Chapter 3 Functional neuroanatomy of the basal ganglia
Introduction
The basal ganglia comprise a collection of nuclear structures deep in the brain and have been defined anatomically and functionally. Anatomically, the basal ganglia are the deep nuclei in the telencephalon. Functionally, three closely associated structures, the subthalamic nucleus (in the diencephalon), the substantia nigra and pedunculopontine nucleus (both in the mesencephalon), are also included as part of the motor part of the basal ganglia. The definition of which structures are included has varied over the years and depends also in part on a preconceived notion of their function. Most of the time, and for the purposes of the study of movement disorders, the basal ganglia are viewed as having primarily a motor function. Indeed, the early movement disorders included in the concept, such as Parkinson disease (PD) (see Table 3.1 for all abbreviations in this chapter) and Huntington disease (HD), were primarily basal ganglia related, and interested neuroscientists would meet at “basal ganglia clubs.” It is now clear, however, that the basal ganglia also play a role in cognitive, behavioral, and emotional functions. For example, the limbic system interacts extensively with the basal ganglia, and some components of the basal ganglia, such as the amygdala (archistriatum), nucleus accumbens, and ventral pallidum, serve these functions (Haber and Knutson, 2010).
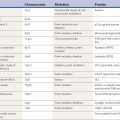
| AAADC | Aromatic L-amino acid decarboxylase |
| ACh | Acetylcholine |
| AChE | Acetylcholinesterase |
| ADP | Adenosine diphosphate |
| AMPA | α-Amino-3-hydroxyl-5-methyl-4-isoxazole-propionate |
| ATP | Adenosine triphosphate |
| BuChE | Butyrylcholinesterase (pseudocholinesterase) |
| cAMP | Cyclic adenosine monophosphate |
| ChAT | Choline acetyltransferase |
| CM | Centrum medianum nucleus of the thalamus |
| COMT | Catechol-O-methyltransferase |
| DA | Dopamine |
| DAG | Diacylglycerol |
| DAT | Dopamine transporter |
| DBH | Dopamine beta-hydroxylase |
| DBS | Deep brain stimulation |
| DOPAC | 3,4-Dihydroxyphenylacetic acid |
| EAAT | Excitatory amino acid transporter |
| GABA | Gamma-amino butyric acid |
| GABA-T | GABA-transaminase |
| GAD | Glutamic acid decarboxylase |
| GAT | GABA transporter |
| Glu | Glutamate |
| GP | Globus pallidus |
| GPe | Globus pallidus externa |
| GPi | Globus pallidus interna |
| HD | Huntington disease |
| 5-HT | 5-Hydroxytryptamine, serotonin |
| 5-HTP | 5-Hydroxytryptophan |
| HVA | Homovanillic acid |
| IP3 | Inositol triphosphate |
| LC | Locus coeruleus |
| L-dopa | Levodopa |
| LFP | Local field potential |
| M1 | Primary motor cortex |
| MAO | Monoamine oxidase |
| mAChR | Muscarinic acetylcholine receptor |
| MEA | Midbrain extrapyramidal area |
| MPTP | 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MRN | Median raphe nucleus |
| 3-MT | 3-Methoxytyramine (3-O-methydopamine) |
| nAChR | Nicotinic acetylcholine receptor |
| NE | Norepinephrine |
| NMDA | N-methyl-D-aspartic acid |
| PD | Parkinson disease |
| Pf | Parafascicular nucleus of the thalamus |
| PMv | Premotor cortex, ventral division |
| PPN | Pedunculopontine nucleus |
| PPNc | Pedunculopontine nucleus, pars compacta |
| PPNd | Pedunculopontine nucleus, pars dissipatus |
| SERT | Serotonin transporter |
| SMA | Supplementary motor area |
| SN | Substantia nigra |
| SNc | Substantia nigra, pars compacta |
| SNr | Substantia nigra, pars reticulata |
| STN | Subthalamic nucleus |
| TANs | Tonically active neurons |
| TH | Tyrosine hydroxylase |
| VA | Ventral anterior nucleus of thalamus |
| VAChT | Vesicular ACh transporter |
| VL | Ventral lateral nucleus of thalamus |
| VMAT2 | Vesicular monoamine transporter 2 |
| VTA | Ventral tegmental area |
| ZI | Zona incerta |
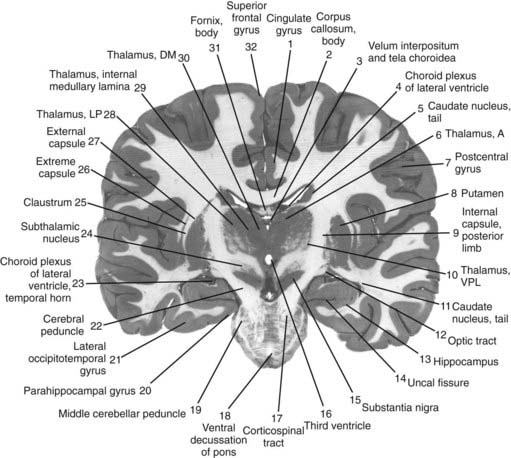
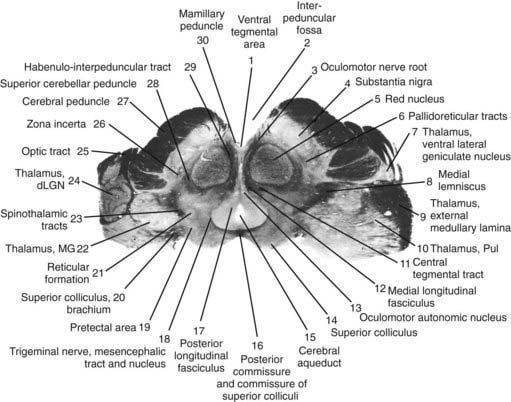
The core motor structures of the basal ganglia include the caudate and putamen, collectively called the neostriatum (commonly abbreviated as the striatum), the globus pallidus (GP) (paleostriatum), the subthalamic nucleus (STN), the substantia nigra (SN), and the pedunculopontine nucleus (PPN) (Figs 3.1, 3.2, and 3.3). The putamen and globus pallidus together are sometimes called the lenticular nucleus. The main informational processing loop of the basal ganglia comes from the cortex and goes back to the cortex via the thalamus. The substantia nigra pars compacta (SNc) is largely a modulator of this main loop, with dopamine as its neurotransmitter. Other modulators are the locus coeruleus (LC), with norepinephrine as neurotransmitter, and the median raphe nucleus (MRN), which uses serotonin as neurotransmitter. The notion that the basal ganglia provide an “extrapyramidal” control of movement separate from the cortical-pyramidal control is not correct since the main output of the basal ganglia projects to the cortex. Therefore, the term “extrapyramidal disorders” for disorders arising from dysfunction of the basal ganglia is a misnomer.
Neurotransmitters
Dopamine (DA)
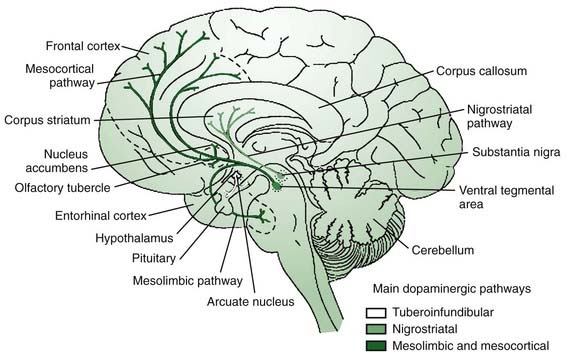
It is appropriate to start out the discussion of neurotransmitters with a consideration of dopamine, the most “prominent” neurotransmitter since it is depleted in PD and because we have the means to manipulate this transmitter in therapeutics. The main sources of dopamine are the lateral SNc (A9), the medial ventral tegmental area (VTA, A10), and the retrorubral area (A8) (Fig. 3.2). The SNc innervates the striatum via the nigrostriatal pathway, while the VTA and retrorubal areas give rise to the mesolimbic innervation of the ventral striatum (nucleus accumbens) and the mesocortical innervation of the dorsolateral and ventromedial prefrontal cortex regions (Fig. 3.4) (Van den Heuvel and Pasterkamp, 2008).
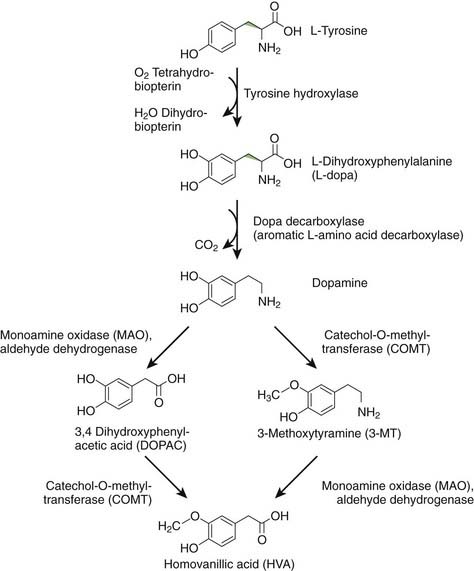
DA is formed from levodopa (L-dopa) by the enzyme aromatic L-amino acid decarboxylase (AAADC), which is commonly called dopa decarboxylase (Fig. 3.5) (Stahl, 2008). Once synthesized, DA is taken up into synaptic vesicles by the vesicular monoamine transporter 2 (VMAT2). In vivo, levodopa is synthesized from L-tyrosine by the enzyme tyrosine hydroxylase (TH). L-tyrosine is an essential amino acid in the brain, because it cannot be synthesized from L-phenylalanine, as it can in the rest of the body. DA can be metabolized by monoamine oxidase (MAO) to 3,4-dihydroxyphenylacetic acid (DOPAC), by catechol-O-methyltransferase (COMT) to 3-methoxytyramine (3-MT) (also called 3-O-methydopamine), and by both enzymes serially to homovanillic acid (HVA). MAO exists in two forms, MAO-A and MAO-B, both found in the mitochondria of neurons and glia (Bortolato et al., 2008). COMT is a membrane-bound enzyme (Bonifacio et al., 2007). Physiologically, DA action is terminated by reuptake back into the dopaminergic nerve terminal by action of the dopamine transporter (DAT). Once in the cytosol, it can be taken back up into synaptic vesicles by VMAT2. Dopamine neurons have MAO-A (Demarest et al., 1980), but virtually no COMT. DA not taken up into vesicles will therefore be metabolized to DOPAC. If DA remains non-metabolized in the cytosol, it might contribute to oxidative stress, as discussed in Chapter 5.
The exact biology of DA differs in different parts of the body and even different parts of the brain. For example, in the cerebral cortex there is not much DAT so that after DA release, COMT is much more important in terminating DA action (Matsumoto et al., 2003).
There are five subtypes of dopamine receptors, D1–D5, in two families, D1-like and D2-like (Missale et al., 1998; Beaulieu and Gainetdinov, 2011). The D1-like family, composed of D1 and D5, activates adenyl cyclase and causes conversion of adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP). Raising the concentration of cAMP is typically excitatory. The D2-like family, composed of D2, D3, and D4, inhibits adenyl cyclase and reduces the concentration of cAMP. Lowering cAMP is typically inhibitory. Some D2 receptors, called autoreceptors, are on the presynaptic side of dopamine synapses, regulating release by negative feedback.
Acetylcholine (ACh)
Cholinergic neurons have two different types of roles (Pisani et al., 2007). One is as an interneuron, and the “giant aspiny interneuron” of the striatum is cholinergic. A second role is as a projection neuron. There are two prominent cholinergic projection systems in the brain. The best known are the neurons of the basal forebrain, such as the nucleus basalis of Meynert, that innervate wide areas of cortex, are involved with functions such as memory, and are deficient in Alzheimer disease. The other is the set of projections from the meso-pontine tegmental complex, which includes the PPN. These are importantly involved in the basal ganglia motor system.
Acetylcholine is synthesized in neurons from choline and acetyl-CoA by the enzyme choline acetyltransferase (ChAT). After synthesis it is collected into vesicles by the enzyme vesicular ACh transporter (VAChT). Once released from the nerve terminals it is broken down by acetylcholinesterase (AChE), which is both pre- and postsynaptic, and butyrylcholinesterase (BuChE), also called pseudocholinesterase, that resides in glia (Cooper et al., 2003; Siegel et al., 2006). The resultant choline is taken back up into the presynaptic cell by a choline transporter (Stahl, 2008).
There are two broad classes of ACh receptors, nicotinic and muscarinic. Nicotinic receptors (nAChR) are ionotropic and are prominent outside the brain at the neuromuscular junction and autonomic ganglia, but are also in the brain (Albuquerque et al., 2009). Activation at an nAChR will open a nonselective cation channel allowing flow of sodium, potassium, and sometimes calcium. Muscarinic receptors (mAChR) are metabotropic and also found both inside and outside the brain. Activation at an mAChR couples to a variety of types of G proteins (Eglen, 2005, 2006). There are many types of nAChR and these are generally described by their subunit composition. Designations of M1–M5 are given to the mAChRs. Both nAChR and mAChR are found in the basal ganglia, and there are both excitatory and inhibitory effects.
Glutamate (Glu)
Glutamate is the primary excitatory neurotransmitter in the brain and as such it has a prominent role in the excitatory cortical-striatal input and in the excitatory projection from the STN to the globus pallidus interna (GPi). Glutamate is a central molecule in many cellular processes, and is also the precursor for the most important inhibitory neurotransmitter in the brain, GABA. Glutamate is made from glutamine in mitochondria by glutaminase. It is then taken up into synaptic vesicles by the vesicular glutamate transporter. Upon release, its action is terminated by its being taken up into glial cells via an excitatory amino acid transporter (EAAT) and then converted to glutamine by glutamine synthetase. Glutamine transporters then move the glutamine from the glial cell into the neuron (Siegel et al., 2006; Stahl, 2008).
Glutamate receptor biology is very complex and the details are well beyond this chapter. There are three groups of metabotropic glutamate receptors, groups I, II, and III, depending on mGluR composition. There are also three classes of ionotropic receptors, α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA), N-methyl-D-aspartic acid (NMDA), and the kainate (KA) receptors. Hence, glutamate not only transmits an excitatory signal by opening calcium channels, but also sets many metabolic processes in action, such as creating short- and long-term changes in synaptic excitability. Such changes are thought to be fundamental in brain plasticity (Lovinger, 2010).
Gamma-amino butyric acid (GABA)
There are three classes of GABA receptors: A, B, and C (Stahl, 2008). GABA-A and GABA-C are ionotropic, and have inhibitory action by opening chloride and potassium channels. There is much known about GABA-A, but only little about GABA-C. GABA-A channels have many subclasses depending on the subunit makeup. An important distinction between subclasses is whether they are sensitive to benzodiazepines or not, depending on whether the benzodiazepines bind to them or not. In the sensitive channels, benzodiazepines can increase the inhibitory action of a GABA-A synapse. GABA-B is a metabotropic receptor (Filip and Frankowska, 2008), and produces a longer duration inhibition than GABA-A by promoting potassium channels and inhibiting calcium channels.
Norepinephrine (NE)
There are a large number of NE receptors; the different classes are alpha 1A, 1B, 1D, alpha 2A, 2B, 2C, and beta 1, 2 and 3 (Stahl, 2008). All can be postsynaptic, and the alpha 2 receptors can also be presynaptic. Activation of the presynaptic receptors inhibits further NE release. The alpha 1 receptors are G protein coupled, and increase levels of phospholipase C, inositol trisphosphate (IP3), and calcium. The alpha 2 receptors are G protein coupled, with an action to inactivate adenylate cyclase and reduce concentrations of cAMP. The beta receptors couple to G proteins that activate adenylate cyclase and increase cAMP.
Serotonin (5-hydroxytryptamine, 5-HT)
There are many subtypes of 5-HT receptors, categorized into seven families, 5-HT1 to 5-HT7. 5-HT3 is a ligand-gated Na+ and K+ channel that depolarizes membranes. The other family members are G protein coupled. 5-HT1 and 5-HT5A decrease cAMP; 5-HT4, 5-HT6, and 5-HT7 increase cAMP; 5-HT2 increases inositol triphosphate (IP3) and diacylglycerol (DAG). 5-HT1A and 5-HT1B/D receptors are presynaptic and act to reduce 5-HT release, a negative feedback influence. Postsynaptic receptors include 5-HT1A, 5-HT1B/D, 5-HT2A, 5-HT2C, 5-HT3, 5-HT4, 5-HT5, 5-HT6, and 5-HT7. Serotonin actions are complex. Activation of the 5-HT1A receptor is generally inhibitory but also increases dopamine release. Activation of the 5-HT2A receptor is generally excitatory but also inhibits dopamine release (Stahl, 2008). Monoamine interactions in general are very complex; for example, NE can influence 5-HT release, and 5-HT can influence NE release.
Adenosine
Adenosine is a purine nucleoside and is an endogenous molecule in the brain (Benarroch, 2008a). Part of ATP, ADP, and cAMP, adenosine is a critical molecule in cellular energy metabolism, but it also plays a role as a neurotransmitter. Adenosine is found both intra- and extracellularly, and the concentration in the synaptic area is regulated by adenosine transporters (Hasko et al., 2008). There are four subtypes of adenosine receptors, A1, A2A, A2B, and A3, all G protein coupled. Caffeine is an important antagonist at the adenosine receptors. The A1 receptor is generally inhibitory, while the A2 receptors are excitatory, increasing levels of cAMP. Adenosine A2A receptors are colocalized with striatal DA D2 receptors on GABAergic medium spiny neurons which project via the “indirect” striatopallidal pathway to the globus pallidus externa (GPe) (Fuxe et al., 2007). Adenosine at the A2A receptor reduces binding of DA to the D2 receptor, and an antagonist of adenosine, like caffeine, therefore enhances dopamine binding (Simola et al., 2008; Stahl, 2008).
Components of the basal ganglia
Striatum
A large majority of cells in the striatum (80–95%) are medium spiny neurons (MSN), primarily affected in HD (Martinez-Torres et al., 2008). These are GABAergic cells that project out of the striatum to the GP. They receive glutamatergic input from the cortex and the thalamus. The centrum medianum (CM) nucleus of the thalamus projects to the putamen and the parafascicular (Pf) nucleus to the caudate. These cells also receive important dopaminergic input from the SNc. Additional input from the LC is noradrenergic and from the MRN is serotonergic. The glutamatergic input comes to the dendritic spines on these cells, and the dopaminergic input comes to the neck of these spines (Fig. 3.6). It certainly appears that DA regulates the glutamatergic influence on these cells. There are two types of the MSNs that are differentiated by the DA receptors on their surface. Those that have D1 receptors, in addition to GABA, also contain the polypeptide neurotransmitters substance P and dynorphin. These cells project directly to the GPi. Those that have D2 receptors, in addition to GABA, also contain the polypeptide neurotransmitter enkephalin. These cells project to the GPe, as the first step of the circuit to the GPi known as the indirect pathway.
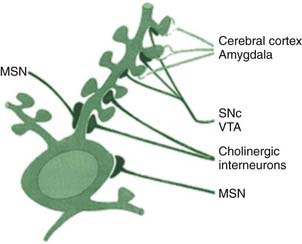
The striatum also contains interneurons, which are aspiny, and do not project outside the striatum. There are at least four classes of these cells. One of these neurons is the giant aspiny cholinergic cell that has axons with large terminal fields (Pisani et al., 2007). These cells receive their principal input from the cortex (glutamate) and the SNc (dopamine). The cortical input activates the cells and the nigral input inhibits them. The cells have autonomous spontaneous activity and are also known as the tonically active neurons (TANs). This spontaneous action means that there is a tonic release of ACh in the striatum. The extracellular level of ACh will be modulated by AChE and by negative feedback mediated by presynaptic muscarinic receptors. These interneurons are also influenced by adenosine, GABA, NE, and 5-HT (Pisani et al., 2007). The cells play a role in reward processing and modulation of dopamine-dependent neuroplasticity.
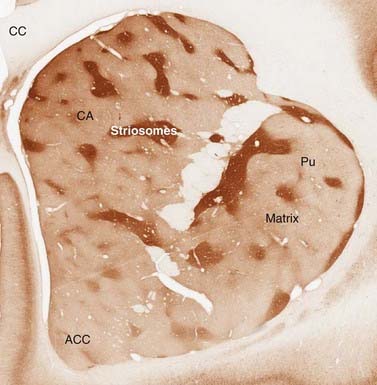
Staining of the striatum for AChE revealed an interesting organization of the cells, which had not been anticipated by simple histology. There are regions called patches or striosomes that are AChE-poor, embedded in a matrix that is AChE-rich (Fig. 3.7). This organization presumably comes from segregated influences of the cholinergic interneurons. The matrix appears to receive more sensorimotor and associative input, while the patches receive more limbic input (Eblen and Graybiel, 1995). The output of the two compartments also differs slightly (Fujiyama et al., 2011).
Globus pallidus (GP)
The GP is divided into the dorsal part and the ventral part (ventral striatum) and into the internal and external divisions, GPi and GPe, respectively, which are separated by the medial medullary lamina. There are only a few interneurons as most neurons are large, parvalbumin-positive, GABAergic neurons with large arbors of dendrites. The cells are shaped as flat disks that are parallel to each other (Yelnik et al., 1984). The GP gets input from all parts of the striatum, and the motor part is posterolateral. The pars reticulata of the SN (SNr) is similar in histology and connectivity to the GPi, from which it is separated by the internal capsule.
Subthalamic nucleus (STN)
The main neurons of the STN are glutamatergic with long dendrites (Yelnik and Percheron, 1979; Hamani et al., 2004). There are about 7.5% GABAergic interneurons (Levesque and Parent, 2005). The dorsolateral part of STN is motor, whereas the ventral part is associative and the medial part projects to limbic areas (Benarroch, 2008b).
Substantia nigra (SN)
The two parts of the SN are rather different from each other and will be described separately.
Substantia nigra pars compacta (SNc)
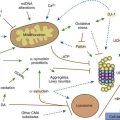
The majority of the neurons of the SNc are dopaminergic and are the cells of origin of the nigrostriatal projection. It is clear that the SNc facilitates movement, and there is good evidence as well for a role of DA in facilitating specific reward behaviors. These cells contain neuromelanin which makes them dark, giving rise to the name of the nucleus (“nigra”). As the death of these cells gives rise to the motor symptoms in Parkinson disease, their cell biology has been studied extensively. Neuromelanin is derived from conjugation of dopamine-quinone, an oxyradical, thereby protecting the dopaminergic neurons from oxidative stress (Sulzer et al., 2000). Neuromelanin can chelate iron and can bind a variety of toxins (Zecca et al., 2001). Some of the dendrites of the dopaminergic cells extend into the SNr, where they have GABAergic receptors. About 15% of cells are interneurons, at least some of which are GABAergic (Hebb and Robertson, 2000).
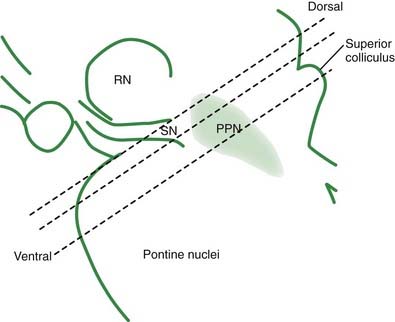
Pedunculopontine nucleus (PPN)
The PPN has important reciprocal connections with other parts of the basal ganglia, and it is crucial to understand its role. It appears to be a critical component in the midbrain locomotor area, among other functional activities. The PPN region is rather complex, and is composed of a number of subregions that are not always completely distinct from each other. Many of the details of the subregions, their exact localization in humans, their connectivities, and their neurotransmitters are still being worked out. The PPN itself can be divided into the compacta (PPNc) and dissipatus (PPNd) (Pahapill and Lozano, 2000; Hamani et al., 2007; Zrinzo et al., 2008; Jenkinson et al., 2009). Other nuclei in the vicinity include the midbrain extrapyramidal area (MEA) (Steininger et al., 1992), the peripeduncular nucleus (Zrinzo and Hariz, 2007), and the sub-cuneiform nucleus (Piallat et al., 2009).
Lateral habenula
As we learn more about the basal ganglia, it becomes apparent that there are many structures with important influence on their function. There has been considerable recent interest in the lateral habenula (Hikosaka et al., 2008). The habenula is located above the posterior thalamus near the midline. The cells have a mixture of neurotransmitters. The lateral part of the habenula has a strong inhibitory influence on the SNc as well as the MRN. It appears to exert its inhibition when there is a negative result from action, thus inhibiting a possible favorable effect of dopamine in facilitating rewarded behavior (Bromberg-Martin et al., 2010).
Zona incerta (ZI)
The ZI is a distinct nucleus, which appears to be an extension of the reticular nucleus of the thalamus, sitting ventral to the thalamus and between the fields of Forel, the fiber tracts conveying the pallidal output to the thalamus (Plaha et al., 2008). It receives input from the GPi and SNr, the ascending reticular activating system, the cerebellum, and different regions of the cerebral cortex. Its output cells are GABAergic and go to the CM/Pf and the VA/VL thalamic nuclei, as well as the MEA, medial reticular formation, and reciprocal connections to the cerebellum, GPi/SNr and cerebral cortex. The ZI may act to help synchronize activity across the many regions that it contacts (Plaha et al., 2008).
Circuitry of the basal ganglia
General circuitry
The connectivity of the basal ganglia is clearly crucial in carrying out its functions. The connections are complex, and it is necessary to have some general model for how they are organized. Such a model was proposed about two decades ago independently by three groups of investigators, each using a different technique: Crossman (Crossman, 1987); Albin, Young, and Penney (Albin et al., 1989); and DeLong (DeLong, 1990). This model has been extremely helpful in organizing thinking, planning pharmaceutical strategies for basal ganglia disorders, and even developing surgical approaches to patients. However, there were always difficulties with the model, and in more recent years, it has become apparent that this model is not sufficient to explain what we now know. Hence, a new model is emerging that takes into account many more of the known connections and basal ganglia functions. It is worthwhile to present the older, “classic” model first, since it does form a foundation for the new model and much current thinking is still based on it.
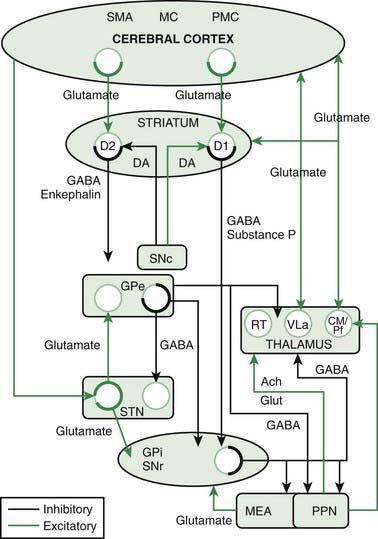
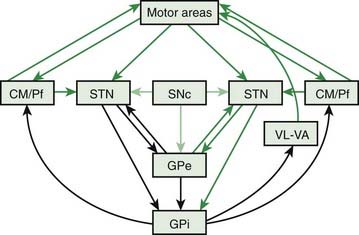
The classic model is shown in Chapter 2, Figure 2.2A, and is a part of the model shown in Figure 3.8. In this model, described also in Chapter 2, there are two parallel loops from the cortex through the basal ganglia and back to the cortex, the direct and indirect pathway. The direct pathway starts with cortical glutamatergic input to the striatal cells bearing D1 receptors. These GABAergic neurons project directly to the GPi. The GABAergic neurons of the GPi project to the VA/VL nuclei of the thalamus, and the thalamic cells return glutamatergic input to the cortex. This four-neuron circuit has two inhibitory neurons and would be net excitatory. The indirect pathway starts with cortical glutamatergic input to the striatal cells bearing D2 receptors. These GABAergic cells project to the GPe, which has a GABAergic projection to the STN, which has a glutamatergic projection to the GPi. The final part of the path from GPi through thalamus to cortex is the same as the direct pathway. This is a six-neuron pathway with three inhibitory neurons, and therefore this would be net inhibitory. The SNc is a modulator of both direct and indirect pathways. Via its influence on D1 and D2 receptors, it will facilitate those striatal neurons of the direct pathway and inhibit those striatal neurons of the indirect pathway. Thus the influence of the SNc is to facilitate the facilitatory pathway and inhibit the inhibitory pathway. While it is not the role of this chapter to discuss pathophysiology, it is immediately apparent why dysfunction of the SNc should give rise to bradykinesia as seen in Parkinson disease. Other movement disorders appear superficially to be similarly easily explained.
There are many connections that are left out of the classic model, and it appears that many of them are rather important. Figure 3.8 (and Chapter 2, Fig. 2.2B) shows the connections of the more complete model, and Figure 3.9 shows the model in a different form to emphasize different connections. This model does not dispute any of the old connections, it adds new, apparently important connections. A missing node in the network is the CM/Pf nucleus of the thalamus. The GPi projects to it as well as to VA/VL, and the CM/Pf in turn projects back both to striatum and STN. CM projects mainly to the putamen, and Pf to caudate and ventral pallidum. CM/Pf also has reciprocal connections to the cortex. There is a strong connection from the cortex directly to the STN; this is called the hyperdirect pathway. Additionally, the SNc projects to STN, and the STN projects back to the GPe, as a reciprocal connection to what was in the classic model. The GPe itself plays a more critical role, now not only getting input from the striatum, but also from STN and SNc. The major new aspects are increased importance of STN and GPe as integrative nodes, and more widespread direct influence of SNc (Obeso et al., 2008).
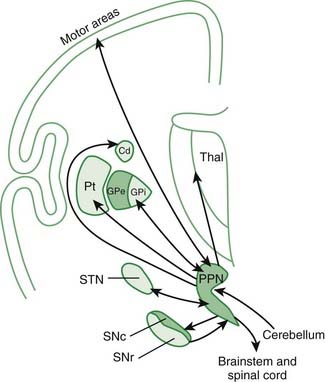
Even this newer model, discussed in the last paragraph, does not include the brainstem influences. These come from the LC (NE), the MRN (5-HT), the ZI, the lateral habenula, and the PPN. The lateral habenula and the PPN have several different neurotransmitters, but the PPN is the main source of cholinergic input to the basal ganglia. The PPN has reciprocal connections with virtually every part of the basal ganglia circuitry (Fig. 3.10). The most important inputs come from GPi and STN, and the most important outputs go to STN, GPi, SNc, thalamus, and brainstem. The latter output to the brainstem is now thought to be the major directly descending motor influence from the basal ganglia. The PPN is also reciprocally connected directly to the cortex.
Parallel pathways
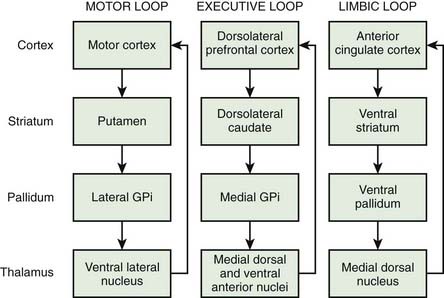
Another important principle of basal ganglia circuitry is its parallel organization (Alexander et al., 1986). Each part of the cortex has a separate pathway through the whole circuit (Fig. 3.11). In broad brush, the motor loop would run from motor cortex, to putamen, to lateral GPi, to VL, back to cortex. An executive or cognitive loop would run from dorsolateral prefrontal cortex, to dorsolateral caudate, to medial GPi, to medial dorsal and VA nuclei of the thalamus, back to cortex. A limbic loop would run from anterior cingulate cortex, to ventral striatum, to ventral pallidum, to medial dorsal nucleus of thalamus, back to cortex. These loops do not interact with each other, or only in a very limited way. The loops are even more fine-grained, so that, for example, in the motor system, different parts of the motor system have different loops and somatotopy of different body parts is maintained throughout the loop (Middleton and Strick, 2000). For example, the primary motor area (M1), the supplementary motor area (SMA), and the ventral premotor area (PMv) will have separate loops. The cerebellum has similar isolated loops, and generally the cerebellar and basal ganglia loops do not interact either. However, there is at least some connection between cerebellar and basal ganglia loops in the GPe (Hoshi et al., 2005).
Physiology
Cellular activity
The medium spiny neurons (MSN) in the striatum have a low firing rate, on average 0.5–2 Hz (van Albada and Robinson, 2009). Cells are often in a hyperpolarized, or down state, because of an intrinsic inwardly rectifying K+ current (Hammond et al., 2007). Cells will fire largely only when there is a convergence of inputs onto the cell. Excitatory inputs come mainly from cortex and thalamus. The excitatory input will be modulated by the striatal interneurons and the nigrostriatal dopamine neurons that have input at the base of the dendritic spine and on the shaft of the dendrite itself (Fig. 3.6). The cholinergic interneurons, the tonically active neurons (TANs), appear to be the most spontaneously active neurons in the striatum. They fire at 2–10 Hz, and are modulated to some extent during learning (Aosaki et al., 1995).
Cellular activity in the GPi is the opposite of the striatum; cells are tonically active at 60–90 Hz (van Albada and Robinson, 2009). Activity in the GPe shows two types of behavior. About 85% of cells have high-frequency bursts together with long intervals of silence for up to several seconds, and an average firing rate of 55 Hz. The other 15% have a slower average rate of about 10 Hz, with occasional bursts (van Albada and Robinson, 2009). Cells in the STN also show spontaneous activity, at about 20–30 Hz, and spikes may be in pairs or triplets (van Albada and Robinson, 2009). Cells in the VA/VL of the thalamus fire at about 18–19 Hz (van Albada and Robinson, 2009).
Circuit physiology
The basal ganglia clearly provide important signal processing, but there is strong evidence that they are also involved in motor learning. The basal ganglia show significant plasticity of synaptic connections. DA plays an important role in this plasticity, conveying reward signals that indicate the importance of what should be learned (Schultz, 2010).
Another measure of cellular activity is called a local field potential (LFP). LFPs summate large number of neurons and synaptic activity, similar to the EEG. Like the EEG, LFPs can sometimes show rhythmic behavior in different frequency ranges. Cellular activity in the basal ganglia nuclei is not typically synchronized and LFPs do not show prominent oscillations. In Parkinson disease, there is synchronization and oscillations in both STN and GPi in the 10–30 Hz (beta) range (Brown, 2007; Galvan and Wichmann, 2008; Israel and Bergman, 2008). The origin of this beta rhythm is not completely clear, but it appears to correlate with bradykinesia and tremor (Chen et al., 2010; Tass et al., 2010). The beta rhythm may also be coupled to even higher-frequency oscillations (Lopez-Azcarate et al., 2010).
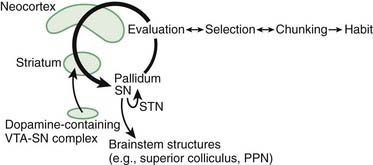
There are a number of theories as to what the basal ganglia contribute to movement processing, and by analogy to processing of cognitive and limbic information as well. One such theory is movement selection, which has been discussed in Chapter 2. According to this theory the direct pathway selects a movement to be facilitated, and the inhibitory influences by the indirect and hyperdirect pathways inhibit undesired movements. Other concepts include the automatic running of motor programs (Marsden, 1982), and learning and release of habits (Graybiel, 2008). The latter idea is that certain behaviors that get rewarded and get repeated, eventually become automatic (Fig. 3.12). As is abundantly clear in this book, considering all the hyperkinetic and hypokinetic disorders that derive from basal ganglia dysfunction, the basal ganglia must have something to do with the regulation of amount of motor output. It seems also to be the case that the function of the basal ganglia must to some extent be a parallel pathway of brain function, since a large stroke destroying most of the basal ganglia or an ablative lesion of the GPi, such as done with pallidotomy in Parkinson disease, can allow the patient to perform reasonably well.
Albin R.L., Young A.B., Penney J.B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12(10):366-375.
Albuquerque E.X., Pereira E.F., Alkondon M., Rogers S.W. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89(1):73-120.
Alexander G.E., DeLong M.R., Strick P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357-381.
Aosaki T., Kimura M., Graybiel A.M. Temporal and spatial characteristics of tonically active neurons of the primate’s striatum. J Neurophysiol. 1995;73(3):1234-1252.
Beaulieu J.M., Gainetdinov R.R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63(1):182-217.
Benarroch E.E. Adenosine and its receptors: multiple modulatory functions and potential therapeutic targets for neurologic disease. Neurology. 2008;70(3):231-236.
Benarroch E.E. Subthalamic nucleus and its connections: Anatomic substrate for the network effects of deep brain stimulation. Neurology. 2008;70(21):1991-1995.
Bonifacio M.J., Palma P.N., Almeida L., Soares-da-Silva P. Catechol-O-methyltransferase and its inhibitors in Parkinson’s disease. CNS Drug Rev. 2007;13(3):352-379.
Bortolato M., Chen K., Shih J.C. Monoamine oxidase inactivation: from pathophysiology to therapeutics. Adv Drug Deliv Rev. 2008;60(13–14):1527-1533.
Bromberg-Martin E.S., Matsumoto M., Hong S., Hikosaka O. A pallidus-habenula-dopamine pathway signals inferred stimulus values. J Neurophysiol. 2010;104(2):1068-1076.
Brown P. Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Curr Opin Neurobiol. 2007;17(6):656-664.
Chen C.C., Hsu Y.T., Chan H.L., et al. Complexity of subthalamic 13–35 Hz oscillatory activity directly correlates with clinical impairment in patients with Parkinson’s disease. Exp Neurol. 2010;224(1):234-240.
Cooper J.R., Bloom F.E., Roth R.H. The Biochemical Basis of Neuropharmacology, eighth ed. Oxford: Oxford University Press; 2003.
Crossman A.R. Primate models of dyskinesia: the experimental approach to the study of basal ganglia-related involuntary movement disorders. Neuroscience. 1987;21(1):1-40.
DeLong M.R. Primate models of movement disorders of basal ganglia origin. Trends in Neuroscience. 1990;13:281-285.
Demarest K.T., Smith D.J., Azzaro A.J. The presence of the type A form of monoamine oxidase within nigrostriatal dopamine-containing neurons. J Pharmacol Exp Ther. 1980;215(2):461-468.
Eblen F., Graybiel A.M. Highly restricted origin of prefrontal cortical inputs to striosomes in the macaque monkey. J Neurosci. 1995;15(9):5999-6013.
Eglen R.M. Muscarinic receptor subtype pharmacology and physiology. Prog Med Chem. 2005;43:105-136.
Eglen R.M. Muscarinic receptor subtypes in neuronal and non-neuronal cholinergic function. Auton Autacoid Pharmacol. 2006;26(3):219-233.
Filip M., Frankowska M. GABA(B) receptors in drug addiction. Pharmacol Rep. 2008;60(6):755-770.
Fujiyama F., Sohn J., Nakano T., et al. Exclusive and common targets of neostriatofugal projections of rat striosome neurons: a single neuron-tracing study using a viral vector. Eur J Neurosci. 2011;33(4):668-677.
Fuxe K., Marcellino D., Genedani S., Agnati L. Adenosine A(2A) receptors, dopamine D(2) receptors and their interactions in Parkinson’s disease. Mov Disord. 2007;22(14):1990-2017.
Galvan A., Wichmann T. Pathophysiology of parkinsonism. Clin Neurophysiol. 2008;119(7):1459-1474.
Graybiel A.M. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359-387.
Groenewegen H.J. The basal ganglia and motor control. Neural Plast. 2003;10(1–2):107-120.
Haber S.N., Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4-26.
Hamani C., Saint-Cyr J.A., Fraser J., et al. The subthalamic nucleus in the context of movement disorders. Brain. 2004;127(Pt 1):4-20.
Hamani C., Stone S., Laxton A., Lozano A.M. The pedunculopontine nucleus and movement disorders: anatomy and the role for deep brain stimulation. Parkinsonism Relat Disord. 2007;13(Suppl 3):S276-S280.
Hammond C., Bergman H., Brown P. Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci. 2007;30(7):357-364.
Hasko G., Linden J., Cronstein B., Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7(9):759-770.
Hebb M.O., Robertson H.A. Identification of a subpopulation of substantia nigra pars compacta gamma-aminobutyric acid neurons that is regulated by basal ganglia activity. J Comp Neurol. 2000;416(1):30-44.
Hikosaka O., Sesack S.R., Lecourtier L., Shepard P.D. Habenula: crossroad between the basal ganglia and the limbic system. J Neurosci. 2008;28(46):11825-11829.
Hoshi E., Tremblay L., Feger J., et al. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8(11):1491-1493.
Israel Z., Bergman H. Pathophysiology of the basal ganglia and movement disorders: from animal models to human clinical applications. Neurosci Biobehav Rev. 2008;32(3):367-377.
Jenkinson N., Nandi D., Muthusamy K., et al. Anatomy, physiology, and pathophysiology of the pedunculopontine nucleus. Mov Disord. 2009;24(3):319-328.
Kopell B.H., Rezai A.R., Chang J.W., Vitek J.L. Anatomy and physiology of the basal ganglia: implications for deep brain stimulation for Parkinson’s disease. Mov Disord. 2006;21(Suppl 14):S238-S246.
Levesque J.C., Parent A. GABAergic interneurons in human subthalamic nucleus. Mov Disord. 2005;20(5):574-584.
Lopez-Azcarate J., Tainta M., Rodriguez-Oroz M.C., et al. Coupling between beta and high-frequency activity in the human subthalamic nucleus may be a pathophysiological mechanism in Parkinson’s disease. J Neurosci. 2010;30(19):6667-6677.
Lovinger D.M. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology. 2010;58(7):951-961.
Marsden C.D. The mysterious motor function of the basal ganglia: the Robert Wartenberg Lecture. Neurology. 1982;32(5):514-539.
Martinez-Torres I., Tisch S., Limousin P. The basal ganglia. In: Conn P.M., editor. Neuroscience in Medicine. third ed. Totowa, NJ: Humana Press; 2008:401-414.
Matsumoto M., Weickert C.S., Akil M., et al. Catechol O-methyltransferase mRNA expression in human and rat brain: evidence for a role in cortical neuronal function. Neuroscience. 2003;116(1):127-137.
Middleton F.A., Strick P.L. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31(2–3):236-250.
Missale C., Nash S.R., Robinson S.W., et al. Dopamine receptors: from structure to function. Physiol Rev. 1998;78(1):189-225.
Obeso J.A., Marin C., Rodriguez-Oroz C., et al. The basal ganglia in Parkinson’s disease: current concepts and unexplained observations. Ann Neurol. 2008;64(Suppl 2):S30-S46.
Pahapill P.A., Lozano A.M. The pedunculopontine nucleus and Parkinson’s disease. Brain. 2000;123(Pt 9):1767-1783.
Piallat B., Chabardes S., Torres N., et al. Gait is associated with an increase in tonic firing of the sub-cuneiform nucleus neurons. Neuroscience. 2009;158(4):1201-1205.
Pisani A., Bernardi G., Ding J., Surmeier D.J. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci. 2007;30(10):545-553.
Plaha P., Khan S., Gill S.S. Bilateral stimulation of the caudal zona incerta nucleus for tremor control. J Neurol Neurosurg Psychiatry. 2008;79(5):504-513.
Purves D., Augustine G.J., Fitzpatrick D., et al. Neuroscience, second ed. Sunderland, MA: Sinauer Associates, Inc.; 2001.
Schultz W. Dopamine signals for reward value and risk: basic and recent data. Behav Brain Funct. 2010;6:24.
Siegel G.J., Albers R.W., Brady S.T., Price D.L. Basic Neurochemistry. Molecular, cellular and Medical Aspects, seventh ed. Amsterdam: Elsevier; 2006.
Simola N., Morelli M., Pinna A. Adenosine A2A receptor antagonists and Parkinson’s disease: state of the art and future directions. Curr Pharm Des. 2008;14(15):1475-1489.
Stahl S.M. Stahl’s Essential Psychopharmacology, third ed. Cambridge: Cambridge University Press; 2008.
Steininger T.L., Rye D.B., Wainer B.H. Afferent projections to the cholinergic pedunculopontine tegmental nucleus and adjacent midbrain extrapyramidal area in the albino rat. I. Retrograde tracing studies. J Comp Neurol. 1992;321(4):515-543.
Sulzer D., Bogulavsky J., Larsen K.E., et al. Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc Natl Acad Sci USA. 2000;97(22):11869-11874.
Tass P., Smirnov D., Karavaev A., et al. The causal relationship between subcortical local field potential oscillations and Parkinsonian resting tremor. J Neural Eng. 2010;7(1):16009.
van Albada S.J., Robinson P.A. Mean-field modeling of the basal ganglia-thalamocortical system. I Firing rates in healthy and parkinsonian states. J Theor Biol. 2009;257(4):642-663.
Van den Heuvel D.M., Pasterkamp R.J. Getting connected in the dopamine system. Prog Neurobiol. 2008;85(1):75-93.
Woolsey T.A., Hanaway J., Gado M.H. The Brain Atlas. A Visual Guide to the Human Central Nervous System, third ed. Hoboken, NJ: John Wiley & Sons, Inc.; 2008.
Yelnik J., Percheron G. Subthalamic neurons in primates: a quantitative and comparative analysis. Neuroscience. 1979;4(11):1717-1743.
Yelnik J., Percheron G., Francois C. A Golgi analysis of the primate globus pallidus. II. Quantitative morphology and spatial orientation of dendritic arborizations. J Comp Neurol. 1984;227(2):200-213.
Zecca L., Tampellini D., Gerlach M., et al. Substantia nigra neuromelanin: structure, synthesis, and molecular behaviour. Mol Pathol. 2001;54(6):414-418.
Zrinzo L., Hariz M. The peripeduncular nucleus: a novel target for deep brain stimulation? Neuroreport. 2007;18(15):1631-1632. author reply 2–3
Zrinzo L., Zrinzo L.V., Tisch S., Limousin P.D., et al. Stereotactic localization of the human pedunculopontine nucleus: atlas-based coordinates and validation of a magnetic resonance imaging protocol for direct localization. Brain. 2008;131(Pt 6):1588-1598.