Chapter 113 High Myopia and the Vitreoretinal Complications
Introduction
The incidence of high myopia varies among ethnic groups, races, and countries, but there is a higher incidence in Asian countries. Some of the incidence rates worldwide range from 1% in Black Americans,1 2% in Caucasian Americans,1 2.6% in Chinese individuals,2 to 5.5% in Japanese individuals.3 The prevalence of myopia seems to be increasing,4 and it is one of the major causes of visual impairment, especially in Europe and Asia.5,6
Examiners often have difficulties obtaining clear and high-contrast OCT images in highly myopic eyes. The characteristics of the OCT image in these eyes are: (1) a relatively low signal to noise ratio; (2) deep posterior staphyloma in the presence of which the peripheral tissue often drops off from the top edge of the image; (3) poor fixation due to a large central scotoma from chorioretinal atrophy; and (4) critical signs that are mostly outside the fovea. The pearls for the OCT examination are as follows. First, it is much better to use spectral domain (SD-) OCT, which provides much higher sensitivity and scanning speed than time domain (TD-) OCT. TD-OCT is acceptable; however, micropathologies such as small macular holes or columns (glial thin tissue bridging the inner and outer retina in the area of retinoschisis) that are clearly depicted in SD-OCT images might be overlooked. Second, the pathology of interest must be located near the top of the OCT image. SD-OCT has the strongest signal at the top and the weakest at the bottom because of the so-called signal decay. This procedure maximizes the signal and enhances the contrast of the pathology being targeted. Third, large internal fixation or external fixation must be used to avoid unnecessary ocular movement in cases with a large central scotoma. Finally, attention must be paid to pathologies outside the fovea. For instance, a small macular hole is sometimes outside the fovea,7 and other micropathologies such as retinal vascular microfolds, internal limiting membrane detachments, and paravascular microholes are sometimes far outside the macula. The use of the 5-raster scan has a significantly higher rate of detection of these pathologies than the use of a single B-scan alone.8 The use of raster or grid scan is highly recommended to understand all of these micropathologies.
Imaging technologies have identified myopic foveoschisis, which is a relatively new pathology that was recognized about 10 years ago.9 It is now generally agreed that this pathology is not uncommon in highly myopic eyes, and myopic foveoschisis is regarded as a precondition for development of a macular hole and retinal detachment. OCT studies of myopic foveoschisis have revealed many preclinical and pathological conditions underlying the myopic–macular diseases, as discussed below. This information is also helpful for understanding the process and pathophysiology of macular holes and retinal detachments, which are the most problematic complications for vitreoretinal surgeons. In this chapter, we review the recent studies and shed light on the vitreoretinal complications of high myopia.
Retinal detachment from peripheral breaks
Retinal detachments from peripheral retinal breaks are common in high myopia. The prevalence of any type of retinal detachment increases in association with the degree of negative refractive error.10 The distribution of high myopia exceeding −6.0 D is about 16% in overall cases and the lifetime risk is more than 20-fold compared with emmetropia.11 In addition, the onset of retinal detachment is at a younger age, based on the degree of myopia.11 The frequency of retinal peripheral degeneration increases along with the axial length.12 The development of posterior vitreous detachment (PVD) according to the higher liquefaction of the vitreous body occurs at a younger age in eyes with severe high myopia.13 These facts seem to account for the increased prevalence of retinal detachment.
Epidemiology of surgical macular complications
The incidence rates of myopia-specific macular complications such as myopic foveoschisis and macular holes with or without retinal detachments have not been well documented, because these complications are rare. Myopic foveoschisis was identified in 9% of patients with posterior staphyloma.14 In patients with a macular hole and retinal detachment, 8.5% develop the same pathology in the fellow eye within 4 years.15
Rhegmatogenous retinal detachment after refractive surgeries
Rhegmatogenous retinal detachment is a major complication after refractive surgeries. The incidence is not as high after laser in situ keratomileusis and was reported to be 0.25%16; however, it is much higher after refractive lens exchange, reportedly 7.3% within 3 years17 and 8.1% in 7 years.18 Retinal detachment is a rare but general complication after cataract surgery, with incidence rates of 0.93% in the general population19 and 2.2% after phacoemulsification in highly myopic eyes.20 A high likelihood of PVD occurring within 5 years after surgery21 seems to be associated with a higher incidence.
Etiology and pathophysiology
Myopic foveoschisis
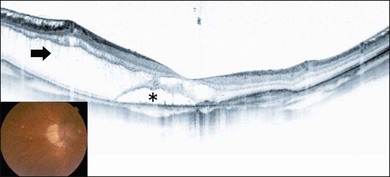
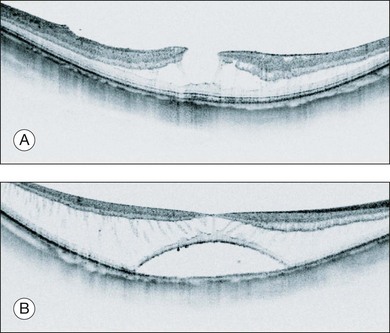
Myopic foveoschisis is characterized by retinoschisis with or without localized retinal detachment and is specific to high myopia. This pathology was identified recently (Fig. 113.1).9 Myopic foveoschisis is also referred to as a posterior retinal detachment without a macular hole in highly myopic eyes, described by Phillips in 1958, who reported a case with a retinal detachment within posterior staphyloma but no apparent macular hole.22 Perhaps some patients might have this pathology; however, it is difficult to rule out small macular holes or paravascular microholes. The detailed pathology and its mechanism awaited the development of OCT for decades. Takano and Kishi examined 32 highly myopic eyes with OCT and found retinoschisis in nine eyes and a foveal detachment in one eye, suggesting that retinoschisis and foveal detachment are not uncommon in highly myopic eyes.9 It has been reported that myopic foveoschisis is characterized by variations in the foveal architecture, including a foveal cyst in 47%, a lamellar hole in 29%, and a foveal detachment in 29%.23
The pathogenesis of myopic foveoschisis has been disclosed recently. Various OCT images from these myopic eyes have led to the hypothesis that the inner retina is less flexible than the outer retina.24 Factors limiting the inner retinal flexibility include the vitreous cortex adhering to the retina, epiretinal membranes (ERMs), internal limiting membrane (ILM), and retinal vessels. Preretinal membranes, which can be hard to recognize clinically and are found only at the microscopic level in highly myopic eyes,25 cause deterioration in the retinal flexibility.
Histologic studies have shown retinoschisis at multiple levels in the outer plexiform layer, inner plexiform layer, ganglion cell layer, and nerve fiber layer.26 There also is a preretinal fibrous membrane. The retinoschisis is bridged and a lamellar hole sometimes can be observed in the foveal region. An electron microscopic study on excised ILM samples during vitrectomy has shown that collagen fibers and cell debris were present on the inner surface of the ILM in 70% of the myopic foveoschisis, substantially more than that found in idiopathic nonmyopic macular holes (0%).25 Moreover, more fibrous glial cells were likely to be found on the inner surface of the ILM.26 Microvascular traction can be seen as arteriolar separation from the retina.27
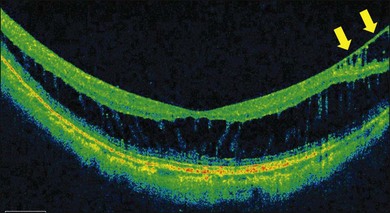
ILM detachments, sometimes recognized as “inner retinoschisis,” are often seen in highly myopic eyes, which explains the underlying traction from the ILM on the other retinal layers (Fig. 113.2).28 Inner retinal elevations along with retinal vessels recognized as tent-like lesions on OCT images, so-called retinal vascular microfolds, are observed especially in vertical sections (Fig. 113.3).29 This finding represents traction from the vessels on the retinal surface. A large OCT study of 200 highly myopic eyes reported a 6% incidence of ILM detachments, a 13.5% incidence of retinoschisis, and a 20% incidence of retinal vascular microfolds.30 These components generate inward tractional force on the inner retina, which potentially splits the retina, causing retinoschisis and finally leads to a retinal detachment at the fovea.
Macular hole with or without retinal detachment
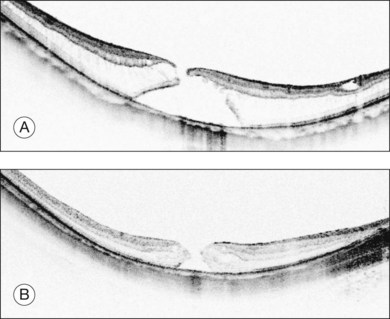
Retinal detachments from a macular hole are a typical complication in highly myopic eyes. The vitreous cortex adhering to the retinal surface around the hole causes tangential traction that generates an inward vector component in deep staphyloma in highly myopic eyes, resulting in a retinal detachment.31 Releasing the retinal traction is critical to successful reattachment; thus, vitrectomy with vitreous cortex and membrane removal is effective.31 Physicians sometimes observe macular holes without a retinal detachment,32 indicating that macular hole formation does not always lead to retinal detachment and that there might be two distinct types of macular holes in high myopia. A recent OCT study reported that persistent traction at the macular hole edge after opening is critical for initiating a retinal detachment.33 A macular hole with retinoschisis has a higher likelihood of progressing to a retinal detachment than those with a retinal cyst, resembling idiopathic non-myopic macular holes (Fig. 113.4).
A macular hole with retinoschisis typically presents with deeper posterior staphyloma, which explains the lower anatomic success rate in this subtype.33 Deep posterior staphyloma generates a larger vector component from the tangential traction exerted by the ERM or the ILM, which acts on the retina as an inward tractional force. This is why the retinoschisis is present in the retinoschisis type. In addition, with deep staphyloma it is more difficult to close the macular hole because of excess stretching in the retina. While shallower posterior staphyloma generates less tractional force and the retina is flat as seen in non-myopic eyes. This flat configuration exerts less stretching in the retina, and, thus, there is a higher likelihood of macular hole closure. The type of macular hole is highly dependent on the depth of the posterior staphyloma and underlying tractional force, which affect the anatomic success rate. OCT observation of the macular hole edge provides one clue to the prognosis.33
In highly myopic eyes, multiple components adhere to the retinal surface in most cases and are often recognized during vitreous surgery. Electron microscopic studies have shown that they are the vitreous cortex, cellular ERMs, and ILMs,34 suggesting that complete removal of these tractional forces is essential in reattachment surgery.
Posterior retinal detachments from paravascular microholes
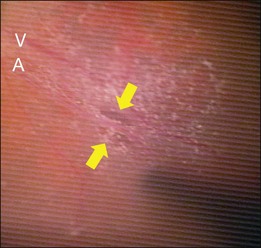
Retinal detachment from a paravascular microhole is also specific to highly myopic eyes (Fig. 113.5). Microholes are typically small, round or oval retinal holes associated with posterior major vessels.35 There are sometimes multiple holes that are adjacent to the major vessels. An OCT study of highly myopic eyes reported that the incidence rates of retinal cysts and paravascular holes were 50% and 27%, respectively.36 The vitreoretinal adhesion is often strong at the paravascular region, and traction from the vitreous at the site is believed to be a main cause of retinal rarefaction, resulting in retinal cysts and breaks.37 Paravascular microholes often colocalize with vascular microfolds and retinoschisis,8,29 suggesting a close relationship with the retinal vascular traction.
Symptoms of myopic foveoschisis and macular holes with or without retinal detachments
Myopic foveoschisis and macular holes with or without retinal detachments typically occur in highly myopic, middle-aged to older women. In the authors’ clinic, 44 of 52 patients were women during the period 2000–2005.38 Patients are normally aware of central visual distortion if they have only retinoschisis and a relative central scotoma corresponding to the involved area when the retinal detachment starts. Patients may be aware of an absolute scotoma at the center of the relative scotoma when a macular hole opens. Patients also report visual loss at the involved area if an extensive retinal detachment is complicated. Even if patients present with a macular hole, the Watzke–Allen test is usually negative.
Clinical findings
Optical coherence tomography features
Myopic foveoschisis presents with retinoschisis in multiple retinal layers. The split retinal layers normally have a bridge between them, the so-called column, which is presumed to be residual Müller cells (Fig. 113.1). In cases with a very atrophic retina, it can be difficult to distinguish retinoschisis from a retinal detachment, and the presence of the column is an important clue for diagnosing retinoschisis but not a detachment. Tracing the junction of the inner segment and outer segment (IS/OS) line of the photoreceptors is also useful for differentiating a retinal detachment from retinoschisis as shown in an early OCT study.39 ILM separation from the other retinal layers also is observed, i.e., a so-called ILM detachment, and is an indicator of the tractional force from the ILM.28 The tent-like peak of the inner retina is observed on the OCT image. This is coincident with retinal vessels, and the so-called retinal microvascular traction.29 Because the retinal vessels normally run horizontally around the macular area, there is a higher chance of finding this in a vertical than a horizontal section because of the higher chance of observing a cross-section of the retinal vessels. The IS/OS junction line of the photoreceptors sometimes disappears in the area of the retinal detachment40; however, the IS/OS junction line is typically well preserved in the area of retinoschisis, suggesting that the photoreceptor function is well preserved in this subtype.
Macular hole formation associated with retinoschisis has two stages (Fig. 113.6).36 The first stage is the development of the so-called retinoschisis type, in which only retinoschisis is present and not a retinal detachment. Several months (sometimes several years) later a retinal detachment begins around the fovea. This stage is the so-called foveal detachment type, and a retinal detachment involving the fovea and retinoschisis around the macula are present. After a while, the inner retina above the detachment is stretched and torn. This is how a macular hole appears as a consequence of retinoschisis with a retinal detachment. Small macular holes are often difficult to visualize in a B-scan image, because the fixation point has shifted.
The edge of a macular hole provides valuable information. As discussed previously, there are two types of macular holes in highly myopic eyes.33 One is similar to an idiopathic macular hole, as normally seen in non-myopic eyes (Fig. 113.4B). In these, the edge of the hole is thickened with retinal cysts. There is no retinal detachment around the hole clinically, and this type usually does not progress for months or years. The other type has surrounding retinoschisis instead of retinal cysts around the hole (Fig. 113.4A). This type of macular hole results from myopic foveoschisis and can be considered a transition from foveoschisis to a macular hole with a retinal detachment. This type of macular hole typically progresses rapidly and is likely to complicate the retinal detachment because of underlying traction (see Etiology and pathophysiology, above). The typical OCT appearance of this type is characterized by a relatively high and rectilinear wall of the macular hole edge with an acute angle to the RPE line, and a sharp-angled edge of the top of the macular hole.33 Therefore, observing the macular hole edge is essential for exact diagnosis, which is critical for decision-making and planning of the vitreous surgery.
Fundus autofluorescence
Fundus autofluorescence stimulates emission of light from the lipofuscin accumulated in the RPE and is an indicator of the oxidative stress level.41 Fundus autofluorescence also indicates the functioning of the RPE. In the atrophic fundus in myopic eyes, fundus autofluorescence helps distinguish the retinal detachment from the retinoschisis, because loss of the contact between the photoreceptors and the RPE results in hypoautofluorescence.42 This hypofluorescent area indicates the area of the retinal detachment and is a good indicator for monitoring its progression.
Treatment of foveoschisis
The natural course of myopic foveoschisis is unfavorable. Investigators have reported that the vision decreased in 69% of patients, a macular hole developed in 31% after 3 years of follow-up,43 and in 50% of patients with retinoschisis a macular hole or retinal detachment developed after 2 years.44 These observations encourage surgery to treat myopic foveoschisis as prophylaxis to prevent macular holes with consequent development of a retinal detachment.
Surgical indications
Myopic foveoschisis is sometimes asymptomatic, especially in cases with simple retinoschisis and no retinal detachment. Those cases are not good indications for vitreous surgeries. Even though patients are aware of a visual disturbance, turbulence, or visual loss, surgery can be postponed until the vision decreases to about 20/40 because there is still a chance of visual worsening after vitrectomy. The chance of visual improvement after surgery is about 80% in cases with a foveal detachment and 50% with retinoschisis alone.45 The chance of visual improvement is substantially smaller if a macular hole is present before surgery,45,46 which is partly because macular holes have a poor closure rate after surgery. Hence, surgical intervention before macular hole formation is considered beneficial, although there is not yet strong evidence for this. The best indications for preventing macular hole development in these cases, are the presence of the foveal detachment type, moderate and severe visual loss, and high retinal detachment. These manifestations are typical and high risk for macular hole formation.
A macular hole with an extensive retinal detachment is a good indication for surgery. The surgical goal is to remove the entire component that is generating the tractional force on the retina and causing a detachment, including the vitreous cortex, ERMs, and the ILM. However, sometimes the traction persists from the retinal vessels even after a complete vitrectomy, leading to recurrence of the retinal detachment. Treating the posterior staphyloma is theoretically required for these cases and placing a macular buckle might be considered. Simple gas injection was attempted in an early case series especially for eyes with a PVD; however, this procedure cannot relieve the traction exerted by ERMs, for instance. Therefore, the expectation for anatomic success is not as high as that associated with vitrectomy with complete removal of any traction.47
Surgical prognosis
The retina usually reattaches to the retinal pigment epithelium (RPE) slowly after the surgery (Fig. 113.7). The visual outcome associated with myopic foveoschisis is favorable if no macular hole develops. We reported that a substantial visual gain was achieved after vitrectomy in either group and that the final vision was similar between the foveal detachment type and the retinoschisis type; however, the visual change was significantly greater in the foveal detachment group than in the retinoschisis group.45 Another study reported a similar trend and showed that 70% of eyes with a foveal detachment had a significant visual improvement compared with 42% in the retinoschisis group.48 Once a macular hole develops, the hole is hard to close after vitrectomy. The macular hole closure rate with retinoschisis or retinal detachment ranges from 30% to 50% on OCT images.46,49 The macular hole closes in most cases32,33 without retinoschisis or a detachment. The postoperative visual level varies depending on the degree of the chorioretinal atrophy. The mean visual acuity was below 20/200, and the initial reattachment rate was about 70% after vitrectomy.50 A longer axial length is generally a poor prognostic factor.50,51 Macular buckling can compensate for any tractional force. For this reason, a comparative study showed a slightly higher initial reattachment rate than vitrectomy alone (90% versus 70%).52
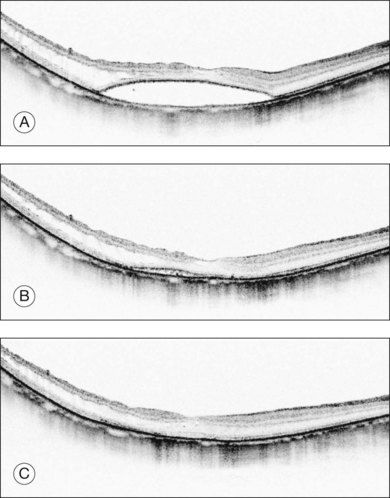
Fig. 113.7 Optical coherence tomography (OCT) appearance of a patient who underwent vitrectomy with internal limiting membrane (ILM) peeling and gas tamponade for myopic foveoschisis in Figure 113.6 (lower panel, foveal detachment type). (A) OCT image 2 months after surgery shows flattening of the retina. (B) An OCT image at the same position, 6 months after surgery shows that the retinoschisis has resolved; however, there is still substantial subretinal fluid. (C) An OCT image 12 months after surgery shows complete resolution of the retinal detachment.
Surgical procedures
Vitreous separation
A 25-gauge vitrectomy system is the first-line treatment for highly myopic eyes. No encircling band is required for this surgery. The vitreous plays an important role; therefore, creating a PVD is critical for reattaching the retina. The vitreous tightly and extensively adheres to the retinal surface in highly myopic eyes. Use of triamcinolone acetonide is essential for visualization and to identify residual cortex. To create posterior vitreous separation, a vitreous cutter and silicone-tipped backflush needle with active suction are normally used. A diamond-dusted membrane scraper53 is another option for an extremely thin vitreous cortex. The vitreoretinal adhesion is normally extremely tight around the optic nerve disc, and, therefore, the surgeon might consider starting temporally where the adhesion is normally the weakest. Importantly, great care is needed to separate the vitreous at the macula, because the macula is tightly adhered to the vitreous; placing stress on the macula may lead to macular hole formation. This procedure is difficult to perform in the detached retina, and a bimanual technique can be considered for a safe separation.
Internal limiting membrane peeling
The necessity for ILM peeling remains controversial in myopic foveoschisis, and one group reported a high success rate even without this procedure.54 However, the ILM is separated from the other retinal layers on OCT images in most eyes,28 suggesting that rigidity of the ILM plays a major role in this pathology. This fact warrants the necessity of ILM peeling for myopic foveoschisis at least in selected cases.
ILM peeling also can enhance macular hole closure and remove any traction on the retina in myopic macular holes with a retinal detachment,55 and this raises the initial success rate.51,56 Indocyanine green was used to stain the ILM in the initial case series; however, it remains in the eye for longer than 6 months postoperatively.57 Brilliant Blue G can selectively stain the ILM and is less toxic.58 This might be a useful option.
Tamponade
In cases of macular holes with or without a retinal detachment, gas tamponade must be performed at the end of surgery. Long-acting gas tamponade (i.e., perfluoropropane) must be used because it has a higher anatomic success rate.59 Because there is no coagulation maneuver around the macula, it is critical to support the retina for a long time to recover the integrity between the RPE and photoreceptors in atrophic eyes. Silicone oil tamponade is also an effective option.60 The use of heavy silicone oil is reportedly effective as well.61
Macular buckling
In cases of recurrent retinal detachments, placing a buckle can be considered to fix the dimensional abnormality of the posterior staphyloma. Direct macular buckling was attempted in the initial cases; however, the modified silver clip62 method seems to be commonly used. The superotemporal conjunctiva is cut down and a mattress suture placed to fixate the top of the buckle. The indented part is on the other side. The arm is adjusted to fit the scleral curve so that the tip of the buckle reaches the macula.
Macular buckling reportedly has a higher success rate for retinal reattachment than vitrectomy,52 likely because of the change in the vector force. Tangential traction generates the inward vector force in the posterior staphyloma, which is concave in shape. Macular buckling changes the macular area to a convex shape, and the vector force changes in direction to reattach the retina. Therefore, macular buckling is supposed to provide stronger contact between the neural retina and the RPE.
Postoperative complications
Opening of a macular hole occurs in less than 5% of patients who undergo vitrectomy for myopic foveoschisis.45,48,54 Cases in which ILM peeling was performed in the presence of an extremely thin, stretched fovea are at risk of developing this complication. Attention must be paid to detecting any small macular holes before vitrectomy, because the holes are normally stretched and enlarged postoperatively. Simply using a long-acting gas injection is not well accepted for postoperative macular holes, because this complication results from a shortage of retinal redundancy. Reoperation and removing any residual traction might be useful.63
1 Katz J, Tielsch JM, Sommer A. Prevalence and risk factors for refractive errors in an adult inner city population. Invest Ophthalmol Vis Sci. 1997;38:334–340.
2 Xu L, Li J, Cui T, et al. Refractive error in urban and rural adult Chinese in Beijing. Ophthalmology. 2005;112:1676–1683.
3 Sawada A, Tomidokoro A, Araie M, et al. Refractive errors in an elderly Japanese population: the Tajimi Study. Ophthalmology. 2008;115:363–370. e3
4 Rose K, Smith W, Morgan I, et al. The increasing prevalence of myopia: implications for Australia. Clin Experiment Ophthalmol. 2001;29:116–120.
5 Iwase A, Araie M, Tomidokoro A, et al. Prevalence and causes of low vision and blindness in a Japanese adult population: the Tajimi Study. Ophthalmology. 2006;113:1354–1362.
6 Klaver CC, Wolfs RC, Vingerling JR, et al. Age-specific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam Study. Arch Ophthalmol. 1998;116:653–658.
7 Coppé AM, Ripandelli G, Parisi V, et al. Prevalence of asymptomatic macular holes in highly myopic eyes. Ophthalmology. 2005;112:2103–2109.
8 Sayanagi K, Morimoto Y, Ikuno Y, et al. Spectral-domain optical coherence tomographic findings in myopic foveoschisis. Retina. 2010;30:623–628.
9 Takano M, Kishi S. Foveal retinoschisis and retinal detachment in severely myopic eyes with posterior staphyloma. Am J Ophthalmol. 1999;128:472–476.
10 Ogawa A, Tanaka M. The relationship between refractive errors and retinal detachment – analysis of 1,166 retinal detachment cases. Jpn J Ophthalmol. 1988;32:310–315.
11 Burton TC. The influence of refractive error and lattice degeneration on the incidence of retinal detachment. Trans Am Ophthalmol Soc. 1989;87:143–155.
12 Pierro L, Camesasca FI, Mischi M, et al. Peripheral retinal changes and axial myopia. Retina. 1992;12:12–17.
13 Akiba J. Prevalence of posterior vitreous detachment in high myopia. Ophthalmology. 1993;100:1384–1388.
14 Baba T, Ohno-Matsui K, Futagami S, et al. Prevalence and characteristics of foveal retinal detachment without macular hole in high myopia. Am J Ophthalmol. 2003;135:338–342.
15 Oie Y, Emi K. Incidence of fellow eye retinal detachment resulting from macular hole. Am J Ophthalmol. 2007;143:203–205.
16 Ruiz-Moreno JM, Pérez-Santonja JJ, Alió JL. Retinal detachment in myopic eyes after laser in situ keratomileusis. Am J Ophthalmol. 1999;128:588–594.
17 Barraquer C, Cavelier C, Mejía LF. Incidence of retinal detachment following clear-lens extraction in myopic patients. Retrospective analysis. Arch Ophthalmol. 1994;112:336–339.
18 Colin J, Robinet A, Cochener B. Retinal detachment after clear lens extraction for high myopia: seven-year follow-up. Ophthalmology. 1999;106:2281–2284.
19 Norregaard JC, Thoning H, Andersen TF, et al. Risk of retinal detachment following cataract extraction: results from the International Cataract Surgery Outcomes Study. Br J Ophthalmol. 1996;80:689–693.
20 Neuhann IM, Neuhann TF, Heimann H, et al. Retinal detachment after phacoemulsification in high myopia: analysis of 2356 cases. J Cataract Refract Surg. 2008;34:1644–1657.
21 Friedman Z, Neumann E. Posterior vitreous detachment after cataract extraction in non-myopic eyes and the resulting retinal lesions. Br J Ophthalmol. 1975;59:451–454.
22 Phillips CI. Retinal detachment at the posterior pole. Br J Ophthalmol. 1958;42:749–753.
23 Benhamou N, Massin P, Haouchine B, et al. Macular retinoschisis in highly myopic eyes. Am J Ophthalmol. 2002;133:794–800.
24 Ikuno Y. Pathogenesis and treatment of myopic foveoschisis. Nippon Ganka Gakkai Zasshi. 2006;110:855–863.
25 Bando H, Ikuno Y, Choi JS, et al. Ultrastructure of internal limiting membrane in myopic foveoschisis. Am J Ophthalmol. 2005;139:197–199.
26 Tang J, Rivers MB, Moshfeghi AA, et al. Pathology of macular foveoschisis associated with degenerative myopia. J Ophthalmol. 2010. Epub:175613
27 Grossniklaus HE, Green WR. Pathologic findings in pathologic myopia. Retina. 1992;12:127–133.
28 Sayanagi K, Ikuno Y, Tano Y. Tractional internal limiting membrane detachment in highly myopic eyes. Am J Ophthalmol. 2006;142:850–852.
29 Ikuno Y, Gomi F, Tano Y. Potent retinal arteriolar traction as a possible cause of myopic foveoschisis. Am J Ophthalmol. 2005;139:462–467.
30 Forte R, Cennamo G, Pascotto F, et al. En face optical coherence tomography of the posterior pole in high myopia. Am J Ophthalmol. 2008;145:281–288.
31 Stirpe M, Michels RG. Retinal detachment in highly myopic eyes due to macular holes and epiretinal traction. Retina. 1990;10:113–114.
32 García-Arumí J, Martinez V, Puig J, et al. The role of vitreoretinal surgery in the management of myopic macular hole without retinal detachment. Retina. 2001;21:332–338.
33 Jo Y, Ikuno Y, Nishida K. Retinoschisis: a predictive factor in vitrectomy for macular holes without retinal detachment in highly myopic eyes. Br J Ophthalmol. 2011;96:197–200.
34 Sakaguchi H, Ikuno Y, Choi JS, et al. Multiple components of epiretinal tissues detected by triamcinolone and indocyanine green in macular hole and retinal detachment as a result of high myopia. Am J Ophthalmol. 2004;138:1079–1081.
35 Favre M. Trous parapapillaires comme cause du décollement de la rétine. Ophthalmologica (Basel). 1954;127:351–356.
36 Shimada N, Ohno-Matsui K, Nishimuta A, et al. Detection of paravascular lamellar holes and other paravascular abnormalities by optical coherence tomography in eyes with high myopia. Ophthalmology. 2008;115:708–717.
37 Spencer LM, Foos RY. Paravascular vitreoretinal attachments. Role in retinal tears. Arch Ophthalmol. 1970;84:557–564.
38 Soga K, Sayanagi K, Ikuno Y, et al. [Foveal anatomical profile of myopic foveoschisis in high myopia clinic.] Atarashii. Ganka. 2011;28:739–741.
39 Ip M, Garza-Karren C, Duker JS, et al. Differentiation of degenerative retinoschisis from retinal detachment using optical coherence tomography. Ophthalmology. 1999;106:600–605.
40 Sayanagi K, Ikuno Y, Soga K, et al. Photoreceptor inner and outer segment defects in myopic foveoschisis. Am J Ophthalmol. 2008;145:902–908.
41 Yannuzzi LA, Ober MD, Slakter JS, et al. Ophthalmic fundus imaging: today and beyond. Am J Ophthalmol. 2004;137:511–524.
42 Sayanagi K, Ikuno Y, Tano Y. Different fundus autofluorescence patterns of retinoschisis and macular hole retinal detachment in high myopia. Am J Ophthalmol. 2007;144:299–301.
43 Gaucher D, Haouchine B, Tadayoni R, et al. Long-term follow-up of high myopic foveoschisis: natural course and surgical outcome. Am J Ophthalmol. 2007;143:455–462.
44 Shimada N, Ohno-Matsui K, Baba T, et al. Natural course of macular retinoschisis in highly myopic eyes without macular hole or retinal detachment. Am J Ophthalmol. 2006;142:497–500.
45 Ikuno Y, Sayanagi K, Soga K, et al. Foveal anatomical status and surgical results in vitrectomy for myopic foveoschisis. Jpn J Ophthalmol. 2008;52:269–276.
46 Ikuno Y, Tano Y. Vitrectomy for macular holes associated with myopic foveoschisis. Am J Ophthalmol. 2006;141:774–776.
47 Li X, Wang W, Tang S, et al. Gas injection versus vitrectomy with gas for treating retinal detachment owing to macular hole in high myopes. Ophthalmology. 2009;116:1182–1187.
48 Kumagai K, Furukawa M, Ogino N, et al. Factors correlated with postoperative visual acuity after vitrectomy and internal limiting membrane peeling for myopic foveoschisis. Retina. 2010;30:874–880.
49 Ikuno Y, Sayanagi K, Oshima T, et al. Optical coherence tomographic findings of macular holes and retinal detachment after vitrectomy in highly myopic eyes. Am J Ophthalmol. 2003;136:477–481.
50 Nakanishi H, Kuriyama S, Saito I, et al. Prognostic factor analysis in pars plana vitrectomy for retinal detachment attributable to macular hole in high myopia: a multicenter study. Am J Ophthalmol. 2008;146:198–204.
51 Lam RF, Lai WW, Cheung BT, et al. Pars plana vitrectomy and perfluoropropane (C3F8) tamponade for retinal detachment due to myopic macular hole: a prognostic factor analysis. Am J Ophthalmol. 2006;142:938–944.
52 Ripandelli G, Coppé AM, Fedeli R, et al. Evaluation of primary surgical procedures for retinal detachment with macular hole in highly myopic eyes: a comparison of vitrectomy versus posterior episcleral buckling surgery. Ophthalmology. 2001;108:2258–2264.
53 Lewis JM, Park I, Ohji M, et al. Diamond-dusted silicone cannula for epiretinal membrane separation during vitreous surgery. Am J Ophthalmol. 1997;124:552–554.
54 Kwok AK, Lai TY, Yip WW. Vitrectomy and gas tamponade without internal limiting membrane peeling for myopic foveoschisis. Br J Ophthalmol. 2005;89:1180–1183.
55 Kadonosono K, Yazama F, Itoh N, et al. Treatment of retinal detachment resulting from myopic macular hole with internal limiting membrane removal. Am J Ophthalmol. 2001;131:203–207.
56 Uemoto R, Yamamoto S, Tsukahara I, et al. Efficacy of internal limiting membrane removal for retinal detachments resulting from a myopic macular hole. Retina. 2004;24:560–566.
57 Sayanagi K, Ikuno Y, Soga K, et al. Residual indocyanine green fluorescence pattern after vitrectomy with internal limiting membrane peeling in high myopia. Am J Ophthalmol. 2007;144:600–607.
58 Enaida H, Hisatomi T, Goto Y, et al. Preclinical investigation of internal limiting membrane staining and peeling using intravitreal Brilliant blue G. Retina. 2006;26:623–630.
59 Uemoto R, Saito Y, Sato S, et al. Better success of retinal reattachment with long-standing gas tamponade in highly myopic eyes. Graefes Arch Clin Exp Ophthalmol. 2003;241:792–796.
60 Nishimura A, Kimura M, Saito Y, et al. Efficacy of primary silicone oil tamponade for the treatment of retinal detachment caused by macular hole in high myopia. Am J Ophthalmol. 2011;151:148–155.
61 Avitabile T, Bonfiglio V, Buccoliero D, et al. Mistretta A. Heavy versus standard silicone oil in the management of retinal detachment with macular hole in myopic eyes. Retina. 2011;31:540–546.
62 Klöti R. Silver clip for central retinal detachments with macular hole. Mod Probl Ophthalmol. 1974;12:330–336.
63 Sayanagi K, Ikuno Y, Tano Y. Reoperation for persistent myopic foveoschisis after primary vitrectomy. Am J Ophthalmol. 2006;141:414–417.