Chapter 81 HIV-Associated Infections
Acquired immunodeficiency syndrome (AIDS) is a potentially fatal multisystem syndrome characterized by profound disruption of the immune system and a propensity for various opportunistic infections and neoplasms. AIDS is caused by either of two human immunodeficiency viruses (HIV-1 [formerly HTLV-3] or HIV-2).1–3 HIV-1 entered humans from chimpanzees nearly a century ago and, after passing unrecognized among West Africans,4,5 came to the United States via Haiti in the 1970s. AIDS was recognized first in 1981 as outbreaks of usual opportunistic infections (OIs) in homosexual men in three American cities.
Ocular involvement occurs in up to 73% of AIDS patients,6,7 with the most common lesions being a retinal vasculopathy consisting of cotton-wool spots, retinal hemorrhages, and infectious retinopathy such as cytomegalovirus (CMV), herpetic, toxoplasmic, or luetic retinitis.
Epidemiology of hiv infection and aids
By 2010 an estimated 1.1 million people were living with HIV in the United States, approximately 56 000 new HIV infections have occurred annually for more than a decade, and about 15 000 patients die annually. This difference between incidence and mortality increases the prevalence of HIV infection by about 40 000 persons annually. Since 1996 AIDS has been converted from an inevitably progressive and fatal disease into a manageable chronic condition by three-drug regimens referred to as highly active or combination antiretroviral therapy (HAART or CART). HIV is spread by homo- and heterosexual intercourse, blood exposures through shared needles among intravenous drug users, or peripartum or by breast feeding of infants.8–11
Occupational exposure to HIV
The average risk of HIV transmission in healthcare workers after percutaneous exposure to HIV-infected blood is approximately 0.3% without treatment.12,13 This risk is further lowered by double-gloving and is probably much lower in the ophthalmic setting.14 Postexposure prophylaxis (PEP) with two or three antiretroviral drugs appears likely to dramatically reduce the transmission of HIV even after high-risk injuries.15 Because PEP should be started as soon as possible after injury, healthcare institutions should have well-developed procedures that provide for expert consultation and ready access to a combination of drugs for persons at high risk of injuries, such as surgeons, invasive proceduralists, and phlebotomists.
Currently, for percutaneous injuries, the US Public Health Service recommends 4 weeks of treatment with a two-drug regimen if the exposure is less severe (solid needle and superficial injury) and the source patient has asymptomatic HIV infection or known low viral load (<1500 RNA copies/ml) [class I patient]. An expanded three-drug regimen is recommended if the exposure is severe (large-bore hollow needle, deep puncture, visible blood on device, or needle used in patient’s artery or vein) or if the source patient has symptomatic HIV infection, AIDS, acute seroconversion, or known high viral load [class II patient]. The recommended two-drug regimens for HIV/PEP are: (a) tenofavir plus emtricitabine or (b) zidovudine plus lamivudine. Tenofovir is generally better tolerated, but should not be used in persons with renal insufficiency. Three-drug regimens involve adding ritonavir-boosted (/r) lopinavir/r or darunavir/r. Alternatives for constructing an expanded regimen in cases of resistance, drug interactions, or intolerance include darunavir/r, atazanavir/r, or raltegravir. For mucous membrane or nonintact skin exposures, the two-drug regimen is recommended for all small volume exposures (a few drops) and large volume (major blood splash) exposure in a class I patient, with three-drug expanded regimens recommended for large volume exposure in a class II patient.13
HIV virology and pathogenesis
HIV infection usually gradually depletes CD4 lymphocytes, resulting in decreased blood levels of this crucial subset of “helper” T cells.16 AIDS patients typically become ill only after CD4+ helper T cells reach less than 200/ml, a level that no longer supports cell-mediated immunity at levels needed to contain infections by select opportunistic viral, bacterial, or fungal pathogens.
Understanding of HIV infection was revolutionized by the development in the mid-1990s of assays that measured the levels of HIV in the blood. All stages of HIV infection were seen to be characterized by a high rate of viral replication in most patients. Levels of plasma HIV-1 RNA predict the rate of clinical progression in HIV patients.17–23 Both high replication and an error-prone reverse transcription process promote frequent mutations in the HIV genome that result in the emergence of variants that can better resist either control by the hosts antibody and cell-mediated immune responses or by antiretroviral drugs, if drugs are present in insufficient numbers or at low blood levels that fail to fully suppress HIV replication. Under selective pressure from antiretroviral agents, mutations that confer a decreased sensitivity to individual drugs are selected and stored in latent cells where they last for decades.24–27
Therapy of HIV infections
Treatment of HIV has evolved rapidly over the past 25 years (1986–2011) with the development of more than 30 drugs that fall into at least four classes based on which of the steps in HIV replication they inhibit. These steps include: (a) binding to receptors and entry into the host cell; (b) reverse transcription of HIV RNA to proviral DNA; (c) integration of proviral DNA into the host cell genome; and (d) maturation of HIV after budding by the action of HIV protease.28 Coadministration of drugs from several of the classes delays or prevents the emergence of drug-resistant HIV by minimizing viral replication. However, HIV may develop resistance to all available therapy because (a) patients have difficulty maintaining high levels of adherence over long periods and (b) cross-resistance is common to drugs within a class.29–32 After failure of a regimen, the next combination of drugs is less likely to be successful in fully suppressing HIV replication which is the necessary condition for prolonged success. For details of recent expert guidelines on HIV therapy consult Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents, which is constantly updated and available at this NIH website: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf (cited February 2012).
Clincial spectrum of HIV
HIV infection and disease is highly variable in presentation and includes asymptomatic, various chronic or recurring constitutional signs and symptoms, and a plethora of opportunistic conditions. The “acute retroviral syndrome,” that is, early or primary infection with HIV, is characterized by fever, pharyngitis, skin rash, arthralgias, malaise, mucosal ulcerations, and neurologic manifestations such as aseptic meningitis.33
Patients chronically infected with HIV may present with a prodrome of generalized, nontender lymph node enlargement, fevers and night sweats, weight loss, and diarrhea for weeks or months, formerly termed AIDS-related complex (ARC). Nearly all HIV-seropositive patients will progress to AIDS, but a small minority, called “long-term nonprogressors” or “elite controllers,” suppress their infections naturally (without treatment) to the point of very low or undetectable levels of plasma HIV. Although highly active antiretroviral therapy (HAART) often reduces plasma viremia of HIV-1 to undetectable levels, latent viral reservoirs of resting CD4 lymphocytes persist for years and reappear in the blood if therapy is stopped.34
Opportunistic infections are responsible for the deaths of most AIDS patients, but consequences of coinfections with hepatitis C and B, such as hepatic insufficiency or hepatocellular carcinoma, have become increasingly important, in part because HIV promotes progression of these infections.35 The most common pathogens encountered in AIDS patients are cytomegalovirus (CMV), Candida albicans, Pneumocystis jiroveci (formerly carinii), Mycobacterium tuberculosis and M. avium-intracellulare, Cryptococcus neoformans, herpes simplex virus (HSV), Cryptosporidium spp., Toxoplasma gondii, and varicella zoster virus (VZV).3,36 CMV retinitis can be the initial sign of tissue-invasive systemic CMV infection in these patients, although it is restricted to patients with advanced immunosuppression (CD4+ count less than 50). CMV may present also in the gastrointestinal tract, brain and spinal cord, or other organs.37
Infection control related to HIV
The US Centers for Disease Control and Prevention (CDC) recommendations for universal precautions to prevent the occupational transmission of HIV and other bloodborne viruses in the healthcare setting were provided in 1988 and have not been amended.38
Sterilization of all instruments and equipment that come into contact with the eye in all patients is necessary using gas or steam autoclaving or a 5- to 10-minute soak in one of the following solutions: 3% hydrogen peroxide solution, 10% solution of sodium hypochlorite (common household bleach), or 70% ethanol or isopropanol. Instruments disinfected in this manner should be rinsed in water and dried before reuse.39,40 Damage to tonometer tips has been reported with the use of 70% isopropanol; thus a 5- to 10-minute soak in 3% H2O2 or 1 : 10 dilution of household bleach may be preferable.41 It should be noted that there is no evidence of HIV transmission through contact with tears or instruments used to examine these patients.39
Contact lenses used in trial fittings on all patients should be disinfected by use of any commercially available cleaning method or solution.42 Inactivation of HIV by various disinfectants on surfaces has been reviewed.43 Guidelines for preventing transmission of HIV through transplantation of human tissue and organs (including corneal transplants) have been set forth.44 Specific recommendations for postexposure management of needle-stick injuries or mucosal membrane exposures to secretions from patients with HIV infection have been published.45
Ocular findings in aids: an overview
HIV has been detected in the cornea,46 conjunctival epithelium,47 and in tears,46 but at very low titers. Ocular manifestations of AIDS may be seen in up to 100% of patients. They are less common, but may be seen in patients with earlier, symptomatic HIV infection.48 Most common are cotton-wool spots and other noninfectious retinopathies,49 CMV retinitis, and conjunctival Kaposi sarcoma, followed less frequently by herpes zoster ophthalmicus,50,51 retinal toxoplasmosis, choroidal P. carinii infection, herpes simplex and herpes zoster retinitis (acute retinal necrosis [ARN]), and cryptococcal choroiditis.6,52–59
Iritis may occur in association with viral retinitis but especially with CMV; it is mild. Acute iritis may be associated with the use of oral rifabutin (used for the treatment and prophylaxis of mycobacterial infections) or intravenous cidofovir used for CMV retinitis.60
Choroidal infection with Cryptococcus, Pneumocystis, M. tuberculosis, Aspergillus, Toxoplasma, Histoplasma, and M. avium-intracellulare usually is associated with systemic infection.61,62 Histoplasma capsulatum chorioretinitis and endophthalmitis,63 Paracoccidioidomycosis brasiliensis chorioretinitis,64 keratitis sicca, cranial nerve paralysis, Roth’s spots, papilledema, perivasculitis, and fungal corneal ulcers are rare but have been reported.65
Noninfectious retinopathy
Noninfectious retinopathy refers to cotton-wool spots, retinal hemorrhages, and microvascular abnormalities that do not progress, enlarge, or cause visual symptoms. No infectious cause of these lesions has been demonstrated, and they appear to represent nonspecific retinal microvascular disease. A correlation between the number of cotton-wool spots and decreased cerebral blood flow (as shown by technetium 99m hexamethylpropyleneamine oxime single photon emission computed tomography) was shown in 25 patients with AIDS or symptomatic HIV infection.66
Cotton-wool spots are the most common ocular lesion seen in AIDS, occurring in 25–50% of patients6,67 and in up to 75% of cases by autopsy examination.57 In one study, up to 92% of AIDS patients were found to have evidence of retinovascular disease when examined using fluorescein angiography.67
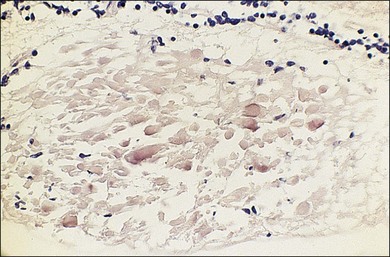
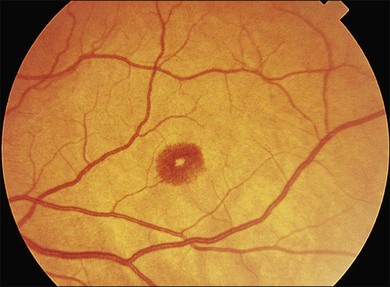
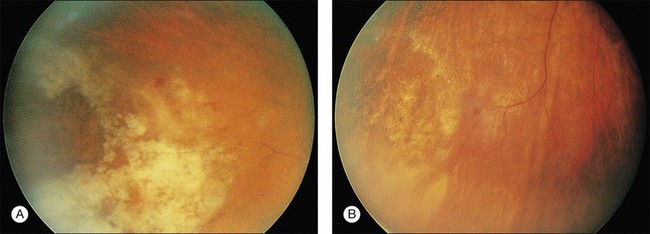
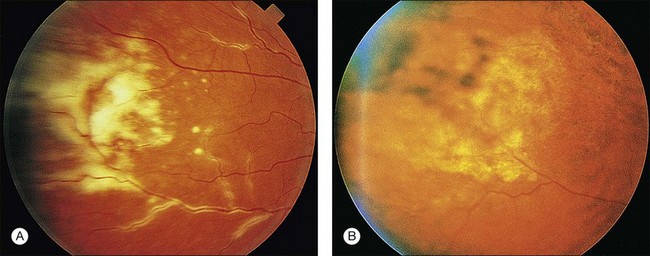
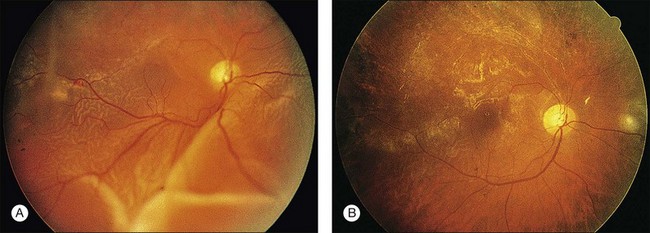
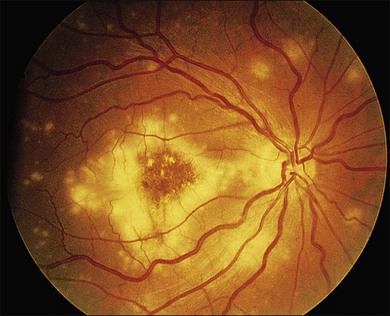
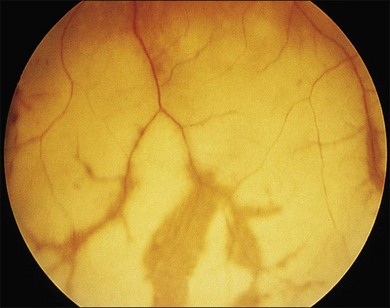
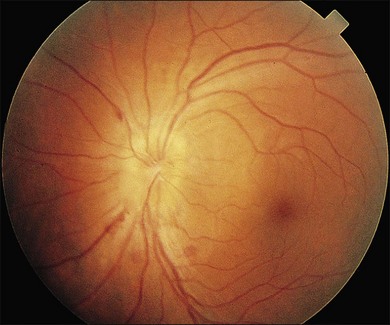
Cotton-wool spots seen by ophthalmoscopy are a result of microinfarction of the nerve fiber layer of the retina. In AIDS these lesions usually are confined to the posterior pole near the optic disc67 (Fig. 81.1). Histopathologic study of retinal cotton-wool spots in AIDS patients has demonstrated that these lesions have pathologic features identical to those seen in cotton-wool spots of other cause. Similar to the cotton-wool spots seen in other systemic diseases, this lesion in AIDS demonstrates no associated inflammation, no cells in the vitreous, and no vascular leakage on fluorescein angiography (Fig. 81.2). Attempts to isolate organisms from cotton-wool spots in the hope of explaining their cause in AIDS as infectious have been unsuccessful, and the cause of this lesion in AIDS remains elusive.6,57,68,69
Cotton-wool spots have been speculated to be harbingers of CMV retinitis or perhaps sites of susceptibility to CMV infection, but substantiation of these ideas is lacking. Histopathologic studies of eyes at autopsy have failed to show clear evidence of a viral cause of cotton-wool spots.70–72
We have reported that noninfectious retinopathy is not seen in HIV-seronegative men and is rare in ARC, but it is very common in patients with AIDS even in the absence of active opportunistic ophthalmic infection.48 It is striking that this lesion may be seen in 50–75% of AIDS patients, and studies using multiple examinations indicate that the more frequently these patients are examined, the higher the incidence may be.6,49 Cotton-wool spots probably are ophthalmoscopically visible for 6–12 weeks, and owing to the transient nature of the lesion and its apparent noninfectious cause, treatment is not indicated at this time.73
In a cross-sectional study, the median CD4 count (per microliter [µl]) in patients with cotton-wool spots was 14 cells (range 0–160) and was 8 cells (range 0–42) in patients with CMV retinitis.74 In the absence of other systemic vascular disease, such as hypertension or diabetes mellitus, AIDS must be considered in the differential diagnosis of cotton-wool spots owing to their very high prevalence in these patients. Whether these lesions are an early manifestation of AIDS remains to be elucidated, but they may be apparent in HIV-infected persons before the onset of opportunistic infections.48
Morphologic studies have shown that the number of retrobulbar optic nerve fibers in patients with AIDS is decreased compared with the number of optic nerve fibers in normal control eyes as a result of axonal degeneration and an associated decrease in the number of optic nerve axons.75–77 Infarctions of the nerve fiber layer develop in most patients with AIDS, and the number of such infarctions increases over time.6,49 Visual dysfunction associated with multiple nerve fiber layer infarctions may be manifested by defects in color-vision and contrast-sensitivity testing in patients with AIDS.78 Interestingly, in vivo studies of the retinal nerve fiber layer have shown both broad and slit-like defects, suggesting that retinal nerve fiber loss and optic nerve fiber loss are related to subclinical vision loss in HIV patients without infectious retinitis.79 Electroretinographic studies of HIV patients without retinitis also have shown retinal dysfunction.80,81
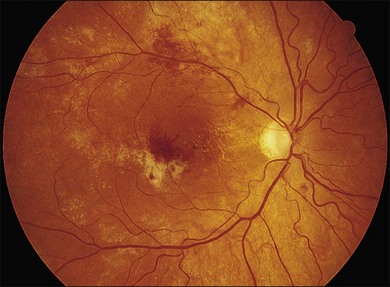
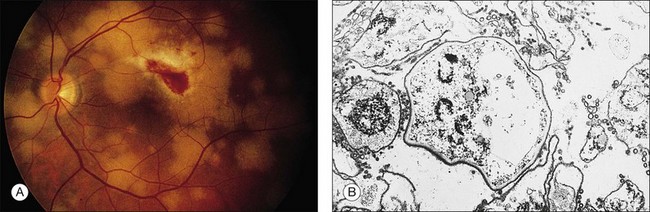
Retinal hemorrhages are seen in AIDS in association with CMV retinitis, cotton-wool spots, and as an isolated finding. These lesions have been reported in up to 30%52,67,82 of AIDS patients, and autopsy evidence of retinal hemorrhages has been reported to be as high as 40%. Retinal hemorrhages usually take the form of flame-shaped lesions in the posterior pole, dot-blot hemorrhages, or as punctate intraretinal hemorrhages peripherally (Fig. 81.3). Occasionally the hemorrhage is manifested as Roth’s spots (hemorrhage with a white central area).52,67 The pattern of retinal hemorrhages changes over time. The hemorrhages do not appear to be related to a bleeding diathesis or coagulopathy, but rather seem to be a manifestation of AIDS itself.6 Vision loss from retinal hemorrhage has not been described, and treatment is conservative if the lesions are not associated with CMV retinitis or septicemia.
Microvascular pathologic findings in AIDS, as demonstrated by fluorescein angiography, include microaneurysms, telangiectasias, focal areas of nonperfusion, and capillary loss.67 These changes are similar to the changes seen in diabetes mellitus. The histologic findings of periodic acid–Schiff (PAS)-positive thickening of blood vessels and precapillary arteriolar closure also correlate with the findings in diabetes mellitus.
Branch or central retinal vein occlusion, branch retinal artery occlusion, and ischemic maculopathy have been reported in HIV patients without infectious retinitis. The incidence is unknown. It is possible that the cause may be related to lupus anticoagulant and other clotting abnormalities seen in HIV-infected patients.83,84 Abnormalities of retinal blood flow have also been reported in HIV patients and may contribute to the pathogenesis of microvascular abnormalities.85–88
Infectious retinopathy
Cytomegalovirus retinitis
Pathogenesis, diagnosis, and clinical manifestations
Cytomegalovirus (CMV) infection is a major cause of morbidity and mortality in AIDS. CMV retinitis has been reported to occur in 15–40% of AIDS patients with the rate declining since the arrival of HAART.37,52,89,90 In contrast to the noninfectious lesions of AIDS, CMV retinitis demands aggressive treatment to prevent severe visual loss.6,91,92 Patients with active CMV disease may have systemic symptoms of fever, arthralgia, and pneumonitis, or leukopenia, retinitis, or hepatitis; blood cultures and urine specimens may be positive for CMV. CMV retinitis often is the presenting sign of systemic CMV infection, and all patients should be thoroughly evaluated for systemic disease.
The clinical presentation of CMV retinitis in AIDS is similar in many respects to CMV retinitis found in iatrogenically immunosuppressed patients and infants with cytomegalic inclusion disease.93,94 Correlation of the clinical and typical pathologic findings at autopsy has been demonstrated.95 Specifically, it is known that CMV is a neurotropic virus with a tendency to infect neural tissues and the retina. Necrosis of the retina in AIDS-associated CMV retinitis is typical, with pathognomonic cytomegaly and minimal inflammatory cells present in the lesions. Choroidal involvement is rare, and whether vascular endothelium is involved is unclear. These lesions also may appear as noncontiguous patches rather than the more commonly seen contiguous spreading lesion. Antigens to CMV have been found by immunofluorescence, immunoperoxidase staining, and DNA hybridization techniques.96,97 The most distinctive anterior segment finding is the presence of fine stellate keratic precipitates on the corneal endothelium.98 Retinal vascular nonperfusion and retinal neovascularization resulting from CMV retinitis and choroiditis also have been reported.99
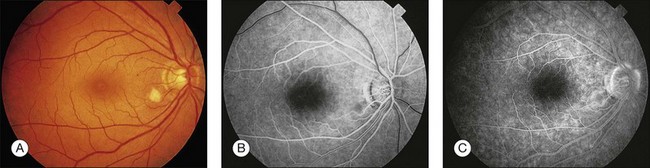
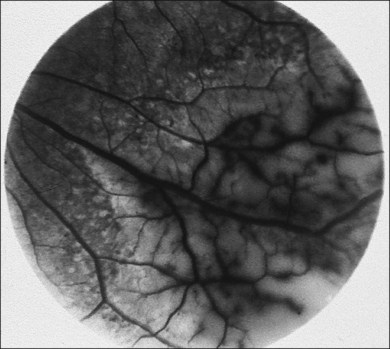
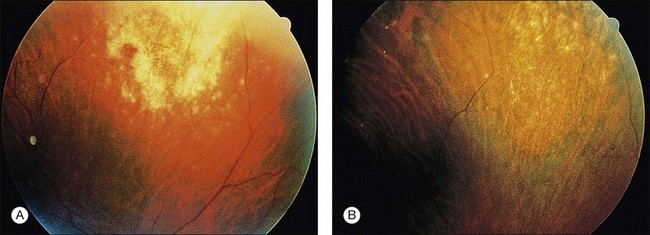
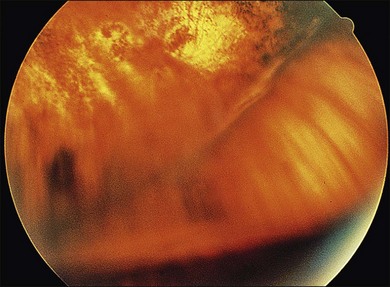
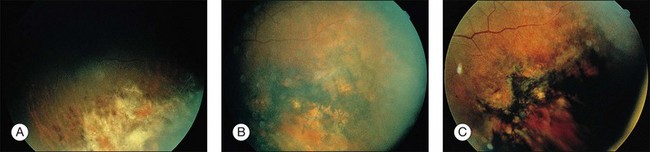
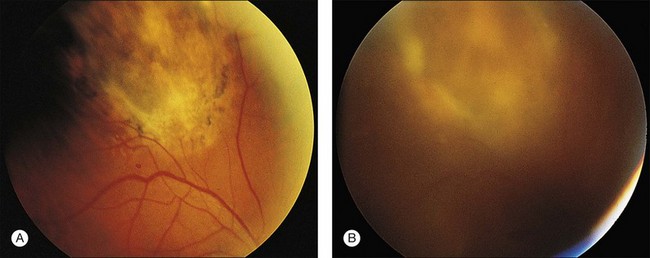
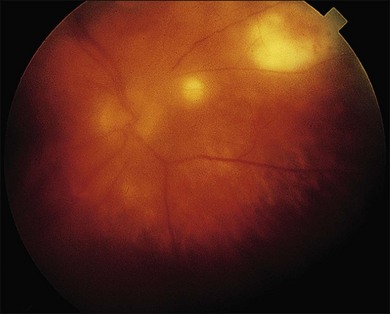
CMV is a slowly progressive necrotizing retinitis that may affect the posterior pole, the periphery, or both, and may be unilateral or bilateral. Involved retinal areas appear as white intraretinal lesions, areas of infiltrate, and often necrosis along the vascular arcades in the posterior pole. In addition, prominent retinal hemorrhages often are present within the necrotic area or along its leading edge (Fig. 81.4). Peripherally, CMV retinitis occurs commonly; it tends to have a less intense white appearance, with areas of granular, white retinitis that may or may not demonstrate associated retinal hemorrhage (Fig. 81.5). As the retinitis progresses, an area of atrophic, avascular retina may remain with underlying retinal pigment epithelial atrophy or hyperplasia.6,37,100 Peripheral CMV retinitis is common in AIDS patients who initially may report only floaters with or without a visual field deficit. Wide-angle fundus photography and fluorescein angiography may be of benefit if the diagnosis is uncertain. These techniques may be used to document progression of retinitis, and fluorescein leakage in areas of retinitis may be helpful in confirming the diagnosis (Fig. 81.6).
Reactivation of CMV retinitis is characterized by reopacification of the border of the lesion followed by advancement. Smoldering retinitis (Fig. 81.7) and subtle reactivation may be difficult to recognize without prior fundus photographs. Several studies have shown that wide-angle fundus photographs are a more sensitive indicator of retinitis progression than is clinical examination by indirect ophthalmoscopy.101,102
Several investigators have shown that untreated CMV retinitis is inexorably progressive in AIDS patients.6,52,94,96,102,103 As in our experience, untreated CMV becomes bilateral in the vast majority of patients. In an observational study of 26 patients treated for CMV retinitis, vision scores decreased with greater abnormalities found on ophthalmologic examination. Visual symptoms were most strongly related to findings in the worse eye. Patients reported considerable impairment, including blurred vision (42%), difficulty reading (40%), difficulty driving (44%), treatment interference with social activities (40%), and substantial trouble with vision (50%).104 Treatment of AIDS-related CMV retinitis minimizes loss of vision and may protect previously uninfected eyes, prolonging visual independence.105
Recurrent CMV retinitis exhibiting a foveal-sparing pattern within 1500 mm of the foveola has been described and occurs primarily in patients with recurrent CMV retinitis resistant to treatment (“clinically resistant”), particularly that which has arisen temporally. Despite its foveolar proximity and ultimate significant loss of function, the pattern of progression allows for preservation of useful foveal vision for longer periods than would have been expected.106
Other manifestations of CMV retinitis include retinal edema, attenuated vessels, perivascular sheathing, and exudative retinal detachment107 (Fig. 81.8). In addition, vitritis and anterior uveitis are often seen, and optic atrophy may occur as a late manifestation resulting from widespread retinal destruction. CMV occasionally may be demonstrated in vitreous biopsy specimens in these patients.95 The yield may be higher in the presence of marked vitritis because CMV is a cell-associated virus. Other causes of retinitis, including herpes simplex retinitis,93,108 toxoplasmosis,62 candidal infection, Behçet disease, syphilis, acute retinal necrosis,109,110 and subacute sclerosing panencephalitis, usually can be distinguished from CMV on clinical grounds, although this may not be the case in retinitis caused by other members of the herpesvirus family.109 CMV has a very characteristic clinical appearance, but the lesions in CMV retinitis vary from patient to patient, and it is important to maintain a high index of suspicion for the above infections, especially in light of frequent superinfection of AIDS patients with multiple organisms.6,82,83
CMV retinitis is a reflection of underlying active systemic CMV infection. In almost all cases it is a blinding disease if not controlled. Thus, in the face of changing mental status, development of focal signs on neurologic examination, or other symptoms consistent with subacute encephalitis in AIDS patients, a comprehensive ophthalmologic examination is indicated, and an increased index of suspicion of CMV infection of the CNS and possible CMV retinitis is warranted. There is also evidence that patients with CMV retinitis, especially peripapillary disease, have a much higher incidence of CMV encephalitis. CMV infection of the brain, optic nerves, and retinas from 47 consecutive autopsies of patients with AIDS was examined.111 Immunocytochemistry demonstrated CMV infection in 11 (23%) brains, 2 (2%) of 94 optic nerves, and 38 (40%) of 94 retinas. Ten (91%) of 11 patients with CMV encephalitis had concurrent retinitis. While 10 (42%) of 24 patients with CMV retinitis had CMV encephalitis, when the retinitis included the peripapillary region, 75% had encephalitis. The optic nerve parenchyma usually was not infected histologically despite extensive peripapillary retinitis. The strength of these associations suggests that CMV retinitis defines a group of patients with AIDS at risk for development of CMV encephalitis (relative risk, 9.5%), especially when the retinitis involves the peripapillary region (relative risk, 13%). Furthermore, in patients with AIDS without CMV retinitis, central nervous system symptoms are unlikely to be attributable to CMV encephalitis.111 The pathologic correlation between ocular and cerebral lesions in patients with AIDS has been reviewed.112
CMV retinitis is less frequent in children with AIDS, with reported rates of approximately 5–6%, though rates of extraocular CMV are higher than in adults. CMV retinitis has been reported in young children with high absolute CD4 counts, though these counts are low relative to the child’s age. Older children tend to have low absolute CD4 counts similar to adults. There is a higher incidence of bilateral and posterior pole disease in children, however this is likely due in part to delays in diagnosis in children from lack of subjective vision complaints.113–115
Screening techniques for retinal and systemic CMV infection
Screening for CMV retinitis is a difficult problem. Many patients who are CMV-viremic or -viruric may not have end-organ disease, and studies employing quantitative CMV polymerase chain reaction (PCR) in plasma or CMV antigenemia have not been able to definitively predict the development of CMV retinitis.116 Currently no laboratory marker exists that reliably predicts the occurrence of clinical CMV retinitis.
Urine is culture-positive for CMV in more than 50% of homosexual men and the majority of AIDS patients; thus urine culture may not be of diagnostic value. Serology in AIDS patients is nonspecific, and documentation of rising CMV titers is unusual.6,96 Studies of newly diagnosed CMV retinitis patients indicate that many are CMV culture-negative in the blood. Positive blood cultures for CMV, fever, and weight loss are associated with more extensive CMV retinitis at the time of diagnosis.117 The results of virologic blood assays for CMV also have been associated with clinical outcome in patients with CMV retinitis.118 Thus assays for the detection of CMV antigenemia may be a simple and rapid means of identifying those patients with unilateral retinitis at highest risk of developing CMV retinitis of the fellow eye or of visceral CMV disease if intravitreal injections or implants are used as the sole treatment for CMV retinitis.119
A positive PCR result supports the clinical diagnosis and may be useful for monitoring response to antiviral treatment. By prospective monitoring for increases in plasma CMV DNA copy number, it may be possible to identify HIV-seropositive patients who are at imminent risk for development of symptomatic CMV retinitis.120
It is also reasonable and practical to use the CD4 cell count as a threshold below which to screen patients, since the risk of CMV retinitis increases at CD4 cell counts below 50/mm3.74 The incidence and prevalence of CMV retinitis in a cohort study of patients with CD4 cell counts below 0.10 × 109/L (100/µl) revealed a 25% chance for the development of CMV retinitis by 4 years of follow-up. Among those subjects in whom CMV retinitis developed, about 19% had retinitis before a CD4 cell count of less than 0.05 × 109/L (<50/µl) was observed, and 81% had CMV retinitis after the CD4 cell count reached this threshold.121 In the HAART era, some patients may develop CMV retinitis with CD4 counts above 100/ml, probably because of incomplete restoration of the immune repertoire against CMV.122
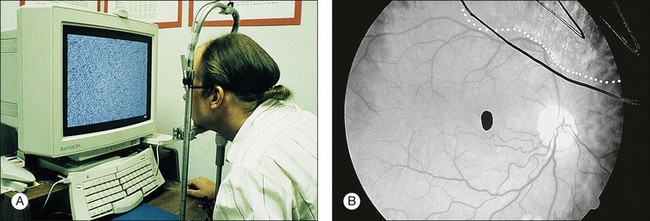
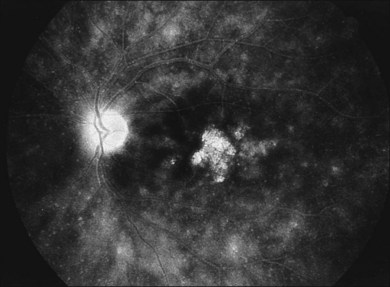
A technique for screening for central CMV retinitis, entoptic perimetry, employs patient visualization of moving particles on a computer monitor, appears to have a very high sensitivity and specificity (over 90%) in detecting CMV retinitis within the central 30-degrees radius of fixation79 (Fig. 81.9).
Techniques for detection of CMV DNA based on PCR are increasingly being applied to ocular fluids; however, the clinical significance of such findings can sometimes be unclear. The application of PCR-based methods to ocular fluids made a useful contribution to the treatment of the patients.123
This appears to be a sensitive and specific diagnostic assay that could assist in the diagnosis of CMV retinitis.124 PCR detection of CMV DNA has been reported to be a more sensitive method than analysis of locally produced antibodies by calculating a Goldman–Witmer coefficient to determine local ocular antibody production. There is also an immune predisposition to the development of CMV retinitis in patients with AIDS.125,126
Treatment of CMV retinitis
The treatment of CMV retinitis has been reviewed.127,128 Treatment may be systemic, local, or a combination of the two. There are currently five medications approved by the US Food and Drug Administration (FDA) for the treatment of CMV retinitis: ganciclovir, valganciclovir, cidofovir, foscarnet. Fomivirsen, the first antisense drug with a relatively long duration of action, is no longer available in the United States.
Systemic therapy of CMV retinitis
CMV retinitis may be treated systemically or intravitreally. However, systemic treatment is associated with less spread of CMV retinitis from one eye to the other.128 In addition, local treatment including the sustained release ganciclovir implant has been shown to be associated with higher risk of the development of systemic CMV.129,130
Systemic CMV may cause gastrointestinal disease, with colitis being the most common manifestation as well as esophagitis. Systemic CMV diagnosis may be difficult and usually requires histopathologic evidence of CMV infection. The cumulative incidence of systemic CMV disease that becomes clinically apparent is approximately 25%.131 Therefore, although systemic CMV disease may not be clinically apparent at the time of diagnosis of CMV retinitis, some experts believe that initial systemic therapy may be warranted despite the inconvenience, expense, and potential toxicities.
Intravenous ganciclovir
Ganciclovir is a nucleoside analog of 2-deoxyguanosine, similar to acyclovir.132 Despite its structural similarities with acyclovir, ganciclovir is much more active in vitro against CMV than acyclovir.133 Ganciclovir inhibits all herpesviruses, including CMV, by preventing DNA elongation. CMV lacks the virally specified thymidine kinase (TK) that converts ganciclovir (or acyclovir) to its monophosphate form.134 TK-altered strains resistant to acyclovir are as sensitive to ganciclovir as the unaltered parent strains. Thus ganciclovir is phosphorylated to its triphosphate form much more efficiently than acyclovir, which accounts for the greater activity of ganciclovir against CMV.135
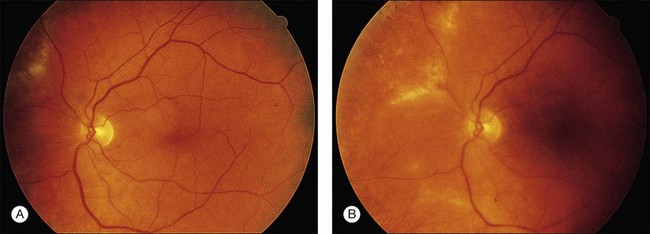
The majority of AIDS patients treated with ganciclovir respond within 2–4 weeks with decreased retinal opacification and stabilization of the retinitis89 (Fig. 81.10). Ganciclovir is commercially available as an intravenous and oral formulation (and also is included in an intraocular device), and indefinite maintenance therapy is necessary as long as the patient remains in immune failure with a CD4 count below 50/µl. An intravenous loading dose of 5 mg/kg every 12 hours for 14–21 days should be followed by maintenance doses of 5 mg/kg/day. If the drug is discontinued, retinitis often recurs within 10–21 days, continuing its progression at the borders of healed areas.89 Recurrences have been common, even during maintenance therapy, being reported 3 weeks to 5 months after institution of therapy and occurring in 30–40% of patients.89 Many investigators have found that discontinuation or delay of ganciclovir therapy results in nearly 100% recurrence of retinitis, at which time reinstitution of the loading dose regimen often is necessary.134,136,137 Multiple series in patients with AIDS and CMV retinitis have shown response rates of 80–100%, with 60–80% of patients achieving a remission with ganciclovir therapy.37,137–141
The treatment of CMV retinitis usually includes an induction phase followed by a maintenance phase to prevent relapse. Before the advent of HAART, relapse occurred almost universally, given a sufficiently long period after induction courses of ganciclovir. Therapy therefore continued for a lifetime. The median time to relapse in patients treated with just an induction dose is 3–4 weeks.137
Side-effects of ganciclovir include granulocytopenia, neurologic dysfunction, abnormal liver function tests, and rarely, thrombocytopenia. The most serious toxicity is granulocytopenia, which may occur in up to one-third of patients when defined as less than 500 neutrophils per microliter.137 Granulocytopenia is generally reversible, and this adverse effect is exacerbated when used with AZT.142 The use of colony-stimulating factors rGM-CSF (recombinant granulocyte–macrophage colony-stimulating factor) and rG-CSF (recombinant granulocyte colony-stimulating factor) for reversing or preventing neutropenia may be useful.
The prevalence of CMV resistance to ganciclovir is unknown, but ever-increasing induction regimens may be necessary to control CMV retinitis. Strains of CMV that develop resistance to ganciclovir may remain susceptible to foscarnet. Because of the question of ganciclovir resistance, a trial of combined vs alternating foscarnet–ganciclovir maintenance therapy has been reported to be effective.143
Visual acuity depends on the location of the involved retina, and involvement of the fovea or optic nerve may result in decreased visual acuity even if there has been a response to therapy. Ganciclovir has been shown to be effective in preserving visual acuity. For example, 73% of eyes maintained a visual acuity of 20/40 or better when treated with ganciclovir.144
Oral ganciclovir
Perhaps the most common use of oral ganciclovir is in patients being treated with intravitreal therapy (injections of cidofovir or ganciclovir implant) to prevent systemic CMV disease. Such use of oral ganciclovir also may reduce the incidence of CMV in the fellow eye if it is not involved.145,146 Oral ganciclovir for prophylaxis or treatment of CMV retinitis has been replaced by oral valganciclovir, which provides much higher blood levels.
Valganciclovir
Valganciclovir, the valine ester of ganciclovir, is an orally administered formulation of ganciclovir. The valine ester confers enhanced permeability and absorption of the molecule through the cell membranes of the gut. Once in the bloodstream, the valine ester is cleaved from the molecule by esterases, rendering plasma levels of ganciclovir comparable to those achieved with intravenous ganciclovir administration. A single-dose randomized crossover pharmacokinetic study reported the absolute ganciclovir bioavailability after oral valganciclovir administration is 60.9% compared to 5.6% bioavailability of oral ganciclovir.147 A randomized crossover dose-ranging study determined that plasma levels of ganciclovir after an 875 mg dose of valganciclovir are similar to the plasma levels achieved with a dose of 5 mg/kg of intravenous ganciclovir (AUC 24.8 mg/ml per h vs 26 mg/ml per h). The authors suggested the 900 mg dose of valganciclovir would approximate the AUC value of the 5 mg/kg dose of IV ganciclovir.148
Valganciclovir has been studied for induction therapy for CMV retinitis. Martin et al. (Valganciclovir Study Group) reported a multicenter randomized, controlled clinical trial comparing oral valganciclovir 900 mg twice a day for 3 weeks’ induction therapy followed by 900 mg daily for 1 week maintenance therapy with intravenous ganciclovir 5 mg/kg twice a day for 3 weeks’ induction therapy followed by 5 mg/kg once daily for one week of maintenance therapy. After 4 weeks both groups received continued maintenance therapy with 900 mg valganciclovir daily. Eighty patients with newly diagnosed CMV retinitis were randomized to each group in a 1 : 1 ratio. Primary endpoint was photographically determined progression of CMV retinitis within 4 weeks after the initiation of treatment. In the valganciclovir group, 9.9% of patients had progression of CMV retinitis within the first 4 weeks compared to 10.0% of patients assigned to IV ganciclovir. This 0.1 percentage point difference was not significant (95% CI −9.7 to 10.0). Secondary endpoints included the achievement of a prospectively defined successful response to induction therapy and the time to progression of CMV retinitis. Seventy-seven percent of patients receiving IV ganciclovir and 71.9% of patients receiving oral valganciclovir achieved a satisfactory response to induction therapy. This 5.2 percentage point difference was not significant (95% CI −20.4 to 10.1). The median times to progression of retinitis were 125 days in the IV ganciclovir group and 160 days in the oral valganciclovir group. The relative risk of progression of retinitis in the valganciclovir group compared to the ganciclovir group was 0.90 (95 CI 0.58–1.38). Diarrhea was the most common adverse effect during the study and occurred more frequently in the valganciclovir group compared to the ganciclovir group (19% vs 10%, P = 0.11). Neutropenia occurred with similar frequency in each group. Catheter-related side-effects were seen more frequently in the IV ganciclovir group than in the valganciclovir group.149 No clinical trials have been published specifically comparing efficacy of valganciclovir for maintenance therapy of CMV retinitis.
Lalezari et al. (Roche Valganciclovir Study Group) reported a large safety study of valganciclovir. The adverse event profile was similar to that reported from previous studies of intravenous and oral ganciclovir. Adverse events of note were diarrhea (38%), nausea (23%), fever (18%), neutropenia (absolute neutrophil count <500 cells/mm3) (10%), and anemia (hemoglobin <8.0 g/dl) (12%), thrombocytopenia (platelet count <25 000 cells/mm3) (2%).150
Foscarnet
The second drug for the treatment of CMV retinitis in patients with AIDS was licensed by the FDA in 1993. Foscarnet is a pyrophosphate analog with broad antiviral activity via inhibition of viral polymerases, such inhibition not being dependent on activation or phosphorylation by viral or cellular enzymes. Foscarnet inhibits DNA chain elongation by preventing pyrophosphate exchange.151
Foscarnet inhibits the DNA polymerase of CMV and other herpesviruses (HSV-1, HSV-2, VZV, and Epstein–Barr virus [EBV]) and the replication of HIV in vitro and in vivo.152 Both herpesvirus and HIV replication may be inhibited by therapeutically achievable concentrations of foscarnet. Since the drug is not metabolized and is excreted by the kidney, the dosage must be adjusted for renal insufficiency. Foscarnet also has been used successfully to treat HIV-infected patients with acyclovir-resistant HSV and VZV infections, in addition to CMV retinitis. Foscarnet acts directly on the viral polymerase of all herpesviruses and on the reverse transcriptase of HIV-1. Resistance of CMV to foscarnet is associated with mutations in the genes of these polymerases. Cross-resistance to antiviral drugs is likely to be an increasing problem, since patients with AIDS are living longer as a result of HAART and of the drugs used in the prophylaxis of various opportunistic infections, as well as because of the experience gained in the management of HIV-related problems.
Foscarnet has been shown to be useful against ganciclovir-resistant herpesviruses, such as CMV, because a mutation in a DNA polymerase gene conferring resistance to ganciclovir and acyclovir differs from the region conferring resistance to foscarnet.153 Foscarnet also is an effective inhibitor of the HIV reverse transcriptase enzyme and acts in a dose-dependent manner. AZT and foscarnet have synergistic activity in vitro against HIV, and in vivo, foscarnet has activity against HIV as measured by surrogate markers.154
The use of foscarnet salvage therapy in patients with CMV retinitis who are intolerant of or resistant to ganciclovir was studied in AIDS patients with CMV retinitis who had documented hematologic intolerance or resistance to ganciclovir therapy. This study showed that in patients intolerant of ganciclovir, salvage foscarnet therapy resulted in a longer time to retinitis progression than reported previously in historic controls who terminated ganciclovir therapy. In patients who exhibited clinical resistance to ganciclovir, foscarnet appeared to have efficacy in controlling retinitis. No significant differences in either efficacy or toxicity were observed in the range of foscarnet maintenance doses studied.155
A large, randomized, multicenter, blinded clinical trial (the Foscarnet–Ganciclovir Cytomegalovirus Retinitis Trial) compared ganciclovir with foscarnet in the treatment of CMV retinitis in patients with AIDS. No difference was reported between the treatment groups in the rate of progression of retinitis; however, the median survival was 8.5 months in the ganciclovir group and 12.6 months in the foscarnet group. Excess mortality was reported in a subset of patients in the foscarnet group whose renal function was compromised at entry. Differences in mortality could not be explained entirely on the basis of less antiretroviral therapy in the ganciclovir group, which suggests beneficial interactions between foscarnet and antiretroviral nucleosides. These results indicate that, for patients with AIDS and CMV retinitis, treatment with foscarnet initially offers a survival advantage over treatment with ganciclovir, although foscarnet was not as well tolerated as ganciclovir.156
A marginally prolonged survival seen in patients treated with foscarnet compared with those treated with ganciclovir may have been due to a direct effect on HIV replication. Both drugs had a suppressive effect on circulating p24 antigen, which was predictive of improved survival. The inhibitory effect on CMV replication also may have a beneficial effect on limiting HIV replication.157
A randomized, controlled, comparative trial of foscarnet and ganciclovir demonstrated that they equally controlled CMV retinitis but that foscarnet was associated with a longer survival. However, foscarnet was less well tolerated than ganciclovir, primarily because of the nature of its side-effects. Since foscarnet and ganciclovir have different side-effects, initial treatment of CMV retinitis should be individualized.158
The most frequently reported major adverse effect associated with foscarnet administration is nephrotoxicity, with dose-limiting toxicity occurring frequently and cases of acute renal failure having been observed. Symptomatic hypocalcemia has been reported and may be responsible for arrhythmias and seizures, and the risk is increased by concurrent administration of intravenous pentamidine. Bone marrow suppression with neutropenia, anemia, and thrombocytopenia can be seen with foscarnet administration. Neutropenia was reported to be less common with foscarnet than with ganciclovir (14% vs 34%).156
Practical guidelines for the use of foscarnet include administration through an infusion pump to avoid the potential consequences of overdose or too rapid infusion, adequate hydration of patients with saline loading159 to reduce the risk of nephrotoxicity, avoidance of administration of other potentially nephrotoxic agents, and monitoring of renal function two or three times per week during induction therapy and once per week during maintenance therapy, with the dosage being recalculated on the basis of patient weight and serum creatinine. Studies of foscarnet doses have suggested that patients receiving high maintenance doses (120 mg/kg/day) have slower rates of retinitis progression.160,161
Foscarnet is active against HIV, and studies have shown that it raises the CD4 count transiently and decreases viral antigenemia (p24 antigen). Because of its efficacy against CMV and HIV, it would appear to be a potentially effective agent for treating HIV-infected patients; however, it is currently only available for intravenous administration, and its use is associated with substantial toxicity (see above).154,162
Cidofovir
Cidofovir, (S)-1-[3-hydroxy-2-(phosphonylmethoxy)propyl]cytosine, formerly known as HPMPC, was the first antiviral nucleotide analog available for the treatment of CMV retinitis. Cidofovir is active in uninfected cells, may act preemptively, and may retain activity against ganciclovir-resistant strains. Preclinical studies showed the major toxicity of cidofovir to be dose-, schedule-, and species-dependent nephrotoxicity. The concomitant administration of probenecid protects animal models against cidofovir-induced nephrotoxicity. Four treatment modifications are indicated clinically to reduce the incidence of cidofovir-related nephrotoxicity: dose reduction or interruption for changes in renal function; concomitant administration of probenecid; administration of 1 liter of normal saline 1 hour before infusion; and extension of the dosing interval.163
The treatment of CMV retinitis with intravenous cidofovir was demonstrated to be effective in slowing the progression of peripheral CMV retinitis in patients with previously untreated CMV retinitis and AIDS. Intravenous cidofovir also has been used for long-term suppression of CMV retinitis. Biweekly therapy (after induction therapy) was reported to have a time to progression of CMV retinitis of 120 days in one randomized, controlled trial164 and 2.5 months in another randomized, controlled trial.165,166 Treatment and subsequent maintenance of CMV retinitis with 20 µg of intravitreously injected cidofovir, given at 5- to 6-week intervals, also is safe and highly effective.167
Unfortunately parenteral cidofovir has also been found to have ocular toxicity, including a high incidence of iritis (up to 50%), including recurrent iritis, and a risk of profound ocular hypotony with vision loss, similar to the iritis and hypotony seen with intravitreal injections of cidofovir.168–170 It has been estimated in one study that cidofovir-related iritis developed in half of patients within approximately 4 months. The long-term reports from the HPMPC Peripheral Cytomegalovirus Retinitis Trial showed a rate of cidofovir-associated uveitis of 0.20 per person-year and a rate of significant ocular hypotony of 0.16 per patient-year.166 Thus in the setting of iritis in HIV-infected patients, use of systemic cidofovir or rifabutin should be considered potential causes of iritis, and these drugs may need to be discontinued.
CMV resistance
Many patients taking chronic maintenance therapy for CMV retinitis develop resistant virus. Development of in vitro resistance of CMV to ganciclovir and foscarnet and disease progression has been shown in several small studies,171,172 and mechanisms of resistance to ganciclovir have been described.173 In one prospective, randomized study of 207 patients with newly diagnosed CMV retinitis, drug-resistant CMV occurred in four of nine ganciclovir-treated patients and in none of five foscarnet-treated patients.118 In patients with CMV retinitis and AIDS treated with either oral or intravenous ganciclovir, isolates of CMV after a median exposure of 75 and 165 days, respectively, showed increasing resistance in vitro.174 Jabs et al. reported that the cumulative incidence of ganciclovir resistance at 9 months was 27.5%.172 Similar incidence rates of resistance occur for foscarnet and cidofovir.175 In addition, the incidence of resistance to valganciclovir appears to be similar to that for ganciclovir.176
Resistance to an anti-CMV drug can be described as phenotypic, expressed as an inhibitory concentration 50% greater than a certain threshold (IC50). This is determined typically via plaque reduction assays, DNA hybridization assays, or antigen-reduction assays that require large amounts of viable virus often requiring culturing.174,177–179 Genotypic resistance is defined by the presence of a mutation in the CMV genome conferring resistance to a particular drug. PCR amplification techniques allow fast detection of resistance-conferring mutations in the viral genome, requiring only small amounts of viral nucleic acids and can use nonviable virus.180–182 Low-level ganciclovir resistance is typically associated with mutations in the CMV UL97 gene. UL97 codes for a phosphotransferase that catalyzes the first step of ganciclovir activation to the triphosphate form. High-level ganciclovir resistance is typically caused by mutations in both the CMV UL97 and UL54 genes. UL54 codes for the cytomegalovirus DNA polymerase.173 Mutations in the UL54 gene are also responsible for foscarnet and cidofovir resistance.173,183–186 UL54 mutations responsible for foscarnet resistance are usually distinct from those causing ganciclovir–cidofovir resistance. However, low-grade ganciclovir–foscarnet cross-resistance has been reported, plus Chou et al. reported a DNA polymerase mutation causing resistance to ganciclovir, cidofovir, and foscarnet.172,173,182–184
Treatment strategies in resistant CMV
When clinically resistant retinitis appears, many clinicians employ an alternative antiviral agent systemically; intravenous cidofovir or foscarnet are alternatives. Unfortunately, as mentioned above, there can be cross-resistance between CMV isolates resistant to ganciclovir and resistant to cidofovir and/or foscarnet; this must be borne in mind in such patients. The probability of developing foscarnet or cidofovir resistance while taking these drugs appears similar to the rates of development of resistance to ganciclovir.175 For this reason, clinicians often employ intravitreal therapies including the ganciclovir intraocular device when systemic therapy begins to fail. Intravitreal therapies appear to be more effective in such circumstances, largely because they deliver higher doses of anti-CMV medication to the retina.187 In such circumstances, it is recommended to continue to treat the patient with some form of systemic therapy, often oral valganciclovir, to help prevent systemic CMV infection or infection of the fellow eye. Studies have shown that treatment with the ganciclovir implant alone is associated with a higher risk of contralateral CMV retinitis and extraocular CMV.188,189
Combination therapies: ganciclovir–foscarnet
Several studies have shown that combinations of foscarnet and ganciclovir are more effective in the treatment of recurrent or resistant retinitis than is continued monotherapy.91,189 Such combination intravenous therapy also has been shown to be safe and effective in children with CMV retinitis.190 Unfortunately, combination intravenous therapy with these two drugs necessitates multiple intravenous infusions daily and has a marked negative effect on patients’ lifestyle. Combination of IV foscarnet and oral valganciclovir has supplanted this combination intravenous therapy.
The combination of foscarnet and ganciclovir in patients with AIDS and CMV retinitis who have relapsed has been shown to be more effective than either agent given alone;143 however, combination therapy was associated with the greatest negative impact of treatment on quality-of-life measures.
To determine the best therapeutic systemic regimen for treatment of relapsed CMV retinitis, a multicenter randomized controlled clinical trial of 279 patients with AIDS and either persistently active or relapsed CMV retinitis was reported. Patients were randomized to one of three therapeutic regimens: induction with foscarnet sodium at 90 mg/kg intravenously every 12 hours for 2 weeks, followed by maintenance at a dosage of 120 mg/kg per day (foscarnet group); induction with ganciclovir sodium at 5 mg/kg intravenously every 12 hours for 2 weeks followed by maintenance at 10 mg/kg per day (ganciclovir group); or continuation of previous maintenance therapy plus induction with the other drug (either ganciclovir or foscarnet) for 2 weeks followed by maintenance therapy with both drugs, ganciclovir sodium at 5 mg/kg per day and foscarnet sodium at 90 mg/kg per day (combination therapy group). The mortality rate was similar among the three groups. Median survival times were as follows: foscarnet group, 8.4 months; ganciclovir group, 9.0 months; and combination therapy group, 8.6 months (P = 0.89). Comparison of retinitis progression revealed that combination therapy was the most effective regimen for controlling the retinitis. The median times to retinitis progression were as follows: foscarnet group, 1.3 months; ganciclovir group, 2.0 months; and combination therapy group, 4.3 months (P = 0.001). Although no difference could be detected in visual acuity outcomes, visual field loss and retinal area involvement on fundus photographs both paralleled the progression results, with the most favorable results in the combination therapy group. The rates of visual field loss were as follows: foscarnet group, 28 degrees per month; ganciclovir group, 18 degrees per month; combination therapy group, 16 degrees per month (P = 0.009). The rates of increase of retinal area involved by CMV were as follows: foscarnet group, 2.47% per month; ganciclovir group, 1.40% per month; and combination therapy group, 1.19% per month (P = 0.04). Although side-effects were similar among the three treatment groups, combination therapy was associated with the greatest negative impact of treatment on quality-of-life measures. This study suggests that for patients with AIDS and CMV retinitis whose retinitis has relapsed and who can tolerate both drugs, combination therapy appears to be the most effective therapy for controlling CMV retinitis.143 Small series suggest that combined intravitreal injections of ganciclovir and foscarnet may be effective in treating CMV retinitis when the infection is clinically resistant to either intravitreal drug alone.191
Intraocular therapy of viral retinitis
Ganciclovir
In 40 patients with primary CMV retinitis involving 57 eyes, all had received one 14-day course of intravenous ganciclovir and all were free of other end-organ CMV disease. All affected eyes received weekly intravitreal injections of 400 mg of ganciclovir for maintenance therapy. Median survival of patients was at least 13 months. Fifteen patients had 19 new opportunistic infections during the observation period, but none developed new nonocular CMV disease. Active retinitis recurred in 68.4% of the eyes while receiving maintenance therapy, with a median time to progression of 14.7 weeks. CMV retinitis occurred in 30.4% of the previously uninvolved eyes (follow-up 3.1 years). Bacterial endophthalmitis complicated treatment in one eye, and retinal detachment developed in five eyes. Thus the long-term treatment of CMV retinitis with weekly intraocular injections of ganciclovir was associated with survival and ocular outcomes similar to those reported with systemic ganciclovir.192
Intravitreal ganciclovir also was shown to be an effective alternative to systemic ganciclovir in patients with severe neutropenia and in patients who chose to continue receiving systemic zidovudine or didanosine.193 Injections of high-dose intravitreal ganciclovir using a 2 mg dose revealed that weekly 2 mg injections appear to offer superior control of retinitis for periods of months or longer.194 Highly concentrated ganciclovir solution for intravitreal injection also reduced repeated amaurosis and ocular pain and was reported by patients to have improved their comfort and quality of life, thus increasing their compliance to treatment and reducing side-effects, as compared with usual protocols.195
Foscarnet
Intravitreal foscarnet at a dose of 2.4 mg per injection given one or two times weekly also appears to be a safe and effective treatment method for CMV retinitis. Resistance to this treatment regimen may develop, however.196 High-dose intravitreal foscarnet for CMV retinitis was shown to be a safe, effective, and useful alternative in patients with intolerance to intravenous therapy.197
Ganciclovir intraocular device
An intraocular sustained-release ganciclovir delivery implant that releases drug into the vitreous is commercially available.198 These surgically implanted, time-release implants have been shown to be more effective than intravenous ganciclovir alone in delaying the progression of CMV retinitis.129,199,200
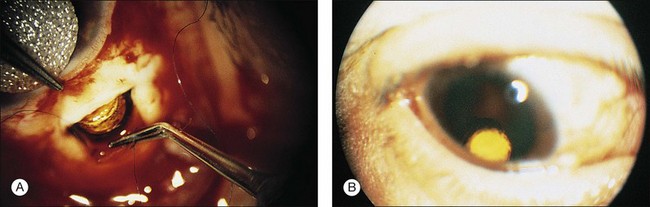
Insertion of the device requires a pars plana incision and a partial vitrectomy. The implant is sewn into the pars plana behind the lens.198 Insertion of the ganciclovir intraocular device (GIOD) requires trimming the strut of the device so that it is nearly flush with the drug pellet. A 5.5 mm incision can be made 4 mm posterior to the limbus with a microvitreoretinal blade or similar instrument (Fig. 81.11). A unimanual bipolar intraocular cautery can be used to coagulate bleeding choroid. It is important to ensure that the incision is full-thickness, since the device can be inserted inadvertently under the pars plana. A suture is placed through the preplaced hole (the surgeon must make the hole) in the strut of the device; 8–0 Prolene can be used. The device is anchored in the middle of the wound, and running or interrupted sutures can be used to close the wound. Astigmatism can result from overzealous wound closure; this is usually transient. This procedure can be performed in an outpatient setting under local anesthesia.
Despite the relative ease of insertion, it has become clear that the risk of retinal detachment in the first 2 months after insertion is substantially higher than if other methods are used to control retinitis, though in the long term there is no statistical difference in retinal detachment rate.198,201–203 In addition, the risk of postoperative endophthalmitis appears to be a real one, with incidences on the order of 1% or sometimes higher.204 The intravitreal levels attained by this drug are over twice those after intravenous administration, and this appears to be associated with a lower incidence of resistance and progression of retinitis. This is particularly true in newly diagnosed cases, but failure can occur in up to 25% of such cases within the first 2 months. In a study of 91 implants in 70 eyes, GIOD was effective as an adjunct to continued systemic therapy in those patients with recurrent CMV retinitis.200 Intraocular sustained-release implants have been used to treat acute CMV disease and to prevent recurrence. Pathology studies of eyes having undergone implantation with the GIOD have shown no evidence of intraocular toxicity.136 It is not certain whether implants should be exchanged at the 7-month time period or whether retinitis should be allowed to reactivate before replacing the implant.
Intravitreal cidofovir
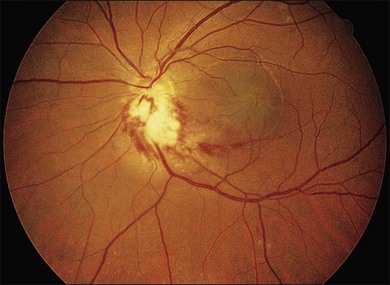
Another form of intraocular therapy is intravitreal cidofovir (HPMPC), which is injected every 6 weeks. This work was initiated after discovery of long-acting properties of the drug in the eye. The safety and efficacy of intravitreal cidofovir for CMV retinitis in humans were reported in a phase I/II unmasked, consecutive case series in a single-center institutional referral practice. Eligible patients with AIDS had active CMV retinitis in at least one eye, despite adequate intravenous therapy with ganciclovir or foscarnet, were intolerant to intravenous therapy, were noncompliant with intravenous therapy, or refused intravenous therapy. In a preliminary safety study (group 1), 10 eyes of nine patients received 14 injections of cidofovir while being treated concurrently with intravenous ganciclovir. In a dose-escalating efficacy study (group 2), eight eyes of seven patients received 11 injections of cidofovir as sole treatment for CMV retinitis. The primary outcome was time to retinitis progression. In group 1 eyes receiving 20 µg of cidofovir, the median time to retinitis progression was between 49 and 92 days (mean, 78 days). In group 2 eyes treated with 20 µg of cidofovir with probenecid, the median time to retinitis progression was 64 days (mean 63 days). Hypotony occurred in the two eyes treated with a 100 µg dose of cidofovir and in one of three eyes receiving a 40 µg dose. No adverse effects resulted from the remaining 20 µg cidofovir injections. Cidofovir was found to be safe and effective for the local treatment of CMV retinitis, providing a long duration of antiviral effect (Fig. 81.12).205
It was then shown that injections of 20 µg of intravitreal HPMPC resulted in complete suppression of CMV replication with no advancement of retinitis borders when given every 6 weeks.170,205–209 This medication must be given with oral probenecid. Probenecid 2 g is given orally 2 hours before, and 1 g 2 hours and 8 hours after injection.
Two types of adverse events may occur after intravitreal cidofovir injection: iritis and hypotony. The incidence of these is not dissimilar to what is seen after intravenous administration. The incidence of iritis can be reduced from 70% to 18% if oral probenecid is used, and it is now recommended universally. Iritis can be managed with topical steroids and cycloplegia; however, it may lead to cataract and synechiae in the long term. A mild, asymptomatic 20% reduction in intraocular pressure (IOP) is seen almost universally after cidofovir injection, and this appears to be of no concern. The mechanism of this has been defined by ultrasound biomicroscopy, which has disclosed that severe hypotony after cidofovir injections is associated with ciliary body atrophy.206 Reduction in aqueous flow has been demonstrated by aqueous fluorophotometry. This effect on secretory epithelia also is probably responsible for the nephrotoxicity of the drug when given intravenously. Indeed, probenecid also is given before and after each intravenous infusion to prevent uptake by the proximal tubule of the kidney and associated nephrotoxicity. Profound hypotony with vision loss occurs in approximately 1% of injections.
A retrospective, cohort study described iritis and hypotony after treatment with intravenous cidofovir for CMV retinitis in association with intraocular inflammation.168 Eleven cases of iritis (26%) occurred among 43 patients. In six cases the iritis was bilateral. Patients who experienced iritis were more likely to have been previously treated for CMV retinitis (P = 0.03), to be diabetic (P = 0.05), or to be receiving protease inhibitors (P = 0.001). The onset of iritis occurred at a mean (±SD) of 4.9 ± 1.8 days after a cidofovir dose and after a mean (±SD) of 4.2 ± 1.6 doses of cidofovir. Six eyes of four patients had hypotony. Five eyes of five patients had a persistent decrease in visual acuity of at least 2 Snellen lines. Acute intraocular inflammation may occur with or without hypotony after intravenous cidofovir therapy, similar to the reactions seen after intravitreous administration. Cidofovir therapy can be continued in some patients if medical necessity warrants, but inflammation may recur or permanent hypotony develop.
The efficacy and safety of multiple intravitreal cidofovir (HPMPC) injections given every 5–6 weeks for the maintenance treatment of CMV retinitis with 20 µg intravitreally injected was shown to be highly effective, with only rare episodes of reactivation and progression.170
A correlation between IOP and CD4 T-lymphocyte counts in patients with HIV with and without CMV retinitis has been described.210 IOP was measured with calibrated Goldmann applanation tonometers in two groups of patients. Group A included 84 HIV patients (120 eyes) with CMV retinitis, and group B included 110 HIV patients (183 eyes) without CMV retinitis; 33 patients without HIV (66 eyes) were included as a control group. Step-wise regression analysis of IOP included correlation with CMV retinitis (presence, extent, and activity), CD4 T-lymphocyte count, age, and gender. The mean IOP was 9.8 mmHg in group A, 12.6 mmHg in group B, and 16.1 mmHg in the control group. All three groups were statistically different from each other when intraocular pressure was compared (P < 0.0001). Step-wise regression showed that low CD4 T-lymphocyte count and extent of CMV retinitis both correlated to low IOP. These results demonstrate that IOP is lower than normal in patients with HIV and that decreased CD4 T-lymphocyte count is the major factor associated with low IOP, accounting for 20% of the effect. The extent of CMV retinitis accounts for 8% of the effect.
Fomivirsen
Fomivirsen, formerly called ISIS 2922, was approved by the FDA in August 1998 for the treatment of CMV retinitis in AIDS patients intolerant of or who have a contraindication to other CMV regimens or who were insufficiently responsive to previous treatments for CMV retinitis. Fomivirsen is the first of a class of antisense oligonucleotides. This compound possesses potent anti-CMV activity, but does not target the CMV viral DNA polymerase. Fomivirsen is a 21-base synthetic phosphorothioate oligonucleotide designed to be complementary to CMV mRNA that encodes for the major immediate early region (IE2) proteins of CMV. Binding to this location results in specific inhibition of gene expression that is critical to production of essential viral proteins.211–213
Following intravitreal administration, the rate of vitreous clearance of fomivirsen is first-order with a half-life of approximately 55 hours in humans. Measurable concentrations of drug are not detected in the systemic circulation after intravitreal injection making the interaction of fomivirsen with systemic drugs unlikely. Preclinical studies of fomivirsen by Freeman and associates suggested that this type of antiviral antisense compound does inhibit viral replication; however, it did cause changes in the RPE and intraocular inflammation at doses only moderately higher than the dose needed to treat retinitis by the intravitreal route.214
The Vitravene Study Group published the data from the clinical trials involving fomivirsen. Two prospective randomized open-label controlled clinical trials (USA/Brazilian and EuroCanadian Studies) compared two fomivirsen regimens for the treatment of reactivated CMV retinitis or CMV retinitis that was persistently active despite other anti-CMV treatments. The more intense schedule (regimen A) included 61 patients (67 eyes) and consisted of three weekly 330 µg (0.05 ml) intravitreal injections for induction, then 330 µg every 2 weeks for maintenance therapy. The less intense schedule (regimen B) included 32 patients (39 eyes) and utilized a 330 µg injection for induction on day 1 and day 15, then 330 µg injections every 4 weeks for maintenance therapy. The study endpoint was time to progression based on masked evaluation of serial fundus photos. Eligibility criteria included AIDS patients with active CMV retinitis who had failed prior treatment with ganciclovir, foscarnet, or cidofovir.215
In the USA/Brazilian study, median time to progression was 106 days (interpolated median 88.6 days) for regimen A; 267 days (interpolated median 111.3 days) for regimen B (P = 0.2179 Wilcoxon rank sum test; 0.2950 log rank). In the EuroCanadian study the median time to progression was not determinable for regimen A; only four patients progressed (25th percentile 91 days). The median time to progression for regimen B was 403 days (interpolated median 182 days).216
Independent of the randomized clinical trials, there have been reports of Vitravene-induced peripheral retinal toxicity and serious inflammation with vision loss. In clinical practice fomivirsen has been used as a fourth-line drug for CMV retinitis resistant to other therapy. The approved dose of fomivirsen is 330 µg intravitreally every 2 weeks for induction therapy for two doses followed by 330 µg intravitreally every month for maintenance therapy. Fomivirsen is no longer available in the United States.217
Investigational agents for CMV retinitis
Maribavir
Maribavir (1263W94), which completed phase II trials but failed to achieve study goals in a phase III trial in 2009, is a benzimidazole compound with in vitro activity against CMV. It does not require intracellular activation and has demonstrated activity against clinical isolates resistant to ganciclovir or foscarnet. The mechanism of action of maribavir is mediated through inhibition of the CMV UL97 gene product. The drug inhibits viral DNA synthesis through blocking of terminal DNA processing. UL97 is involved in the monophosphorylation of ganciclovir and is essential for viral growth. Maribavir has no effect on the metabolism of HIV protease inhibitors. UL97 mutants that are compromised in their ability to phosphorylate ganciclovir are fully susceptible to maribavir. In vitro IC50 of maribavir was 0.12 mM compared to ganciclovir at 0.53 mM.218,219
Tomeglovir
Tomeglovir is a 4-sulphonamide substituted naphthalene derivative with good activity in vitro against laboratory-adapted and clinical strains of CMV (IC50 1.17 µM vs 5.77 µM ganciclovir). Tomeglovir is also active against ganciclovir-resistant strains. Preclinical studies showed a significant decrease in mortality at all dosages in CMV-infected immunodeficient mice treated with tomeglovir. Metabolism occurs via the CYP3A4 system, although the effects of tomeglovir on the metabolism of HIV protease inhibitors has not been formally reported. Drug-resistant strains of CMV generated by in vitro passage in the presence of tomeglovir contained mutations in the UL89 and UL104 genes, suggesting that this novel non-nucleoside class of compounds inhibits CMV by preventing cleavage of the polygenic concatameric viral DNA into unit-length genomes. Safety and tolerability studies of single oral doses (up to 2000 mg) of tomeglovir in healthy male volunteers have been performed without significant adverse events being observed.220,221
Rhegmatogenous retinal detachment in CMV retinitis
Retinal detachment is a common cause of vision loss in patients with CMV retinitis. In the pre-HAART era, the incidence rate of retinal detachment in patients with CMV retinitis was approximately 33% per eye per year.137,144,203,222–224 The incidence of retinal detachment in immunosuppressed patients with CMV retinitis was believed to be higher in patients treated with anti-CMV therapies, specifically ganciclovir.225–227 These retinal detachments were characterized by multiple peripheral breaks in areas of healed atrophic retinitis; and in some patients severe proliferative vitreoretinopathy resulted (Fig. 81.13).228 Detachment occurred from weeks to months after institution of intravenous ganciclovir therapy and was frequently bilateral. Retinal detachment may also complicate the course of CMV retinitis.
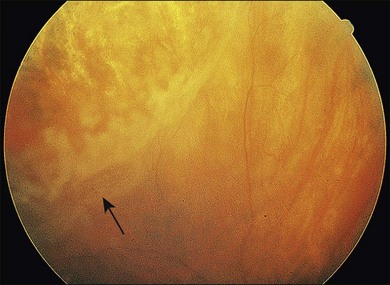
Fig. 81.13 Retinal breaks are seen just peripheral to the area of border opacification; the retina is detached.
However, it now appears that rhegmatogenous retinal detachment is associated with healed or active CMV retinitis due to breaks in the necrotic retina.229 Results of a multicenter, prospective, randomized, controlled clinical trial analyzing incidence and risk factors for rhegmatogenous retinal detachment in a population of patients with newly diagnosed CMV retinitis treated with foscarnet vs ganciclovir revealed that retinal detachment in patients with CMV retinitis is unrelated to the type of intravenous therapy used or to refractive error. The median time to retinal detachment in an involved eye with CMV retinitis and free of retinal detachment at baseline was 18.2 months.223
Studies have confirmed that the risk factors for retinal detachment in eyes with CMV retinitis include the extent of peripheral CMV disease, as well as retinitis activity and involvement of the anterior retina near the vitreous base.203,223,230 This is logical, considering that in most cases the causative retinal breaks are within or at the border of healed CMV retinitis lesions. In addition, any intervention that violates the vitreous (e.g. vitreous biopsy or insertion of the ganciclovir implant) would be expected to accelerate the development of vitreous detachment or liquefaction, which would increase the risk of retinal detachment.199,201,203
With the advent of HAART therapy the incidence of CMV retinitis-related retinal detachment has decreased by 60%. The success of HAART in the reduction of retinal detachment risk may be related to the improved immune control over CMV replication, thus protecting against progression of disease to larger lesion sizes. The altered pattern of inflammation with HAART-mediated immune improvement may also change the course of vitreous detachment, a key step in the development of CMV-related detachments, thus altering the retinal detachment risk.199,203,231
Patients with AIDS and CMV retinitis are surviving longer as a result of the use of HAART and improved treatment of opportunistic infections. As a result, though the incidence rate of retinal detachment is lower, the overall prevalence of retinal detachment may become an increasingly common cause of visual morbidity in these patients. In the pre-HAART era, the incidence and outcome of retinal detachment complicating CMV retinitis were studied at two London AIDS centers. Patients with CMV retinitis were identified prospectively and underwent standard treatment. Retinal detachments were diagnosed during regular follow-up. If retinal reattachment surgery was performed, a standard procedure of vitrectomy and internal tamponade with silicone oil was employed. Of 147 patients with CMV retinitis, 41 (28%) developed retinal detachments (47 eyes); 43 detachments were rhegmatogenous and four were exudative. At the last clinic visit, eight eyes (53%) maintained a visual acuity of 6/60 or better. The visual results of surgery are good in selected patients, bearing in mind the progressive nature of the underlying disease and poor life expectancy.232
Vitrectomy with silicone oil tamponade also was studied in eyes with retinal detachments related to CMV retinitis or acute retinal necrosis.233 Anatomic reattachment was achieved in all eyes, and preservation of ambulatory vision was achieved in most eyes. Visual acuity was limited by concomitant optic nerve disease in some eyes. The authors noted that surgical repair employing silicone oil produces excellent results and that prognosis for vision is strongly related to preoperative visual acuity.
Treatment of retinal detachment consists of vitrectomy, posterior hyaloid removal, and intraocular tamponade with silicone oil or long-acting gas.234 Retinal reattachment surgery in 29 eyes of 24 patients with AIDS and retinal detachment associated with CMV retinitis was described by Freeman et al.229 In this study the total retinal reattachment rate was 76%, and the macular attachment rate was 90% after one operation. The mean postoperative visual acuity (best corrected) was 20/60, but in some patients visual acuity decreased because of progressive CMV retinitis. Prophylactic laser photocoagulation of fellow eyes did not appear to prevent retinal detachment (Fig. 81.14).
The repair of retinal detachment in eyes with viral retinitis is complex and is performed using a combination of pars plana vitrectomy, internal tamponade (usually with silicone oil or a long-acting gas such as perfluoropropane), and endolaser often combined with scleral buckling.229 Pneumatic retinopexy can cause retinal traction and seldom is useful in these eyes. The most common causes of rhegmatogenous retinal detachment in AIDS patients with viral retinitis are acute retinal necrosis syndrome and previously treated CMV retinitis. In these eyes proliferative vitreoretinopathy occasionally is established at the time of detachment or has the potential to occur as a result of multiple retinal breaks and necrosis combined with intraocular inflammation. Scleral buckling alone often is unsuccessful in these cases because of the numerous areas of retinal necrosis and break formation. Retinal breaks are often not apparent until the time of vitrectomy, and the configurations of the retinal detachments are atypical because of peripheral retinal scarring and adhesion to the pigment epithelium and choroid. Thus in these eyes rhegmatogenous retinal detachments may not extend to the ora serrata. In eyes with CMV retinitis, we have favored an approach using complete delamination of the posterior hyaloid combined with endodrainage and permanent tamponade with silicone oil, although we have had good success with intraocular long-acting gases in cases of more limited retinitis and retinal detachment. We have had a very high surgical success rate with this approach. Patients with AIDS and CMV retinitis appear to be surviving longer, and survival after retinal reattachment surgery has been increased to between 6 months and 2 years.229
To determine if scleral buckling is of any benefit in surgical repair of CMV-associated retinal detachment if combined with vitrectomy, silicone oil, and inferior midperipheral endolaser, 22 consecutive eyes with CMV-associated retinal detachments were repaired with vitrectomy and endolaser to all breaks and to the inferior midperipheral retina using silicone oil without scleral buckling. Results were compared with another series of 56 consecutive eyes undergoing vitrectomy, silicone oil injection, endolaser to all breaks, and 360 degrees encircling scleral buckling. Total retinal reattachment rates were 84% for group 1 and 86% for group 2. Rates of macular reattachment were 91% for group 1 and 91% for group 2. Mean best postoperative refracted visual acuity was 20/66 for group 1 and 20/67 for group 2. Median best postoperative refracted visual acuity was 20/74 for group 1 and 20/80 for group 2. These differences between the two groups were not statistically significant. Patients who underwent surgery with the macula attached had a better postoperative visual outcome. Thus, scleral buckling may not be necessary in CMV-related retinal detachment if repaired with vitrectomy, silicone oil, and inferior midperipheral endolaser.235 Elimination of scleral buckling may reduce intraoperative time, patient morbidity, and the risk of an accidental needle-stick. Patients with macula-on-retinal detachments also should be considered for surgery before macular detachment occurs.236
The long-term visual results of CMV retinal detachment surgery are still in question, however, and visual acuity may be limited by factors such as refractive problems resulting from silicone oil and cataract228,237–241 (Fig. 81.15). In addition, posterior capsule fibrosis is very common if subsequent cataract surgery is performed in the presence of silicone oil. Methods to reduce visual acuity loss from cataract include judicious use of gas tamponade with scleral buckling instead of silicone oil, and removal of silicone oil prior to or at the time of cataract surgery. Posterior capsule fibrosis can be treated with Nd : YAG capsulotomy, though success is higher if the silicone oil has been previously removed.238
The general operative approach to these eyes is by pars plana vitrectomy, and the surgeon should leave the lens intact whenever possible. After the vitreous gel is removed, all epiretinal membranes are segmented and traction is removed, allowing the retina to become mobile. In some cases the peripheral vitreous gel is adherent to the necrotic peripheral retina and cannot be removed without causing further retinal damage. The use of a soft-tipped extrusion needle may allow the surgeon to remove the posterior hyaloid over broad areas of the retina. A posterior retinotomy is made, and, if an endoretinal biopsy is to be performed, it is done at the location of the posterior retinotomy that will be used for internal drainage.229,234 A pneumohydraulic exchange is made through the retinotomy site, attaching the retina and filling the eye with air using a constant-pressure, sterile, air-delivery pump. Retinopexy is placed around all breaks in eyes to be treated with a long-acting gas. The peripheral retina may be encircled with either a small buckle or a band to relieve vitreous base traction, which may become a problem later in these inflamed eyes. In eyes with widespread retinal necrosis, most surgeons use silicone oil because it permanently tamponades all retinal breaks, including future sites of retinal necrosis and break formation.
In the HAART era, PVR may be seen in CMV detachment. This may be due to immune recovery uveitis causing a propensity to intraocular inflammation.242 The management of CMV-related rhegmatogenous retinal detachments has been reviewed.243 Certainly, it may be useful in acute retinal necrosis (ARN) because rhegmatogenous retinal detachment develops in a large number of patients with ARN. Similar considerations apply in bilateral healed CMV retinitis. The difficulty in both diseases is that all areas of retinal involvement must be surrounded with three rows of argon laser treatment. It is often impossible to carry out treatment to the ora serrata, however, and fluid may leak anteriorly and cause retinal detachment despite treatment. The widespread availability of the indirect laser ophthalmoscopic delivery system should solve this problem. In addition, subretinal fluid may break through a wall of laser treatment if the mass of detached retina and subretinal fluid is relatively large. For this reason most surgeons advocate placement of a panretinal type of pattern within the area of healing retinitis as well.244–247
HIV disease/CMV retinitis in the HAART era
Since the advent of HAART many patient have had dramatic restoration of immune system function. This also may be associated with a sustained drop in the plasma HIV viral load to low or undetectable levels. This suppression of plasma viremia may be prolonged; however, the HIV genome may still be found.37,248 As mentioned earlier, with the prevalent use of HAART, the incidence of CMV retinitis has decreased approximately 75%.249–252 For patients with CMV retinitis on HAART the risk of vision loss is lower,249 the risk of retinal detachment is approximately 60% less, and long term survival is much higher.203,249
In fact, for patients who have healed CMV retinitis and respond to HAART, discontinuation of maintenance therapy for CMV disease has been shown to be safe in a subset of patients.249,253–257 We have found that some of these patients may discontinue anti-CMV therapy without reactivation of retinitis (Fig. 81.16). These data suggest that HAART therapy also is permitting at least partial immune reconstitution in some patients. Thus a trial of withdrawal of CMV therapy may be indicated in some patients with good response to HAART therapy and well-healed CMV retinitis. Patients should have a sustained CD4 count elevation of over 100 cells/mm3 for at least 3–6 months before discontinuing anti-CMV treatment and should be carefully monitored for reactivation. Reactivation of CMV retinitis may occur after successful HAART therapy when the CD4 count diminishes.258 In addition, some patients may develop CMV retinitis on HAART with CD4 counts above 100/µl, probably because of incomplete restoration of the immune repertoire against CMV.122
The effects of HAART on the natural history of other AIDS-related opportunistic disorders have been summarized259,260 and reflect the improvement or resolution of changes in the natural history of these disorders with inflammatory syndromes. The development of CMV retinitis relatively soon after initiation of HAART has been described.261
Immune recovery uveitis
In conjunction with the dramatic improvements in the immune system reported in some patients on HAART therapy, inflammation at sites of OIs is common, and related to recovery of immunity with effective antiretroviral therapy.262 The syndrome has been described in the eye as “immune recovery vitritis” or “immune recovery uveitis” (IRU).263–269
Immune recovery uveitis appears to occur in eyes with healed CMV lesions in patients with immune reconstitution on HAART. The incidence rate of this phenomenon has varied, with reports from 0.11 to 0.83 per person-year in HAART responders with CMV retinitis.268,269 Jabs et al. reported the frequency of IRU at 15.5% of 200 prevalent cases of CMV retinitis.270 Arevalo et al. reported IRU in 37.5% of 32 patients.271 Eyes in which CMV retinitis lesions involve large surface areas of retina seem to be at higher risk for the development of IRU.272 Previous treatment with cidofovir may also be a risk factor.273 Patients with IRU exhibit signs of inflammation such as iritis, vitritis, macular edema, and epiretinal membrane formation (Fig. 81.17).274–277 Cataract, vitreomacular traction, proliferative vitreoretinopathy, optic disc and retinal neovascularization, panuveitis with hypopyon, and uveitic angle closure glaucoma with posterior synechia have also been reported in IRU.278–284 Vision loss from these inflammatory sequelae may range from mild to moderate and is usually associated with macular edema and associated macular surface changes or cataract in most cases.
The pathophysiology of IRU is not well understood. One hypothesis is that once the CMV retinitis is healed and the immune system is reconstituted, the patient can mount an inflammatory response to residual CMV antigens in retinal glial cells in or adjacent to the necrotic CMV lesion. Another hypothesis is that control of CMV retinitis is incomplete in certain individuals with continued subclinical virus or viral protein production that stimulates the immune system. It has been reported that CMV antigens persist in cells of all retinal layers at the borders of clinically healed CMV lesions and in CMV infected retinal glial cells after treatment with ganciclovir.283–285
Periocular steroids may be used successfully to treat this disorder, but ophthalmologists should be aware of CD4 cell counts. It appears that if the CD4 cell count is elevated above 60/mm3, treatment of immune recovery vitritis can be carried out without reactivation of retinitis (Fig. 81.13).262,278 Recently, one case of reactivated CMV retinitis has been reported after treatment of IRU with periocular steroids.286 Intravitreal triamcinolone is also effective in reducing macular edema, however, caution is needed not to reactivate retinitis.287,288
Other complications of CMV retinitis
Central visual loss in AIDS patients with CMV retinitis occurs in two forms: direct macular tissue destruction and secondary involvement as part of rhegmatogenous retinal detachment. We treated 32 patients (35 eyes) with macular exudation that caused reversible visual loss and initially manifested as neurosensory retinal detachment and lipid exudates. Of 35 eyes, 25 showed papillary or peripapillary active retinitis and 10 showed retinitis 1500–3000 µm from the fovea. Of 23 eyes with reduced vision that were followed up until healing of the retinitis and resolution of subretinal fluid and lipid exudates, 22 (96%) showed visual improvement with anti-CMV treatment. Our findings suggest that macular exudation is a reversible cause of visual loss in patients with CMV retinitis.107
Cystoid macular edema can occur in the setting of resolving CMV retinitis in patients with immunodeficiency other than AIDS. This entity is distinct from serous macular exudation, which can occur in patients with AIDS with active CMV retinitis involving the posterior pole.289
Herpetic retinitis
Acute retinal necrosis in HIV patients
Acute retinal necrosis (ARN) has been reported in AIDS patients.290 It is a devastating disease characterized by the acute onset of a fulminant panuveitis with confluent, well-demarcated areas of retinitis, plus prominent anterior uveitis, occlusive retinal and choroidal vasculitis, vitritis, and papillitis.54,109,291,292 In most cases the cause of the clinical syndrome of ARN is varicella zoster but HSV can also cause ARN. The retinitis is characterized by deep retinal whitening, minimal hemorrhage, and a rapid progression. In some cases, ARN in AIDS patients may be preceded by VZV optic neuropathy.293 A history of preceding cutaneous zoster infection may be helpful in making the diagnosis in such cases.294,295 In addition, the CD4 count is usually above 60/ml.292 The diagnosis of ARN is clinical based on criteria established by the American Uveitis Society that do not include immune status of the patient.296
No evidence of retinal vascular abnormalities may be present either clinically or angiographically early in the course of ARN. Retinal detachment is a common sequela, with multiple retinal breaks evident within areas of retinal necrosis. Retinal atrophy, often accompanied by proliferative vitreoretinopathy, is a common endstage finding, and there may be associated anterior uveitis, scleritis, and ocular hypotension.109
Large numbers of herpesvirus particles in retinal tissue affected with ARN have been demonstrated by electron microscopy using endoretinal biopsy techniques. Virus may be detectable only during the acute phase of the disease.109 Necrotic retinal tissue or retina reduced to thin glial remnants may not demonstrate virus. The difficulty in growing virus from these specimens is consistent with the hypothesis of VZV as the causative agent, since VZV is difficult to isolate and grow in vitro. CMV initially was believed to be the presumed infectious agent of ARN, but subsequent studies have not confirmed this.297
Studies employing endoretinal biopsy and PCR techniques have enabled definitive identification and culture of the causative virus, which has important diagnostic and therapeutic implications. Recent studies have suggested that combination antiviral therapy given intravenously (usually acyclovir or ganciclovir in combination with foscarnet), if given promptly, can arrest the disease and salvage vision.189 Retinal detachment in VZV retinitis is common (up to two-thirds of patients) and may be associated with PVR or retinal shortening. Repair with vitrectomy and silicone oil after relief of traction with membrane segmentation and sometimes retinotomy may result in useful vision.189,298 Prophylactic barrier laser around lesions should be considered to lower the risk of retinal detachment in ARN.
Ganciclovir has good efficacy against all herpesviruses but has a lower therapeutic index and must be given indefinitely because it acts as a virostatic agent. Determination of a specific viral cause early in the course of the disease when large numbers of viral particles are present is therefore imperative. Both HSV and VZV may be sensitive to acyclovir. However, VZV requires higher serum concentrations than HSV. Treatment for ARN in AIDS patients is usually based on established treatment for non-HIV-infected patients. Acyclovir IV 500 mg/m2 or 10 mg/kg every 8 hours is effective followed by oral famciclovir 500 mg TID, acyclovir 800 mg five times a day or valacyclovir 1000 mg TID for maintenance therapy.85 Duration of maintenance therapy is controversial, with reports of contralateral ARN infection decades later.299 This may support lifetime maintenance therapy, especially in immunosuppressed AIDS patients. Valacyclovir 1000 mg TID has been reported effective for initial treatment of ARN in a small series of immunocompetent individuals.300 Intravenous foscarnet may be used in acyclovir resistant cases.301,302 Corticosteroids have been used to decrease vitritis in immunocompetent patients with ARN, but steroids are usually contraindicated in HIV patients with advanced immunosuppression.85,294
Progressive outer retinal necrosis
Progressive outer retinal necrosis (PORN) is another variant of herpetic retinitis in AIDS patients and is nearly always caused by VZV. The incidence of PORN has decreased during the HAART era.303 It has been described in association with VZV as the onset of retinitis either succeeded by or coincident with an eruption of dermatomal zoster.304 Most patients with this syndrome have had low CD4 cell counts (i.e., below 50/ml). PORN syndrome is an extremely rapid progressive necrotizing retinitis characterized by early patchy multifocal deep outer retinal lesions (Fig. 81.18) with late diffuse thickening of the retina, absence of vascular inflammation and minimal to no vitreous inflammation.305 Severe vision loss develops as a result of a widespread retinal necrosis and from retinal detachment, the latter reported in up to 70% of patients in early studies.305–312 Perivascular clearing of the retinal opacification is characteristic of PORN syndrome (Fig. 81.19).305
Therapy of PORN often requires immediate high-dose anti-zoster or -HSV therapy. The earliest reports of treatment of PORN with single intravenous antivirals, primarily acyclovir, showed poor visual results. Engstrom et al. reported final vision of no light perception (NLP) in 67% of 63 eyes within 4 weeks.305 Poor outcomes with IV acyclovir were possibly due to development of HSV or VZV resistance to acyclovir in patients who developed PORN while on prophylactic anti-HSV therapy with acyclovir. Recent studies have shown improved visual outcomes employing combination intravenous and intravitreal antiviral treatment. Scott et al. reported final vision of 20/80 or better in 5 of 11 eyes (45%) with only two of 11 eyes (18%) progressing to NLP vision utilizing a regimen of intravitreal ganciclovir and foscarnet plus IV foscarnet and IV ganciclovir or oral valganciclovir. In addition, the authors’ data suggested that laser demarcation may be beneficial to decrease the rate of retinal detachment.306 Combination of antiviral drugs and HAART preserved vision in a report by Kim et al.312
Nonviral intraocular infections in AIDS patients
Nonviral intraocular infections have been reported in AIDS patients. Autopsy studies have documented numerous infections. Many of the opportunistic infections seen in patients with AIDS can be prevented with appropriate prophylactic agents.313
Pneumocystis carinii choroidopathy
In 1987 Macher and associates314 described a patient with AIDS with disseminated pneumocystosis, and choroidal P. carinii was found at autopsy; no clinical correlation was reported. In 1989 Rao and colleagues58 reported the histopathologic findings in an autopsy series of three patients with AIDS who clinically demonstrated yellow choroidal infiltrates while receiving aerosolized pentamidine for P. carinii pneumonia (PCP) prophylaxis. In two cases a presumptive diagnosis of disseminated pneumocystosis was made by ophthalmologic examination. Histopathologically, the choroidal infiltrates were eosinophilic, acellular, vacuolated, and frothy, with the infiltrates within the choroidal vessels and choriocapillaris. Both Gomori’s methenamine-silver stain and electron microscopy demonstrated organisms.
In 1989 Freeman et al. described a woman with AIDS with multifocal, slowly enlarging, round-to-oval lesions in the choroid.315 Fluorescein angiography revealed early hypofluorescence with late staining of the lesions, which appeared deep to the retinal circulation, without evidence of retinal involvement or inflammation (Fig. 81.20). A transscleral choroidal biopsy revealed, by electron microscopy, cystic structures characteristic of P. carinii within necrotic choroid.
A multicenter study of pneumocystis choroidopathy in 1991 reported 21 patients with AIDS and presumed P. carinii choroidopathy.316 The lesions were characteristically yellow to pale yellow, appeared in the choroid, and were found in the posterior pole. They enlarged slowly before systemic anti-pneumocystis therapy and eventually resolved. Of 21 patients, 18 were receiving topical therapy with aerosolized pentamidine. There was little evidence of retinal destruction by visual acuity and visual field testing. The choroidal infiltrates were not associated with vitreous inflammation unless another infectious retinitis was present. Resolution of choroiditis took from 6 weeks to 4 months after systemic therapy. Survival after the diagnosis ranged from 2 to 36 weeks.
The CDC recommends double strength trimethoprim-sulfamethoxazole (TMP-SMX) daily or three times weekly for primary prophylaxis of PCP when CD4 counts are below 200/ml, with alternatives including dapsone, dapsone plus pyrimethamine and leucovorin, aerosolized pentamidine administered by the Respirgard II nebulizer, and atovaquone.313 PCP is the most common AIDS-defining opportunistic infection. Choroidal P. carinii infection appears to have been more common when the prophylactic use of nonsystemically absorbed aerosolized pentamidine for PCP was widespread. The choroidal lesions of P. carinii appear as pale, cream- or orange-colored, space-occupying lesions from several hundred to several thousand microns in size and they rarely are symptomatic or lead to a decrease in visual acuity. The lesions may be unilateral or bilateral.58,315,317–319 P. carinii choroidopathy appears to be a marker for disseminated pneumocystosis and should be treated systemically. The need for maintenance systemic therapy for choroidopathy has not been established.
Although the diagnosis of disseminated pneumocystosis may be suggested by the characteristic appearance of P. carinii choroidopathy, isolated retinal disease may rarely be the earliest clinical manifestation of disseminated pneumocystosis. The incidence of P. carinii choroiditis has decreased, probably because of more widespread use of systemic PCP prophylaxis such as trimethoprim–sulfamethoxazole, immune restoration from HAART, and decreased use of aerosolized pentamidine for prophylaxis and therapy.320
Ocular toxoplasmosis
Toxoplasmosis is a common CNS opportunistic infection in patients with AIDS. Ocular toxoplasmosis is much less common,62,321,322 with reported incidence of 3% in HIV-infected patients in France.323 US incidence is decreased in the HAART era by immune restoration and primary toxoplasmosis prophylaxis with TMP-SMX for those with CD4 counts less than 200/ml.85 Eight patients with presumed ocular toxoplasmic retinochoroiditis were described in 1988 by Holland et al.62 In two cases the diagnosis was confirmed histologically. Lesions were usually bilateral (5/8) and multifocal, with vitreous inflammation noted clinically. Therapy resulted in remission, but reactivation and disease progression followed in two of three patients when therapy was stopped. Three patients had retinal tears or detachment as a result of severe retinal necrosis. The ocular lesions were the first manifestation of toxoplasmosis in four of five patients with disseminated disease, although all patients had pre-existing HIV infection, and in four the diagnosis of AIDS had not been made. In four of five patients with no evidence of nonocular infection, evidence of Toxoplasma was demonstrated in the CNS (encephalitis or brain abscess). No patient had evidence of pre-existing chorioretinal scars, and all had IgG antibodies to T. gondii at the time of diagnosis. Ocular disease was believed to be secondary to reactivation of Toxoplasma or to newly acquired or newly disseminated disease to the eye from nonocular sites of disease (Fig. 81.21).
Retinal toxoplasmosis in HIV infection may present as a focal necrotizing nonhemorrhagic retinitis that does not heal spontaneously and may simulate ARN, CMV, or luetic retinitis.85,324 Prominent vitreous and anterior chamber reaction, relative absence of retinal hemorrhage, and thick, densely opaque yellow-white lesions with smooth nongranular borders suggest toxoplasmosis. Endoretinal biopsy or PCR techniques may be useful if diagnosis is difficult.85,325,326
Ocular toxoplasmosis often presents differently in AIDS compared to immunocompetent individuals, spreading as a contiguous or multifocal retinitis. AIDS patients more often have extensive areas of retinal necrosis plus multiple areas of active infection.327,328 Histopathologic studies show absent to scant inflammatory cells in the infected retina of immunocompromised patients. AIDS patients can develop ocular toxoplasmosis in the absence of preexisting chorioretinal scars. This pattern combined with frequent systemic toxoplasmosis at diagnosis suggests that acquired disease is more common than reactivation of congenital disease.85,329 For immunocompetent individuals, current evidence also suggests that most patients with ocular toxoplasmosis were infected postnatally, even though the risk of ocular toxoplasmosis is higher from congenital infection330 (Fig. 81.21). Ocular toxoplasmosis in AIDS patients has also been reported to cause miliary disease, optic neuritis, panophthalmitis, and acute unilateral iridocyclitis without retinal lesions.331,332 Ocular toxoplasmosis is diagnosed clinically and using PCR from a vitreous fluid sample.
Ocular, as well as disseminated, toxoplasmosis in patients with AIDS is treated with standard antitoxoplasma regimens used in immunocompetent patients such as sulfadiazine (4–6 g/day) or clindamycin in sulfa allergic patients plus pyrimethamine/leucovorin, with apparent response rates of 80%.333 TMP-SMX was reported in a small (77 patients) randomized trial to be effective and better tolerated than pyrimethamine–sulfadiazine.334 Other treatments for patients unable to tolerate sulfa drugs such as azithromycin or atovaquone have been primarily studied for CNS toxoplasmosis in AIDS patients and ocular toxoplasmosis in immunocompetent patients.335–337 Atovaquone was originally synthesized as an antimalarial and has been shown to have activity against both P. carinii and T. gondii. Ocular toxoplasmosis in patients with AIDS frequently recurs when medical therapy is terminated so maintenance therapy generally is given. Corticosteroids may be given as adjunctive therapy for intracranial toxoplasmosis to reduce cerebral edema, although this is unproved; systemic corticosteroids are sometimes given to reduce inflammation in ocular disease, although these should be administered cautiously in HIV-infected patients. In addition, resolution of ocular toxoplasmosis in AIDS patients has been seen without corticosteroid therapy.62
Toxoplasma retinitis is decreasing because of more widespread use of HAART and of prophylaxis, such as TMP-SMX.320 Currently the CDC recommends consideration of discontinuing maintenance therapy (sulfadiazine plus pyrimethamine/leucovorin with clindamycin used in sulfa-allergic patients) for toxoplasma encephalitis if a patient maintains a CD4 count above 200/ml for greater than 6 months.
Fungal diseases
Candida albicans
Since HIV can be a consequence of intravenous drug use, which is also associated with candidemia, it is surprising that candida endophthalmitis is not seen more frequently in HIV patients. Focal retinal and chorioretinal lesions and endophthalmitis from Candida spp. have been described in patients with AIDS.55,338 Traditional therapy for candida endophthalmitis has been systemic amphotericin B. However, limited vitreous penetration has resulted in treatment failures, plus adverse effects including nephrotoxicity discourage its use.339 Trials comparing oral fluconazole to IV amphotericin B for systemic candidemia in immunocompetent patients suggested equivalent efficacy with fluconazole being the less toxic.340 Vitrectomy and intravitreal amphotericin B can be helpful in cases failing systemic therapy.
Cryptococcus neoformans
Cryptococcal infections may occur in 5–10% of patients with AIDS341 and are associated with both direct and indirect ocular complications. Cryptococcal infection is a common occurrence in AIDS, resulting in meningitis and secondary ocular involvement. Chorioretinitis, endophthalmitis, or both, caused by direct intraocular invasion of the organism, have been described in immunosuppressed patients.342,343 Visual loss caused by cryptococcal infection has been demonstrated to result from invasion of the visual pathways, including the optic nerve, tract, and chiasm.
Histoplasmosis
Histoplasmosis was initially reported in patients with AIDS in 1982, and in the next eight years more than 100 systemic cases were reported,344 mostly disseminated disease that presents as fevers without localized symptoms. The treatment of histoplasmosis in patients with AIDS usually consists of an induction phase with amphotericin B followed by a lifelong maintenance phase with either amphotericin B or itraconazole.345
Gonzales et al. reported bilateral endogenous endophthalmitis in an HIV-positive patient presenting with severe subretinal exudation, choroidal granulomas, and intraretinal hemorrhage leading to bilateral exudative retinal detachments. Vitreous cultures grew H. capsulatum var. capsulatum. Treatment involved systemic and bilateral intravitreal amphotericin B plus vitrectomy/scleral buckle in one eye.346 Ala-Kauhaluoma et al. reported a case of panuveitis in disseminated histoplasmosis in an HIV patient treated with liposomal amphotericin B, HAART, and topical steroids that ended up with anterior segment scarring.347
Aspergillosis
Endogenous endophthalmitis caused by Aspergillus fumigatus rarely has been described in AIDS and can arrive in the eye hematogenously or through extension to the orbit from adjacent sinuses. One case of disseminated, invasive aspergillosis with ocular involvement noted at autopsy was described in 13 patients with pulmonary aspergillosis; no clinical correlation of ocular findings was reported.348 Whereas four cases of orbital aspergillosis in HIV were reported, ocular aspergillosis is very rare in the HIV population.349
Coccidioidomycosis
To our knowledge, no cases of ocular coccidioidomycosis have been described in patients with AIDS. In a retrospective review of 77 patients with HIV infection and coccidioidomycosis, no case of endogenous endophthalmitis secondary to coccidioidomycosis was described, although disseminated disease (including meningitis) was described in a majority of patients.350 Other unusual mycotic infections causing endophthalmitis, some in association with chorioretinitis, but not necessarily HIV-seropositive patients, are reviewed by McDonnell and Green.351
Paracoccidioidomycosis
Severe CNS plus ocular infection with Paracoccidioides brasiliensis that simulated CNS and ocular toxoplasmosis has been reported in a pregnant HIV-positive patient. The infection caused severe iridocyclitis, vitritis, plus a granulomatous chorioretinal lesion also involving the optic nerve that ultimately progressed to retinal detachment, NLP vision, and enucleation despite treatment.352
Advances in antifungal therapy
Fungal endophthalmitis traditionally has been treated with intravenous amphotericin B. Limitation of vitreous penetration plus systemic toxicity limit its effectiveness.353 Flucytosine and fluconazole have higher vitreous penetration but are limited by lack of broad coverage against many of the organisms typically seen in fungal endophthalmitis. As a result, vitrectomy and intravitreal amphotericin B is usually recommended in serious fungal endophthalmitis or cases unresponsive to systemic therapy.354
The triazole antifungal medications fluconazole, itraconazole, voriconazole, posaconazole, and ravuconazole are orally available and are less toxic drugs that can treat a variety of fungal organisms.355
Older drugs such fluconazole, which treats candida, and cryptococci and itraconazole, which treat histoplamosis and aspergillus, remain valuable. Voriconazole has been shown to have mean aqueous and vitreous minimum inhibitory concentrations for 90% of isolates (MIC90) for a wide spectrum of yeast and molds, including Candida and Aspergillus spp. after oral administration. Voriconazole and posaconazole have also been reported to successfully treat fungal endophthalmitis that was unresponsive to traditional antifungal agents.356,357 Voriconazole and posaconazole have been shown to have efficacy against Candida albicans isolates from HIV patients.358
Bacterial retinitis
Syphilis
Concurrent ocular syphilis appears to be more common in HIV-infected than in uninfected persons.359 Ophthalmic manifestations of syphilis usually occur during or shortly after the secondary stage. Syphilitic uveitis and chorioretinitis in HIV-infected patients have been described. Nine patients with ocular syphilis and concurrent HIV infection were described by McLeish and colleagues in 1990.360 They found iridocyclitis in three of 15 eyes, vitritis in one eye, retinitis or neuroretinitis in five eyes, papillitis in two eyes, optic perineuritis in two eyes, and retrobulbar neuritis in two eyes. The three of nine patients with AIDS had the worst initial visual acuities. Six of nine had concomitant neurosyphilis. Benzathine benzylpenicillin, the only treatment in three of the patients, led to relapses in all three. Seven of nine patients treated with high-dose intravenous penicillin responded dramatically to therapy with no evidence of relapse.360
Concurrent ocular syphilis and neurosyphilis reported in two patients with HIV infection, in addition to a review of 13 other HIV-infected patients with ocular syphilis, revealed that 11 of the 13 HIV-infected patients with ocular syphilis had neurosyphilis.361 The authors stress that neuro-ophthalmic syphilis may be the presenting feature of HIV infection and that ocular syphilis is strongly associated with concurrent neurosyphilis in patients that are HIV-seropositive (Fig. 81.22).
Necrotizing retinitis has been reported in a patient with HIV and syphilis. In some cases the appearance of luetic retinitis as a focal expanding white lesion may simulate CMV retinitis, ARN, or toxoplasmic retinitis. Marked inflammation of the vitreous and anterior chamber usually accompanies syphilitic retinal disease with posterior synechiae and keratic precipitates.82,362
Standard treatment of primary and secondary syphilis with 2.4 MU of intramuscular benzathine penicillin in patients with and without HIV infection produce similar excellent clinical responses. Serolological responses to therapy (reducing nontreponemal antibody to unreactive levels), the primary method for ascertaining adequate treatment, are less certain in HIV patients.363
For retinitis, treatment for neurosyphilis with intravenous penicillin should be used and ceftriaxone may be used when penicillin is contraindicated. With regard to diagnosis, false-negative serum rapid plasma reagin (RPR) and Venereal Disease Research Laboratories (VDRL) may occur uncommonly with HIV-infection. Thus additional, more specific tests are recommended in this population (i.e. fluorescent treponemal antibody, absorbed [FTA-ABS]).363
Invasive diagnostic techniques for retinal disease
Appropriate processing of vitreous obtained as part of a diagnosis is mandatory, and the testing performed should reflect the differential diagnosis. Where infection is suspect, plating of undiluted vitreous on appropriate culture media for aerobic bacteria (chocolate and blood agar, brain–heart media), anaerobic bacteria (thioglycolate media and cooked meat broth), media for acid-fast bacilli, and fungal culture media is imperative and should be done in the operating room. Smears of the undiluted gel should be stained for these etiologic agents, and cytologic smears also should be obtained. In addition, histopathologic stains are useful in ruling out intraocular neoplasms, particularly intraocular lymphoma. Other important diagnostic aids include the use of cytospin preparations that concentrate the cells in vitreous washings, and the use of cell blocks, should enough cellular material be present in a vitreous specimen. The choice of fixatives should be considered carefully. In general, the use of electron microscopic fixatives such as glutaraldehyde may destroy the antigenicity of proteins, rendering immunostaining impossible. Buffered paraformaldehyde will preserve many antigens, although in some cases, frozen, nonfixed tissue is required. In situ hybridization may work on fixed or fresh tissue and can be valuable in determining the presence of pathogenic DNA.364
PCR techniques also may be useful in analyzing aqueous or vitreous specimens in difficult cases. Nonfixed fluids are best; they can be frozen for later evaluation or processed fresh. In cases of active retinitis, the test is very sensitive, although it may be somewhat nonspecific because it may detect CMV in the blood in the absence of retinal infection. Choroidal biopsies may be indicated when infiltrative processes of the choroid are seen clinically. Fungal, bacterial, protozoal, or parasitic disease may cause metastatic focal or diffuse infiltrative lesions in the choroid.365 Tuberculous disease and neoplastic disorders, including lymphoma, also may produce such lesions. All such diseases respond temporarily, if at all, to steroid therapy, and in many cases the diagnosis cannot be made by study of vitreous cells. Before undertaking this diagnostic procedure, prior arrangements for appropriate histologic examination of all materials obtained must be made, since the amount of material that can be obtained is usually small. Any cultures to be taken should be performed by direct plating of the tissue onto the appropriate media in the operating room, as outlined above.
Endoretinal biopsy has been reported to be of value in the diagnosis of viral retinitis. Freeman et al.109 first reported this technique in 1986. We pursued this technique in patients with CMV retinitis (Fig. 81.23). After healing of retinitis with ganciclovir, rhegmatogenous retinal detachment developed in a large number of these patients as a result of numerous breaks in areas of necrotic and healed retina. We showed in several eyes that persistent infection was present, as viral particles were seen. At this time we do not recommend endoretinal biopsy for all cases of viral retinitis. Rather, we treat such cases with the appropriate antiviral drug and await a response. Response may not indicate a specific viral cause, however, because some drugs treat multiple viruses. In certain cases it is helpful to obtain an etiologic diagnosis. As new antiviral and immunostimulating drugs become available, viral retinitis may not take on the so-called classic clinical appearance, and aggressive diagnostic techniques may become more important. Currently we obtain endoretinal biopsies at the time of pars plana vitrectomy to repair rhegmatogenous retinal detachments in these patients. During these procedures, undiluted vitreous specimens are taken for viral cultures, and in some cases in situ nucleic acid hybridization studies are done. The retinal biopsy (described below) is divided into three small pieces, and each is placed on a small wedge of sterile paper or on an agar sandwich in the operating room using the operating microscope. Tissue is fixed in glutaraldehyde for electron microscopy, as well as in buffered formalin for light microscopy and some immunologic studies. The third piece of tissue may be frozen for further immunologic studies or cultured for virus. It is important to remember that the choice of fixative and tissue preparation technique is critically important; if an incorrect choice is made, it may not be possible to arrive at a diagnosis. Even when obtaining an endoretinal biopsy, the vitreous should also be examined because vitreous biopsy in cases of infectious retinitis may be positive for the causative organism. One can also perform endoretinal biopsy in nondetached retinas. The preliminary results in animals have been encouraging, and refinement of these techniques in the future may shed light on a variety of retinal disorders.
Any ocular tissue, but particularly small endoretinal biopsy specimens, must be processed with care. The surgeon must decide in advance on the location of the area to be biopsied and the fixatives and numbers of specimens to be processed. We have developed a mount that allows more facile mounting of retinal biopsy specimens.366 The agar–albumin sandwich technique allows the small piece of retinal tissue to be floated onto a slab of clear agar and then “glued” to it with liquid agar that has been warmed in a microwave oven. When the tissue is draped on the agar, it can be readily identified and not lost during processing. Multiple sections of the tissue can then be cut for processing and immunostaining. The technique also works well for electron microscopy and other morphologic studies.
Antiretroviral therapy
Expert guidelines for use of these drugs are continuously updated.367 Many other antiretroviral agents are undergoing preclinical and clinical studies. Currently available drugs do not eradicate latent HIV infection which returns when drugs are stopped (Table 81.1). When used in combination, they usually decrease viral replication, improve immunologic status, reduce risk of infectious complications, and prolong life. Drugs active against the patient’s HIV strain should always be used in combination for full potency and prevention of resistance.
Table 81.1 Currently licensed antiretroviral drugs in the United States in 2011 by class
| Brand name | Generic name | Manufacturer name |
|---|---|---|
| Multi-class combination products | ||
| Atripla | efavirenz, emtricitabine and tenofovir disoproxil fumarate | Bristol–Myers Squibb and Gilead Sciences |
| Nucleoside reverse transcriptase inhibitors (NRTIs) | ||
| Combivir | lamivudine and zidovudine | GlaxoSmithKline |
| Emtriva | emtricitabine, FTC | Gilead Sciences |
| Epivir | lamivudine, 3TC | GlaxoSmithKline |
| Epzicom | abacavir and lamivudine | GlaxoSmithKline |
| Retrovir | zidovudine, azidothymidine, AZT, ZDV | GlaxoSmithKline |
| Trizivir | abacavir, zidovudine, and lamivudine | GlaxoSmithKline |
| Truvada | tenofovir disoproxil fumarate and emtricitabine | Gilead Sciences, Inc. |
| Videx EC | enteric coated didanosine, ddI EC | Bristol–Myers Squibb |
| Videx | didanosine, dideoxyinosine, ddI | Bristol–Myers Squibb |
| Viread | tenofovir disoproxil fumarate, TDF | Gilead |
| Zerit | stavudine, d4T | Bristol–Myers Squibb |
| Ziagen | abacavir sulfate, ABC | GlaxoSmithKline |
| Nonnucleoside reverse transcriptase inhibitors (NNRTIs) | ||
| Edurant | rilpivirine | Tibotec Therapeutics |
| Intelence | etravirine | Tibotec Therapeutics |
| Rescriptor | delavirdine, DLV | Pfizer |
| Sustiva | efavirenz, EFV | Bristol–Myers Squibb |
| Viramune (immediate release) | nevirapine, NVP | Boehringer Ingelheim |
| Viramune XR (extended release) | nevirapine, NVP | Boehringer Ingelheim |
| Protease inhibitors (PIs) | ||
| Agenerase | amprenavir, APV | GlaxoSmithKline |
| Aptivus | tipranavir, TPV | Boehringer Ingelheim |
| Crixivan | indinavir, IDV, | Merck |
| Fortovase | saquinavir (no longer marketed) | Hoffmann–La Roche |
| Invirase | saquinavir mesylate, SQV | Hoffmann–La Roche |
| Kaletra | lopinavir and ritonavir, LPV/RTV | Abbott Laboratories |
| Lexiva | fosamprenavir calcium, FOS-APV | GlaxoSmithKline |
| Norvir | ritonavir, RTV | Abbott Laboratories |
| Prezista | darunavir | Tibotec, Inc. |
| Reyataz | atazanavir sulfate, ATV | Bristol–Myers Squibb |
| Viracept | nelfinavir mesylate, NFV | Agouron Pharmaceuticals |
| Fusion inhibitors | ||
| Fuzeon | enfuvirtide, T-20 | Hoffmann–La Roche & Trimeris |
| Entry inhibitors – CCR5 co-receptor antagonist | ||
| Selzentry | maraviroc | Pfizer |
| HIV integrase strand transfer inhibitors | ||
| Isentress | raltegravir | Merck & Co., Inc. |
1 Review of draft for revision of HIV infection classification system and expansion of AIDS surveillance case definition. MMWR Morb Mortal Wkly Rep. 1991;40:787.
2 Revision of the CDC surveillance case definition for acquired immunodeficiency syndrome. Council of State and Territorial Epidemiologists; AIDS Program, Center for Infectious Diseases. MMWR Morb Mortal Wkly Rep. 1987;36(Suppl 1):1S–15S.
3 CDC. HIV prevalence estimates. MMWR Morb Mortal Wkly Rep. 2008;57(39):1073–1076.
4 Deeks SG, Smith M, Holodniy M, et al. HIV-1 protease inhibitors: a review for clinicians. JAMA. 1997;277:145–153.
5 Zhu T, Korber BT, Nahmias AJ, et al. An African HIV-1 sequence from 1959 and implications for the origin of the epidemic. Nature. 1998;391:594–597.
6 Freeman WR, Lerner CW, Mines JA, et al. A prospective study of the ophthalmologic findings in the acquired immune deficiency syndrome. Am J Ophthalmol. 1984;97:133–142.
7 Jabs DA, Green R, Fox R, et al. Ocular manifestations of acquired immune deficiency syndrome. Ophthalmology. 1989;96:1092–1099.
8 Centers for Disease Control and Prevention [Internet]. Atlanta, GA: CDC; 2002 [cited 2010 Jan 26]. Cases of HIV infection and AIDS in the United States, 2002, HIV/AIDS surveillance report, Vol. 14. Available from http://www.cdc.gov/hiv/surveillance/resources/reports/2002report/
9 Hogg RS, Health KV, Yi B, et al. Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. JAMA. 1998;279:450–454.
10 Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860.
11 Padian NS, Shiboski SC, Jewell NP. Female-to-male transmission of human immunodeficiency virus. JAMA. 1991;266:1664–1667.
12 Centers for Disease Control and Prevention [Internet]. Atlanta, GA: CDC; 2002 [cited 2012 Jan 26]. Surveillance of healthcare personnel with HIV/AIDS, as of December 2002. Available from http://www.cdc.gov/ncidod/hip/BLOOD/hivpersonnel.htm
13 US Public Health Service. Updated US public health service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2005:541–547.
14 Marcus R. Surveillance of health care workers exposed to blood from patients infected with the human immunodeficiency virus. N Engl J Med. 1988;319:1118–1123.
15 Cardo DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. N Engl J Med. 1997;137:1485–1490.
16 Fauci AS. Immunopathogenic mechanisms of HIV infection. Ann Intern Med. 1996;124:654–663.
17 Coombs RW, Welles SL, Hooper C, et al. Association of plasma human immunodeficiency virus type 1 RNA level with risk of clinical progression in patients with advanced infection. J Infect Dis. 1996;174:704–712.
18 Hughes MD, Johnson VA, Hirsch MS. Monitoring plasma HIV-1RNA levels in addition to CD4 lymphocyte count improves assessment of antiretroviral therapeutic response. Ann Intern Med. 1997;127:929–938.
19 Mellors JW, Kingsley LA, Rinaldo CR, Jr., et al. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann Intern Med. 1995;122:573–579.
20 Mellors JW, Rinaldo CR, Jr., Gupta P, et al. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170.
21 Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4 lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–954.
22 O’Brien WA, Hartigan PM, Martin D, et al. Changes in plasma HIV-1 RNA and CD4 lymphocyte counts and the risk of progression to AIDS. N Engl J Med. 1996;334:426–431.
23 Welles SL, Jackson JB, Yen-Lieberman B, et al. Prognostic value of plasma human immunodeficiency virus type 1 (HIV-1) RNA levels in patients with advanced HIV-1 disease and with little or no zidovudine therapy. J Infect Dis. 1996;174:696–703.
24 Feinberg MB. Changing the natural history of HIV disease. Lancet. 1996;348:239–246.
25 Havlir DV, Richman DD. Viral dynamics of HIV: implications for drug development and therapeutic strategies. Ann Intern Med. 1996;124:984–994.
26 Ho DD, Neumann AU, Perelson AS, et al. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126.
27 Perelson AS, Newmann AU, Markowitz M, et al. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586.
28 McDonald CK, Kuritzkes DR. Human immunodeficiency virus type 1 protease inhibitors. Arch Intern Med. 1997;157:951–959.
29 Gulick RM, Mellor JW, Havlir D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739.
30 Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N Engl J Med. 1997;337:725–733.
31 Ledergerber B, Egger M, Opravil M, et al. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: a prospective cohort study. Swiss HIV Cohort Study. Lancet. 1999;353:863–881.
32 Cervia JS, Smith MA. Enfuvirtide (T-20): a novel human immunodeficiency virus type 1 fusion inhibitor. Clin Infect Dis. 2003;37:1102–1106.
33 Pedersen C, Lindhardt BO, Jensen BL, et al. Clinical course of primary HIV infection: consequences for subsequent course of infection. Br Med J. 1989;299:154–157.
34 Chun T, Stuyver L, Mezell S, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 1997;94:13193–13197.
35 Joshi D, O’Grady J, Dietrich D, et al. Increasing burden of liver disease in patients with HIV infection. Lancet. 2011;377(9772):1198–1209.
36 Update: Acquired Immunodeficiency Syndrome – United States. MMWR Morb Mortal Wkly Rep. 1986;35:757–766.
37 Henderly DE, Freeman WR, Smith RE, et al. Cytomegalovirus retinitis as the initial manifestation of the acquired immune deficiency syndrome. Am J Ophthalmol. 1987;103:316–320.
38 Update: Universal precautions for prevention of transmission of human immunodeficiency virus, hepatitis B virus, and other bloodborne pathogens in health-care settings. MMWR Morb Mortal Wkly Rep. 1988;37:377–388.
39 Leads from the MMWR. Recommendations for preventing possible HTLV-III/LAV virus from tears. JAMA. 1985;254:1429.
40 Update: Acquired Immunodeficiency Syndrome – United States. MMWR Morb Mortal Wkly Rep. 1985;35:17–21.
41 Key CB, Whitman J. Alcohol soaking damages applanation tonometer heads. Arch Ophthalmol. 1986;104:800.
42 Vogt M, Ho DD, Bakar S, et al. Safe disinfection of contact lenses after contamination with HTLV-III. Ophthalmology. 1986;93:771–774.
43 Sattar SA. Springthorpe vs Survival and disinfectant inactivation of the human immunodeficiency virus: a critical review. Rev Infect Dis. 1991;13:430–447.
44 Centers for Disease Control and Prevention, Department of Health and Human Services. Guidelines for preventing transmission of human immunodeficiency virus through transplantation of human tissue and organs. MMWR Morb Mortal Wkly Rep. 1994;43:1–17.
45 Centers for Disease Control and Prevention. Update: provisional Public Health Service recommendations for chemoprophylaxis after occupational exposure to HIV. MMWR Morb Mortal Wkly Rep. 1996;45:468–472.
46 Ablashi DV, Sturzenegger S, Hunter EA, et al. Presence of HTLV-III in tears and cells from the eyes of AIDS patients. J Exp Pathol. 1987;3:693–703.
47 Fujikawa LS, Salahuddin SZ, Ablashi D, et al. Human T-cell leukemia/ lymphotropic virus type III in the conjunctival epithelium of a patient with AIDS. Am J Ophthalmol. 1985;100:507–509.
48 Freeman WR, Chen A, Henderly D, et al. Prognostic and systemic significance of non-infectious AIDS associated retinopathy. Invest Ophthalmol Vis Sci. 1987;28(suppl):9.
49 Freeman WR, Chen A, Henderly DE, et al. Prevalence and significance of acquired immunodeficiency syndrome-related retinal microvasculopathy. Am J Ophthalmol. 1989;107:229–235.
50 Cole EL, Meisler DM, Calabrese LH, et al. Herpes zoster ophthalmicus and acquired immune deficiency syndrome. Arch Ophthalmol. 1984;102:1027–1029.
51 Sandor EV, Millman A, Croxson TS, et al. Herpes zoster ophthalmicus in patients at risk for the acquired immune deficiency syndrome (AIDS). Am J Ophthalmol. 1986;101:153–155.
52 Holland GN, Pepose JS, Pettit TH, et al. Acquired immune deficiency syndrome, ocular manifestations. Ophthalmology. 1983;90:859–873.
53 Kestelyn P, Van de Perre P, Rouvroy D, et al. A prospective study of the ophthalmologic findings in the acquired immune deficiency syndrome in Africa. Am J Ophthalmol. 1985;100:230–238.
54 Lipson BK, Freeman WR, Beniz J, et al. Optic neuropathy associated with cryptococcal arachnoiditis in AIDS patients. Am J Ophthalmol. 1989;107:523–527.
55 Morinelli EN, Dugel PU, Riffenburgh R, et al. Infectious multifocal choroiditis in patients with acquired immune deficiency syndrome. Ophthalmology. 1993;100:1014–1021.
56 Pepose JS, Hilborne LH, Cancilla PA, et al. Concurrent herpes simplex and cytomegalovirus retinitis and encephalitis in the acquired immune deficiency syndrome (AIDS). Ophthalmology. 1984;91:1669–1677.
57 Pepose JS, Nestor MS, Holland GN, et al. An analysis of retinal cotton-wool spots and cytomegalovirus retinitis in the acquired immunodeficiency syndrome. Am J Ophthalmol. 1983;95:118–119.
58 Rao NA, Zimmerman PL, Boyer D, et al. A clinical, histopathologic, and electron microscopic study of Pneumocystis carinii choroiditis. Am J Ophthalmol. 1989;107:218–228.
59 Weiss A, Margo CE, Ledord DK, et al. Toxoplasmic retinochoroiditis as an initial manifestation of the acquired immune deficiency syndrome. Am J Ophthalmol. 1986;101:248–249.
60 Arevalo JF, Russack V, Freeman WR. New ophthalmic manifestations of presumed rifabutin-related uveitis. Ophthalmic Surg Lasers. 1997;28:321–324.
61 Glasgow BJ, Engstrom RE, Jr., Holland GN, et al. Bilateral endogenous fusarium endophthalmitis associated with acquired immunodeficiency syndrome. Arch Ophthalmol. 1996;114:873–877.
62 Holland GN, Engstrom RE, Glasgow BJ, et al. Ocular toxoplasmosis in patients with the acquired immunodeficiency syndrome. Am J Ophthalmol. 1988;106:653–667.
63 Gonzales CA, Scott IU, Chaudhry NA, et al. Endogenous endophthalmitis caused by Histoplasma capsulatum var. capsulatum: a case report and literature review. Ophthalmology. 2000;107:725–729.
64 Finamor LP, Muccioli C, Martins MC, et al. Ocular and central nervous system paracoccidioidomycosis in a pregnant woman with acquired immunodeficiency syndrome. Am J Ophthalmol. 2002;134:456–459.
65 Santos C, Parker J, Dawson C, et al. Bilateral fungal corneal ulcers in a patient with AIDS-related complex. Am J Ophthalmol. 1985;102:118–119.
66 Geier SA, Schielke E, Klauss V, et al. Retinal microvasculopathy and reduced cerebral blood flow in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1992;113:100–101.
67 Newsome DA, Green W, Miller ED, et al. Microvascular aspects of acquired immune deficiency syndrome retinopathy. Am J Ophthalmol. 1984;98:590–601.
68 Freeman WR, O’Connor GR. Acquired immune deficiency syndrome retinopathy, pneumocystis, and cotton-wool spots. Am J Ophthalmol. 1984;98:235–237.
69 Kwok S, O’Donnell JJ, Wood IS. Retinal cotton-wool spots in a patient with Pneumocystis carinii infection. N Engl J Med. 1982;307:184–185.
70 Faber DW, Wiley CA, Bergeron-Lynn G, et al. Role of human immunodeficiency virus and cytomegalovirus in the pathogenesis of retinitis and retinal vasculopathy in AIDS patients. Invest Ophthalmol Vis Sci. 1992;33:2345–2353.
71 Gonzalez CR, Wiley CA, Arevalo JF, et al. Polymerase chain reaction detection of cytomegalovirus and human immunodeficiency virus-1 in the retina of patients with acquired immune deficiency syndrome with and without cotton-wool spots. Retina. 1996;16:305–311.
72 Honrubia FM, Ferrer E, Torron C, et al. Study of the retinal fiber layer in patients with acquired immunodeficiency syndrome. Ger J Ophthalmol. 1994;3:1–4.
73 Mansour AM, Rodenko G, Dutt R. Half-life of cotton wool spots in the acquired immunodeficiency syndrome. Int J STD AIDS. 1990;1:132–133.
74 Kuppermann BD, Petty JG, Richman DD, et al. Cross-sectional prevalence of CMV retinitis in AIDS patients: correlation with CD4 counts. Invest Ophthalmol Vis Sci. 1992;33:750.
75 Sadun AA, Pepose JS, Madigan MC, et al. AIDS-related optic neuropathy: a histological, virological and ultrastructural study. Graefes Arch Clin Exp Ophthalmol. 1995;233:387–398.
76 Sadun AA, Tenhula WN, Heller KB. Optic nerve pathology associated with AIDS: ultrastructural changes. [ARVO Abstract]. Invest Ophthalmol Vis Sci (Suppl). 1990.
77 Tenhula WN, Sadun AA, Heller KB, et al. Optic nerve axon losses in AIDS. Morphometric comparisons. [ARVO Abstract]. Invest Ophthalmol Vis Sci (Suppl). 1990;31:1793.
78 Quiceno JI, Capparelli E, Sadun AA, et al. Visual dysfunction without retinitis in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1992;113:8–13.
79 Plummer DJ, Arevalo JF, Fram N, et al. Effectiveness of entoptic perimetry for locating peripheral scotomas caused by cytomegalovirus retinitis. Arch Ophthalmol. 1996;114:828–831.
80 Latkany PA, Holopigian K, Lorenzo-Latkany M, et al. Electroretinographic and psychophysical findings during early and late stages of human immunodeficiency virus infection and cytomegalovirus retinitis. Ophthalmology. 1997;104:445–453.
81 Falkenstein IA, Bartsch DU, Azen SP, et al. Multifocal electroretinography in HIV-positive patients without infectious retinitis. Am J Ophthalmol. 2008;146(4):579–588.
82 Holland GN, Gottlieb MS, Foos RY. Retinal cotton-wool patches in acquired immunodeficiency syndrome. N Engl J Med. 1982;307:1702.
83 Conway MD, Tong P, Olk RJ. Branch retinal artery occlusion (BRAO) combined with branch retinal vein occlusion (BRVO) and optic disc neovascularization associated with HIV and CMV retinitis. Int Ophthalmol. 1995–1996;19:249–252.
84 Cunningham ET, Jr., Levinson RD, Jampol LM, et al. Ischemic maculopathy in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 2001;132:727–733.
85 Levinson RD, Dunn JP, Holland GN. Ophthalmic disorders associated with selected primary and acquired immunodeficiency diseases. CD-ROM. Duane’s clinical ophthalmology. Hagerstown: Harper and Row; 2004. ch. 40
86 Dejaco-Ruhswurm I, Kiss B, Rainer G, et al. Ocular blood flow in patients infected with human immunodeficiency virus. Am J Ophthalmol. 2001;132:719–725.
87 Lim MC, Cumberland WG, Minassian SL, et al. Decreased macular leukocyte velocity in HIV-infected individuals. Am J Ophthalmol. 2001;132:710–718.
88 Dadgostar H, Holland GN, Huang X, et al. Hemorheologic abnormalities associated with HIV infection: in vivo assessment of retinal microvascular blood flow. Invest Ophthalmol Vis Sci. 2006;47(9):3933–3938.
89 Henderly DE, Freeman WR, Causey DM, et al. Cytomegalovirus retinitis and response to therapy with ganciclovir. Ophthalmology. 1987;94:425–434.
90 Jabs DA, Bartlett JG. AIDS and ophthalmology: a period of transition. Am J Ophthalmol. 1997;124:227–233.
91 Studies of Ocular Complications of AIDS Research Group in collaboration with the AIDS Clinical Trials Group. Foscarnet–ganciclovir cytomegalovirus retinitis trial 4 – visual outcomes. Ophthalmology. 1994;101:1250–1261.
92 Collaborative DHPG Treatment Study Group. Treatment of serious cytomegalovirus infections with 9-(1,3-dihydroxy-2-propoxymethyl) guanine in patients with AIDS and other immunodeficiencies. N Engl J Med. 1986;314:801–805.
93 Cogan DG. Immunosuppression and eye disease. Am J Ophthalmol. 1977;83:777–788.
94 Egbert PR, Pollard RB, Gallagher JG, et al. Cytomegalovirus retinitis in immunosuppressed hosts. II. Ocular manifestations. Ann Intern Med. 1980;93:664.
95 DeVenecia G, Zu Rhein GM, Pratt MV, et al. Cytomegalic inclusion retinitis in an adult, a clinical, histopathologic, and ultrastructural study. Arch Ophthalmol. 1971;86:44.
96 Friedman AH, Orellana J, Freeman WR, et al. Cytomegalovirus retinitis: a manifestation of the acquired immune deficiency syndrome (AIDS). Br J Ophthalmol. 1983;67:372–380.
97 Kennedy PGE, Newsome DA, Hess J, et al. Cytomegalovirus but not human T lymphotropic virus type III/lymphadenopathy associated virus detected by in-situ hybridization in retinal lesions in patients with the acquired immune deficiency syndrome. Br Med J. 1986;293:162–164.
98 Brody JM, Butrus SI, Laby DM, et al. Anterior segment findings in AIDS patients with cytomegalovirus retinitis. Graefes Arch Clin Exp Ophthalmol. 1995;233:374–376.
99 Saran BR, Pomilla PV. Retinal vascular nonperfusion and retinal neovascularization as a consequence of cytomegalovirus retinitis and cryptococcal choroiditis. Retina. 1996;16:510–512.
100 Palestine AG, Stevens G, Lane HC, et al. Treatment of cytomegalovirus retinitis with dihydroxy propoxymethyl guanine. Am J Ophthalmol. 1986;101:95–101.
101 Studies of Ocular Complications of AIDS Research Group in collaboration with the AIDS Clinical Trials Group. Assessment of cytomegalovirus retinitis – clinical evaluation vs centralized grading of fundus photographs. Arch Ophthalmol. 1996;114:791–805.
102 Studies of Ocular Complications of AIDS Research Group in collaboration with the AIDS Clinical Trials Group. Clinical vs photographic assessment of treatment of cytomegalovirus retinitis. Foscarnet–ganciclovir cytomegalovirus retinitis trial report 8. Arch Ophthalmol. 1996;114:848–855.
103 Roarty JD, Fisher EJ, Nussbaum JJ. Long-term visual morbidity of cytomegalovirus retinitis in patients with acquired immune deficiency syndrome. Ophthalmology. 1993;100:1685–1688.
104 Wu AW, Coleson LC, Holbrook J, et al. Measuring visual function and quality of life in patients with cytomegalovirus retinitis: development of a questionnaire. for Studies of Ocular Complications of AIDS Research Group. Arch Ophthalmol. 1996;114:841–847.
105 Bloom PA, Sandy CJ, Migdal CS, et al. Visual prognosis of AIDS patients with cytomegalovirus retinitis. Eye. 1995;9:697–702.
106 Luckie AP, Ai E. A foveal-sparing pattern of cytomegalovirus retinitis in the acquired immunodeficiency syndrome. Aust NZ J Ophthalmol. 1996;24:53–59.
107 Gangan PA, Besen G, Munguia D, et al. Macular serous exudation in patients with acquired immunodeficiency syndrome and cytomegalovirus retinitis. Am J Ophthalmol. 1994;118:212–219.
108 Minkler DS, Mcleon EB, Shaw CM, et al. Herpes virus hominis encephalitis and retinitis. Arch Ophthalmol. 1976;94:89–95.
109 Freeman WR, Thomas EL, Rao NA, et al. Demonstration of herpes group virus in the acute retinal necrosis syndrome. Am J Ophthalmol. 1986;102:701–709.
110 Young SJ, Bird AC. Bilateral acute retinal necrosis. Br J Ophthalmol. 1978;62:581–590.
111 Bylsma SS, Achim CL, Wiley CA, et al. The predictive value of cytomegalovirus retinitis for cytomegalovirus encephalitis in acquired immunodeficiency syndrome. Arch Ophthalmol. 1995;113:89–95.
112 Leger F, Vital C, Vital A, et al. Pathologic correlations between ocular and cerebral lesions in 36 AIDS patients. Clin Neuropathol. 1997;16:45–48.
113 Livingston PG, Kerr NC, Sullivan JL. Ocular disease in children with vertically acquired human immunodeficiency virus infection. J AAPOS. 1998;2:177–181.
114 Du LT, Coats DK, Kline MW, et al. Incidence of presumed cytomegalovirus in HIV-infected pediatric patients. J AAPOS. 1999;3:245–249.
115 Baumal CR, Levin AV, Read SE. Cytomegalovirus retinitis in immunosuppressed children. Am J Ophthalmol. 1999;127:550–558.
116 Rasmussen L, Zipeto D, Wolitz RA, et al. Risk for retinitis in patients with AIDS can be assessed by quantitation of threshold levels of cytomegalovirus DNA burden in blood. J Infect Dis. 1997;176:1146–1155.
117 Studies of Ocular Complications of AIDS Research Group in collaboration with the AIDS Clinical Trials Group. Foscarnet–ganciclovir cytomegalovirus retinitis trial 5 – clinical features of cytomegalovirus retinitis at diagnosis. Am J Ophthalmol. 1997;124:141–157.
118 Studies of Ocular Complications of AIDS Research Group in collaboration with the AIDS Clinical Trials Group. Cytomegalovirus (CMV) culture results, drug resistance, and clinical outcome in patients with AIDS and CMV retinitis treated with foscarnet or ganciclovir. J Infect Dis. 1997;176:50–58.
119 Pannuti CS, Kallas EG, Muccioli C, et al. Cytomegalovirus antigenemia in acquired immunodeficiency syndrome patients with untreated cytomegalovirus retinitis. Am J Ophthalmol. 1996;122:847–852.
120 Rasmussen L, Morris S, Zipeto D, et al. Quantitation of human cytomegalovirus DNA from peripheral blood cells of human immunodeficiency virus-infected patients could predict cytomegalovirus retinitis. J Infect Dis. 1995;171:177–182.
121 Hoover DR, Peng Y, Saah A, et al. Occurrence of cytomegalovirus retinitis after human immunodeficiency virus immunosuppression. Arch Ophthalmol. 1996;114:821–827.
122 Song MK, Schrier RD, Smith IL. Paradoxical activity of CMV retinitis in patients receiving highly active antiretroviral therapy. Retina. 2002;22:262–267.
123 Mitchell SM, Fox JD. Aqueous and vitreous humor samples for the diagnosis of cytomegalovirus retinitis. Am J Ophthalmol. 1995;120:252–253.
124 McCann JD, Margolis TP, Wong MG, et al. A sensitive and specific polymerase chain reaction-based assay for the diagnosis of cytomegalovirus retinitis. Am J Ophthalmol. 1995;120:219–226.
125 Schrier RD, Freeman WR, Wiley CA, et al. Immune predispositions for cytomegalovirus retinitis in AIDS. The HNRC Group. J Clin Invest. 1995;95:1741–1746.
126 Dunn JP, Martin DF. Medscape from WebMD Ophthalmology; 2003 Sep 30 [cited 2012 Jan 26]. Treatment of cytomegalovirus (CMV) retinitis in the era of highly active antiretroviral therapy. Available (with account) from [Internet] http://medscape.com/viewprogram/663
127 See RF, Rao NA. Cytomegalovirus retinitis in the era of combined highly active antiretroviral therapy. Ophthalmol Clin North Am. 2002;15:529–536. viii
128 Stalder N, Sudre P, Olmari M, et al. Cytomegalovirus retinitis: decreased risk of bilaterality with increased use of systemic treatment. Swiss HIV Cohort Study Group. Clin Infect Dis. 1997;24:620–624.
129 Martin DF, Kuppermann BD, Wolitz RA, et al. Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. Roche Ganciclovir Study Group. N Engl J Med. 1999;340:1063–1070.
130 Jabs DA, Martin BK, Forman MS. Cytomegalovirus resistance to ganciclovir and clinical outcomes of patients with cytomegalovirus retinitis. Ophthalmol. 2003;135:26–34.
131 Chiron Ganciclovir Implant Study Group. A randomized, controlled, multicenter clinical trial of sustained-release intraocular ganciclovir implant in AIDS patients with CMV retinitis (abstract 1215). Program and abstracts of the Thirty-fifth Interscience Conference on Antimicrobial Agents and Chemotherapy (San Francisco). Washington, DC: American Society for Microbiology; 1995.
132 Bach MC, Bagwell SP, Knapp NP, et al. 9-(1,3-dihydroxy-2-propoxymethyl) guanine for cytomegalovirus infections in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1985;103:381.
133 Sha BE, Benson CA, Deutsch TA, et al. Suppression of cytomegalovirus retinitis in persons with AIDS with high-dose intravenous acyclovir. J Infect Dis. 1991;164:777–780.
134 Biron KK, Stanat SC, Sorrell JB, et al. Metabolic activation of the nucleoside analog 9-[[2-hydroxy-1-(hydroxymethyl) ethoxy]methyl] guanine in human diploid fibroblasts infected with human cytomegalovirus. Proc Natl Acad Sci U S A. 1985;82:2473–2477.
135 Field AK, Daview ME, Dewitt C, et al. 9-[[2-hydroxy-1-(hydroxymethyl)ethoxy]methyl] guanine: a selective inhibitor of herpes group virus replication. Proc Natl Acad Sci U S A. 1983;80:4139–4143.
136 Anand R, Font RL, Fish RH, et al. Pathology of cytomegalovirus retinitis treated with sustained release intravitreal ganciclovir. Ophthalmology. 1993;100:1032–1039.
137 Jabs DA, Enger C, Bartlett JG. Cytomegalovirus retinitis and acquired immunodeficiency syndrome. Arch Ophthalmol. 1989;107:75–80.
138 Holland GN, Sidikaro Y, Kreiger AE, et al. Treatment of cytomegalovirus retinopathy with ganciclovir. Ophthalmology. 1987;94:815–823.
139 Jacobson MA, O’Donnell JJ, Porteous D, et al. Retinal and gastrointestinal disease due to cytomegalovirus in patients with the acquired immune deficiency syndrome: prevalence, natural history, and response to ganciclovir therapy. Q J Med. 1988;67:473–486.
140 Orellana J, Teich SA, Friedman AH, et al. Combined short- and long-term therapy for the treatment of cytomegalovirus retinitis using ganciclovir (BW B759U). Ophthalmology. 1987;94:831–838.
141 Studies of Ocular Complications of AIDS Research Group in collaboration with the AIDS Clinical Trials Group. Mortality in patients with the acquired immunodeficiency syndrome treated with either foscarnet or ganciclovir for cytomegalovirus retinitis. N Engl J Med. 1992;326:213–220.
142 Hoechst H, Dieterich D, Bozzette S, et al. Toxicity of combined ganciclovir and zidovudine for cytomegalovirus disease associated with AIDS. Ann Intern Med. 1990;113:111–117.
143 Studies of Ocular Complications of AIDS Research Group in collaboration with the AIDS Clinical Trials Group. Combination foscarnet and ganciclovir therapy vs monotherapy for the treatment of relapsed cytomegalovirus retinitis in patients with AIDS: the cytomegalovirus retreatment trial. Arch Ophthalmol. 1996;114:23–33.
144 Gross JG, Bozzette SA, Mathews WC, et al. Longitudinal study of cytomegalovirus retinitis in acquired immune deficiency syndrome. Ophthalmology. 1990;97:681–686.
145 Spector SA, McKinley GF, Lalezari JP, et al. Oral ganciclovir for the prevention of cytomegalovirus disease in persons with AIDS. N Engl J Med. 1996;334:1491–1497.
146 Oral Ganciclovir European and Australian Cooperative Study Group. Intravenous vs oral ganciclovir: European/Australian comparative study of efficacy and safety in the prevention of cytomegalovirus retinitis recurrence in patients with AIDS. The Oral Ganciclovir European and Australian Cooperative Study Group. AIDS. 1995;9:471–477.
147 Jung D, Dorr A. Single-dose pharmacokinetics of valganciclovir in HIV- and CMV-seropositive subjects. J Clin Pharmacol. 1999;39:800–804.
148 Brown F, Banken L, Saywell K, et al. Pharmacokinetics of valganciclovir and ganciclovir following multiple oral dosages of valganciclovir in HIV- and CMV-seropositive volunteers. Clin Pharmacokinet. 1999;37:167–176.
149 Martin DF, Sierra-Madero J, Walmsley S, et al. A controlled trial of valganciclovir as induction therapy for cytomegalovirus retinitis. N Engl J Med. 2002;346:1119–1126.
150 Lalezari J, Lindley J, Walmsley S, et al. A safety study of oral valganciclovir maintenance treatment of cytomegalovirus retinitis. J Acquir Immune Defic Syndr. 2002;30:392–400.
151 Crumpacker CS. Mechanism of action of foscarnet against viral polymerases. Am J Med. 1992;92(Suppl 2A):3–7.
152 Bergdahl S, Sonnerbor A, Larsson A, et al. Declining levels of HIV p24 antigen in serum during treatment with foscarnet. Lancet. 1988;1:1052.
153 Crumpacker CS, Kowalsky PN, Oliver SA, et al. Resistance of herpes simplex virus to 9-[[2-(hydroxymethyl) ethoxy]methyl] guanine (2-NDG); physical mapping of drug synergism within the viral DNA polymerase locus. Proc Natl Acad Sci U S A. 1984;81:1556–1560.
154 Jacobson MA, Crowe S, Levy J, et al. Effect of foscarnet therapy on infection with human immunodeficiency virus in patients with AIDS. J Infect Dis. 1988;158:862–865.
155 Jacobson MA, Wulfsohn M, Feinberg JE, et al. Phase II dose-ranging trial of foscarnet salvage therapy for cytomegalovirus retinitis in AIDS patients intolerant of or resistant to ganciclovir (ACTG protocol 093). AIDS Clinical Trials Group of the National Institute of Allergy and Infectious Diseases. AIDS. 1994;8:451–459.
156 Studies of Ocular Complications of AIDS Research Group in collaboration with the AIDS Clinical Trials Group. Morbidity and toxic effects associated with ganciclovir or foscarnet therapy in a randomized cytomegalovirus retinitis trial. Arch Intern Med. 1995;155:65–74.
157 Studies of Ocular Complications of AIDS Research Group in collaboration with AIDS Clinical Trials Group. Antiviral effects of foscarnet and ganciclovir therapy on human immunodeficiency virus p24 antigen in patients with AIDS and cytomegalovirus retinitis. J Infect Dis. 1995;172:613–621.
158 Jabs DA. Controversies in the treatment of cytomegalovirus retinitis: foscarnet vs ganciclovir. Infect Agents Dis. 1995;4:131–142.
159 Deray G, Martinez F, Katlama C, et al. Foscarnet nephrotoxicity: mechanism, incidence, and prevention. Am J Nephrol. 1989;9:316–321.
160 Holland GN, Levinson RD, Jacobson MA. Dose-related difference in progression rates of cytomegalovirus retinopathy during foscarnet maintenance therapy. AIDS Clinical Trials Group Protocol 915 Team. Am J Ophthalmol. 1995;119:576–586.
161 Berthe P, Baudouin C, Garraffo R, et al. Toxicologic and pharmacokinetic analysis of intravitreal injections of foscarnet, either alone or in combination with ganciclovir. Invest Ophthalmol Vis Sci. 1994;35:1038–1045.
162 Palestine AG, Polis MA, De Smet MD, et al. A randomized controlled trial of foscarnet in the treatment of cytomegalovirus retinitis in patients with AIDS. Ann Intern Med. 1991;115:665–673.
163 Lalezari JP. Cidofovir: a new therapy for cytomegalovirus retinitis. J Acquir Immune Defic Syndr. 1997;14(Suppl 1):22–26.
164 Lalezari JP, Stagg RJ, Kuppermann BD, et al. Intravenous cidofovir for peripheral cytomegalovirus retinitis in patients with AIDS: a randomized, controlled trial. Ann Intern Med. 1997;126:257–263.
165 Studies of Ocular Complications of AIDS Research Group in collaboration with the AIDS Clinical Trials Group. Long-term follow-up of patients with AIDS treated with parenteral cidofovir for cytomegalovirus: the HPMPC Peripheral Cytomegalovirus Retinitis Trial. AIDS. 2000;14:1571–1581.
166 Studies of Ocular Complications of AIDS Research Group in collaboration with the AIDS Clinical Trials Group. Parenteral cidofovir for cytomegalovirus retinitis in patients with AIDS: the HPMPC peripheral cytomegalovirus retinitis trial – a randomized, controlled trial. Ann Intern Med. 1997;126:264–274.
167 Rahhal FM, Arevalo JF, Chavez de la Paz E, et al. Treatment of cytomegalovirus retinitis with intravitreous cidofovir in patients with AIDS: a preliminary report. Ann Intern Med. 1996;125:98–103.
168 Davis JL, Taskintuna I, Freeman WR, et al. Iritis and hypotony after treatment with intravenous cidofovir for cytomegalovirus retinitis. Arch Ophthalmol. 1997;115:733–737.
169 Kirsch LS, Arevalo JF, Chavez de la Paz E, et al. Intravitreal cidofovir (HPMPC) treatment of cytomegalovirus retinitis in patients with acquired immune deficiency syndrome. Ophthalmology. 1995;102:533–543.
170 Rahhal FM, Arevalo JF, Munguia D, et al. Intravitreal cidofovir for the maintenance treatment of cytomegalovirus retinitis. Ophthalmology. 1996;103:1078–1083.
171 Jabs DA, Dunn JP, Enger C, et al. Cytomegalovirus retinitis and viral resistance: prevalence of resistance at diagnosis, 1994. Cytomegalovirus Retinitis and Viral Resistance Study Group. Arch Ophthalmol. 1996;114:809–814.
172 Jabs DA, Enger C, Dunn JP, et al. Cytomegalovirus retinitis and viral resistance: 4. Ganciclovir resistance. J Infect Dis. 1998;177:770–773.
173 Smith KL, Cherrington JM, Jiles RE, et al. High-level resistance of cytomegalovirus to ganciclovir is associated with alterations in both the UL97 and DNA polymerase genes. J Infect Dis. 1997;176:69–77.
174 Drew WL, Stempton MJ, Andrews J, et al. Cytomegalovirus (CMV) resistance in patients with CMV retinitis and AIDS treated with oral or intravenous ganciclovir. J Infect Dis. 1999;179:1352–1355.
175 Jabs DA, Enger C, Forman M, et al. Incidence of foscarnet resistance and cidofovir resistance in patients treated for cytomegalovirus retinitis. The Cytomegalovirus Retinitis and Viral Resistance Study Group. Antimicrob Agents Chemother. 1998;42:2240–2241.
176 Boivin G, Gilbert C, Gaudreau A, et al. Rate of emergence of cytomegalovirus (CMV) mutations in leukocytes of patients with acquired immunodeficiency syndrome who are receiving valganciclovir as induction and maintenance therapy for CMV retinitis. J Infect Dis. 2001;184:1598–1602.
177 Drew WL, Miner RC, Saleh E, et al. Antiviral susceptibility of cytomegalovirus: criteria for detecting resistance to antivirals. Clin Diagn Virol. 1993;1:179–185.
178 Landry ML, Stanat S, Biron K, et al. A standardized plaque reduction assay for determination of drug susceptibilities of cytomegalovirus clinical isolates. Antimicrob Agents Chemother. 2000;44:688–692.
179 Cihlar T, Fuller MD, Cherrington JM. Characterization of drug resistance-associated mutations in the human cytomegalovirus DNA polymerase gene by using recombinant mutant viruses generated from overlapping DNA fragments. J Virol. 1998;72:5927–5936.
180 Weinberg A, Jabs DA, Chou S, et al. Mutations conferring foscarnet resistance in a cohort of patients with acquired immunodeficiency syndrome and cytomegalovirus retinitis. J Infect Dis. 2003;187:777–784.
181 Spector SA, Hsia K, Wolf D, et al. Molecular detection of human cytomegalovirus and determination of genotypic ganciclovir resistance in clinical specimens. Clin Infect Dis. 1995;21(Suppl 2):S170–S173.
182 Erice A. Resistance of human cytomegalovirus to antiviral drugs. Clin Microbiol Rev. 1999;12:286–297.
183 Chou S, Lurain NS, Weinberg A, et al. Interstrain variation in the human cytomegalovirus DNA polymerase sequence and its effect on the genotypic diagnosis of antiviral drug resistance. Antimicrob Agents Chemother. 1999;43:1500–1502.
184 Chou S, Marousek G, Parenti DM, et al. Mutation in region III of the DNA polymerase gene conferring foscarnet resistance in cytomegalovirus isolates from 3 subjects receiving prolonged antiviral therapy. J Infect Dis. 1998;178:526–530.
185 Gilbert C, Bestman-Smith J, Boivin G. Resistance of herpesvirus to antiviral drugs: clinical impacts and molecular mechanisms. Drug Resist Update. 2002;5:88–114.
186 Hu H, Jabs DA, Forman MS, et al. Comparison of cytomegalovirus (CMV) UL97 gene sequences in the blood and vitreous of patients with acquired immunodeficiency syndrome and CMV retinitis. J Infect Dis. 2002;185:861–867.
187 Arevalo JF, Gonzalez C, Capparelli EV, et al. Intravitreous and plasma concentrations of ganciclovir and foscarnet after intravenous therapy in patients with AIDS and cytomegalovirus. J Infect Dis. 1995;172:951–956.
188 Jabs DA, Martin BK, Forman MS, et al. Cytomegalovirus resistance to ganciclovir and clinical outcomes of patients with cytomegalovirus retinitis. Am J Ophthalmol. 2003;135:26–34.
189 Kuppermann BD, Flores-Aguilar M, Quiceno J, et al. Combination ganciclovir and foscarnet therapy in the treatment of clinically resistant cytomegalovirus retinitis in patients with the acquired immune deficiency syndrome. Am J Ophthalmol. 1993;111:1359–1366.
190 Walton RC, Whitcup SM, Mueller BU, et al. Combined intravenous ganciclovir and foscarnet for children with recurrent cytomegalovirus retinitis. Ophthalmology. 1995;102:1865–1870.
191 Desatnik HR, Foster RE, Lowder CY. Treatment of clinically resistant cytomegalovirus retinitis with combined intravitreal injections of ganciclovir and foscarnet. Am J Ophthalmol. 1996;122:121–123.
192 Hodge WG, Lalonde RG, Sampalis J, et al. Once-weekly intraocular injections of ganciclovir for maintenance therapy of cytomegalovirus retinitis: clinical and ocular outcome. J Infect Dis. 1996;174:393–396.
193 Montero MC, Pastor M, Buenestado C, et al. Intravitreal ganciclovir for cytomegalovirus retinitis in patients with AIDS. Ann Pharmacother. 1996;30:717–723.
194 Young S, Morlet N, Besen G, et al. High-dose (2000-microgram) intravitreous ganciclovir in the treatment of cytomegalovirus retinitis. Ophthalmology. 1998;105:1404–1410.
195 Baudouin C, Chassain C, Caujolle C, et al. Treatment of cytomegalovirus retinitis in AIDS patients using intravitreal injections of highly concentrated ganciclovir. Ophthalmologica. 1996;210:329–335.
196 Tognon MS, Turrini B, Masiero G, et al. Intravitreal and systemic foscarnet in the treatment of AIDS-related CMV retinitis. Eur J Ophthalmol. 1996;6:179–182.
197 Diaz-Llopis M, Espana E, Munoz G, et al. High dose intravitreal foscarnet in the treatment of cytomegalovirus retinitis in AIDS. Br J Ophthalmol. 1994;78:120–124.
198 Sandorn GE, Anand R, Torti RE, et al. Sustained-release ganciclovir therapy for treatment of cytomegalovirus retinitis: use of an intravitreal device. Arch Ophthalmol. 1992;110:188–195.
199 Martin DF, Parks DJ, Mellow SD, et al. Treatment of cytomegalovirus retinitis with an intraocular sustained-release ganciclovir implant: a randomized controlled clinical trial [see comments]. Arch Ophthalmol. 1994;112:1531–1539.
200 Marx JL, Kapusta MA, Patel SS, et al. Use of the ganciclovir implant in the treatment of recurrent cytomegalovirus retinitis. Arch Ophthalmol. 1996;114:815–820.
201 Martin DF, Ferris FL, Brothers RJ, et al. Retinal detachment in eyes treated with a ganciclovir implant [Letter]. Arch Ophthalmol. 1995;113:1355.
202 Martin DF, Dunn JP, Davis JL, et al. Use of the ganciclovir implant for the treatment of cytomegalovirus retinitis in the era of potent antiretroviral therapy: recommendations of the International AIDS Society – USA panel. Am J Ophthalmol. 1999;127:329–339.
203 Kempen JH, Jabs DA, Dunn JP, et al. Retinal detachment risk in cytomegalovirus retinitis related to the acquired immunodeficiency syndrome. Arch Ophthalmol. 2001;119:33–40.
204 Shane TS, Martin DF. Endophthalmitis after ganciclovir implant in patients with AIDS and cytomegalovirus retinitis. Am J Ophthalmol. 2003;136:649–654.
205 Kirsch LS, Arevalo JF, De Clercq E, et al. Phase I/II study of intravitreal cidofovir for the treatment of cytomegalovirus retinitis in patients with the acquired immunodeficiency syndrome. Am J Ophthalmol. 1995;119:466–476.
206 Banker AS, Arevalo JF, Munguia D, et al. Intraocular pressure and aqueous humor dynamics in patients with AIDS treated with intravitreal cidofovir (HPMPC) for cytomegalovirus retinitis. Am J Ophthalmol. 1997;124:168–180.
207 Besen B, Flores-Aguilar M, Assil KK, et al. Long-term therapy for herpes retinitis in an animal model with high-concentrated liposome-encapsulated HPMPC. Arch Ophthalmol. 1995;113:661–668.
208 Kirsch LS, Arevalo JF, Chavez de la Paz E, et al. Intravitreal cidofovir (HPMPC) treatment of cytomegalovirus retinitis in patients with acquired immune deficiency syndrome [published erratum appears in Ophthalmology 102:702, 1995]. Ophthalmology. 1995;102:533–542. discussion 542–3
209 Taskintuna I, Rahhal FM, Arevalo JF, et al. Low-dose intravitreal cidofovir (HPMPC) therapy of cytomegalovirus retinitis in patients with acquired immune deficiency syndrome. Ophthalmology. 1997;104:1049–1057.
210 Arevalo JF, Munguia D, Faber D, et al. Correlation between intraocular pressure and CD4 T-lymphocyte counts in patients with human immunodeficiency virus with and without cytomegalovirus retinitis. Am J Ophthalmol. 1996;122:91–96.
211 Azad RF, Driver VB, Tanaka K, et al. Antiviral activity of a phosphorothioate oligonucleotide complementary to RNA of the human cytomegalovirus major immediate-early region. Antimicrob Agents Chemother. 1993;37:1945–1954.
212 Anderson KP, Fox MC, Brown-Driver V, et al. Inhibition of human cytomegalovirus immediate-early gene expression by an antisense oligonucleotide complementary to immediate-early RNA. Antimicrob Agents Chemother. 1996;40:2004–2011.
213 The Vitravene Study Group. A randomized controlled clinical trial of intravitreous fomivirsen for treatment of newly diagnosed peripheral cytomegalovirus retinitis in patients with AIDS. Am J Ophthalmol. 2002;133:467–474.
214 Freeman WR. Retinal toxic effects associated with intravitreal fomivirsen. Arch Ophthalmol. 2001;119:458.
215 The Vitravene Study Group. Randomized dose-comparison studies of intravitreous fomivirsen for treatment of cytomegalovirus retinitis that has reactivated or is persistently active despite other therapies in patients with AIDS. Am J Ophthalmol. 2002;133:475–483.
216 The Vitravene Study Group. Safety of intravitreous fomivirsen for treatment of cytomegalovirus retinitis patients with AIDS. Am J Ophthalmol. 2002;133:484–498.
217 Jabs DA, Griffiths PD. Fomivirsen for the treatment of cytomegalovirus retinitis. Am J Ophthalmol. 2002;133:552–556.
218 Biron KK, Harvey RG, Chamberlain SS. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole L-riboside with a unique mode of action. Antimicrob Agents Chemother. 2002;46:2365–2372.
219 Koszalka GW, Johnson NW, Good SS, et al. Preclinical and toxicology studies of 1263W94, a potent and selective inhibitor of human cytomegalovirus replication. Antimicrob Agents Chemother. 2002;46:2373–2380.
220 Wang LH, Peck RW, Yin Y, et al. Phase I safety and pharmacokinetic trials of 1263W94, a novel oral anti-human cytomegalovirus agent, in healthy and human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother. 2003;47:1334–1342.
221 Lalezari JP, Aberg JA, Wang LH, et al. Phase I dose escalation trial evaluating the pharmacokinetics, anti-human cytomegalovirus (HCMV) activity, and safety of 1263W94 in human immunodeficiency virus-infected men with asymptomatic HCMV shedding. Antimicrob Agents Chemother. 2002;46:2969–2976.
222 Kempen JH, Jabs DA, Wilson LA, et al. Risk of vision loss in patients with cytomegalovirus retinitis and the acquired immunodeficiency syndrome. Arch Ophthalmol. 2003;121:466–476.
223 Freeman WR, Friedberg DN, Berry C, et al. Risk factors for development of rhegmatogenous retinal detachment in patients with cytomegalovirus retinitis. Am J Ophthalmol. 1993;116:713–720.
224 Studies of Ocular Complications of AIDS (SOCA) Research Group in Collaboration with the AIDS Clinical Trials Group (ACTG). Rhegmatogenous retinal detachment in patients with cytomegalovirus retinitis. Foscarnet–Ganciclovir Cytomegalovirus Retinitis Trial. Am J Ophthalmol. 1997;124:61–70.
225 Broughton WL, Cupples HP, Parver LM. Bilateral retinal detachment following cytomegalovirus retinitis. Arch Ophthalmol. 1978;96:618–624.
226 Freeman WR, Henderly DE, Wan WL. Rhegmatogenous retinal detachment in treated cytomegalovirus retinitis: prevalence, pathophysiology, and treatment. Am J Ophthalmol. 1987;103:527–536.
227 Sidikaro Y, Silver L, Holland GN, et al. Rhegmatogenous retinal detachments in patients with AIDS and necrotizing retinal infections. Ophthalmology. 1991;98:129–135.
228 Irvine AR. Treatment of retinal detachment due to cytomegalovirus retinitis in patients with AIDS. Trans Am Ophthalmol Soc. 1991;89:349–363.
229 Freeman WR, Quiceno JI, Crapotta JA, et al. Surgical repair of rhegmatogenous retinal detachment in immunosuppressed patients with cytomegalovirus retinitis. Ophthalmology. 1992;99:466–474.
230 Holland GN, Buhles WC, Mastre B, et al. A controlled retrospective study of ganciclovir treatment for cytomegalovirus retinopathy: use of a standardized system for the assessment of disease outcome. Arch Ophthalmol. 1989;107:1759–1766.
231 Freeman WR. Retinal detachment in cytomegalovirus retinitis: should our approach be changed? Retina. 1999;19:27–33.
232 Sandy CJ, Bloom PA, Graham EM, et al. Retinal detachment in AIDS-related cytomegalovirus retinitis. Eye. 1995;9:277–281.
233 Regillo CD, Vander JF, Duker JS, et al. Repair of retinitis-related retinal detachments with silicone oil in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1992;113:21–27.
234 Freeman WR. Application of vitreoretinal surgery to inflammatory and infectious diseases of the posterior segment. Int Ophthalmol Clin. 1992;32:15–33.
235 Nasemann JE, Mutsch A, Wiltfang R, et al. Early pars plana vitrectomy without buckling procedure in cytomegalovirus retinitis-induced retinal detachment. Retina. 1995;15:111–116.
236 Garcia RF, Flores-Aguilar M, Quiceno JI, et al. Results of rhegmatogenous retinal detachment repair in cytomegalovirus retinitis with and without scleral buckling. Ophthalmology. 1995;102:236–245.
237 Davis JL, Chuang EL. Management of retinal detachment associated with CMV retinitis in AIDS patients. Eye. 1992;6:28–34.
238 Tanna AP, Kempen JH, Dunn JP. Incidence and management of cataract after retinal detachment repair with silicone oil in immune compromised patients with cytomegalovirus retinitis. Am J Ophthalmol. 2003;136:1009–1015.
239 Irvine AR, Lonn L, Schwartz D, et al. Retinal detachment in AIDS: long-term results after repair with silicone oil. Br J Ophthalmol. 1997;81:180–183.
240 Meldrum ML, Aaberg TM, Patel A, et al. Cataract extraction after silicone oil repair of retinal detachments due to necrotizing retinitis. Arch Ophthalmol. 1996;114:885–892.
241 Azen SP, Scott IU, Flynn HW, Jr., et al. Silicone oil in the repair of complex retinal detachments. A prospective observational multicenter study. Ophthalmology. 1998;105:1587–1597.
242 Karavellas MP, Song M, MacDonald JC, et al. Long-term posterior and anterior segment complications of immune recovery uveitis associated with cytomegalovirus retinitis. Am J Ophthalmol. 2000;130:57–64.
243 Baumal CR, Reichel E. Management of cytomegalovirus-related rhegmatogenous retinal detachments. Ophthalmic Surg Lasers. 1998;29:916–925.
244 Althaus C, Loeffler KU, Schimkat M, et al. Prophylactic argon laser coagulation for rhegmatogenous retinal detachment in AIDS patients with cytomegalovirus retinitis. Graefes Arch Clin Exp Ophthalmol. 1998;236:359–364.
245 Davis JL, Hummer J, Feuer WJ. Laser photocoagulation for retinal detachment and retinal tears in cytomegalovirus retinitis. Ophthalmology. 1997;104:2053–2060. discussion 2060–1
246 McCluskey P, Grigg J, Playfair TJ. Retinal detachments in patients with AIDS and CMV retinopathy: a role for laser photocoagulation. Br J Ophthalmol. 1995;79:153–156.
247 Meffert SA, Ai E. Laser photocoagulation prophylaxis for CMV retinal detachments. Ophthalmology. 1998;105:1353–1355.
248 Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300.
249 Kempen JH, Martin BK, Wu AW, et al. The effect of cytomegalovirus retinitis on the quality of life of patients with AIDS in the era of highly active antiretroviral therapy. Ophthalmology. 2003;110:987–995.
250 Palella FJ, Jr., Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860.
251 Varani S, Spezzacatena P, Manfredi R, et al. The incidence of cytomegalovirus (CMV) antigenemia and CMV disease is reduced by highly active antiretroviral therapy. Eur J Epidemiol. 2000;16:433–437.
252 Deayton JR, Wilson P, Sabin CA, et al. Changes in the natural history of cytomegalovirus retinitis following the introduction of highly active antiretroviral therapy. AIDS. 2000;14:1163–1170.
253 MacDonald JC, Torriani FJ, Morse LS, et al. Lack of reactivation of cytomegalovirus (CMV) retinitis after stopping CMV maintenance therapy in AIDS patients with sustained elevations in CD4 T cells in response to highly active antiretroviral therapy. J Infect Dis. 1998;177:1182–1187.
254 Jabs DA, Bolton SG, Dunn JP, et al. Discontinuing anticytomegalovirus therapy in patients with immune reconstitution after combination antiretroviral therapy. Am J Ophthalmol. 1998;126:817–822.
255 MacDonald JC, Karavellas MP, Torriani FJ, et al. Highly active antiretroviral therapy-related immune recovery in AIDS patients with cytomegalovirus retinitis. Ophthalmology. 2000;107:877–881.
256 Curi AL, Muralha A, Muralha L, et al. Suspension of anticytomegalovirus maintenance therapy following immune recovery due to highly active antiretroviral therapy. Br J Ophthalmol. 2001;85:471–473.
257 Whitcup SM, Fortin E, Lindblad AS, et al. Discontinuation of anticytomegalovirus therapy in patients with HIV infection cytomegalovirus. JAMA. 1999;282:1633–1671.
258 Vrabec TR, Baldassano VF, Whitcup SM. Discontinuation of maintenance therapy in patients with quiescent cytomegalovirus retinitis and elevated CD4 counts. Ophthalmology. 1998;105:1259–1264.
259 Sepkowitz KA. Effect of HAART on natural history of AIDS-related opportunistic disorders. Lancet. 1998;351:228–230.
260 Michelet C, Arvieux C, Francois C, et al. Opportunistic infections occurring during highly active antiretroviral treatment. AIDS. 1998;12:1815–1822.
261 Jacobson MA, Zegans M, Pavan PR, et al. Cytomegalovirus retinitis after initiation of highly active antiretroviral therapy. Lancet. 1997;349:1443–1445.
262 Elston JW, Thaker H. Immune reconstitution inflammatory syndrome. Int J STD AIDS. 2009;23:221–224.
263 Karavellas MP, Lower CY, Macdonald JC, et al. Immune recovery vitreitis associated with inactive cytomegalovirus retinitis: a new syndrome. Arch Ophthalmol. 1998;116:169–175.
264 Otiti-Sengeri J, Meenken C, van den Horn GJ, et al. Ocular immune reconstitution inflammatory syndromes. Curr Opin HIV AIDS. 2008;3:432–437.
265 Holland GN. Pieces of a puzzle: toward better understanding of intraocular inflammation associated with human immunodeficiency virus infection. Am J Ophthalmol. 1998;125:383–385.
266 Zegans ME, Walton RC, Holland GN, et al. Transient vitreous inflammatory reactions associated with combination antiretroviral therapy in patients with AIDS and cytomegalovirus retinitis. Am J Ophthalmol. 1998;125:292–300.
267 Holland GN. Immune recovery uveitis. Ocul Immunol Inflamm. 1999;7:215–221.
268 Karavellas MP, Plummer DJ, Macdonald JC, et al. Incidence of immune recovery vitreitis in cytomegalovirus retinitis patients following institution of successful highly active antiretroviral therapy. J Infect Dis. 1999;179:697–700.
269 Nguyen QD, Kempen JH, Bolton SG, et al. Immune recovery uveitis in patients with AIDS and cytomegalovirus retinitis after highly active antiretroviral therapy. Am J Ophthalmol. 2000;129:634–639.
270 Jabs DA, Van Natta ML, Kempen JH, et al. Characteristics of patients with cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Am J Ophthalmol. 2002;133:48–61.
271 Arevalo JF, Mendoza AJ, Ferretti Y. Immune recovery uveitis in AIDS patients with cytomegalovirus retinitis treated with highly active antiretroviral therapy in Venezuela. Retina. 2003;23:495–502.
272 Karavellas MP, Azen SP, Macdonald JC, et al. Immune recovery vitreitis in AIDS: clinical predictors, sequella, and treatment outcomes. Retina. 2001;21:1–9.
273 Song M, Azen SP, Buley A, et al. Effect of anti-cytomegalovirus therapy on the incidence of immune recovery uveitis in AIDS patients with healed cytomegalovirus retinitis. Am J Ophthalmol. 2003;136:696–702.
274 Newsome R, Casswell T, O’Moore E, et al. Cystoid macular oedema in patients with AIDS and cytomegalovirus retinitis on highly active antiretroviral therapy. Br J Ophthalmol. 1998;82:456–457.
275 Silverstein BE, Smith JH, Sykes SO, et al. Cystoid macular edema associated with cytomegalovirus retinitis in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1998;125:412–415.
276 Whitcup SM. Cytomegalovirus retinitis in the era of highly active antiretroviral therapy. JAMA. 2000;283:653–657.
277 Karavellas MP, Song MK, Macdonald JC, et al. Long-term posterior and anterior segment complications of immune recovery uveitis associated with cytomegalovirus retinitis. Am J Ophthalmol. 2000;130:57–64.
278 Sanislo SR, Lowder CY, Kaiser PK. Optic nerve head neovascularization in a patient with inactive cytomegalovirus retinitis and immune recovery. Am J Ophthalmol. 1998;126:318–320.
279 Canzano JC, Reed JB, Morse LS. Vitreomacular traction syndrome following highly active antiretroviral therapy in AIDS patients with cytomegalovirus retinitis. Retina. 1998;18:443–447.
280 Robinson MR, Csasky KG, Lee SS, et al. Fibrovascular changes misdiagnosed as cytomegalovirus retinitis reactivation in a patient with immune recovery. Clin Infect Dis. 2004;38:139–141.
281 Biswas J, Choudhry S, Kumarasamy, et al. Immune recovery vitreitis presenting as panuveitis following therapy with protease inhibitors. Indian J Ophthalmol. 2000;48:313–315.
282 Goldberg DE, Freeman WR. Uveitic angle closure glaucoma in a patient with inactive cytomegalovirus and immune recovery uveitis. Ophthalmic Surg Lasers. 2002;33:421–425.
283 Goldberg DE, Wang H, Azen SP, et al. Long term visual outcome of patients with cytomegalovirus retinitis treated with highly active antiretroviral therapy. Br J Ophthalmol. 2003;87:853–855.
284 Kuppermann BD, Holland GN. Immune recovery uveitis. Am J Ophthalmol. 2000;130:103–106.
285 Pepose JS, Newman C, Bach MC, et al. Pathologic features of cytomegalovirus retinopathy after treatment with the antiviral agent ganciclovir. Ophthalmology. 1987;94:414–424.
286 D’Alessandro L, Bottaro E. Reactivation of CMV retinitis after treatment with subtenon corticosteroids for immune recovery uveitis in patients with AIDS. Scand J Infect Dis. 2002;34:780–782.
287 Morrison VL, Kozak I, LaBree LD, et al. Intravitreal triamcinolone acetate for the treatment of immune recovery uveitis macular edema. Ophthalmology. 2007;114(2):334–339.
288 Shah AM, Oster SF, Freeman WR. Viral retinitis after intravitreal triamcinolone injection in patients with predisposing medical comorbidities. Am J Ophthalmol. 2010;149(3):433–440.
289 Maguire AM, Nichols CW, Crooks GW. Visual loss in cytomegalovirus retinitis caused by cystoid macular edema in patients without the acquired immune deficiency. Ophthalmology. 1996;103:601–605.
290 Omerod LD, Larkin JA, Margo CA, et al. Rapidly progressive herpetic retinal necrosis: a blinding disease characteristic of advanced AIDS. [see commentary by C.L. Lowder, pp. 46–47]. Clin Infect Dis. 1998;26:34–35.
291 Forster DJ, Dugel PU, Frangieh GT, et al. Rapidly progressive outer retinal necrosis in the acquired immunodeficiency syndrome. Am J Ophthalmol. 1990;110:341–348.
292 Duker JS, Blumenkranz MS. Diagnosis and management of the acute retinal necrosis (ARN) syndrome. Surv Ophthalmol. 1991;35:327–343.
293 Friedlander S, Rahhal FM, Ericson L, et al. Optic neuropathy preceding acute retinal necrosis in acquired immunodeficiency syndrome. Arch Ophthalmol. 1996;114:1481–1485.
294 Culbertson WW, Atherton SS. Acute retinal necrosis and similar retinitis syndromes. Int Ophthalmol Clin. 1993;33:129–143.
295 Sellitti TP, Huang AJ, Schiffman J, et al. Association of herpes zoster ophthalmicus with acquired immunodeficiency syndrome and acute retinal necrosis. Am J Ophthalmol. 1993;116:297–301.
296 Holland GN. Standard diagnostic criteria for the acute retinal necrosis syndrome. Executive Committee of the American Uveitis Society. Am J Ophthalmol. 1994;117:663–667.
297 Abe T, Tsuchida K, Tamai M. A comparative study of the polymerase chain reaction and local antibody production in acute retinal necrosis syndrome and cytomegalovirus retinitis. Graefes Arch Clin Exp Ophthalmol. 1996;234:419–424.
298 Weinberg DV, Lyon AT. Repair of retinal detachments due to herpes varicella-zoster virus retinitis in patients with acquired immune deficiency syndrome. Ophthalmology. 1997;104:279–282.
299 Schlingemann RO, Bruinenberg M, Wertheim-Van Dillen P, et al. Twenty years’ delay of fellow eye involvement in herpes simplex virus type 2-associated bilateral acute retinal necrosis syndrome. Am J Ophthalmol. 1996;122:891–892.
300 Aslanides IM, De Souza S, Wong DTW, et al. Oral valacyclovir in the treatment of acute retinal necrosis syndrome. Retina. 2002;22:352–354.
301 Chatis PA, Miller CH, Schrager LE, et al. Successful treatment with foscarnet of an acyclovir-resistant mucocutaneous infection with herpes simplex virus in a patient with the acquired immunodeficiency syndrome. N Engl J Med. 1989;320:297–300.
302 Safrin S, Berger TG, Gilson I, et al. Foscarnet therapy in five patients with AIDS and acyclovir-resistant varicella-zoster virus infection. Ann Intern Med. 1991;115:19–21.
303 Dunn JP. Viral retinitis. Ophthalmol Clin North Am. 1999;12:109.
304 Pavesio CE, Mitchell SM, Barton K, et al. Progressive outer retinal necrosis (PORN) in AIDS patients: a different appearance of varicella-zoster retinitis. Eye. 1995;9:271–276.
305 Engstrom RJ, Holland GN, Margolis TP, et al. The progressive outer retinal necrosis syndrome. A variant of necrotizing herpetic retinopathy in patients with AIDS. Ophthalmology. 1994;101:1488–1502.
306 Scott IU, Luu KM, Davis JL. Intravitreal antivirals in the management of patients with acquired immunodeficiency syndrome with progressive outer retinal necrosis. Arch Ophthalmol. 2002;120:1219–1222.
307 Ciulla TA, Rutledge BK, Morley MG, et al. The progressive outer retinal necrosis syndrome: successful treatment with combination antiviral therapy. Ophthalmic Surg Lasers. 1998;29:198–206.
308 Margolis TP, Lowder CY, Holland GN, et al. Varicella-zoster virus retinitis in patients with the acquired immunodeficiency syndrome. Am J Ophthalmol. 1991;112:119–131.
309 Spaide RF, Martin DF, Teich SA, et al. Successful treatment of progressive outer retinal necrosis syndrome. Retina. 1996;16:479–487.
310 Meffert SA, Kertes PJ, Lim P, et al. Successful treatment of progressive outer retinal necrosis using high-dose intravitreal ganciclovir. Retina. 1997;17:560–562.
311 Perez-Blasquez E, Traspas R, Marin IM, et al. Intravitreal ganciclovir treatment in progressive outer retinal necrosis. Am J Ophthalmol. 1997;124:418–421.
312 Kim SJ, Equi R, Belair ML, et al. Long-term preservation of vision in progressive outer retinal necrosis treated with combination antiviral drugs and highly active antiretroviral therapy. Ocul Immun Inflamm. 2007;15:425–427.
313 Kaplan JE, Benson C, Holmes KK, et al. Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Recommendations of the U.S. Public Health Service and the Infectious Disease Society of America. MMWR Recomm Rep. 2009;58(RR04):1–198.
314 Macher AM, Bardenstein DS, Zimmerman LE. Pneumocystis carinii choroiditis in a male homosexual with AIDS and disseminated pulmonary and extrapulmonary P. carinii infection. N Engl J Med. 1987;236:1092.
315 Freeman WR, Gross JG, Labelle J, et al. Pneumocystis carinii choroidopathy: a new clinical entity. Arch Ophthalmol. 1989;107:863–867.
316 Shami MJ, Freeman WR, Friedberg D, et al. A multicenter study of pneumocystis choroidopathy. Am J Ophthalmol. 1991;112:15–22.
317 Dugel PU, Rao NA, Forster DJ, et al. Pneumocystis carinii choroiditis after long-term aerosolized pentamidine therapy. Am J Ophthalmol. 1990;110:113–117.
318 Foster RE, Lowder CY, Meisler DM, et al. Presumed Pneumocystis carinii choroiditis: unifocal presentation, regression with intravenous pentamidine, and choroiditis recurrence. Ophthalmology. 1991;98:1360–1365.
319 Sneed SR, Blodi CF, Berger BB, et al. Pneumocystis carinii choroiditis in patients receiving inhaled pentamidine. N Engl J Med. 1989;322:936–937.
320 Centers for Disease Control and Prevention. HIV/AIDS surveillance report 1999;11:2–38.
321 Parke DW, II., Font RL. Diffuse toxoplasmic retinochoroiditis in a patient with AIDS. Arch Ophthalmol. 1986;104:571–575.
322 Heinemann MH, Gold JMW, Maisel J. Bilateral toxoplasma retinochoroiditis in a patient with acquired immune deficiency syndrome. Retina. 1986;6:224.
323 Cochereau-Massin I, LeHoang P, Lautier-Frau M, et al. Ocular toxoplasmosis in human immunodeficiency virus-infected patients. Am J Ophthalmol. 1992;114:130–135.
324 Elkins BS, Holland GN, Opremcak EM, et al. Ocular toxoplasmosis misdiagnosed as cytomegalovirus retinopathy in immunocompromised patients. Ophthalmology. 1994;101:499–507.
325 Berger BB, Egwuagu CE, Freeman WR, et al. Miliary toxoplasmic retinitis in acquired immunodeficiency syndrome. Arch Ophthalmol. 1993;111:373–376.
326 Cano-Parra JL, Diaz-Llopis ML, Cordoba JL, et al. Acute iridocyclitis in a patient with AIDS diagnosed as toxoplasmosis by PCR. Ocul Immunol Inflamm. 2000;8:127–130.
327 Moshfeghi DM, Dodds EM, Couto CA, et al. Diagnostic approaches to severe, atypical toxoplasmosis mimicking acute retinal necrosis. Ophthalmology. 2004;111:716–725.
328 Moorthy RS, Smith RE, Rao NA. Progressive ocular toxoplasmosis in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1993;115:742–747.
329 Holland GN. Ocular toxoplasmosis: a global reassessment. Part II: Disease manifestations and management. Am J Ophthalmol. 2004;137:1–17.
330 Holland GN. Ocular toxoplasmosis: a global reassessment. Part I: Epidemiology and course of disease. Am J Ophthalmol. 2003;136:973–988.
331 Grossniklaus HE, Specht CS, Allaire G, et al. Toxoplasma gondii retinochoroiditis and optic neuritis in acquired immune deficiency syndrome. Report of a case. Ophthalmology. 1990;97:1342–1346.
332 Rehder JR, Burnier MBJ, Pavesio CE, et al. Acute unilateral toxoplasmic iridocyclitis in an AIDS patient. Am J Ophthalmol. 1988;106:740–741.
333 Tate GW, Jr., Martin RG. Clindamycin in the treatment of human ocular toxoplasmosis. Can J Ophthalmol. 1977;12:188–195.
334 Torre D, Casari S, Speranza F, et al. Randomized trial of trimethoprim-sulfamethoxazole versus pyrimethamine-sulfadiazine for therapy of toxoplasmic encephalitis in patients with AIDS. Antimicrob Agents Chemother. 1998;2:1346–1349.
335 Jacobson JM, Hafner R, Remington J, et al. Dose-escalation, phase I/II study of azithromycin and pyrimethamine for the treatment of toxoplasmic encephalitis in AIDS. AIDS. 2001;15:583–589.
336 Bosch-Driessen LH, Verbraak FD, Suttorp-Schulten MSA, et al. A prospective, randomized trial of pyrimethamine and azithromycin vs pyrimethamine and sulfadiazine for the treatment of ocular toxoplasmosis. Am J Ophthalmol. 2002;134:34–40.
337 Pearson PA, Piracha AR, Sen H, et al. Atovaquone for the treatment of toxoplasma retinochoroiditis in immunocompetent patients. Ophthalmology. 1999;106:148–153.
338 Friedman AH. The retinal lesions of the acquired immune deficiency syndrome. Trans Am Ophthalmol Soc. 1984;82:447–491.
339 Edwards JE, Foos RY, Montomerie JZ, et al. Ocular manifestations of candida septicemia: review of seventy-six cases of hematogenous candida endophthalmitis. Medicine (Baltimore). 1974;53:47–75.
340 Rex JH, Bennet JE, Sugar AM, et al. A randomized trial comparing fluconazole with amphoteracin B for the treatment of candidemia in patients without neutropenia. N Engl J Med. 1994;331:1325–1330.
341 Panther LA, Sande MA. Cryptococcal meningitis in AIDS. Sande MA, Volberding PA. The medical management of AIDS, 2nd ed, Philadelphia: WB Saunders, 1997.
342 Shields JA, Wright DM, Augsburger JJ, et al. Cryptococcal chorioretinitis. Am J Ophthalmol. 1980;89:210.
343 Wykoff CA, Albini TA, Couvillion SS, et al. Intraocular Cryptococcoma. Arch Ophthalmol. 2009;127(5):700–702.
344 Wheat LJ, Connolly-Stringfield PA, Baker RL, et al. Disseminated histoplasmosis in the acquired immune deficiency syndrome: clinical findings, diagnosis and treatment, and review of the literature. Medicine (Baltimore). 1990;69:361–374.
345 Wheat J, Hafner R, Wulfsohn M, et al. Prevention of relapse of histoplasmosis with itraconazole in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1993;118:610–616.
346 Gonzales CA, Scott IU, Chaudhry NA, et al. Endogenous endophthalmitis caused by Histoplasma capsulatum var. capsulatum: a case report and literature review. Ophthalmology. 2000;107:725–729.
347 Ala-Kauhaluoma M, Aho I, Ristola M, et al. Involvement of intraocular structures in disseminated histoplasmosis. Acta Ophthalmol. 2010;88(4):493–496.
348 Denning DW, Follansbee SE, Scolaro M, et al. Pulmonary aspergillosis in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:654–662.
349 Kronish JW, Johnson TE, Gilberg SM, et al. Orbital infections in patients with human immunodeficiency virus infection. Ophthalmology. 1996;103(9):1483–1492.
350 Fish DG, Ampel NM, Galgiani JN, et al. Coccidioidomycosis during human immunodeficiency virus infection: a review of 77 patients. Medicine (Baltimore). 1990;69:384–391.
351 McDonnell PJ, Green WR, Endophthalmitis, Mandell GL, Gordon RG, Bennett JE. Principles and practice of infectious diseases, 4th ed, New York: Churchill Livingstone, 1995.
352 Finamor LP, Muccioli C, Martins MC, et al. Ocular and central nervous system paracoccidioidomycosis in a pregnant woman with acquired immunodeficiency syndrome. Am J Ophthalmol. 2002;134:456–459.
353 Hariprasad SM, Mieler WF, Holz ER, et al. Determination of vitreous, aqueous, and plasma concentration of orally administered voriconazole in humans. Arch Ophthalmol. 2004;122:42–47.
354 Brod RD, Flynn HW, Miller D. Endogenous fungal endophthalmitis. In: Duane’s clinical ophthalmology. Hagerstown: Harper and Row; 2004. CD-ROM. ch 11
355 Riddell J, 4th., Comer GM, Kauffman CA. Treatment of endogenous fungal endophthalmitis: focus on new antifungal agents. Clin Infect Dis. 2011;52:648–653.
356 Garbino J, Ondrusova A, Baligvo E, et al. Successful treatment of Paecilomyces lilacinus endophthalmitis with voriconazole [published correction appears in Scand J Infect Dis 2003; 35:79]. Scand J Infect Dis. 2002;34:701–703.
357 Kim JE, Perkins SL, Harris GJ. Voriconazole treatment of fungal scleritis and epibulbar abscess resulting from scleral buckle infection. Arch Ophthalmol. 2003;121:735–737.
358 Ruhnke M, Schmidt-Westhausen A, Trautmann M. In vitro activities of voriconazole (UK-109,496) against fluconazole-susceptible and -resistant Candida albicans isolates from oral cavities of patients with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1997;41:575–577.
359 Tucker JD, Li JZ, Robbins GK, et al. Ocular syphilis among HIV-infected patients: a systematic analysis of the literature. Sex Transm Infect. 2011;87:4–8.
360 McLeish WM, Pulido JS, Holland S, et al. The ocular manifestations of syphilis in the human immunodeficiency virus type 1-infected host. Ophthalmology. 1990;97:196–203.
361 Levy JH, Liss RA, Maguire AM. Neurosyphilis and ocular syphilis in patients with concurrent human immunodeficiency virus infection. Retina. 1989;9:175–180.
362 Gass JDM, Braunstein RA, Chenoweth RG. Acute syphilitic posterior placoid chorioretinitis. Ophthalmology. 1990;97:1288–1297.
363 Farhi D, Benhaddou N, Grange P, et al. Clinical and serologic baseline and follow-up features of syphilis according to HIV status in the post-HAART era. Medicine (Baltimore). 2009;88:331–340.
364 Freeman WR, Wiley CA. In situ nucleic acid hybridization. Surv Ophthalmol. 1988;34:187–192.
365 de Smet MD. Differential diagnosis of retinitis and choroiditis in patients with acquired immunodeficiency syndrome. Am J Med. 1992;92:17S–21S.
366 Schneiderman TE, Faber DW, Gross JG, et al. The agar–albumin sandwich technique for processing retinal biopsy specimens. Am J Ophthalmol. 1989;108:567–571.
367 HHS Panel on Antiretroviral Guidelines for Adults and Adolescents [Internet]. Department of Health and Human Services; 2011 Oct 14 [cited 2012 Jan 26]. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available from http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf