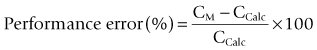Chapter 19 Infusion equipment and intravenous anaesthesia
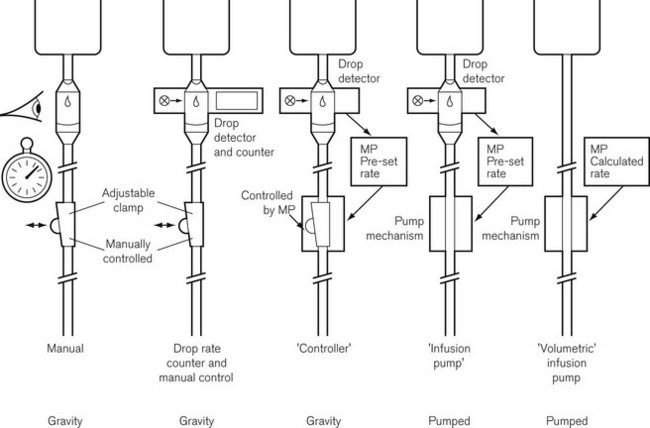
Evolution of infusion systems
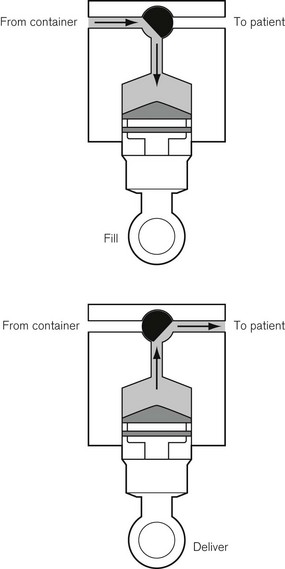
This was superseded first by electronic drip counters, which were subsequently made to control the adjustable clamp on a gravity-fed giving set (infusion controllers) and, ultimately, by microprocessor-controlled infusion pumps (Fig. 19.1), able to generate flow irrespective of the effects of gravity, and incorporating many features such as sensing infusion line pressure and the facility for being programmed in a variety of units and even languages to give stepped infusions based on patient weight. The first devices to not rely on a drip counter used a syringe type cassette to reliably control the volumes delivered and were hence known as ‘volumetric pumps’ to differentiate them (Fig. 19.2). This design is less common now, and most devices rely on a peristaltic mechanism to push fluid along. The term should now probably be made obsolete to be replaced by ‘infusion pump’.
Microprocessor controlled/software driven
• simple ml/h request giving rise to the simplest infusion pump, say for ward use
• unit of drug per unit patient weight per unit time with variable units used for each; for example mg/kg/h or µg/kg/min. Clearly when dosing drug in this manner, the drug concentration in use and the patient weight must be input to the pump to allow automatic calculation of the flow rate. Such pumps may be used in the intensive care unit setting, but are more often termed ‘anaesthesia pumps’ as liberal discretion for choice of units is now usually only entrusted to anaesthetists
• simple infusion rate with an additional bolus volume when commanded by a separately attached control handle, thus constituting a PCA pump
• the in-built processor (or an additional piggybacked processor) can contain algorithms for the pharmacokinetics of particular drugs, allowing automated drug delivery based on achieving any desired theoretical patient plasma or effect site concentration of that drug. Such a configuration is termed a ‘target-controlled infusion (TCI) pump’. These systems are currently only commercially available for use in anaesthesia and are principally limited to four drugs: propofol, remifentanil, sufentanil and alfentanil. Patient variables such as age, sex, weight and height may need to be inputted to complete the algorithm. The algorithm, using this information together with the known drug concentration in the syringe, thereafter allows the anaesthetist to simply select a target patient concentration of drug. The pump automatically alters the infusion rate to most rapidly attain and maintain the calculated drug concentration in the patient at the set target. Such systems are very simple to use and obviate the need for complex calculations and detailed knowledge of pharmacokinetics by the user. They are largely responsible for a revolution in the administration of anaesthesia in a trend away from volatile agent maintenance towards total intravenous anaesthesia (TIVA).
Simple infusion systems
• simple fluid administration, no filter, droplet size approximately 15 drops/ml (0.07 ml)
• blood and fluid administration with clot filter at about 200 μm mesh size. These giving sets use a larger-bore tubing and a double drip chamber containing a float: by squeezing the bottom chamber, the float jams the inlet and acts as a one-way ball valve allowing fluid to be pumped. Droplet size is usually approximately 15 drops/ml
• burette, 100–150 ml in volume, droplet size either 15 drops/ml or, for paediatric usage, 60 drops/ml. A flap or ball valve at the bottom of the burette prevents air entering the drip chamber when the burette is empty
• platelet giving sets, designed to reduce the risk of aggregation in the giving set as would occur with conventional blood giving sets.
Some giving sets are now also designed so as to also be compatible with volumetric infusion pumps. This necessitates a narrower-bore tube made from softer plastic to function with the peristaltic pumps (see below). Flow rates are, therefore, lower and the tubing is less kink-resistant. These sets usually have a 15 μm filter at the base of the drip chamber and are not suitable for infusing blood or for use in adult resuscitation.
The rate of infusion in a simple gravity fed system depends on:
• the height of the fluid container above the infusion site
• the resistance to flow caused by the infusion set
• occlusion of the tubing from a rate controlling device
• the physical properties (i.e. viscosity) of the fluid to be administered
Rapid infusion
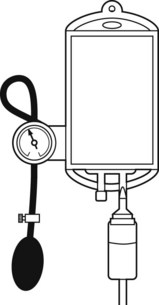
When rapid infusion of blood or other fluids is required, the rate of administration may be increased by the use of an inflatable pressure bag (Fig. 19.3), which may even be contained in a rigid box to increase the speed at which pressure may be applied.
When fluids are administered at a rapid rate, provision should be made for warming them to body temperature, otherwise significant cooling of the body may ensue (see Chapter 30).
Principles of infusion devices
The stepper motor
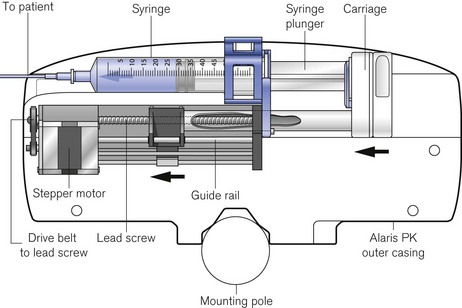
The driving force in the majority of infusion pumps and electronic syringe drivers is provided by an electronic stepper motor, which is directly controlled from a digital microprocessor system. The speed of a conventional electric motor driven from either an AC supply or a DC supply may vary with mechanical load, the voltage or the frequency of the supply. It is, therefore, difficult, without electronic feedback, to control such a motor accurately. The stepper motor (Fig. 19.4) is designed so that a series of pulses applied to the stator windings of the motor cause the shaft to rotate by a fixed amount for each pulse, typically 1.8°, 2.5°, 3.75°or 7.5°, irrespective (within certain limits) of the load. Infusion systems are designed so that a pulse generator, whose output frequency is varied by the microprocessor, can produce accurate control of an infusion by varying the speed of the stepper motor.

Figure 19.4 The stepper motor and lead screw assembly of an Alaris PK syringe pump during manufacture.
Infusion pumps
Cassette type
Originally, the most accurate (and expensive) infusion pumps used syringe type cassettes (see Fig. 19.2) and were referred to as volumetric infusion pumps. This, like the newer peristaltic pumps, is driven by a stepper motor controlled directly by a microprocessor. The volume of the cassette is typically about 5 ml, with the dedicated disposable ‘syringe cassette’ for each manufacturer’s pump being supplied separately as a sterile product. A valve operating in harmony with the piston directs flow from the infusate bag to the reservoir or from there to the patient. Fluid is drawn rapidly from the reservoir bag into the cassette in less than 1 s. The valve is then actuated such that on the piston upstroke the contents are expelled at the required rate into the patient, and the cycle is repeated. Although effectively this produces an intermittent flow, it also gives overall extremely accurate infusion rates, with only infrequent 1 s interruptions.
Peristaltic pumps
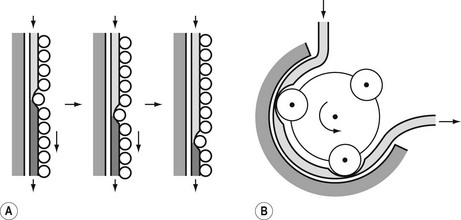
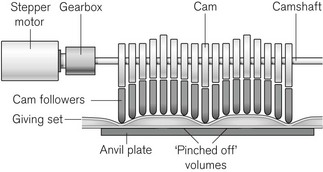
The principle of the peristaltic infusion pump is shown in Fig. 19.5. The tubing of a giving set is compressed by a series of rotating rollers or by a wave of mechanical ‘fingers’ or cam followers. The section of tubing in the peristaltic mechanism must be hard wearing, of known and consistent internal volume, and have no memory after compression so that it easily fills on being released. Depending on the manufacturer, specific proprietary tubing with dedicated fittings may be needed to allow it to be loaded into the pumping mechanism. Precision silicone tubing is often used in this section of the giving set.
Linear peristaltic mechanisms allow much easier loading of the infusion tubing and are now by far the commonest design of infusion pump. The driving force is again a stepper motor. The rotary motion from the motor is translated into a linear peristalsis by the use of cams and cam followers as shown in Fig. 19.6.
Because such infusion pumps have the theoretical capacity to inject limitless quantities of air into a patient should air ingress occur upstream of the pump (for example due to an empty infusion bottle), these devices incorporate sophisticated ultrasonics (Fig. 19.7) or optics-based ‘air in line’ detection systems capable of sensing air bubbles as small as 0.1 ml volume. These are usually placed downstream of the pump mechanism. Further protection is conferred by setting target delivery volumes smaller than the volume in the bag of infusate.
To detect obstructed or extravasated catheters, electrically powered infusion devices must have some measure of the pressure generated in the infusion line beyond the device. Peristaltic intravenous pumps, therefore, use a sensing piston pressing on the infusion line immediately downstream of the pumping chamber. This is calibrated to indirectly measure line pressure and can be programmed to alarm for occlusion at different pre-set levels.
Syringe drivers
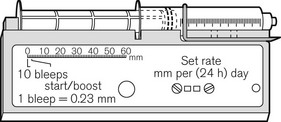
There continues to be a range of small simple battery-operated syringe drivers (Fig. 19.8). The driving mechanism is a miniature DC motor that is switched on and off intermittently and drives a screw-threaded rod (lead screw), which is linked to the syringe plunger, causing its advancement. They may have a variable rate that is altered by adjusting a recessed control using a small screwdriver. These pumps are small and light enough to be worn in a holster by an ambulant patient and are now used chiefly for narcotic infusions for the relief of cancer pain. Great care must be taken in calculating drug dilutions and to ascertain that the correct units are used for setting the infusion rates, as the pumps are available in different models with rates set either as mm per 24 h or mm per h of plunger movement (Fig. 19.9).
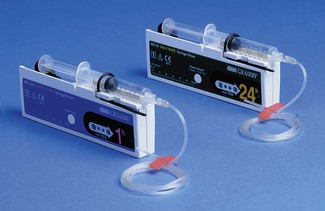
Figure 19.9 Graseby battery operated syringe drivers calibrated in mm per h and mm per 24 h.
Photo courtesy M Stewart, Graseby Medical Ltd.
Virtually all other syringe drivers for hospital use and particularly those used in intensive care and anaesthesia use microprocessor controlled stepper motors, again connected to the syringe plunger by a carriage on a lead screw (Fig. 19.10). Thus, each pulse applied to the stepper motor causes the advancement of the syringe plunger by a known amount. The pulse generator may be calibrated from 0.1 ml h−1 to 1200 or occasionally 1800 ml h−1, the higher rates being used only for delivering a bolus (often of predetermined volume) or for purging the infusion line.
Syringe pumps (the term is synonymous with syringe drivers) are now designed to automatically recognize a variety of syringes by virtue of the calibre of the barrel using some form of spring-loaded arm; some manufacturers’ models nonetheless require manual confirmation of the detector. Infusion line pressure (and empty syringe detection) is calculated indirectly from the force acting on the syringe plunger by sensors, which may be incorporated into the carriage or lead screw assembly (Fig. 19.11). This is a more popular option than the use of specialized infusion sets with in-built diaphragm and corresponding transducer housing on the syringe pump which remains largely confined to pumps used on neonatal ICUs. The facility in some devices to also alarm for low infusion line pressures is intended to allow recognition of disconnection of an infusion line (with the aim of, for example, preventing awareness in intravenous anaesthesia).
Rechargeable batteries
Although mains-driven, electrical infusion devices must have battery back-up both to cover mains failure and for patient transfer and emergency situations. The performance of the in-built rechargeable batteries is an important consideration when purchasing such equipment, but it must be remembered that this is also influenced by the battery maintenance procedures. Poor battery life can render otherwise excellent devices unreliable and unusable. Pumps should be kept connected to the mains when not in use and batteries should be replaced appropriately. Microprocessor-driven infusion devices are susceptible to bizarre error conditions when rechargeable batteries begin to fail.
Safety
Microprocessor-driven infusion devices are used for the administration of many potent drugs with narrow therapeutic windows where maladministration can have lethal consequences. The drugs are used in a variety of dilutions and dosed in units that can vary by several orders of magnitude (ηg/ml, µg/ml, µg/kg/min, mg/kg/h). The devices themselves are highly versatile and capable of being instructed to perform in many different manners. Given these factors it is evident that there is significant potential for user error with disastrous consequences.
The machines have built-in alarms for occlusion, low battery, mains failure, disengagement of drive mechanism, failure to load infusion set and other common fault conditions. In spite of this, they remain high-risk devices capable ultimately of delivering drugs dangerously: they have a recognized associated morbidity and mortality. In at least 27% of the 1495 incidents involving infusion pumps reported to the Medical Devices Agency in the UK between 1990 and 2000, the cause was found to be user error (including failure to maintain the device appropriately).1 Only in 20%, were problems device-related with issues such as performance, degradation, quality assurance and design and labelling. In the 53% of cases where no cause was established it is likely that a very large number represent user error. The MHRA report for the year 2009 again reports that infusion and feeding pumps ‘continues to be one of our busiest areas’ with 375 adverse incident reports.2 The user must, therefore, be ever vigilant and particularly aware of the following problems:
• Unitary programming errors. The simplest and most common error is a mistake or slip in selecting the correct dosing unit or drug concentration, e.g. mg/kg/min instead of µg/kg/min or µg/ml instead of mg/ml. These issues are addressed to a large extent by the use of drug libraries (v.i.).
• Wrong drug errors. Where more than one infusion pump is used particularly when they are controlled through a common interface it is relatively easy to confuse the drugs. Propofol available as identical looking 2% and 1% solutions is also easy to confuse especially as one manufacturer of open label TCI pumps allows a change in concentration during a TCI (Fresenius) and one does not (Alaris), and hence does not prompt for concentration when new syringes are loaded. The Diprifusor system automatically senses the drug in the prefilled syringe and hence does not have this problem but this may now predispose doctors to errors when using other TCI systems.
• Failure to restart infusion. This is a very common error after refilling syringes during intravenous anaesthesia. Software changes across generations of the same device may require additional key presses for the same function (Diprifusor) and can contribute further to this error.
• Siphoning. This is the term used to describe the uncontrolled flow of fluid from a syringe into the patient under gravity. Modern drivers clamp the plunger and have a detector or mechanism built in to protect against incorrectly mounting the syringe plunger. It is best, even with modern designs, to not have the syringe driver higher than the patient, as small amounts of siphoning can still occur. Anti-siphon valves – essentially a one-way valve with a high opening pressure – may be incorporated into syringe pump giving sets (see below, Infusion lines). The possibility of inadvertent administration with infusion pumps as a result of administration sets not being properly clamped off when the pump is not in operation or when the infusion set has not loaded into the device properly still exists but should be largely designed out in new devices.
• Reflux. Where multiple infusion lines are connected to a single intravascular device and there is a distal obstruction it is possible for drugs from pumped lines to reflux up an attached gravity fed line. It is imperative that such lines have anti-reflux valves incorporated (see below, Infusion lines). It is worth stating that this fault situation can and does also frequently occur when drugs are injected into side ports of intravascular catheters or simple giving sets administering fluids by gravity feed. (Classically muscle relaxants injected into giving sets with blocked intravascular catheters, which are then cleared by postoperative recovery staff, resulting in a paralyzed awake patient.)
• Common deadspace and multiple infusions. See ‘Infusion lines’ below.
• Infusion of air. More commonly now due to unprimed infusion lines.
• Extravasation injuries. Continued infusion after a cannula has ‘tissued’ or become extravascular are not prevented by the use of low infusion line pressure limits.
• Electrical hazards. These are present as with all powered devices, but the common liquid spills on to infusion pumps can be particularly dangerous in the presence of cracked machine casings or frayed mains electricity leads.
• Software revisions in microprocessor-driven devices. These can produce a wholly new machine within the familiar appearance of the old. Although features may be added or improved, there is also the possibility of introducing new problems and errors, particularly for those familiar with previous versions. Manufacturers should treat all but the most trivial software revisions as new devices and issue new instruction and training/maintenance manuals.
Error traps and drug libraries
Drug libraries (Fig. 19.12)
• These are pre-programmed usually through a personal or networked computer.
• Setting up is a laborious process often requiring an upper and lower limit for each drug with regard to: patient weight, drug concentration, bolus size, bolus rate and infusion rate.
• They must be set up with great thought and attention to detail so as to:
 militate against inadvertent maladministration by having tight dosage limits and a restricted number of drugs to prevent confusion
militate against inadvertent maladministration by having tight dosage limits and a restricted number of drugs to prevent confusion not cause systematic and institution wide forced errors, e.g. wrong dosing unit or range pre-programmed.
not cause systematic and institution wide forced errors, e.g. wrong dosing unit or range pre-programmed.• They need careful delineation of responsibility for maintenance, e.g. named super-users or the manufacturer, so that it is clear when and by whom changes are made and who carries ultimate responsibility.
• They are particularly appropriate where the user of the device is not the drug prescriber as in ward areas and the ICU.
• They do inherently decrease flexibility of infusion devices. Users in anaesthetic areas often programme in a series of ‘unnamed’ drugs administered in a number of differing mass units per unit patient weight per units of time to allow for unexpected eventualities. Pumps working through libraries are intrinsically slower to set up for use, especially as they also often do not have a numeric keyboard to allow direct entry of values. This may be a significant issue in anaesthetic areas with a high turnover of cases.
Bar code scanners
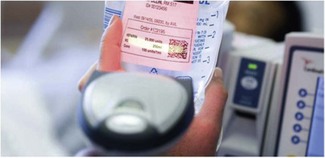
Where drugs can be purchased or prepared in advance with imprinted bar codes or machine readable code, it is possible to combine this with the in-built library of the infusion device to give a simpler to operate and more error-proof system for drug administration (Fig. 19.13). In conjunction with connection to automated record keeping systems such a set-up also allows high-quality record keeping with obvious associations between medication changes and clinical effect. Such systems do currently exist but are not widespread in UK practice.
Line pressure limits
1. It will take longer to alarm for occlusion (alarm time is increased) particularly at low infusion rates and at the start of an infusion – most commonly due to leaving a stopcock closed.
2. On release of the obstruction a proportionately larger bolus will be delivered to the patient; this may be clinically significant.
Electromagnetic interference
Microprocessors are susceptible to electromagnetic interference (EMI) from wireless telecommunication devices such as mobile telephones. EMI can cause serious malfunction in medical devices. The strength of the electromagnetic field is proportional to the power output of the telecommunication device and inversely proportional to the square of the distance from the source. The possibility of EMI is greatest with emergency radios (as used by ambulance, police and fire services) followed by security radios (as used by hospital maintenance and portering staff) and, finally, by mobile phones (both analogue and digital). The Medical Devices Agency of the UK reports 41% of the medical devices they tested suffered interference from emergency radio handsets at a distance of 1 m with 49% of these being classed as serious. By comparison, the figures for security radio handsets were 35% and 49%, respectively, and those for mobile phones 4% and 0.1%.3 It is important to note that these devices can cause interference even in standby mode and must, therefore, be fully switched off to be considered safe. Cordless telephones and wireless computer local area networks do not appear to cause significant interference.
Target-controlled infusion (tci)
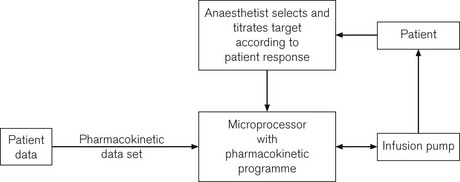
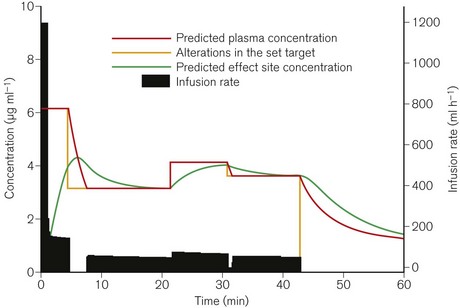
Although the drugs used for intravenously maintained anaesthesia are of rapid onset and have short half-lives and durations of action, in order to rapidly achieve and maintain a given clinical effect it is still necessary with currently available agents to administer boluses and then to reduce progressively the infusion rate. TCI describes a system whereby a computer controls the rate of infusion of a drug to achieve (in as short a time as possible) and maintain any given target concentration. In use this means that the anaesthetist is relieved of having to continuously make complex calculations and adjustments of the infusion rate, thus lending the ability to endlessly vary the drug concentration to achieve the desired effect (Fig. 19.14).
Investigators in the fields of anaesthesia and pharmaco-kinetics have considerable experience in programming computers to achieve constant concentrations of drug by driving infusion pumps at variable rates. Many different terms have been used to describe this process since the description of the bolus elimination and transfer (BET) infusion scheme with a system called CATIA (computer assisted total intravenous anaesthesia) in 1983.4 The aim throughout has been to emulate the simplicity of administration of inhalational agents, in effect to design a ‘calibrated vaporizer’ for intravenous agents, i.e. a system akin to that for inhalational agents wherein a given setting on the ‘vaporizer’ will, within certain limits, result in a similar and constant drug concentration in the patient.
Although the original experimental systems were cumbersome, in 1990 workers from the University of Glasgow described a system for the delivery of propofol using a conventional syringe pump driven by a Psion II hand-held computer.5 There was good correlation between measured and predicted plasma concentrations, and users found the device easy to use. This was the basis for the Diprifusor system, for many years the only commercial system for delivering a drug using a pharmacokinetic algorithm. Since 2005 various device manufacturers have incorporated TCI algorithms into their infusion pumps, these are examined under ‘open label TCI’.
Key components
A TCI system must have the following key components:
• a computer, and a pharmacokinetic algorithm for each drug which translates predictions from the model into instructions to control the infusion device, the algorithm must have the correct pharmacokinetic data set (constants) for the patient group in which it will be used
• patient-specific data: usually weight (the Diprifusor system does not alter the data set for patient age or circumstance); some algorithms may need other co-parameters such as age, sex and height
• a user interface to allow and confirm data input and to inform the user of the algorithm’s predictions
• a fail-safe system: a safety mechanism to shut down the system in the event of computer failure; this may be in the form of a second microprocessor calculating the drug concentration based upon the output of the pump and comparing this with the predicted value from the first microprocessor’s control algorithm, with instructions to shut down the system if there is greater than a preset discrepancy (5% for Diprifusor).
Diprifusor
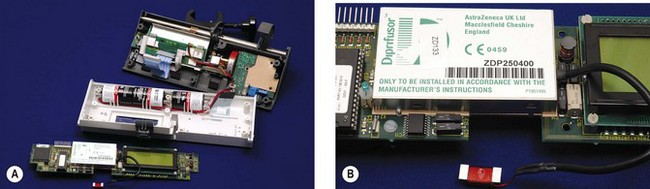
In 1996 the manufacturer Graseby produced anaesthesia syringe drivers bearing the ‘Diprifusor’ TCI Subsystem (Fig. 19.15) licensed from Zeneca (now AstraZeneca), the makers of propofol. The Diprifusor chip has subsequently been incorporated into other makes of syringe driver, the devices being readily identified by the associated green and white logo. An advantage of this modular approach to the design of TCI systems, where the pharmaceutical company retains responsibility for the Diprifusor module, is the standardization of propofol delivery, such that all pumps bearing that module will deliver the same amount of drug for any given target setting because the same pharmacokinetic model is operating (cf. open label TCI issues). To date, in excess of 20 000 such modules (Fig. 19.16) have been sold worldwide (personal communication, AstraZeneca). The Subsystem comprises components designed to:

Figure 19.15 Graseby 3500 Anaesthesia Pump incorporating the Diprifusor chip and demonstrating the associated logo.
• recognize electronically tagged pre-filled syringes of propofol produced by AstraZeneca in order to identify drug and concentration and enable TCI mode when appropriate
• control syringe pump in TCI mode to deliver propofol by TCI.
In order to ensure that the syringe driver operates in TCI mode only when loaded with the correct drug (proprietary propofol as Diprivan), the pre-filled syringes carry a small electronic tag in the finger grip of the barrel. When the syringe is correctly located the ‘Programmable Magnetic Resonance’ tag lies within a recess close to an aerial in the body of the pump. In response to signals from the aerial the magnets within the tag oscillate at particular frequencies generating a signal, which is picked up by the aerial. To discourage refilling of the syringe the tag is subsequently ‘wiped’ by the pump when the actuator pushing the syringe plunger reaches a pre-set travel.
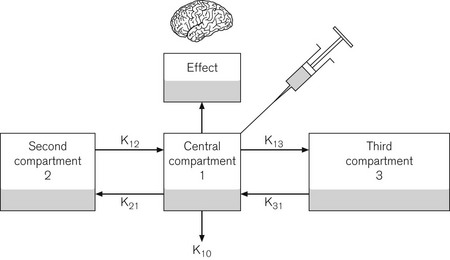
The pharmacokinetic model used is based on the Marsh open three-compartment model6 (Fig. 19.17) with parameters as shown in Table 19.1. Fig. 19.18 shows a typical infusion scheme from a TCI device to demonstrate the bolus and decreasing infusion rate needed to maintain an increased concentration. Diprifusor software (which targets the plasma concentration as the control variable) also allows the predicted effect site concentration to be demonstrated at any time. This facility was added slightly later using a rate constant for equilibration of the effect site (ke0) of 0.26 min−1 derived from a separate study.7 Effect site display is useful, both in terms of demonstrating for impatient anaesthetists the magnitude of the time lag between the compartments and for correlating clinical effect with theoretical concentration when plasma compartment ‘overpressure’ is used to speed up the onset of effect.
Table 19.1 Pharmacokinetic parameters for propofol incorporated in Diprifusor software
| V1 Volume of central compartment | 228 ml kg−1 |
| Half life of delay central to effect site (t1/2Keo) | 2.6 min |
| Rate constants: | |
| K10 (elimination rate constant) | 0.119 min−1 |
| K12 | 0.114 min−1 |
| K21 | 0.055 min−1 |
| K13 | 0.042 min−1 |
| K31 | 0.003 min−1 |
© University of Glasgow
Accuracy
Two independent factors contribute to the overall accuracy of a TCI system:
1. Delivery performance of the infusion system: how well the syringe pump outputs the desired volume of drug
Syringe pumps incorporating Diprifusor are required by the manufacturer to have a performance such that the infusion error at specific time points is within ±5% of the ideal volume.8
• inter-patient pharmacokinetic variability must always be expected and the need to titrate drug target concentration to achieve the desired effect is inescapable
• performance is likely to vary with time and chosen target concentrations
• errors in blood sampling technique or variability in the measurement of blood concentrations; this may at times be due to inadequate mixing at high infusion rates
• in clinical assessments of accuracy the co-administration of other drugs may affect the handling of the target drug.
There is an attempt to standardize measurement of the accuracy of computer-controlled infusion systems.9 Performance error expressed as a percentage is calculated as shown below:
where CM and CCALC are the measured and calculated blood drug concentrations. Other indices are derived from this. The median performance error (MDPE) is a measure of the tendency of the system to over- or underestimate the measured blood concentration in a given patient or scenario. This is the degree of bias and has direction as well as value, thus if bias has a positive value the measured concentrations tend to be greater than predicted. The precision, or size, of the error is represented by the median absolute performance error (MDAPE). Divergence and wobble reflect on time-related changes in performance and intra-subject variability in performance respectively. Typical figures for MDPE of 16.2% and MDAPE of 24.1% were reported for Diprifusor in one study,10 with measured concentrations tending to be higher, particularly after induction or an increase in target concentration and at higher targets. In context these figures compare favourably with the performance of inhalational anaesthesia, where after 15 min of isoflurane administration a ratio of 0.78 is reported between arterial and end-tidal partial pressures11 with the concentration remaining 20% lower at 1 h. In this context there is an even greater difference between vaporizer setting and arterial concentration.12
Open label TCI
Currently in the UK, open label pumps are available from four manufacturers. All offer a choice of Schnider13 and Marsh pharmacokinetic (PK) models to drive the propofol TCI and the same single model (Minto14) for TCI remifentanil.
TCI sufentanil using the Gepts15 model is also available from all the device manufacturers mentioned here except B Braun. At the time of writing, two companies offer further drug models. CareFusion in the Alaris PK pump also offer paediatric propofol pharmacokinetic models – Kataria16 and Paedfusor17 – and a model for alfentanil (Maitre18). Arcomed AG (Switzerland) can offer two versions of the Marsh model as well as Kataria and Maitre models and have expressed a willingness to consider implementing other models (with due licensing disclaimers) at customers’ specific requests (personal communication). B Braun and Arcomed AG are able to implement the TCI algorithms into their peristaltic infusion pumps as well as the more traditional syringe pumps, adding another degree of flexibility.
Ultimately the various devices all offer much the same functionality whether they are controlled through one interface as in the Orchestra Base Primea from Fresenius (Fig. 19.19) or programmed and controlled individually as in the Alaris (Fig. 19.20) and B Braun (Fig. 19.21) devices. For most prospective users the choice between them is probably more a matter of balancing personal preference for the input method and display against the irritating interface and programming quirks. The devices all suffer from not having a numeric keyboard – thus having to scroll through numbers for entering values – and needing preloading with infusion schemes to allow use in anything other than TCI mode or simple ml/h control.
• having two or more pumps side by side with no automatic drug identification
• differing user interfaces and set up procedures between devices
• issues with pharmacokinetic models: their limitations and varied implementations by manufacturers, as below.
Other pharmacokinetic models
Additional confusion surrounds the issue of effect site control of the infusion, with multiple models and manufacturers updating the Marsh model with their own interpretations of available data (Table 19.2). The rate constant (ke0) that was originally added to Diprifusor to calculate and display the effect site concentration of propofol was felt by some to be too slow, but because of the predominance of Diprifusor it is a figure that has clinical relevance for many and is used elsewhere. Fresenius uses a Marsh model with a much a larger (ke0) for effect site control, but this is felt now to be too fast.19 Arcomed offer two versions of Marsh for effect site control, each effectively using figures at opposite ends of the spectrum. The Alaris PK uses the same value as Diprifusor, and Braun use an intermediate figure for their Marsh model, but neither currently allows effect site TCI using the Marsh model.
Table 19.2 The differing rate constants for equilibration of the effect site with the plasma propofol concentration (also expressed as half time) used by different manufacturers
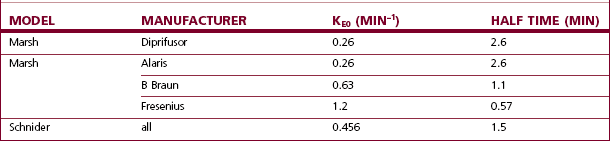
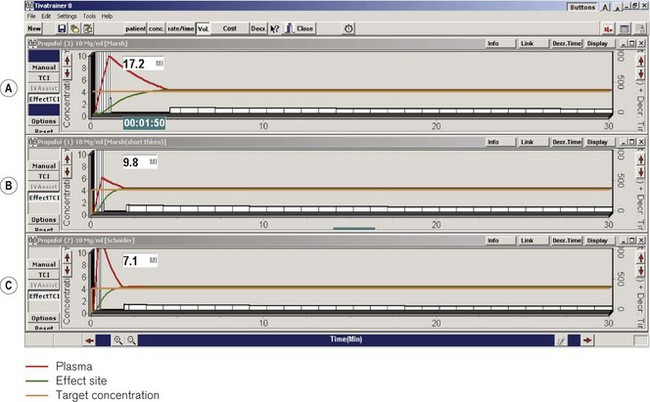
Thus, in effect site targeting mode a given target concentration can result in different volumes of drug being delivered from different manufacturers even when nominally using the same model (see Fig. 19.30). Even for those with an interest in these issues, it is difficult to learn or remember how a PK model and device combination will behave under different circumstances, particularly once patient variability is also considered!
Extremes of weight also present a problem for the current PK models. This is most notable in the obese, where the James equations20 used by the Schnider and Minto models in the calculation of lean body mass (LBM) for their algorithms, generate decreasing values above a certain body weight depending on height and gender. As a result Fresenius pumps will not progress with TCI for body mass index (BMI) >42 for males and >35 for females, these being the inflection points on the James equation graphs after which the LBM starts to decrease. Alaris pumps handle the problem differently; they simply will not allow a greater weight entry then the corresponding figure for the above BMIs. The Marsh model (and hence Diprifusor) markedly underestimates plasma concentrations for morbidly obese patients (i.e. delivers too much drug for a given concentration) and the patient weight used in the algorithm needs to be manipulated significantly towards the ideal body weight of 70 kg for both the obese and the underweight.
Future developments
Open TCI initiative
The ‘Open TCI initiative’ is an attempt by workers in the field of drug pharmacokinetics and pharmacodynamics to come up with a single unifying model for each drug on which all workers are agreed and which would be the basis for all dosing by TCI.19 Manufacturers of medical devices would, thus, not be in the position of having to dictate drug dosing. It will also become possible to compare effects and outcomes between studies once drug delivery is based on a common dosing regimen.
Experimental systems
A logical development from the TCI system, for maintaining constant concentrations of anaesthetic, has been to link this with some form of depth of anaesthesia monitor to automatically alter the chosen target to maintain a constant depth of anaesthesia. Closed loop anaesthesia (CLAN) has been successfully used to anaesthetize patients breathing spontaneously during surgery.21,22
TCI may also be used as the basis for an advanced form of PCA (or sedation). Here, the patient’s button presses may, for example, result in an increase or prevent a decrease in the target concentration of drug rather than a drug bolus.23 Such systems are so far experimental only.
Patient-controlled analgesia (pca)
• analgesia/dosage tailored to patient’s requirements, with the patient able to balance analgesia and side-effects.
Key points in any PCA system are:
PCA devices (Fig. 19.22) are mostly variations of the infusion devices discussed earlier. They may be syringe drivers or peristaltic type pumps, which are microprocessor-controlled; hence, again the aspects of those devices mentioned elsewhere are still pertinent. Because of the trend towards increased patient mobility and patient-controlled epidural analgesia (PCEA), a number of manufacturers now produce miniaturized PCA pumps, often using a small compressible pumping chamber designed as an integral part of the giving set (Fig. 19.23). Size and portability are limited by the size of the drug reservoir and the need to encase this in a lockable anti-tamper shell.

Figure 19.23 A Baxter PCA pump which may be used for epidural continuous infusion or even epidural PCA.
Elastomeric pumps
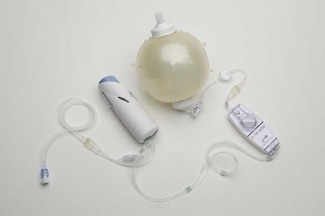
Given the issues surrounding microprocessor-controlled infusion pumps and the specific problems associated with the PCA configurations, there is much to be said for the use of simple low-cost disposable PCA devices, as shown in Fig. 19.24.
Elastomeric devices are in effect powered by the energy stored in the stretching of the balloon holding the reservoir of drug. A small compressible chamber of preset volume (usually 0.5 or 1 ml), on a wrist strap or attached to the reservoir, holds the bolus dose which is delivered to the patient through a one-way valve when the chamber is squeezed. The lock-out time is predetermined by a flow restrictor connecting the reservoir and chamber, which governs the rate of refilling of the chamber, thus giving a maximal flow rate through the device, typically 5 ml h−1. Drug demands, before the effective lock-out time is reached, result in proportionately smaller doses. PCA regimen alterations are, therefore, made by altering the drug concentration in the reservoir. Some newer models have a user (prescriber) variable flow restrictor and or bolus size (Fig. 19.25).
Related equipment
Filtration
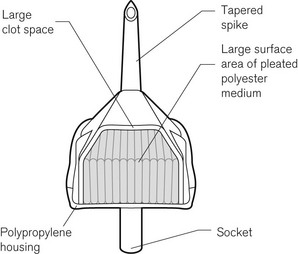
Intravenous fluids should be filtered to protect the patient from microscopic foreign material. Blood for transfusion that is more than 24 h old should be filtered (Fig. 19.26) to remove micro-aggregates that form from the breakdown products of the cellular components and platelets. Blood filters are of two basic forms: screen filters and depth filters. Screen filters function as ‘sieves’ and are usually constructed from a woven mesh. They have a regular pore size, often of 40 µm. The efficiency of a screen-type filter at removing foreign matter increases progressively with each unit of blood passed as the pore size tends to decrease progressively down to 20 µm. Screen-type filters are said to be less damaging to the red cells than are depth filters. Depth filters consist of a pack of synthetic fibre, often Dacron, not formed into a mesh. The mechanism of filtration is actually by adsorption of unwanted material, down to a size of about 10 µm onto the surface of the fibres. This adsorption is probably due to electrical charge differences between the particles and the fibres. With the depth filter, the efficiency at removal of unwanted material decreases with each unit of blood, probably as a result of channelling in the pack of fibres.
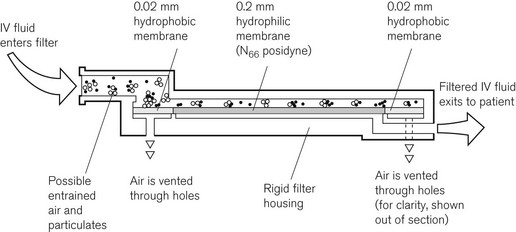
Intravenous crystalloids may be filtered with much finer sieve-type filters to remove foreign particulate matter, including bacteria. The Pall intravenous ‘Site Saver’ extends the life of the giving set, which should normally be replaced every 24 h, to up to 96 h. The construction of the ‘Site Saver’ is shown in Fig. 19.27. All particulate matter larger than 0.2 µm is removed by the 0.2 µm filter membrane. Air can be vented through a hydrophobic membrane, which has 0.02 µm pores.
Infusion lines
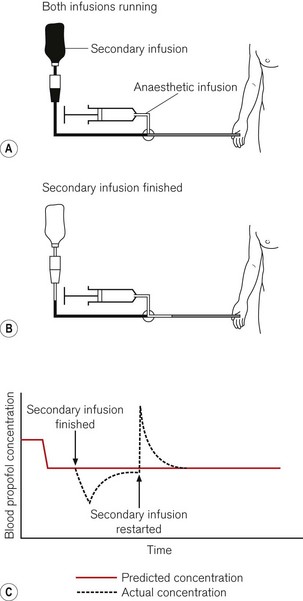
When more than one fluid or drug is infused through a common intravascular device, alterations in the rate of infusion of one of these substances will temporarily affect the rate of administration of the remainder. The degree of perturbation is a function of the shared volume (dead-space) of intravenous device and the infusion rates (Fig. 19.28). For drugs with short half-lives this can have a dramatic effect. Ideally, each intravenous infusion of drug would be given through a dedicated infusion line, so that alterations in the rate of infusion of one drug do not affect the others. For simplicity and patient comfort, it is preferable to insert only one intravenous catheter and use a many-tailed infusion set. Such infusion sets are available specifically for TIVA with one or two long narrow calibre ‘pump lines’ and one wider bore line to connect to a giving set for fluid administration under gravity (Fig. 19.29). Each line should incorporate a one-way or anti-reflux valve to prevent backflow as well as anti-siphon valves on the pump lines. The shared volume, where the lines join before the intravenous catheter, should be as small as possible. Because such lines are made up of a number of components assembled together it should be borne in mind that they may leak or be obstructed at the bonded junctions. This may not always be obvious.
There were previously the beginnings of a move towards yellow-coloured infusion lines for local anaesthetic applications; this is probably in abeyance now following recent developments. In response to previous well-publicized cases of maladministration of intrathecal cytotoxics and intravenous local anaesthetics, the National Patient Safety Agency of the UK issued an alert, in November 2009, requiring all UK hospitals to introduce syringes and needles with specific connections aiming to prevent ‘wrong route’ errors. In spite of the commercial absence of such devices at present, it is required that, by April 2011, when intrathecal access is required this will be achieved using systems that do not connect with intravenous devices, i.e. will not be compatible with the ubiquitous Luer connectors.24 A second part to the alert requires that this system is extended to include all epidural and other regional anaesthesia devices, ‘but not local anaesthetics for small parts of the body’, by April 2013.25 Quite apart from the unknown risks and the resource implications, and the mayhem that is likely to result from needing a duplicate system of syringes and needles available in clinical areas, it is also untested and unproven whether this approach will ultimately reduce the, thankfully, small number of significant wrong-route errors.
TIVAtrainer©
For the purpose of understanding and predicting the disposition of intravenous drugs, no amount of theoretical knowledge of pharmacokinetics or practical experience of intravenous anaesthesia is as effective as seeing graphical representations of drug concentrations and the changes in response to interventions. TIVAtrainer© (copyright F. Engbers, Leiden University Hospital) is a pharmacokinetic simulation programme for intravenous anaesthetics developed as shareware available from the web site for the European Society for Intravenous Anaesthesia – EuroSIVA. TIVAtrainer (Fig. 19.30) is not the first simulation software of this type,26 but it is perhaps the most developed and ‘user friendly’. It is an ideal teaching tool for use in the operating room or the lecture theatre, allowing simultaneous modelling of several different drugs used in anaesthesia.
In use, a drug is chosen from the menu and a method of administration is chosen, which may be:
1 Medical Devices Agency. Infusion Systems, Device Bulletin. MDA DB 2003(02). London: Medical Devices Agency; 2003.
2 Medical Devices Agency. MHRA DB 2010(03). London: Medical Devices Agency; 2010. Available online at www.mhra.gov.uk
3 Medical Devices Agency. Electromagnetic Compatibility of Medical Devices with Mobile Communications. DB9702. London: Medical Devices Agency; 1997.
4 Schüttler J, Schwilden H, Stoeckel H. Pharmacokinetics as applied to total intravenous anaesthesia. Anaesthesia. 1983;38(suppl):53–56.
5 Kenny GNC, White M. A portable computerized control system for propofol infusion. Anaesthesia. 1990;45:692–693.
6 Marsh B, White M, Morton N, Kenny GN. Pharmacokinetic model driven infusion in children. Br J Anaesth. 1991;67:41–48.
7 White M, Engbers FHM, Schenkels MJ, Burm AGL, Bovill JG. The pharmacodynamics of propofol determined by auditory evoked potientials. Abstract presented in Sydney at the 11th World Congress of Anaesthesiologists 1996;A608.
8 Glen JB. The development of ‘Diprifusor’: a TCI system for propofol. Anaesthesia. 1998;53(suppl 1):13–21.
9 Varvel JR, Donoho DL, Shafer SL. Measuring the performance of computer-controlled infusion pumps. J Pharmacokinet Biopharm. 1992;20:63–94.
10 Swinhoe CF, Peacock JE, Glen JB, Reilly CS. Evaluation of the predictive performance of a ‘Diprifusor’ TCI system. Anaesthesia. 1998;53(suppl 1):61–67.
11 Frei FJ, Zbinden AM, Thomson DA, Reider HU. Is the end-tidal partial pressure of isoflurane a good predictor of its arterial partial pressure? Br J Anaesth. 1991;66:331–339.
12 Dwyer RC, Fee JPH, Howard PJ, Clarke RSJ. Arterial wash-in of halothane and isoflurane in young and elderly adult patients. Br J Anaesth. 1991;66:572–579.
13 Schnider TW, Minto CF, Gambus PL, Andresen C, Goodale DB, Shafer SL, et al. The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology. 1998;88:1170–1182.
14 Minto CF, Schnider TW, Shafer SL. Pharmacokinetics and pharmacodynamics of remifentanil. II. Model application. Anaesthesiology. 1997;86:24–33.
15 Gepts E, Shafer SL, Camu F, Stanski DR, Woestenborghs R, Van Peer A, et al. Linearity of pharmacokinetics and model estimation of sufentanil. Anaesthesiology. 1995;83:1194–1204.
16 Kataria BK, Ved SA, Nicodemus HF, Hoy GR, Lea D, Dubois MY, et al. The pharmacokinetics of propofol in children using three different data analysis approaches. Anaesthesiology. 1994;80:104–122.
17 Absalom A, Amutike D, Lal A, White M, Kenny GN. Accuracy of the ‘Paedfusor’ in children undergoing cardiac surgery or catheterization. Br J Anaesth. 2003;91:507–513.
18 Maitre PO, Vozeh S, Heykants J, Thomson DA, Stanski DR. Population pharmacokinetics of alfentanil:the average dose – plasma concentration relationship and interindividual variability in patients. Anaesthesiology. 1987;66:3–12.
19 Glen JB. Minutes of the World Society of Intravenous Anaesthesia Open TCI Initiative, Copenhagen. http://opentci.org/doku.php?id=minutes:copenhagen, 1 June 2008. accessed 15/7/10
20 James WPT. Research on obesity. London: Her Majesty’s Stationery Office; 1976.
21 Kenny GN, Mantzaridis H. Closed-loop control of propofol anaesthesia. Br J Anaesth. 1999;83:223–228.
22 Morley A, Derrick J, Mainland P, Lee BB, Short TG. Closed loop control of anaesthesia: an assessment of the bispectral index as the target of control. Anaesthesia. 2000;55:953–959.
23 Irwin MG, Jones RD, Visram AR, Kornberg JP. A patient’s experience of a new postoperative patient-controlled analgesic technique. European Journal of Anaesthesia. 1994;11:413–415.
24 National Patient Safety Agency. Safer spinal (intrathecal) epidural and regional devices- Part A. 24 November 2009. NPSA/2009/PSA004A. London: National Patient Safety Agency; 2009. Available at http://www.nrls.npsa.nhs.uk/alerts/?entryid45=65259
25 National Patient Safety Agency. Safer spinal (intrathecal) epidural and regional devices- Part B. 24 November 2009. NPSA/2009/PSA004B. London: National Patient Safety Agency; 2009. Available at http://www.nrls.npsa.nhs.uk/alerts/?entryid45=65259
26 Stanford PK/PD server, http://anesthesia.stanford.edu/pkpd/default.aspx. accessed 08/08/2010
Absalom AR. Struys MMRF. Overview of target-controlled infusions and total intravenous anaesthesia. Gent: Academia Press; 2005.
Buchmann I. Batteries in a portable world: a handbook on rechargeable batteries for non-engineers, 2nd ed. Richmond: Cadex Electronics Inc.; 2010. Available at http://www.buchmann.ca/default.asp accessed 12/07/2010)
Medical Devices Agency. Infusion Systems, Device Bulletin. MDA DB2003(02). London: Medical Devices Agency; 2003.
White PF, ed. Textbook of intravenous anaesthesia. Baltimore: Williams & Wilkins, 1997.