Intracranial Monitoring
Intracranial Pressure Monitoring
The history of ICP monitoring dates to 1891, when Quincke measured the cerebrospinal fluid (CSF) pressure via a lumbar puncture.1 Soon afterward, Cushing showed that as ICP increases and approaches systemic arterial pressure in an animal model, hypertension, bradycardia, and respiratory changes become evident.2 The use of continuous ICP monitoring was first described by Guillaume and Janny3 using an intraventricular catheter in 1951. Nine years later, Lundberg4 published the first systematic observations of ICP and its response to medical and physiologic interventions. Using ventricular catheters, he showed the clinical value of direct ICP monitoring and described pressure waveforms, of which the Lundberg A wave has the most practical importance in the intensive care unit (ICU). These A, or plateau, waves are characterized by a steep increase in ICP to 60 to 80 mm Hg lasting 2 to 5 minutes or longer, followed by a rapid decrease to near initial baseline pressures. This represents a pathologic response to decompensation of pressure controlling mechanisms.4–6 Since these early investigations, CSF pressure and ICP measurement have been developed and refined further.
An ICP monitor is an invaluable research and clinical tool, contributing to the understanding of intracranial pathologic conditions and the assessment of therapeutic interventions. ICP monitoring can be used for patients with intracerebral hemorrhage, Reye syndrome, hepatic encephalopathy, encephalitis, stroke, hydrocephalus, near-drowning, and subarachnoid hemorrhage, but most of the clinical experience with ICP monitoring involves traumatic brain injury (TBI). In severe TBI, it is important to know if ICP is elevated. Early signs and symptoms of increased ICP include headache, lethargy, nausea, and vomiting. In critically ill patients, these clinical signs may be nonspecific and unreliable.6 In addition, the positive trend toward early intubation and sedation, if not pharmacologic paralysis, eliminates the neurologic assessment of a patient with the exception of pupils. Even papilledema, a hard physical sign of increased ICP, is rarely seen acutely in patients with TBI.7 Computed tomography (CT) is arguably the most useful diagnostic tool in patients with TBI, but may not reliably determine the ICP.
ICP monitoring in severe TBI has become routine because it facilitates rational management, provides prognostic information, and improves outcomes.8–11 ICP monitoring can provide crucial information relative to cerebral perfusion pressure (CPP); detect the development or enlargement of a mass lesion, such as contusion or hematoma; facilitate the estimation of intracranial compliance; and be the only parameter to follow in a pharmacologically paralyzed patient, apart from the pupillary examination. It is rarely justifiable to treat a patient for intracranial hypertension empirically without a mechanism for measuring the effect of treatment, such as a clinical examination or ICP.
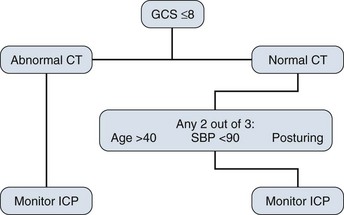
There are well-defined guidelines for the use of ICP monitoring in TBI, which include patients with an abnormal CT scan and a Glasgow Coma Scale score of 8 or less after cardiopulmonary resuscitation. An abnormal CT scan is defined as one that reveals hematomas, contusion, edema, or compressed cisterns. It also is recommended to consider monitoring head-injured patients with a Glasgow Coma Scale score of 8 or less even if the head CT scan is negative if two of the following three criteria are met on admission: age older than 40, systolic blood pressure less than 90 mm Hg, or signs of posturing (Fig. 16.1).12
Although ICP monitoring can be a useful tool in the ICU, this technology has limitations. It is crucial not to assign undue weight to a normal ICP if other clinical information suggests otherwise. ICP does not always increase in the presence of midline shift.13,14 More specifically, a temporal lobe mass can herniate over the tentorial edge and cause brainstem compression without a concomitant increase in ICP.15,16 Likewise, there is not good correlation between supratentorial and infratentorial pressures,17 so it is imperative to remember that patients with a posterior fossa mass can deteriorate rapidly without a significant increase in ICP measured in the supratentorial compartment.6
ICP waveform analysis includes systolic and diastolic pressures with superimposed respiratory variation; however, the mean pressure is of practical importance. A normal adult mean ICP is 10 mm Hg or less with transient physiologic elevations above this value seen in a head-down position or during a Valsalva maneuver (see discussion of secondary injury in Chapter 67).6 The Association for the Advancement of Medical Instrumentation developed standards for ICP monitoring devices that require a pressure range from 0 to 100 mm Hg, an accuracy of ±2 mm Hg in the range of 0 to 20 mm Hg, and a maximal error of 10% between 20 mm Hg and 100 mm Hg.18 There are four basic types of clinically useful monitoring systems: ventricular catheter, subarachnoid bolt, fiberoptic device, and catheter tip strain gauge.
Ventricular Catheter
A ventriculostomy is the gold standard for ICP monitoring. It can be inserted through a twist drill craniostomy at the bedside and can also be used to drain CSF or for the estimation of intracranial compliance. The catheter is connected to a fluid-filled system, which is connected to an external transducer. The transducer converts the measured pressure to an electrical signal, which provides a waveform and numerical value displayed on a monitor through a signal processor. A three-way stopcock is used to divert CSF from the monitor to a drainage bag if needed. This setup allows the catheter to be zeroed as frequently as necessary with the transducer positioned at the level of the center of the brain, which generally corresponds to the external auditory meatus. This system has the potential to be opened and contaminated and infected. The most significant risk of a ventriculostomy is infection; rates of 27% have been cited,10,19–22 although most reported rates are in the 1% to 10% range.21,23–26 Infection rates are similar regardless of procedure location (ICU or the operating room).20,21,27 Tunneling the catheter subcutaneously to a distant skin exit site seems to reduce the infection risk. Other risk factors for infection include irrigation of the catheter or drainage system and the presence of intraventricular blood.20,21,28 Duration of monitoring also may be a risk factor for infection. Some studies have found an increase in infection rates when ventriculostomies were left in for longer than 5 days,20,21,25 but more recent data reveal no significant reduction in infection rates when catheters were replaced before the fifth day.29 Likewise, other investigators have found no significant relationship between duration of monitoring and rate of daily infection for 2 weeks.30 The literature on the role of prophylactic antibiotics during external CSF drainage is also variable.20,26,28,31 A Brain Trauma Foundation Level III recommendation discourages routine ventricular catheter exchange as well as prophylactic antibiotics for ventricular catheter placement.32 Antibiotic-impregnated catheters have been shown to reduce infection rates33 and are being used routinely in some centers.
Hemorrhage at the time of placement occurs about 1% to 2% of the time and only rarely needs to be surgically evacuated.6,25,34 A more common problem with placement is difficulty in accessing small, compressed, or shifted ventricles, resulting in malposition and a poor waveform. Although a ventriculostomy is generally considered to be the most precise and accurate method of measuring ICP,35 malfunction occurs if the ventriculostomy becomes clogged with air, blood, or debris, or if the ventricles are collapsed around the fenestrations in the catheter tip.
In addition to its primary use as an ICP monitor, a ventriculostomy is commonly used in the ICU as a drain for patients with TBI or hydrocephalus. Common causes of acute hydrocephalus in an adult ICU include cerebellar stroke or hemorrhage, intraventricular hemorrhage, and aneurysmal subarachnoid hemorrhage. A common and often debated concern regarding CSF drainage with a ventriculostomy is that it can cause subfalcine herniation in the presence of a hemispheric mass or upward herniation of the cerebellum in the presence of an infratentorial lesion.36 Under these circumstances, we believe that surgical decompression of the primary mass also should be considered. Another potential risk of ventricular catheter insertion is aneurysmal rebleeding37 after an acute subarachnoid hemorrhage. Our group’s opinion is that the benefit of treating hydrocephalus with high ICP far outweighs the small, potential risk of aneurysmal rebleeding, and we have a low threshold for placing a ventriculostomy in these patients.
Subarachnoid Bolt
The subarachnoid bolt technique for ICP monitoring was developed because of concern about the infection rate associated with ventriculostomies, and because small ventricular size after head trauma often makes catheter insertion difficult.38 A subarachnoid bolt is a self-tapping metal or plastic tube that is screwed into a twist drill craniostomy at the bedside. The dura at the base of the bolt is perforated with a spinal needle to allow CSF to fill the bolt, which is connected to pressure tubing filled with preservative-free (nonbacteriostatic) saline that leads to an external transducer leveled to the ear. In contrast to a ventriculostomy, the subarachnoid bolt is only a monitoring instrument; CSF is not withdrawn from it. It usually provides a reliable ICP waveform and pressure reading, but is susceptible to error if the dural perforations become obstructed with blood or debris, or if brain swelling obliterates communication with CSF. Uncapping the bolt to flush debris with 0.2 mL of preservative-free saline solution can restore accurate ICP readings and is unlikely to cause dangerous ICP elevation.39 The subarachnoid bolt tends to underestimate ICP, particularly when ICP is elevated.39,40 Because the subarachnoid bolt measures the local ICP at the surface of the hemisphere, it can be inaccurate if a pressure gradient is present between the left and right supratentorial compartments.19 The existence of compartmental pressure differences has been debated, but such gradients can occur between the left and right hemispheres or the supratentorial and infratentorial compartments and sometimes are only transient.19,41–43 This is an important phenomenon to consider, and if a discrepancy exists between an apparently normal ICP and the patient’s clinical condition or CT scan or both, treatment of elevated ICP may be warranted.
The infection risk for subarachnoid bolts is extremely low, and infections are nearly always superficial and rarely involve the brain or meninges.20 No local or systemic infection was reported with the use of subarachnoid bolts in 124 comatose children.44 Risk of subarachnoid bolt infection is increased when the bolt is opened and flushed to improve the waveform.20 Subarachnoid bolts are rarely associated with brain injury; however, intracerebral hematoma may occur45 if there is a mishap with the drill, or if the needle used to puncture the dura is passed too deeply. Since the introduction of fiberoptic catheters, subarachnoid bolts are being used less often.
Fiberoptic Intracranial Pressure Monitors
Fiberoptic ICP monitors use miniature transducers that are coupled via fiberoptic cables to an external instrument. These monitors can be placed at the bedside through a standard twist drill craniostomy or through a smaller opening made with a 2.71-mm bit. The transducer is incorporated into the end of a tube and can be used alone or in combination with a ventriculostomy. Fiberoptic systems operate by projecting light through an optic fiber to a miniature, displaceable mirror in the catheter tip.46 The amount of light reflected to a collecting optic fiber depends on the mechanical displacement of the mirror, which is a function of ICP. Fiberoptic devices can be inserted into the lateral ventricle, the brain parenchyma, or the subdural space. The greatest advantage of fiberoptic catheters is that they do not require fluid coupling for pressure transduction, which avoids the problems of waveform dampening and artifacts from poor coupling. Because they do not require fluid coupling, there also is less opportunity for contamination. The mechanism that does the actual pressure transduction is what is inserted into the patient; the system functions independent of head position, and the monitor is zeroed once before it is placed. This feature also is a disadvantage because the transducer cannot be recalibrated to zero after insertion. System accuracy compared with a ventriculostomy has been shown in the subdural space, brain parenchyma, and ventricles, although the parenchymal fiberoptic pressures may consistently exceed ventriculostomy pressures by nearly 10 mm Hg.47–49 The fiberoptic device has an average daily drift of ±0.6 mm Hg. Over a 5-day period, there is an average drift of 2.1 mm Hg with a maximal drift of ±6 mm Hg. This drift over time may be enough to necessitate replacement if ICP monitoring is required for more than 5 days.6,49,50
Complications with the use of fiberoptic catheters relating to hemorrhage51,52 and infection51 have been reported, but our experience is that clinically relevant problems are unusual, particularly with intraparenchymal monitors. We do exercise caution, however, in patients with coagulopathy.
Catheter Tip Strain Gauge
The catheter tip strain gauge consists of a miniaturized solid-state pressure sensor mounted in a titanium case at the tip of a long, thin, flexible nylon tube. The transducer tip contains a silicon microchip with diffuse piezoresistive strain gauges that connect to tiny wires that travel the length of the tube. This is a small wire with a diameter of 1.2 mm that can be placed at the bedside. It can be incorporated into a ventricular catheter and used in any intracranial space. This device is accurate with a low daily drift range between −0.125 mm Hg and +0.110 mm Hg. It shares many of the advantages and disadvantages of fiberoptic devices,6,53,54 but we have found it more cumbersome to place and secure through a bedside twist drill craniostomy.
Cerebral Blood Flow Monitoring
Measuring CBF outside the ICU began with Kety and Schmidt in 1948 and more recently has been done with xenon-enhanced CT,55,56 but these methods only give snapshots of CBF and do not allow for continuous monitoring at the bedside.57 Continuous monitoring techniques in the ICU are classified as direct or indirect. Direct and continuous monitoring of CBF at the bedside includes laser Doppler flowmetry and thermal diffusion.
The laser Doppler flow sensor (1.5 mm diameter), which emits a monochromatic light, is placed into the white matter through a burr hole. The sensor measures the volume or concentration of red blood cells and their velocity and generates a flow signal. Although laser Doppler flow does allow continuous measurements of perfusion, the sample volume is small (1 mm3), and only relative changes can be determined. Laser Doppler flow provides a qualitative estimate of regional CBF displayed in arbitrary units.58 A quantitative estimate of regional CBF can be acquired with the thermal diffusion method.59 With this technique, a probe with two small thermistors is inserted into the brain to measure the tissue’s ability to dissipate heat, and a microprocessor converts this into CBF displayed in the standard units of mL/100 g/minute.57
An indirect method for measuring blood flow at the bedside is transcranial Doppler ultrasound, which measures mean blood flow velocity in the basal cerebral vessels.60 Although not equivalent to volume flow, changes in CBF can be inferred from changes in blood flow velocity.61 Continuous transcranial Doppler monitoring is still cumbersome, however, because of problems of probe fixation to the head and computer interfacing.57 Another indirect way of continuously monitoring CBF at the bedside is by measuring jugular venous bulb oxygenation.
AJDO2 is proportional to CMRO2 or inversely proportional to CBF or both.62–65 Assuming that the CMRO2 is constant, an increase in the amount of oxygen extraction as reflected by a decrease in jugular oxygen saturation (increased AJDO2) implies that CBF has been compromised. Conversely, if AJDO2 decreases and metabolism remains constant, we can infer an increase in CBF (i.e., hyperemia). Measuring jugular bulb oxygen can be a useful way to monitor a patient who requires hyperventilation for ICP control (see Chapter 66). Hyperventilation is a powerful tool for reducing ICP and enhancing CPP, but at the expense of increasing cerebrovascular resistance, with the consequent reduction of blood in the brain. The goal of treatment is to prevent ischemia, the ultimate and proximate cause of secondary injury. There is an obvious conflict when attempting to prevent ischemia by reducing the amount of blood delivered to the brain. AJDO2 is sensitive to changes in cerebrovascular resistance; jugular vein monitoring may reveal inappropriate reductions of CBF as a result of hyperventilation.62
Most of the data concerning jugular bulb monitoring come from the trauma literature. In a prospective study of 353 patients with severe TBI, Cruz66 found that outcome at 6 months was significantly better in the patients who had monitoring and management of cerebral extraction of oxygen along with CPP compared with the patients undergoing monitoring and management of CPP alone.
Increased AJDO2 also can be detected during the early phases of head injury when CBF is pathologically reduced. Typically, the patient is being rescued at the scene, transported, and triaged during this early phase of injury, when CBF is low and ventilation is not carefully titrated. Hyperventilation is frequently used during this vulnerable period, often empirically and sometimes inadvertently.62,67 Complicating this scenario is superimposed hypotension and hypoxia, which can occur during the early phases of resuscitation and later in the ICU.68–70 Despite its usefulness, there are significant limitations to this monitoring technique. Measurements can be done by intermittent sampling, which is accurate but is limited by intermittent information, or continuous monitoring of oxygen saturation using fiberoptic catheters, which require careful maintenance and have reliability issues.63,71
It is easier to use the oxygen saturation of the venous blood in the jugular, rather than direct measurements of oxygen content, but because the percentage of oxygen saturation in the jugular bulb depends on hemoglobin concentration, it cannot be used independently for estimating the relationship between CMRO2 and CBF. If the patient is anemic, more oxygen may be extracted from the available blood, even under conditions of normal CBF. When measuring oxygen saturation, AJDO2 needs to be calculated, taking the hemoglobin concentration into consideration. Also, AJDO2 is an overall estimate of the global relationship between CBF and CMRO2 and cannot differentiate focal abnormalities. In addition, there is a limit to how much oxygen can be extracted from the available blood. If maximal extraction is reached, and CBF continues to deteriorate, the AJDO2 does not continue to increase. Under this circumstance, AJDO2 appears stable despite a progressively worsening situation. Other pitfalls in monitoring AJDO2 are observed under conditions in which oxygen extraction itself is impaired, such as in cases of mitochondrial dysfunction or a large stroke in which oxygen is not extracted at all.62
Several important technical factors are associated with AJDO2 monitoring. The side chosen (right or left jugular bulb) is important and can be affected by the side of the brain with the most injury whether or not it is the side of dominant venous drainage.72–74 How high the tip of the catheter is positioned is another consideration and requires x-ray confirmation.75 The speed at which samples are drawn also may affect the results.76 Complications are uncommon and include carotid puncture63,77 and subclinical internal jugular vein thrombosis.77
Ultimately, jugular bulb monitoring is geared toward the assessment of global CBF and cerebral oxygenation and can be useful in guiding therapeutic hyperventilation.78,79 In severe TBI, there is enough evidence to recommend its use for titrating the level of hyperventilation;80 however, some clinicians find jugular bulb monitoring to be cumbersome, to be prone to artifact, and to have other potential problems with poor data quality.81–83 Because of all of the problems involved with this technique, monitoring of AJDO2 has not been universally accepted as a useful tool in the routine management of head-injured patients. There remain strong arguments for and against the use of jugular bulb monitoring.66,84
Brain Tissue Oxygenation Monitoring
Oxygen tension in brain tissue is as close to a gold standard of cerebral oxygenation as we have at the bedside. Brain tissue oxygen tension can be directly measured using a small flexible microcatheter (<0.5 mm in diameter) that is usually inserted into the frontal white matter and fixed onto a special bolt. The normal brain tissue oxygen tension is approximately 40 mm Hg.85–89 Cerebral oxygen tension is generally reflective of CBF and local oxygen extraction. In this sense, brain tissue oxygen tension may represent the “pool of oxygen” in brain tissue.57,90,91 Changes in brain tissue oxygen tension can be used to monitor evolving disturbances of tissue metabolism.81,92–94
Ischemic damage seems to correlate to a brain tissue oxygen tension less than 8 to 10 mm Hg as measured by a Licox catheter (Integra Neuroscience, Plainsboro, NJ).81 A meta-analysis of three studies including 158 patients with severe TBI found overall cerebral hypoxia, defined as a monitored brain tissue oxygen tension less than 10 mm Hg, was associated with worse outcome and increased mortality rate.95 Another study treated 70 severe head injury patients with management directed to maintaining brain tissue oxygenation greater than 25 mm Hg in addition to conventional ICP and CPP management. The results were compared with 53 matched, historical controls treated with conventional management only. The mean daily ICP and CPP and the frequency of episodes of ICP greater than 20 mm Hg and CPP less than 60 mm Hg were similar in both groups. Forty percent of patients with management guided by only ICP and CPP had a favorable outcome compared with 70% of patients with management guided by brain tissue oxygenation.96 Additional studies demonstrate a relationship between brain tissue oxygen tension, ICP, and outcome. Recent97,98 guidelines suggest a treatment threshold of less than 15 mm Hg,99 but the author’s preference is to consider treatment at values less than 20 to 25 mm Hg, and a recent study demonstrated a relationship between mortality rate and values less than 29 mm Hg.100
Local cerebral hypoxia in the presence of normal ICP, CPP, and mean blood pressure may be caused by insufficient arterial oxygenation,92 a mismatch between supply (CBF) and demand (CMRO2),101 or hyperventilation-induced hypocapnia.92,102 Hyperventilation can decrease brain tissue oxygen tension because of decreased CBF, which can negate the perceived benefit of improving ICP and CPP.81,93,102–108 Hyperventilation has a particular risk of causing cerebral hypoxia when PCO2 is less than or equal to 30 mm Hg, or within the first 24 hours104,105 because of a further reduction of an already reduced CBF.109 Brain tissue oxygen tension has been shown not to be independently influenced by increases in CPP greater than 60 mm Hg,83,93,108 which supports more recent evidence suggesting that maintaining a CPP greater than 60 mm Hg may be unnecessary,110,111 and is consistent with current guidelines to keep CPP in the range of 50 to 70 mm Hg in severe TBI.112
When strategically placed, these monitors are very sensitive in detecting local tissue hypoxia at the tip of the probe. An inherent problem with this technique (as with all focal monitors) is that placement of the probe into the area of interest becomes important.57 There are rational arguments as to the best region to monitor. Brain tissue oxygen tension values close to a contusion are smaller relative to a region that looks normal on a CT scan.113 Some authors recommend monitoring the zone surrounding a contusion because of its high risk of tissue death. Other authors place the probes in uninjured brain with the idea being that a reduction in the brain tissue oxygen tension in the uninjured hemisphere is reflecting a diffuse decrease in arterial oxygenation, an increase in ICP, a decrease in CPP, overaggressive hyperventilation, or some other global event.81
References
1. Quincke, H. Die Lumbarpunktion des Hydrocephalus. Klin Wochenschr. 1891; 28:965.
2. Cushing, H. Some experimental observations concerning states of increased intracranial tension. Ann J Med Sci. 1902; 124:375.
3. Guillaume, J, Janny, P. Manometrie intracranienne continue: Interet de la methode et premiers resultants. Rev Neurol. 1951; 84:131.
4. Lundberg, N. Continuous recording and control of ventricular fluid pressure in neurosurgical practice. Acta Psychiatr Neurol Scand. 1960; 36:1.
5. Lundberg, N, Troupp, H, Lorin, H. Continuous recording of the ventricular-fluid pressure in patients with severe acute traumatic brain injury: A preliminary report. J Neurosurg. 1965; 22:581–590.
6. Feldman, Z, Narayan, RK. Intracranial pressure monitoring: Techniques and pitfalls. In: Cooper PR, Golfinos JG, eds. Head Injury. 4th ed. New York: McGraw-Hill; 2000:265–292.
7. Selhorst, JB, Gudeman, SK, Butterworth, JF. Papilledema after acute head injury. Neurosurgery. 1985; 16:357–363.
8. Gopez, JJ, Meagher, RJ, Narayan, RK. When and how should I monitor intracranial pressure? In: Valadka AB, Andrews BT, eds. Neurotrauma. New York: Thieme; 2005:53–57.
9. Marmarou, A, Anderson, RL, Ward, JD, et al. Impact of ICP instability and hypotension on outcome in patients with severe head injury. J Neurosurg. 1991; 75:S59–S66.
10. Rosner, MJ, Rosner, SD, Johnson, AH. Cerebral perfusion pressure: Management protocol and clinical results. J Neurosurg. 1995; 83:949–962.
11. Farahvar, A, Gerber, L, Chiu, YL, et al, Increased mortality in patients with severe traumatic brain injury treated without intracranial pressure monitoring. J Neurosurg. 2012; 117(4):729–734. http://thejns.org/doi/abs/10.3171/2012.7.JNS111816
12. Brain Trauma Foundation, American Association of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care. Management and prognosis of severe traumatic brain injury: Part I. Guidelines for the management of severe traumatic brain injury. Indications for intracranial pressure monitoring. J Neurotrauma. 2000; 17:47–69.
13. Galbraith, S, Teasdale, GM. Predicting the need for operation in patients with an occult traumatic intracranial hematoma. J Neurosurg. 1981; 55:75–81.
14. Murphy, A, Teasdale, E, Matheson, M, et al. Relationship between CT indices of brain swelling and intracranial pressure after head injury. In: Ishii S, Nagai H, Brock M, eds. Intracranial Pressure. 5th ed. Berlin: Springer-Verlag; 1983:562–566.
15. Andrews, BT, Chiles, BW, Olsen, WL. The effect of intracerebral hematoma location on the risk of brainstem compression and on clinical outcome. J Neurosurg. 1988; 69:518–522.
16. Solonik, D, Pitts, LH, Lovely, M. Traumatic intracerebral hematomas: Timing of appearance and indications for operative removal. J Trauma. 1986; 26:787–794.
17. Rosenwasser, RH, Kleiner, LI, Krzeminski, JP. Intracranial pressure monitoring in the posterior fossa: A preliminary report. J Neurosurg. 1989; 71:503–505.
18. Brown, E. Intracranial Pressure Monitoring Devices. Arlington, VA: Association for the Advancement of Medical Instrumentation; 1988.
19. Ohrstrom, JK, Skou, JK, Ejlertsen, T, et al. Infected ventriculostomy: Bacteriology and treatment. Acta Neurochir. 1989; 100:67.
20. Aucoin, PJ, Kotilainen, HR, Gantz, NM, et al. Intracranial pressure monitors: Epidemiologic study of risk factors and infections. Am J Med. 1986; 80:369–376.
21. Mayhall, CG, Archer, NH, Lamb, VA, et al. Ventriculostomy-related infections: A prospective epidemiologic study. N Engl J Med. 1984; 310:553–559.
22. Smith, RW, Alksne, JF. Infections complicating the use of external ventriculostomy. J Neurosurg. 1976; 44:567.
23. Clark, CW, Muhlbauer, MS, Lowery, R, et al. Complications of intracranial pressure monitoring in trauma patients. Neurosurgery. 1989; 25:20–24.
24. Kanter, RK, Weiner, LB. Ventriculostomy-related infection. N Engl J Med. 1984; 311:987.
25. Narayan, RK, Kishore, PRS, Becker, DP, et al. Intracranial pressure: To monitor or not to monitor? J Neurosurg. 1982; 56:650–659.
26. Rosner, MJ, Becker, DP. ICP monitoring: Complications and associated factors. Clin Neurosurg. 1976; 23:494–519.
27. Friedman, WA, Vries, JK. Percutaneous tunnel ventriculostomy: Summary of 100 procedures. J Neurosurg. 1980; 53:662.
28. Wyler, AR, Kelly, WA. Use of antibiotics with external ventriculostomies. J Neurosurg. 1972; 37:185.
29. Holloway, KL, Barnes, T, Choi, S, et al. Ventriculostomy infection: The effect of monitoring duration and catheter exchange in 584 patients. J Neurosurg. 1996; 85:419–424.
30. Winfield, JA, Rosenthal, P, Kantner, RK, et al. Duration of intracranial pressure monitoring does not predict daily risk of infectious complications. Neurosurgery. 1993; 33:424–431.
31. Poon, WS, Ng, S, Wai, S. CSF antibiotic prophylaxis for neurosurgical patients with ventriculostomy: A randomised study. Acta Neurochir Suppl. 1998; 71:146.
32. Brain Trauma Foundation. Infection prophylaxis. J Neurotrauma. 2007; 24(Suppl 1):S26–S31.
33. Harrop, J, Sharan, A, Ratliff, J, et al. Impact of a standardized protocol and antibiotic-impregnated catheters on ventriculostomy infection rates in cerebrovascular patients. Neurosurgery. 2010; 67:187–191.
34. Paramore, CG, Turner, DA. Relative risk of ventriculostomy infection and morbidity. Acta Neurochir (Wien). 1994; 127:79–84.
35. Brain Trauma Foundation. Recommendations for intracranial pressure monitoring technology. J Neurotrauma. 1996; 13:685.
36. Cuneo, RA, Caronna, JJ, Pitts, L, et al. Upward transtentorial herniation: Seven cases and a literature review. Arch Neurol. 1979; 36:618.
37. Pare, L, Delfino, R, Leblanc, R. The relationship of ventricular drainage to aneurysmal rebleeding. J Neurosurg. 1992; 76:422.
38. Vries, JK, Becker, DP, Young, HF. A subarachnoid screw for monitoring intracranial pressure: Technical note. J Neurosurg. 1973; 39:416.
39. Dearden, NM, McDowall, DG, Gibson, RM. Assessment of Leeds device for monitoring intracranial pressure. J Neurosurg. 1984; 60:123.
40. Mendelow, AD, Rowan, JO, Murray, L, et al. A clinical comparison of subdural screw pressure measurements with ventricular pressure. J Neurosurg. 1983; 58:45.
41. Gambardella, G, d’Avella, D, Tomasello, F. Monitoring of brain tissue pressure with a fiberoptic device. Neurosurgery. 1992; 31:918.
42. Johnston, IH, Rowan, JO. Raised intracranial pressure and cerebral blood flow: 4. Intracranial pressure gradients and regional cerebral blood flow. J Neurol Neurosurg Psychiatry. 1974; 37:585.
43. Yano, M, Ikeda, Y, Kobayashi, S, et al. Intracranial pressure in head-injured patients with various intracranial lesions is identical throughout the supratentorial intracranial compartment. Neurosurgery. 1987; 21:688.
44. Nussbaum, E, Maggi, JC. Intracranial pressure monitoring by subarachnoid bolt in comatose children. Clin Pediatr. 1985; 24:329.
45. Bobo, H, Miller, JD, Evans, OB, et al. Delayed intracerebral hematoma at the site of a subarachnoid bolt pressure monitor: Case report. J Neurosurg. 1986; 64:673.
46. Barnett, G, Chapman, P. Insertion and care of intracranial pressure monitoring devices. In: Ropper A, Kennedy S, eds. Neurological and Neurosurgical Intensive Care. Aspen: Rockville, MD, 1988.
47. Crutchfield, JS, Narayan, RK, Robertson, CS, et al. Evaluation of a fiberoptic intracranial pressure monitor. J Neurosurg. 1990; 72:482.
48. Ostrup, RC, Luerssen, TG, Marshall, LF, et al. Continuous monitoring of intracranial pressure with a miniaturized fiberoptic device. J Neurosurg. 1987; 67:206.
49. Chambers, IR, Mendelow, AD, Sinar, EJ, et al. A clinical evaluation of the Camino subdural screw and ventricular monitoring kits. Neurosurgery. 1990; 26:421.
50. Price, DJ, Van Hille, PT, Mason, J. Evaluation of a fiberoptic system for monitoring ventricular pressure. In: Hoff JT, Betz AL, eds. Intracranial Pressure. 7th ed. Berlin: Springer-Verlag; 1989:52–54.
51. Bekar, A, Goren, S, Korfali, E, et al. Complications of brain tissue pressure monitoring with a fiberoptic device. Neurosurg Rev. 1998; 21:254.
52. Blei, AT, Olafsson, S, Webster, S, et al. Complications of intracranial pressure monitoring in fulminant hepatic failure. Lancet. 1993; 341:157.
53. Gopinath, SP, Robertson, CS, Contant, CF, et al. Clincal evaluation of a miniature strain-gauge transducer for monitoring intracranial pressure. Neurosurgery. 1995; 36:1137–1141.
54. Gray, WP, Palmer, JD, Gill, J, et al. A clinical study of parenchymal and subdural miniature strain gauge transducer for monitoring intracranial pressure. Neurosurgery. 1996; 39:927–931.
55. Kety, SS, Schmidt, CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: Theory, procedure, and normal values. J Clin Invest. 1948; 27:476–483.
56. Yonas, H, Johnson, D, Pindzola, RR. Xenon-enhanced CT of cerebral blood flow. Sci Am Sci Med. 1995; 2:58–67.
57. De Georgia, MA, Deogaonkar, A. Multimodal monitoring in the neurological intensive care unit. Neurologist. 2005; 11:45–54.
58. Bolognese, P, Miller, JI, Heger, IM, et al. Laser Doppler flowmetry in neurosurgery. J Neurosurg Anesthesiol. 1993; 5:151–158.
59. Carter, LP, Weinand, ME, Oommen, KJ. Cerebral blood flow (CBF) monitoring in intensive care by thermal diffusion. Acta Neurochirurg Suppl. 1993; 59:43–46.
60. Aaslid, R. Cerebral hemodynamics. In: Newell DW, Aaslid R, eds. Transcranial Doppler. New York: Raven Press; 1992:49–58.
61. Kontos, HA. Validity of cerebral arterial blood calculations from velocity measurements. Stroke. 1989; 20:1–3.
62. Stocchetti, N. Should I monitor jugular venous oxygen saturation? In: Valadka AB, Andrews BT, eds. Neurotrauma. New York: Thieme; 2005:58–61.
63. Macmillan, CS, Andrews, PJ. Cerebrovenous oxygen saturation monitoring: Practical considerations and clinical relevance. Intensive Care Med. 2000; 26:1028–1036.
64. Robertson, CS, Narayan, RK, Gokaslan, ZL, et al. Cerebral arteriovenous oxygen difference as an estimate of cerebral blood flow in comatose patients. J Neurosurg. 1989; 70:222–230.
65. Obrist, WD, Langfitt, TW, Jaggi, JL, et al. Cerebral blood flow and metabolism in comatose patients with acute head injury: Relationship to intracranial hypertension. J Neurosurg. 1984; 61:241–253.
66. Cruz, J. The first decade of continuous monitoring of jugular bulb oxyhemoglobin saturation: Management strategies and clinical outcome. Crit Care Med. 1998; 26:344–351.
67. Schneider, GH, von Helden, A, Lanksch, WR, et al. Continuous monitoring of jugular bulb oxygen saturation in comatose patients: Therapeutic implications. Acta Neurochir (Wien). 1995; 134:71–75.
68. Vespa, P. Should I monitor cerebral blood flow after traumatic brain injury? In: Valadka AB, Andrews BT, eds. Neurotrauma. New York: Thieme; 2005:68–72.
69. Chestnut, RM, Marshall, LF, Klauber, MR, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993; 34:216–222.
70. Stocchetti, N, Furlan, A, Volta, F. Hypoxemia and arterial hypotension as the accident scene in head injury. J Trauma. 1996; 40:764–767.
71. Rossi, S, Cormio, M, Marmarou, A. Internal jugular vein oxygen saturation: Clinical usefulness and limitations in the management of head-injured patients. Crit Rev Neurosurg. 1996; 6:202–208.
72. Dearden, NM. Jugular bulb venous oxygen saturation in the management of severe head injury. Curr Opin Anaesthesiol. 1991; 4:279–286.
73. Stocchetti, N, Paparella, A, Bridelli, F, et al. Cerebral venous oxygenation saturation studied with bilateral samples in the internal jugular veins. Neurosurgery. 1994; 34:38–44.
74. Metz, C, Holzschuh, M, Bein, T, et al. Monitoring of cerebral oxygen metabolism in the jugular bulb: Reliability of unilateral measurments in severe head injury. J Cereb Blood Flow Metab. 1998; 18:332–343.
75. Bankier, AA, Fleisschmann, D, Windisch, A, et al. Position of jugular oxygen saturation catheter in patients with head trauma: Assessment by use of plain films. AJR Am J Roentgenol. 1995; 164:437–441.
76. Matta, BF, Lam, AM. The rate of blood withdrawal affects the accuracy of jugular venous bulb: Oxygen saturation measurements. Anesthesiology. 1997; 86:806–808.
77. Coplin, WM, O’Keefe, GE, Grady, MS, et al. Thrombotic, infectious, and procedural complications of the jugular bulb catheter in the intensive care unit. Neurosurgery. 1997; 41:101–109.
78. Sheinberg, M, Kantner, MJ, Robertson, CS, et al. Continuous monitoring of jugular venous oxygen saturation in head-injured patients. J Neurosurg. 1992; 76:212–217.
79. Fandino, J, Stocker, R, Prokop, S, et al. Cerebral oxygenation and systemic trauma related factors determining neurologic outcome after brain injury. J Clin Neurosci. 2000; 7:226–233.
80. Brain Trauma Foundation, American Association of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care. Guidelines for the management of severe traumatic brain injury: Hyperventilation. J Neurotrauma. 2000; 17:513–520.
81. Kiening, KL, Sarrafzadeh, AS, Stover, JF, et al. Should I monitor brain tissue PO2? In: Valadka AB, Andrews BT, eds. Neurotrauma. New York: Thieme; 2005:62–67.
82. Deardon, NM, Midgley, S. Technical considerations in continuous jugular venous oxygen saturation measurement. Acta Neurochir Suppl (Wien). 1993; 59:91–97.
83. Kiening, KL, Unterberg, AW, Bardt, TF, et al. Monitoring of cerebral oxygenation in patients with severe head injuries: Brain tissue PO2 versus jugular vein oxygen saturation. J Neurosurg. 1996; 85:751–757.
84. Latronico, N, Beindorf, AE, Rasulo, FA, et al. Limits of intermittent jugular bulb oxygen saturation monitoring in the management of severe head trauma patients. Neurosurgery. 2000; 46:1131–1139.
85. Maas, AI, Fleckenstein, W, de Jong, DA, et al. Monitoring cerebral oxygenation: Experimental studies and preliminary clinical results of continuous monitoring of cerebrospinal fluid and brain tissue oxygen tension. Acta Neurochirurg Suppl. 1993; 59:50–57.
86. Meixensberger, J, Dings, J, Kuhnigk, H, et al. Studies of tissue PO2 in normal and pathological human brain cortex. Acta Neurochirurg Suppl. 1993; 59:58–63.
87. van den Brink, WA, Haitsma, IK, Avezaat, CJ, et al. Brain parenchyma/PO2 catheter interface: A histopathological study in the rat. J Neurotrauma. 1998; 15:813–824.
88. Leniger-Follert, E. Oxygen supply and microcirculation of the brain cortex. Adv Exp Med Biol. 1985; 191:3–19.
89. Hoffman, WE, Charbel, FT, Edelman, G, et al. Brain tissue oxygen pressure, carbon dioxide pressure and pH during ischemia. Neurol Res. 1996; 18:54–56.
90. Hemphill, JC, 3rd., Knudson, MM, Derugin, N, et al. Carbon dioxide reactivity and pressure autoregulation of brain tissue oxygen. Neurosurgery. 2001; 48:377–383.
91. Scheufler, KM, Rohrborn, HJ, Zentner, J. Does tissue oxygen-tension reliably reflect cerebral oxygen delivery and consumption? Anesth Analg. 2002; 95:1042–1048.
92. Gopinath, SP, Valadka, AB, Uzura, M, et al. Comparison of jugular venous oxygen saturation and brain tissue PO2 as monitors of cerebral ischemia after head injury. Crit Care Med. 1999; 27:2337–2345.
93. Kiening, KL, Hartl, R, Unterberg, AW, et al. Brain tissue PO2-monitoring in comatose patients: Implications for therapy. Neurol Res. 1997; 19:223–240.
94. Manley, GT, Pitts, LH, Morabito, D, et al. Brain tissue oxygenation during hemorrhagic shock, resuscitation, and alterations in ventilation. J Trauma. 1999; 46:261–267.
95. Maloney-Wilensky E, Gracias V, Christian S, et al: Brain tissue oxygen and outcome after severe traumatic brain injury: A meta-analysis. Digital Poster 584, Congress of Neurological Surgeons 55th Annual Meeting, 2005, Boston.
96. Spiotta AM, Stiefel MF, Gracias VH, et al: Brain tissue oxygen directed management improves outcome after severe traumatic brain injury. Oral Poster 41, Congress of Neurological Surgeons 55th Annual Meeting, 2005, Boston.
97. Oddo, M, Levine, J, Mackenzie, L, et al. Brain hypoxia is associated with short-term outcome after severe traumatic brain injury independently of intracranial hypertension and low cerebral perfusion pressure. Neurosurgery. 2011; 69:1037–1045.
98. Rohlwink, U, Zwane, E, Fieggen, AG, et al. The relationship between intracranial pressure and brain oxygenation in children with severe traumatic brain injury. Neurosurgery. 2012; 70:1220–1231.
99. Brain Trauma Foundation. Brain oxygen monitoring and thresholds. J Neurotrauma. 2007; 24(Suppl 1):S65–S70.
100. Eriksson, E, Barletta, J, Figueroa, B, et al. The first 72 hours of brain tissue oxygenation predicts patient survival with traumatic brain injury. J Trauma. 2012; 72(5):1345–1349.
101. Grohn, OH, Kauppinen, RA. Assessment of brain tissue viability in acute ischemic stroke by BOLD MRI. NMR Biomed. 2001; 14:432–440.
102. Imberti, R, Bellinzona, G, Langer, M. Cerebral tissue PO2 and SjvO2 changes during moderate hyperventilation in patients with severe traumatic brain injury. J Neurosurg. 2002; 96:97–102.
103. Zhi, DS, Zhang, S, Zhou, LG. Continuous monitoring of brain tissue oxygen pressure in patients with severe head injury during moderate hypothermia. Surg Neurol. 1999; 52:393–396.
104. Carmona Suazo, JA, Maas, AI, van den Brink, WA, et al. CO2 reactivity and brain oxygen pressure monitoring in severe head injury. Crit Care Med. 2000; 28:3268–3274.
105. Dings, J, Meixensberger, J, Amschler, J, et al. Brain tissue PO2 in relation to cerebral perfusion pressure, TCD findings and TCD-CO2-reactivity after severe head injury. Acta Neurochir (Wien). 1996; 138:425–434.
106. Dings, J, Meixensberger, J, Amschler, J, et al. Continuous monitoring of brain tissue PO2: A new tool to minimize the risk of ischemia caused by hyperventilation therapy. Zentralbl Neurochir. 1996; 57:177–183.
107. Schneider, GH, Sarrafzadeh, AS, Kiening, KL, et al. Influence of hyperventilation on brain tissue PO2, PCO2, and pH in patients with intracranial hypertension. Acta Neurochir Suppl (Wien). 1998; 71:62–65.
108. Unterberg, AW, Kiening, KL, Hartl, R, et al. Multimodal monitoring in patients with head injury: Evaluation of the effects of treatment on cerebral oxygenation. J Trauma. 1997; 42:S32–S37.
109. Bouma, GJ, Muizelaar, JP, Stringer, WA, et al. Ultraearly evaluation of regional cerebral blood flow in severely head-injured patients using xenon-enhanced computerized tomography. J Neurosurg. 1992; 77:360–368.
110. Brain Trauma Foundation, American Association of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2000; 17:507–511.
111. Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care. Update notice: Guidelines for the management of severe traumatic brain injury. Cerebral Perfusion Pressure. New York: Brain Trauma Foundation/American Association of Neurological Surgeons; 2000.
112. Brain Trauma Foundation. Cerebral perfusion thresholds. J Neurotrauma. 2007; 24(Suppl 1):S59–S64.
113. Sarrafzadeh, AS, Kiening, KL, Bardt, TF, et al. Cerebral oxygenation in contusioned vs. nonlesioned brain tissue: Monitoring of BtiO2 with Licox and Paratrend. Acta Neurochir Suppl (Wien). 1998; 71:186–189.