General Principles of Postoperative Intensive Care Unit Care
Generally, the surgical ICU is where experience, staffing, skills, and technology converge to provide services that cannot be provided anywhere else within the hospital. Highly skilled nurses, often greater in number than the patients themselves, work intimately with intensivists and ancillary staff in an environment designed to stabilize, diagnose, and simultaneously treat the most acutely ill patients. ICU management by intensivists allows for improved staff and family satisfaction, reduced complication rates, lower costs, shorter length of stay, improved processes of care, and a morbidity and mortality risk advantage.1–4 ICU systems focused on an environment of safety and compliance with evidence-based standards promote improvement in many outcome metrics.5 Safe and efficient patient throughput allows for greater institutional procedural volume, which, when paired with surgeon procedural volume, has been shown to be associated with reduced mortality risk.6
Classic postoperative indications for ICU admission include advanced age or prolonged duration of the operation, both criteria without specifically defined thresholds. Other factors, such as the need for mechanical ventilation, volume resuscitation, or administration of vasoactive medications, make ICU care unavoidable. Monitoring of level of consciousness, airway, bleeding, pulses, rhythm, acidosis, urine output, and global perfusion also is facilitated by ICU admission. Identifying patients who may need postoperative ICU care can be difficult. Although there are scoring systems to assess risk and fatality (APACHE, SAPS, MPM, SOFA), it is difficult to apply these predictions to specific disease states or individual patients. Some prediction models utilize physiologic data for patients after admission to the ICU and have not been validated as preadmission screening tools.7,8 Physicians may predict mortality risk even better than scoring systems.9 In practice, most physicians do not use these tools to determine postoperative ICU admission. Admission criteria based on priority, diagnosis, and objective parameter models have been published by the Task Force of the American College of Critical Care Medicine and the Society of Critical Care Medicine.10
Postoperative Evaluation
Obtaining a comprehensive medical and surgical history is a fundamental step in understanding a patient in the surgical ICU. The medical record, traditionally written but now more commonly electronic, should contain all of the elements necessary to assemble the story up until the time of ICU admission, although deciphering a chart, particularly when it is long, requires time, patience, and detective skills. Data gathering usually begins by word of mouth from the providers delivering the patient. Effective “hand-off” is essential to maintain the continuity of care and to ascertain important operative events that may have escaped documentation. It is in fact a standard expected by The Joint Commission.11 Certain questions are common to virtually all admissions:
2. What are the highlights of the medical/surgical history?
3. Was the operation elective or emergent?
4. What operation was performed, and what are the details of the surgery?
6. What are the current ventilator settings if the patient is intubated?
7. What medications is the patient receiving currently?
8. Where are the vascular access points? Were they placed under sterile conditions?
The physical examination of the patient completes the initial postoperative evaluation. It starts as a cursory survey and concludes as a detailed examination. The examination should expose all parts of the patient that can be accessed, and the examiner should inspect and palpate the patient. Areas that are not under examination should be kept covered to preserve body temperature. If the bed sheets are being changed, it presents an opportunity to examine the back of the patient. An initial assessment of the vital signs, skin, pulses, and urine output provides preliminary insight into clinical perfusion (Box 35.1).
Recovery From Anesthesia
Postoperative Resuscitation
Assessment
“Adequate resuscitation” is a state, often temporary, that allows for good clinical perfusion and physiologic stability. Patients with good clinical perfusion (expected heart rates, blood pressures, and urine outputs; absence of acidosis) may require no further resuscitation other than maintenance intravenous fluids. The correct maintenance fluid rate will be just enough to match intravascular losses out of proportion to that which is mobilizable from the interstitium but not so much as to needlessly expand the third space or interstium with edema. Subtle abnormalities in any of these parameters of perfusion may suggest a more serious physiologic derangement warranting further investigation and intervention. Resuscitation is the process of optimizing macroscopic and microscopic metabolic substrate delivery with the goal of avoiding an imbalance between supply and demand. The most fundamental concept is to ensure adequate oxygen delivery (DO2) and meet the oxygen consumption (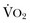 ) needs of tissues and organelles. Because the moment when
) needs of tissues and organelles. Because the moment when 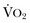 exceeds DO2 is difficult to determine, resuscitation “targets” serve as proxy markers of adequate DO2. Resuscitation targets are reproducible, quantifiable values, such as pressures, outputs, metabolites, inflammatory mediators, or oxygen saturations, which represent therapeutic goals. Resuscitation targets provide an important opportunity for study and outcome validation. Despite the seemingly simple logic of employing resuscitation targets, few of these therapeutic goals have been shown to improve clinical outcome. Even routine data derived from a pulmonary artery catheter have not been shown to improve outcome in patients undergoing surgery with decompensated cardiogenic shock or acute lung injury.12,13
exceeds DO2 is difficult to determine, resuscitation “targets” serve as proxy markers of adequate DO2. Resuscitation targets are reproducible, quantifiable values, such as pressures, outputs, metabolites, inflammatory mediators, or oxygen saturations, which represent therapeutic goals. Resuscitation targets provide an important opportunity for study and outcome validation. Despite the seemingly simple logic of employing resuscitation targets, few of these therapeutic goals have been shown to improve clinical outcome. Even routine data derived from a pulmonary artery catheter have not been shown to improve outcome in patients undergoing surgery with decompensated cardiogenic shock or acute lung injury.12,13
Management Theory
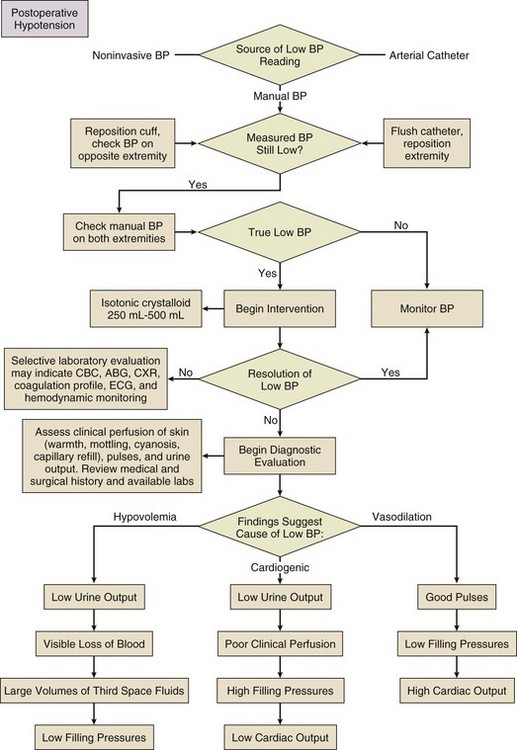
Evaluation and optimization of blood pressure, filling pressures, DO2, heart rate, and rhythm often occur simultaneously, particularly in unstable patients (Fig. 35.1). This may require ongoing volume resuscitation and support with vasopressors and inotropes. Restoration of “normal” blood pressure, heart rate, and urine output, however, do not ensure adequate DO2 at the level of the microvasculature.14 Overzealous resuscitation and supranormal DO2 not only do not improve outcome but also may be detrimental.15 Not all patients require the same type of resuscitation. Although the fundamental principles are the same, the particular resuscitation technique end points may differ among the different types of shock.16,17 Crystalloid resuscitation may be appropriate in septic shock but detrimental in the early resuscitation of penetrating traumatic injury.18,19 Even low-volume resucitation plays a role in the management of patients with penetrating traumatic injury or severe intraoperative hemorrhage.20 Early goal-directed therapy with parameter-specific targets has not completely survived prospective validation. However, the principle of timely intervention remains a cornerstone for virtually all types of resuscitation. End points specific to particular mechanisms of injury can vary significantly.21–23
Targeted resuscitation strategies provide an orderly approach to resuscitation, monitoring, and outcome validation. In general, such strategies optimize cardiovascular performance and concurrently measure markers of adequate global DO2 and 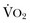 . Increased serum lactate concentration, decreased mixed venous oxygen saturation, and decreased central venous oxygen saturation are the proxy markers for inadequate global DO2. However, normal values of mixed venous oxygen saturation and central venous oxygen saturation do not guarantee normal use of oxygen in the tissues, particularly at the regional level. Appropriate targets for microcirculatory resuscitation remain elusive. Noninvasive techniques have reduced the need to obtain physiologic data by the use of a pulmonary artery catheter.24 Pulse and pressure wave analysis along with their derivitives (cardiac output and stroke volume variation) offer a less invasive way of measuring hemodynamic performance and predict volume responsiveness in the appropriate patient population.25 Gastric tonometry, sublingual capnography, near-infrared spectroscopy, and orthogonal polarization spectral imaging are less mainstream technologies available to assess the effectiveness of resuscitation at the regional level.26
. Increased serum lactate concentration, decreased mixed venous oxygen saturation, and decreased central venous oxygen saturation are the proxy markers for inadequate global DO2. However, normal values of mixed venous oxygen saturation and central venous oxygen saturation do not guarantee normal use of oxygen in the tissues, particularly at the regional level. Appropriate targets for microcirculatory resuscitation remain elusive. Noninvasive techniques have reduced the need to obtain physiologic data by the use of a pulmonary artery catheter.24 Pulse and pressure wave analysis along with their derivitives (cardiac output and stroke volume variation) offer a less invasive way of measuring hemodynamic performance and predict volume responsiveness in the appropriate patient population.25 Gastric tonometry, sublingual capnography, near-infrared spectroscopy, and orthogonal polarization spectral imaging are less mainstream technologies available to assess the effectiveness of resuscitation at the regional level.26
Resuscitation products should target the intravascular components that are inadequate, including red blood cell concentrates, platelets, coagulation factors, and acellular resuscitation fluids. Fluid type, bolus volume, and maintenance rate must be individualized. The optimal resuscitation fluid effectively should expand the intravascular space and minimize the inflammatory response (particularly in hemorrhagic shock27,28). All resuscitation fluids leak to some degree out of the intravascular space into the interstitium of the extracellular space. Hypotonic resuscitation fluids are inappropriate for volume resuscitation because of their inability to remain exclusively in the extracellular space. Volume per volume, hypertonic fluids cause more intravascular expansion than isotonic fluids. Hypertonic fluids yield no better outcomes than isotonic crystalloids, however, in the resuscitation of trauma patients.29 Similarly, isotonic crystalloids are at least as efficacious or may be better than colloids to reach the same end points.14 In trauma, burn, and general surgery patients, resuscitation with colloids, as compared to crystalloids, has not been shown to reduce the risk of death.30
Metabolic consequences are associated with virtually all resuscitation fluids. Ringer’s lactate can activate neutrophils and cause a potent inflammatory response.31 Hypertonic saline and dextran combinations cause less of an inflammatory response but any mortality benefit is unproved.32,33 Greater than 1 L of hypertonic saline typically results in the development of hypernatremia. Resuscitation exclusively with isotonic NaCl results in a hyperchloremic acidosis. Recent literature has suggested that hetastarch is associated with greater adverse events when compared to saline resuscitation.34 Hetastarch can cause coagulopathy if greater than 1.5 L is given. All acellular resuscitation fluids, if given in sufficient quantities, cause dilutional anemia. As one can infer from this confusing and sometimes contradictory collection of recommendations, no single resuscitation fluid is satisfactory on its own.
Temperature Control
Postoperative patients can come to the ICU with moderate to severe hypothermia. Heat is lost in the operating room as a result of vasodilation from volatile anesthetics, cool intravenous fluids and air temperature, large open surfaces, and evaporation. Excluding patients with potentially anoxic central nervous system injuries,35 hypothermia complicates initial postoperative care by creating an in vivo coagulopathy, even when in vitro coagulation studies (normalized to 37° C) are normal. In trauma patients, reduction in enzyme activity and platelet function, leading to abnormal fibrin polymerization, occurs at temperatures less than 34° C.36 Care must be taken when administering large volumes of cold blood products or even room temperature crystalloids. Fluid warming devices are available not only to prevent but also to treat hypothermia. All patients with postoperative hypothermia less than 36° C should be actively warmed with forced air blankets, and when normothermia has been achieved, patients should be kept covered to prevent heat loss. Active warming does not cause peripheral vasodilation and subsequent hypotension, and it does not paradoxically cause core cooling owing to heat exchange in cold extremities.
Awakening from Anesthesia
Before completing a successful resuscitation, sedation, analgesia, and anxiolysis should be maintained to facilitate patient comfort and to prevent interference with medical care (e.g., mechanical ventilation or motor activity jeopardizing airway, drains, and intravenous catheters). Selected agents should have minimal hemodynamic sequelae and relatively short duration of action so that frequent neurologic assessment can be performed. Daily interruption of continuous sedation has been shown to reduce ICU length of stay, duration of mechanical ventilation, and incidence of posttraumatic stress disorder.37,38
Narcotics such as fentanyl, morphine, and hydromorphone make ideal first-line analgesics. Delivered by continuous infusion and supplemented as needed, successful analgesia reduces pain-driven tachycardia and hypertension and facilitates cough and deep breathing. The sensation of anxiety is a potent dysphoric stimulus that can result in restlessness and interfere with care. Anxiety can be treated with short-acting intravenous benzodiazepines, such as lorazepam. Very short-acting benzodiazepines, such as midazolam, are less useful because of the dosing frequency necessary to prevent symptoms from returning. It is important not to use scheduled benzodiazepines to treat restlessness due to delirium. This practice can exacerbate delirium and worsen outcomes. Delirium can be identified using simple evaluation tools such as the Confusion Assessment Method for the ICU (CAM-ICU). Competitive restlessness due to delirium is best managed with atypical antipsychotics such as haloperidol, ziprazadone, and quetiepine.39 Persistant restlessness, agitation, or delirium can compete with mechanical ventilation, confound hemodynamic stability, and impede the provision of care. If further reduction of level of consciousness is necessary, propofol or dexmedetomidine can be added and titrated to desired effect. Dexmedetomidine, a weak analgesic, can reduce narcotic requirements.40 Propofol, however, has no intrinsic analgesic properties. In a patient who has serious pain, neither propofol nor dexmedetomidine should be used without the concurrent administration of a narcotic. The use of most agents mentioned can be limited by their tendency to reduce blood pressure and, in the case of dexmedetomidine, decrease heart rate.
Reentry into consciousness may be accompanied by disorientation, anxiety, pain, and varying degrees of restlessness. In the absence of underlying encephalopathy, it is usually possible to get patients to follow commands, answer questions, and participate in the extubation process. The discomfort of an endotracheal tube can lead to unplanned self-extubation. It is important for the bedside care provider to maintain control of the recovery process by ensuring analgesia and anxiolysis. Small doses of narcotic or benzodiazepine or both can usually correct these problems without inducing further sedation and delay of extubation.41 Patients with encephalopathy resulting from sepsis or shock may not recover a level of consciousness that allows participation in the weaning process. It is controversial whether such a patient should be extubated (avoiding the complications of prolonged extubation) or remain intubated until the ability to protect the airway is more certain. Dexmedetomidine can reduce restlessness without respiratory suppression and may be useful to facilitate extubation of a restless patient. Patients who require sedation for an extended time should receive doses of medication no higher than necessary to achieve the therapeutic target. Sedation scales, such as the Ramsay and Richmond Agitation Sedation Scale,42 are useful to avoid oversedation and ultimately promote earlier liberation from mechanical ventilation.
Postoperative Extubation
Liberation from mechanical ventilation requires clinical readiness to begin weaning and demonstration of adequate physiologic reserve before extubation. Clinical readiness assesses completion of perioperative tasks at hand and questions any need for early return to the operating room. Resuscitation should be complete, hemostasis should be achieved, metabolic acidosis should be resolving, vasoactive support and gas exchange abnormalities should be minimized, anesthetic agents should be cleared, the ability to protect the airway should be present, and the patient should be awake and reasonably cooperative. These criteria have not been validated clinically, but similar consensus guidelines have been published.43 Daily, if not more frequent, reassessment of clinical readiness is necessary to determine if it is reasonable to consider weaning.44
Patients who do not achieve these basic criteria may require continued mechanical ventilation that maximizes patient comfort and unloads the respiratory muscles. These patients require a structured, evidence-based approach to ventilator weaning and assessment of adequate physiologic reserve. For more detailed information on weaning, refer to Chapter 43.
Best Practices
Prevention of Venous Thromboembolism and Deep Venous Thrombosis
All postoperative ICU patients should be considered for venous thromboembolism (VTE) or deep venous thrombosis (DVT) prophylaxis. The risk of postoperative VTE depends upon both the type of procedure and modifying attributes such as age, prior VTE, history of cancer, obesity, or hypercoagulable state. Risk has been quantified and grouped based on the Modified Caprini Risk Assessment Model.45 Low-risk general and abdominal-pelvic surgery patients should receive intermittent pneumatic compression (IPC) over no prophylaxis or anticoagulant-based prophylaxis. Moderate-risk general and abdominal-pelvic surgery patients should receive anticoagulant-based prophylaxis. Low-dose unfractionated heparin, low-molecular-weight heparin, or fondaparinux should be started in the absence of postoperative bleeding. High-risk general and abdominal-pelvic surgery patients should receive low-dose unfractionated heparin three times a day, low-molecular-weight heparin, or fondaparinux. The highest risk patients should receive mechanical prophylaxis via IPC devices, in addition to low-dose unfractionated heparin, low-molecular-weight heparin, or fondaparinux. In general surgery patients with a high risk of postoperative bleeding, mechanical prophylaxis should be the initial preventive modality until the risk of bleeding has decreased enough to allow for anticoagulant prophylaxis.46
Trauma patients constitute an extremely heterogeneous group, making it difficult to study the strategies of VTE/DVT prophylaxis. There is disagreement in the literature about valid independent risk factors for VTE/DVT in trauma patients. Older age, spinal fractures, spinal cord injuries, traumatic brain injuries, prolonged mechanical ventilation, pelvic fractures, venous injuries, and multiple major operative procedures are often cited. In trauma patients, there are few large, prospective, randomized studies validating the efficacy of any method of VTE/DVT prevention.47 Low-dose unfractionated heparin, which has proven efficacy in the general surgery population, is no better than absence of prophylaxis in a trauma patient.48 Low-molecular-weight heparin given twice daily does offer a statistical benefit, however, in the prevention VTE/DVT in trauma patients.49 Trauma patients without significant risks for bleeding should begin anticoagulant prophylaxis or postoperatively. Data are insufficient to make recommendations as to when anticoagulant prophylaxis in trauma patients with brain injury or liver or spleen fracture is safe. Waiting 24 hours after bleeding has ceased is a conservative time to delay.50 In trauma patients at high risk for bleeding, mechanical prophylaxis can be used, although benefit is unproved. Note that IPCs cannot be applied to lower extremities with fractures, fasciotomies, or external fixators. Compression devices applied to the feet may be used as a substitute for IPC but have not been shown to be as efficacious as leg devices. In selected trauma patients expected to have prolonged immobilization or with significant risks for bleeding, inferior vena cava filters may be placed as VTE prophylaxis.30 Inferior vena cava filters should not be used as a primary prophylactic strategy in trauma patients.29 If available, removable filters should be considered, despite the low removal rates. In a trauma patient at high risk for VTE/DVT, the addition of mechanical prophylaxis to anticoagulant prophylaxis may be useful, but synergistic benefit is unproved.
Stress Ulcer Prophylaxis
Stress-related mucosal disease (SRMD) is manifest as diffuse gastric mucosal petechiae, erosions (loss of epithelium, necrosis, and hemorrhage), and discrete ulcers. SRMD can progress to clinically significant bleeding resulting in hemodynamic instability and need for transfusion. It can develop as early as 24 hours after ICU admission. Patients at risk for SRMD include critically ill patients who require mechanical ventilation for greater than 48 hours; patients with coagulopathy, traumatic brain or spinal cord injury, or severe burns; and patients with a history of gastrointestinal bleeding or ulceration within the past year. Minor risks include sepsis, corticosteroids, and prolonged ICU admission.51 The risk of clinically significant bleeding increases with the severity of illness, duration of mechanical ventilation, increased length of stay, and low intragastric pH. Hemodynamic compromise secondary to acute blood loss occurs in only a small percentage of patients with SRMD, but it is associated with a significantly increased mortality rate.52
Because of the morbidity and mortality rates associated with the complications of SRMD, it is important to identify patients at risk for SRMD and employ effective prophylaxis before bleeding occurs. Although early enteral nutrition has many benefits, the effects of enteral nutrition on SRMD are controversial and should not be used as a sole prophylactic strategy.53 Pharmacologic prophylaxis targets mucosal protection or the suppression of acid secretion. Proton-pump inhibitors may be a good first choice for SRMD prophylaxis owing to degree of acid suppression, duration of action, lack of tolerance, and cost. Parenteral H2 receptor antagonists may offer a cost advantage over proton-pump inhibitors. Prophylaxis with sucralfate is not preferred because of the efficacy profile of acid-suppression therapies and a higher rate of bleeding with sucralfate prophylaxis.
Preventing Nosocomial Pneumonia
The most significant risk for hospital-acquired pneumonia (HAP) in the postoperative patient is mechanical ventilation. Other significant risks include age more than 70 years, chronic lung disease, and depressed levels of consciousness. Though gastric acid suppression is also associated with an increased incidence of HAP, withholding ulcer prophylaxis can hardly be avoided in the patient mechanically ventilated for more than 48 hours.54 Postoperative patients should be encouraged to take deep breaths, cough, ambulate, and use incentive spirometry. Semirecumbent body positioning, keeping the head of bed elevated more than 30 degrees, has been shown to reduce ventilator-associated pneumonia in mechanically ventilated patients.55 Placing the bed in reverse Trendelenburg position can simulate this elevation without flexing the back, as could be difficult in trauma patients or patients with large open abdomens. Iatrogenic spread of bacteria that can cause pneumonia can be reduced by the enforcement of handwashing and by the use of appropriate barrier protection when performing procedures.56 Before deflating the cuff of an endotracheal tube for tube removal or position change, ensure that secretions are suctioned clear from above the cuff.36 Endotracheal tubes designed to provide drainage to the subglottic area above the tube’s cuff have been shown to reduce the risk of ventilator-associated pneumonia.57,58 The use of 0.12% chlorhexidine oral rinse has been associated with reductions in the rate of ventilator-associated pneumonia in surgical ICU patients and should be part of good oral hygiene.59 Although there is evidence that selective digestive decontamination beyond the oropharynx also can reduce the risk of ventilator-associated pneumonia, it is unclear how the routine use of this technique would affect antimicrobial resistance.60 The use of noninvasive ventilation in patients with exacerbations of chronic obstructive pulmonary disorder and congestive heart failure is associated with reductions in rates of nosocomial pneumonia, but there are few studies evaluating application of this technique in the management of postoperative respiratory failure.61
Management of Agitation and Delirium
Delirium is a major problem in postoperative ICU patients.62 Previously believed to be an expected and unavoidable result of critical illness that resolves with clinical improvement, it is now known to be a significant marker of increased morbidity,63 resource use, and long-term cognitive deficit. Delirium is an acute, variable change in mental status with inattention and either altered level of consciousness or disorganized thinking. Delirium can be hypoactive or hyperactive, the majority of patients being in the former group. Occurring in about 70% to 80% of ICU patients, delirium had been underdiagnosed until validated assessment tools such as the CAM-ICU became available.64 Delirium is believed to be due to imbalances between the stimulatory and inhibitory neurotransmitters, particularly an increase in dopaminergic and decrease in γ-aminobutyric acid and cholinergic activity. Risk factors include age, preexisting dementia, sepsis, metabolic abnormalities, and medications. The use of benzodiazepines, narcotics, anticholinergics, and antipsychotics is associated with a substantial increase in risk. It is currently unclear whether prevention or treatment of delirium changes clinical outcomes such as fatality and long-term cognitive deficits.
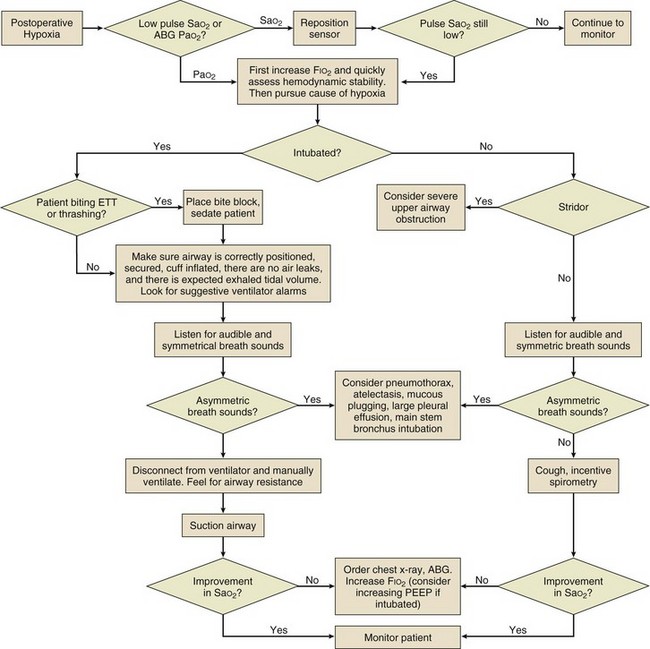
Preventive strategies include avoidance of hypoxemia (Fig. 35.2), correction of metabolic disturbances, and adequate pain control. In addition, environmental normalization with minimization of unnecessary physical and auditory stimulation, restoration of sleep/wake cycles, frequent reorientation (particularly with family involvement), and early mobilization can help decrease rates of ICU delirium.42 Pharmacologic treatment of delirium is suboptimal because the same medications intended to reduce disorganized thought may simultaneously increase sedation, prolonging the undesired state. Benzodiazepines may aggravate disorganized thought and should not be used to treat delirium. Haloperidol is the most commonly prescribed neuroleptic to treat delirium,65 although its efficacy is yet to be validated. Other atypical antipsychotics such as olanzapine, quetiapine, ziprasidone, and risperidone have also recently gained popularity.66,67 Until efficacy of any pharmacologic intervention is shown, medications should be used in the lowest doses possible for as brief a time as possible.
Management of Blood Glucose Level
Hyperglycemia in a critically ill patient can be due to diabetes mellitus (established or new) or stress-induced release of counterregulatory mediators. It is associated with increased mortality risk after acute myocardial infarction, stroke, and severe traumatic brain injury. Hyperglycemia also is associated with reduced functional outcome after neurologic injury, the development of polyneuropathy in critically ill patients, increased rates of infectious complications in the postoperative period, and defective collagen formation in wound healing. Earlier studies68–70 about the benefits of intensive insulin therapy had been published touting improved outcomes, but more contemporary evidence has shown results to the contrary.
Blood glucose less than 60 mg/dL occurs up to 32% of the time when intensive insulin strategies are utilized. Hypoglycemia can have a negative impact on mortality risk and neurologic outcome.71 Identification of appropriate blood glucose target ranges and management techniques has required the prospective study of thousands (NICE-SUGAR)72 of medical and surgical patients. From this data, and other meta-analyses, we can make some observations and logical management recommendations. Intensive insulin treatment, targeting a blood glucose of 80 to 110 mg/dL, increases the incidence of episodes of severe hypoglycemia and either has no effect on mortality risk or increases mortality risk when compared to more liberal blood glucose target ranges of 140 to 180 mg/dL and 180 to 200 mg/dL.
Postoperative Nutrition
Postoperative surgical patients are exposed to unique nutritional challenges as a result of the enhanced metabolic demands of wound healing and the abnormalities of bowel motility, anastomotic function, and swallowing. Nutritional support provides calories for metabolic processes, reduces catabolism of protein stores as an energy source, supplies substrate for anabolic processes, and provides an opportunity to reduce net protein losses in the face of ongoing protein catabolism. In an otherwise well-nourished postoperative patient, beginning nutritional support may be unnecessary, unless it is anticipated that oral intake at nutritional goal would be delayed for 7 days.73 There are considerably fewer studies showing nutritional support strategies that work in the postoperative patient than ones that do not work.74
Timing and Route
There are three routes of nutritional support—enteral nutrition (including nasogastric tube or postpyloric tube), parenteral nutrition, and oral feedings. With respect to outcomes, it is important to consider not only the route of administration but also the timing. Neither enteral nutrition nor parenteral nutrition seems to have an effect on mortality rates whether given preoperatively or postoperatively.75 Preoperative nutritional support seems to benefit only severely malnourished patients by reducing complication rates.76,77 Parenteral nutrition, which requires vascular access, is associated with complications related to non–catheter-related infection and catheter-related bloodstream infection. In addition to avoiding the complications associated with parenteral nutrition, enteral nutrition possibly reduces gut mucosal atrophy and up-regulates gut-associated immunity. In theory this protects against infections elsewhere by the common mucosal immune hypothesis.78 In perioperative patients, sufficient evidence is lacking, however, to suggest that the effect of enteral nutrition on the gut barrier has any outcome advantage over parenteral nutrition.79,80 Enteral nutrition has been shown to be associated with a lower risk of infection compared with parenteral nutrition.81 Early enteral nutrition also has been shown to be associated with a shorter length of stay and lower incidence of infections compared with delayed enteral nutrition.82 Enteral nutrition is the preferred route over parenteral nutrition because of the reduction in complications and cost. Early postoperative parenteral nutrition does not improve clinical outcomes and should be reserved only for patients who are unable to receive timely enteral nutrition.83
The combination of parenteral nutrition and early enteral nutrition has no advantage over early enteral nutrition alone in patients who are not malnourished.84 Patients who are malnourished or are not expected to be tolerating enteral feedings at nutritional goal by about postoperative day 7 should begin parenteral nutrition. If otherwise adequately nourished postoperative ICU patients are expected to be tolerating enteral feedings at nutritional goal by postoperative day 7, early parenteral nutrition may not provide substantial benefit. Finally, patients who are able to tolerate enteral feedings but are unable to tolerate an amount equal to the nutritional goal require supplemental nutrition, typically parenteral nutrition.
When the decision is made to deliver enteral nutrition, tube feedings should be increased quickly in volume to reach nutritional goal. The initial destination for enteral nutrition is the stomach. Nothing about laparotomy itself precludes enteral nutrition with the return of bowel function (e.g., bowel sounds, flatus). Although bowel motility continues through surgery or returns shortly thereafter, gastroparesis is common postoperatively and may result in delayed gastric emptying. It may be recognized by abdominal distention, high daily nasogastric output (>500 mL/day), or high residual volume in the stomach (>300 mL). Gastroparesis has the potential to delay achieving delivery of adequate enteral nutrition and has resulted in a trend toward delivering enteral nutrition via a postpyloric route. There are recent data to suggest that postpyloric feedings in patients with severe traumatic brain injury reduces the incidence of overall and late pneumonia and in addition improves nutritional efficacy.85 Other data suggest that there is no clinical benefit to postpyloric feeding with respect to incidence of pneumonia, ICU length of stay, mortality rate, or time to reach nutritional goal compared with the prepyloric route.86 Evidence to demonstrate the clinical benefit of postpyloric to prepyloric feedings is possibly still equivocal. Gastroparesis often can be improved with prokinetic agents, such as metoclopramide or erythromycin.87 It is reasonable to continue gastric enteral nutrition in the presence of gastric residual volumes of 150 to 300 mL as long as the patient is not experiencing nausea, vomiting, or progressive abdominal distention or has any evidence of functional gastric outlet obstruction or ileus. The nasogastric route of feeding is preferred, but if establishing stomach function is anticipated to be problematic, implantation placement of a jejunostomy feeding tube should be considered during laparotomy.
Feeding Considerations in General Surgery Patients
Small and Large Intestine
Postoperative ICU patients with manipulation, resection, or diversion of the bowel may have a transient ileus. Small bowel hypomotility, if present, resolves 6 to 8 hours after surgery, and some absorptive capacity is present even without normal peristalsis.88,89 Large bowel hypomotility, if present, begins to resolve 24 hours postoperatively, heralded by the passage of flatus. Recognized postoperatively as abdominal distention on physical examination or a nonobstructed gas pattern on abdominal x-ray study, ileus usually resolves over 24 to 72 hours with conservative therapy including nasogastric suctioning. Refractory ileus in the absence of mechanical obstruction should suggest some unresolved inflammatory process. In the absence of such unresolved problems, ileus also can be improved with prokinetic agents. Neostigmine has been successful in decompressing acute colonic pseudo-obstruction.90 The presence of enterotomy repairs, bowel anastomoses, or new ostomies should not be barriers to enteral nutrition with the return of bowel function.91
Pancreatitis
Acute pancreatitis is treated commonly in the surgical ICU. In mild acute pancreatitis, enteral nutrition has no effect on outcome and is recommended only in patients who cannot tolerate oral nutrition after 5 to 7 days.92 In severe acute pancreatitis, the therapeutic pendulum has swung from bowel rest and parenteral nutrition back toward early enteral nutrition. Although no differences in mortality rate have been shown in severe acute pancreatitis between groups treated with enteral nutrition and parenteral nutrition, the early enteral nutrition group has significant reductions in stress response, infections, surgical interventions, and length of stay.93,94 The theoretical benefit of feeding beyond the ligament of Treitz versus gastric feeding in patients with severe acute pancreatitis remains controversial in the available literature.
Nutrition in Wound Healing
Nutritional deficiencies can impede wound healing. Large open wounds are metabolically demanding and may be a source of substantial protein loss. Daily dietary goals of calorie and protein need to be increased accordingly. Deficiencies of vitamins and minerals (micronutrients) are infrequent, but should be suspected in malnourished (including unusual dietary habits) patients, elderly patients, and patients who have been receiving parenteral nutrition. Vitamin and mineral supplementation should accompany dietary calorie and protein in patients with deficiencies, but the benefit of pharmacologic doses of these micronutrients in the absence of deficiency is unproved. Vitamin A has been shown to antagonize the detrimental effects of corticosteroids on inflammation, epithelialization, and collagen synthesis. However, it does not lower infection rates or ameliorate impaired wound contraction associated with corticosteroid therapy.95,96 Currently, vitamin A is not routinely used used to treat patients with corticosteroid-induced immunosuppression because evidence for benefit in clinical practice is lacking. Vitamin C is needed for hydroxylation of lysine and proline in collagen formation (see earlier discussion). The benefit of vitamin C supplementation in patients receiving a normal diet is not validated. Zinc is an essential trace mineral for protein synthesis, cell division, and protein synthesis; however, its supplementation has not been shown to be beneficial in patients who are not zinc deficient.75 Glucosamine is required for the synthesis of hyaluronic acid, an abundant component of the extracellular matrix, but also lacks clinical validation of benefit.
Wound Healing and Care
Physiology and Biology of Wound Healing
Many tissues in the body respond to injury by undergoing a reparative process, which can be described histologically, biochemically, chronologically, or functionally. There are many ways to label these processes, but a simple and useful paradigm includes inflammatory, proliferative, and remodeling phases.97,98 The process begins with hemostasis, inflammation, and generation of an extracellular matrix on which proliferating cells can attach. Wound healing is locally coordinated by cytokines and facilitated by systemically mobilized cellular elements and noncellular substrate. Ultimately, the normal healing process ends with collagen maturation. Collagen develops its tensile strength through intermolecular cross-linking of fibrils into larger and longer bundles. The collagen mass undergoes continual synthesis and degradation as weaker, randomly oriented collagen fibers are reorganized into stronger, linear, highly cross-linked bundles aligned toward mechanical stress placed on the wound. This remodeling process may last 6 to 12 months, with the tissue never fully recovering its original strength. In normal circumstances, these phases tend to be sequential with generous overlap between the end of one phase and the beginning of the next.
Surgical site infection (SSI), the presence of necrotic tissue, the presence of a foreign body, an immunocompromised state, ischemia, and poor surgical closure technique all can contribute to failed wound healing and possibly wound dehiscence. Wounds are classified by their native propensity for infection as clean, clean contaminated, contaminated, and dirty. Clean wounds are uninfected with little or no inflammation, and dirty wounds are those with gross contamination such as fecal matter. Clean-contaminated and contaminated wounds lie somewhere in the middle of this spectrum.99 Although a clean or clean-contaminated surgical wound may be purposely closed by primary intention, a contaminated or dirty wound is left open to close slowly by granulation and wound contraction (secondary intention). Alternatively, a contaminated wound may be left open for several days prior to being closed (delayed primary closure) to prevent infection. The healing processes are similar in these various approaches to wound management. Successful healing of a closed surgical wound yields mechanical integrity by virtue of high tensile strength. Successful healing in an open wound may be measured by epithelialization with the promise of satisfactory mechanical integrity (scarring) over time. Understanding these interrelated processes facilitates logical wound care and helps to avoid diversions from normal wound healing.
Epithelialization and Wound Care
Without moist, occlusive dressings over superficial wounds, eschar forms, delaying epithelialization. Only with clot proteolysis can the wound be resurfaced successfully. If the wound is kept moist with an occlusive dressing,100 however, and accumulated exudates and necrotic tissue are removed frequently, epithelialization can occur. Small amounts of wound exudates and necrotic tissue can be removed with frequent, moist dressing changes and water irrigation; larger amounts may require surgical débridement. The optimal wound dressing provides a moist environment, has absorptive reserve to trap wound exudates, possesses bacteriostatic properties, and does not adhere to the wound. Large, open wounds may be dressed with moist gauze at the surface and reinforced with dry gauze packing (wet-to-dry dressing). Absorptive capacity is limited, however, and frequent dressing changes are required. Dressings made of hydrocolloids, materials that incorporate high-capacity absorptive materials into a self-adhering occlusive backing, are useful for open wounds of moderate size and allow for less frequent dressing changes. More recently, the vacuum-assisted closure has gained popularity for the management of large open wounds. Vacuum-assisted closure therapy is the combination of moderate suction applied above an absorptive surface, such as a towel or sponge, which is covered by an occlusive plastic drape. The application provides for increased blood flow, the promotion of angiogenesis, a reduction of wound surface area in certain types of wounds, and induction of cell proliferation. However, at this time, vacuum-assisted closure therapy has not been shown to reduce edema, improve bacterial clearance, or increase the speed of healing in chronic wounds.101,102
Optimizing Wound Healing
Antibiotics
The routine use of systemic antibiotics to aid wound healing, in the absence of actual SSI, should be avoided. Wound surfaces are typically colonized by bacteria, and this colonization is not detrimental to wound healing. An increased bacterial load, more than the typical colonization, may impede wound healing, however. Distinguishing between common colonization and an increased bacterial burden requires microbiologic confirmation. Simple swab cultures lack specificity, and quantitative tissue cultures revealing greater than 105 organisms per gram are necessary to identify true bacterial infection. Topical antibiotics are commonly applied to wound surfaces, but the benefits of topical antibiotics are not well documented.72,103 The incorporation of silver into dressing materials adds bacteriostatic properties and may be useful to limit bacterial overgrowth in the wound.
Surgical Site Infections
Infections of surgical incisions are referred to as surgical site infections (SSIs).104,105 SSIs are superficial incisional SSIs when limited to skin and subcutaneous tissues above the fascia or deep incisional SSIs if extending below. Intracavitary SSIs are referred to as organ-space SSIs. The surgical site becomes inoculated either inward from the skin or outward from the structures beneath the incision. Most SSIs are caused by the gram-positive cocci found on the skin, such as Staphylococcus aureus, Staphylococcus epidermidis, and Enterococcus species. The type of operation also can influence the causative organisms of the SSI such that enteric aerobic gram-negative rods (Escherichia, Enterobacter) and anaerobic organisms (Bacteroides) are more likely after intestinal or head and neck surgery.76
Although it was once believed that mechanical bowel preparation would decrease postoperative infectious complication rates, this practice has not survived prospective validation.106 The use of skin preparations, in addition to the use of narrow-spectrum “prophylactic” systemic antibiotics, has reduced the incidence of SSIs by decreaseing bacterial numbers. However, the administration of prophylactic antibiotics beyond 24 hours, even in the presence of colonic perforation or shock, does not contribute further to reducing the rate of SSIs.107 In addition, prolonged use of prophylactic antibiotics may result in the emergence of multiple drug-resistant strains of organisms, Clostridium difficile colitis, nosocomial pneumonia, and catheter-related infections.108 It is important to discontinue prophylactic antibiotics before the benefits of such therapy are overshadowed by the risks that their continuation brings with them.
A daily wound evaluation is necessary to identify early signs of wound infections. Nonpurulent drainage is not likely to be infected. Clear drainage from the wound may simply be escaping subcutaneous edema fluid or may signify seroma formation. However, wounds with an enlarging border of erythema and induration, without fluctuance or drainage, particularly when painful to palpation, suggest cellulitis or infection of deeper structures. Fluctuance and drainage may be from an abscess beneath the wound. Drainage that is turbid or frankly purulent should suggest true SSI. SSIs require opening of the incision for irrigation and drainage. Antibiotics may not be needed for uncomplicated SSIs, which respond to this intervention and local care.76
Drains
Drains can be classified on many levels.109 Drains with one end open to the atmosphere are known as “open” systems and constitute most early devices. Before the recognition of germ theory, it was not appreciated that open systems provided a free route for entrance of infectious agents into the body. Some open systems employed a filter at the open end to limit the ability of microorganisms to enter the system. “Closed” systems of drainage have no opening to the atmosphere directly; fluid collection terminates in a bag or canister.
Drains can be classified as “passive” or “active.” Passive drains provide a route of low resistance to the body’s exterior and are driven by capillary action and pressure gradients. Capillary-type drains are classified as passive drains. Active drains use an external source of negative pressure to establish a pressure gradient. Active drainage of deep recesses is classified as sump drainage. Sump drains were ultimately modified so that an additional lumen running alongside the primary lumen supplied atmospheric gas into the drainage site to prevent the intestine and omentum from occluding the fenestra.110 Sump drains are used to drain the gastrointestinal tract and abscess cavities. Active drainage employing a closed system is used to obliterate potential spaces, particularly under skin/muscle flaps or other wounds.
Drains also are classified as therapeutic or prophylactic.111 Therapeutic drainage is intended to remove necrotic debris, pus, or fistula drainage or to prevent premature closure of wounds. Prophylactic drainage is intended to prevent the accumulation of blood, pus, bile, pancreatic secretions, intestinal contents, and fluids. In the historical literature of medicine and surgery, it was noted that patients with ovariotomy developed accumulations of blood and fluid in the pelvis. It was believed that this fluid, in stagnation, would decompose and release toxins whose absorption resulted in fatal outcomes. In 1882, drains were used to “remove from cavities fluids liable to undergo putrefactive changes if retained and to cleanse such cavities by injection of disinfectants.”80
The popularity of drainage in certain applications waxed and waned owing to its controversial effect on outcome, particularly mortality risk. When surgeons abandoned the use of abdominal drains during World War II, mortality rates decreased by 50% compared with those of World War I.112 The use of prophylactic drains, particularly in abdominal surgery, was equally controversial. Capillary-based systems, which did more to prevent drainage of necrotic or purulent material than facilitate its removal, ultimately fell out of favor. Complications increased from the use of multiple or unnecessary drains and included ventral hernias, pain on removal, omental penetration of the drain’s fenestrations, intestinal obstruction, adhesions (occasionally pulling omentum or bowel into the abdominal wall), fecal fistulas, and persistent sinus tracts. The pioneering surgeon Halsted believed that good surgical technique and obliteration of dead space obviated the need for drainage in nonseptic instances. He believed that drains “invariably produce some necrosis of tissue with which it comes in contact and enfeebles the power of resistance of tissues toward organisms. But given necrotic tissue plus infections, drains become almost indispensable.”80 Prophylactic drainage ultimately gave way to therapeutic drainage. In the 1920s, indications for drains included the “presence of free purulent material in considerable quantity . . . and the presence of an abscess sac.”80
Currently, the indications for drainage include the following:
• Removal of cerebrospinal fluid (CSF) from the brain’s ventricles or spinal cord for the purpose of reducing pressure in a closed space and improving perfusion pressure
• Removal of blood or fluid from the subdural space to prevent compression or shift of intracranial contents
• Closure of certain soft tissue wounds to minimize dead space and remove excess fluid and debris; often seen in neck surgery, breast surgery, and certain reconstructive procedures
• Drainage of the pleural space in the event of pneumothorax, hemothorax, or large pleural effusions
• Drainage of the pericardium to treat large pericardial effusions
• Drainage of abscess cavities; drains can be placed directly in the operating room or percutaneously with the guidance of imaging technologies
• Drainage of existing fistulas to create a controlled route of elimination; includes drainage of bile or pancreatic secretions, succus, or stool
• Surveillance drainage over the sites of complicated procedures involving the stomach, duodenum, pancreas, and rerouting anastomoses
Placement of surveillance drains is controversial because of the risk of creating a fistula by the drains themselves. However, in the event of a catastrophic breach in enteral integrity, such as the highly morbid duodenal stump “blow-out,” early identification and controlled drainage may be facilitated by placement of such a drain.
In general, the following questions must be answered for all drains:
References
1. Pronovost, PJ, Needham, DM, Waters, H, et al. Intensive care unit physician staffing: Financial modeling of the Leapfrog standard. Crit Care Med. 2004; 32:1247–1253.
2. Reriani, M, Biehl, M, Sloan, JA, et al. Effect of 24-hour mandatory vs on-demand critical care specialist presence on long-term survival and quality of life of critically ill patients in the intensive care unit of a teaching hospital. J Crit Care. 2012; 27(4):421.
3. Wallace, DJ, Angus, DC, Barnato, AE, et al. Nighttime intensivist staffing and mortality among critically ill patients. N Engl J Med. 2012; 366(22):2093–2101.
4. McMillen, MA, Boucher, N, Keith, D, et al. Maintaining quality of care 24/7 in a nontrauma surgical intensive care unit. J Trauma Acute Care Surg. 2012; 73(1):202–208.
5. Kurlansky, PA, Argenziano, M, Dunton, R, et al. Quality, not volume, determines outcome of coronary artery bypass surgery in a university-based community hospital network. J Thorac Cardiovasc Surg. 2012; 143(2):287–293.
6. Peterson, ED, Coombs, LP, DeLong, ER, et al. Procedural volume as a marker of quality for CABG surgery. JAMA. 2004; 291:195–201.
7. Arabi, Y, AI Shirawi, N, Memish, Z, et al. Assessment of six mortality prediction models in patients admitted with severe sepsis and septic shock to the intensive care unit: A prospective cohort study. Crit Care. 2003; 7:R116–R122.
8. Nassar, AP, Jr., Mocelin, AO, Nunes, AL, et al. Caution when using prognostic models: A prospective comparison of 3 recent prognostic models. J Crit Care. 2012; 27(4):423.
9. Sinuff, T, Adhikari, NK, Cook, DJ, et al. Mortality predictions in the intensive care unit: Comparing physicians with scoring systems. Crit Care Med. 2006; 34:878–885.
10. Task Force of the American College of Critical Care Medicine, Society of Critical Care Medicine. Guidelines for intensive care unit admission, discharge, and triage. Crit Care Med. 1999; 27:633–638.
11. Petrovic, MA, Martinez, EA, Aboumatar, H. Implementing a perioperative handoff tool to improve postprocedural patient transfers. Jt Comm J Qual Patient Saf. 2012; 38(3):135–142.
12. Shah, MR, Hasselblad, V, Stevenson, LW, et al. Impact of the pulmonary artery catheter in critically ill patients: Metaanalysis of randomized clinical trials. JAMA. 2005; 294:1664–1670.
13. Wheeler, AP, Bernard, GR, Thompson, BT, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. N Engl J Med. 2006; 354:2213–2224.
14. Vincent, JL, De Backer, D. Microvascular dysfunction as a cause of organ dysfunction in severe sepsis. Crit Care. 2005; 9(Suppl 4):S9–S12.
15. Gattinoni, L, Brazzi, L, Pelosi, P, et al. A trial of goal-oriented hemodynamic therapy in critically ill patients. Svo2 Collaborative Group. N Engl J Med. 1995; 333:1025–1032.
16. Bickell, WH, Wall, MJ, Pepe, P, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994; 331:1105–1109.
17. Kowalenko, T, Stern, S, Dronen, S, et al. Improved outcome with hypotensive resuscitation of uncontrolled hemorrhagic shock in a swine model. J Trauma. 1992; 33:349–353.
18. Rowell, SE, Barbosa, RR, Diggs, BS, et al. Effect of high product ratio massive transfusion on mortality in blunt and penetrating trauma patients. Trauma Outcomes Group. J Trauma. 2011; 71(2 Suppl 3):S353–S357.
19. Mabry, R, McManus, JG. Prehospital advances in the management of severe penetrating trauma. Crit Care Med. 2008; 36(7 Suppl):S258–S266.
20. Duchesne, JC, Guidry, C, Hoffman, JR, et al. Low-volume resuscitation for severe intraoperative hemorrhage: A step in the right direction. Am Surg. 2012; 78(9):936–941.
21. Rivers, E, Nguyen, B, Havstad, S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001; 345:1368–1377.
22. Dellinger, RP, Levy, MM, Carlet, JM, et al. International Surviving Sepsis Campaign Guidelines Committee; American Association of Critical-Care Nurses; American College of Chest Physicians; American College of Emergency Physicians; Canadian Critical Care Society; European Society of Clinical Microbiology and Infectious Diseases; European Society of Intensive Care Medicine; European Respiratory Society; International Sepsis Forum; Japanese Association for Acute Medicine; Japanese Society of Intensive Care Medicine; Society of Critical Care Medicine; Society of Hospital Medicine; Surgical Infection Society; World Federation of Societies of Intensive and Critical Care Medicine. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008; 36(1):296–327.
23. Marik, PE. Surviving sepsis: Going beyond the guidelines. Ann Intensive Care. 2011; 1(1):17.
24. Pinsky, MR, Vincent, JL. Let us use the pulmonary artery catheter correctly and only when we need it. Crit Care Med. 2005; 33:1119–1122.
25. Vasdev, S, Chauhan, S, Choudhury, M, et al. Arterial pressure waveform derived cardiac output FloTrac/Vigileo system (third generation software): Comparison of two monitoring sites with the thermodilution cardiac output. J Clin Monit Comput. 2012; 26(2):115–120.
26. Bilkovski, RN, Rivers, EP, Horst, HM. Targeted resuscitation strategies after injury. Curr Opin Crit Care. 2004; 10:529–538.
27. Kristiansson, M, Soop, M, Shanwell, A, et al. Prestorage versus bedside white blood cell filtration of red blood cell concentrates: Effects on the content of cytokines and soluble tumor necrosis factor receptors. J Trauma. 1996; 40:379–383.
28. Alam, HB, Stanton, K, Koustova, E, et al. Effect of different resuscitation strategies on neutrophil activation in a swine model of hemorrhagic shock. Resuscitation. 2004; 60:91–99.
29. Bulger, EM, Hoyt, DB. Hypertonic resuscitation after severe injury: Is it of benefit? Adv Surg. 2012; 46:73–85.
30. Perel, P, Roberts, I. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. (6):2012.
31. Rhee, P, Burris, D, Kaufmann, C, et al. Lactated Ringer’s solution resuscitation causes neutrophil activation after hemorrhagic shock. J Trauma. 1998; 44:313–319.
32. Wade, CE, Kramer, GC, Grady, JJ, et al. Efficacy of hypertonic 7. 5% saline and 6% dextran-70 in treating trauma: A meta-analysis of controlled clinical studies. Surgery. 1997; 122:609–616.
33. Choi, SH, Lee, SW, Hong, YS, et al. Selective inhibition of polymorphonuclear neutrophils by resuscitative concentration of hypertonic saline. Emerg Med J. 2006; 23:119–122.
34. Perner, A, Haase, N, Guttormsen, AB, et al. Hydroxyethyl starch 130/0. 42 versus Ringer’s acetate in severe sepsis. 6S Trial Group; Scandinavian Critical Care Trials Group. N Engl J Med. 2012; 367(2):124–134.
35. Sanders, AB. Therapeutic hypothermia after cardiac arrest. Curr Opin Crit Care. 2006; 12:213–217.
36. Watts, DD, Trask, A, Soeken, K, et al. Hypothermic coagulopathy in trauma: Effect of varying levels of hypothermia on enzyme speed, platelet function, and fibrinolytic activity. J Trauma. 1998; 44:846–854.
37. Kress, JP, Gehlbach, B, Lacy, M, et al. The long-term psychological effects of daily sedative interruption on critically ill patients. Am J Respir Crit Care Med. 2003; 168:1457–1461.
38. Schweickert, WD, Gehlbach, BK, Pohlman, AS, et al. Daily interruption of sedative infusions and complications of critical illness in mechanically ventilated patients. Crit Care Med. 2004; 32:1272–1276.
39. Pandharipande, PP, Sanders, RD, Girard, TD, et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: An a priori-designed analysis of the MENDS randomized controlled trial. MENDS Investigators. Crit Care. 2010; 14(2):R38.
40. Coursin, DB, Coursin, DB, Maccioli, GA. Dexmedetomidine. Curr Opin Crit Care. 2001; 7:221–226.
41. Guler, T, Unlugenc, H, Gundogan, Z, et al. A background infusion of morphine enhances patient-controlled analgesia after cardiac surgery. Can J Anaesth. 2004; 51:718–722.
42. Ely, EW, Truman, B, Shintani, A, et al. Monitoring sedation status over time in ICU patients: Reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA. 2003; 289:2983–2991.
43. MacIntyre, NR, Cook, DJ, Ely, EW, et al. Evidence-based guidelines for weaning and discontinuing ventilatory support: A collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. American College of Chest Physicians; American Association for Respiratory Care; American College of Critical Care Medicine. Chest. 2001; 120(6 Suppl):375S–395S.
44. MacIntyre, NR. Evidence-based ventilator weaning and discontinuation. Respir Care. 2004; 49:830–836.
45. Bahl, V, Hu, HM, Henke, PK, et al. A validation study of a retrospective venous thromboembolism risk scoring method. Ann Surg. 2010; 251(2):344–350.
46. Guyatt, GH, Akl, EA, Crowther, M, et al. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Chest. 2012; 141(2 Suppl):7S–47S.
47. Knudson, MM, Ikossi, DG, Khaw, L, et al. Thromboembolism after trauma: An analysis of 1602 episodes from the American College of Surgeons National Trauma Data Bank. Ann Surg. 2004; 240:490–496.
48. Rogers, FB, Cipolle, MD, Velmahos, G, et al. Practice management guidelines for the prevention of venous thromboembolism in trauma patients: The EAST practice management guidelines work group. J Trauma. 2002; 53:142–164.
49. Geerts, WH, Jay, RM, Code, KI, et al. A comparison of low-dose heparin with low-molecular-weight heparin as prophylaxis against venous thromboembolism after major trauma. N Engl J Med. 1996; 335:701–707.
50. Saadeh, Y, Gohil, K, Bill, C, et al. Chemical venous thromboembolic prophylaxis is safe and effective for patients with traumatic brain injury when started 24 hours after the absence of hemorrhage progression on head CT. J Trauma Acute Care Surg. 2012; 73(2):426–430.
51. Cook, DJ, Fuller, HD, Guyatt, GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group. N Engl J Med. 1994; 330:377–381.
52. Steinberg, KP. Stress-related mucosal disease in the critically ill patient: Risk factors and strategies to prevent stress-related bleeding in the intensive care unit. Crit Care Med. 2002; 30(6 Suppl):S362–S364.
53. Stollman, N, Metz, DC. Pathophysiology and prophylaxis of stress ulcer in intensive care unit patients. J Crit Care. 2005; 20:35–45.
54. Herzig, SJ, Howell, MD, Ngo, LH, Marcantonio, ER. Acid-suppressive medication use and the risk for hospital-acquired pneumonia. JAMA. 2009; 301(20):2120–2128.
55. Tablan, OC, Anderson, LJ, Besser, R, et al. Guidelines for preventing health-care-associated pneumonia, 2003: Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep. 2004; 53(RR-3):1–36.
56. Drakulovic, MB, Torres, A, Bauer, TT, et al. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: A randomised trial. Lancet. 1999; 354:1851–1858.
57. Dezfulian, C, Shojania, K, Collard, HR, et al. Subglottic secretion drainage for preventing ventilator-associated pneumonia: A meta-analysis. Am J Med. 2005; 118:11–18.
58. Muscedere, J, Rewa, O, McKechnie, K, et al. Subglottic secretion drainage for the prevention of ventilator-associated pneumonia: A systematic review and meta-analysis. Crit Care Med. 2011; 39(8):1985–1991.
59. Genuit, T, Bochicchio, G, Napolitano, LM, et al. Prophylactic chlorhexidine oral rinse decreases ventilator-associated pneumonia in surgical ICU patients. Surg Infect (Larchmt). 2001; 2:5–18.
60. Flanders, SA, Collard, HR, Saint, S. Nosocomial pneumonia: State of the science. Am J Infect Control. 2006; 34:84–93.
61. Esteban, A, Frutos-Vivar, F, Ferguson, ND, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med. 2004; 350:2452–2460.
62. Pandharipande, P, Jackson, J, Ely, EW. Delirium: Acute cognitive dysfunction in the critically ill. Curr Opin Crit Care. 2005; 11:360–368.
63. Ely, EW, Shintani, A, Truman, B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004; 291:1753–1762.
64. Ely, EW, Inouye, SK, Bernard, GR, et al. Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001; 286:2703–2710.
65. Ely, EW, Stephens, RK, Jackson, JC, et al. Current opinions regarding the importance, diagnosis, and management of delirium in the intensive care unit: A survey of 912 healthcare professionals. Crit Care Med. 2004; 32:106–112.
66. Hughes, CG, Patel, MB, Pandharipande, PP. Pathophysiology of acute brain dysfunction: What’s the cause of all this confusion? Curr Opin Crit Care. 2012; 18(5):518–526.
67. Banh, HL. Management of delirium in adult critically ill patients: An overview. J Pharm Sci. 2012; 15(4):499–509.
68. Khoury, W, Klausner, JM, Ben-Abraham, R, et al. Glucose control by insulin for critically ill surgical patients. J Trauma. 2004; 57:1132–1138.
69. Furnary, AP, Zerr, KJ, Grunkemeier, GL, et al. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999; 67:352–360.
70. van den Berghe, G, Wouters, P, Weekers, F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001; 345:1359–1367.
71. Finfer, S, Liu, B, Chittock, DR, et al. Hypoglycemia and risk of death in critically ill patients. NICE-SUGAR Study Investigators. N Engl J Med. 2012; 367(12):1108–1118.
72. Finfer, S, Chittock, DR, Su, SY, et al. Intensive versus conventional glucose control in critically ill patients. NICE-SUGAR Study Investigators. N Engl J Med. 2009; 360(13):1283–1297.
73. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. J Parenter Enteral Nutr. 2002; 26(1 Suppl):1SA–138SA.
74. Huckleberry, Y. Nutritional support and the surgical patient. Am J Health Syst Pharm. 2004; 61:671–682.
75. Peter, JV, Moran, JL, Phillips-Hughes, J. A meta-analysis of treatment outcomes of early enteral versus early parenteral nutrition in hospitalized patients. Crit Care Med. 2005; 33:213–220.
76. The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. Perioperative total parenteral nutrition in surgical patients. N Engl J Med. 1991; 325:525–532.
77. Heyland, DK, MacDonald, S, Keefe, L, et al. Total parenteral nutrition in the critically ill patient: A meta-analysis. JAMA. 1998; 280:2013–2019.
78. Kudsk, K. Effect of route and type of nutrition on intestine-derived inflammatory responses. Am J Surg. 2003; 185:16–21.
79. MacFie, J. Enteral versus parenteral nutrition: The significance of bacterial translocation and gut-barrier function. Nutrition. 2000; 16:606–611.
80. Lipman, TO. Grains or veins: Is enteral nutrition really better than parenteral nutrition? A look at the evidence. J Parenter Enteral Nutr. 1998; 22:167–182.
81. Braunschweig, CL, Levy, P, Sheean, PM, et al. Enteral compared with parenteral nutrition: A meta-analysis. Am J Clin Nutr. 2001; 74:534–542.
82. Marik, PE, Zaloga, GP. Early enteral nutrition in acutely ill patients: A systematic review. Crit Care Med. 2001; 29:2264–2270.
83. Silk, DB, Green, CJ. Perioperative nutrition: Parenteral versus enteral. Curr Opin Clin Nutr Metab Care. 1998; 1:21–27.
84. Dhaliwal, R, Jurewitsch, B, Harrietha, D, Heyland, DK. Combination enteral and parenteral nutrition in critically ill patients: Harmful or beneficial? A systematic review of the evidence. Intensive Care Med. 2004; 30:1666–1671.
85. Acosta-Escribano, J, Fernandez-Vivas, M, Grau Carmona, T, et al. Gastric versus transpyloric feeding in severe traumatic brain injury: A prospective, randomized trial. Intensive Care Med. 2010; 36:1532–1539.
86. Marik, PE, Zaloga, GP. Gastric versus postpyloric feeding: A systematic review. Crit Care. 2003; 7:R46–R51.
87. Lacy, BE, Weiser, K. Gastric motility, gastroparesis, and gastric stimulation. Surg Clin North Am. 2005; 85:967–987.
88. Woods, JH, Erickson, LW, Condon, RE, et al. Postoperative ileus: A colonic problem? Surgery. 1978; 84:527–533.
89. Ward, N. Nutrition support to patients undergoing gastrointestinal surgery. Nutr J. 2003; 2:18.
90. Ponec, RJ, Saunders, MD, Kimmey, MB. Neostigmine for the treatment of acute colonic pseudo-obstruction. N Engl J Med. 1999; 341:137–141.
91. Malhotra, A, Mathur, AK, Gupta, S. Early enteral nutrition after surgical treatment of gut perforations: A prospective randomised study. J Postgrad Med. 2004; 50:102–106.
92. Meier, R, Ockenga, J, Pertkiewicz, M, et al. ESPEN guidelines on enteral nutrition: Pancreas. Clin Nutr. 2006; 25:275–284.
93. Marik, PE, Zaloga, GP. Meta-analysis of parenteral nutrition versus enteral nutrition in patients with acute pancreatitis. BMJ. 2004; 328:1407.
94. McClave, SA, Chang, WK, Dhaliwal, R, Heyland, DK, et al. Nutrition support in acute pancreatitis: A systematic review of the literature. J Parenter Enteral Nutr. 2006; 30:143–156.
95. Anstead, GM. Steroids, retinoids, and wound healing. Adv Wound Care. 1998; 11(6):277–285.
96. Wicke, C, Halliday, B, Allen, D, et al. Effects of steroids and retinoids on wound healing. Arch Surg. 2000; 135(11):1265–1270.
97. Deodhar, AK, Rana, RE. Surgical physiology of wound healing: A review. J Postgrad Med. 1997; 43:52–56.
98. Cohen, IK, Diegelman, RF. Wound healing. In Mulholland MW, Lillemoe KD, Doherty GM, et al, eds. : Greenfield’s Surgery: Scientific Principles and Practice, 4th ed, Philadelphia: Lippincott Williams & Wilkins, 2006.
99. Altemeier, WA, Burke, JF, Pruitt, BA, Sandusky, WR. Manual on Control of Infection in Surgical Patients. Philadelphia: Lippincott Williams & Wilkins; 1984.
100. Winter, GD, Scales, JT. Effect of air drying and dressings on the surface of a wound. Nature. 1963; 197:91–92.
101. Mouës, CM, Heule, F, Hovius, SE. A review of topical negative pressure therapy in wound healing: Sufficient evidence? Am J Surg.. 2011; 201(4):544–556.
102. Ubbink, DT, Westerbos, SJ, Evans, D, et al. Topical negative pressure for treating chronic wounds. Cochrane Database Syst Rev. (3):2008.
103. Smack, DP, Harrington, AC, Dunn, C, et al. Infection and allergy incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment. A randomized controlled trial. JAMA. 1996; 276(12):972–977.
104. Barie, PS, Eachempati, SR. Surgical site infections. Surg Clin North Am. 2005; 85:1115–1135.
105. Horan, TC, Gaynes, RP, Martone, WJ, et al. CDC definitions of nosocomial surgical site infections, 1992: A modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992; 13:606–608.
106. Eskicioglu, C, Forbes, SS, Fenech, DS, McLeod, RS, Best Practice in General Surgery Committee. Preoperative bowel preparation for patients undergoing elective colorectal surgery: A clinical practice guideline endorsed by the Canadian Society of Colon and Rectal Surgeons. Can J Surg. 2010; 53(6):385–395.
107. Velmahos, GC, Toutouzas, KG, Sarkisyan, G, et al. Severe trauma is not an excuse for prolonged antibiotic prophylaxis. Arch Surg. 2002; 137:537–541.
108. Namias, N, Harvill, S, Ball, S, et al. Cost and morbidity associated with antibiotic prophylaxis in the ICU. J Am Coll Surg. 1999; 188:225–230.
109. Moss, JP. Historical and current perspectives on surgical drainage. Surg Gynecol Obstet. 1981; 152:517–527.
110. Robinson, JO. Surgical drainage: An historical perspective. Br J Surg. 1986; 73:422–426.
111. Memon, MA, Memon, MI, Donohue, JH. Abdominal drains: A brief historical review. Isr Med J. 2001; 94:164–166.
112. Smith, SR, Gilmore, OJ. Surgical drainage. Br J Hosp Med. 1985; 33:308.

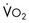 needs of tissues and organelles.
needs of tissues and organelles.





