Chapter 65 Hepatic steatosis, steatohepatitis, and chemotherapy-related liver injury
Overview
With the expansion of the indications for liver surgery in patients with hepatic malignancies, liver surgeons have become concerned with steatosis, steatohepatitis, and other liver damage from chemotherapy. Preoperative chemotherapy is widely used before resection of colorectal cancer liver metastases (see Chapters 81A and 87), and can induce different types of hepatic parenchymal injuries. Although most of these changes can be observed outside the context of preoperative chemotherapy, they have become more relevant in patients receiving preoperative chemotherapy for colorectal cancer liver metastase because they can affect perioperative outcome after hepatic resection. This chapter focuses on risk factors and prevention of chemotherapy-related liver damage as it affects liver resection.
Types and Etiology of Liver Parenchymal Damage
Hepatic parenchymal damage from chemotherapy (see Chapter 87) can be divided into two main categories: nonalcoholic fatty liver disease (NAFLD) and sinusoidal injury. NAFLD describes a large spectrum of histologic liver changes unrelated to alcohol consumption. It is important to note that these lesions may also be observed in the context of alcohol abuse. Steatosis is defined by an intracellular hepatocyte lipid accumulation, and it is the hallmark of NAFLD. Histologic evidence of an inflammatory component associated with steatosis defines steatohepatitis. Upon operative exploration, a liver that has undergone steatotic changes is recognized by its yellow-tan color, often referred to as yellow liver. NAFLD is typically graded according to the scoring system of Kleiner and colleagues (2005), which considers the degree of steatosis (0 to 3 points), lobular inflammation (0 to 3 points), and hepatocellular ballooning (0 to 2 points; Fig. 65.1). A score of 5 or more indicates steatohepatitis.
Increased BMI is the main risk factor of nonalcoholic steatosis (Brouquet et al, 2009; Kooby et al, 2003; Vauthey et al, 2006). The use of chemotherapy may increase the risk of steatosis (Kooby et al, 2003), which can be detected in 30% to 47% of patients who have received systemic chemotherapy (Peppercorn et al, 1998; Sorensen et al, 1995). Although no specific chemotherapy regimen has been definitively linked to the occurrence of steatosis, it is generally attributed to the use of 5-FU or irinotecan in patients with liver metastases (Moertel et al, 1993; Sorensen et al, 1995; Vauthey et al, 2006). However, in these patients, it is difficult to ascertain whether chemotherapy directly induces steatosis or more likely aggravates preexisting NAFLD.
Steatohepatitis has long been described as a “second hit” in patients with preexisting steatosis, but the continuum between these two different types of NAFLD is uncertain. Macroscopically, no specific features distinguish the steatohepatitis from other types of NAFLD. Chemotherapy-related steatohepatitis was initially believed to be related to the use of any chemo- therapy (Bilchik et al, 2005; Fong et al, 2006), but only irinotecan has been confirmed as a risk factor for steatohepatitis (Vauthey et al, 2006). The effect of an elevated BMI on the development of chemotherapy-associated steatohepatitis is additive to the risk imposed by the use of irinotecan (Vauthey et al, 2006), and this risk can rise to up to 25% in patients with a BMI of 25 kg/m2 or more who received irinotecan-based chemotherapy (Attal et al, 1992).
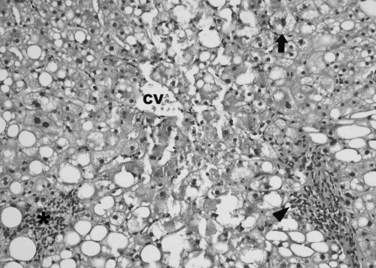
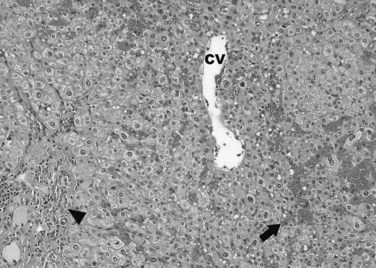
Sinusoidal injury is a variant of the sinusoidal obstruction syndrome first described in association with bone marrow transplantation. In the operating room, a liver that has sustained sinusoidal injury is characterized by vascular ectasia, commonly referred as blue liver. The initial damage affects endothelial cells of the sinusoids, leading to a rupture of the continuity of the sinusoidal wall, which triggers a coagulation cascade and induces the deposition of coagulation factors, erythrocyte aggregates, and cytoplasmic fragments into the perisinusoidal space, ultimately leading to sinusoidal obstruction (Attal et al, 1992; Rubbia-Brandt et al, 2004).
Histologically, the severity of sinusoidal injury can be graded according to a scoring system developed by Rubbia-Brandt and colleagues (2004). This system evaluates the proportion of centrilobular involvement relative to the entire lobular surface (Fig. 65.2). A score of 0 indicates no sinusoidal dilation; 1 indicates mild sinusoidal dilation, characterized by centrilobular changes limited to one third of the lobular surface; 2 indicates moderate sinusoidal dilation, characterized by centrilobular changes extending to two thirds of the lobular surface; and 3 indicates severe sinusoidal dilation, characterized by evidence of complete lobular involvement. There is strong evidence that sinusoidal injury is caused by the use of platinum compounds, including oxaliplatin (Aloia et al, 2006; Brouquet et al, 2009; Nakano et al, 2008; Rubbia-Brandt et al, 2004; Vauthey et al, 2006), and the incidence of sinusoidal injury can range from 19% to 52% in patients who have received preoperative oxaliplatin-based chemotherapy (Aloia et al, 2006; Brouquet et al, 2009; Nakano et al, 2008; Rubbia-Brandt et al, 2004; Vauthey et al, 2006).
Pathophysiology of Chemotherapy-Associated Liver Damage
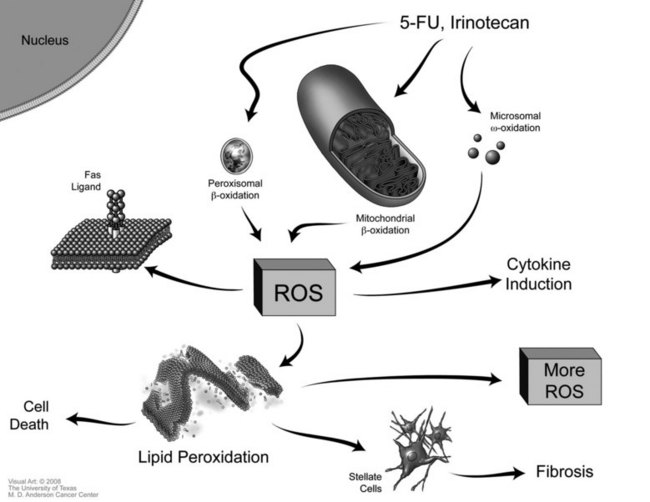
Although histologically distinct, the NAFLD and sinusoidal injury pathways both involve chemical damage by reactive oxygen species (ROS) (Fig. 65.3). The initial event in the NAFLD sequence is likely drug-related damage to the mitochondrial membrane that interferes with oxidation of fatty acids and ultimately leads to the accumulation of ROS within hepatocytes (Laurent et al, 2004; Pessayre et al, 2001). Fluoro-β-alanine, a fluorouracil metabolite, is also believed to impair metabolism in hepatocytes, thus prolonging their exposure to toxic substances (Miyake et al, 2005).
The pathophysiology of sinusoidal injury also involves the accumulation of ROS. Wang and colleagues (2000) developed a rodent model of sinusoidal injury using exposure to pyrrolizidine alkaloids, which deplete the sinusoidal endothelial cells’ glutathione stores, leaving them susceptible to subsequent ROS injury. Pyrrolizidine alkaloids also decrease nitric oxide concentrations, resulting in the upregulation of matrix metalloproteinase activity, which can be prevented by pretreatment with a nitric oxide donor (DeLeve et al, 2003). Damage induced by exposure to pyrrolizidine compounds can also be abrogated in a dose-dependent manner by pretreating sinusoidal endothelial cells with exogenous glutathione or N-acetyl-L-cysteine in this model (Wang et al, 2000). However, these preventive pharmacologic strategies have not yet been tested in humans.
Preoperative Detection of Liver Injury
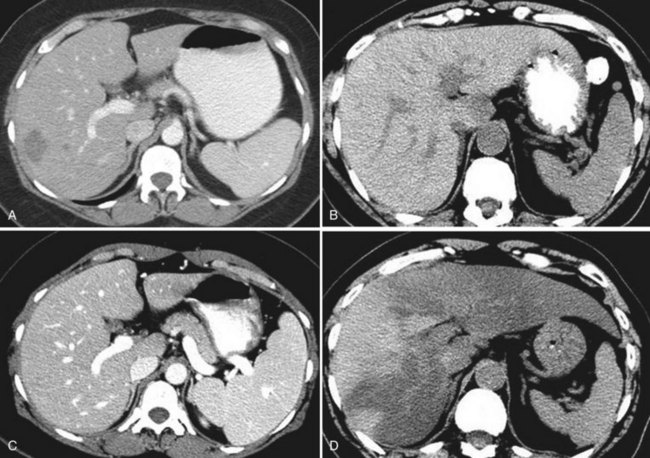
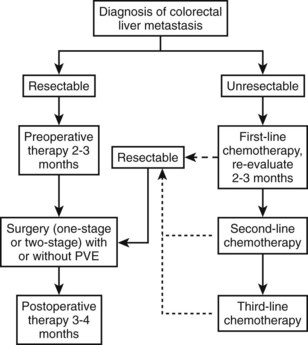
The definitive diagnosis of hepatic injury relies on the pathologic examination of non–tumor-bearing liver tissue after liver resection. The preoperative detection of liver injury is useful to devise the best surgical strategy in patients with severe hepatotoxicity (Fig. 65.4). Numerous modalities have been proposed to detect chemotherapy-related liver injuries preoperatively, but no consensus has been reached. Although abnormal liver function tests may suggest hepatic injury, assessment of the severity of hepatic injury is not possible based only on these laboratory values (Brouquet et al, 2009; Nakano et al, 2008). The combination of macroscopic and microscopic examinations of the liver provided by preoperative laparoscopy with intraoperative liver biopsies probably offers the best assessment of the effects of preoperative chemotherapy in patients at high risk for surgical complications (Vauthey et al, 2006). However, this is an invasive approach, which limits its routine use. Image-guided liver biopsy has also been proposed to assess liver injuries; however, the practicality of such an approach is questionable because of the heterogeneity of hepatic lesions and because of interobserver variations in the histologic identification of chemotherapy-related liver injuries.
A number of noninvasive imaging methods have been evaluated to preoperatively determine the presence and degree of liver injuries. Ultrasound (US) is ineffective in differentiating hepatic steatosis from fibrosis because it is highly operator dependent and therefore prone to interobserver variations (Mazhar et al, 2009). Computed tomography (CT) offers the advantage of being part of the preoperative workup of liver metastases, and its performance to detect steatosis has been evaluated (Kodama et al, 2007; Cho et al, 2008). Non–contrast-enhanced CT images more accurately estimate hepatic steatosis than do contrast-enhanced images. Unenhanced liver parenchyma with an attenuation value less than 40 Hounsfield units (HU) is indicative of more than 30% hepatic steatosis. However, marked steatosis cannot be excluded on non–contrast-enhanced CT (Cho et al, 2008; Kodama et al, 2007).
Magnetic resonance imaging (MRI) can also be useful for characterizing certain types of liver injuries. Super paramagnetic iron oxide (SPIO) agents are preferentially taken up in Kupffer cells (Ward et al, 2008) and can be used to increase the accuracy of MRI to detect sinusoidal injury because this lesion is associated with a change in Kupffer cell phagocytic behavior. Ward and colleagues (2008) evaluated the performance of SPIO-enhanced MRI for the assessment of sinusoidal injury and found a sensitivity of 87% and specificity of 89%; however, these MRI techniques are investigational and therefore have limited utility. Furthermore, MRI lacks specificity (63%) and is less accurate than non–contrast-enhanced CT for detecting steatosis (Cho et al, 2008). Because of these limitations and the high cost, MRI is not routinely used to detect hepatic injury.
Chemotherapy-Associated Liver Damage and Surgical Resection
Table 65.1 summarizes the results of several studies on the relationship between the presence of hepatic injury and the postoperative complication rate. Although the presence of steatosis does not increase 90-day postoperative mortality rates (Vauthey et al, 2006), Kooby and colleagues (2003) reported in a series of 485 patients that marked steatosis was an independent risk factor for postoperative infectious complications. In this study, the presence of marked steatosis was independently associated with preoperative chemotherapy.
Table 65.1 Studies Examining the Effects of Preoperative Chemotherapy and Chemotherapy-Related Liver Injury on Postoperative Morbidity and Mortality
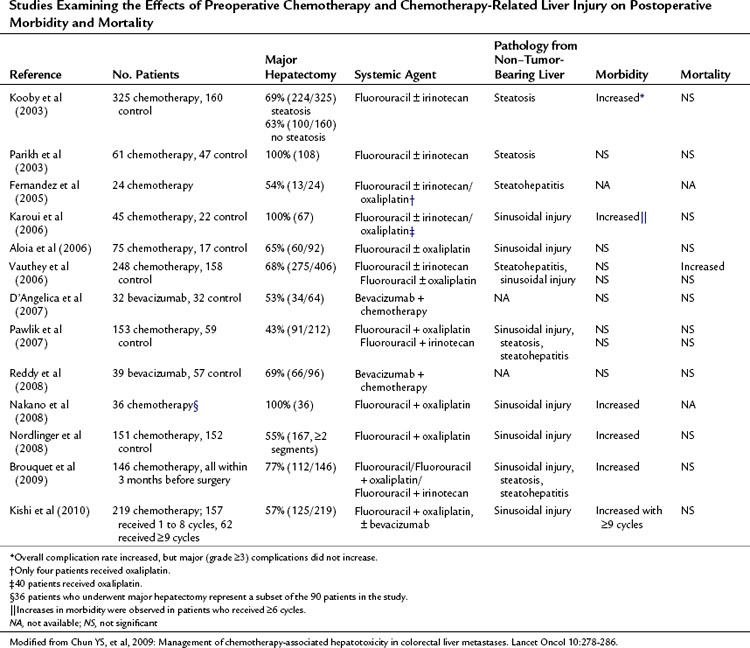
Irinotecan-related steatohepatitis, although less frequent, is potentially more dangerous in candidates for liver resection. Vauthey and colleagues (2006) evaluated the outcomes of 406 patients who underwent liver resection for colorectal cancer liver metastases; 248 of them had received preoperative chemotherapy, and 158 had not. The presence of steatohepatitis, observed in 8.4% of patients, was associated with an increase in the 90-day postoperative mortality rate as a result of liver failure (15% in patients with steatohepatitis vs. 2% in those without; P = .001). The presence of severe sinusoidal injuries has been reported to be closely associated with the risk of bleeding and the requirement for blood transfusion during resection in patients who received oxaliplatin-based chemotherapy for more than 6 months (Aloia et al, 2006). Others have suggested that sinusoidal injuries increase the risk of postoperative complications after major liver resection (Nakano et al, 2008).
Overall, the effect of a sinusoidal lesion on posthepatectomy outcome is real but probably less severe than steatohepatitis, as demonstrated in the European Organisation for Research and Treatment of Cancer (EORTC) 40983 trial (Nordlinger et al, 2008). In this prospective randomized trial comparing patients treated by surgery plus perioperative FOLFOX (folinic acid, fluorouracil, oxaliplatin) versus surgery alone for resectable colorectal liver metastases, the 151 patients randomized to receive perioperative FOLFOX were more likely to develop reversible postoperative complications than the 152 patients who underwent resection without chemotherapy (25% vs. 16%, respectively; P = .04; Nordlinger et al, 2008). However, perioperative mortality rate was low (1%), and it was similar in the two groups.
In addition to increasing the risk for postoperative complications, chemotherapy-related liver injuries appear to impair the radiographic detection of liver metastases. Angliviel and colleagues (2009) assessed the ability of CT to detect liver metastases in patients with or without preoperative chemotherapy before planned liver resection. Preoperative CT findings were compared with intraoperative and histologic findings. In this study, preoperative chemotherapy, more than three liver metastases, and steatosis greater than 30% were independent risk factors of inaccurate CT staging.
Minimizing the Risks and Consequences of Hepatic Injury
A better knowledge of the determinants of chemotherapy hepatotoxicity has led to various measures proposed to minimize the incidence and consequences of hepatic injury. The duration of chemotherapy exposure and the time interval between the cessation of chemotherapy and liver resection have been recognized as risk factors of hepatic injury (Aloia et al, 2006; Karoui et al, 2006; Kishi et al, 2010; Nakano et al, 2008). In a series of 45 patients who underwent major liver resection, Karoui and colleagues (2006) showed that receiving six or more cycles of preoperative chemotherapy was a predictor of postoperative morbidity (54% vs. 19%; P = .047). Another study found that when compared with shorter duration treatment, preoperative chemotherapy of more than 12 cycles increased the risk of reoperation (11% vs. 0%, respectively; P = .04) and resulted in a longer hospital stay (11 vs. 7 days; P = .02) after liver resection for colorectal liver metastases (Aloia et al, 2006). In this study, hemorrhagic centrilobular necrosis was more common in patients treated with more than 12 cycles of chemotherapy than in patients with a shorter chemotherapy duration (50%, 25%, and 10%, respectively; P = .01). Prolonged preoperative chemotherapy has been confirmed as an independent risk factor for grade 2 and grade 3 sinusoidal injuries in a series of 90 patients who underwent liver resection for colorectal cancer liver metastases (Nakano et al, 2008). Prolonged chemotherapy is associated with an increased rate of postoperative morbidity, but it does not appear to increase the rate of major or complete pathologic response (Kishi et al, 2010); therefore it is desirable to pursue liver resection after a short course (e.g., six cycles or less) of preoperative chemotherapy and to cease chemotherapy as soon as the lesions are resectable in patients with initially unresectable liver metastases.
The time interval between cessation of chemotherapy and liver resection also influences the risk of postoperative complications and the incidence of hepatic injury (Brouquet et al, 2009; Welsh et al, 2007). Welsh and colleagues (2007) found in a series of 497 patients who underwent resection of colorectal cancer liver metastases with and without preoperative chemotherapy that an interval of 4 or fewer weeks between chemotherapy and surgery was a risk factor for the development of postoperative complications. More recently, a time interval of less than 4 weeks was identified as an independent risk factor for chemotherapy-related liver injury in a series of 146 patients who received preoperative chemotherapy for colorectal cancer liver metastases (Brouquet et al, 2009). The influence of the time interval on the severity of hepatic injury supports the current recommendation to wait at least 5 weeks between the cessation of chemotherapy and liver resection.
Other strategies to minimize liver injury have focused on the use of additional pharmacologic agents. Bevacizumab is a monoclonal antibody directed against the vascular endothelial growth factor receptor (VEGF-R); when used with 5-FU–based chemotherapy for metastatic colorectal adenocarcinoma, it is associated with improvements in both radiologic response rates and survival (Hurwitz et al, 2004). However, bevacizumab increases the risk of various tumor-related complications, including bleeding and perforation (Chen et al, 2006; Shah et al, 2005); therefore its use during the perioperative period in patients with colorectal cancer liver metastases was initially thought to be potentially deleterious. Several studies have since shown that the addition of bevacizumab to preoperative chemotherapy is safe, provided a sufficient time interval is allowed to elapse between the cessation of chemotherapy and liver resection (i.e., 6 weeks, which is two half-lives) (D’Angelica et al, 2007; Gruenberger et al, 2008; Kesmodel et al, 2008).
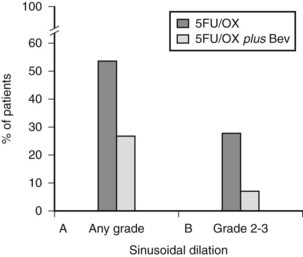
Ribero and colleagues (2007b) evaluated the influence of bevacizumab in a series of 105 patients who underwent liver resection for colorectal liver metastases after preoperative chemotherapy. They found that preoperative bevacizumab with oxaliplatin had higher rates of pathologic response, assessed by the proportion of patients with less than 25% viable tumor cells (45% with bevacizumab vs. 23% without bevacizumab) and a lower incidence of sinusoidal injury (any grade: 27.4% with bevacizumab vs. 53.5% without; moderate or severe: 8.1% with bevacizumab vs. 27.9% without; both P < .01) (Fig. 65.5). This protective effect of bevacizumab against oxaliplatin-induced sinusoidal injury has been confirmed in two other studies (Gruenberger et al, 2008; Rubbia-Brandt et al, 2010).
Other pharmacologic agents may also have a protective effect against liver injury. Brouquet and colleagues (2009) retrospectively explored the risk factors for chemotherapy-related liver injuries in a group of 146 patients who had undergone resection of liver metastases within 3 months of preoperative chemotherapy and found that low, daily “cardiac prophylaxis” doses of aspirin (75 to 360 mg) may have a protective effect against sinusoidal injury and other chemotherapy-related liver injuries. This beneficial effect of aspirin has yet to be confirmed by other studies.
In patients with advanced liver disease who receive prolonged chemotherapy, hepatic injury cannot be avoided, and the objective is to minimize its consequences. Portal vein embolization (PVE) was initially proposed to increase resectability in patients who require extended resections but with an inadequate liver remnant (see Chapter 93A, Chapter 93B ). It can also be used to increase the hepatic reserve in patients suspected to have hepatic injury who may require extended liver resection (Fig. 65.6) (Abdalla et al, 2002; Azoulay et al, 2000; Kishi et al, 2009; Ribero et al 2007a). Hypertrophy of the liver remnant after PVE seems to not be significantly affected by the preoperative chemotherapy even if associated with bevacizumab (Goere et al, 2006; Zorzi et al, 2008). This is a test to assess the regenerative capacities of the liver because the degree of hypertrophy correlates with the postoperative mortality rate (Farges et al, 2003; Ribero et al 2007a). At the University of Texas M.D. Anderson Cancer Center, in patients who have received extended chemotherapy (>4 months) or in those with a preexisting hepatic injury, PVE is performed if the future liver volume is 30% or less of the total liver volume. A degree of hypertrophy of 5% or less after PVE is considered a contraindication to major liver resection (Ribero et al, 2007a).
Conclusion
The increasing use of preoperative chemotherapy has led to an increased incidence of hepatic injury, including NAFLD and sinusoidal injury. Although the consequences of hepatic injury can vary widely according to its severity, it is of major concern to liver surgeons. Overall, hepatic injury rarely affects surgical outcomes if the lesions are identified preoperatively. In patients receiving preoperative chemotherapy who are at risk for developing hepatic injury, the use of simple guidelines regarding the duration of the treatment (2 to 3 months in patients with resectable metastases) and the time interval between chemotherapy and surgery (>5 weeks) should optimize surgical outcomes (Fig. 65.7).
Abdalla EK, et al. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675-681.
Aloia T, et al. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol. 2006;24:4983-4990.
Angliviel B, et al. Impact of chemotherapy on the accuracy of computed tomography scan for the evaluation of colorectal liver metastases. Ann Surg Oncol. 2009;16:1247-1253.
Azoulay D, et al. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg. 2000;231:480-486.
Bilchik AJ, et al. Neadjuvant chemotherapy for metastatic colon cancer: a cautionary note. J Clin Oncol. 2005;23(36):9073-9078.
Brouquet A, et al. Risk factors for chemotherapy-associated liver injuries: a multivariate analysis of a group of 146 patients with colorectal metastases. Surgery. 2009;145:362-371.
Chen HX, et al. Phase II multicenter trial of bevacizumab plus fluorouracil and leucovorin in patients with advanced refractory colorectal cancer: an NCI Treatment Referral Center trial TRC-0301. J Clin Oncol. 2006;24:3354-3360.
Cho CS, et al. Preoperative radiographic assessment of hepatic steatosis with histologic correlation. J Am Coll Surg. 2008;206:480-488.
D’Angelica M, et al. Lack of evidence for increased operative morbidity after hepatectomy with perioperative use of bevacizumab: a matched case-control study. Ann Surg Oncol. 2007;14:759-765.
DeLeve LD, et al. Decreased hepatic nitric oxide production contributes to the development of rat sinusoidal obstruction syndrome. Hepatology. 2003;38:900-908.
Farges O, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Sur. 2003;237:208-217.
Fernandez FG, et al. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg. 2005;200:845-853.
Fong Y, Bentrem DJ. CASH (chemotherapy-associated-steatohepatitis) costs. Ann Surg. 2006;243:8-9.
Goere D, et al. Chemotherapy does not impair hypertrophy of the left liver after right portal vein obstruction. J Gastrointest Surg. 2006;10:365-370.
Gruenberger B, et al. Bevacizumab, capecitabine, and oxaliplatin as neoadjuvant therapy for patients with potentially curable metastatic colorectal cancer. J Clin Oncol. 2008;26:1830-1835.
Hurwitz H, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342.
Karoui M, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243:1-7.
Kesmodel SB, et al. Preoperative bevacizumab does not significantly increase postoperative complication rates in patients undergoing hepatic surgery for colorectal cancer liver metastases. J Clin Oncol. 2008;26:5254-5260.
Kishi Y, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250:540-547.
Kishi Y, et al. Extended preoperative chemotherapy does not improve pathologic response and increases postoperative liver insufficiency after hepatic resection for colorectal liver metastases. Ann Surg Oncol. 2010;17:2870-2876.
Kleiner DE, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321.
Kodama Y, et al. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol. 2007;188:1307-1312.
Kooby DA, et al. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034-1044.
Laurent A, et al. Pivotal role of superoxide anion and beneficial effect of antioxidant molecules in murine steatohepatitis. Hepatology. 2004;39:1277-1285.
Mazhar SM, Shiehmorteza M, Sirlin CB. Noninvasive assessment of hepatic steatosis. Clin Gastroenterol Hepatol. 2009;7:135-140.
Miyake K, et al. Effects of oral 5-fluorouracil drugs on hepatic fat content in patients with colon cancer. Acad Radiol. 2005;12:722-727.
Moertel CG, et al. Hepatic toxicity associated with fluorouracil plus levamisole adjuvant therapy. J Clin Oncol. 1993;11:2386-2390.
Nakano H, et al. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg. 2008;247:118-124.
Nordlinger B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007-1016.
Parikh AA, et al. Perioperative complications in patients undergoing major liver resection with or without neoadjuvant chemotherapy. J Gastrointest Surg. 2003;7:1082-1088.
Pawlik TM, et al. Preoperative chemotherapy for colorectal liver metastases: impact on hepatic histology and postoperative outcome. J Gastrointest Surg. 2007;11:860-868.
Peppercorn PD, et al. Demonstration of hepatic steatosis by computerized tomography in patients receiving 5-fluorouracil–based therapy for advanced colorectal cancer. Br J Cancer. 1998;77:2008-2011.
Pessayre D, et al. Mitochondria in steatohepatitis. Semin Liver Dis. 2001;21:57-69.
Reddy SK, et al. Addition of bevacizumab to irinotecan- and oxaliplatin-based preoperative chemotherapy regimens does not increase morbidity after resection of colorectal liver metastases. J Am Coll Surg. 2008;206:96-106.
Ribero D, et al. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386-1394.
Ribero D, et al. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer. 2007;110:2761-2767.
Rubbia-Brandt L, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460-466.
Rubbia-Brandt L, et al. Sinusoidal obstruction syndrome and nodular regenerative hyperplasia are frequent oxaliplatin-associated liver lesions and partially prevented by bevacizumab in patients with hepatic colorectal metastasis. Histopathology. 2010;56:430-439.
Shah SA, et al. Analysis and outcomes of right lobe hepatectomy in 101 consecutive living donors. Am J Transplant. 2005;5:2764-2769.
Sorensen P, et al. Reversible hepatic steatosis in patients treated with interferon alfa-2a and 5-fluorouracil. Cancer. 1995;75:2592-2596.
Vauthey JN, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065-2072.
Wang X, Kanel GC, DeLeve LD. Support of sinusoidal endothelial cell glutathione prevents hepatic veno-occlusive disease in the rat. Hepatology. 2000;31:428-434.
Ward J, et al. Sinusoidal obstructive syndrome diagnosed with superparamagnetic iron oxide–enhanced magnetic resonance imaging in patients with chemotherapy-treated colorectal liver metastases. J Clin Oncol. 2008;26:4304-4310.
Welsh FKS, et al. Safe liver resection following chemotherapy for colorectal metastases is a matter of timing. Br J Cancer. 2007;96:1037-1042.
Zorzi D, et al. Chemotherapy with bevacizumab does not affect liver regeneration after portal vein embolization in the treatment of colorectal liver metastases. Ann Surg Oncol. 2008;15:2765-2772.