Chapter 86 Helminthic Disease
Introduction
Helminths have plagued mankind since before the era of our earliest recorded history.1 It is possible to recognize features of helminthic infections from the ancient writings of Hippocrates, Egyptian medical papyri, and the Bible.2 Nematode worms or roundworms are one of the two major phyla of the Helminths and include the major intestinal human worms (e.g., Ascaris lumbricoides, Trichuris trichiura, Necator americanus, Ancylostoma duodenale, Strongyloides stercoralis), animal worms (e.g., Toxocara canis, Toxocara cati, Ancylosoma caninum, Bayliascaris procyonis), and the filarial worms that can cause lymphatic filariasis (e.g., Wuchereria bancrofti, Brugia malayi) or onchocerciasis (Onchocerca volvulus).1 The other phylum of the Helminths is called Platyhelminths, which is divided into trematodes (e.g., Schistosoma mansoni) and cestodes (e.g., Taenia solium, Taenia saginata). Many kinds of adult worms cannot develop on their own and parasitize human intestine; some in their larval stage can infect humans directly, causing tissue damage that includes the eye.3 It is estimated that almost 1 billion people worldwide, especially in tropical areas and developing countries, are infected with one or more helminths.4 This chapter discusses the most common helminthic diseases that affect the posterior segment of the eye: ocular toxocariasis, diffuse unilateral subacute neuroretinitis (DUSN), ocular onchocerciasis, and cysticercosis.
Ocular toxocariasis
Toxocariasis is a infection caused by the nematode T. canis and less frequently by other roundworms such as T. cati.3,5,6 Systemic effects of Toxocara infestation in the human are termed visceral larval migrans (VLM). VLM is usually a self-limited disease and typically affects young children from 6 months to 4 years of age.5 Findings associated with the disorder range from a mild to moderate eosinophilia in an asymptomatic patient, fever, pallor, anorexia, malaise, hepatomegaly, and transient infiltrates of the lungs in symptomatic VLM to, more infrequently, a fulminating and fatal disease associated with pneumonia, congestive heart failure, or convulsions.3,5,7 Ocular toxocariasis is unusual in adults but is an important cause of visual impairment during childhood, accounting for approximately 1% of all cases of uveitis among all ages in referral centers in the United States and Japan8,9 and 3% of panuveitis in Switzerland.10 Exact correlation between VLM and ocular toxocariasis is not well determined but patients with systemic disease rarely present with ocular involvement. In one study of 245 cases of VLM, only 5% had any indication of ocular disease.11
History
In 1907 Leiper first described the infestation of humans with canine nematodes.12 Later, Wilder, in 1950, described nematode ocular infection.13 In her study, Wilder reviewed the histopathology of 46 eyes primarily enucleated for suspicion of intraocular malignancy and nematode larvae were found in 24 eyes. Two years later Beaver and coworkers identified a Toxocara species larva in a liver biopsy from a child with visceral lesions and eosinophilia, identifying the etiological agent with this previously reported syndrome in children.14 Nichols, in 1956, reported identifying T. canis (Toxocaridae family) in specimens originally studied by Wilder who first thought the worm to be part of Ancylostomidae family.15 These studies demonstrated a common etiology for systemic and ocular diseases. In 1959 Irvine and Irvine16 described histologic findings in a 4-year-old child presenting with strabismus, decreased vision, and retinal detachment. The eye was enucleated for suspicion of a tumor but an eosinophilic abscess with Toxocara canis larva on the pars plana region was demonstrated. This patient differed from cases reported by Wilder since the patient presented with clear media and the retina could be examined.13 In 1960 Ashton17 described four histopathologically proven cases of Toxocara endophthalmitis that presented with a posterior pole granuloma in a distinct fashion that represented a third classic form of this disorder. Duguid, in 1961,18 described a case series of ocular toxocariasis and emphasized the difference between the posterior pole granuloma and diffuse chronic endophthalmitis, suggesting that clinical diagnosis could be perfomed in certain cases. In 1971 Wilkinson and Welch19 reported 41 cases of proven or presumed ocular toxocariasis. Eyes with chronic endophthalmitis and leukocoria were much more likely to have been enucleated, whereas eyes with the posterior or peripheral isolated and visible granulomas were usually diagnosed clinically, and enucleation was not performed to confirm the diagnosis. Since then, several other relatively unusual forms of ocular Toxocara have been described and these are briefly discussed later. Association of T. canis and diffuse unilateral subacute neuroretinitis will be discussed separately in this chapter.
Parasitology
The Toxocara genus comprises 21 species including T. canis which is the most common cause of toxocariasis.3,5,6,20 T. canis is a natural parasite of dogs and occasionally can infest human and other paratenic hosts such as rodents and rabbits.21 Adult worms are cylindrical and long, measuring from 75 to 120 mm. Male worms are longer than female and in their development find five different stages from larvae to adult parasite.20 Adult forms are found only on small intestine of puppies until 6 months of age (definitive host) and pregnant and lactating bitches. In older dogs and humans the cycle is not complete and because of this only the definitive host where worms reach sexual maturation can shed eggs in feces. To understand the pathogenesis of T. canis and the development of VLM and ocular toxocariasis it is necessary to understand the parasite life cycle.
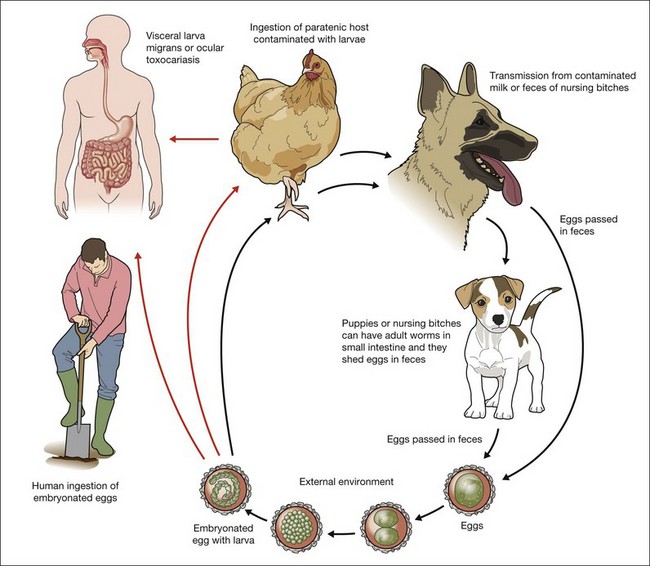
Eggs lacking an embryo are shed in the feces of the definitive host (puppies). The eggs then develop an embryo and become infective in the environment in 2–6 weeks. Following ingestion by dogs or other parasite hosts, the infective eggs hatch and larvae penetrate the intestine wall and are carried by the circulation to a wide variety of tissues (liver, heart, lungs, brain, muscle, eyes). In the human and in adult dogs, this completes the life cycle, and the parasites are usually enclosed by an inflammatory granulomatous response that is primarily eosinophilic, although the larvae may remain viable for years under such conditions.3,5,22,23 During pregnancy, the host response in the infected bitch is altered, and previously encysted larvae may resume migration,23 crossing the placenta and affecting the canine fetus. In puppies, larvae reaching the lungs are coughed up and swallowed and, reaching the small intestine, they quickly mature into adult worms that produce infectious ova that are passed in fecal material. Prenatal infection is the primary route of transmission to puppies, however they can become infected after birth by ingestion of milk or feces from an infected nursing bitch.6,22 The infection rate in puppies may approach 100%24,25 and they start to shed over 200 000 eggs per day in feces from 3 to 6 weeks after initial infestation and may shed eggs until they are about 6 months old when the mature worms die20 (Fig. 86.1).
Pathophysiology
Humans can be infested by ingestion of embryonated eggs or larvae. Eggs can be ingested accidentally after contact with puppies or by geophagia of soil containing embryonated eggs.26–28 Outdoor parks and playgrounds (sandboxes) can be highly contaminated with embryonated eggs of Toxocara, since in this environment people routinely walk their pets.29–33 Under optimal conditions embryonated eggs may remain viable for years.34 Direct contact with untreated puppies is also an important form of transmission. Amaral et al. have shown that perianal hair from stray as well as owned dogs had embryonated eggs in 24% of cases and 99% of the total number of eggs were found in puppies.25 A less frequent method of transmission is the ingestion of larvae from contamined meat. Several studies described infection by eating raw meat or liver from animals such as cows, chicken, and ducks.35,36,37
After ingesting embryonated ova, the parasites mature in the small intestine and transform to second stage larvae which penetrate the intestine wall, enter the lymphatic and portal circulation, and are disseminated to liver and lungs. Then the larvae reach the heart and are disseminated to other organs such as brain, kidney, heart, muscle, and eyes.20 In human beings the parasites are enclosed by an inflammatory granulomatous reaction and the parasites do not evolve further.22 The eosinophilic granuloma comprise the larva enveloped by a central core of eosinophils surrounded by mononuclear cells, histiocytes, epithelioid cells and giant cells. Because of the inability of the parasite to mature into an adult form in humans, a search for T. canis ova in human feces is inevitably unrewarding.38
T. canis can cause either VLM and ocular toxocariasis and the clinical manifestation depends on factors such as host immunological condition, inflammatory response, number of eggs/larvae ingested, frequency of ingestion, and localization of the second stage larvae in human tissues.20 A large amount of ingested embryonated eggs may lead to a rapid increase in eosinophilia and rising antibody levels.20 One study has shown that the degree of systemic eosinophilia in VLM has been correlated with level of exposure to Toxocara larvae.39 A lower exposure to embryonated eggs may produce lower levels of antibodies and eosinophilia and may permit wider larval migration, reaching the eye.20 Intraocular involvement by T. canis tends to occur in young patients, but in individuals who have developed ocular toxocariasis the age of onset is usually older than patients with VLM.7,13,22,40 Mean age of presentation for systemic VLM is 2 years5 versus 7.5 years for ocular toxocariasis.11
Clinical presentations
Although the average age of presentation is around 8 years for ocular toxocariasis,5,11 the age at presentation may range from 2 to 30 years11,41 and individual cases have been reported in adults with age up to 62 years.41,42 Toxocara canis infection in adults and very young children is rare, probably because of better hygiene habits of adults and less contact with puppies, sandboxes, and geophagia.
The ocular involvement is typically unilateral, and bilateral disease is quite rare.20,43 Patients usually present with unilateral visual impairment, leukokoria, and or strabismus in a relatively quiet eye. Careful slit-lamp examination can show cells in the anterior chamber (73%) and vitreous (100%),44 and vitritis causing decrease in visual acuity was reported in 53% in one series.9
The presence of a retinal granuloma is the most common finding in ocular toxocariasis. Wilkinson and Welch,19 in a study with 41 eyes diagnosed with the disease, reported that a peripheral granulomatous mass with relatively clear media was the most common form of presentation, being seen in 18 of 41 (44%) cases.19 Stewart et al.,9 in 2005, described 22 cases of ocular toxocariasis and in 50% of them peripheral granuloma was diagnosed. However, an isolated posterior pole inflammatory mass was the most common form of presentation in a series of 100 cases reported by Hagler et al.45 and in 53% of 30 cases published by Oréfice and coworkers.44
Shields,7 in a review in 1984, divided ocular toxocariasis into nine different categories, including DUSN.7 Despite T. canis being described as one of the possible nematodes to cause DUSN, other roundworms are also causes of DUSN and therefore the disorder will be discussed separately in this chapter.
Peripheral granuloma
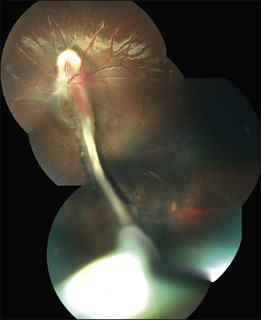
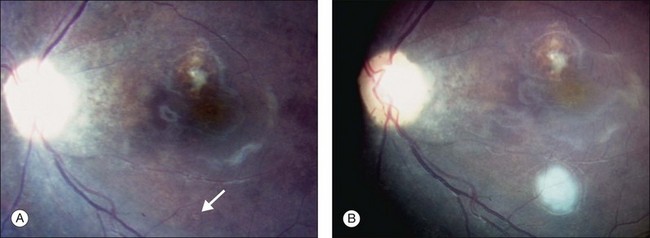
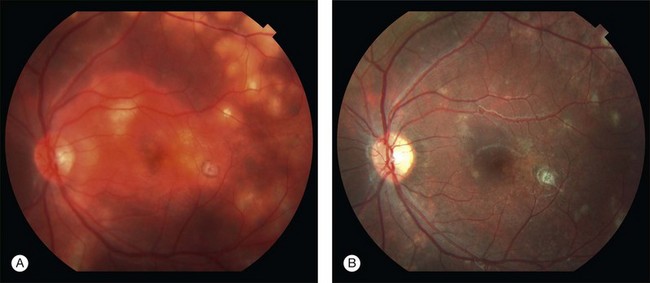
The peripheral granuloma, also described as the peripheral inflammatory mass form of Toxocara endophthalmitis, is usually seen in a quiet eye with varying levels of decreased vision and strabismus.19 The vitreous and anterior chamber show mild to moderate reaction. Intense vitritis can occur, however at diagnosis the vitreous is generally clear. Peripheral granuloma presents as a dense, white inflammatory mass in the periphery of the retina. Localized traction on the retina may result in the production of a typical retinal fold from the periphery to the optic nerve (Fig. 86.2). This mass may be quite localized, spherical, and similar to those observed in the posterior pole. Fibrocellular bands may be observed running from a peripheral inflammatory mass to the more posterior retina or the optic nerve (Fig. 86.3). The prognosis in eyes with peripheral granulomatous inflammation is usually relatively good and visual acuity can be preserved. By the time this diagnosis is made, active inflammation is usually not progressive.19 Ultrasound biomicroscopy study of 15 eyes with peripheral toxocariasis has shown alterations such as vitreous membranes in 86.6%, granuloma in 73.3%, pseudocysts in 53.3%, and thickening of ciliary body in 40% of studied eyes.46 Intra- and epiretinal traction bands can lead to production of both traction and rhegmatogenous retinal detachments, macular displacement and distortion, and optic nerve dysfunction.
Posterior pole granuloma
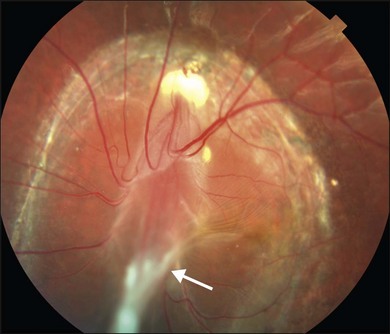
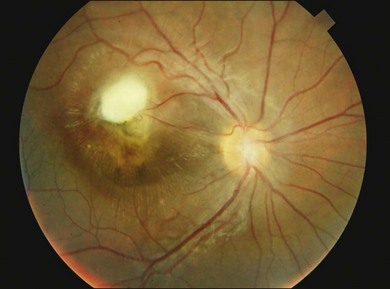
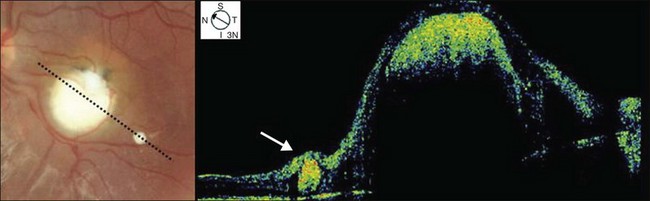
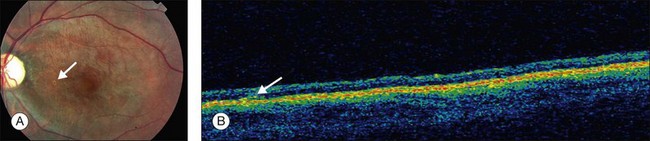
Posterior pole granuloma presents as white or gray spherical intraretinal or subretinal granuloma affecting the posterior pole. Most of the time it is diagnosed in a cicatricial stage when the lesion appears as a well-defined subretinal mass, measuring from 500 to 3000 µm, with no hemorrhage or exudates. Traction bands running from the mass to the surrounding retina may be observed. With cicatricial granulomas, there is very mild or no anterior chamber reaction and a low amount of cells in the vitreous cavity. Perilesional retina can show hyperpigmentation of retinal pigmented epithelium (RPE) and wrinkling of internal limiting membrane (Fig. 86.4). An optical coherence tomography case report has shown that the granuloma presents as a well-defined subretinal mass above the RPE (Fig. 86.5).47 Active disease can show different stages of severity from mild anterior chamber and vitreous reaction to a very intense vitritis as seen in the chronic endophthalmitis form of the disease. During the active phase the posterior pole granuloma is observed as an ill-defined hazy mass surrounded by retinal exudates or hemorrhages.19,20 A granuloma is usually seen in the posterior pole temporal to the optic nerve head; however, the occurrence of optic nerve granuloma has been described.48 Due to posterior pole involvement, patients present with central visual impairment and can have strabismus and leukokoria. Macular lesions may be observed in association with peripheral inflammatory masses and choroidal neovascularization may occur as a late complication.45,49,50
Chronic endophthalmitis
Patients with chronic endophthalmitis are typically younger than those with more localized granulomatous forms of the disease. There is usually relatively clear media.19 Localized granulomatous forms may be a late presentation of an acute form of the disease that had healed without development of retinal detachment or phthisis bulbi. As the vitreous clears it is possible to visualize the retinal lesions.19 The history is usually negative for trauma, intraocular surgery, or bacterial and fungal endophthalmitis. However, a history of playing in sandboxes in public places and geophagia may be obtained.19,27,28 External inspection usually reveals a quiet eye but severe intraocular inflammation can be present. In such cases leukokoria may be seen. Patients present with anterior chamber reaction that can vary from moderate to intense granulomatous reaction and can lead to hypopyon and posterior synechiae. The vitreous shows dense cellular infiltration with hazy media that can clear or develop cyclitic membranes, retinal traction and detachment that can be diagnosed ultrasonographically.51 If vitreous clears, a localized granuloma can be seen. The prognosis in this form of endophthalmitis depends primarily on the degree of intravitreal organization and the development of complications such as retrolental membranes, cataract, glaucoma, retinal detachment, and phthisis bulbi.
Atypical presentations
Atypical forms of ocular toxocariasis have been reported. Most of them had a presumed diagnosis. These include: optic nerve granuloma and optic neuritis;48 motile subretinal nematode,52 which more recently many consider to be an early phase of DUSN; diffuse chorioretinitis;7 conjunctivitis; keratitis; and lens involvement, including motile intralenticular larva and cataract.7,22
Diagnosis
The enzyme-linked immunosorbent assay (ELISA) is the current serum test of choice to detect exposure to T. canis.7 Intraocular fluids (aqueous humor, vitreous) can also be used to detect antibodies in a suspected case of ocular toxocariasis with a negative serum ELISA test.7,41,53 The ELISA test employs antigens secreted by the second-stage larva and recombinant antigens have been produced from them that add even greater specificity to an already-reliable test (approximately 92%). The ELISA has a reasonably high degree of sensitivity as well (approximately 78%), at a titer greater than 1 : 32.54 The immunodiagnostic tests used for VLM are not as reliable for ocular toxocariasis, and eosinophil count is not usually increased in these patients. Centers for Disease Control and Prevention consider serum ELISA titers less than 1 : 32 to be insignificant in the diagnosis of systemic toxocariasis55 but others have stated that a serum titer of 1 : 8 is sufficient to support a diagnosis of ocular toxocariasis if the patient has signs and symptoms compatible with that disorder.45 A positive test may represent a previous contact with the parasite and it does not necessarily mean that the patient suffers from either VLM or ocular toxocariasis. Ellis and coworkers have shown that 23% of 333 kindergarten children in rural North Carolina without signs of ocular toxocariasis exhibited a serum titer ≥1 : 32, and 32% had a titer ≥1 : 16.55 Prestes-Carneiro and coworkers have shown that 14% of 182 ramdomly studied patients in rural Sao Paulo, Brazil, presented positive ELISA tests for T. canis.56 Thus a positive serum titer cannot be used to confirm absolutely a diagnosis of ocular toxocariasis, although the absence of any serologic evidence of Toxocara infestation does not exclude the diagnosis but may assist in reducing the odds of this organism being the cause of ocular disease. Oréfice and coworkers studying 30 cases of presumed ocular toxocariasis found a positive ELISA test in 88% of them.44 Another study has shown that only 45% of patients with clinically diagnosed ocular toxocariasis had titers higher than 1 : 32.54
Cytologic study of aqueous humor or vitrectomy samples may also be helpful in confirming the diagnosis of ocular toxocariasis. The presence of eosinophils in intraocular fluids is consistent with intraocular Toxocara. Remnants of Toxocara organisms have occasionally been recovered from vitrectomy specimens obtained at surgery.57
Differential diagnosis
Retinoblastoma
Retinoblastoma is the most common intraocular malignancy of childhood and also the most important entity frequently confused with ocular toxocariasis.7 The first report of nematode intraocular endophthalmitis by Wilder13 included enucleated eyes for suspicion of intraocular retinoblastoma. Shields and coworkers58 reported that among 500 consecutive patients referred to them with leukokoria 42% had pseudoretinoblastoma and among them 15.6% had a final diagnosis of ocular toxocariasis. Patients with retinoblastoma are usually diagnosed before 2 years of age, being younger than a typical child with ocular toxocariasis. Sporadic retinoblastomas are more frequently confused with ocular toxocariasis since most of them are unilateral, and lack a family history of the malignancy. On ophthalmic evaluation, retinoblastoma usually does not present signs of intraocular inflammation, with clear vitreous and lack of posterior synechiae, cataract or cyclitic membranes, and the tumor presents a growing pattern that does not occur in toxocara granulomas.7 In cases with opaque media the differential diagnosis is harder. Ultrasonography and CT scan may be very valuable in demonstrating an intraocular mass and calcifications of a retinoblastoma, or vitreous organization and tractional membranes, which is more frequently observed in ocular toxocariasis. In cases where the differential diagnosis is difficult, evaluation of intraocular fluids by ELISA or cytology may be of particular importance. If retinoblastoma is suspected, however, biopsy should be avoided and consultation with other experts may be a better path to reliable diagnosis. Risks and concerns relating to sampling retinoblastoma tissue are discussed in the oncology section of this book.
Toxoplasmosis
The typical lesion seen with protozoal Toxoplama gondii infection is focal granulomatous and necrotizing retinochoroiditis. It appears as a whitish or yellowish lesion, slightly elevated and with poorly defined limits.20 During active disease, severe vitritis frequently occurs and in this case a differential diagnosis with chronic endophthalmitis caused by Toxocara can be very difficult. Ultrasonographic study can show vitreous membranes and an elevated mass that are more common in toxocariasis. Serological studies of antibodies to both parasites can be helpful in establishing differential diagnosis.
Other forms of endophthalmitis and uveitis
Bacterial endophthalmitis is frequently related to a recent history of trauma or intraocular surgery. These acute infections produce much more intraocular inflammation than a typical toxocariasis case. Endogenous endophthalmitis is rare, but an indolent infection may be nearly impossible to distinguish from nematode endophthalmitis. In such cases, laboratory diagnostic methods may be of value in determining the etiology. Pars planitis or chronic cyclitis is a condition that typically occurs in an older age group than that in which ocular toxocariasis occurs. Hogan and coworkers59 described a case of pars planitis in which subsequent histologic examination demonstrated that the etiology was T. canis.
Treatment
Medical therapy should be considered in cases of active inflammation. Topical and systemic corticosteroids are useful in managing acute inflammatory reaction and may reduce vitreous opacification and reduce or prevent membrane formation.7,19,48,60,61 Topical mydriatics may prevent posterior synechia and secondary angle closure secondary to seclusio pupillae.20 The exact role of anthelmintic therapy in ocular toxocariasis remains unclear and it is not proven that the use of this therapy can kill intraocular larva.7 Despite this, the use of anthelmintic drugs or combination of them with corticosteroids has been reported with favorable results.52 Anthelmintic drugs such as thiabendazole and diethylcarbamazine have been recommended in selected cases of VLM60 and nowadays albendazole is the current drug of choice in the treatment of VLM,30 showing its superiority over thiabendazole.62 For the treatment of VLM currently recommended therapy is albendazole 400 mg twice a day for 5 days.63 Treatment of ocular disease was described as a dose of 200 mg albendazole twice a day for one month.20 If a subretinal larva is observed it can be destroyed with photocoagulation.7 Intravitreal ranibizumab has been described to treat choroidal neovascularization secondary to ocular toxocariasis.64
Ocular surgery is indicated mainly in cases of ocular toxocariasis with retinal detachment43,65,66 and pars plana vitrectomy is the preferred technique, allowing release of membrane traction and retinal reattachment in more than 70% of cases in some series.45,66,67 Other indications for surgery include management of vitreous opacification, cataract, and glaucoma. Hagler and coworkers45 reported 17 consecutive cases of retinal detachment secondary to ocular toxocariasis. In this report the retina was successfully reattached in 12 (71%) cases, and vision remained stable or improved in 15 (88%) of the 17 eyes. Small et al.66 reported 12 cases of retinal detachment with a postoperative reattachment after vitreous surgery in 10 (83%), and visual acuity improved in 7 (70%) of the anatomic successes. Recently Giuliari and coworkers67 presented 45 cases treated surgically for complicated ocular toxocariasis. Pars plana vitrectomy was the technique of choice in 58% of the cases, 38% had peripheral granuloma and postoperative visual acuity was equal or better than 20/300 in 60% of the eyes studied.
Diffuse unilateral subacute neuroretinitis
History and etiology
In 1978 Gass and Scelfo first described a clinical syndrome in which only one eye of a healthy child or young adult was affected.68 Features of the syndrome include: insidious, usually severe loss of peripheral and central vision; vitritis; diffuse and focal pigment epithelial derangement with relative sparing of the macula; narrowing of the retinal vessels; optic atrophy; increased retinal circulation time; and subnormal electroretinographic findings.68 Initially the syndrome was termed “unilateral retinal wipeout syndrome.” Later, after recognition of signs of acute stages of the disease, the syndrome was named diffuse unilateral subacute neuroretinitis. The nematodal etiology of the syndrome was not yet clear, but from 36 patients reported, two revealed the presence of a subretinal worm at fundus examination, and both larvae were suspected to be from the Toxocara genus.68,69 Later, in 1983, Gass and Braunstein described two different-sized nematodes causing the disease and postulated that the cause was probably not T. canis. The geographic distribution of both was different and the smaller worm (from 400 to 1000 µm in length) was proposed to be a larva of Ancylostoma caninum, while the larger one (from 1500 to 2000 µm in length) remained uncertain.70 One year later, Kazacos and coworkers suggested that the larger larva previously described70 was Bayliascaris procyonis, an intestinal nematode of raccoons and squirrels associated with central nervous system infections.71,72 Association of DUSN and neural larva migrans has been reported and indirect immunofluorescence assays on serum and cerebrospinal fluid of one patient were positive for B. procyonis.73 Other case reports of DUSN with confirmed serological evidence of B. procyonis infestation were reported and support its etiology as the larger nematode to cause DUSN.74,75
The exact etiology of the smaller larva remains unclear. Despite the first impression of the larvae being Toxocara,69 later Gass and Braunstein70 opined that T. canis might not be the cause of DUSN due to lack of consistent serologic evidence, the length of second-stage larva of T. canis being smaller than the small worm described, the clinical features being different than other forms of ocular toxocariasis, and the worldwide prevalence of T. canis did not fit with the limited distribution of DUSN. Gass theorized that A. caninum, a common parasite of dogs, could be the causative agent because it is a frequent cause of cutaneous larval migrans in the southeastern United States, its infective third-stage larva measures approximately 650 µm, it can survive in host tissues for months to years without changing size or shape, and cutaneous larval migrans had immediately preceded the onset of DUSN in some patients.76 Casella and coworkers77 also described cases of DUSN and cutaneous larva migrans, reinforcing that A. caninum could be a probable cause of the disease in Brazil. Cunha de Sousa and Nakashima78 successfully extracted a subretinal worm in a 9-year-old Brazilian boy through a retinotomy after pars plana vitrectomy. This larva was morphologically similar to third-stage T. canis based on length of body and esophagus, tapered tail, and mouth shape. Toxocara canis causes visceral larva migrans and A. caninum causes cutaneous larva migrans, and there is evidence that both can be related to DUSN; thus confirmation of one as causative agent does not necessarily exclude the other as a possible cause. Cases of DUSN caused by non-nematode worm were reported by McDonald and coworkers. In such cases the larva related to DUSN was Alaria mesocercaria, a trematode worm.79 Despite this report, nematodes are described as the main etiologic factors in DUSN. Among them, some filariae such as Dirofilaria,80–82 have been proposed to cause the disease but B. procyonis as the large worm, A. caninum and/or T. canis as the small worms are the most probably etiologic factors.
Epidemiology
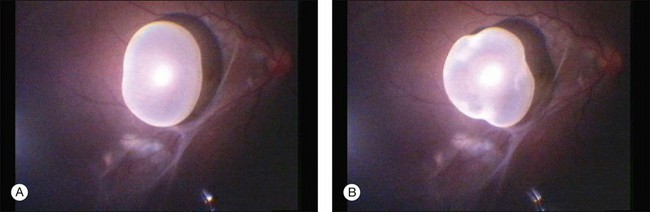
DUSN is an endemic disease in the southeasthern United States where the smaller worm (400–1000 µm) is most often seen and in the northern-midwestern part of the country where the larger worm (1500–2000 µm) is mostly related to the disease.70,76 Its occurrence has been described also in the Caribbean,9 Canada,83 Germany,84 Spain,85 China,86 and India.81,82,87 It is endemic in South America, with most cases described in Brazil77,88–91 and Venezuela.92 In South America, DUSN is caused by a smaller worm, although in one case report a larger worm was found as the etiologic factor (Fig. 86.6).93
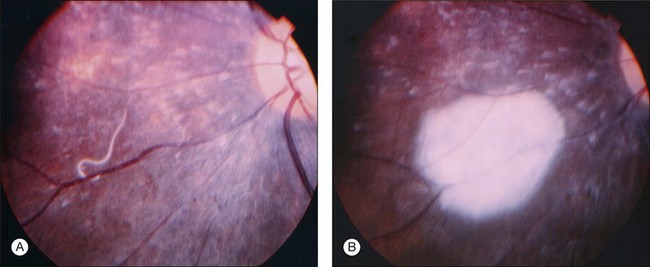
Fig. 86.6 Subretinal large nematode (A). Retinal aspect after photocoagulation of the nematode (B).
(Reproduced with permission from Cialdini AP, de Souza EC, Avila MP. The first South American case of diffuse unilateral subacute neuroretinitis caused by a large nematode. Arch Ophthalmol. 1999;117:1431–2.
DUSN typically affects healthy patients in the second and third decades of life,68,70 with a higher incidence in the male gender. In the typical disease only one eye is affected and a single motile subretinal nematode can be identified. Cases of nematodes infesting both eyes89 or two nematodes infesting the same eye85 are less frequently reported.
Pathophysiology
The complete pathophysiology of DUSN is not clearly determined but it is presumed that DUSN develops as a local reaction of outer retina to toxic products released by subretinal worm.68 These products would also produce a toxic reaction to inner retina. Through the years there is a progressive loss of ganglion cells, secondary arterial narrowing, and optic atrophy.76 Gass and Scelfo68 reported histopathological findings from one eye of a patient with clinical evidence of DUSN. Histopathological analysis has shown a nongranulomatous vitritis, retinitis, and retinal and optic nerve perivasculitis. An extensive degeneration of the peripheral retina, mild degeneration of the posterior retina, retinal pigmented epithelium (RPE), choroiditis, and mild optic nerve atrophy were also observed. There was no eosinophilic reaction as usually seen in the typical granuloma of ocular toxocariasis. Histopathologic data were not sufficient to explain severe visual loss in this case (light perception), which contributed to speculation about the role of functional mechanisms in causing visual damage such as an inflammatory and/or toxic aggression towards retinal bipolar cells.94 Recently Gomes and coworkers95 and Casella and coworkers96 described two case series of patients with DUSN studied by means of time domain optical coherence tomography (OCT). In both series the OCT main finding was diffuse atrophy of the retinal nerve fiber layer. The variability of the inflammatory signs and tissue damage seen in patients with the disease may reflect differences in host immune response to the organism or the characteristics of the nematode itself.97
Clinical presentation
Clinical features of DUSN can be divided into early and late stages.
Early stage
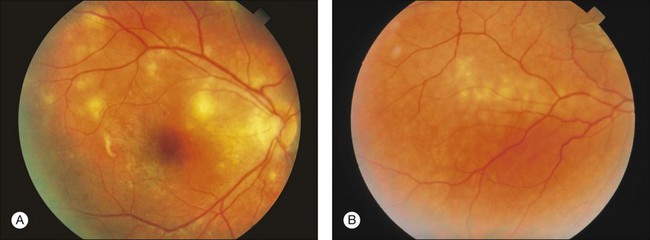
Most patients with DUSN are asymptomatic or have mild symptoms in this stage of the disease.90 Main symptoms include central or paracentral scotoma, floaters, mild to moderate visual loss, and ocular discomfort.69 Signs include: afferent pupillary defect; mild to moderate vitritis; optic disc swelling; vasculitis and/or narrowing of retinal vessels. Less frequently, posterior segment signs include retinal and subretinal hemorrhages, serous exudation, and choroidal neovascularization.69 The anterior segment is typically calm, although occasionally anterior uveitis may be present. The most characteristic retinal finding is the presence of recurrent crops of evanescent, multifocal, gray-white or yellow lesions at the level of the outer retina (Fig. 86.7). These active lesions are typically clustered in posterior pole, and the larva may be found in the surrounding area.76 These lesions disappear in 1–2 weeks as the worm moves to other retinal area. Less than 1% of the lesions can lead to a focal chorioretinal scar that simulates that seen in the presumed ocular histoplasmosis syndrome.97
Late stage
Frequently in children, visual loss is insidious, and the patient comes to medical attention during the late stages of the disease.97 If the nematode is not recognized and the condition is not treated, the disease evolves to its late stage. Main symptoms include a dense central or paracentral scotoma and severe permanent visual loss, while signs include an afferent pupillary defect in the affected eye, progressive optic atrophy, narrowing of the retinal arteries, and marked focal and/or diffuse degenerative changes in the RPE. The nematode may survive for 4 years or longer and may be found in the subretinal space even after the development of retinal and optic disc changes97 (Fig. 86.8).
Diagnosis
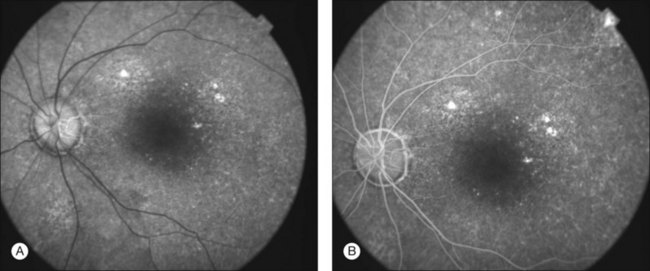
Definitive diagnosis of DUSN is based on identification of subretinal nematode after observation of clinical aspects that raise suspicion of the disease. Indirect ophthalmoscopy is important in making the correct diagnosis and in locating the cluster of active gray-white outer retinal lesions. These lesions are typically found in acute stages of the disease and, if they are present, the nematode will usually be found biomicroscopically in their vicinity.97 If theses lesions are absent, a detailed search of the entire fundus using a contact lens may locate the worm (Fig. 86.9). The rates of nematode identification in previous studies has varied from 33 to 52%.92,95,98 A case report has shown that scanning laser ophthalmoscopy enhances contrast between the nematode and the ocular fundus and improves visualization and location of a moving worm.99 In cases in which nematode is not located on fundus examination, a presumed diagnosis is established based on clinical findings, epidemiology, and ancillary exams.
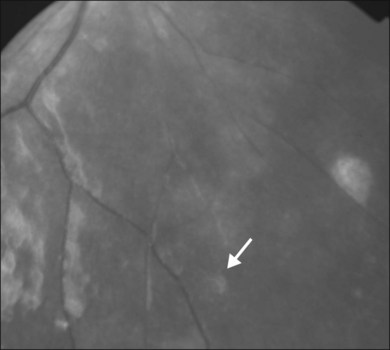
Fig. 86.9 Red-free retinography showing subretinal nematode (arrow) located on nasal-inferior retina.
Fluorescein angiography (FA) findings are different in acute and late stages of DUSN. During the early stages of the disease FA shows leakage of dye from the optic nerve head and perivenous phlebitis.69 The active gray-white lesions exhibit early hypofluorescence and hyperfluorescence with staining in late phases of the angiogram. The aspect of the lesions on FA is very similar to the fluorescein pattern found in other white-dot syndromes, however in multiple evanescent white-dot syndrome (MEWDS) the lesions are hyperfluorescent on early angiogram. In early stages of the disease there are also minimal changes in RPE and cystoid macular edema may occur. In late stages of DUSN, FA shows diffuse hyperfluorescence from RPE window defects and delay in retinal perfusion (Fig. 86.10). Indocyanine green angiography (ICG) shows multiple small round hypofluorescent lesions that persist during all angiograms. Their aspect is similar to lesions found in birdshot chorioretinopathy.100
The electroretinogram (ERG) may be normal in the very early stage of the disease, however it typically varies from subnormal to severe decreased as the retinopathy progress. During all stages of the disease the b-wave is more affected than a-wave, possibly due to a toxic effect of the nematode and its products to retinal bipolar cells.94 Rarely the ERG is extinguished69,70 and the unaffected eye presents normal ERG. Eradication of the nematode in one case resulted in improvement in multifocal ERG amplitudes.101
Optical coherence tomography (OCT) shows diffuse retinal thinning and diffuse retinal nerve layer atrophy.95,96 Casella and coworkers also described focal retinal edema in areas affected by the worm and have shown the topography of subretinal worm by means of OCT96 (Fig. 86.11). Gomes et al., studying 38 patients with DUSN, described statistical significance between retinal nerve fiber thickness and worse visual acuity.95
Serologic studies, stool examinations, and peripheral blood smears are of little value in making the diagnosis of DUSN.69 Toxocaral and B. procyonis antibody titers have been suggested as diagnostic tools to try to characterize which nematode is producing the disease.71 Recent study showed that ELISA cross-reativity between them may be present and suggests a Western blot analysis to complete serological differentiation between Toxocara and Baylisacaris as cause of larva migrans.102 ELISA test has been also used for serological characterization of cutaneous larva migrans caused by A. caninum.103
Differential diagnosis
DUSN can mimic several other chorioretinal diseases.76 The disease presents differently in early and late stages and the differential diagnosis will be described separately for better comprehension.
In the early stages of DUSN, the typically seen gray-white lesions can mimic many other infectious and inflammatory conditions (Fig. 86.7). In DUSN, typical lesions occur in outer retina and after healing they normally present few or no RPE scars. In other infections such as toxoplasmosis, cytomegalovirus, and bacterial or fungal retinitis generally the inner retina is affected and there is prominent chorioretinal scarring left in the area of the retinitis. DUSN may also mimic white-dot syndromes such as acute posterior multifocal placoid pigment epitheliopathy (APMPPE), serpiginous choroiditis, multiple evanescent white-dot syndrome (MEWDS), multifocal choroiditis with panuveitis (MFC), and birdshot chorioretinopathy.100 These entities are discussed in other chapters in more detail. Briefly, unlike DUSN, the active lesions in APMPPE and serpiginous choroiditis are accompanied by loss of visual acuity only when the lesions involve the center of the fovea, and they always cause permanent visible alterations in the pigment epithelium. APMPPE and serpiginous choroiditis occur in adults and are generally bilateral. In APMPPE, the electrooculogram may be abnormal but the ERG is normal, and visual prognosis is good. Serpiginous choroiditis has a worse prognosis than APMPPE and electrophysiologic tests are normal. MEWDS occurs in young adults, it is more frequent in women and may be distinguished from DUSN by a frequent history of an antecedent flu-like illness and photopsia, widely scattered gray-white outer retinal lesions, an enlarged blind spot, hyperfluorescent dots seen from the early phases of fluorescein angiography (in DUSN lesions are initially hypofluorescent), a decrease in the ERG a-wave that normalizes as symptoms fade, and return of the fundus and visual function to normal in most patients within several months.97 Birdshot chorioretinopathy often occurs in middle-aged women, is bilateral, and frequently associated with positive HLA-A29. Fundus changes include moderate vitritis, multiple scattered yellowish rounded lesions that may resemble DUSN lesions. Fluorescein angiography shows hypofluorescent lesions and may demonstrate active retinovascular leakage along the large retinal vessels, small vessel leakage, and cystoid or diffuse macular edema. Circulation times are often delayed and the vessels empty much more rapidly than in a normal eye.100 Other uveitis can mimic DUSN. In Behçet disease, the white lesions involve the inner retina and the patient usually has other systemic manifestations of the disorder, including oral and/or genital ulcers, arthritis, and erythema nodosum. Patients with DUSN occasionally may demonstrate perivenous candle-wax-dripping exudate or subretinal exudate similar to that seen in patients with sarcoidosis.76
In late stage of the disease after fading of gray-white lesions and development of scattered focal chorioretinal scars, the fundus of patients with DUSN may be mistaken for the presumed ocular histoplasmosis syndrome (POHS). POHS is frequently bilateral, shows normal RPE between the focal scars, presents clear vitreous without inflammatory cells, and the lack of optic atrophy and retinal vessel narrowing. Retinitis pigmentosa (RP) is another important differential diagnostic entity. The presence of bone-spicule RPE hyperpigmentation, bilaterality, and posterior subcapsular cataract are common in RP but rare in DUSN.97 Other causes of optic atrophy may mimic DUSN, such as trauma, retrobulbar or intracranial tumors, and sustained occlusion of ophthalmic artery.
Treatment
Prompt identification of the early signs of the disease allows treatment that can prevent late manifestations and encourage visual recovery/preservation. Treatment of DUSN can be performed with either photocoagulation of the nematode when it is identified during fundus examination (careful searching often with a contact lens is needed) or systemic anthelmintic drugs when the diagnosis is presumed. Photocoagulation of the subretinal worm is the current treatment of choice (Fig 86.6 and 86.8). It causes minimal or no post-treatment exacerbation of inflammation and is successful in causing prompt and permanent inactivation of the disease. Visual acuity improvement depends on the stage of the diagnosis.
Initially the use of oral anthelmintic was considered ineffective70 but further studies have shown that thiabendazole is effective in the treatment of patients with a moderate degree of vitritis associated with a breakdown in the blood–retinal barrier.104–106 Subsequently, ivermectin has been tried as a less toxic alternative to thiabendazole.107 However, Casella and coworkers reported that ivermectin failed to kill a nematode that was subsequently identified and destroyed with laser photocoagulation.108 Recent studies report case series of successful treatment of DUSN with albendazole98,109 (Fig. 86.12). Cunha-de-Souza and coworkers98 and Malaguido and coworkers109 report the use of albendazole 400 mg/daily for 30 days with fading of active lesions and visual recovery. In patients with late-stage DUSN some degree of improvement of vision was observed. No adverse side-effects were observed in both studies, and this therapy has been advocated in cases of presumed DUSN where the nematode is not located.90
Onchocerciasis
Onchocerciasis (river blindness) is caused by the microfilarial stage of the nematode Oncocerca volvulus and is endemic in West Africa, but also in Yemen and in six countries of the Americas (Brazil, Colombia, Ecuador, Guatemala, Mexico, and Venezuela).91,110 According to the World Health Organization, onchocerciasis is the world’s second leading cause of preventable blindness and its prevalence is estimated at 37 million individuals worldwide, most of them living on the African continent.111 The filariae cause dermatitis, subcutaneous nodules due to encapsulation of parasites in fibrous tissue and eye disease. The disease is transmitted by an insect of the genus Simulium (Blackflies) which breed in fast-flowing rivers and streams, lending the name “river blindness.” The vector ingests microfilariae during a blood-meal from a sick human. Then the parasites develop into infectious stages and are transmitted to other persons on subsequent bites. Humans are the only definitive host known.110
Clinical presentation
Ocular onchocerciasis can involve any part of the eye and disease manifestations are characterized either as posterior or anterior eye disease.112
Anterior eye disease prevalence has been described ranging from 43 to 48% of affected individuals in studied populations. The most frequently diagnosed anterior eye disease is the presence of microfilariae in the anterior chamber associated or not with iridocyclitis, followed by punctate epithelial keratitis, corneal microfilariae, sclerosing keratitis, and neovascularization leading to visual impairment and blindness in one or both eyes in 2–4% of patients.113,114
Posterior eye disease prevalence ranges from 34 to 75% of affected patients.113,114 Posterior segment lesions include atrophy of RPE and choroid mainly in the posterior pole, hyperpigmentation clumping of RPE, white and shiny intraretinal deposits, retinitis, subretinal fibrosis, neuritis and optic nerve atrophy.113–116 Patients may present with visual field loss and poor night vision and in patients with hyperpigmentation of RPE a differential diagnosis with retinitis pigmentosa is important. Another important differential diagnostic consideration is hypovitaminosis A, which can cause also night blindness and intraretinal white spots.
Treatment and prevention
Ivermectin is a safe and effective microfilaricidal drug which has been donated by Merck and Company since 1987 to be delivered through mass drug administration programs to control onchocerciasis. Ivermectin rapidly kills the microfilariae and reduces the lifespan of adult worms. This therapeutic scheme is administered twice each year and the goal of these programs is to treat during the years at least 85% of the population at risk and completely eliminate transmission of the parasite in Africa and America by the year 2012.110
Cysticercosis
Cysticercosis is the infestation of human tissues by Cysticercus cellulosae, the larval form of Taenia solium, a common pork parasite that can cause taeniasis in man. The ingestion of contaminated and uncooked meat containing cysticerci leads to development of taeniasis, the infestation of human small intestine by the adult tapeworm of T. solium (definitive host). Cysticercosis occurs when the intermediate host (e.g., pork, man) ingests eggs of T. solium from contaminated food or water, or by autoinfection of the definitive host when there is accidental ingestion of eggs shed in the host feces.117,118 The ingested eggs develop into larvae, which penetrate the small intestine wall reaching lymphatics and vascular system. The larvae then spread to highly vascularized organs such as brain, heart, muscles, and eyes, where they become a cystic structure containing the scolex named cysticercus (metacestode).119 Cysticerci may remain viable in the ocular and central nervous system for years and ocular manifestations can be devastating as the cysticercus increases in size or dies releasing toxic products leading to a profound inflammatory reaction, causing blindness in 3–5 years.120
Clinical presentation
The central nervous system is the most affected system and neurocysticercosis is the most commonly diagnosed form of cysticercosis.121 Neurocysticercosis is the main cause of acquired seizures worldwide. The disease can also lead to encephalopathy, meningeal signs, obstructive hydrocephalus, and other neurological abnormalities.122 Cysticerci appear as cystic lesions on computed tomography (CT) or magnetic resonance imaging (MRI), and when calcified appear as hyperdense spots on radiographs.
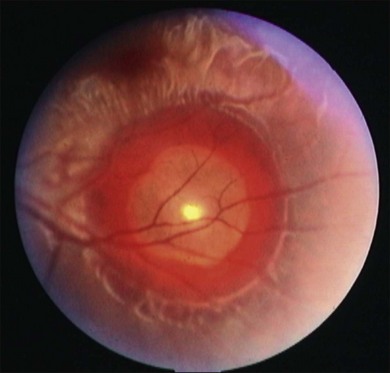
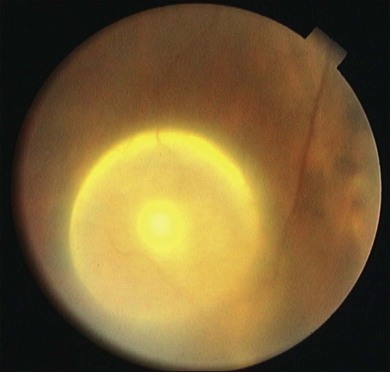
Eyes and adnexial tissues are affected in 13–46% of infected individuals.117 Cysticerci may be present in virtually all ocular and adnexial structures such as in the eyelids, orbit, extraocular muscles, subconjuntival space, anterior chamber, vitreous and subretinal space.118,121,123–125 Vitreous and subretinal locations are the most often diagnosed locations of ocular cysticercosis, ranging from 68 to 74% of studied cases and 41% occured either subretinally or intraretinally in one series.117,121 The parasite reaches the eye through posterior cilliary arteries to the subretinal space. A subretinal cysticercus is seen ophthalmoscopically as a cyst in subretinal space containing one scolex that usually presents as a white spot inside the cyst (Fig. 86.13). Associated findings may be observed, such as retinal pigmented epithelium hyperpigmentation, serous retinal detachment, retinal edema and/or hemorrhages, and severe uveitis in later stages (Fig. 86.14). Intravitreous cysticercus presents as a well-defined translucent floating cyst that can produce undulating movement, especially when stimulated by light. Epiretinal membranes or RPE changes in the spot where the parasites traverse the retina may be observed (Fig. 86.15).119,123,126,127 Ultrasonography may be used to confirm the cystic nature of the parasite and MRI has been suggested to confirm ultrasonographic findings as well as determine the presence of possible other cysts in orbit or brain.125
Treatment
Intraocular cysticercosis is treated with surgical removal.126–128 Treatment with systemic drugs has not been succesful129 and killing the cyst without removing it might produce severe uveitis. An intravitreal cysticercus can be removed by pars plana vitrectomy and the subretinal cyst removal can be performed by either vitrectomy or transcleral removal.126 The cysticercus should be removed unharmed, avoiding its rupture and release of intracystic material in the vitreous cavity, however there are reports of successful suction of the cyst during vitrectomy with the vitreous cutter,126,127 and rupture of intravitreal or partial removal of the subretinal cyst during vitrectomy followed by administration of intravitreous and systemic cortisteroids without postoperative significant inflammation.119,128
1 Hotez PJ, Brindley PJ, Bethony JM, et al. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118:1311–1321.
2 Cox FEG. History of human parasitology. Clin Microbiol Rev. 2002;15:595–612.
3 Glickman LT, Magnaval J. Zoonotic roundworm infections. Infect Dis Clin North Am. 1993;7:717–732.
4 Hotez PJ, Molyneux DH, Fenwick A, et al. Control of neglected tropical diseases. N Engl J Med. 2007;357:1018–1027.
5 Zinkham WH. Systemic visceral larval migrans. In: Ryan SJ, Smith RE. Selected topics on the eye in systemic disease. New York: Grune & Stratton; 1979:167–175.
6 Schantz PM. Of worms, dogs, and human hosts: continuing challenges for veterinarians in prevention of human diseases. J Am Vet Med Assoc. 1994;204:1023–1028.
7 Shields JA. Ocular toxocariasis: a review. Surv Ophthalmol. 1984;28:361–381.
8 Goto H, Mochizuki M, Yamaki K, et al. Epidemiological survey of intraocular inflammation in Japan. Jpn J Ophthalmol. 2007;51:41–44.
9 Stewart JM, Cubillan LD, Cunningham ET, Jr. Prevalence, clinical features, and causes of vision loss among patients with ocular toxocariasis. Retina. 2005;25:1005–1013.
10 Tran VT, Auer C, Guex-Crosier Y, et al. Epidemiological characteristics of uveitis in Switzerland. Int Ophthalmol. 1994–1995;18:293–298.
11 Brown DH. Ocular toxocara canis II. Clinical review. J Pediatr Ophthalmol. 1970;7:182–191.
12 Leiper RT. Two new genera of nematodes occasionally parasitic in man. Br Med J. 1907;1:1296–1298.
13 Wilder HC. Nematode endophthalmitis. Trans Am Acad Ophthalmol Otolaryngol. 1950;55:99–109.
14 Beaver PC, Snyder CH, Carrera GM, et al. Chronic eosinophilia due to visceral larva migrans. Pediatrics. 1952;9:7–19.
15 Nichols RL. The etiology of visceral larva migrans. I. Diagnostic morphology of infective second-stage Toxocara larvae. J Parasitol. 1956;42:349–362.
16 Irvine WC, Irvine AR, Jr. Nematode endophthalmitis: Toxocara canis – report of one case. Am J Ophthalmol. 1959;47:185–191.
17 Ashton N. Larval granulomatosis of the retina due to Toxocara. Br J Ophthalmol. 1960;44:129–148.
18 Duguid IM. Features of ocular infestation by Toxocara. Br J Ophthalmol. 1961;45:789–796.
19 Wilkinson CP, Welch RB. Intraocular Toxocara. Am J Ophthalmol. 1971;71:921–930.
20 Rocha IML, Oréfice F, Soares IP, et al. Toxocaríase. Oréfice F, ed. Uveíte clínica e cirúrgica, 2nd ed, Rio de Janeiro: Cultura Médica, 2005.
21 Chieffi PP, Santos SV, Queiroz ML, et al. Human toxocariasis: contribution by Brazilian researchers. Rev Inst Med Trop S Paulo. 2009;51:301–308.
22 Molk R. Ocular toxocariasis: a review of the literature. Ann Ophthalmol. 1983;15:216–231.
23 Ottesen EA. Visceral larval migrans and other unusual helminth infections. Mandell GL, Douglas RG, Bennett JE. Principles and practice of infectious diseases, 2nd ed, New York: John Wiley, 1979.
24 Mok CH. Visceral larva migrans: a discussion based in review of literature. Clin Pediatr. 1968;7:565–573.
25 Amaral HL, Rassier GL, Pepe MS, et al. Presence of Toxocara canis eggs on the hair of dogs: a risk factor for visceral larva migrans. Vet Parasitol. 2010;174:115–118.
26 Marmor M, Glickman L, Shofer F, et al. Toxocara canis infection of children: epidemiological and neuropsychological findings. Am J Public Health. 1987;77:554–559.
27 Schantz PM, Meyer D, Glickman LT. Clinical, serologic, and epidemiologic characteristics of ocular toxocariasis. Am J Trop Med Hyg. 1979;28:24–28.
28 Schantz PM, Weis PE, Pollard ZF, et al. Risk factors for toxocaral ocular larva migrans: a case–control study. Am J Public Health. 1980;70:1269–1272.
29 Queiroz ML, Simonsen M, Paschoalotti MA, et al. Frequency of soil contamination by Toxocara canis eggs in the south region of São Paulo municipality (SP, Brazil) in a 18 month period. Rev Inst Med Trop Sao Paulo. 2006;48:317–319.
30 Despommier D. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev. 2003;16:265–272.
31 Castillo D, Paredes C, Zanartu C. Environmental contamination with Toxocara spp. eggs in public squares and parks from Santiago, Chile, 1999. Bol Chil Parasitol. 2000;55:86–91.
32 Giacometti A, Cirioni O, Fortuna M. Environmental and serological evidence for the presence of toxocariasis in the urban area of Ancona, Italy. Eur J Epidemiol. 2000;16:1023–1026.
33 Akdemir C. Visceral larva migrans among children in Kütahya (Turkey) and an evaluation of playgrounds for T. canis eggs. Turk J Pediatr. 2010;52:158–162.
34 Borg OA, Woodruff AW. Prevalence of ova of Toxocara species in public places. Br Med J. 1973;4:470–472.
35 Yoshikawa M, Nishiofuku M, Moriya K, et al. A familial case of visceral toxocariasis due to consumption of raw bovine liver. Parasitol Int. 2008;57:525–529.
36 Hoffmeister B, Glaeser S, Flick H, et al. Cerebral toxocariasis after consumption of raw duck liver. Am J Trop Med Hyg. 2007;76:600–602.
37 Morimatsu Y, Akao N, Akiyoshi H, et al. A familial case of visceral larva migrans after ingestion of raw chicken livers: appearance of specific antibody in bronchoalveolar lavage fluid of the patients. Am J Trop Med Hyg. 2006;75:303–306.
38 Wilkinson CP. Ocular toxocariasis. Ryan SJ, ed. Retina, 4th ed, London: Elsevier, 2005.
39 Glickman LT, Schantz PM. Epidemiology and pathogenesis of zoonotic toxocariasis. Epidemiol Rev. 1981;3:230–250.
40 Bass JL, Mehta KA, Glickman LT, et al. Clinically inapparent Toxocara infection in children. N Engl J Med. 1983;308:723–724.
41 Alabiad CR, Albini TA, Santos CI, et al. Ocular toxocariasis in a seronegative adult. Ophthalmic Surg Lasers Imaging. 2010;2:1–3.
42 Raistrick ER, Hart JC. Ocular toxocariasis in adults. Br J Ophthalmol. 1976;60:365–370.
43 Benitez-del Castillo JM, Herreros G, Guillen JL, et al. Bilateral ocular toxocariasis demonstrated by aqueous humor enzyme-linked immunosorbent assay. Am J Ophthalmol. 1995;119:514–516.
44 Oréfice F, Boratto LM, Silva HF. Presumível toxocaríase ocular – revisão de 30 casos (1978–1989) – Relato de dois casos atípicos. Rev Bras Oftalmol. 1991;50:92–101.
45 Hagler WS, Pollard ZF, Jarrett WH, et al. Results of surgery for ocular Toxocara canis. Ophthalmology. 1981;88:1081–1086.
46 Cella W, Ferreira E, Torigoe AM, et al. Ultrasound biomicroscopy findings in peripheral vitreoretinal toxocariasis. Eur J Ophthalmol. 2004;14(2):132–136.
47 do Lago A, Andrade R, Muccioli C, et al. Optical coherence tomography in presumed subretinal toxocara granuloma: case report. Arq Bras Oftalmol. 2006;69:403–405.
48 Bird AC, Smith JL, Curtin VT. Nematode optic neuritis. Am J Ophthalmol. 1970;69:72–77.
49 Gass JDM. Stereoscopic atlas of macular diseases, 4th ed. St Louis: Mosby; 1997.
50 Monshizadeh R, Ashrafzadeh MT, Rumelt S. Choroidal neovascular membrane: a late complication of inactive toxocara chorioretinitis. Retina. 2000;20:219–220.
51 Wan WL, Cano MR, Pince KJ, et al. Echographic characteristics of ocular toxocariasis. Ophthalmology. 1991;98:28–32.
52 Rubin ML, Kaufman HE, Tierney JP, et al. An intraretinal nematode (a case report). Trans Am Acad Ophthalmol Otolaryngol. 1968;72:855–866.
53 Sharkey JA, McKay PS. Ocular toxocariasis in a patient with repeatedly negative ELISA titers to Toxocara canis. Br J Ophthalmol. 1993;77:253–254.
54 Schantz PM. Toxocara larva migrans now. Am J Trop Med Hyg. 1989;41:21–34.
55 Ellis GS, Jr., Pakalnis VA, Worley G, et al. Toxocara canis infestation: clinical and epidemiological associations with seropositivity in kindergarten children. Ophthalmology. 1986;93:1032–1037.
56 Prestes-Carneiro LE, Souza DH, Moreno GC, et al. Toxocariasis/cysticercosis seroprevalence in a long-term rural settlement, São Paulo, Brazil. Parasitology. 2009;136:681–689.
57 Maguire AM, Green WR, Michels RG, et al. Recovery of intraocular Toxocara canis by pars plana vitrectomy. Ophthalmology. 1990;97:675–680.
58 Shields JA. Differential diagnosis of retinoblastoma. In: Guyer DR, Yannuzzi LA, Chang S, et al. Retina–vitreous–macula. Philadelphia: W.B. Saunders, 1999.
59 Hogan MJ, Kimura SJ, Spencer WH. Visceral larval migrans and peripheral retinitis. JAMA. 1965;194:1345–1347.
60 O’Connor GR. Chemotherapy of toxoplasmosis and toxocariasis. In: Srinivasan BD, ed. Ocular therapeutics. New York: Masson, 1980.
61 Barisan-Asenbauer T, Maca SM, Hauff W, et al. Treatment of ocular toxocariasis with albendazole. J Ocul Pharm Therapeut. 2001;17:287–294.
62 Sturchler D, Schubarth P, Gualzata M, et al. Thiabendazole vs albendazole in treatment of toxocariasis: a clinical trial. Ann Trop Med Parasitol. 1989;83:473–478.
63 Hotez PJ. Toxocara canis. In: Burg FD, Wald ER, Ingelfinger JR, et al. Gellis and Kaganís current pediatric therapy. 15th ed. Philadelphia: W.B. Saunders; 1995:683–684.
64 Lyall DA, Hutchison BM, Gaskell A, et al. Intravitreal ranibizumab in the treatment of choroidal neovascularisation secondary to ocular toxocariasis in a 13-year-old boy. Eye. 2010;24:1730–1731.
65 Amin HI, McDonald HR, Han DP, et al. Vitrectomy update for macular traction in ocular toxocariasis. Retina. 2000;20:80–85.
66 Small KW, McCuen BW, de Juan E, et al. Surgical management of retinal traction caused by ocular toxocariasis. Am J Ophthalmol. 1989;108:10–14.
67 Giuliari GP, Ramirez G, Cortez RT. Surgical treatment of ocular toxocariasis: anatomic and functional results in 45 patients. Eur J Ophthalmol. 2011;21(4):490–494.
68 Gass JDM, Scelfo R. Diffuse unilateral subacute neuroretinitis. J R Soc Med. 1978;71:95–111.
69 Gass JD, Gilbert WR, Jr., Guerry RK, et al. Diffuse unilateral subacute neuroretinitis. Ophthalmology. 1978;85:521–545.
70 Gass JD, Braunstein RA. Further observations concerning the diffuse unilateral subacute neuroretinitis syndrome. Arch Ophthalmol. 1983;101:1689–1697.
71 Kazacos KR, Raymond LA, Kazacos EA, et al. The raccoon ascarid. A probable cause of human ocular larva migrans. Ophthalmology. 1985;92:1735–1744.
72 Kazacos KR, Vestre WA, Kazacos EA, et al. Diffuse unilateral subacute neuroretinitis syndrome: probable cause. Arch Ophthalmol. 1984;102:967–968.
73 Mets MB, Noble AG, Basti S, et al. Eye findings of diffuse unilateral subacute neuroretinitis and multiple choroidal infiltrates associated with neural larva migrans due to Baylisascaris procyonis. Am J Ophthalmol. 2003;135:888–890.
74 Goldberg MA, Kazacos KR, Boyce WM, et al. Diffuse unilateral subacute neuroretinitis. Morphometric, serologic and epidemiologic support for Baylisascaris as a causative agent. Ophthalmology. 1993;100:1695–1701.
75 Brasil OF, Lewis H, Lowder CY. Migration of Baylisascaris procyonis into the vitreous. Br J Ophthalmol. 2006;90:1203–1204.
76 Gass JDM. Stereoscopic atlas of macular diseases: diagnosis and treatment. vol. 4. St Louis: Mosby; 1997. p. 622–8
77 Casella AMB, Machado RA, Tsuro A, et al. Would Ancylostoma caninum be one of the agents of diffuse unilateral subacute neuroretinitis (D.U.S.N) in Brazil? Arq Bras Oftalmol. 2001;64:473–476.
78 Cunha de Souza E, Nakashima Y. Diffuse unilateral subacute neuroretinitis. Report of transvitreal surgical removal of a subretinal nematode. Ophthalmology. 1995;102:1183–1186.
79 McDonald HR, Kazacos KR, Schatz H, et al. Two cases of intraocular infection with Alaria mesocercaria (Trematoda). Am J Ophthalmol. 1994;117:447–455.
80 Parsons HE. Nematode chorioretinitis: report of a case, with photographs of a viable worm. Arch Ophthalmol. 1952;47:799–800.
81 Nath R, Gogoi R, Bordoloi N, et al. Ocular dirofilariasis. Indian J Pathol Microbiol. 2010;53:157–159.
82 Myint K, Sahay R, Mon S, et al. “Worm in the eye”: the rationale for treatment of DUSN in south India. Br J Ophthalmol. 2006;90(9):1125–1127.
83 Yuen VH, Chang TS, Hooper PL. Diffuse unilateral subacute neuroretinitis syndrome in Canada. Arch Ophthalmol. 1996;114:1279–1282.
84 Kuchle M, Knorr HL, Medenblik-Frysch S, et al. Diffuse unilateral subacute neuroretinitis syndrome in a German most likely caused by the raccoon roundworm, Baylisascaris procyonis. Graefes Arch Clin Exp Ophthalmol. 1993;231:48–51.
85 Harto MA, Rodriguez-Salvador V, Avino JA, et al. Diffuse unilateral subacute neuroretinitis in Europe. Eur J Ophthalmol. 1999;9:58–62.
86 Cai J, Wei R, Zhu L, et al. Diffuse unilateral subacute neuroretinitis in China. Arch Ophthalmol. 2000;118:721–722.
87 Natesh S, Harsha K, Nair U, et al. Subretinal worm and repeat laser photocoagulation. Middle East Afr J Ophthalmol. 2010;17:183–185.
88 de Souza EC, da Cunha SL, Gass JD. Diffuse unilateral subacute neuroretinitis in South America. Arch Ophthalmol. 1992;110:1261–1263.
89 de Souza EC, Abujamra S, Nakashima Y, et al. Diffuse bilateral subacute neuroretinitis: first patient with documented nematodes in both eyes. Arch Ophthalmol. 1999;117:1349–1351.
90 Oréfice F, Garcia CAA, Paranhos FRL, et al. Neuroretinite subaguda unilateral difusa (DUSN). Oréfice F, ed. Uveíte clínica e cirúrgica, 2nd ed, Rio de Janeiro: Cultura Médica, 2005.
91 Sabrosa NA, Zajdenweber M. Nematode infections of the eye: toxocariasis, onchocerciasis, diffuse unilateral subacute neuroretinitis, and cysticercosis. Ophthalmol Clin North Am. 2002;15:351–356.
92 Cortez R, Denny JP, Muci-Mendoza R, et al. Diffuse unilateral subacute neuroretinitis in Venezuela. Ophthalmology. 2005;112(12):2110–2114.
93 Cialdini AP, de Souza EC, Avila MP. The first South American case of diffuse unilateral subacute neuroretinitis caused by a large nematode. Arch Ophthalmol. 1999;117:1431–1432.
94 Oréfice F, Gonçalves ER, Siqueira RC, et al. Estudo de 21 casos de neuroretinite subaguda unilateral difusa (DUSN): dois casos com larva móvel sub-retiniana. Rev Bras Oftalmol. 1994;53:23–45.
95 Gomes AH, Garcia CA, Segundo P de S, et al. Optic coherence tomography in a patient with diffuse unilateral subacute neuroretinitis. Arq Bras Oftalmol. 2009;72:185–188.
96 Casella AM, Farah ME, Souza EC, et al. Retinal nerve fiber layer atrophy as relevant feature for diffuse unilateral subacute neuroretinitis (DUSN): case series. Arq Bras Oftalmol. 2010;73:182–185.
97 Davis JL, Gass JDM, Olsen KR. Diffuse unilateral subacute neuroretinitis. Ryan SJ, ed. Retina, 4th ed, London: Elsevier, 2005.
98 Souza EC, Casella AM, Nakashima Y, et al. Clinical features and outcomes of patients with diffuse unilateral subacute neuroretinitis treated with oral albendazole. Am J Ophthalmol. 2005;140:437–445.
99 Moraes LR, Cialdini AP, Avila MP, et al. Identifying live nematodes in diffuse unilateral subacute neuroretinitis by using the scanning laser ophthalmoscope. Arch Ophthalmol. 2002;120:135–138.
100 Quillen DA, Davis JB, Gottlieb JL, et al. The white dot syndromes. Am J Ophthalmol. 2004;137:538–550.
101 Martidis A, Greenberg PB, Rogers AH, et al. Multifocal electroretinography response after laser photocoagulation of a subretinal nematode. Am J Ophthalmol. 2002;133:417–419.
102 Dangoudoubiyam S, Kazacos KR. Differentiation of larva migrans caused by Baylisascaris procyonis and Toxocara species by Western blotting. Clin Vaccine Immunol. 2009;16:1563–1568.
103 Kwon IH, Kim HS, Lee JH, et al. A serologically diagnosed human case of cutaneous larva migrans caused by Ancylostoma caninum. Korean J Parasitol. 2003;41:233–237.
104 Gass JD, Callanan DG, Bowman CB. Successful oral therapy for diffuse unilateral subacute neuroretinitis. Trans Am Ophthalmol Soc. 1991;89:97–112.
105 Maguire AM, Zarbin MA, Conner TB, et al. Ocular penetration of thiabendazole. Arch Ophthalmol. 1952;108:1675.
106 Gass JDM, Callanan DG, Bowman CB. Oral therapy in diffuse unilateral subacute neuroretinitis. Arch Ophthalmol. 1992;110:675–680.
107 Callanan DG, Davis JL, Cohen SM, et al. The use of ivermectin in diffuse unilateral subacute neuroretinitis. Ophthalmology. 1993;100(9 Suppl):114–115.
108 Casella AM, Farah ME, Belfort R, Jr. Anthelmintic drugs in diffuse unilateral subacute neuroretinitis. Am J Ophthalmol. 1998;125:109–111.
109 Malaguido MR, Casella AM, Malaguido DR. [Clinical treatment of diffuse unilateral subacute neuroretinitis with albendazole.]. Arq Bras Oftalmol. 2007;70:814–822.
110 Report from the 2009 Inter-American Conference on Onchocerciasis: progress towards eliminating river blindness in the Region of the Americas. Wkly Epidemiol Rec. 2010;85(33):321–326.
111 Reddy M, Gill SS, Kalkar SR, et al. Oral drug therapy for multiple neglected tropical diseases: a systematic review. JAMA. 2007;298:1911–1924.
112 Hall LR, Pearlman E. Pathogenesis of onchocercal keratitis (river blindness). Clin Microbiol Rev. 1999;12:445–453.
113 Newland HS, White AT, Greene BM, et al. Ocular manifestations of onchocerciasis in a rain forest area of west Africa. Br J Ophthalmol. 1991;75:163–169.
114 Kayembe DL, Kasonga DL, Kayembe PK, et al. Profile of eye lesions and vision loss: a cross-sectional study in Lusambo, a forest-savanna area hyperendemic for onchocerciasis in the Democratic Republic of Congo. Trop Med Int Health. 2003;8:83–89.
115 Semba RD, Murphy RP, Newland HS, et al. Longitudinal study of lesions of the posterior segment in onchocerciasis. Ophthalmology. 1990;97:1334–1341.
116 Bird AC, Anderson J, Fuglsang H. Morphology of posterior segment lesions of the eye in patients with onchocerciasis. Br J Ophthalmol. 1976;60:2–20.
117 Duke-Elders S, Perkins ES. System of ophthalmology. Inflammations of the uveal tract: uveitis. St Louis: Mosby; 1966. ch. 3, v. 9, p. 478–87
118 Orefice F, ed. Uveíte clínica e cirúrgica: texto e atlas. Rio de Janeiro: Cultura Médica; 2000:705–713. ch. 40. v. 2
119 Pantaleão GR, Borges de Souza AD, Rodrigues EB, et al. [The use of systemic and intravitreous steroid in inflammation secondary to intraocular cysticercosis: case report.]. Arq Bras Oftalmol. 2007;70:1006–1009.
120 Junior L. Ocular cysticercosis. Am J Ophthalmol. 1949;32:523–548.
121 Wittig EO. Ocular cysticercosis: an epidemiological study. Arq Neuropsiquiatr. 2001;59:693–701.
122 Shandera WX, Kass JS. Neurocysticercosis: current knowledge and advances. Curr Neurol Neurosci Rep. 2006;6:453–459.
123 Kruger-Leite E, Jalkh AE, Quiroz H, et al. Intraocular cysticercosis. Am J Ophthalmol. 1985;99:252–257.
124 Rath S, Honavar SG, Naik M, et al. Orbital cysticercosis: clinical manifestations, diagnosis, management, and outcome. Ophthalmology. 2010;117:600–605.
125 Chung GW, Lai WW, Thulborn KR, et al. Magnetic resonance imaging in the diagnosis of subretinal cysticercosis. Am J Ophthalmol. 2002;134:931–932.
126 Sharma T, Sinha S, Shah N, et al. Intraocular cysticercosis: clinical characteristics and visual outcome after vitreoretinal surgery. Ophthalmology. 2003;110:996–1004.
127 Avila MP, Cialdini AP, Pereira AE, et al. Live cysticercus/Giant intraocular foreign bodies. Best of Show Video Awards. Annual Meeting, American Academy of Ophthalmology – Final Program. 2003. 301
128 Passos E, Frasson MC, Nehemy MB. Vitrectomia para o tratamento de cisticerco intrarretiniano. Rev Bras Oftalmol. 1996;55:841–845.
129 Santos R, Chavarria M, Aguirre AE. Failure of medical treatment in two cases of intraocular cysticercosis. Am J Ophthalmol. 1984;97:249–250.