Chapter 15 Function and Anatomy of the Mammalian Retina
![]() For additional online content visit http://www.expertconsult.com
For additional online content visit http://www.expertconsult.com
The vertebrate retina forms a thin sheet of neural tissue at the back of the eye that converts light to an electrical signal. The neural retina is approximately 100–200 µm thick, depending on the species, and represents a triumph of miniaturization.1 The retina is a spatial information processor built upon a mosaic of rod and cone photoreceptors, which are the light-responsive elements that initiate signaling using graded electrical signals.2 The spatial information content is preserved through the lateral geniculate nucleus, retinotopically mapped on to area V1 (area 17) of the primary visual cortex and from there on to a number of higher visual processing areas. There is considerable processing both within the retina as well as in higher brain structures and we interpret these electrical signals as vision. While vision is an analog system, a rough approximation to a digital system would result in a resolution in excess of 500 megapixels. Although initially conceived to be a simple model for the brain, more probably, the retina approaches limits imposed by metabolism, blood flow, and diffusion. This pressure to pack more function into a small volume of neural tissue leads to increased complexity.
Visual illusions and multiple channels
At first glance, Figure 15.1 looks like a random array of large pixels with three bright areas. Now, screw up your eyes, glance aside or look at it from the other side of the room, and suddenly you may recognize da Vinci’s Mona Lisa. The bright areas are the face, chest, and hands. But why does the image seem to be more recognizable when visual input is distorted? The high-spatial-frequency components have been removed by greatly reducing the pixel number. What detail does remain is a confusing array of boxes. However, this detailed view can be blurred by squinting, which removes the high-frequency components, leaving an easily recognized low-acuity version. So this is a direct demonstration that the visual system operates on at least two channels of information at different spatial scales simultaneously. In fact, there is empirical evidence that vision is composed of at least 15 parallel channels or streams of information that are transmitted simultaneously throughout the visual system. It is the purpose of this chapter to describe the functional anatomy of the retina that leads to the formation of some of these independent channels of visual information.
The retina is a piece of brain
Like the rest of the central nervous system (CNS), the retina is embryologically derived from the neural tube. It is formed from the same components as the rest of the CNS and like all other sensory systems has specialized structures, photoreceptors that transduce environmental energy into electrical potentials. As such, one can divide the retina in two parts3: (1) the sensory retina, concerned with phototransduction of light by rod and cone photoreceptors; and (2) the neural retina, consisting of more typical interneurons (bipolar, horizontal, and amacrine cells) and projection neurons (ganglion cells) that carry out the first steps in processing visual information.
The retina has been characterized by Dowling as an approachable part of the brain,4 because it is a ready-made brain slice with few barriers to the penetration of drugs or antibodies. In addition, its natural stimulus, light, is easily controlled and the same stimuli can be presented either to the intact animal or to the retina removed from the eye and placed in vitro. This chapter focuses on processing in the mammalian retina. However, many of the pioneering studies in retinal function were initially performed in fish and amphibian retina. In particular, the salamander retina has been a long-standing model because its large cells enhance the ease of electrophysiological recording.
Neuronal communication: chemical and electrical
Many neurons also are directly connected via electrical synapses known as gap junctions.5–9 Gap junctions are named after the narrow gap formed by docked hemichannels, or connexons aligned on either side of two cell membranes. Each hemichannel is built from six connexins that surround a central pore, forming an intercellular channel, which passes ions and small molecules (≤1 kDa). Gap junctions are not static pores; they are modulated by light and contribute to neural processing.
The retina is a layered structure
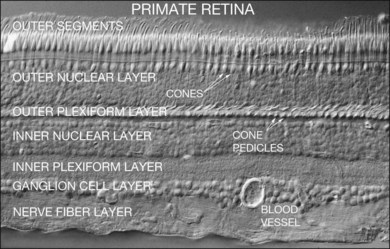
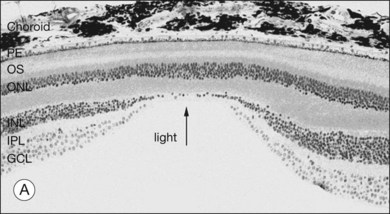
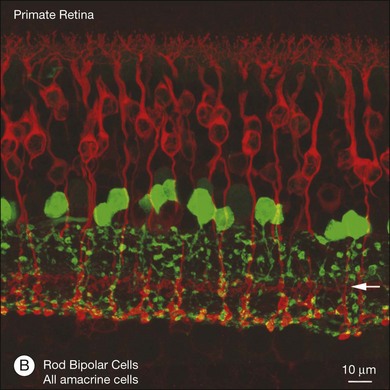
The retina is a beautifully layered structure in which three layers of neurons can be visualized without staining (Fig. 15.2). The outer nuclear layer (ONL) contains the cell bodies of photoreceptors, both rods and cones. The inner nuclear layer (INL) contains the cell bodies of horizontal, bipolar, amacrine and radial glial (or Müller) cells. The ganglion cell layer (GCL) contains displaced amacrine and ganglion cells. Ganglion cells are the projection neurons of the retina: their axons form the optic nerve and project to a variety of subcortical nuclei. The three nuclear layers are separated by two synaptic (plexiform) layers that contain the dendrites and synapses. The outer plexiform layer (OPL) lies between the ONL and the INL. This is where the photoreceptors, horizontal and bipolar cell dendrites interact. The inner plexiform layer (IPL) separates the INL and the GCL and this is where the bipolar cell axons, amacrine, and ganglion cells interact. When we speak of the retina, outer or distal refers to the scleral side of the retina and inner or proximal refers to the vitreal side of the retina.
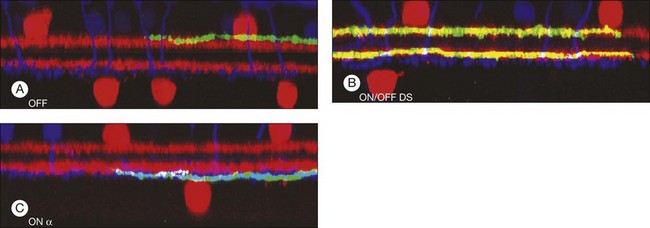
The functional stratification of the retina extends to the IPL, which is organized according to the polarity of bipolar cell inputs.10 Since the time of Cajal, the IPL has been divided into five layers: 1 and 2 for sublamina a and layers 3–5 for sublamina b. In mammalian species, there are 9–11 morphological types of bipolar cell, plus the rod bipolar cell, which terminate at different depths in the IPL.11–13 The polarity of a bipolar cell response is determined by the differential expression of postsynaptic glutamate receptors on their dendrites. The dendrites of OFF cone bipolar cells carry α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate receptors and they ramify in sublamina a of the IPL.14 In contrast, ON cone bipolar cells and rod bipolar cells express mGluR6 receptors and their axons ramify in sublamina b.15–17 The separation of ON and OFF pathways appears to be a fundamental principle of retinal organization, which is reflected throughout the visual system.18,19
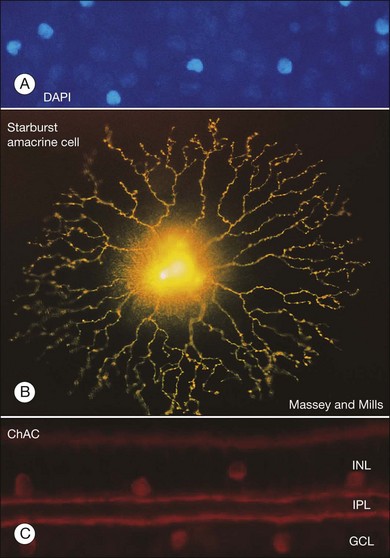
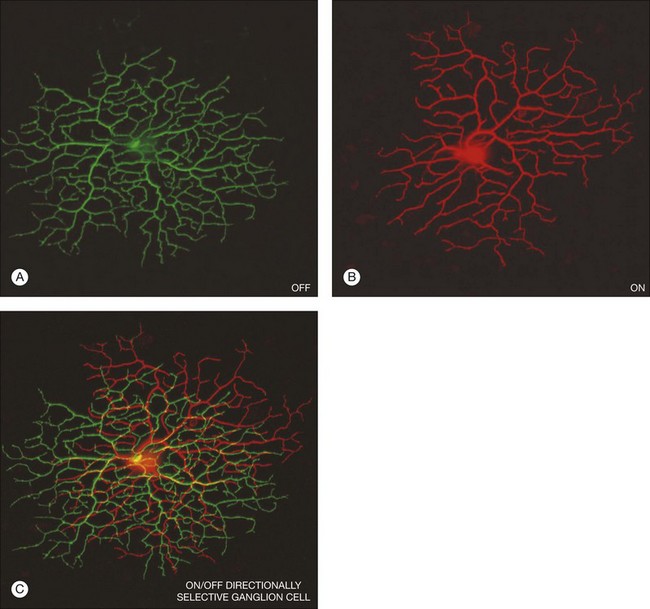
There is a wealth of evidence to support the functional division of the IPL into ON and OFF sublaminae. For example, cholinergic amacrine cells (ChACs), also known as starburst amacrine cells on account of their unique morphology, are present as mirror-image pairs.20 The conventionally placed ChACs have somas in the innermost layer of the INL. They have OFF responses to light stimulation and ramify in sublamina a. In contrast, the displaced ChACs reside in the GCL, produce ON responses, and ramify in sublamina b. Likewise, alpha ganglion cells are present as paramorphic pairs such that the dendritic trees of OFF alpha ganglion cells are stratified in sublamina a to receive input from OFF bipolar cells while ON alpha ganglion cells ramify in sublamina b to make contact with ON bipolar cells.21,22 In primate retina, both midget and parasol ganglion cells are present as paramorphic pairs, which conform to the stratification rules of the IPL. ON/OFF directionally selective (DS) ganglion cells produce both ON and OFF responses of short latency, indicating direct input, and they are bistratified with dendrites in sublaminae 2 and 4, coincident with the cholinergic bands.
The ON and OFF stratification of the IPL is a fundamental tenet of retinal organization which applies to many, if not most, cell types. However, several exceptions to this rule have now been identified. For example, the dopaminergic amacrine cells (DACs) stratify predominantly in sublamina a, the OFF layer, yet they apparently produce ON responses to light.23,24 Likewise, the intrinsically photosensitive retinal ganglion cells (ipRGCs), which stratify in sublamina a of the primate retina, were all ON cells.25 These cell types, among others, receive input from ON bipolar axons as they traverse sublamina a of the IPL. These axonal ribbons provide a set of inputs that break the stratification rules of the IPL. Thus, there is an additional accessory ON sublayer in the outer portion of the IPL26,27 (see sections on DACs and ipRGCs) (Table 15.1).
| Layer | Contains: |
|---|---|
| Outer nuclear layer (ONL) | Photoreceptors, rods, and cones |
| Inner nuclear layer (INL) | Horizontal cells, bipolar cells, amacrine cells, Müller cells |
| Ganglion cell layer (GCL) | Ganglion cells, displaced amacrine cells |
| Outer plexiform layer (OPL) | Photoreceptors talk to horizontal cells and bipolar cells |
| Inner plexiform layer (IPL) | Bipolar cells talk to amacrine cells and ganglion cells |
Gross retinal morphology
The fovea
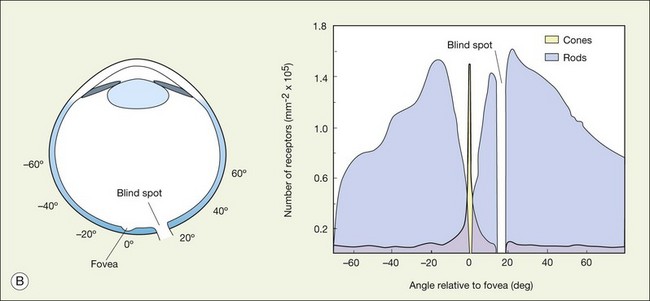
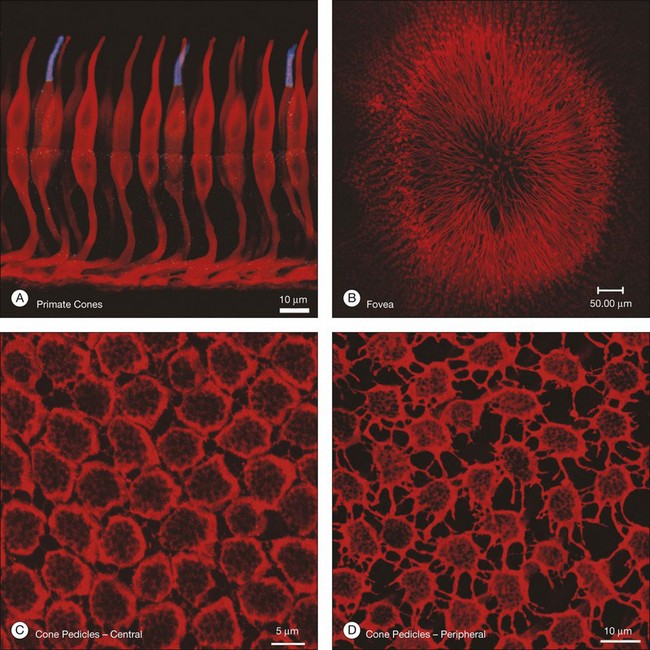
When a peripheral visual stimulus gets our attention, we automatically focus the retinal image in the center of our gaze. In the primate retina this central region of highest visual acuity is known as the fovea. Here there is a depression in the retina, known as the foveal pit, where cone photoreceptor density is highest and there are no rods. The pit results from the lack of overlying neurons (Fig. 15.3). Instead, cone axons, Henle fibers, run obliquely to cone pedicles away from the fovea where the bipolar and ganglion cells connected to the central cones are “piled up” in an annular zone with the GCL 6–8 cells thick. The foveal structure is thought to maximize sensitivity because light cannot be scattered by passing through other retinal layers. It also optimizes acuity by packing the maximum number of cones and reducing their size. In human retina, the peak cone density approaches 200 000/mm2 and the ONL is slightly thicker to accommodate these extra cells.28 There are no blood vessels in the fovea and in the central fovea there are no blue cones. The low density of blue cones lowers their acuity,29 to match the blurring caused by chromatic aberration in the lens.30 Other mammals have an area centralis (cat) or a visual streak (rabbit, swine) with similar high cell density but these structures lack the central depression. A consequence of the exclusion of rods from the fovea is that in dark-adapted conditions, say looking for a dim star, it is necessary to look slightly off the visual axis to focus the image in the region of high rod density.
The blind spot and how to find it
There are no photoreceptors where the optic nerve exits the eye and so any image that falls on this region cannot be processed by the retina. Curiously, we do not perceive a hole in the visual scene because the visual system fills in. To demonstrate the blind spot, hold the page level at normal reading distance. Look at the O in Figure 15.4, and then close your right eye. The X on the left should disappear because the image of the cross falls on the optic nerve head. You can reverse this demonstration: look at the X, then close the left eye to make the O disappear. Remember, the blind spot is nasal to the fovea. The light rays cross over at the lens so the blind spot is lateral to the point of focus.
Painting the retina – techniques to label and visualize retinal neurons
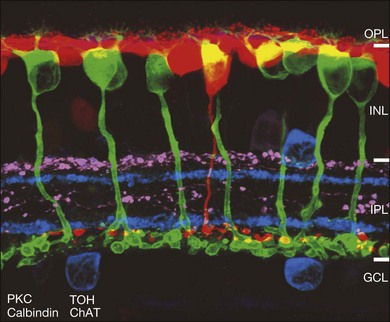
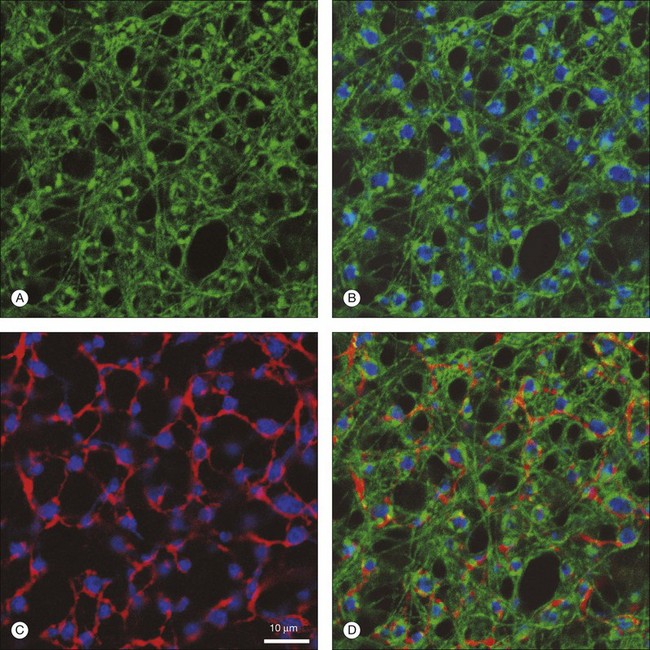
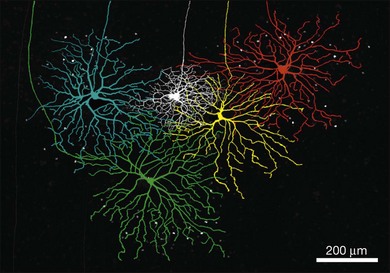
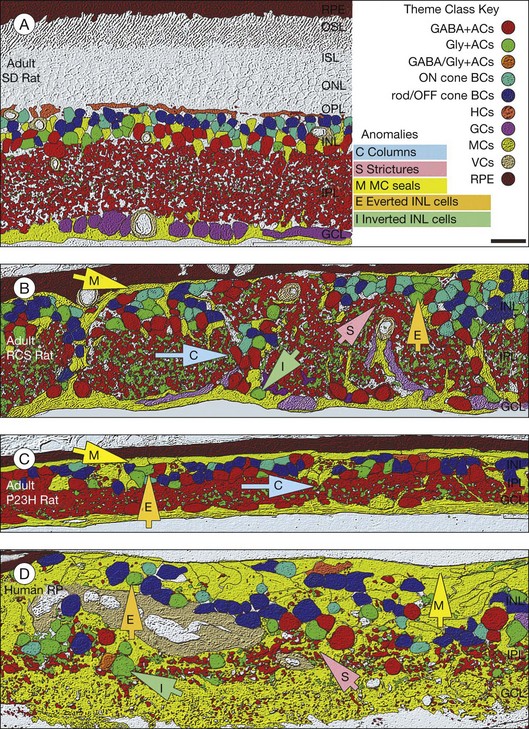
The major landmarks of the retina, two synaptic layers sandwiched between three nuclear layers, are obvious even in the unstained section of macaque retina shown in Figure 15.2. In fact, there is an immense amount of detail present in this image. For example, individual cones can be seen with their descending axons terminating in a row of cone pedicles in the OPL. However, the somas of all the other retinal neurons are indistinguishable and the details of their structures cannot be visualized. One goal of vision scientists is to have a complete map of all the interconnections between the retinal neurons so that the functional outputs can be fully understood. To uncover the hidden pathways and circuits through the retina, we need to stain specific cell types selectively (Fig. 15.5), so that we can differentiate between rod and cone pathways, for example. Furthermore, it is very useful to visualize several cell types at once so that the number and position of one cell type relative to another can be determined. This can be achieved by confocal microscopy, which permits the simultaneous acquisition of three or more different labels in addition to providing improved resolution and three-dimensional visualization (Fig. 15.5). With care, confocal microscopy can be used to visualize both chemical and electrical synapses in the retina.31 Two-photon confocal microscopy permits imaging using an infrared laser, which does not stimulate the visual pigments. It has been used to measure changes in intracellular calcium concentration of single neurons or dendrites in response to stimulation with visible light.32,33 One disadvantage of these methods is that individual synapses cannot easily be visualized because of the inherent resolution limits of confocal microscopy. However, high-throughput electron microscopy methods have been developed that allow three-dimensional reconstruction of blocks of retina, albeit in fixed tissue. By careful analysis and multiple staining approaches it is possible to identify all the synapses and cells to which an individual neuron makes connections.34
The mainstay of structure, function, and morphology studies is immunocytochemical methods, which can be used to stain structural components, enzymes, neurotransmitters, synaptic proteins, and postsynaptic receptors (Fig. 15.5). Specific primary antibodies are currently available for many of these components or they can be generated. Secondary antibodies conjugated to an ever-increasing variety of fluorochromes are readily available as standard reagents and allow for multiple staining. Single neurons can also be filled with fluorescent dyes via glass microelectrodes. Diffusible tracers such as Neurobiotin can be used to label a network of coupled cells.5 The optic nerve can be back-filled with fluorescent dyes, which in one useful variant are concentrated in vacuoles that explode when excited to release dye, which diffuses throughout the dendritic structure of individual ganglion cells.35 Certain ganglion cell types can be selectively labeled by stereotactically injecting a central target with retrograde tracers.36 Another method to label single cells involves labeling the whole retina with dihydrorhodamine, which can then be photoconverted to a fluorescent product in selected cells by illumination.37,38 Individual neurons of all types can be randomly labeled with ballistic particles coated with fluorescent dyes or DNA to synthesize a specific marker.39 Finally, there is increasing use of transgenic animals such as mice engineered to express green fluorescent protein (GFP) variants under the control of a specific promoter.40
Six major neuronal cell classes
The retina contains six major neuronal classes. Photoreceptors are located in the ONL and can be subdivided into rods and cones. Bipolar cells take the signals from photoreceptors and transmit them to the inner retina. Horizontal cells and amacrine cells are laterally extensive interneurons in the outer and inner retina, respectively. Ganglion cells receive input from bipolar and amacrine cells and form the output from the retina. In addition, interplexiform cells share many properties with amacrine cells but project back to the outer retina. Finally, the radially oriented Müller cells are the predominant glial cells (Table 15.2).
| Neuronal types | Role | Types |
|---|---|---|
| Photoreceptors | Rod and cones | 2 |
| Horizontal cells | Lateral interneurons, OPL | 2 |
| Bipolar cells | Vertical connection | 10–12 |
| Amacrine cells | Lateral interneurons, IPL | ~30 |
| Ganglion cells | Output neurons | ~20 |
| Interplexiform cells | Feedback, IPL to OPL | ? |
| Total | ~65 |
OPL, outer plexiform layer; IPL, inner plexiform layer.
Classification of retinal cells
Although the retina contains six major cell classes, these may be further divided into many distinct subtypes for a total of about 65 different neurons. Thus, the retina is certainly complex, but it is not chaotic. Cells of a given type form nonrandom mosaics across the retina.41 A single type shares essential morphological features and uses the same neurotransmitter(s). Furthermore, they ramify at a characteristic depth in the retina and make stereotyped synaptic connections, including the same type and number of postsynaptic cells, and even the same number of synapses.42,43 As we learn more about neuronal circuits and the functional anatomy we see repeated patterns across the retina, and we are reminded that it is a two-dimensional array of recurring circuits.
The classification of retinal neurons, which dates back to the work of Cajal more than a century ago, is not a simple matter.44 However, it is important that we are at least aware of all the major cell types in the retina. Even a basic understanding of a circuit cannot be achieved if critical parts are missing. Classification is complicated by the large number of different cell types, the low probability of obtaining rare types, variation in size with eccentricity, and the normal morphological variation within a cell type. While the staining and sampling issues have largely been resolved by modern methods, the question of what constitutes a separate cell type, as opposed to subtle differences between examples from the same type, is still a problem. The resolution lies partly in understanding the significance of some morphological features and partly in considering more variables, i.e., independent criteria to classify a specific cell type. Finally, as a kind of parity check, it is necessary to consider the properties of a whole population for a given cell type to see if it is consistent.
Neurons may be classified into unique cell types on the basis of several properties:
1. their morphology, meaning the size, shape, and structure of a neuron, especially its dendritic field. This may include measures of dendritic density and branching patterns
2. the depth within the retina, particularly the level in the IPL where its dendrites or axons stratify. We have come to realize that depth in the IPL functions as a simple addressing system to determine which target neurons are in synaptic contact
3. their electrophysiology, particularly their excitatory and inhibitory inputs
4. their biochemistry, particularly with regard to the different neurotransmitters used to communicate between neurons and structural proteins or receptors
5. the pattern of connections with other neurons, which should be consistent for a unique cell type
6. finally, the properties of the whole population for a specific cell type.
A group of cells that are all the same type have certain special properties. The retina is a two-dimensional array and most cell types tile the retina in a consistent manner so that an even coverage is obtained. Cells of the same type are usually not adjacent. The position of the cells relative to others of the same class is also an indicator of cell type because unique populations form nonrandom mosaics.41 Consider a handful of marbles dropped on the floor. Some will stop close by but others may roll into a corner. If a measure is taken between the nearest neighbors, it will be found to have a high variation. In contrast, for a nonrandom mosaic of neurons, the spacing is controlled such that the distance to the nearest cell of the same type is relatively constant and the variance of this measure is low. The ratio of mean nearest neighbor distance to the variance gives an index of regularity for a cell population. We would expect the ratio to be low for the randomly scattered marbles, but indices of three or higher are common for the typical nonrandom, orderly mosaics of retinal neurons. The most satisfying results for classification come from the convergence of these methods to indicate the function of a retinal cell in the process of vision.
Photoreceptors
Cones
The mammalian retina contains two types of photoreceptor, rods and cones.45 Rods account for 95% of all photoreceptors. They are numerous, with slender outer segments, densely packed and specialized for high sensitivity under dark or starlight conditions. Cones are larger, with tapering outer segments, and they are found in the top row of the ONL (see Fig. 15.3). Cones make up only ~5% of photoreceptors28,45 but they provide high-acuity color vision in daylight conditions when photons are abundant. This versatile combination of rods and cones and their associated circuits covers an intensity range of around 10 log units from the darkest night to bright sunlight.46 While the average visual scene has a range of intensities covering 2–3 log units, the continual adaptation of retinal sensitivity slides this operating range through the entire range of light intensities. This is a critical function of the retina because outside the normal operating range we are functionally blind. Common examples include the inability to see momentarily when entering a dark cinema or driving into the setting sun. Much of the adaptation takes place in photoreceptors but, as we shall see below, this is accompanied by major changes in the neural pathways through the retina. This is arguably the most important function of the retina, after light detection itself.
There are approximately 5 million cones in the human retina and 190 000 in the mouse retina.47,48 Cones make up approximately 5% of the total photoreceptors in humans, compared to 2.8% in the mouse,49 so we are all rod-dominated in terms of absolute numbers. One exception is the ground squirrel, which is truly cone-dominated. Importantly, cones are not evenly distributed. In human retina, there is a massive peak at the fovea (Fig. 15.3) where the density reaches around 200 000/mm2, approximately 100 times the density in the periphery.28,47 This is the region of maximum acuity, although the peak density slightly exceeds experimentally measured visual acuity due to blurring by the optics of the eye.50–52 Where the ganglion cell axons gather to form the optic nerve there are no photoreceptors and their absence from this location is the cause of the blind spot (Fig. 15.3).
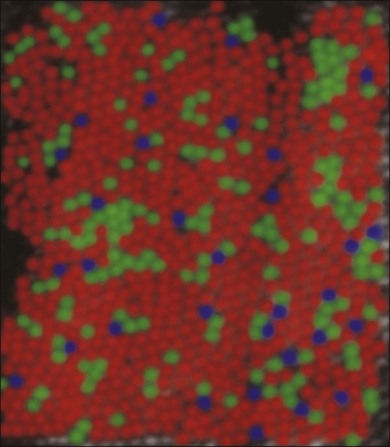
Cones support color vision and, in old-world primates and humans, there are three classes: blue, green and red. They are maximally sensitive to 430, 530, and 561 nm light, respectively.53–55 Other mammals, including cats, rabbits, and rodents, have an evolutionary ancient form of color vision based on green and blue cones only. The presence of red and green cone opsins in a tandem array on the X chromosome is thought to be due to a recent gene duplication and underlies the preponderance of color blindness among males.56 Using adaptive optics to correct for blurring in the lens and cornea, the distribution of red, green, and blue cones can be mapped in the living human eye.57 Surprisingly, the distribution of cones was random and clumpy (Fig. 15.6). In addition, there appears to be enormous variation in the red/green cone ratio among individuals with normal color vision.
Blue cones are present as a minority: they make up approximately 10% of the cone population (Fig. 15.6). This is not enough to support high acuity but calculations show that the blue cone density is sufficient to support the reduced resolution caused by visual aberration at the blue end of the spectrum.30 At the very center of the fovea, blue cones are absent.29,58 The fact that this is not readily apparent is due to the relatively poor spatial acuity of color vision compared to the luminance-driven pathways.
Red and green cones cannot be differentiated morphologically and the red and green cone opsins are so closely related that they are also indistinguishable. However, blue cones can be mapped with a selective antibody against blue cone opsin29,59 or by their selective accumulation of certain dyes.58
Rods
The ONL contains photoreceptor cell bodies, both rods and cones.45 Even in the cone-dominated human retina, rods far outnumber cones, by a factor of 20 : 1, so they account for most of the ONL except at the fovea. The human retina contains approximately 100 million rods, and they pool signals to provide high sensitivity for dark-adapted vision, say starlight, which appears monochromatic. A lack of color vision is the hallmark of rod-mediated vision. Rods are absent within 350 µm of the fovea but reach a peak density in an annular region at about 20° eccentricity (Fig. 15.3). This does not match the area of maximum scotopic acuity, which occurs around 5°,60 so it has been suggested that another component of the rod pathway, such as the AII amacrine cells, present at a much lower density, forms a bottleneck to limit acuity61,62 (see below).
Cone pedicles and rod spherules
Cones and rods make contact with bipolar and horizontal cells in the OPL. Cone axons descend through the massed ranks of rod somas in the ONL to terminate in a two-dimensional array of cone pedicles at the OPL (Fig. 15.7). Near the fovea in primate retinas, the cone axons are splayed out radially so that the pedicles form an annular array around the center (Fig. 15.7B). Cones form two specialized contacts, ribbon synapses and flat contacts, with postsynaptic neurons. Ribbon synapses are so named because of electron-dense structures at invaginations where they contact depolarizing (or ON) bipolar and horizontal cells (Fig. 15.8). Cone pedicles also make flat contacts along the base of the pedicle with hyperpolarizing (or OFF) bipolar cells. Rod axons also descend to form synapses with a single type of depolarizing bipolar cell as well as horizontal cells. The terminals of rods are called spherules, and, similar to cones, they use a ribbon synapse. In contrast to cones, there is only a single invagination in each, containing two rod bipolar and two horizontal cell dendrites.
Each cone pedicle is 6–8 µm in diameter and, near the fovea, contains 20 synaptic ribbons and, in the periphery, around 40.63 Cones release glutamate constantly in the dark and the synaptic ribbons are thought to support this high rate of release. As we will describe further below, the multiple ribbon and flat synapses on each cone pedicle form connections with many different types of bipolar cell.
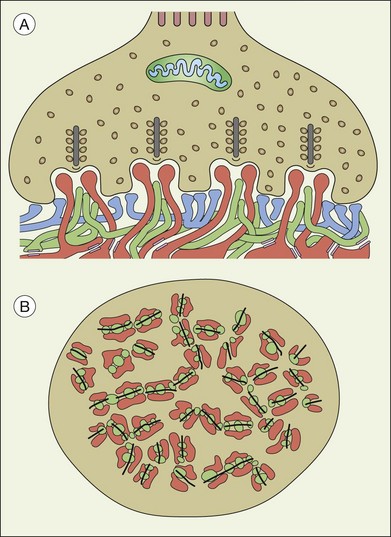
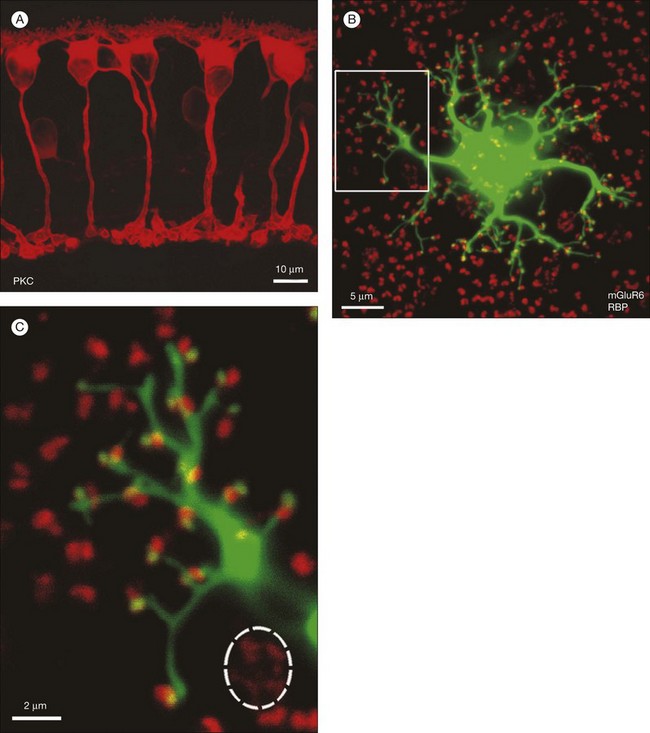
A sense of the complexity of these synapses can be obtained by labeling some of the individual components. Antibodies reactive against a vesicle protein such as synaptophysin provide a way to label photoreceptor terminals in the OPL, because photoreceptor axon terminals are filled with vesicles. To visualize the synaptic ribbons, antibodies to kinesin II can be used. Finally, antibodies to the mGluR6 receptor, which is located on the dendritic tip of the depolarizing bipolar cells, mark one of the postsynaptic processes. When visualizing just the cone pedicle, there are a number of indentations or invaginations (Fig. 15.8). Within each invagination, horizontal cell dendrites are lateral and high, approaching the synaptic ribbons. ON cone bipolar cell processes are central but slightly lower at the synaptic ribbon. In contrast, OFF bipolar dendrites form basal synapses with the cone pedicle at a distinctly lower level. Staining a piece of retina for synaptophysin, kinesin II, and mGluR6, begins to show the complexity of the structures (Fig. 15.9). Rod spherules contain a single large horseshoe-shaped ribbon with two bipolar cell dendrites nestled within. The cones have numerous and slightly smaller ribbons with each also nestled against an mGluR6-labeled dendrite. What is not visible are the horizontal cell dendrites that also invaginate, but are slightly lower than the bipolar cells.
Around 10–12 cone bipolar cells plus multiple horizontal cell dendrites contact each cone, so under the pedicle there may be as many as 200 postsynaptic processes.64 In fact, the cone pedicle may be the most complex synaptic structure in the brain. The distribution of postsynaptic processes under the cone pedicle is laminated in a stereotyped fashion (Fig. 15.8).63,64
The fundamental problem for rods is to detect a small brief hyperpolarization due to a single photon against a noisy background of thermal noise and the stochastic nature of transmitter release.65,66 One strategy is to minimize background variation by maintaining a high sustained-release rate in the dark. The synaptic terminal of a rod, or rod spherule, is about 2 µm in diameter and contains a very large synaptic ribbon (Fig. 15.9), a specialization thought to be associated with a high rate of transmitter release. The spherule is packed with synaptic vesicles, containing the neurotransmitter glutamate, to support sustained transmitter release. There is a single invagination (imagine a closed fist and insert a finger between thumb and forefinger to make a pocket) containing a tetrad of processes, two from horizontal cells and two rod bipolar dendrites.67 This structure brings the postsynaptic processes close to the release site and may prevent spillover to adjacent rods. These anatomical specializations appear to reduce variation in rod signaling so that small single-photon responses can be detected with high reliability.
Photoreceptor coupling
While the synapses between photoreceptors and second-order neurons are extremely complex, there are still additional connections between photoreceptors. These take the form of electrical coupling, mediated by gap junctions. Close to the fovea, the cone pedicles are densely packed, almost touching, and connected by very fine processes known as telodendria (Fig. 15.7C).45 Rod terminals are either absent or displaced slightly higher, towards the outer segments, in this region. More peripherally, the cone pedicles are widely spaced and the telodendria are much more prominent (Fig. 15.7D). The contact points between telodendria of neighboring cones are the sites of connexin (Cx)36 gap junctions, which mediate electrical coupling between cones.68,69 Recordings between neighboring cones also show that they are coupled.68,70 This may seem puzzling at first because it should lead to a loss of acuity and blurring. However, the cone array actually oversamples the signal so this leads to little or no loss. Instead, the coupling is thought to reduce noise, which is random, while the light-driven signals, which are correlated between close neighbors, will be reinforcing. It has been calculated that this may improve the signal-to-noise ratio by 77% – a large gain for little loss.71 Coupling between red/green cones is indiscriminate, which may reflect the close absorption curves of red and green cones.70 Morphologically, blue cone pedicles are slightly smaller than those of red/green cones and they have only a few withered dendrites, which rarely touch neighboring cones.72 Hence, blue cones are not coupled into the red/green network.68,70 This may serve to preserve spectral information in the color pathways.
There are also gap junctions between cone pedicles and rods.73 These allow rod signals to enter cones, and rod-mediated signals can be detected in second-order neurons that are thought to be connected exclusively to cones.74 Responses with the signature of rod origin have also been recorded in primate cones.75 Because rods far outnumber cones, the influence of many rods on a single cone may be substantial. It is now thought that rod–cone coupling forms an alternative rod-driven pathway that may be important at intermediate light intensities, in the mesopic range.76,77 This influence may relate to the enhancement of cone electroretinogram (ERG) amplitudes in humans and mice by light adaptation, where the cone ERG gradually increases in amplitude following the onset of a steady adapting field.78–81 Blocking gap junctions inhibits this effect in the mouse retina. In the human, however, the adaptation dependence of these changes has a photopic (cone) signature. Rod–cone coupling also is very dynamic and influenced by circadian rhythms, increasing at night and decreasing during the day.82
Photoreceptors release glutamate in the dark
The first intracellular recordings from cones were surprising because they showed that photoreceptors hyperpolarize in response to light.4 This means they are relatively depolarized and release their neurotransmitter, glutamate, in the dark. From the viewpoint of signal information, the sign of the photoreceptor response makes no logical difference; photoreceptors produce graded responses modulated around the mean light level. When a photon is absorbed, the visual pigment is activated and then a cascade of other biochemical events is triggered.83 This is known as phototransduction and it leads to the closure of a cGMP-gated cation channel in the cell membrane to produce hyperpolarization and a reduction in the release of glutamate.84 The postsynaptic responses to light are in fact due to a decrease of glutamate release. Thus, glutamate uptake also plays a key role in retinal neurotransmission because the clearance of glutamate must be rapid to provide a fast postsynaptic response to light.85 The hyperpolarizing light response of photoreceptors is now well established and it is supported by studies of vesicle turnover, which is much higher in the dark.86 Furthermore, synaptic blocking studies produce the appropriate responses in the different second-order neurons and there is a stereotyped array of distinct glutamate receptors associated with specific cell types. Glutamate, with its library of postsynaptic receptors, seems particularly suitable to orchestrate the large variety of postsynaptic responses at the cone pedicle.
Rods operate in a manner essentially similar to cones: they hyperpolarize in response to light increases, albeit there are many molecular differences. However, everything about the rod pathway in the retina is designed for maximum sensitivity. This is reflected in the anatomical details and synaptic connections of rods.66 Rods can respond to single photons, obviously the design limit, with a binary (all or nothing) signal, but visual threshold requires a signal in 5–10 rods. Depending on the species, about 20–100 rods converge on to a single rod bipolar cell87,88 and this high convergence also contributes to the high sensitivity of the rod pathway. If 100 rods converge to a single rod bipolar cell and 100 rod bipolar cells converge to a ganglion cell, then the absolute threshold for vision is determined by the product, approximately 1 photoisomerization per 10 000 rods.
Second-order neurons: horizontal and bipolar cells
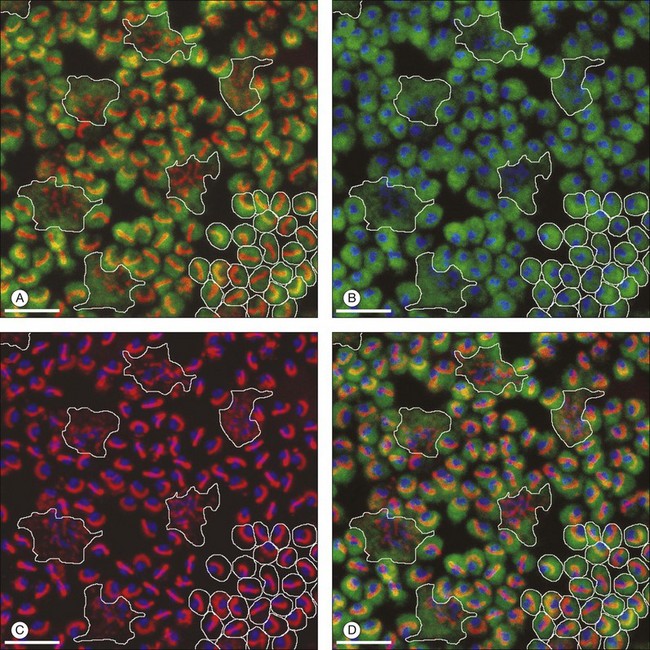
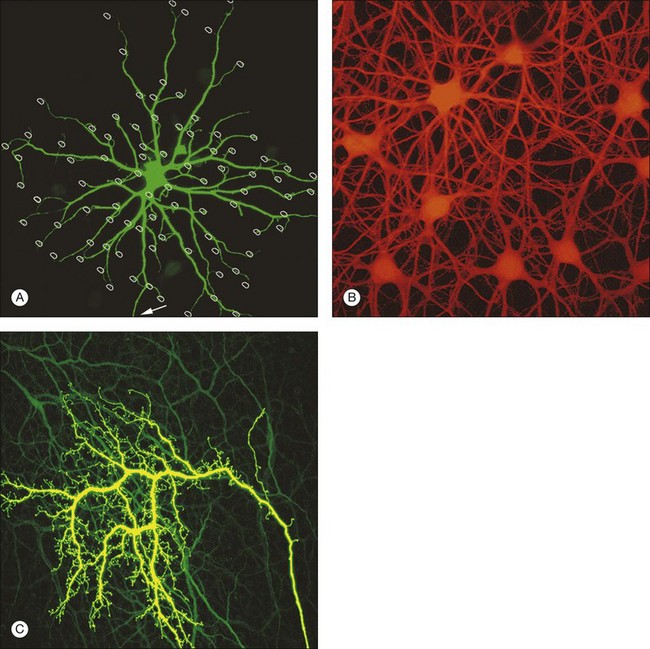
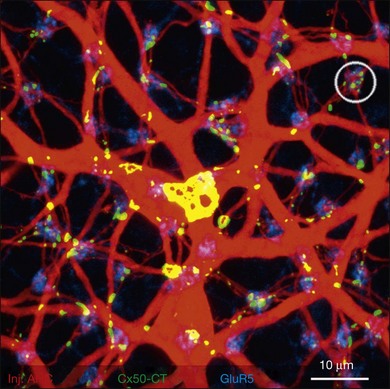
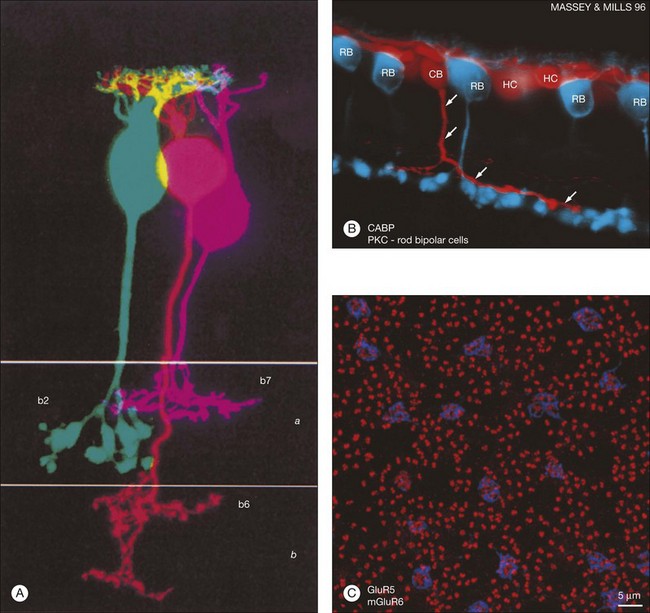
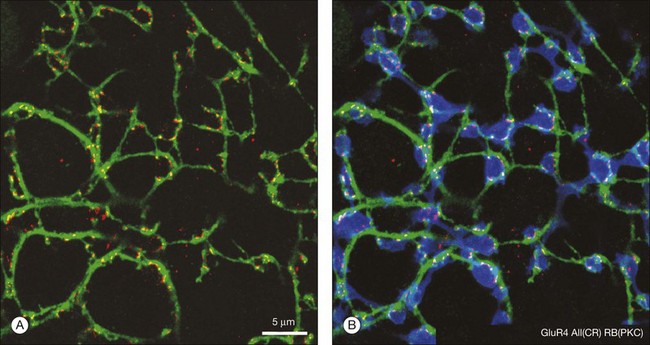
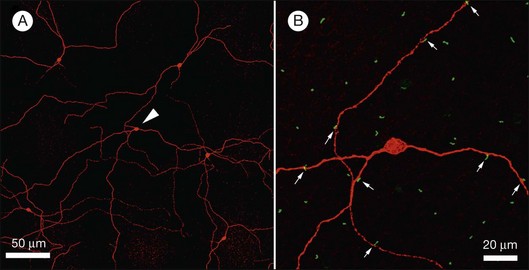
Rods and cones make synaptic connections with bipolar and horizontal cells. Horizontal cells are laterally extensive interneurons located in the outer row of the INL, adjacent to the OPL. They respond to diffuse light with a large hyperpolarization. This is the same as cones so we say that the input is sign-conserving. It appears to be mediated by excitatory glutamate receptors of the AMPA subtype, which have been located on horizontal cell dendrites.89,90 In most mammals there are two morphological types of horizontal cell, but only one in rodents.91–94 In all species, horizontal cells are extensively coupled, which dramatically increases the size of the receptive field. In Figure 15.10 the entire A-type horizontal cell network in the rabbit retina has been labeled by injecting several cells with a diffusible tracer, Neurobiotin, which readily passes through gap junctions.95 A-type horizontal cells are axonless and have a large irregular shape, giving rise to many fine terminals which contact every cone pedicle within the dendritic field. A high-resolution image shows how fine horizontal cell dendrites converge at cone terminals while ignoring the numerous rod spherules (Fig. 15.10). The cone terminals were marked by the labeling of two different glutamate receptors, GluR5, which marks the basal contacts of certain OFF bipolar cells,96 and mGluR6, which is expressed by ON cone bipolar cells17,97 (see below). It should be noted that the three labels are nonoverlapping at the cone terminal, consistent with the presence of three separate neurons, horizontal cells, ON cone bipolar cells, and OFF cone bipolar cells, which all converge independently at the cone pedicles (Fig. 15.10).64
B-type horizontal cells in the rabbit retina are smaller and radially symmetrical (Fig. 15.11). While the somatic dendrites of the B-type horizontal cell also contact cones, each cell gives rise to a long axon that meanders randomly before branching into an elaborate terminal structure (Fig. 15.11C).95 The electrotonic length of the axon apparently isolates the somatic region from the axon terminal whose branches contact rods instead of cones.98 B-type horizontal cells are also coupled by gap junctions and so are the axon terminals (Fig. 15.11B).95,99–101 For both types of horizontal cell, as the cell density falls with eccentricity, the dendritic field size increases so that an even coverage of 6–8 is maintained across the retina. B-type horizontal cells are always smaller and more numerous – two to three times the A-type density.95
In primates, there are also two kinds of horizontal cell, both axon-bearing, but the H2 only contacts cones and the axon is not well developed.102 H1 is a large, well-coupled cell that contacts all red/green cones but not blue cones.103 H2 has a smaller soma and finer dendrites that make sparse contacts with red/green cones but densely innervate blue cone pedicles.103 Recording from both horizontal cell types shows that they receive sign-conserving inputs from both red and green cones (plus blue for H2).103 The wiring of the H2 horizontal cell suggests it plays a role in blue/yellow (red +green) processing.
In central retina, there are four times as many H1s as H2s, decreasing to twice as many in peripheral retina.104 Increasing size with eccentricity compensates for decreasing density so coverage for both types is 3–5 evenly across most of the retina. The peak density for H1 horizontal cells reaches about 18 000/mm2 near the fovea. This is an order of magnitude higher than rabbit retina. Packer and Dacey105 have recorded from many primate H1 cells and they make the interesting observation that central cells are not only smaller but less coupled, perhaps because they overlap less. Thus, the H1 receptive field in the fovea may be small enough, 20–30 µm, to match the receptive field surround of midget ganglion cells.
Horizontal cells are extensively coupled by gap junctions and their molecular identity has been determined for many species. In the rabbit retina, the gap junctions between A-type horizontal cells are labeled with an antibody against Cx50 at many contact points in the matrix (Fig. 15.12).106 Some of the gap junction plaques are very large and the unitary conductance of Cx50 channels is also high. This explains why A-type horizontal cells form an electrical syncytium. B-type horizontal cells are not labeled for Cx50. These gap junctions have different properties so they are likely to be assembled from different connexins. In rodents, there is only one type of axon-bearing horizontal cell.94 In a Cx57 knockout mouse, these cells are no longer coupled.107 This suggests that multiple neuronal connexins are present in the retina and that Cx57 gap junctions may be responsible for coupling in axon-bearing horizontal cells. The situation in the primate retina is still unknown but future progress should make it possible to test theories concerning horizontal cell coupling.
Horizontal cell function
As pointed out by Sterling, reading high-contrast images in bright artificial light is one thing,108 but step into the outside world of the naturalist and visual objects often have very low contrast from the background. What we need is a way to subtract the common background so we can focus on the small, low-contrast details in the image. At least in part, this role is accomplished by horizontal cells in the outer retina. The horizontal cell network holds a big slow picture of the visual scene that is subtracted by feedback to cones. Because horizontal cells have a large receptive field, due to their size and coupling, they subtract the mean illumination, allowing the photoreceptors to respond to local changes in visual illumination. Because they are slow, horizontal cells always lag behind and damp the visual signal without blocking the rapidly changing peaks that carry the most important visual information. In one in silico model of the retina, the bipolar cells amplify the difference between the cone signal and the horizontal cell network.109 Horizontal cells also may have other specific roles in vision. Recent studies have led to the hypothesis that they are responsible for red-green opponency in the primate midget ganglion cells.110
There is general consensus that horizontal cells provide a negative-feedback signal to cones and rods. When a light flash hyperpolarizes the photoreceptors and the horizontal cells, the horizontal cell input results in sign-inverted voltage change in rods.111 This negative feedback results in an antagonistic center surround receptive field in the photoreceptors. The mechanism is thought to consist of a shift in the voltage sensitivity of the calcium channels in the photoreceptors, which results in a leftward shift of the calcium channels I/V relationship and increased currents. Increased currents in photoreceptors inhibit the light-mediated hyperpolarization and alter glutamate release.
The mechanism that mediates feedback remains under considerable debate. Three models have been proposed as the basis of negative feedback at this synapse. The first proposed that horizontal cells release GABA to hyperpolarize the cone photoreceptor.112 There are data to support this idea. GABA has been found in some mammalian horizontal cells,113,114 as has VGAT, the vesicular GABA transporter,115 and there is mounting evidence that many components required for GABA release also are present.116–118 In addition, GABA receptors are located on bipolar cell dendrites119 or perhaps cone pedicles themselves,120 providing another mechanism for feedback. However, GABA antagonists do not block surround antagonism either in photoreceptors121 or at the level of the ganglion cell receptive field.122 An ephaptic mechanism is proposed in which a change in the transmembrane potential of the cone photoreceptor occurs via hemichannels and glutamate receptors on the horizontal cell dendrites and creates a voltage drop in the synapse; a subsequent increase in photoreceptor calcium channel activity modifies the gain in the cone synapse.123,124 Finally, a model is proposed in which horizontal cell depolarization produces an efflux of protons, which acidifies the extracellular space and inhibits cone voltage-gated calcium channels.125,126 While these discrepancies in the mechanism of negative feedback remain to be resolved, results of a recent publication suggest that a positive-feedback mechanism may also contribute to signaling at this synapse.127
Studies of this important mechanism have been undertaken primarily in species other than mouse, which has meant the analyses have required pharmacological manipulations, rather than genetic approaches. However, recently a zebrafish model, in which a specific connexin was thought to make up the horizontal cell hemichannels, was identified. In these fish feedback was reduced, providing support for the ephaptic hypothesis.128 Studies in mice also have shown that feedback is present. This model organism provides the possibility that the underlying mechanism can be dissected genetically. No doubt in the coming years the details of this feedback mechanism, which is critical to vision, will be fully characterized.
Bipolar cell function
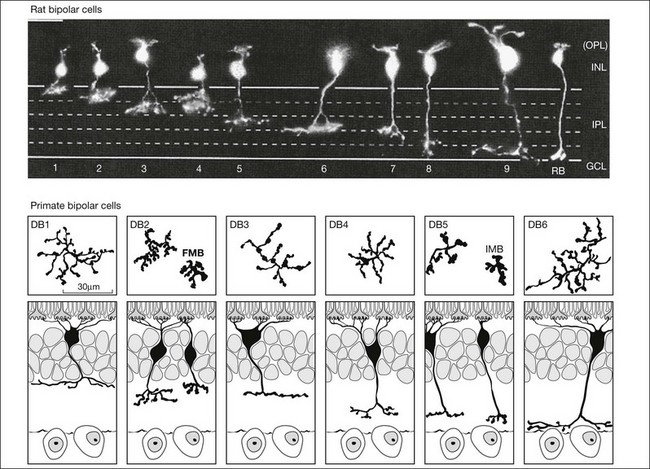
Bipolar cells receive input from photoreceptors and then conduct the visual signal to the inner retina. They are excitatory interneurons, using glutamate as a neurotransmitter, specialized for sustained transmitter release, with terminals containing synaptic ribbons, similar to those of photoreceptors, only smaller. There are approximately 9–12 kinds of cone bipolar cell in the mammalian species that have been studied thoroughly, i.e., primates, rabbits, cats, rats, and mice (Fig. 15.13).12,129–132 In contrast to the cone bipolar cells, there is only one type of rod bipolar cell, which is morphologically and physiologically distinct from the cone bipolar cells. Rod bipolar cells are numerous – three times the density of any diffuse cone bipolar type. However, there are around 10 types of cone bipolar cell so, in total, they outnumber the rod bipolar cells by a factor of three to four.
Cone bipolar cells are difficult to identify in a retinal slice preparation. Once filled with a tracer they can be distinguished by the locations of their somas in the INL, by the morphology of their dendritic and axon terminals, and by the depth of termination in the IPL. An increasing number of selective antibodies are now available to aid in the identification of specific cone bipolar cells.133–136 In addition, several transgenic reporter mouse lines that mark specific types of bipolar cells have been created.137,138
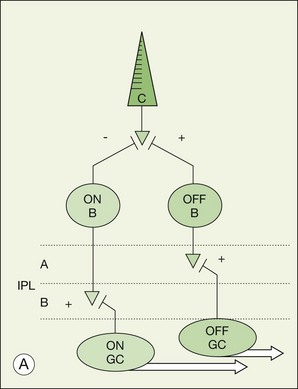
There is a major functional division in the cone pathways that arises at the first synapse and is preserved in all higher visual centers. The retina is divided into ON and OFF channels served by ON and OFF cone bipolar cells (Fig. 15.13).15 The ON pathway is optimized to detect increases in intensity and the OFF pathway decreases in intensity. Reflecting the importance of depth in the retina, these two types of bipolar cells terminate at different levels in the IPL. OFF bipolar cells ramify in the top half, sublamina a, where they synapse with OFF ganglion cells. ON bipolar cells descend further in the IPL to sublamina b, where they synapse with ON ganglion cells. Now, ON and OFF bipolar cells produce opposite signals in response to changes in light intensity. But how is this achieved if both bipolar types contact the same cones? The short answer is via different postsynaptic receptors and, indeed, glutamate produces opposing responses in ON and OFF bipolar cells15,139,140 This simple trick, dividing the cone signal into ON and OFF components, is thought to double the dynamic range of the retina. Half the bipolar cells carry signals greater than the local mean and the other half dimmer than the average.
OFF cone bipolar cells
The dendrites of OFF bipolar cells branch in the OPL and contact every cone within the dendritic field at basal synapses (Fig. 15.14). OFF bipolar cells, like cones themselves, are hyperpolarized by light. So these are sign-conserving excitatory synapses, shown by a + in Figure 15.14, served by ionotropic glutamate receptors, i.e., conventional glutamate receptors of the AMPA/kainate type. Thus, the dark release of glutamate from cones holds OFF bipolar cells at a relatively depolarized potential and the reduction of glutamate release in response to light produces a hyperpolarization in OFF bipolar cells. In this regard, the photoreceptor/OFF bipolar cell synapse is similar to the photoreceptor/horizontal cell synapse.
Recordings from cone and OFF bipolar cell pairs in ground squirrel, which has very large cones particularly suitable for recordings, has shown that three different OFF bipolar cells use three different glutamate receptors – one AMPA receptor and two kainate receptors (Fig. 15.15).141 This dovetails very well with the differential distribution of glutamate receptors at the cone pedicle complex.90,96 Furthermore, the characteristic rate at which each receptor recovers from desensitization effectively divides the cone signal into different bandwidths. The fast AMPA receptors are well suited to convey transient signals, while the slower kainate receptors may transmit sustained responses.141 All three OFF bipolar types terminate in sublamina a, as expected, but each one branches at a different depth within the OFF sublamina (Fig. 15.15). Thus, the division of the visual signal into different channels, for delivery to different addresses in the IPL, begins at the first synapse in the retina.
ON cone bipolar cells
The dendrites of ON bipolar cells invaginate into the cone pedicle and approach the synaptic ribbons in a central position. ON bipolar cells are depolarized by light. This is opposite to the cone signal so we refer to this as a sign-inverting synapse, hence the minus sign at the cone/ON bipolar cell synapse in Figure 15.14.15 The dark release of glutamate from the photoreceptors holds ON bipolar cells relatively hyperpolarized. Light turns off the cone transmitter, and the decrease of glutamate release produces a depolarization in ON bipolar cells. Bipolar cells obey the division of the IPL and so ON bipolar cells are stratified in sublamina b (Fig. 15.13).
A hyperpolarizing response to glutamate is unusual and this is an unusual receptor, which is only expressed in the retina. It has now been identified as mGluR6, one in a series of eight metabotropic glutamate receptors, so called because activation of these receptors turns on an intracellular signaling cascade.142,143 The mGluR6 receptor is selectively activated by glutamate or the glutamate analog 2-amino-4-phosphonobutyrate (APB).15 Activation of the mGluR6 receptor leads to the closure of a cation channel, producing a hyperpolarization in ON bipolar cells. Light decreases glutamate release from photoreceptors and produces the opposite response. The cation channel is now known to be the transient receptor melastatin 1 (TRPM1) channel.144–146 The exact mechanism by which mGluR6 activation closes TRPM1 is not known, but it does not seem to use a cyclic nucleotide-mediated mechanism.147,148 While the details of the mechanisms remain uncertain, the signal inversion that occurs at the ON bipolar mGluR6 receptor underlies the separation between ON and OFF channels throughout the visual system.
Labeling the retina with an antibody against the mGluR6 receptor stains a narrow band at the level of bipolar dendrites in the OPL.17,97 In whole mount, the distribution of mGluR6 labeling shows a distinct pattern with two types of terminals (Fig. 15.15C). There are lightly labeled mGluR6-positive clusters of fine ON cone bipolar terminals at each cone pedicle and bright mGluR6-positive terminals, which insert into each rod spherule. These are the terminal dendrites of rod bipolar cells. This mGluR6 pattern also provides a simple way to map the location of rod and cone terminals in the outer retina. All ON bipolar cells, which include ON cone bipolar cells, blue cone bipolar cells, and rod bipolar cells, express mGluR6 at the tips of their dendrites.17 This is quite different from the multiple glutamate receptors used by OFF cone bipolar cells. However, a variety of responses may still be produced by the different types of ON cone bipolar cell due to modulation of the mGluR6 cascade or to the action of calcium channels or amacrine cells at the bipolar cell terminal.149
Midget bipolar cells
The primate retina is unusual in that the central retina is dominated by midget bipolar cells. Within the central 10 mm, there is one OFF midget bipolar cell and one ON midget bipolar cell for each cone. In this area, they account for more than 80% of all cone bipolar cells.130,150 Midget bipolar cells have very small dendritic fields and receive input from single cones and make output to single midget ganglion cells. This is the so-called private line, one cone to one midget bipolar cell to one midget ganglion cell. The ON midget bipolar cells invaginate the cone pedicle, ramify in sublamina b of the IPL, and contact ON midget ganglion cells. OFF midget bipolar cells make flat or basal contacts with cones and terminate in sublamina a where they contact OFF midget ganglion cells. Most investigators think this specialization of the primate retina was designed to achieve maximum acuity at high cone density. It may also, by virtue of the single cone connections, which are automatically color-coded, serve red/green color vision.
Blue cone bipolar cells
In general, diffuse cone bipolar cells contact every cone within the dendritic field and this gives them a characteristic appearance. However, in the primate retina, one bipolar cell type is distinctly different in that it has long dendrites that bypass many cones to seek out only blue cones.151,152 The dendrites are labeled for mGluR6 and the axon descends deep into sublamina b so the blue cone bipolar cells are ON cells.17,153
Rod bipolar cells
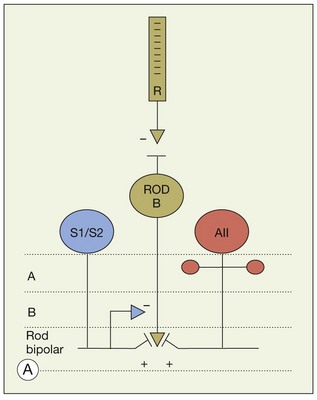
In contrast to the cone bipolar cells, there is only one morphological type of rod bipolar cell. They are numerous, with a mop-head of fine dendrites that may receive input from as many as 80–120 rods in the rabbit retina (Fig. 15.16A).88 This very high convergence contributes to the sensitivity of the primary rod pathway. A single dye-injected rod bipolar cell is shown in Figure 15.16B. The dendrites branch profusely but avoid the cone pedicles within the dendritic field. Instead, all the terminal dendrites invaginate a rod spherule and they are double-labeled for mGluR6. Only one terminal at each rod spherule comes from this rod bipolar cell. The other half of each mGluR6 doublet is claimed by another unlabeled rod bipolar dendrite. Thus, each rod contacts two different rod bipolar cells.97 Each rod bipolar cell gives rise to a long slender axon that descends to sublamina 5 of the IPL (Fig. 15.16). Physiologically, rod bipolar cells give ON responses to light stimulation and this is consistent with the labeling for mGluR6 and the depth of stratification.13,154 Rod bipolar cells do not usually contact ganglion cells directly. Instead, the primary output of rod bipolar cells is to AII amacrine cells, which pass on the rod signal, or to S1 and S2 amacrine cells42,154 that provide a powerful negative-feedback signal to rod bipolar terminals (Fig. 15.17; see below). More than 90% of the rod bipolar output is on to these amacrine cells.
The high convergence allows the rod bipolar cell to collect signals from many rods but is also potentially noisy. However, the rod-to-rod bipolar synapse has a nonlinearity by which small signals are thresholded.155 The price for this is that many small signals are rejected but the reduction in noise is worth it. Some near-threshold signals may be lost but when a photon signal is captured it has a high signal-to-noise ratio and is transmitted very reliably.156
Multiple rod pathways
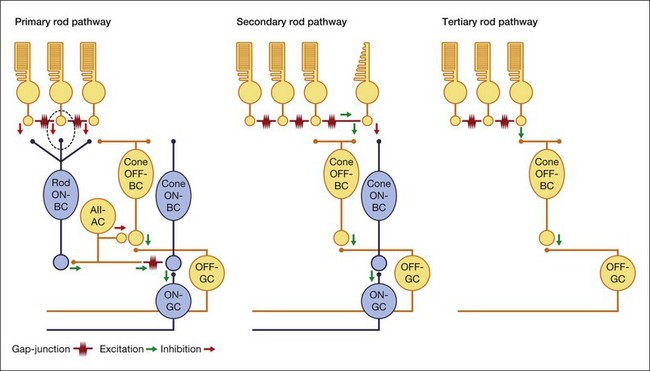
Rods are utilized under low light conditions and provide information over 5 log units. It is now clear that there are at least three pathways, referred to as primary, secondary, and tertiary, by which rod signals reach ganglion cells (Fig. 15.18). They are thought to process signals under slightly different conditions, the primary being the most sensitive and the tertiary being the least sensitive.
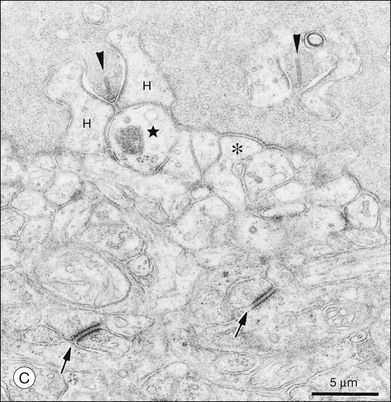
The primary rod pathway utilizes rod bipolar cells, which make ribbon synapses with two postsynaptic amacrine cells in sublamina 5 of the IPL (Fig. 15.17). One of these postsynaptic neurons is the AII amacrine cell, and the other is either an S1 (A17 in the cat) or S2 amacrine cell (Fig. 15.17).42,154 These two wide-field GABA amacrine cells both make reciprocal synapses with rod bipolar terminals and thus provide another stage of negative feedback. The conspicuous terminals of rod bipolar cells literally plug into holes in the meshwork of dendrites provided by these amacrine cell types. Rod bipolar cells, like other bipolar cells, release glutamate and the contact points with the AII matrix are covered with glutamate receptors of the AMPA subtype (Fig. 15.19).157,158 The glutamate receptors of S1/S2 amacrine cells have not been completely clarified, but the A17 (probably the S1 equivalent in the rodent) uses a Ca2+-permeable AMPA glutamate receptor via L-type Ca2+ and Ca2+-activated potassium (BK) channels159 to modulate feedback inhibition on to the rod bipolar cell and control glutamate release at this synapse. On the rod bipolar cell the postsynaptic targets are the GABAA and GABAC receptors.160–162
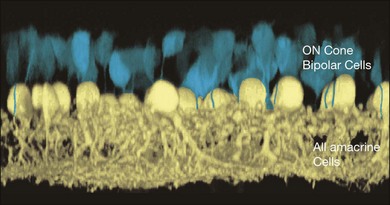
So, there are two obvious questions: how do rod signals reach ganglion cells and, if there is only one type of rod bipolar cell, how are both ON and OFF signals generated in dark-adapted conditions? The answer lies in the way the rod pathway is integrated into the cone pathways via the AII amacrine cell (Fig. 15.18). AII amacrine cells are glycinergic neurons and they also make conventional inhibitory glycinergic synapses with the axon terminals of OFF cone bipolar cells in sublamina a via alpha 1 glycine receptors.163 In turn, the AII itself is modulated by a glycinergic input at synapses expressing alpha 3 glycine receptors.164 Their distal processes make electrical synapses or gap junctions with ON cone bipolar cells in sublamina b and provide a direct, presumably sign-conserving, input signal to the ON cone bipolar cells.43,165,166 The signal transfer via gap junctions is presumed to be sign-conserving. Thus, while the cone pathways split via different postsynaptic glutamate receptors in the outer retina, the rod pathway bifurcates at the level of the AII amacrine cell. It is often said that the rod signals piggyback on the cone pathways.
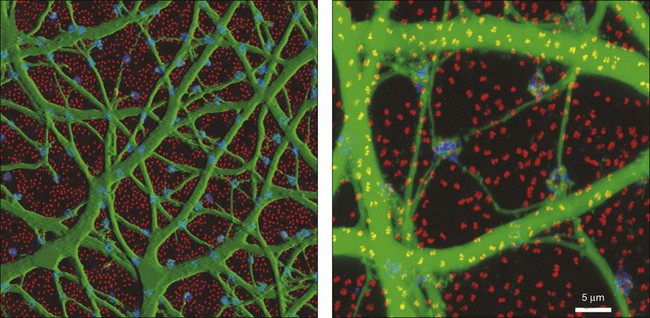
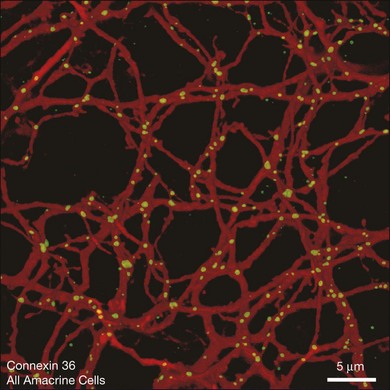
AII amacrine cells themselves are well coupled by gap junctions, as can be demonstrated by injecting a single AII amacrine cell with the diffusible tracer Neurobiotin, which passes through gap junctions to label all the coupled cells.166,167 Figure 15.20 shows that many AII amacrine cells are coupled as well as an overlying group, consisting of four or five different types, of ON cone bipolar cells. The AII-to-AII gap junctions occur preferentially at dendritic crossings (Fig. 15.21) and AII coupling is absent in a Cx36 knockout mouse.168 Coupling in this complex heterocellular network is modulated by dopamine and, perhaps more importantly, by light.169 The underlying mechanism is by phosphorylation of Cx36, and this regulation can be achieved on a cell-by-cell basis.170
These gap junctions are bidirectional. This means that electrical signals and tracers can pass through the gap junction in both directions.171,172 One consequence is that glycine from the AII amacrine cell can enter ON cone bipolar cells by this route and indeed most ON bipolar cells contain glycine, even though they use glutamate as a neurotransmitter. The source of the bipolar cell glycine was definitively established by blocking gap junctions with carbenoxolone. This changed the labeling pattern for glycine, which was subsequently diminished in bipolar cells.167 Another more relevant consequence of bidirectional coupling is that not only do rod signals pass into the cone pathways but, in fact, cone signals can pass from ON bipolar cells into the AII network. The implication is that the AII network can also influence cone signals.
The importance of these gap junction pathways has been elegantly demonstrated by the development of a Cx36 knockout mouse.168 In these animals, filling an AII amacrine cell with Neurobiotin yielded only one cell: there was no coupling without the expression of Cx36. In contrast to the wild type, in the knockout animals, no rod signals are detectable in recordings from ON ganglion cells. In the absence of Cx36 gap junctions, rod level signals do not pass into the ON cone pathways. Of course, the OFF pathways are not dependent on transmission via gap junctions and so rod-driven OFF signals are maintained. One obvious explanation is the absence of AII/ON bipolar gap junctions, described above. However, the Cx36 knockout may also interfere with rod/cone coupling in the outer retina. In fact, both these pathways must be missing to eliminate the transmission of rod signals to ON pathways. In either case, this is the first time that a gap junction connection has been shown to be essential for the function of a neuronal pathway in the mammalian CNS.
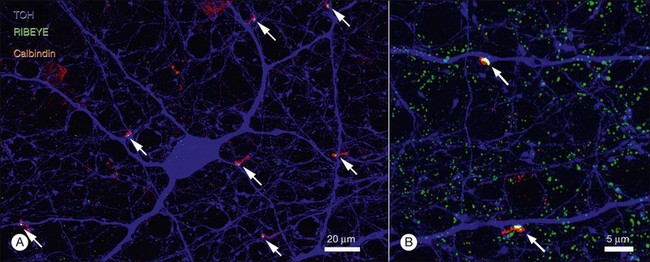
In the rabbit retina, the gap junctions between one type of ON cone bipolar cell that is selectively labeled by antibodies against the calcium-binding protein calbindin also involve Cx36.173 However, the properties of AII/bipolar gap junctions are different from AII/AII gap junctions,166 suggesting that the bipolar side of the gap junction is different in some way, perhaps due to phosphorylation or even the expression of a different connexin by bipolar cells. There is some evidence that bipolar cells express Cx36.168 However, there is convincing evidence that in some ON bipolar cells the coupling is mediated by Cx36 (AII)/Cx45 (BC) heterotypic gap junctions.174
Secondary and tertiary rod pathways
The primary rod pathway is used under threshold conditions such as starlight108,154 and it is absolutely dependent on synaptic transmission between rods and rod bipolar cells via mGluR6 receptors. However, when these synapses are blocked with APB it was still possible to record rod level signals in OFF ganglion cells.175 This alternative pathway was attributed to rod/cone coupling, for which there is both anatomical and physiological evidence. This second rod pathway runs from rods via gap junctions to cones and then into cone bipolar cells and ganglion cells in the usual way (Fig. 15.18). It has been suggested that this pathway operates in mesopic conditions of intermediate light intensity.76
This pathway has also been reported for the mouse retina. However, the rod-driven responses persisted even in a transgenic mouse line in which cones were eliminated.176 The absence of cones would seem to rule out rod/cone coupling as a pathway. Therefore, a third rod pathway was proposed involving direct connections between rods and OFF cone bipolar cells and subsequently these connections were found.177,178 This pathway was first thought to be a specialization of the rodent retina but it has now been identified in cats and rabbits.97,179 It also is present in ground squirrel, where a specific type of OFF bipolar cell, the b2, was shown to contact rods and provide a means for rods to provide fast photoreceptor signaling.180 Thus, this third rod pathway may be a general feature of the mammalian retina.
It has been suggested that the rod to OFF bipolar pathway operates at high mesopic/low photopic conditions, around the transition from rod to cone vision. In general, the different rod pathways may be designed to cover different intensity ranges and perhaps they are selectively connected to specific ganglion cell types but, as yet, there are no data on this point. There is physiological evidence from the mouse retina that different ganglion cell types have different intensity response functions but the ganglion cell types have not been identified and the contributing pathways are unknown.168 The function of these novel retinal pathways under different light intensities is an important and active area of current research.
Amacrine cells
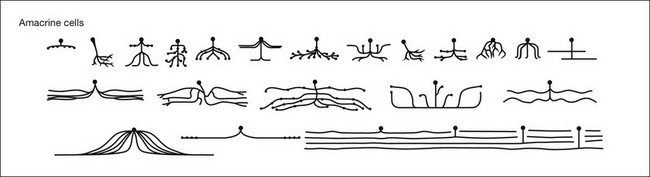
Amacrine cells form a morphologically and physiologically diverse group of mostly inhibitory interneurons. Approximately 30 types of amacrine cell have been identified morphologically in mammalian retina (Fig. 15.22).37,38,181 The cell bodies of the amacrine cells are primarily located in the innermost portion of the INL, unofficially known as the amacrine cell layer. Amacrine cells are also found in the GCL, where they are known as displaced amacrine cells. Most are GABAergic medium- to wide-field cells49,182 and many recognized classes of amacrine cells are found in both layers. In fact, in the mouse retina, more than half the cells in the GCL are amacrine cells. In general, the three major connections made by amacrine cells are feedback inhibition to bipolar cell terminals, feedforward inhibition to ganglion cells and serial inhibitory connections where amacrine cells are the postsynaptic targets of other amacrine cells.183
Amacrine cells can be divided by their dendritic arbor size into narrow- (<200 µM) and wide-field. The narrow-field amacrine cells are generally glycinergic and frequently provide inputs across the ON/OFF strata in the IPL.184 Wide-field amacrine cells are mostly GABAergic.181,182,185–187 Some provide lateral inhibition within a substratum184 and others provide reciprocal feedback.188 They are involved in surround inhibition189 or contrast adaptation.190 Several of the wide-field cells also release catecholamines or other neuropeptides.114 The best characterized in this group are the dopaminergic wide-field amacrine cells that are involved in light adaptation, and those releasing ACh.
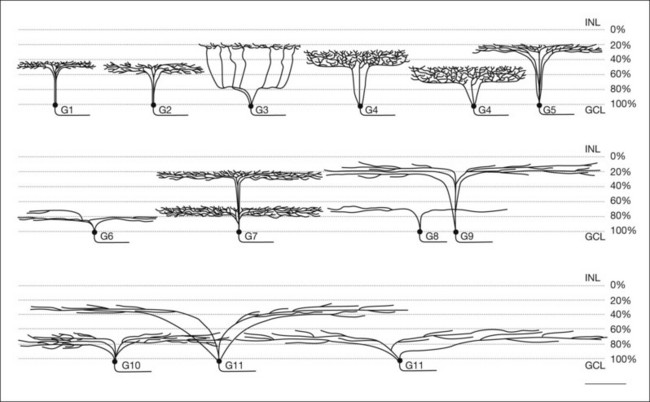
In a large population of rabbit amacrine cells, acquired randomly using modern imaging methods, 24 different morphological types were identified (Fig. 15.22).37,38 Similar studies have been extended to the mouse, where several groups have identified large numbers of narrow-, medium-, and wide-field amacrine cells.181,182,185–187
The reasons for such a variety of amacrine cells are unknown. However, two functions seem to be commonly repeated. First, all bipolar cell terminals seem to receive GABA-mediated negative feedback. In the case of the rod bipolar cell, feedback is mediated by two amacrine cell types, S1 and S2.191 Bipolar cells (approximately 10 types) are narrowly stratified and if different amacrine cells mediate feedback to each one, this could account for 10–20 different cell types. Secondly, about half the ganglion cells (approximately eight types) are coupled to one or two amacrine cell types by gap junctions.192–194 Again, if different amacrine cells are coupled to each ganglion cell type, this could account for 10–15 types. These two groups need not be mutually exclusive and doubtless amacrine cells have many functions. In another calculation, we estimate there are 15 ganglion cell types forming independent circuits or channels. If each channel required two amacrine cells, that would produce a total of 30 amacrine cell types. While necessarily vague, these simple estimates indicate the variety of amacrine cell types may not be extravagant.
General functions attributed to amacrine cells include feedback inhibition, surround inhibition, some forms of adaptation, signal averaging, and noise reduction.195,196 In a rapid eye movement or saccade, the visual image does not blur. This is because a wave of inhibition sweeps the inner retina, like a vertical blank on a television screen. In one study, the inhibitory wave associated with saccades was attributed to wide-field amacrine cells.197 However, in terms of specific connections and neuronal circuits, the functional anatomy of amacrine cells, it has only been possible to study a few types, four of which are selected below. We know almost nothing except the shape of 75% of the amacrine cells, not their connections, physiology, or circuit functions – in other words, little more than Cajal knew 100 years ago. As the means of targeting, imaging, and recording from visually identified cell types improves, we can expect further progress in this area.
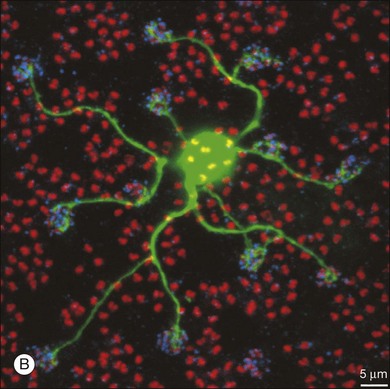
An example of these new approaches was demonstrated by Knop and colleagues,198 who used a GFP transgenic mouse line that marked a type 2 wide-field amacrine cell. They characterized the inputs, stratification, and physiology of the cell, showing it was ON-OFF and GABAergic and that its function was maintained over a wide range of light adaptation conditions. A major goal of visual neuroscientists is to characterize fully all the amacrine cells and integrate their function into the retinal circuitry. Several amacrine cells have been studied extensively, and their characteristics are described below.
AII amacrine cells
The AII amacrine cell, also known as the rod amacrine cell, is the most numerous of the amacrine cells, accounting for 11% of the total. It is correctly written with a Roman numeral, which is retained from an early classification scheme, while other numbered amacrine cells use Arabic numerals. AII amacrine cells have a distinctive bistratified morphology, which makes them easy to identify, even in retinal slices.199 The soma protrudes into the IPL and turns into a thick axon that descends to sublamina b of the IPL and branches into an overlapping matrix.200 This is the site of glutamate input from rod bipolar cells (Fig. 15.20) and output, via gap junctions to ON cone bipolar cells. There are also fine dendrites, which terminate in lobules in sublamina a. At this level, AII amacrine cells make glycinergic inhibitory contacts with OFF cone bipolar cells. They also receive input from OFF cone bipolar cells at this level, the function of which is unclear.154
Primate AII amacrine cells are similar in morphology to those of other mammalian species.61,62 The density of rods reaches a maximum at about 20° from the fovea but psychophysical measurements in humans indicate that, in dark-adapted conditions, the maximum acuity occurs at approximately 5° from the fovea, a substantial mismatch. The rod pathway for maximum acuity is thought to be rods → rod bipolar cells → AII → midget bipolar cells → midget ganglion cells (Fig. 15.18). There is good evidence that AII amacrine cells contact midget bipolar cells and other ganglion cell types are too sparse to support the maximum resolution. Now, rods and rod bipolar cells far outnumber AII amacrine cells so they will not present a limit to scotopic acuity. In addition, both ON and OFF midget bipolar cells and ON and OFF midget ganglion cells have a 1 : 1 : 1 correspondence with the tightly packed cones of the central retina. That leaves the AII amacrine cell as the lowest density, the bottleneck, in the pathway. In macaque retina, the peak density of AII amacrine cells is about 5000 cells/mm2 around 5°, which matches the peak of dark-adapted acuity. If we consider the mosaic of AII amacrine cells and, from sampling theory, calculate the maximum resolution for such an array, we find that it closely matches the peak acuity from psychophysical experiments. Together, these results indicate that the density of AII amacrine cells sets the limit of dark-adapted resolution in central retina.
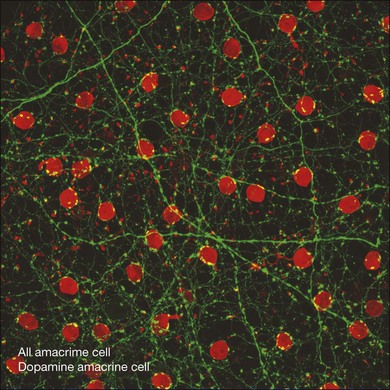
The AII network seems to be very important since it is found in all mammalian retinas. In general terms, the AII network is thought to reduce noise by canceling out random events which are not correlated and by reinforcing light-driven events that are synchronized.201,202 Coupling is also strongly modulated by dopamine169 and processes from the DACs make a ring around the neck of each AII where it protrudes into the IPL (Fig. 15.23). Of course, we think that dopamine is the general signal for light adaptation and, in fact, light itself seems to modulate the strength of coupling in the AII network.203 Disrupting the AII network interrupts the rod-driven ON pathways, as described above. However, even in the rod-driven OFF pathways, which are not routed via AII gap junctions, the sensitivity is reduced by approximately 1 log unit in the Cx36 knockout mouse.203 This indicates that coupling in the AII network adapts to ambient lighting conditions to optimize rod signaling over a large dynamic range.
The AII amacrine cells also appear to have a day job.204–206 During the day, dark objects that approach the viewer activate a particular class of ganglion cells. An important component of the circuit is the AII. However, unlike in the dark, the information flow is effectively reversed. Depolarization of cone ON bipolar cells results in depolarization of the AII through gap junctions, which increases inhibition by the AII on to OFF bipolar cells. This is a crossover inhibition pathway by which ON channels inhibit OFF pathways.207 The dual use of the AII amacrine depending on lighting condition maximizes circuit efficiency.
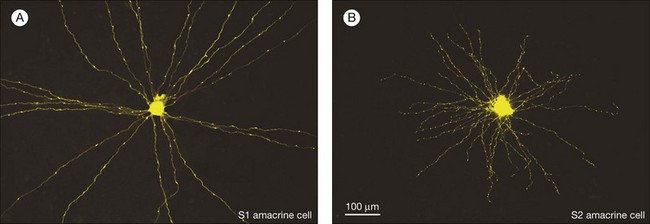
S1 and S2 amacrine cells
The S1 amacrine cell of the rabbit retina (A17 in the cat) is a wide-field GABA amacrine cell with straight radiating dendrites decorated with prominent varicosities (Fig. 15.24A). The S2 GABA amacrine cell is smaller, the dendrites are more tangled, and the varicosities are smaller but more numerous (Fig. 15.24B).208,209 Both cell types contribute to a dense overlapping meshwork at the level of the rod bipolar terminals (Fig. 15.25). These large cells are relatively numerous so the dendritic overlap is huge with coverage factors as high as 500. Although the rabbit retina contains little endogenous serotonin, for some unknown reason these cells take up serotonin. Therefore, they are sometimes known as indoleamine-accumulating amacrine cells and this provides a simple way to label the entire population. Electron microscopy shows that S1/S2 amacrine cells make reciprocal synapses with rod bipolar terminals, i.e., they receive input at a rod bipolar ribbon synapse and nearby they synapse back on to the rod bipolar terminal.42
The varicosities contain presynaptic markers; they are wrapped around rod bipolar terminals (alternating with AII dendrites) and opposed by synaptic ribbons and GABA receptors (Fig. 15.26). In fact, the varicosities are synaptic sites. Confocal analysis of double-label material shows that every varicosity is in synaptic contact with a rod bipolar terminal.209 This means that the functional role of the S1/S2 amacrine cells is to provide a level of GABA-mediated negative feedback to rod bipolar terminals. This is their job and they do nothing else; they have no output to any other cell besides the rod bipolar cell.
These cells provide a massive inhibitory input to rod bipolar terminals. S1/S2 amacrine cells carry about 300 and 500 varicosities, respectively, and the density of varicosities has been calculated as 330 000/mm2. Each rod bipolar terminal receives input from about 25 S1 and 50 S2 varicosities. They are both apposed by GABAA and GABAC receptors.210 Thus, the inhibitory input to bipolar terminals will have two components: a large sustained component from GABAC receptors, which are only expressed by bipolar terminals, and rapid initial transient input from GABAA receptors, which are more widespread.138,160,189,211–213 The S1 and S2 amacrine cells clearly have different spatial and coupling properties.191 Hence, the S2 input dominates within 200 µm and provides an inhibitory signal that matches the size of the surround recorded from AII amacrine cells. The larger, well-coupled S1 amacrine cell may provide a more distant network or global signal. This analysis suggests that the presence of both S1 and S2 amacrine cells is not redundant: each cell contributes different components of lateral inhibition in the rod pathway. Together, these components will summate to modulate the spatial and temporal properties of rod bipolar output. Further, each of the synaptic sites of S1 (A17) amacrine cells may operate independently of the whole. This is possible because each varicosity is electrically isolated from its neighbor. This was tested recently by Diamond and colleagues, who showed that at this reciprocal synapse of the rod ON bipolar cell and the A17 amacrine cell, BK channels control calcium influx via L-type voltage-dependent Ca2+ channels. In conjunction with the cable properties of the A17 neurites the outputs are compartmentalized, keeping individual reciprocal synaptic dyads independent.159,188,214 Such local processing has an important consequence: it allows a single A17 to process upwards of 500 signals independently of each other. This represents a large increase in processing power based on what amounts to parallel circuits in a single cell.188
It should be emphasized that the reciprocal feedback described at the rod bipolar terminal is, in fact, a general case. It is likely that the terminals of all bipolar cells make reciprocal synapses with GABA amacrine cells and bipolar terminals are literally surrounded by GABA-positive profiles. In the mouse retina, as well as other species, GABAC receptors, a postsynaptic target of feedback inhibition, are expressed on cone ON and OFF bipolar cells. The current mediated by this receptor is slow and sustained, making it a likely target of feedback control on the cone bipolar cells, as it does in rod ON bipolar cell. The absence (in GABAC receptors knockout mice) or pharmacological block of this receptor increases both the spontaneous activity and gain of ON-center ganglion cells relative to wild-type.213,215,216
There seem to be GABAC receptors on all bipolar cell terminals, although the density for each cell type varies.211–213 If each bipolar cell type, with its unique address, had one GABA amacrine cell type, that would account for 10–12 of the amacrine cell types. The “sharing” of some broadly stratified amacrine cells would tend to reduce this number while those cases, like the rod bipolar cell, which receive feedback inhibition from two amacrine cell types, will inflate it. In either case, negative feedback provides greater stability, increased frequency response, and a wider bandwidth at the level of bipolar cell terminals. A major role for at least some of the multiple types of GABA amacrine cells is to provide negative feedback for all bipolar cells.
Dopaminergic amacrine cells
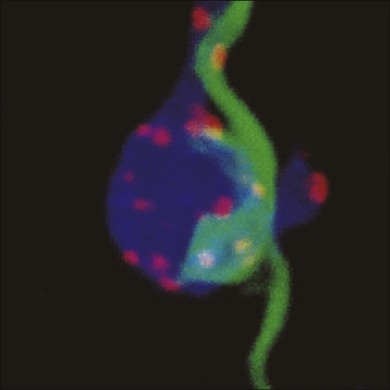
A small number of amacrine cells, less than 0.1%, contain dopamine. To make up for their low density, these cells have very long axon-like processes that can run for millimeters across the retina.217 The net result is a dense high-coverage plexus of dopaminergic dendrites, which covers the entire retina. Most of the plexus is in sublamina 1, adjacent to the INL, but there are minor bands in sublaminae 3 and 5 of the IPL. In some species, particularly fish, the dopamine neurons project to the OPL. In other words, they are interplexiform cells. In the rabbit retina, a few stunted processes run towards the OPL but they do not form a plexus. In the macaque retina, some dopaminergic dendrites run into the INL where they surround the somas of AII amacrine cells (Fig. 15.23).62
Electron microscopy shows that the dendrites in layer 1 receive direct bipolar input at ribbon synapses, many of which appear to be monads, as opposed to the usual dyadic arrangement with two postsynaptic processes.218 Yet, recordings from GFP-labeled cells in the mouse retina have shown that DACs produce a variety of light responses: ON transient, ON sustained and light independent.23,24 But how do these ON responses arise if most dopaminergic processes are stratified in the OFF sublamina? If DACs are ON cells, then they break the rules of stratification for the IPL.
Several different mechanisms have been proposed to overcome this difficulty. For example, it has been shown that ON bipolar inputs to the DACs occur on the minor bands in layer 3.219 However, it is also clear that there are bipolar ribbon inputs to the DAC in sublamina 1.218 Most recently, it has been suggested that the melanopsin-containing ipRGCs may provide an input to the DACs, because the sustained features of the DAC response and the spectral signature match those of the melanopsin ganglion cells.24 Furthermore, the light-evoked DAC response is maintained when photoreceptors degenerate, although transient light responses are blocked by APB, suggesting they arise from the ON bipolar pathway.23 In addition, melanopsin ganglion cell dendrites that ramify in sublamina a also have ON responses, thus breaking the stratification rules themselves.25,27 Most recent evidence suggests that many ON cone bipolar cells make axonal ribbon or ectopic synapses as their axons descend through stratum 1 (Fig. 15.27). These axonal ribbons provide ON excitatory input via AMPA-type glutamate receptors to DACs (and to ipRGCs) in the OFF layer of the IPL. Thus, axonal ribbons break the stratification rules of the IPL and provide an additional accessory ON sublayer in the outer IPL.26,27
In the mouse retina, DACs also contain GABA220 but DACs from the rabbit retina do not display a GABA signature.34 Dissociated DACs are spontaneously active221 and, in contrast to GABA, dopamine seems to be steadily released in a paracrine fashion to diffuse throughout the retina.222 Dopamine levels are higher in the daytime and seem to be under circadian control. The most recent work indicates that the dopaminergic cells themselves express clock genes.223–226 This is appropriate because dopamine is intimately involved in the diurnal rhythms associated with the processes of light and dark adaptation. Dopamine acts at a variety of sites in the retina, many of which seem obviously linked to light or dark adaptation.227 For example, coupling in the AII network is dramatically reduced by dopamine169,170 and, in the outer retina, dopamine acts to promote cone input to second-order neurons. Conversely, in the dark and absence of dopamine, rod inputs predominate. Specifically, by controlling rod/cone coupling, it seems like dopamine in the light, or its absence in the night, presets rod and cone pathways as appropriate for the lighting conditions.82,228
A role for D1 receptors has recently been shown to be critical in the GABAergic control of the rod ON bipolar cell circuit that enhances light sensitivity and increases the dynamic range of their responses at dim-light levels.229 The location of this control remains to be discovered and could occur via a novel dopaminergic to GABAergic amacrine serial synapse in the IPL or a horizontal cell-mediated input modulated by dopamine in the outer retina. The retina is like a self-optimizing network controlled, at least in part, by the circadian release of dopamine.
Starburst amacrine cells
The ChACs, also known as starburst amacrine cells on account of their unique morphology, form two mirror-symmetric populations on either side of the IPL in all mammals.20 They are the only source of ACh in the retina. In whole-mount view, they are radially symmetric with dendrites radiating from the soma in all directions (Fig. 15.28). The bipolar cell inputs, via AMPA-type glutamate receptors, occur all along the branches but the outputs seem to be restricted to the varicosities in the terminal third of each dendrite. The cells in the INL produce OFF responses and stratify in sublamina a while the displaced starburst amacrine cells are ON cells branching in sublamina b. They are relatively numerous wide-field amacrine cells. Consequently, they have a very high coverage factor and in cross-section their dendrites form two continuous bands in the IPL that look like train tracks in cross-section (Fig. 15.28). This is a very dense, narrowly stratified matrix of overlapping dendrites that are cofasciculated with the direction selective (DS) ganglion cell types.230,231
Starburst amacrine cells also contain GABA.232 In fact, we should probably think of these cells as wide-field GABA amacrine cells, which also contain ACh. The presence of two classical neurotransmitters in the same cell is unusual and it has aroused a great deal of interest. The release of both transmitters seems to be dependent on calcium, which indicates a conventional mechanism using synaptic vesicles. But GABA and ACh release have a differential sensitivity to calcium. This implies that the two transmitters may be released from different populations of vesicles but whether cotransmission occurs at the same sites is unknown.233
The starburst amacrine cells have been a focus of attention because they seem to be directly involved in neuronal circuits that produce DS responses in certain ganglion cells. DS ganglion cells were discovered nearly 50 years ago and, although the mechanism has been under intense investigation, the details have still not been resolved. However, starburst amacrine cells are in the middle of the puzzle. Ablating starburst amacrine cells, in a mouse line engineered to respond to an immunotoxin, blocked DS responses in ganglion cells.234 Furthermore, optokinetic nystagmus, a type of reflex eye movement elicited by a moving stimulus, was also blocked. This combination of physiological and behavioral data provides convincing evidence that starburst amacrine cells are required for directional selectivity and optokinetic eye movements.
Starburst amacrine cells are found at the same depth as the bistratified ON/OFF DS ganglion cells, which are the most sensitive of all ganglion cells to ACh. But cholinergic input alone is not sufficient to produce DS responses.235 Rather, the mechanism seems to depend on asymmetrical GABA inhibition that comes from the starburst amacrine cells. In dual recordings, stimulating a starburst amacrine cell on the null side produced an inhibitory GABA input to DS ganglion cells.233,236
Furthermore, calcium imaging by two-photon microscopy showed that individual dendrites of starburst amacrine cells can themselves produce directional responses, even though recordings from the soma do not.33 The dendrites respond better to a centrifugal stimulus, moving away from the soma, than a centripetal stimulus, towards the soma. The directional signals are due to the intrinsic morphological properties of starburst amacrine cells and a voltage gradient generated by the asymmetric distribution of voltage-dependent channels.237,238 It has also been suggested that a chloride gradient generated by the regional expression of chloride transporters may contribute to the directional asymmetry.239 In addition, there is GABA-mediated starburst-to-starburst inhibition, which may enhance or fine-tune the DS responses.233 These important results confirm a long-held suspicion that there is a great deal of local processing among amacrine cell dendrites and it suggests that starburst amacrine cells are the source of directional signals in the retina.
Given that starburst amacrine cells generate directional signals, they release GABA, and they synapse directly with ON/OFF DS ganglion cells, then the final requirement for specific wiring is that individual starburst dendrites with the appropriate directional asymmetry are connected to ON/OFF DS ganglion cells with the same orientation of the null/preferred axis. (Remember, there are four subsets of ON/OFF DS ganglion cells with different axes, roughly aligned with the four main compass points.) By a combination of calcium imaging to identify the directional preferences combined with serial block-face electron microscopic imaging to reconstruct the underlying retinal circuits, the specific synaptic connections have now been identified.240 Starburst dendrites with a specific orientation make synaptic connections, identified by varicosities containing presynaptic machinery, with ON/OFF DS ganglion cells with an antiparallel null axis. Thus, preferred excitation in starburst dendrites can provide the GABA input for null inhibition to ON/OFF DS ganglion cells. Often, the starburst dendrites and the ON/OFF DS ganglion cell dendrites are oriented nearly parallel and it is well known that these two cell types are cofasciculated at the level of the two cholinergic bands. Once a local DS response is generated in the ganglion cell, dendritic spikes propagate to the soma with a high probability of generating somatic spikes.241 After 50 years, the neuronal circuitry underlying directional selectivity has finally been revealed. How this arises developmentally and what the role is for cholinergic input are further important questions that remain.
Finally, the network of starburst amacrine cells appears to play a key role in retinal development.242 Imaging of neonatal retina shows the presence of calcium waves, which propagate across the retina and elicit bursts of firing in all ganglion cells. These waves apparently are not required to establish directional selectivity, which arises independently of both waves and visual experience.243 However, in one model, wave activity is thought to promote the development of ganglion cell connections in a retinotopic manner. The cholinergic plexus is present surprisingly early in development and, in the early stages, the calcium waves are blocked by cholinergic antagonists.244–246 This suggests that spontaneous activity in the ChACs may initiate the calcium waves. This was thought to be light-independent; however, it now appears that light input via ipRGCs (light-sensitive ganglion cells: see below) is required for aspects of the spiking that are important for retinogeniculate segregation.247
Ganglion cells
Ganglion cells are the output neurons of the retina and they receive input from bipolar cells, at ribbon synapses, via AMPA-type glutamate receptors.248 In addition, there are even more amacrine cell inputs, through conventional synapses and via gap junctions, which are thought to tune ganglion cell properties in a complex way that is not well understood. Ganglion cells obey the stratification rules governing the inner retina. Thus OFF ganglion cells receive input from OFF bipolar cells in sublamina a and ON ganglion cells receive input from ON bipolar cells in sublamina b. There are a few ON/OFF types of ganglion cell, such as the famous DS type, and they are bistratified.
Ganglion cell axons run across the vitreal surface of the retina and unite to form the optic nerve. Unlike many other retinal neurons, ganglion cells produce conventional action potentials suitable for transmission over the relatively long distance to the brain. The vast majority of ganglion cells use the excitatory neurotransmitter glutamate to communicate with higher visual centers. Everything you see about the visual world comes to you through the responses of ganglion cells. If they don’t signal it, you don’t see it! If there are about 20 ganglion cell types (see below) and the coverage factors range from one to three, then the total coverage of the retina by the whole ganglion cell population is about 50. In other words, each point on the retina is covered by about 50 ganglion cells. The number of ganglion cells in each human retina is approximately 1.5 million, equal to the number of axons in each optic nerve. In macaque, there are 1.8 million,108 in rabbit, 380 000,249 and in mouse retina approximately 60 000 ganglion cells.49 The actual number of cells has a large genetic component, which produces up to threefold variation in numbers, as demonstrated by quantitative analyses of a large number of mouse strains.250
Ganglion cells are physiologically and morphologically diverse. Best estimates are in the range of 15–20 different ganglion cell types. We think the number of distinct types is extremely important because each ganglion cell type is thought to represent an independent visual channel. They can be classified by many physiological and anatomical criteria, including size, response, receptive field, color, bandwidth, ON or OFF, conduction velocity, morphology, branch pattern, stratification, coupling, coverage, and central projections. The rabbit retina has long been favored for physiological recording and the classic studies of Levick251 and, later, Caldwell and Daw252 identified about 12 different physiological types. An array of microelectrodes makes it possible to record from a large number of ganglion cells simultaneously. Using this technique, DeVries and Baylor253 identified 11 different physiological types in the rabbit retina, many of which were arranged in independent mosaics with a characteristic spacing, such that a gaussian fit of the receptive fields touched at the border. This corresponds to a coverage of 1.8 and suggests a general principle for the optimal spacing of ganglion cells. OFF α cells, in particular, showed synchronized firing patterns consistent with the presence of gap junction coupling in this cell type.193,254,255
The classification of ganglion cell types, especially when based on morphological details, has been difficult and contentious. However, a quiet revolution in staining techniques and digital imaging has produced a surprising consensus on the number of ganglion cell types. The Masland lab used four different staining strategies (intracellular dye injection, photofilling, and gene gun-mediated entry of DiI particles or DNA-coated particle coding for GFP expression) to label over 700 ganglion cells in the rabbit retina.256 Based on dendritic field size and structure and, most importantly, the depth of stratification in the IPL, this set of ganglion cells was divided into 13 different types (Fig. 15.29). A few cells were unclassified, which could allow additional ganglion cell types of low density. A calculation based on the expected coverage and density for each cell type showed that this number of classes would fit within the total number of rabbit ganglion cells. In the macaque retina, 13 different ganglion cell types were labeled by back-filling from the lateral geniculate nucleus with rhodamine-linked dextrans followed by photostaining.35 In summary, large-scale surveys have now been conducted for human,257–259 macaque,260 rabbit,256 cat, rat, and mouse,261,262 and some morphological varieties of ganglion cell are common to all these species. An important goal now is to characterize fully each RGC type with respect to physiology and synaptic connections. An understanding of the central projections of each ganglion cell type will be necessary before we have a true understanding of the visual process.
New tools by way of transgenic mouse lines that express a marker protein such as GFP in a single type of cell are becoming available.40,263–268 A particularly striking example of the power of this approach was the ability of Michal Rivlin-Etzion and colleagues to show that two ON/OFF DS RGCs with similar direction selectivity had different projection patterns to the brain. These data suggest there are at least eight ON/OFF DS RGCs with indistinguishable dendritic arbors. If a similar situation arises with other classes of RGCs then the number of independent cell types may increase dramatically, also increasing the number of visual channels leaving the retina. In fact, the total number of ganglion cell types has not yet been determined for any species. Seemingly rare cell types which account for a small fraction of the total, e.g., ipRGCs, make up less than 1%, but may be split into five subtypes with different morphology and different central projections, further adding to the types of RGCs. There seems to be general agreement that the number of ganglion cell types will be more than 20.269
The importance of depth in the IPL was emphasized when Roska and Werblin showed that at least 10 ganglion cell types, most narrowly stratified at different depths, received distinct physiological inputs in the rabbit retina.270,271 Apparently, the IPL contains a stack of parallel versions of the visual scene coded by depth. Each layer receives a different combination of excitatory and inhibitory inputs to produce at least 10 spatiotemporal channels. Ganglion cells with transient or sustained responses were located at different levels of the IPL. Some ganglion cells received inhibitory inputs that originated in another layer. This type of vertical inhibition could be carried by narrow-field amacrine cells, many of which are broadly stratified.38 The functional anatomy of the IPL is very precise. In the rabbit retina, OFF and ON alpha ganglion cells are stratified just below the two cholinergic bands (Fig. 15.30).10,272 They are separated by as little as 1–3 µm but they are clearly different addresses serving different ganglion cells with distinct morphological and physiological properties. If a single stratum can be as narrow as this, there is certainly room for a dozen or more different layers within the IPL, each serving one or more different ganglion cell types.
A parallel attempt to classify rabbit ganglion cells relied on an entirely different strategy. The molecular signature for six different amino acids and a glutamate excitation signal (1-amino-4-guanidobutane: AGB) allowed a set of 465 cells in the GCL to be sorted into 14 different ganglion cell types and three small amacrine cell types.193 All ganglion cells contained glutamate, but the levels of GABA and glycine, which are thought to reflect gap junction coupling to amacrine cells, were more variable. Importantly, cluster analysis in multivariate space showed that the different ganglion cell types are statistically separate.
Does each ganglion cell type represent a visual channel?
The simple answer to this question is yes, we think so. However, we still do not have a definitive value for the number of channels and, for those ganglion cell types that have been classified morphologically, it is not always clear which physiological class they belong to. The correspondence between functional anatomy and physiological type is only partially complete.273 A problem that has yet to be adequately resolved is what features of the functional responses of the ganglion cells are the ones that set individual classes apart. In fact, function alone is probably not sufficient and projection pattern, axon diameter, and terminal morphology may also be important. Regardless, it is widely believed that the retina, with the ganglion cells as the output, produces a large set of neural codes, most of which have not been deciphered, that are transmitted to the brain in parallel both on a temporal scale and to a variety of central structures. These are the visual channels, some of which carry different versions of Mona Lisa (Fig. 15.1), where we began. Below are some examples of different ganglion cells/visual channels that we are beginning to understand.
In a few cases, a particular ganglion cell type seems to have a specific role. Midget ganglion cells in the primate retina are a good example. There are two types, ON and OFF, which are broadly stratified towards the middle of the IPL. Midget ganglion cells are numerically dominant in central retina, where they may account for as many as 95% of all ganglion cells.150 Importantly, we know that in central retina there is a 1 : 1 : 1 correspondence from cones → midget bipolar cells → midget ganglion cells. Beyond 2 mm or 10°, the midget ganglion cells form separate dendritic clusters that receive input from multiple midget bipolar cells. Sampling theory, based on the density of midget ganglion cells, closely predicts the curve describing a psychophysical measure of human acuity, except close to the fovea where the cone pedicles are laterally displaced.274 Thus, it seems clear that midget ganglion cells transmit a high-acuity version of the visual scene from the center of your gaze, the central retina. The 1 : 1 : 1 ratio also means that individual midget ganglion cells will be color-coded according to the type of cone at the beginning of the chain. Midget ganglion cells project to the parvocellular layers of the lateral geniculate nucleus which also carry color signals. Midget ganglion cells are a specialization of the primate retina, perhaps the final point in the relentless survival-driven goal of maximum acuity. There is no exact match in other mammalian species but an equivalent role could be played by beta ganglion cells in the cat retina. In the rabbit retina, there are neither midget nor beta ganglion cells but an ON and OFF pair of relatively small ganglion cells, G4, which are rather broadly stratified toward the middle of the IPL.256 These are at least morphologically similar. Consistent with the idea that the midget/beta cells are a specialization of animals with a fovea or foveal-like specialization, the rodent retina lacks these types of ganglion cells.
Some of the most convincing evidence that ganglion cells carry specific channels of visual information comes from comparative anatomy, where it can be seen that certain ganglion cell types recur frequently across species. Perhaps the best example is the alpha ganglion cell, which has been reported in many mammalian retinas.275 Alpha ganglion cells have large somas, come in paramorphic ON and OFF pairs, make up less than 5% of all ganglion cells, and have a large dendritic field with a characteristic radiate branching pattern (Fig. 15.31). In the rabbit retina, alpha ganglion cells are narrowly stratified just outside the cholinergic bands. Physiologically, they have transient nonlinear (Y-like) properties, they respond to stimuli of high temporal frequency, and project to the magnocellular layers of the lateral geniculate nucleus. Their responses are not color-coded. Alpha ganglion cells seem well suited for detection of low luminance contrast over large areas of the visual field, such as a blurred copy of the Mona Lisa. Their occurrence over such a wide range of species suggests they play a fundamental role common to the visual needs of many animals, e.g., detection of motion. Parasol ganglion cells in the primate retina and A-type cells of rodents have similar properties, although whether they correspond exactly to alpha cells is an open question. There appear to be wide-field ganglion cells in the primate retina that are morphologically closer to alpha ganglion cells in other species.260
The ON/OFF DS ganglion cells of the rabbit retina, reported by Barlow and Levick nearly 50 years ago,276are one of the best characterized of the retinal ganglion cells. In these ganglion cells, a bar moving in the preferred direction through their receptive field produces a strong burst of spikes, but the same stimulus moving in the opposite direction produces little or no response. As first shown by Amthor and colleagues,277 bistratified cells fasciculate extensively within the two bands of starburst amacrine cell processes and they have a distinct retroflexive branching pattern (Fig. 15.32). DS ganglion cells are exquisitely sensitive to nicotinic cholinergic agonists but, surprisingly, directional selectivity is not dependent on this cholinergic input.235
Paired recordings showed that starburst amacrine cells on the null side of the DS ganglion cells provide asymmetric GABA inhibition to DS ganglion cells.236,278 The source of the GABAergic input is from starburst amacrine cells, which contain GABA as well as ACh.232 Individual dendrites of the starburst amacrine cells generate directional responses due to their intrinsic properties and this is the origin of directional signals in several other types of DS ganglion cells.33 This difference is consistent with recent morphological results using an extensive three-dimensional reconstruction of functionally identified DS ganglion cells by serial block-face electron microscopy that showed specific inhibitory inputs from starburst dendrites with the appropriate orientation.240
At present, four ganglion cell types with different null/preferred axes have been described, one of which is dye-coupled and shows a precise dendrite-to-dendrite tiling pattern with a coverage close to 1.279 DS cells with the other preferred axes are thought to tile the retina independently so that if the dendrites of two DS ganglion cells overlap, they must have different preferred directions. They can also be differentiated by molecular markers and have slightly different central projections.280 The four null/preferred axes are aligned with the extraocular muscles281 so it is often suggested that the ON/OFF DS cells play a role in the control of eye movements. Cells with a similar morphology have been noted in mouse,261,262 rat, cat (the iota cell),282 and even primate retina.260 In the mouse two posteriorly tuned, essentially morphologically identical DS cells have been defined that have subtle receptive field differences, and different central projection patterns.268 If each compass point is duplicated in the same manner, this would predict there should be a total of eight ON/OFF DS ganglion cells. It is not unreasonable to assume a similar situation will exist in most other mammals.
There is another DS cell in the rabbit retina that has interesting properties. Its dendrites are monostratified in the ON starburst band and have a similar retroflexive branch pattern. They also receive specific null-side inhibition from starburst amacrine cells.283 However, these ON DS cells respond to much slower movements and its three variants have different null/preferred axes, which are aligned with the semicircular canals.284 Consistent with the hypothesis that DS ganglion cells are critical to eye movement control, the ON DS ganglion cells project to the medial terminal nucleus of the accessory optic system.284,285 Ganglion cells with a strikingly similar morphology have been reported in mouse, rat, and primate retina.260 The value of this comparative anatomy becomes clear when we consider an experiment in the mouse retina. When starburst amacrine cells are ablated, DS responses in ganglion cells are abolished and the optokinetic nystagmus is also blocked.234 This is a type of reflex eye movement involved with tracking moving objects with the same velocity range as the ON DS ganglion cell. It suggests that ON DS cells provide the input to a system for tracking or stabilizing the retinal image. At least one gene associated with an eye movement disorder is exclusively expressed in starburst amacrine cells, which provide the directional input to the ON DS ganglion cell.267
A second type of ON DS ganglion cell has recently been identified in the rabbit retina.285–287 This cell type, originally reported by Ackert and colleagues,288 is distinct from the cell described above in terms of morphology and dendritic stratification, just above the lower band of ChAC processes. If the starburst amacrine cells are the source of directional signals in the retina, it is not clear how DS signals are generated in this cell type. Finally, in a stunning example of a cell type-specific molecular marker, a whole population of ganglion cells from the mouse retina was stained using a junctional adhesion molecule B (JAM-B)-driven GFP mouse line.265 This procedure identified a uniform population of ganglion cells with asymmetrical dendritic trees aligned dorsal to ventral. A similar OFF cell type, G3 in the Masland catalog, has been reported for the rabbit retina.256,289 In the mouse, this cell type has been characterized as OFF DS, although in the rabbit retina it was identified as orientation-selective.290 If it is truly DS, then the mechanism may be unusual because it does not stratify with the starburst amacrine cells. Together, these observations suggest that there may be multiple circuits to generate directional signals.291
A ganglion cell for the control of pupil diameter and circadian rhythm
It has long been known that certain visually driven activities persist in mice where rods and cones have completely degenerated. These apparently blind mice retain pupillary light reflexes as well as circadian rhythms and a similar profile has been reported in totally blind humans.292 It was concluded that these reflexes are driven by connections separate from the usual image-forming pathways. This issue was resolved when a set of ganglion cells were found to express an additional visual pigment called melanopsin. They form a relatively sparse mosaic (only 2000 per mouse retina) with loopy dendrites (Fig. 15.33) in sublamina a and b and they respond with a slow sustained discharge dependent on the light intensity.36 Even when these cells are isolated, they still produce light responses.293 In other words, the melanopsin-containing ganglion cells are intrinsically photosensitive and they are referred to as ipRGCs.
The melanopsin-containing ganglion cells project to the suprachiasmatic nucleus (SCN), the brain’s circadian pacemaker.36,294 Labeled axons were also found in the olivary pretectal nucleus (OPN), which is involved with the circuit controlling pupil diameter. These are referred to as nonimage-forming pathways. In mice lacking melanopsin, photoentrainment of the circadian rhythm and the control of the pupillary light reflex were attenuated at high light intensities.295
However, melanopsin ganglion cells also receive the usual rod and cone inputs, which can drive these circuits at low light intensities. The M1 subtype is stratified in sublamina a but it still has ON-driven light responses due to the presence of ectopic or axonal ribbon synapses from ON cone bipolar cells as they traverse sublamina a (Fig. 15.33).27,28,296 In crossbred mice with photoreceptor degeneration as well as no melanopsin, the pupillary light reflex and the photoentrainment of the circadian clock were completely eliminated.297 The conclusion is that, at low irradiance, the normal rod/cone inputs drive these circuits but at higher light levels the intrinsic melanopsin system drives these accessory visual functions. These experiments provide anatomical, physiological, and behavioral evidence that the ganglion cell type defined by the expression of melanopsin forms a specific channel in the mammalian visual system that is connected to the SCN and the OPN among other targets. This is a prime example that different ganglion cell types provide parallel channels in the visual system that serves specific functions.
Based on a combination of morphology and physiology, five subtypes of melanopsin-containing ganglion cells, M1–M5, have been identified in the mouse retina.298,299 The M1 subtype is the only one stratified in sublamina a; it contains the most melanopsin and has strong intrinsic photoresponses and relatively weak rod/cone input. M2 cells have dendrites in sublamina b but less melanopsin and weaker intrinsic responses. M3 cells are bistratified and do not form a uniform population.300 M4 and M5 cells have very low levels of melanopsin and correspondingly weak intrinsic responses.298 Using combinations of markers, the M1-class ipRGCs can be further divided into two types, one projecting to the shell of the OPN, which is required for the pupillary light reflex, and one targeting the SCN, required for circadian photo entrainment.301 This Brn-3b-negative group of M1 ipRGCs comprises approximately only 10% of all melanopsin ganglion cells. Thus, the specific nonimage-forming visual pathway that resets the circadian clock every morning consists of only 200 ganglion cells.301 It is very surprising that a distinct visual pathway can be formed by such a small number of cells.
The roles of non-M1 types are still unclear but they may contribute to light avoidance in mouse pups, sleep regulation and, in humans, the light sensitivity of migraine and seasonal affective disorder.298 The activity of ipRGCs is also important for retinogeniculate segregation, which is now known to involve light sensitivity prior to function of the photoreceptors.247
Color vision and ganglion cells
Humans are trichromatic, with red, green, and blue cones. Color vision provides an additional variable to improve visual discrimination. It is organized into two opponent systems, red-green and blue-yellow (where yellow = red + green).302 Hence, there are no red/green hues and no mixtures of blue and yellow; they are said to be color-opponent. The blue-yellow system is found widely in mammals and is thought to be older on an evolutionary timescale.56 In primates, once the midget system had reached maximum resolution in the 1 : 1 : 1 pathway, a comparatively recent gene duplication produced red and green pigments. By reason of the connection with a single cone, midget ganglion cells are automatically color-coded.
Midget ganglion cells are concentrically organized as red-green or green-red. The center input is spectrally pure because of the 1 : 1 : 1 connection with single cones. There has been some controversy concerning the origin of the color-opponent surround, whether it is spectrally pure, and whether it arises from the outer or inner retina. Recent recordings from midget ganglion cells in the primate retina indicate that the surround is chromatically mixed. In other words, it is the average of the surrounding cones. On average, the ratio of red to green in the surround was approximately 50%.110 Furthermore, both center and surround inputs modulated an excitatory conductance with a reversal potential around 0 mV. This means that the surround signal does not arise from amacrine cell inhibition in the inner retina. Rather, the color opponency must be generated in the outer retina by horizontal cell feedback.110 Horizontal cells are suitably wired to provide the spectrally mixed surround signal because they contact both red and green cones indiscriminately.303 Enhanced buffering to block pH-dependent horizontal cell feedback eliminated both the spatial and chromatic surround of midget ganglion cells. Although the exact mechanism of horizontal cell feedback is controversial, this strongly indicates that color opponency is produced by horizontal cell feedback in the outer retina.110,304 Finally, neither GABA nor glycine antagonists blocked the surround inputs. Thus inhibition in either the outer or inner retina is not required. Nor does horizontal cell feedback in the primate retina depend on GABA or glycine.110
Large-scale multielectrode array recording has also been used to analyze the color inputs to primate ganglion cells.305 Hundreds of ganglion cells may be recorded simultaneously and then sorted by spike size and shape to identify individual ganglion cells. Types of ganglion cell may be identified by their receptive field sizes, center/surround organization, and the mosaic properties by which they tile the retina. Using a fine-grained textual stimulus, local hot spots were found within each receptive field that matched the distribution of the underlying cone array. The spectral characteristics of each hot spot, recorded in several nearby ganglion cells, were used to identify each cone locus as red, green, or blue and then the contributions of each individual cone were mapped to the overlying array of ganglion cells. Parasol ganglion cells sampled uniformly from red and green cones but not blue cones. In contrast, many midget ganglion cells showed color-opponent responses. As above, the surrounds were spectrally mixed but the center inputs to both ON and OFF midget ganglion cells had a small nonrandom tendency to sample from more red or green cones.305Obviously, in central retina, where midget bipolar cells may contact a single cone, this would provide a spectrally pure center with a mixed color-opponent surround. Intellectually, we understand that tracing back from ganglion cells through the retinal circuitry must lead to cones. However, the direct demonstration of ganglion cell sampling from the complete array of identified cones is an experimental triumph. These experiments indicate that parasol ganglion cells are not color-coded while color signals are carried by the midget ganglion cells.305
In contrast to the red-green system, the circuits underlying blue-yellow opponency have been relatively well described. Some of this success is due to the morphological details which make cells in the blue pathway more recognizable. This begins with blue cones themselves, which can be labeled with antibodies against blue cone opsin.59 Red and green cone opsins are so close they cannot be discriminated by current antibodies. In addition, blue cone pedicles are noticeably smaller, with fewer telodendria.72 The blue cone bipolar cells can also be recognized because of their long dendrites, which bypass many cones in search of the blue ones.152,306,307 Blue ON cone bipolar cells have direct input to a small bistratified ganglion cell that gives blue ON/yellow OFF responses.308 Blue-yellow small bistratified ganglion cells have also been identified in multielectrode array recordings from primate retina. These experiments suggested that most of the color opponency arose in the outer retina.309,310 Further physiological evidence suggests that blue-yellow color opponency originates in the outer retina.311 Indeed, blue cones themselves are blue-yellow color-opponent, indicating that the yellow-opponent signal is generated by horizontal cell feedback.312
In nonprimate mammalian retina, blue-driven ganglion cells were reported as monostratified ON cells.313 This is quite different from the small bistratified blue-yellow ganglion cell of the primate retina. The color opponency of this monostratified ganglion cell could arise in the outer retina, as suggested for primate retina.309,312
Gene therapy to cure color blindness
Mice are dichromats, expressing a short-wavelength photopigment and a single medium-wavelength pigment on the X chromosome. Jacobs and colleagues314 showed that adding a human long-wavelength photopigment allowed mice to have a form of color vision. This idea has been extended to primates and could potentially be used in humans. About 5–8% of the population has red-green color blindness, because they lack either the long- or middle-wavelength visual pigments. Some squirrel monkeys also are dichromats and recently Mancuso and colleagues315 showed that adding a third photopigment, using gene therapy approaches could restore color discrimination, even though the monkeys had been dichromats since birth.
New tools to identify ganglion cell types
However, new molecular techniques are starting to provide a way to mark certain ganglion cell types as a complete population. Thus, it is becoming possible to study a specific ganglion cell type as opposed to a spectrum of mixed cell types with variable properties. One research group has screened a library of bacterial artificial chromosome (BAC) transgenic mice with GFP expressed under the control of different promoters. In particular, they looked for GFP expression in a nonrandom mosaic. This is one of the known properties of specific ganglion cell types, which tile the retina in a uniform manner.41 In calretinin-enhanced GFP (EGFP) mice, a mosaic of large ganglion cells, stratified in sublamina a, with OFF transient responses to light was found. Collectively, these properties identify the GFP-labeled cells as OFF α ganglion cells.263 Centrally, this specific ganglion cell type projects to both the superior colliculus, an area integrating sensory input and guiding visual attention, and the dorsal lateral geniculate nucleus, a relay station on the way to the visual cortex. Furthermore, the axonal projections in both areas are precisely organized in columns at a specific laminar depth. Importantly, disrupting cholinergic retinal waves during development prevented the columnar organization of OFF α ganglion cell axons.263 This suggests that spontaneous activity during retinal development controls the development of a retinotopic map. It is worth repeating that these experiments were made possible because a genetic marker for a single ganglion cell type, the OFF α ganglion cell, was identified.
In a cadherin-3 GFP mouse line, a small percentage of ganglion cells was labeled, some of which also expressed melanopsin. They also contained cadherin-6 and projected to nonimage-forming visual nuclei such as the ventral lateral geniculate nucleus, the intergeniculate leaflet, and the OPN. These nuclei, known targets of ipRGCs. Importantly, in cadherin-6-deficient mice (cadherin-3/GFP crossed with a cadherin-6 knockout), the cadherin-3-labeled ganglion cells failed to innervate the appropriate visual nuclei. Instead, the GFP-labeled axons projected through and past their usual targets. These experiments, utilizing genetically labeled mouse lines, indicate that cadherin-6 is necessary for certain ganglion cell types to recognize their synaptic targets and form functional circuits.316
As mouse lines in which different cell types are labeled by molecular methods become widely available, the methods to differentiate among retinal cell types will continue to expand. One group has published a “genetic address book” of retinal cell types.40 Moreover, several groups have used GFP-labeled cell types to develop single-cell expression libraries for specific cell types.280,317 This advance in technique is likely to be a game-changer. It will enable a molecular fingerprint to be developed for each cell type, as well as identifying the transcription factors and signaling molecules which govern development and circuit connections.317 In particular, a transcription factor-based code was used to sort the cells into relevant groups. Perhaps this methodology will provide the definitive answer to how many ganglion cell types are present in the mammalian retina. Finally, by correlating these single-cell libraries against a database of gene mutations associated with visual system disorders, it may be possible to identify defects arising in specific cell types or circuits. For example, an eye movement disorder was correlated with starburst amacrine cells, which may be the source of DS signals required to control reflex eye movements.317
Clinical relevance of functional anatomy
In glaucoma, the basic defect is in the GCL. Increased intraocular pressure apparently induces programmed cell death in retinal ganglion cells. Factors involved may include anoxia, reduced axonal transport, and excess glutamate, leading to excitatory damage known as excitotoxicity. Ganglion cells appear to be particularly sensitive while other retinal neurons such as amacrine cells are unaffected. Figure 15.34 shows a retinal section from the same eccentricity of a control macaque eye compared to the operated eye with experimentally induced high intraocular pressure. All retinal layers appear normal, with the exception of the GCL, which is severely depleted compared to the control eye. The absence of retinal ganglion cells may be correlated with dramatically reduced visual acuity, especially in the peripheral retina.318
A large number of photoreceptor mutations, often in the phototransduction cascade, lead to photoreceptor degeneration in conditions such as retinitis pigmentosa (RP). The defect often arises in rods but eventually both rods and cones degenerate. Whether this occurs because rods secrete a maintenance factor for cones, or via rod/cone coupling, known as the bystander effect, or due to the accumulation of cellular debris is a matter of debate beyond the scope of this chapter. However, photoreceptor degeneration has severe consequences for the organization of the remaining retina. The neural retina apparently requires input from the sensory retina to maintain its well-organized structure. When the neural retina has been deafferented, the second-order neurons, such as rod bipolar cells, produce new dendrites and sprout profusely, making many inappropriate contacts.3 Many neurons develop novel processes that invade other layers where they proliferate to produce tangled microneuromas. Glial cells are extensively remodeled; there is mass neuronal migration and almost total disruption of the layered structure of the retina. Figure 15.35 shows examples from two rodent lines and a human RP case. In each case, the resulting tangle of misplaced cells and inappropriate contacts is essentially unrecognizable as a section of retina. The basic layered organization of the retina has been destroyed. One consequence of this neural reorganization is that the remaining retina is unlikely to retain sufficient organization to operate in any kind of coherent manner. Thus, in the absence of photoreceptors, strategies aimed at stimulating the remaining neural retina by means of a retinal implant seem unlikely to succeed, unless the process of retinal remodeling can be controlled.
In addition to the above diseases that disrupt the gross morphology of the retina, there also are diseases with less severe effects. One group results in night blindness, without any degeneration of retinal cell types. These are called congenital stationary night blindness (CSNB). The result is a greatly diminished amplitude of the electroretinogram b-wave, indicating lack of or poor signal transmission between photoreceptors and bipolar cells. Careful analyses of the electroretinogram indicate that there are two forms, incomplete and complete.319 The genetic defects that give rise to these defects are now known to be caused by mutations in genes that are involved in release of glutamate from photoreceptors (incomplete, iCSNB) or absence of signaling in depolarizing bipolar cells (complete, cCSNB). Mouse models for iCSNB reveal that the dendritic arbors of ON bipolar and horizontal cells are abnormal. They extend across the OPL into the ONL and often reach the outer limiting membrane.320 By contrast, models of cCSNB have no morphological abnormalities, although in some there appears to be some abnormal expression of synaptic proteins, resulting in ribbon synapses being present in dendrites of depolarizing bipolar cells.321
1 Masland RH. The functional architecture of the retina. Sci Am. 1986;255:102–111.
2 Rodieck R. The first steps in seeing. Sunderland, MA: Sinauer Associates; 1998.
3 Marc RE, Jones BW, Watt CB, et al. Neural remodeling in retinal degeneration. Prog Retin Eye Res. 2003;22:607–655.
4 Dowling JE. The retina: an approachable part of the brain. Cambridge, MA: Belknap Press of Harvard University Press; 1987.
5 Vaney DI. Patterns of neuronal coupling in the retina. Prog Retin Eye Res. 1994;13:301–355.
6 Vaney DI, Weiler R. Gap junctions in the eye: evidence for heteromeric, heterotypic and mixed-homotypic interactions. Brain Res Brain Res Rev. 2000;32:115–120.
7 Galarreta M, Hestrin S. Electrical synapses between GABA-releasing interneurons. Nat Rev Neurosci. 2001;2:425–433.
8 Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys. 2001;34:325–472.
9 Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–294.
10 Zhang J, Yang Z, Wu SM. Development of cholinergic amacrine cells is visual activity-dependent in the postnatal mouse retina. J Comp Neurol. 2005;484:331–343.
11 Wassle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757.
12 Hartveit E. Functional organization of cone bipolar cells in the rat retina. J Neurophysiol. 1997;77:1716–1730.
13 Dacheux RF, Raviola E. The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. J Neurosci. 1986;6:331–345.
14 DeVries SH, Schwartz EA. Kainate receptors mediate synaptic transmission between cones and ‘Off’ bipolar cells in a mammalian retina. Nature. 1999;397:157–160.
15 Slaughter MM, Miller RF. 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981;211:182–185.
16 Nakanishi S, Masu M, Bessho Y, et al. Molecular diversity of glutamate receptors and their physiological functions. EXS. 1994;71:71–80.
17 Vardi N, Duvoisin R, Wu G, et al. Localization of mGluR6 to dendrites of ON bipolar cells in primate retina. J Comp Neurol. 2000;423:402–412.
18 Nelson R, Famiglietti EV, Jr., Kolb H. Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in cat retina. J Neurophysiol. 1978;41:472–483.
19 Schiller PH, Sandell JH, Maunsell JH. Functions of the ON and OFF channels of the visual system. Nature. 1986;322:824–825.
20 Tauchi M, Masland RH. The shape and arrangement of the cholinergic neurons in the rabbit retina. Proc R Soc Lond B Biol Sci. 1984;223:101–119.
21 Peichl L, Buhl EH, Boycott BB. Alpha ganglion cells in the rabbit retina. J Comp Neurol. 1987;263:25–41.
22 Peichl L, Ott H, Boycott BB. Alpha ganglion cells in mammalian retinae. Proc R Soc Lond B Biol Sci. 1987;231:169–197.
23 Zhang DQ, Zhou TR, McMahon DG. Functional heterogeneity of retinal dopaminergic neurons underlying their multiple roles in vision. J Neurosci. 2007;27:692–699.
24 Zhang DQ, Wong KY, Sollars PJ, et al. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc Natl Acad Sci USA. 2008;105:14181–14186.
25 Dacey DM, Liao HW, Peterson BB, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754.
26 Hoshi H, Liu WL, Massey SC, et al. ON inputs to the OFF layer: bipolar cells that break the stratification rules of the retina. J Neurosci. 2009;29:8875–8883.
27 Dumitrescu ON, Pucci FG, Wong KY, et al. Ectopic retinal ON bipolar cell synapses in the OFF inner plexiform layer: contacts with dopaminergic amacrine cells and melanopsin ganglion cells. J Comp Neurol. 2009;517:226–244.
28 Curcio CA, Sloan KR, Kalina RE, et al. Human photoreceptor topography. J Comp Neurol. 1990;292:497–523.
29 Curcio CA, Allen KA, Sloan KR, et al. Distribution and morphology of human cone photoreceptors stained with anti-blue opsin. J Comp Neurol. 1991;312:610–624.
30 Wandell B. Foundations of vision. Sunderland, MA: Sinauer Associates; 1995.
31 Masland RH, Raviola E. Confronting complexity: strategies for understanding the microcircuitry of the retina. Annu Rev Neurosci. 2000;23:249–284.
32 Denk W, Detwiler PB. Optical recording of light-evoked calcium signals in the functionally intact retina. Proc Natl Acad Sci USA. 1999;96:7035–7040.
33 Euler T, Detwiler PB, Denk W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature. 2002;418:845–852.
34 Anderson JR, Jones BW, Watt CB, et al. Exploring the retinal connectome. Mol Vis. 2011;17:355–379.
35 Dacey DM, Peterson BB, Robinson FR, et al. Fireworks in the primate retina: in vitro photodynamics reveals diverse LGN-projecting ganglion cell types. Neuron. 2003;37:15–27.
36 Hattar S, Liao HW, Takao M, et al. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070.
37 MacNeil MA, Masland RH. Extreme diversity among amacrine cells: implications for function. Neuron. 1998;20:971–982.
38 MacNeil MA, Heussy JK, Dacheux RF, et al. The shapes and numbers of amacrine cells: matching of photofilled with Golgi-stained cells in the rabbit retina and comparison with other mammalian species. J Comp Neurol. 1999;413:305–326.
39 Gan WB, Grutzendler J, Wong WT, et al. Multicolor “DiOlistic” labeling of the nervous system using lipophilic dye combinations. Neuron. 2000;27:219–225.
40 Siegert S, Scherf BG, Del Punta K, et al. Genetic address book for retinal cell types. Nat Neurosci. 2009;12:1197–1204.
41 Wassle H, Riemann HJ. The mosaic of nerve cells in the mammalian retina. Proc R Soc Lond B Biol Sci. 1978;200:441–461.
42 Strettoi E, Dacheux RF, Raviola E. Synaptic connections of rod bipolar cells in the inner plexiform layer of the rabbit retina. J Comp Neurol. 1990;295:449–466.
43 Strettoi E, Raviola E, Dacheux RF. Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. J Comp Neurol. 1992;325:152–168.
44 Masland RH. Neuronal cell types. Curr Biol. 2004;14:R497–R500.
45 Ahnelt PK, Kolb H. The mammalian photoreceptor mosaic-adaptive design. Prog Retin Eye Res. 2000;19:711–777.
46 Sterling P, Demb JB. Retina. In: Shepard GM, ed. The synaptic organization of the brain. New York: Oxford University Press; 2004:217–293.
47 Curcio CA, Sloan KR, Jr., Packer O, et al. Distribution of cones in human and monkey retina: individual variability and radial asymmetry. Science. 1987;236:579–582.
48 Whitney IE, Raven MA, Lu L, et al. A QTL on chromosome 10 modulates cone photoreceptor number in the mouse retina. Invest Ophthalmol Vis Sci. 2011;52:3228–3236.
49 Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18:8936–8946.
50 Williams DR. Visibility of interference fringes near the resolution limit. J Opt Soc Am A. 1985;2:1087–1093.
51 Williams DR. Aliasing in human foveal vision. Vis Res. 1985;25:195–205.
52 Hirsch J, Curcio CA. The spatial resolution capacity of human foveal retina. Vis Res. 1989;29:1095–1101.
53 Baylor DA, Nunn BJ, Schnapf JL. Spectral sensitivity of cones of the monkey Macaca fascicularis. J Physiol. 1987;390:145–160.
54 Schnapf JL, Kraft TW, Baylor DA. Spectral sensitivity of human cone photoreceptors. Nature. 1987;325:439–441.
55 Schnapf JL, Kraft TW, Nunn BJ, et al. Spectral sensitivity of primate photoreceptors. Vis Neurosci. 1988;1:255–261.
56 Nathans J. The evolution and physiology of human color vision: insights from molecular genetic studies of visual pigments. Neuron. 1999;24:299–312.
57 Roorda A, Williams DR. The arrangement of the three cone classes in the living human eye. Nature. 1999;397:520–522.
58 DeMonasterio FM, Schein SJ, McCrane EP. Staining of blue-sensitive cones of the macaque retina by a fluorescent dye. Science. 1981;213:1278–1281.
59 Szel A, Diamantstein T, Rohlich P. Identification of the blue-sensitive cones in the mammalian retina by anti-visual pigment antibody. J Comp Neurol. 1988;273:593–602.
60 Lennie P, Fairchild MD. Ganglion cell pathways for rod vision. Vis Res. 1994;34:477–482.
61 Wassle H, Grunert U, Chun MH, et al. The rod pathway of the macaque monkey retina: identification of AII-amacrine cells with antibodies against calretinin. J Comp Neurol. 1995;361:537–551.
62 Mills SL, Massey SC. AII amacrine cells limit scotopic acuity in central macaque retina: A confocal analysis of calretinin labeling. J Comp Neurol. 1999;411:19–34.
63 Chun MH, Grunert U, Martin PR, et al. The synaptic complex of cones in the fovea and in the periphery of the macaque monkey retina. Vis Res. 1996;36:3383–3395.
64 Haverkamp S, Grunert U, Wassle H. The cone pedicle, a complex synapse in the retina. Neuron. 2000;27:85–95.
65 Sterling P. Needle from a haystack. Optimal signaling by a nonlinear synapse. Neuron. 2002;34:670–672.
66 Sterling P, Smith RG. Design for a binary synapse. Neuron. 2004;41:313–315.
67 Rao-Mirotznik R, Harkins AB, Buchsbaum G, et al. Mammalian rod terminal: architecture of a binary synapse. Neuron. 1995;14:561–569.
68 Li W, DeVries SH. Separate blue and green cone networks in the mammalian retina. Nat Neurosci. 2004;7:751–756.
69 O’Brien J, Nguyen HB, Mills SL. Cone photoreceptors in bass retina use two connexins to mediate electrical coupling. J Neurosci. 2004;24:5632–5642.
70 Hornstein EP, Verweij J, Schnapf JL. Electrical coupling between red and green cones in primate retina. Nat Neurosci. 2004;7:745–750.
71 DeVries SH, Qi X, Smith R, et al. Electrical coupling between mammalian cones. Curr Biol. 2002;12:1900–1907.
72 Ahnelt P, Keri C, Kolb H. Identification of pedicles of putative blue-sensitive cones in the human retina. J Comp Neurol. 1990;293:39–53.
73 Raviola E, Gilula NB. Gap junctions between photoreceptor cells in the vertebrate retina. Proc Natl Acad Sci U S A. 1973;70:1677–1681.
74 Nelson R. Cat cones have rod input: a comparison of the response properties of cones and horizontal cell bodies in the retina of the cat. J Comp Neurol. 1977;172:109–135.
75 Schneeweis DM, Schnapf JL. The photovoltage of macaque cone photoreceptors: adaptation, noise, and kinetics. J Neurosci. 1999;19:1203–1216.
76 Smith RG, Freed MA, Sterling P. Microcircuitry of the dark-adapted cat retina: functional architecture of the rod-cone network. J Neurosci. 1986;6:3505–3517.
77 Hornstein EP, Verweij J, Li PH, et al. Gap-junctional coupling and absolute sensitivity of photoreceptors in macaque retina. J Neurosci. 2005;25:11201–11209.
78 Peachey NS, Alexander KR, Fishman GA. Visual adaptation and the cone flicker electroretinogram. Invest Ophthalmol Vis Sci. 1991;32:1517–1522.
79 Peachey NS, Arakawa K, Alexander KR, et al. Rapid and slow changes in the human cone electroretinogram during light and dark adaptation. Vis Res. 1992;32:2049–2053.
80 Peachey NS, Ball SL. Electrophysiological analysis of visual function in mutant mice. Adv Ophthalmol. 2003;107:13–36.
81 Heikkinen H, Vinberg F, Nymark S, et al. Mesopic background lights enhance dark-adapted cone ERG flash responses in the intact mouse retina: a possible role for gap junctional decoupling. J Neurophysiol. 2011;105:2309–2318.
82 Ribelayga C, Cao Y, Mangel SC. The circadian clock in the retina controls rod–cone coupling. Neuron. 2008;59:790–801.
83 Arshavsky VY, Lamb TD, Pugh EN, Jr. G proteins and phototransduction. Annu Rev Physiol. 2002;64:153–187.
84 Copenhagen DR, Jahr CE. Release of endogenous excitatory amino acids from turtle photoreceptors. Nature. 1989;341:536–539.
85 Sarantis M, Mobbs P. The spatial relationship between Müller cell processes and the photoreceptor output synapse. Brain Res. 1992;584:299–304.
86 Schaeffer SF, Raviola E. Membrane recycling in the cone cell endings of the turtle retina. J Cell Biol. 1978;79:802–825.
87 Grunert U, Martin PR. Rod bipolar cells in the macaque monkey retina: immunoreactivity and connectivity. J Neurosci. 1991;11:2742–2758.
88 Young HM, Vaney DI. Rod-signal interneurons in the rabbit retina: 1. Rod bipolar cells. J Comp Neurol. 1991;310:139–153.
89 Vardi N, Morigiwa K, Wang TL, et al. Neurochemistry of the mammalian cone ‘synaptic complex. Vis Res. 1998;38:1359–1369.
90 Haverkamp S, Grunert U, Wassle H. The synaptic architecture of AMPA receptors at the cone pedicle of the primate retina. J Neurosci. 2001;21:2488–2500.
91 Kolb H. The connections between horizontal cells and photoreceptors in the retina of the cat: electron microscopy of Golgi preparations. J Comp Neurol. 1974;155:1–14.
92 Boycott BB, Peichl L, Wassle H. Morphological types of horizontal cell in the retina of the domestic cat. Proc R Soc Lond B Biol Sci. 1978;203:229–245.
93 Dacheux RF, Raviola E. Horizontal cells in the retina of the rabbit. J Neurosci. 1982;2:1486–1493.
94 Peichl L, Gonzalez-Soriano J. Morphological types of horizontal cell in rodent retinae: a comparison of rat, mouse, gerbil, and guinea pig. Vis Neurosci. 1994;11:501–517.
95 Mills SL, Massey SC. Distribution and coverage of A- and B-type horizontal cells stained with Neurobiotin in the rabbit retina. Vis Neurosci. 1994;11:549–560.
96 Haverkamp S, Grunert U, Wassle H. Localization of kainate receptors at the cone pedicles of the primate retina. J Comp Neurol. 2001;436:471–486.
97 Li W, Keung JW, Massey SC. Direct synaptic connections between rods and OFF cone bipolar cells in the rabbit retina. J Comp Neurol. 2004;474:1–12.
98 Nelson R, von Litzow A, Kolb H, et al. Horizontal cells in cat retina with independent dendritic systems. Science. 1975;189:137–139.
99 Vaney DI. Many diverse types of retinal neurons show tracer coupling when injected with biocytin or Neurobiotin. Neurosci Lett. 1991;125:187–190.
100 Vaney DI. The coupling pattern of axon-bearing horizontal cells in the mammalian retina. Proc Biol Sci. 1993;252:93–101.
101 Mills SL, Massey SC. The kinetics of tracer movement through homologous gap junctions in the rabbit retina. Vis Neurosci. 1998;15:765–777.
102 Boycott BB, Hopkins JM, Sperling HG. Cone connections of the horizontal cells of the rhesus monkey’s retina. Proc R Soc Lond B Biol Sci. 1987;229:345–379.
103 Dacey DM, Lee BB, Stafford DK, et al. Horizontal cells of the primate retina: cone specificity without spectral opponency. Science. 1996;271:656–659.
104 Wassle H, Dacey DM, Haun T, et al. The mosaic of horizontal cells in the macaque monkey retina: with a comment on biplexiform ganglion cells. Vis Neurosci. 2000;17:591–608.
105 Packer OS, Dacey DM. Receptive field structure of H1 horizontal cells in macaque monkey retina. J Vis. 2002;2:272–292.
106 Massey SC, O’Brien JJ, Trexler EB, et al. Multiple neuronal connexins in the mammalian retina. Cell Commun Adhes. 2003;10:425–430.
107 Hombach S, Janssen-Bienhold U, Sohl G, et al. Functional expression of connexin57 in horizontal cells of the mouse retina. Eur J Neurosci. 2004;19:2633–2640.
108 Sterling P. How retinal circuits optimize the transfer of visual information. In: Chalupa LM, ed. The visual neurosciences. Cambridge, MA: MIT Press; 2004:234–259.
109 Mahowald MA, Mead C. The silicon retina. Sci Am. 1991;264:76–82.
110 Crook JD, Manookin MB, Packer OS, et al. Horizontal cell feedback without cone type-selective inhibition mediates “red–green” color opponency in midget ganglion cells of the primate retina. J Neurosci. 2011;31:1762–1772.
111 Baylor DA, O’Bryan PM. Electrical signaling in vertebrate photoreceptors. Fed Proc. 1971;30:79–83.
112 Wu SM. Feedback connections and operation of the outer plexiform layer of the retina. Curr Opin Neurobiol. 1992;2:462–468.
113 Vardi N, Sterling P. Subcellular localization of GABAA receptor on bipolar cells in macaque and human retina. Vis Res. 1994;34:1235–1246.
114 Marc R. Retinal neurotransmitters. In: Claw JS, ed. The visual sciences. Cambridge, MA: MIT Press; 2004:301–319.
115 Cueva JG, Haverkamp S, Reimer RJ, et al. Vesicular gamma-aminobutyric acid transporter expression in amacrine and horizontal cells. J Comp Neurol. 2002;445:227–237.
116 Hirano AA, Brandstatter JH, Morgans CW, et al. SNAP25 expression in mammalian retinal horizontal cells. J Comp Neurol. 2011;519:972–988.
117 Mercer AJ, Rabl K, Riccardi GE, et al. Location of release sites and calcium-activated chloride channels relative to calcium channels at the photoreceptor ribbon synapse. J Neurophysiol. 2011;105:321–335.
118 Zampighi GA, Schietroma C, Zampighi LM, et al. Conical tomography of a ribbon synapse: structural evidence for vesicle fusion. PloS ONE. 2011;6:e16944.
119 Shields CR, Tran MN, Wong RO, et al. Distinct ionotropic GABA receptors mediate presynaptic and postsynaptic inhibition in retinal bipolar cells. J Neurosci. 2000;20:2673–2682.
120 Picaud S, Pattnaik B, Hicks D, et al. GABAA and GABAC receptors in adult porcine cones: evidence from a photoreceptor-glia co-culture model. J Physiol. 1998;513:33–42.
121 Verweij J, Hornstein EP, Schnapf JL. Surround antagonism in macaque cone photoreceptors. J Neurosci. 2003;23:10249–10257.
122 McMahon MJ, Packer OS, Dacey DM. The classical receptive field surround of primate parasol ganglion cells is mediated primarily by a non-GABAergic pathway. J Neurosci. 2004;24:3736–3745.
123 Kamermans M, Fahrenfort I, Schultz K, et al. Hemichannel-mediated inhibition in the outer retina. Science. 2001;292:1178–1180.
124 VanLeeuwen M, Fahrenfort I, Sjoerdsma T, et al. Lateral gain control in the outer retina leads to potentiation of center responses of retinal neurons. J Neurosci. 2009;29:6358–6366.
125 Hirasawa H, Kaneko A. pH changes in the invaginating synaptic cleft mediate feedback from horizontal cells to cone photoreceptors by modulating Ca2+ channels. J Gen Physiol. 2003;122:657–671.
126 Vessey JP, Stratis AK, Daniels BA, et al. Proton-mediated feedback inhibition of presynaptic calcium channels at the cone photoreceptor synapse. J Neurosci. 2005;25:4108–4117.
127 Jackman SL, Babai N, Chambers JJ, et al. A positive feedback synapse from retinal horizontal cells to cone photoreceptors. PLoS Biol. 2011;9:e1001057.
128 Klaassen LJ, Sun Z, Steijaert MN, et al. Synaptic transmission from horizontal cells to cones is impaired by loss of connexin hemichannels. PLoS Biol. 2011;9:e1001107.
129 Boycott BB, Wassle H. Morphological classification of bipolar cells of the primate retina. Eur J Neurosci. 1991;3:1069–1088.
130 Grunert U, Martin PR, Wassle H. Immunocytochemical analysis of bipolar cells in the macaque monkey retina. J Comp Neurol. 1994;348:607–627.
131 Ghosh KK, Bujan S, Haverkamp S, et al. Types of bipolar cells in the mouse retina. J Comp Neurol. 2004;469:70–82.
132 MacNeil MA, Heussy JK, Dacheux RF, et al. The population of bipolar cells in the rabbit retina. J Comp Neurol. 2004;472:73–86.
133 Haverkamp S, Ghosh KK, Hirano AA, et al. Immunocytochemical description of five bipolar cell types of the mouse retina. J Comp Neurol. 2003;455:463–476.
134 Haverkamp S, Specht D, Majumdar S, et al. Type 4 OFF cone bipolar cells of the mouse retina express calsenilin and contact cones as well as rods. J Comp Neurol. 2008;507:1087–1101.
135 Puller C, Haverkamp S. Cell-type-specific localization of protocadherin beta16 at AMPA and AMPA/Kainate receptor-containing synapses in the primate retina. J Comp Neurol. 2011;519:467–479.
136 Puthussery T, Gayet-Primo J, Taylor WR. Localization of the calcium-binding protein secretagogin in cone bipolar cells of the mammalian retina. J Comp Neurol. 2010;518:513–525.
137 Dhingra A, Sulaiman P, Xu Y, et al. Probing neurochemical structure and function of retinal ON bipolar cells with a transgenic mouse. J Comp Neurol. 2008;510:484–496.
138 Schubert T, Kerschensteiner D, Eggers ED, et al. Development of presynaptic inhibition onto retinal bipolar cell axon terminals is subclass-specific. J Neurophysiol. 2008;100:304–316.
139 Murakami M, Otsuka T, Shimazaki H. Effects of aspartate and glutamate on the bipolar cells in the carp retina. Vis Res. 1975;15:456–458.
140 Slaughter MM, Miller RF. The role of excitatory amino acid transmitters in the mudpuppy retina: an analysis with kainic acid and N-methyl aspartate. J Neurosci. 1983;3:1701–1711.
141 DeVries SH. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron. 2000;28:847–856.
142 Nomura A, Shigemoto R, Nakamura Y, et al. Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell. 1994;77:361–369.
143 Dhingra A, Lyubarsky A, Jiang M, et al. The light response of ON bipolar neurons requires Gαo. J Neurosci. 2000;20:9053–9058.
144 Shen Y, Heimel JA, Kamermans M, et al. A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. J Neurosci. 2009;29:6088–6093.
145 Morgans CW, Zhang J, Jeffrey BG, et al. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc Natl Acad Sci U S A. 2009;106:19174–19178.
146 Koike C, Numata T, Ueda H, et al. TRPM1: a vertebrate TRP channel responsible for retinal ON bipolar function. Cell Calcium. 2010;48:95–101.
147 Nawy S. The metabotropic receptor mGluR6 may signal through G(o), but not phosphodiesterase, in retinal bipolar cells. J Neurosci. 1999;19:2938–2944.
148 Dhingra A, Faurobert E, Dascal N, et al. A retinal-specific regulator of G-protein signaling interacts with Galpha(o) and accelerates an expressed metabotropic glutamate receptor 6 cascade. J Neurosci. 2004;24:5684–5693.
149 Awatramani GB, Slaughter MM. Intensity-dependent, rapid activation of presynaptic metabotropic glutamate receptors at a central synapse. J Neurosci. 2001;21:741–749.
150 Dacey DM. The mosaic of midget ganglion cells in the human retina. J Neurosci. 1993;13:5334–5355.
151 Mariani AP. Bipolar cells in monkey retina selective for the cones likely to be blue-sensitive. Nature. 1984;308:184–186.
152 Kouyama N, Marshak DW. Bipolar cells specific for blue cones in the macaque retina. J Neurosci. 1992;12:1233–1252.
153 Breuninger T, Puller C, Haverkamp S, et al. Chromatic bipolar cell pathways in the mouse retina. J Neurosci. 2011;31:6504–6517.
154 Bloomfield SA, Dacheux RF. Rod vision: pathways and processing in the mammalian retina. Prog Retin Eye Res. 2001;20:351–384.
155 Field GD, Rieke F. Nonlinear signal transfer from mouse rods to bipolar cells and implications for visual sensitivity. Neuron. 2002;34:773–785.
156 Sampath AP, Rieke F. Selective transmission of single photon responses by saturation at the rod-to-rod bipolar synapse. Neuron. 2004;41:431–443.
157 Ghosh KK, Haverkamp S, Wassle H. Glutamate receptors in the rod pathway of the mammalian retina. J Neurosci. 2001;21:8636–8647.
158 Li W, Trexler EB, Massey SC. Glutamate receptors at rod bipolar ribbon synapses in the rabbit retina. J Comp Neurol. 2002;448:230–248.
159 Chavez AE, Singer JH, Diamond JS. Fast neurotransmitter release triggered by Ca influx through AMPA-type glutamate receptors. Nature. 2006;443:705–708.
160 Eggers ED, McCall MA, Lukasiewicz PD. Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina. J Physiol. 2007;582:569–582.
161 Eggers ED, Lukasiewicz PD. Interneuron circuits tune inhibition in retinal bipolar cells. J Neurophysiol. 2010;103:25–37.
162 Chavez AE, Grimes WN, Diamond JS. Mechanisms underlying lateral GABAergic feedback onto rod bipolar cells in rat retina. J Neurosci. 2010;30:2330–2339.
163 Ivanova E, Müller U, Wassle H. Characterization of the glycinergic input to bipolar cells of the mouse retina. Eur J Neurosci. 2006;23:350–364.
164 Weiss J, O’Sullivan GA, Heinze L, et al. Glycinergic input of small-field amacrine cells in the retinas of wildtype and glycine receptor deficient mice. Mol Cell Neurosci. 2008;37:40–55.
165 Strettoi E, Dacheux RF, Raviola E. Cone bipolar cells as interneurons in the rod pathway of the rabbit retina. J Comp Neurol. 1994;347:139–149.
166 Mills SL, Massey SC. Differential properties of two gap junctional pathways made by AII amacrine cells. Nature. 1995;377:734–737.
167 Vaney DI, Nelson JC, Pow DV. Neurotransmitter coupling through gap junctions in the retina. J Neurosci. 1998;18:10594–10602.
168 Deans MR, Volgyi B, Goodenough DA, et al. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron. 2002;36:703–712.
169 Hampson EC, Vaney DI, Weiler R. Dopaminergic modulation of gap junction permeability between amacrine cells in mammalian retina. J Neurosci. 1992;12:4911–4922.
170 Kothmann WW, Massey SC, O’Brien J. Dopamine-stimulated dephosphorylation of connexin 36 mediates AII amacrine cell uncoupling. J Neurosci. 2009;29:14903–14911.
171 Trexler EB, Li W, Mills SL, et al. Coupling from AII amacrine cells to ON cone bipolar cells is bidirectional. J Comp Neurol. 2001;437:408–422.
172 Veruki ML, Hartveit E. Electrical synapses mediate signal transmission in the rod pathway of the mammalian retina. J Neurosci. 2002;22:10558–10566.
173 Mills SL, O’Brien JJ, Li W, et al. Rod pathways in the mammalian retina use connexin 36. J Comp Neurol. 2001;436:336–350.
174 Maxeiner S, Dedek K, Janssen-Bienhold U, et al. Deletion of connexin45 in mouse retinal neurons disrupts the rod/cone signaling pathway between AII amacrine and ON cone bipolar cells and leads to impaired visual transmission. J Neurosci. 2005;25:566–576.
175 DeVries SH, Baylor DA. An alternative pathway for signal flow from rod photoreceptors to ganglion cells in mammalian retina. Proc Natl Acad Sci U S A. 1995;92:10658–10662.
176 Soucy E, Wang Y, Nirenberg S, et al. A novel signaling pathway from rod photoreceptors to ganglion cells in mammalian retina. Neuron. 1998;21:481–493.
177 Hack I, Frech M, Dick O, et al. Heterogeneous distribution of AMPA glutamate receptor subunits at the photoreceptor synapses of rodent retina. Eur J Neurosci. 2001;13:15–24.
178 Tsukamoto Y, Morigiwa K, Ueda M, et al. Microcircuits for night vision in mouse retina. J Neurosci. 2001;21:8616–8623.
179 Fyk-Kolodziej B, Qin P, Pourcho RG. Identification of a cone bipolar cell in cat retina which has input from both rod and cone photoreceptors. J Comp Neurol. 2003;464:104–113.
180 Li W, Chen S, DeVries SH. A fast rod photoreceptor signaling pathway in the mammalian retina. Nat Neurosci. 2010;13:414–416.
181 Lin B, Masland RH. Populations of wide-field amacrine cells in the mouse retina. J Comp Neurol. 2006;499:797–809.
182 Perez De Sevilla Müller L, Shelley J, Weiler R. Displaced amacrine cells of the mouse retina. J Comp Neurol. 2007;505:177–189.
183 Zhang C, Mccall MA. Receptor targets of amacrine cells. Vis Neurosci. 2012. in press
184 Hsueh HA, Molnar A, Werblin FS. Amacrine-to-amacrine cell inhibition in the rabbit retina. J Neurophysiol. 2008;100:2077–2088.
185 Badea TC, Nathans J. Quantitative analysis of neuronal morphologies in the mouse retina visualized by using a genetically directed reporter. J Comp Neurol. 2004;480:331–351.
186 Dedek K, Breuninger T, de Sevilla Müller LP, et al. A novel type of interplexiform amacrine cell in the mouse retina. Eur J Neurosci. 2009;30:217–228.
187 Majumdar S, Weiss J, Wassle H. Glycinergic input of widefield, displaced amacrine cells of the mouse retina. J Physiol. 2009;587:3831–3849.
188 Grimes WN, Zhang J, Graydon CW, et al. Retinal parallel processors: more than 100 independent microcircuits operate within a single interneuron. Neuron. 2010;65:873–885.
189 Lukasiewicz PD. Synaptic mechanisms that shape visual signaling at the inner retina. Prog Brain Res. 2005;147:205–218.
190 Demb JB. Functional circuitry of visual adaptation in the retina. J Physiol. 2008;586:4377–4384.
191 Li W, Zhang J, Massey SC. Coupling pattern of S1 and S2 amacrine cells in the rabbit retina. Vis Neurosci. 2002;19:119–131.
192 Xin D, Bloomfield SA. Tracer coupling pattern of amacrine and ganglion cells in the rabbit retina. J Comp Neurol. 1997;383:512–528.
193 Marc RE, Jones BW. Molecular phenotyping of retinal ganglion cells. J Neurosci. 2002;22:413–427.
194 Hu EH, Pan F, Volgyi B, et al. Light increases the gap junctional coupling of retinal ganglion cells. J Physiol. 2010;588:4145–4163.
195 Berry MJ, 2nd., Brivanlou IH, Jordan TA, et al. Anticipation of moving stimuli by the retina. Nature. 1999;398:334–338.
196 Olveczky BP, Baccus SA, Meister M. Segregation of object and background motion in the retina. Nature. 2003;423:401–408.
197 Roska B, Werblin F. Rapid global shifts in natural scenes block spiking in specific ganglion cell types. Nat Neurosci. 2003;6:600–608.
198 Knop GC, Feigenspan A, Weiler R, et al. Inputs underlying the ON-OFF light responses of type 2 wide-field amacrine cells in TH::GFP mice. J Neurosci. 2011;31:4780–4791.
199 Famiglietti EV, Jr., Kolb H. A bistratified amacrine cell and synaptic circuitry in the inner plexiform layer of the retina. Brain Res. 1975;84:293–300.
200 Massey SC, Mills SL. Antibody to calretinin stains AII amacrine cells in the rabbit retina: double-label and confocal analyses. J Comp Neurol. 1999;411:3–18.
201 Smith RG, Vardi N. Simulation of the AII amacrine cell of mammalian retina: functional consequences of electrical coupling and regenerative membrane properties. Vis Neurosci. 1995;12:851–860.
202 Vardi N, Smith RG. The AII amacrine network: coupling can increase correlated activity. Vis Res. 1996;36:3743–3757.
203 Bloomfield SA, Volgyi B. Function and plasticity of homologous coupling between AII amacrine cells. Vis Res. 2004;44:3297–3306.
204 Manookin MB, Beaudoin DL, Ernst ZR, et al. Disinhibition combines with excitation to extend the operating range of the OFF visual pathway in daylight. J Neurosci. 2008;28:4136–4150.
205 Munch TA, da Silveira RA, Siegert S, et al. Approach sensitivity in the retina processed by a multifunctional neural circuit. Nat Neurosci. 2009;12:1308–1316.
206 Oesch NW, Kothmann WW, Diamond JS. Illuminating synapses and circuitry in the retina. Curr Opin Neurobiol. 2011;21:238–244.
207 Werblin FS. Six different roles for crossover inhibition in the retina: correcting the nonlinearities of synaptic transmission. Vis Neurosci. 2010;27:1–8.
208 Vaney DI. Morphological identification of serotonin-accumulating neurons in the living retina. Science. 1986;233:444–446.
209 Zhang J, Li W, Trexler EB, et al. Confocal analysis of reciprocal feedback at rod bipolar terminals in the rabbit retina. J Neurosci. 2002;22:10871–10882.
210 Fletcher EL, Wassle H. Indoleamine-accumulating amacrine cells are presynaptic to rod bipolar cells through GABA(C) receptors. J Comp Neurol. 1999;413:155–167.
211 Eggers ED, Lukasiewicz PD. Receptor and transmitter release properties set the time course of retinal inhibition. J Neurosci. 2006;26:9413–9425.
212 Eggers ED, Lukasiewicz PD. GABA(A), GABA(C) and glycine receptor-mediated inhibition differentially affects light-evoked signalling from mouse retinal rod bipolar cells. J Physiol. 2006;572:215–225.
213 Lukasiewicz PD, Eggers ED, Sagdullaev BT, et al. GABAC receptor-mediated inhibition in the retina. Vis Res. 2004;44:3289–3296.
214 Grimes WN, Li W, Chavez AE, et al. BK channels modulate pre- and postsynaptic signaling at reciprocal synapses in retina. Nat Neurosci. 2009;12:585–592.
215 Freed MA, Smith RG, Sterling P. Timing of quantal release from the retinal bipolar terminal is regulated by a feedback circuit. Neuron. 2003;38:89–101.
216 Sagdullaev BT, McCall MA, Lukasiewicz PD. Presynaptic inhibition modulates spillover, creating distinct dynamic response ranges of sensory output. Neuron. 2006;50:923–935.
217 Dacey DM. The dopaminergic amacrine cell. J Comp Neurol. 1990;301:461–489.
218 Hokoc JN, Mariani AP. Synapses from bipolar cells onto dopaminergic amacrine cells in cat and rabbit retinas. Brain Res. 1988;461:17–26.
219 Contini M, Lin B, Kobayashi K, et al. Synaptic input of ON-bipolar cells onto the dopaminergic neurons of the mouse retina. J Comp Neurol. 2010;518:2035–2050.
220 Contini M, Raviola E. GABAergic synapses made by a retinal dopaminergic neuron. Proc Natl Acad Sci U S A. 2003;100:1358–1363.
221 Feigenspan A, Gustincich S, Bean BP, et al. Spontaneous activity of solitary dopaminergic cells of the retina. J Neurosci. 1998;18:6776–6789.
222 Puopolo M, Hochstetler SE, Gustincich S, et al. Extrasynaptic release of dopamine in a retinal neuron: activity dependence and transmitter modulation. Neuron. 2001;30:211–225.
223 Witkovsky P, Veisenberger E, LeSauter J, et al. Cellular location and circadian rhythm of expression of the biological clock gene Period 1 in the mouse retina. J Neurosci. 2003;23:7670–7676.
224 Gustincich S, Contini M, Gariboldi M, et al. Gene discovery in genetically labeled single dopaminergic neurons of the retina. Proc Natl Acad Sci U S A. 2004;101:5069–5074.
225 Dorenbos R, Contini M, Hirasawa H, et al. Expression of circadian clock genes in retinal dopaminergic cells. Vis Neurosci. 2007;24:573–580.
226 Pozdeyev N, Tosini G, Li L, et al. Dopamine modulates diurnal and circadian rhythms of protein phosphorylation in photoreceptor cells of mouse retina. Eur J Neurosci. 2008;27:2691–2700.
227 Witkovsky P. Dopamine and retinal function. Adv Ophthalmol. 2004;108:17–40.
228 Ribelayga C, Mangel SC. Identification of a circadian clock-controlled neural pathway in the rabbit retina. PloS ONE. 2010;5:e11020.
229 Herrmann R, Heflin SJ, Hammond T, et al. Rod vision is controlled by dopamine-dependent sensitization of rod bipolar cells by GABA. Neuron. 2011;72:101–110.
230 Vaney DI, Pow DV. The dendritic architecture of the cholinergic plexus in the rabbit retina: selective labeling by glycine accumulation in the presence of sarcosine. J Comp Neurol. 2000;421:1–13.
231 Dacheux RF, Chimento MF, Amthor FR. Synaptic input to the on-off directionally selective ganglion cell in the rabbit retina. J Comp Neurol. 2003;456:267–278.
232 Brecha N, Johnson D, Peichl L, et al. Cholinergic amacrine cells of the rabbit retina contain glutamate decarboxylase and gamma-aminobutyrate immunoreactivity. Proc Natl Acad Sci U S A. 1988;85:6187–6191.
233 Lee S, Kim K, Zhou ZJ. Role of ACh-GABA cotransmission in detecting image motion and motion direction. Neuron. 2010;68:1159–1172.
234 Yoshida K, Watanabe D, Ishikane H, et al. A key role of starburst amacrine cells in originating retinal directional selectivity and optokinetic eye movement. Neuron. 2001;30:771–780.
235 Kittila CA, Massey SC. Pharmacology of directionally selective ganglion cells in the rabbit retina. J Neurophysiol. 1997;77:675–689.
236 Fried SI, Munch TA, Werblin FS. Mechanisms and circuitry underlying directional selectivity in the retina. Nature. 2002;420:411–414.
237 Borg-Graham LJ. The computation of directional selectivity in the retina occurs presynaptic to the ganglion cell. Nat Neurosci. 2001;4:176–183.
238 Hausselt SE, Euler T, Detwiler PB, et al. A dendrite-autonomous mechanism for direction selectivity in retinal starburst amacrine cells. PLoS Biol. 2007;5:e185.
239 Gavrikov KE, Nilson JE, Dmitriev AV, et al. Dendritic compartmentalization of chloride cotransporters underlies directional responses of starburst amacrine cells in retina. Proc Natl Acad Sci U S A. 2006;103:18793–18798.
240 Briggman KL, Helmstaedter M, Denk W. Wiring specificity in the direction-selectivity circuit of the retina. Nature. 2011;471:183–188.
241 Oesch N, Euler T, Taylor WR. Direction-selective dendritic action potentials in rabbit retina. Neuron. 2005;47:739–750.
242 Wei W, Feller MB. Organization and development of direction-selective circuits in the retina. Trends Neurosci. 2011;34:638–645.
243 Elstrott J, Anishchenko A, Greschner M, et al. Direction selectivity in the retina is established independent of visual experience and cholinergic retinal waves. Neuron. 2008;58:499–506.
244 Feller MB. The role of nAChR-mediated spontaneous retinal activity in visual system development. J Neurobiol. 2002;53:556–567.
245 Syed MM, Lee S, Zheng J, et al. Stage-dependent dynamics and modulation of spontaneous waves in the developing rabbit retina. J Physiol. 2004;560:533–549.
246 Syed MM, Lee S, He S, et al. Spontaneous waves in the ventricular zone of developing mammalian retina. J Neurophysiol. 2004;91:1999–2009.
247 Renna JM, Weng S, Berson DM. Light acts through melanopsin to alter retinal waves and segregation of retinogeniculate afferents. Nat Neurosci. 2011;14:827–829.
248 Lukasiewicz PD, Wilson JA, Lawrence JE. AMPA-preferring receptors mediate excitatory synaptic inputs to retinal ganglion cells. J Neurophysiol. 1997;77:57–64.
249 Vaney DI. A quantitative comparison between the ganglion cell populations and axonal outflows of the visual streak and periphery of the rabbit retina. J Comp Neurol. 1980;189:215–233.
250 Williams RW, Strom RC, Rice DS, et al. Genetic and environmental control of variation in retinal ganglion cell number in mice. J Neurosci. 1996;16:7193–7205.
251 Levick WR. Receptive fields of rabbit retinal ganglion cells. Am J Optom. 1965;42:337–343.
252 Caldwell JH, Daw NW. New properties of rabbit retinal ganglion cells. J Physiol. 1978;276:257–276.
253 DeVries SH, Baylor DA. Mosaic arrangement of ganglion cell receptive fields in rabbit retina. J Neurophysiol. 1997;78:2048–2060.
254 DeVries SH. Correlated firing in rabbit retinal ganglion cells. J Neurophysiol. 1999;81:908–920.
255 Hu EH, Bloomfield SA. Gap junctional coupling underlies the short-latency spike synchrony of retinal alpha ganglion cells. J Neurosci. 2003;23:6768–6777.
256 Rockhill RL, Daly FJ, MacNeil MA, et al. The diversity of ganglion cells in a mammalian retina. J Neurosci. 2002;22:3831–3843.
257 Dacey DM. Physiology, morphology and spatial densities of identified ganglion cell types in primate retina. Ciba Found Symposium. 1994;184:12–28. discussion -34, 63–70
258 Peterson BB, Dacey DM. Morphology of human retinal ganglion cells with intraretinal axon collaterals. Vis Neurosci. 1998;15:377–387.
259 Dacey DM. Primate retina: cell types, circuits and color opponency. Prog Retin Eye Res. 1999;18:737–763.
260 Yamada ES, Bordt AS, Marshak DW. Wide-field ganglion cells in macaque retinas. Vis Neurosci. 2005;22:383–393.
261 Sun W, Li N, He S. Large-scale morophological survey of rat retinal ganglion cells. Vis Neurosci. 2002;19:483–493.
262 Sun W, Li N, He S. Large-scale morphological survey of mouse retinal ganglion cells. J Comp Neurol. 2002;451:115–126.
263 Huberman AD, Manu M, Koch SM, et al. Architecture and activity-mediated refinement of axonal projections from a mosaic of genetically identified retinal ganglion cells. Neuron. 2008;59:425–438.
264 Huberman AD, Wei W, Elstrott J, et al. Genetic identification of an ON-OFF direction-selective retinal ganglion cell subtype reveals a layer-specific subcortical map of posterior motion. Neuron. 2009;62:327–334.
265 Kim IJ, Zhang Y, Yamagata M, et al. Molecular identification of a retinal cell type that responds to upward motion. Nature. 2008;452:478–482.
266 Kim IJ, Zhang Y, Meister M, et al. Laminar restriction of retinal ganglion cell dendrites and axons: subtype-specific developmental patterns revealed with transgenic markers. J Neurosci. 2010;30:1452–1462.
267 Yonehara K, Ishikane H, Sakuta H, et al. Identification of retinal ganglion cells and their projections involved in central transmission of information about upward and downward image motion. PloS ONE. 2009;4:e4320.
268 Rivlin-Etzion M, Zhou K, Wei W, et al. Transgenic mice reveal unexpected diversity of ON-OFF direction-selective retinal ganglion cell subtypes and brain structures involved in motion processing. J Neurosci. 2011;31:8760–8769.
269 Volgyi B, Chheda S, Bloomfield SA. Tracer coupling patterns of the ganglion cell subtypes in the mouse retina. J Comp Neurol. 2009;512:664–687.
270 Roska B, Werblin F. Vertical interactions across ten parallel, stacked representations in the mammalian retina. Nature. 2001;410:583–587.
271 Roska B, Molnar A, Werblin FS. Parallel processing in retinal ganglion cells: how integration of space-time patterns of excitation and inhibition form the spiking output. J Neurophysiol. 2006;95:3810–3822.
272 van Wyk M, Wassle H, Taylor WR. Receptive field properties of ON- and OFF-ganglion cells in the mouse retina. Vis Neurosci. 2009;26:297–308.
273 Farrow K, Masland RH. Physiological clustering of visual channels in the mouse retina. J Neurophysiol. 2011;105:1516–1530.
274 Dacey DM. Morphology of a small-field bistratified ganglion cell type in the macaque and human retina. Vis Neurosci. 1993;10:1081–1098.
275 Peichl L. Alpha ganglion cells in mammalian retinae: common properties, species differences, and some comments on other ganglion cells. Vis Neurosci. 1991;7:155–169.
276 Barlow HB, Levick WR. The mechanism of directionally selective units in rabbit’s retina. J Physiol. 1965;178:477–504.
277 Amthor FR, Oyster CW, Takahashi ES. Morphology of ON-OFF direction-selective ganglion cells in the rabbit retina. Brain Res. 1984;298:187–190.
278 Wei W, Elstrott J, Feller MB. Two-photon targeted recording of GFP-expressing neurons for light responses and live-cell imaging in the mouse retina. Nature protocols. 2010;5:1347–1352.
279 Vaney DI. Territorial organization of direction-selective ganglion cells in rabbit retina. J Neurosci. 1994;14:6301–6316.
280 Kay JN, De la Huerta I, Kim IJ, et al. Retinal ganglion cells with distinct directional preferences differ in molecular identity, structure, and central projections. J Neurosci. 2011;31:7753–7762.
281 Oyster CW, Barlow HB. Direction-selective units in rabbit retina: distribution of preferred directions. Science. 1967;155:841–842.
282 Isayama T, Berson DM, Pu M. Theta ganglion cell type of cat retina. J Comp Neurol. 2000;417:32–48.
283 Yonehara K, Balint K, Noda M, et al. Spatially asymmetric reorganization of inhibition establishes a motion-sensitive circuit. Nature. 2011;469:407–410.
284 Oyster CW, Simpson JI, Takahashi ES, et al. Retinal ganglion cells projecting to the rabbit accessory optic system. J Comp Neurol. 1980;190:49–61.
285 Buhl EH, Peichl L. Morphology of rabbit retinal ganglion cells projecting to the medial terminal nucleus of the accessory optic system. J Comp Neurol. 1986;253:163–174.
286 Hoshi H, Tian LM, Massey SC, et al. Two distinct types of ON directionally selective ganglion cells in the rabbit retina. J Comp Neurol. 2011;519:2509–2521.
287 Kanjhan R, Sivyer B. Two types of ON direction-selective ganglion cells in rabbit retina. Neurosci Lett. 2010;483:105–109.
288 Ackert JM, Farajian R, Volgyi B, et al. GABA blockade unmasks an OFF response in ON direction selective ganglion cells in the mammalian retina. J Physiol. 2009;587:4481–4495.
289 Hoshi H, Mills SL. Components and properties of the G3 ganglion cell circuit in the rabbit retina. J Comp Neurol. 2009;513:69–82.
290 Venkataramani S, Taylor WR. Orientation selectivity in rabbit retinal ganglion cells is mediated by presynaptic inhibition. J Neurosci. 2010;30:15664–15676.
291 Trenholm S, Johnson K, Li X, et al. Parallel mechanisms encode direction in the retina. Neuron. 2011;71:683–694.
292 Klerman EB, Shanahan TL, Brotman DJ, et al. Photic resetting of the human circadian pacemaker in the absence of conscious vision. J Biol Rhythms. 2002;17:548–555.
293 Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073.
294 Berson DM. Strange vision: ganglion cells as circadian photoreceptors. Trends Neurosci. 2003;26:314–320.
295 Lucas RJ, Hattar S, Takao M, et al. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–247.
296 Grunert U, Jusuf PR, Lee SC, et al. Bipolar input to melanopsin containing ganglion cells in primate retina. Vis Neurosci. 2011;28:39–50.
297 Hattar S, Lucas RJ, Mrosovsky N, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81.
298 Schmidt TM, Chen SK, Hattar S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 2011;34:572–580.
299 Schmidt TM, Do MT, Dacey D, et al. Melanopsin-positive intrinsically photosensitive retinal ganglion cells: from form to function. J Neurosci. 2011;31:16094–16101.
300 Berson DM, Castrucci AM, Provencio I. Morphology and mosaics of melanopsin-expressing retinal ganglion cell types in mice. J Comp Neurol. 2010;518:2405–2422.
301 Chen SK, Badea TC, Hattar S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature. 2011;476:92–95.
302 Lennie P. Color vision: putting it together. Curr Biol. 2000;10:R589–R591.
303 Dacey DM. Circuitry for color coding in the primate retina. Proc Natl Acad Sci U S A. 1996;93:582–588.
304 Fahrenfort I, Steijaert M, Sjoerdsma T, et al. Hemichannel-mediated and pH-based feedback from horizontal cells to cones in the vertebrate retina. PloS ONE. 2009;4:e6090.
305 Field GD, Gauthier JL, Sher A, et al. Functional connectivity in the retina at the resolution of photoreceptors. Nature. 2010;467:673–677.
306 Mariani AP. The neuronal organization of the outer plexiform layer of the primate retina. Int Rev Cytol. 1984;86:285–320.
307 Haverkamp S, Wassle H, Duebel J, et al. The primordial, blue-cone color system of the mouse retina. J Neurosci. 2005;25:5438–5445.
308 Dacey DM, Lee BB. The ‘blue-on’ opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature. 1994;367:731–735.
309 Field GD, Sher A, Gauthier JL, et al. Spatial properties and functional organization of small bistratified ganglion cells in primate retina. J Neurosci. 2007;27:13261–13272.
310 Field GD, Greschner M, Gauthier JL, et al. High-sensitivity rod photoreceptor input to the blue-yellow color opponent pathway in macaque retina. Nat Neurosci. 2009;12:1159–1164.
311 Crook JD, Davenport CM, Peterson BB, et al. Parallel ON and OFF cone bipolar inputs establish spatially coextensive receptive field structure of blue-yellow ganglion cells in primate retina. J Neurosci. 2009;29:8372–8387.
312 Packer OS, Verweij J, Li PH, et al. Blue-yellow opponency in primate S cone photoreceptors. J Neurosci. 2010;30:568–572.
313 Yin L, Smith RG, Sterling P, et al. Physiology and morphology of color-opponent ganglion cells in a retina expressing a dual gradient of S and M opsins. J Neurosci. 2009;29:2706–2724.
314 Jacobs GH, Williams GA, Cahill H, et al. Emergence of novel color vision in mice engineered to express a human cone photopigment. Science. 2007;315:1723–1725.
315 Mancuso K, Hauswirth WW, Li Q, et al. Gene therapy for red-green colour blindness in adult primates. Nature. 2009;461:784–787.
316 Osterhout JA, Josten N, Yamada J, et al. Cadherin-6 mediates axon-target matching in a non-image-forming visual circuit. Neuron. 2011;71:632–639.
317 Siegert S, Cabuy E, Gross Scherf B, et al. Transciptional code and disease map for adult retinal cell types. Nat Neurosci. 2012;15:487–495.
318 Harwerth RS, Carter-Dawson L, Shen F, et al. Ganglion cell losses underlying visual field defects from experimental glaucoma. Invest Ophthalmol Vis Sci. 1999;40:2242–2250.
319 Miyake Y, Yagasaki K, Horiguchi M, et al. Congenital stationary night blindness with negative electroretinogram. A new classification. Arch Ophthalmol. 1986;104:1013–1020.
320 Chang B, Heckenlively JR, Bayley PR, et al. The nob2 mouse, a null mutation in Cacna1f: anatomical and functional abnormalities in the outer retina and their consequences on ganglion cell visual responses. Vis Neurosci. 2006;23:11–24.
321 Ishii M, Morigiwa K, Takao M, et al. Ectopic synaptic ribbons in dendrites of mouse retinal ON- and OFF-bipolar cells. Cell Tissue Res. 2009;338:355–375.