Table 25-1
Key Tumor System Processes and Biomarkers
| System Process | Example Biomarkers | References |
| Proliferation | Ki-67, Aurora A kinase | 294, 295 |
| Apoptosis | p53, Apo-1/Fas, FasL, TRAIL receptors, caspases, pAKT, Survivin, MCL-1, Bcl-2 | 296–303 |
| Cell cycle control | p53, p21, p27, p16, cyclins D1, E | 304–310 |
| Adhesion | E-cadherin, beta-catenin, CD44, CD24, Claudin-1 | 311–315 |
| Migration/motility | CXCR4, alpha6beta4 integrin, Net1, matrix metalloproteinases | 316–318 |
| Angiogenesis | VEGF, Flt-4, HIF-1alpha, pericyte markers | 319–323 |
| Immune responses | CD68, CD45RO, CD3zeta, CD4, CD8, PD-L1, FOXP3, CD1a, cytokines | 224, 324–327 |
| Inflammation | NF-kappaB, COX2, CSF-1R | 214, 328, 329 |
| Fibroblasts | Fibroblast activation protein-alpha, PDGF-beta | 330, 331 |
Adapted from Critchley-Thorne RJ, Miller SM, Taylor DL, et al. Applications of cellular systems biology in breast cancer patient stratification and diagnostics. Comb Chem High Throughput Screen. 2009;12:860-869.
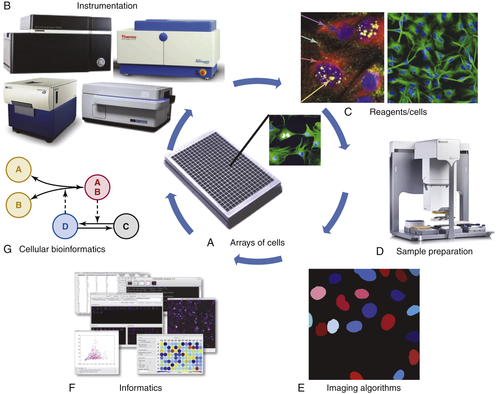
Imaging Live Cells and Model Organisms with HCA
Imaging Fixed Cells and Model Organisms with HCA
Table 25-2
Classes of Fluorescence-Based Reagents, Readouts, and Online Resources for HCA
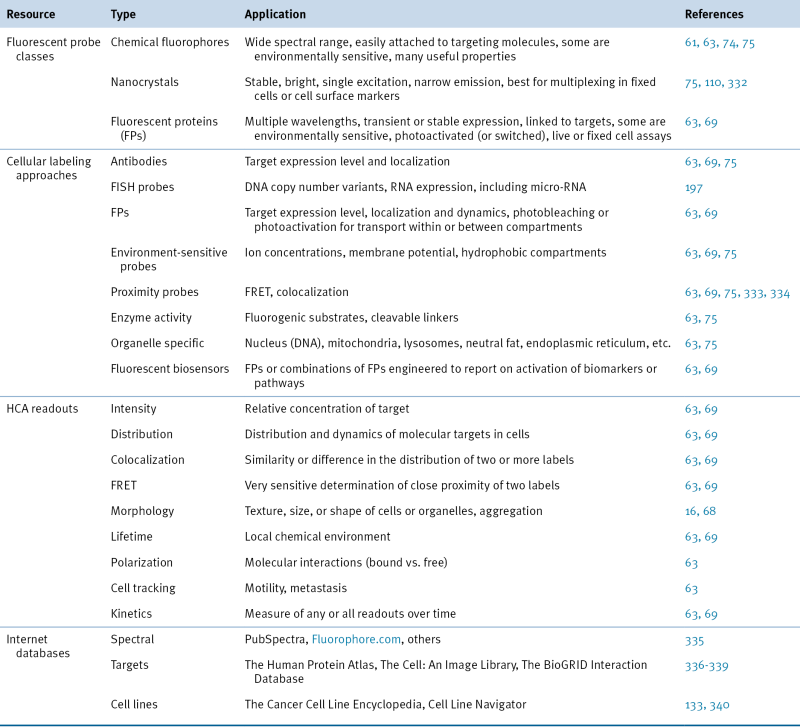
FISH, Fluorescence in situ hybridization; FRET, fluorescence resonance energy transfer; HCA, high-content analysis.
Table 25-3
Comparison of Fluorescent and Chromogenic Readouts 177,341–344
| Reporter Type | Advantages | Disadvantages |
| Fluorescent | Present standard in cell analysis High sensitivity and specificity Quantitative readout Multiplex targets that are colocalized and/or in close proximity Broad spectrum of wavelengths Higher resolution with confocal imaging |
Reagents are less stable for long-term storage More expensive fluorescence, more expensive imaging systems More expensive reagents |
| Chromogenic | Present standard in tissue analysis Long-term stability of labeling Brightfield microscopes Greater amplification |
Variable sensitivity and specificity Multiplexed targets must be spatially separated Precipitates cause fuzziness around target |