Initiating and Adjusting Invasive Ventilatory Support
After reading this chapter you will be able to:
 Discuss the goals of ventilatory support.
Discuss the goals of ventilatory support.
 Describe how to choose an appropriate ventilator to begin ventilatory support.
Describe how to choose an appropriate ventilator to begin ventilatory support.
 Explain how to select an appropriate mode of ventilation given a patient’s specific condition and ventilatory requirements.
Explain how to select an appropriate mode of ventilation given a patient’s specific condition and ventilatory requirements.
 Choose appropriate initial ventilator settings, based on patient assessment.
Choose appropriate initial ventilator settings, based on patient assessment.
 Describe how to assess a patient after initiation of ventilation.
Describe how to assess a patient after initiation of ventilation.
 Discuss how to adjust ventilatory support based on oxygenation and ventilation status.
Discuss how to adjust ventilatory support based on oxygenation and ventilation status.
 Discuss how to ventilate using the concept of lung protective ventilation.
Discuss how to ventilate using the concept of lung protective ventilation.
 Discuss asynchrony and how ventilator adjustments in pressure and volume ventilation improve asynchrony.
Discuss asynchrony and how ventilator adjustments in pressure and volume ventilation improve asynchrony.
 Explain how to adjust the ventilator on the basis of the patient’s response.
Explain how to adjust the ventilator on the basis of the patient’s response.
Goals of Mechanical Ventilation
The goals of mechanical ventilatory support are to maintain adequate alveolar ventilation and oxygen (O2) delivery, restore acid-base balance, and reduce the work of breathing (WOB) with minimum harmful side effects and complications.1 Mechanical ventilation also may reduce increased myocardial work secondary to hypoxemia and an increased WOB.1 Other physiologic objectives of mechanical ventilatory support include increasing or maintaining lung volume with positive end expiratory pressure (PEEP) and continuous positive airway pressure (CPAP) for promotion, improvement, or maintenance of lung recruitment.1
A lung protective ventilatory strategy is an approach to mechanical ventilation that includes the use of small tidal volume (VT) and appropriate levels of PEEP.2 This approach is usually employed in patients with acute lung injury (ALI) or the acute respiratory distress syndrome (ARDS). However, the concept of lung protection should be applied to all patients requiring ventilatory support for acute respiratory failure. Lung injury is primarily caused by an elevated transpulmonary pressure during positive pressure ventilation.3 Transpulmonary pressure is the difference between alveolar pressure and pleural pressure. A safe transpulmonary pressure during mechanical ventilation is not firmly established, but most clinicians would agree that the lower the transpulmonary pressure, the less likely the development of ventilator-induced lung injury.4 High transpulmonary pressures are associated with alveolar overdistention and lung injury.3
Plateau pressure (Pplat), the end inspiratory equilibration pressure, measures the mean peak alveolar pressure and is the best bedside clinical reflection of transpulmonary pressure.2,4,5 Although Pplat is not an accurate measurement of transpulmonary pressure, the transpulmonary pressure never exceeds the Pplat.4 Pplat provides an excellent bedside assessment of the level of potentially dangerous ventilating pressure. Limiting Pplat reduces the likelihood of ventilator-induced lung injury. Generally, the lower the Pplat, the better the patient outcome.2,5 Ideally, Pplat should be less than 30 cm H2O.5 However, a Pplat greater than 30 cm H2O may be applied in patients with a decreased thoracic compliance without resulting in overdistention5 because a decrease in chest wall compliance (obesity, massive fluid resuscitation, abdominal distention, elevated bladder pressure) increases the pleural pressure, decreasing the transpulmonary pressure. Generally, the lowest possible Pplat is maintained by selecting a VT of 4 to 8 ml/kg of ideal body weight (IBW). The higher the Pplat, the smaller the VT should be. Generally, VT greater than 10 ml/kg IBW is never indicated in critically ill patients.
Lung injury can also be caused by repetitive opening and closing of unstable lung units.6 The application of an appropriate level of PEEP ensures that unstable lung units are maintained in the open position reducing the likelihood of additional lung injury.
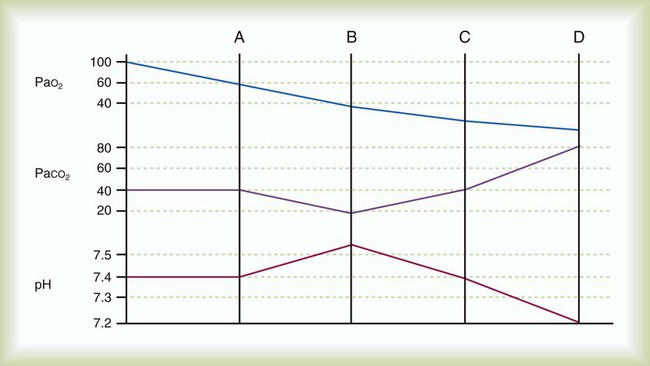
Specific clinical objectives of mechanical ventilation include reversal of hypoxemia, hypercapnia, and associated respiratory acidosis and prevention or reversal of ventilatory muscle dysfunction. The general trajectory of pH, PCO2, and PO2 during the progression of acute respiratory failure is depicted in Figure 44-1. Mechanical ventilation may be used to allow sedation or paralysis for certain procedures, to decrease myocardial and ventilatory muscle O2 consumption to maximize O2 delivery to the tissues, to decrease intracranial pressure acutely in the presence of closed head injury or cerebral edema (by reducing PaCO2 to 25 to 30 mm Hg for a short period and promoting cerebral vasoconstriction), to prevent or reverse atelectasis, and to stabilize the chest wall in the case of a massive flail or chest wall resection. Table 44-1 lists the most common causes of acute respiratory failure leading to ventilatory support in the United States and Canada. Hazards of mechanical ventilation include decreased venous return and cardiac output, patient-ventilatory asynchrony, and ventilatory muscle dysfunction owing to inappropriate ventilator settings, ventilator-associated pneumonia, and ventilator-induced lung injury.1 Box 44-1 lists the goals of ventilatory support, and Box 44-2 lists specific objectives of mechanical ventilation.
TABLE 44-1
| Condition | Rank | Percentage |
| Postoperative respiratory failure | 1 | 17 |
| Sepsis | 1 | 17 |
| Other | 2 | 16 |
| Heart failure | 3 | 13 |
| Pneumonia | 3 | 13 |
| Trauma | 3 | 13 |
| ARDS | 4 | 9 |
| Aspiration | 5 | 3 |
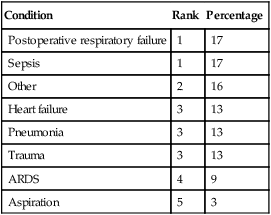
Modified form Esteban A, Anzueto A, Alia I, et al: How is mechanical ventilation employed in the intensive care unit? An international utilization review. Am J Respir Crit Care Med 161:1450, 2000.
Ventilator Initiation
When the decision to begin mechanical ventilatory support is made, one must choose the mode of ventilation, select an appropriate device, and establish the initial ventilator settings. In the selection of initial ventilator settings, the goal is to optimize the patient’s oxygenation, ventilation, and acid-base balance, while avoiding harmful side effects. This goal is achieved by choosing an appropriate mode of ventilation, fractional inspired oxygen (FiO2), VT (volume ventilation) or pressure level (pressure ventilation), rate, peak flow and flow waveform, inspiratory time, and PEEP level. Appropriate trigger sensitivity, pressure limit, alarms, backup ventilation, and humidification must be selected. After initial ventilator setup, adjustments must be made on the basis of the patient’s response and the patient-specific clinical objectives of ventilatory support. Most patients who need mechanical ventilatory support receive invasive positive pressure ventilation; however, an increasing number of patients are being ventilated noninvasively (see Chapter 46). Next, the clinician must choose the mode of ventilation (e.g., volume assist/control [VA/C], pressure assist/control [PA/C], pressure support ventilation [PSV], pressure regulated volume control [PRVC], volume support, adaptive support ventilation, proportional assist ventilation [PAV], or neurally adjusted ventilatory assist [NAVA]) and initial ventilator settings (e.g., rate, VT or pressure level, FiO2, PEEP). Finally, the clinician must choose appropriate alarm and apnea settings. Box 44-3 summarizes key decisions that must be made as a part of initial ventilator setup.
Noninvasive Ventilation
Although more than 75% to 80% of all patients receiving ventilatory assistance receive it invasively, the use of noninvasive ventilation should be considered in select patients requiring ventilatory assistance. Noninvasive ventilation is preferred in some patients because the outcomes are better. Chapter 46 provides details on all aspects on noninvasive ventilation.
Establishment of the Airway
Conventional mechanical ventilatory support requires the establishment of an artificial airway. Initially, nearly 100% of patients receiving positive pressure ventilation are intubated, and of these, 99% have oral endotracheal tubes, and only about 1% are intubated nasally.7 Approximately 5% to 10% of patients receiving mechanical ventilation have a tracheotomy performed at some point.7 Airway management is described in detail in Chapter 33.
Pressure-Controlled versus Volume-Controlled Ventilation
The next decision to be made regarding initiation of mechanical ventilation is whether to use a primarily pressure-targeted or volume-targeted mode of ventilation. Volume-targeted ventilation essentially includes VA/C and synchronized intermittent mandatory ventilation (SIMV). Pressure ventilation includes PA/C, SIMV, PRVC, volume support, and airway pressure release ventilation. In addition, the clinician can select the patient controlled modes PAV or NAVA. However, most patients are initially ventilated with pressure or volume forms of ventilation. The operational capabilities of these modes are described in detail in Chapter 42, and the indications, benefits, and concerns regarding these modes are discussed in Chapter 43.
Full Ventilatory Support versus Partial Ventilatory Support
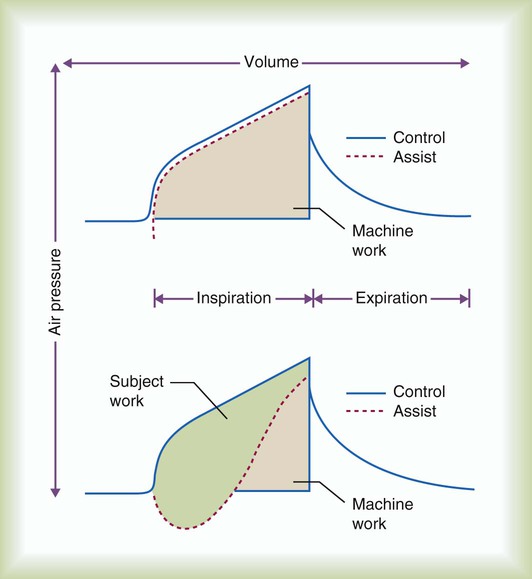
Full ventilatory support can be defined as the application of mechanical support such that all or most of the energy necessary for effective alveolar ventilation is provided by the ventilator.8 When a ventilator is set to deliver full ventilatory support, the patient is either passive or simply triggers the breath to initiate inspiration allowing the ventilator to perform most of the work of breathing. However, it is very difficult to set the ventilator to assume all of the work of breathing without significantly sedating the patient. In most patient-triggered approaches to ventilatory support, patient-ventilatory synchrony is a major issue, and very careful titration of the ventilator settings is necessary to ensure synchrony and minimize patient WOB. Patient-ventilatory synchrony is discussed in detail later in the chapter.
Partial ventilatory support implies that only a percentage of the WOB is provided by the ventilator.8 Normally, when partial ventilatory support is indicated, SIMV, PSV, volume support, PAV, and NAVA are the modes of choice. However, as with full ventilatory support, care in setting the ventilatory is critical to ensure that patient-ventilator synchrony is maximized. Partial ventilatory support strategies minimize the loss of ventilatory muscle function, require less sedation, assist in recruiting and stabilizing alveolar units, and generally move patients closer to ventilator discontinuance than full ventilatory support approaches.
Initial Ventilator Settings
Choice of Mode
Assist-Control Ventilation (Patient-Triggered or Time-Triggered Continuous Mandatory Ventilation)
Assist-control ventilation can be delivered in either pressure-targeted or volume-targeted ventilation. Suggested initial settings for assist-control volume ventilation in the care of adults are listed in Box 44-4. Advantages of assist-control volume ventilation include the assurance that a minimum safe level of ventilation is achieved, yet the patient can still set his or her own breathing rate. In the event of sedation or apnea, a minimum safe level of ventilation is guaranteed by the selection of an appropriate backup rate, usually approximately 4 to 6 breaths/min less than the patient’s assist rate but not less than the rate necessary to provide a minimum safe level of ventilation (e.g., a backup rate of at least 12 to 14 breaths/min).8
Because assist-control ventilation usually provides full ventilatory support, it may result in less WOB than partial support modes. However, less WOB should not be assumed just because the patient is in assist-control ventilation. Trigger work may be significant if inappropriate sensitivity settings are selected. In addition, when a breath is triggered, inspiratory muscle activity persists.9,10 If the inspiratory flow rate during volume ventilation does not meet or exceed the patient’s inspiratory demand, or inspiratory time is too lengthy, the patient’s WOB may be greater, equaling or exceeding the work of a spontaneous unassisted breath.9,10 In pressure ventilation, lengthy inspiratory times, inadequate rise time, and improperly set pressure levels can also cause asynchrony. (See later sections focusing on patient-ventilator synchrony.)
If properly applied and tolerated by the patient, assist-control ventilation may provide ventilatory muscle rest that allows the ventilatory muscles to recover from ventilatory muscle dysfunction. Disadvantages of assist-control mode include an increase in WOB.8,11 Assist control also may be poorly tolerated by awake, nonsedated patients. The patient may fight the ventilator, or asynchronous patient-to-ventilator breathing patterns may develop. Because flow is based on patient demand in pressure-targeted ventilation, synchrony is generally better achieved during PA/C than with VA/C ventilation. Advantages and disadvantages of pressure-controlled ventilation (PCV) are described in Box 44-5.
Assist-control volume ventilation is the most common ventilator mode used throughout the world as the primary initial mode of ventilatory support.7,12 Regardless of the indication for ventilatory support or underlying disease, this mode is able when properly adjusted and the patient is properly managed to provide adequate ventilatory support for all indications for ventilatory support.6,12
Controlled Ventilation (Time-Triggered Continuous Mandatory Ventilation)
Controlled ventilation, pressure, or volume is achieved using the assist-control mode when the patient is apneic because of a medical condition, anesthesia, or use of sedative drugs and paralytic agents. Ventilators in use today do not prevent a patient with sufficient effort from triggering the ventilator, a situation always to be avoided. Controlled ventilation can be achieved only with pharmacologic agents. Advantages of controlled ventilation include eliminating WOB and complete control over the patient’s ventilatory pattern. In cases in which WOB is high, controlled ventilation may allow for ventilatory muscle rest, reduce O2 consumption of the ventilatory muscles, and “free up” O2 for delivery to the tissues.8
Controlled ventilation is a common initial approach in situations of severe acute respiratory failure especially if the primary problem is hypoxemia. Figure 44-2 depicts the effects of inspiratory time on VT during controlled ventilation. Disadvantages of controlled ventilation include the need for sedatives and perhaps paralytic drugs. All patients given paralytic drugs must be sedated adequately because paralysis does not alter the patients’ perception of their surroundings. All patients’ senses are active; none are affected by paralysis; only voluntary muscles are paralyzed. In addition, in the care of apneic patients, ventilator malfunction or disconnection can lead to death.
Synchronized Intermittent Mandatory Ventilation
Synchronized intermittent mandatory ventilation (SIMV) may be used as a means of providing partial or full ventilatory support.8 With SIMV, the machine breath may be volume or pressure targeted; in adults, it is typically a volume-targeted breath. SIMV often is combined with pressure support to overcome the imposed work of breathing (WOBI) during spontaneous breathing owing to the artificial airway. SIMV allows the clinician to vary the amount of support provided from minimal to full ventilatory support. Disadvantages of SIMV include possible development of respiratory muscle dysfunction, especially in patients with rapid, shallow spontaneous breathing patterns; acute hypoventilation with use of low rates if patients do not continue to do their share of breathing; and an increase in WOB secondary to lack of ventilatory support during spontaneous breaths unless pressure support is applied.8 SIMV also delays weaning compared with spontaneous breathing trials or pressure support.13,14 The advantages and disadvantages of assist-control and SIMV modes are summarized in Table 44-2. Outside of the United States, SIMV is an infrequently used mode of ventilation because of the above-mentioned problems.
TABLE 44-2
Advantages and Disadvantages of Synchronized Intermittent Mandatory Ventilation
| Advantages | Disadvantages |
| Lower mean airway pressure may result than is achieved with assist-control ventilation | SIMV with PSV may increase mean airway pressure |
| Ventilatory muscle activity, strength, and coordination are maintained | Ventilatory muscle fatigue may occur |
| Level of support to maintain adequate levels of alveolar ventilation is easy to titrate | Acute hypoventilation may occur, especially with lower machine rates (<8-10 breaths/min) |
| Weaning protocols are easy to apply | Weaning may be prolonged |
| Spontaneous breathing, which is physiologic, is incorporated | Addition of pressure support often is required to overcome WOBI |
| Patients tend not to hyperventilate and may not fight the ventilator, as they may do with assist mode | Patients may have difficulty adjusting to the ventilator; breath stacking is possible with intermittent mandatory ventilation |
| Sedation or paralysis is not required, as it is in control mode | Patients may experience or continue a rapid, shallow breathing pattern or continue to make spontaneous breathing efforts during delivery of a “machine breath” |
| Full or partial ventilatory support and level of support can be titrated according to patient’s need | Patient’s workload increases considerably when SIMV rate decreases to approximately 50% of full ventilatory support value |
Pressure Support Ventilation
Pressure support ventilation (PSV) assumes minimal control over the patient’s ventilatory pattern. Specifically, only the level of pressure applied is controlled by the ventilator, and all other aspects of gas delivery are controlled by the patient. However, PSV is very similar to PA/C. The primary difference is that in PSV flow terminates the breath, whereas in PA/C time terminates the breath. Other than this, PA/C has a backup rate, and with pressure support an apnea mode of ventilation is set.15 PSV can reduce work of breathing and may improve patient-ventilator synchrony by placing more control with the patient.16 Many clinicians use PSV simply to overcome WOB imposed by the artificial airway.16 The PSV level needed to overcome WOBI may be estimated as follows:
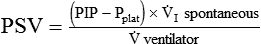
where PSV is the pressure support level needed to overcome WOBI, PIP is the peak inspiratory pressure during a volume-control machine breath, Pplat is the plateau pressure after an inspiratory pause (usually >1 second), 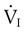 is the patient’s spontaneous peak inspiratory flow (L/sec), and
is the patient’s spontaneous peak inspiratory flow (L/sec), and 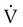 ventilator is the ventilator peak inspiratory flow rate (L/sec) with a square wave inspiratory flow waveform. An example of the calculations for PSV needed to overcome WOBI is presented in Box 44-6.
ventilator is the ventilator peak inspiratory flow rate (L/sec) with a square wave inspiratory flow waveform. An example of the calculations for PSV needed to overcome WOBI is presented in Box 44-6.
High-Frequency Oscillatory Ventilation
High-frequency oscillatory ventilation (HFOV) is the primary approach to high-frequency ventilation used in adults. Respiratory rates range from about 3 Hz (180/min) to about 8 Hz (480/min), and very small VT, often approaching anatomic dead space, is delivered.17 Gas transport during HFOV is due to conventional bulk flow, longitudinal (Taylor) dispersion, pendelluft, asymmetric velocity profiles, cardiogenic mixing, or enhanced molecular diffusion.17 Although high-frequency ventilation has been shown to be safe and effective in maintaining oxygenation and ventilation in various patients,17–19 HFOV has not been shown to be superior to conventional ventilation. The primary setting where HFOV has been used is in the treatment of ARDS, but randomized trials comparing the two indicate no difference in outcome. Most clinicians consider HFOV and conventional ventilation equivalent in their ability to manage ARDS. Many clinicians wait until the patient reaches a critical level of illness before instituting HFOV; however, if one considers HFOV the better approach to managing ARDS, it should be started early in the course of ARDS instead of waiting and using it only as a rescue technique. High-frequency ventilation may allow for reduction in airway pressure and may have value as part of a lung protective strategy for the management of ARDS.19
Initial Choice of Mode
Most patients who need mechanical ventilation in the acute care setting initially are managed with volume or pressure ventilation in the assist-control mode or with PSV.7,12 SIMV may also be used, but it has no advantage over these modes, and it has considerable disadvantages. However, there is no evidence suggesting any of the modes are more beneficial in terms of patient outcomes except that weaning is delayed with SIMV.13,14 Consequently, the choice of initial ventilator mode is primarily one of clinician preference and patient tolerance. Once the patient is stabilized on a ventilator mode, decisions can be made regarding the use of other, newer modes of ventilation, such as PRVC, volume support, adaptive support ventilation, PAV, or NAVA.
Tidal Volume and Rate
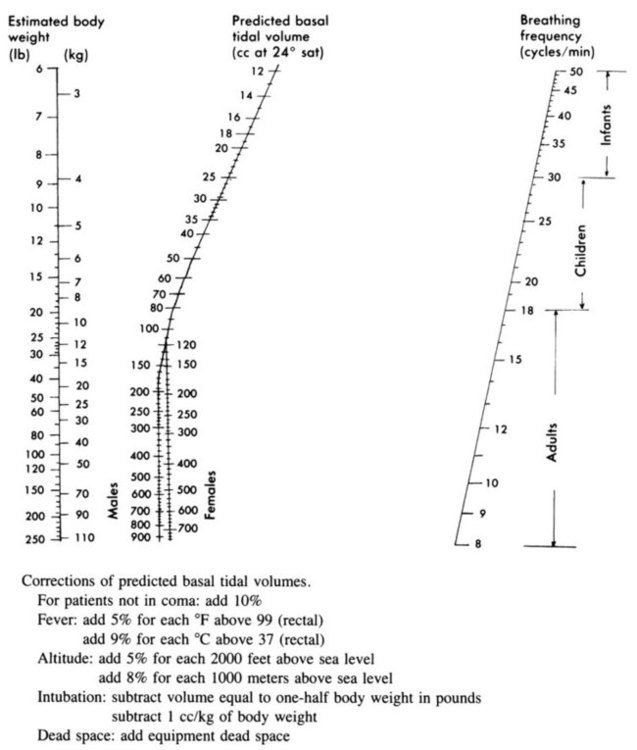
VT and machine rate should be chosen concurrently because these are the two major determinants of minute ventilation. Normal spontaneous VT for unstressed adults is on average 6.3 ml/kg IBW (approximately 5 to 7 ml/kg IBW) with a respiratory rate of 12 to 18 breaths/min establishing a minute ventilation of approximately 100 ml/kg IBW per minute.20 In the past, the Radford nomogram was used to estimate VT and rate on the basis of estimated body weight (Figure 44-3). In modern practice, acceptable VT for mechanical ventilation usually ranges from 4 to 10 ml/kg IBW,1,5 although VT larger than 8 ml/kg IBW is harmful in patients with ALI/ARDS2,21,22 and is mostly harmful to any patient in acute respiratory failure regardless of the cause of the failure.
Generally, regardless of mode, an initial VT of 6 to 8 ml/kg IBW with a rate of 12 to 16 breaths/min is suggested for patients without acute restrictive disease.5,23 After initiation of ventilation, the Pplat can be assessed, and VT can be adjusted downward, as needed, for maintenance of a Pplat less than 30 cm H2O. A smaller initial VT (4 to 6 ml/kg IBW) is appropriate for patients with ALI/ARDS2,20,21 with high Pplat and is usually necessary in patients with severe acute asthma. Table 44-3 lists VT values for men and women according to calculated IBW.
TABLE 44-3
Tidal Volume Based on Ideal Body Weight*
| Height (in) | Height (ft) | Weight (lb) | Weight (kg) | 6 ml/kg | 8 ml/kg | 10 ml/kg | 12 ml/kg |
| Men | |||||||
| 58 | 4′10″ | 94 | 43 | 260 | 340 | 430 | 520 |
| 59 | 4′11″ | 100 | 45 | 270 | 360 | 450 | 540 |
| 60 | 5′0″ | 106 | 48 | 290 | 380 | 480 | 580 |
| 61 | 5′1″ | 112 | 51 | 310 | 410 | 510 | 610 |
| 62 | 5′2″ | 118 | 54 | 320 | 430 | 540 | 650 |
| 63 | 5′3″ | 124 | 56 | 340 | 450 | 560 | 670 |
| 64 | 5′4″ | 130 | 59 | 350 | 470 | 590 | 710 |
| 65 | 5′5″ | 136 | 62 | 370 | 500 | 620 | 740 |
| 66 | 5′6″ | 142 | 65 | 390 | 520 | 650 | 780 |
| 67 | 5′7″ | 148 | 67 | 400 | 540 | 670 | 800 |
| 68 | 5′8″ | 154 | 70 | 420 | 560 | 700 | 840 |
| 69 | 5′9″ | 160 | 73 | 440 | 580 | 730 | 880 |
| 70 | 5′10″ | 166 | 75 | 450 | 600 | 750 | 900 |
| 71 | 5′11″ | 172 | 78 | 470 | 620 | 780 | 940 |
| 72 | 6′0″ | 178 | 81 | 490 | 650 | 810 | 970 |
| 73 | 6′1″ | 184 | 84 | 500 | 670 | 840 | 1010 |
| 74 | 6′2″ | 190 | 86 | 520 | 690 | 860 | 1030 |
| 75 | 6′3″ | 196 | 89 | 530 | 700 | 890 | 1070 |
| 76 | 6′4″ | 202 | 92 | 550 | 740 | 920 | 1100 |
| 77 | 6′5″ | 208 | 95 | 570 | 760 | 950 | 1140 |
| Women | |||||||
| 55 | 4′7″ | 80 | 36 | 220 | 290 | 360 | 430 |
| 56 | 4′8″ | 85 | 39 | 230 | 310 | 390 | 470 |
| 57 | 4′9″ | 90 | 41 | 250 | 330 | 410 | 500 |
| 58 | 4′10″ | 95 | 43 | 260 | 340 | 430 | 520 |
| 59 | 4′11″ | 100 | 45 | 270 | 360 | 450 | 540 |
| 60 | 5′0″ | 105 | 48 | 290 | 380 | 480 | 580 |
| 61 | 5′1″ | 110 | 50 | 300 | 400 | 500 | 600 |
| 62 | 5′2″ | 115 | 52 | 310 | 416 | 520 | 620 |
| 63 | 5′3″ | 120 | 55 | 330 | 440 | 550 | 660 |
| 64 | 5′4″ | 125 | 57 | 340 | 460 | 570 | 680 |
| 65 | 5′5″ | 130 | 59 | 350 | 470 | 590 | 710 |
| 66 | 5′6″ | 135 | 61 | 370 | 490 | 610 | 730 |
| 67 | 5′7″ | 140 | 64 | 380 | 510 | 640 | 770 |
| 68 | 5′8″ | 145 | 66 | 400 | 530 | 660 | 790 |
| 69 | 5′9″ | 150 | 68 | 410 | 540 | 680 | 820 |
| 70 | 5′10″ | 155 | 70 | 420 | 560 | 700 | 840 |
| 71 | 5′11″ | 160 | 73 | 440 | 580 | 730 | 876 |
| 72 | 6′0″ | 165 | 75 | 450 | 600 | 750 | 900 |
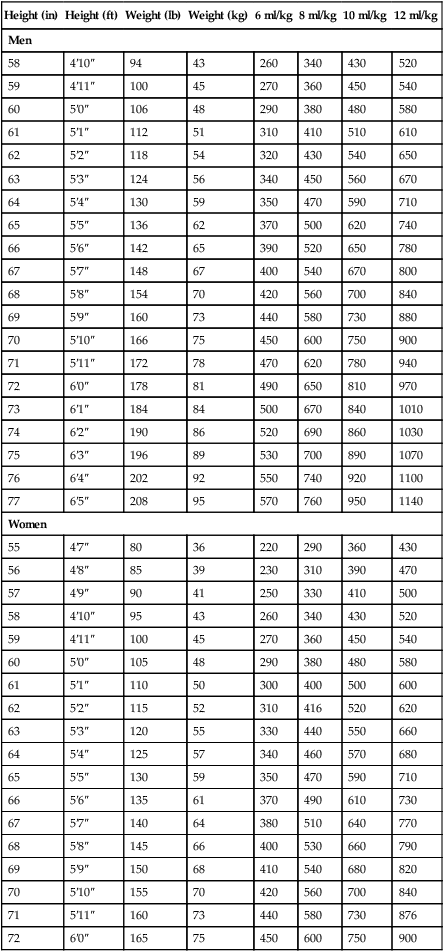
*Ideal body weight (lb): Men, 106 + [6(H − 60)]; women, 105 + [5(H − 60)], where H is height in inches.
VT times rate (f) determines minute ventilation (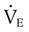 ) As a rule, for adult patients, the resultant minute ventilation should be approximately 80 to 100 ml/kg IBW per minute.8 A 70-kg adult (IBW) would have a minute ventilation of approximately 7000 ml/min. Patients with elevated CO2 production (
) As a rule, for adult patients, the resultant minute ventilation should be approximately 80 to 100 ml/kg IBW per minute.8 A 70-kg adult (IBW) would have a minute ventilation of approximately 7000 ml/min. Patients with elevated CO2 production (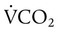 ) or increased physiologic dead space (VDphys) need a larger minute ventilation to maintain acceptable PaCO2. Minute volume should be increased by increasing the rate, not the VT.
) or increased physiologic dead space (VDphys) need a larger minute ventilation to maintain acceptable PaCO2. Minute volume should be increased by increasing the rate, not the VT.
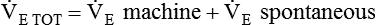
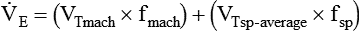
For PA/C or PSV, the delivered VT depends on the pressure limit, the inspiratory time, and the patient’s lung mechanics. Generally, the pressure limit is increased or decreased to achieve a target VT while a Pplat of less than 30 cm H2O is maintained. A good initial pressure setting is to start at 15 cm H2O (above baseline pressure) and observe the resulting VT. Pressure is increased or decreased to achieve the desired volume. As with VA/C, minute ventilation with PA/C is simply rate multiplied by 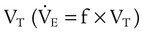 . Recommended initial VT and frequency for various patient types are described in Table 44-4.
. Recommended initial VT and frequency for various patient types are described in Table 44-4.
TABLE 44-4
| Patient Type | Tidal Volume (ml/kg) | Frequency (breaths/min) |
| Adults | ||
| Normal lungs | 6-8 | 12-16 |
| Neuromuscular disease, postoperative period, or with normal pulmonary mechanics in which maintaining lung volume is a concern | 6-10 | 12-16 |
| Acute restrictive disease, ALI/ARDS | 4-8* | 20-35 |
| Obstructive lung disease (COPD) | 6-8 | 10-12† |
| Acute severe asthma exacerbation | 4-6 | 10-12 |
| Children | ||
| Age 8-16 yr | 6-8 | 20-30 |
| Age 0-8 yr | 6-8 | 25-35 |
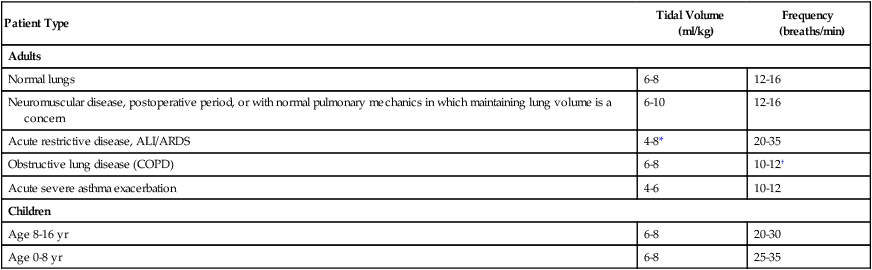
*For ALI/ARDS, maintain Pplat at ≤30 cm H2O. VT begins at 8 ml/kg and is gradually reduced to 6 ml/kg. VT of 4 ml/kg may be required in the care of these patients to avoid ventilator-induced lung injury.
†For patients with obstructive disease, ensure a short inspiratory time and long expiratory time to avoid air trapping and minimize auto-PEEP. Lower VT and rate may be necessary in acute asthma to avoid further lung overinflation.
Patients with ALI/ARDS may need lower VT to avoid further lung injury and a higher rate to maintain effective alveolar ventilation while Pplat is maintained less than 30 cm H2O. Results of multicenter studies suggest VT of 4 to 8 ml/kg IBW for patients with ARDS.2,20,21 In these studies, patients receiving a VT of 4 to 8 ml/kg had lower mortality and morbidity than patients receiving a VT of 10 to 12 ml/kg. For patients with ALI/ARDS, one may begin with VT of 8 ml/kg and decrease the volume as needed to maintain Pplat less than 30 cm H2O.2 Machine rates of 25 to 35 breaths/min may be needed in patients with ALI/ARDS to maintain adequate minute ventilation. Box 44-7 summarizes the ARDS Clinical Network guidelines for initial ventilator setup.2,24
Trigger Sensitivity
Trigger sensitivity for patient-triggered ventilation should be set at the most sensitive level avoiding autotriggering to minimize trigger work and missed triggering. With flow triggering, the trigger should be set at 1 to 2 L/min, and with pressure triggering, the range is generally −0.5 to −1.5 cm H2O. However, because of pin holes in disposable ventilator circuits, the sensitivity may need to be adjusted to 3 or 4 L/min or −2 cm H2O to avoid autotriggering. The increased use of ventilator graphics packages has led to the recognition that patients’ inspiratory efforts often are insufficient to trigger the ventilator.8 Factors that can prolong ventilator response time include large VT, low trigger sensitivity, auto-PEEP, high bias circuit flow, and abdominal paradox (Box 44-8). (See later discussion of asynchrony.)
Many ventilators offer the option of a pressure or a flow trigger. With older generation intensive care unit (ICU) ventilators, flow triggering offered slightly lower trigger work than pressure triggering,25–27 although the gain in terms of the patient’s total WOB was slight. Newer ventilators with fast pressure-triggering capabilities are as sensitive as flow-triggered devices.28 However, no triggering mechanism can reduce the WOB that is a result of auto-PEEP. Auto-PEEP must be addressed by other means (see later section).27 Flow-trigger settings vary by ventilator. Generally, for flow triggering, the trigger flow should be set 1 to 2 L/min below baseline or bias flow.
Inspiratory Flow, Time, and Inspiratory-to-Expiratory Ratio for Volume Ventilation
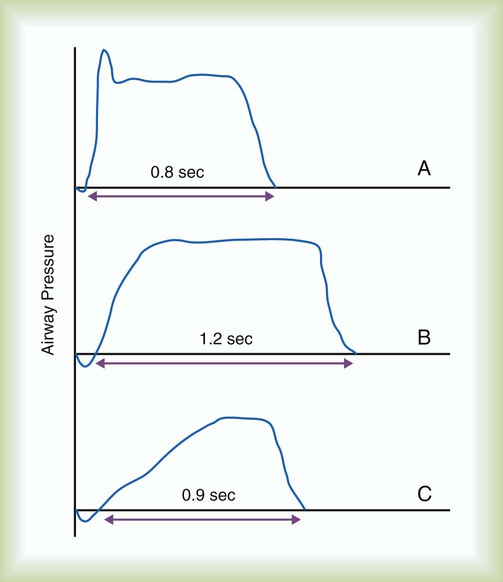
Most modern critical care ventilators allow the clinician to select peak flow, VT, and rate or inspiratory time (or percentage inspiratory time, VT, and rate). For most adults, an initial inspiratory time of approximately 0.8 second (range 0.6 to 1.0 second) with a resultant inspiratory-to-expiratory (I : E) ratio of 1 : 2 or lower is a good starting point. This value corresponds to an initial peak flow setting of approximately 60 L/min (range 40 to 80 L/min) and a down ramp or square flow waveform.29 Higher flow (≤100 L/min) may improve gas exchange in patients with chronic obstructive pulmonary disease (COPD), probably because of the resulting better synchrony and increase in expiratory time.9,29
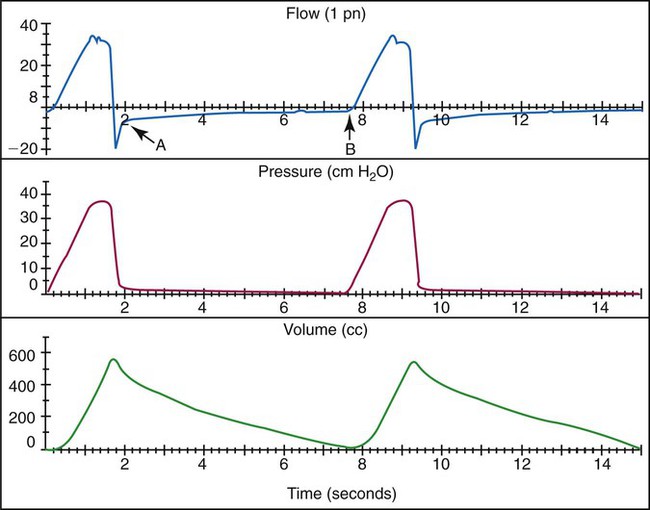
Inspiratory flow rate should be adjusted to ensure that the flow provided meets or exceeds the patient’s spontaneous inspiratory flow.29 This rate can be achieved by observing the resultant pressure-time curve contour on a graphics monitor. Ideally, the pressure curve should show a linear increase during inspiration.9,29 A large negative deflection indicates inadequate trigger sensitivity, and excessive scalloping of the waveform indicates inadequate ventilatory flow settings (Figure 44-4).9,10 A less sensitive trigger level and lower ventilator inspiratory flow tend to increase the patient’s WOB. Common ventilator configurations and related controls that determine inspiratory flow, time, and I : E ratio are described in Figure 44-5.
For ventilators with VT, peak flow, and rate controls, inspiratory time is determined by VT, peak flow, and flow pattern. To decrease inspiratory time, one may increase peak flow, decrease VT, or change from a down ramp to a square wave flow pattern. Expiratory time and I : E ratio are determined by inspiratory time and rate. To increase expiratory time (and decrease I : E ratio), one may decrease the inspiratory time as described earlier or increase the expiratory time by decreasing the rate.29
For ventilators with VT (or minute ventilation), percentage inspiratory time, and rate controls, the inspiratory time and VT determine the inspiratory flow rate. On these ventilators, one can directly increase or decrease the percentage inspiratory time. At the same rate, as inspiratory time (or percentage inspiratory time) decreases, expiratory time and inspiratory flow rate increase, and I : E ratio decreases. An increase in VT at the same percentage inspiratory time and rate also increases inspiratory flow rate with no change in I : E ratio. Box 44-9 shows the calculation of inspiratory flow rate based on percentage inspiratory time settings. To alter I : E ratio on these ventilators, one simply adjusts inspiratory percent time. Decreasing rate at the same inspiratory percent time setting does not affect I : E ratio, and both inspiratory time and expiratory time increase owing to a longer respiratory cycle. Changing the inspiratory flow waveform on these ventilators has no effect on inspiratory time, expiratory time, or I : E ratio; however, flow waveform changes affect peak and mean airway pressure.
Flow Waveform
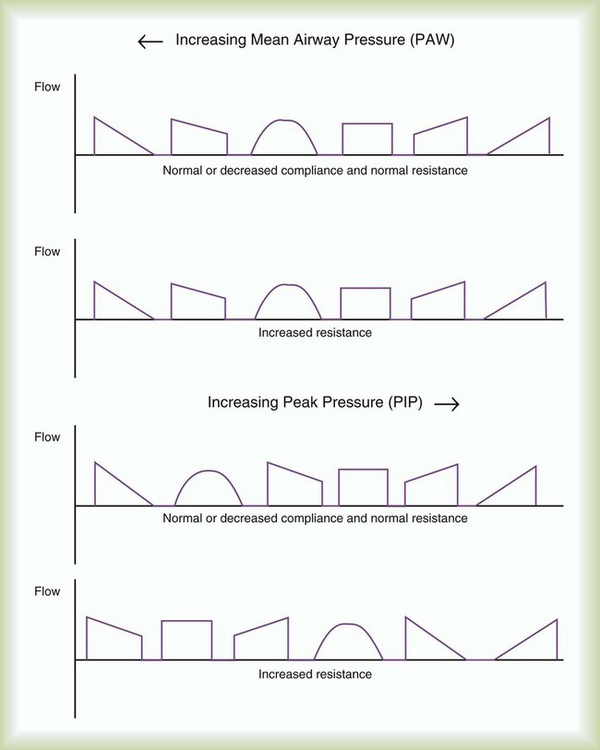
Flow waveform options on mechanical ventilators vary from a preset square wave to seven adjustable waveforms on older ventilators. Common choices available on current generation ventilators for waveform are square or down ramp (decreasing or “decelerating” waveform). Pressure support and pressure-controlled modes also deliver decreasing flow waveforms, but the decrease is patient-specific and not programmed into the gas delivery. The literature on clinical application of specific waveforms is mixed.30 However, as one moves from an increasing (“accelerating”) flow waveform to a square wave to a decreasing flow waveform, while holding inspiratory time constant, there tends to be a predictable decrease in peak airway pressure and a corresponding increase in mean airway pressure.30 Increases in mean airway pressure may improve oxygenation, while further impeding venous return to the heart.30 At least as far as inspiratory flow patterns are concerned, what is good for the lungs may be bad for the heart. We suggest a decreasing, or down ramp, flow waveform when the goal is optimization of the distribution of inspired air and improvement in oxygenation. A square waveform may be useful in reducing mean airway pressure in patients with severe hypotension or cardiovascular instability. Figure 44-6 compares the effect of ventilator flow waveforms on peak and mean airway pressure. Box 44-10 describes guidelines for selecting flow waveform during volume ventilation.
Inspiratory Pause
In addition to inspiratory time or flow, most ventilators have an option for setting an inspiratory pause or hold in the volume-control mode. A brief inspiratory pause (up to 10%) has been recommended in the past for improving the distribution of the inspired air and PaO2.31,32 Use of an inspiratory pause has been suggested for administration of bronchodilators to improve medication delivery. However, in the treatment of patients with COPD, a 5-second inspiratory pause did not result in significant improvement in measures related to the effectiveness of the bronchodilator.31 If a brief inspiratory pause is used, I : E ratio and mean airway pressure increase. An inspiratory pause of 0.5 to 2 seconds applied for a single breath is used for measurement of Pplat and in estimation of airway resistance (Raw):
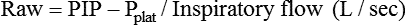
An inspiratory pause can also be used to ensure a full inspiration before a chest radiograph is obtained, and this step may improve the quality of the resulting radiograph.33 Use of an extended inspiratory pause should be limited because of the resultant increase in mean airway pressure and risk of impeding venous return and cardiac output, especially in patients who are hypovolemic or hypotensive or whose condition is hemodynamically unstable.
Positive End Expiratory Pressure and Continuous Positive Airway Pressure
PEEP and CPAP are effective techniques for improving and maintaining lung volume and improving oxygenation for patients with acute restrictive disease such as ALI, pneumonia, pulmonary edema, and ARDS.8,29 PEEP and CPAP should be cautiously applied in the treatment of patients with an already elevated FRC, such as patients with COPD or acute asthma, except at levels that are applied to offset auto-PEEP and air trapping.8 Generally, the indications for PEEP or CPAP in the critical care setting are inadequate arterial O2 level with moderate to high concentrations of O2 caused by unstable lung units that are collapsed. PaO2 less than 50 to 60 mm Hg with FiO2 greater than 0.40 to 0.50 is a good general starting place for considering use of PEEP or CPAP.
In terms of ventilator initiation, initial PEEP or CPAP levels usually are 5 cm H2O even in the absence of unstable lung units or auto-PEEP. Most experts advocate for the use of 5 cm H2O “physiologic” PEEP for all patients who have an artificial airway in place. Intubation results in small reductions in FRC,8,29 which can be balanced with the application of PEEP or CPAP. Whether the application of physiologic PEEP has important benefits in terms of patient outcome is unknown.
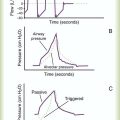
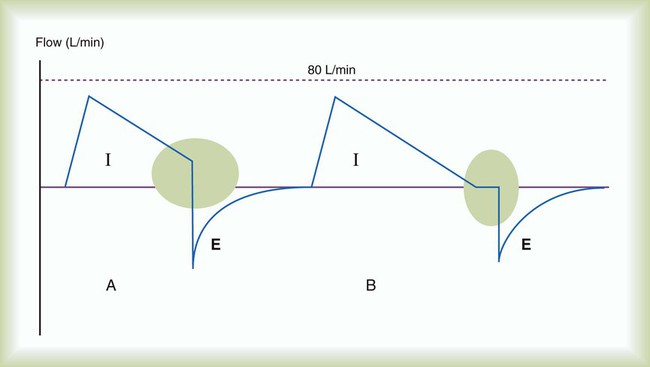
PEEP has been advocated in the presence of auto-PEEP, in particular, in the care of patients with obstructive lung disease.34Applied PEEP in the presence of auto-PEEP is indicated only if the patient has difficulty triggering the ventilator. During controlled ventilation, increasing PEEP in the presence of auto-PEEP is usually not indicated. Because the patient is spontaneously breathing, the measurement of auto-PEEP (end expiratory pause) is very difficult; the patient frequently forces exhalation negating the measurement. The presence of auto-PEEP is generally indicated by the expiratory flow waveform (Figure 44-7) or the fact that there are missed triggered breaths. That is, the patient inspires but is unable to trigger the ventilator. In this case, PEEP is slowly applied in increments of 1 to 2 cm H2O, rechecking the patient and ventilator response rate with each adjustment. When the patient is able to trigger the ventilator with each inspiratory effort, the PEEP level is set properly.29 The exact auto-PEEP level may never be known in these patients; however, this is unimportant. What is important is that each patient’s effort triggers the ventilator. The literature indicates that if the amount of PEEP applied does not exceed approximately 80% of the measured auto-PEEP, the patients’ lung mechanics are not affected by the application of PEEP.35 Auto-PEEP ideally should be rechecked to ensure intrinsic PEEP does not increase as PEEP is applied. Box 44-11 summarizes methods for minimizing the effects of auto-PEEP. An absolute contraindication to PEEP is an uncontrolled tension pneumothorax. However, PEEP should be cautiously applied in any patient with severe intrinsic lung disease, hypotension, and elevated intracranial pressure.
Open Lung Strategy, Recruitment Maneuvers, and Positive End Expiratory Pressure
In the care of patients with ALI/ARDS, it is usually necessary to initiate PEEP at 10 to 15 cm H2O.21,22,35,36 However, many clinicians use the ARDS Clinical Network PEEP/FiO2 tables to set PEEP initially during the establishment of ventilatory support (see Box 44-7). When patients are stabilized, the use of an open lung ventilation strategy in early-stage ARDS has been recommended.5,29 Such a strategy incorporates VT of 4 to 8 ml/kg IBW with either pressure-targeted or volume-targeted ventilation and a PEEP level set after a lung recruitment maneuver using a decremental PEEP trial.5,29,37 The lung recruitment maneuver is intended to open collapsed lung units, and the setting of PEEP using a decremental PEEP trial is intended to apply PEEP based on the patient’s lung mechanics to keep the lung units recruited open.
Although all patients with ARDS/ALI require PEEP, not all patients with ALI respond to PEEP, and patients with pulmonary (vs. nonpulmonary) causes of ALI/ARDS, such as pneumonia, may be less likely to respond to low to moderate levels of PEEP.11 Nonpulmonary causes of ALI/ARDS (e.g., extrathoracic trauma, intraabdominal sepsis) seem to respond well to PEEP.11 In practice, some authors have suggested that higher levels of PEEP (>15 cm H2O) be reserved for patients with a high percentage of recruitable lung.38 High levels of PEEP have been shown to improve outcomes in ARDS in patients with the most severe forms of ARDS (PaO2/FiO2 < 150 mm Hg).39,40 (See later section on the performance of recruitment maneuvers and the setting of PEEP by decremental trial.)
Pressure Rise Time or Slope
Most newer critical care ventilators include an inspiratory pressure rise time or pressure slope. This control functions only with pressure-limited breaths (PSV, PCV, PRVC, volume support, airway pressure release ventilation, pressure SIMV). The purpose of this control is to adjust the rate at which flow increases from baseline to peak.41–43 Generally, rise time should be set at a value that ensures adequate inspiratory gas flow (meeting or exceeding patient demand) without an excessive “overshoot” of the pressure at the beginning of inspiration. A slow or low rise time may increase the patient’s WOB.41–43 The effect of changing inspiratory rise time is described in Figure 44-8 and discussed more in the section on patient-ventilator synchrony.
Limits and Alarms
Patient status alarms usually are set by the respiratory therapist (RT). These include maximum inspiratory pressure, low-pressure and low-PEEP alarms, high-volume and low-volume and rate alarms, O2 and humidification alarms, and apnea alarms. After initiation of ventilation, alarms and limits are readjusted as needed. Alarms usually are set so that they warn the clinician of important changes or problems. Without proper setting, these alarms can become a nuisance by falsely signaling problems that are not real.8
In volume ventilation, a pressure limit should be set. Generally, before the patient is connected to the ventilator, the limit should be set at 40 cm H2O to avoid overpressuring the system when the patient is connected. After the patient is connected to the ventilator, the peak and plateau pressures should be assessed. If Pplat is greater than 30 cm H2O, consideration should be given to decreasing the set VT. If Pplat is less than 30 cm H2O, the high pressure limit can be adjusted to 10 to 15 cm H2O above PIP. One can decrease peak pressure by decreasing the peak flow rate, increasing the inspiratory time, changing the inspiratory flow waveform from a square to a down ramp, or decreasing the delivered VT. For spontaneously breathing patients, inspiratory flow and time must meet or exceed the patient’s inspiratory demand to ensure one does not increase the patient’s WOB further (see the section on Patient-Ventilator Interaction). Preset or adjustable alarms common to most ventilators include pressure (high-low), volume (high-low VT, minute ventilation), apnea, O2 percentage, and temperature. Suggested initial settings for these alarms and backup ventilator settings are presented in Table 44-5.
TABLE 44-5
Alarm and Backup Ventilation Setting of Initial Ventilatory Setup (Adults)
| Low pressure | 8 cm H2O or 5-10 cm H2O below PIP |
| Low PEEP/CPAP | 3-5 cm H2O below PEEP |
| High pressure limit | 50 cm H2O, which is adjusted to 10-20 cm H2O above PIP |
| Low exhaled VT | 100 ml or 10%-15% below set VT |
| Low exhaled minute ventilation | 2-5 L/min or 10%-15% below minimum SIMV or assist-control backup minute ventilation |
| High minute ventilation | 5 L/min or 10%-15% above baseline minute ventilation |
| O2 percentage (FiO2) | 5% above and below set O2 percentage |
| Temperature | 2° C above and below set temperature, high temperature not to exceed 37° C |
| Apnea delay | 20 sec |
| Apnea values | VT and rate set to achieve full ventilatory support (VT 8-10 ml/kg; rate 10-12 breaths/min) with 100% O2 |
Humidification
Humidification is required during both invasive and noninvasive mechanical ventilation. A heated humidifier or a heat and moisture exchanger (HME) should provide a minimum of 30 mg/L of water with a temperature of 30° C or greater.44 Use of HMEs should be avoided in the care of patients with secretion problems and patients with low body temperature (<32° C), high spontaneous minute ventilation (>10 L/min), or air leaks in which exhaled VT is less than 70% of delivered VT.44 Heated humidifiers may be used to deliver 100% body humidity at 37° C. Current clinical practice guidelines suggest an inspired gas temperature of 33° C ± 2° C, although inspiratory gas temperatures of 33° C to 37° C are acceptable in most patients.44 We prefer an optimal humidity approach and use of a heated humidifier to deliver gas in the range of 33° C to 37° C at the airway in most intubated patients and at a temperature consistent with patient comfort during noninvasive ventilation. However, in patients without primary pulmonary dysfunction and short-term ventilation, HMEs are very useful; generally, these are postoperative patients after elective surgery, patients in the emergency department, and patients recovering from an overdose.
Periodic Sighs
Constant, monotonous tidal ventilation at a small volume (<7 ml/kg) may result in progressive atelectasis.8,45 Periodic deep breaths or sighs taken every 6 to 10 minutes reverse this trend.8,45 During the 1960s and 1970s, it was common to ventilate patients with a smaller VT (5 to 7 ml/kg) and no PEEP. As a result, an intermittent sigh function was incorporated into most volume ventilators. Sighs were programmed at 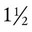 to 2 times the set VT at an interval of every 6 to 10 minutes. Sometimes multiple sighs were included at a preset interval of up to 10 times per hour. Because of the use of PEEP, sighs are no longer routinely included. PEEP prevents the formation of atelectasis in a patient on ventilation with constant small VT. This is the primary reason why 5 cm H2O of PEEP is routinely used on patients, including patients with healthy lungs. General guidelines for the initial ventilator settings for most adult patients are described in Box 44-12.
to 2 times the set VT at an interval of every 6 to 10 minutes. Sometimes multiple sighs were included at a preset interval of up to 10 times per hour. Because of the use of PEEP, sighs are no longer routinely included. PEEP prevents the formation of atelectasis in a patient on ventilation with constant small VT. This is the primary reason why 5 cm H2O of PEEP is routinely used on patients, including patients with healthy lungs. General guidelines for the initial ventilator settings for most adult patients are described in Box 44-12.

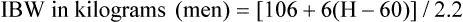
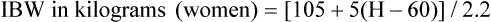
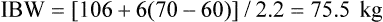
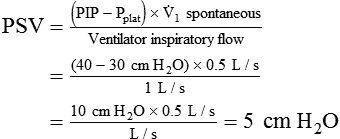
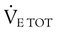 ) is described as follows:
) is described as follows: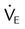 ). Do not exceed 35 breaths/min.
). Do not exceed 35 breaths/min.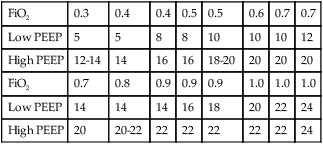

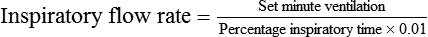
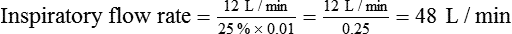
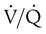 ) or a
) or a 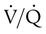 imbalance without shunt (
imbalance without shunt (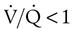 but >0). Patients who often do well with low to moderate concentrations of O2 include patients with acute exacerbation of COPD, emphysema, chronic bronchitis, drug overdose without aspiration, or neuromuscular disease and postoperative patients with normal lungs. For example, a patient with an acute exacerbation of COPD who needs mechanical ventilatory support may have had PaO2 of 50 mm Hg with a nasal cannula at 4 L/min before intubation and mechanical ventilation. This patient would probably do well with FiO2 of about 0.50 when adequate ventilation is restored. The patient can begin with 50% O2 and be immediately assessed for assurance of adequate SpO2. FiO2 can be adjusted according to the patient’s response.
but >0). Patients who often do well with low to moderate concentrations of O2 include patients with acute exacerbation of COPD, emphysema, chronic bronchitis, drug overdose without aspiration, or neuromuscular disease and postoperative patients with normal lungs. For example, a patient with an acute exacerbation of COPD who needs mechanical ventilatory support may have had PaO2 of 50 mm Hg with a nasal cannula at 4 L/min before intubation and mechanical ventilation. This patient would probably do well with FiO2 of about 0.50 when adequate ventilation is restored. The patient can begin with 50% O2 and be immediately assessed for assurance of adequate SpO2. FiO2 can be adjusted according to the patient’s response.