Chapter 73 Impairment of Consciousness and Coma
The study of consciousness and causes of alteration of consciousness represents one of the oldest areas of neuroscience. The Edwin Smith Surgical Papyrus (circa 1700 b.c.) is the oldest historical document to use the term “brain” and contains the first description of the meninges, cerebrospinal fluid, and the convolutions of the brain surface. It contains vivid analytical descriptions of injury to the nervous system and deficits in neurological function caused by the injuries [Sanchez and Burridge, 2007]. However, in Ancient Egyptian medicine, the heart and the mind were synonymous [Mohamed, 2008]. Therefore, from the earliest recorded neuroscience document until current times, the location of the mind and the nature of consciousness have been the subject of study and debate based on the state of philosophy and science of the time.
The French mathematician, physicist, and philosopher, René Descartes (1596–1650), associated the mind with consciousness and self-awareness, and distinguished the mind from the brain. He postulated that consciousness and self-awareness were separate from the physical brain and sought to identify the “seat” of their convergence. He postulated that the mind and the brain were integrated at the pineal gland, a theory based more on basic knowledge of the gross anatomy of the brain than an understanding of neurophysiology. His writings perpetuated the concept of dualism: that the mind and mental processes are not physical and are separate from the brain. His is one of the earliest and most articulate descriptions of the mind–body problem as it exists today. Stated differently, how can consciousness and awareness be resolved within the structure and function of the brain [Gierer, 2008]? Dualism continues to have scientific, philosophical, and practical implications in medical practice today [Demertzi et al., 2009].
Multiple philosophical, metaphysical, and psychologic theories of consciousness have been proposed [Zeman, 2001]. William James, in 1890, wrote that, when the word consciousness is limited to the personal self of the individual, the cortex is the sole organ of consciousness in man [James, 1890]. He also emphasized attention (internal and external) and the ability to direct the focus of attention as fundamental to consciousness. None the less, reconciliation of the concept of mind (consciousness and awareness) and the structure and function of the brain has long been elusive [Sperry, 1952]. Evidence that there is a neural correlate of consciousness, however, is clearly established [Brain, 1950; Crick and Koch, 1998; Crick and Koch, 2003; Damasio, 2003; Evans, 2003; Negrao and Viljoen, 2009].
The modern understanding of wakefulness and consciousness begins with the work of von Economo, who first characterized encephalitis lethargica in 1917 and subsequently studied the clinical course and neuropathology of its victims for the remainder of his career. He did not think that encephalitis lethargica was caused by influenza, which was pandemic at the time, but acknowledged that influenza might predispose to its development. His intuition about etiology of encephalitis lethargica has been subsequently borne out [McCall et al., 2008]. In 1929, von Economo addressed the College of Physicians and Surgeons at Columbia University in New York and proposed discreet subcortical centers that control wakefulness and sleep, based on his studies [von Economo, 1930]. He showed that wakefulness arose from the upper brainstem and that hypothalamic lesions predispose to sleep. The extraordinary accuracy of his observations has been recently reviewed and confirmed [Saper et al., 2005].
Also in 1929, Hans Berger published his electroencephalographic (EEG) study in humans [Berger, 1929; Gloor, 1969]. The convergence of neuropathology and neurophysiology with the newer ability to stimulate discreet areas of the brain electrically led to Moruzzi and Magoun’s discovery that stimulation of the brainstem reticular formation produced EEG desynchronization in the cerebral cortex and behavioral alerting [Moruzzi and Magoun, 1949]. Subsequently, many studies have shown the importance of the ascending arousal system and other closely allied systems in the control of wakefulness and sleep and in the pathophysiology of coma [Saper et al., 2001, 2005; Parvizi and Damasio, 2003; Hobson, 2005; Schwartz and Roth, 2008; Perlis et al., 2009].
The ascending arousal system (reticular activating system, ascending reticular activating system) has been the subject of research concerning its role in attention and consciousness for more than 50 years. However, advances in understanding of neuroanatomy restimulated interest in the role of the thalamus and neocortex in attention and consciousness [Brodal, 1964]. Mesulam wrote, in 1985: “Today, even though vestiges of predominately subcortical theory of attention can occasionally still be detected, there is an emerging sense that neocortex, thalamus and brainstem are inextricably linked in the modulation of attention and more complex aspects of this function are executed predominately by neocortical mechanism” [Mesulam, 1985].
Many studies have confirmed that the midbrain reticular formation provides much of its cortical activation through the thalamus, and that reciprocal connections between the thalamus and cortex play a pivotal role in attention and consciousness [Crick, 1984; Newman, 1995; Steriade, 1997; Van der Werf, 2002; Evans, 2003; Llinás and Steriade, 2006; Pinault, 2004].
The historical understanding of the thalamus as the major synapse point for environmental sensory information on the way to the cortex is unchallenged. However, the larger role of the thalamus is that of the major source of extracortical activation for the cortex. The thalamic reticular nuclei provide connections with the cortex that allow cortical regulation of noncortical output. Thus the action of the midbrain reticular formation is primarily excitatory, causing increased but relatively nonspecific arousal, while the thalamic reticular nuclei have a more inhibiting role, allowing focus [Crick, 1984; Newman, 1995].
The complex interplay among the brainstem reticular core, the thalamus, and the cortex form the basis of attention, awareness, and consciousness [Boveroux et al., 2008]. The existence of cortical activation from subcortical centers and cortical regulation of input across hundreds of thousands of reciprocal networks (in parallel activated through the thalamus and within the cortex) are the basis for cognitive function, including attention and arousal, awareness of self and environment, and consciousness. Several convergent lines of evidence suggest that this complicated interaction is, to some degree, conducted by rhythmic fast activity (40 Hz) originating in the thalamus [Min, 2010; Llinas, et al., 1998]. How large numbers of neuron networks are orchestrated to provide sufficient degree of information integration necessary for consciousness is a field of on-going study [Lundervold, 2010].
Functional neuroimaging of brain activity using positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) has substantially contributed to our understanding of the brain’s functional networks [Monti et al., 2009; Owen, 2008]. PET is a form of in vivo autoradiography that utilizes water radiolabeled 15oxygen as the positron emitter to measure brain blood flow. Once produced, positrons almost immediately combine with electrons and, by destroying one another, they emit two high-energy gamma rays that travel in opposite directions. Radiation detectors, arranged around the head, detect these paired gamma rays and, utilizing computers, localize the source with great accuracy.
fMRI measures a complex function of blood flow related to the observation that the amount of oxygen in a certain area of the brain influences its magnetic properties (see Chapter 11). This change, the blood oxygen level dependent or BOLD effect, can be detected by fMRI [Raichle, 2009]. fMRI measures blood oxygenation, which indirectly reflects neuronal activity. Multiple studies have shown that the fMRI signal is primarily related to synaptic activity from the input and processing of information locally, and not related to signal transmission to other areas of the brain. Thus, focal changes in the fMRI signal reflect focal neuronal processing and are not a byproduct of a broader or more diffuse process [Raichle and Mitun, 2006; Khader et al., 2008; He and Raichle, 2009; Logothetis, 2002; Lippert, 2010].
The brain is not, however, simply driven reflexively, quietly waiting to react to stimuli. Rather, the brain has substantial on-going metabolic needs that account for approximately 20 percent of all energy consumed by the body, while representing only about 2 percent of total body weight in the average adult. Moreover, 60–80 percent of overall brain energy use is related to neuronal signaling [Raichle and Mintun, 2006]. None the less, changes in brain activity produce relatively small fluctuations of energy utilization, often less than 5 percent, compared to the high energy consumption of the resting state [Raichle, 2010].
PET and fMRI studies have shown the existence of areas of high resting baseline activity wherein metabolic activity actually decreases during certain goal-directed tasks. This is called the resting state or default mode of brain function [Raichle et al., 2001]. This leads to the conclusion that most brain function is intrinsic and involves integration of information for interpreting, responding to, and predicting environmental demands [Raichle, 2010].
Coordination of the default mode network and other brain functional systems is a subject of intense study. Spontaneous fluctuations in the BOLD signals of fMRI demonstrate consistent temporal correlations within brain networks that are associated with different functions [He et al., 2008]. In different situations and conditions, local field potentials across a wide frequency range (5–90 Hz) have been correlated with the spontaneous fluctuation of the fMRI signal. Recently, however, lower-frequency local field potentials (slow cortical potentials) have been studied and correlated with fMRI signal fluctuations. Because their temporal scale (<4 Hz) overlaps with the fMRI signal (<0.5 Hz) they seem to be a likely source for broadly distributed information integration in the brain [He and Raichle, 2009].
To be part of consciousness, information has to be integrated because conscious experience is a unitary and undivided whole [Tononi, 2008]. Slow cortical potentials, already studied in cognitive neuroscience as event-related potentials, may be the ideal neural mechanism for information integration across large areas of cortex. Slow cortical potentials take their origin from the more superficial cortical layers where far-reaching intracortical and corticocortical connections preferentially terminate, allowing influence of the slow cortical potentials across large areas of cortex. The relatively slow time scale of the slow cortical potentials would facilitate synchronization across wide areas independent of axonal conduction delay [He and Raichle, 2009]. Slow cortical potentials may subserve information integration across large cortical areas contributing to conscious awareness.
Because neurobiological processes that are realized in brain structures are responsible for consciousness [Baars et al., 2003; Neylan, 1995; Searle, 2000], neurologic disorders of the cortex and its brainstem activators result in impairment of consciousness and coma. Understanding neurologic disease depends on the clinical assessment and interpretation of consciousness, the content of consciousness, and alterations of consciousness. The evaluation of all disorders of higher cortical function fundamentally begins with consciousness assessment, and all signs and symptoms of neurologic impairment must be interpreted based on the state of consciousness and awareness [Giacino, 1997].
Consciousness refers to the state of awareness of self and environment [James, 1890; Plum and Posner, 1982]. Evaluation of consciousness in the pediatric patient must take into account age and the appropriate developmental level. Conscious individuals may have abnormal content of consciousness, such as hallucinations, delirium, or dementia. Therefore, normal consciousness must be distinguished from abnormal consciousness as objectively as possible. Unconsciousness is unawareness of self and environment, and may be physiologic (sleep) or pathologic (coma or the vegetative state). The fundamental difference between sleep and coma is that, with appropriate stimulus intensity and duration, a sleeping person can be aroused to a normal state of consciousness, whereas a comatose patient can not. Thus, the diagnosis of coma and other impairments of consciousness involves state and reactivity. The physiologic differences between sleep and coma are actually more profound. Sleep is a biologically active state with identifiable behavioral and EEG stages, whereas coma is a state of reduced neuronal activity [Moruzzi, 1972].
Along the continuum from normal consciousness to coma or unarousable unconsciousness, many terms are used to describe mental state and reactivity. When there is doubt about the appropriate use of one of these terms, it is far better to describe the state and reactivity of the individual rather than label it. Definitions of consciousness, impairment of consciousness, coma, and related states have been proposed throughout the history of medicine and have been reviewed, refined, and stated systematically during the past several decades [Ashwal, 1996; Ashwal and Cranford, 2002; Bates, 1993; Bozza Marrubini, 1984; Medical Research Council Brain Injuries Committee, 1941; Michelson and Ashwal, 2004; Plum and Posner, 1982; Owen, 2008].
Definitions
Consciousness is the spontaneously occurring state of awareness of self and environment. Consciousness has two dimensions – wakefulness and awareness [Multi-Society Task Force on the Persistent Vegetative State, 1994]. Normal consciousness requires arousal, an independent, autonomic-vegetative brain function subserved by ascending stimuli – emanating from pontine tegmentum, posterior hypothalamus, and thalamus – that activate mechanisms inducing wakefulness. Awareness is subserved by cerebral cortical neurons and their reciprocal projections to and from the major subcortical nuclei. Awareness requires wakefulness, but wakefulness can be present without awareness.
 impairment of consciousness along the continuum of coma–vegetative state–minimally conscious state and related conditions.
impairment of consciousness along the continuum of coma–vegetative state–minimally conscious state and related conditions.Impairment of Consciousness with Reduced Mental State
Coma is a state of deep, unarousable, sustained pathologic unconsciousness with the eyes closed that results from dysfunction of the ascending reticular-activating system in the brainstem or in both cerebral hemispheres [Multi-Society Task Force on Persistent Vegetative State, 1994; Young, 2009]. Coma usually requires the period of unconsciousness to persist for at least 1 hour to distinguish coma from syncope, concussion, or other states of transient unconsciousness. The term unconsciousness implies global or total unawareness, and applies equally to patients in coma or vegetative states. Patients in coma are unconscious because they lack both wakefulness and awareness. In contrast, patients in vegetative states are unconscious because, although they have retained wakefulness, they lack awareness. The depth of coma may be further specified by assessment of brainstem reflexes, breathing pattern, change of pulse or respiratory rate to stimulation, or stimulus-induced nonspecific movement.
Vegetative State, Minimally Conscious State, and Related Conditions
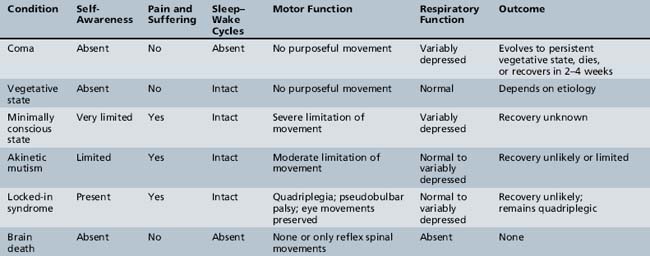
Table 73-1 lists several of the major neurologic conditions that the clinician must be capable of differentiating from coma. This table was modified from the report issued by the Multi-Society Task Force on the Persistent Vegetative State [1994] and more recent work that is defining the concept of the minimally conscious state [American Congress of Rehabilitation Medicine, 1995; Giacino et al., 1997]. The principal neurologic conditions in which there may be overlap of clinical findings on examination that should be differentiated from coma are discussed in the next sections.
Vegetative State
The vegetative state can be described as a condition of complete unawareness of the self and the environment, accompanied by sleep–wake cycles with either complete or partial preservation of hypothalamic and brainstem autonomic (vegetative) functions [Zeman, 1997; Monti et al., 2010]. Criteria to diagnose the vegetative state have been recommended for adults and children by the Multi-Society Task Force on Persistent Vegetative State and are listed in Box 73-1. Children in a vegetative state lack evidence of self-awareness or recognition of external stimuli. Rather than being in a state of “eyes-closed” coma, they remain unconscious but have irregular periods of wakefulness alternating with periods of sleeping. Vegetative patients demonstrate a variety of sounds, emotional expressions, and body movements, and they may smile or shed tears. They have inconsistent head- and eye-turning movements to sounds and inconsistent, nonpurposeful trunk and limb movements. The most objective sign is lack of sustained visual fixation or visual tracking.
Box 73-1 Criteria for the Diagnosis of the Vegetative State
 No evidence of awareness of themselves or their environment; they are incapable of interacting with others
No evidence of awareness of themselves or their environment; they are incapable of interacting with others No evidence of sustained, reproducible, purposeful, or voluntary behavioral responses to visual, auditory, tactile, or noxious stimuli
No evidence of sustained, reproducible, purposeful, or voluntary behavioral responses to visual, auditory, tactile, or noxious stimuliIt is estimated that there are about 4000–10,000 children in a vegetative state in the United States [Ashwal, 1996] and about 100,000 worldwide [Ashwal, 2005]. The etiology of the vegetative state in children can be classified into the following three broad groups of disorders:
The percentage of children having these etiologies of the vegetative state is as follows: acute traumatic and nontraumatic injuries (30 percent), perinatal insults (17.7 percent), chromosomal disorders or congenital malformations (13.0 percent), infections (10.3 percent), and unknown causes (28 percent) [Ashwal et al., 1994].
The most common causes of acute brain injury leading to the vegetative state in children are head trauma and hypoxic-ischemic encephalopathies [Adams et al., 2000]. Severe traumatic brain injury in children is often due to nonaccidental trauma but also occurs after a motor vehicle crash, particularly when infant restraints have not been used or when a pedestrian versus motor vehicle crash occurs. Hypoxic-ischemic injuries after cardiorespiratory arrest occur at birth, after episodes of near-miss sudden infant death syndrome or near-drowning, and after other acute life-threatening episodes, including shock due to systemic trauma or infection.
The clinical course of evolution to a vegetative state after an acute injury usually begins with eyes-closed coma for several days to weeks, followed by the appearance of sleep–wake cycles. Gillies and Seshia retrospectively determined that the transition from eyes-closed coma to the vegetative state averaged 8.6 ± 1.7 days in 17 children aged 1 month to 6 years [Gillies and Seshia, 1980]. Other responses, such as decorticate and decerebrate posturing (1.7 days), roving eye movements (1.8 days), and eye blinking (3.3 days), appeared earlier than sleep–wake cycles [Gillies and Seshia, 1980].
Diagnosis of the vegetative state is made clinically [AAN Quality Standards Subcommittee, 1995]. There are no confirmatory laboratory tests, in contrast to establishing the diagnosis of brain death. However, the absence of cortical (scalp-recorded) potentials of the somatosensory-evoked response to median nerve stimulation has been associated with the vegetative state in some studies [Beca et al., 1995; Frank et al., 1985]. Neuroimaging studies usually demonstrate diffuse or multifocal cerebral disease involving the gray and white matter or major CNS malformations [Ashwal, 1996]. In patients with traumatic and nontraumatic brain injury, serial imaging studies may demonstrate progressive atrophy. PET studies may demonstrate significant global reductions of cerebral glucose metabolism in infants [Larsen et al., 1993]. Proton magnetic resonance spectroscopy measurement of increased cerebral lactate in newborns, infants, and children with nervous system disease is associated with severe disability, survival in the vegetative state, or death [Ashwal et al., 1997].
Issues have been raised for several of the disorders of consciousness with regard to the diagnostic accuracy of the clinical assessment versus the use of advanced imaging or neurophysiological methods to assess such severely affected patients [McKinney and Giacino, 2008; Owen and Coleman, 2007; Giacino et al., 2009; Gawryluk et al., 2010]. In addition, several recent studies in adult patients thought to be in a vegetative state have raised considerable interest, as investigations with fMRI have shown evidence of awareness and presumably some level of higher cortical function [Owen and Coleman, 2008]. In addition, deep brain stimulation in minimally conscious-state patients [Schiff et al., 2009] has resulted in clinical improvement and has raised additional issues as to the role of functional imaging in evaluating vegetative state patients and also in potentially new approaches to treatment.
Recovery from the vegetative state in children depends on the etiology and is reviewed in more detail in the Multi-Society Task Force on the Persistent Vegetative State report [1994]. In children with severe closed-head injuries who were vegetative 1 month after injury, follow-up at 1 year found that 29 percent remained in a vegetative state, 9 percent had died, and 62 percent recovered consciousness. Recoveries after 12 months were not reported, although one study found that 2 of 40 children with traumatic brain injury began to recover after 1 year in a vegetative state [Kriel et al., 1993]. In contrast, children in a nontraumatic vegetative state have a much poorer potential for recovery than those in a traumatic vegetative state. At 1 year, most children remained in a vegetative state (65 percent) or died (22 percent); only 13 percent demonstrated recovery, usually to a severe disability. It was based on these data and consensus opinion that the Task Force came to the conclusions that, in children (as well as in adults), the vegetative state could be judged permanent 12 months after traumatic brain injury and 3 months after nontraumatic injury. The chance for recovery after these time periods was exceedingly low, and the patient almost always progressed to a severe disability. This perspective has received general acceptance, although concerns have been raised about the certainty of diagnosis and whether there is a greater potential for patients to recover than previously realized [Giacino et al., 1997; Jennett, 1997].
It is important to identify children who are in a vegetative state correctly because of the implications for continued care, family expectations, and the need for rehabilitation. Specialty programs for coma recovery are usually directed at patients who are severely disabled or are in a minimally conscious or vegetative state. Management issues are confounded by a lack of understanding of expected recovery patterns, terminology inconsistency, lack of or inappropriate use of outcome measures, and inadequate knowledge concerning medical management based on scientifically sound practices for this special patient population [Zasler et al., 1991]. Children in a vegetative state have been reported to have a considerably shorter than normal life expectancy [Ashwal et al., 1994]. Children in a vegetative state who are younger than 1 year of age had a median survival of 2.6 years, whereas the median survival of children aged 2–6 years was 5.2 years. In children 7–18 years of age, the reported median survival was 7 years.
Minimally Conscious State
The minimally conscious state has been defined as a condition of severely altered consciousness in which the person demonstrates minimal but definite behavioral evidence of self- or environmental awareness [Giacino et al., 1997]. Recovery from coma depends on the cause of coma, extent of brain injury, and ability of injured areas to recover or of uninjured areas to assume the functions of injured areas. Not all comatose patients recover rapidly or completely. A variety of terms have been used to define these states, and there is often confusion between recovery states and prognostic categories. The term minimally conscious state has been proposed to describe those patients who were in coma or a vegetative state and who begin to demonstrate minimal signs of awareness. The term minimally conscious state may also be used to describe patients with degenerative disorders who are no longer functionally interactive but are not in the vegetative state. Patients in a minimally conscious state are able to do the following:
Criteria to diagnose the minimally conscious state are shown in Box 73-2 [Giacino et al., 2002].
Box 73-2 Diagnostic Criteria for the Minimally Conscious State
 Purposeful behavior, including movements or effective behaviors that occur in contingent relation to relevant environmental stimuli and are not due to reflexive activity. Some behavioral examples of qualifying purposeful behaviors include the following:
Purposeful behavior, including movements or effective behaviors that occur in contingent relation to relevant environmental stimuli and are not due to reflexive activity. Some behavioral examples of qualifying purposeful behaviors include the following:
 Appropriate smiling or crying in response to the linguistic or visual content of emotional but not to neutral topics or stimuli
Appropriate smiling or crying in response to the linguistic or visual content of emotional but not to neutral topics or stimuli(Adapted from Giacino JT et al. The minimally conscious state. Definition and diagnostic criteria. Neurology 2002;58:349.)
Children in the minimally conscious state are usually victims of acquired brain injury (traumatic and nontraumatic), neurodegenerative and neurometabolic disorders, or congenital or developmental disorders [Ashwal, 2003]. Based on limited literature on this disorder in adults [Giacino and Kalmar, 2005], it is possible that such children, depending on the etiology of the insult, may have a better potential for neurologic recovery and a longer life expectancy than children in the vegetative state. In a study of 5075 children aged 3–15 years in a vegetative state or minimally conscious state, 564 were in the vegetative state, 705 in the minimally conscious state and immobile, and 3806 in the minimally conscious state and mobile. The 8-year survival rates were 63 percent for vegetative state, 65 percent for immobile minimally conscious state, and 81 percent for mobile minimally conscious state. Thus, presence of consciousness was not a critical variable in determining life expectancy in this study, and mobility was more important than consciousness in predicting survival [Strauss et al., 2000].
Locked-In Syndrome
The locked-in syndrome refers to a condition in which patients retain consciousness and cognition but are unable to move or communicate because of severe paralysis. This condition is a result of diseases involving the descending corticospinal and corticobulbar pathways at or below the pons or of severe involvement of the peripheral nervous system. By definition, patients in coma or a vegetative state are unconscious and differ from patients in a locked-in syndrome, who retain awareness, although this difference may be difficult to determine. The locked-in syndrome is quite rare in children [Habre et al., 1996; Scott et al., 1997]. Some patients with this condition can establish limited communication using eye movements.
Akinetic Mutism
Akinetic mutism is a rare condition consisting of pathologically slowed or nearly absent bodily movement, accompanied by a similar loss of speech [Plum and Posner, 1982]. The original description of this condition in 1941 was in an adolescent with symptoms of intermittent depressed states of consciousness secondary to a craniopharyngioma [Cairns et al., 1941]. Since then, akinetic mutism has been seen with bacterial and viral CNS infections, other tumors of the nervous system, and hydrocephalus, and occasionally as a postoperative phenomenon, especially with posterior fossa and cerebellar tumors [Lin et al., 1997; Melton et al., 1991]. Wakefulness and self-awareness are usually preserved in most patients, but the level of mental function is reduced. The condition characteristically accompanies gradually developing or subacute, bilateral damage to the paramedian mesencephalon, basal diencephalon, or inferior frontal lobes. The long-term outlook for children with akinetic mutism is unknown because few patients with this disorder have been reported. It is most likely related to the etiology and severity of the associated disease.
Brain Death
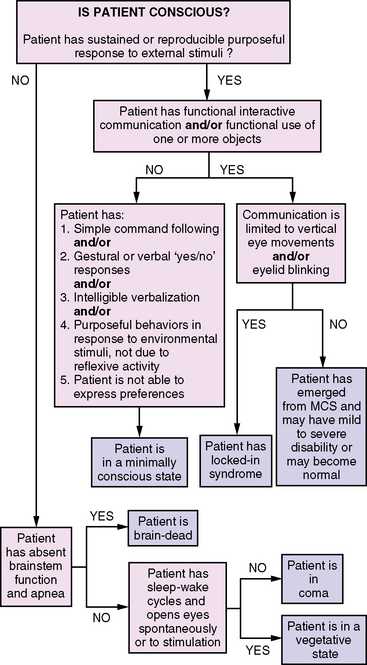
Brain death describes the permanent absence of all brain functions, including those of the brainstem [Task Force for the Determination of Brain Death in Children, 1987; Nakagawa et al., 2011]. Brain-dead patients are irreversibly comatose and apneic, and they have absent brainstem reflexes, including the loss of all cranial nerve functions. The appearance of brain death can be imitated by deep anesthesia, sedative overdose, or severe hypothermia. Patients who are brain-dead differ from patients who are in a coma, in that comatose patients usually have preserved brainstem functions and some degree of respiratory drive. Guidelines for the diagnosis of brain death in infants and children were originally published in 1987 and were recently revised in 2011; they are reviewed in greater detail in Chapter 79. A useful algorithm for distinguishing among brain death, vegetative state, locked-in syndrome, and higher-order consciousness is presented in Figure 73-1 [Ashwal and Cranford, 2002].
Consciousness Rating Scales
Several behavior rating scales have been developed because of the difficulty with imprecise use of terminology and because of the need for a truly reproducible assessment of consciousness with good inter-rater reliability [Kirkham et al., 2008]. Although typically referred to as coma scales, these scales have value in assessing consciousness alterations other than coma.
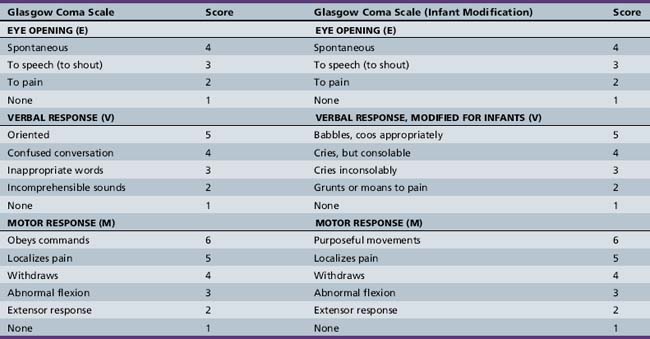
The best known and most widely used is the Glasgow Coma Scale, which yields a score of 3–15, based on best response to stimuli in the following three categories: eye opening, verbal response, and motor response (Table 73-2) [Teasdale and Jennett, 1974]. In its original form, the Glasgow Coma Scale was not developmentally suitable for assessment of newborns, infants, and younger children, and a variety of alternate scales have been proposed [Duncan et al., 1981; Hahn et al., 1988; Raimondi and Hirschauer, 1984; Reilly et al., 1988; Rubenstein, 1994; Simpson et al., 1991; Simpson and Reilly, 1982]. An ideal pediatric modification of the Glasgow Coma Scale would meet the following criteria:
The Pediatric Coma Scale [Reilly et al., 1988; Simpson et al., 1991; Simpson and Reilly, 1982] makes minor changes in the verbal scale of the Glasgow Coma Scale and redefines the “best” score based on developmental and age-appropriate norms (Table 73-3). It has the advantage of prospective evaluation and inter-rater reliability and the disadvantage of different maximum scores based on age, thus making outcome prediction and comparison difficult to study using the Glasgow Coma Scale.
| Response | Score |
|---|---|
| Eye Opening | |
| Spontaneous | 4 |
| To speech | 3 |
| To pain | 2 |
| None | 1 |
| Best Verbal Response | |
| Oriented | 5 |
| Words | 4 |
| Vocal sounds | 3 |
| Cries | 2 |
| None | 1 |
| Best Motor Response | |
| Obeys commands | 5 |
| Localizes pain | 4 |
| Flexion to pain | 3 |
| Extension to pain | 2 |
| None | 1 |
| Normal Aggregate Score | |
| Birth to 6 months | 9 |
| Less than 6–12 months | 11 |
| Less than 1–2 years | 12 |
| Less than 2–5 years | 13 |
| More than 5 years | 14 |
The Children’s Coma Scale redefines intracategory criteria and the “maximum” score, and changes the eye-opening category to “ocular response” [Raimondi and Hirschauer, 1984]. The Children’s Coma Scale meritoriously included pupillary reflexes, extraocular movements, and apnea in its categories, and is therefore substantially different from the Glasgow Coma Scale and requires different training and forms to be used (Table 73-4).
Table 73-4 Children’s Coma Scale
| Sign | Score |
|---|---|
| Ocular Response | |
| Pursuit | 4 |
| Extraocular movement intact, pupils react appropriately | 3 |
| Fixed pupils or extraocular movement impaired | 2 |
| Fixed pupils and extraocular movement paralyzed | 1 |
| Verbal Response | |
| Cries | 3 |
| Spontaneous respiration | 2 |
| Apneic | 1 |
| Motor Response | |
| Flexes and extends | 4 |
| Withdraws from painful stimuli | 3 |
| Hypertonic | 2 |
| Flaccid | 1 |
| Best Total Score | 11 |
The Glasgow Coma Scale – Modified for Children maintains the same categories as the Glasgow Coma Scale and the same maximum and minimum scores, while allowing developmental and age-appropriate scoring [Hahn et al., 1988; Rubenstein, 1994]. It does, however, equate spontaneous movement with following commands to achieve 6 points in the best motor response category to allow inclusion of infants and young children (see Table 73-2). None the less, the Glasgow Coma Scale – Modified for Children allows the best direct comparison with the Glasgow Coma Scale for scoring of consciousness impairment and assessment of outcome.
The Glasgow Coma Scale and its modifications for children are useful for evaluating and quantifying the continuum from consciousness to coma more objectively, and for allowing serial reassessment that meaningfully reflects the state of the patient at a particular moment in time with good inter-rater reliability. The scores also allow a relatively large amount of information to be conveyed quickly and concisely, with minimal need to remember cumbersome definitions or to write long descriptions. Their ease of use makes them even more valuable in the critical care and emergency department settings. The Glasgow Coma Scale and its pediatric modifications do not take into account the important brainstem reflexes, such as pupillary reactivity or oculocephalic, oculovestibular, or corneal reflexes. These should be a routine part of the assessment of the patient with impaired consciousness [Narayan et al., 1981].
The sum of the Glasgow Coma Scale may convey less useful information than the ocular, motor, and verbal responses individually [Teasdale et al., 1983]. In a study of 270 children aged 2 months to 12 years with acute nontraumatic coma, multivariate analysis suggests that the ocular and motor response scores, and not the total score, were predictors of short-term survival. Additionally, the absence of one or more brainstem reflexes (oculocephalic, oculovestibular, or pupillary reactivity) predicted short-term adverse outcomes [Nayana Prabha et al., 2003].
In children, the Glasgow Coma Scale motor subscore has equal predictive value with the full scale score and is more easily and reliably obtained [Van de Voorde, 2008]. In a retrospective study of 130 children 3 months to 16 years old admitted for traumatic brain injury, the motor response of the Glasgow Coma Scale predicted outcome as well as the full Glasgow Coma Scale, had a more linear relationship to mortality, and was easier to collect accurately [Fortune and Shann, 2010]. Multiple studies have shown the value of the motor subscore of the Glasgow Coma Scale and the additive value of brainstem reflex data in predicting outcome in children with traumatic and nontraumatic coma.
The Full Outline of Unresponsiveness (FOUR) Score Coma Scale (Table 73-5) was created to address these areas of weakness in the use of the Glasgow Coma Scale and its derivatives [Wijdicks et al., 2005]. The FOUR Score is divided into four categories (eye response, motor response, brainstem reflexes, and respiration), each with a maximum score of four (range 0–4). By providing a measure of consciousness that does not require patient vocalization, assessment of motor function, evaluation of brainstem reflexes at the mesencephalic, pontine, and medullary levels, and observation of respiration, the FOUR Score improves upon the Glasgow Coma Scale and incorporates other important features of the neurologic exam. Addition of this neurologic scoring system allows recognition of brain death, the vegetative state, the locked-in syndrome, and progressive changes seen in herniation syndromes [Wijdicks, 2006].
Table 73-5 “FOUR” Score Coma Scale
| Category and Response | Score |
|---|---|
| Eye Response | |
| Eyelids open or opened, tracking or blinking to command | 4 |
| Eyelids open but not tracking | 3 |
| Eyelids closed, opens to loud voice, not tracking | 2 |
| Eyelids closed, opens to pain, not tracking | 1 |
| Eyelids remain closed with pain | 0 |
| Motor Response | |
| Thumbs up, fist, or peace sign to command | 4 |
| Localizing to pain | 3 |
| Flexion response to pain | 2 |
| Extensor posturing | 1 |
| No response to pain or generalized myoclonus status epilepticus | 0 |
| Brainstem reflexes | |
| Pupil and corneal reflexes present | 4 |
| One pupil wide and fixed | 3 |
| Pupil or corneal reflexes absent | 2 |
| Pupil and corneal reflexes absent | 1 |
| Absent pupil, corneal, and cough reflex | 0 |
| Respiration | |
| Not intubated, regular breathing pattern | 4 |
| Not intubated, Cheyne–Stokes breathing pattern | 3 |
| Not intubated, irregular breathing pattern | 2 |
| Breathes above ventilator rate | 1 |
| Breathes at ventilator rate or apnea | 0 |
(From Wijdicks E et al. Validation of a new Coma Scale: The FOUR Score. Annals of Neurology 2005;58[4]:584–593.)
In adults, the FOUR Score has excellent inter-rater reliability when performed by nurses or physicians, and is suitable for use in neurosurgical and medical critical care and in the emergency department [Wolf, 2007; Akavipat, 2009; Stead, 2009; Fischer et al., 2010]. In a study of 60 children (ages 2–18 years) admitted for traumatic and nontraumatic injuries, the inter-rater reliability of the Glasgow Coma Scale was good and that of the FOUR Score was excellent [Cohen, 2009]. Both the FOUR Score and the Glasgow Coma Scale were predictive of in-hospital mortality and poor outcome at the conclusion of hospitalization.
The FOUR Score Coma Scale has comparable predictive value and inter-rater reliability to the Glasgow Coma Scale and is easier to use. Further studies in children will be necessary to determine the role of the FOUR Score in prediction of long-term outcome.The FOUR Score, however, may not adequately distinguish the vegetative state from the minimally conscious state when compared to the Coma Recovery Scale – Revised score [Schnakers et al., 2006]. This distinction may be more important in chronic critical care and acute and chronic rehabilitation settings than in the acute critical care setting. The Coma Recovery Scale – Revised is more complex than the FOUR Score Coma Scale, requires more training to use, and is more appropriately employed as an outcome measure [Giacino et al., 2004].
Pathophysiology
Consciousness is the result of the complex interplay between the cerebral cortex and the ascending reticular-activating system. The ascending reticular-activating system is a somewhat diffuse and widely circumscribed group of neurons that lie in the reticular formation of the brain that extends from the lower medulla to the midbrain and diencephalon [Moruzzi and Magoun, 1949; Plum and Posner, 1982]. Coma is produced by diseases or conditions that cause bilateral cerebral cortical dysfunction, ascending reticular-activating system dysfunction, or both. Pathologically, these can be subdivided as follows [Adams et al., 1982; Gennarelli et al., 1982; Plum and Posner, 1982]:
 Metabolic, toxic, or infectious encephalopathies that diffusely affect the cerebral hemispheres, the ascending reticular-activating system, or both
Metabolic, toxic, or infectious encephalopathies that diffusely affect the cerebral hemispheres, the ascending reticular-activating system, or both Subtentorial mass or destructive lesions that compress or damage the ascending reticular-activating system
Subtentorial mass or destructive lesions that compress or damage the ascending reticular-activating systemEtiologies
There may be considerable overlap between these categories, and it is always necessary to consider combined causes of impaired consciousness and coma. Disorders that produce abnormalities of osmolality, such as hyponatremia, hypernatremia, and diabetic ketoacidosis, may also lead to structural complications, such as intracranial hemorrhage or stroke as a consequence of sinovenous thrombosis. Both have been reported as rare complications of diabetic ketoacidosis [Atlluru, 1986]. Cerebral edema may complicate treatment of ketoacidosis, causing alterations of consciousness or herniation [Green et al., 1990].
Children with psychiatric disorders, such as conversion, panic, or anxiety disorders, may present with neurologic symptoms including genuine or apparent impairment of consciousness. Symptoms may include dizziness, hyperventilation, paresthesias, agitation, or restlessness. The episodic and paroxysmal nature of these episodes and the complete recovery between episodes should suggest the diagnosis [Herskowitz, 1986].
Nutritional causes of alteration of consciousness or coma are relatively uncommon in the United States but represent a significant problem in other areas of the world. These are reviewed in Chapter 103. Wernicke’s encephalopathy is related to a deficiency of thiamine. In addition to consciousness alteration, patients may have eye-movement abnormalities, ataxia, and deep tendon reflex changes. Wernicke’s encephalopathy has been reported in children and adolescents as a result of systemic malignancy and because of thiamine deficiency in total parenteral nutrition, and it may occur related to anorexia nervosa, acquired immunodeficiency syndrome (AIDS), and prolonged fasting [Bruck et al., 1991; Hahn et al., 1998; Nautiyal et al., 2004; Pihko et al., 1989; Salas-Salvado et al., 2000; Vasconcelos et al., 1999].
Niacin or nicotinic acid deficiency (pellagra), with the typical features of mental changes, dermatitis, and diarrhea, is rarely seen in the United States. However, disorders of the synthetic pathway from tryptophan to niacin or nicotinic acid or impaired intestinal absorption of tryptophan in Hartnup’s disease may produce symptoms in children or adolescents [Clayton et al., 1991; Freundlich et al., 1981].
Evaluation
History
Coma may manifest as the progression of a known underlying illness, unpredictable consequence, or complication of a known disease, or it may be the result of a totally unexpected event or illness [Bates, 1993; Michelson and Ashwal, 2004]. An accurate history of the events and circumstances before the onset of symptoms, and basic information concerning past medical history and medications, may be invaluable in determining the cause of coma and may quickly lead to the most appropriate diagnostic testing and treatment [Bates, 1993; Plum and Posner, 1982; Vannucci and Wasiewski, 1993].
Sudden onset of coma in an otherwise normal and awake child suggests convulsions or intracranial hemorrhage. Coma preceded by sleepiness or unsteadiness suggests ingestion of a drug or toxin in an otherwise well child. Fever is typical when coma is due to an infectious process but may not be present when shock is present or if the ambient temperature is low. A history of headache may suggest elevated intracranial pressure resulting from hydrocephalus or neoplasm, but may also be seen in migraine syndromes with alteration of consciousness. However, in patients who were in coma after a traumatic brain injury and in whom CT scanning was normal or could not explain the severity of clinical symptoms, MRI should be performed to evaluate for diffuse axonal injury [Ashwal et al., 2006].
Evaluation of accidental versus nonaccidental trauma in children can be especially difficult. In a study of 152 children aged 2 years or younger with traumatic brain injury, who died or were admitted to a pediatric intensive care unit, 80 (52.6 percent) had inflicted (i.e., nonaccidental) injury, and 72 (47.3 percent) had noninflicted traumatic brain injury [Keenan et al., 2004]. Clinical history may make the cause relatively obvious, such as a history of an automobile accident or plausible history of a fall. Retinal hemorrhage, metaphyseal fracture, rib fracture, and subdural hemorrhage are more commonly seen in children with inflicted traumatic brain injury. Skeletal survey and ophthalmologic examination alone missed 10 percent of inflicted traumatic brain injury cases in one recent study [Keenan et al., 2004]. When there is a high degree of suspicion of inflicted injury, CT or MRI of the head, or both, should be performed. A history of fever or recent illness suggests an acute infectious etiology but should also lead to consideration of complications from infectious disease, such as acute disseminated encephalomyelitis, Reye’s syndrome, and metabolic or mitochondrial disorders. Children with diabetes may have coma because of hypoglycemia or ketoacidosis. Children with congenital heart disease may be susceptible to brain abscess or infarction. Intermittent episodes of coma should suggest ingestion, drug overdose, inborn errors of metabolism, or Munchausen’s syndrome by proxy. Review of current medications should include use of ophthalmic drops, often overlooked as unlikely to have systemic effects. Brimonidine, an α2-adrenergic antagonist used in the treatment of glaucoma, caused recurrent episodes of coma at home and in hospital in a 1-month-old infant [Berlin et al., 2001]. A history of the use of a kerosene stove or heater should suggest carbon monoxide poisoning.
General Physical Examination
Inspection of the head, scalp, and skin can be most helpful. Cyanosis suggests poor oxygenation, and jaundice is seen in liver failure. Extreme pallor may be seen in anemia and shock, and a cherry-red color suggests carbon monoxide poisoning. The presence of a cephalohematoma, boggy or swollen areas of the scalp, or head bruises suggests cranial trauma. Bleeding or clear fluid leaking from the nose or ears suggests a basilar skull fracture. Certain types of burns and multiple bruises of characteristic shape and location or varied age may suggest child abuse. Various rashes may be seen with infectious causes of coma, such as meningococcemia or rickettsial disease. The presence of neurocutaneous lesions, such as the depigmented areas of tuberous sclerosis, suggests seizures or intracranial mass as the cause of coma. Generalized increased pigmentation may be seen in Addison’s disease or in adrenoleukodystrophy [Ravid et al., 2000].
The cardiovascular examination may suggest congenital heart disease or endocarditis, both of which are sources of intracranial abscess dissemination. Abdominal discoloration or rigidity may suggest intra-abdominal bleeding as a source of shock. A palpable linear abdominal mass may lead to the diagnosis of intussusception encephalopathy and underscores the need to include a thorough general examination in the evaluation of the child with an altered state of consciousness or coma [Goetting et al., 1990].
Neurologic Examination
The neurologic examination is of paramount importance in the assessment of the comatose patient. In particular, examination of the optic fundi, pupillary size and reactivity, eye movement control, corneal reflexes, motor responses, body posture, and the presence or absence of meningeal signs gives important information about the potential causes and localization of brain dysfunction in the comatose patient [Bates, 1993; Plum and Posner, 1982; Vannucci and Wasiewski, 1993]. Localization may be especially aided by detailed understanding of brainstem pathways involved in the eye examination. Funduscopic examination offers important clues about the etiology of coma, including papilledema as an indicator of raised intracranial pressure and retinal hemorrhages in occult trauma. In acute increased intracranial pressure, there may not have been time for papilledema to develop at the time of presentation in the emergency department.
Pupil size and reactivity are controlled by reciprocal sympathetic and parasympathetic innervation. Sympathetic pupil innervation originates in the hypothalamus and descends to the lower cervical and upper thoracic spinal cord. After synapsing in the intermediolateral columns, preganglionic sympathetic fibers exit the spinal cord and ascend in the cervical sympathetic chain, course near the apex of the lung, and end in the superior cervical ganglion, where postganglionic fibers pass along the internal carotid artery. Intracranially, the fibers travel with branches of the ophthalmic division of the trigeminal nerve to the pupil. Sympathetic innervation to sweat glands of the upper face also follows the internal carotid arteries, and other fibers innervate the smooth muscles of the eyelid. The sympathetic pathways are vulnerable at several locations, and an appropriately placed injury may produce unilateral pupillary constriction, ptosis, and facial anhidrosis (Horner’s syndrome). Parasympathetic pupil innervation originates in the midbrain at or near the Edinger–Westphal nucleus and travels as a component of the oculomotor nerve to the pupils [Montgomery et al., 1986].
Brain Herniation
Historical Perspective
In his Hunterian Lectures of 1896, Leonard Hill presented experimentally obtained data supporting the argument that increased pressure in the supratentorial compartment of the brain was not evenly distributed in all directions but eventually caused the brain to shift, blocking the isthmus tentorium cerebelli and the foramen magnum. He called this phenomenon translocation of the brain and noted its association with progressive neurological deterioration from agitation to paralysis and apnea. He reported that the neurological deterioration was due to diminished blood supply and not the compression itself [Hill, 1896].
Pierre Marie, in 1899, reported two cases of cerebral hemorrhage causing cerebellar compression. In one of the two cases, there was protrusion of the cerebellar tonsils into the foramen magnum. He referred to the works of Leonard Hill, noting that the viscosity of the brain and the position of the falx cerebri and the tentorium cerebelli causes increased intracranial pressure to be directed unevenly, leading to crowding at the tentorial notch and the foramen magnum [Simonetti et al., 1997].
Harvey Cushing, in 1902, related herniation of the cerebellar tonsils into the foramen magnum to neurological disturbances and cardiorespiratory failure, emphasizing the physiological changes of hypertension, bradycardia, and respiratory irregularity. He is often acknowledged as the first clinician to make these observations, although his experimental studies derived from the work of Leonard Hill, and others had made similar observations [Howell, 1959; Simonetti et al., 1997; Fodstad et al., 2006].
In 1904, Collier described brain distortions due to cerebral trauma or tumors seen at autopsy. He described herniation of the cerebellar tonsils and medulla as a cone-shaped plug in the foramen magnum. He also noted that the edge of the tentorium cerebelli caused a crease in the medial temporal lobe, an early description of transtentorial herniation. He reported false localizing signs in 20 of 161 cases of intracranial tumor evaluated clinically and pathologically [Pearce, 2006a].
In 1929, Kernohan and Woltman described compression of the contralateral cerebral peduncle against the tentorial edge, leading to a paradoxical hemplegia in intracranial mass lesions. Their work emphasized horizontal displacement of the brainstem as cause of symptoms [Kernohan and Woltman, 1929; Pearce, 2006b]. Adolph Meyer, in 1920, used the term herniation when describing displacement of brain tissue from brain tumor in postmortem material [Meyer, 1920]. McNealy and Plum, in 1962, systematically correlated clinical observation with pathological confirmation, emphasizing recognizable clinical signs, their pathological correlation, and the opportunity to intervene to improve outcome [McNealy and Plum, 1962; Posner et al., 2007].
Herniation Syndromes
Central or Transtentorial Downward Herniation
With generalized increases in intracranial pressure, there is gradual downward displacement of the diencephalon (thalamus and hypothalamus) through the tentorium cerebelli, producing progressive compression and ischemia of the brainstem from mesencephalon (rostral brainstem) to medulla (caudal brainstem). The traditional clinical-pathologic correlation is progressive deterioration of consciousness and brainstem function in a rostral to caudal direction [Plum and Posner, 1982; Young et al., 1998]. In the diencephalic stage, patients do not follow instructions but will localize to noxious stimuli and have small reactive pupils and preserved oculocephalic and oculovestibular reflexes. Respiration may be regular, with yawns or sighs, or Cheyne–Stokes respirations may appear. Cheyne–Stokes respirations consist of a cyclical gradual build-up and decrease in the volume of air inhaled with each breath. Increased rigidity or decorticate posturing may appear. In the midbrain–upper pons stage, patients have decerebrate rigidity or no movement, midposition pupils that may be irregular in shape and demonstrate no reactivity, and abnormal or absent oculocephalic and oculovestibular reflexes. Patients usually hyperventilate, although Cheyne–Stokes respirations may still be noted. In the lower pontine–medullary stage, there is no spontaneous motor activity or activity in response to stimuli, midposition fixed pupils, absent oculocephalic and oculovestibular reflexes, and shallow and rapid or slow and irregular (ataxic) respirations. The lower extremities may withdraw to plantar stimulation. Finally, in the medullary stage, there is generalized flaccid tone, absence of pupillary reflexes and ocular movements, further slowing and irregularity of respiration, and ultimately death.
Clinical-radiologic correlation studies have indicated that horizontal brainstem displacement may be likely to produce progressive impairment of consciousness and coma [Fisher, 1995; Ropper, 1986; Ross et al., 1989]. Horizontal shift of midline structures may be greater than their downward vertical displacement in patients who are in coma [Ropper, 1993]. Additionally, significant downward displacement of brainstem structures may be seen in individuals with low spinal fluid pressure syndromes without consciousness alteration [Reich et al., 1993]. Other studies have found downward herniation of the diencephalon in consciousness alteration and coma by MRI, seeming to confirm the traditional clinical-pathologic correlation [Feldmann et al., 1988; Wijdicks and Miller, 1997].
Infratentorial (Cerebellar) Herniation Syndromes
Space-occupying lesions in the posterior fossa can produce upward herniation of brainstem structures through the tentorial notch, and may result in obstructive hydrocephalus, brainstem ischemia, and death. Progressive alteration of consciousness or coma, associated with miotic pupils, gaze paresis, decerebrate posturing, and asymmetric or absent caloric response, with relative preservation of respiration, suggests upward transtentorial herniation in the setting of posterior fossa tumor, hemorrhage, stroke, or cerebellar edema [Cuneo et al., 1979].
The lesions that cause upward or downward cerebellar herniation in children are often subacute or chronic, and are discovered before herniation as a result of widespread use of neuroimaging. However, some acute illnesses, such as meningitis or encephalitis, may be associated with either cerebellar herniation syndrome [Gohlich-Ratmann et al., 1998; Roulet Perez et al., 1993]. Early identification of impending herniation by CT or MRI criteria may allow effective treatment and improved outcome [Karantanas et al., 2002].
However, in children with meningitis, the cerebellar pressure cone effect has been implicated as a cause of death after lumbar puncture, even with a normal CT scan of the head. In cases of suspected bacterial meningitis, lumbar puncture should be avoided if there is evidence of increased intracranial pressure or early coning. When lumbar puncture is performed, measurement of opening pressure is essential to allow early treatment if increased intracranial pressure persists. In all such cases, concern about risks for lumbar puncture should not delay appropriate antibiotic treatment [Oliver et al., 2003; Rennick et al., 1993; Shetty et al., 1999].
The practice of placing preoperative ventriculostomy or a ventriculoperitoneal shunt before surgery for posterior fossa tumor with hydrocephalus has fallen out of favor in most situations because of the risk for precipitation of upward transtentorial herniation [Cuneo et al., 1979; Epstein and Murali, 1978; Raimondi and Tomita, 1981]. Infratentorial herniation, either upward transtentorial herniation or downward cerebellar pressure coning, should be considered in the clinical setting of deterioration of consciousness or coma associated with posterior fossa mass lesion, cerebellar edema, or cerebrospinal fluid drainage, whether by ventriculostomy, ventriculoperitoneal shunt, or lumbar puncture. Cerebellar herniation associated with lumbar puncture in the clinical setting of a child with meningitis and with evidence of increased intracranial pressure is an especially important consideration.
Diagnostic Testing
When infection is suspected, blood and urine cultures should be obtained.
Lumbar puncture should be performed when there is a suggestion of infection of the CNS, with or without fever. Depending on the clinical findings and evaluation, CT needs to be performed before the lumbar puncture. When medically stable, all patients in coma and most, if not all, patients with impairment of consciousness of undetermined etiology should have a CT performed as rapidly as possible. In children with closed-head injury, CT or preferably MRI may be critical in identifying the specific cause of impaired loss of consciousness [Ashwal and Holshouser, 1997]. MRI is also invaluable in identifying evidence of herpes simplex encephalitis or an acute demyelinating process, such as acute disseminated encephalomyelitis.
The EEG is essential to diagnose clinically inapparent status epilepticus (i.e., nonconvulsive status epilepticus). Certain EEG patterns, such as periodic lateralized epileptiform discharges, may suggest herpes simplex encephalitis, especially in the setting of a febrile illness [Misra and Kalita, 1998]. In addition, the EEG is useful in the serial re-assessment and evaluation of patients in status epilepticus or persistent coma, as well as in patients requiring pharmacologic paralysis or sedation.
Treatment
Treatment of the child with impaired consciousness or coma requires scrupulous attention to certain basic principles while definitive diagnostic tests are obtained and therapy initiated. Specific etiologies of many individual causes of disordered consciousness are listed in Box 73-3 and are reviewed by specific diagnostic category in other sections of this book. The following principles of management are similar in most patients and are outlined in Box 73-4.
6 Reduce Increased Intracranial Pressure
When intracranial pressure elevation is not caused by a surgically treatable lesion, a variety of therapeutic interventions can be considered. Fluid administration can be limited to half or two-thirds of maintenance, provided that cardiovascular function and systemic blood pressure are maintained. Fluid restriction as a treatment for increased intracranial pressure should only be used when cerebral perfusion is deemed adequate. Positioning of the head at 30 degrees above the horizontal to maximize venous outflow from the head has merit so long as cerebral perfusion pressure is not harmed. Hyperventilation through mechanical ventilation reduces intracranial pressure by lowering cerebral blood flow and volume and may have short-term merit if cerebral perfusion pressure and delivery of metabolic substrate are maintained. Reduction of intracranial volume by reduction of brain water content using mannitol, furosemide, or hypertonic saline may be beneficial. Sedation is valuable to reduce Valsalva response and resulting reduction in brain venous outflow during noxious, but necessary, critical care such as suctioning. Barbiturate therapy to reduce cellular metabolic demand may help control intracranial pressure but does not seem to influence outcome. Reduction of fever also may be valuable in lowering brain metabolic demands. High-dose corticosteroid therapy may have a role in treating increased intracranial pressure due to brain tumors and in some infections, but is not useful in managing traumatic brain injury, infarction, and hemorrhage [Smith and Madsen, 2004a].
Optimal treatment of increased intracranial pressure must take into consideration the fact that metabolic substrate supply must equal critical metabolic requirement to prevent secondary brain injury. Simply controlling intracranial pressure and cerebral perfusion pressure by using diuretics to reduce brain water content and hyperventilation to reduce cerebral blood flow and volume does not adequately take into account strategies to prevent secondary injury [Meyer et al., 2001]. Therefore, monitoring of intracranial pressure is essential, and monitoring cerebral blood flow and brain tissue PO2 adds monitoring power. Draining cerebrospinal fluid through a surgically placed external ventricular drain can be particularly useful in reducing intracranial pressure.
Decompressive craniectomy, with or without opening the dura, lowers intracranial pressure and is increasingly used for refractory increased intracranial pressure in certain situations [Figaji et al., 2003; Smith and Madsen, 2004b; Taylor et al., 2001; Ruf et al., 2003].
7 Stop Seizures
Treatment of status epilepticus and other seizure emergencies is discussed in Chapter 58. It is always important to consider seizures, even when there are no obvious outward seizure manifestations. EEG is essential in identifying subclinical nonconvulsive status epilepticus or other forms of seizure activity, and currently, continuous bedside EEG monitoring is commonly used for patients in coma who are in critical care units.
9 Adjust Body Temperature
Usually, normal body temperature is best for recovery and prevention of acidosis. Patients with fever should have appropriate antipyretic agents administered. The use of hypothermia is being re-evaluated as a potential treatment for coma [Bernard et al., 1997; Biagas and Gaeta, 1998; Clifton, 1995; Safar and Kochanek, 2002]. This is discussed in more detail in Chapter 74.
Monitoring of the Comatose Patient
 as an extension of the neurologic examination to identify nonconvulsive status epilepticus or EEG patterns that suggest a diagnosis, and to evaluate the integrity of certain neural pathways
as an extension of the neurologic examination to identify nonconvulsive status epilepticus or EEG patterns that suggest a diagnosis, and to evaluate the integrity of certain neural pathways to improve assessment of the neurologic condition of patients who are sedated, or sedated and paralyzed, and on mechanical ventilation
to improve assessment of the neurologic condition of patients who are sedated, or sedated and paralyzed, and on mechanical ventilation to assist in assessment of availability of metabolic substrate critical to maintain brain integrity and function
to assist in assessment of availability of metabolic substrate critical to maintain brain integrity and functionTraditionally, the EEG has been used to identify presence of or risk for seizures, and has additive value in that certain patterns may suggest specific pathology, such as triphasic waves in metabolic causes of coma and periodic lateralized epileptiform discharges in focal injury and infection. EEG reactivity to sensory stimulation is considered a favorable sign in coma recovery [Young, 2000]. EEG has the advantage of ready availability in most centers and is usually definitive in the evaluation for on-going seizures. However, there is no universal agreement about criteria to diagnose nonconvulsive status epilepticus in obtunded or comatose patients [Brenner, 2002]. Other drawbacks to the use of long-term EEG monitoring include technical training necessary to apply and maintain the electrode–scalp interface, specialized training necessary for interpretation, and EEG susceptibility to artifact in the critical care setting. Processed derivations of EEG activity, therefore, have been successfully used to monitor brain activity.
The Bispectral Index monitor is a recorder that uses processed EEG data to measure sedation depth on a scale from 0 to 100 (0, coma; 40–60, general anesthesia; 60–90, sedated; 100, awake). The Bispectral Index monitor is useful in monitoring depth of sedation during conscious sedation [Agrawal et al., 2004] and neurologic status in critically ill unsedated patients [Gilbert et al., 2001]. It is also easily applied to children in critical care settings, and has been used to monitor children in barbiturate coma, sedation to assist mechanical ventilation, and procedural sedation, and to evaluate sedation effects of multiple medications [Grindstaff and Tobias, 2004]. Although the Bispectral Index Scale does not have the monitoring power of a full-montage EEG, it has the advantage of easier electrode application and more ready interpretation. In a study of 16 patients between 10 and 192 months of age with consciousness alteration, who were admitted to a pediatric intensive care unit, the Bispectral Index Scale correlated well with the Glasgow Coma Scale [Hsia et al., 2004]. Amplitude-integrated EEG is an alternative methodology that has been used for the evaluation of the neonate but is now being used in older children and adults to assess the severity of neurologic injury [Scheuer and Wilson, 2004; ter Horst et al., 2004].
Sensory-evoked potentials allow assessment of the integrity of visual, auditory, and somatosensory pathways in the unconscious patient. Somatosensory-evoked potentials have been especially valuable because their neural pathways extend from brainstem through thalamus and subcortical white matter to cortex. Somatosensory-evoked potentials are relatively resistant to sedation effect and are useful in predicting outcome in comatose patients. Visual-evoked potentials have not been as valuable as somatosensory-evoked potentials for outcome prediction in comatose children [Shewman, 2000a, b; Taylor and Farrell, 1989]. Brainstem auditory-evoked potentials are valuable for monitoring brainstem function but do not predict cortical function [Shewman, 2000a, b]. The presence of normal brainstem auditory-evoked potentials with abnormal somatosensory-evoked potentials is seen in patients who are in a vegetative state [Frank et al., 1985]. Middle latency auditory-evoked potentials have cortical representation and have been shown to improve coma outcome prediction in adults, although they may be less resistant to environmental artifact and sedation than somatosensory-evoked potentials. Event-related potentials (auditory P300 response) may assist in evaluating severity of injury and may correlate with closed-head injury outcome in adults [Fischer et al., 2004; Keren et al., 1998; Litscher, 1995; Logi et al., 2003].
Motor-evoked potentials have limited value in evaluation of the comatose patient and have not been studied in children for this purpose. Adult studies have found that electrically elicited, but not magnetoelectrically elicited, motor-evoked potentials may predict postcoma pyramidal motor deficit but do not allow prediction of severity of motor deficit. Motor-evoked potentials do not have the predictive value of somatosensory-evoked potentials for coma outcome. Motor-evoked potentials may be valuable in evaluating clinical deterioration in the acute phase of coma [Rohde et al., 1999; Zentner and Rohde, 1992, 1994].
Measurement of cerebral blood flow is a direct way to determine delivery of essential metabolic substrate to the brain but is not useful for continuous monitoring. Brain-tissue PO2 measurement, however, is suitable for continuous monitoring. In 25 patients older than 16 years with severe traumatic brain injury (Glascow Coma Scale value less than 8), it was found that intraparenchymal brain PO2 favorably compared with stable xenon CT for measurement of cerebral blood flow and allowed continuous monitoring of this critical substrate [Doppenberg et al., 1998]. Another study of 22 severe head-injury patients aged 11–52 years found that continuous monitoring of partial pressure of brain-tissue oxygen was practical and safe, allowed monitoring of ischemia on a continuous basis, allowed demonstration of abnormalities of oxygen autoregulatory mechanisms, and was superior to jugular oximetry [Van Santbrink et al., 1996]. Low brain-tissue PO2, as a measure of cerebral hypoxia, is predictive of poor neurologic outcome and is an important independent measurement parameter in adults with severe traumatic brain injury [Bardt et al., 1998]. Monitoring of brain tissue PO2 may also allow early detection of critical and potentially irreversible worsening, and allow measurement of intervention effect on a continuous basis. This is exemplified in a case report of a 15-year-old patient with severe traumatic brain injury and uncontrollable intracranial hypertension, who developed bilateral fixed and dilated pupils and critically low brain-tissue PO2 on the third day after injury; this patient who underwent bilateral decompressive craniotomy with immediate normalization of intracranial pressure and complete recovery of brain-tissue PO2 to the normal level [Kiening et al., 1997].
Local sampling of energy substrates by microdialysis during ischemia is feasible and allows measurement of glucose, lactate, pyruvate, and glutamate in brain tissue serially over time. Glucose concentration closely follows brain-tissue PO2, lactate concentration significantly increases when brain-tissue O2 falls below critical level, and glutamate is significantly elevated with very low brain-tissue PO2 levels in adult head-trauma patients [Hlatky et al., 2004]. Microdialysis measurements have thus far only been applied in children for monitoring of severe traumatic brain injury. In one study of nine children aged 2–14 years, a low glutamine to glutamate ratio was associated with increased morbidity [Tolias et al., 2002].
Near-infrared spectroscopy (NIRS) noninvasively monitors brain-tissue oxygenation by measuring the distinct absorption spectra of oxygenated and deoxygenated hemoglobin in the frontal cortex. NIRS compares favorably to brain-tissue PO2 monitoring in adults with severe traumatic brain injury or aneurysmal subarachnoid hemorrhage [Brawanski et al., 2002]. Additionally, in a study of 43 children monitored after corrective surgery for noncyanotic congenital heart defects, noninvasive measurement of the cerebral tissue oxygenation index by NIRS correlated with central venous oxygen saturation measured with a catheter placed in the right atrium [Nagdyman et al., 2004]. At least one commercially available device for NIRS monitoring is approved by the U.S. Food and Drug Administration for children [Andropoulos et al., 2004], and has been used extensively to monitor brain-tissue oxygenation during surgery for congenital heart disease. In a study of 26 infants and children having heart surgery with bypass and deep hypothermic circulatory arrest, low cerebrovascular hemoglobin oxygen saturation was associated with postoperative neurologic complications in 3 children (1 with seizure and 2 with prolonged coma) [Kurth et al., 1995].
Cerebral oxygenation measured by NIRS has been found to be valuable in two pediatric patients (ages 4 months and 16 years) being monitored for seizures in the pediatric intensive care unit and the epilepsy monitoring unit, and demonstrated preictal increase in cerebral oxygenation and suggested perfusion–metabolism mismatch during seizure activity. Thus, the monitoring of cerebral oxygenation by NIRS has value in the critical care setting for continuous monitoring of dynamic changes in cerebral oxygenation in relation to changes in metabolic need [Adelson et al., 1999]. None the less, use of NIRS to monitor brain-tissue oxygenation has not been studied extensively in children, except in surgery for congenital heart defects, and a recent study in children indicated poor agreement between two commercially available cerebral oximeters using NIRS [Dullenkopf et al., 2003].
Transcranial Doppler ultrasonography measures real-time cerebral blood flow velocity and is useful for monitoring middle cerebral artery flow velocity transcranially in all ages and through the anterior fontanel in infants. Transcranial Doppler has been used in pediatric cardiac surgery to evaluate cerebrovascular response to cardiopulmonary bypass, hypothermia, and deep hypothermia circulatory arrest [Andropoulos et al., 2004]. It has also been used to evaluate detectable cerebral perfusion threshold in low-flow cardiopulmonary bypass in neonates [Zimmerman et al., 1997]. It is important to note, however, that cerebral blood flow velocity depends on vessel diameter and is not the same measurement as cerebral blood flow, which depends on cerebral vascular resistance and varies with temperature, CO2 pressure, cerebral perfusion pressure, and bypass flow. Therefore, cerebral blood flow velocity may correlate best with cerebral blood flow in deep hypothermia when autoregulation is lost and blood vessel diameter does not change.
Brain-tissue PO2 monitoring and microdialysis to evaluate energy substrates are complementary, and both monitoring probes require neurosurgical installation into brain parenchyma. Therefore, they are primarily used clinically to evaluate and monitor victims of severe traumatic brain injury. NIRS and transcranial Doppler are currently used to monitor brain oxygenation in children during and after cardiac surgery. The devices are small and relatively portable, and can be used in the pediatric critical care environment safely, effectively, and continuously. Their ultimate value in monitoring of children in coma and guiding therapeutic interventions has yet to be delineated. However, in a prospective study of 250 patients undergoing pediatric cardiac surgery, it was found that interventions based on neurophysiologic monitoring (EEG, transcranial Doppler, NIRS) appeared to decrease the incidence of postoperative neurologic sequelae and reduce length of stay [Austin et al., 1997].
Outcome Measurement
When consciousness is impaired, outcome is related to the etiology of the insult and rapid identification and treatment of the underlying cause. Early appropriate treatment may prevent further deterioration of the mental state and injury to the nervous system and improve outcome. Outcome studies in childhood coma are available for traumatic and nontraumatic causes, and typically emphasize predictive value of signs and symptoms at the time of initial medical intervention. These studies are described in detail in Chapters 74–76.
A commonly used and widely accepted measurement of outcome after severe closed-head injury is the Glasgow Outcome Scale [Jennett and Bond, 1975]. The Glasgow Outcome Scale has the following five broad outcome categories:
Early studies using the Glasgow Outcome Scale as an outcome measure after closed-head injury reported that patients advance to the highest functional level by 6 months after injury [Jennett et al., 1976, 1979; Jennett and Bond, 1975; Lange-Cossack et al., 1981]. Meaningful recovery, however, may occur for months to years after injury [Mahoney et al., 1981, 1983]. Because there may be significant functional improvement within the three highest Glasgow Outcome Scale categories (i.e., good recovery, moderate disability, and severe disability) and because long-term improvement is possible, especially when injury occurs during early childhood, alternative outcome scales have been developed.
Another scoring system, the Pediatric Cerebral Performance Category Scale, was published by Fiser in 1992 and is a modified form of the Glasgow Outcome Scale. This six-point outcome scoring system was validated in 1469 pediatric patients suffering acute CNS injuries. It has also been correlated with neuropsychological test scores [Fiser et al., 2000]. The score includes the following outcomes:
This scale has been used in outcome prediction studies after acute brain injury in children who were acutely evaluated with proton MR spectroscopy [Ashwal et al., 2000; Brenner et al., 2003; Holshouser et al., 1997]. In a prospective study of 53 children less than 3 years old with severe brain injury or hypoxic-ischemic encephalopathy, the Glasgow Outcome Scale indicated good recovery in 46 percent of 50 children with known outcome [Robertson et al., 2002]. However, only 16 percent had average or above-average scores on both the mental and motor developmental sections of the Bayley Scales of Infant Development II at 18–36 months of age. Thus, a commonly used developmental assessment tool, the Bayley Scales of Infant Development II, provides a greater distinction between normal and delayed development than does the good recovery level of the Glasgow Outcome Scale [Robertson et al., 2002].
Another scoring system, also adapted from the adult Glasgow Outcome Scale, that was developed for evaluating children with traumatic brain injury and likely has wider usage is the King’s Outcome Scale for Childhood Head Injury (KOSCHI) [Crouchman et al., 2001]. The KOSCHI expands the five-category Glasgow Outcome Scale to provide increased sensitivity at the milder end of the disability range (Table 73-6). The Glasgow Outcome Scale category of “persistent vegetative state” was replaced by “vegetative.” “Good recovery” was allocated two categories, in acknowledgment of the long-term importance of relatively minor sequelae in a developing child. The scale is easy to use and reliable, and is very helpful in differentiating children with milder outcomes into subcategories. Although studies have suggested limited value when it is used at the time of hospital discharge in predicting outcome [Calvert et al., 2008], it remains a practical and straightfoward method to classify outcomes.
Table 73-6 King’s Outcome Scale for Childhood Head Injury (KOSCHI)
| Category | Definition | |
|---|---|---|
| 1 | Death | |
| 2 | Vegetative | The child is breathing spontaneously and may have sleep–wake cycles. He may have nonpurposeful or reflex movements of limbs or eyes. There is no evidence of ability to communicate verbally or nonverbally, or to respond to commands |
| 3 | Severe disability | (a) The child is at least intermittently able to move part of the body/eyes to command or make purposeful spontaneous movements; e.g., confused child pulling at nasogastric tube, lashing out at carers, rolling over in bed. May be fully conscious and able to communicate but not yet able to carry out any self-care activities, such as feeding |
| (b) Implies a continuing high level of dependency, but the child can assist in daily activities; e.g., can feed self or walk with assistance or help to place items of clothing. Such a child is fully conscious but may still have a degree of post-traumatic amnesia | ||
| 4 | Moderate disability | (a) The child is mostly independent but needs a degree of supervision/actual help for physical or behavioral problems. Such a child has overt problems; e.g., a 12-year-old with moderate hemiplegia and dyspraxia, insecure on stairs or needing help with dressing |
| (b) The child is age-appropriately independent but has residual problems with learning/behavior or neurological sequelae affecting function. He probably should have special needs assistance but his special needs may not have been recognized/met. Children with symptoms of post-traumatic stress are likely to fall into this category | ||
| 5 | Good recovery | (a) This should only be assigned if the head injury has resulted in a new condition that does not interfere with the child’s well-being and/or functioning; e.g., Minor headaches not interfering with social or school functioning Abnormalities on brain scan without any detectable new problem Prophylactic anticonvulsants in the absence of clinical seizures Unsightly scarring of face/head likely to need cosmetic surgery at some stage Mild neurological asymmetry but no evidence of affect on function of limb. Includes isolated change in hand dominance in young child |
| (b) Implies that the information available is that the child has made a complete recovery with no detectable sequelae from the head injury. |
(From Crouchman M et al. A practical outcome scale for paediatric head injury. Arch Dis Child 2001;84:120–124.)
The Disability Rating Scale consists of eight items divided into four categories:
The Disability Rating Scale is a more sensitive measure of improvement during inpatient rehabilitation than the Glasgow Outcome Scale [Hall et al., 1985], and is suitable for use in adolescents and some older children.
The Functional Independence Measure and its pediatric counterpart, the Wee Functional Independence Measure (WeeFIM), further refine outcome assessment using six items and 18 categories [Data Management Service of the Uniform Data System of Medical Rehabilitation and the Center for Functional Assessment Research, 1989]. The WeeFIM items and categories are as follows:
The Pediatric Evaluation of Disabilities Inventory is designed for use from 6 months to 7 years of age, but is appropriate for older children whose functional level is within the intended age range. Specialized derivations of the Pediatric Evaluation of Disabilities Inventory specific to acquired brain injury do not improve the assessment value of the generic inventory, although further refinement may allow better functional recovery scoring in acquired brain injury [Kothari et al., 2003]. Pediatric Evaluation of Disabilities Inventory score change of about 11 percent has been found clinically meaningful during inpatient rehabilitation in a cohort of 53 children aged 1–19 years [Iyer et al., 2003].
The WeeFIM and the Pediatric Evaluation of Disabilities Inventory are sensitive measures of functional outcome and are suitable for inpatient rehabilitation monitoring and long-term outpatient follow-up. They are two of the most commonly used functional outcome measures in children [Ziviani et al., 2001], and are superior to the Glasgow Outcome Scale and its pediatric variants for assessment of long-term functional improvement in children across more relevant domains of recovery.
The Barthel Index is commonly used as a prognostic indicator in adult clinical stroke trials [Huybrechts et al., 2007]. It has functional disability categories (Table 73-7) that lend themselves to use in children who are emerging from coma, but it has not been studied in detail [Mahoney et al., 1965; Jöhnk et al., 1999].
| Activity | Score |
|---|---|
| Feeding | |
| 0 = unable; 5 = needs help cutting, spreading butter, etc., or requires modified diet; 10 = independent | 0, 5, 10 |
| Bathing | |
| 0 = dependent; 5 = independent (or in shower) | 0, 5 |
| Grooming | |
| 0 = needs to help with personal care; 5 = independent face/hair/teeth/shaving (implements provided) | 0, 5 |
| Dressing | |
| 0 = dependent; 5 = needs help but can do about half unaided; 10 = independent (including buttons, zippers, laces, etc.) | 0, 5,10 |
| Bowels | |
| 0 = incontinent (or needs to be given enemas); 5 = occasional accident; 10 = continent | 0, 5, 10 |
| Bladder | |
| 0 = incontinent, or catheterized and unable to manage alone; 5 = occasional accident; 10 = continent | 0, 5, 10 |
| Toilet use | |
| 0 = dependent: 5 = needs some help, but can do something alone; 10 = independent (on and off, dressing, wiping) | 0, 5, 10 |
| Transfers (bed to chair and back) | |
| 0 = unable, no sitting balance; 5 = major help (one or two people, physical), can sit; 10 = minor help (verbal or physical); 15 = independent | 0, 5, 10, 15 |
| Mobility (on level surfaces) | |
| 0 = immobile or <50 yards; 5 = wheelchair-independent, including corners, >50 yards; 10 = walks with help of one person (verbal or physical) >50 yards; 15 = independent (but may use any aid; e.g., stick) >50 yards | 0, 5, 10, 15 |
| Stairs | |
| 0 = unable; 5 = needs help (verbal, physical, carrying aid); 10 = independent | 0, 5 |
| Total (0–100) |
(From Mahoney and Barthel: Functional evaluation: the Barthel Index, MD State Med J 14:2, 1965; Jöhnk K et al. Assessment of sensorimotor functions after traumatic brain injury (TBI) in childhood – Methodological aspects. Restor Neurol Neurosci 1999;14[2–3]:143.)
Other standardized measures used to assess intellectual, cognitive, language, and behavioral development in children without injury are useful in evaluating and assessing outcomes in children after severe acquired brain injury. In children old enough and cooperative enough, neuropsychologic testing is the most sensitive and specific way to measure and monitor change in these domains. Valuable reviews of these issues are available [Fletcher et al., 2001; Hannay and Sherer, 1996; Levin, 1994; Ylvisaker et al., 1990]. In a retrospective analysis of 83 children aged 1–18 years admitted to hospital after head injury, 70 percent had severe head injury and 13 died. Forty-five patients had at least one cognitive assessment, and 17 were evaluated with intelligence testing less than 4 months after injury, 5–15 months after injury, and 16–38 months after injury. Continued improvement in cognitive functioning was seen at 16–38 months’ follow-up assessment [Campbell et al., 2004]. This improvement underscores the fact that continued cognitive recovery may progress for years and that more long-term studies are necessary.
Prognosis
Traumatic Injury
Children who survive traumatic injury have a better prognosis than children who suffer a global hypoxic-ischemic injury [Johnson et al., 2009; see also Chapters 74 and 75). How prognosis is defined, however, is significant. For example, in a study of 127 children said to be in the persistent vegetative state for at least 30 days, 84 percent of the traumatic brain injury group, but only 55 percent of the hypoxic-ischemic injury group, improved beyond the vegetative state [Heindl and Laub, 1996]. This study highlights the fact that hypoxic-ischemic injury is worse than traumatic brain injury, but uses the term persistent vegetative state (a statement of prognosis) rather than vegetative state (a moment-in-time description).
In studies of coma in children resulting from traumatic brain injury, substantially better outcome is seen in children when compared with adults [Broman and Michel, 1995]. Up to 80–90 percent of children with severe traumatic brain injury have good outcomes or recover to a moderate disability, based on studies using the Glasgow Outcome Scale. In a small retrospective review of eight survivors of severe traumatic brain injury at a mean age of 14 years, neuropsychologic evaluation was performed 1, 7, and 14 years after the injury. Three of the eight subjects went from a school situation with no adjustments to adult life without ability to be employed [Jonsson et al., 2004]. In a review of 65 children and adolescents with acquired brain injury aged 6 months to 18 years (mean age, 9.5 years), 82 percent were discharged home, but only 3–27 percent were deemed ready for age-expected participation in home, school, and community life [Bedell et al., 2002a]. Social behavioral activity performance at discharge explained 74 percent of the variance associated with participation at discharge. In the same cohort of patients, functional assessment scales of daily and social behavioral activity allowed assessment of response to treatment [Bedell et al., 2002b].
Brink and associates reported a large series of 344 pediatric patients (younger than 18 years) with severe closed-head injury and coma for a minimum of 24 hours, who were admitted to a rehabilitation center between 1959 and 1977 [Brink et al., 1970, 1980]. Median coma duration was 5–6 weeks. One year after injury, 73 percent were independent in ambulation and self-care, but only 10 percent were physically normal. Coma duration of less than 6 weeks was associated with better outcome than coma duration of longer than 12 weeks. However, less than one-third of these children had normal intelligence; about half had significant behavior problems, and most required special education services at school.
A study of 33 survivors of severe closed-head injury enrolled at 17 years of age or younger demonstrated the following Glasgow Outcome Scale distribution 5–7 years after injury: 27 percent good recovery, 55 percent moderate disability, and 18 percent severe disability. At the time of follow-up, 70 percent were independent in self-care, 64 percent in mobility, and only 24 percent in cognitive items on the Functional Independence Measure [Massagli et al., 1996]. More importantly, 70 percent received special education services, and employment histories were poor. This clearly illustrates the better specificity of the Functional Independence Measure over the Glasgow Outcome Scale when evaluating long-term quality of life and community re-entry. Moreover, most of these individuals were not involved in neurologic or rehabilitation follow-up, suggesting the need for better long-term programs of monitoring and care.
Both the WeeFIM and the Pediatric Evaluation of Disabilities Inventory measure functional ability, taking into account the amount of caregiver assistance necessary and use of special equipment. The WeeFIM assesses 18 variables in self-care, mobility, and cognition, whereas the Pediatric Evaluation of Disabilities Inventory evaluates 197 functional items in self-care, mobility, and social function. In a study of 205 children with disability aged 11–87 months, the WeeFIM measured similar skill areas to those assessed by the Battelle Developmental Inventory Screening Test or the Vineland Adaptive Behavior Scale. The WeeFIM requires less administration time than the Pediatric Evaluation of Disabilities Inventory, the Battelle Developmental Inventory Screening Test, or the Vineland Adaptive Behavior Scale, and provides directly relevant functional outcome information for children and their families [Ottenbacher et al., 1999]. Further, the WeeFIM was an improvement over the Battelle and the Vineland scales in predicting the amount of assistance that parents or teachers must provide for basic activities of daily living [Ottenbacher et al., 2000].
Nontraumatic Injury
Outcome after coma resulting from nontraumatic causes has been extensively reviewed [Abend et al., 2008; Hoesch et al., 2008] and available information suggests that, as with closed-head injury, outcome in children is better than in adults (see Chapter 76). In a cohort of 104 children ranging in age from 1 month to 17 years with nontraumatic coma (average duration of coma was 3 hours to 35 days), 32 percent died, 50 percent were normal, and the remaining 18 percent had handicaps ranging from mild to severe. Stepwise multivariate discriminant analysis within 12 hours of onset of coma classified 75 percent of 102 cases correctly into one of five outcome groups and misclassified 8 percent of patients. When the discriminant analysis was used on data collected 24 hours after the onset of coma, 67 percent of the cases were correctly classified, and only 3 percent were misclassified. Variables that correlated best with outcome were coma severity, motor patterns, blood pressure, and seizure type. Age correlated well with outcome based on data obtained at 24 but not at 12 hours. Extraocular movements, pupillary responses, and temperature correlated with outcome at less than 12 hours but not at 24 hours. Mortality rate was highest in patients less than 1 year of age (44 percent), and only 24 percent were considered normal at follow-up. For patients aged 6–17 years, 24 percent died, and 73 percent were normal. Patients who died all had hypothermia, inability to maintain body temperature, and absent pupillary responses or extraocular movements [Johnston and Seshia, 1984; Seshia et al., 1983].
In a retrospective population study of nontraumatic coma in children aged 1 month to 16 years, 278 individuals were identified, representing 283 episodes of coma. The most common cause of coma was infection, and the most common cause of recurrent episodes of coma was epilepsy. Causes of nontraumatic coma were infection, 37.9 percent; unknown, 14.5 percent; intoxication, 10.3 percent; epilepsy, 9.6 percent; congenital malformation, 8.2 percent; other, 7.8 percent; accident, 6.7 percent; and metabolic, 5.0 percent. Within the unknown group, half of the cases were suspected to be due to infection because of the clinical course, but pathogens were not identified. At 12 months’ follow-up, the overall mortality rate was 46 percent, and the etiology-specific mortality rate varied from 3 percent (intoxication) to 84 percent (accident). Most individuals were either intact (normal) at 12 months’ follow-up or were deceased. Highest intact survival rates were seen in patients with epilepsy (71.4 percent), metabolic etiologies (60 percent), or unknown causes of coma (47.5 percent). Mortality was highest in patients in coma from accidents (84.2 percent), congenital malformations (72.7 percent), and infections (60 percent) [Wong et al., 2001]. In this study of 278 individuals, cognitive and adaptive outcomes were evaluated 6 weeks and 12 months after admission. Age-appropriate psychometric instruments were employed as follows: Bayley Scales of Infant Development (younger than 3.5 years); Wechsler Preschool and Primary Scale of Intelligence – Revised (3.5–6 years), and the Wechsler Intelligence Scale for Children III (older than 6 years). When appropriate, adaptive behavior was evaluated using the Vineland Adaptive Behavior Scales. Of 278 patients, 151 survived to late follow-up, and 141 (93 percent of survivors) completed follow-up assessment. Significant differences in outcome based on etiology could not be established, but children younger than 2 years of age at admission had no improvement, and children older than 2 years of age had improvement when evaluated 6 weeks and 12 months after initial admission. The overall incidence of moderate to severe disability (cognitive quotient less than 70 and moderate to severe behavioral disturbances) was 70 percent. The relation between early age at first coma episode and poor outcome was particularly evident in children with nontraumatic coma caused by epilepsy [Fletcher et al., 1987; Hannag and Sherer, 1996; Levin, 1994; Ylvisaker et al., 1990; Forsyth et al., 2001].
Clinical Neurophysiology
In the comatose patient, clinical neurophysiology evaluation (EEG, somatosensory-evoked potential) is the most valuable method for prediction of survival, brain death, and functional recovery beyond the vegetative state [Guerit et al., 2009; Beca et al., 1995; DeMeirleir and Taylor, 1986, 1987; Fischer et al., 2004; Goodwin et al., 1991; Mandel et al., 2002; Mewasingh et al., 2003; Shewman, 2000b; Wohlrab et al., 2001]. There are, however, a few reports of contrary findings, such as normal somatosensory-evoked potentials in a child in a vegetative state following near-drowning [Tsao and Ellingson, 1989]. Therefore, the clinical neurophysiology assessment is used as an adjunct to the clinical examination and other outcome predictors, such as neuroimaging and cerebral blood flow. To date, there are no studies linking neurophysiologic tests to very long-term functional outcome measures, such as the Wee Functional Independence Measure or the Pediatric Evaluation of Disabilities Inventory.
MRI has been explored to help evaluate patients with disorders of consciousness [Lescot et al., 2009; Gawryluk et al., 2010; Monti et al., 2009] and this is also reviewed in depth in Chapters 74–76. Several studies also have indicated the prognostic value of proton MR spectroscopy in newborns, infants, and children with CNS insult or injury of different types [Holshouser et al., 1997, 2000]. Patients with elevated cerebral lactate are more likely to die or have serious long-term disability [Ashwal et al., 1997]. Metabolic ratios of N-acetylaspartate/choline-containing compounds and N-acetylaspartate/creatine and phosphocreatine were significantly lower in patients with bad outcomes. Outcome measurement was performed at 6–12 months using the Pediatric Cerebral Performance Scale, and outcomes were expressed as good/moderate outcome (good, mild, moderate disability) or poor outcome (severe disability, persistent vegetative state, death). When MR spectroscopy data were combined with clinical data and MRI scores, outcome was correctly predicted in 91 percent of neonates and 100 percent of infants and children [Ashwal et al., 1997; Auld et al., 1995; Holshouser et al., 1997; Shu et al., 1997].
In an MR spectroscopy study of 26 infants (1–18 months old) and 27 children (18 months or older) with nonaccidental or other forms of traumatic brain injury, lactate was evident in 91 percent of infants and 80 percent of children with poor outcome, whereas none of the patients with good outcome had elevated lactate. Metabolic ratios were abnormal (low N-acetylaspartate/creatine and phosphocreatine, low N-acetylaspartate/choline-containing compounds, and high choline-containing compounds/creatine and phosphocreatine) in patients with poor outcome. Clinical variables alone predicted outcome in 77 percent of infants and 86 percent of children. Lactate alone predicted outcome in 96 percent of infants and 96 percent of children. Addition of metabolic ratios and clinical variables did not improve outcome prediction over lactate alone [Ashwal et al., 2000]. Myoinositol, a marker of astrocyte activity, is measurable by MR spectroscopy, and in a study of 38 children with traumatic brain injury, aged 1.6–17 years, myoinositol was higher in patients with poor outcomes than in patients with good outcomes [Ashwal et al., 2004].
Susceptibility-weighted imaging, a new high-resolution gradient-echo MRI technique, is superior to conventional T2-weighted two-dimensional gradient-recalled echo imaging in identifying hemorrhagic lesions associated with diffuse axonal injury [Tong et al., 2003]. In 40 children and adolescents with traumatic brain injury and suspected diffuse axonal injury, those with Glasgow Coma Scale scores of 8 or lower, prolonged coma of more than 4 days’ duration, or poor neurologic outcomes 6–12 months after injury had significantly increased numbers and volume of hemorrhagic lesions. More accurate evaluation of diffuse axonal damage soon after injury may provide improved prognostic information about coma duration and long-term outcome in children with traumatic brain injury [Tong et al., 2004].
Conclusions
The outcome for children with coma is substantially better than for adults and may be affected by the speed and appropriateness of intervention. Even with the best and most appropriate care, some children die, some survive on mechanical ventilation and become brain-dead, and some survive beyond mechanical ventilation and have minimal consciousness or severe disability, or remain in a vegetative state. Decisions concerning basic life sustenance, type and location of care, and need for rehabilitation should take into consideration the type of injury, time from injury, recovery potential, family wishes, and appropriate ethical guidelines and standards [Michelson and Ashwal, 2004; Ashwal and Cranford, 2002].
References
![]() The complete list of references for this chapter is available online at www.expertconsult.com.
The complete list of references for this chapter is available online at www.expertconsult.com.
AAN Quality Standards Subcommittee. Practice parameters: Assessment and management of patients in the persistent vegetative state. Neurology. 1995;45:1015.
Abend N.S., Licht D.J. Predicting outcome in children with hypoxic ischemic encephalopathy. Pediatr Crit Care Med. 2008;9:32-39.
Adams J.H., Graham D.I., Jennett B. The neuropathology of the vegetative state after an acute brain insult. Brain. 2000;123:1327.
Adams J.H., Graham D.I., Murray L.S., et al. Diffuse axonal injury due to nonmissile head injury in humans: An analysis of 45 cases. Ann Neurol. 1982;12:557.
Adelson P.D., Nemoto E., Scheuer M., et al. Noninvasive continuous monitoring of cerebral oxygenation periictally using near-infrared spectroscopy: A preliminary report. Epilepsia. 1999;40:1484.
Agrawal D., Feldman M.A., Krauss B., et al. Bispectral Index monitoring quantifies depth of sedation during emergency department procedural sedation and analgesia in children. Ann Emerg Med. 2004;43:247.
Akavipat P. Endorsement of the FOUR Score for Consciousness Assessment in Neurosurgical Patients. Neurol Med Chir. 2009;49:565-571. (Tokyo)
American Congress of Rehabilitation Medicine. Recommendations for use of uniform nomenclature pertinent to patients with severe alterations in consciousness. Arch Phys Med Rehabil. 1995;76:205.
Andropoulos D.B., Stayer S.A., Diaz L.K., et al. Neurological monitoring for congenital heart surgery. Anesth Analg. 2004;99:1365.
Ashwal S. The persistent vegetative state in infancy and childhood. In: Frank Y., editor. Pediatric behavioral neurology. New York: CRC Press, 1996.
Ashwal S. Medical aspects of the minimally conscious state in children. Brain Dev. 2003;25:535.
Ashwal S. Recovery of consciousness and life expectancy of children in a vegetative state. Neuropsychol Rehabil. 2005;15:190.
Ashwal S., Cranford R. The minimally conscious state in children. Semin Pediatr Neurol. 2002;9:19.
Ashwal S., Eyman R.K., Call T.L. Life expectancy of children in a persistent vegetative state. Pediatr Neurol. 1994;10:27.
Ashwal S., Holshouser B.A. New neuroimaging techniques and their potential role in patients with acute brain injury. J Head Trauma Rehabil. 1997;12:13.
Ashwal S., Holshouser B.A., Shu S.K., et al. Predictive value of proton magnetic resonance spectroscopy in pediatric closed head injury. Pediatr Neurol. 2000;23:114.
Ashwal S., Holshouser B.A., Tomasi L.G., et al. 1H-magnetic resonance spectroscopy-determined cerebral lactate and poor neurological outcomes in children with central nervous system disease. Ann Neurol. 1997;41:470.
Ashwal S., Holshouser B.A., Tong K., et al. Proton spectroscopy detected myoinositol in children with traumatic brain injury. Pediatr Res. 2004;56:630.
Ashwal S., Holshouser B.A., Tong K.A. Use of advanced neuroimaging techniques in the evaluation of pediatric traumatic brain injury. Dev Neurosci. 2006;28(4-5):309-326.
Atlluru V.L. Spontaneous intracerebral hematomas in juvenile diabetic ketoacidosis. Pediatr Neurol. 1986;2:167.
Auld K.L., Ashwal S., Holshouser B.A., et al. Proton magnetic resonance spectroscopy in children with acute central nervous system injury. Pediatr Neurol. 1995;12:323.
Austin E.H., Edmons H.L., Auden S.M., et al. Benefit of neurophysiologic monitoring for pediatric cardiac surgery. J Thorac Cardiovasc Surg. 1997;114:707.
Baars B.J., Banks W.P., Newman J.B., editors. Essential sources in the scientific study of consciousness. Cambridge, MA: MIT Press (Bradford Books), 2003.
Bardt T.F., Unterberg A.W., Hartl R., et al. Monitoring of brain tissue PO2 in traumatic brain injury: Effect of cerebral hypoxia on outcome. Acta Neurochir Suppl. 1998;71:153.
Bates D. The management of medical coma. J Neurol Neurosurg Psychiatry. 1993;56:589.
Beca J., Cox P.N., Taylor M.J., et al. Somatosensory evoked potentials for prediction of outcome in acute severe brain injury. J Pediatr. 1995;126:44.
Bedell G.M., Haley S.M., Coster W.J., et al. Participation readiness at discharge from inpatient rehabilitation in children and adolescents with acquired brain injuries. Pediatr Rehabil. 2002;5:107.
Bedell G.M., Haley S.M., Coster W.J., et al. Developing a responsive measure of change for paediatric brain injury inpatient rehabilitation. Brain Inj. 2002;16:659.
Berger H. Uber des Elektrenkephalogramm des Menschen. Arch Psychiatr Nervenkr. 1929;87:572-580.
Berlin R.J., Lee U.T., Samples J.R., et al. Ophthalmic drops causing coma in an infant. J Pediatr. 2001;138:441.
Bernard S.A., Jones B.M., Horne M.K. Clinical trial of induced hypothermia in comatose survivors of out-of-hospital cardiac arrest. Ann Emerg Med. 1997;30:146.
Biagas K.V., Gaeta M.L. Treatment of traumatic brain injury with hypothermia. Curr Opin Pediatr. 1998;10:271.
Boveroux P., Bonhomme V., Boly M., et al. Brain function in physiologically, pharmacologically, and pathologically altered states of consciousness. Int Anesthesiol Clin. 2008;46:131-146.
Bozza Marrubini M. Classifications of coma. Int Care Med. 1984;10:271.
Brain W.. The Cerebral Basis of Consciousness. [Abridged]. Section of Neurology Proc R Soc Med. 1950;44:37-42.
Brawanski A., Faltermeier R., Rothoerl R.D., et al. Comparison of near-infrared spectroscopy and tissue O2 time series in patients after severe head injury and aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2002;22:605.
Brenner R.P. Is it status? Epilepsia. 2002;43(Suppl 3):103.
Brenner T., Freier M.C., Holshouser B.A., et al. Predicting neuropsychologic outcome after traumatic brain injury in children. Pediatr Neurol. 2003;28:104.
Brink J., Garrett A.L., Hale W.R., et al. Recovery of motor and intellectual function in children sustaining severe head injuries. Dev Med Child Neurol. 1970;12:565.
Brink J.D., Umus M.D., Woo-San J. Physical recovery after severe closed head trauma in children and adolescents. J Pediatr. 1980;97:721.
Brodal A. Anatomical points of view on the alleged morphological basis of consciousness. Acta Neurochir (Wien). 1964;12:166-186.
Broman S.H., Michel M.E. Traumatic head injury in children. New York: Oxford University Press; 1995.
Bruck W., Christen H.J., Lakomele M., et al. Wernicke’s encephalopathy in a child with acute lymphoblastic leukemia treated with polychemotherapy. Clin Neuropathol. 1991;3:134.
Cairns H., Oldfield R.C., Pennybacker J.B., et al. Akinetic mutism with an epidermoid cyst of the third ventricle. Brain. 1941;64:273.
Calvert S., Miller H.E., Curran A., et al. The King’s Outcome Scale for Childhood Head Injury and injury severity and outcome measures in children with traumatic brain injury. Dev Med Child Neurol. 2008;50(6):426-431.
Campbell C.G.N., Kuehn S.M., Richards P.M.P., et al. Medical and cognitive outcome in children with traumatic brain injury. Can J Neurol Sci. 2004;31:213.
Clayton P.T., Bridges N.A., Atherton D.J., et al. Pellagra with colitis due to a defect in tryptophan metabolism. Eur J Pediatr. 1991;150:498.
Clifton G.L. Systemic hypothermia in treatment of severe brain injury: A review and update. J Neurotrauma. 1995;12:923.
Cohen J. Interrater Reliability and Predictive Validity of the FOUR Score Coma Scale in a Pediatric Population. J Neurosci Nurs. 2009;41(5):273-1267.
Coster W.J., Deeney T., Haltiwanger J., et al. School function assessment. San Antonio, TX: The Psychology Corporation; 1998.
Crick F. Function of the thalamic reticular complex: The searchlight hypothesis. Proc Natl Acad Ser USA. 1984;81:4586.
Crick F., Koch C. Consciousness and neuroscience. Cereb Cortex. 1998;8:97.
Crick F., Koch C. A framework for consciousness. Nat Neurosci. 2003;6(2):119-126.
Crouchman M., Rossiter L., Colaco T., et al. A practical outcome scale for paediatric head injury. Arch Dis Child. 2001;84:120-124.
Cuneo R.A., Caronno J.J., Pitts L., et al. Upward transtentorial herniation: Seven cases and a literature review. Arch Neurol. 1979;36:618.
Damasio A. Feelings of emotion and the self. Ann N Y Acad Sci. 2003;1001:253.
Data Management Service of the Uniform Data System for Medical Rehabilitation and the Center for Functional Assessment. Guide for use of the functional independence measure for children (WeeFIM) of the uniform data set for medical rehabilitation. Buffalo: State University of New York at Buffalo; 1989.
DeMeirleir L.J., Taylor M.J. Evoked potentials in comatose children: Auditory brainstem responses. Pediatr Neurol. 1986;2:31.
DeMeirleir L.J., Taylor M.J. Prognostic utility of SEPs in comatose children. Pediatr Neurol. 1987;3:78.
Demertzi A., et al. Dualism Persists in the Science of Mind. Disorders of Consciousness. Ann N Y Acad Sci. 2009;157:1-9.
Doppenberg E.M.R., Zauner A., Bullock R., et al. Correlations between brain tissue oxygen tension, carbon dioxide tension, pH and cerebral blood flow: A better way of monitoring the severely injured brain? Surg Neurol. 1998;49:650.
Dullenkopf A., Frey B., Baenziger O., et al. Measurement of cerebral oxygenation state in anaesthetized children using the INVOS 5100 cerebral oximeter. Paediatr Anaesth. 2003;13:384.
Duncan C.C., Ment L.R., Smith B., et al. A scale for the assessment of neonatal neurologic status. Child Brain. 1981;8:299.
Epstein F., Murali R.L. Pediatric posterior fossa tumors: Hazards of the “preoperative” shunt. Neurosurgery. 1978;3:348.
Evans B.M. Sleep, consciousness and the spontaneous and evoked electrical activity of the brain. Is there a cortical integrating mechanism? Neurophysiol Clin/Clin Neurophysiol. 33(1), 2003.
Feldmann E., Gandy S.E., Becker R., et al. MRI demonstrates descending transtentorial herniation. Neurology. 1988;38:697.
Figaji A.A., Fiegen A.G., Peter J.C. Early decompressive craniotomy in children with severe traumatic brain injury. Childs Nerv Syst. 2003;19:666.
Fischer C., Luaute J., Adeleine P., et al. Predictive value of sensory and cognitive evoked potentials for awakening from coma. Neurology. 2004;63:669.
Fischer M., Reugg S., Czaplinski A., et al. Inter-rater reliability of the Full Outline of Unresponsiveness score and the Glasgow Coma Scale in critically ill patients: a prospective observational study. Crit Care. 2010;14:R64.
Fiser D.H. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121:68.
Fiser D.H., Long N., Roberson P.K., et al. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000;28:2616.
Fisher C.M. Brain herniation: A revision of classical concepts. Can J Neurol Sci. 1995;22:83.
Fletcher J.M., Miner M.E., Ewing-Cobb S.L.. Age and recovery from head injury in children: Developmental issues. Levin H.S., Grafman J., Eisenberg H.M, editors. Neurobehavioral recovery from head injury. Oxford: Oxford University Press; 1987.
Fodstad H., Kelly P.J., Buchfelder M. History of the Cushing Reflex. Neurosurgery. 2006;59:1132.
Forsyth R.J., Wong C.P., Kelly T.P., et al. Cognitive and adaptive outcomes and age at insult effects after non-traumatic coma. Arch Dis Child. 2001;84(3):200.
Fortune P., Shann F. The motor response to stimulation predicts outcome as well as the full Glasgow Coma Scale in children with severe head injury. Pediatric Critical Care Medicine. 2010;11(3):339-342.
Frank L.M., Furgiuele T.L., Etheridge J.E.Jr. Prediction of chronic vegetative state in children using evoked potentials. Neurology. 1985;35:931.
Freundlich E., Slatter M., Yatz V.S. Familial pellagra-like skin rash with neurological manifestations. Arch Dis Child. 1981;56:146.
Gawryluk J.R., D’Arcy R.C., Connolly J.F., et al. Improving the clinical assessment of consciousness with advances in electrophysiological and neuroimaging techniques. BMC Neurol. 2010;10:11.
Gennarelli T.A., Thibault L.D., Adams J.H., et al. Diffuse axonal injury and traumatic coma in the primate. Ann Neurol. 1982;12:564.
Giacino J., Kalmar K., Whyte J., et al. Coma Recovery Scale-Revised: Measurement Characteristics and Diagnostic Utility. Arch Phys Med Rehabil. 2004;85:2020-2029.
Giacino J.T. Disorders of consciousness: Differential diagnosis and neuropathologic features. Semin Neurol. 1997;17:105.
Giacino J.T., Ashwal S., Childs N., et al. The minimally conscious state. Definition and diagnostic criteria. Neurology. 2002;58:349.
Giacino J.T., Kalmar K. The vegetative and minimally conscious states: A comparison of clinical features and functional outcome. J Head Trauma Rehabil. 1997;12:36.
Giacino J.T. Kalmar K: Diagnostic and prognostic guidelines for the vegetative state and minimally conscious states. Neuropsychol Rehabil. 2005;15(3–4):166.
Giacino J.T., Schnakers C., Rodriguez-Moreno D., et al. Behavioral assessment in patients with disorders of consciousness: gold standard or fool’s gold? Prog Brain Res. 2009;177:33-48.
Giacino J.T., Zasler N.D., Katz D.L., et al. Development of practice guidelines for assessment of the vegetative and minimally conscious states. J Head Trauma Rehab. 1997;12:79.
Gierer A. Brain, mind and limitations of a scientific theory of hyman consciousness. Bioessays. 2008;30(5):499-505.
Gilbert T.T., Wagner M.R., Halukurike V., et al. Use of bispectral electroencephalogram monitoring to assess neurologic status in unsedated, critically ill patients. Crit Care Med. 2001;29:1996.
Gillies J.D., Seshia S.S. Vegetative state following coma in childhood: Evolution and outcome. Dev Med Child Neurol. 1980;22:642.
Gloor P.. Hans Berger on the Electroencephalogram of man. The Fourteen Original Reports on the Human Electroencephalogram. Electroencephalogr Clin Neurophysical. 1969(Suppl 28):1-350.
Goetting M.G., Tiznado-Garcia E., Bakdash T.F. Intussusception encephalopathy: An underrecognized cause of coma in children. Pediatr Neurol. 1990;6:419.
Gohlich-Ratmann G., Wallot M., Baethmann M., et al. Acute cerebellitis with near-fatal cerebellar swelling and benign outcome under conservative treatment with high dose steroids. Eur J Paediatr Neurol. 1988;2:157.
Goodwin S.R., Friedman W.A., Bellefleur M. Is it time to use evoked potentials to predict outcome in comatose children and adults? Crit Care Med. 1991;19:518.
Green S.A., Jefferson I.G., Baum J.D. Cerebral edema complicating diabetic ketoacidosis. Dev Med Child Neurol. 1990;32:633.
Grindstaff R.J., Tobias J.D. Applications of Bispectral Index monitoring in the pediatric intensive care unit. J Intensive Care Med. 2004;19:111.
Guerit J.M., Amantini A., Amodio P., et al. Consensus on the use of neurophysiological tests in the intensive care unit (ICU): electroencephalogram (EEG), evoked potentials (EP), and electroneuromyography (ENMG). Neurophysiol Clin. 2009;39:71-83.
Habre W., Caffisch M., Chaves-Vischer V., et al. Locked-in syndrome in an adolescent patient with pneumococcal meningitis. Neuropediatrics. 1996;27:323.
Hahn J.S., Berquist W., Alcorn D.M., et al. Wernicke encephalopathy and beriberi during total parenteral nutrition attributable to multivitamin infusion shortage. Pediatrics. 1998;101:E10.
Hahn Y.S., Chyung C., Barthel M.J., et al. Head injuries in children under 36 months of age. Childs Nerv Syst. 1988;4:34.
Haley S.M., Coster W.J., Ludlow L.M., et al. Pediatric evaluation of disability inventory: Development, standardization and administration manual, Version 1.0. Boston: Trustees of Boston University, Center for Rehabilitation Effectiveness; 1992.
Hall K., Cope N., Rappaport M. Glasgow outcome scale and disability rating scale: Comparative usefulness in following recovery in traumatic head injury. Arch Phys Med Rehabil. 1985;66:35.
Hannay H.J., Sherer M. Assessment of outcome from head injury. In: Narayan R.K., Wilberger J.E.Jr, Povlishock J.T., editors. Neurotrauma. New York: McGraw-Hill, 1996.
He B.J., Raichle M.E. The fMRI signal, slow cortical potential and consciousness. Trends Cogn Sci. 2009;13(7):302-309.
He B.J., Snyder A., Zempel J.M., et al. Electrophysiological correlates of the brain’s intrinsic large-scale functional architecture. PNAS. 2008;105(41):16039-16044.
Heindl U.T., Laub M.C. Outcome of persistent vegetative state following hypoxic or traumatic brain injury in children and adolescents. Neuropediatrics. 1996;27:94.
Herskowitz J. Neurologic presentations of panic disorder in childhood and adolescence. Dev Med Child Neurol. 1986;28:617.
Hill L. The physiology and pathology of the cerebral circulation in experimental research. London: J&A Churchill; 1896.
Hlatky R., Valadka A.B., Goodman J.C., et al. Patterns of energy substrates during ischemia measured in the brain by microdialysis. J Neurotrauma. 2004;21:894.
Hobson J. Sleep is of the brain, by the brain and for the brain. Nature. 2005;437:1254-1256.
Hoesch R.E., Koenig M.A., Geocadin R.G. Coma after global ischemic brain injury: pathophysiology and emerging therapies. Crit Care Clin. 2008;24:25-44. vii–viii
Holshouser B.A., Ashwal S., Luh G.Y., et al. Proton MR spectroscopy after acute central nervous system injury: Outcome prediction in neonates, infants and children. Radiology. 1997;202:487.
Holshouser B.A., Ashwal S., Shu S., et al. Proton MR spectroscopy in children with acute brain injury: Comparison of short and long echo time acquisitions. J Magn Reson Imaging. 2000;11:9.
Howell D.A. Upper brainstem compression and foraminal impaction with intracranial space-occupying lesions and brain swelling. Brain. 1959;82:525.
Hsia S.H., Wu C.T., Wang H.S., et al. The use of Bispectral Index to monitor unconscious children. Pediatr Neurol. 2004;31:20.
Huybrechts K.F., Caro J.J. The Barthel Index and modified Rankin Scale as prognostic tools for long-term outcomes after stroke: a qualitative review of the literature. Curr Med Res Opin. 2007;23(7):1627-1636.
Iyer L.V., Haley S.M., Watkins M.P., et al. Establishing minimal clinically important differences for scores on the Pediatric Evaluation of Disability Inventory for Inpatient Rehabilitation. Phys Ther. 2003;83:888.
James W. The principles of psychology. London: MacMillan; 1890.
Jennett B., Bond M. Assessment of outcome after severe brain damage, a practical scale. Lancet. 1975;1:480.
Jennett B., Teasdale G., Brookman R., et al. Predicting outcome in individual patients after severe head injury. Lancet. 1976;1:1031.
Jennett B., Teasdale G., Brookman R., et al. Prognosis of patients with severe head injury. Neurosurgery. 1979;4:283.
Jennett B.A. quarter century of the vegetative state: An international perspective. J Head Trauma Rehabil. 1997;12:1.
Jöhnk K., Kuhtz-Buschbeck J.P., Stolze H., et al. Assessment of sensorimotor functions after traumatic brain injury (TBI) in childhood – Methodological aspects. Restor Neurol Neurosci. 1999;14(2–3):143.
Johnson A.R., DeMatt E., Salorio C.F. Predictors of outcome following acquired brain injury in children. Dev Disabil Res Rev. 2009;15:124-132.
Johnston B., Seshia S.S. Prediction of outcome in non-traumatic coma in childhood. Acta Neurol Scand. 1984;69:417.
Jonsson C.A., Horneman G., Emanuelson I. Neuropsychological progress during 14 years after severe traumatic brain injury in childhood and adolescence. Brain Inj. 2004;18:921.
Karantanas A.H., Hadjigeorgiou G.M., Paterakis K., et al. Contribution of MRI and MR angiography in early diagnosis of brain death. Eur Radiol. 2002;12:2710.
Keenan H.T., Runyan D.K., Marshall S.W., et al. A population-based comparison of clinical and outcome characteristics of young children with serious inflicted and noninflicted traumatic brain injury. Pediatrics. 2004;114:633.
Keren O., Ben-Dror S., Stern M.J., et al. Event-related potentials as an index of cognitive function during recovery from severe closed head injury. J Head Trauma Rehabil. 1998;13:15.
Kernohan J.W., Woltman H.W. Incisura of the crus due to contralateral brain tumor. Arch Neurol Psychiatry. 1929;21:274.
Khader P., Schicke T., Roder B., et al. On the relationship between slow cortical potentials and BOLD signal changes in humans. Int J Psychophysiol. 2008;67:252-261.
Kiening K.L., Hartl R., Unterberg A.W., et al. Brain tissue PO2-monitoring in comatose patients: Implications for therapy. Neurol Res. 1997;19:233.
Kirkham F.J., Newton C.R., Whitehouse W. Paediatric coma scales. Dev Med Child Neurol. 2008;50:267-274.
Kothari D.H., Haley S.M., Gill-Body K.M., et al. Measuring functional change in children with acquired brain injury (ABI): Comparison of generic and ABI-specific scales using the Pediatric Evaluation of Disability Inventory (PEDI). Phys Ther. 2003;83:776.
Kriel R.L., Jrach L.E., Jones-Saese C. Outcome of children with prolonged unconsciousness and vegetative states. Pediatr Neurol. 1993;9:362.
Kurth C.D., Steven J.M., Nicolson S.C. Cerebral oxygenation during pediatric cardiac surgery using deep hypothermic circulatory arrest. Anesthesiology. 1995;82:74.
Lange-Cossack H., Riebel U., Grumme T., et al. Possibilities and limitation of rehabilitation after traumatic apallic syndrome in children and adolescents. Neuropediatrics. 1981;12:338.
Larsen P.D., Gupta N.C., Lefkowitz D.M., et al. PET of infants in persistent vegetative state. Pediatr Neurol. 1993;9:323.
Lescot T., Galanaud D., Puybasset L. Exploring altered consciousness states by magnetic resonance imaging in brain injury. Ann N Y Acad Sci. 2009;1157:71-80.
Levin H.S. A guide to clinical neuropsychological testing. Arch Neurol. 1994;51:854.
Lin K.L., Wang H.S., Chou M.L., et al. Role of cavum septum pellucidum in akinetic mutism of hydrocephalic children. Pediatr Neurol. 1997;16:156.
Llinas R., Ribary U., Contreras D., et al. The neuronal basis for consciousness. Phil Trans R Soc Lond B. 1998;353:1841.
Llinás R.R., Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol. 2006;95(6):3297-3308.
Lippert M.T., Steudel T., Ohi F., et al. Coupling of neural activity and fMRI-Bold in the motion area MT. Magn Reson Imaging. 2010;28(8):1087.
Litscher G. Middle latency auditory evoked potentials in intensive care patients and normal controls. Int J Neurosci. 1995;83:253.
Logi F., Fischer C., Murri L., et al. The prognostic value of evoked responses from primary somatosensory and auditory cortex in comatose patients. Clin Neurophysiol. 2003;114:1615.
Logothetis N.K. The neural basis of the blood-oxygen-level dependent functional magnetic resonance imaging signal. Philos Trans R Soc Lond B. 2002;357:103-1037.
Lundervold A. On consciousness, resting state fMRI, and neurodynamics. Nonlinear Biomed Phys. 2010;4(Suppl 1):59.
Mahoney Fl, Barthel D.W. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:2.
Mahoney W.J., D’Souza B.J., Freeman J.M. Surprising good outcome of prolonged coma after severe head injury. Ann Neurol. 1981;10:286.
Mahoney W.J., D’Souza B.J., Haller J.A., et al. Long-term outcome of children with severe head trauma and prolonged coma. Pediatrics. 1983;71:756.
Mandel R., Martinot A., Delepoulle F., et al. Prediction of outcome after hypoxic-ischemic encephalopathy: A prospective clinical and electrophysiologic study. J Pediatr. 2002;141:45.
Massagli T.L., Michaud L.J., Rivara F.P. Association between injury indices and outcome after severe traumatic brain injury in children. Arch Phys Med Rehabil. 1996;77:125.
McCall S., Vilensky J., Gilman S., et al. The relationship between encphalitis lethargica and influenza: A critical analysis. J Neuro Virol. 2008;14(3):177.
McKinney J.S.3rd, Giacino J.T. Diagnostic accuracy in disorders of consciousness. Neurologist. 2008;14:340.
McNealy D.E., Plum F. Brainstem dysfunction with supratentorial mass lesions. Arch Neurol. 1962;7:26.
Medical Research Council Brain Injuries Committee. A glossary of psychological terms commonly used in cases of head injury. London: HM Stationery Office; 1941. Medical Research Council War Memorandum Number 4
Melton A.F., Appleton R.E., Gardner-Medwin D., et al. Encephalitis lethargica-like illness in a five-year-old. Dev Med Child Neurol. 1991;33:158.
Mesulam M.M. Principles of Behavioral Neurology. Philadelphia: FA Davos; 1985.
Mewasingh L.D., Christophe C., Fonteyne C., et al. Predictive value of electrophysiology in children with hypoxic coma. Pediatr Neurol. 2003;28:178.
Meyer A. Herniation of the Brain. Arch Neurol Psychiatry. 1920;4:387.
Meyer P.G., Ducrocq S., Carli P. Pediatric neurologic emergencies. Curr Opin Crit Care. 2001;7:81.
Michelson D., Ashwal S. Evaluation of coma and brain death. Semin Pediatr Neurol. 2004;11:103.
Min B. A thalamic reticular networking model of consciousness. Theor Biol Med Model. 2010;7:10.
Misra U.K., Kalita J. Neurophysiological studies in herpes simplex encephalitis. Electromyogr Clin Neurophysiol. 1998;38:177.
Mohamed W.. The Edwin Smith Surgical Papyrus: Neuroscience in Ancient Egypt. IBRO History of Neuroscience. 2008.http://www.ibro.info/Pub/Pub_Main_Display.Asp?LC_Docs_ID-3199. Accessed 03-07-2010
Montgomery E.B., Wall M., Henderson V.W. Principles of neurologic diagnosis. Boston/Toronto: Little, Brown; 1986.
Monti M.M., Coleman M.R., Owen A.M. Neuroimaging and the vegetative state: resolving the behavioral assessment dilemma? Ann N Y Acad Sci. 2009;1157:81-89.
Monti M.M., Laureys S., Owen A.M. The vegetative state. BMJ. 2010;341:c3765.
Moruzzi G. The sleep-waking cycle. Rev Physiol. 1972;64:1.
Moruzzi G., Magoun H.W. Brainstem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455.
Multi-Society Task Force on Persistent Vegetative State. Medical aspects of the persistent vegetative state. N Engl J Med. 1994;330:1499. 1572
Nagdyman N., Fleck T., Barth S., et al. Relation of cerebral tissue oxygenation index to central venous oxygen saturation in children. Intensive Care Med. 2004;30:468.
Nakagawa T.A., Ashwal S., Mathur M., et al. Guidelines for the determination of brain death in infants and children: an update of the 1987 Task Force recommendations. Crit Care Med. 2011. in press
Narayan R.K., Greenberg R.P., Miller J.D., et al. Improved confidence of outcome prediction in severe head injury. A comparative analysis of the clinical examination, multimodality evoked potentials, CT scanning, and intracranial pressure. J Neurosurg. 1981;54:751.
Nautiyal A., Singh S., Alaimo D.J. Wernicke encephalopathy: An emerging trend after bariatric surgery. Am J Med. 2004;10:804.
Nayana Prabha P.C., Nalini P., Tiroumouronugane Serane V. Role of Glasgow Coma Scale in pediatric nontraumatic coma. Indian Pediatr. 2003;40:620.
Negrao B., Viljoen M. Neural correlates of consciousness. Afr J Psychiatry. 2009:265-269. November
Newman J. Thalamic contributions to attention and consciousness. Conscious Cogn. 1995;4(2):172-193.
Neylan T.C. Physiology of arousal: Moruzzi and Magoun’s ascending reticular activating system. J Neuropsychiatry Clin Neurosci. 1995;7:250.
Oliver W.J., Shope T.C., Kuhns L.R. Fatal lumbar puncture: Fact versus fiction – an approach to a clinical dilemma. Pediatrics. 2003;112:174.
Ottenbacher K.J., Msall M.E., Lyon N., et al. Measuring developmental and functional status in children with disabilities. Dev Med Child Neurol. 1999;41:186.
Ottenbacher K.J., Msall M.E., Lyon N., et al. Functional assessment and care of children with neurodevelopmental disabilities. Am J Phys Med Rehabil. 2000;29:114.
Owen A.M. Disorders of consciousness. Ann N Y Acad Sci. 2008;1124:225-238.
Owen A.M., Coleman M.R. Functional MRI in disorders of consciousness: advantages and limitations. Curr Opin Neurol. 2007;20:632-637.
Owen A.M., Coleman M.R. Detecting awareness in the vegetative state. Ann N Y Acad Sci. 2008;1129:130-138.
Pearce J.M.S. James Collier (1870-1935) and Uncal herniation. J Neurol Neurosurg Psychiatry. 2006;77:883.
Pearce J.M.S. Kernohan’s Notch. Eur Neurol. 2006;55:230.
Perlis M., et al. Neurobiologic Mechanisms in Chronic Insomnia. Sleep Med Clin. 2009;4:549-558.
Pihko H., Saarinen U., Paetau A. Wernicke encephalopathy: A preventable cause of death. Report of 2 children with malignant disease. Pediatr Neurol. 1989;4:237.
Plum F., Posner J.B. The diagnosis of stupor and coma, ed 3. Philadelphia: FA Davis; 1982. revised
Posner J.B., et al. Plum and Posner’s Diagnosis of Stupor and Coma, ed 4. Oxford: Oxford University Press; 2007.
Parvizi J., Damasio A. Neuroanatomical correlates of brainstem coma. Brain. 2003;126:1524-1536.
Pinault D. The thalamic reticular nucleus: Structure, function and concept. Brain Res Brain Res Rev. 2004;46(1):1-31.
Raichle M.E. Two views of brain function. Trends Cogn Sci. 2010;14:180-190.
Raichle M.E., MacLeod A.M., Snyder A.Z., et al. A default mode of brain function. PNAS. 2001;98:676-682.
Raichle M.E., Mintun M.A. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449-476.
Raichle M.E. A Paradigm Shift in Functional Brain Imaging. The Journal of Neuroscience. 2009;29(41):12729.
Raimondi A.J., Hirschauer J. Head injury in the infant and toddler. Coma scoring and outcome scale. Child Brain. 1984;11:12.
Raimondi A.J., Tomita T. Hydrocephalus and infratentorial tumors. Incidence, clinical picture and treatment. J Neurosurg. 1981;55:174.
Rappaport M., Hall K.M., Hopkins K., et al. Disability rating scale for severe head trauma: Coma to community. Arch Phys Med Rehabil. 1982;63:118.
Ravid S., Diamond A.S., Eviatar L. Coma as an acute presentation of adrenoleukodystrophy. Pediatr Neurol. 2000;22:237.
Reich J.B., Sierra J., Camp W., et al. Magnetic resonance imaging measurement and clinical changes accompanying transtentorial and foramen magnum brain herniation. Ann Neurol. 1993;33:159.
Reilly P.L., Simpson D.A., Sprod R., et al. Assessing the conscious level in infants and young children: A pediatric version of the Glasgow Coma Scale. Child Nerv Syst. 1988;4:30.
Rennick G., Shann F., deCampo J. Cerebral herniation during bacterial meningitis in children. BMJ. 1993;306:953.
Robertson C.M., Joffe A.R., Moore A.J., et al. Neurodevelopmental outcome of young pediatric intensive care survivors of serious brain injury. Pediatr Crit Care Med. 2002;3:345.
Rohde V., Irle S., Hassler W.E. Prediction of the post-comatose motor function by motor evoked potentials obtained in the acute phase of traumatic and non-traumatic coma. Acta Neurochir. 1999;141:841.
Ropper A.H. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. N Engl J Med. 1986;314:953.
Ropper A.H. Syndrome of transtentorial herniation: Is vertical displacement necessary? J Neurol Neurosurg Psychiatry. 1993;56:932.
Ross D.A., Olsen W.L., Ross A.M., et al. Brain shift, level of consciousness and restoration of consciousness in patients with acute intracranial hematoma. J Neurosurg. 1989;71:498.
Roulet Perez E., Maeder P., Cotting J., et al. Acute fatal parainfectious cerebellar swelling in two children. A rare or an overlooked situation? Neuropediatrics. 1993;24:346.
Rubenstein J.S. Initial management of coma and altered consciousness in the pediatric patient. Pediatr Rev. 1994;15:204.
Ruf B., Heckmann M., Schroth I., et al. Early decompressive craniectomy and duraplasty for refractory intracranial hypertension in children: Results of a pilot study. Crit Care. 2003;7:409.
Safar P.J., Kochanek P.M. Therapeutic hypothermia after cardiac arrest. N Engl J Med. 2002;346:612.
Salas-Salvado J., Garcia-Lorda P., Cuatrecasas G., et al. Wernicke’s syndrome after bariatric surgery. Clin Nutr. 2000;5:371.
Sanchez G., Burridge O.A. Decision making in head injury management in the Edwin Smith papyrus. Neurosurg Focus. 23(1), 2007.
Saper C., Chou T., Scammell T. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726-731.
Saper C., Scammell T., Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257-1263.
Scheuer M.L., Wilson S.B. Data analysis for continuous EEG monitoring in the ICU: Seeing the forest and the trees. J Clin Neurophysiol. 2004;21:353.
Schiff N.D., Giacino J.T., Fins J.J. Deep brain stimulation, neuroethics, and the minimally conscious state: moving beyond proof of principle. Arch Neurol. 2009;66:697-702.
Schnakers C., Giacino J., Kalmar K., et al. Does the FOUR Score Correctly Diagnose the Vegetative and Minimally Conscious States? In Letters and Replies. Ann Neurol. 2006;60(6):744.
Schwartz J., Roth T. Neurophysiology of Sleep and Wakefulness: Basic Science and Clinical Implications. Current Neuropharmacology. 2008;6:367-378.
Scott J.S., Ockey R.R., Holmes G.E., et al. Autonomic dysfunction associated with locked-in syndrome in a child. Am J Phys Med Rehabil. 1997;76:200.
Searle J.R. Consciousness. Annu Rev Neurosci. 2000;23:557.
Seshia S.S., Johnston B., Kasian G. Non-traumatic coma in childhood: Clinical variables in prediction of outcome. Dev Med Child Neurol. 1983;25:493.
Shetty A.K., Dessell B.C., Craver R.D., et al. Fatal cerebral herniation after lumbar puncture in a patient with a normal computed tomography scan. Pediatrics. 1999;103:1284.
Shewman D.A. Coma prognosis in children. Part I: Definition and methodological challenges. J Clin Neurophysiol. 2000;17:457.
Shewman D.A. Coma prognosis in children. Part II: Clinical application. J Clin Neurophysiol. 2000;17:467.
Shu S.K., Ashwal S., Holshouser B.A., et al. Prognostic value of 1H-MRS in perinatal CNS insults. Pediatr Neurol. 1997;17:309.
Simonetti F., Pergami P., Ceroni M., et al. About the original description of cerebellar tonsil herniation by Pierre Marie. J Neurol Neurosurg Psychiatry. 1997;63(3):412.
Simpson D.A., Cockington R.A., Heniej A., et al. Head injuries in infants and young children: The value of the pediatric coma scale. Childs Nerv Syst. 1991;7:183.
Simpson D.A., Reilly P.L. Pediatric coma scale [Letter]. Lancet. 1982;2:450.
Smith E., Madsen J. Cerebral pathophysiology and critical care neurology: Basic hemodynamic principals, cerebral perfusion and intracranial pressure. Semin Pediatr Neurol. 2004;11:89.
Smith E., Madsen J. Neurosurgical aspects of critical care neurology. Semin Pediatr Neurol. 2004;11:169.
Sperry R.W. Neurology and the mind-brain problem. Am Scientist. 1952;40:291.
Stead L., Wijdicks E., Bhagra A., et al. Validation of a New Coma Scale, The FOUR Score in the Emergency Department. Neurocrit Care. 2009;10:50-54.
Steriade M. Synchronized activities of coupled oscillators in the cerebral cortex and thalamus at different levels of vigilance. CerebCortex. 1997;7(6):583-604.
Strauss D.J., Ashwal S., Day S.M., et al. Life expectancy of children in vegetative and minimally conscious states. Pediatr Neurol. 2000;23:312.
Task Force for the Determination of Brain Death in Children. Guidelines for the determination of brain death in children. Pediatrics. 1987;80:298.
Taylor A., Butt W., Rosenfeld J., et al. A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Childs Nerv Syst. 2001;17:154.
Taylor M.R., Farrell E.J. Comparison of the prognostic utility of VEPs and SEPs in comatose children. Pediatr Neurol. 1989;5:145.
Teasdale G., Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81.
Teasdale G., Jennett B., Murray L., et al. Glasgow coma scale: To sum or not to sum. Lancet. 1983;2:678.
ter Horst H.J., Sommer C., Bergman K.A., et al. Prognostic significance of amplitude-integrated EEG during the first 72 hours after birth in severely asphyxiated neonates. Pediatr Res. 2004;55:1026.
Tolias C.M., Richards D.A., Bowery N.G. Extracellular glutamate in the brains of children with severe head injuries: A pilot microdialysis study. Childs Nerv Syst. 2002;18:368.
Tong K.A., Ashwal S., Holshouser B.A., et al. Hemorrhagic shearing lesions in children and adolescents with post-traumatic diffuse axonal injury: Improved detection and initial results. Radiology. 2003;22:332.
Tong K.A., Ashwal S., Holshouser B.A., et al. Diffuse axonal injury in children: Clinical correlation with hemorrhagic lesions. Ann Neurol. 2004;56:36.
Tononi G. Consciousness as integrated information: a provisional manifesto. Biol Bull. 2008;215:216-242.
Tsao C.Y., Ellingson R.J. Normal somatosensory evoked potentials in a child in persistent coma. Pediatr Neurol. 1989;5:257.
Van der Werf Y.D., Witter M.P., Groenewegen H.J. The interlaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness, Brain Research Reviews. 2002;39:107-140.
Van de Voorde P., Sabbe M., Rizopoulos D., et al. Assessing the level of consciousness in children: A plea for the Glasgow Coma Motor subscore. Resuscitation. 2008;76:175-179.
Vannucci R.C., Wasiewski W.W. Diagnosis and management of coma in children. In: Pellock J.M., Meyer E.D., editors. Neurologic emergencies in infancy and childhood. ed 2. London: Butterworth-Heinemann; 1993:103-122.
Van Santbrink H., Maas A.I.R., Avezaat C.J.J. Continuous monitoring of partial pressure of brain tissue oxygen in patients with severe head injury. Neurosurgery. 1996;38:21.
Vasconcelos M.M., Silva K.P., Vidal G., et al. Early diagnosis of pediatric Wernicke’s encephalopathy. Pediatr Neurol. 1999;4:289.
von Economo C. Sleep as a Problem of Localization. J Nerv Ment Dis. 1930;7(3):1-5.
Wijdicks E. Clinical Scales for Comatose Patients: The Glasgow Coma Scale in Historical Context and the New FOUR Score. Rev Neurol Dis. 2006;3(3):109-117.
Wijdicks E., Bamlet W., Maramattom B., et al. Validation of a new Coma Scale: The FOUR Score. Ann Neurol. 2005;58(4):584-593.
Wijdicks E.F., Miller G.M. MR imaging of progressive downward herniation of the diencephalons. Neurology. 1997;48:1456.
Wohlrab G., Boltshauser E., Schmitt B. Neurological outcome in comatose children with bilateral loss of cortical somatosensory evoked potentials. Neuropediatrics. 2001;32:271.
Wolf C., Wijdicks E., Bamlet W., et al. Further Validation of the FOUR Score Coma Scale by Intensive Care Nurses. Mayo Clinic Proc. 2007;82(4):435-438.
Wong C.P., Forsyth R.J., Kelly T.P., et al. Incidence, aetiology and outcome of non-traumatic coma: A population based study. Arch Dis Child. 2001;84:193.
Ylvisaker M., Chorazy A.J.L., Cohen S.B., et al. Rehabilitation assessment following head injury in children. In Rosenthal M., Griffith E.R., Bond M.R., editors: Rehabilitation of the adult and child with traumatic brain injury, ed 2, Philadelphia: FA Davis, 1990.
Young G.B. The EEG in coma. J Clin Neurophysiol. 2000;17:473.
Young G.B. Coma. Ann N Y Acad Sci. 2009;1157:32-47.
Young G.B., Ropper A.H., Bolton C.F. Coma and impaired consciousness: A clinical perspective. New York: McGraw-Hill; 1998.
Zasler N.D., Kreutzer J.S., Taylor D.A. Coma stimulation and coma recovery: A critical review. Neurorehabilitation. 1991;1:33.
Zeman A. Persistent vegetative state. Lancet. 1997;350:795.
Zeman A. Consciousness. Brain. 2001;124:1263.
Zentner J., Rohde V. The prognostic value of somatosensory and motor evoked potentials in comatose patients. Neurosurgery. 1992;31:429.
Zentner J., Rohde V. SEP and MEP in comatose patients. Neurol Res. 1994;16:89.
Zimmerman A.A., Burrows F.A., Jones R.A., et al. The limits of detectable cerebral perfusion by transcranial Doppler sonography in neonates undergoing deep hypothermic low-flow cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1997;114:594.
Ziviani J., Ottenbacher K.J., Shephard K., et al. Concurrent validity of the Functional Independence Measure for Children (WeeFIM) and the Pediatric Evaluation of Disabilities Inventory in children with developmental disabilities and acquired brain injuries. Phys Occup Ther Pediatr. 2001;22:91.