Intracellular Signaling and T Cell Activation
Learning Objectives
• Draw the immunologic synapse showing primary and secondary components
• Explain the function of adaptor proteins
• Discuss the advantages of T cell receptor (TCR) clustering
• Identify the three transcription factors necessary for interleukin 2 (IL-2) synthesis
• Identify the signaling pathway activated by CD28–B7 interactions
• Identify the signaling pathways activated by CD28–B7 engagement
• Compare the mechanisms by which cyclosporine and tacrolimus inhibit T cell signaling
• Recognize the mechanism by which sirolimus inhibits T cell signaling
• Compare and contrast the structure of high-affinity and low-affinity IL-2 receptors
• Identify the immunologic defect in X-linked severe combined immunodeficiency (SCID)
Key Terms
AP-1 complex
Autocrine signaling
Cyclosporine A
Fos
Immunologic synapse
Jun
NFAT
Nuclear paracrine factor-κB (NF-κB)
Phospholipase C
Phosphokinase C
Ras
Sirolimus
Tacrolimus
Introduction
An immune response requires the activation and proliferation of antigen-stimulated T cell clones. Surface interactions initiate intracellular signaling that results in the synthesis of numerous proinflammatory cytokines, survival factors, and growth factors. One of the most important growth factors is interleukin 2 (IL-2), a 15,500-kDal protein produced by activated T cells. IL-2 has both autocrine and paracrine functions. In autocrine signaling, activated T cells produce IL-2, which binds to receptors on the same cell to initiate T cell growth and proliferation. Soluble IL-2 can react with nearby activated T cells expressing IL-2 receptors (paracrine signaling) as well. IL-2 is also required for the survival and function of regulatory T cells that damper the response to self-antigens.
Signaling Pathways for Interleukin 2 Synthesis
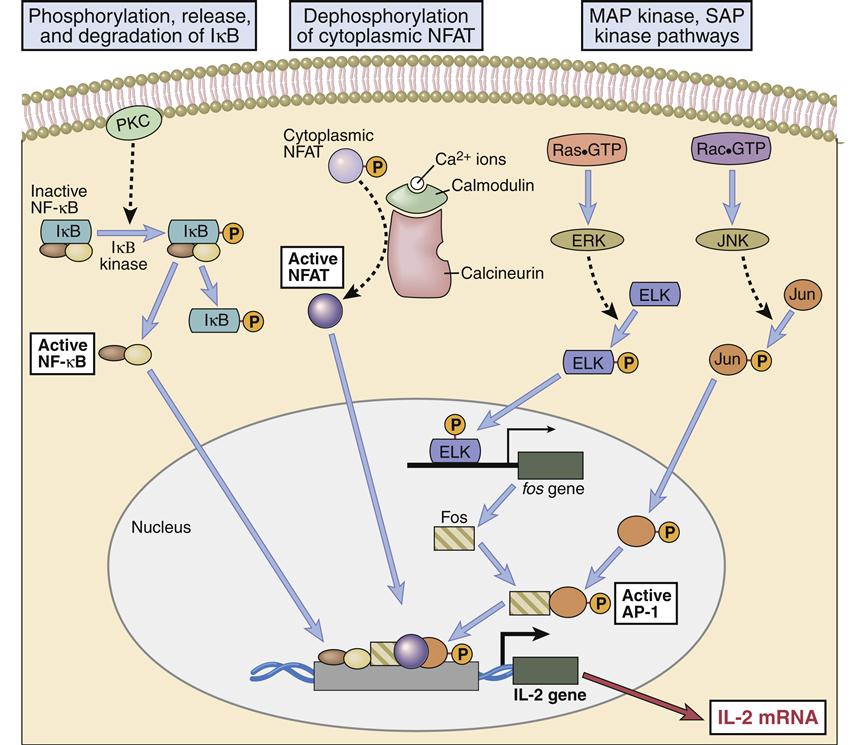
Engagement of the TCR–HLA molecules and CD28–B7 molecules activates several signaling pathways, which culminate in the translocation of three nuclear transcription factors (NFAT, NF-κB, and an AP-1 complex consisting of Fos and Jun proteins). In the nucleus, transcription factors bind to the promoter regions of IL-2 genes, which begin the transcription of messenger ribonucleic acid (RNA) and the translation of IL-2 protein. The pathways involved in the synthesis of IL-2 are shown below.
Pathways Activated by TCR–HLA Interactions
Pathways Activated by CD28–B7 Interactions
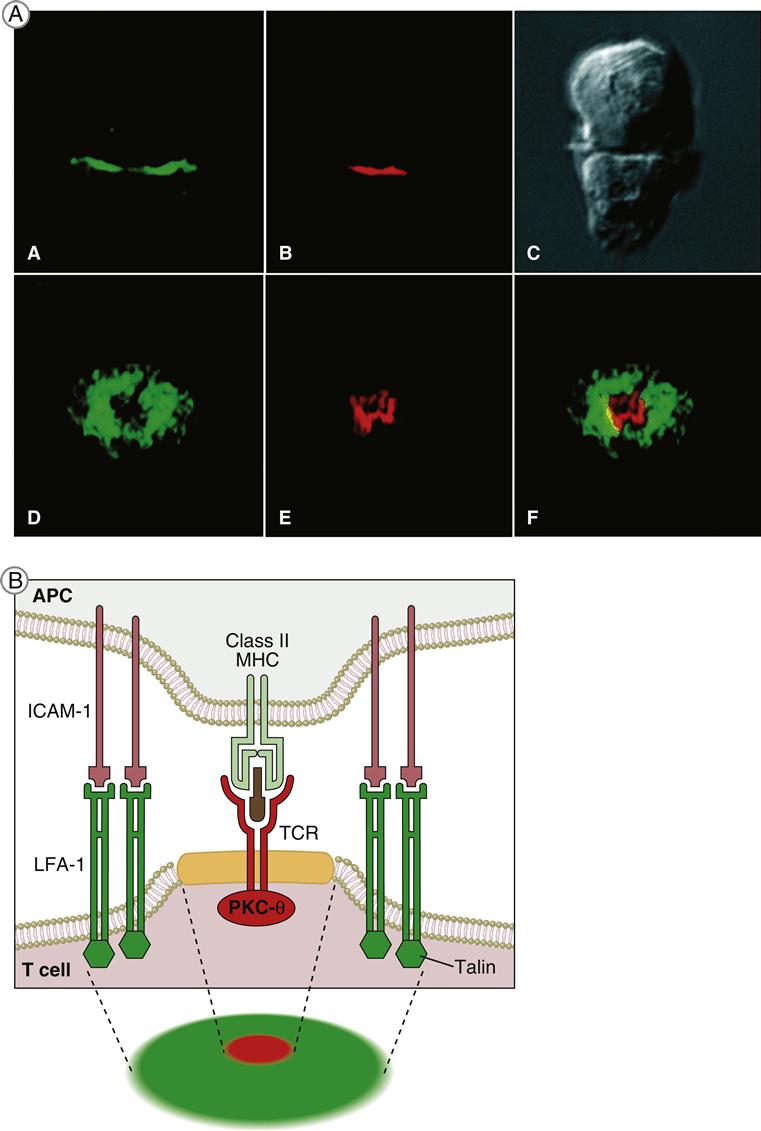
Immunologic Synapse
T cell receptor (TCR)–mediated signaling is initiated by a structure known as the immunologic synapse or the supramolecular activation cluster (SMAC). The synapse is a “bull’s eye–like” structure with the engaged TCR–HLA class I or II molecules and CD28–B7 molecules clustered in the center (Figure 7-1).
Molecular clustering serves three purposes: (1) TCRs engage antigen-loaded HLA molecules with an intermediate affinity. Although these complexes only survive for short periods, they can transduce an activation signal to the nucleus. However, successful activation of T cells requires serial and sustained engagement of TCR–HLA complexes. (2) In the clustered arrangement, multiple TCRs interact with small numbers of antigen-loaded HLA molecules on antigen-presenting cells (APCs). (3) Clustering also congregates multiple cytoplasmic immuno receptor tyrosine-based activation motifs (ITAMs) near adaptor proteins, which are necessary for downstream signaling. Following the engagement of CD4 or CD8 with invariant HLA molecule domains, leukocyte-specific protein tyrosine (lck kinase) is activated and phosphorylates ITAMs. Activated ITAMs serve as “docking stations” for adaptor proteins.
Adaptor Proteins
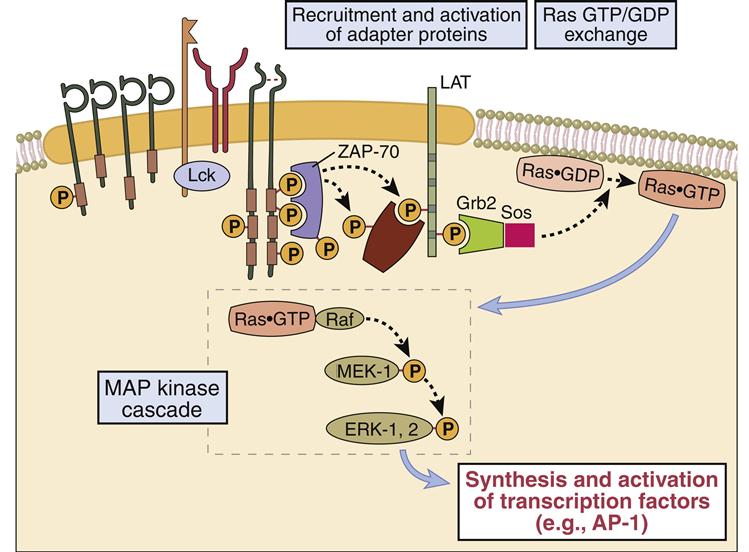
Adaptor proteins form short-lived complexes with other proteins to transduce membrane activation signals to the major cytoplasmic signaling pathways. The most studied adaptor protein is zeta (ζ)-chain associated protein of 70 kDal (Zap-70). Phosphorylation of two ITAMs on TCR ζ-molecules creates a “docking site” for ZAP-70. CD4-activated or CD8-activated lck phosphorylates ZAP-70, which becomes an active kinase. ZAP-70 phosphorylates phospholipase Cγ1 and another adaptor protein called linker for activation of T cells (LAT).
Phosphorylated LAT serves two functions: (1) It provides a “docking site” for growth factor receptor-bound protein 2 (Grb-2) and its associated protein son of sevenless (SOS). (2) The GrB-2–SOS complex activates Ras, the initiating signal in the MAPK pathway. LAT also activates phosphokinase Cγ1 (PKC), the initiating signal for the NF–κB pathway.
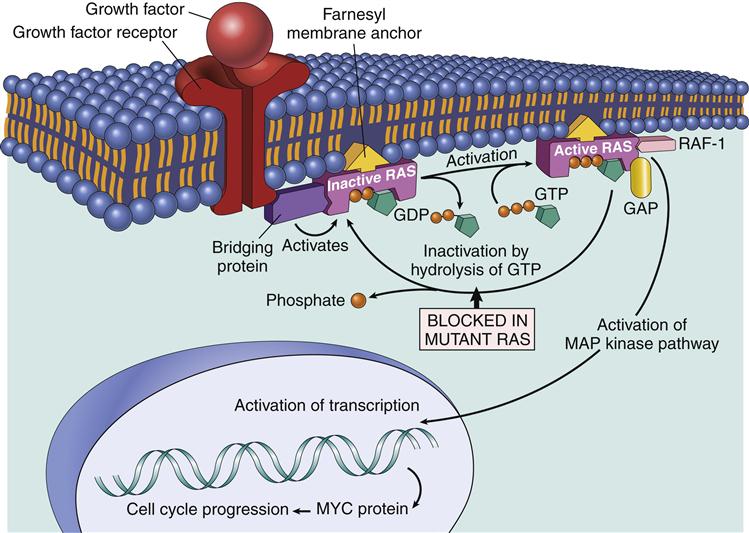
The Ras–Mapk Pathway
One component of the AP-1 transcription factor complex necessary for the synthesis of IL-2 is a product of the Ras–MAPK pathway. Rat sarcoma protein (Ras) is a small G protein, which is regulated by guanosine diphosphate (GDP) and guanosine triphosphate (GTP) in the cytoplasm. GTP activates the Ras protein. Hydrolysis of GTP and removal of a phosphate inactivates Ras (Figure 7-2). In T cell activation, Ras transduces signals from the surface receptor to the MAPK pathway. Hydrolysis of GTP is controlled by the presence or absence of Grb–SOS.
When activated, Ras attaches to the membrane and undergoes a conformational change that allows the activation of MAP kinases. The principal kinase in the pathway is an extracellular signal-regulated kinase called ERK. ERK phosphorylates a small protein called ELK, which initiates the transcription of the Fos protein—one component of the AP-1 complex (Figure 7-3).
The Rac–Jnk Pathway
The second component (Jun protein) of the AP-1 complex is activated in the Rac–JNK pathway. Protein kinase B, also known as Rac protein kinase, is activated by a GDP–GTP exchange protein called Vav. Activated Rac (GATP) phosphorylates the c-Jun N terminal kinase (JNK) which subsequently adds a phosphate to c-Jun. The association of Fos and Jun creates a functional AP-1 complex, which acts as an early transcriptional activator.
AP-1 regulates the production of gene products necessary for T cell division (see Figure 7-2) and the synthesis of IL-2. The creation of the AP-1 provides the first of the three separate nuclear transcription signals necessary for IL-2 synthesis.
Calcium and Plc Signaling Pathway
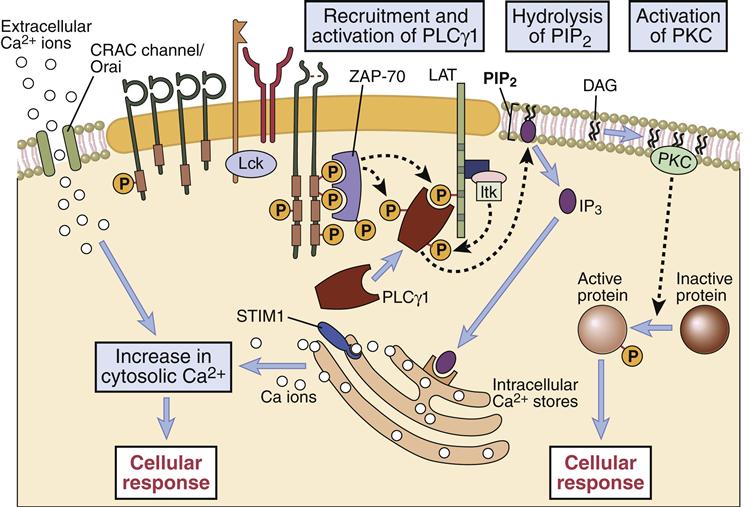
The second nuclear transcription factor (NFAT) necessary for synthesis of IL-2 is a product of the calcium–PLC signaling pathway. In the pathway, phospholipaseγ1 activated by LAT catalyzes the cleavage of membrane phosphatidylinositol-4, 5-bisphosphate (PIP2) to yield two second messengers: inositol-1, 4, 5-trisphosphate (IP3) and diacylglycerol (DAG). In turn, these molecules activate two distinct pathways.
In the calcium-dependent pathway, IP3 translocates to the endoplasmic reticulum and releases stored intracellular calcium. To maintain cellular homeostasis and replenish intracellular calcium stores, the cell activates a membrane ion channel called calcium release activated channel (CRAC), which allows an influx of calcium from the external milieu. Some calcium binds to a regulatory protein called calmodulin. The calcium–calmodulin complex activates a phosphatase called calcineurin. This enzyme activates the nuclear factor of activated T cells (NFAT) protein, which translocates to the nucleus. NFAT provides the second transcription signal for IL-2 synthesis (Figure 7-4).
PLCγ1–DAG/PKC Pathway
Nucleotide factor–κB (NF-κB), the third nuclear transcription factor, is a product of the calcium-independent PLCγ1–DAG/PKC pathway. Membrane-attached DAG activates phosphokinase C (PKC). The activated PKC kinase adds phosphates to a number of different target molecules, which form a trimolecular complex that removes an inhibitor from NF-κB. NF-κB is a peliotropic transcription factor that promotes cell growth, cell survival, and the synthesis of IL-2 (Figures 7-4 and 7-5).
CD28–B7 Signaling Pathway
NF-κB is also the downstream target of CD28–B7 signaling. In the activation pathway, ITAMs on the cytoplasmic tails of CD28 are phosphorylated and recruit PI-3 kinase. The PI-3 kinase activates a number of different pathways, including the Ras–MAP and Jak–STAT (Janus tyrosine kinase–signal transducer activator of transcription) signaling pathways. In the Jak–STAT pathway, PI-3 kinase phosphorylates Jak1 and recruits STATs to the cytoplasmic tail of CD28. In turn, Jak1 phosphorylates STATs, which are released into the cytoplasm. Dimerization of phosphorylated STATs allows translocation to the nucleus and activation of multiple genes. The Jak–STAT pathway is much simpler and shorter than other pathways, and the cellular response is extremely rapid. The PI-3 phosphorylates at position 3 on the inositol ring creating PI-3, 4, 5-triphosphate (PI-3, 4, 5), which accelerates the removal of the inhibitor of NF-κB.
Agents that Inhibit T Cell Receptor Signaling
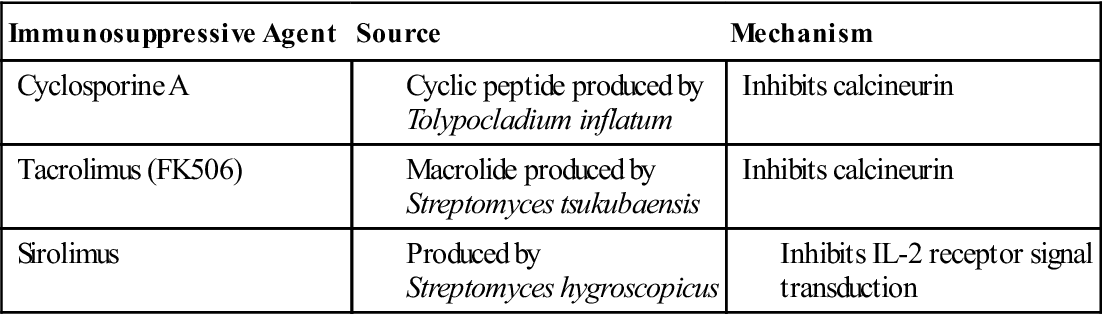
Several therapeutic agents inhibit T cell signaling and downregulate immune responses. Cyclosporine A (CsA) is used as prophylaxis to prevent organ rejection or severe, active rheumatoid arthritis. Although the exact mechanism has not been clearly defined, it is believed that CsA binds to a protein called cyclophilin in the cytoplasm. The complex blocks T cell activation by inhibiting calcineurin phosphatase. As a result, NFAT is not translocated to the nucleus, and IL-2 is not synthesized.
Cyclosporine has serious and life-threatening side effects, including neural, hepatic, and renal toxicity. Neurotoxicity is usually mild and manifests as tremors, but seizures have been described. Cyclosporine A–induced cholestasis and hepatocyte destruction are characteristic of hepatotoxicity. In the kidney, CsA produces a dose-dependent vasoconstriction of renal arteries, which results in mild-to-moderate renal dysfunction. Manifestations of renal dysfunction include a reduced glomerular filtration rate, increased sodium resorption, and hypertension.
Tacrolimus (FK506) has replaced CsA as an anti-rejection agent. It is 10- to 100-fold more active than CsA and has less toxicity. Tacrolimus binds to a cytoplasmic protein called FK506-binding protein 12 (FKBP-12) and blocks the activity of calcineurin (Table 7-1) in the calcium–PKC signaling pathway.
Table 7-1
Fungal Immunosuppressive Agents That Inhibit T Cell Activation and Signaling
< ?comst?>
| Immunosuppressive Agent | Source | Mechanism |
| Cyclosporine A | Inhibits calcineurin | |
| Tacrolimus (FK506) | Inhibits calcineurin | |
| Sirolimus |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Modifed from Actor JA: Immunology and immunobiology, Philadelphia, 2007, Elsevier.
Interleukin 2 Receptors
Autocrine IL-2 stimulation of high-affinity IL-2 receptors is necessary for T cell growth and proliferation. High-affinity IL-2 receptors are only expressed on activated T cells and consist of three chains (α, β, and γ). α-chains and β-chains bind IL-2 and are involved in intracellular signaling. The γ-chain serves as a support structure and is common to a number of cytokine receptors (IL-2, IL-4, IL-7, IL-9, and IL-15).
Agents that Block Autocrine Interleukin 2 Signaling
Sirolimus has a biochemical structure similar to CsA and tacrolimus, but it blocks T cell activation by preventing autocrine IL-2 signaling from the high-affinity receptor to the nucleus. In the signaling pathway, sirolimus binds to cytoplasmic FKBP-12 but does not block the activity of calcineurin. Rather, a bi-molecular complex binds to a third protein called rapamycin-associated protein (RAP). The three-component complex inactivates the protein target of rapamycin (TOR), which is important in accelerating cell division.
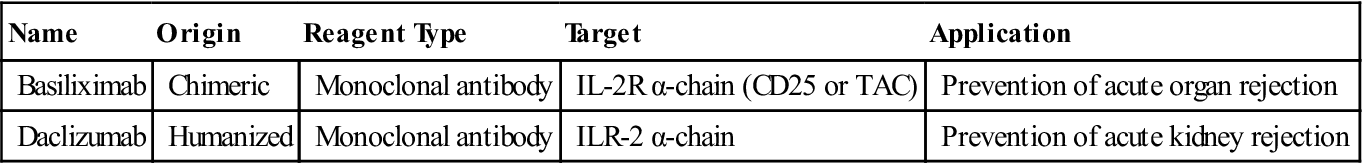
Monoclonal antibodies are also used to block the interaction between the high-affinity receptor and the soluble IL-2. Antibody blockade of the IL-2 receptor prevents T cell growth, and proliferation and induces T cell apoptosis (Table 7-2).
Table 7-2
Monoclonal Antibodies That Inhibit Soluble Interleukin 2 Binding to Receptors
< ?comst?>
| Name | Origin | Reagent Type | Target | Application |
| Basiliximab | Chimeric | Monoclonal antibody | IL-2R α-chain (CD25 or TAC) | Prevention of acute organ rejection |
| Daclizumab | Humanized | Monoclonal antibody | ILR-2 α-chain | Prevention of acute kidney rejection |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Basiliximab is a monoclonal antibody directed at the IL-2 receptor α-chain (also known as IL-2R-α-chain, CD25, or TAC). Another humanized monoclonal antibody, called daclizumab, also is directed at the IL-2R-α-chain. Both antibodies have proven useful in the treatment of acute rejection of transplanted kidneys.
Defective T Cell Signaling and Immunodeficiencies
X-Linked Severe Combined Immunodeficiency
X-linked severe combined immunodeficiency (SCID) is a life-threatening primary immunodeficiency, which is characterized by a lack of T and B lymphocytes or by dysfunctional lymphocytes. Most infants with the disease die within 1 year as a result of recurrent infections by opportunistic pathogens. Several defects in cellular signaling have been described. In X-linked SCID, the major defect is an inability to synthesize a receptor γ-chain, which is part of the IL-2 high-affinity receptor and a number of different IL receptors (IL-2, IL-4, IL-7, IL-9, and IL-15). Engagement of these receptors is critical to lymphocyte activation, synthesis of different types of antibodies, TCR re-arrangements, and natural killer (NK) cell development. X-linked SCID is the most common form of immunodeficiency, affecting 1 in 50,000 to 100,000 births.
ZAP-70 Immunodeficiency
The ZAP-70 protein kinase is expressed on T cells and is associated with the CD3 ζ-chain of the TCR complex. Dysfunctional ZAP proteins cannot phosphorylate ITAMs, which are critical to the initiation of the signaling process and for T cell differentiation. Mutations in Zap-70 are responsible for a rare autosomal recessive form of SCID in humans. This syndrome is characterized by CD8 cells and CD4 cells that are refractory to T cell stimulation.
Treatment of Severe Combined Immunodeficiency
Unfortunately, treatment options for SCID are limited to isolating the patient to protect against microbial infections. Aggressive broad-spectrum antimicrobial treatment must be initiated at the first sign of infection. Affected individuals may also receive intravenous immunoglobulin replacement therapy. The only cure for the disease is bone marrow transplantation or, in select cases, gene therapy.
Summary
• Interleukin 2 (IL-2) synthesis is essential for T cell stimulation.
• Cyclosporine and tacrolimus block the activation of the transcription factors for IL-2.
• Autocrine IL-2 stimulation is required for the growth and proliferation of T cells.
• Sirolimus blocks the signaling from the high-affinity IL-2 receptor.