs. Spine. 1993;18:28-34.
166. Benoist M., Deburge A., Heripret G., et al. Treatment of lumbar disc herniation by chymopapain chemonucleolysis. A report on 120 patients. Spine. 1982;7:613-617.
167. Gogan W.J., Fraser R.D. Chymopapain. A 10-year, double-blind study. Spine. 1992;17:388-394.
168. Fraser R.D. Chymopapain for the treatment of intervertebral disc herniation. A preliminary report of a double-blind study. Spine. 1982;7:608-612.
169. Fraser R.D. Chymopapain for the treatment of intervertebral disc herniation. The final report of a double-blind study. Spine. 1984;9:815-818.
170. Couto J.M., Castilho E.A., Menezes P.R. Chemonucleolysis in lumbar disc herniation: a meta-analysis. Clinics. 2007;62:175-180.
171. Wittenberg R.H., Oppel S., Rubenthaler F.A., et al. Five-year results from chemonucleolysis with chymopapain or collagenase: A prospective randomized study. Spine (Phila Pa 1976). 2001;26:1835-1841.
172. Gibson J.N., Waddell G. Surgical interventions for lumbar disc prolapse: updated Cochrane Review. Spine. 2007;32:1735-1747.
173. van Alphen H.A., Braakman R., Bezemer P.D., et al. Chemonucleolysis versus discectomy: a randomized multicenter trial. J Neurosurg. 1989;70:869-875.
174. Murphy K.J. Oxygen/ozone as effective as discectomy for herniated disc pain. Society of Interventional Radiology (SIR) 34th Annual Scientific Meeting. Abstract. 37, 2009. Conference Proceeding
175. Alexandre A., Coro L., Azuelos A., et al. Intradiscal injection of oxygen-ozone gas mixture for the treatment of cervical disc herniations. Acta Neurochir Suppl. 2005;92:79-82.
176. Muto M., Andreula C., Leonardi M. Treatment of herniated lumbar disc by intradiscal and intraforaminal oxygen-ozone (O2-O3) injection. J Neuroradiol. 2004;31:183-189.
177. Paradiso R., Alexandre A. The different outcomes of patients with disc herniation treated either by microdiscectomy, or by intradiscal ozone injection. Acta Neurochir Suppl. 2005;92:139-142.
178. Wu Z., Wei L.X., Li J., et al. Percutaneous treatment of non-contained lumbar disc herniation by injection of oxygen-ozone combined with collagenase. Eur J Radiol. 2009;72(3):499-504.
179. Felder-Puig R., Gyimesi M., Mittermayr T., Geiger-Gritsch S., et al. [Chemonucleolysis and intradiscal electrothermal therapy: what is the current evidence?]. Rofo. 2009;181:936-944.
180. Bo W., Longyi C., Jian T., et al. A pyogenic discitis at C3-C4 with associated ventral epidural abscess involving C1-C4 after intradiscal oxygen-ozone chemonucleolysis: a case report. Spine. 2009;34:E298-E304.
181. Gazzeri R., Galarza M., Neroni M., et al. Fulminating septicemia secondary to oxygen-ozone therapy for lumbar disc herniation: case report. Spine. 2007;32:E121-E123.
182. Kanai A. Treatment of lumbar disk herniation by percutaneous intradiscal high-pressure injection of saline. Pain Med. 2009;10:76-84.
183. Gerszten P.C., Welch W.C., King J.T.Jr. Quality of life assessment in patients undergoing nucleoplasty-based percutaneous discectomy. J Neurosurg Spine. 2006;4:36-42.
184. Manchikanti L., Derby R., Benyamin R.M., et al. A systematic review of mechanical lumbar disc decompression with nucleoplasty. Pain Physician. 2009;12:561-572.
185. Yan D., Li J., Zhu H., et al. Percutaneous cervical nucleoplasty and percutaneous cervical discectomy treatments of the contained cervical disc herniation. Arch Orthop Trauma Surg. 2010;130:1371-1376.
186. Nardi P.V., Cabezas D., Cesaroni A. Percutaneous cervical nucleoplasty using coblation technology. Clinical results in fifty consecutive cases. Acta Neurochir Suppl. 2005;92:73-78.
187. Birnbaum K. Percutaneous cervical disc decompression. Surg Radiol Anat. 2009;31:379-387.
188. Persson L.C., Carlsson C.A., Carlsson J.Y. Long-lasting cervical radicular pain managed with surgery, physiotherapy, or a cervical collar. A prospective, randomized study. Spine. 1997;22:751-758.
189. Bhagia S.M., Slipman C.W., Nirschl M., et al. Side effects and complications after percutaneous disc decompression using coblation technology. Am J Phys Med Rehabil. 2006;85:6-13.
190. Singh V., Manchikanti L., Benyamin R.M., et al. Percutaneous lumbar laser disc decompression: a systematic review of current evidence. Pain Physician. 2009;12:573-588.
191. Lee S.H., Kang H.S. Percutaneous endoscopic laser annuloplasty for discogenic low back pain. World Neurosurg.. 2010;73:198-206.
192. Krugluger J., Knahr K. Chemonucleolysis and automated percutaneous discectomy—a prospective randomized comparison. Int Orthop. 2000;24:167-169.
193. Revel M., Payan C., Vallee C., et al. Automated percutaneous lumbar discectomy versus chemonucleolysis in the treatment of sciatica. A randomized multicenter trial. Spine. 1993;18:1-7.
194. Chatterjee S., Foy P.M., Findlay G.F. Report of a controlled clinical trial comparing automated percutaneous lumbar discectomy and microdiscectomy in the treatment of contained lumbar disc herniation. Spine. 1995;20:734-738.
195. Haines S.J., Jordan N., Boen J.R., et al. Discectomy strategies for lumbar disc herniation: results of the LAPDOG trial. J Clin Neurosci. 2002;9:411-417.
196. Haines S.J., Jordan N., Boen J.R., et al. Discectomy strategies for lumbar disc herniation: Results of the LAPDOG trial. J Clin Neurosci. 2002;9:411-417.
197. Appleby D., Andersson G., Totta M. Meta-analysis of the efficacy and safety of intradiscal electrothermal therapy (IDET). Pain Med. 2006;7:308-316.
198. Saal J.A., Saal J.S. Intradiscal electrothermal treatment for chronic discogenic low back pain: Prospective outcome study with a minimum 2-year follow-up. Spine. 2002;27:966-973.
199. Spruit M., Jacobs W.C. Pain and function after intradiscal electrothermal treatment (IDET) for symptomatic lumbar disc degeneration. Eur Spine J. 2002;11:589-593.
200. Lee M.S., Cooper G., Lutz G.E., et al. Intradiscal electrothermal therapy (IDET) for treatment of chronic lumbar discogenic pain: A minimum 2-year clinical outcome study. Pain Physician. 2003;6:443-448.
201. Lutz C., Lutz G.E., Cooke P.M. Treatment of chronic lumbar diskogenic pain with intradiskal electrothermal therapy: A prospective outcome study. Arch Phys Med Rehabil. 2003;84:23-28.
202. Cohen S.P., Larkin T., Abdi S., et al. Risk factors for failure and complications of intradiscal electrothermal therapy: A pilot study. Spine. 2003;28:1142-1147.
203. Davis T.T., Delamarter R.B., Sra P., Goldstein T.B., et al. The IDET procedure for chronic discogenic low back pain. Spine. 2004;29:752-756.
204. Gerszten P.C., Welch W.C., McGrath P.M., et al. A prospective outcomes study of patients undergoing intradiscal electrothermy (IDET) for chronic low back pain. Pain Physician. 2002;5:360-364.
205. Freedman B.A., Cohen S.P., Kuklo T.R., et al. Intradiscal electrothermal therapy (IDET) for chronic low back pain in active-duty soldiers: 2-Year follow-up. Spine J. 2003;3:502-509.
206. Sharma S.K., Jones J.O., Zeballos P.P., et al. The prevention of discitis during discography. Spine J. 2009;9:936-943.
207. Osti O.L., Fraser R.D., Vernon-Roberts B. Discitis after discography. The role of prophylactic antibiotics. J Bone Joint Surg Br. 1990;72:271-274.
208. Langer R.D., Usmani A., VAN Gorkom K.N., et al. In vitro assessment of the antibiotic efficacy of contrast media and antibiotics and their combinations at various dilutions. Br J Radiol. 2010;83:394-400.
209. Willems P.C., Jacobs W., Duinkerke E.S., De Kleuver M. Lumbar discography: should we use prophylactic antibiotics? A study of 435 consecutive discograms and a systematic review of the literature. J Spinal Disord Tech. 2004;17:243-247.
210. Carragee E.J., Chen Y., Tanner C.M., et al. Can discography cause long-term back symptoms in previously asymptomatic subjects? Spine. 2000;25:1803-1808.
211. Simmons J.W., Emery S.F., McMillin J.N., et al. Awake discography. A comparison study with magnetic resonance imaging. Spine. 1991;16(suppl 6):S216-S221.
212. Jackson R.P., Becker G.J., Jacobs R.R., et al. The neuroradiographic diagnosis of lumbar herniated nucleus pulposus: I. A comparison of computed tomography (CT), myelography, CT-myelography, discography, and CT-discography. Spine. 1989;14:1356-1361.
213. Guyer R.D., Collier R., Stith W.J., et al. Discitis after discography. Spine. 1988;13:1352-1354.
214. Fraser R.D., Osti O.L., Vernon-Roberts B. Discitis after discography. J Bone Joint Surg Br. 1987;69:26-35.
215. Milette P.C., Melanson D. A reappraisal of lumbar discography. J Can Assoc Radiol. 1982;33:176-182.
216. Colquhoun J. The role of lumbar discography in diagnosis of lumbar spine lesions. Orthop Rev. 1977;6:51-56.
217. Patrick B.S. Lumbar discography: a five year study. Surg Neurol. 1973;1:267-273.
218. Collis J.S.Jr., Gardner W.J. Lumbar discography. An analysis of one thousand cases. J Neurosurg. 1962;19:452-461.
219. Kim K.S., Yoon S.T., Li J., et al. Disc degeneration in the rabbit: a biochemical and radiological comparison between four disc injury models. Spine. 2005;30:33-37.
220. Carragee E.J., Don A.S., Hurwitz E.L., et al. Does discography cause accelerated progression of degeneration changes in the lumbar disc: A ten-year matched cohort study. Spine. 2009;34:2338-2345.