Head and spinal injuries
HEAD INJURY
The severity of head injury is often assessed using the Glasgow coma scale (Table 11.1). This yields scores of 3 (the worst score) to 15 (the best score) based on an assessment of ocular, verbal, and motor responses.
Table 11.1
| Best eye response (maximum = 4) | |||
| 1. | No eye opening | 3. | Eyes opening to verbal command |
| 2. | Eye opening to pain | 4. | Eyes open spontaneously |
| Best verbal response (maximum = 5) | |||
| 1. | No verbal response | 4. | Confused |
| 2. | Incomprehensible sounds | 5. | Oriented |
| 3. | Inappropriate words | ||
| Best motor response (maximum = 6) | |||
| 1. | No motor response | 4. | Withdrawal from pain |
| 2. | Extension to pain | 5. | Localizing to pain |
| 3. | Flexion to pain | 6. | Obeys commands |
| Coma score | Clinical correlate |
| 13 or above | Mild brain injury |
| 9–12 | Moderate brain injury |
| 8 or less | Severe brain injury |
From: Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974; 2:81–84.
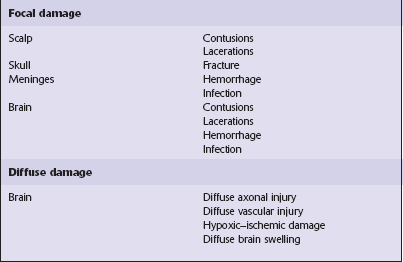
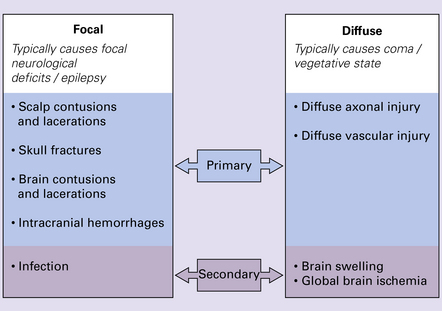
NATURE OF LESIONS IN HEAD INJURY
Type of head injury
 Non-missile or blunt head injury, associated with acceleration or deceleration forces to the head that cause brain movement within the skull. This is the commonest pattern of head trauma seen clinically.
Non-missile or blunt head injury, associated with acceleration or deceleration forces to the head that cause brain movement within the skull. This is the commonest pattern of head trauma seen clinically.
 Missile head injury results from penetration of the skull or brain by an external object such as a bullet.
Missile head injury results from penetration of the skull or brain by an external object such as a bullet.
Time course
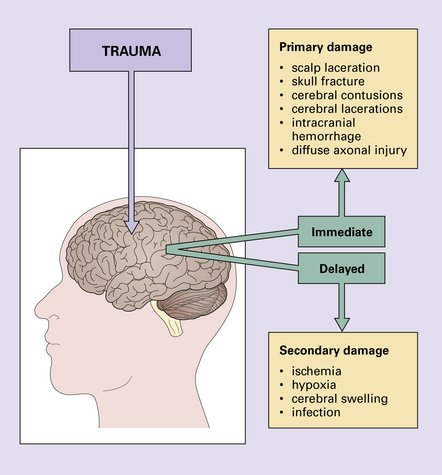
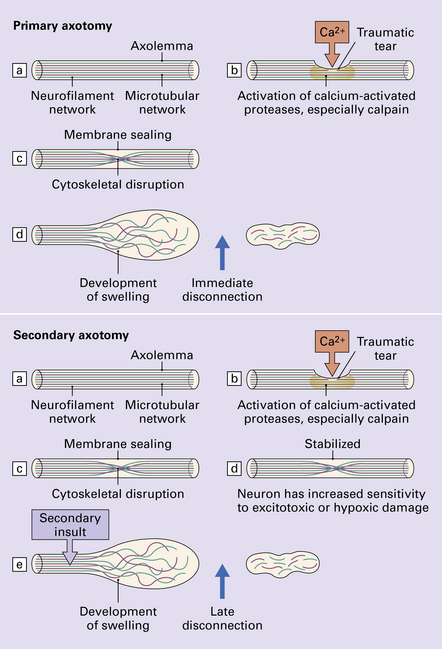
Brain damage following trauma can be viewed as occurring in two phases (Fig. 11.1):
 Primary damage resulting from the immediate physical stresses involved.
Primary damage resulting from the immediate physical stresses involved.
 Secondary damage, initiated by the process of injury, but presenting later and mainly involving physiologic responses to trauma, the effects of hypoxia/ischemia, or infection.
Secondary damage, initiated by the process of injury, but presenting later and mainly involving physiologic responses to trauma, the effects of hypoxia/ischemia, or infection.
Progressive neurologic deterioration over many years has been noted in 15% of patients who have suffered severe head trauma (Fig. 11.2).
NON-MISSILE HEAD INJURY
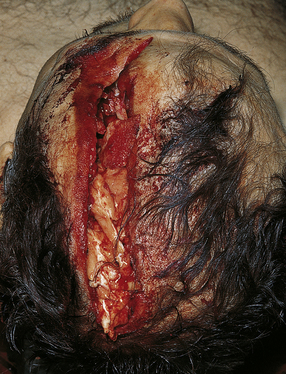
Laceration or bruising of the scalp is a common form of focal injury and a good indicator of the site of the trauma (Fig. 11.3).
FOCAL LESIONS OF THE SKULL
 Contact with a flat surface tends to produce closed fissure fractures, which often extend into the base of the skull.
Contact with a flat surface tends to produce closed fissure fractures, which often extend into the base of the skull.
 Contact with angled or pointed objects tends to produce localized fractures, which are often open and depressed (i.e. displaced inwards by at least the thickness of the skull).
Contact with angled or pointed objects tends to produce localized fractures, which are often open and depressed (i.e. displaced inwards by at least the thickness of the skull).
 Contrecoup fractures (i.e. fractures located at a distance from the point of injury that are not direct extensions of the fracture originating at the point of injury) occur principally in the roofs of the orbits, and the cribriform plates, following falls or other blunt impact involving the back of the skull.
Contrecoup fractures (i.e. fractures located at a distance from the point of injury that are not direct extensions of the fracture originating at the point of injury) occur principally in the roofs of the orbits, and the cribriform plates, following falls or other blunt impact involving the back of the skull.
The risk of intracranial hematoma is significantly increased in patients with skull fractures.
Fractures of the base of the skull predispose to:
 Infection, as they often pass through the petrous temporal bone or the anterior cranial fossa and cause leakage of cerebrospinal fluid (CSF) through the ear, nose, or nasopharynx. These breaks in the integrity of the skull are also routes for the spread of infection.
Infection, as they often pass through the petrous temporal bone or the anterior cranial fossa and cause leakage of cerebrospinal fluid (CSF) through the ear, nose, or nasopharynx. These breaks in the integrity of the skull are also routes for the spread of infection.
 Aeroceles (trapping of air in the cranial cavity), which occur in up to 30% of patients with leakages of CSF and can cause raised intracranial pressure and displacement of the brain.
Aeroceles (trapping of air in the cranial cavity), which occur in up to 30% of patients with leakages of CSF and can cause raised intracranial pressure and displacement of the brain.
Depressed fractures may tear the dura and associated blood vessels, leading to intracranial hemorrhage (see p. 274).
MACROSCOPIC APPEARANCES
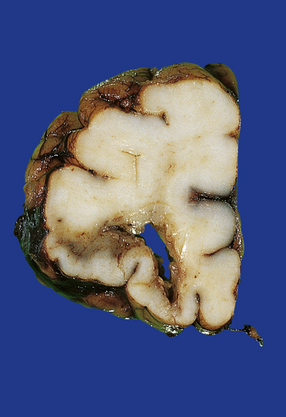
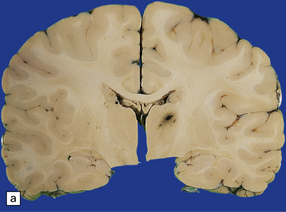
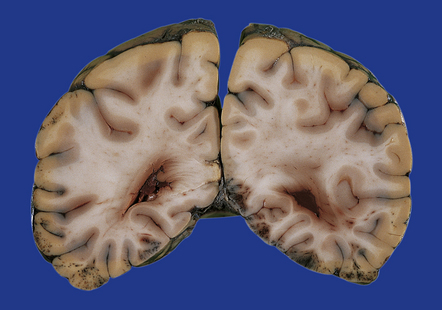
Acutely, contusions appear as superficial hemorrhagic areas associated with some hemorrhage into the overlying leptomeninges and variable brain swelling (Figs 11.4–11.6). Over subsequent days and weeks their color changes to brown or orange, and involved gyri become indented or superficially cavitated as necrotic tissue is resorbed. In brain slices, old contusions often have a triangular shape, with the point in the depths of the cortex or underlying white matter and a wide base at the surface of the crest of the gyrus (Fig. 11.7, see also Fig. 11.31). Contusions can be distinguished from foci of old ischemic damage, which are almost invariably more severe within the depths of sulci.
11.4 Superficial contusions on the underside of the occipital lobes.
The contusions in the left occipital lobe are mostly superficial whereas those on the right are more hemorrhagic and involve the full thickness of the cortex.
11.5 Severe cerebral contusions.
Severe frontal and temporal lobe contusions associated with extensive hemorrhage into the overlying subarachnoid space.
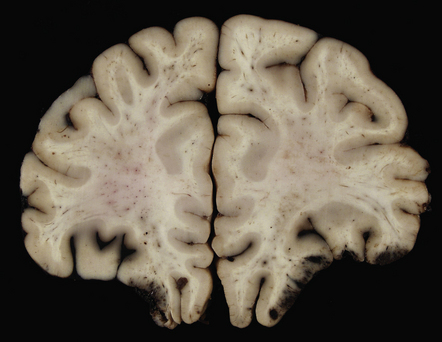
11.6 Superficial contusions of orbital frontal cortex.
The contusions are more extensive in the right frontal lobe.
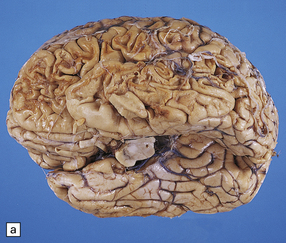
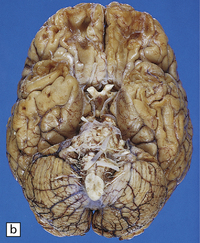
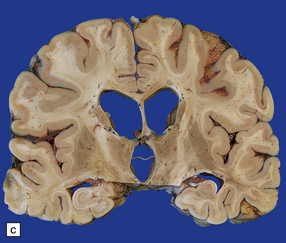
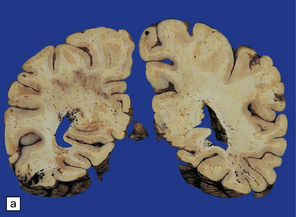
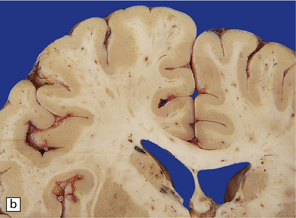
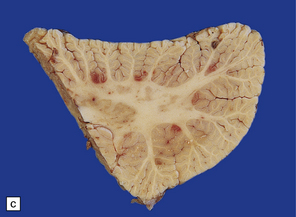
11.7 Old cerebral contusions.
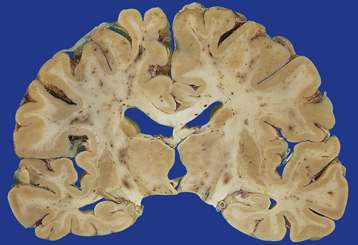
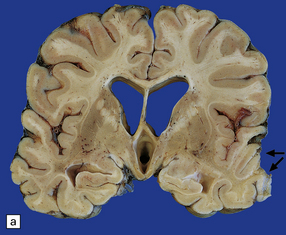
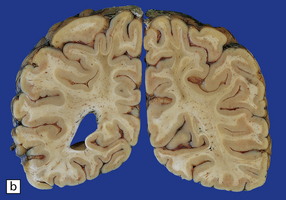
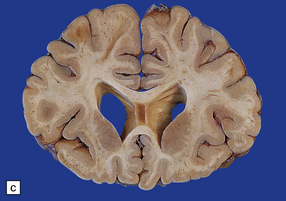
(a) Old contusions over the lateral aspect of frontal, temporal and parietal lobes. These appear as brown or orange indentations in the crests of gyri. (b) Old contusions on inferior aspect of frontal lobes (c) Old wedge-shaped contusion of the left inferior temporal gyrus (arrow). There is also evidence of old diffuse axonal injury: the ventricles are dilated, the corpus callosum is thinned and yellow, and the temporal and right parasagittal frontal white matter show gray discoloration.
11.31 Long-term effects of DAI.
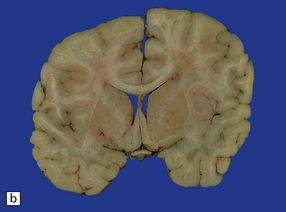
(a) Old trauma with contusions of the right middle and superior temporal gyri (arrows) and ventricular dilatation. Note the ill-defined gray discoloration of the white matter due to DAI. This is most marked in the temporal lobes (including the non-contused left temporal lobe), but also involves the corpus callosum and internal capsule. (b) Gray discoloration of the parasagittal white matter in the posterior part of the parietal lobes. (c) The gray-brown streaks in the parasagittal white matter in this brain are old gliding contusions.
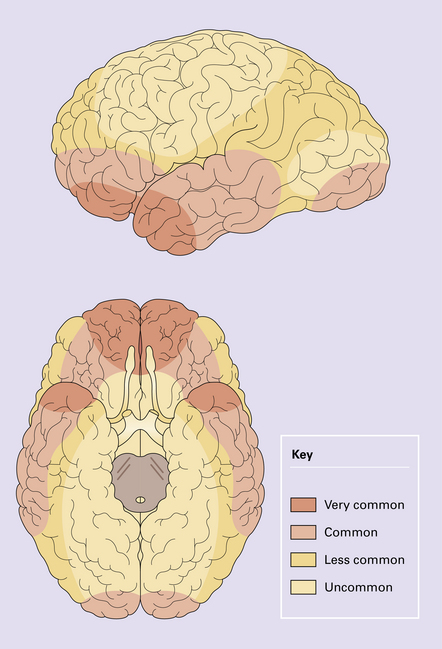
Contusions may occur in the region of impact (a form of contact damage), particularly if this causes fracturing, but also occur elsewhere over the brain in a stereotyped distribution that tends to be much the same whatever the site of the original injury. The contusions involve the crests of gyri that come into contact with protuberances within the skull (Figs 11.8, 11.9). Contusions tend therefore, to be located in the following regions:
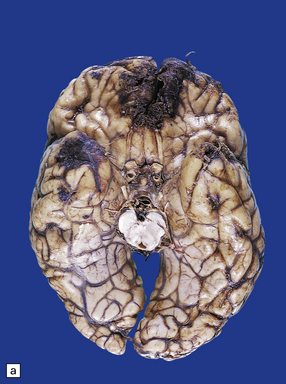
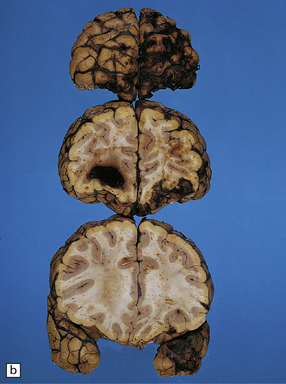
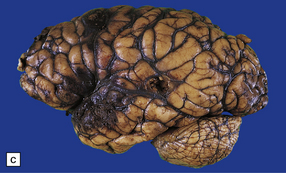
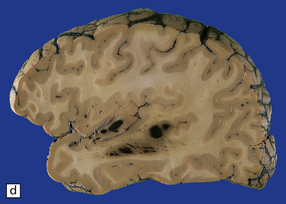
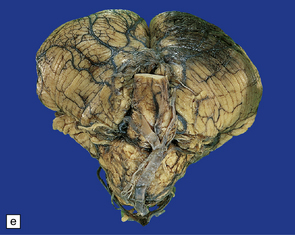
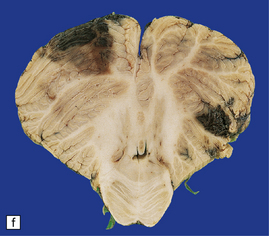
11.9 Common sites for contusions.
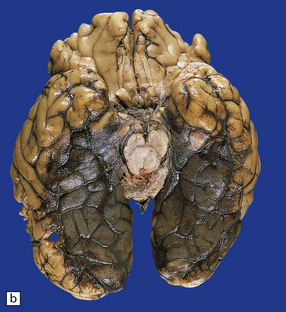
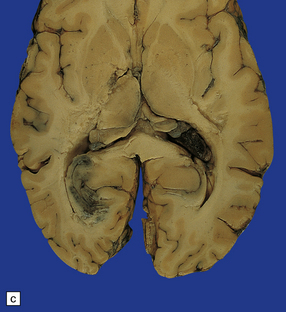
(a) Contusions of the orbital surface of the frontal lobes and tips of the temporal lobes. (b) Cut surface of brain showing contusions of the orbital surfaces of frontal lobes and tip of left temporal lobe. There is a hematoma in the right frontal lobe. (c) and (d) Contusions on either side of the sylvian fissure. (e) Contusions of the right cerebellar hemisphere. (f) A horizontal slice through the cerebellum shows that the contusions extend into the cerebellar white matter.
Contusions may also involve the cerebellar hemispheres (Fig. 11.9e,f).
Lacerations develop when the severity of trauma has been sufficient to cause tearing of the pia. This may occur in association with a depressed skull fracture. The most severe pattern of cerebral laceration is embraced by the term ‘burst lobe’. This is often associated with a skull fracture and most commonly involves the frontal and temporal lobes, which show confluent intracerebral and subarachnoid bleeding (sometimes even extending into the subdural or extradural space), associated with massive disruption of the affected lobe (Fig. 11.10).
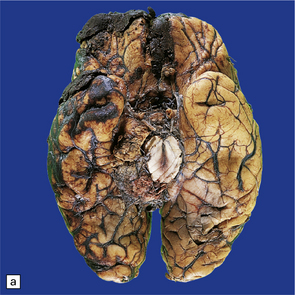
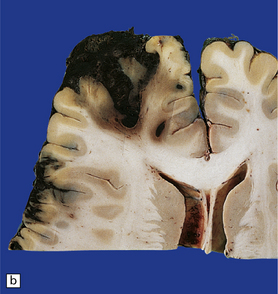
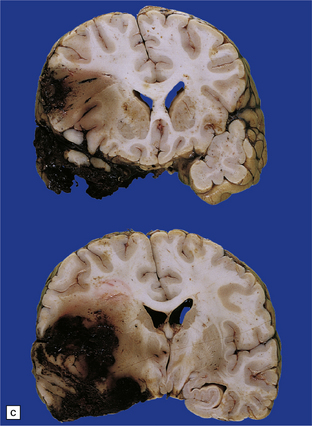
11.10 Severe contusion.
(a) Severe contusion of the right frontal and temporal poles with blood clot over the frontal pole suggesting cerebral laceration. (b) Sectioning confirms laceration of the frontal lobe associated with severe contusional damage and extension of bleeding into the subarachnoid and subdural spaces. (c) Another common site of ‘burst lobe’ is the temporal region (as shown here).
MICROSCOPIC APPEARANCES
The appearance of contusions evolves with time through several stages:
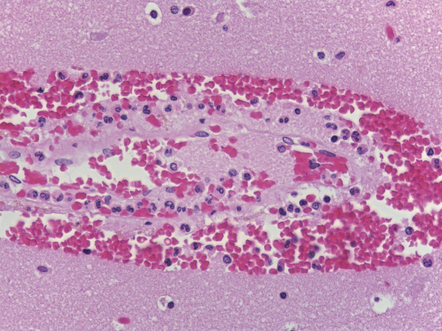
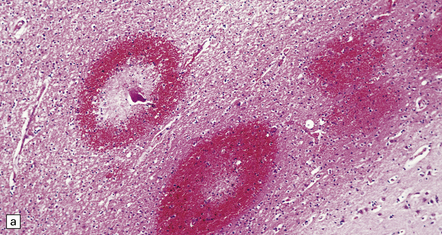
 Initially the contusion is visible as microscopic regions of perivascular hemorrhage (Fig. 11.11a) following the track of small vessels in the cortex and usually running perpendicular to the cortical surface. The hemorrhage occurs within seconds or minutes of the injury.
Initially the contusion is visible as microscopic regions of perivascular hemorrhage (Fig. 11.11a) following the track of small vessels in the cortex and usually running perpendicular to the cortical surface. The hemorrhage occurs within seconds or minutes of the injury.
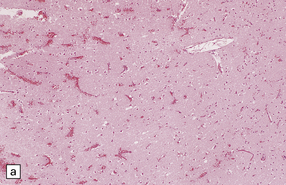
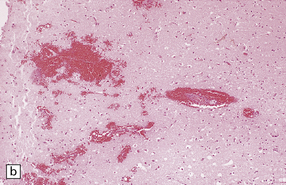
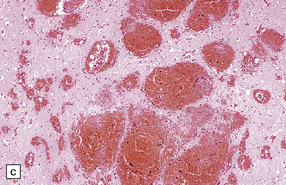
11.11 Microscopic appearance of early contusions.
(a) Early contusions with small perivascular hemorrhages. (b) Early contusions showing extravasation of blood into the cortex associated with acute degeneration of the surrounding neurons. (c) Later evolution leads to more extensive perivascular hemorrhages in the cortex and superficial white matter.
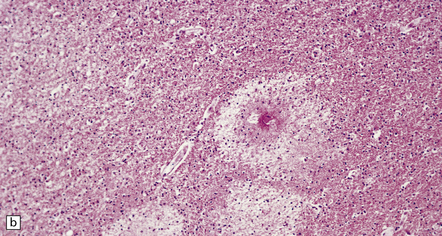
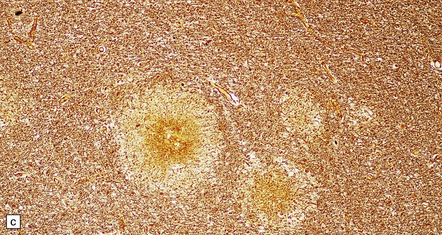
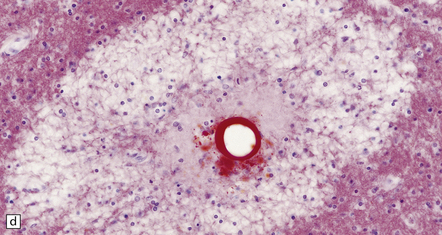
 Over several hours blood continues to seep into the adjacent cortex, which shows local swelling and confluent hemorrhage. If the contusion is severe there is extension into the underlying white matter. During this phase, neurons in the immediate vicinity begin to degenerate (Fig. 11.11b,c). Blood vessels within the contused tissue may show margination by neutrophils (Fig. 11.12), which later infiltrate the adjacent parenchyma.
Over several hours blood continues to seep into the adjacent cortex, which shows local swelling and confluent hemorrhage. If the contusion is severe there is extension into the underlying white matter. During this phase, neurons in the immediate vicinity begin to degenerate (Fig. 11.11b,c). Blood vessels within the contused tissue may show margination by neutrophils (Fig. 11.12), which later infiltrate the adjacent parenchyma.
 If there is survival, the damaged area undergoes progressive organization characterized by activation and proliferation of astrocytes and microglia, infiltration of blood-derived phagocytes, and removal of necrotic material.
If there is survival, the damaged area undergoes progressive organization characterized by activation and proliferation of astrocytes and microglia, infiltration of blood-derived phagocytes, and removal of necrotic material.
 What remains eventually is a superficial, roughly wedge-shaped, region of neuronal loss and glial scarring (Fig. 11.13). Hemosiderin pigment is found in scattered residual macrophages and astrocytes. The remaining neurons may become mineralized. The subjacent white matter is rarefied and gliotic.
What remains eventually is a superficial, roughly wedge-shaped, region of neuronal loss and glial scarring (Fig. 11.13). Hemosiderin pigment is found in scattered residual macrophages and astrocytes. The remaining neurons may become mineralized. The subjacent white matter is rarefied and gliotic.
INTRACRANIAL HEMORRHAGES
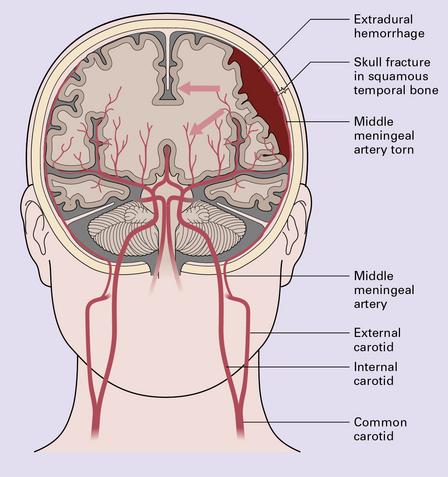
Extradural hematoma
Extradural hematomas are relatively localized and may accumulate slowly over a period of hours because of the adherence between the dura and the inner aspect of the calvarium (Fig. 11.14). They are evident macroscopically as a biconvex accumulation of clotted blood between the skull and the dura (Fig. 11.15).
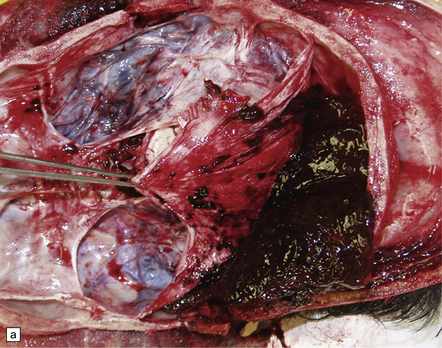
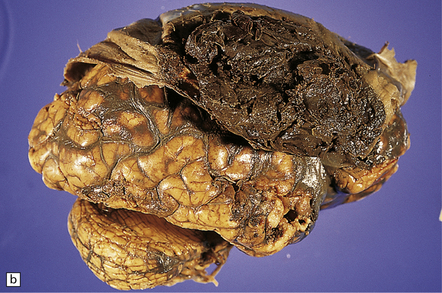
11.15 Extradural hematoma.
(a) Fracture of the right squamous temporal bone with an associated extradural hematoma. Extradural blood is visible through the dura in the left middle and anterior cranial fossae. (b) Extradural hematoma in right frontoparietal region. ((b) Courtesy of Professor Michael A. Farrell, Dublin, Ireland.)
Subdural hematoma
Subdural hematoma usually results from tearing of bridging veins that cross the subdural space, especially those related to the superior sagittal sinus. Blood from the ruptured vessels spreads freely through the subdural space and can envelop the entire hemisphere (Fig. 11.16).
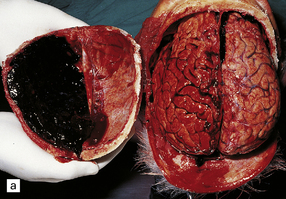
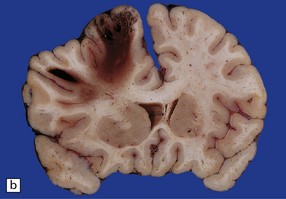
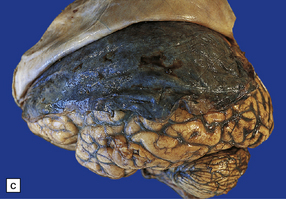
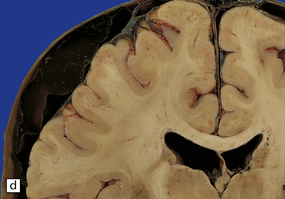
11.16 Subdural hematoma.
(a) Removal of the calvarium at necropsy reveals a left subdural hematoma that has compressed the underlying brain. The arachnoid surface of the left cerebral hemisphere is blood-stained and the gyri are flattened. (b) Indentation of the left cerebral hemisphere due to a subdural hematoma. Note the contusion, hemorrhage, and focal infarction in the underlying brain tissue. (c) Chronic subdural hematoma enclosed in a thin ‘membrane’ of granulation tissue. Although the hematoma was known to be more than 2 weeks old, it consisted of clotted blood. (d) Subdural hematoma showing compression of brain.
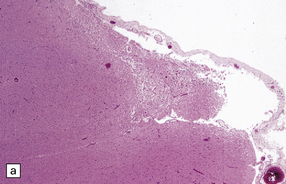
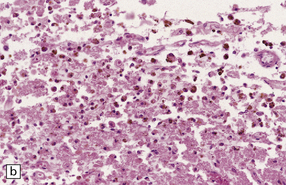
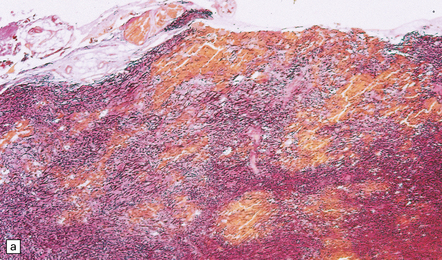
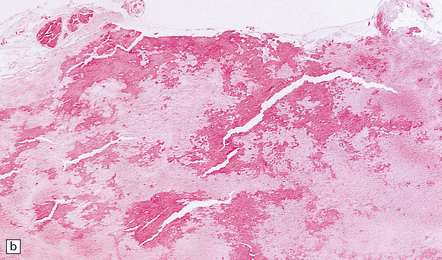
Chronic subdural hematomas become surrounded by a ‘membrane’ of organizing granulation tissue (Fig. 11.17). This is usually evident on the dural aspect of the hematoma within about 1 week, and later on its deep surface. Progressive enlargement of the hematoma may occur, mainly due to recurrent bleeding from friable blood vessels in the granulation tissue, although an osmotic effect of blood breakdown products may also contribute. Attempted dating of subdural hematomas by histologic examination of the ‘membranes’ has proven unreliable.
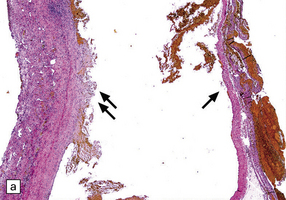
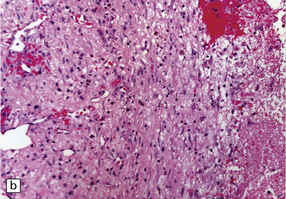
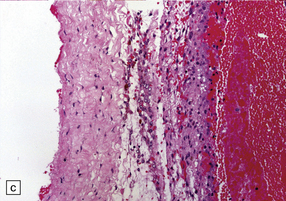
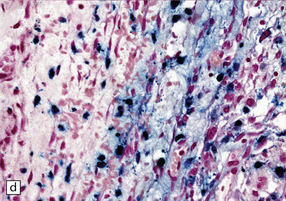
11.17 Chronic subdural hematoma.
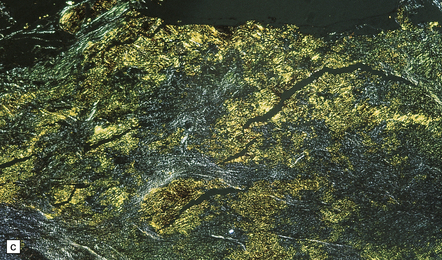
(a) This hematoxylin-van Gieson-stained section through a chronic subdural hematoma illustrates the differing thickness of the outer (twin arrows) and inner walls (single arrow). The collagen within the hematoma walls is stained red. Very little blood (stained orange) remains within the hematoma cavity, which macroscopic examination had shown to contain a mixture of loose blood clot and turbid fluid. (b) A higher-magnification view of the outer wall (here stained with H&E) shows it to consist of collagenous connective tissue within which are numerous thin-walled blood vessels, some with adjacent microscopic foci of hemorrhage. The tissue also includes scattered lymphocytes and hemosiderin-laden macrophages. (c) The outer wall is much thinner and less vascular. In this case there has also been more recent hemorrhage into the underlying subarachnoid space (to the right of the image). (d) The accumulation of hemosiderin pigment in the outer membrane is well demonstrated in a section stained by Perls’ method.
Traumatic subarachnoid hemorrhage
There are several possible sources of subarachnoid bleeding:
 Subarachnoid blood may derive from severe contusions and lacerations (Fig. 11.18, see also Figs 11.5, 11.10); the hemorrhage is usually localized to the region of cortical damage.
Subarachnoid blood may derive from severe contusions and lacerations (Fig. 11.18, see also Figs 11.5, 11.10); the hemorrhage is usually localized to the region of cortical damage.
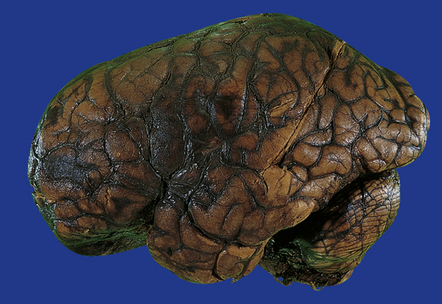
11.18 Traumatic subarachnoid hemorrhage.
Blood is present over the cerebral convexity and in the sylvian fissure.
 Fractures of the skull base can tear large vessels at the base of the brain.
Fractures of the skull base can tear large vessels at the base of the brain.
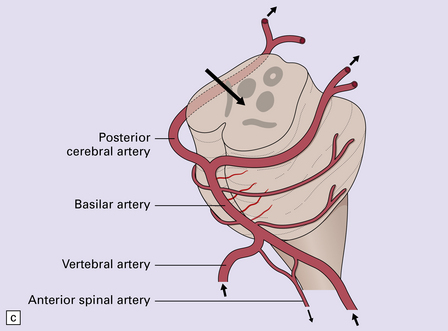
 Even in the absence of basal fractures, cranial trauma occasionally causes rupture or dissection of the vertebral arteries, resulting in arterial occlusion or subarachnoid hemorrhage; the tear usually originates near where the arteries pass through the dura into the cranial cavity (Fig. 11.19). More rarely, subarachnoid hemorrhage results from a tear in the intraosseous part of the vertebral arteries within the cervical spine (Fig. 11.20).
Even in the absence of basal fractures, cranial trauma occasionally causes rupture or dissection of the vertebral arteries, resulting in arterial occlusion or subarachnoid hemorrhage; the tear usually originates near where the arteries pass through the dura into the cranial cavity (Fig. 11.19). More rarely, subarachnoid hemorrhage results from a tear in the intraosseous part of the vertebral arteries within the cervical spine (Fig. 11.20).
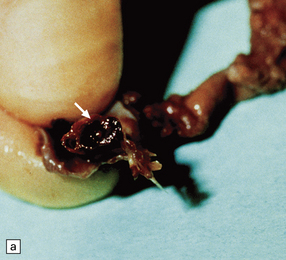
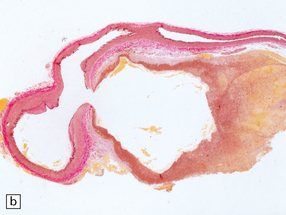
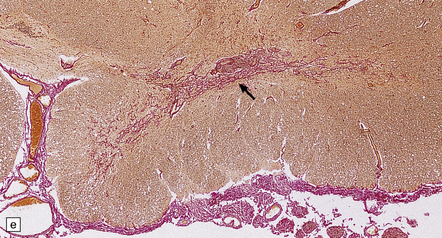
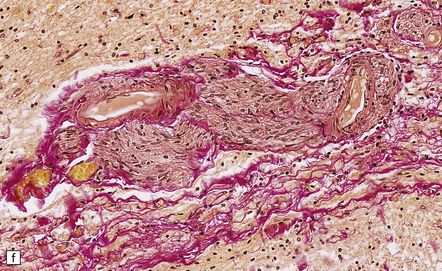
11.19 Traumatic rupture of the vertebral artery in the region of entry into the cranial cavity.
(a) A pseudoaneurysm (arrow) is present at the site of vertebral artery rupture after head injury. The patient presented with delayed subarachnoid hemorrhage several days later. (b) The pseudoaneurysm is clearly visible in an elastic/van Gieson-stained section. (Courtesy of Dr Hugh White, North Bristol NHS Trust, UK.)
11.20 Subarachnoid hemorrhage originating from a tear in the vertebral artery.
Note the basal distribution of blood clot in the subarachnoid space.
 Blood from intraventricular hemorrhage enters the subarachnoid space through the exit foramina of the fourth ventricle.
Blood from intraventricular hemorrhage enters the subarachnoid space through the exit foramina of the fourth ventricle.
Cerebral and cerebellar hematomas
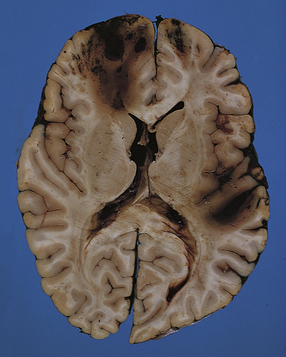
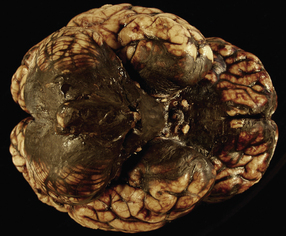
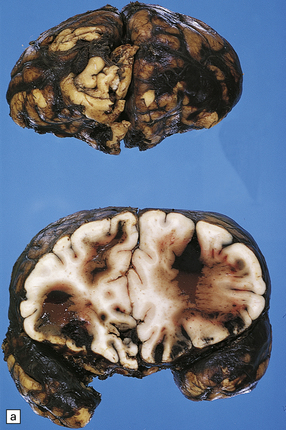
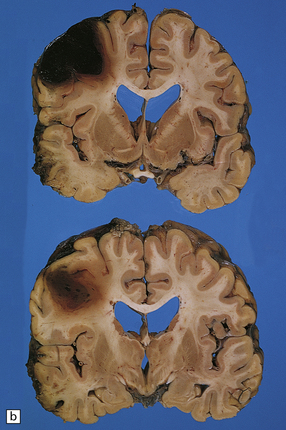
Superficial lobar hematomas are generally related to overlying contusional damage and are mainly seen in the frontal and temporal lobes (Fig. 11.21). They also occur in association with contusions in the cerebellum (Fig. 11.22). Deep hematomas tend to be related to the basal ganglia and thalamus (Fig. 11.23, see also Fig. 11.30a) and are often associated with diffuse axonal injury.
11.21 Cerebral hematomas.
(a) Hematomas in the white matter of the frontal lobe associated with severe contusions. (b) Superficial lobar hematoma in the posterior frontal region. The hematoma was related to a cortical laceration associated with a depressed skull fracture.
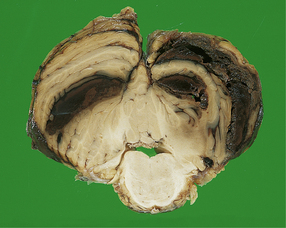
11.22 Cerebellar hematoma.
Hematomas in the cerebellum are usually associated with severe contusional damage to the cerebellar hemispheres, which is often related to a skull fracture in the posterior fossa.
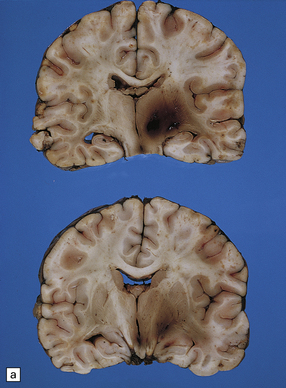
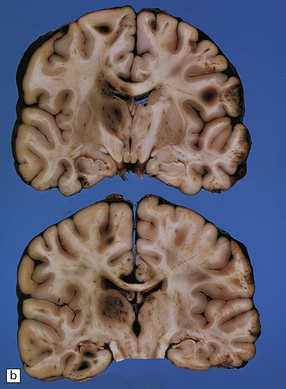
11.23 Hematomas of the basal ganglia and thalamus.
(a) Traumatic hematoma in the right basal ganglia associated with mass effect and compression of the ventricular system. The appearance resembles that of a primary intracerebral bleed. Histologic examination revealed diffuse axonal injury in a wide distribution. (b) A cluster of hematomas in the left thalamus caused by head injury. There is other damage in the form of cortical contusions, and a small hematoma in the hippocampus. A hemorrhagic lesion in the corpus callosum is indicative of diffuse axonal injury.
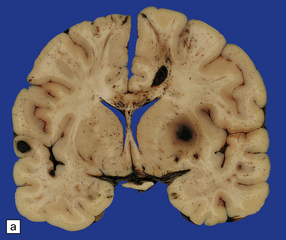
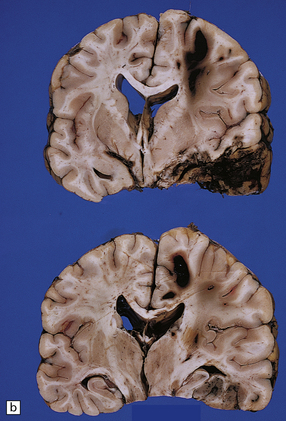
11.30 Gliding contusions.
(a) Bilateral gliding contusions in the parasagittal white matter. The one on the left is no more than a perivascular streak of hemorrhagic white matter discoloration. The one on the right includes several hemorrhagic foci and a larger hematoma. There are also typical DAI-related lesions in the corpus callosum, contusions of both unci, and several other hematomas, including one in the right putamen. (b) Large lesions extending through the parasagittal white matter towards the corpus callosum. In this case there is also severe contusion of the temporal lobe and petechial hemorrhages are present in the corpus callosum in the lower slice.
UNCOMMON TYPES OF FOCAL BRAIN DAMAGE
Uncommon types of focal brain damage include:
 Ischemic brain damage due to traumatic arterial dissection and thrombosis resulting from stretching of the vertebral or carotid arteries by hyperextension of the neck (Fig. 11.24).
Ischemic brain damage due to traumatic arterial dissection and thrombosis resulting from stretching of the vertebral or carotid arteries by hyperextension of the neck (Fig. 11.24).
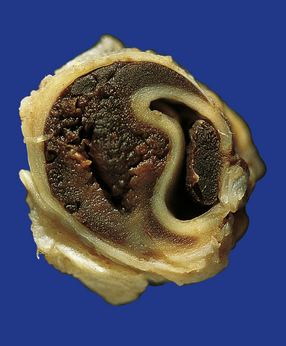
11.24 Traumatic arterial dissection.
Traumatic dissection and associated thrombosis of the vertebral artery in a patient who developed brain stem infarction after head injury.
 Infarction of the pituitary gland due to traumatic transection of the pituitary stalk or complicating severely raised intracranial pressure.
Infarction of the pituitary gland due to traumatic transection of the pituitary stalk or complicating severely raised intracranial pressure.
 A pontomedullary rent resulting from severe injury associated with hyperextension of the neck (Fig. 11.25).
A pontomedullary rent resulting from severe injury associated with hyperextension of the neck (Fig. 11.25).
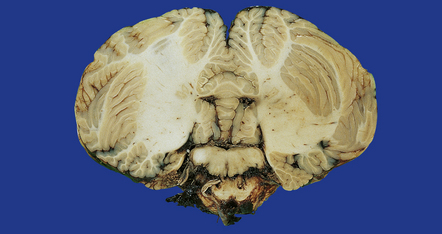
11.25 Pontomedullary rent.
Tearing of the brain stem at the junction between the upper medulla and lower pons has produced a hemorrhagic lesion termed a pontomedullary rent. In this case the injury resulted from a high-speed motor-cycle collision in which the patient suffered a hyperextension injury to the neck. At necropsy there was a large quantity of subarachnoid blood in the posterior fossa, but no clear site of origin of the hemorrhage. Once the brain had been fixed and sliced it became clear that the site of bleeding had been this injury to the brain stem.
 Cranial nerve avulsion (most commonly the olfactory nerve fibers, the optic nerve, the facial nerve, or the auditory nerve).
Cranial nerve avulsion (most commonly the olfactory nerve fibers, the optic nerve, the facial nerve, or the auditory nerve).
INFECTION
Infection is predominantly a complication of skull fractures:
 Fractures of the calvarium or skull base can provide a route for bacteria to pass from the major air sinuses into the subarachnoid space and cause meningitis. Such fractures are often associated with a leakage of CSF or the formation of an aerocele.
Fractures of the calvarium or skull base can provide a route for bacteria to pass from the major air sinuses into the subarachnoid space and cause meningitis. Such fractures are often associated with a leakage of CSF or the formation of an aerocele.
 Open head injuries predispose to the development of brain abscesses, particularly if the brain is lacerated or sustains a missile injury.
Open head injuries predispose to the development of brain abscesses, particularly if the brain is lacerated or sustains a missile injury.
The incidence of brain abscesses is, however, increased even after closed head injuries, presumably because the devitalized tissues are prone to colonization in the event of a transient bacteremia (see Chapter 15).
DIFFUSE DAMAGE
TAI is the term given to axonal damage that is directly attributable to trauma, usually involving acceleration and deceleration of the head. There is a spectrum of severity from the mildest, subclinical TAI, involving only a few, scattered axons, to the widespread axonal damage that is seen in diffuse axonal injury (DAI) (Table 11.3). Patients who have sustained DAI are typically unconscious from the moment of impact, do not experience a lucid interval, and remain unconscious, vegetative, or at least severely disabled until death. Lesser degrees of TAI are compatible with recovery of consciousness, with or without persisting neurologic deficits of varying severity.
Table 11.3
Traumatic axonal injury – terminology
| Axonal injury | A non-specific term referring to damage to axons of any etiology |
| Traumatic axonal injury (TAI) | Damage to axons caused by trauma. This may vary from small foci of axonal injury to more widespread brain damage |
| Diffuse TAI | Originally termed ‘DAI’, this is the most severe form of traumatic axonal damage |
| Diffuse axonal injury (DAI) | First described as a clinicopathologic syndrome in which there is widespread traumatic axonal damage throughout the brain, including the brain stem. However, because axonal injury may be caused by other pathological processes, the etiology of the axonal damage should always be indicated when the term DAI is used as a neuropathologic diagnosis |
From: Geddes JF, Whitwell HL, Graham DI. Traumatic axonal injury: practical issues for diagnosis in medicolegal cases. Neuropathol Appl Neurobiol 2000; 26:105–116.
MACROSCOPIC APPEARANCES
 Focal lesions in the corpus callosum, which appear as clusters of petechial hemorrhages or soft hemorrhagic foci (Fig. 11.28). The lesions may disrupt the interventricular septum, in which case there is often associated intraventricular hemorrhage.
Focal lesions in the corpus callosum, which appear as clusters of petechial hemorrhages or soft hemorrhagic foci (Fig. 11.28). The lesions may disrupt the interventricular septum, in which case there is often associated intraventricular hemorrhage.
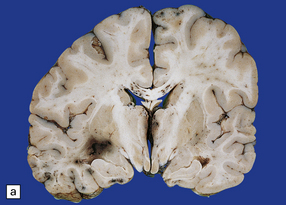
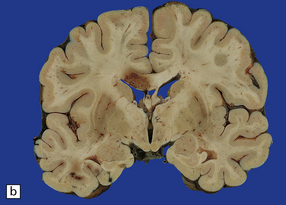
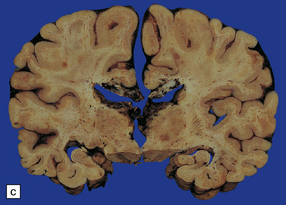
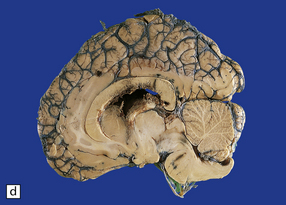
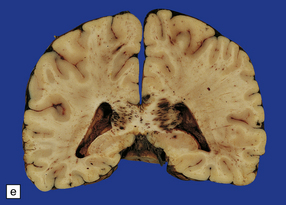
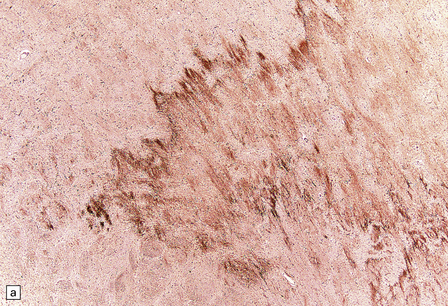
11.28 Lesions in the corpus callosum in DAI.
These may vary from small petechiae to larger foci of hemorrhagic discoloration and softening and extensive hemorrhagic disruption. (a) Small petechiae. (b) Larger focus of hemorrhagic discoloration and softening. (c) Extensive hemorrhagic disruption. (d) In the sagittal plane lesions typically involve the corpus callosum for several centimeters along its length. (e) The lesions may occur anywhere along the corpus callosum, but there is a tendency for them to be concentrated in the splenium.
 Focal lesions in the dorsolateral quadrants of the rostral brain stem. Small lesions appear as discrete foci of petechial hemorrhage in and adjacent to the superior cerebellar peduncles. More severe damage results in hemorrhagic softening of the dorsal part of the midbrain and rostral pons (Fig. 11.29).
Focal lesions in the dorsolateral quadrants of the rostral brain stem. Small lesions appear as discrete foci of petechial hemorrhage in and adjacent to the superior cerebellar peduncles. More severe damage results in hemorrhagic softening of the dorsal part of the midbrain and rostral pons (Fig. 11.29).


11.29 Brain stem lesions associated with DAI.
These consist of clusters of petechial hemorrhages or foci of hemorrhagic softening in the dorsolateral part of the midbrain and dorsolateral part of the rostral pons. (a) The right dorsolateral part of the midbrain contains petechiae. (b) The dorsolateral part of the rostral pons includes petechiae bilaterally.
 ‘Gliding contusions,’ which are hemorrhagic lesions affecting the parasagittal white matter in the superior part of the cerebral hemispheres. Small lesions occur close to the junction between the white matter and the overlying cortex, but larger lesions curve downwards through the parasagittal white matter towards the corpus callosum (Fig. 11.30). Gliding contusions are often bilateral, but are not usually symmetric.
‘Gliding contusions,’ which are hemorrhagic lesions affecting the parasagittal white matter in the superior part of the cerebral hemispheres. Small lesions occur close to the junction between the white matter and the overlying cortex, but larger lesions curve downwards through the parasagittal white matter towards the corpus callosum (Fig. 11.30). Gliding contusions are often bilateral, but are not usually symmetric.
The brains of long-term survivors of DAI typically show moderate to marked cerebral atrophy with dilatation of the lateral and third ventricles, thinning of the corpus callosum (sometimes marked), ill-defined gray discoloration of the cerebral white matter, and, in some cases, atrophy of the cerebral peduncles, base of the pons, and medullary pyramids (Fig. 11.31). In the absence of other injuries such as cerebral contusions the cortical ribbon usually appears normal.
MICROSCOPIC APPEARANCES
Histology is needed to substantiate the diffuse damage to axons. The main regions affected are:
 superior parasagittal cerebral white matter
superior parasagittal cerebral white matter
 subcortical fiber tracts (fornix, internal and external capsules)
subcortical fiber tracts (fornix, internal and external capsules)
 brain stem (corticospinal tracts, medial lemnisci, medial longitudinal bundles, central tegmental tracts).
brain stem (corticospinal tracts, medial lemnisci, medial longitudinal bundles, central tegmental tracts).
The time course of histologic changes is as follows:
 Within about 2–4 hours there are focal axonal accumulations of β-amyloid precursor protein (APP) and other anterogradely transported proteins. These can be identified immunohistochemically at a time when conventional tinctorial stains do not show definite abnormalities (Fig. 11.32).
Within about 2–4 hours there are focal axonal accumulations of β-amyloid precursor protein (APP) and other anterogradely transported proteins. These can be identified immunohistochemically at a time when conventional tinctorial stains do not show definite abnormalities (Fig. 11.32).
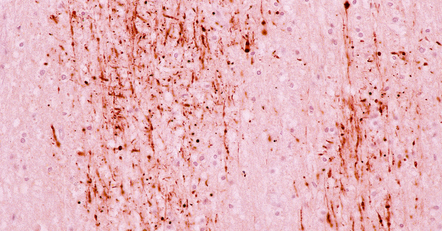
11.32 Clusters of axons with abnormal accumulation of APP early after head injury.
A few axons show focal swelling, but this is still mild and many of the axons appear to be intact.
 By 12–24 hours axonal varicosities may be evident on conventional histologic examination (Fig. 11.33).
By 12–24 hours axonal varicosities may be evident on conventional histologic examination (Fig. 11.33).
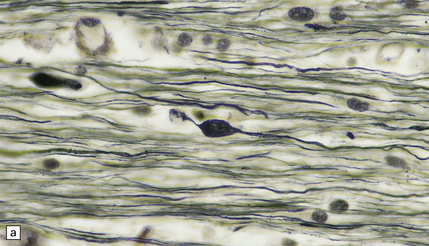
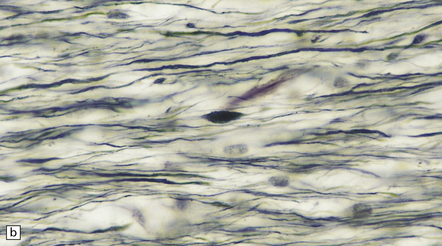
11.33 Microscopic changes 12–24 hours after head injury with DAI.
(a) and (b) Silver impregnation may reveal axonal varicosities, as shown in these illustrations.
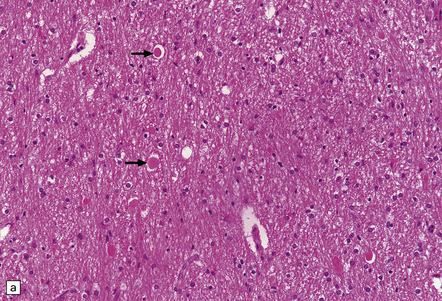
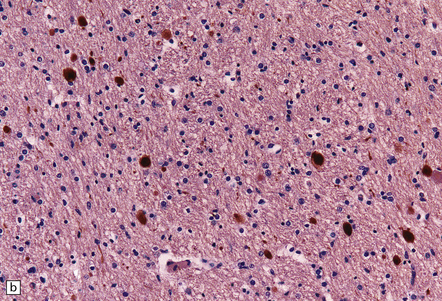
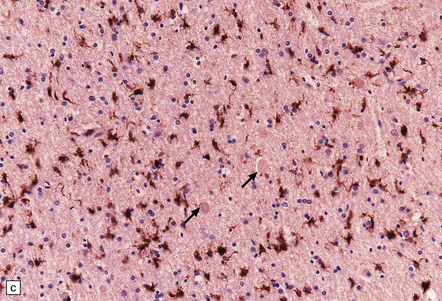
 From 24 hours to 2 months after the injury conventional histology reveals axonal swellings. These are eosinophilic (Fig. 11.34a,c), but are best demonstrated by silver impregnation techniques (Fig. 11.34b,d) or immunohistochemistry for APP (Fig. 11.35, see also Fig. 11.26). Axonal swellings can also be detected by immunohistochemistry for ubiquitin, APP, or neurofilament protein, but these methods are less sensitive at later times.
From 24 hours to 2 months after the injury conventional histology reveals axonal swellings. These are eosinophilic (Fig. 11.34a,c), but are best demonstrated by silver impregnation techniques (Fig. 11.34b,d) or immunohistochemistry for APP (Fig. 11.35, see also Fig. 11.26). Axonal swellings can also be detected by immunohistochemistry for ubiquitin, APP, or neurofilament protein, but these methods are less sensitive at later times.
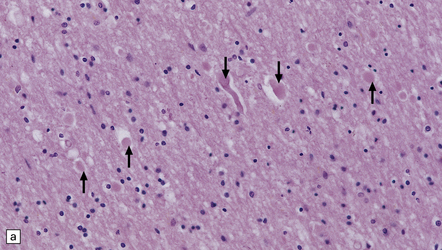
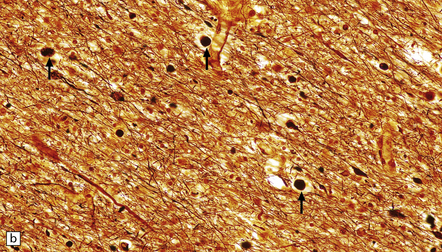
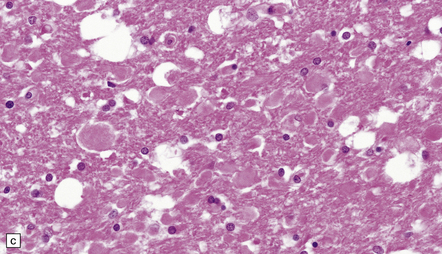
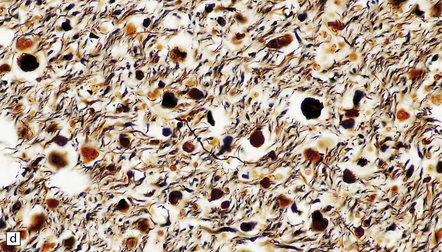
11.34 Microscopic changes after head injury with DAI.
In sections stained with hematoxylin and eosin, the axonal swellings are generally small and inconspicuous, but they are well visualized by silver impregnation. (a) Hematoxylin and eosin section. The axonal swellings (arrows) are small and inconspicuous. (b) Silver impregnation highlights the axonal swellings (arrows). (c) The axonal swellings may be obvious in a hematoxylin and eosin section in severe DAI, particularly if the patient survives for several days as here. (d) Palmgren silver impregnation of section adjacent to (c) showing large axonal swellings.
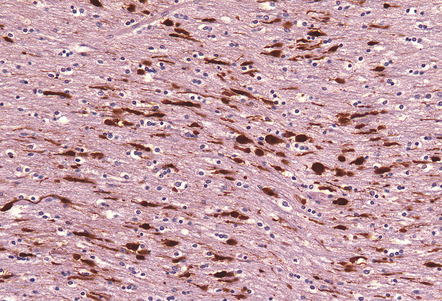
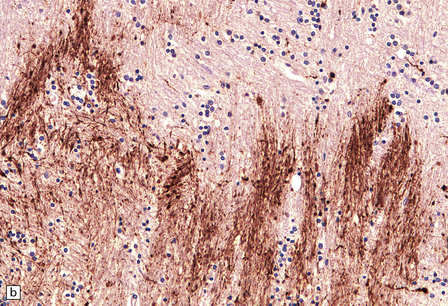
11.35 Demonstration of traumatic axonal injury by immunostaining for APP.
In this case the patient had DAI, the pathologic manifestations of which included the presence of numerous APP-immunopositive axons and axonal swellings in the corpus callosum, as illustrated.
 Two weeks to 5 months after the injury clusters of microglia are seen in the affected regions (Fig. 11.36).
Two weeks to 5 months after the injury clusters of microglia are seen in the affected regions (Fig. 11.36).
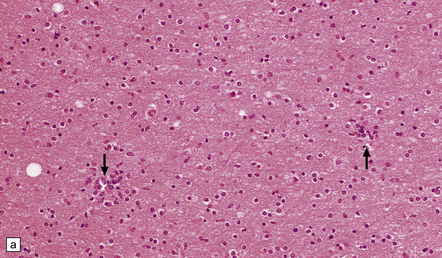
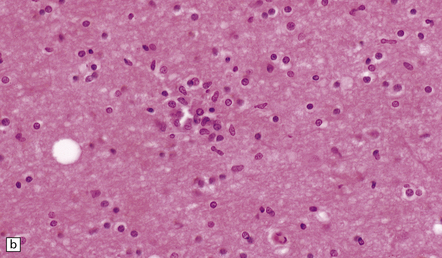
11.36 Clustering of microglial cells after head injury with DAI. From about 2 weeks after the injury and persisting for many months, the white matter contains numerous clusters of microglial cells.
(a) Clustering of cells, seen at low magnification (arrows). This is particularly well demonstrated in thick sections cut at 20 μm, as here. (b) At higher magnification, the clusters are seen to consist of microglia.
 From 2 months to years after the injury wallerian degeneration leads to loss of myelinated fibers (Fig. 11.37).
From 2 months to years after the injury wallerian degeneration leads to loss of myelinated fibers (Fig. 11.37).
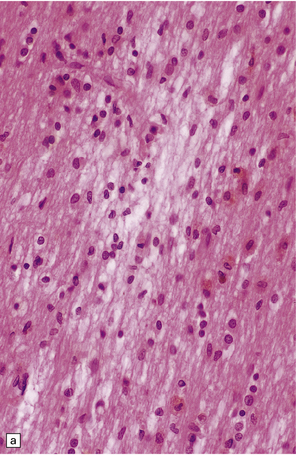
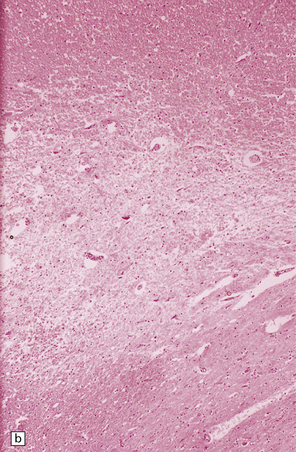
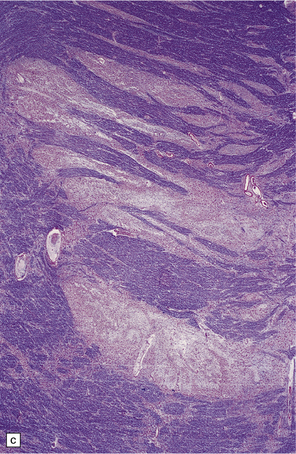
11.37 Long-term microscopic appearance of head injury with DAI.
(a) Focal glial scarring in a long-term survivor of head injury with DAI. (b) Focal rarefaction of the white matter in a long-term survivor of head injury with DAI. (c) Patchy depletion of corticospinal fibers in the pons of a patient who survived several months with severe DAI.
Grading of DAI is shown in Table 11.4.
Table 11.4

From: Adams JH, Doyle D, Ford I, et al. Diffuse axonal injury in head injury: definitions, diagnosis and grading. Histopathology 1989; 15:49–59.
DIFFUSE VASCULAR INJURY
In a small proportion of patients who die within minutes after head injury, the only discernible structural abnormalities are petechial hemorrhages, mostly in the frontal and temporal white matter, diencephalon and brainstem (Fig. 11.40). This diffuse vascular damage probably results from acute deformation, stretching and tearing of small blood vessels.
BRAIN SWELLING AND RAISED INTRACRANIAL PRESSURE
 cerebral vasodilatation and an increase in cerebral blood volume (i.e. congestive brain swelling)
cerebral vasodilatation and an increase in cerebral blood volume (i.e. congestive brain swelling)
 damage to blood vessels and extravasation of edema fluid through the defective blood–brain barrier (i.e. vasogenic edema)
damage to blood vessels and extravasation of edema fluid through the defective blood–brain barrier (i.e. vasogenic edema)
 an increase in the water content of neurons and glia (i.e. cytotoxic cerebral edema)
an increase in the water content of neurons and glia (i.e. cytotoxic cerebral edema)
Three patterns of brain swelling are encountered in patients who have sustained a head injury:
 Swelling adjacent to contusions. Physical disruption of blood vessels in the surrounding brain tissue results in increased capillary permeability and a loss of normal blood flow regulation at the arteriolar level.
Swelling adjacent to contusions. Physical disruption of blood vessels in the surrounding brain tissue results in increased capillary permeability and a loss of normal blood flow regulation at the arteriolar level.
 Diffuse swelling of one cerebral hemisphere. This is especially common after evacuation of an ipsilateral subdural hematoma (Fig. 11.43), which may also be followed by delayed intracerebral hemorrhage, probably due to reperfusion of infarcted tissue.
Diffuse swelling of one cerebral hemisphere. This is especially common after evacuation of an ipsilateral subdural hematoma (Fig. 11.43), which may also be followed by delayed intracerebral hemorrhage, probably due to reperfusion of infarcted tissue.
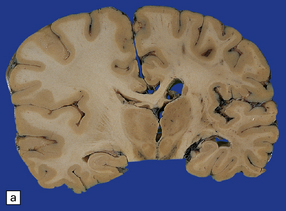
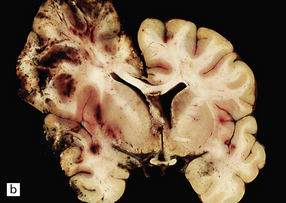
11.43 Brain swelling after evacuation of subdural hematoma.
(a) Swelling of the left cerebral hemisphere after evacuation of an ipsilateral subdural hematoma. (b) Brain swelling during evacuation of a subdural hematoma has led to external herniation of brain through the craniotomy, and focal parenchymal hemorrhage and infarction.
 Diffuse swelling of both cerebral hemispheres (Fig. 11.44). Children and adolescents are particularly susceptible and may develop brain swelling after relatively minor head injury. The swelling is thought to result from a global increase in brain blood volume. It may be associated with epileptic activity and even status epilepticus. In children the onset of swelling may occasionally be delayed for several hours.
Diffuse swelling of both cerebral hemispheres (Fig. 11.44). Children and adolescents are particularly susceptible and may develop brain swelling after relatively minor head injury. The swelling is thought to result from a global increase in brain blood volume. It may be associated with epileptic activity and even status epilepticus. In children the onset of swelling may occasionally be delayed for several hours.
HERNIATION
The cranial cavity is subdivided by the relatively rigid tentorium and falx cerebri into three compartments with limited capacity to accommodate accumulations of blood or swelling due to edema without an increase in pressure. Differences in pressure between two adjacent intracranial compartments, or between an intracranial compartment and the spinal canal, cause displacement of the soft substance of the brain into the lower-pressure compartment (i.e. internal herniation). There are three sites where this tends to occur, resulting in subfalcine, tentorial, or tonsillar herniation (Figs 11.45–11.48). Tentorial herniation may involve the medial part of the temporal lobe (the uncus and sometimes the parahippocampal gyrus), or diencephalic structures (the inferior part of the hypothalamus and thalamus); the latter type of tentorial herniation is also known as diencephalic or central transtentorial herniation. Raised intracranial pressure can also lead to external herniation of brain tissue through a skull fracture or craniotomy.
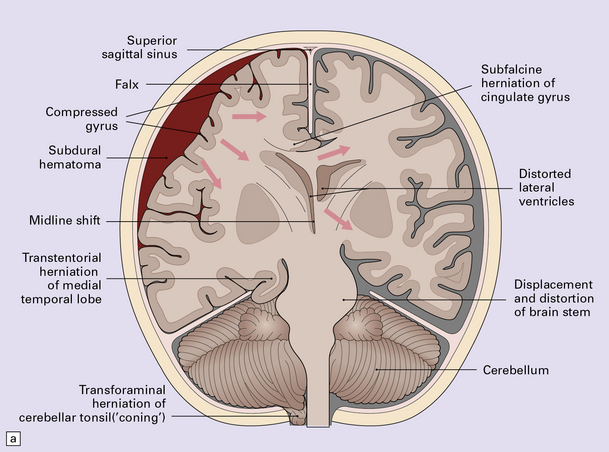
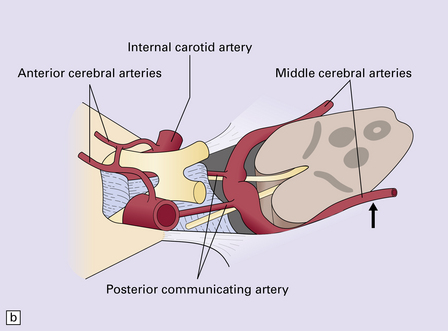
11.45 Internal brain herniation.
(a) Space occupancy by an expanding lesion causes herniation at several sites. (b) Transtentorial herniation of the medial part of the temporal lobe may compress the posterior cerebral artery where it crosses the free edge of the tentorium and cause infarction (usually hemorrhagic) in the corresponding perfusion territory. (c) Downward displacement of the brain stem causes deformity and traction of perforating branches (shown in light red) of the basilar artery and this may result in foci of infarction and hemorrhage (Duret hemorrhages).
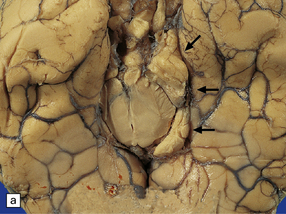
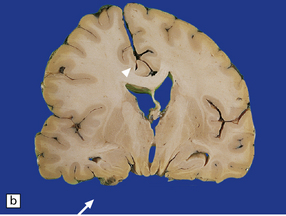
11.46 Transtentorial herniation.
(a) Herniation of the parahippocampal gyrus through the tentorial hiatus. The free edge of the tentorium cerebelli has indented the cerebrum (arrows) along the margin of the herniated brain tissue. (b) Herniation contusions (arrow) may be visible where the uncus or parahippocampal gyrus has been pressed against the edge of the tentorium, in this case by a subdural hematoma (not visible in figure). Histologic examination of the contusions reveals focal necrosis and small hemorrhages. The subdural hematoma has also displaced midline structures to the right, caused subfalcine herniation of the left cingulate gyrus (arrowhead) and downward displacement of the floor of the third ventricle (central transtentorial herniation).
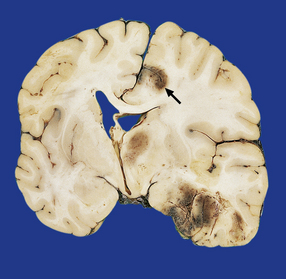
11.47 Cerebral herniation.
In this case, contusional damage and hemispheric swelling have caused subfalcine herniation of the right cingulate gyrus. This has resulted in adjacent hemorrhagic infarction (arrow). Note also the shift of midline structures and herniation of the right uncus.

11.48 Tonsillar herniation and necrosis.
In this case head injury has resulted in severe tonsillar herniation and necrosis. The medulla is partly ensheathed by the necrotic tonsillar tissue.
Internal herniation may result in compression or stretching of blood vessels, leading to secondary ischemic damage (Figs 11.49, 11.50). It is important to recognize the secondary nature of these lesions and not attribute any bleeding to primary hemorrhage. Compression of the oculomotor nerves by downwards displacement of the posterior cerebral arteries can cause focal nerve contusion.
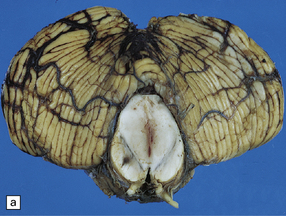
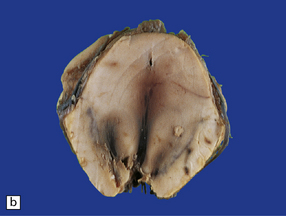
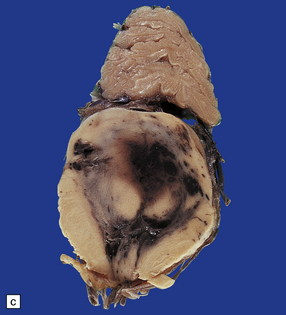
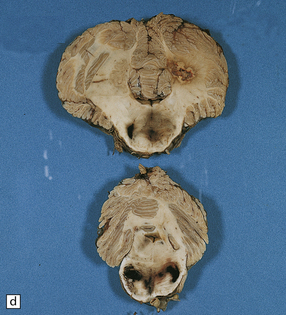
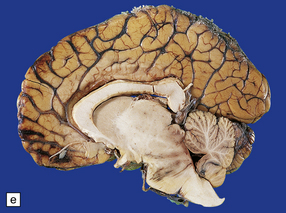
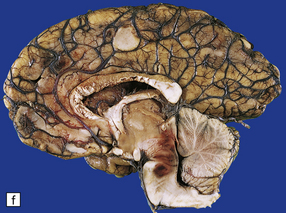
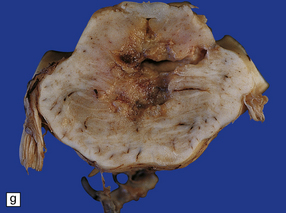
11.49 Effects of internal herniation.
With severe persisting herniation there is downwards displacement of diencephalic structures and descent of the pons, resulting in anteroposterior kinking or buckling of the brain stem with traction on the extramedullary segments of penetrating arteries and compression of their intramedullary segments. This results in foci of necrosis, and hemorrhages termed Duret hemorrhages, in the midbrain and upper pons. (a) Small Duret hemorrhage appearing as a linear area of midline hemorrhage. (b) A more severe lesion associated with hemorrhage in the midline and substantia nigra. (c) Duret hemorrhages appearing as extensive foci of hemorrhagic necrosis. (d) Marked hemorrhage and necrosis in the pons. (e) and (f) Sagittal sections, which demonstrate the buckling of the upper brain stem and the distribution of hemorrhagic lesions. (e) Early Duret hemorrhages. (f) Later, more extensive Duret hemorrhages. (g) Although internal herniation and brain stem hemorrhage are usually terminal events, rarely survival is long term, in which case the lesions in the midbrain and pons undergo organization and appear as soft cystic areas of cavitation.
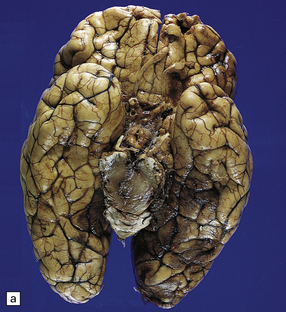
11.50 Posterior cerebral infarction due to herniation of the parahippocampal gyrus.
During herniation of the parahippocampal gyrus the posterior cerebral artery is compressed against the free edge of the tentorium. This can cause occipital lobe infarction, in some cases largely confined to the primary visual cortex in the calcarine fissure, but much more extensive in others. (a) Extensive infarction of inferomedial part of the left occipital lobe and a small region of the right occipital lobe. (b) Bilateral inferomedial occipital and adjacent temporal infarction. (c) Cut surface of brain showing medial occipital infarction.
Focal contusion or hemorrhagic infarction of the hippocampus or parahippocampal gyrus may result from compression of these structures against the edge of the tentorium during transtentorial herniation (Figs 11.46, 11.47). Similar damage may result from subfalcine herniation of the cingulate gyrus (Fig. 11.47). Transtentorial herniation also produces distortion of the midbrain and compression of the cerebral peduncles (Fig. 11.51). In some cases this causes necrosis of the contralateral cerebral peduncle due to its compression against the edge of the tentorium or of the ipsilateral cerebral peduncle due to its compression by the herniated uncus.
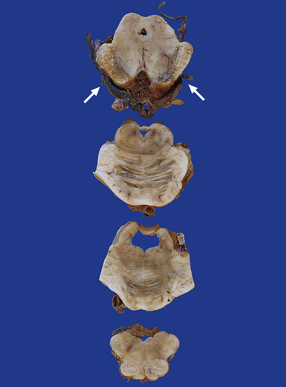
11.51 Long-term damage to brain stem after transtentorial herniation.
Sections through the brain stem of a patient who survived several months after head injury with bilateral uncal herniation. There is bilateral necrosis of the cerebral peduncles (arrows), and associated gray discoloration and focal cavitation of the descending fiber tracts in the base of the pons and medullary pyramids.
ISCHEMIC DAMAGE
 hypotension with a systolic blood pressure less than 80 mmHg for at least 15 minutes
hypotension with a systolic blood pressure less than 80 mmHg for at least 15 minutes
 episodes of raised intracranial pressure (i.e. over 30 mmHg).
episodes of raised intracranial pressure (i.e. over 30 mmHg).
Evidence of hypoxic–ischemic damage may be confined to the hippocampus (Fig. 11.52) or associated with more widespread changes in the cerebral cortex (Fig. 11.53) and deep gray matter. Cortical damage is often accentuated in the border zones between the major cerebral arterial territories, particularly between the anterior and middle cerebral arteries (Fig. 11.54), but may be diffuse. The damage is bilateral in most cases. Infarction may be restricted to the territory supplied by a single artery, but this is comparatively rare.
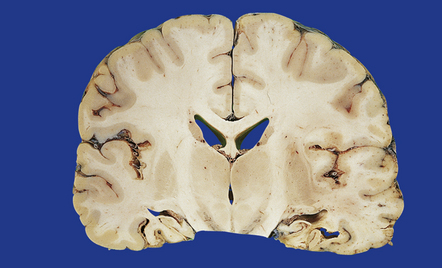
11.52 Ischemic brain damage.
Bilateral hippocampal necrosis caused by ischemic damage resulting from head injury.
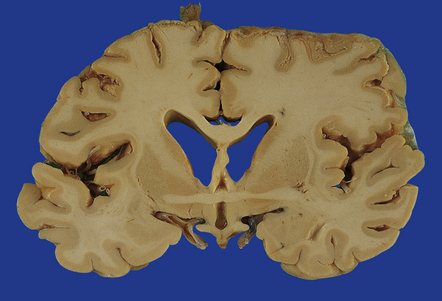
11.53 Ischemic damage caused by head injury.
Appearance of bilateral cortical necrosis in the perfusion territories of the middle cerebral arteries after survival of several weeks.
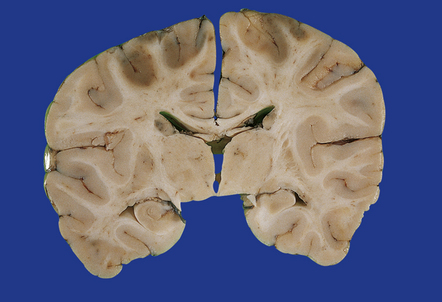
11.54 Ischemic damage caused by head injury.
Acute boundary zone infarction in the watershed between the anterior and middle cerebral arterial territories bilaterally.
Internal herniation may be complicated by secondary infarction, especially of the occipital lobes (Fig. 11.50) and upper brain stem (Fig. 11.49).
MISSILE HEAD INJURY
In perforating injuries, the missile (usually a bullet) enters and exits the skull, in most cases passing through the brain (Figs 11.55–11.57).
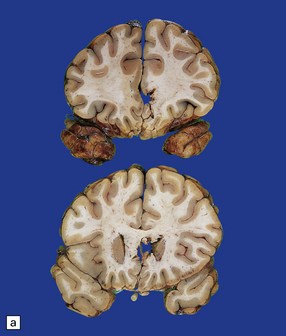
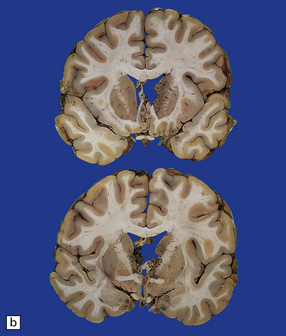
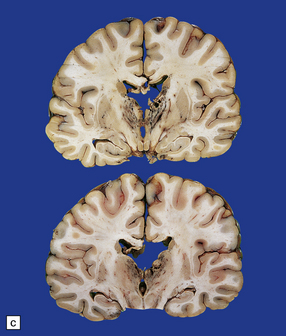
11.55 Penetrating injury due to bullet from a small-bore handgun.
Note that the slices are viewed from the front. The entry wound was in the forehead in the midline. The exit wound was in the occipital region of the skull. (a) The missile has passed through the corpus callosum and head of caudate nucleus on the left. (b) There is hemorrhagic disruption of the caudate nucleus and interventricular septum. (c) There is also bilateral thalamic damage.
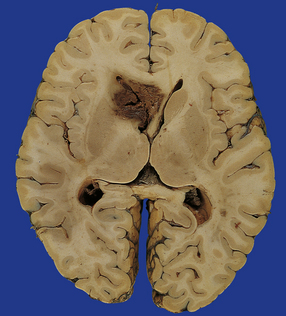
11.56 Suicide resulting from discharge of a small-bore handgun in the mouth.
The bullet passed through the nasopharynx and base of skull and produced a hemorrhagic lesion in the caudate nucleus and corpus callosum.
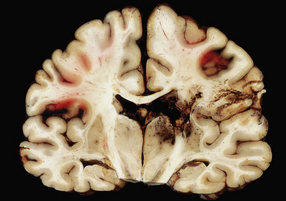
11.57 Penetrating injury due to bullet from a small-bore handgun.
The bullet entered the skull in the right temporoparietal region, traversed both cerebral hemispheres, and exited through the left posterior temporal region of the skull. This brain slice shows the hemorrhagic disruption caused by the bullet in the right parietal cortex and white matter, the posterior part of the right putamen, internal capsule and thalamus.
 Low-velocity missiles are more likely to fragment or ricochet within the skull, producing a penetrating rather than a perforating injury, and to drive fragments of cloth, bone, and scalp into the brain.
Low-velocity missiles are more likely to fragment or ricochet within the skull, producing a penetrating rather than a perforating injury, and to drive fragments of cloth, bone, and scalp into the brain.
 High-velocity bullets can pass through the skull without knocking the victim down or causing an immediate loss of consciousness. However, the brain damage is often severe. Exit wounds are generally larger than entry wounds. The shock wave produced by high-velocity missiles tends to cause severe brain injury that extends well away from the missile track and includes remote contusions of the frontal and temporal poles and the undersurface of the cerebellum, and contrecoup fractures of the orbital plates.
High-velocity bullets can pass through the skull without knocking the victim down or causing an immediate loss of consciousness. However, the brain damage is often severe. Exit wounds are generally larger than entry wounds. The shock wave produced by high-velocity missiles tends to cause severe brain injury that extends well away from the missile track and includes remote contusions of the frontal and temporal poles and the undersurface of the cerebellum, and contrecoup fractures of the orbital plates.
CHRONIC TRAUMATIC ENCEPHALOPATHY
MACROSCOPIC AND MICROSCOPIC APPEARANCES
 degeneration of the substantia nigra with neuronal loss
degeneration of the substantia nigra with neuronal loss
 neuronal loss from the cortex and cerebellum
neuronal loss from the cortex and cerebellum
 neurofibrillary tangle formation in the cerebral cortex (Fig. 11.58).
neurofibrillary tangle formation in the cerebral cortex (Fig. 11.58).
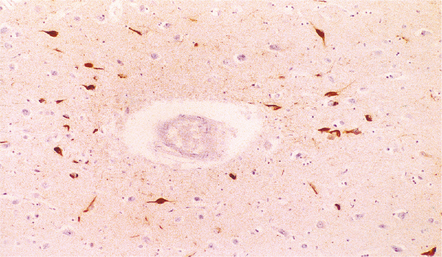
11.58 Punch-drunk syndrome.
Neurofibrillary tangles in the cerebral cortex of a 23-year-old boxer. The tangles were most numerous in the vicinity of the cortical blood vessels and were immunoreactive for tau protein, as shown here. In older boxers tangles may be present in large numbers in the cerebral cortex and brain stem. (Courtesy of Dr J. Geddes, Royal London Hospital.)
In some cases, patients have also developed a widespread TDP-43 (TAR DNA-binding protein of approximately 43 kd) proteinopathy (see Chapters 27, 28 and 31), associated with numerous glial and neuronal inclusions in the cerebral cortex, basal ganglia, diencephalon, and brainstem. A few patients have developed a motor neuron disease resembling amyotrophic lateral sclerosis and have had many TDP-43-positive inclusions in the primary motor cortex and spinal cord.
SPINAL INJURY
Included in this chapter are injuries to the spinal cord resulting from trauma and degenerative disease of the spinal column (Fig. 11.59). Neoplastic, ischemic, infectious, toxic, and metabolic disorders that may affect the spinal cord are considered in the appropriate chapters elsewhere in this book.
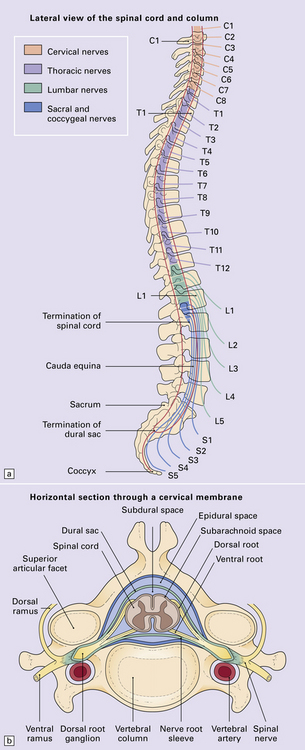
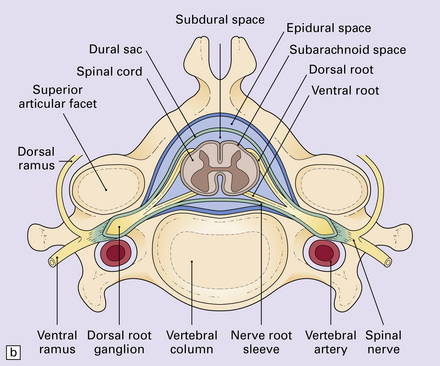
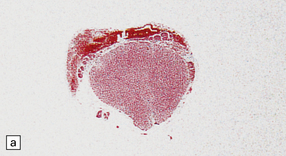
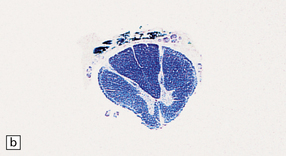
11.59 Spinal anatomy.
(a) Lateral view of the spinal cord and column. (b) Horizontal section through a cervical membrane.
TRAUMATIC SPINAL CORD INJURY
NATURE OF LESIONS
Distribution
The nature and site of injury vary according to the underlying etiology:
Time course
Cord damage after trauma can be viewed as occurring in two phases:
 Primary damage resulting from cord compression, contusion, laceration, and hemorrhage.
Primary damage resulting from cord compression, contusion, laceration, and hemorrhage.
 Secondary damage initiated by the trauma, but developing over several hours or even days and mainly involving physiologic responses to trauma, hypoxia, and ischemia. Systemic hypotension, loss of autoregulation of blood flow in the injured cord, local edema, generation of free radicals, and release of excitotoxic neurotransmitters may all be contributory factors.
Secondary damage initiated by the trauma, but developing over several hours or even days and mainly involving physiologic responses to trauma, hypoxia, and ischemia. Systemic hypotension, loss of autoregulation of blood flow in the injured cord, local edema, generation of free radicals, and release of excitotoxic neurotransmitters may all be contributory factors.
MACROSCOPIC APPEARANCES
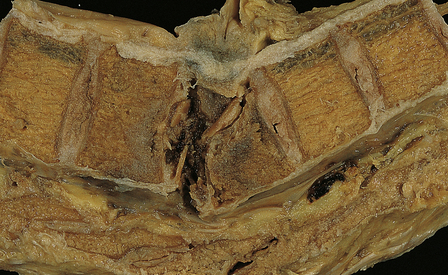
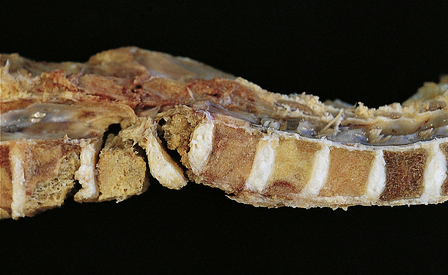
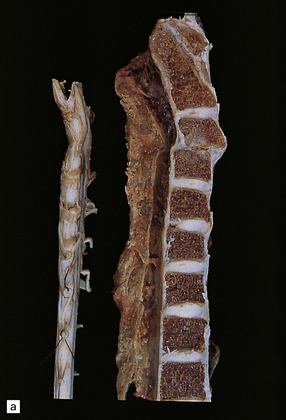
In most cases the soft tissues around the vertebral column are hemorrhagic and the fracture or fracture–dislocation is visible or at least palpable on external examination of the column. The site of the fracture is obvious on dividing the column (Figs 11.60, 11.61). Blood is usually visible in the epidural space and may also be present in the subarachnoid space around the cord. The macroscopic damage to the adjacent cord varies from mild focal indentation to severe hemorrhagic disruption that may extend several segments above and below the level of the fracture (Fig. 11.62). Even in the absence of obvious hemorrhage, the injured cord is usually swollen and congested.
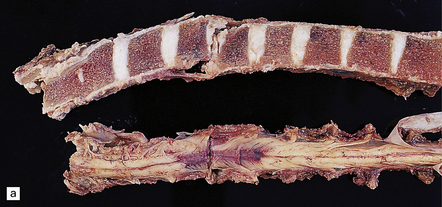
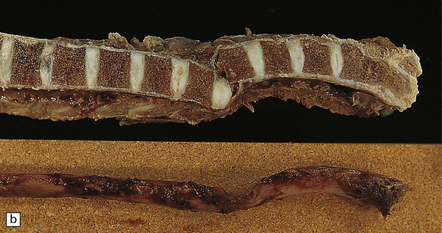
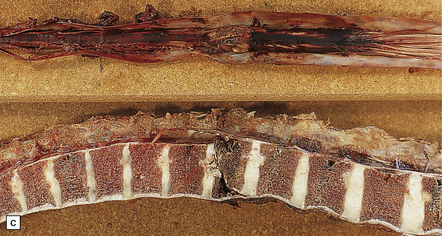
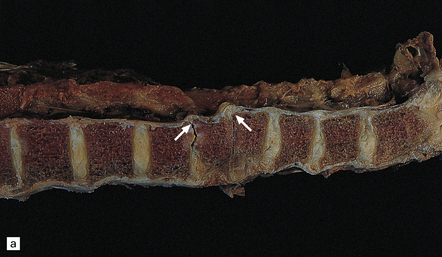
11.60 Fracture–dislocations demonstrated by sawing midsagittally through the vertebral bodies.
(a) Fracture–dislocation of the cervical column. The anterior longitudinal ligament is torn, but despite the displacement, the posterior longitudinal ligament is still intact and there is only scanty hemorrhage into the soft tissues around the spinal column or extradural space. The horizontal slice through the cord was made during the course of the examination. (b) Fracture–dislocation of the cervical column. The marked displacement has caused severe compression of the cord and is associated with more marked soft tissue and extradural hemorrhage than in (a). (c) Fracture–dislocation of the lumbar column. The fractured vertebral body has compressed and lacerated the cord and is associated with extensive extradural and subarachnoid hemorrhage. (Courtesy of Dr T. Moss, Frenchay Hospital, Bristol.)
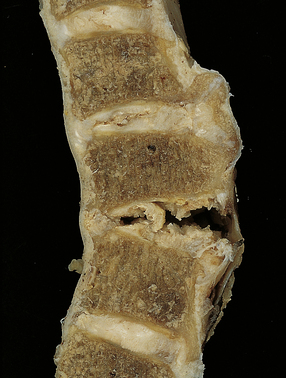
11.61 Hyperextension injury of the cervical cord.
In this case, the patient had degenerative spinal disease with anterior osteophytes. As a result of a fall, he sustained a cervical hyperextension injury causing cord compression. There has been tearing of the anterior longitudinal ligament and intervertebral disc space, but the posterior longitudinal ligament is intact and there is no horizontal displacement of the column.

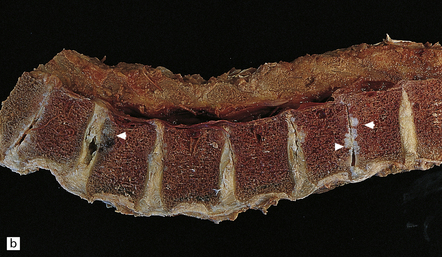
11.62 Hemorrhagic cord lesion.
Hemorrhagic disruption extending several segments above the level of cord compression at the site of the vertebral fracture–dislocation. The rostral and caudal ends of the hemorrhagic lesion have a typical tapering spindle shape. (Courtesy of Dr T. Moss, Frenchay Hospital, Bristol.)
MICROSCOPIC APPEARANCES
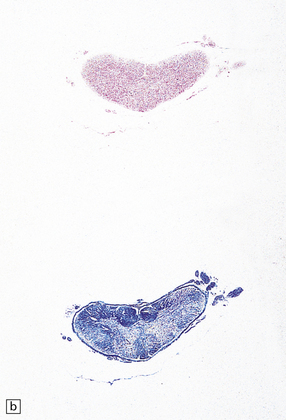
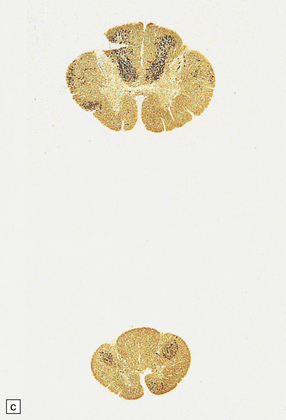
During the acute phase after spinal injury, histology reveals variable combinations of edema, disruption of long tracts with prominent axonal swellings, hemorrhage, and foci of infarction (Figs 11.63–11.65). Over the next few weeks there is infiltration by macrophages, and gradual removal of myelin and neuronal debris (Figs 11.66, 11.67). Rostral and caudal segments of cord show degeneration of the ascending and descending long tracts that were damaged at the level of cord injury.
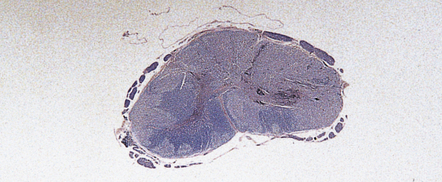
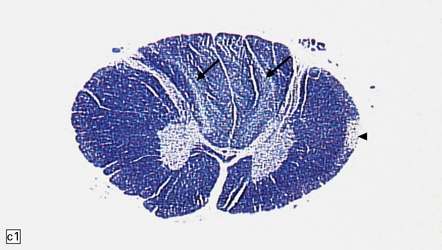
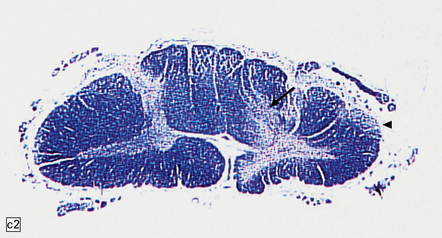
11.63 Acute cord injury.
Hemorrhagic necrosis of gray matter and disruption and extensive infarction of white matter in the compressed cord at the level of a cervical fracture–dislocation.
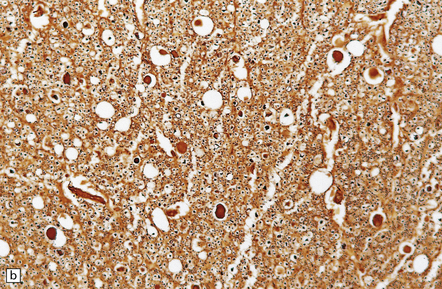
11.64 Microscopic appearance of spinal cord in a patient who survived several days after cord injury.
(a) There are scattered axonal spheroids in the lateral columns. (b) The axonal spheroids are well demonstrated by Palmgren silver impregnation.
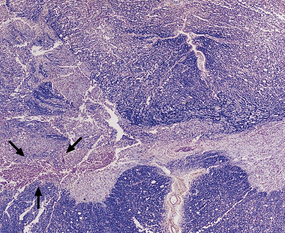
11.65 Microscopic appearance of spinal cord one week after cord injury.
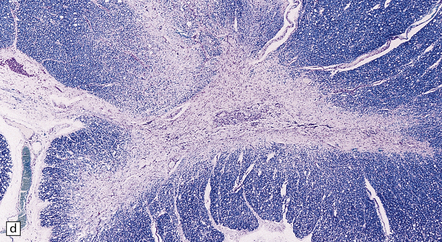
At the level of injury the cervical cord is disrupted and there are foci of gray matter necrosis with infiltration by macrophages (arrows).
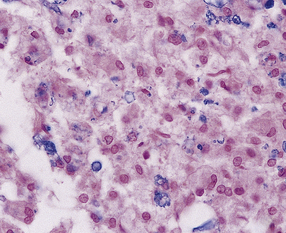
11.66 Infiltration of injured cord by macrophages.
Removal of myelin debris from the posterior columns of an injured cord by infiltrating macrophages.
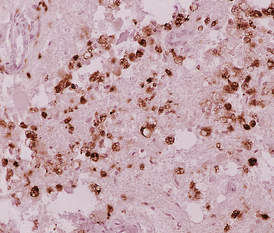
11.67 Infiltration of injured cord by macrophages.
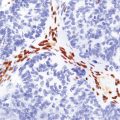
CD68-immunoreactive macrophages in an injured spinal cord.
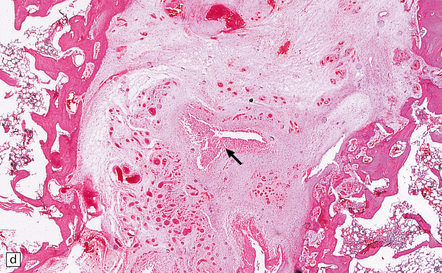
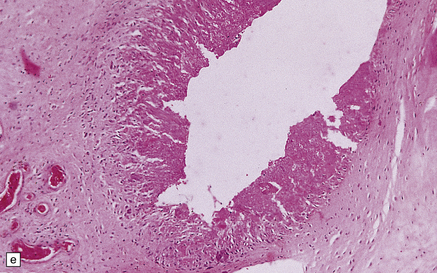
The gray matter at the level of injury may become necrotic and, later, cavitated (Fig. 11.68), and often shows prominent proliferation and later hyaline thickening of small blood vessels. With time there is often infiltration by fibroblasts and associated collagenous fibrosis. Frequently, cavitation also involves the anterior part of the posterior columns. Such cavitation is usually maximal at the level of gray matter injury, but can extend several segments above and below (post-traumatic syringomyelia). The cavitation may continue to extend rostrally and caudally within the cord over many years.
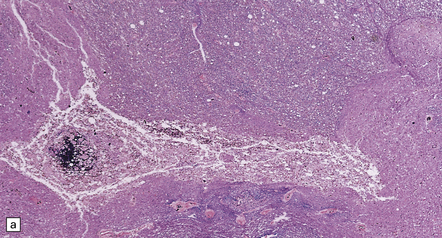
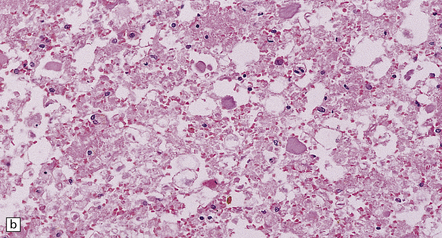
11.68 Necrosis of cord after trauma.
(a) Necrosis of central gray matter and immediately adjacent part of the posterior columns above the level of a spinal fracture. (b) Higher magnification of a hematoxylin and eosin-stained section reveals necrotic hemorrhagic tissue, scattered macrophages, and a few axonal spheroids.
REFERENCES
Adams, J.H., Jennett, B., Murray, L.S., et al. Neuropathological findings in disabled survivors of a head injury. J Neurotrauma.. 2011;28:701–709.
Bramlett, H.M., Dietrich, W.D. Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J Cereb Blood Flow Metab.. 2004;24:133–150.
Contostavlos, D.L. Isolated basilar traumatic subarachnoid hemorrhage: an observer’s 25 year re-evaluation of the pathogenetic possibilities. Forensic Sci Int.. 1995;73:61–74.
Dowling, G., Curry, B. Traumatic basal subarachnoid hemorrhage. Report of six cases and review of the literature. Am J Forensic Med Pathol.. 1988;9:23–31.
Elder, G.A., Cristian, A. Blast-related mild traumatic brain injury: mechanisms of injury and impact on clinical care. Mt Sinai J Med.. 2009;76:111–118.
Eriksson, E.A., Pellegrini, D.C., Vanderkolk, W.E., et al. Incidence of pulmonary fat embolism at autopsy: an undiagnosed epidemic. J Trauma.. 2011;71:312–315.
Finnie, J.W., Blumbergs, P.C. Traumatic brain injury. Vet Pathol.. 2002;39:679–689.
Fusco, M.R., Harrigan, M.R. Cerebrovascular dissections: a review. Part II: blunt cerebrovascular injury. Neurosurgery.. 2011;68:517–530;. [discussion 530].
Geddes, J.F., Whitwell, H.L. Head injury in routine and forensic pathological practice. Curr Top Pathol.. 2001;95:101–124.
Geddes, J.F., Whitwell, H.L., Graham, D.I. Traumatic axonal injury: practical issues for diagnosis in medicolegal cases. Neuropathol Appl Neurobiol.. 2000;26:105–116.
Graham, D.I., Smith, C., Reichard, R., et al. Trials and tribulations of using β-amyloid precursor protein immunohistochemistry to evaluate traumatic brain injury in adults. Forensic Sci Int.. 2004;146:89–96.
Gross, A. Traumatic basal subarachnoid hemorrhages: autopsy material analysis. Forensic Sci Int.. 1990;45:53–61.
Johnson, V.E., Stewart, W., Smith, D.H. Widespread tau and amyloid-β pathology many years after a single traumatic brain injury in humans. Brain Pathol. 2011. [[E-pub ahead of print]].
Kocsis, J.D., Tessler, A. Pathology of blast-related brain injury. J Rehabil Res Dev.. 2009;46:667–672.
Larson, P.S., Reisner, A., Morassutti, D.J., et al. Traumatic intracranial aneurysms. Neurosurg Focus.. 2000;8:e4.
Maxwell, W.L., Povlishock, J.T., Graham, D.L. A mechanistic analysis of nondisruptive axonal injury: a review. J Neurotrauma.. 1997;14:419–440.
McKee, A.C., Cantu, R.C., Nowinski, C.J., et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol.. 2009;68:709–735.
McKee, A.C., Gavett, B.E., Stern, R.A., et al. TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol. 2010;69:918–929.
Oehmichen, M., Meissner, C., Konig, H.G. Brain injury after gunshot wounding: morphometric analysis of cell destruction caused by temporary cavitation. J Neurotrauma.. 2000;17:155–162.
Oehmichen, M., Meissner, C., Konig, H.G. Brain injury after survived gunshot to the head: reactive alterations at sites remote from the missile track. Forensic Sci Int.. 2001;115:189–197.
Oehmichen, M., Meissner, C., Konig, H.G., et al. Gunshot injuries to the head and brain caused by low-velocity handguns and rifles. A review. Forensic Sci Int.. 2004;146:111–120.
Saing, T., Dick, M.C., Nelson, P.T., et al. Frontal cortex neuropathology in dementia pugilistica. J Neurotrauma.. 2011. [[E-pub ahead of print]].
Accidental and non-accidental pediatric brain injury
Case, M.E. Accidental traumatic head injury in infants and young children. Brain Pathol.. 2008;18:583–589.
Case, M.E. Inflicted traumatic brain injury in infants and young children. Brain Pathol.. 2008;18:571–582.
Geddes, J.F., Hackshaw, A.K., Vowles, G.H., et al. Neuropathology of inflicted head injury in children. I. Patterns of brain damage. Brain.. 2001;124:1290–1298.
Geddes, J.F., Vowles, G.H., Hackshaw, A.K., et al. Neuropathology of inflicted head injury in children. II. Microscopic brain injury in infants. Brain.. 2001;124:1299–1306.
Matschke, J., Voss, J., Obi, N., et al. Nonaccidental head injury is the most common cause of subdural bleeding in infants < 1 year of age. Pediatrics.. 2009;124:1587–1594.
Oehmichen, M., Schleiss, D., Pedal, I., et al. Shaken baby syndrome: re-examination of diffuse axonal injury as cause of death. Acta Neuropathol.. 2008;116:317–329.
Squier, W. The “Shaken Baby” syndrome: pathology and mechanisms. Acta Neuropathol.. 2011;122:519–542.
Traumatic and non-traumatic spinal cord injury
Cornish, R., Blumbergs, P.C., Manavis, J., et al. Topography and severity of axonal injury in human spinal cord trauma using amyloid precursor protein as a marker of axonal injury. Spine (Phila Pa 1976). 2000;25:1227–1233.
Henderson, F.C., Geddes, J.F., Crockard, H.A. Neuropathology of the brainstem and spinal cord in end stage rheumatoid arthritis: implications for treatment. Ann Rheum Dis.. 1993;52:629–637.
Ito, T., Oyanagi, K., Takahashi, H., et al. Cervical spondylotic myelopathy. Clinicopathologic study on the progression pattern and thin myelinated fibers of the lesions of seven patients examined during complete autopsy. Spine (Phila Pa 1976).. 1996;21:827–833.
Kakulas, B.A. A review of the neuropathology of human spinal cord injury with emphasis on special features. J Spinal Cord Med.. 1999;22:119–124.
Kakulas, B.A. Neuropathology: the foundation for new treatments in spinal cord injury. Spinal Cord.. 2004;42:549–563.
O’Brien, M.F., Casey, A.T., Crockard, A., et al. Histology of the craniocervical junction in chronic rheumatoid arthritis: a clinicopathologic analysis of 33 operative cases. Spine (Phila Pa 1976).. 2002;27:2245–2254.