Chapter 60 HIV and the acquired immunodeficiency syndrome
HIV REPLICATION
Human immunodeficiency viruses (HIVs) 1 and 2 are retroviruses of the Lentivirus group. Like other lentiviruses, they exhibit tropism for cells of the immune system and cause immunological disorders, particularly immunodeficiency. Infection of immune cells in the nervous system may also cause neurological disease. Entry of HIV into cells of the immune system is via cell surface receptors. The major receptor is the CD4 molecule but the chemokine receptors CCR5 and CXCR4 play an important role as co-receptors. Inside the cell, the viral RNA is reverse-transcribed into DNA by a viral reverse transcriptase enzyme, and the DNA is integrated into the DNA of the host cell as proviral DNA by a viral integrase enzyme. The proviral DNA remains there until the cell is activated, when it is transcribed into RNA, which provides the template for assembly of new HIVs under the control of viral enzymes such as proteases. Budding of new virus from the cell is followed by infection of new cells and a repeat of the replication cycle.
PRIMARY HIV INFECTION
Initial infection by HIV-1 is associated with a primary HIV infection syndrome (also known as a seroconversion illness) in 50–70% of patients. This syndrome is similar to infectious mononucleosis, being characterised by fever, lymphadenopathy, headache, photophobia, fatigue and myalgia. However, mucocutaneous lesions, neurological disease and even transient immunodeficiency may also occur and, when present, this differentiates it from infectious mononucleosis. It may take a number of weeks for HIV antibody (detected by enzyme-linked immunosorbent assay (ELISA)) to become positive during seroconversion; however the level of plasma HIV RNA is very high. The diagnostic test of choice during this period is the measurement of HIV viral load utilising HIV RNA RT/PCR (reverse transcription of HIV RNA and amplification by polymerase chain reaction). HIV RNA peaks at a median of 10 days.1
DIAGNOSIS
A diagnosis of HIV infection can usually be made by demonstrating anti-HIV antibodies in the patient’s serum. However, the serological diagnosis of HIV infection can sometimes be problematic. A small minority of individuals who are not infected by HIV have serum antibodies which are reactive with some HIV proteins, and give false-positive results with some ELISAs. Many laboratories use two different types of ELISA to identify such sera. To ensure that HIV infection is not incorrectly diagnosed, an antibody test should only be considered positive if antibody is also detected according to defined criteria, using a confirmatory antibody test such as a Western blot immunoassay.2 Anti-HIV antibodies may be absent from the serum of patients with primary HIV infection. They are usually detectable by 2–6 weeks after infection, and almost always detectable by 12 weeks. After this time, absence of HIV antibodies excludes HIV infection in all but the most advanced cases of AIDS, or in a patient with an antibody deficiency disorder.
VIROLOGICAL MONITORING
HIV VIRAL LOAD MEASUREMENT
The major advance in viral diagnostics has been the development of polymerase chain reaction (PCR) and the application of this to the detection and quantification of viral nucleic acid in body fluids. Measurement of the blood ‘HIV load’ can be done by quantitating HIV RNA in plasma. Knowledge of the HIV load is useful in prognostication and essential when making decisions to commence and optimise therapy3 (Figure 60.1).
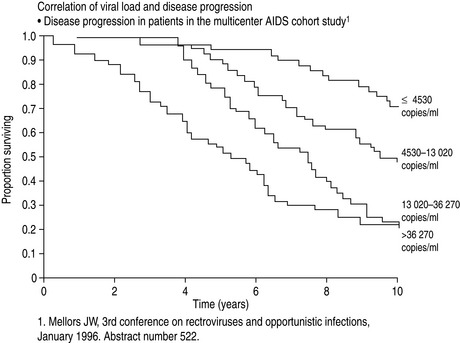
Figure 60.1 Correlation of viral load with disease progression. AIDS, acquired immunodeficiency syndrome.
(From Mellors JW, Rinaldo CR, Gupta P et al. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 1996; 272: 1167–70. Copyright: American Association for the Advancement of Science; reproduced with permission.)
GENOTYPING HIV TO DETECT DRUG RESISTANCE MUTATIONS
Drug susceptibility testing of HIV from a clinical specimen is performed in order to detect mutations in the genome of HIV that predict failure of individual antiviral therapeutic agents. The predominant genotype from an individual’s plasma or cerebrospinal fluid sample is determined by sequencing the reverse transcriptase and protease genes of complementary DNA (cDNA) obtained following RT/PCR. This information is used to assist the clinician and patient in the choice of an antiretroviral drug combination with the best chance of successful suppression of HIV replication, especially where resistance is suspected, i.e. in a failing regimen.4
IMMUNOLOGICAL MONITORING
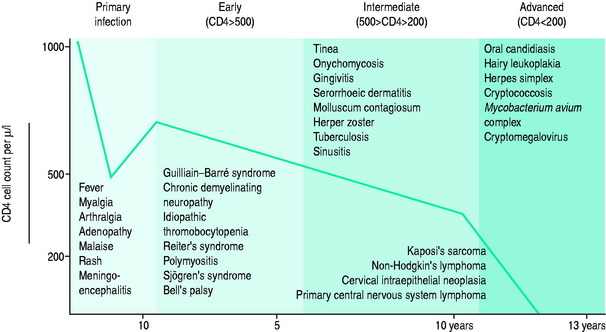
The blood CD4+ T-cell count or percentage is the best indicator of the severity of HIV-induced immunodeficiency and, therefore, of the patient’s susceptibility to opportunistic infections. Measurement of the blood CD4+ T-cell count or percentage is therefore critical in determining if the symptoms of an HIV-infected patient are likely to be caused by an opportunistic infection, and if so, what type of infection (Figure 60.2; and see text below).5
MANAGEMENT OF THE HIV-INFECTED PATIENT
The impact of combination antiretroviral therapy on the morbidity and mortality associated with HIV infection has been dramatic. The HIV Outpatient Study demonstrated a stepwise reduction in opportunistic infections and mortality with increasing intensity of antiretroviral therapy.6 In Australia, a comparison of cohorts before and after the introduction of combination antiretroviral therapy documented the effectiveness of these agents in reducing the risk of progression to AIDS and death.7 This refiects similar epidemiological studies conducted in Switzerland,8 France9 and the USA.10 A 70–80% reduction in mortality over 5 years has been the norm. AIDS-defining illnesses in Australia now occur predominantly in those without a past diagnosis of HIV infection.
Six classes of antiretroviral drugs are currently in use (Table 60.1). Fusion inhibitors block fusion of the virus with the cell membrane and CCR5 inhibitors block binding of CCR5-tropic strains of virus with CCR5, a co-receptor for HIV. Reverse transcriptase inhibitors are of three types. Firstly, nucleoside analogues and nucleotide analogues act by substituting for natural nucleosides or nucleotides during HIV replication, thereby inhibiting DNA chain elongation and the effects of the reverse transcriptase enzyme. Secondly, non-nucleoside reverse transcriptase inhibitors inhibit the reverse transcriptase enzyme by a different mechanism. Thirdly, integrase inhibitors block integration of viral DNA into host DNA and protease inhibitors inhibit the viral protease. All antiretroviral drugs have a limited duration of efficacy if used alone because HIV eventually develops resistance to them. The use of drug combinations is much more effective than single drugs, partly because drug resistance develops more slowly. Adherence to antiretroviral therapy is critical for the success of treatment.
Table 60.1 Antiretroviral drugs used to treat human immunodeficiency virus (HIV) infection
| Fusion inhibitors |
| Enfuvirtide (T20) |
| CCR5 inhibitors |
| Maraviroc |
| Nucleoside/nucleotide analogue reverse transcriptase inhibitors |
| Nucleoside analogues |
| Abacavir (ABV) |
| Didanosine (ddI) |
| Emtricitabine (FTC) |
| Lamivudine (3TC) |
| Stavudine (d4T) |
| Zidovudine (AZT) |
| Nucleotide analogues |
| Tenofovir (TNF) |
| Nucleoside/nucleotide fixed-dose combination tablets |
| Combivir (AZT + 3TC) |
| Trizivir (AZT + 3TC + ABV) |
| Kivexa (ABV + 3TC) |
| Truvada (TNF + FTC) |
| Non-nucleoside reverse transcriptase inhibitors |
| Delavirdine |
| Efavirenz |
| Nevirapine |
| Integrase inhibitors |
| Raltegravir |
| Protease inhibitors |
| Atazanavir* |
| Darunavir* |
| Fosamprenavir* |
| Indinavir* |
| Lopinivir* |
| Nelfinavir |
| Saquinavir* |
| Tipranavir* |
* Administered with low-dose ritonavir to increase serum levels (lopinavir + low-dose ritonavir are co-formulated as Kaletra).
DRUG TOXICITY
Recently, nucleoside analogue reverse transcriptase inhibitors (NRTIs) have been implicated in the development of syndromes that include fatigue, fat wasting, lactic acidosis and peripheral neuropathy. It has been suggested that these symptoms may be due to an inhibition of mitochondrial DNA (mtDNA) synthesis.11,12 Pancreatitis has occurred uncommonly with ddI (5–7%) and d4T (1–2%).
The most common adverse effect of NNRTIs is a skin rash. This occurs in up to 25% of subjects started on nevirapine and can range from a mild rash to Stevens–Johnson syndrome. Combination antiretroviral therapy is associated with a syndrome characterised by redistribution of fat (lipodystrophy) and fat atrophy (lipoatrophy). The biological mechanism responsible for the development of this syndrome is still unclear,13 although protease inhibitors in association with d4T have been implicated.
The use of antiretroviral therapy is also associated with hepatotoxicity in about 10% of patients.14 Co-infection with hepatitis C virus is one risk factor for hepatotoxicity and at least some cases are probably a type of immune restoration disease.15 Restoration of immune responses against pathogens also appears to be a cause of other types of inflammatory disease after the use of combination antiretroviral therapy.16,17
HIV-INDUCED IMMUNODEFICIENCY
Patients who are not treated with antiretroviral therapy or whose antiretroviral therapy is ineffective, for whatever reason, often become immunodeficient. Both CD4+ T-cell depletion and the effects of other immune defects lead to the development of an immunodeficiency syndrome. This syndrome is characterised by impaired cellular immunity and an increased propensity to opportunistic infections. In addition, some patients have impaired antibody responses and phagocyte dysfunction, which results in infections with encapsulated bacteria. As mentioned previously, during chronic HIV infection there is a strong relationship between the degree of immunodeficiency and susceptibility to opportunistic infections. The CD4+ T-cell count (or percentage) predicts the likely pathogens and is a guide to the need for prophylactic antimicrobials (see Figure 60.2).
MILD IMMUNODEFICIENCY (CD4 T-CELL COUNT > 200/ml, 20%)
Infectious complications of cellular immunodeficiency may occur when there is relatively mild impairment of cellular immune responses (CD4+ T-cell counts of 200–500/ml). Mucocutaneous infections occur most commonly (Table 60.2) but infections with bacteria such as Campylobacter jejuni, Salmonella spp. or Shigella spp. are a cause of diarrhoea, and occasionally bacteraemia. Bacteraemic pneumococcal disease is also more common in this group. Most of these infections are not restricted to patients with HIV infection, and are therefore not considered to be AIDS-defining opportunistic infections. However, when they present atypically, are severe or are recurrent, they may be the first indication of underlying HIV-induced immunodeficiency. In contrast to the other infections, oral hairy leukoplakia (due to Epstein–Barr virus infection of epithelial cells) is almost always indicative of HIV infection.
Table 60.2 Mucocutaneous opportunistic infections in patients with human immunodeficiency virus (HIV)-induced immunodeficiency
| Herpes zoster (varicella-zoster virus infection) |
| Mucosal candidiasis |
| Oral hairy leukoplakia (Epstein–Barr virus infection) |
| Seborrhoeic dermatitis (Pityrosporon spp. yeast infection) |
| Molluscum contagiosum (poxvirus infection) |
| Genital and cutaneous warts (human papillomavirus infection) |
| Fungal infections of the skin and nails |
| Recurrent mucocutaneous herpes simplex virus infections |
| Folliculitis (Staphylococcus aureus, Pityrosporon spp.) |
MODERATE IMMUNODEFICIENCY (CD4 T-CELL COUNT 50–200/UL, 10–20%)
PNEUMOCYSTIS JIROVECI PNEUMONITIS
Infection of the lungs by Pneumocystis jiroveci causes an interstitial pneumonitis. Patients with this condition usually have a history of subacute progressive dyspnoea, cough, fever and weight loss. Examination often reveals basal pulmonary crackles. The chest X-ray usually shows interstitial infiltrates but is occasionally normal. Other findings which would support a diagnosis of P. jiroveci pneumonitis (PJP) are hypoxaemia, an increased serum lactate dehydrogenase (LDH) concentration, diffuse uptake of radiolabelled gallium into the lungs on a gallium scan, ground-glass pulmonary opacities on a high-resolution computed tomographic (CT) scan of the lungs, and detection of Pneumocystis by smear or DNA by PCR in induced sputum specimens. A definitive diagnosis can be made by demonstrating Pneumocystis cysts in an induced sputum specimen, bronchoalveolar lavage fluid or a transbronchial biopsy.18
PJP is treated with co-trimoxazole (trimethoprim-sulfamethoxazole), given orally or intravenously depending on disease severity. However, many patients develop a hypersensitivity reaction to co-trimoxazole which is usually mild and transient but, if systemic and severe, alternative medications, including oral dapsone and trimethoprim or intravenous pentamidine, can be used with equal efficacy. Steroid therapy should also be used if the PaO2 is < 70 mmHg (9.3 kPa)19 with an FiO2 of 0.21.
Pneumocystis infection can be prevented by prophylactic medications, which should be offered to all patients with a CD4 T-cell count of < 200/ml. The most effective drug is co-trimoxazole. Alternatives for patients who are sensitive to co-trimoxazole include dapsone (with or without pyrimethamine) or inhaled pentamidine.20
CRYPTOCOCCAL MENINGITIS
Meningitis is the most common manifestation of infection with Cryptococcus neoformans in patients with AIDS. It usually presents with headache and fever, but sometimes confusion or behavioural abnormalities are the predominant abnormalities. Neck stiffness is often minimal or absent. Cerebrospinal fluid examination may reveal little evidence of infiammation, particularly in the most severe cases, but cryptococcal antigen is virtually always present in both serum and cerebrospinal fluid and cultures for cryptococci are positive.21 Most patients also have cryptococcal antigen in their serum. Intravenous amphotericin is the treatment of choice, and must be followed by suppressive therapy or oral fluconazole, until immune reconstitution is achieved with antiretroviral therapy, to prevent relapses.22
TOXOPLASMA ENCEPHALITIS
Reactivation of Toxoplasma gondii infection most commonly presents as a focal encephalitis. This may cause headaches, fever, focal neurological deficits, convulsions and even coma. One or more brain lesions may be present. They usually produce ring-enhancing lesions with surrounding oedema on a contrast brain CT scan, and can occur in many sites, with a predilection for the basal ganglia. Serological evidence of previous Toxoplasma infection is present in virtually all patients, and absence of serum Toxoplasma antibody is strongly against the diagnosis. Treatment is with intravenous sulfadiazine or clindamycin and oral pyrimethamine. Hypersensitivity reactions to sulfadiazine and clindamycin are common, and an alternative drug regimen may be necessary. A brain CT scan should be repeated after 2–3 weeks of therapy, and an alternative diagnosis considered if there has been no resolution of the lesions. Cerebral lymphoma can produce very similar lesions to Toxoplasma encephalitis. A brain biopsy is often necessary to make the diagnosis.23
SEVERE IMMUNODEFICIENCY (CD4 T CELLS < 50/UL, 10%)
CRYPTOSPORIDIOSIS
Infection of the gastrointestinal tract by Cryptosporidium parvum causes a severe and intractable secretory diarrhoea, which is often associated with a malabsorption syndrome. It can also cause cholangitis. Diagnosis is by demonstrating Cryptosporidium oocysts in faeces and/or a rectal or duodenal biopsy. There is no satisfactory treatment, but nitozoxanide is of use in some patients. Resolution is common following successful antiretroviral therapy.
MYCOBACTERIUM AVIUM COMPLEX (MAC) INFECTION
Infection with MAC is usually disseminated and affects blood leukocytes, liver, spleen and lymph nodes, and the gastrointestinal tract. This infection often results in weight loss, fatigue, fevers, anaemia and diarrhoea. The diagnosis is usually made by culturing MAC from blood, but sometimes stool microscopy and culture or biopsy of affected tissues are necessary. Treatment with multiple-drug therapy is often successful. Commonly used drugs are clarithromycin, rifabutin and ethambutol. Suppressive therapy should be continued until there is immune reconstitution following the use of combination antiretroviral therapy.24 An unusual painful necrotising lymphadenopathy following initiation of antiretroviral therapy has been identified as MAC immune restoration disease.16,17
AIDS-RELATED NEOPLASMS
Lymphomas are also a complication of HIV-induced immunodeficiency. The great majority are B-cell lymphomas (non-Hodgkin’s), and reactivation of Epstein–Barr virus infection is implicated in the pathogenesis of many cases. Primary cerebral lymphoma or extracerebral lymphoma, which often has extranodal involvement, is common in patients with severe immunodeficiency. These lymphomas are usually high-grade with poor prognosis (stage 3/4, CD4 < 100, median survival 44 weeks).25
HIV-RELATED NEUROLOGICAL DISEASE
In addition to the opportunistic infections of the nervous system discussed above, HIV infection of macrophages and microglial cells in the nervous system often results in neurological disease by incompletely understood mechanisms. Encephalopathy, myelopathy and peripheral neuropathy are all possible. In a small number of patients, the neurological disease is more problematic than the immunodeficiency. The encephalopathy usually develops insidiously in individuals with advanced immunodeficiency and eventually results in cognitive, motor and behavioural abnormalities. Myelopathy, which is now rare, results in an ataxic spastic paraparesis.26
Investigation of HIV patients with space-occupying cerebral lesions requires analysis of serology, cerebrospinal fluid and neuro-imaging investigations. Analysis of cerebrospinal fluid includes PCR for Epstein–Barr virus (indicative of lymphoma), herpes simplex virus, CMV, varicella-zoster virus (viral encephalitis), JC virus (indicative of progressive multifocal leukoencephalopathy), toxoplasmosis and Mycobacterium tuberculosis.27
HIV/AIDS AND ADMISSION TO THE INTENSIVE CARE UNIT (ICU)
HIV infection remains an incurable condition but is controllable by antiretroviral therapy for long periods of time. The prognosis has improved significantly over the past 10 years, with mathematical models indicating a median survival of 30 years. The admission of a patient with AIDS to an ICU is therefore often indicated.28,29
The most common indications are:
Respiratory failure complicating PJP has been the most common reason for admitting an HIV-infected patient to an ICU. Survival following ventilation for PJP was poor in the early 1980s, but improved with treatment advances. In recent years, survival has worsened again in some cohorts, particularly for patients with a CD4 T-cell count of < 50/ml and for those who develop pneumothorax as a result of barotrauma. The poor outcome in recent years may reflect the fact that patients are living longer because of improved therapy, and are hence often more immunodeficient when PJP develops. A first episode of PJP, a CD4 T-cell count of > 50/ml and no previous antiretroviral therapy are all favourable factors for survival. Furthermore, ventilation may be avoided by the use of continuous positive airways pressure (CPAP) or bilevel positive airways pressure (BPAP), thereby reducing the risks of a pneumothorax, airway obstruction and nosocomial infection. Prolonged illness antedating ICU admission, low serum albumin and few options for antiretroviral therapy should be considered when assessing predictors of survival.
Drug–drug interactions are of particular importance, especially when ART includes protease inhibitors or efaverenz (metabolised via P450 hepatic enzyme system). Web-based guidelines are very useful (www.HIV-druginteractions.org) when prescribing antimicrobials, antiemetics and lipid-lowering drugs.
NEEDLESTICK INJURIES AND POSTEXPOSURE PROPHYLAXIS
There is evidence that antiretroviral prophylaxis is associated with a reduction in transmission rates. On the basis of this, antiretroviral prophylaxis guidelines have been published.30 A protocol for dealing with blood and body fluid exposure should therefore be developed for every health-care institution.
1 Tindall B, Cooper DA. Primary HIV infection: host responses and intervention strategies. AIDS. 1991;5:1-14.
2 Robertson P, Dwyer D. Western blot assay. In: Lee N, editor. Clinical Microbiology Update. Sydney: University of New South Wales; 1993:9-16. no. 35
3 Mellors JW, Rinaldo CR, Gupta P, et al. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167-1170.
4 Durant J, Clevenbergh P, Halfon P, et al. Drug resistance genotyping in HIV-1 therapy: the VIRADAPT randomised controlled trial. Lancet. 1999;353:2195-2199.
5 Mellors JW, Munoz A, Giogi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946-954.
6 Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853-860.
7 Correll PK, Law MG, McDonald AM, et al. HIV disease progression in Australia at the time of combination antiretroviral therapies. Med J Aust. 1998;169:469-472.
8 Egger M, Hirschel B, Francioli P, et al. Impact of new antiretroviral combination therapies in HIV infected patients in Switzerland: prospective multicentre study: HIV cohort study. Br Med J. 1997;315:1194-1199.
9 Mouton Y, Alfandari S, Valette M, et al. Impact of protease inhibitors on AIDS defining events and hospitalizations in 10 French AIDS reference centres. AIDS. 1997;11:F101-F105.
10 Dore GJ, Cooper DA. HAART’s first decade: success brings further challenges. Lancet. 2006;368:427-428.
11 Brinkman K, Smeitink JA, Romijn JA, et al. Mitochondrial toxicity induced by nucleoside-analogue reverse-transcriptase inhibitors is a key factor in the pathogenesis of antiretroviral-therapy-related lipodystrophy. Lancet. 1999;354:1112-1115.
12 Carr A, Miller J, Law M, et al. A syndrome of lipoatrophy, lactic acidaemia and liver dysfunction associated with HIV nucleoside analogue therapy: contribution to protease inhibitor-related lipodystrophy syndrome. AIDS. 2000;14:F25-F32.
13 Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet. 2000;356:1423-1430.
14 Monforte Ade A, Bugarini R, Pezzotti P, et al. The ICONA (Italian Cohort of Naive for Antiretrovirals) Study Group. Low frequency of severe hepatotoxicity and association with HCV coinfection in HIV-positive patients treated with HAART. J Acquir Immune Defic Syndr. 2001;28:114-123.
15 John M, Flexman J, French MA. Hepatitis C virus-associated hepatitis following treatment of HIV-infected patients with HIV protease inhibitors: an immune restoration disease? AIDS. 1998;12:2289-2293.
16 French MA, Lenzo N, John M, et al. Immune restoration disease after the treatment of immunodeficient HIV-infected patients with highly active antiretroviral therapy. HIV Med. 2000;1:107-115.
17 French MA. Disorders of immune reconstitution in patients with HIV infection responding to antiretroviral therapy. Curr HIV/AIDS Rep. 2007;4:16-21.
18 Frame P, Wilkin A. Pneumocystis carinii pneumonia. In: Crowe S, Hoy J, Mills J, editors. Management of the HIV-Infected Patient. London: Martin Dunitz; 2002:421-442.
19 Consensus statement on the use of corticosteroids as adjunctive therapy for Pneumocystis pneumonia in the acquired immunodeficiency syndrome. The National Institutes of Health University of California expert panel for corticosteroids as adjunctive therapy for Pneumocystis pneumonia. N Engl J Med. 1990;323:500-504.
20 Kovacs JA, Masur H. Prophylaxis against opportunistic infections in patients with human immunodeficiency virus infection. N Engl J Med. 2000;342:1416-1429.
21 Dismukes WE. Cryptococcal meningitis in patients with AIDS. J Infect Dis. 1998;157:624-628.
22 Powderly WG, Sagg MS, Cloud GA, et al. A controlled trial of fiuconazole or amphotericin B to prevent relapse of cryptococcal meningitis in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1992;326:793-798.
23 Porter SB, Sande M. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N Engl J Med. 1992;327:1643-1648.
24 Aberg AJ, Yajko DM, Jacobson MA. Eradication of AIDS-related disseminated Mycobacterium avium complex infection after twelve months of antimycobacterial therapy combined with highly active antiretroviral therapy. J Infect Dis. 1998;178:1446-1449.
25 Straus DJ, Huang J, Testa MA, et al. Prognostic factors in the treatment of human immunodeficiency virus-associated non-Hodgkin’s lymphoma: analysis of AIDS Clinical Trials Group Protocol 142-low-dose versus standard-dose m-BACOD plus granulocyte-macrophage colony-stimulating factor. J Clin Oncol. 1998;16:3601-3606.
26 McArthur JC, Brew B, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005;4:543-555.
27 Antinori A, Ammassari A, De Luca A, et al. Diagnosis of AIDS-related focal brain lesions: a decision-making analysis based on clinical and neuroradiologic characteristics combined with polymerase chain reaction assays in CSF. Neurology. 1997;48:687-694.
28 Nickas G, Wachter RM. Outcomes of intensive care for patients with human immunodeficiency virus infection. Arch Intern Med. 2000;169:541-547.
29 Gill JK, Greene L, Miller R, et al. ICU admission in patients infected with the human immunodeficiency virus – a multicentre survey. Anaesthesia. 1999;4:727-732.
30 Young TN, Arens FJ, Kennedy GE, et al. Antiretroviral post-exposure prophylaxis (PEP) for occupational HIV exposure. Cochrane Database Syst Rev. 1, 2007. CD002835