Gastrointestinal Endoscopy
History
The first documented endoscopic foray into the human body was performed by Philip Bozinni in the early 1800s, when he used a speculum fitted with a candle and mirror to examine the urinary tract.1 The first gastroscopy was performed in 1868 by German physician Adolf Kussmaul, with a rigid metal tube passed carefully down a patient’s esophagus into his stomach. In 1932, Rudolph Schindler, in collaboration with a German engineer, Georg Wolff, developed a semiflexible instrument with a flexible distal shaft. Although this device was hailed as the first safe workable gastroscope, it was not without limitation, including incomplete visualization of the esophagus and stomach, patient discomfort, and absence of photographic documentation.2 Fiberoptics were introduced into the endoscope in 1957 by Basil Hirschowitz, and in the 1960s and 1970s further advancements were made with endoscope length, improved visualization, and greater control. Video cameras and monitors were subsequently incorporated into endoscopic technology, allowing others to view what was previously available only to the endoscopist.
Early experience with rigid and semiflexible proctosigmoidoscopes and colonoscopes was disappointing because of the tortuous nature of the sigmoid and colon, and early fiberoptic instruments fared no better. Bergein Overholt made adjustments in torque and control to develop a prototype flexible fiberoptic instrument in 1963.3 The first total colonoscopy was performed in Sardinia, Italy, in 1965. Luciano Provenzale and Antonio Revignas instructed a patient to swallow a piece of polyvinyl tubing, which ultimately emerged from the anus. They attached a side-viewing gastroscope and gently pulled it through the entire colon to the cecum.3 Further refinements were carried out in England, the United States, and Japan, and in 1969 Hiromi Shinya performed the first polypectomy, removing a 1.5 cm pedunculated polyp from the sigmoid colon of a 70-year-old Chinese gentleman.4 Shortly thereafter, colonoscopy became a routine procedure performed by gastroenterologists and other health care providers all over the world.
Endoscopic cannulation of the duodenal ampulla was first accomplished in Chicago by William S. McCune and colleagues in 1968 and is considered the first reported case of endoscopic retrograde cholangiopancreatography (ERCP).5 Sphincterotomy was performed in 1974 facilitating removal of two common bile duct stones6 and ushering in a new era of therapeutic pancreaticobiliary endoscopy. Today, ERCP remains an invaluable procedure in evaluating and treating diseases of the pancreas and biliary tract.
The first ultrasound examination within the gastrointestinal (GI) lumen was performed by physician John Julian Wild and electrical engineer John Reid in 1956, when they developed the transrectal ultrasound probe.7 The incorporation of ultrasound into a standard endoscope occurred in 1976, when Lutz and Rosch passed an ultrasound probe through an accessory port of an endoscope. Further improvements were achieved by Strohm and colleagues and Eugene DiMagno and coworkers, who introduced their own prototype echoendoscopes in 1980. The first endoscopic ultrasound (EUS)–guided fine-needle aspiration was performed on submucosal lesions of the stomach in 1991 by Giancarlo Caletti.8
Endoscopic Equipment
EUS equipment differs from the standard endoscope in that an ultrasound transducer is incorporated into the distal end of the insertion tube. The transducer emits sound waves that are directed at adjacent tissues and deflected back to the transducer. Individual tissues have different acoustic qualities. Radial and linear echoendoscopes are currently available. Interventional procedures, such as fine-needle aspiration and injections, may be performed safely with the latter echoendoscope.9
All video endoscopes have an image sensor called a charge-coupled device (CCD) mounted at the tip of the endoscope, which transmits an image to a video processor for display. Advances in CCD technology have resulted in the current high-resolution or high-definition (HD) endoscopes, which produce signal image resolutions that range from 850,000 pixels to more than 1 million pixels, allowing for detailed inspection of the GI mucosa.10
The wireless video capsule is a small disposable unit containing a small camera, short focal length lens, light source, two batteries, and a radio telemetry transmitter.11 There are presently three types of video capsules: an esophagus-specific capsule incorporating two CCD chips oriented at 180 degrees, a small bowel video capsule employing a single CCD chip with an 8-hour battery capacity, and experimental colonic video capsules utilizing time-sensitive deactivation and reactivation of the illumination and telemetry elements in order to preserve battery power during small bowel transit. Accessory devices have allowed endoscopic advancement of activated video capsules into the small intestine of patients who have dysfunctional esophageal and gastric motility or altered upper GI anatomy due to prior surgery. The capsule is activated by removal from a magnetic holder, and battery life is approximately 8 hours. Two frames per second are captured by the camera and transmitted to a data recorder that is carried by the patient. Data are downloaded from the recorder to a personal computer and interpreted. Handheld devices have also recently been developed to allow real-time monitoring of video capsule images.
Anesthesia
Choice of anesthesia is based on patient profile, the endoscopic procedure, and preference of the endoscopist, anesthesiologist, and patient. Essential patient information includes prior adverse events from anesthesia, current medications, pertinent medical history, cardiopulmonary status, age, allergies, body habitus, and social history. Patients with alcohol or narcotic dependency may require high doses of opiates and benzodiazepines. Agents such as propofol may facilitate their sedation. Pregnancy should be excluded in any woman of childbearing age. The level of sedation also depends on the endoscopic procedure. Flexible sigmoidoscopy and esophagogastroduodenoscopy (EGD) may require minimal or moderate sedation, whereas more complex and lengthier procedures, such as ERCP and EUS, may require deep sedation or even general anesthesia. Table 17.1 illustrates the different depths of sedation as defined by The American Society of Anesthesiologists Task Force.12
Table 17.1
Levels of Sedation and Anesthesia
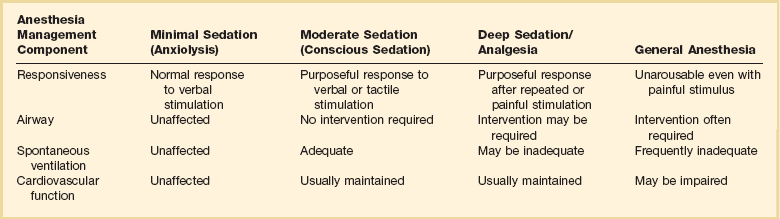
Adapted from Gross JB, Bailey PL, Connis RT, et al. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology 2002;96:1004-1017.
Regardless of the type of sedation, cardiopulmonary status should be monitored at all times. Standard equipment should include a pulse oximeter, continuous electrocardiogram, and cyclical blood pressure monitoring. Personnel trained in airway support should always be present. The cardiopulmonary complication rate is 0.005% for EGD and 0.01% for colonoscopy.13
Four drug types are commonly used in GI endoscopies: pharyngeal anesthesia, benzodiazepines, opiates, and propofol. Pharyngeal anesthetics, such as lidocaine, benzocaine, and tetracaine, are used to suppress the gag reflex during upper GI tract procedures. These agents, applied by spray or gargling, are active for approximately 1 hour. Potential risks include aspiration owing to loss of gag reflex and, rarely, methemoglobinemia.14
Benzodiazepines, such as midazolam and diazepam, are used to induce relaxation and amnesia by binding to receptors of the postsynaptic γ-aminobutyric acid neurons. Both have similar properties, with the latter possessing a longer half-life and milder amnestic properties.15 Onset of action occurs in 1 to 2.5 minutes with intravenous midazolam and 8 minutes with diazepam.16 Adverse reactions include respiratory depression and hypotension. Overdoses can be reversed with flumazenil, although caution should be used because seizures secondary to acute withdrawal may occur.
Opiates such as fentanyl and meperidine are used for analgesia and sedation. A synergistic effect occurs when opiates are given concurrently with intravenous benzodiazepines. Fentanyl has a rapid onset (1.5 minutes) with a short duration of action (0.5 to 1 hour), whereas meperidine has an onset of 5 minutes and lasts 3 to 5 hours.16 Common adverse reactions include respiratory depression, hypotension, constipation, nausea, and vomiting. Overdosage can be reversed with naloxone, an opioid antagonist. Long-term opiate users may experience acute withdrawal symptoms with naloxone. Serotonin syndrome may occur if monoamine oxidase inhibitors are used with meperidine.
Propofol, an ultra-short-acting anesthetic agent, has been increasingly used in recent years.17 Propofol has a rapid onset of action, deeper levels of sedation, and faster recovery time compared with narcotics and benzodiazepines.18 Propofol use during colonoscopy has been shown to carry a lower risk of cardiopulmonary complications compared with traditional agents.19 Controversies exist as to its cost-effectiveness and the requirement that it be administered exclusively by an anesthesiologist.
Esophagogastroduodenoscopy
EGD is one of the most commonly performed procedures in the world and has become the primary tool for evaluating the esophagus, stomach, and proximal portion of the duodenum. EGD is performed for a wide variety of indications and has a diagnostic and therapeutic role (Box 17.1). There are relatively few contraindications to upper endoscopy (Box 17.2).
EGD is a safe procedure. Perforation occurs in approximately 0.05% to 0.70% of patients,20 with the higher incidence in patients undergoing therapeutic intervention (i.e., biopsy, dilation, mucosal resection). Bleeding may occur as a result of Mallory-Weiss tears, cautery injury, or sclerotherapy injection and after biopsy or polypectomy.
Prior to undergoing elective EGD, patients should be fasting for at least 6 hours. Motility agents, such as erythromycin, may be beneficial in clearing the stomach of blood or food.21 In situations of possible airway compromise, elective intubation is reasonable. A 20% incidence of aspiration pneumonia was initially demonstrated after emergent EGD for upper GI bleed.22 A subsequent retrospective study of 220 patients failed to show any significant difference in post-EGD pulmonary infiltrates, witnessed aspiration, cardiopulmonary complications, or in-hospital mortality rate.23 Despite the lack of a conclusive double-blinded randomized trial, endotracheal intubation may be appropriate in patients with active hematemesis, altered mental status, unstable cardiopulmonary function, or agitation. Alternatives to intubation may include pre-endoscopy lavage, overtube placement, or the use of large-caliber endoscopes for suction.
Thermal cautery probes deliver predetermined pulses of heat (250° C) to an endoscopic catheter tip, which is transferred to tissue on contact.24 Thermal probe coagulation can be applied to peptic ulcers, vascular lesions, and Mallory-Weiss tears. Another option for contact thermal coagulation is monopolar or bipolar electrocautery. With electrocautery, electrical current flows from electrode tip through contacted tissue. Monopolar cautery requires attaching an electrical ground to the patient and may cause extensive burn injuries and tissue stickiness. Monopolar cautery is typically not used for hemostasis, but serves a role in snare polypectomy. Bipolar cautery consists of two active electrodes incorporated into a single catheter probe, allowing electrical current to pass from one electrode through the tissue and back to the other electrode. Consequently, bipolar cauterization allows for improved control of coagulation depth.
Injection therapy for nonvariceal and variceal bleeding is performed with sclerotherapy injector needles. Solutions commonly employed are epinephrine in saline (1 : 10,000) and sclerosing agents, such as polidocanol and ethanolamine. Epinephrine reduces bleeding by vasoconstriction, vessel tamponade, and platelet aggregation.25,26 The potential exists for systemic side effects from submucosal injections because plasma epinephrine levels can transiently increase four to five times above basal levels.27 To date, only a single case of hypertension and ventricular tachycardia after epinephrine injection has been reported.28 Sclerosing agents achieve hemostasis through inflammation and sclerosis and have been employed in peptic ulcer hemorrhage and variceal bleeding. Mediastinitis, perforation, stricture formation, and infection are among the reported complications.29
Injector needles are also used in nonbleeding situations. Polyps and superficial tumors can be raised with submucosal injection of saline or epinephrine prior to polypectomy. This technique reduces the likelihood of postpolypectomy hemorrhage or perforation.30 Lesions requiring surgery can be tattooed with ink to facilitate localization by the surgeon.
Rubber band ligation is an effective tool for hemostasis. The delivery system is loaded onto the endoscope tip, and current models allow for deployment of multiple rubber bands before reloading. For variceal bleeding, endoscopic variceal ligation has become the treatment of choice and is superior to endoscopic sclerotherapy in speed of variceal eradication, decreased risk of recurrent bleeding, and fewer complications.31 Other uses of banding include gastric varices, peptic ulcers, Dieulafoy’s lesions, postpolypectomy hemorrhage, and internal hemorrhoids.
Metal clips, or endoclips, have been used successfully for GI bleeding,32 closure of perforations,33 anastomotic leaks,34 and prevention of postpolypectomy bleeding.35 The potential for significant tissue injury is small as only the mucosal and submucosal layers are involved in the grasping.36 The procedure may be technically challenging if massive bleeding is present, or the angle of approach is tangential to the lesion.37
Laser therapy, utilizing neodymium:yttrium-aluminum-garnet (Nd:YAG) or argon, is delivered through probes passed via the endoscope to treat bleeding lesions and for tumor ablation. Nd:YAG and argon lasers differ in the width and depth of tissue effect, with the former having the greater effect.38 Advantages of laser therapy include improved accuracy and not requiring direct contact with the desired target.
Argon plasma coagulation is a noncontact method of hemostasis that delivers argon gas through a catheter probe. The argon gas is ionized, delivering thermal energy to adjacent target tissue. Large areas and tissue not in direct view, due to the tangential arcing nature of the argon gas, can be treated rapidly. Clinical uses include adjunctive ablative therapy after piecemeal resection of colonic polyps, radiation proctopathy, GI vascular lesions, bleeding peptic ulcers, Barrett’s esophagus ablation, and palliation of GI malignancies.39,40
Tissue adhesives constitute a newer class of agents for GI hemostasis. The major types of tissue adhesives are fibrin sealants and cyanoacrylate. Fibrin sealants form a coagulum through the interaction of fibrinogen, factor XIII, and thrombin.41 Extensively used in the surgical fields for tissue adhesion, hemostasis, and wound care, fibrin sealants also have been used endoscopically in bleeding peptic ulcers,42 variceal bleeding,43 and GI fistulas.44 Cyanoacrylate is synthetic glue that rapidly polymerizes into a solid complex when in contact with water or blood.45 Cyanoacrylate has been used with success for esophageal and gastric varices.46,47 A serious complication of tissue adhesives is embolization and infarction.48
In addition to hemostasis, upper endoscopy is routinely employed for other therapeutic situations. Foreign object ingestion and food bolus impaction occur commonly. Although most foreign bodies pass spontaneously, up to 20% of cases may require endoscopic intervention. Various types of endoscopy, ranging from rigid to flexible, and equipment (Box 17.3) are available for foreign body retrieval. An overtube is available for airway protection and frequent esophageal intubations. Retrieval should be performed within 24 hours or more urgently if the ingested object is sharp, is a disc battery, or is causing the patient pain or difficulty in handling secretions. If it is not possible to remove the object endoscopically and it is less than 2.5 cm, the object can be gently maneuvered into the stomach, from which spontaneous passage usually occurs.49 Unsuccessful removal or obstruction requires surgical evaluation.
Esophageal narrowing is a common reason for recurrent food impaction. Narrowing may occur from benign conditions, such as peptic strictures and Schatzki’s rings, or malignancy compressing the lumen. Endoscopic dilation can be performed on anatomic narrowings of the esophagus, pylorus, and anastomotic strictures. Four types of dilators are currently available: tip-weighted push bougies (Maloney or Hurst), wire-guided dilators (Savary-Gilliard or American), through-the-scope dilating balloons, and clear optical dilators that allow direct endoscopic visualization. Dilation also is indicated in patients with achalasia, although recurrence is common, and clinical efficacy is decreased with subsequent dilations.50 In general, endoscopic dilation increases the risk of perforation, with reported rates between 0.1% and 0.4%.51
Endoscopic stenting with endoprosthesis can be performed in a wide variety of clinical scenarios. Stenting is performed for fistulas, anastomotic leaks,52 and malignant and nonmalignant perforations.53,54 In addition, malignant obstructive lesions of the esophagus, stomach, duodenum, and colon can be stented for palliation. Stents vary in size, in material (plastic or metal mesh), and by the presence or absence of a covering. Complications include increased reflux if the gastroesophageal junction is involved, bleeding, perforation, and stent migration.
Photodynamic therapy involves pretreatment of a desired target lesion with an injected photosensitizing agent, which is subsequently activated by the application of a light source. The activated photosensitizer achieves an excited state with reactive oxygen radicals that result in cellular injury.55 In addition to high-grade dysplasia of Barrett’s esophagus and esophageal cancer, photodynamic therapy has been employed for neoplasms throughout the GI tract, including the stomach, bile duct, pancreas, and colon.56
Radiofrequency ablation of the distal esophagus has been developed as a treatment for high-grade dysplasia in Barrett’s esophagus. The HALO system (Barrx Medical, Inc., Sunnyvale, CA) consists of a balloon that contains 60 separate 250-µm electrodes circumferentially oriented on its outer surface, with electrodes separated by a distance of 250 µm. Immediately adjacent electrodes function as bipolar devices that deliver heat to the mucosa at a controlled depth. Radiofrequency energy is delivered through the electrodes, which causes superficial tissue destruction circumferentially over a length of 3 cm. A 90-degree model allows for more focal ablation.57
Percutaneous endoscopic gastrostomy (PEG) tube placement is a common procedure for gastroenterologists. The purpose of PEG tube placement is to improve quality of life, shorten hospitalization, prevent aspiration, improve nutritional and functional status, and prolong survival.58 Controversy exists as to whether PEG tube placement is beneficial in patients with terminal anorexia-cachexia syndromes or in permanent vegetative states.59,60 In addition to providing nutritional support, PEG placement has been used for long-term gastric decompression and recurrent gastric volvulus management. Placement is contraindicated if the anterior abdominal wall cannot be brought into contact with the anterior gastric wall, such as in morbid obesity and significant ascites. Complications, although infrequent, include wound infection, necrotizing fasciitis, peritonitis, septicemia, peristomal leakage, device dislodgement, bowel perforation, and fistula formation.60 Possible implantation metastasis in patients with head and neck cancer also has been reported.61 Pneumoperitoneum is seen in 40% of cases, but most are asymptomatic and eventually resolve.62
Wireless Capsule Endoscopy
Wireless video capsule endoscopy (VCE) is a safe, noninvasive method for visualizing the entire small bowel. The capsule examination typically is performed in an ambulatory setting. Preparation involves an overnight fast. Although data are conflicting, a bowel preparation has been shown to improve visualization and result in a higher rate of capsules reaching the small intestine.63,64 Metoclopramide also may be beneficial in ensuring a complete small bowel evaluation before expiration of the battery life.65 After swallowing the pill, the patient can leave the outpatient office, resume nonstrenuous daily activity, and eat 4 hours later. The data recorder is returned after 8 hours.
Common indications for VCE are evaluating obscure GI bleeding, suspected Crohn’s disease, small intestinal tumors and polyps, diarrhea, malabsorption disorders, and abdominal pain.66,67 VCE has been shown to be superior to push enteroscopy and small bowel barium radiography in detecting sources of obscure GI bleeding.68 Superiority also was shown when VCE was compared with double-balloon enteroscopy.69 A major limitation of VCE is the inability to perform therapeutic interventions.
In general, VCE is a safe procedure. Contraindications for VCE include swallowing disorders, known or suspected GI obstruction, stricture, fistula, pregnancy, and possibly cardiac pacemakers or implantable defibrillators. (Although listed as a contraindication by the manufacturer, more recent studies have shown no interference with cardiac pacemakers and implantable defibrillators by VCE.70,71) The capsule does not reach the cecum within recording time in 16% of cases. Abdominal x-ray study should be performed to evaluate for capsule retention, which occurs in 1.9% of all examinations, usually secondary to an anatomic abnormality, and may require endoscopic or surgical removal.72,73 A patency system similar in size to a video capsule, but dissolvable if retained in the body, may be useful in screening high-risk patients for possible small bowel stenosis.74 Patients with swallowing disorders or delayed gastric emptying can have the capsule endoscopically placed into the small bowel.
A variation of the small bowel video capsule exists to evaluate the esophagus. Although similar in design to the small bowel capsule, the esophageal video capsule incorporates a camera at each end, with each camera taking 7 frames per second for a total of 14 frames per second.11 Fasting time is only 2 hours, and the examination time is less than 1 hour. The patient ingests the pill in a supine position and is gradually raised to an upright position at 2-minute intervals. The esophageal video capsule can be used to evaluate for Barrett’s disease, esophageal varices, and complications of gastroesophageal reflux disease.
Enteroscopy
Double-balloon enteroscopy uses two balloons, one at the tip of an enteroscope, and the other at the end of an overtube backloaded onto the enteroscope. Using alternating inflation and deflation of the balloons during sequential advancement of the scope and the overtube, the double-balloon enteroscope is progressively advanced through the small bowel. Utilizing both oral and anal intubation, evaluation of the entire small bowel is possible. Single-balloon enteroscopy, like double-balloon enteroscopy, incorporates a backloaded overtube with a balloon at the tip. Unlike the double-balloon enteroscope, the tip of the scope is deflected to anchor the scope against the bowel wall and permit advancement of the overtube. After advancement, the balloon on the overtube can be inflated, the intestine pleated over the overtube, and the scope advanced once again. Severe complications from double-balloon enteroscopy are described in 1% to 1.7% of patients, with pancreatitis being the most common (0.3%), and bleeding and perforation also encountered.75 Fewer data are available regarding the complications of single-balloon enteroscopy.76
Spiral enteroscopy was developed with the potential advantages of decreased time and increased control in examining the small bowel. The enteroscope is advanced into the small bowel by continuous rotation of the raised helix-fitted overtube, which pleats the small bowel mucosa over the overtube. An inner sleeve allows the independent motion of the overtube during advancement and withdrawal.77 Spiral enteroscopy has demonstrated a complication rate of 0.4% with perforation found to be the leading severe complication.76
Several studies have been performed comparing various combinations of each of the three modalities. Studies comparing single- and double-balloon enteroscopy have consistently demonstrated that double-balloon enteroscopy offers deeper penetration into the small bowel. Studies are conflicting, however, on whether there is any difference in diagnostic and therapeutic outcomes.78–80 Spiral enteroscopy offers shorter procedure time when compared to balloon-assisted enteroscopy but no apparent difference in diagnostic yield.81
Colonoscopy
The colonoscope is used by general practitioners, surgeons, and gastroenterologists to evaluate the colon and distal ileum. A shorter version, the flexible sigmoidoscope, is available for sigmoid examination. Colonoscopy has replaced the routine sigmoidoscopy and barium studies as the gold standard for large bowel evaluation. Indications range from colorectal screening and evaluation of anemia to therapeutic interventions such as polypectomy and palliative stenting (Box 17.4). Relative contraindications to colonoscopy are acute diverticulitis, and suspected perforation.
Before colonoscopy, the patient should be on a clear liquid diet with subsequent fasting after bowel preparation. Several bowel preparations are commercially available, including polyethylene glycol, sodium-free polyethylene glycol, low-volume polyethylene glycol with bisacodyl, and tablet sodium phosphate.82 Nausea, vomiting, and abdominal discomfort are common side effects among all bowel preparations. Sodium phosphate, owing to inducement of rapid volume changes, is contraindicated in patients with serum electrolyte abnormalities, advanced hepatic dysfunction, renal failure, recent myocardial infarction, unstable angina, congestive heart failure, ileus, malabsorption, and ascites.82 A recent meta-analysis demonstrated that the use of a split-dose polyethylene glycol for bowel preparation before colonoscopy significantly improved the number of satisfactory bowel preparations, increased patient compliance, and decreased nausea compared with the full-dose polyethylene glycol.83 Inadequate bowel preparation has been attributed to failure to follow preparation instructions; later colonoscopy start time; inpatient status; procedural indication of constipation; use of tricyclic antidepressants; male gender; and history of cirrhosis, stroke, or dementia.84 Patients undergoing flexible sigmoidoscopy usually do not require complete bowel purgation. An enema before the procedure usually is sufficient to clear the distal colon.
Complete colonoscopic examination is achieved in approximately 94% of patients.85 Advanced age, female gender, body mass index less than 25 kg/m2, diverticular disease in women, and a history of constipation or reported laxative abuse in men are predictors of a technically difficult colonoscopy.86 In general, complications from diagnostic colonoscopy are rare. Hemorrhage and perforation occur in 0.001% to 0.008% and 0.005% to 0.14% of patients, respectively.87,88 Interventional procedures, such as polypectomy, can increase the risk of bleeding and perforation to 2% and 0.3%.89 There is a theoretical risk of colonic explosion during cautery from accumulation of colonic gases, usually as a result of a carbohydrate-based bowel preparation such as mannitol.90
Common causes of colonic hemorrhage include diverticulosis, postpolypectomy bleeding, vascular malformations, and hemorrhoids. Diverticular and postpolypectomy bleeding may be controlled with epinephrine injection, heater probe, electrocautery, or metallic clips. Band ligation also may be effective in hemostasis of postpolypectomy bleeds. Vascular malformations may be ablated with heater probe, electrocautery, laser, argon plasma coagulation, and metallic clips. Hemorrhoidal bleeds are effectively controlled with elastic band ligation, either with a rigid proctoscope or with a flexible video endoscope.91
Anastomotic strictures may occur from inflammatory bowel disease or postsurgical resection. These strictures can be dilated with balloon dilators or managed with self-expanding metallic stents.92 Endoluminal stenting may be used as palliation or as a bridge to surgery for near obstructive malignant lesions.93 Laser therapy is another option for tumor ablation.94
Endoscopic Retrograde Cholangiopancreatography
ERCP is used to evaluate and treat diseases of the pancreas, bile ducts, gallbladder, and liver. With the advent of highly diagnostic alternative modalities, such as magnetic resonance imaging and EUS, the role of ERCP has slowly evolved into a therapeutic rather than diagnostic tool (Box 17.5).95 The procedure is performed under anesthesia with the patient lying on the left side or prone. Significant coagulopathy should be corrected if sphincterotomy is to be performed. Antibiotic prophylaxis is indicated in patients undergoing ERCP for suspected biliary obstruction where incomplete drainage is anticipated or in patients with sterile pancreatic fluid collections that communicate with the pancreatic duct. Patients undergoing EUS with aspiration of a cystic lesion along the GI tract and patients undergoing percutaneous feeding tube placement are also recommended to receive antibiotic prophylaxis.96 Glucagon may be beneficial to reduce peristalsis of the small bowel, facilitating cannulation of the bile duct. Secretin may be administered to assist in identification of the papilla of Vater in the setting of ulceration, scarring, or malignancy, or the minor papilla in cases of pancreas divisum.
After oral intubation, the side-viewing duodenoscope is advanced into the second portion of the duodenum, where the papilla of Vater is located, and the papilla is subsequently cannulated. Visualization of the common bile duct or pancreatic duct is achieved with injection of contrast dye and radiographic fluoroscopy. Biliary obstruction, usually secondary to choledocholithiasis, may be treated with ERCP. Stone extraction is successful in 90% of cases.97 Techniques for stone extraction involve biliary sphincterotomy or balloon sphincteroplasty, followed by stone removal by soft balloon or wire basket. Large stones may be fragmented before removal with mechanical, laser, or electrohydraulic lithotripsy. Inadequate bile drainage may require biliary stenting to prevent ascending cholangitis.
Bile duct stenting is used to alleviate obstruction caused by malignant and benign disorders and to treat bile duct injuries and leaks.98 Pancreatic stents are used for pancreatic duct disruptions99 and pseudocysts that communicate with the pancreatic duct.100 Stents vary in diameter, length, material (plastic, metallic, and biodegradable), and occlusion rates.
Malignant and benign strictures may be dilated with hydrostatic balloons. ERCP also is used to obtain brush cytology, fine-needle aspiration, or biopsy specimens of a suspected malignancy. Sensitivity is typically low, ranging from 30% with brushings to 60% with all three methods combined.101 Reports of photodynamic therapy for nonresectable cholangiocarcinoma have been described.102 Manometry, the measurement of biliary and pancreatic sphincter pressures, may be used to evaluate sphincter of Oddi dysfunction, postcholecystectomy pain, and idiopathic pancreatitis.
Choledochoscopes and pancreatoscopes are small endoscopes that can be passed through a duodenoscope channel port into the common bile duct or pancreatic duct. This placement allows direct visualization of the duct lumen. Direct visualization of vasculature within a biliary stricture may help differentiate benign from malignant lesions.103
ERCP carries a substantial morbidity risk. Pancreatitis is the most common complication, occurring in 7% of cases.104 Although the benefits of prophylactic administration of gabexate mesylate are controversial,105,106 pancreatic stenting of high-risk patients seems to be efficacious.107 Stenting decreases papillary hindrance to pancreatic duct drainage. Other reported complications include hemorrhage, cholangitis, and perforation.108
Endoscopic Ultrasound
EUS, a combination of endoscopy and ultrasonography, is used for evaluation of luminal walls and structures adjacent to the GI tract. Dedicated ultrasound endoscopes with linear or radial viewing can be used. In addition, high-frequency ultrasound probes that can be passed through the channel port of standard endoscopes are commercially available.109
A common application of EUS is to evaluate benign and malignant mucosal and submucosal lesions. EUS is employed routinely for detection and staging of esophageal, gastric, ampullary, pancreaticobiliary, colorectal, and lung neoplasms. EUS also is used to evaluate chronic pancreatitis and biliary disorders, such as calculi. In general, EUS is an extremely sensitive tool and is often superior to computed tomography or magnetic resonance imaging for diagnosis and staging of neoplasia.110 An advantage of EUS over these noninvasive modalities is the ability to perform therapeutic interventions when needed. Fine-needle aspiration can be done with EUS, which also can be used for pseudocyst drainage, celiac plexus blocks, cholangiography, pancreatography, and tumor ablation.111
Preparation of the patient is similar to that for standard endoscopy. Complications from instrumentation vary, depending on the clinical scenario. Perforation rates, usually cervical esophageal in origin, occur in 0.03%.112 Despite the low risk of bacteremia after EUS fine-needle aspiration, prophylactic antibiotics are recommended for pancreatic cystic lesions and perhaps the perirectal space. Pancreatitis, hemorrhage, and bile peritonitis also have been reported.113
Endoscopy in the Pregnant Patient
Endoscopy in the general population is commonplace and widely regarded as safe. Endoscopy during pregnancy, however, raises the unique concern of fetal safety and is generally avoided when possible. Potential risks of endoscopy include teratogenesis or premature induction of labor from medications, hypoxemia, and hemodynamic fluctuations, all of which could cause fetal harm. Also, a lack of quality research into the safety of these procedures during pregnancy adds to the uncertainty of performing endoscopic procedures in this population.114
In controlled studies, no differences were seen between pregnant and nonpregnant patients undergoing EGD115 or in fetal outcomes regardless of a history of EGD during pregnancy.116 Gross acute upper GI bleeding, dysphagia associated with involuntary weight loss, and suspected GI mass have all been suggested as acceptable indications for EGD in the pregnant patient. Data surrounding colonoscopy during pregnancy are less robust, limited to small studies, case series, and case reports, and therefore accurate estimation of risk is not possible. Colonoscopy should be considered for unknown colonic mass or stricture, severe uncontrolled colonic hemorrhage, as an alternative to surgery in colonic pseudo-obstruction, and when required before colonic surgery.114
ERCP is associated with unique risks that must be appreciated when considering performing the procedure on a pregnant patient. Procedural time is often longer and increased doses of anesthetic medications are required as compared to EGD. ERCP also places the patient at risk for postprocedural complications including bleeding and perforation from sphincterotomy and post-ERCP pancreatitis. ERCP during pregnancy also introduces a theoretical risk from fetal radiation exposure during fluoroscopy. Despite these risks, it is felt that ERCP can safely be performed during pregnancy as the literature has consistently demonstrated a high maternal success rate, a low procedural complication rate, and generally favorable fetal outcomes.114 Additionally, although estimates of fetal radiation exposure vary, with careful technique, doses of radiation can be limited to less than the 5 rad threshold often used to minimize risk of fetal anomalies or pregnancy loss.117 Indications for ERCP during pregnancy include choledocholithiasis with obstructive jaundice, ascending cholangitis, or gallstone pancreatitis, or in the presence of biliary or pancreatic ductal injury.114
When considering GI endoscopy in the pregnant patient the physician must ultimately weigh the risks of performing the procedure to the fetus against the benefits to the mother. Guidelines exist offering suggestions to help minimize these risks, such as limiting procedures to patients with strong indications, choosing medications that are safe during pregnancy, close involvement of obstetric staff, and performance of the procedure by an experienced endoscopist. In situations in which therapeutic intervention is necessary, endoscopy may offer a safe alternative to surgery.118
References
1. Edmonson, JM. History of the instruments for gastrointestinal endoscopy. Gastrointest Endosc. 1991; 37:S27–S56.
2. Haubrich, WS, Edmonson, JM. History of endoscopy. In: Sivak MV, ed. Gastroenterologic Endoscopy. 2nd ed. Philadelphia: WB Saunders; 2000:2–15.
3. Edmonson, JM. Focus on fiberoptic colonoscope. Gastrointest Endosc. 2000; 52:17A–20A.
4. Sivak, MV. Polypectomy: Looking back. Gastrointest Endosc. 2004; 60:977–982.
5. McCune, WS, Shorb, PE, Moscovitz, H. Endoscopic cannulation of the ampulla of Vater: A preliminary report. Ann Surg. 1968; 167:752–756.
6. Kawai, K, Akasaka, Y, Murakami, K, et al. Endoscopic sphincterotomy of the ampulla of Vater. Gastrointest Endosc. 1974; 20:148–151.
7. Edmonson, JM. Endoscopic ultrasound. Gastrointest Endosc. 2000; 52:13A–14A.
8. Yamao, K, Sawaki, A, Mizuno, N, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNAB: past, present, and future). J Gastroenterol. 2005; 40:1013–1023.
9. Roesch, T. Endoscopic ultrasonography: Equipment and technique. Gastrointest Endosc Clin N Am. 2005; 15:13–31.
10. Varadarajulu, S, Banerjee, S, Barth, BA, et al. GI endoscopes. Gastrointest Endosc. 2011; 74:1–6.
11. Mishkin, DS, Chuttani, R, Croffie, J, et al. ASGE technology status evaluation: Wireless capsule endoscopy. Gastrointest Endosc. 2006; 63:539–545.
12. American Society of Anesthesiologists Task Force. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002; 96:1004–1017.
13. Sieg, A, Hachmoeller-Eisenbach, U, Eisenbach, T. Prospective evaluation of complications in outpatient GI endoscopy: A survey among German gastroenterologists. Gastrointest Endosc. 2001; 53:620–627.
14. Gunaratnam, NT, Vazquez-Sequeiros, E, Gostout, CJ, et al. Methemoglobinemia related to topical benzocaine use: Is it time to reconsider the empiric use of topical anesthesia before sedated EGD? Gastrointest Endosc. 2000; 52:692–693.
15. Lichtenstein, DR, Jagannath, S, Baron, T, et al. Sedation and anesthesia in GI endoscopy. Gastrointest Endosc. 2008; 68:815–926.
16. Horn, E, Nesbit, SA. Pharmacology and pharmokinetics of sedatives and analgesics. Gastrointest Endosc Clin North Am. 2004; 14:247–268.
17. Rex, D. The science and politics of propofol. Am J Gastroenterol. 2004; 99:2080–2083.
18. Ulmer, BJ, Hansen, JJ, Overley, CA, et al. Propofol versus midazolam/fentanyl for outpatient colonoscopy: Administration by nurses supervised by endoscopists. Clin Gastroenterol Hepatol. 2003; 1:425–432.
19. Qadeer, MA, Vargo, JJ, Khandwaa, F, et al. Propofol versus traditional sedative agents for gastrointestinal endoscopy: A meta-analysis. Clin Gastroenterol Hepatol. 2005; 3:1049–1056.
20. Wolfsen, HC, Hemminger, LL, Achem, SR, et al. Complications of endoscopy of the upper gastrointestinal tract. Mayo Clin Proc. 2004; 79:1264–1267.
21. Hwang, JH, Fisher, DA, Ben-Menachem, T, et al. Role of endoscopy in the management of acute non-variceal upper GI bleeding. Gastrointest Endosc. 2012; 75:1132–1138.
22. Lipper, B, Simon, D, Cerrone, F. Pulmonary aspiration during emergency endoscopy in patients with upper gastrointestinal hemorrhage. Crit Care Med. 1991; 19:330–333.
23. Rudolf, SJ, Landsverk, BK, Freeman, ML. Endotracheal intubation for airway protection during endoscopy for severe upper GI hemorrhage. Gastrointest Endosc. 2003; 57:58–61.
24. Jensen, DM. Thermal contact methods for endoscopic hemostasis. In: Sivak MV, ed. Gastroenterologic Endoscopy. 2nd ed. Philadelphia: WB Saunders; 2000:317–329.
25. Chung, SCS, Leung, FW, Leung, JWC. Is vasoconstriction the mechanism of hemostasis in bleeding ulcers with adrenalin? A study using reflectance spectrophotometry. Gastrointest Endosc. 1998; 34:A174.
26. O’Brien, JR. Some effects of adrenaline and anti-adrenaline compounds on platelets in vitro and in vivo. Nature. 1963; 200:763–764.
27. Sung, JY, Chung, SC, Low, LM, et al. Systemic absorption of epinephrine after endoscopic submucosal injection in patients with bleeding peptic ulcers. Gastrointest Endosc. 1993; 39:20–22.
28. Stevens, PD, Lebwohl, O. Hypertensive emergency and ventricular tachycardia after endoscopic epinephrine injection of a Mallory Weiss tear. Gastrointest Endosc. 1994; 40:77–78.
29. Truesdale, RA, Wong, RK. Complications of esophageal variceal sclerotherapy. Gastroenterol Clin North Am. 1991; 20:859–870.
30. Shirai, M, Nakamura, T, Matsuura, A, et al. Safer colonoscopic polypectomy with local submucosal injection of hypertonic saline-epinephrine solution. Am J Gastroenterol. 1994; 89:334–338.
31. Qureshi, W, Adler, DG, Davila, R, et al. ASGE guideline: The role of endoscopy in the management of variceal hemorrhage. Gastrointest Endosc. 2005; 62:651–655.
32. Binmoeller, KF, Thonke, F, Soehendra, N. Endoscopic hemoclip treatment for gastrointestinal bleeding. Endoscopy. 1993; 25:167–170.
33. Minami, S, Gotoda, T, Ono, H, et al. Complete endoscopic closure of gastric perforation induced by endoscopic resection of early gastric cancer using endoclips can prevent surgery (with video). Gastrointest Endosc. 2006; 63:596–601.
34. Rodella, L, Laterza, E, De Manzoni, G, et al. Endoscopic clipping of anastomotic leakages in esophagogastric surgery. Endoscopy. 1998; 30:453–456.
35. Iida, Y, Miura, S, Munemoto, Y, et al. Endoscopic resection of large colorectal polyps using a clipping method. Dis Colon Rectum. 1994; 37:179–180.
36. Devereaux, CE, Binmoeller, KF. Endoclip: Closing the surgical gap. Gastrointest Endosc. 1999; 50:440–442.
37. Lo, CC, Hsu, PI, Lo, GH, et al. Comparison of hemostatic efficacy for epinephrine injection alone and injection combined with hemoclip therapy in treating high-risk bleeding ulcers. Gastrointest Endosc. 2006; 63:767–773.
38. Swain, CP. Laser therapy. In: Sivak MV, ed. Gastroenterologic Endoscopy. 2nd ed. Philadelphia: WB Saunders; 2000:330–344.
39. Barr, H, Stone, N, Rembacken, B. Endoscopy therapy for Barrett’s oesophagus. Gut. 2005; 54:875–884.
40. Vargo, JJ. Clinical applications of the argon plasma coagulator. Gastrointest Endosc. 2004; 59:81–88.
41. Albala, DM. Fibrin sealants in clinical practice. Cardiovasc Surg. 2003; 11:5–11.
42. Pescatore, P, Jornod, P, Borovicka, J, et al. Epinephrine versus epinephrine plus fibrin glue injection in peptic ulcer bleeding: A prospective randomized trial. Gastrointest Endosc. 2002; 55:348–353.
43. Heneghan, MA, Byrne, A, Harrison, PM. An open pilot study of the effects of a human fibrin glue for endoscopic treatment of patients with acute bleeding from gastric varices. Gastrointest Endosc. 2002; 56:422–426.
44. Huang, CS, Hess, DT, Lichtenstein, DR. Successful endoscopic management of postoperative GI fistula with fibrin glue injection: Report of two cases. Gastrointest Endosc. 2004; 60:460–463.
45. Seewald, S, Sriram, PV, Naga, M, et al. Cyanoacrylate glue in gastric variceal bleeding. Endoscopy. 2002; 34:926–932.
46. Maluf-Filho, F, Sakai, P, Ishioka, S, et al. Endoscopic sclerosis versus cyanoacrylate endoscopic injection for the first episode of variceal bleeding: A prospective, controlled and randomized study in Child-Pugh class C patients. Endoscopy. 2001; 22:421–427.
47. Lo, GH, Lai, KH, Cheng, JS, et al. A prospective, randomized trial of butyl cyanoacrylate injection versus band ligation in the management of bleeding gastric varices. Hepatology. 2001; 33:1060–1064.
48. Petersen, B, Barkun, A, Carpenter, S, et al. Tissue adhesives and fibrin glues: Technology status evaluation report. Gastrointest Endosc. 2004; 60:327–333.
49. Ikenberry, S, Jue, TL, Anderson, MA, et al. Management of ingested foreign bodies and food impactions. Gastrointest Endosc. 2011; 73(6):1085–1091.
50. Karamanolis, G, Sgouros, S, Karatzias, G, et al. Long-term outcome of pneumatic dilation in the treatment of achalasia. Am J Gastroenterol. 2005; 100:270–274.
51. Egan, JV, Baron, TH, Adler, DG, et al. Esophageal dilation. Gastrointest Endosc. 2006; 63:755–760.
52. Siersema, PD. Treatment of esophageal perforations and anastomotic leaks: The endoscopist is stepping into the arena. Gastrointest Endosc. 2005; 61:897–900.
53. Ferri, L, Lee, JK, Law, S, et al. Management of spontaneous perforation of esophageal cancer with covered self expanding metallic stents. Dis Esophagus. 2005; 18:67–69.
54. Siersema, PD, Homs, MY, Haringsma, J, et al. Use of large diameter metallic stents to seal traumatic nonmalignant perforations of the esophagus. Gastrointest Endosc. 2003; 58:356–361.
55. Dougherty, JJ, Gomer, CT, Henderson, BW, et al. Photodynamic therapy. J Natl Cancer Inst. 1998; 90:889–905.
56. Wolfsen, HC. Uses of photodynamic therapy in premalignant and malignant lesions of the gastrointestinal tract beyond the esophagus. J Clin Gastroenterol. 2005; 39:653–664.
57. Rodriguez, S, Adler, D, Chand, B, et al. Gastrointest Endosc. 2008; 68:1031–1042.
58. Niv, Y, Abuksis, G. Indications for percutaneous endoscopic gastrostomy insertion: Ethical aspects. Dig Dis. 2002; 20:253–256.
59. Rabeneck, L, McCullough, LB, Wray, NP. Ethically justified, clinically comprehensive guidelines for percutaneous endoscopic gastrostomy tube placement. Lancet. 1997; 349:496–498.
60. Nicholson, FB, Korman, MG, Richardson, MA. Percutaneous endoscopic gastrostomy: A review of indications, complications, and outcomes. J Gastroenterol Hepatol. 2000; 15:21–25.
61. Cruz, I, Mamel, JJ, Brady, PG, et al. Incidence of abdominal wall metastasis complicating PEG tube placement in untreated head and neck cancer. Gastrointest Endosc. 2005; 62:708–711.
62. Gottfried, EB, Plummer, AB, Clair, MR. Pneumoperitoneum following percutaneous endoscopic gastrostomy: A prospective study. Gastrointest Endosc. 1986; 32:397–399.
63. Ben-Soussan, E, Savoye, G, Antonietti, M, et al. Is a 2-liter PEG preparation useful before capsule endoscopy? J Clin Gastroenterol. 2005; 39:381–384.
64. Dai, N, Gubler, C, Hengstler, P, et al. Improved capsule endoscopy after bowel preparation. Gastrointest Endosc. 2005; 61:28–31.
65. Selby, W. Complete small-bowel transit in patients undergoing capsule endoscopy: Determining factors and improvement with metoclopramide. Gastrointest Endosc. 2005; 61:80–85.
66. Sturniolo, GC, Leo, VC, Vettorato, MG, et al. Small bowel exploration by wireless capsule endoscopy: Results from 314 procedures. Am J Med. 2006; 119:341–347.
67. Tatar, EL, Shen, EH, Palance, AL, et al. Clinical utility of wireless capsule endoscopy: Experience of 200 cases. J Clin Gastroenterol. 2006; 40:140–144.
68. Treister, SL, Leighton, JA, Leontiadis, GI, et al. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005; 100:2407–2418.
69. Hadithi, M, Heine, GD, Jacobs, MA, et al. A prospective study comparing video capsule endoscopy with double-balloon enteroscopy in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2006; 101:52–57.
70. Payeras, G, Piqueras, J, Moreno, VJ, et al. Effects of capsule endoscopy on cardiac pacemakers. Endoscopy. 2005; 37:1181–1185.
71. Leighton, JA, Srivathsan, K, Carey, EJ, et al. Safety of wireless capsule endoscopy in patients with implantable cardiac defibrillators. Am J Gastroenterol. 2005; 100:1732–1735.
72. Liao, Z, Gao, R, Xu, C, et al. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: A systematic review. Gastrointest Endosc. 2010; 71:280–286.
73. Rondonotti, E, Herrerias, JM, Pennazio, M, et al. Complications, limitations, and failures of capsule endoscopy: A review of 733 cases. Gastrointest Endosc. 2005; 62:712–716.
74. Signorelli, C, Rondonotti, E, Villa, F, et al. Use of the given patency system for the screening of patients at high risk for capsule retention. Dig Liver Dis. 2006; 38:326–330.
75. Kopaova, M, Tacheci, I, Rejchrt, S, et al. Double balloon enteroscopy and acute pancreatitis. World J Gastroenterol. 2010; 16:2331–2340.
76. May, A. How to approach the small bowel with flexible enteroscopy. Gastroenterol Clin North Am. 2010; 39:797–806.
77. Morgan, D, Upchurch, B, Draganov, P, et al. Spiral enteroscopy: Prospective U. S. multicenter study in patients with small-bowel disorders. Gastrointest Endosc. 2010; 72:992–998.
78. May, A, Farber, M, Aschmoneit, I, et al. Prospective multicenter trial comparing push-and-pull enteroscopy with the single- and double-balloon techniques in patients with small-bowel disorders. Am J Gastroenterol. 2010; 105:575–581.
79. Takano, N, Yamada, A, Watabe, H, et al. Single-balloon versus double-balloon endoscopy for achieving total enteroscopy: A randomized controlled trial. Gastrointest Endosc. 2011; 73:734–739.
80. Domagk, D, Mensink, P, Aktas, H, et al. Single- vs. double-balloon enteroscopy in small-bowel diagnostics: A randomized multicenter trial. Endoscopy. 2011; 43:472–476.
81. Kashab, M, Lennon, AM, Dunbar, K, et al. A comparative evaluation of single-balloon enteroscopy and spiral enteroscopy for patients with mid-gut disorders. Gastrointest Endosc. 2010; 72:766–772.
82. Wexner, SD, Beck, DE, Baron, TH, et al. A consensus document on bowel preparation before colonoscopy: Prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Dis Colon Rectum. 2006; 49:1–18.
83. Kilgore, T, Abinoor, A, Szary, N, et al. Bowel preparation with split-dose polyethylene glycol before colonoscopy: A meta-analysis of randomized controlled trials. Gastrointest Endosc. 2011; 73:1240–1245.
84. Ness, RM, Manam, R, Hoen, H, et al. Predictors of inadequate bowel preparation for colonoscopy. Am J Gastroenterol. 2001; 96:1797–1802.
85. Church, JM. Complete colonoscopy: How often? And if not, why? Am J Gastroenterol. 1994; 89:556–560.
86. Anderson, JC, Messing, CR, Cohn, W, et al. Factors predictive of difficult colonoscopy. Gastrointest Endosc. 2001; 54:558–562.
87. Fruhmorgen, P, Demling, L. Complications of diagnostic and therapeutic colonoscopy in the Federal Republic of Germany: Results of an inquiry. Endoscopy. 1979; 11:146–150.
88. Sieg, A, Hachmoeller-Eisenbach, U, Eisenbach, T. Prospective evaluation of complications in outpatient GI endoscopy: A survey among German gastroenterologists. Gastrointest Endosc. 2001; 53:620–627.
89. Waye, JD, Lewis, BS, Yessayan, S. Colonoscopy: A prospective report of complications. J Clin Gastroenterol. 1992; 15:347–351.
90. Bigard, MA, Gaucher, P, Lassalle, C. Fatal colonic explosion during colonoscopic polypectomy. Gastroenterology. 1979; 77:1307–1310.
91. Wehrmann, T, Riphaus, A, Feinstein, J, et al. Hemorrhoidal elastic band ligation with flexible videoendoscopes: A prospective, randomized comparison with the conventional techniqued that uses rigid proctoscopes. Gastrointest Endosc. 2004; 60:191–195.
92. Forshaw, MJ, Sankararajah, D, Stewart, M, et al. Self-expanding metallic stents in the treatment of benign colorectal disease: Indications and outcomes. Colorect Dis. 2006; 8:102–111.
93. Baron, TH. Colonic stenting: Technique, technology, and outcomes for malignant and benign disease. Gastrointest Endosc Clin North Am. 2005; 15:757–771.
94. Wood, JW, Innes, JW. Tumor ablation by endoscopic Nd:YAG laser. Am J Gastroenterol. 1985; 80:715–718.
95. Cohen, S, Bacon, BR, Berlin, JA, et al. National Institutes of Health State-of-the-Science Conference Statement: ERCP for diagnosis and therapy, January 14-16, 2002. Gastrointest Endosc. 2002; 56:803–809.
96. Banerjee, S, Shen, B, Baron, TH, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2008; 67:791–798.
97. Carr-Locke, DL. Therapeutic role of ERCP in the management of suspected common bile duct stones. Gastrointest Endosc. 2002; 56:S170–S174.
98. Katsinelos, P, Kountouras, J, Paroutoglou, G, et al. The role of endoscopic treatment in postoperative bile leaks. Hepatogastroenterology. 2006; 53:166–170.
99. Cay, A, Imamoglu, M, Ozdemir, O, et al. Nonoperative treatment of traumatic pancreatic duct disruption in children with an endoscopically placed stent. J Pediatr Surg. 2005; 40:9–12.
100. Sharma, SS, Bhargawa, N, Govil, A. Endoscopic management of pancreatic pseudocyst: A long-term follow-up. Endoscopy. 2002; 34:203–207.
101. Jailwala, J. Triple tissue sampling at ERCP in malignant biliary obstruction. Gastrointest Endosc. 2000; 51:383–390.
102. Chahal, P, Baron, TH. Endoscopic palliation of cholangiocarcinoma. Curr Opinion Gastroenterol. 2006; 22(5):551–560.
103. Kim, HJ, Kim, MH, Lee, SK, et al. Tumor vessel: A valuable cholangioscopic clue of malignant biliary stricture. Gastrointest Endosc. 2000; 52:635–638.
104. Anderson, MA, Fisher, L, Jain, R, et al. Complications of ERCP. Gastrointest Endosc. 2012; 75:467–473.
105. Cavallini, G, Tittobello, A, Frulloni, L, et al. Gabexate for the prevention of pancreatic damage related to endoscopic retrograde cholangiopancreatography. N Engl J Med. 1996; 335:919–923.
106. Andriulli, A, Clemente, R, Solmi, L, et al. Gabexate or somatostatin administration before ERCP in patients with high risk for post-ERCP pancreatitis: A multicenter placebo-controlled randomized clinical trial. Gastrointest Endosc. 2002; 56:488–495.
107. Testoni, PA. Preventing post-ERCP pancreatitis: Where are we? J Pancreas. 2003; 4:22–32.
108. Masci, E, Toto, G, Mariani, A, et al. Complications of diagnostic and therapeutic ERCP: A prospective multicenter study. Am J Gastroenterol. 2001; 96:417–423.
109. Carpenter, S, Chuttani, R, Croffie, J, et al. Endoscopic ultrasound probes. Gastrointest Endosc. 2006; 63:751–754.
110. Lowe, AS, Kay, CL. Noninvasive competition for endoscopic ultrasound. Gastrointest Endoscopy Clin North Am. 2005; 15:209–224.
111. Shami, VM, Waxman, I. Technology insight: Current status of endoscopic ultrasonography. Nat Clin Pract Gastroenterol Hepatol. 2005; 2:38–45.
112. Das, A, Sivak, MV, Chak, A. Cervical esophageal perforation during EUS: A national survey. Gastrointest Endosc. 2001; 53:599–602.
113. Adler, DG, Davila, RE, Hirota, WK, et al. ASGE guideline: Complications of EUS. Gastrointest Endosc. 2005; 61:8–12.
114. Cappell, M. Risks versus benefits of gastrointestinal endoscopy during pregnancy. Nat Rev Gastroenterol Hepatol. 2011; 8:610–634.
115. Debby, A, Golan, A, Sadan, O, et al. Clinical utility of esophagogastroduodenoscopy in the management of recurrent and intractable vomiting in pregnancy. J Reprod Med. 2008; 53:347–351.
116. Cappell, M, Colon, V, Sidhom, O. A study of eight medical centers of the safety and clinical efficacy of esophagogastroduodenoscopy in 83 females with follow-up of fetal outcome with comparison to control groups. Am J Gastroenterol. 1996; 91:348–354.
117. ACOG Committee on Obstetrical Practice. Guidelines for diagnostic imaging during pregnancy. Obstet Gynecol. 2004; 104:647–651.
118. Shergill, AK, Ben-Menachem, T, Chandrasekhara, V, et al. Guidelines for endoscopy in pregnant and lactating women. Gastrointest Endosc. 2012; 76:18–24.