43 Imaging for Chronic Spinal Pain
Back pain remains the most common and most expensive cause of work disability in the United States.1 Recent data suggest that approximately 26% of U.S. citizens have experienced low back pain, and 14% have experienced neck pain, within the past 3 months.2 The course of acute or persistent low back pain is not as optimistic as has been generally conveyed.3 Von Korff and colleagues studied patients with a recent (within 6 months) history of low back pain and found that at 6 months, 76% of patients either had no pain (21%) or mild pain and low disability, but that 14% had high disability with moderate-to-severe limitation of function.4 A British study of acute low back pain noted full recovery in only 21% and 25% at 3 and 12 months, respectively.5 A Dutch study found that 70% of acute low back pain patients still had pain at 4 weeks, with a median time to recovery of 7 weeks; however, back pain tended to persist with residual prevalence of 35% at 12 weeks and 10% at 1 year, and 76% sustained a median number of two relapses of pain within that 1 year.6 An Australian study was more encouraging; at 3 months 49% to 67% (two study groups) had recovered, at 12 months 56% to 71% had recovered, with a relapse rate within that 1 year of 7% to 27%.7 Back pain accounted for more than 890 million physician office visits in the United States in 2002; however, the back pain proportion of total physician visits has remained stable since the early 1990s.2 Despite this, utilization of imaging has increased dramatically; U.S. Medicare use of lumbar magnetic resonance imaging (MRI) rose 307% in the 12-year interval 1994 to 2005.8 Imaging utilization is often unreasoned. There are large variations in rates of imaging across the United States; from one-third to two-thirds of spine computed tomography (CT) and MRI studies are inappropriate when measured against established guidelines.8 The relatively unregulated U.S. medical marketplace has served as a laboratory for the mass consumption of imaging technology, with no detectable benefit in patient outcomes. Despite incurring the greatest per capita expenditures for the diagnosis and treatment of back pain, including dramatically increased use of imaging, the United States continues to have the highest rate of back pain-related work disability of all industrialized societies.
It is well established that there is no role for imaging early in the course of a low back or limb pain syndrome. In a recent meta-analysis in Lancet, Chou and coworkers identified six randomized controlled trials evaluating the early use of imaging versus clinically directed care.9 In the pooled data, there were no significant outcome differences in pain or function in imaged versus nonimage patients in the short- (3 months) or long-term (6 to 12 months). These data apply primarily to acute or subacute back pain in primary care settings, and encompass radiographs and advanced imaging. Multiple studies have included economic analyses, which make it clear that radiographs obtained early in the course of a back pain syndrome are not cost effective; a study by Jarvik and associates suggested that radiographs performed on an initial visit for acute back pain in the absence of signs of systemic disease will ultimately cost $2000 (1982 U.S. dollars) to alleviate a single day of pain.1
A study by Carragee10 reinforced the futility of early advanced imaging. This 5-year prospective observational study obtained baseline MRI examinations on a large cadre of asymptomatic patients at risk for back pain due to strenuous vocations. These patients were then contacted periodically; when a subset ultimately presented to their physician with back or leg pain, a second lumbar MRI was performed. The study noted that fewer than 5% of the MRI scans obtained at the time of acute presentation showed clinically relevant new findings; the vast majority of the “abnormalities” were present on the imaging performed when the patient was asymptomatic. Only direct evidence of neural compression in patients with corresponding radicular pain syndromes was useful. Also of note, psychosocial factors were the primary predictors of the degree of disability, not the morphology identified on imaging.
Early Imaging Recommendations
Based on such data, there is broad international consensus on the lack of efficacy of imaging in acute pain syndromes of spinal origin. The American College of Radiology11,12 considers that imaging is inappropriate in acute low back pain unless there are complicating (“red flag”) features including the following:
Similarly, the Agency for Healthcare Research and Policy (U.S.) guidelines advises no imaging in patients younger than 50 years of age in the absence of systemic disease, or until pain persists for at least 4 weeks.13 The Australian National Health and Medical Research Council recommends against imaging in nonspecific low back pain of less than 12 weeks’ duration in the absence of red flag features suggesting underlying systemic disease.14 The American College of Physicians and the American Pain Society released a joint guideline in 2007 stating that imaging should not be obtained in patients with nonspecific low back pain unless there is a severe or progressive neurologic deficit, or when serious underlying systemic disease is suspected.15 Furthermore, patients with signs or symptoms of radiculopathy or spinal stenosis should be imaged with MRI (preferred) or CT only if they are candidates for surgery or epidural steroid injection.15
The primary role of imaging in the back pain patient is to aid in the detection of systemic disease that is causal of back or back-related leg pain. In essence, imaging is undertaken to detect neoplasm, infection, or manifestations of unsuspected traumatic injury. In a patient presenting with back pain to a primary care physician, these conditions are highly uncommon. In this population, only 0.7% of patients will have metastatic neoplasm, 0.01% spine infection, 0.3% ankylosing spondylitis, and 4% will have osteoporotic compression fractures.1 The differential diagnosis of back pain, and the relative frequency of causal conditions, are displayed in Table 43-1.1 The imperfect sensitivity and specificity of all imaging modalities makes diagnostic accuracy problematic, given the low pretest probability of systemic disease. According to Jarvik and Deyo, there are three essential questions that must be answered for the patient with back pain: (1) is there evidence of underlying systemic disease? (2) Is there neurologic impairment requiring surgical intervention? (3) Are social/psychological factors exacerbating the pain? Imaging may help to identify systemic disease and anatomic neural impingement, but its role should not be overstated.1 As Jarvik and colleagues note, depression is a more significant predictor of new low back pain than MRI imaging findings.16
| Mechanical Low Back or Leg Pain (97%)† | Nonmechanical Spinal Conditions (−1%) | Visceral Disease (2%) |
|---|---|---|
| Lumbar strain or sprain (70%)‡ | Neoplasia (0.7%) | Pelvic organ involvement |
| Degenerative processes of disc and facets (usually related to age) (10%) | Multiple myeloma | Prostatitis |
| Herniated disc (4%) | Metastatic carcinoma | Endometriosis |
| Spinal stenosis (3%) | Lymphoma and leukemia | Chronic pelvic inflammatory disease |
| Osteoporotic compression fracture (4%) | Spinal cord tumors | Renal involvement |
| Spondylolisthesis (2%) | Retroperitoneal tumors | Nephrolithiasis |
| Traumatic fractures (<1%) | Primary vertebral tumors | Pyelonephritis |
| Congenital disease (<1%) | Infection (0.01%) | Perinephric abscess |
| Severe kyphosis | Osteomyelitis | Aortic aneurysm |
| Severe scoliosis | Septic discitis | Gastrointestinal involvement |
| Transitional vertebrae | Paraspinous abscess | Pancreatitis |
| Spondylolysis§ | Epidural abscess | Cholecystitis |
| Internal disc disruption or discogenic back pain¶ | Shingles | Penetrating ulcer |
| Presumed instability∗∗ | Inflammatory arthritis (often HLA-B27 associated) (0.3%) | |
| Ankylosing spondylitis | ||
| Psoriatic spondylitis | ||
| Reiter syndrome | ||
| Inflammatory bowel disease | ||
| Scheuermann disease (osteochondrosis) | ||
| Paget disease |
∗ Diagnoses in italics are often associated with neurogenic leg pain. Figures in parentheses indicate estimated percentage of patients with these conditions among all adult patients with signs and symptoms of low back pain. Percentages may vary substantially according to demographic characteristics or referral patterns in a practice. For example, spinal stenosis and osteoporosis will be more common in geriatric practices and spinal infection will be more common in injection drug users.
† The term mechanical is used here to designate an anatomic or functional abnormality without an underlying malignant, neoplastic, or inflammatory disease.
‡ Strain and sprain are nonspecific terms with no pathoanatomic confirmation. Idiopathic low back pain may be a preferable term.
§ Because spondylolysis is equally common in asymptomatic persons and those with low back pain, its etiologic role remains ambiguous.
¶ Internal disc disruption is diagnosed by provocative discography (injection of contrast material into a degenerative disc, with assessment of pain at the time of injection). However, discography often generates pain in asymptomatic adults, and many patients with positive discogram results improve spontaneously. Thus, the significance and appropriate management of this disorder remain unclear. Discogenic back pain is often used synonymously with internal disc disruption.
∗∗ Presumed instability is loosely defined as >10 degrees of angulation or 4 mm of vertebral displacement on lateral flexion and extension radiographs. However, diagnostic criteria, natural history, and surgical indications remain controversial.
Data obtained from Deyo,4 Hart et al.,6 Deyo et al.,7 and Deyo et al.8 Reproduced with permission Deyo RA, Weinstein JN: Low back pain. N Engl J Med 2001;344:363-370. Copyright© 2001. Massachusetts Medical Society. All rights reserved.
Risk/Benefit Analysis
There are, however, risks involved in imaging. These include the labeling effect, radiation exposure, monetary cost, and the provocation of intervention. The labeling effect refers to the inevitable identification of degenerative phenomenon on any spine imaging test. Degenerative findings on spine imaging are almost always inconsequential. If this is not made very clear to patients, they may identify themselves as suffering from a degenerative process from which there is no ultimate recovery. This can lead to fear avoidance behaviors, deconditioning, and depression. The pain practitioner is obligated to educate the patient that degenerative findings seen on imaging have little if any prognostic value, and actively contradict common misconceptions regarding back pain. A recent Cochrane review concluded that intensive patient education is effective in patients with acute and subacute low back pain; its value in chronic pain patients is less clear.17 The Australian media studies of Buchbinder also document the effectiveness of carefully planned educational efforts in changing patient and physician attitudes and reducing disability related to back pain.18
Radiation exposure from radiographs or CT must also be carefully considered. The effective radiation dose (biologic effect) is measured by the Sievert (Sv). The average natural background exposure is approximately 3.0 mSv per year.19 A three- view lumbar spine radiographic series exposes the patient to approximately 1.5 mSv; lumbar spine CT carries an exposure of approximate 6 mSv. An abdominal and pelvic CT delivers an average of 14 mSv; a technetium bone scan results in an average of 6.3 mSv exposure. Frontal and lateral chest radiographs, by contrast, expose the patient to 0.1 mSv.19 Radiation exposure is cumulative; repeated radiographic or CT examinations without clear expectation of patient benefit through improved decision-making cannot be condoned.
Imaging is costly. In the United Kingdom, imaging is known to comprise approximately 5% of the direct medical costs related to back pain.20 This is likely significantly higher in the less regulated U.S. marketplace. The 2009 Medicare reimbursements for lumbar spine imaging in the United States are: lumbar radiographs, $36; lumbar spine CT (noncontrast), $241; CT myelography, $525; MRI (noncontrast), $402, and bone scan with SPECT, $239.21 Nominal fees may be three to five times Medicare reimbursements. These costs directly affect our patients and our society and should be incurred only through reasoned decision-making.
Finally, imaging is likely to provoke intervention. Jarvik noted that early MRI imaging of the spine leads to increased surgical interventions despite equivalent pain and disability profiles when compared with nonimaged patients.22 Similarly, a study by Lurie and associates found that the intensity of CT and MRI use can account for virtually all of the extensive (12-fold) regional variation in surgical rates for spinal stenosis in the United States.23 When we image, we intervene. This occurs with standard of care interventions (many of which have little evidence basis) and totally unproven interventions with significant risks that have found their way into our highly imperfect medical marketplace. The single most important imaging precept in the back pain patient is this: treat the patient, do not treat the images.
Specificity and Sensitivity Considerations
Specificity shortcomings of spine imaging apply to all modalities from simple radiographs to the most elaborate MRI study, and have been well documented for decades. Hitselberger and associates noted in 1968 that myelographic studies were abnormal in 24% of asymptomatic volunteers.24 Wiesel and associates subsequently showed that CT demonstrated “significant” abnormalities in 50% of asymptomatic volunteers over the age of 40.25 Boden and associates showed that MRI of asymptomatic volunteers over the age of 60 demonstrated “significant” findings in 57% of asymptomatic volunteers.26 Numerous studies of asymptomatic volunteers can be summarized as follows: (1) imaging demonstrates abnormalities in the lumbar spine of 1/3 to 2/3 of asymptomatic persons; (2) annular fissures, disc bulges or protrusions, facet degeneration, and low-grade antero- or retrolisthesis are common, usually asymptomatic, and their prevalence increases with increasing age; (3) disc extrusions, severe central canal stenosis, and direct evidence of a neural compression are more likely to be symptomatic.16,25,27–38 Only clear concordance of an individual patient’s pain syndrome and imaging findings can suggest causation. Imaging cannot prove causation.
Imaging also suffers from sensitivity shortcomings. It is well documented that neuroclaudicatory pain is exacerbated by extension and axial load. The cross-sectional area of the lumbar and cervical central canal, lateral recesses, and neural foramina are known to decrease with extension and axial load.39 Studies by Danielson and Willem have shown that axial loading and extension will reduce the cross-sectional dimension of the lumbar dural sac in 56% of asymptomatic persons, but in up to 80% of patients with neuroclaudicatory pain syndromes.40,41 Unfortunately, most advanced imaging occurs with the patient in a supine, psoas relaxed (nonextended) position. This position diminishes imaging sensitivity to dynamic processes in which neural compression and pain are experienced only in specific postures. Upright and seated MRI imaging of the spine is commercially available, but suffers from modest image quality at best, due to the low field strength magnets available in this configuration. This may ultimately prove to be a valuable tool, but current devices excessively compromise image quality, and their cost precludes their use being appropriately limited to a problem-solving application.
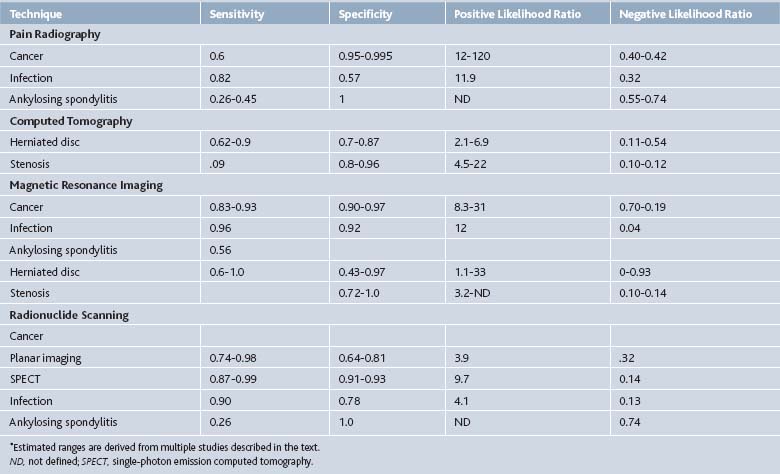
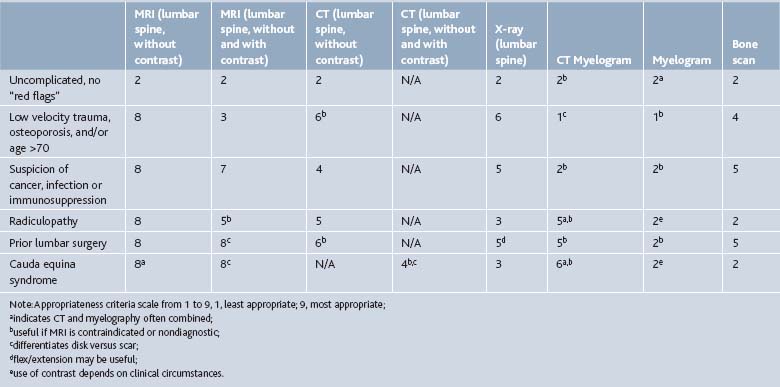
Multiple imaging modalities can be used to investigate the spine pain patient, from simple radiographs to elegant MRI scans. The efficacy of the several imaging modalities used in evaluating common spine disorders has been summarized by Jarvik and Deyo in Table 43-2.1 The American College of Radiology Appropriateness Criteria grade the utility of these imaging modalities in common clinical scenarios, presented in Table 43-3.42
Radiographs
In the setting of clinical red flags suggestive of systemic disease, pain unresponsive to conservative measures, or progressive neurologic deficit, imaging should commence with plain radiographs.11 Radiographs are a modest sensitivity screening tool for sinister processes such as neoplasm, infection, or fracture. They also provide vertebral enumeration, and allow assessment of sagittal and coronal balance. To provide the physiologic information of balance, radiographs should be obtained while the patient is weight-bearing. Single frontal and lateral views of the lumbar spine are adequate. Spot images of the lumbosacral junction, oblique views, and flexion-extension views should not be obtained on a routine basis. They provide little additional information, and significantly increase radiation exposure.43 The significance of vertebral enumeration should not be underestimated. There are anomalies of segmentation at the lumbosacral junction in 7% to 12% of the back pain population.44 Radiographs should establish the convention of vertebral numbering for all subsequent advanced imaging in the individual patient, preventing wrong level minimally invasive or surgical interventions.
Radiographic identification of morphologic changes of disc degeneration or osteoarthritis in lumbar zygapophyseal or sacroiliac joints has no value in identifying the cause of an individual patient’s pain.45 Radiographic manifestations of disc degeneration include loss of disc space height, nitrogen gas within the disc, and end plate hypertrophs; these findings have no predictive value for discogenic pain. Similarly, radiographic findings of osteoarthritis, including hypertrophic change, sclerosis, joint space narrowing, gas within the joint space and subchondral cyst formation do not correlate with response to intraarticular anesthetic injections. Morphologically degenerated joints are most commonly asymptomatic, and joints that appear normal radiographically may be painful.
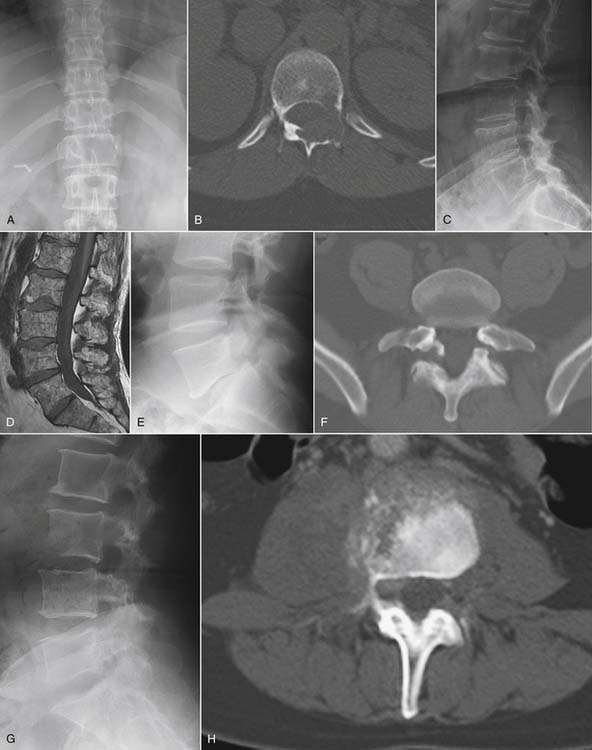
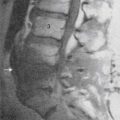
Radiographs provide a modest sensitivity screen for systemic disease causal of back pain, which may require advanced imaging to characterize and establish extent of disease (Fig. 43-1). The most common neoplastic condition is metastatic disease. Skeletal metastases are primarily blastic (bone forming), lytic (bone destroying), or of mixed type. Radiographs primarily image cortical bone. Metastases most commonly involve trabecular bone; at least 50% of trabecular bone mass must be destroyed to be visible on a radiograph. Radiographs therefore have only modest sensitivity (60%) in the detection of metastatic disease.1 Myeloma may also be initially suggested by radiographic evaluation; myeloma typically manifests as small discrete lytic lesions without sclerotic margination. When diffuse, myeloma may be indistinguishable from metabolic osteoporosis. Primary bone tumors are rare causes of spine-related pain. Osteoid osteoma deserves mention because it commonly presents with nocturnal pain.46 This neoplasm of adolescents and young adults has a posterior element predilection, and it generates a lesion consisting of a central lytic nidus with considerable surrounding sclerotic reaction. The diagnosis is seldom made with plain films; CT imaging, with image directed biopsy and radio frequency ablation constitute standard of care. Osteoid osteomas may also be identified on MRI examinations or bone scans as focal intensely enhancing (or metabolically active) posterior element lesions.47
Radiographs may also suggest infectious or inflammatory disease. Radiographic findings of infectious spondylodiscitis (disc space infection) only become apparent several weeks after the onset of disease.48 Because disc space infection in the adult begins as a hematologically seeded vertebral osteomyelitis, radiographs initially demonstrate rarefaction in the anterior subchondral bone adjacent to the end plates within a single disc space. Over time, there will be frank end-plate destruction with loss of disc space height.48 These findings should provoke an urgent MRI examination with gadolinium enhancement. Granulomatous disease (TB, brucellosis) may be indistinguishable from pyogenic disc space infection. Granulomatous discitis more commonly spares the disc space with vertebral collapse and kyphotic deformity at the site of infection.48
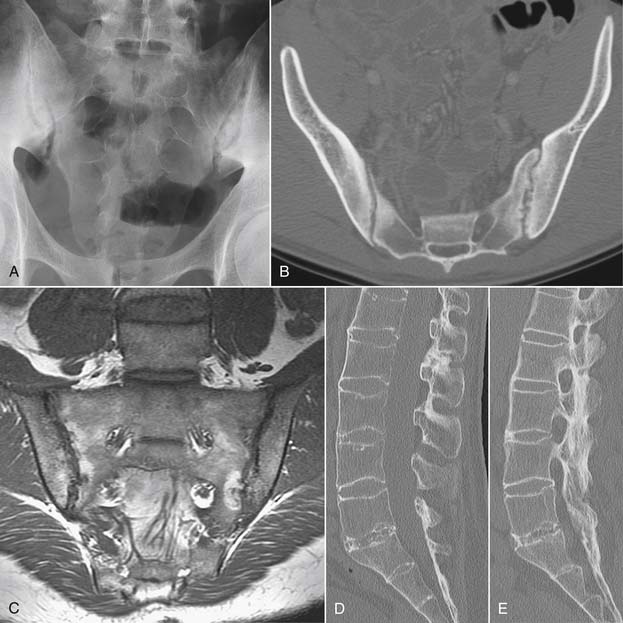
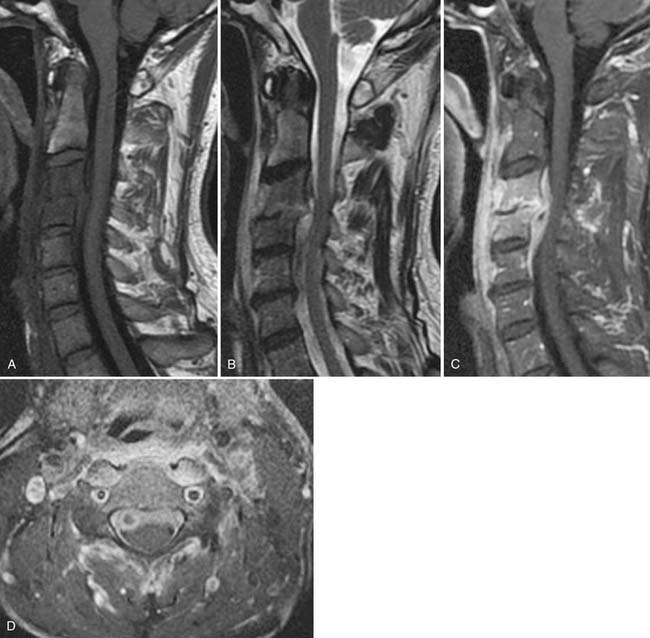
Inflammatory spondyloarthropathies may be suggested or diagnosed with plain radiographs. Rheumatoid arthritis primarily involves the cervical spine, with inflammatory destruction of the transverse ligament binding the dens to C1 with consequent atlantoaxial subluxation.48 There is frequently multilevel cervical disc space narrowing with low grade anterior subluxations resulting in a stair-step appearance to the cervical spine. The seronegative spondyloarthropathies are more commonly apparent in the sacroiliac joints, lumbar and thoracic regions (Fig. 43-2). Narrowing of the sacroiliac joints with associated indistinct cortical surfaces and adjacent sclerosis may herald ankylosing spondylitis, psoriatic arthritis, or Reiter syndrome. The ankylosing spondylitis patient may also have fusion of portions of the thoracolumbar or cervical spine with vertically oriented, gracile syndesmophytes bridging adjacent vertebral bodies. The long-segment spine rigidity in these patients makes them susceptible to catastrophic fracture dislocation, often with neurologic compromise, even with modest trauma. Such fractures may be difficult to visualize with plain radiographs, and the threshold for use of advanced imaging should be very low in ankylosing spondylitis patients suffering trauma.49
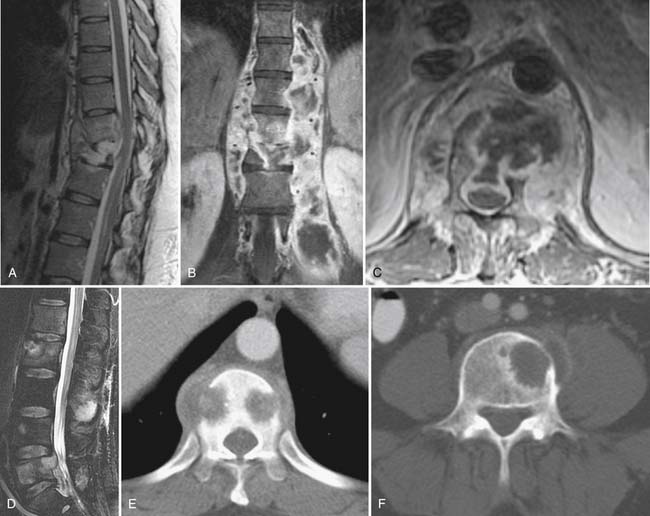
Compression fractures can be identified on plain radiographs, but the acuity of a fracture may not be apparent in the absence of serial images. MRI or technetium bone scan can characterize fractures as acute or subacute, metabolically active, and therefore more likely to respond to bone augmentation procedures such as vertebroplasty (Fig. 43-3). However, recent randomized controlled trials have raised questions regarding the efficacy of vertebroplasty.50,51 Similarly, pelvic insufficiency fractures may be apparent on radiographs within the sacral ala, pubic rami, pubic symphysis, and periacetabular regions of the pelvis. The plain film finding of a vertical sclerotic band paralleling the sacroiliac joint in the sacral ala strongly suggests insufficiency fracture. CT, technetium bone scan, or MRI are all more sensitive than radiographs in detection of insufficiency fractures.52,53
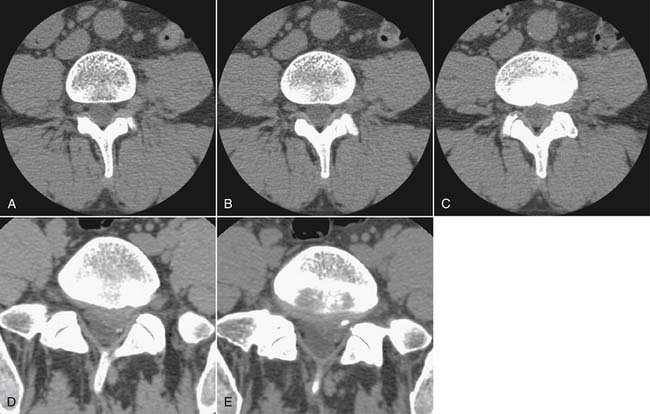
Another traumatic entity in which plain films play a role is spondylolysis. Defects in the pars interarticularis may be detected on lateral plain films, with a prevalence of approximately 3% to −10% of the population.54 Most cases are asymptomatic. If pars defects are suspected as the cause of an axial pain syndrome, a localized CT will confirm or refute the presence of such a defect and aid in characterizing the defect as acute or chronic. There is no role for oblique radiographs, which double the gonadal radiation dose over the standard images, and do not advance the diagnosis.43
Computed Tomography
Computed tomography has undergone a technologic renaissance in the last decade with the evolution of multidetector scanners. Even the most uncomfortable patient can tolerate the few seconds necessary to obtain a high quality data set, which can then be reconstructed in any plane without loss of spatial resolution. CT provides superior imaging of cortical and trabecular bone. It has reasonable contrast resolution, allowing the identification of most root compressive lesions (Fig. 43-4). It is less costly than MRI, more widely available, and is not subject to imaging disqualification due to indwelling magnetically susceptible devices.
As with plain radiographs, morphologic changes of disc degeneration, zygapophyseal joint arthrosis and sacroiliac joint osteoarthritis seen on CT do not correlate with pain syndromes, and are of no significance.55,56 Disc herniations are readily identifiable with computed tomography. There is no literature comparing current generation CT scanners with MRI in the detection of root-compressive lesions. A study by Thornbury in 1993 showed little difference in MRI, CT myelography, or CT alone in detecting root-compressive disease.57 Spine MRI has advanced modestly in the intervening 15 years; CT has been reborn. A more recent study from 2006 showed no evidence that CT is inferior to MRI in detecting disc herniation, although there was more interobserver variability with CT.58 This study used two-slice CT scanners; current scanners in general practice are 64-slice scanners with 128-slice machines being introduced. It can be argued that CT is underused in evaluating the younger patient with root-compressive symptoms and no clinical likelihood of systemic disease. In this setting, CT can provide adequate imaging at a lesser cost than MRI. This is, of course, dependent on the local medical ecosystem; if the patient is a surgical candidate and the surgeon involved demands an MRI for planning purposes, then CT becomes an additive rather than a lower cost substitute test.
CT is more sensitive than plain radiographs, but less sensitive than MRI, in the detection of infectious or inflammatory spinal lesions.1 Discitis may be suggested when end-plate destructive changes are seen, often accompanied by a paraspinal inflammatory mass.59 CT alone may be insufficient to assess central canal compromise, and CT myelography or MRI may be necessary to assess the need for surgical intervention. Noninfectious inflammatory spondyloarthropathies are more conspicuous on CT than on plain films.60 Like radiographs, however, CT is limited to displaying static morphology and cannot assess physiologic inflammation.
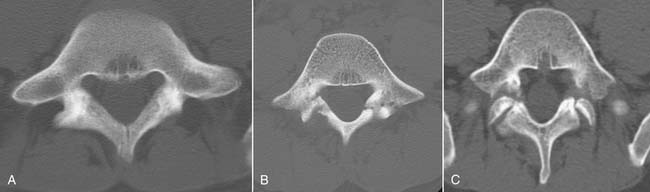
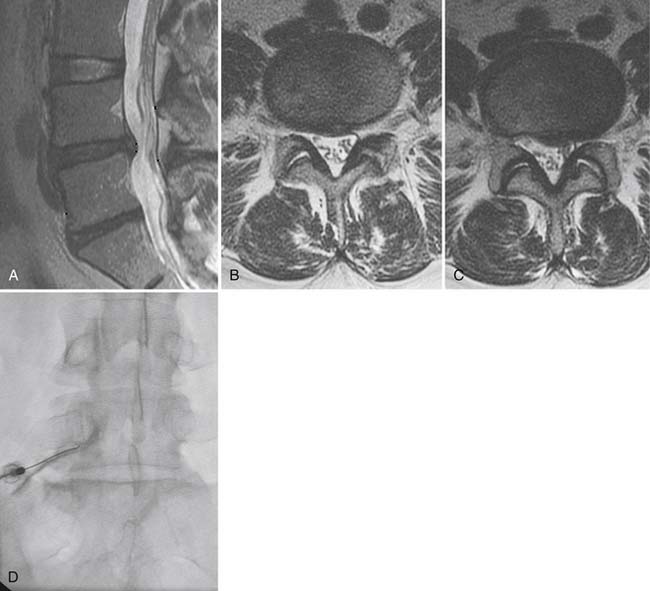
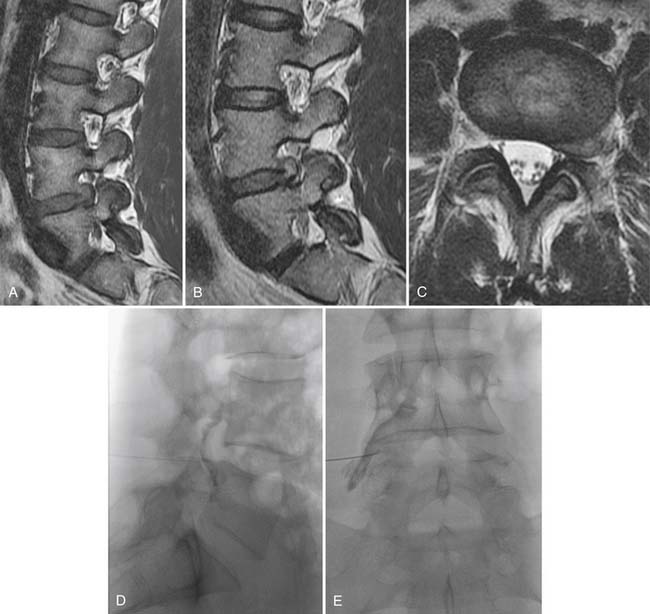
Traumatic or insufficiency fractures are more apparent on CT than on radiographs, particularly with the assistance of multiplanar reconstructions. Assessment of fracture acuity with CT requires evaluation of the fracture line and fragments. Very angular fracture lines and fragments with no marginal sclerosis suggest acuity, whereas smooth remodeling of bone implies chronicity. MRI or technetium bone scans provide more specific, physiologic assessment of fracture acuity. When fatigue fractures of the pars interarticularis are suspected on plain radiographs, CT can assist in further characterization of the fractures and the prognosis for healing (Fig. 43-5). If sclerosis is present within the pars without a discrete defect, this represents a stress reaction with healing potential. An ill-defined lucent zone with marginal sclerosis is an incomplete fracture—also with healing potential.61 A chronic defect with well-defined rounded and sclerotic margins has no healing potential. The combination of SPECT technetium scan with CT is generally considered the standard of care for complete characterization and treatment planning of spondylotic defects.61
Bony destruction or blastic response as a manifestation of tumor is much more evident on CT than on radiographs; MRI, however, is even more sensitive.1 CT exceeds MRI in its ability to assess the degree of structural compromise caused by neoplastic spinal involvement and, therefore, is critical in determining if surgical stabilization is necessary. Neoplasms that infiltrate marrow without trabecular or cortical destruction, such as lymphoma, may be difficult to identify with CT alone. Neoplastic soft tissue lesions involving the central canal or paraspinal regions may be detected by CT, but will be far more conspicuous on MRI.
CT Myelography
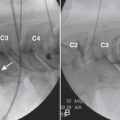
The demise of CT myelography has been predicted for more than 2 decades; it nonetheless remains a viable imaging adjunct with a problem-solving role in the lumbar region and a dominant role in the cervical spine (Fig. 43-6).48 The addition of radio-opaque contrast material to the subarachnoid space allows detection of subtle epidural lesions of benign or malignant etiology, which encroach on the thecal sac and root sleeves. Intrathecal lesions may also be identified, although they are less well characterized than with MRI. The spatial resolution of CT myelography exceeds that of MRI, a critical parameter in the tight confines of the cervical spine. CT myelography excels at the discrimination between soft disc and bony osteophyte, which may alter the surgical approach in the cervical spine. Myelography is minimally invasive, and it adds considerable cost above CT alone.
MRI
MRI remains the preeminent spine imaging modality, a status it has enjoyed over much of the last 2 decades, owing to its superior contrast resolution. MRI remains unchallenged in its ability to discriminate between soft tissues of varying character. Alterations of disc contour (herniations) are evaluated with substantial interobserver reliability.62 Lesions compressing the thecal sac, root sleeves, and exiting nerve roots can be directly visualized and the cross-sectional dimension of the thecal sac can be quantified without invasion. Interobserver reliability for these parameters is moderate.63 The soft tissue contrast characteristics of MRI allow detection of neoplastic or inflammatory marrow infiltrative lesions that do not destroy trabecular or cortical bone. MRI is more sensitive than CT or CT myelography in the detection of neoplastic or infectious processes.1 With the use of heavily T2 weighted imaging sequences (short tau inversion recovery, fat-saturated fast spin echo) or contrast enhancement, MRI can image an inflammatory response, allowing potential identification of degenerative or noninfectious inflammatory pain generators.
MRI: Degenerative Disease
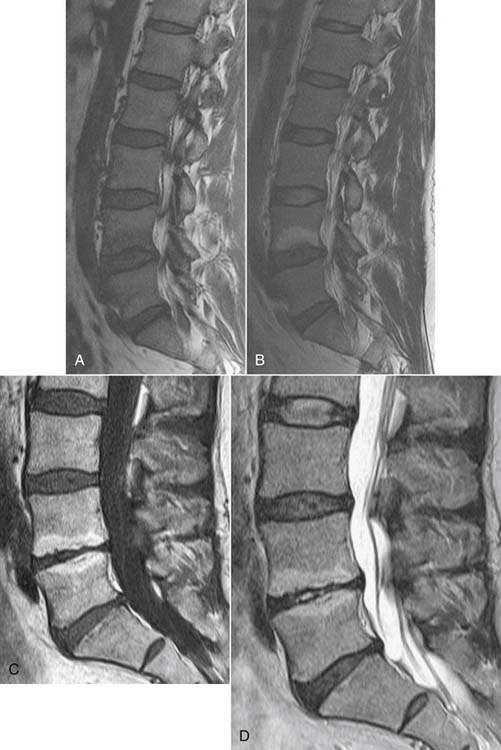
MRI will identify disc degeneration as loss of the normally high T2 signal in the nuclear compartment of the disc before there is detectable loss of disc space height on either CT or radiographs. This finding alone is not predictive of a painful disc. The disc and the adjacent vertebral cartilaginous end plate are a functional unit; end-plate inflammatory changes (Modic changes) frequently accompany disc degeneration.64 Modic type 1 changes are an inflammatory state consisting of vascularized granulation tissue within the sub–end-plate marrow. MRI demonstrates low T1 signal and high T2 signal with enhancement if gadolinium is administered. Modic 1 change may progress to Modic type 2, in which there is fatty infiltration of sub–end-plate marrow, evidenced by elevated T1 and T2 signal (Fig. 43-7). Immunohistochemistry studies suggest that end-plate abnormalities are related to inflammation and axon growth induced by tumor necrosis factor (TNF); such nerve in-growth in abnormal end plates may be a cause of low back pain.65 This is more prominent in Modic 1 change than Modic 2 change. Modic 3 changes represent sclerosis and are seen as low T1 and low T2 signal on MRI. It is a postinflammatory state.66 Inflammatory end-plate changes have been correlated with a painful disc, with a positive predictive value of 91% in a single small study.67
MRI may also identify annular fissures. As the nuclear-annular junction deteriorates, radial fissures may extend to the outer aspect of the disc annulus. Such fissures, called high intensity zones (HIZs), may be visualized as a focal zone of elevated T2 signal, which may enhance due to the presence of vascularized granulation tissue (Fig. 43-8).68 The initial study showed that an HIZ increases the odds that provocation discography (PD) will be positive in a patient at that level by a factor of 6.5.68 Studies on HIZs since that time have all shown that an HIZ has high specificity (range 0.74 to 0.93)69–73 but variable sensitivity, from as low as 0.0973 to as high as 0.7871 for positive PD. Thus, it is uncommon for an HIZ to occur in a disc that is not painful to PD, and the calculated likelihood ratios (ranging from 1.3 to 6.5 and averaging 4.1) indicate that an HIZ increases the odds that PD will be positive by at least 50%.74 Additionally, although HIZs are present in asymptomatic and symptomatic subjects, they are found significantly more in symptomatic subjects (prevalence 60% ±15%) than asymptomatic subjects (24% ± 11%).75 The interobserver reliability in identifying a HIZ is fair to good,72,76 with reported kappa values of 0.4476 and 0.57.72
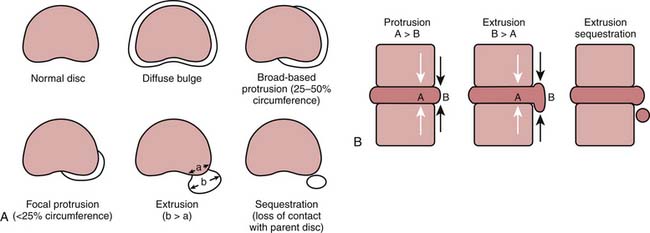
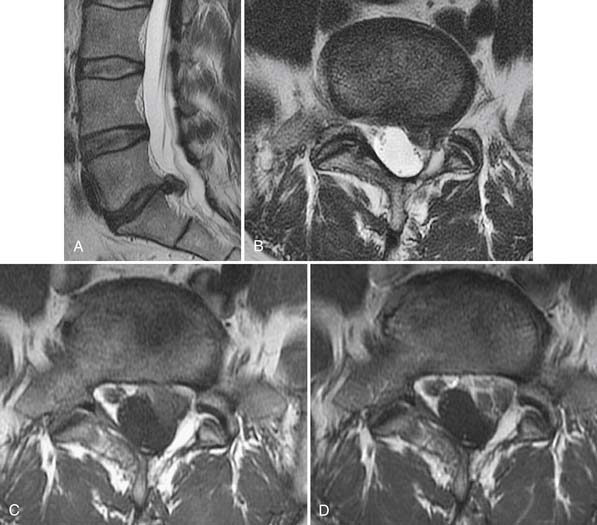
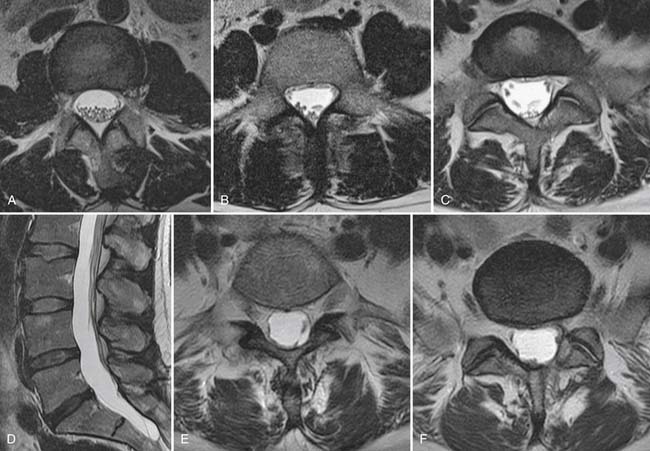
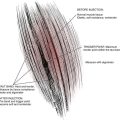
Degenerative discs may undergo herniation, which is the displacement of disc material beyond the normal confines of the intervertebral disc space. The accepted nomenclature for disc herniation has recently been codified (Fig. 43-9).77 Disc herniation by definition involves less than 50% of the circumference of the disc; displacement of greater than 50% of the circumference of the disc may be termed a diffuse bulge. A broad-based herniation encompasses 25% to 50% of disc circumference, whereas a focal herniation involves less than 25% of the disc circumference. Disc herniations may be subdivided into protrusions and extrusions, which are shape definitions but carry implications regarding the integrity of the outer annulus. A protrusion remains bounded by an intact outer annulus; herniated material never exceeds the dimension of the parent disc in any plane (Fig. 43-10). An extrusion is uncontained by an intact annulus and may migrate cephalad or caudal to the disc space level, attaining a dimension greater than the annular defect in the disc from which it originated. A sequestration is a free disc fragment which has lost continuity with its parent disc and undergone migration away from it (Fig. 43-11). The location of herniated disc material in the axial plane may be described as central, subarticular (lateral recess), foraminal, or extraforaminal (far lateral), in progression from the midline laterally.
In the patient with radicular pain or radiculopathy, in whom a disc herniation is suspected, imaging should begin with radiographs but may progress to MRI if conservative measures fail and epidural steroid injections or surgical intervention are contemplated.12,15 Ninety percent of lumbar disc herniations occur at the L4-5 and L5-S1 interspaces.71 Disc herniations most commonly have a posterolateral vector, impinging on the nerve root (traversing root) which will exit under the next lowest pedicle (L4 disc herniation causing L5 radicular pain syndrome). For a lumbar herniation to affect the like-numbered nerve root, herniated material must migrate cephalad into the neural foramen (Fig. 43-12). In the cervical region, the nerve root exits low in the foramen, directly over the subadjacent pedicle, and is vulnerable to disc herniation from the intervertebral disc at the same level as its exit. Remembering that in the cervical spine the numbering of the exiting root matches the subadjacent vertebral body, most cervical herniations will affect the next lower numbered root (e.g., disc herniation at the C6-7 interspace will affect the exiting C7 root [Fig. 43-13]). Herniations are most evident on T2 weighted images, where the mass effect of the herniation can be directly visualized on the thecal sac or nerve root. The herniated disc material is often of higher T2 signal than the parent disc from which it arose. The imaging natural history of disc herniation in both the lumbar and cervical regions is resolution. Numerous longitudinal imaging studies demonstrate that most disc herniations resolve spontaneously, with larger herniations and extrusions most likely to undergo resorption.78–80 The functional disability incurred by disc herniations is better predicted by psychosocial factors than the size or imaging characteristics of the herniation.81
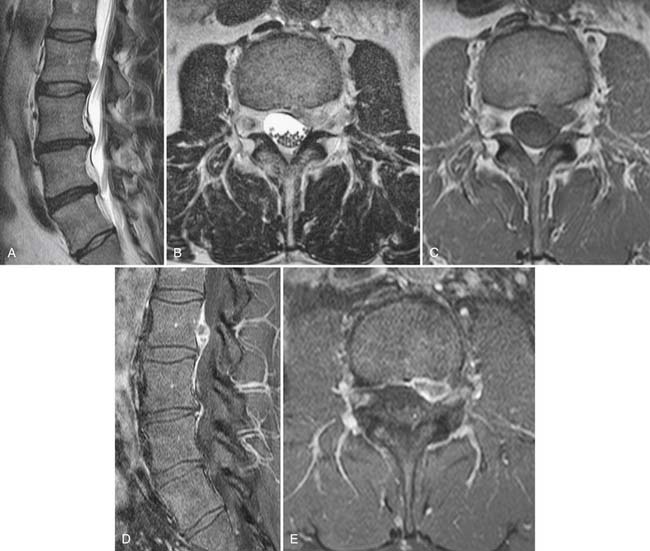
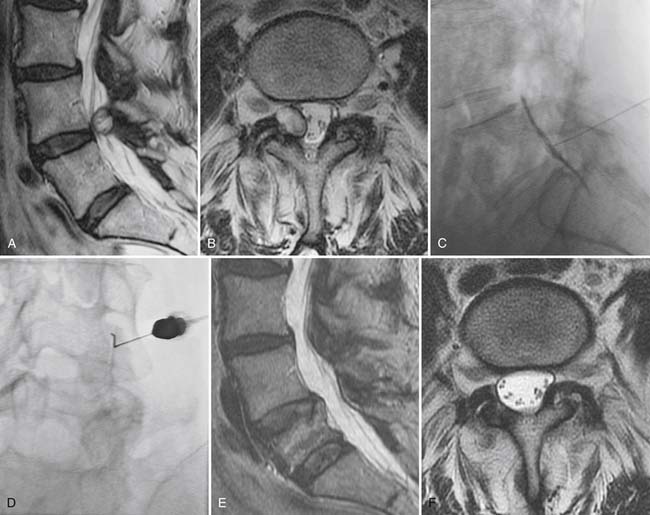
Gadolinium is not typically used in the nonoperated cervical or lumbar spine; when used, it will often show that the volume of herniated disc material (which does not enhance) is relatively small compared to accompanying enhancing granulation tissue. In the postoperative spine, contrast enhancement is mandatory. Imaging is essentially blind to the detection of recurrent disc herniation in the 6 weeks immediately following surgical intervention. Beyond this perioperative period, the use of gadolinium enhancement should allow accuracy of up to 96% in distinguishing recurrent herniation from epidural fibrosis (scar).82,83 Epidural fibrosis enhances uniformly and rapidly following gadolinium administration, whereas herniated disc material enhances minimally and slowly (Fig. 43-14). Extensive epidural fibrosis is in itself a negative prognostic sign in the postoperative patient and is associated with an increased incidence of persistent postoperative radiculopathy.84 In the postoperative setting, the thecal sac should also be examined for evidence of arachnoiditis, usually attributed to hemorrhage within the thecal sac. In this condition, the roots of the cauda equina either clump or are adherent to the neural tube (Fig. 43-15). The dural tube may appear empty of roots which are smoothly scarred to its wall. Clumped roots in arachnoiditis may exhibit enhancement. In severe cases, the subarachnoid space is completely obliterated by scarring.
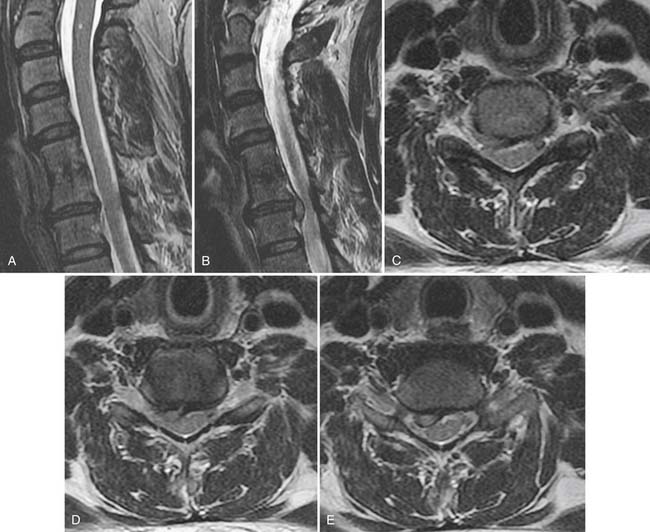
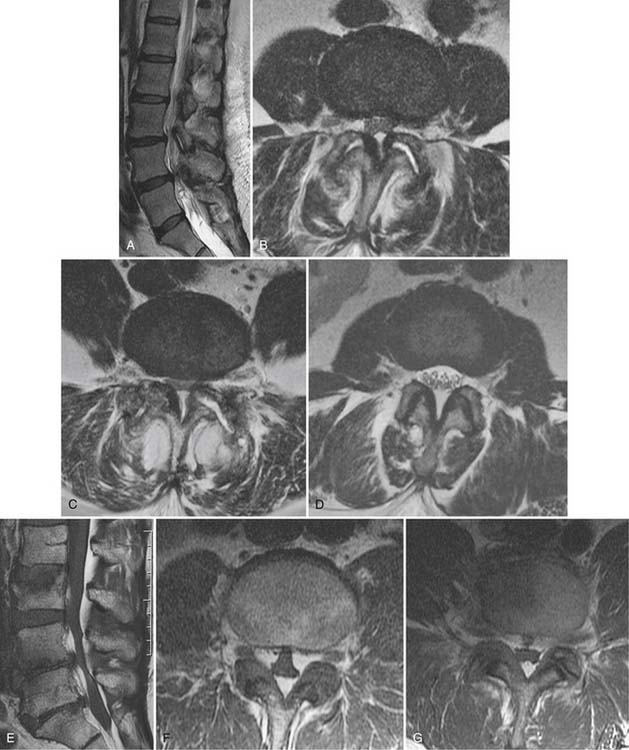
Spinal stenosis describes a clinical syndrome characterized by neurogenic claudication with exertion. Imaging identifies the morphologic substrate of central canal narrowing. Patients who exhibit a narrowed central canal on imaging frequently are asymptomatic and cannot carry the diagnosis of spinal stenosis. Central canal compromise is well displayed on T2 weighted MRI (Fig. 43-16), although CT can also make this diagnosis; CT myelography provides the best spatial resolution in this regard. Central canal narrowing in the lumbar region is usually primarily the result of posterior element impingement: central buckling of the ligamentum flavum, zygapophyseal joint arthropathy, and synovial cysts. Disc herniation and disc-osteophyte complexes may also contribute from the ventral aspect of the canal. Sagittal T2 weighted MRI or CT myelography images also often demonstrate serpiginous redundant nerve roots or dilated veins within the subarachnoid space in the stenotic central canal. Multilevel central canal stenosis is more likely to provoke clinical symptoms. It must be remembered that advanced imaging obtained on a recumbent patient may be insensitive to dynamic lesions.40
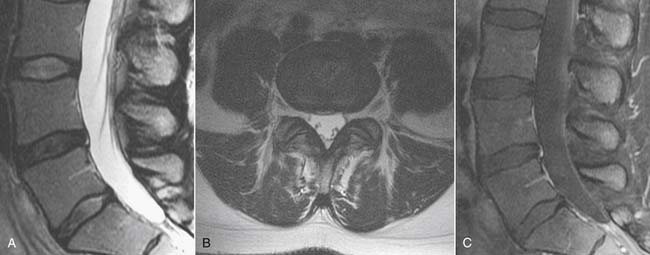
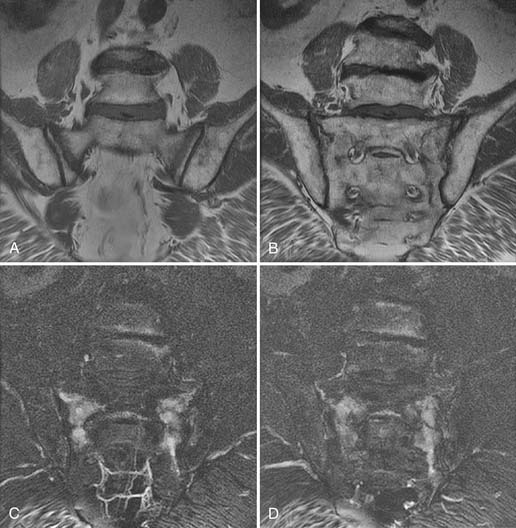
MRI, like radiographs and CT, can identify the morphologic features of zygapophyseal joint osteoarthritis, which have no predictive value for pain generation.56 MRI can, however, also identify inflammatory change using heavily T2 weighted imaging sequences such as short tau inversion recovery (STIR) or fat saturated fast spin echo. In these sequences, T2 signal abnormality is white against a dark background and is highly conspicuous (Fig. 43-17). Gadolinium enhancement, best seen with fat saturated sequences, is equivalent in its identification of inflammation, but more costly. A recent paper by Czervionke and associates suggests that such T2 signal abnormality may be useful in identifying painful joints.85 This paradigm may apply to the sacroiliac joint as well, although published work to date describes such MRI findings in inflammatory spondyloarthropathy such as ankylosing spondylitis rather than osteoarthritis.
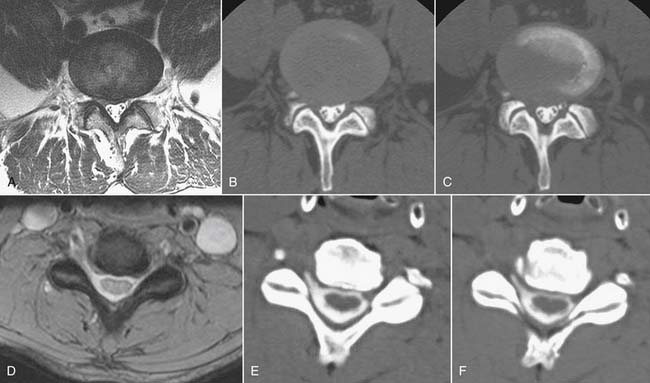
Synovial cysts, a manifestation of zygapophyseal joint osteoarthritis, may be a cause of radicular pain syndromes and are readily identified on MRI (Fig. 43-18).86 A rent in the fibrous joint capsule allows the synovial membrane and its contained fluid to form a juxtaarticular cyst, most common at the L4 level. Synovial cysts are present in approximately 10% of patients undergoing MRI imaging for lumbar pain issues; most of the cysts are dorsal, arising from the inferior recess of the joint and project harmlessly into the multifidus musculature.87 Cysts, which project ventrally into the spinal canal, have a prevalence of slightly greater than 2% and may compress neural elements in the central canal, lateral recess, or neural foramen.86 On MRI, synovial cysts may signal as CSF does, or be heterogeneous owing to hemorrhage or inspissated proteinaceous material. Ultimately, synovial cysts may calcify. If the cyst wall is very thin, this suggests the cyst may collapse with aspiration under image guidance; cysts with a thick fibrous wall of low T2 signal are less amenable to minimally invasive treatment.
MRI: Systemic Disease
MRI is the most sensitive modality for the detection of systemic disease, which presents as back or leg pain. This broadly consists of infection, unsuspected trauma, or neoplasm. As noted previously, MRI has superior sensitivity in the early detection of pyogenic or granulomatous spondylitis.59,88–90 Early MRI findings consist of marrow edema (low T1 signal, high T2 signal) about a disc which will likely have zones of elevated T2 signal within it. Gadolinium administration is essential in cases of spondylodiscitis; it will typically reveal enhancement both within the index disc and its adjacent vertebral bodies as well as in the epidural space and paraspinal soft tissues (Fig. 43-19).59,88 The combination of gallium and technetium nuclear medicine studies has a sensitivity equal to that of MRI in the detection of spondylodiscitis, but will not provide the anatomic information necessary to make a clinical decision regarding medical versus surgical therapy.91 The presence of an epidural abscess in the setting of neurologic compromise may provoke emergent surgical decompression. Only MRI can provide this anatomic information, as well as provide high sensitivity in the detection of less fulminant cases of spondylodiscitis.59,88 The imaging appearance of discitis on MRI lags behind the clinical state. Imaging findings may continue to be dramatic when the patient has clearly demonstrated clinical response to therapy. In the author’s clinical experience, therapy should be followed with biochemical markers such as C-reactive protein, not MRI; repeat imaging should be reserved for situations where there is concern for failure of therapy and compromise of the central spinal canal.
Granulomatous spondylodiscitis may be indistinguishable from pyogenic disease, but is more likely to spare the disc with vertebral collapse and kyphotic deformity (Fig. 43-20).48 Up to 50% of cases of granulomatous spondylitis have no disc space involvement, and the isolated vertebral lesions can mimic metastatic disease.92 CT may provide a clue to the infectious origin of such lesions by demonstrating a broad sclerotic margin about the lytic lesions, implying a more indolent course then is seen with most neoplasms. Diagnosis frequently depends on image-guided biopsy and culture. Assay for tuberculous RNA can provide rapid (24 hour) diagnosis of tuberculous infection while awaiting cultures to provide sensitivity information.
MRI can detect the characteristic inflammatory response about the sacroiliac joints in the seronegative spondyloarthropathies before morphologic changes are detectable on radiographs or CT. Technetium bone scan is equally sensitive in this regard. In rheumatoid arthritis, MRI can directly evaluate the integrity of the transverse ligament at C1-2, the presence of pannus formation, and potential compromise of the cervical spinal canal.48
MRI is not typically used in the acute traumatic setting, but has a role in evaluation of traumatic lesions as subacute and chronic pain generators. Osteoporotic compression fractures are a relatively common cause of back pain in the elderly.1 Many patients respond well to analgesics and perhaps bracing. Vertebroplasty has been shown to be no better than placebo,50,51 and although these fractures can be severely painful initially, the natural history is relatively good.93 Unfortunately, a small percentage of vertebral compression fractures are the result of metastatic disease or hematologic malignancy; vertebral body expansion with associated paraspinal or epidural enhancing mass suggests neoplasm. Image guided biopsy can then establish a diagnosis.
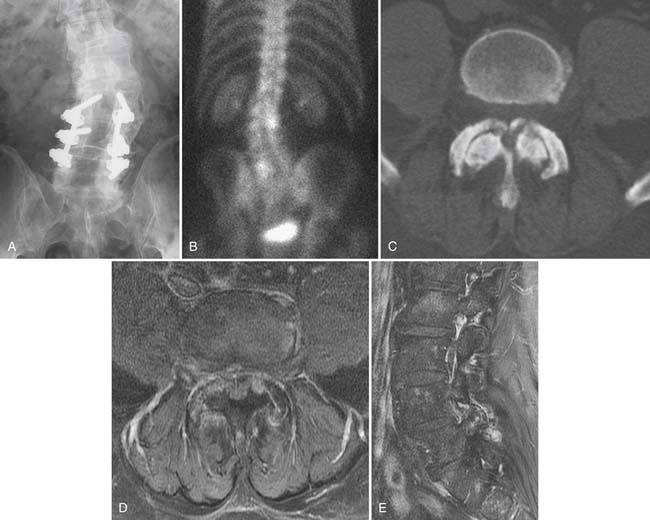
Insufficiency fractures are also common in the pelvis, most often seen in the sacrum (Fig. 43-21) but also in the pubic rami, body of the pubic bone, or periacetabular regions. Linear zones of elevated T2 signal or diminished T1 signal in characteristic locations (Honda sign in sacral lesions) suggest insufficiency fracture; MRI signal abnormality will be apparent before reactive sclerosis is detectable by CT or radiographs.94 Technetium bone scan is similar in sensitivity to MRI for insufficiency fracture detection.95,96
Neoplasm, whether extradural or intradural, is best detected and characterized by MRI.1 Most extradural lesions will be metastases and manifest as vertebral lesions with low T1, high T2 signal enhancement with gadolinium, perhaps with extension into the epidural space. Hematologic malignancies may diffusely replace marrow fat, with uniformly low T1 signal that may not be readily apparent; marrow should always be brighter than disc on T1 images. Intradural lesions include meningiomas, schwannomas, leptomeningeal metastases; the intramedullary neoplasms include ependymoma, astrocytoma, and hemangioblastoma. These will be detected as enhancing mass lesions.
Nuclear Medicine
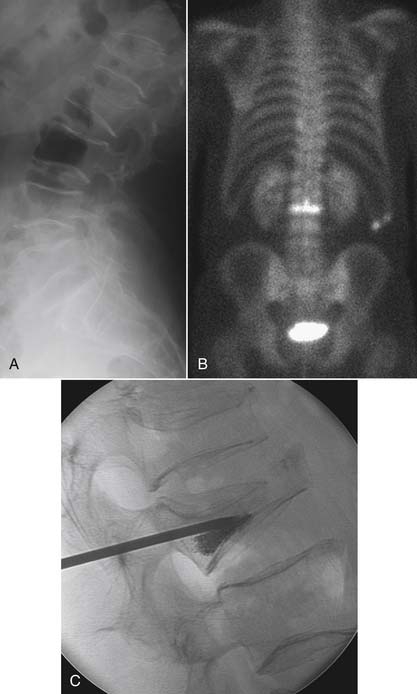
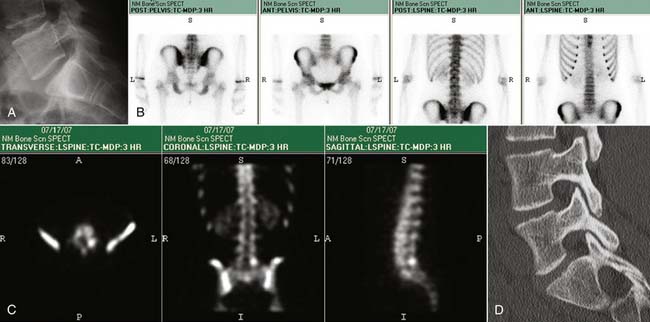
Characterization of compression fractures in terms of chronicity can be readily accomplished by bone scan; this is particularly useful in the non–MRI-compatible patient. Bone scanning is highly sensitive in the detection of insufficiency fractures of the pelvis. Technetium scans also play a role in the detection of spondylolysis; the presence of increased activity in a pars interarticularis implies an actively metabolic lesion, a likely source of pain (Fig. 43-22).61 Limited CT may be necessary for further characterization of healing potential and therapy planning. SPECT bone scans are very useful in the detection of pseudarthroses in patients who present with pain following fusion surgery.97 The combination of gallium plus technetium scans is highly sensitive and specific for the detection of spine infection.98 It suffers from its lack of anatomic information, which will likely be necessary for planning therapy and result in additive tests; MRI is generally preferred when spine infection is suspected. Technetium scanning room retains its longstanding role in assessing tumor burden.
The assessment of tumor burden is now being assumed by PET scanning. Integrated PET/CT scanners provide metabolic and anatomic information, hopefully obviating the need for additional imaging in following the patient with neoplastic disease.
Imaging Principles and Strategies
The best understanding of the relevance of imaging findings will occur when there is close communication between the spine clinician and the imaging professional, and their skill sets overlap. The spine clinician must understand the physical principles underlying imaging and its inherent strengths and weaknesses. The imaging professional must be knowledgeable of clinical pain syndromes, their pathophysiology, and imaging manifestations.
1. Jarvik J.G., Deyo R.A. Diagnostic evaluation of low back pain with emphasis on imaging. Ann Intern Med. 2002;137:586-597.
2. Deyo R.A., Mirza S.K., Martin B.I. Back pain prevalence and visit rates: estimates from U.S. national surveys. Spine. 2002;2006(31):2724-2727.
3. Coste J., Delecoeuillerie G., Cohen de Lara .A., et al. Clinical course and prognostic factors in acute low back pain: an inception cohort study in primary care practice. BMJ. 1994;308:577-580.
4. Von Korff M., Deyo R.A., Cherkin D., et al. Back pain in primary care. Outcomes at 1 year. Spine. 1993;18:855-862.
5. Croft P.R., Macfarlane G.J., Papageorgiou A.C., et al. Outcome of low back pain in general practice: a prospective study. BMJ. 1998;316:1356-1359.
6. van den Hoogen H.J., Koes B.W., van Eijk J.T., et al. On the course of low back pain in general practice: a one year follow up study. Ann Rheum Dis. 1998;57:13-19.
7. McGuirk B., King W., Govind J., et al. Safety, efficacy, and cost effectiveness of evidence-based guidelines for the management of acute low back pain in primary care. Spine. 2001;26:2615-2622.
8. Deyo R.A., Mirza S.K., Turner J.A., et al. Overtreating chronic back pain: time to back off? J Am Board Fam Med. 2009;22:62-68.
9. Chou R., Fu R., Carrino J.A., et al. Imaging strategies for low-back pain: systematic review and meta-analysis. Lancet. 2009;373:463-472.
10. Carragee E., Alamin T., Cheng I., et al. Are first-time episodes of serious LBP associated with new MRI findings? Spine J. 2006;6:624-635.
11. Davis P.C., Wippold F.J.II, Brunberg J.A., et al. Expert Panel on Neurologic Imaging. ACR Appropriateness Criteria® low back pain. Reston, Va: American College of Radiology (ACR); 2008.
12. Bradley W.G.Jr. Low back pain. AJNR Am J Neuroradiol. 2007;28:990-992.
13. Acute low back problems in adults. assessment and treatment. Agency for Health Care Policy and Research. Clin Pract Guidel Quick Ref Guide Clin. 1994. iii-25
14. Evidence-based Management of Acute Musculoskeletal Pain. NHMRC (Australian Government); 2003.
15. Chou R., Qaseem A., Snow V., et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478-491.
16. Jarvik J.G., Hollingworth W., Heagerty P.J., et al. Three-year incidence of low back pain in an initially asymptomatic cohort: clinical and imaging risk factors. Spine. 2005;30:1541-1548.
17. Engers A., Jellema P., Wensing M., et al. Individual patient education for low back pain. Cochrane Database of Syst Rev.. 2008. CD004057
18. Buchbinder R. Self-management education en masse: effectiveness of the Back Pain: Don’t Take It Lying Down mass media campaign. Med J Aust. 2008;189:S29-S32.
19. Mettler F.A.Jr., Huda W., Yoshizumi T.T., Mahesh M., et al. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248:254-263.
20. Maniadakis N., Gray A. The economic burden of back pain in the UK. Pain. 2000;84:95-103.
21. Centres for Medicare & Medicaid Services. [http://www.cms.hhs.gov/]. 2009.
22. Jarvik J.G., Hollingworth W., Martin B., et al. Rapid magnetic resonance imaging vs. radiographs for patients with low back pain: a randomized controlled trial. JAMA. 2003;289:2810-2818.
23. Lurie J.D., Birkmeyer N.J., Weinstein J.N. Rates of advanced spinal imaging and spine surgery. Spine. 2003;28:616-620.
24. Hitselberger W.E., Witten R.M. Abnormal myelograms in asymptomatic patients. J Neurosurg. 1968;28:204-206.
25. Wiesel S.W., Tsourmas N., Feffer H.L., et al. A study of computer-assisted tomography. I. The incidence of positive CAT scans in an asymptomatic group of patients. Spine. 1984;9:549-551.
26. Boden S.D., Davis D.O., Dina T.S., et al. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403-408.
27. Boden S.D., McCowin P.R., Davis D.O., et al. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:1178-1184.
28. Alyas F., Turner M., Connell D. MRI findings in the lumbar spines of asymptomatic, adolescent, elite tennis players. Br J Sports Med. 2007;41:836-841.
29. Jensen M.C., Brant-Zawadzki M.N., Obuchowski N., et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69-73.
30. Boos N., Rieder R., Schade V., et al. 1995 Volvo Award in clinical sciences. The diagnostic accuracy of magnetic resonance imaging, work perception, and psychosocial factors in identifying symptomatic disc herniations. Spine. 1995;20:2613-2625.
31. Jarvik J.J., Hollingworth W., Heagerty P., et al. The Longitudinal Assessment of Imaging and Disability of the Back (LAIDBack) Study: baseline data. Spine. 2001;26:1158-1166.
32. Hellstrom M., Jacobsson B., Sward L., Peterson L., et al. Radiologic abnormalities of the thoraco-lumbar spine in athletes. Acta Radiol. 1990;31:127-132.
33. Weinreb J.C., Wolbarsht L.B., Cohen J.M., et al. Prevalence of lumbosacral intervertebral disk abnormalities on MR images in pregnant and asymptomatic nonpregnant women. Radiology. 1989;170:125-128.
34. Burns J.W., Loecker T.H., Fischer J.R.Jr., et al. Prevalence and significance of spinal disc abnormalities in an asymptomatic acceleration subject panel. Aviat Space Environ Med. 1996;67:849-853.
35. Stadnik T.W., Lee R.R., Coen H.L., et al. Annular tears and disk herniation: prevalence and contrast enhancement on MR images in the absence of low back pain or sciatica. Radiology. 1998;206:49-55.
36. Weishaupt D., Zanetti M., Hodler J., Boos N. MR imaging of the lumbar spine: prevalence of intervertebral disk extrusion and sequestration, nerve root compression, end plate abnormalities, and osteoarthritis of the facet joints in asymptomatic volunteers. Radiology. 1998;209:661-666.
37. Carragee E.J., Paragioudakis S.J., Khurana S. 2000 Volvo Award winner in clinical studies: Lumbar high-intensity zone and discography in subjects without low back problems. Spine. 2000;25:2987-2992.
38. Chung C.B., Vande Berg B.C., Tavernier T., et al. End plate marrow changes in the asymptomatic lumbosacral spine: frequency, distribution and correlation with age and degenerative changes. Skeletal Radiol. 2004;33:399-404.
39. Schmid M.R., Stucki G., Duewell S., et al. Changes in cross-sectional measurements of the spinal canal and intervertebral foramina as a function of body position: in vivo studies on an open-configuration MR system. AJR Am J Roentgenol. 1999;172:1095-1102.
40. Danielson B., Willén J. Axially loaded magnetic resonance image of the lumbar spine in asymptomatic individuals. Spine. 2001;26:2601-2606.
41. Willen J., Danielson B., Gaulitz A., et al. Dynamic effects on the lumbar spinal canal: axially loaded CT-myelography and MRI in patients with sciatica and/or neurogenic claudication. Spine.. 1997;22:2968-2976.
42. Davis P.C., Wippold F.J.II, Brunberg J.A., et al. Expert Panel on Neurologic Imaging. ACR Appropriateness Criteria® low back pain. Reston, Va: American College of Radiology (ACR); 2008.
43. Hall F.M. Back pain and the radiologist. Radiology. 1980;137:861-863.
44. Elster A.D. Bertolotti’s syndrome revisited. Transitional vertebrae of the lumbar spine. Spine. 1989;14:1373-1377.
45. Schwarzer A.C., Derby R., Aprill C.N., et al. The value of the provocation response in lumbar zygapophyseal joint injections. Clin J Pain. 1994;10:309-313.
46. Resnick D., Kyriakos M., Greenway G. Tumors and tumor-like lesions of bone. Diagnosis of Bone and Joint Disorders, 3rd ed. Philadelphia: WB Saunders; 1995. 3363-3369
47. Davies M., Cassar-Pullicino V.N., Davies A.M., et al. The diagnostic accuracy of MR imaging in osteoid osteoma. Skeletal Radiol. 2002;31:559-569.
48. Ross J.S., Brant-Zawadzki M., Moore K.R., et al. Diagnostic Imaging. Spine Salt Lake City, UT: Amirsys; 2007.
49. Fordham S., Lloyd G. Clinical management of injured patients with ankylosing spondylitis. BMJ. 339, 2009. b2568
50. Kallmes D.F., Comstock B.A., Heagerty P.J., et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361:569-579.
51. Buchbinder R., Osborne R.H., Ebeling P.R., et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361:557-568.
52. Grangier C., Garcia J., Howarth N.R., et al. Role of MRI in the diagnosis of insufficiency fractures of the sacrum and acetabular roof. Skeletal Radiol. 1997;26:517-524.
53. Fujii M., Abe K., Hayashi K., et al. Honda sign and variants in patients suspected of having a sacral insufficiency fracture. Clin Nucl Med. 2005;30:165-169.
54. Belfi L.M., Ortiz A.O., Katz D.S. Computed tomography evaluation of spondylolysis and spondylolisthesis in asymptomatic patients. Spine. 2006;31:E907-E910.
55. Schwarzer A.C., Wang S.C., O’Driscoll D., et al. The ability of computed tomography to identify a painful zygapophysial joint in patients with chronic low back pain. Spine. 1995;20:907-912.
56. Elgafy H., Semaan H.B., Ebraheim N.A., et alCoombs R.J. Computed tomography findings in patients with sacroiliac pain. Clin Orthop Relat Res. 2001:112-118.
57. Thornbury J.R., Fryback D.G., Turski P.A., et al. Disk-caused nerve compression in patients with acute low-back pain: diagnosis with MR, CT myelography, and plain CT. Radiology. 1993;186:731-738.
58. van Rijn J.C., Klemetso N., Reitsma J.B., et al. Observer variation in the evaluation of lumbar herniated discs and root compression: Spiral CT compared with MRI. Br J Radiol. 2006;79:372-377.
59. Maiuri F., Iaconetta G., Gallicchio B., et al. Spondylodiscitis. Clinical and magnetic resonance diagnosis. Spine. 1997;22:1741-1746.
60. Bennett D.L., Ohashi K., el-Khoury G.Y. Spondyloarthropathies: ankylosing spondylitis and psoriatic arthritis. Radiol Clin North Am. 2004;42:121-134.
61. Gregory P.L., Batt M.E., Kerslake R.W., et al. The value of combining single photon emission computerised tomography and computerised tomography in the investigation of spondylolysis. Eur Spine J. 2004;13:503-509.
62. Lurie J.D., Tosteson A.N., Tosteson T.D., et al. Reliability of magnetic resonance imaging readings for lumbar disc herniation in the Spine Patient Outcomes Research Trial (SPORT). Spine. 2008;33:991-998.
63. Ohtori S., Inoue G., Ito T., et al. Tumor necrosis factor-immunoreactive cells and PGP 9.5-immunoreactive nerve fibers in vertebral endplates of patients with discogenic low back pain and Modic Type 1 or Type 2 changes on MRI. Spine. 2006;31:1026-1031.
64. Modic M.T., Steinberg P.M., Ross J.S., et al. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193-199.
65. Ohtori S., Inoue G., Ito T., et al. Tumor necrosis factor-immunoreactive cells and PGP 9.5-immunoreactive nerve fibers in vertebral endplates of patients with discogenic low back pain and modic type 1 or type 2 changes on MRI. Spine. 2006;31:1026-1031. (Phila Pa 1976.)
66. Modic M.T., Steinberg P.M., Ross J.S., et al. Degenerative disk disease: Assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193-199.
67. Braithwaite I., White J., Saifuddin A., et al. Vertebral end-plate (Modic) changes on lumbar spine MRI: correlation with pain reproduction at lumbar discography. Eur Spine J. 1998;7:363-368.
68. Aprill C., Bogduk N. High-intensity zone: a diagnostic sign of painful lumbar disc on magnetic resonance imaging. Br J Radiol. 1992;65:361-369.
69. Ito M., Incorvaia K.M., Fredrickson B.E., et al. Predictive signs of discogenic lumbar pain on magnetic resonance imaging with discography correlation. Spine. 1998;23:1252-1260.
70. Saifuddin A., Braithwaite I., White J., et al. The value of lumbar spine magnetic resonance imaging in the demonstration of anular tears. Spine. 1998;23:453-457.
71. Schellhas K.P., Pollei S.R., Gundry C.R., Heithoff K.B. Lumbar disc high-intensity zone. Correlation of magnetic resonance imaging and discography. Spine. 1996;21:79-86.
72. Smith B.M., Hurwitz E.L., Solsberg D., et al. Interobserver reliability of detecting lumbar intervertebral disc high-intensity zone on magnetic resonance imaging and association of high-intensity zone with pain and anular disruption. Spine. 1998;23:2074-2080.
73. Ricketson R., Simmons J.W., Hauser B.O. The prolapsed intervertebral disc. The high-intensity zone with discography correlation. Spine. 1996;21:2758-2762.
74. Bogduk N. Why I pursue discogenic pain. Annual Scientific Meeting of the German Pain Society; 2010. 20-10-2005
75. Carragee E.J., Paragioudakis S.J., Khurana S. 2000 Volvo Award winner in clinical studies: Lumbar high-intensity zone and discography in subjects without low back problems. Spine. 2000;25:2987-2992.
76. Carrino J.A., Lurie J.D., Tosteson A.N., et al. Lumbar spine: reliability of MR imaging findings. Radiology. 2009;250:161-170.
77. Fardon D.F., Milette P.C. Nomenclature and classification of lumbar disc pathology. Recommendations of the Combined task Forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Spine. 2001;26:E93-E113.
78. Teplick J.G., Haskin M.E. Spontaneous regression of herniated nucleus pulposus. AJR Am J Roentgenol. 1985;145:371-375.
79. Saal J.A., Saal J.S., Herzog R.J. The natural history of lumbar intervertebral disc extrusions treated nonoperatively. Spine. 1990;15:683-686.
80. Bush K., Cowan N., Katz D.E., Gishen P. The natural history of sciatica associated with disc pathology. A prospective study with clinical and independent radiologic follow-up. Spine. 1992;17:1205-1212.
81. Carragee E.J., Alamin T.F., Miller J.L., Carragee J.M. Discographic, MRI and psychosocial determinants of low back pain disability and remission: a prospective study in subjects with benign persistent back pain. Spine J. 2005;5:24-35.
82. Ross J.S., Zepp R., Modic M.T. The postoperative lumbar spine: enhanced MR evaluation of the intervertebral disk. AJNR Am J Neuroradiol. 1996;17:323-331.
83. Ross J.S. MR imaging of the postoperative lumbar spine. Magn Reson Imaging Clin N Am. 1999;7:513-524.
84. Ross J.S., Robertson J.T., Frederickson R.C., et al. Association between peridural scar and recurrent radicular pain after lumbar discectomy: magnetic resonance evaluation. ADCON-L European Study Group. Neurosurgery. 1996;38:855-861.
85. Czervionke L.F., Fenton D.S. Fat-saturated MR imaging in the detection of inflammatory facet arthropathy (facet synovitis) in the lumbar spine. Pain Med. 2008;9:400-406.
86. Phuong L.K., Atkinson J.L., Thielen K.R. Far lateral extraforaminal lumbar synovial cyst: report of two cases. Neurosurgery. 2002;51:505-507.
87. Apostolaki E., Davies A.M., Evans N., Cassar-Pullicino V.N. MR imaging of lumbar facet joint synovial cysts. Eur Radiol. 2000;10:615-623.
88. Modic M.T., Feiglin D.H., Piraino D.W., et al. Vertebral osteomyelitis: assessment using MR. Radiology. 1985;157:157-166.
89. Slipman C.W., Shin C.H., Patel R.K., et al. Etiologies of failed back surgery syndrome. Pain Med. 2002;3:200-214.
90. Perronne C., Saba J., Behloul Z., et al. Pyogenic and tuberculous spondylodiskitis (vertebral osteomyelitis) in 80 adult patients. Clin Infect Dis. 1994;19:746-750.
91. Hadjipavlou A.G., Cesani-Vazquez F., Villaneuva-Meyer J., et al. The effectiveness of gallium citrate Ga 67 radionuclide imaging in vertebral osteomyelitis revisited. Am J Orthop. 1998;27:179-183.
92. Whalen J.L., Brown M.L., McLeod R., Fitzgerald R.H.Jr. . Limitations of indium leukocyte imaging for the diagnosis of spine infections. Spine. 1991;16:193-197.
93. Lyritis G.P., Mayasis B., Tsakalakos N., et al. The natural history of the osteoporotic vertebral fracture. Clin Rheumatol. 1989:66-69.
94. Lourie H. Spontaneous osteoporotic fracture of the sacrum. An unrecognized syndrome of the elderly. JAMA. 1982;248:715-717.
95. Grangier C., Garcia J., Howarth N.R., et al. Role of MRI in the diagnosis of insufficiency fractures of the sacrum and acetabular roof. Skeletal Radiol. 1997;26:517-524.
96. Stabler A., Beck R., Bartl R., et al. Vacuum phenomena in insufficiency fractures of the sacrum. Skeletal Radiol. 1995;24:31-35.
97. Cannada L.K., Scherping S.C., Yoo J.U., et al. Pseudoarthrosis of the cervical spine: a comparison of radiographic diagnostic measures. Spine. 2003;28:46-51.
98. Hadjipavlou A.G., Cesani-Vazquez F., Villaneuva-Meyer J., et al. The effectiveness of gallium citrate Ga 67 radionuclide imaging in vertebral osteomyelitis revisited. Am J Orthop. 1998;27:179-183.