Chapter 22 Intensive care after cardiac surgery
Coronary artery bypass surgery is one of the most frequently undertaken surgical procedures. Because of the prevalence of cardiac disease, cardiac surgery has significant health and economic implications. Intensive care may account for up to 40% of the total hospital costs for these patients and much of the short-term morbidity and mortality is based on perioperative events.
CARDIOVASCULAR MANAGEMENT
Management is conveniently dictated by standardised protocols, which should cover investigations, fluid and electrolyte management, vasoactive and other drug administration and mechanical ventilation. Standardisation is probably more important than the particulars of the protocol, which might vary considerably among institutions. Optimal cardiovascular management requires a sound knowledge of normal and abnormal cardiovascular physiology. It also requires an understanding of the haemodynamic changes usually seen in the postoperative patient (Figure 22.1).1
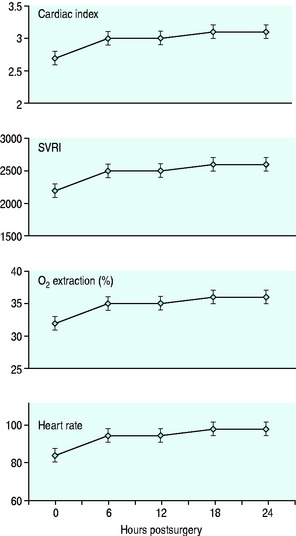
Figure 22.1 Haemodynamic changes following cardiac surgery. Systemic vascular resistance index (SVRI).
MONITORING
Electrocardiography and continuous invasive blood pressure and central venous pressure monitoring are standard. Flotation pulmonary artery (PA) catheterisation has become somewhat controversial. It is now reasonably well established that PA catheterisation is safe but may not alter outcomes.2
FLUID AND ELECTROLYTE MANAGEMENT
RESUSCITATION FLUID
HYPOTENSION
Hypotension is both a consequence and a cause of myocardial dysfunction. The major causes include:
Other causes of low cardiac output state (Table 22.1) should be considered and excluded. Whatever the cause, hypotension frequently causes myocardial ischaemia and consequent heart failure, especially if it occurs in the first 12–24 hours postoperatively. Angiography during this time almost always shows a significant reduction in the calibre of native coronary arteries, indicating increased coronary resistance and, presumably, altered coronary vasodilator reserve. Treatment of hypotension is urgent if a spiral of ischaemia and heart failure is to be averted.
| Preload |
HYPERTENSION
Complications associated with hypertension include:
Significant postoperative hypertension is more common in patients having a history of hypertension and with cessation of β-blockade, but is reasonably common in the early postoperative period. Both absolute pressure and dp/dt are important factors in vascular injury. Vascular resistance declines over the first few hours (see Figure 22.1), so that therapy during this period is best undertaken with agents with a short duration of action. Nitroglycerine is theoretically more appropriate than nitroprusside because of the possibility of coronary steal with the latter agent.6 In practice, this is almost never apparent and nitroprusside appears to be more effective. Simple measures such as the provision of adequate analgesia and sedation should also be considered.
The target blood pressure varies with the indication. Excessive reduction of blood pressure risks reducing myocardial oxygen supply more than demand. Under most circumstances, a mean arterial pressure between 90 and 100 mmHg (12.0 and 13.5 kPa) seems optimal.7 Much lower target pressures may be applicable for the management of heart failure or in the presence of a vulnerable aorta, such as occurs with aortic trauma, dissection or aneurysm. Reduction of dp/dt with beta-blockade is more important than absolute blood pressure control in the management of aortic dissection.
LOW CARDIAC OUTPUT
The aetiology of a low cardiac output following heart surgery is diverse (see Table 22.1). The most common causes include:
Transient, reversible myocardial depression may follow an episode of acute ischaemia (preoperative or intraoperative) and is especially problematic where intraoperative myocardial protection has been difficult or suboptimal. Recognition of a low-output state in the absence of invasive monitoring may be difficult. Many of the usual signs of low output are also consequences of anaesthesia and surgery. Tachycardia may be obscured by drugs, hypothermia and heart disease, and even lactic acidosis may be an unreliable marker in this patient group.1
In the early postoperative period, a relatively low cardiac output may not warrant intervention, providing tissue oxygen delivery is adequate. Since beta-blockade is beneficial in the postoperative cardiac patient, beta-agonists should not be used unquestioningly. Nevertheless, optimisation of cardiac function does confer some benefit8 and intervention is clearly required when tissue oxygen delivery is inadequate.
MANAGEMENT OPTIONS
INTRA-AORTIC BALLOON COUNTERPULSATION
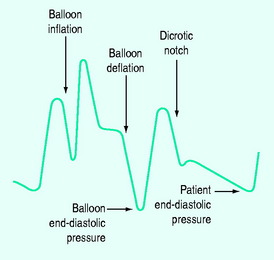
IABC has an established role in support of cardiac surgery. Its two actions are: (1) augmentation of diastolic coronary perfusion pressure; and (2) left ventricular afterload reduction. This is achieved by balloon inflation (30–50 ml capacity) within the aorta during diastole and rapid deflation of the balloon immediately before aortic valve opening. The catheter is usually inserted using a Seldinger technique, but can be placed by femoral artery cutdown or directly into the descending thoracic aorta. Timing of inflation and deflation is critical to optimal function. This is best achieved using the pressure waveform and a 1:2 ratio (Figure 22.2).
Inflation is timed to coincide with the dicrotic notch. Deflation is timed to occur as late as possible in diastole,ensuring that the IABC end-diastolic pressure is lower than the patient’s end-diastolic pressure. IABC increases cardiac index and coronary perfusion and reduces left ventricular filling pressure, myocardial lactate production and oxygen extraction percentage.
Indications for IABC are summarised in Table 22.2.
Table 22.2 Indications for intra-aortic balloon counterpulsation
| Prophylactic |
ISCHAEMIA
Postoperative myocardial ischaemia predicts a more complicated course.18 Recognition is enhanced by automated multilead ST analysis with confirmatory electrocardiography, although diagnosis may be difficult in the presence of preoperative electrocardiographic abnormalities.
DIASTOLIC DYSFUNCTION
Ventricular hypertrophy is the commonest cause of diastolic dysfunction, and is exacerbated by poor myocardial protection during surgical procedures. Myocardial ischaemia, right ventricular (RV) dilatation and pericardial abnormalities (including tamponade) can also reduce left ventricular volume in spite of elevated filling pressures.19 Recognition and management can be very difficult.
DYSRHYTHMIAS
ATRIAL FIBRILLATION (AF)
AF is the most common complication of cardiac surgery.20,21 Its incidence varies from 10 to 40% in patients undergoing coronary artery surgery and up to 50% with some valvular procedures. Predisposing factors include a history of AF, valvular heart disease (especially mitral valve pathology), increasing age and prolonged P-wave duration. AF can be effectively treated and its recurrence prevented with intraoperative radiofrequency ablation and left atrial reduction during cardiac surgery.22 AF is probably less frequently observed following ‘off-pump’ surgery, and is most frequently encountered around the second and third postoperative days, but may occur weeks after surgery and hospital discharge.
Several strategies are beneficial in preventing AF:23
Treatment should be tempered with an understanding that spontaneous reversion to sinus rhythm is frequent. A treatment strategy is summarised in Table 22.3.
| Rate control |
RIGHT VENTRICULAR DYSFUNCTION
RV failure following cardiac surgery is reasonably common. Aetiological factors include:
Useful afterload-reducing agents include nitric oxide,24 prostaglandins and sildenafil.25 Conventional vasodilators tend to produce excessive systemic vasodilatation. Occasionally, RV balloon counterpulsation or an RV assist device may be required. Delayed sternal closure has an established role.
EMERGENCY REOPERATION
Emergency re-sternotomy is indicated as part of resuscitation when haemodynamic stability cannot be rapidly re-established with conventional means.26,27 Advantages compared to closed-chest resuscitation include:
RESPIRATORY MANAGEMENT
Postoperative mechanical ventilation remains routine in cardiac surgical patients. Immediate extubation appears to offer little patient benefit28 but may be expedient. Neither is routine ventilation beyond 12 hours essential.29 Extubation can be safely undertaken with simple protocols. Reintubation is rarely required, but is more likely in older patients with pre-existing lung and vascular disease and impaired ventricular function. Reoperative surgery and bleeding requiring massive transfusion also increase the likelihood of early extubation failure.30
Hypoxia is very common in the early postoperative period. It is mostly attributable to atelectasis and responds well to simple measures such as positive end-expiratory pressure (PEEP), prolonged inspiration and simple recruitment manoeuvres. Atelectasis is a consequence of cardiopulmonary bypass and intraoperative ventilation with high inspired oxygen and without PEEP. Long-term sequelae are rare. Incentive spirometry and chest physiotherapy are commonly utilised in the postoperative period, without good supportive evidence. Early mobilisation appears most beneficial. Adequate analgesia facilitates physiotherapy and mobilisation. Local analgesic initiatives may be helpful31 whereas some non-steroidal agents may increase the risk of complications.32
Pulmonary embolism is an uncommon complication of cardiac surgery, probably due to bypass-induced platelet dysfunction, routine postoperative antiplatelet therapy and thromboprophylaxis.33 ‘Off-pump’ surgery may be associated with an increase in this complication and so may warrant a more aggressive prophylactic regimen.34
POSTOPERATIVE COMPLICATIONS
HAEMORRHAGE
Most studies of pharmacological strategies to minimise postoperative blood loss have involved preoperative or intraoperative intervention. Extrapolation to the postoperative phase is intuitive rather than established. Aprotinin and the lysine analogues aminocaproic acid and tranexamic acid reduce bleeding and exposure to blood and blood products. Aprotinin has been associated with an increased risk of serious end-organ damage.35 Desmopressin is probably less effective and has been associated with an increased risk of myocardial infarction.36
Effective postoperative measures include reversal of residual heparin (including ‘heparin rebound’) and correction of coagulopathy with blood products. Application of PEEP has been shown to be effective in some studies, but not others. Retransfusion of shed blood reduces autologous transfusion requirements without apparent side-effects. Controlled tamponade with discontinuation of drain suction and even clamping of drains has also been reported.37 Finally, at least in reasonably stable patients, reduction of the transfusion threshold to at least 80 g/l reduces exposure to autologous blood transfusion38 without adverse consequences.
RENAL FAILURE
Depending on definition and patient groups, acute renal failure is observed in 1–5% of patients undergoing cardiac surgery. Risk factors include increasing age, heart failure, prolonged bypass, diabetes, pre-existing renal impairment and postoperative shock.39 Surgery without cardiopulmonary bypass may be relatively protective.40 Morbidity, mortality and costs are significantly increased. Effective preventive strategies beyond careful haemodynamic management and minimisation of associated nephrotoxic insults have not been established. Nevertheless, attention to urine output in the presence of known risk factors appears warranted.
STERNAL INFECTION
Deep sternal wound infection is uncommon (0.5–2.5%). Associated morbidity and mortality are significant. Risk factors have been variously reported but consistently include diabetes, obesity and use of internal mammary arteries, especially if bilateral. Other contributing factors include prolonged surgery, chronic lung disease, male sex, low postoperative cardiac output, blood transfusion, sternal reopening and dialysis. Rigid control of blood sugar levels in diabetics41 and preoperative nasal and oropharyngeal decontamination42 may help prevent infection.
NEUROLOGICAL COMPLICATIONS
The most devastating neurological complication is cerebral infarction. The incidence varies with patient selection, but ranges from 1 to 5%. The pathophysiology is mostly embolic. Risk factors include carotid artery stenosis, hypertension, AF, aortic atheroma, impaired ventricular function and peripheral vascular disease.43 ‘Off-pump’ surgery is almost certainly protective and other operative techniques may also reduce the incidence.
GASTROINTESTINAL COMPLICATIONS
Peptic ulcer disease, pancreatitis, cholecystitis, gut ischaemia, ileus and hepatic dysfunction are uncommon (< 1%) after cardiac surgery. Morbidity, however, is quite high. Risk factors include increased age, more complicated surgery and postoperative shock. The incidence of gastrointestinal complications may be reduced with ‘off-pump’ surgery.44
LONG-TERM CONSIDERATIONS
A small percentage of patients require long-term ICU management for a variety of complications. Excellent long-term survival and quality of life are possible in this group of patients, especially if the prolonged ICU stay results from pulmonary complications rather than severe heart failure or neurological dysfunction.45
NON-CARDIAC SURGERY
Non-cardiac surgery poses a significant risk to the patient with cardiac disease.46,47 Postoperative cardiac complications result in significantly increased immediate and late mortality. Simple preoperative assessment enables risk stratification which might lead to deferment or cancellation of non-essential surgery. Indications for specific investigations and treatments are probably not different in the preoperative patient. However, this might be the first time that a cardiac assessment has been undertaken and hence significant cardiac pathology identified. Risk factors are summarised in Table 22.4. Cumulative factors are more than additive. Patients undergoing vascular procedures have a high risk of associated coronary artery disease and should be carefully assessed preoperatively. Perioperative risk can be modified by anaesthetic technique and by optimal medical (or surgical) management of heart disease, which might include myocardial revascularisation.46
Table 22.4 Risk factors for cardiac patients undergoing non-cardiac surgery
High risk: three or more factors.
1 Raper RF, Cameron G, Walker D, et al. Type B lactic acidosis following cardiopulmonary bypass. Crit Care Med. 1997;25:46-51.
2 Shah MR, Hasselbad V, Stevenson LW, et al. Impact of the pulmonary artery catheter in critically ill patients. Maeta-analysis of randomized clinical trials. JAMA. 2005;294:1664-1670.
3 Leibowitz AB, Beilin T. Pulmonary artery catheters and outcome in the perioperative period. N Horiz. 1997;5:214-222.
4 Taylor RW. Controversies in pulmonary artery catheterization. N Horiz. 1997;5:173-296.
5 Finfer S, Bellomo R, Boyce N, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247-2256.
6 Fremes C, Weisel RD, Mickle DAG, et al. A comparison of nitroglycerine and nitroprusside: 1. Treatment of postoperative hypertension. Ann Thorac Surg. 1985;39:53-60.
7 Fremes C, Weisel RD, Baird RJ, et al. Effects of postoperative hypertension and its treatment. J Thorac Cardiovasc Surg. 1983;86:47-56.
8 Polonen P, Ruokonen E, Hippelainen M, et al. A prospective randomized study of goal-oriented haemodynamic therapy in cardiac surgical patients. Anesth Analg. 2000;90:1052-1059.
9 Price S, Prout J, Gibson DG, et al. ‘Tamponade’ following cardiac surgery: terminology and echocardiography may both mislead. Eur J Cardiothporacic Surg. 2004;26:1156-1160.
10 Totaro RJ, Raper RF. Epinephrine-induced lactic acidosis following cardiopulmonary bypass. Crit Care Med. 1997;25:1693-1699.
11 Raja SG, Raven BS. Levosimendan in cardiac surgery: curent best available evidence. Ann Thorac Surg. 2006;81:1536-1546.
12 Morales DLS, Mehmet CO. Mechanical circulatory assist devices in critical care management. N Horiz. 1999;7:489-503.
13 Samuels LE, Kaufman MS, Thomas MP, et al. Pharmacological criteria for ventricular assist device insertion following postcardiotomy shock: experience with the Abiomed BVS system. J Card Surg. 1999;14:288-293.
14 Furnary AP, Magovern JA, Simpson KA, et al. Prolonged open sternotomy and delayed sternal closure after cardiac operations. Ann Thorac Surg. 1992;54:233-239.
15 Dietl CA, Berkheimer MD, Woods EL, et al. Efficacy and cost effectiveness of preoperative IABP in patients with ejection fraction of 0.25 or less. Ann Thorac Surg. 1996;62:401-409.
16 Christenson JT, Simonet F, Badel P, et al. Optimal timing of preoperative intraaortic balloon support in high-risk coronary patients. Ann Thorac Surg. 1999;68:934-939.
17 Ohman EM, George BS, White CJ, et al. Coronary heart disease/myocardial infarction/peripheral vascular disease: use of aortic counterpulsation to improve sustained coronary artery patency during acute myocardial infarction: results of a randomised trial. Circulation. 1994;90:792-799.
18 Yazigi A, Richa F, Gebara S, et al. Prognostic importance of automated ST-segment monitoring after coronary artery bypass graft surgery. Acta Anaesth Scand. 1998;42:532-535.
19 Raper RF, Sibbald WJ. Misled by the wedge? The Swan Ganz catheter and left ventricular preload. Chest. 1986;89:427-434.
20 Bharucha DB, Kowey PR. Management and prevention of atrial fibrillation after cardiovascular surgery. Am J Cardiol. 2000;85:24D. –4D
21 Ommen SR, Odell JA, Stanton MS. Current concepts: atrial arrhythmias after cardiothoracic surgery. N Engl J Med. 1997;336:1429-1434.
22 Scherer M, Therapidis P, Miskovic A, et al. Left atrial size reduction improves the sinus rhythm conversion rate after radiofrequency ablation for continuous atrial fibrillation in patients undergoing concomitant cardiac surgery. Thorac Cardiovasc Surg. 2006;54:34-38.
23 Crystal E, Garfinkle MS, Connolly SS, et al. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Systenaic Rev. 4, 2004. CD003611
24 Argenziano M, Choudhri AF, Moazami N, et al. Randomized, double-blind trial of inhaled nitric oxide in LVAD recipients with pulmonary hypertension. Ann Thorac Surg. 1998;65:340-345.
25 Trachte AL, Lobato EB, Urdaneta F, et al. Oral sildenafil reduces pulmonary hypertension after cardiac surgery. Ann Thorac Surg. 2005;79:194-197.
26 Birdi I, Chaudhuri N, Lenthall K, et al. Emergency reinstitution of cardiopulmonary bypass following cardiac surgery: outcome justifies the cost. Eur J Cardiothorac Surg. 2000;17:743-746.
27 Raman J, Saldanha RF, Branch J, et al. Open cardiac compression in the postoperative cardiac intensive care unit. Anaesth Intens Care. 1989;17:129-135.
28 Montes FR, Sanchez SI, Giraldo JC, et al. The lack of benefit of tracheal extubation in the operating room after coronary artery bypass surgery. Anesth Analg. 2000;91:776-780.
29 Hickey RF, Cason BA. Timing of tracheal extubation in adult cardiac surgery patients. J Cardiac Surg. 1995;10:340-348.
30 Rady MY, Ryan T. Perioperative predictors of extubation failure and the effect on clinical outcome after cardiac surgery. Crit Care Med. 1999;27:340-347.
31 McDonald SB, Jacobshn E, Kopacz DJ, et al. Parasternal block and local anaesthetic infiltration with levobupivocaine after cardiac surgery with desflurane: the efect on postoperative pain, pulmonary function, and tracheal extubation times. Anesth Analg. 2005;100:25-32.
32 Nussmeier NA, Whelton AA, Brown MT, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med. 2005;352:1133-1135.
33 Shammas NW. Pulmonary embolus after coronary artery bypass surgery: a review of the literature. Clin Cardiol. 2000;23:637-644.
34 Mariani MA, Gu J, Boonstra PW, et al. Procoagulant activity after off-pump coronary operation: is the current antocoagulation adequate? Ann Thorac Surg. 1999;67:1370-1375.
35 Mangano DT, Tudor IC, Dietzel C. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354:353-365.
36 Levi M, Cromheecke ME, deJonge E, et al. Pharmacological strategies to decrease excessive blood loss in cardiac surgery: a meta-analysis of clinically relevant endpoints. Lancet. 1999;354:1940-1947.
37 Aravot DJ, Barak J, Vidne BA. Induction of controlled tamponade in the management of massive unexplained postcardiotomy bleeding. Case report and review of the literature. J Cardiovasc Surg. 1986;27:613-617.
38 Bracey AW, Radovancevic P, Riggs SA, et al. Lowering the haemoglobin threshold for transfusion in coronary artery bypass procedures: effect on patient outcome. Transfusion. 1999;39:1070-1077.
39 Mangano CM, Diamondstone LS, Ramsay J, et al. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. Ann Intern Med. 1998;128:194-203.
40 Ascione R, Lloyd CT, Underwood MJ, et al. On-pump versus off-pump coronary revascularization: evaluation of renal function. Ann Thorac Surg. 2000;68:493-498.
41 Furnary AP, Zerr KJ, Grunkemeier GL, et al. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67:352-360.
42 Segers P, Speekenbrink RGH, Ubbink DT, et al. Prevention of nosocomial infection in cardiac surgery by decontamination of the nasopharynx and oropharynx with chlorhexidine gluconate. A randomized controlled trial. JAMA. 2006;296:2460-2466.
43 Das SK, Brow TD, Pepper J. Continuing controversy in the management of concomitant coronary and carotid disease: an overview. Int J Cardiol. 2000;74:47-65.
44 Raja SG, Haider Z, Ahmad M. Predictors of gastrointestinal complications after conventional and beating heart coronary surgery. Surgeon. 2003;1:221-228.
45 Wahl GW, Swinburne AJ, Fedullo AJ, et al. Long-term outcome when major complications follow coronary artery bypass graft surgery. Chest. 1996;110:1394-1398.
46 Eagle KA, Charanjit S, Mickel MC, et al. Cardiac risk of noncardiac surgery: influence of coronary disease and type of surgery in 3368 operations. Circulation. 1997;96:1882-1887.
47 Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043-1049.