33 Head and Facial Trigeminal Neuralgia
Trigeminal neuralgia is a disease characterized by brief, stereotypical episodes of lancinating pain in the trigeminal nerve distribution on a single side. The vast majority of cases affect either the second or third division (V2 or V3), alone or in combination. In only 4% to 5% of patients, symptoms occur solely in the first division (V1, Table 33-1). Historically, trigeminal neuralgia is considered one of the most painful disorders known to mankind. The name “tic douloureux” was coined by Nicholaus Andre, referring to the spasm of the face that follows an attack of pain. John Fothergill first codified the clinical characteristics of the disease in his paper “On a Painful Affliction of the Face,” which was published in London in 1775.
Table 33-1 Distribution of Pain in Trigeminal Neuralgia
| Distribution | % |
|---|---|
| V1 alone | 4 |
| V2 alone | 17 |
| V3 alone | 15 |
| V2 + V3 | 32 |
| V1 + V2 | 14 |
| V1 + V2 + V3 | 17 |
Afflicted patients typically describe the pain as electric, shooting, and shocklike. The pain occurs in attacks, each of which lasts only seconds or less; however, attacks tend to cluster so that pain-free episodes may not be appreciated.1,3 Pain can be precipitated by light mechanical stimulation to small trigger zones in the face or oral mucosa. Frequent triggers include light touch, wind, brushing teeth, speaking, eating, and drinking. There may be ipsilateral muscle spasm described in the condition termed tic douloureux. The disease typically takes a sporadic course, with remissions that may last months or even years. Most people have normal neurological examinations and are symptom free between attacks. The annual incidence is between 4 and 5 in 100,000. It typically affects people older than 50 years, although instances of the disease in young adults and even children have been reported. There is a slight female predominance.2 Strong environmental or genetic predisposing factors are not apparent.
Anatomy
The nerve then separates into three divisions (Fig. 33-1), each of which can be compressed by local inflammatory conditions or primary neoplastic or metastatic lesions. The ophthalmic nerve (V1) courses anteriorly through the cavernous sinus, where it is accompanied by cranial nerves III, IV, VI, and V.2 It exits the skull through the superior orbital fissure and passes into the orbit. From there, it divides to supply the eyeball, lacrimal glands, conjunctiva, part of the nasal mucosa, and skin of the nose, eyelid, and forehead.
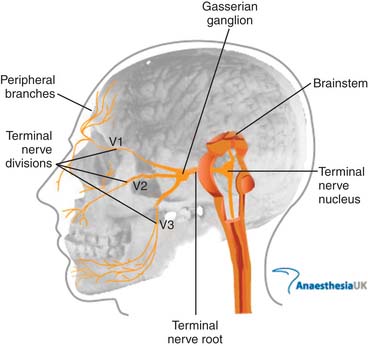
Figure 33-1 Anatomy of the trigeminal nerve.
Image from http://www.frca.co.uk/images/trigeminal_nerve.jpg, accessed 2010, March 14, courtesy of AnaesthesiaUK.
The mandibular nerve (V3) exits the skull more proximally through the foramen ovale and passes into the masticator space. It supplies the lower third of the face as well as the tongue and floor of the mouth and jaw. The motor root, which bypasses the gasserian ganglion but then rejoins the mandibular nerve provides innervation to the muscles of mastication (see Fig. 33-1).
Pathophysiology
Although Jannetta and colleagues showed that surgical decompression of the nerve root can effectively alleviate the symptoms of trigeminal neuralgia, a pathophysiologic connection between trigeminal nerve compression and the intermittent nature of the disease has not been well elucidated.4 A disease mechanism must also explain how nonnoxious peripheral mechanical stimuli trigger pain within different regions of the face. In a number of patients who undergo surgical exploration, no compressing vessel or lesion has been identified.
Pathologic rhizotomy specimens have demonstrated focal loss of myelin with close apposition of the demyelinated axons.5,6 Hilton found five patients without microvascular compression who did not show demyelinating features. In the study by Devor and colleagues, 1 of the 12 patients was not found to have vascular compression, and the rhizotomy specimen from this patient showed only “modest dysmyelination”.
Devor and his colleagues proposed their “ignition hypothesis” by which nerve compression leads to an increase in neuronal activity and reduced firing thresholds.6 In support of this hypothesis, Vos and colleagues showed in rats that mild compression of the infraorbital nerve produces a facial pain syndrome.7
Diagnosis
The White and Sweet criteria for trigeminal neuralgia8 were a major advance that facilitated research and enabled early and accurate clinical recognition of the syndrome (Tables 33-2 through 33-4). The criteria were incorporated, largely unchanged, into the official research diagnostic framework criteria published by the International Association for the Study of Pain (IASP)9 and the International Headache Society (IHS).3
Table 33-2 Sweet Diagnostic Criteria for Trigeminal Neuralgia
From White JC, Sweet WH: Pain and the Neurosurgeon. Springfield, Ill. Charles C Thomas, 1969.
Table 33-3 ICHD Criteria for Classic Trigeminal Neuralgia
ICHD, International Classification of Headache Disorders.
Table 33-4 ICHD Criteria for Symptomatic Trigeminal Neuralgia
ICHD, International Classification of Headache Disorders.
The current IHS criteria (International Classification of Headache Disorders II [ICHD-II])3 establishes trigeminal neuralgia as a discrete clinical diagnosis under the general classification of “cranial neuralgias and central causes of facial pain” (ICHD-II diagnostic code 13). The diagnosis ‘‘trigeminal neuralgia’’ (diagnostic code 13.1) replaces the earlier term, tic douloureux. ICHD-II further subdivides trigeminal neuralgia into ‘‘classic trigeminal neuralgia’’ and ‘‘symptomatic trigeminal neuralgia.’’ Classic trigeminal neuralgia (Table 33-3) is the most common idiopathic form of the disorder (although it also includes cases associated with vascular compression). Classic trigeminal neuralgia is defined as ‘‘a unilateral disorder characterized by brief electric shocklike pains, abrupt in onset and termination, limited to the distribution of one or more divisions of the trigeminal nerve. Pain is commonly evoked by trivial stimuli including washing, shaving, smoking, talking and/or brushing the teeth (trigger factors) and frequently occurs spontaneously. Small areas in the nasolabial fold and/or chin may be particularly susceptible to the precipitation of pain (trigger areas). The pains usually remit for variable periods.’’ The ICHD-II specific diagnostic criteria for classic trigeminal neuralgia are listed in Table 33-3. Symptomatic trigeminal neuralgia has the same key features of trigeminal neuralgia but results from another disease process (such as multiple sclerosis or a cerebellopontine angle tumor). Symptomatic trigeminal neuralgia is defined by IHS as ‘‘Pain indistinguishable from classic trigeminal neuralgia but caused by a demonstrable structural lesion other than vascular compression.’’ The ICHD-II diagnostic criteria for symptomatic trigeminal neuralgia are listed in Table 33-4.
Because a significant percentage of patients have symptomatic trigeminal neuralgia resulting from another disease process, diagnostic brain imaging studies should be part of the initial evaluation of any patient with trigeminal neuralgia symptoms.10,11 The study should visualize the anatomic landmarks around the trigeminal (gasserian) ganglion and the cerebellopontine angle. Although a routine brain CT scan is usually adequate to screen for a cerebellopontine tumor, an MRI scan often better demonstrates multiple sclerosis plaques and the anatomic relationships of the trigeminal root. High-resolution imaging techniques are also increasingly able to visualize subtle vascular anomalies that may be the source of root compression in certain patients with classic trigeminal neuralgia. Other diagnostic studies, such as blood studies, lumbar puncture, and evoked potentials, are generally not necessary.
Medical Treatment
Carbamazepine has been the mainstay of medical treatment for trigeminal neuralgia for many years.12 A Cochrane review on this subject confirmed that carbamazepine with a number needed to treat (the number of people who would need to be treated to see benefit in one person) of 2.5 was the recommended initial treatment for trigeminal neuralgia.13 Antiepileptic drugs were first used in the treatment of trigeminal neuralgia when Bergouignan in 1942 noted that the anticonvulsant phenytoin effectively controlled attacks of pain in the condition.14 Studies on various antiepileptic drugs have revealed that although carbamazepine and lamotrigine are superior to placebo in trials, most others drugs thought to be useful do not have trials to support their use.15 Baclofen is another choice for monotherapy for trigeminal neuralgia as was evidenced in data from Fromm and colleagues.16 In many instances where carbamazepine is not tolerated, initial therapy with baclofen has been successful.
Although the drugs are classified as anticonvulsants, they have differing mechanisms of action as well as side effect patterns. In a retrospective study of anticonvulsant therapy, Scrivani and coworkers found that 50% of patients reported satisfactory pain relief while taking a single antiepileptic drug, whereas 70% of those taking two drugs reported a satisfactory response.17
Interventional Approaches
There is a paucity in the literature directly comparing medical management with surgical management. Given inconsistency in outcome measurements, poor quality of studies (most do not have independent assessment), and lack of direct comparisons, this leads to a limited ability to truly assess. Zakrzewska and associates compared the medical and surgical management of intractable trigeminal neuralgia. This is a long-term prospective longitudinal study comparing 15 patients who were followed for a mean duration of 15 years on the effectiveness of medical (oxcarbazepine) versus surgical therapy. The study indicated that mean time to recurrence of pain following oxcarbazepine therapy was 8 months and with surgical therapy it was 28 months. Clinical and patient global analysis of the outcome measures suggested that patients could benefit substantially from having surgery earlier, rather than later, in the disease process to improve the quality of life, freedom from medication, and the need for regular follow-up. Remember: this was a study group of only 15 patients.19
Block of the Ophthalmic Nerve
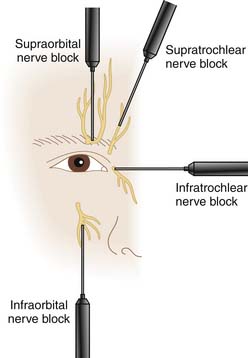
The ophthalmic nerve per se is not blocked in the treatment of trigeminal neuralgia because it leads to keratitis. The supraorbital and supratrochlear branches can be individually blocked. The supraorbital nerve block is described elsewhere in this text. The supratrochlear nerve can be injected at the superior medial corner of the orbital ridge with 1 mL of local anesthetic with or without corticosteroid. The inferior orbital nerve can be injected at the inferior orbital foramen which is 1 cm below the orbit and is usually located with a needle inserted about 2 cm lateral to the nasal and directed superiorly, posteriorly, and slightly laterally (Fig. 33-2).
Block of the Maxillary Nerve and its Branches
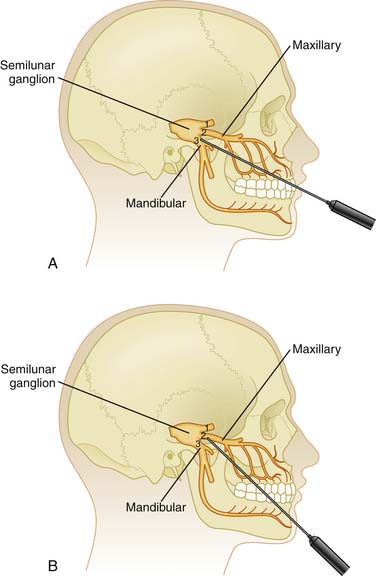
With the patient’s mouth opened, a 3.5-inch, 25-gauge needle can be inserted between the zygomatic arch and the notch of the mandible. At about 3 to 4 cm in depth, contact will be made with the lateral pterygoid plate. Withdraw the needle 1 cm and angle it superiorly and anteriorly to pass into the pterygopalatine fossa. Local anesthetic (4 to 6 mL) can then be instilled here after negative aspiration. This technique anesthetizes the maxillary nerve and the sphenopalatine ganglion. There is a risk of hemorrhage when blocking the maxillary nerve with this technique (Fig 33-3).
Mandibular Nerve Block
Local Anesthetics and Streptomycin
Trigeminal nerve blocks with local anesthetics, usually lidocaine, have been described in multiple reports to provide relief but usually are very short lived. A study on intranasal application of lidocaine to the pterygopalatine fossa showed brief improvement in 25 patients with V2 treated with intranasal 8% lidocaine spray compared to placebo; however, the relief lasted only 4 hours on average, with a maximum duration of 24 hours.18 An uncontrolled report using a high concentration of lidocaine for trigeminal nerve blocks documented much longer durations of improvement with many patients obtaining months of pain control.20 Studies looking at the use of streptomycin and lidocaine for trigeminal nerve block have largely shown no significant improvement.21
Neurectomy
Neurectomy is probably the oldest recorded surgical procedure for trigeminal neuralgia. Most of the studies done for neurectomy were retrospective and published more than 20 years ago with only one recent paper by Murali and Rovit. They reported on a case series of 40 patients, 12 with neurectomies performed as the primary procedure, and 28 as a secondary procedure to treat pain recurrence after radiofrequency thermocoagulation.22
Cryotherapy
Cryotherapy is the therapeutic use of extremely low temperatures to destroy cells by crystallizing the cytosol to obtain pain relief. Under local anesthesia, the affected nerve is exposed surgically and a cryoprobe is placed directly on the nerve for three 2-minute freeze-thaw cycles. Few complications have been reported; sensation, although initially lost, returns before pain recurs.23,24 The procedure can be repeated, and the results have been similar.
Neurolytic Peripheral Blocks
Neurolytic peripheral blocks of the trigeminal nerve and its branches are usually done with phenol or alcohol. Most studies on these techniques are older retrospective analyses. A more recent paper by Fardy and Patton, reported on a series of 413 alcohol blocks administered over a 20-year period. The mean period of pain relief was 13 months, and only three (0.73%) significant complications were noted.25 These included local tissue necrosis, diplopia, and sensory loss. Disadvantages to this procedure include sensory loss in the distribution of the treated nerve and a high rate of recurrence of pain owing to nerve regeneration with subsequent deafferentation pain.
Gasserian Ganglion Techniques
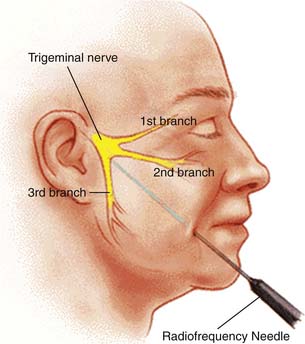
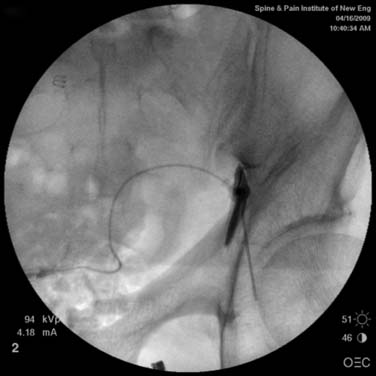
Techniques targeting the gasserian ganglion can broadly be classified into ablative and decompressive approaches. Percutaneous trigeminal ablation of the gasserian ganglion is usually performed by a specially designed device inserted into the cheek or through the mouth. Under radiographic guidance with fluoroscopy or CT, the device is directed through the foramen ovale into the gasserian ganglion or retrogasserian rootlets. The methods of ablation typically include radiofrequency coagulation, glycerol injection, and mechanotrauma by balloon ablation. The results from any of these modalities depends on the duration and intensity of denervation (Fig. 33-4).
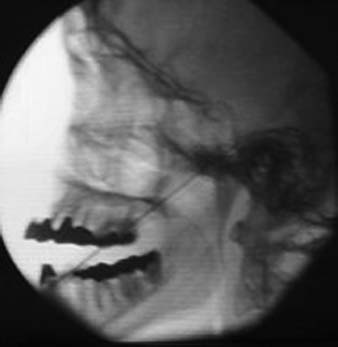
Figure 33-4 Percutaneous needle to the gasserian ganglion.
(Image from http://www.umanitoba.ca/cranial_nerves/trigeminal neuralgia/manuscript/rhizotomies.html; © 2001, The Centre for Cranial Nerve Disorders.)
Radiofrequency Thermocoagulation
Radiofrequency thermocoagulation is the most common surgical treatment for trigeminal neuralgia. A meta-analysis of the literature confirmed the efficacy of this procedure.26 The recurrence rate quoted ranges from 4% to 65%.This wide variation in part was due to differing standards for defining recurrence. Some studies classified recurrence as pain severe enough to require surgery, and others as in recurrence of symptoms. The most important factors influencing recurrence are the duration of follow-up and the degree of denervation.
Taha and Tew reviewed the therapy in 500 patients that were followed for 2 to 12 years with patients who had undergone other surgical treatments for trigeminal neuralgia.27 The authors found a 100% technical success rate for completion of the radiofrequency procedure, the highest rates for initial pain relief, and one of the lowest pain recurrence rates for RT (radiofrequency treatment) when compared with other surgical procedures. A recurrence rate of only 20% in 9 years was reported (Fig 33-5).
Glycerol Injections
Percutaneous injection of glycerol was described initially by Hakanson.28 Glycerol injection involves the injection of sterile glycerol into the gasserian ganglion and retrogasserian rootlets. Placement of the needle adjacent to the ganglion is confirmed with contrast cisternogram. The procedure results in significant initial pain relief.
North, in 1990, reviewed 109 percutaneous retrogasserian glycerol rhizotomies in 85 patients, 11 with atypical features (constant pain and/or significant sensory loss unexplained by prior procedures).29 They were followed for 6 to 54 months. The endpoints were recurrence requiring medication and recurrence refractory to medication requiring further intervention. Mean duration for primary endpoint was 2 years, and 3 years for secondary endpoint. Mean time to failure of repeat treatment was 1 year. There were two reported cases of corneal anesthesia and three of hyperesthesia.
As initially described, there was a low incidence of complications, dysesthesia, or keratitis, and little loss of sensation. In fact, glycerol injection replaced neurolysis by ethanol and phenol, both of which caused significant sensory loss. However, many recent larger series have shown that complications do occur frequently, and the recurrence rate is relatively high, up to 50%.30–32
Balloon Microcompression
Lichtor and colleagues reported a 10-year follow-up in a series of 100 patients. At 5 years, the recurrence rate was 20%, and at 10 years, it is estimated that 70% of patients will still be pain free.33 One of the main advantages of this procedure is that the corneal reflex is maintained in most cases. However, dysesthesia occurs in 7% to 19% of the cases and may be related to compression time.
Skirving and coworkers in a retrospective review of balloon compression found there was prompt pain relief in 521/522 patients.34 The recurrence rate was 19.2% within 5 years. There were symptomatic dysesthesias in 3.8% of patients (Fig 33-6).
Stereotactic Radiosurgery
Most patient series enrolled fewer than 100 patients with follow-up of less than 1 year. Kondziolka and associates reported 80% initial pain relief in 106 patients who were followed for 18 months.35 Ten percent of the patients developed dysesthesia as a complication (Fig 33-7).
Decompressive Procedures
These procedures are based on the concept that compression of the trigeminal nerve causes trigeminal neuralgia.36 The compression of the trigeminal nerve from blood vessels or tumors is thought to result in demyelination of the nerve. There is evidence from clinical and anatomic studies that supports the neurovascular compression theory of trigeminal neuralgia.37,38 However, there are many patients who do not experience pain relief following trigeminal nerve decompression, suggesting that there are etiologies other than compression of the nerve that could cause trigeminal neuralgia (Fig 33-8).
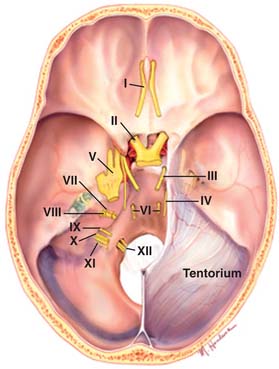
Figure 33-8 Posterior fossa with trigeminal nerve (V) shown.
(Image from http://www.mayfieldclinic.com/PE-AnatBrain.htm, accessed 2010, March 14; © Mayfield Clinic.)
Microvascular Decompression
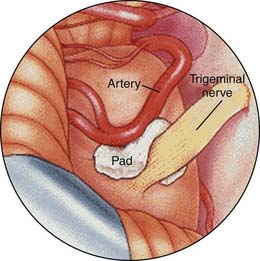
Walter Dandy was the first to put forth the posterior fossa approach for the treatment of trigeminal neuralgia.39 In 1934, Dandy proposed his theory of vascular compression as a cause, but also acknowledged that vascular contact occasionally occurs without the production of pain and may be absent when neuralgia is present. Dandy identified the major compressing vessel as the anterior inferior cerebellar artery. Peter Jannetta documented the frequent occurrence of vascular channels compressing the trigeminal nerve in patients with trigeminal neuralgia and devised a technique for nondestructive microvascular decompression of the nerve.40
Barker and colleagues have reported an excellent long-term (20 years) prospective longitudinal study on the outcome of microvascular decompression for trigeminal neuralgia in 1185 patients. Ten years after surgery, 70% of the patients had excellent final results; they were free of pain without need for medication. Major complications included deaths (0.2%), brainstem infarction (0.1%), and ipsilateral hearing loss (1%). Initial relief of neuralgia pain was present in 98% of patients41 (Fig. 33-9).
Neurostimulation for Trigeminal Neuralgia
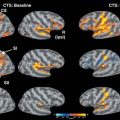
Neuromodulation in the treatment of trigeminal neuralgia has been studied in multiple ways. Deep brain stimulation of well-defined targets in the sensory thalamus and periaqueductal or periventricular gray matter with stereotactic placement of electrodes has been studied but has not proven to be efficacious.42
In a few studies, excellent results have been documented in the treatment of trigeminal neuropathic pain, with 75% to 100% of patients achieving good or excellent pain relief.43–45 The effect of motor cortex stimulation can wane over time, requiring reprogramming. Even in patients who undergo intensive reprogramming, pain relief cannot always be achieved.46 Although the development of epilepsy has not been reported, there is a risk of seizures during stimulator programming. In summary, of the neuromodulatory techniques, motor cortex stimulation shows some early promise.
1. Katusic S., Williams D.B, Beard C.M., et al. Epidemiology and clinical features of idiopathic trigeminal neuralgia and glossopharyngeal neuralgia: similarities and differences, Rochester, Minnesota, 1945-1984. Neurepidemiology. 1991;10:276-281.
2. Katusic S., Beard C.M., Bergstralh E., Kurland L.T. Incidence and clinical features of trigeminal neuralgia. Rochester, Minnesota, 1945-1984. Ann Neurol. 1990;27(1):89-95.
3. Headache Classification Subcommittee of the International Headache Society. The international classification of headache disorders. 2nd ed. Cephalalgia. 2004;24(suppl 1):9-160.
4. Jannetta P.J. Neurovascular compression in cranial nerve and systemic disease. Ann Surg. 1980;192(4):518-525.
5. Hilton D.A., Love S., Gradidge T., Coakham H.B. Pathological findings associated with trigeminal neuralgia caused by vascular compression. Neurosurgery. 1994;35(2):299-303.
6. Devor M., Amir R., Rappaport Z.H. Pathophysiology of trigeminal neuralgia: The ignition hypothesis. Clin J Pain. 2002;18:4-13.
7. Vos B.P., Strassman A.M., Maciewicz R.J., et al. Behavioral evidence of trigeminal neuropathic pain following loose constrictive ligation of the rat’s infraorbital nerve. J Neurosci. 1994;14:2708-2723.
8. White J.C., Sweet W.H. Pain and the Neurosurgeon. Springfield, Ill: Charles C Thomas; 1969.
9. Merskey H., Bogduk N. Classification of chronic pain: Descriptions of chronic pain syndromes and definitions of pain terms. IASP Press; 1994.
10. Vitte E., Bensimon J.L., Baulac M. Radiological studies in trigeminal nerve pathology. Arch Otorhinolaryngol. 1989;246:262-264.
11. Yang J., Simonson T.M., Ruprecht A., et al. Magnetic resonance imaging used to assess patients with trigeminal neuralgia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:343-350.
12. McQuay H., Carroll D., Jadad A.R., et al. Anticonvulsant drugs for management of pain: A systematic review. BMJ. 1995;311(7012):1047-1052.
13. Wiffen P.J., McQuay H.J., Moore R.A. Carbamazepine for acute and chronic pain. Cochrane Database Syst Rev, 3. 2005;:CD005451.
14. Bergouignan M. Cures heureuses de ne´vralgies facials essentielles par le diphe´nylhydantoinate de soude. Rev Laryng (Bordeaux). 1942;63:34-41.
15. Sindrup S.H., Jensen T.S. Pharmacotherapy of trigeminal neuralgia. Clin J Pain. 2002;18:22-27.
16. Fromm G.H., Terrence C.F., Chattha A.S. Baclofen in the treatment of trigeminal neuralgia: Double-blind study and long-term follow-up. Ann Neurol. 1984;15(3):240-244.
17. Scrivani S.J., Mathews E.S., Maciewics R.J. Trigeminal neuralgia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:527-538.
18. Kanai A., Suzuki A., Kobayashi M., Hoka S. Intranasal lidocaine 8% spray for second-division trigeminal neuralgia. Br J Anaesth. 2006;97:559-563.
19. Zakrzewska J.M., Patsalos P.N. Long-term cohort study comparing medical (oxcarbazepine) and surgical management of intractable trigeminal neuralgia. Pain. 2002;95:259-266.
20. Han K.R, Kim C., Chae Y.J., Kim D.W. Efficacy and safety of high concentration lidocaine for trigeminal nerve block in patients with trigeminal neuralgia. Int J Clin Pract. 2008;62:248-254.
21. Bittar GT, Graff-Radford SB. The effects of streptomycin/lidocaine block on trigeminal neuralgia: A double blind crossover placebo controlled study. Headache. 1993;33:155-160.
22. Murali R., Rovit R.L. Are peripheral neurectomies of value in the treatment of trigeminal neuralgia? An analysis of new cases and cases involving previous radiofrequency gasserian thermocoagulation. J Neurosurg. 1996;85:435-437.
23. Zakrzewska J.M., Nally F.F. The role of cryotherapy (cryoanalgesia) in the management of paroxysmal trigeminal neuralgia: A six year experience. Br J Oral Maxillofac Surg. 1988;26:18-25.
24. Zakrzewska J.M. Cryotherapy for trigeminal neuralgia: A 10 year audit. Br J Oral Maxillofac Surg. 1991;29:1-4.
25. Fardy M.J., Patton D.W. Complications associated with peripheral alcohol injections in the management of trigeminal neuralgia. Br J Oral Maxillofac Surg. 1994;32:387-391.
26. Zakrzewska J.M. Trigeminal Neuralgia. London: WB Saunders; 1995. 125-156
27. Taha J.M., Tew J.M. Comparison of surgical treatments for trigeminal neuralgia: Evaluation of radiofrequency rhizotomy. Neurosurg. 1996;38:865-871.
28. Hakanson S. Trigeminal neuralgia treated by the injection of glycerol into the trigeminal cistern. Neurosurgery. 1981;9:638-646.
29. North R.B., Kidd D.H., Piantadosi S., Carson BS. Percutaneous retrogasserian glycerol rhizotomy. Predictors of success and failure in treatment of trigeminal neuralgia. J Neurosurg. 1990;72:851-856.
30. Burchiel K.J. Percutaneous retrogasserian glycerol rhizolysis in the management of trigeminal neuralgia. J Neurosurg. 1988;69:361-366.
31. Saini S.S. Retrogasserian glycerol injection therapy in trigeminal neuralgia. J Neurol Neurosurg Psych. 1987;50:1536-1538.
32. Fujimiki T., Fukushima T., Miyazaki S. Percutaneous retrogasserian glycerol injection in the management of trigeminal neuralgia: Long-term follow-up results. J Neurosurg. 1990;3:212-216.
33. Lichtor T., Mullan J.F. A 10-year follow-up review of percutaneous microcompression of the trigeminal ganglion. J Neurosurg. 1990;72:49-54.
34. Skirving D.J., Dan N.G. A 20-year review of percutaneous balloon compression of the trigeminal ganglion. J Neurosurg. 2001;94(6):913-917.
35. Kondziolka D., Lunsford L.D., Flickinger J.C. Stereotactic radiosurgery for the treatment of trigeminal neuralgia. Clin J Pain. 2002;18:42-47.
36. Jannetta P.J. Treatment of trigeminal neuralgia by suboccipital and transtentorial cranial operations. Clin Neurosurg. 1977;24:538-549.
37. Hamilyn P.J., King T.T. Neurovascular compression in trigeminal neuralgia: A clinical and anatomical study. J Neurosurg. 1992;76:948-954.
38. Devor M., Lippman R.G., Rappaport Z.H. Mechanism of trigeminal neuralgia: An ultrastructural analysis of trigeminal root specimens obtained during microvascular decompression surgery. J Neurosurg. 2002;96:532-543.
39. Dandy W.E. Concerning the cause of trigeminal neuralgia. Am J Surg. 1934;24:447-455.
40. Jannetta P.J. Microvascular decompression of the trigeminal nerve for tic douloreux. In: winn R.H., editor. Youmans—Neurological Surgery. 4th ed. Philadelphia: Saunders; 1996:3404-3415.
41. Barker F.G.2nd, Jannetta P.J., Bissoneete D.J., et al. The long-term outcome of microvascular decompression for trigeminal neuralgia. N Eng J Med. 1996;334:1077-1083.
42. Coffey R.J. Deep brain stimulation for chronic pain: Results of two multicenter trials and a structured review. Pain Med. 2001;2:183-192.
43. Meyerson B.A., Lindblom U., Linderoth B., et al. Motor cortex stimulation as treatment of trigeminal neuropathic pain. Acta Neurochir Suppl (Wien). 1993;58:150-153.
44. Ebel H., Rust D., Tronnier V., et al. Chronic precentral stimulation in trigeminal neuropathic pain. Acta Neurochir (Wien). 1996;138:1300-1306.
45. Rainov N.G., Fels C., Heidecke V., Burkert W. Epidural electrical stimulation of the motor cortex in patients with facial neuralgia. Clin Neurol Neurosurg. 1997;99:205-209.
46. Henderson J.M., Boongird A., Rosenow J.M., et al. Recovery of pain control by intensive reprogramming after loss of benefit from motor cortex stimulation for neuropathic pain. Stereotact Funct Neurosurg. 2004;82:207-213.