Screening for Colorectal Cancer
Colorectal cancer (CRC) is a leading cause of death from cancer in the United States. In 2010 there are 143,000 new cases of CRC expected with more than 51,000 people dying from the disease. More than a million individuals worldwide are diagnosed with CRC every year and half a million die of CRC in the same time period [1].
CRC is a preventable disease and effective CRC screening may reduce CRC incidence, mortality, and the cost associated with CRC treatment. The goal of screening is to select those patients who need a colonoscopy for the resection of significant polyps at risk for following the adenoma to carcinoma sequence while avoiding unnecessary colonoscopy in patients with normal findings. With the current financial constraints on health care, cost-effectiveness is an essential component of any screening modality.
Screening rates for colorectal cancer have steadily increased in the past 10 years. This increase is accompanied with decreasing trends in distal CRC rates since 1985. Currently, 55% of insured individuals in the United States aged older than 50 years are screened. The remaining 45% of eligible individuals are not screened by any method. Twenty-two million people aged 50 to 75 years are not screened for CRC. This compliance rate is even worse for those patients without insurance coverage, where only 24% of eligible patients are screened for CRC [2]. The focus of this review is to discuss the current techniques available for CRC screening and their relative effectiveness at early detection and prevention.
Endoscopy
Endoscopy has both screening and therapeutic value. It enables inspection and detection of precancerous and cancerous lesions, with immediate resection of reasonably sized lesions by polypectomy. The two most important outcomes when examining a screening procedure for CRC are mortality reduction by early detection and cancer prevention by identification and removal of advanced adenomas. Sigmoidoscopy and colonoscopy are two widely used screening examinations for CRC. Choice of screening procedure is patient and physician dependent. A successful endoscopy requires an excellent bowel preparation, identification, and possible removal of adenomas with minimal morbidity. Colonoscopy has a higher perforation rate, greater need for sedation, requires increased time and commitment by patients, and has an overall increased cost when compared to sigmoidoscopy. Which screening endoscopic procedure patients undergo depends on their access to specialists, their insurance coverage, potential difficulties in performing the test, and their experience with the test [3].
Numerous studies underscore the cost-effectiveness of every colorectal screening test (at an estimated cost of less than $20,000 per year of life saved), including colonoscopy. Though colonoscopy is the most expensive test, it may ultimately cost less than other alternatives because it prevents more cancers and does not need to be performed frequently [4,5]. Rabeneck and colleagues [6] examined a database of 2,412,077 persons aged 50 to 90 years who underwent colonoscopy. They showed that for every 1% increase in utilization of colonoscopy, there was a 3% reduction in colon cancer death.
Even though colonoscopy and sigmoidoscopy are equally acceptable options in guidelines from the US Preventive Services Task Force (USPSTF) and the US Multisociety Task Force, colonoscopy was granted preferred status in guidelines published by the American College of Gastroenterology [7]. Colonoscopy has achieved a predominant role in colon cancer screening since Medicare initiated reimbursement in 2001. Although colonoscopy is considered the gold standard for colon cancer screening by most physicians, its superiority has been challenged by several recent publications. In a case-control study from Canada, colonoscopy was associated with a reduction in colorectal cancer mortality (odds ratio [OR], 0.63; 95% confidence interval [CI], 0.57–0.69), but this reduction was limited to left-sided cancers (OR, 0.33; 95% CI, 0.28–0.39) with no reduction in right-sided cancers (OR, 0.99; 95% CI, 0.86–1.14) [8]. In two other observational studies, the association between colonoscopy and reduced colorectal cancer risk was limited to the distal colon [9,10]. These two studies suggest that the colon cancer prevention benefit for colonoscopy is primarily for the left colon and not the right. Both of these case-control studies and other randomized trials establish that sigmoidoscopy was also associated with a reduced incidence and mortality from distal CRC [11–14]. Based on these recent data it appears colonoscopy does not provide added screening benefit over sigmoidoscopy. Sigmoidoscopy seems to be as accurate as colonoscopy in detecting distal CRC for average-risk individuals and is more cost-effective [15].
Distal CRC rates in the United States have been steadily decreasing since 1985, whereas rates for proximal colon cancers have remained largely unchanged [6,8,16]. Whether this trend is caused by increased incidence of right colon cancer or missed diagnosis of right colon cancer is still under debate [17]. There are several potential explanations for the high miss rate of right-sided lesions in patients undergoing colonoscopy. It could be the biology of cancers in the right side of the colon, especially tumors characterized by inactivation of a mismatch repair gene, which makes right-sided cancers grow more rapidly than left-sided ones. This biological difference, and hard-to-detect flat lesions, may explain the difference in cancer reduction observed between the right and left side of the colon in patients undergoing programmatic colonoscopy screening every 10 years [18].
The technical challenge and quality of colonoscopy may also account for missed right-sided lesions. Studies have shown that gastroenterologists do much better as far as colon cancer prevention than primary care physicians. Having the colonoscopy done by a low-volume endoscopist was independently associated with colonoscopy-related bleeding and perforation [19]. When colonoscopy is performed by a properly trained endoscopist, the risk of serious adverse events is 3 to 5 events per 1000 colonoscopies. These serious complications include perforation, bleeding, diverticulitis, and postpolypectomy syndrome. In a retrospective cohort study from Kaiser Permanente, 82 serious complications occurred (5.0 per 1000 colonoscopies [95% CI, 4.0–6.2 per 1000 colonoscopies]) in 16,318 patients aged 40 years older undergoing colonoscopy between January 1994 and July 2002 [20]. In another retrospective cohort study, using the California Medicaid program claims database, the investigators showed a total of 228 perforations after 277,434 colonoscopies, which corresponded to a cumulative 7-day incidence of 0.082%. On multivariate analysis, when comparing the group that had a perforation after a colonoscopy (n = 216) with those who did not (n = 269,496), increasing age, significant patient comorbidity, obstruction as an indication for the colonoscopy, and performance of invasive interventions during colonoscopy were significant positive predictors of complications [21]. Similar results were demonstrated using the Surveillance, Epidemiology, and End Results database [22]. In fact, the chance of death from colon cancer was 10 to 12 times higher in the patients who underwent examinations by those endoscopists who had low adenoma detection rates (defined as less than 20% detection of adenomas in screening colonoscopies), in contrast to endoscopists who were achieving an adenoma detection rate of more than 20% [23]. These data demonstrate that quality is important. Evidence suggests that the number of qualified endoscopists may be inadequate to provide colonoscopy (or even sigmoidoscopy) to all eligible US citizens [24]. In such a situation, unqualified examiners could absorb the colonoscopy overflow. This potential increased inaccuracy and higher complication rate may negate the small incremental benefit that colonoscopies might offer over other tests [25].
To improve the technical limitation of current endoscopy, several strategies, including high-definition magnification, imaging enhancers, narrow band imaging, and chromoendoscopy, are being investigated. Limited data are available on these techniques as adjunct screening tools for the general population.
Alternative to colonoscopy
Blood markers
Blood sampling may be more convenient and acceptable for patients. In addition to the obvious displeasure patients have with obtaining stool samples, blood samples provide the added benefit of not having bacteria present, which degrade the potential biomarkers or hamper analysis. The sample processing for blood is also easier than for stool. Biomarkers in blood include carbohydrate antigens, proteins, cytologic markers, DNA, and mRNA markers.
Carbohydrate antigens, including CA 19-9, CA 195, CA M26, CA M29, CA50, CA72-4, CA M43, and CA 242, have been investigated for CRC screening [26–31]. Most have specificity greater than 90% and low sensitivity rates, ranging from 18% to 65%.
The protein marker carcinoembryonic antigen (CEA) was the first blood marker proposed in connection with CRC [32]. In most studies, an elevated CEA level has specificity greater than 90%, with its sensitivity increasing with increasing tumor stage. It therefore varies between 43% and 69% [33,34]. The sensitivity and specificity of insulinlike growth factor-binding protein-2 for diagnosing CRC were 80.2% and 64.0%, respectively [35]. Promising results were also observed for cancer procoagulant [36], serum CD26 [37], fibrin degradation products [38,39], and prolactin [40]. Other protein markers, such as Sialylated Lewis antigen and CO 29.11, showed sensitivity less than 50% [41]. Vascular endothelial growth factor [42,43], insulinlike growth factor II [44], and interleukins-3 [45] also have low sensitivity in detecting CRC. Circulating autoantibodies have also been studied for the detection of CRC. Although their specificity was close to 100%, the sensitivity of these autoantibodies was less than 30% [46–48].
Evidence is suggesting that primary CRC may shed neoplastic cells in the circulation at an early stage. In a study from Taiwan, a high-sensitivity colorimetric membrane-array method has been devised to detect circulating tumor cells in the peripheral blood of patients with CRC. Eighty-eight subjects with CRC and 50 healthy subjects were compared. The sensitivity and specificity of membrane-arrays for the detection of CRCs were 94.3% (95% CI, 86.4%–102.2%) and 94% (95% CI, 85.9%–102.1%), respectively [49,50]. This promising technique requires further examination in the general population.
Both genetic and epigenetic alterations of genes have been investigated for detection of CRC. No current genomic alteration showed adequate sensitivity [51,52]. However, mRNA in circulating tumor cells may be detected by reverse transcriptase-polymerase chain reaction and are showing some promise. Numerous mRNA molecules coding for CEA [53], human telomerase reverse transcriptase [54], guanylyl cyclase C (GCC), carcinoembryonic gene member 2 [55], melanoma-associated antigen family A [56], tumor-associated antigen L6, and thymidylate synthase [57] have also been analyzed for CRC detection. Only GCC mRNA [58] and L6 mRNA [59] showed sensitivity greater than 80% and specificity greater than 95%.
A significant limitation of studies investigating blood markers as a screening tool for CRC is the small sample size of those studies. Large-scale screening studies examining blood markers are missing from the published literature. Cost and practical issues, such as standardized sample collection or processing and storage, need to be considered before a population-based screening program is implemented [60].
Stool markers
Developing alternative, less expensive, and accurate tests to endoscopy is thought to be necessary to decrease screening costs and improve compliance. Alternative tests, such as fecal occult blood test (FOBT), are available and can target those in the population most likely to harbor advanced neoplasm, therefore, identifying those who would most likely benefit from colonoscopy. FOBT complies well with the World Health Organization’s criteria of a screening tool: “A screening test should be inexpensive, rapid, and simple, and is not intended to be diagnostic; those with positive tests require further evaluation [61].” FOBT is rapid and inexpensive, but lacks sensitivity and specificity.
There are two types of fecal occult-blood tests. The standard guaiac FOBT detects pseudoperoxidase activity of heme or hemoglobin and is not specific for human blood. One-time testing with a standard guaiac test has sensitivity for detecting cancer of only 33% to 50%, whereas a more sensitive guaiac test (Hemoccult SENSA; Beckman Coulter, Brea, CA, USA) has sensitivity for detecting cancer of 50% to 75%. Three separate stool samples per test have superior sensitivity, as compared with one or two samples. One must make sure the patients have stopped taking all supplements containing iron and limit their red meat intake during the time of testing to improve sensitivity. Although these tests are rapid and have improved compliance, they are poor detectors of precancerous lesions.
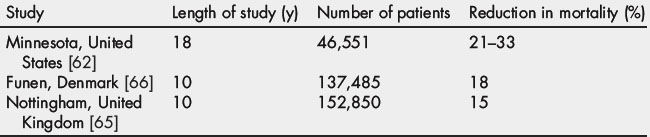
Most CRC screening programs are based on the FOBT or colonoscopy. Although FOBT is poorly accepted and has a low sensitivity, the use of either annual or biennial FOBT significantly reduces the incidence and mortality of CRC [62,63]. FOBT is the only test shown in randomized trials to lower mortality from CRC [64–66] and the mortality benefits of FOBT were similar for right- and left-sided colon lesions (Table 1) [67]. By one estimate, 1173 persons must undergo fecal occult-blood screening to prevent 1 death in 10 years (a 0.09% probability of preventing death for the individual patient) [25].
Immunochemical FOBT (iFOBT) is a newly developed type of FOBT that uses specific antibodies against human blood components to overcome the problem of diet or medication restriction. In a recent randomized trial of the Dutch population, the detection rate of advanced tumors was significantly higher in the iFOBT group (2.4%; OR, 2.0; CI, 1.3–3.1) than the global FOBT arm (1.1%) [68]. Another prospective screening study evaluated the utility of a different iFOB kit (CAREdiagnostica, Voerde, Germany). The sensitivity for the detection of advanced adenomas was 25% (95% CI, 18%–34%); specificity was 97% (CI, 95%–98%); and the positive likelihood ratio was 3.5 (CI, 2.2–5.4) [69].
More specific stool marker studies have been developed that attempt to identify genetic mutational tissue within the stool. The stool DNA test detects mutations in the genes involved in the adenoma-to-carcinoma sequence. The goal of this test is to identify cancers and adenomas with high malignant potential with reasonable sensitivity and specificity, thereby identifying patients who require colonoscopy. They are based on the research findings that specific mutations are associated with colorectal cancer and that cellular DNA is excreted in stool and can be detected with the use of sensitive polymerase-chain-reaction (PCR) methods. Mutant DNA fragments are detectable in the stool of more than 90% of patients with colorectal cancer. DNA purified from stool provides a better template for mutation testing than plasma [70]. However, there are many technical difficulties in detecting abnormal DNA shed from tumor cells into the colon and subsequently excreted in the stool. These difficulties include collection and storage of the stool samples, extraction of the DNA from the stool, removal of PCR inhibitors (food digestion products and bacterial contaminants) from the stool, and the not-always-successful amplification of mutant DNA to a detectable amount.
One of the genes that was identified early on as present in stool was p53. Although identified in stool DNA tests, its role in early CRC detection is still in question. The functional inactivation of p53 occurs later in colorectal cancer development [71], and mutations in p53 are found in only 4% to 26% of adenomas and up to 75% of colon cancers [72,73]. Both of these facts eliminate p53 mutations as a screening tool for a significant number of adenomas and carcinomas.
APC mutations can also be detected in fecal DNA from patients with early colorectal tumors [74]. Although single gene mutation can be detected in stool, it has limited sensitivity for molecular screening for CRC. Multi-target assay panels, with the capability to identify many point mutations, have been successfully studied on freezer-archived stools [75]. The Stool DNA test 1 (SDT1) is a precommercial fecal DNA assay that consists of 21 tumor-specific point mutations: 3 in the K-ras gene, 10 in the APC gene, and 8 in the p53 gene, the microsatellite-instability marker BAT-26, and a marker of long DNA thought to reflect disordered apoptosis of cancer cells sloughed into the colonic lumen. In a study using SDT1 in asymptomatic patients undergoing colonoscopy, 51% of patients with cancer and 18% of patients with advanced cancer-precursor lesions were correctly identified [76]. It also has better sensitivity than FOBT with comparable specificity [76]. A newer version of the test, SDT2, uses gel electrophoresis to detect 3 tumor-specific markers broadly informative for both colorectal cancer and adenomas: K-ras mutations, scanning of APC mutator cluster regions, and methylation of the vimentin gene [77]. SDT2 appears to have a greater sensitivity then SDT1, but it has not yet been carefully evaluated in population-based screening cohorts [78]. Thus, the overall test performance of SDT2 remains uncertain, as does the appropriate management of patients with positive SDT1 or SDT2 tests and negative colonoscopic findings. Similarly, the appropriate screening interval and the cost-effectiveness of these tests are unknown.
Virtual colonoscopy or compute tomographic colonography
Colonoscopy is thought to be invasive by patients and has risks of complications, such as perforation, bleeding, or difficulties resulting from the sedation. As a screening test, colonoscopy has a low compliance rate and its accuracy is dependent on the quality of the patients’ bowel preparation and the skill of the endoscopist. Computed tomographic (CT) colonography renders 2-dimensional and 3-dimensional images of the colon. It also requires complete bowel preparation, but no sedation. After positioning the patient, air is inserted through the anus until the colon is distended and the patient is uncomfortable, but not in pain. CT colonography is a less-invasive option in screening for colorectal cancer.
In a multicenter study of CT colonography with 2531 asymptomatic adults, aged 50 years or older, CT colonographic screening identified 90% of the subjects with adenomas or cancers measuring 10 mm or more in diameter, with a false positive rate of 14% [79]. For large (10 mm in diameter or larger) adenomas and cancers, the mean (standard error) per-patient estimates of the sensitivity, specificity, positive and negative predictive values, and area under the receiver-operating-characteristic curve for CT colonography were 90% (0.03), 86% (0.02), 23% (0.02), 99% (<0.01), and 0.89 (0.02), respectively. A sensitivity of 90% indicates that CT colonography failed to detect lesions measuring 10 mm or greater in 10% of patients. The detection rate for polyps that are 6 mm or larger in diameter, the threshold used to refer patients for colonoscopy, is 78% (a specificity of 88%). CT colonography is less sensitive and specific for polyps smaller than 6 mm in diameter. By using the 6-mm polyp size as a cutoff point for referral to colonoscopy, 15% to 25% of persons undergoing screening colonography would be referred for colonoscopy.
This rate of referral for colonoscopy is an important element of program cost when examining colonography as a screening test. In comparison with colonoscopy, it was not cost-effective to offer CT colonography prior to colonoscopy as screening for patients unless the price per test was approximately $108 to $205 per case, or if the adherence increased by 25% over any of the other modalities with respect to getting more people to be screened [80]. This increased adherence over colonoscopy is not anticipated given patients still have to perform preprocedure bowel cleansing, and the insertion of the anal catheter and air within the colon is uncomfortable.
The treatment plan for patients in whom the largest polyp is smaller than 6 mm in diameter is controversial. Less than 2% of these patients will have adenomas with advanced features, and cancer is rare. No studies have demonstrated the safety of following such patients with repeat CT colonography. There is also uncertainty about whether CT colonography can be used to identify flat polyps, some of which may harbor malignant cells. Appropriate screening intervals after negative examinations or in cases of growths that are smaller than 6 mm in diameter and that may be polyps are uncertain.
Several unknowns with respect to colonography are concerning. The sensitivity and specificity of CT colonography in routine clinical practice settings are unknown. Radiation exposure associated with CT colonography could increase the risk of cancer. Although low-dose regimens are used, there is concern about cumulative radiation exposure, and some countries will not allow imaging for screening purposes. The rate of extracolonic findings that require further evaluation is an important driver of cost. Studies show that 27% to 69% of persons who undergo screening with CT colonography have at least one finding outside the colon, requiring further evaluation in 5% to 16% of persons undergoing screening.
In 2008, in a joint guideline, the Multi-Society Task Force, the American Cancer Society, and the American College of Radiology (ACS–MSTF–ACR) did add CT colonography to its list of recommended colorectal cancer screening tests [81]. However, in an observational study from the International Colorectal Cancer Screening Network, 35 organized screening initiatives were identified in 17 countries, including 10 routine population-based screening programs, 9 pilots, and 16 research projects. Fecal occult blood tests were the most frequently used screening modality and total colonoscopy was seldom used as a primary screening test [82]. Population screening with optical colonoscopy would overwhelm national colonoscopy resources and potentially lead to significant complications in patients who are otherwise healthy [83].
Video capsule endoscopy: pillcam colon
The aim of using a noninvasive capsule endoscopy for screening is to recruit individuals who are unwilling or unable to undertake an invasive procedure, such as a colonoscopy, but who may accept the invasive procedure once a polyp or a cancer is detected. In this fashion, the noninvasive test will ultimately improve the CRC prevention rate in the general population, including those patients who otherwise would not have adequate screening.
Capsule endoscopy is effective in detecting colonic polyps and significant findings in patients with an indication for colonoscopy. Two pilot studies have compared the diagnostic accuracy of capsule endoscopy and colonoscopy for the detection of colonic polyps in symptomatic patients and demonstrated that capsule endoscopy is feasible, safe, and shows promising sensitivity and specificity indexes [84,85]. In a recent study of second generation PillCam Colon 2 (Given Imaging, Yoquinean, Israel) the capsule sensitivity for the detection of patients with polypsgreater than or equal to 6 mm was 89% (95% CI, 70–97) and for those with polyps greater than or equal to 10 mm it was 88% (95% CI, 56–98), with specificities of 76% (95% CI, 72–78) and 89% (95% CI, 86–90), respectively [86]. Another prospective, multicenter study compared capsule endoscopy with optical colonoscopy (the standard for comparison) in a cohort of patients with known or suspected colonic disease. In a total of 328 patients, the sensitivity and specificity of capsule endoscopy for detecting polyps that were 6 mm in size or bigger were 64% (95% CI, 59–72) and 84% (95% CI, 81–87), respectively, and for detecting advanced adenoma, the sensitivity and specificity were 73% (95% CI, 61–83) and 79% (95% CI, 77–81), respectively. The use of capsule endoscopy of the colon allows visualization of the colonic mucosa in most patients, but its sensitivity for detecting colonic lesions is low as compared with the use of optical colonoscopy [87].
Capsule endoscopy is a cost-effective option compared with no screening. However, it is cost-ineffective when compared to colonoscopy assuming there is equal compliance. The cost-effectiveness of capsule endoscopy in CRC screening will mainly depend on its ability to improve compliance in the general population [88].
Summary
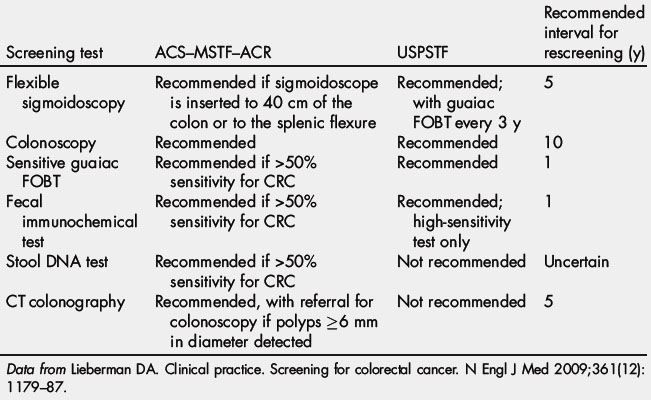
March is national colorectal cancer awareness month. It is estimated that as many as 60% of colorectal cancer deaths could be prevented if all men and women aged 50 years or older were screened routinely. In 2000, Katie Couric’s televised colonoscopy led to a 20% increase in screening colonoscopies across America, a stunning rise called the “Katie Couric Effect” [89]. This event demonstrated how celebrity endorsement affects health behavior. Currently, discussion is ongoing about the optimal strategy for CRC screening, particularly the costs of screening colonoscopy. The current CRC screening guidelines are summarized in Table 2 [81]. Debates over the optimum CRC screening test continue in the face of evidence that 22 million Americans aged 50 to 75 years are not screened for CRC by any modality and 25,000 of those lives may have been saved if they had been screened for CRC.
It is clear that improving screening rates and reducing disparities in underscreened communities and population subgroups could further reduce colorectal cancer morbidity and mortality. National Institutes of Health consensus identified the following priority areas to enhance the use and quality of colorectal cancer screening [90]:
Encouraging population adherence to screening tests and allowing patients to select the tests they prefer may do more good (as long as they choose something) than whatever procedure is chosen by the medical profession as the preferred test [25].
References
[1] D.M. Parkin, F. Bray, J. Ferlay, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74-108.
[2] J.A. Shapiro, L.C. Seeff, T.D. Thompson, et al. Colorectal cancer test use from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2008;17(7):1623-1630.
[3] D.A. Lieberman, D.G. Weiss, J.H. Bond, et al. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343(3):162-168.
[4] A.L. Frazier, G.A. Colditz, C.S. Fuchs, et al. Cost-effectiveness of screening for colorectal cancer in the general population. JAMA. 2000;284(15):1954-1961.
[5] A. Sonnenberg, F. Delco, J.M. Inadomi. Cost-effectiveness of colonoscopy in screening for colorectal cancer. Ann Intern Med. 2000;133(8):573-584.
[6] L. Rabeneck, L.F. Paszat, R. Saskin, et al. Association between colonoscopy rates and colorectal cancer mortality. Am J Gastroenterol. 2010;105(7):1627-1632.
[7] D.A. Lieberman. Clinical practice. Screening for colorectal cancer. N Engl J Med. 2009;361(12):1179-1187.
[8] N.N. Baxter, M.A. Goldwasser, L.F. Paszat, et al. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150(1):1-8.
[9] H. Brenner, M. Hoffmeister, V. Arndt, et al. Protection from right- and left-sided colorectal neoplasms after colonoscopy: population-based study. J Natl Cancer Inst. 2010;102(2):89-95.
[10] H. Singh, Z. Nugent, S.M. Mahmud, et al. Predictors of colorectal cancer after negative colonoscopy: a population-based study. Am J Gastroenterol. 2010;105(3):663-673. [quiz: 674]
[11] W.S. Atkin, R. Edwards, I. Kralj-Hans, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375(9726):1624-1633.
[12] G. Hoff, T. Grotmol, E. Skovlund, et al. Risk of colorectal cancer seven years after flexible sigmoidoscopy screening: randomised controlled trial. BMJ. 2009;338:b1846.
[13] P.A. Newcomb, B.E. Storer, L.M. Morimoto, et al. Long-term efficacy of sigmoidoscopy in the reduction of colorectal cancer incidence. J Natl Cancer Inst. 2003;95(8):622-625.
[14] J.V. Selby, G.D. Friedman, C.P. Quesenberry Jr., et al. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326(10):653-657.
[15] A.I. Neugut, B. Lebwohl. Colonoscopy vs sigmoidoscopy screening: getting it right. JAMA. 2010;304(4):461-462.
[16] J. Lakoff, L.F. Paszat, R. Saskin, et al. Risk of developing proximal versus distal colorectal cancer after a negative colonoscopy: a population-based study. Clin Gastroenterol Hepatol. 2008;6(10):1117-1121. [quiz: 1064]
[17] J.E. Allison. The best screening test for colorectal cancer is the one that gets done well. Gastrointest Endosc. 2010;71(2):342-345.
[18] J.E. Allison, M.B. Potter. New screening guidelines for colorectal cancer: a practical guide for the primary care physician. Prim Care. 2009;36(3):575-602.
[19] L. Rabeneck, L.F. Paszat, R.J. Hilsden, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology. 2008;135(6):1899-1906. 1906. e1891
[20] T.R. Levin, W. Zhao, C. Conell, et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145(12):880-886.
[21] G. Arora, A. Mannalithara, G. Singh, et al. Risk of perforation from a colonoscopy in adults: a large population-based study. Gastrointest Endosc. 2009;69(3 Pt 2):654-664.
[22] J.L. Warren, C.N. Klabunde, A.B. Mariotto, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009;150(12):849-857. W152
[23] M.F. Kaminski, J. Regula, E. Kraszewska, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362(19):1795-1803.
[24] L.C. Seeff, D.L. Manninen, F.B. Dong, et al. Is there endoscopic capacity to provide colorectal cancer screening to the unscreened population in the United States? Gastroenterology. 2004;127(6):1661-1669.
[25] S.H. Woolf. The best screening test for colorectal cancer–a personal choice. N Engl J Med. 2000;343(22):1641-1643.
[26] M. Carpelan-Holmstrom, J. Louhimo, U.H. Stenman, et al. CA 19-9 and CA 72-4 improve the diagnostic accuracy in gastrointestinal cancers. Anticancer Res. 2002;22(4):2311-2316.
[27] P. Pasanen, M. Eskelinen, A. Kulju, et al. Tumour-associated trypsin inhibitor (TATI) in patients with colorectal cancer: a comparison with CEA, CA 50 and CA 242. Scand J Clin Lab Invest. 1995;55(2):119-124.
[28] A. Spila, P. Ferroni, M. Cosimelli, et al. Comparative analysis of CA 242 and CA 19-9 serum tumor markers in colorectal cancer patients. A longitudinal evaluation. Anticancer Res. 2001;21(2B):1263-1270.
[29] A. Spila, P. Ferroni, M. Cosimelli, et al. Evaluation of the CA 242 tumor antigen as a potential serum marker for colorectal cancer. Anticancer Res. 1999;19(2B):1363-1368.
[30] G.J. van Kamp, S. von Mensdorff-Pouilly, P. Kenemans, et al. Evaluation of colorectal cancer-associated mucin CA M43 assay in serum. Clin Chem. 1993;39(6):1029-1032.
[31] K.A. Yedema, P. Kenemans, T. Wobbes, et al. Carcinoma-associated mucin serum markers CA M26 and CA M29: efficacy in detecting and monitoring patients with cancer of the breast, colon, ovary, endometrium and cervix. Int J Cancer. 1991;47(2):170-179.
[32] D.M. Thomson, J. Krupey, S.O. Freedman, et al. The radioimmunoassay of circulating carcinoembryonic antigen of the human digestive system. Proc Natl Acad Sci U S A. 1969;64(1):161-167.
[33] F. Castaldi, M. Marino, L. Beneduce, et al. Detection of circulating CEA-IgM complexes in early stage colorectal cancer. Int J Biol Markers. 2005;20(4):204-208.
[34] L.C. Fernandes, S.B. Kim, D. Matos. Cytokeratins and carcinoembryonic antigen in diagnosis, staging and prognosis of colorectal adenocarcinoma. World J Gastroenterol. 2005;11(5):645-648.
[35] J.M. Liou, C.T. Shun, J.T. Liang, et al. Plasma insulin-like growth factor-binding protein-2 levels as diagnostic and prognostic biomarker of colorectal cancer. J Clin Endocrinol Metab. 2010;95(4):1717-1725.
[36] D.L. Kozwich, L.C. Kramer, W.P. Mielicki, et al. Application of cancer procoagulant as an early detection tumor marker. Cancer. 1994;74(4):1367-1376.
[37] O.J. Cordero, D. Ayude, M. Nogueira, et al. Preoperative serum CD26 levels: diagnostic efficiency and predictive value for colorectal cancer. Br J Cancer. 2000;83(9):1139-1146.
[38] A. Kerber, J. Trojan, K. Herrlinger, et al. The new DR-70 immunoassay detects cancer of the gastrointestinal tract: a validation study. Aliment Pharmacol Ther. 2004;20(9):983-987.
[39] A.L. Small-Howard, H. Harris. Advantages of the AMDL-ELISA DR-70 (FDP) assay over carcinoembryonic antigen (CEA) for monitoring colorectal cancer patients. J Immunoassay Immunochem. 2010;31(2):131-147.
[40] A.R. Soroush, H.M. Zadeh, M. Moemeni, et al. Plasma prolactin in patients with colorectal cancer. BMC Cancer. 2004;4:97.
[41] M. Kawahara, D. Chia, P.I. Terasaki, et al. Detection of sialylated LewisX antigen in cancer sera using a sandwich radioimmunoassay. Int J Cancer. 1985;36(4):421-425.
[42] R. Broll, H. Erdmann, M. Duchrow, et al. Vascular endothelial growth factor (VEGF)–a valuable serum tumour marker in patients with colorectal cancer? Eur J Surg Oncol. 2001;27(1):37-42.
[43] W.S. Tsai, C.R. Changchien, C.Y. Yeh, et al. Preoperative plasma vascular endothelial growth factor but not nitrite is a useful complementary tumor marker in patients with colorectal cancer. Dis Colon Rectum. 2006;49(6):883-894.
[44] A.G. Renehan, J.E. Painter, D. O’Halloran, et al. Circulating insulin-like growth factor II and colorectal adenomas. J Clin Endocrinol Metab. 2000;85(9):3402-3408.
[45] B. Mroczko, M. Szmitkowski, U. Wereszczynska-Siemiatkowska, et al. Stem cell factor (SCF) and interleukin 3 (IL-3) in the sera of patients with colorectal cancer. Dig Dis Sci. 2005;50(6):1019-1024.
[46] S.C. Chang, J.K. Lin, T.C. Lin, et al. Genetic alteration of p53, but not overexpression of intratumoral p53 protein, or serum p53 antibody is a prognostic factor in sporadic colorectal adenocarcinoma. Int J Oncol. 2005;26(1):65-75.
[47] P. Hammel, B. Boissier, M.T. Chaumette, et al. Detection and monitoring of serum p53 antibodies in patients with colorectal cancer. Gut. 1997;40(3):356-361.
[48] B.M. Reipert, S. Tanneberger, A. Pannetta, et al. Increase in autoantibodies against Fas (CD95) during carcinogenesis in the human colon: a hope for the immunoprevention of cancer? Cancer Immunol Immunother. 2005;54(10):1038-1042.
[49] G. Sergeant, F. Penninckx, B. Topal. Quantitative RT-PCR detection of colorectal tumor cells in peripheral blood–a systematic review. J Surg Res. 2008;150(1):144-152.
[50] J.Y. Wang, C.S. Yeh, Y.F. Chen, et al. Development and evaluation of a colorimetric membrane-array method for the detection of circulating tumor cells in the peripheral blood of Taiwanese patients with colorectal cancer. Int J Mol Med. 2006;17(5):737-747.
[51] W.K. Leung, K.F. To, E.P. Man, et al. Quantitative detection of promoter hypermethylation in multiple genes in the serum of patients with colorectal cancer. Am J Gastroenterol. 2005;100(10):2274-2279.
[52] J.Y. Wang, J.S. Hsieh, M.Y. Chang, et al. Molecular detection of APC, K-ras, and p53 mutations in the serum of colorectal cancer patients as circulating biomarkers. World J Surg. 2004;28(7):721-726.
[53] F. Guadagni, J. Kantor, S. Aloe, et al. Detection of blood-borne cells in colorectal cancer patients by nested reverse transcription-polymerase chain reaction for carcinoembryonic antigen messenger RNA: longitudinal analyses and demonstration of its potential importance as an adjunct to multiple serum markers. Cancer Res. 2001;61(6):2523-2532.
[54] S.M. Lledo, E. Garcia-Granero, F. Dasi, et al. Real time quantification in plasma of human telomerase reverse transcriptase (hTERT) mRNA in patients with colorectal cancer. Colorectal Dis. 2004;6(4):236-242.
[55] R. Douard, V. Le Maire, P. Wind, et al. Carcinoembryonic gene member 2 mRNA expression as a marker to detect circulating enterocytes in the blood of colorectal cancer patients. Surgery. 2001;129(5):587-594.
[56] I. Miyashiro, C. Kuo, K. Huynh, et al. Molecular strategy for detecting metastatic cancers with use of multiple tumor-specific MAGE-A genes. Clin Chem. 2001;47(3):505-512.
[57] V. Garcia, J.M. Garcia, C. Pena, et al. Thymidylate synthase messenger RNA expression in plasma from patients with colon cancer: prognostic potential. Clin Cancer Res. 2006;12(7 Pt 1):2095-2100.
[58] S.A. Bustin, V.G. Gyselman, N.S. Williams, et al. Detection of cytokeratins 19/20 and guanylyl cyclase C in peripheral blood of colorectal cancer patients. Br J Cancer. 1999;79(11–12):1813-1820.
[59] T.H. Schiedeck, C. Wellm, U.J. Roblick, et al. Diagnosis and monitoring of colorectal cancer by L6 blood serum polymerase chain reaction is superior to carcinoembryonic antigen-enzyme-linked immunosorbent assay. Dis Colon Rectum. 2003;46(6):818-825.
[60] M.S. Pepe, R. Etzioni, Z. Feng, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93(14):1054-1061.
[61] J.M. Wilson, Y.G. Jungner. Principles and practice of mass screening for disease. Bol Oficina Sanit Panam. 1968;65(4):281-393. [in Spanish]
[62] J.S. Mandel, T.R. Church, J.H. Bond, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343(22):1603-1607.
[63] P. Hewitson, P. Glasziou, E. Watson, et al. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008;103(6):1541-1549.
[64] B. Towler, L. Irwig, P. Glasziou, et al. A systematic review of the effects of screening for colorectal cancer using the faecal occult blood test, hemoccult. BMJ. 1998;317(7158):559-565.
[65] J.D. Hardcastle, J.O. Chamberlain, M.H. Robinson, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348(9040):1472-1477.
[66] O. Kronborg, C. Fenger, J. Olsen, et al. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348(9040):1467-1471.
[67] O.D. Jorgensen, O. Kronborg, C. Fenger. A randomised study of screening for colorectal cancer using faecal occult blood testing: results after 13 years and seven biennial screening rounds. Gut. 2002;50(1):29-32.
[68] L. Hol, M.E. van Leerdam, M. van Ballegooijen, et al. Screening for colorectal cancer: randomised trial comparing guaiac-based and immunochemical faecal occult blood testing and flexible sigmoidoscopy. Gut. 2010;59(1):62-68.
[69] S. Hundt, U. Haug, H. Brenner. Comparative evaluation of immunochemical fecal occult blood tests for colorectal adenoma detection. Ann Intern Med. 2009;150(3):162-169.
[70] F. Diehl, K. Schmidt, K.H. Durkee, et al. Analysis of mutations in DNA isolated from plasma and stool of colorectal cancer patients. Gastroenterology. 2008;135(2):489-498.
[71] T. Mak, F. Lalloo, D.G. Evans, et al. Molecular stool screening for colorectal cancer. Br J Surg. 2004;91(7):790-800.
[72] B. Vogelstein, E.R. Fearon, S.R. Hamilton, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319(9):525-532.
[73] B. Vogelstein, E.R. Fearon, S.E. Kern, et al. Allelotype of colorectal carcinomas. Science. 1989;244(4901):207-211.
[74] G. Traverso, A. Shuber, B. Levin, et al. Detection of APC mutations in fecal DNA from patients with colorectal tumors. N Engl J Med. 2002;346(5):311-320.
[75] D.A. Ahlquist, A.P. Shuber. Stool screening for colorectal cancer: evolution from occult blood to molecular markers. Clin Chim Acta. 2002;315(1–2):157-168.
[76] T.F. Imperiale, D.F. Ransohoff, S.H. Itzkowitz, et al. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med. 2004;351(26):2704-2714.
[77] L. Kann, J. Han, D. Ahlquist, et al. Improved marker combination for detection of de novo genetic variation and aberrant DNA in colorectal neoplasia. Clin Chem. 2006;52(12):2299-2302.
[78] D.A. Ahlquist, D.J. Sargent, C.L. Loprinzi, et al. Stool DNA and occult blood testing for screen detection of colorectal neoplasia. Ann Intern Med. 2008;149(7):441-450. W481
[79] C.D. Johnson, M.H. Chen, A.Y. Toledano, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359(12):1207-1217.
[80] A.B. Knudsen, I. Lansdorp-Vogelaar, C.M. Rutter, et al. Cost-effectiveness of computed tomographic colonography screening for colorectal cancer in the Medicare population. J Natl Cancer Inst. 2010;102(16):1238-1252.
[81] B. Levin, D.A. Lieberman, B. McFarland, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570-1595.
[82] V.S. Benson, J. Patnick, A.K. Davies, et al. Colorectal cancer screening: a comparison of 35 initiatives in 17 countries. Int J Cancer. 2008;122(6):1357-1367.
[83] D.A. Macafee, J.H. Scholefield. Antagonist: population based endoscopic screening for colorectal cancer. Gut. 2003;52(3):323-326.
[84] R. Eliakim, Z. Fireman, I.M. Gralnek, et al. Evaluation of the PillCam Colon capsule in the detection of colonic pathology: results of the first multicenter, prospective, comparative study. Endoscopy. 2006;38(10):963-970.
[85] N. Schoofs, J. Deviere, A. Van Gossum. PillCam colon capsule endoscopy compared with colonoscopy for colorectal tumor diagnosis: a prospective pilot study. Endoscopy. 2006;38(10):971-977.
[86] R. Eliakim, K. Yassin, Y. Niv, et al. Prospective multicenter performance evaluation of the second-generation colon capsule compared with colonoscopy. Endoscopy. 2009;41(12):1026-1031.
[87] A. Van Gossum, M. Munoz-Navas, I. Fernandez-Urien, et al. Capsule endoscopy versus colonoscopy for the detection of polyps and cancer. N Engl J Med. 2009;361(3):264-270.
[88] C. Hassan, A. Zullo, S. Winn, et al. Cost-effectiveness of capsule endoscopy in screening for colorectal cancer. Endoscopy. 2008;40(5):414-421.
[89] P. Cram, A.M. Fendrick, J. Inadomi, et al. The impact of a celebrity promotional campaign on the use of colon cancer screening: the Katie Couric effect. Arch Intern Med. 2003;163(13):1601-1605.
[90] D. Steinwachs, J.D. Allen, W.E. Barlow, et al. National Institutes of Health state-of-the-science conference statement: Enhancing use and quality of colorectal cancer screening. Ann Intern Med. 2010;152(10):663-667.