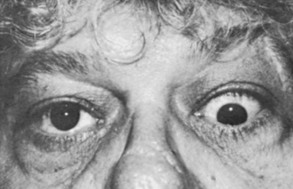
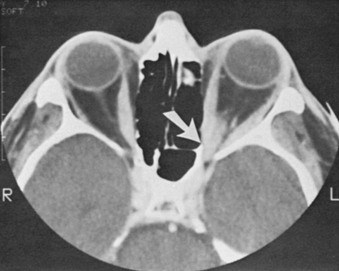
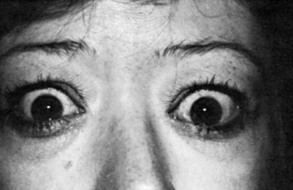
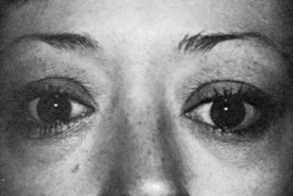
Graves’ Ophthalmopathy
Graves’ ophthalmopathy occurs as a spectrum of disease ranging from subclinical enlargement of the extraocular muscles in most patients with this condition to disfiguring and vision-threatening involvement of the entire orbit in an unfortunate few. Although the diagnosis of ophthalmopathy in patients with Graves’ disease may precede, accompany, or follow that of hyperthyroidism, eye involvement generally is diagnosed concurrently with or after the diagnosis of thyrotoxicosis1–5 (Fig. 10-1).
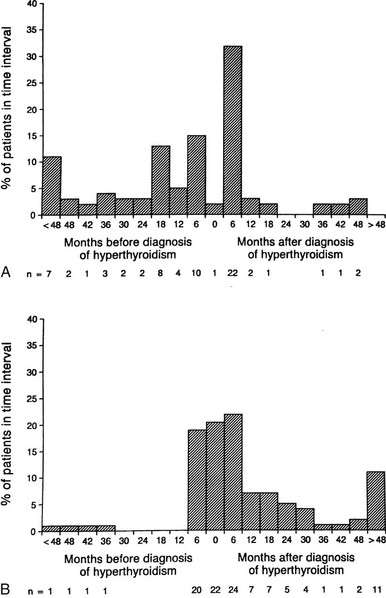
FIGURE 10-1 Onset of eye symptoms and diagnosis of Graves’ ophthalmopathy relative to the time of diagnosis of hyperthyroidism (0 on the horizontal axis). A, The number of patients who first experienced eye symptoms within a given 6-month period is expressed as a percentage of the entire group. B, The number of patients in whom Graves’ ophthalmopathy was first diagnosed within a given 6-month period. (From Bartley GB, Fatourechi V, Kadrmas EF, et al: The chronology of Graves’ ophthalmopathy in an incidence cohort. Am J Ophthalmol 121:426–434, 1996. Published with permission from the American Journal of Ophthalmology. Copyright by the Ophthalmic Publishing Company.)
The concept of a pathophysiologic link between Graves’ thyrotoxicosis and ophthalmopathy has been challenged on the basis of the existence of patients with euthyroid ophthalmopathy or unilateral eye disease and a poor numeric correlation between thyroid-stimulating antibody levels and the presence of eye disease. However, these arguments have been superseded by the demonstration of subtle thyroid abnormalities in most “euthyroid” ophthalmopathy patients,6,7 the finding of contralateral eye muscle abnormalities in most patients with apparent unilateral disease,1 and the demonstration of a definite qualitative association between the severity of ophthalmopathy and other peripheral manifestations of Graves’ disease and thyroid-stimulating antibody titers.8,9
Management of Graves’ ophthalmopathy requires a carefully integrated approach involving the endocrinologist and the ophthalmologist, with the goal of preserving the patient’s vision and restoring favorable self-perception and quality of life.10 In this chapter, we provide an examination of the present state of knowledge regarding the pathogenesis of Graves’ ophthalmopathy and an up-to-date review of current methods in the diagnosis and management of this disorder.
Epidemiology
Clinically evident ophthalmopathy occurs in 10% to 25% of unselected patients with Graves’ disease if lid signs are excluded as a diagnostic feature, in 30% to 45% if lid signs are included,3,11 and in approximately 70% of patients without overt eye disease if computed tomography (CT) or increased intraocular pressure on upgaze is used to establish the diagnosis.12–15 Magnetic resonance imaging (MRI) reveals extraocular muscle enlargement in 71% of patients without overt findings on physical examination.16 Fortunately, fewer than 5% of patients with Graves’ disease have severe ophthalmopathy.17 The age-adjusted incidence of Graves’ ophthalmopathy in the population of Olmsted County, Minnesota, was found to be 16 cases per 100,000 population per year for women and 2.9 for men.18 A bimodal distribution was noted, with peak incidence in the age groups 40 to 44 years and 60 to 64 years in women and 45 to 49 years and 65 to 69 years in men. Additional peripheral manifestations of Graves’ disease such as dermopathy and acropachy occur with lower frequency. Thyroid dermopathy is found in 4% to 15% of patients with clinically evident Graves’ ophthalmopathy, and 7% of dermopathy patients will also have thyroid acropachy.4
Pathogenesis
Orbital imagining in Graves’ ophthalmopathy reveals an increase in the volume of soft tissue within the orbit, including the extraocular muscles and adipose and connective tissues. The bones of the orbit are unyielding in response to the pressure generated by this increase in tissue volume. Therefore, forward displacement of the globe, or proptosis, may result and can serve as a natural means of orbital decompression. The degree of proptosis is limited, to varying degrees, by the orbital septum and tethering action of the extraocular muscles on the globe. Although CT scans show that the increased volume of orbital tissues is due to enlargement of both the orbital fat and the extraocular muscles in most patients, some patients appear to have predominantly adipose tissue or extraocular muscle involvement.19 In addition, the orbital fatty tissue volume appears to be more closely correlated with the degree of proptosis than is the extraocular muscle volume. Besides resulting in forward displacement of the globe, the increase in orbital tissue volume causes impairment of venous and lymphatic outflow from the orbit, leading to periorbital and conjunctival edema.
Predisposing Influences
The autoimmune thyroid diseases, including Graves’ disease and Hashimoto’s thyroiditis, are genetically complex and develop as a result of interactions between susceptibility genes and nongenetic factors. Studies performed in the past decade have identified several genes that either contribute directly to the development of these diseases or appear to be linked to important susceptibility genes.20–23 However, no unique gene associations distinct from those predisposing to Graves’ disease itself have been convincingly identified in patients with severe ophthalmopathy. A study of the families of 114 consecutive patients with Graves’ ophthalmopathy did not support a major role for familial factors in the development of severe ophthalmopathy.24 These findings suggest that environmental factors, rather than major susceptibility genes, are likely to predispose certain individuals with Graves’ disease to the development of ophthalmopathy.
Tobacco Smoking
A striking association between cigarette smoking and Graves’ ophthalmopathy has been noted in several studies. One group of researchers found that current smokers or ex-smokers represented 64% of patients with Graves’ disease and ophthalmopathy, compared with 47.9% of those without overt ophthalmopathy, 23.6% of patients with toxic nodular goiter, 30.4% of individuals with nontoxic goiter, 33.5% of patients with Hashimoto’s thyroiditis, and 27.8% of normal controls25 (Fig. 10-2). These effects do not appear to be related to behavioral changes associated with thyrotoxicosis, nor do they seem to be due to differences in the age, gender, or educational background of the study patients and their controls.26 Although the mechanisms underlying this association remain unknown, smokers have larger thyroids and higher thyroglobulin levels than do nonsmokers. Other contributors might include the effect of orbital hypoxia27 or the action of free radicals contained in tobacco smoke on orbital fibroblast proliferation.28 Smokers have lower levels of interleukin-1 receptor antagonists than do nonsmokers,29 which could lead to enhanced negative effects of interleukin-1 on the orbital inflammatory process.30 Smokers in one study were more likely to experience aggravation of ophthalmopathy after radioiodine therapy than were nonsmokers.17,31 Finally, smoking was shown to adversely influence the course of the eye disease during treatment with corticosteroids and orbital radiotherapy.32 The strong association between smoking and ophthalmopathy might provide important clues to the pathogenesis of this disorder in at least a subset of patients.
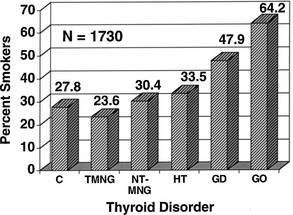
FIGURE 10-2 The prevalence of past and present cigarette smokers among patients with various thyroid disorders. Percentages shown (on the vertical axis) represent the prevalence of cigarette smokers in the group. Normal controls (C), toxic nodular goiter (TMNG), nontoxic goiter (NT-MNG), Hashimoto’s thyroiditis (HT), Graves’ disease without eye involvement (GD), and Graves’ disease with ophthalmopathy (GO) are shown. Smokers represented significantly increased proportions among patients with diagnoses of Graves’ disease and Graves’ ophthalmopathy. (From Burch HB, Wartofsky L: Graves’ ophthalmopathy: current concepts regarding pathogenesis and management. Endocr Rev 14:747–793, © 1993, The Endocrine Society.)
Age and Gender
Patient age and gender may also affect the prevalence and severity of Graves’ ophthalmopathy. The female-to-male ratio in series of ophthalmopathy patients has ranged from 1.8 to 1 to 2.8 to 1, which is considerably lower than the ratio of 8 to 1 generally cited for Graves’ disease in general.33 Men also appear to be disproportionately represented among patients with severe ophthalmopathy. One study found a female-to-male ratio of 9.3 to 1 in patients with mild disease, 3.2 to 1 in patients with moderate disease, and 1.4 to 1 in patients with severe ophthalmopathy.34
Therapy for Thyrotoxicosis
An area of considerable controversy concerns the impact of the choice of therapy for hyperthyroidism on the subsequent course of ophthalmopathy in Graves’ disease.35–37 Several retrospective studies have examined this issue, often with conflicting results.3 More recently, prospective trials have focused on this area.38–42 Two studies allow a direct comparison between the effects of radioiodine, thyroidectomy, or antithyroid drug therapy.39,42 In the first of these studies, 114 patients aged 35 to 55 years were randomized to receive radioiodine, thyroidectomy, or methimazole.39 As assessed with an ophthalmopathy index, new or worsened eye involvement occurred in 10% of patients treated medically, 16% of those treated surgically, and 33% of those treated with 131I. Interpretation of this small study was hampered by a higher prevalence of cigarette smokers among the radioiodine-treated patients, a period of hypothyroidism before thyroid hormone therapy was started in patients treated with radioiodine, and a requirement for multiple doses of radioiodine in nearly half the patients receiving this therapy, which suggests both refractory disease and a mechanism by which repeated release of thyroid antigen may have contributed to the autoimmune response.1,43 However, these authors later showed that elevated TSH levels after 131I treatment did not correlate with worsening eye status,44,45 and that eye changes in smokers were no more frequent than eye changes in nonsmokers. Finally, the authors noted that most patients receiving multiple doses of 131I had worsening of eye status before the second dose of radioiodine was given.46
Another randomized trial compared eye changes in 150 patients treated with radioiodine, 148 patients receiving methimazole alone, and a third group of 145 patients receiving both radioiodine and prophylactic prednisone.42 Patients were monitored for 1 year and were assessed for change by largely objective criteria, as well as an activity score and patient self-assessment. The groups were similar with regard to percentages of smokers or patients with preexisting ophthalmopathy. Hypothyroidism or persistent hyperthyroidism was corrected within 2 to 3 weeks of testing performed every 1 to 2 months. Worsening of eye disease occurred within 6 months after radioiodine therapy in 15% of patients versus 2.7% of patients who received antithyroid drugs alone. Seventy-four percent of the patients who experienced worsening eye status after radioiodine therapy had preexisting ophthalmopathy. The eye changes that occurred were largely mild and returned to baseline within 2 to 3 months in 65% of cases. However, eight patients (5%) in the radioiodine group required orbital radiation or high-dose corticosteroids as compared with one patient in the methimazole group and no patients in the combined prednisone and radioiodine group. Patients who had preexisting ophthalmopathy and those who were smokers were more likely to have progression after radioiodine administration.
A logical interpretation of the two prospective controlled studies in this area is that patients with Graves’ disease who are treated with radioiodine therapy have an increased risk for worsening eye status compared with those who are treated with antithyroid drugs alone. An alternative explanation for these findings is that patients who are treated with antithyroid drugs experience beneficial effects from this therapy47 rather than harmful effects from the radioiodine.48 In any event, the ocular worsening that was noted was generally mild, reversible, and short-lived. Patients with preexisting eye disease and those who smoke or have severe thyrotoxicosis appear to be more likely to experience this complication, but concurrent use of corticosteroids negates this risk.1,49,50
Despite continuing uncertainty concerning the use of radioiodine thyroid ablation, results of these studies appear to have influenced management practices in patients with Graves’ hyperthyroidism. A recent survey of members of the European Thyroid Association revealed that 60% of respondents believe that their choice of therapy would be influenced by the presence of ophthalmopathy, two thirds of whom would avoid radioiodine in the presence of severe eye disease.51 However, many members of this organization would not chose radioiodine as the first therapy even in the absence of eye disease.52
The authors use an approach that is tailored to the individual patient. Thyrotoxicosis in patients without ophthalmopathy or with mild inactive ophthalmopathy generally is managed with radioiodine without concurrent corticosteroids. Patients with active eye disease are managed with antithyroid drugs alone or the combined use of radioiodine and corticosteroids (commencing with 0.3 to 0.5 mg of prednisone/kg bw per day orally 1 to 3 days after radioiodine and tapering the dose until withdrawal about 3 months later).1 Near-total thyroidectomy is occasionally the treatment of choice, such as in a patient who is allergic to thionamides and has progressive ophthalmopathy. Thyroidectomy and total thyroid ablation share the theoretical advantage of removing thyroid antigen, which could potentially serve to bolster the autoimmune response directed against this antigen. However, evidence at present is insufficient to support this as a routine approach. The most important factors in prevention or successful treatment for Graves’ eye disease appear to be early and accurate control of hyperthyroidism (with avoidance of subsequent hypothyroidism) with the use of radioiodine, antithyroid drugs, or surgery, and counseling the patient to refrain from smoking.1,17 Frequent monitoring of thyroid status (every 4 to 6 weeks) is important in the initial stages of treatment to ensure that euthyroidism is restored promptly and maintained stably.1
The Orbital Autoimmune Response
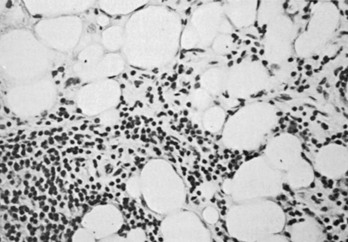
The autoimmune response within the orbit can be dissected into contributions from constituent cells of the inflamed orbit, including extraocular myocytes, connective tissue (fibroblasts, adipocytes, and intercellular matrix), and “professional” immune effector cells and their products.2,3,53 Histologic analysis has revealed largely intact muscle fibers, an expanded extracellular compartment, and infiltration by macrophages, activated T lymphocytes, and to a lesser extent B lymphocytes, as well as natural killer cells54 (Fig. 10-3). Further characterization of the activated retro-ocular T lymphocytes reveals increases in both CD4+ and CD8+ lymphocytes and restriction in the T cell receptor repertoire.55 T cell phenotyping has shown the presence of Th156 or Th257 profiles for cytokine gene expression or no clear preponderance of either phenotype.58,59 Additional evidence suggests that the profile that is found might depend on the stage of disease, Th1 being predominant in early disease and the Th2 profile appearing in later stages.60 Enlargement of the connective tissue compartment can be ascribed in part to a proliferation of retro-ocular fibroblasts and an attendant increase in the secretion of hydrophilic glycosaminoglycans by these cells.53 In addition, recent studies suggest that adipogenesis is active in the orbits of patients with Graves’ ophthalmopathy and results in expansion of the orbital adipose tissues.2,61,62 The increase in orbital tissue volume caused by edema and increased orbital fat causes forward displacement of the globe and venous compression, followed by orbital congestion and further edema.
Orbital Immune Targets
Although extraocular muscle enlargement and subsequent fibrosis play a central role in the mechanics of Graves’ ophthalmopathy, the retro-ocular fibroblast and the embryologically related preadipocyte may have a greater role in the molecular events contributing to orbital autoimmunity. Retro-ocular CD8+ T cells proliferate in response to autologous retro-ocular fibroblasts but not eye muscle extracts, which suggests that fibroblasts might contain antigenic targets for activated T lymphocytes.63 Similar results were found recently with the use of retro-ocular CD4+ T cells against autologous orbital fibroblast protein.64 Retro-ocular fibroblasts respond to various cytokines with proliferation, release of glycosaminoglycan, and expression of several immunomodulatory proteins, including HLA class II molecules, lymphocyte adhesion molecules such as intercellular adhesion molecule-1, and heat shock proteins.2,53 Orbital fibroblasts also appear to have unique characteristics and responses to cytokines that facilitate their participation in the autoimmune response, as opposed to fibroblasts derived from other sources.65–67
Coexpression of thyroid autoantigen in the retro-ocular tissue has received considerable attention in studies investigating the pathogenesis of Graves’ ophthalmopathy. Investigators in studies of this sort have difficulty distinguishing a secondary immune response against previously sequestered antigens released during tissue damage from a primary autoimmune response. It now is generally believed that antibodies against such retro-ocular proteins as the 64 kDa extraocular muscle protein,68 protein 1D,69 and the 23 kDa fibroblast protein70 are secondary phenomena.
Because the autoimmune response against the TSHR is responsible for the hyperthyroidism of Graves’ disease, numerous studies have examined retro-ocular tissue for expression of the TSHR or antigenically related proteins. Most studies show at least a low level of TSHR gene expression in retro-ocular fibroblasts,71,72 preadipocytes, or retro-orbital fat73,74 and either a TSHR or an antigenically related protein in these cells.75–77 In addition, some studies have found higher levels of TSHR gene expression in Graves’ orbital adipose tissues than in normal orbital tissues or tissues from Graves’ patients with inactive eye disease.73,78,79 Insight into the role of TSHR as a thyroid and orbital antigen has come from animal models of Graves’ disease, including one in which increased TSHR expression was noted in the orbits of mice vaccinated with TSHR cDNA or receiving T cells that were primed in animals immunized with a TSHR fusion protein.80 Recent studies of cultured orbital preadipocytes have shown enhanced TSHR expression following stimulation of adipogenesis in these cells,81 suggesting a link between increased TSHR antigen expression and the expanded orbital adipose tissues characteristic of the disease.62 A current model for the pathogenesis of Graves’ ophthalmopathy is shown in Fig. 10-4.
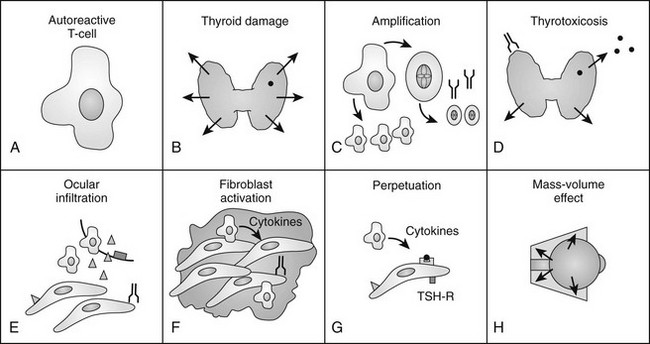
FIGURE 10-4 The authors’ current concept of the pathogenesis of Graves’ ophthalmopathy. A, In the presence of a permissive immunogenetic milieu and given appropriate environmental stimuli, autoreactive T cells directed against thyroid antigens emerge. B, Thyroid damage releases thyroid antigens, including the thyroid-stimulating hormone receptor (TSHR), thyroglobulin, and thyroid peroxidase. C, Release of thyroid antigens leads to further activation of autoreactive T lymphocytes, which results in amplification of both the cellular and humoral immune response against thyroid antigens. D, Thyrotoxicosis results from activation of the TSHR by circulating antibodies against this receptor. E, Circulating activated T lymphocytes infiltrate the orbit in response to the elaboration of specific lymphocyte adhesion molecules by orbital connective tissue cells. F, Activated T lymphocytes synthesize and release cytokines that stimulate fibroblasts to proliferate and to produce glycosaminoglycans. In addition, orbital preadipocytes are stimulated to differentiate into adipocytes that express increased levels of TSHR. G, Presentation of thyroid-eye cross-reactive antigens, such as hTSHR, leads to further activation of the local autoimmune response within the orbit. Enhanced orbital fat cell production and local edema caused by glycosaminoglycan-associated hydrophilic forces result in increased orbital tissue volume. H, The resulting mass-volume mismatch within the orbit produces venous congestion and forward displacement of the globe and leads to periorbital and conjunctival edema, extraocular muscle dysfunction, and proptosis.
History and Examination
In diagnosing the clinical features of Graves’ ophthalmopathy, it is helpful for the physician to be familiar with a problem-focused ophthalmic history and examination.82,83
Examination of The Eye: An Ophthalmic Evaluation for The Nonophthalmologist
Objective measurement and recording of eye findings are essential for assessing both the severity of ophthalmopathy and the response to therapy.84 In addition, an assessment of disease activity and the patient’s self-assessment of the disease state are important.85 The following nine areas should be included in the eye evaluation.
Visual Acuity
Visual acuity usually is measured as a Snellen fraction (e.g., 20/30) for distance vision. During bedside or office examinations, however, one may use a near-vision acuity card, several of which are commercially available. In the absence of a standardized card, the patient may be asked to read any available printed material, for example, the smallest type possible in a newspaper; the size of the print can be recorded, or the material itself can be taped in the patient’s record. Of course, patients should wear their glasses when visual acuity is being checked. Because loss of color perception can be an early sign of optic neuropathy, color vision evaluation is an important diagnostic test.86,87 One simple method for detecting possible early optic neuropathy is to check whether the patient perceives a difference between the two eyes in the color intensity of a red object; the top of a bottle of mydriatic eye drops is commonly used for this purpose. More advanced color vision testing should be performed by an ophthalmologist.
Eyelids
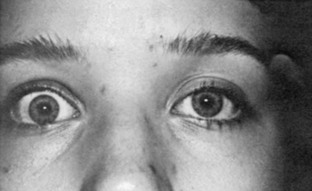
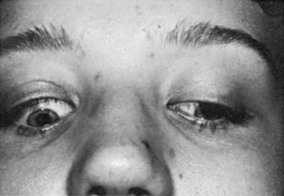
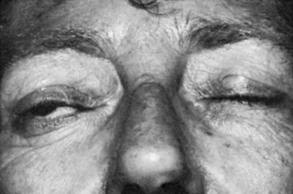
Upper eyelid retraction is a common finding in patients with Graves’ ophthalmopathy.5,88–90 Early in the course of Graves’ disease, eyelid malposition may result from increased sympathetic activity. With chronicity, the eyelid retractors (levator palpebrae superioris and Müller’s muscle) become hypertrophic, eventually fibrotic, and adherent to orbital tissues.91 Lid retraction may be unilateral or bilateral and may be subtle in some instances. The upper lid usually rests 1 to 2 mm below the junction of the cornea and sclera; therefore, if the white of the eye is seen above the corneoscleral limbus, eyelid retraction of at least 1.5 mm is present. The level of the lower eyelid is typically at the inferior corneoscleral limbus. Lower eyelid retraction is a less constant and specific finding and usually is not seen in patients with Graves’ ophthalmopathy without concomitant retraction of the upper lids. Lid lag (Figs. 10-5 and 10-6), which is diagnosed by asking the patient to look down and then observing delayed or restricted excursion of the upper eyelids as they follow the globes, and lagophthalmos (Fig. 10-7), which is an inability to close the eyelids completely, are additional stigmata of Graves’ ophthalmopathy. Eyelid retraction, lid lag, and lagophthalmos frequently interfere with the maintenance of an adequate tear film on the eye. Results include ocular irritation and dryness, reflex tearing, photophobia, and corneal scarring or even ulceration in severe cases.
Conjunctiva and Cornea
The conjunctiva is the clear, thin tissue that covers the sclera. It is normally transparent, except for the small blood vessels that course within it. In Graves’ ophthalmopathy, the conjunctiva may become hyperemic (usually termed injection) or edematous (chemosis) from exposure or from decreased venous drainage secondary to orbital suffusion. A characteristic conjunctival finding in patients with Graves’ eyes is focal injection over the insertions of the lateral or medial rectus muscles (Fig. 10-8). Engorged blood vessels do not extend to the corneoscleral limbus.
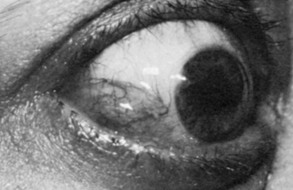
FIGURE 10-8 Chemosis (conjunctival edema) and focal injection (conjunctival blood vessel dilation) over the lateral rectus muscle insertion in a patient with Graves’ ophthalmopathy.
The cornea normally appears transparent and lustrous. Dryness resulting from exposure is difficult to detect without slit-lamp biomicroscopy. Corneal ulceration, which is an ophthalmic emergency, usually can be seen grossly with a penlight and typically is accompanied by severe pain.92
Exophthalmometry
Proptosis may be quantitated with an exophthalmometer, an instrument that measures the position of the globes in relation to the lateral orbital rim. The device is easy to use and is helpful in documenting the results of treatment. Exophthalmometry measurements in most adult eyes are 22 mm or less, and the difference between the patient’s two eyes usually does not exceed 2 mm.93 Caucasians tend to have Hertel exophthalmometry measurements of less than 18 to 20 mm, which is higher than that of Asians (16 to 18 mm) and lower than those seen in many blacks (20 to 22 mm).94–96
Ocular Motility
The range of movement should be evaluated for each eye separately and then for the eyes together, during which time the patient should be asked to state whether double vision is noted. Diplopia is most likely to occur in upgaze or in the extremes of lateral gaze because of restriction of the inferior or medial recti. Any or all of the extraocular muscles may be involved in Graves’ ophthalmopathy, however, and unusual patterns of strabismus may occur (Fig. 10-9).
Disease Activity
It is useful to classify patients with Graves’ ophthalmopathy as to whether they have active or inactive disease, as patients with active disease are more likely to respond to immunosuppressive therapy.1 This can be accomplished by using the Clinical Activity Score (CAS), which most commonly is a scale consisting of seven items, including spontaneous retrobulbar pain, pain on attempted upgaze or downgaze, redness of the eyelids, redness of the conjunctivae, swelling of the eyelids, inflammation of the caruncle and/or plica, and conjunctival edema. Patients who have a CAS ≥3/7 of these items should be considered to have active disease.1
Formal Ophthalmology Testing
Patients with positive findings in any of the above areas (i.e., most patients with Graves’ ophthalmopathy, except the mildest cases) should be referred to an ophthalmologist for additional testing, which may include color vision testing, perimetry (visual fields), measurement of intraocular pressure, and slit-lamp biomicroscopy. It is important to refer urgently any patient with Graves’ ophthalmopathy who experiences unexplained deterioration in vision, change in intensity or quality of color vision, globe subluxation, corneal opacity, disk swelling, or corneal exposure when eyelids are closed.1
Subtle evidence of optic neuropathy may be assessed with visual-evoked potentials,97 color vision testing, or automated perimetry. A Farnsworth-Munsell panel detects subtle acquired color vision defects better than do most pseudoisochromatic color plate systems designed to evince congenital color vision abnormalities.98 It is important to remember that approximately 7% to 8% of the male population has some degree of congenital red-green color “blindness.” Measurement of extraocular muscle dysfunction relies on the Maddox rod test, the alternate cover test, the Hess chart, or the Lancaster red-green test. Exposure keratitis may be detected by slit-lamp examination with rose bengal or fluorescein staining.
Differential Diagnosis
Each major manifestation of Graves’ ophthalmopathy has an associated differential diagnosis. The combination of more than one finding, such as lid retraction and proptosis, or the finding of biochemical thyroid dysfunction increases the likelihood that one is dealing with a manifestation of Graves’ disease.99
Visual loss caused by optic neuropathy in Graves’ ophthalmopathy needs to be distinguished from that resulting from exposure keratitis, cataracts, macular degeneration, intracranial or orbital tumors, diabetic retinopathy, or psychogenic causes. The differential diagnosis for eyelid retraction includes neurogenic, myogenic, and mechanical causes.100 Pseudoretraction of an eyelid may occur in response to aponeurogenic ptosis in the normal appearing contralateral eye.100,101 Proptosis measurements may be affected by systemic nonthyroidal illness, with recession of the orbit in wasting disorders and forward protrusion in obesity. Proptosis of up to 25 mm has been described as a familial trait.102 The differential diagnosis for proptosis is reviewed in Table 10-1.
Extraocular muscle enlargement may be seen with orbital malignancies, orbital “pseudotumor,” and other inflammatory conditions, including sarcoidosis and Wegener’s granulomatosis.3 Vascular anomalies of the orbit that can simulate the clinical features of Graves’ ophthalmopathy include dural-cavernous sinus fistulas (low-flow shunts), carotidcavernous sinus fistulas (high-flow shunts),103 and orbital varices. Historical features and appropriate radiographic studies usually facilitate proper diagnosis. Myasthenia gravis may cause both eyelid malposition and extraocular muscle dysfunction and can be confused with or may add to the severity of Graves’ ophthalmopathy. Patients with ocular myasthenia are more likely to have an associated autoimmune thyroid disease and are less likely to be anticholinesterase antibody positive than are patients with generalized myasthenia.104
Natural History
The natural history of Graves’ ophthalmopathy is characterized by a period of progression over a span of 3 to 6 months, a plateau phase, and then gradual improvement. Individual components of ophthalmopathy have differing natural histories. Lid retraction is unlikely to be present at long-term follow-up, and soft tissue changes such as chemosis and lid edema improve or resolve in the vast majority of patients over short-term follow-up. Strabismus regresses spontaneously in only 30% to 40% of patients without specific therapy, and proptosis persists to some degree in up to 90% of carefully monitored individuals.3 A recent follow-up survey of an incidence cohort of patients with Graves’ ophthalmopathy at a mean of 9.4 years after initial eye examination revealed that 10% had experienced diplopia within the preceding 4 weeks, and 32.6% experienced ocular discomfort within the previous 4 weeks. Sixty-one percent of patients believed that their eyes had not returned to baseline, and 37.9% were dissatisfied with the appearance of their eyes.105 Another study found that among 59 patients with mild Graves’ ophthalmopathy who did not receive disease-modifying eye therapy and who were monitored for a median of 12 months, 64% showed spontaneous improvement, 22% showed no change, and 13.5% showed deterioration.106
Therapy
The vast majority of patients with Graves’ ophthalmopathy experience a mild self-limited disease course that has only minor impact on daily life and requires only local measures for symptomatic improvement. These patients generally have minor lid retraction (<2 mm), mild soft tissue involvement, exophthalmos <3 mm above normal for race and gender, transient or no diplopia, and corneal exposure responsive to lubricants.1 Other patients with more severe signs and symptoms that affect their daily lives to a significant extent may benefit from immunosuppressive therapy (if active) or surgical intervention (generally done in inactive disease). Patients with dysthyroid optic neuropathy or corneal breakdown warrant immendiate treatment. For all patients, it is important to restore thyroid hormone levels to normal before any type of orbital surgery is performed. The single exception to this rule is when sight-threatening ophthalmopathy threatens vision and requires urgent orbital decompression. The effect of ophthalmopathy on the selection of therapy for thyrotoxicosis was discussed earlier in the chapter under the heading “Therapy for Thyrotoxicosis.”
Local Measures
Mild symptoms resulting from corneal drying are treated effectively by instilling methylcellulose-containing eye drops and taping the eyelids shut at night to prevent nocturnal corneal drying.107 Worsening of diplopia and soft tissue changes at night results from dependent edema, which often responds to elevation of the head. The use of sunglasses or tinted lenses can assist in decreasing photophobia. Prisms are occasionally useful for the correction of mild diplopia. In a recent survey of members of the European Thyroid Association on management of an index case of ophthalmopathy, 76% of respondents recommended methylcellulose eye drops, 21% would use diuretics to decrease ocular edema, and 18% would recommend prisms to correct diplopia.108 The topical use of guanethidine eye drops to correct lid retraction has been associated with local irritation and variable effectiveness109 and is rarely encouraged today.
Immunomodulatory Therapy
Corticosteroids have been used for nearly 50 years in the treatment of Graves’ ophthalmopathy. These agents have both antiinflammatory and immunomodulatory effects and may inhibit the synthesis and release of glycosaminoglycan by fibroblasts.110–112 In general, corticosteroid therapy provides rapid relief from the pain, injection, and conjunctival edema associated with inflammatory soft tissue changes in patients with active Graves’ ophthalmopathy. Corticosteroid therapy, especially when given intravenously,1 is also highly effective in the treatment of compressive optic neuropathy, with most patients showing at least some improvement.1 Regression in proptosis and ophthalmoplegia has been reported, but such regression occurs to a lesser extent and with a greater likelihood of exacerbation after drug withdrawal.1
Oral corticosteroid therapy generally is initiated at a relatively high dose, such as 40 to 80 mg of prednisone per day.1 After 2 to 4 weeks, the daily dose is tapered by 2.5 to 10.0 mg every 2 to 4 weeks. In many instances, drug withdrawal results in exacerbation, which requires increased dosage and slowing of the rate of subsequent taper.113,114 Improvement in soft tissue inflammation begins within 1 to 2 days, and typical courses range from 3 to 12 months. Side effects associated with high-dose corticosteroid therapy may include gastrointestinal irritation, weight gain, psychosis, osteoporosis, and glucose intolerance.1
The use of depot subconjunctival or retrobulbar corticosteroid injections has been advocated as a means of attaining a high local concentration of drug and minimizing systemic side effects.115,116 However, the risk,117 patient discomfort, and lack of benefit beyond conventional regimens115 limit the utility of this approach.
Pulse therapy with intravenous methylprednisolone has been studied in patients with Graves’ ophthalmopathy. Using three doses of 500 mg on alternate days intravenously, followed by an oral regimen, one study found clinical improvement in 83% of the patients studied.118 Similar results have been found by others, including one study consisting of five patients with optic neuropathy.119 A recent randomized trial compared pulse therapy with methylprednisolone versus oral prednisolone and found that intravenous pulse therapy was more effective and better tolerated than the oral regimen.120 While using weekly infusions of 500 mg methylprednisolone for 6 weeks, followed by 250 mg weekly for an additional 6 weeks, the authors noted improved disease activity and severity relative to the oral regimen. One important caveat regarding this approach is that the cumulative dose of intravenous methylprednisolone must not exceed 6 to 8 g, because higher doses have been associated with fatal hepatic dysfunction due to either direct toxicity or activation of autoimmune liver disease when these medications are discontinued.121
Predictors of clinical response to corticosteroids include high scores on a disease activity scale,122 signal intensity on T1-weighted MRI (signal intensity [SI] of extraocular muscle: SI of cerebral substantia nigra ≥2.15:1),123,124 orbital uptake of radiolabeled somatostatin analogues,125,126 and duration of disease (<18 months).
Cyclosporine
Cyclosporine inhibits helper T cell proliferation and cytokine production, prevents cytotoxic T cell activation, and suppresses immunoglobulin production by B lymphocytes.127 Two prospective randomized trials have examined the efficacy of cyclosporine in Graves’ ophthalmopathy.128,129 In one study,128 patients who were receiving prednisone alone were compared with those who were given both prednisone and cyclosporine, and changes in an activity score were used to monitor therapy. Combined therapy resulted in a more rapid fall in activity score and a greater decrease in extraocular muscle thickness as seen on CT. Recurrences were seen after corticosteroid therapy was stopped in nearly half the patients in the prednisone-alone group as compared with only 5% in the combined treatment group. In a second study,129 cyclosporine and prednisone were compared directly as single-agent therapies, and patients who failed either drug alone were given combination therapy. Prednisone was superior to cyclosporine as single-agent therapy, but nearly 60% of patients who did not respond to either drug alone subsequently improved with combined therapy. Despite this apparent efficacy, the high cost of cyclosporine and the requirement for frequent drug monitoring, together with an extensive side effect profile, limit the utility of this drug in clinical practice.
Somatostatin Analogue Therapy
The presence of somatostatin receptors on the surface of activated lymphocytes has been suggested as the explanation for orbital uptake of radiolabeled somatostatin analogues126 and has provided a rationale for the therapeutic use of octreotide and lanreotide in Graves’ ophthalmopathy. Despite findings of clinical improvement in nonrandomized trials, two recent randomized controlled trials utilizing a long-acting release formulation of octreotide showed minimal beneficial effect,130,131 and a third randomized trial noted slight improvement in fissure width measurement in patients with lid retraction, with no other clinical benefit.132
Orbital Radiotherapy
The rationale for the use of orbital radiation therapy in Graves’ ophthalmopathy involves the marked radiosensitivity of the lymphocyte, thought to be a primary effector in this disorder. Radiation to the orbits generally is administered at 20 Gy (2000 rad) calculated at the midline and delivered by lateral ports angled 5 degrees posteriorly to prevent inclusion of the anterior chamber and retina. Therapy is delivered in 10 fractions over a 2-week period. Several European centers have used a lower total dosage effectively.133,134 A beneficial effect has been reported within 1 to 4 weeks after the start of therapy135 and can continue for as long as 12 months after completion.
In a review of 14 uncontrolled studies of orbital radiotherapy in Graves’ ophthalmopathy, orbital radiation appeared to be well tolerated and seemed to provide benefit in approximately two thirds of treated patients.1 The largest single-center experience involved more than 300 patients treated with megavoltage irradiation, one third of whom received concurrent corticosteroid therapy.136,137 After orbital irradiation, 80% of patients exhibited improvement in soft tissue changes, 51% had recession of proptosis, 56% had improvement in eye muscle function, and 67% had improvement in vision. Despite this improvement, 29% of patients required one or more eye surgeries after orbital irradiation, most of which were performed to correct strabismus. It has been suggested that rather than obviating the need for corrective surgery, radiation might shorten the interval until stabilization of disease, thereby allowing earlier surgical intervention.
A randomized double-blind trial comparing orbital irradiation with prednisone in patients with Graves’ ophthalmopathy showed that although these two therapies yielded similar results, orbital irradiation had fewer side effects.138 In another study, the combination of orbital irradiation and corticosteroid therapy was deemed to be superior to medical therapy alone.139 These authors found that 26 of 36 (72%) patients who were treated with concurrent corticosteroids and orbital irradiation experienced a good or excellent response, as compared with only 3 of 12 (25%) patients who were treated with steroids alone.
A study in which patients were used as their own controls found little effectiveness of orbital radiotherapy alone in the treatment of Graves’ ophthalmopathy.140 In this prospective, randomized, double-blind, placebo-controlled study, the authors were unable to demonstrate any clinically significant beneficial effect of this treatment in patients with moderately severe disease. Prummel and colleagues examined the use of orbital irradiation in 88 patients with mild ophthalmopathy who were randomized to receive actual or sham irradiation.141 Among treated patients, 57% showed improvement in one or two major criteria such as duction and diplopia, compared with only 27% of patients receiving sham irradiation. No apparent effect on overall quality of life, cost of management, progression to severe disease, or need for corrective surgery was noted in treated patients. Although none of the trials examining orbital irradiation show sustained benefit, it is possible that orbital radiotherapy may be of benefit for selected patients, especially those with particularly active or severe disease, but this remains to be demonstrated.142
Side effects of orbital irradiation include temporary hair loss at the temples and transient worsening of soft tissue changes. Rare cases of retinopathy and cataracts have been described after orbital irradiation, and this underscores the importance of reliance on a center in which staff members have expertise with this application.143,144 Diabetic retinopathy is considered a contraindication to orbital irradiation because this condition increases vascular susceptibility to radiation damage.140
Other Immunomodulatory Therapy
B cell depletion therapy with such drugs as rituximab has been found to provide benefit for patients with rheumatologic disease, and several small uncontrolled studies have applied this therapy to patients with Graves’ disease with and without active ophthalmopathy. Salvi and colleagues treated nine Graves’ disease patients with rituximab at 2-week intervals for 16 weeks.145 Seven patients had active ophthalmopathy, and the remaining two had mild lid changes alone. Improvement in the clinical activity score occurred in all seven patients with active eye disease, with the change from a mean CAS of 4.7 to 1.8 after 30 weeks of follow-up.145 It is interesting to note that thyroid-stimulating hormone (TSH) receptor antibody titers did not change significantly during rituximab therapy. El Fassi and colleagues noted subjective and objective improvement in active ophthalmopathy in two patients treated with retuximab therapy at weekly intervals for 4 weeks.146 These same authors noted higher thyrotoxicosis remission rates in Graves’ disease patients treated with rituximab compared with untreated patients in a nonrandomized study—an effect that was unrelated to changes in TSH receptor antibody titers.147 Adverse effects, including fever, nausea, and a serum sickness–like response requiring corticosteroids, were common with rituximab therapy.
Additional immune therapy for active Graves’ ophthalmopathy has included azathioprine, cyclophosphamide, ciamexon, pentoxifylline, and intravenous immunoglobulin, all used with no benefit or no clear advantage noted over conventional therapy.3
Nonimmunomodulatory Therapy
Nonimmunomodulatory therapy for Graves’ ophthalmopathy, including local injection of botulinum toxin,148 bromocriptine,149 metronidazole,150 and acupuncture, has been tested with varying results.151
Surgical Treatment for Graves’ Ophthalmopathy
A tripartite approach is used most commonly in surgical treatment for Graves’ ophthalmopathy: orbital decompression to relieve optic neuropathy or proptosis, extraocular muscle surgery to reduce diplopia, and eyelid procedures to treat retraction and cosmetic disfigurement.152,153 In general, surgical intervention is performed in inactive disease to relieve significant proptosis or diplopia.1 However, orbital decompression sometimes is performed when the disease is active in patients who are intolerant or nonresponsive to immunosuppressive therapy.154 Although only a small fraction of patients with Graves’ disease require operative intervention, some patients with severe ophthalmopathy need multiple procedures to achieve satisfactory functional and aesthetic results.155
Orbital Decompression
The orbit is decompressed by removal of one or more of its bony walls, which expands the eye socket and increases the potential space for orbital contents.156 Indications for this procedure include optic neuropathy, severe proptosis (which in some patients may cause subluxation of the globe anterior to the lids), vision-threatening ocular exposure, debilitating retrobulbar and periorbital pain, and intolerable corticosteroid side effects.1 Additionally, because some extraocular muscle procedures used in patients with Graves’ ophthalmopathy may worsen exophthalmos, preliminary orbital decompression may be useful in those with severe proptosis. Finally, orbital expansion may be considered in some patients who do not have functional ocular disease but desire enhanced cosmesis.157
Optic neuropathy is the most common indication for orbital decompression. In most instances, the optic nerve is compressed by the enlarged or noncompliant extraocular muscles at the crowded orbital apex158,159 (Fig. 10-10); in some patients, however, the muscles are of essentially normal size. Through removal of one or more walls of the bony orbit, pressure on the nerve is reduced. Numerous approaches to orbital decompression have been described; variations include the number of walls removed (one, two, three, or four) and the avenue used for surgical access: lateral, medial, transpalpebral, transantral, transcranial, through a bicoronal incision, endoscopically, or through a combination of procedures.157 Transantral orbital decompression with removal of a portion of the medial wall and the orbital floor has been the preferred method at the Mayo Clinic over the past 25 years.160 Potential complications of orbital decompression include worsened diplopia, hypoglobus, numbness in the distribution of the infraorbital nerve, eyelid malposition, nasolacrimal duct obstruction, cerebrospinal fluid leakage, meningitis, and even death in rare instances.
Extraocular Muscle Surgery
Diplopia resulting from extraocular muscle involvement in Graves’ ophthalmopathy is often difficult to treat. When the disease is active, ocular alignment may vary from hour to hour and can preclude prism spectacle correction or surgical repair. If the disease is inactive and double vision cannot be corrected with glasses, strabismus surgery is indicated.161 Because the underlying problem is usually a restrictive myopathy (not paralysis, as older reports suggested) from tight, hypertrophied, and eventually fibrotic muscles, strabismus procedures for Graves’ ophthalmopathy most frequently involve weakening the muscles by recessing their insertions onto the globe. The goal of surgery is to allow single vision in primary (straight ahead) gaze, as well as in the reading position; postoperative diplopia in the extremes of lateral gaze or in upgaze is common and does not signify an unsuccessful procedure.
Eyelid Surgery
Eyelid surgery for Graves’ ophthalmopathy typically is performed after orbital decompression and strabismus procedures, if either or both are needed162 (Figs. 10-11 and 10-12). The retractors of the upper eyelid, the levator palpebrae superioris and Müller’s muscle, undergo pathologic changes similar to those seen in the extraocular muscles. Upper lid retraction is relieved by weakening (recessing) the muscles; lower lid retraction is treated with analogous procedures, although spacers of hard palate mucosa, tarsus, donor sclera, or cartilage often are grafted into the lids to counteract the tendency of gravity to pull the lids inferiorly during the postoperative period. Blepharoplasty (removal of excess eyelid and orbital tissue that prolapses anteriorly from the increase in orbital volume) may be of additional cosmetic value in selected patients.
References
1. Bartalena, L, Baldeschi, L, Dickinson, A, et al. Consensus statement of the European group on Graves’ orbitopathy (EUGOGO) on management of Graves’ orbitopathy. Thyroid. 2008;18:333–346.
2. Bahn, RS. Clinical Review 157: pathophysiology of Graves’ ophthalmopathy: the cycle of disease. J Clin Endocrinol Metab. 2003 May;88(5):1939–1946.
3. Burch, HB, Wartofsky, L. Graves’ ophthalmopathy: current concepts regarding pathogenesis and management. Endocr Rev. 1993;14:747–793.
4. Gorman, CA. Temporal relationship between onset of Graves’ ophthalmopathy and diagnosis of thyrotoxicosis. Mayo Clin Proc. 1983;58:515–519.
5. Bartley, GB, Fatourechi, V, Kadrmas, EF, et al. The chronology of Graves’ ophthalmopathy in an incidence cohort. Am J Ophthalmol. 1996;121:426–434.
6. Salvi, M, Zhang, Z-G, Haegert, D, et al. Patients with endocrine ophthalmopathy not associated with overt thyroid disease have multiple thyroid immunological abnormalities. J Clin Endocrinol Metab. 1990;70:89–94.
7. Kasagi, K, Konishi, J, Iida, Y, et al. Scintigraphic findings of the thyroid in euthyroid ophthalmic Graves’ disease. J Nucl Med. 1994;35:811–817.
8. Morris, JC, Hay, ID, Nelson, RE, et al. Clinical utility of thyrotropin receptor antibody assays: comparison of radioreceptor and bioassay methods. Mayo Clin Proc. 1988;63:707.
9. Chang, TC, Chang, TJ, Change, CC, et al. TSH and TSH receptor antibody-binding sites in fibroblasts of pretibial myxedema are related to the extracellular domain of entire TSH receptor. Clin Immunol Immunopathol. 1994;71:113–120.
10. Terwee, C, Wakelkamp, I, Tan, S, et al. Long-term effects of Graves’ ophthalmopathy on health-related quality of life. Eur J Endocrinol. 2002;146:751–757.
11. Werner, SC, Coelho, B, Quimby, EH. Ten year results of I-131 therapy in hyperthyroidism. Bull N Y Acad Med. 1957;33:783–806.
12. Forbes, G, Gorman, CA, Brennan, MD, et al. Ophthalmopathy of Graves’ disease: computerized volume measurements of the orbital fat and muscle. Am J Neuroradiol. 1986;7:651–656.
13. Chang, TC, Huang, KM, Chang, TJ, et al. Correlation of orbital computed tomography and antibodies in patients with hyperthyroid Graves’ disease. Clin Endocrinol. 1990;32:551–558.
14. Gamblin, GT, Harper, DG, Galentine, P, et al. Prevalence of increased intraocular pressure in Graves’ disease—evidence of frequent subclinical ophthalmopathy. N Engl J Med. 1983;308:420–424.
15. Gamblin, GT, Galentine, PG, Eil, C. Intraocular pressure and thyroid disease. In: Gorman CA, Campbell RJ, Dyer JA, eds. The Eye and Orbit in Thyroid Disease. New York: Raven Press; 1984:155–166.
16. Villadolid, MC, Nagataki, S, Uetani, M, et al. Untreated Graves’ disease patients without clinical ophthalmopathy demonstrate a high frequency of extraocular muscle (EOM) enlargement by magnetic resonance. J Clin Endocrinol Metab. 1995;80:2830–2833.
17. Wiersinga, WM, Bartelena, L. Epidemiology and prevention of Graves’ ophthalmopathy. Thyroid. 2002;12:855–860.
18. Bartley, GB, Fatourechi, V, Kadrmas, EF, et al. The incidence of Graves’ ophthalmopathy in Olmsted County, Minnesota. Am J Ophthalmol. 1995;120:511–517.
19. Anderson, RL, Tweeten, JP, Patrinely, JR, et al. Dysthyroid optic neuropathy without extraocular muscle involvement. Ophthalmic Surg. 1989;20:568–574.
20. Ban, Y, Davies, TF, Greenberg, DA, et al. The influence of human leukocyte antigen (HLA) genes on autoimmune thyroid disease (AITD): results of studies in HLA-DR3 positive AITD families. Clin Endocrinol. 2002;57:81–88.
21. Kouki, T, Gardine, CA, Yanagawa, T, et al. Relation of three polymorphisms of the CTLA-4 gene in patients with Graves’ disease. J Endocrinol Invest. 2002;25:208–213.
22. Tomer, Y. Genetic dissection of familial autoimmune thyroid disease using whole genome screening. Autoimmun Rev. 2002;1:198–204.
23. Brix, TH, Kyvik, KO, Hegedus, L. What is the evidence of genetic factors in the etiology of Graves’ disease? A brief review. Thyroid. 1998;8:727–734.
24. Villanueva, R, Inzerillo, AM, Tomer, Y, et al. Limited genetic susceptibility to severe Graves’ ophthalmopathy: no role for CTLA-4 but evidence for an environmental etiology. Thyroid. 2000;10:791–798.
25. Bartalena, L, Martino, E, Marcocci, C, et al. More on smoking habits and Graves’ ophthalmopathy. J Endocrinol Invest. 1989;12:733–737.
26. Prummel, MF, Wiersinga, WM. Smoking and risk of Graves’ disease. JAMA. 1993;269:479–482.
27. Metcalfe, RA, Weetman, AP. Stimulation of extraocular muscle fibroblasts by cytokines and hypoxia: possible role in thyroid-associated ophthalmopathy. Clin Endocrinol (Oxf). 1994;40:67–72.
28. Burch, HB, Lahiri, S, Bahn, R, et al. Superoxide radical production stimulates human retroocular fibroblast proliferation in Graves’ ophthalmopathy. Exp Eye Res. 1997;65:311–316.
29. Hofbauer, LC, Muhlberg, T, Konig, A, et al. Soluble interleukin-1 receptor antagonist serum levels in smokers and nonsmokers with Graves’ ophthalmopathy undergoing orbital radiotherapy. J Clin Endocrinol Metab. 1997;82:2244–2247.
30. Tan, GH, Dutton, CM, Bahn, RS. Interleukin-1 (IL-1) receptor antagonist and soluble IL-1 receptor inhibit IL-1-induced glycosaminoglycan production in cultured human orbital fibroblasts from patients with Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1996;81:449–452.
31. Bartalena, L, Marcocci, C, Tanda, ML, et al. Cigarette smoking and treatment outcomes in Graves ophthalmopathy. Ann Intern Med. 1998;129:632–635.
32. Eckstein, A, Quadbeck, B, Mueller, G, et al. Impact of smoking on the response to treatment of thyroid associated ophthalmopathy. Br Med J. 2003;87:773–776.
33. Vanderpump, MP, Tunbridge, WM, French, JM, et al. The incidence of thyroid disorders in the community: A twenty-year follow-up of the Whickham survey. Clin Endocrinol (Oxf). 1995;43:55–68.
34. Perros, P, Kendall-Taylor, P. Pathogenetic mechanisms in thyroid-associated ophthalmopathy. J Intern Med. 1992;231:205–211.
35. Gorman, CA. Therapeutic controversies: radioiodine therapy does not aggravate Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1995;80:340–342.
36. Pinchera, A, Bartalena, L, Marcocci, C. Therapeutic controversies: radioiodine may be bad for Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1995;80:342–345.
37. Wartofsky, L. Therapeutic controversies: summation, commentary, and overview: concerns over aggravation of Graves’ ophthalmopathy by radioactive iodine treatment and the use of retrobulbar radiation therapy. J Clin Endocrinol Metab. 1995;80:347–349.
38. Bartalena, L, Marcocci, C, Bogazzi, F, et al. Use of corticosteroids to prevent progression of Graves’ ophthalmopathy after radioiodine therapy for hyperthyroidism. N Engl J Med. 1989;321:1349–1352.
39. Tallstedt, L, Lundell, G, Tàrring, O, et al. Occurrence of ophthalmopathy after treatment for Graves’ hyperthyroidism. N Engl J Med. 1992;326:1733–1738.
40. Kung, AW, Cheng, A, Yau, CC. The incidence of ophthalmopathy after radioiodine therapy for Graves’ disease: prognostic factors and the role of methimazole. J Clin Endocrinol Metab. 1994;79:542–546.
41. Fernandez-Sanchez, JR, Vara-Thorbeck, R, Garbin-Fuentes, I, et al. Graves’ ophthalmopathy after subtotal thyroidectomy and radioiodine therapy. Br J Surg. 1993;80:1134–1136.
42. Bartalena, L, Pinchera, A, Martino, E, et al. Relation between therapy for hyperthyroidism and the course of Graves’ ophthalmopathy. N Engl J Med. 1998;338:73–78.
43. Mendlovic, DB, Saeed Zafar, M. Ophthalmopathy after treatment for Graves’ hyperthyroidism. N Engl J Med. 1992;327:1320.
44. Torring, O, Hamberger, B, Saaf, M, et al. Graves’ hyperthyroidism: treatment with antithyroid drugs, surgery, or radioiodine: a prospective, randomized study: Thyroid Study Group. J Clin Endocrinol Metab. 1996;81:2986–2993.
45. Tallstedt, L, Lundell, G. Radioiodine treatment, ablation, and ophthalmopathy: a balanced perspective. Thyroid. 1997;7:241–245.
46. Tallstedt, L. Ophthalmopathy after treatment for Graves’ hyperthyroidism. N Engl J Med. 1992;327:1321.
47. Wartofsky, L. Has the use of antithyroid drugs for Graves’ disease become obsolete? Thyroid. 1993;3:335–344.
48. Wiersinga, WM. Preventing Graves’ ophthalmopathy. N Engl J Med. 1998;338:121–122.
49. Bartalena, L, Marcocci, C, Bogazzi, F, et al. Use of corticosteroids to prevent progression of Graves’ ophthalmopathy after radioiodine therapy for hyperthyroidism. N Engl J Med. 1989;321:1349–1352.
50. Bartalena, L, Pinchera, A, Martino, E, et al. Relation between therapy for hyperthyroidism and the course of Graves’ ophthalmopathy. N Engl J Med. 1998;338:73–78.
51. Weetman, AP, Wiersinga, WM. Current management of thyroid-associated ophthalmopathy in Europe: results of an international survey. Clin Endocrinol (Oxf). 1998;49:21–28.
52. Wartofsky, L, Glinoer, D, Solomon, B, et al. Differences and similarities in the diagnosis and treatment of Graves’ disease in Europe, Japan, and the United States. Thyroid. 1991;1:129–135.
53. Bahn, RS, Heufelder, AE. Pathogenesis of Graves’ ophthalmopathy. N Engl J Med. 1993;329:1468–1475.
54. Delemarre, FG, Drexhage, HA, Simons, PJ. Histomorphological aspects of the development of thyroid autoimmune diseases: consequences for our understanding of endocrine ophthalmopathy. Thyroid. 1996;6:369–377.
55. Heufelder, AE, Scriba, PC, Wenzel, BE. Antigen receptor variable region repertoires expressed by T cells infiltrating thyroid, retroorbital, and pretibial tissue in Graves’ disease. J Clin Endocrinol Metab. 1996;81:3733–3739.
56. de Carli, M, del Prete, G, Romagnani, S, et al. Cytolytic T cells with Th1-like cytokine profile predominate in retroorbital lymphocytic infiltrates of Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1993;77:1120–1121.
57. McLachlan, SM, Rapoport, B, Prummel, MF. Cell-mediated or humoral immunity in Graves’ ophthalmopathy?: Profiles of T-cell cytokines amplified by polymerase chain reaction from orbital tissue. J Clin Endocrinol Metab. 1994;78:1070–1074.
58. Forster, G, Kahaly, G, Ochs, K, et al. Analysis of orbital T cells in thyroid-associated ophthalmopathy. Clin Exp Immunol. 1998;112:427–434.
59. Pappa, A, Lightman, S, Weetman, AP, et al. Analysis of extraocular muscle-infiltrating T cells in thyroid-associated ophthalmopathy (TAO). Clin Exp Immunol. 1997;109:362–369.
60. Aniszewski, JP, Valyasevi, RW, Bahn, RS. Relationship between disease duration and predominant orbital T cell subset in Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2000;85:776–780.
61. Kumar, S, Coenen, M, Scherer, P, et al. Evidence for enhanced adipogenesis in the orbits of patients with Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2003;88:4246–4250.
62. Bahn, RS. Clinical review: pathogenesis of Graves’ ophthalmopathy: the cycle of disease. J Clin Endocrinol Metab. 2003;88:1939–1946.
63. Grubech-Loebenstein, B, Trieb, K, Sztankay, A, et al. Retrobulbar T cells from patients with Graves’ ophthalmopathy are CD8+ and specifically recognize autologous fibroblasts. J Clin Invest. 1994;93:2738–2743.
64. Otto, EA, Kahaly, GJ, Wall, JR, et al. Orbital tissue-derived T lymphocytes from patients with Graves’ ophthalmopathy recognize autologous orbital antigens. J Clin Endocrinol Metab. 1996;81:3045–3050.
65. Smith, TJ. Orbital fibroblasts exhibit a novel pattern of responses to proinflammatory cytokines: potential basis for the pathogenesis of thyroid-associated ophthalmopathy. Thyroid. 2002;12:197–203.
66. Smith, TJ, Koumas, L, Gagnow, AM, et al. Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2002;87:385–392.
67. Koumas, L, Smith, TJ, Feldon, S, et al. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. J Pathol. 2003;163:1291–1300.
68. Kubota, S, Wall, J, Hiromatsu, Y, et al. The 64-kilodalton eye muscle protein is the flavoprotein subunit of mitochondrial succinate dehydrogenase: the corresponding serum antibodies are good markers of an immune-mediated damage to the eye muscle in patients with Graves’ hyperthyroidism. J Clin Endocrinol Metab. 1998;83:443–447.
69. Bernard, NF, Nygen, TN, Tyutyunikov, A, et al. Antibodies against 1D, a recombinant 64-kDa membrane protein, are associated with ophthalmopathy in patients with thyroid autoimmunity. Clin Immunol Immunopathol. 1994;70:225–233.
70. Bahn, RS, Gorman, CA, Johnson, CM, et al. Presence of antibodies in the sera of patients of patients with Graves’ disease recognizing a 23 kilodalton fibroblast protein. J Clin Endocrinol Metab. 1989;69:622–628.
71. Mengistu, M, Lukes, YG, Nagy, EV, et al. TSH receptor gene expression in retroocular fibroblasts. J Endocrinol Invest. 1994;17:437–441.
72. Heufelder, AE, Dutton, CM, Sarkar, G, et al. Detection of TSH receptor RNA in cultured fibroblasts from patients with Graves’ ophthalmopathy and pretibial dermopathy. Thyroid. 1993;3:297–300.
73. Bahn, RS, Heufelder, AE, Spitzweg, C, et al. Thyrotropin receptor expression in Graves’ orbital adipose/connective tissues: potential autoantigen in Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1998;83:998–1002.
74. Feliciello, A, Fenzi, G, Avvedimento, EV, et al. Expression of thyrotropin-receptor mRNA in healthy and Graves’ disease retro-orbital tissue. Lancet. 1993;342:337–338.
75. Burch, HB, Sellitti, D, Barnes, SG, et al. TSH receptor antisera for the detection of immunoreactive protein species in retroocular fibroblasts obtained from patients with Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1994;78:1384–1391.
76. Stadlmayr, W, Heufelder, AE, Bichlmair, AM, et al. TSH receptor transcripts and TSH receptor-like immunoreactivity in orbital and pretibial fibroblasts of patients with Graves’ ophthalmopathy and pretibial myxedema. Thyroid. 1997;7:3–12.
77. Perros, P, Kendall-Taylor, P. Demonstration of thyrotropin binding sites in orbital connective tissue: Possible role in the pathogenesis of thyroid-associated ophthalmopathy. J Endocrinol Invest. 1994;17:163–170.
78. Starkey, KJ, Janezic, A, Jones, G, et al. Adipose thyrotropin receptor expression is elevated in Graves’ and thyroid eye disease ex vivo and indicates adipogenesis in progress in vivo. J Mol Endocrinol. 2003;30:369–380.
79. Wakelkamp, IM, Bakker, O, Baldeschi, L, et al. TSH-R expression and cytokine profile in orbital tissue of active vs. inactive Graves’ ophthalmopathy patients. Clin Endocrinol. 2003;58:280–287.
80. Many, M-C, Costagliola, S, Detrait, M, et al. Development of an animal model of autoimmune thyroid eye disease. J Immunol. 1999;162:4966–4974.
81. Valyasevi, R, Erickson, DA, Harteneck, DA, et al. Differentiation of human orbital preadipocyte fibroblasts induces expression of functional thyrotropin receptor. J Clin Endocrinol Metab. 1999;84:2557–2562.
82. Erie, JC. Ophthalmic history and examination. In: Bartley BG, Liesegang TJ, eds. Essentials of Ophthalmology. Philadelphia: JB Lippincott; 1992:3–25.
83. Bartley, GB, Waller, RR. Graves’ ophthalmopathy. In: van Heerden JA, ed. Common Problems in Endocrine Surgery. Chicago: Year Book; 1989:25–29.
84. Gorman, CA. The measurement of change in Graves’ ophthalmopathy. Thyroid. 1998;8:539–543.
85. Classification of eye changes of Graves’ disease. Thyroid. 1992;2:235–236.
86. Fells, P. Management of dysthyroid eye disease. Br J Ophthalmol. 1991;75:245–246.
87. Neigel, JM, Rootman, J, Belkin, RI, et al. Dysthyroid optic neuropathy: the crowded orbital apex syndrome. Ophthalmology. 1988;95:1515–1521.
88. Bahn, RS, Garrity, JA, Bartley, GB, et al. Diagnostic evaluation of Graves’ ophthalmopathy. Endocrinol Metab Clin North Am. 1988;17:527–545.
89. Bartley, GB, Gorman, CA. Diagnostic criteria for Graves’ ophthalmopathy. Am J Ophthalmol. 1995;119:792–795.
90. Bartley, GB, Fatourechi, V, Kadrmas, EF, et al. Clinical features of Graves’ ophthalmopathy in an incidence cohort. Am J Ophthalmol. 1996;121:284–290.
91. Feldon, SE, Levin, L. Graves’ ophthalmopathy: V. Etiology of upper eyelid retraction in Graves’ ophthalmopathy. Br J Ophthalmol. 1991;74:484–485.
92. Bahn, RS, Bartley, GB, Gorman, CA. Emergency treatment of Graves’ ophthalmopathy. Ballieres Clin Endocrinol Metab. 1992;6:95–105.
93. Bogren, HG, Franti, CE, Wilmarth, SS. Normal variations of the position of the eye in the orbit. Ophthalmology. 1986;93:1072–1077.
94. Werner, SC. Modification of the classification of the eye changes of Graves’ disease: recommendations of the ad hoc committee of the American Thyroid Association. J Clin Endocrinol Metab. 1977;44:203–204.
95. Migliori, ME, Gladstone, GJ. Determination of the normal range of exophthalmometric values for black and white adults. Am J Ophthalmol. 1984;98:438–442.
96. Amino, N, Yuasa, T, Yabu, Y, et al. Exophthalmos in autoimmune thyroid disease. J Clin Endocrinol Metab. 1980;51:1232–1234.
97. Salvi, M, Zhang, Z-G, Haegert, D, et al. Patients with endocrine ophthalmopathy not associated with overt thyroid disease have multiple thyroid immunological abnormalities. J Clin Endocrinol Metab. 1990;70:89–94.
98. Mourits, MPH, Koornneef, L, Wiersinga, WM, et al. Clinical criteria for the assessment of disease activity in Graves’ ophthalmopathy: a novel approach. Br J Ophthalmol. 1989;73:639–644.
99. Waller, RR, Jacobson, DH. Endocrine ophthalmopathy: Differential diagnosis. In: Gorman CA, Campbell RJ, Dyer JA, eds. The Eye and Orbit in Thyroid Disease. New York: Raven Press; 1984:213–220.
100. Bartley, GB. The differential diagnosis and classification of eyelid retraction. Ophthalmology. 1996;103:168–176.
101. Gonnering, RS. Pseudoretraction of the eyelid in thyroid-associated orbitopathy. Arch Ophthalmol. 1988;106:1078–1080.
102. Werner, SC, Coleman, DJ, Frazen, LA. Ultrasonographic evidence of a consistent orbital involvement in Graves’ disease. N Engl J Med. 1974;290:1447–1450.
103. Merlis, AL, Schaiberger, CL, Adler, R. External carotid-cavernous sinus fistula simulating unilateral Graves’ ophthalmopathy. J Comput Assist Tomogr. 1982;6:1006–1009.
104. Marino, M, Mariotti, S, Muratorio, A, et al. Mild clinical expression of myasthenia gravis associated with autoimmune thyroid diseases. J Clin Endocrinol Metab. 1997;82:438–443.
105. Bartley, GB, Fatourechi, V, Kadrmas, EF, et al. Long-term follow-up of Graves ophthalmopathy in an incidence cohort. Ophthalmology. 1996;103:958–962.
106. Perros, P, Kendall-Taylor, P, Crombie, AL. Natural history of thyroid associated ophthalmopathy. Clin Endocrinol (Oxf). 1995;42:45–50.
107. Jacobson, DH, Gorman, CA. Diagnosis and management of Graves’ ophthalmopathy. Med Clin North Am. 1985;69:973–988.
108. Weetman, AP, Wiersinga, WM. Current management of thyroid-associated ophthalmopathy in Europe: results of an international study. Clin Endocrinol. 1998;49:21–28.
109. Martin, B, Jay, B. Use of guanethidine eye drops in dysthyroid lid retraction. Proc R Soc Med. 1969;62:18–19.
110. Sisson, JC. Stimulation of glucose utilization and glycosaminoglycan production by fibroblasts derived from retrobulbar tissue. Exp Eye Res. 1971;12:285–292.
111. Smith, TJ, Bahn, RS, Gorman, CA. Connective tissue, glycosaminoglycans, and diseases of the thyroid. Endocr Rev. 1989;10:366–391.
112. Smith, TJ. Dexamethasone regulation of glycosaminoglycan synthesis in cultured human skin fibroblasts: similar effects of glucocorticoid and thyroid hormone therapy. J Clin Invest. 1984;74:2157–2163.
113. Wiersinga, WM. Immunosuppressive treatment of Graves’ ophthalmopathy. Trends Endocrinol Metab. 1990;1:377–381.
114. Burman, KD. Treatment of autoimmune ophthalmopathy. Endocrinologist. 1991;1:102–110.
115. Marcocci, C, Bartalena, L, Panicucci, M, et al. Orbital cobalt irradiation combined with retrobulbar or systemic corticosteroids for Graves’ ophthalmopathy: a comparative study. Clin Endocrinol. 1987;27:33–42.
116. Yamamoto, K, Saito, K, Takai, T, et al. Treatment of Graves’ ophthalmopathy by steroid therapy, orbital radiation therapy, plasmapheresis, and thyroxine replacement. Endocrinol Jpn. 1982;29:495–501.
117. Kahaly, G, Beyer, J. Immunosuppressant therapy of thyroid eye disease. Klin Wochenschr. 1988;66:1049–1059.
118. Kendall-Taylor, P, Crombie, AL, Perros, P. High-dose intravenous methylprednisolone pulse therapy in severe thyroid-associated ophthalmopathy [abstract]. Thyroid. 1992;2(Suppl 1):29.
119. Guy, JR, Fagien, S, Donovan, JP, et al. Methylprednisolone pulse therapy in severe dysthyroid optic neuropathy. Ophthalmology. 1989;96:1048–1053.
120. Kahaly, GJ, Pitz, S, Hommel, G, et al. Randomized, single blind trial of intravenous versus oral steroid monotherapy in Graves’ orbitopathy. J Clin Endocrinol Metab. 2005 Sep;90(9):5234–5240.
121. Marino, M, Morabito, E, Brunetto, MR, et al. Acute and severe liver damage associated with intravenous glucocorticoid pulse therapy in patients with Graves’ ophthalmopathy. Thyroid. 2004;14:403–406.
122. Mourits, MP, Koornneef, L, Wiersinga, WM, et al. Clinical criteria for the assessment of disease activity in Graves’ ophthalmopathy: a novel approach. Br J Ophthalmol. 1989;73:639–644.
123. Laitt, RD, Hoh, B, Wakeley, C, et al. The value of short tau inversion recovery sequence in magnetic resonance imaging of thyroid eye disease. Br J Radiol. 1994;67:244–247.
124. Hiromatsu, Y, Kojima, K, Ishisaka, N, et al. Role of magnetic resonance imaging in thyroid-associated ophthalmopathy: Its predictive value for therapeutic outcome of immunosuppressive therapy. Thyroid. 1992;2:299–305.
125. Moncayo, R, Donnemiller, E, Kendler, D, et al. Evaluation of immunological mechanisms mediating thyroid-associated ophthalmopathy by radionuclide imaging using the somatostatin analog 111In-octreotide. Thyroid. 1997;7:21–29.
126. Kahaly, G, Bockisch, A, Hommel, G, et al. Indium-111-pentetreotide in Graves’ disease. J Nucl Med. 1998;39:533–536.
127. Wiersinga, WM. Novel drugs for the therapy of Graves’ ophthalmopathy. In: Wall JR, How J, eds. Graves’ Ophthalmopathy. Cambridge: Blackwell; 1990:111–126.
128. Kahaly, G, Schrezenmeir, J, Krause, U, et al. Cyclosporin and prednisone in treatment of Graves’ ophthalmopathy: a controlled, randomized and prospective study. Eur J Clin Invest. 1986;16:415–422.
129. Prummel, MF, Mourits, MP, Berghout, A, et al. Prednisone and cyclosporine in the treatment of severe Graves’ ophthalmopathy. N Engl J Med. 1989;321:1353–1359.
130. Dickinson, AJ, Vaidya, B, Miller, M, et al. Kendall-Taylor double-blind, placebo-controlled trial of octreotide long-acting repeatable (LAR) in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2004;89:5910–5915.
131. Wemeau, JL, Caron, P, Beckers, A, et al. Octreotide (long-acting release formulation) treatment in patients with Graves’ orbitopathy: clinical results of a four-month, randomized, placebo-controlled, double-blind study. J Clin Endocrinol Metab. 2005;90:841–848.
132. Stan, MN, Garrity, JA, Bradley, EA, et al. Randomized, double-blind, placebo-controlled trial of long-acting release octreotide for treatment of Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2006;91(12):4817–4824.
133. Sautter-Bihl, M-L. Orbital radiotherapy: recent experience in Europe. In: Wall JR, How J, eds. Graves’ ophthalmopathy. Cambridge: Blackwell; 1990:145–157.
134. Sautter-Bihl, M-L, Heinze, HG. Radiotherapy of Graves’ ophthalmopathy. Dev Ophthalmol. 1989;20:139–154.
135. Pigeon, P, Orgiazzi, J, Berthezene, F, et al. High voltage orbital radiotherapy and surgical orbital decompression in the management of Graves’ ophthalmopathy. Horm Res. 1987;26:172–176.
136. Kriss, JP, Peterson, IA, Donaldson, SS, et al. Supervoltage orbital radiotherapy for progressive Graves’ ophthalmopathy: results of a twenty year experience. Acta Endocrinol (Copenh). 1989;121(Suppl 2):154.
137. Peterson, IA, Kriss, JP, McDougall, IR, et al. Prognostic factors in the radiotherapy of Graves’ ophthalmopathy. Int J Radiat Oncol Biol Phys. 1990;19:259–264.
138. Prummel, MF, Mourits, MP, Blank, L, et al. Randomized double-blind trial of prednisone versus radiotherapy in Graves’ ophthalmopathy. Lancet. 1993;342:949–954.
139. Bartalena, L, Marcocci, C, Chiovato, L, et al. Orbital cobalt irradiation combined with systemic corticosteroids for Graves’ ophthalmopathy: comparison with systemic corticosteroids alone. J Clin Endocrinol Metab. 1983;56:1139–1144.
140. Gorman, CA, Garrity, JA, Fatourechi, V, et al. A prospective, randomized, double-blind, placebo-controlled study of orbital radiotherapy for Graves’ ophthalmopathy. Ophthalmology. 2001;108:1523–1534.
141. Prummel, MF, Terwee, CB, Gerding, MN, et al. A randomized controlled trial of orbital radiotherapy versus sham irradiation in patients with mild Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2004 Jan;89(1):15–20.
142. Bartalena, L, Marcocci, C, Gorman, CA, et al. Orbital radiotherapy for Graves’ ophthalmopathy: useful or useless? Safe or dangerous? J Endocrinol Invest. 2003;26:5–16.
143. Kinyoun, JL, Kalina, RE, Brower, SA, et al. Radiation retinopathy following orbital irradiation for Graves’ ophthalmopathy. Arch Ophthalmol. 1984;102:1473–1476.
144. Parsons, JT, Fitzgerald, CR, Hood, CI, et al. The effects of irradiation on the eye and optic nerve. Int J Radiat Oncol Biol Phys. 1983;9:609–622.
145. Salvi, M, Vannucchi, G, Campi, I, et al. Treatment of Graves’ disease and associated ophthalmopathy with the anti-CD20 monoclonal antibody rituximab: an open study. Eur J Endocrinol. 2007;156:33–40.
146. El Fassi, D, Nielsen, CH, Hasselbalch, HC, et al. Treatment-resistant severe, active Graves’ ophthalmopathy successfully treated with B lymphocyte depletion. Thyroid. 2006;16(7):709–710.
147. El Fassi, D, Clemmensen, O, Nielsen, CH, et al. Evidence of intrathyroidal B-lymphocyte depletion after rituximab therapy in a patient with Graves’ disease. J Clin Endocrinol Metab. 2007;92:3762–3763.
148. Lyons, CJ, Vickers, SF, Lee, JP. Botulinum toxin therapy in dysthyroid strabismus. Eye. 1990;4:538–540.
149. Lopatynsky, MO, Krohel, GB. Bromocriptine therapy for thyroid ophthalmopathy. Am J Ophthalmol. 1989;107:680–681.
150. Harden, RM, Chisholm, CJS, Cant, JS. The effect of metronidazole on thyroid function and exophthalmos in man. Metabolism. 1967;16:890–898.
151. Rogvi-Hansen, B, Perrild, H, Christensen, T, et al. Acupuncture in the treatment of Graves’ ophthalmopathy: a blinded randomized study. Acta Endocrinol (Copenh). 1991;124:143–145.
152. Bartley, GB, Fatourechi, V, Kadrmas, EF, et al. The treatment of Graves’ ophthalmopathy in an incidence cohort. Am J Ophthalmol. 1996;121:200–206.
153. Fatourechi, V, Garrity, JA, Bartley, GB, et al. Graves’ ophthalmopathy: results of transantral orbital decompression performed primarily for cosmetic indications. Ophthalmology. 1994;101:938–942.
154. Kazim, M, Trokel, S, Moore, S. Treatment of acute Graves’ orbitopathy. Ophthalmology. 1991;98:1443–1448.
155. Wilson, WB, Manke, WF. Orbital decompression in Graves’ disease: the predictability of reduction of proptosis. Arch Ophthalmol. 1991;109:334–345.
156. Mourits, MPH, Koornneef, L, Wiersinga, WM, et al. Orbital decompression for Graves’ ophthalmopathy by inferomedial, by inferomedial plus lateral, and by coronal approach. Ophthalmology. 1990;97:636–641.
157. DeSanto, LW, Gorman, CA. Selection of patients and choice of operation for orbital decompression in Graves’ ophthalmopathy. Laryngoscope. 1973;83:945–959.
158. DeSanto, LW. The total rehabilitation of Graves’ ophthalmopathy. Laryngoscope. 1980;90:1652–1678.
159. Gorman, CA, DeSanto, LW, MacCarty, CS, et al. Optic neuropathy of Graves’s disease: treatment by transantral or transfrontal orbital decompression. N Engl J Med. 1974;290:70–75.
160. Garrity, JA, Fatourechi, V, Bergstralh, EJ, et al. Results of transantral orbital decompression in 428 patients with severe Graves’ ophthalmopathy. Am J Ophthalmol. 1993;116:533–547.
161. Dyer, JA. Ocular muscle surgery. In: Gorman CA, Campbell RJ, Dyer JA, eds. The Eye and Orbit in Thyroid Disease. New York: Raven Press; 1984:253–262.
162. Bartley, GB. The eyelids in Graves’ ophthalmopathy. In: Bosniak S, ed. Principles and Practice of Ophthalmic Plastic Reconstructive Surgery. Philadelphia: WB Saunders; 1997:514–524.