Fever and Infection
Scott Segal MD, MHCM
Chapter Outline
Fever
Definition and Pathophysiology
In 1868, Carl Wunderlich analyzed more than 1 million axillary temperature measurements from 25,000 patients.1 He concluded that the average normal temperature of healthy adults was 37° C (98.6° F). However, he found a range of temperatures, with a nadir of 36.2° C between 2:00 and 8:00 AM and a zenith of 37.5° C between 4:00 and 9:00 PM. He also observed that women had slightly higher mean temperatures than men. A 1992 study using modern oral thermometers largely confirmed Wunderlich’s original data.2
Well-regulated temperature results from hypothalamic integration of afferent thermal information from the skin, spinal cord, and other sites within the central nervous system (CNS). When this integrated temperature deviates from normal, thermoregulatory responses are triggered.3 In humans, the first (and least metabolically “expensive”) response to temperature perturbations is behavioral (e.g., moving to a different environment, putting on appropriate clothing, adjusting room temperature). Such responses obviously are unavailable to an anesthetized patient, although some may be implemented by those caring for the patient. Further responses to temperature perturbations are mediated by the autonomic nervous system. Hypothermia prompts vasoconstriction in peripheral tissues to decrease skin blood flow, decrease heat loss, and retain heat in the core compartment. If vasoconstriction is not adequate to prevent hypothermia, thermoregulatory shivering is triggered to increase heat production. The CNS controls the metabolic activity of skeletal muscle, which converts chemical energy into heat by shivering.
Increased body temperature initially prompts vasodilation. This vasodilation is passive. It results from the release of sympathetic tone, and it is observed in unanesthetized adults exposed to a hot environment before any significant change in central temperature occurs. If vasodilation is not adequate to prevent hyperthermia, thermoregulatory sweating occurs, which increases evaporative heat loss.
An abnormal body temperature can result from drugs or diseases that either change thermoregulatory thresholds or impair thermoregulatory responses. Hypothalamic activity and fever may be triggered by endogenous pyrogens released from immune effector cells in response to invasion by microorganisms (Figure 37-1). Although no single endogenous pyrogen has been conclusively identified as the mediator of the febrile response, tumor necrosis factor seems capable of reproducing many components of the febrile response.4 Endogenous pyrogen activity appears to depend largely on increased endothelial cell production of prostaglandins. Of interest, many of these substances help mediate uterine activity and parturition.5 The central neural pathways leading to changes in thermoregulatory physiology are increasingly well understood and involve pathways coordinated in the preoptic area and projecting to the dorsomedial hypothalamus or to the rostral medullary raphe region.3
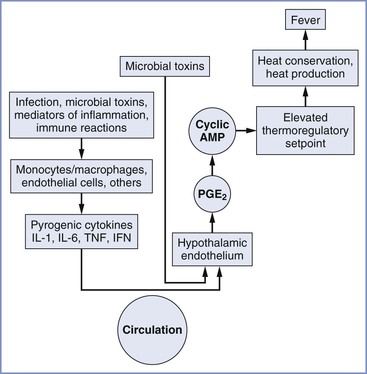
FIGURE 37-1 Chronology of events in the pathophysiology of fever. AMP, adenosine-5′-monophosphate; IFN, interferon; IL, interleukin; TNF, tumor necrosis factor; PGE2, prostaglandin E2. (From Longo DL, Fauci AS, Kasper DL, et al. Harrison’s Principles of Internal Medicine. 18th edition. New York, McGraw-Hill, 2012. Available at http://www.accessmedicine.com/. Accessed July 2013.)
Clinically, temperature measurements greater than 38° C represent fever. During episodes of fever, the thermoregulatory set point is elevated and the normal thermoregulatory mechanisms are used to maintain the elevated temperature. However, there are circumstances in which an abnormally high temperature is measured in the absence of a change in thermoregulatory set point, such as when thermoregulatory responses to hyperthermia are prevented (e.g., block of sympathetically mediated sweating) or overwhelmed (e.g., immersion in hot water, malignant hyperthermia).
The fetus, by virtue of its intra-abdominal location, has a unique problem with heat elimination. The only anatomic routes for egress of heat are the fetal skin surface (through the amniotic fluid) or the uteroplacental circulation. Evidence suggests that the fetus relies on heat exchange across the uteroplacental circulation to dissipate most of its metabolic heat. The normal fetus maintains a temperature that is approximately 0.5° C to 0.75° C higher than maternal temperature.6–8
Consequences of Maternal Fever and Infection
Maternal-fetal infection is associated with increased perinatal morbidity.9,10 The increased morbidity is the result of many factors, including preterm delivery (perhaps related to an increased release of prostaglandins) and direct effects of the infection. Fever, irrespective of its etiology, may be harmful to the fetus, although the mechanisms of injury may differ with various causes. Conversely, some infections may be harmful without producing fever. Infection is the most common cause of fever and involves liberation of inflammatory cytokines, which are implicated in the pathogenesis of many fetal and neonatal injuries (see Chapter 10).11,12 Noninfectious inflammatory fever, also leading to elevated cytokines, may complicate neuraxial analgesia in the absence of clinical infection (see later discussion).
Experimental evidence suggests that extreme levels of hyperthermia, independent of infection or inflammation, may have a deleterious effect on the fetus. Morishima et al.13 reported increased uterine activity and fetal deterioration during maternal hyperthermia produced by radiant heat in anesthetized baboons. However, the extreme degree of hyperthermia (approximately 41.7° C) employed in this study produced maternal as well as fetal deaths. Such extreme hyperthermia exceeds the modest fever that often occurs clinically; thus the clinical relevance of this study is unclear. Similarly, Cefalo and Hellegers14 demonstrated fetal deterioration at levels of hyperthermia that produced maternal cardiovascular collapse in anesthetized gravid ewes. However, the investigators also observed increased umbilical blood flow with clinically relevant, mild to moderate hyperthermia (0.5° C to 1.5° C above baseline). They suggested that increased umbilical blood flow in response to moderate degrees of hyperthermia might be beneficial to the fetus by increasing oxygen delivery and heat removal. Harris et al.15 demonstrated the preservation of fetal oxygenation and acid-base status during moderate degrees of fever (approximately 1° C above baseline) produced by the injection of bacterial pyrogen in awake pregnant ewes. However, they also observed an increase in fetal heart rate (FHR) and a greater incidence of fetal arrhythmias during fever.
Nonetheless, in humans, hot tub and sauna use in pregnancy has been linked epidemiologically to neural tube defects in the fetus with attributable risk comparable to that observed with febrile illness.16 Spontaneous abortion17 and major structural birth defects18 have similarly been associated with the frequency of use of hot tubs and saunas. Neonatal hypoxic encephalopathy has been observed after prolonged immersion in 39.7° C water during otherwise uncomplicated labor.19
Epidemiologic evidence suggests that mild maternal intrapartum fever may not be as benign as has been assumed on the basis of animal studies. Macauley et al.8 measured fetal scalp temperature in utero using a modified intrauterine pressure catheter. They concluded that fetal core temperature may exceed 40° C in some febrile women (Figure 37-2). Lieberman et al.20 retrospectively reviewed the records of 1218 nulliparous women with singleton, term pregnancies in spontaneous labor who were afebrile on admission. They found fever (> 38° C) in 10% of the patients, nearly all of whom had received epidural analgesia. One-minute Apgar scores less than 7 and hypotonia were more common in the newborns of febrile mothers. Fever higher than 38.3° C was associated with more frequent requirement for bag-and-mask ventilation in the delivery room and need for supplemental oxygen in the nursery. There was also a nonsignificant increase in the incidence of neonatal seizures.20 The same group performed a case-control study of unexplained neonatal seizures in term infants and found a strong association with intrapartum fever and seizures (odds ratio [OR], 3.4).21 A similar finding was reported by Perlman,22 who found a high incidence of maternal fever among a cohort of infants with a 5-minute Apgar score of 5 or less and those requiring resuscitation with chest compressions in the delivery room. The same group found a higher incidence of prolonged positive-pressure ventilation in the delivery room, tracheal intubation, admission to a neonatal intensive care unit, and 5-minute Apgar score less than 6 in febrile newborns born to women with chorioamnionitis compared with afebrile newborns born to women with chorioamnionitis.23 Similarly, Greenwell et al.24 found an association between low-grade fever (37.5° C) and adverse neonatal outcomes, including hypotonia and low 1-minute Apgar scores. More extreme fever (> 38.3° C) was also associated with low 5-minutes Apgar scores, assisted ventilation, and seizures.24
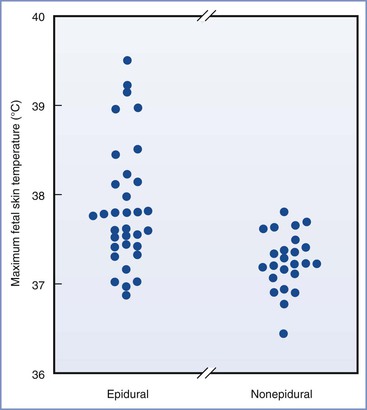
FIGURE 37-2 Maximum fetal scalp temperatures measured in utero in women who selected epidural analgesia and those in women selecting other forms of analgesia. P = .004 epidural compared with nonepidural group. (From Macaulay JH, Bond K, Steer PJ. Epidural analgesia in labor and fetal hyperthermia. Obstet Gynecol 1992; 80:665-9.)
Even more ominous is the suggestion that maternal fever may correlate with neonatal brain injury, particularly when the fever is associated with clinical or pathologically diagnosed chorioamnionitis (see Chapter 10). In several large epidemiologic studies, otherwise unexplained cerebral palsy was two to nine times more common in infants born to mothers with intrapartum fever (> 38° C) than in those born to afebrile mothers.25–29 An equally strong association has been observed between maternal fever and neonatal encephalopathy.30 Even if not apparent at birth or in early infancy, neurologic injury related to maternal fever may appear later in childhood. Dammann et al.31 reported an increased risk for cognitive deficits (> 2 standard deviations below the mean on a nonverbal intelligence scale) at age 9 among children whose mothers were febrile in labor. Fever during pregnancy, not specifically in the intrapartum period, has also been linked to subsequent development of autism32,33 and schizophrenia.34
The mechanism linking neurologic injuries to maternal fever likely involves the liberation of inflammatory cytokines.11,12,29,34 Animal models of chorioamnionitis suggest that fetal brain lesions can be induced by infection and blocked by anti-inflammatory cytokines.35 Infants developing neonatal encephalopathy in the setting of maternal fever do not generally exhibit positive blood cultures, implying that it is neuroinflammation, rather than infection, that causes damage.30,36 It remains unclear whether intrapartum temperature elevation itself can cause neurologic injury, as distinct from underlying infection or other inflammatory processes that cause both elevated inflammatory cytokines and fever.
Fever also produces significant maternal effects. Elevated temperature is associated with increased maternal heart rate, cardiac output, oxygen consumption, and catecholamine production. Evidence has linked fever after cesarean delivery to risk for uterine rupture during subsequent trial of labor after cesarean delivery.37 Women who develop fever are more likely to shiver and experience uncomfortable rigors.38–40 Not surprisingly, obstetricians fearing infection are more likely to treat febrile women with antibiotics.41,42 Even low-grade fever may prompt obstetricians to choose instrumental vaginal or cesarean delivery over expectant labor management. Lieberman et al.43 found a twofold higher incidence of operative vaginal as well as cesarean delivery in a retrospective analysis of nulliparous women who were afebrile at admission but developed fever greater than 37.5° C during labor compared with those who remained afebrile, even after controlling for birth weight, duration of labor, and analgesic choice.
Together, these studies suggest that the fetus acutely tolerates modest degrees of maternal fever. Transient neonatal depression, neonatal seizures, and other neurologic disorders may be associated with inflammatory processes that produce maternal fever, although not clearly with fever per se. Inflammatory processes resulting in fever, and perhaps severe hyperthermia itself, may be risk factors for neonatal brain injury and other abnormalities.
Infections in Pregnant Women
Fever is most often the result of an infectious process. The most common sites for infection in pregnant women are the fetal membranes, urinary tract, respiratory tract, and postpartum uterine cavity.
Chorioamnionitis
Chorioamnionitis is one of the most common infections in pregnant women. It occurs with variable frequency; reported event rates range from 0.5% to 10%, depending on the means of ascertainment and the demographic and obstetric characteristics of the population. In one review, the incidence was 41% for deliveries occurring at less than 27 weeks’ gestation, 15% from 28 to 36 weeks’ gestation, and 2% at term.44 Independent risk factors include low parity, a history of chorioamnionitis in a prior delivery,45 the number of vaginal examinations, duration of total labor, duration of ruptured membranes, and use of internal monitors.45,46 The diagnosis of chorioamnionitis is based on clinical signs, which include temperature greater than 38° C, maternal and/or fetal tachycardia, uterine tenderness, and/or foul-smelling amniotic fluid.9 Unfortunately, the laboratory diagnosis of chorioamnionitis is neither sensitive nor specific and may not correlate with the clinical presentation.9,47–51 Moreover, the classic clinical signs of chorioamnionitis often are absent. Goodman et al.51 reviewed the records of 531 women with pathologically proven chorioamnionitis. They found that only 10% of the patients had abdominal tenderness and only 1% had foul-smelling amniotic fluid. Moreover, histologic evidence of chorioamnionitis can develop without producing clinical signs or symptoms.52,53
In most cases, bacteria gain access to the amniotic cavity and the fetus by ascending through the cervix after rupture of the membranes. Chorioamnionitis develops in a significant number of parturients with premature rupture of the membranes. Alternatively, infectious agents present in the maternal circulation may undergo transplacental transport and gain access to the amniotic cavity.9 Similar to other pelvic infections, chorioamnionitis often is polymicrobial in origin and bacteria normally present in the genital tract most likely are responsible for infections. Bacteroides species, group B streptococci, Mycoplasma and Ureaplasma species, and Escherichia coli are organisms commonly isolated from the amniotic fluid of parturients with chorioamnionitis.9,54 Maternal bacteremia occurs in 7.5% to 12% of women with the clinical diagnosis of chorioamnionitis.9,47–51,55 Candida species occasionally cause chorioamnionitis, especially in preterm labors, and have been associated with severe sequelae, including maternal sepsis and fetal demise.56 Ureaplasma urealyticum has been implicated in intra-amniotic infections even with intact membranes, and infection with this organism is associated with preterm labor.57
Maternal complications of chorioamnionitis include preterm labor,58 placental abruption,59 postpartum infection,60 uterine atony,61 postpartum hemorrhage,62 peripartum hysterectomy,63 sepsis, and death. A large prospective observational study found uterine atony, maternal blood transfusion, septic pelvic thrombophlebitis, pelvic abscess, and maternal admission to the intensive care unit to be independently associated with chorioamnionitis.64 In addition, several studies have noted an increased incidence of cesarean delivery for dystocia in women with chorioamnionitis.* Some investigators have suggested that infection adversely affects uterine contractility and contributes to an increased risk for cesarean delivery.48 However, in some cases, chorioamnionitis may represent an ascending infection developing late in a labor that is already prolonged and dysfunctional.55 Satin et al.55 observed no increase in the incidence of cesarean delivery when chorioamnionitis was diagnosed before the administration of oxytocin. However, they observed a 44% incidence of cesarean delivery when the diagnosis was made after the administration of oxytocin. Thus, the presence of chorioamnionitis may be seen as influencing the clinical decision making of the obstetrician, rather than a direct physiologic cause of dystocia.
Neonatal complications of chorioamnionitis include pneumonia, meningitis, sepsis, and death.9,47 A strong association between chorioamnionitis and cerebral palsy has been identified.25,29,65,66 Meta-analyses of more than 20 studies have demonstrated relative risks of cerebral palsy ranging from 1.9 to 4.7 in preterm and term infants born to mothers with clinical chorioamnionitis.26,66,67 Neonatal stroke has also been linked to chorioamnionitis.68 Chorioamnionitis has been linked epidemiologically to cystic periventricular leukomalacia, which often produces devastating neurologic impairment in the child.66,69 The link between maternal infection and neurologic injury in the neonate appears related to intra-amniotic infection or inflammation, particularly when there is evidence of fetal systemic inflammation (funisitis).29,65
The effect of intra-amniotic inflammation on the fetal lung is complex. Elevated amniotic cytokines may reduce the incidence of acute respiratory distress syndrome in preterm neonates by stimulating surfactant production.70 However, chronic lung disease is increased in infants exposed to chorioamnionitis,71 apparently also related to inflammatory mediators.9,72 Studies in African women suggest chorioamnionitis is associated with an increased risk for peripartum vertical human immunodeficiency virus (HIV) transmission from infected mothers.73
Historically, prompt delivery has been the cornerstone of obstetric management of patients with chorioamnionitis. However, Gibbs et al.47 did not identify a correlation between poor maternal or neonatal outcome and the time interval from diagnosis of chorioamnionitis to delivery. They performed cesarean delivery only for standard obstetric indications and not for the diagnosis of chorioamnionitis alone.47 Similarly, a large prospective observational study found no relationship between the duration of infection and most measures of adverse neonatal outcome among 1965 gestations complicated by chorioamnionitis, although low 5-minute Apgar scores and neonatal mechanical ventilation were correlated with duration of chorioamnionitis.64 No recent studies have reinvestigated this practice as it relates to neonatal neurologic injuries. Monitoring of the FHR pattern is indicated in women with the diagnosis of chorioamnionitis. Many fetuses will exhibit mild tachycardia during maternal fever and infection, but this pattern is not highly predictive of neonatal acidemia and, therefore, by itself, is not an indication for immediate delivery.74
For many years, pediatricians requested that obstetricians delay antibiotic therapy until after delivery. They cited the theoretical concern that intrapartum therapy might “obscure the results of neonatal blood cultures.”75 However, studies suggest that early, antepartum treatment results in decreased maternal and neonatal morbidity compared with delayed, postpartum treatment.76,77 Gibbs et al.76 randomized 45 women with intra-amniotic infection to receive intrapartum or postpartum antibiotic therapy with ampicillin and gentamicin. Intrapartum antibiotic therapy resulted in a decreased incidence of neonatal sepsis and a shorter neonatal hospital stay. Mothers who received intrapartum antibiotics also had a shorter hospitalization, fewer days with fever, and a lower peak postpartum temperature than mothers whose antibiotic therapy was delayed until after delivery. Currently, most obstetricians give antibiotics before delivery in women with chorioamnionitis. The early use of antibiotics also may affect the anesthesiologist’s decision regarding the administration of neuraxial labor analgesia or anesthesia (see later discussion).
Urologic Infections
Urinary tract infections are common during pregnancy, although the incidence of asymptomatic bacteriuria may not be higher than in nonpregnant women. Increased concentrations of progesterone cause the relaxation of ureteral smooth muscle. In addition, the gravid uterus causes partial ureteral obstruction. Both factors cause urinary stasis, which increases the risk for urinary tract infection.9,78,79 Furthermore, these physiologic changes increase the likelihood that asymptomatic bladder infection will ascend into the kidneys and produce pyelonephritis. Approximately 1.3% of pregnant women will develop symptomatic cystitis,80 and up to 25% of women with untreated bacteriuria in pregnancy may develop pyelonephritis.81
Acute pyelonephritis is a serious threat to maternal and fetal well-being and complicates approximately 1.4% of pregnancies. Symptoms of acute pyelonephritis include fever, chills, flank pain, and other symptoms of lower urinary tract infection. Laboratory tests reveal pyuria and leukocytosis with a left shift. Pregnant women with pyelonephritis may appear severely ill. Approximately 14% to 17% of pregnant women with pyelonephritis will develop bacteremia during the course of this infection.82,83 Complications may also include anemia, renal insufficiency, and respiratory insufficiency.83 The most common causative organisms are E. coli, gram-positive organisms, Klebsiella, Enterobacter, and Proteus species.83
Hospitalization is generally required to initiate aggressive parenteral antibiotic treatment of this serious maternal infection, although limited data support outpatient treatment for carefully selected patients in the first and second trimester.84 Nevertheless, treatment failures and septic complications have been reported in patients randomized to outpatient therapy in clinical trials.85
Pyelonephritis is associated with an increased risk for preterm labor and delivery in animal models.86 Thus, obstetricians should observe for evidence of preterm labor, although epidemiologic studies have found the incidence to be less than 5%, approximately the same as in the general pregnant population.83,87
Pyelonephritis may be associated with organ dysfunction. Nearly 20% of affected women have transient renal dysfunction88 and the disease may also be complicated by pulmonary injury. Cunningham et al.89 suggested that “this syndrome was probably caused by permeability pulmonary edema, likely mediated by endotoxin-induced alveolar-capillary membrane injury.”89 Towers et al.90 compared 11 pregnant women who had pyelonephritis and pulmonary injury with 119 women who had pyelonephritis only. They observed that fluid overload and the use of tocolytic therapy were the most significant predictive factors associated with pulmonary injury. The authors suggested that “strict management of fluids should occur so that patients do not have fluid overload.”90 Contemporary authors, however, have questioned fluid restriction in pyelonephritis and instead suggest fluid administration sufficient to generate urine output of 30 to 50 mL/h, while observing respiratory rate, oxygen saturation, and symptoms of dyspnea to identify impending respiratory compromise. Respiratory failure should be investigated with chest radiography and arterial blood gas analysis and managed with appropriate respiratory support.9
Hemodynamic alterations may be present even in infected women who do not demonstrate overt signs of sepsis. Twickler et al.91 used ultrasonographic techniques to evaluate the central hemodynamic measurements in 27 pregnant women with uncomplicated pyelonephritis. They found decreased mean arterial pressure (MAP) and systemic vascular resistance (SVR) and increased heart rate and cardiac output compared with measurements obtained from the same patients after they had recovered from their infection.91
Recent reviews from the Cochrane Database and the United States Preventative Services Task Force strongly support the practice of screening and treating pregnant women for asymptomatic bacteriuria, up to 30% of whom will eventually develop pyelonephritis if left untreated.92,93 Treatment is associated with a reduced incidence of pyelonephritis (OR, 0.23) and low-birth-weight infants (OR, 0.66), although not, as had been previously suggested, in the incidence of preterm delivery.93 Definitive choice of antibiotics could not be determined in a recent systematic review,94 but traditional 7-day regimens are likely more effective than single-dose or other abbreviated antibiotic courses.95 Because pyelonephritis in pregnancy is thought to be preventable, its occurrence may be a marker for poor prenatal care; thus it should alert physicians to other potential pregnancy problems.96
Respiratory Tract Infection
Most respiratory tract infections during pregnancy are upper respiratory tract viral infections that do not pose a serious threat to the mother or fetus. Most lower respiratory tract infections are also viral and self-limiting; pneumonia occurs during pregnancy with an incidence approximating that of the nonpregnant population.97 Pregnancy results in a number of changes that may predispose the pregnant woman to the development of serious respiratory tract infection. Hyperemia and hypersecretion are characteristic of the respiratory tract mucosa during pregnancy, and these changes may intensify the effect of the initial infection.98 In the case of a viral infection, the excess secretions may predispose the patient to bacterial superinfection. Immunologic modulation during pregnancy may also predispose to pulmonary infection.99 Furthermore, the increased oxygen consumption, elevation of the diaphragm, and decreased functional residual capacity characteristic of pregnancy may increase the likelihood that infection will result in maternal hypoxemia.
Benedetti et al.100 emphasized the importance of early diagnosis and treatment as well as the direct measurement of maternal oxygenation in cases of pneumonia during pregnancy. Most community-acquired pneumonias in healthy young women are bacterial in origin. Streptococcus pneumoniae is the most common pathogen.100,101 Mycoplasma pneumoniae and influenza are other common pathogens. Legionella pneumophila, Chlamydia, and varicella are less common pathogens in this population. Varicella pneumonia has been associated with maternal and fetal morbidity and up to 40% maternal mortality. Acyclovir has been used successfully to treat varicella pneumonia during pregnancy.102 Pneumocystis jiroveci (formerly Pneumocystis carinii) pneumonia represents the most common cause of death related to acquired immunodeficiency syndrome (AIDS) in pregnancy; mortality is as high as 50%.103
Because morbidity and mortality from influenza are increased in pregnancy, influenza vaccination is strongly recommended for all pregnant patients, irrespective of gestational age.104 The risk for fetal death is increased by maternal influenza infection and is reduced by vaccination.105 The infant is also passively protected for up to 20 weeks after birth.106 Aggressive treatment with antiviral drugs (oseltamivir, zanamivir) reduces the severity of the ensuing illness; in the 2009-2010 H1N1 influenza pandemic, initiation of therapy in the first 2 days of the infection reduced hospitalization and intensive care unit admission.107,108
Postpartum Infection
The most common source of postpartum infection is the genital tract. The urinary tract and less often the breasts or the lungs may also be infected.9 Postpartum uterine infection typically results in fever, malaise, abdominal pain, and purulent lochia. Bacteremia may occur in as many as 5% to 10% of patients with uterine infection after delivery.49,50,109,110 Although obstetricians typically refer to postpartum uterine infection as endometritis, this infection involves the decidua, myometrium, and parametrial tissues. Bacteria that colonize the cervix and vagina gain access to the amniotic fluid during labor, and they may invade devitalized uterine tissue postpartum.
Patients who undergo cesarean delivery are at increased risk for postpartum endometritis compared with similar patients who deliver vaginally.9 Prolonged rupture of membranes and/or prolonged duration of labor increase the incidence of postpartum uterine infection. Prophylactic administration of antibiotics decreases the incidence of postpartum uterine infection and wound infection after cesarean delivery in all women, whether performed electively or emergently.111 No important differences have been identified among various skin preparation solutions used before cesarean delivery.112 Vaginal preparation before cesarean delivery, however, may reduce endometritis.113 Systematic reviews have found insufficient evidence to recommend prophylactic antibiotics for operative vaginal delivery or for manual removal of the placenta after vaginal delivery; however, consideration may be given to prophylactic antibiotics in the setting of complex perineal repairs.114–116 Endometritis typically responds to appropriate antibiotic therapy, and outcomes are generally excellent. However, serious complications (e.g., peritonitis, abscess, septic thrombophlebitis) may rarely occur.9,49 In future pregnancies, women who have experienced endometritis are at increased risk for uterine rupture, and anesthesiologists and obstetricians should demonstrate extra vigilance for this serious complication.37
Sepsis and Septic Shock
Sepsis is a rare, life-threatening complication of maternal infection that complicates approximately 1 in 8000 deliveries.117 Sepsis is defined by the American College of Chest Physicians and Society of Critical Care Medicine as infection that precipitates the systemic inflammatory response syndrome (SIRS), characterized by two or more of the following criteria: hyperthermia, hypothermia, tachycardia, tachypnea, or leukocytosis (see Chapter 55).118 Pregnancy may complicate the diagnosis, because many healthy pregnant women exhibit some of these signs.119 Noninfectious inflammation may also lead to SIRS; in all cases the syndrome appears to be triggered by mediators released from immune effector cells, including tumor necrosis factor, interleukins, and cyclooxygenase metabolites of arachidonic acid.120
Severe sepsis is defined as sepsis with acute organ dysfunction, hypotension, or sepsis-induced hypoperfusion,118 and septic shock is defined as sepsis-induced hypotension that persists despite adequate fluid resuscitation, along with the presence of hypoperfusion abnormalities or organ dysfunction.118 The incidence of sepsis and sepsis-related maternal mortality appears to be rising; it was the most common cause of death (1.13 per 100,000 maternities) in the most recent (2006-2008) Confidential Enquiries into Maternal Death in the United Kingdom report.121
In pregnant women, sepsis typically is associated with gram-negative bacteremia, although it can occur in association with gram-positive aerobic and anaerobic infections.122 Polymicrobial etiology is common,117,123 particularly when the source of sepsis is a pelvic infection.122 Group A β-hemolytic streptococcal infection appears to predominate in fatal cases or those associated with major morbidity, although Clostridium species have been implicated in deaths of several otherwise healthy women undergoing gynecologic or obstetric procedures.124,125 Untreated pneumonia, chorioamnionitis, pyelonephritis, endometritis, wound infection, incomplete abortion, and self-induced abortion may result in maternal sepsis, severe sepsis, and septic shock; case reports have attributed iatrogenic maternal sepsis to amniocentesis,126–129 medical abortion,125 dental procedures, and assisted reproductive procedures.130 Approximately 45% of cases occur postpartum.131 The clinical course is often fulminant. In a series of nearly 100 cases from The Netherlands, more than half of the patients demonstrated signs of severe sepsis within 48 hours of the first sign of infection and 39% did so within 24 hours.131
Studies have suggested that pregnancy makes laboratory animals more susceptible to infection by a number of mechanisms.132 For example, Beller et al.133 infused E. coli B6 lipopolysaccharide (endotoxin) into both nonpregnant and pregnant minipigs. The average duration of survival for the nonpregnant animals was 16 hours, whereas the average duration of survival for the pregnant animals was only 3.5 hours. The pregnant animals suffered more pronounced cardiovascular abnormalities and metabolic acidosis than the nonpregnant animals.
It is unclear whether pregnant women are more susceptible to sepsis than age-matched nonpregnant women. Case series of maternal sepsis have reported overall case-fatality rates of 6% and 7.7%.122,131 This survival rate may reflect the relative youth of pregnant women and the effectiveness of surgical source control for most pelvic infections. Nonetheless, clinical studies have noted that once septic shock develops in pregnant patients, maternal mortality is as high as 30%.134–136
The mainstay of therapy is elimination and/or aggressive treatment of the source of infection with antibiotics and, if indicated, surgical extirpation. Initial antibiotic therapy should include broad-spectrum coverage for bacteria such as E. coli, enterococcus, and anaerobic organisms. A combination of ampicillin, gentamicin, and clindamycin represents an effective regimen, as does a combination of imipenem, cilastatin, and vancomycin.137 A 2012 review of cases from the United Kingdom Center for Maternal and Child Enquiries (CMACE) project suggested initial broad-spectrum antibiotic coverage (e.g., gentamicin with either piperacillin-tazobactam or ciprofloxacin), consultation with an infectious disease specialist as soon as possible, and appropriate adjustment of antibiotic therapy once the causative organism and antibiotic susceptibilities have been identified.138 Local patterns of microbial resistance should be considered when selecting broad-spectrum empirical therapy. In all cases, aggressive antibiotic initiation without delay (within 1 hour of diagnosis) appears important for a favorable outcome.121,139
Only a few reports have described the management of these seriously ill patients. Timezguid et al.122 summarized 66 intensive care unit admissions for sepsis between 1977 and 2008. Seventeen of 22 women with a pelvic source of infection required surgical source control, including termination of pregnancy (n = 5), uterine exploration for removal of placental fragments (n = 6), drainage of a pelvic abscess (n = 5), or hysterectomy (n = 3). Premature termination of pregnancy was more frequent in 12 cases of chorioamnionitis (83%) than in the 32 cases of extrapelvic infections (31%). Vasopressors, mechanical ventilation, and renal replacement therapy were used in 16 (25%), 19 (28%), and 4 (6%) cases, respectively.
Lee et al.135 reviewed 10 cases of septic shock in obstetric patients between 1984 and 1986, which were caused by pelvic infections in 9 cases and mastitis in 1. Eight of the 10 women required inotropic or vasopressor infusions, or both, guided by pulmonary arterial catheterization. Two women died. The primary hemodynamic abnormalities were decreased systemic vascular resistance and depressed myocardial function.
Mabie et al.136 reported 18 cases of pregnancy-associated septic shock that occurred during 11 years in a single institution, an incidence of 1 per 8338 deliveries. The most common causes were pyelonephritis (n = 6), chorioamnionitis (n = 3), endometritis (n = 2), and toxic shock syndrome (n = 2). Five patients (28%) died. The hemodynamic profiles of the patients in this series were similar to those described by Lee et al.135: four of the five patients who died had mildly or severely depressed myocardial function.136 Eight patients underwent at least one surgical procedure to control the source of infection.
In most cases, concomitant supportive therapy is required to decrease maternal and fetal morbidity. Physicians must give attention to the maintenance of maternal oxygenation, circulation, and coagulation. Most authorities suggest following the Surviving Sepsis guidelines for treatment of sepsis.121,138,139 The guidelines recommend goal-directed therapy aiming to achieve a central venous pressure (CVP) between 8 and 12 mm Hg and a MAP of 65 mm Hg, urine output of 0.5 mL/kg/h, and mixed venous oxygen saturation 65% or higher (see Chapter 55).139 Pregnant women were excluded from trials that evaluated this goal-directed therapy,140,141 and unfortunately no evidence is available to confirm that this approach offers survival benefit for pregnant or recently delivered women. In nonpregnant patients with septic shock, norepinephrine is considered the first choice vasopressor and may be supplemented with epinephrine or vasopressin to maintain adequate blood pressure.139
Evidence from nonobstetric patients receiving intensive care indicates that aggressive glycemic control may not confer a survival advantage in all critically ill patients, although some evidence suggests that avoiding hyperglycemia improves outcomes in surgical patients.142 Overly tight glycemic targets increase the risk for hypoglycemia; current guidelines recommend treatment to avoid hyperglycemia (> 180 mg/dL), hypoglycemia, and wide swings in glucose levels.139 More intensive glucose control may be indicated in the intrapartum period to achieve optimal neonatal outcomes. Corticosteroid supplementation is indicated only when fluid resuscitation and vasopressor therapy fail to restore hemodynamic stability.139,143
Although the effects of maternal therapy on the fetus should be considered, treatment of the mother has first priority. Often what is best for the mother will be best for the fetus. However, in some cases, maternal sepsis may require preterm delivery, or even delivery before the age of viability, particularly if chorioamnionitis is present. Although the American College of Obstetricians and Gynecologists (ACOG) has stated that “delivery usually is not indicated in the septic pregnant patient in whom the pregnancy is not the source of infection,”137 a multidisciplinary team should consider the mother’s response to initial therapeutic interventions, her vasopressor, oxygen and ventilatory requirements, and the gestational age and fetal well-being when considering the optimal timing and route of delivery.
Epidural Analgesia and Maternal Fever
Incidence
Epidural anesthesia administered for surgery—including cesarean delivery—typically results in hypothermia. This effect occurs because vasodilation produced by the sympathetic neuroblockade causes a redistribution of body heat from the core to the periphery, where it is lost to the environment.144
In 1989, Fusi et al.145 first observed that epidural labor analgesia was associated with progressive intrapartum maternal pyrexia. They reported that the vaginal temperatures of 18 parturients who received epidural analgesia increased approximately 1° C over 7 hours, whereas the temperatures of 15 women who received intramuscular meperidine and metoclopramide remained constant. There was no evidence of infection in any of the women. The authors suggested that epidural analgesia may cause an “imbalance between the heat-producing and heat-dissipating mechanisms.”
Fusi et al.145 measured vaginal temperature; this measurement may be affected by the sympathectomy and vaginal mucosal vasodilation associated with epidural analgesia. Tympanic membrane temperature should not be affected by the local vasodilation associated with epidural analgesia, and it may provide a more accurate assessment of core temperature. Camann et al.146 studied the effect of epidural analgesia on maternal oral and tympanic membrane temperature measurements in 53 laboring women. They studied three groups of patients; one group received intravenous nalbuphine, one group received epidural bupivacaine only, and one group received epidural bupivacaine with fentanyl. The patients were not randomized to receive either nalbuphine or epidural analgesia; however, the women who requested epidural analgesia were randomized to receive epidural bupivacaine only or epidural bupivacaine with fentanyl. The authors maintained ambient room temperature between 20° C to 22° C. Epidural analgesia did not affect maternal temperature during the first 4 hours of the study. At 5 hours and thereafter, the mean tympanic membrane temperatures were significantly higher in both of the epidural groups than the intravenous nalbuphine group (Figure 37-3). Among women in the bupivacaine-only group, mean tympanic temperature increased from approximately 36.6° C at 1 hour to 37.1° C at 9 hours, a rate of rise of less than 0.07° C per hour. There was no difference between the epidural bupivacaine-only and the epidural bupivacaine-fentanyl groups in maternal tympanic membrane temperature measurements.
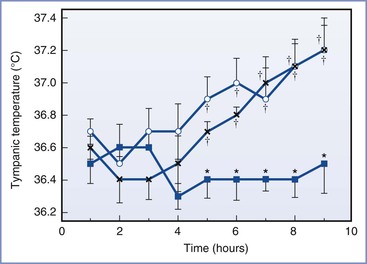
FIGURE 37-3 Mean (SE) tympanic temperatures during labor in three groups of patients: Epidural bupivacaine-fentanyl (open circles), epidural bupivacaine-only (x), and parenteral opioid (blue squares) groups. *P < .01 compared with the epidural group; †P < .01 compared with the pre–epidural analgesia temperature. (From Camann WR, Hortvet LA, Hughes N, et al. Maternal temperature regulation during extradural analgesia for labour. Br J Anaesth 1991; 657:565-8.)
Several other investigators have documented similar patterns of temperature elevation in laboring women receiving epidural analgesia.41,147–152 These studies typically have found the average rate of temperature increase to be approximately 0.1° C per hour of epidural analgesia, usually after a lag time of 4 to 5 hours. Other investigations have documented an increase in clinical fever in women with epidural analgesia, usually defined as temperature greater than 37.5° C or greater than 38° C (Table 37-1).* These studies have employed various study designs, most commonly observational investigations of women self-selecting the mode of analgesia. Selection bias, reflecting greater prevalence of risk factors for fever among women choosing epidural analgesia, undoubtedly has affected the results of these reports.163 However, other study designs have confirmed the finding of greater risk for fever in women who receive epidural analgesia. In a natural experiment, in which epidural analgesia was rapidly introduced in a facility in which it was previously unavailable, clinical fever markedly increased after epidural analgesia was widely available.147 In addition, eight randomized controlled trials, in which women were randomly assigned to receive epidural analgesia or parenteral opioids (or nonpharmacologic analgesia in one study155), also confirmed an increase in clinical fever in women who were randomized to receive epidural analgesia.152,155,156,158–162 Only one study examined fever as a primary outcome.155 Meta-analysis of six trials found a relative risk (RR) for fever of 3.34 (95% confidence interval, 2.63 to 4.23) for epidural compared with nonepidural or no labor analgesia.164 Most trials have investigated epidural labor analgesia, but at least one trial specifically investigated combined spinal-epidural (CSE) analgesia.155
TABLE 37-1
Incidence of Clinical Fever in Women with Epidural Labor Analgesia
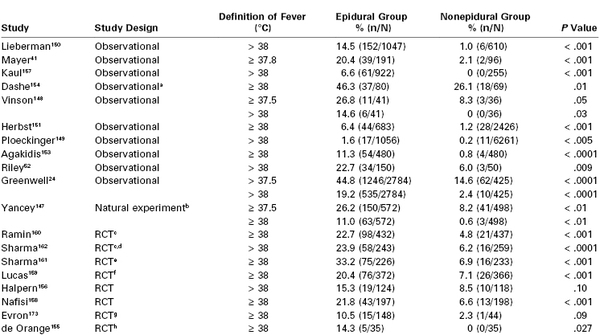
a All patients had ruptured membranes > 6 h; outcome included fever up to 6 h postpartum.
b Natural experiment (impact study) compared two time periods, before and after the introduction of on-demand labor epidural analgesia to a single institution. The “after” period is reported as the epidural group (83% of nulliparous women received epidural analgesia); the “before” period is reported as the nonepidural group (1% received epidural analgesia).
c Fever was reported for protocol-compliant women only.
d Data from this investigation were reanalyzed by Philip et al.152
e Only nulliparous women participated in the study.
f The study included parturients with gestational hypertension or preeclampsia; the percentages were recalculated from n/N reported in the publication.
g The epidural group comprised three groups: (1) epidural analgesia only, (2) epidural analgesia with intravenous remifentanil, and (3) epidural analgesia with intravenous acetaminophen.
h Patients were randomized to receive combined-spinal epidural (CSE) analgesia or nonpharmacologic analgesia.
RCT, randomized controlled trial.
Originally viewed as two separate phenomena, the slow progressive temperature rise observed by Fusi145 and Camann146 and the clinical fever observed in other studies may be manifestations of the same process. Two investigations have concluded that the slow rise may actually be an artifact caused by averaging the temperature curves of women who develop clinical fever with those who remain afebrile (see Figure 23-7).165,166 Frölich et al.167 compared the slope of the temperature-time curves in women before and after receiving epidural analgesia and found no difference. They examined only women who did not develop a temperature greater than 38° C, and thus their finding supports the observation that epidural analgesia affects temperature in only a subset of women who develop clinical fever. These findings imply that an understanding of the nature of the relationship between epidural analgesia and overt fever is the key to understanding hyperthermia during labor in these patients.
Etiology
The mechanisms by which epidural analgesia produces maternal hyperthermia during labor remain unclear, and at least three types of explanation are plausible: thermoregulatory factors, effects of systemic opioids in patients not receiving epidural analgesia, and inflammation.163
Thermoregulatory factors that may play a role include ambient temperature, impaired heat dissipation, and increased heat production. Fusi et al.145 attributed the maternal pyrexia to the high ambient temperature (24° C to 26° C) found in most British delivery rooms. However, other investigators have failed to find an association between ambient temperature and maternal148 or fetal8 temperature. Alternatively, epidural analgesia may impair heat-dissipating mechanisms. Decreased sweating and the lack of hyperventilation that follow the provision of effective epidural pain relief may predispose laboring women to pyrexia.145,146,168 In volunteers, epidural anesthesia raised the sweating threshold by 0.55° C.169 Moreover, epidural analgesia attenuates the significant increase in oxygen consumption observed during uterine contractions and the even greater increase observed during expulsive efforts.170 This lack of increase in ventilation in the setting of increased energy expenditure may manifest as increased temperature in the laboring patient. Finally, the high incidence of shivering among laboring women who receive epidural analgesia may predispose them to the development of fever. Gleeson et al.39 found that laboring patients who shivered after the administration of epidural analgesia developed pyrexia as early as 1 hour after initiation of neuroblockade, compared with more than 4 hours after initiation neuroblockade in patients who did not shiver. Moreover, the maximum temperature was higher and the incidence of clinical fever was three times more common in the women who shivered.39 Some shivering and sweating in labor has been demonstrated to be nonthermoregulatory (i.e., not accompanied by changes in core temperature or vasomotor tone).40 However, it is not clear whether shivering is a cause or an effect of factors increasing body temperature.
Parenteral opioids administered to women for labor analgesia might suppress fever that would otherwise have been apparent. In a nonpregnant volunteer study, fever was induced by the injection of interleukin-2.171 Epidural analgesia with ropivacaine with or without epidural fentanyl did not affect the magnitude of the fever, but intravenous fentanyl markedly attenuated the rise in temperature.171 However, in one large retrospective study,172 differences in intravenous nalbuphine use did not explain the increased occurrence of fever among those receiving epidural analgesia. Evron et al.173 randomized laboring women to receive epidural ropivacaine, intravenous remifentanil, both drugs, or epidural ropivacaine and intravenous acetaminophen. They identified fever (temperature ≥ 38° C) in 14% of the ropivacaine group, 8% in the ropivacaine and remifentanil group, 8% in the ropivacaine and acetaminophen group, and 1% in the remifentanil group. Taken together, these investigations suggest that any fever-suppressing effect of parenteral opioids is relatively weak and is seen only with µ-opioid receptor agonists.
The predominant theory to date holds that inflammation, perhaps of a noninfectious etiology, is responsible for epidural analgesia–associated fever. Several lines of evidence support this mechanism. First, women who are more likely to request epidural analgesia during labor also are more likely to have other risk factors for fever during labor. These may include nulliparity,151,174 premature rupture of membranes,175 prolonged rupture of membranes,148,151,174 induction of labor,175 unfavorable cervical examination at admission,175 prostaglandin exposure,175 prolonged labor,8,146,148,151 obesity, higher temperature on admission,151 early chorioamnionitis,151 and possibly more cervical examinations.46,175,176
Second, women with epidural analgesia and fever more frequently demonstrate placental inflammation. In a case-control study, Vallejo et al.177 compared women without epidural analgesia and with clinical chorioamnionitis (temperature ≥ 38° C and clinical manifestations) to two groups of women who received epidural analgesia, one with and the other without clinical chorioamnionitis. The diagnosis of chorioamnionitis was confirmed histologically. Not surprisingly, fever was far more common in the women with chorioamnionitis. In fact, the incidence of fever in women with epidural analgesia but without chorioamnionitis was only 1%.177 Similarly, Dashe et al.154 analyzed the records and placental pathology of 149 women who delivered more than 6 hours after membrane rupture. They found an increased incidence of fever (temperature ≥ 38° C) in the 54% of their subjects who received epidural analgesia. However, histologic evidence of placental inflammation was also more common among epidural analgesia–exposed women. In the absence of placental inflammation, the incidence of maternal fever was equivalent in the epidural analgesia–exposed and unexposed patients (11% and 9%, respectively).154
Importantly, the placental inflammation associated with epidural analgesia–associated fever may not indicate bacterial infection. Riley et al.52 examined the placentas of 200 women, 150 of whom received epidural analgesia, who had participated in two previous randomized trials of the presence of birthing assistants. The authors attempted to culture bacteria from the placentas, as well as to detect bacterial DNA by the polymerase chain reaction; they also measured serum interleukin concentrations. Fever was more common in women who received epidural analgesia than in those who did not (23% versus 6%, respectively; P = .009), but the incidence of infection was low in both groups (4.7% versus 4.0%, respectively; P > .99). An admission interleukin-6 (IL-6) level greater than 11 pg/mL predicted development of fever, irrespective of analgesic choice, and IL-6 levels increased more over the course of labor in women choosing epidural analgesia, irrespective of development of fever.
A third line of evidence supporting an inflammatory mechanism of epidural analgesia–associated fever is that biomarkers of inflammation are more common in women with epidural analgesia who develop fever. Goetzl et al.178 randomized women with epidural analgesia to receive acetaminophen or placebo in labor. The incidence of fever (temperature > 38° C) was identical in the two groups. They demonstrated elevated maternal serum and umbilical cord blood markers of inflammation (IL-6) in febrile women. Pathologic examination of the placenta was not reported.178 As discussed earlier, Riley et al.52 also found higher IL-6 levels in women receiving epidural analgesia and in women developing fever regardless of whether they received epidural analgesia. In a second trial, Goetzl et al.179 were able to demonstrate suppression of epidural analgesia–associated fever with high-dose maternal corticosteroid therapy (methylprednisolone 100 mg every 4 hours). Umbilical cord blood IL-6 levels were lower in corticosteroid-treated patients, indicating suppression of inflammation. However, neonatal bacteremia was significantly increased by corticosteroid exposure.179 In a small and likely underpowered trial, Wang et al.180 randomized low-risk parturients with epidural analgesia to receive bupivacaine and fentanyl alone or combined with epidural dexamethasone 0.2 mg/mL. They observed no difference in the incidence of overt fever or placental inflammation but a reduction in mean temperature in the dexamethasone group. Maternal and umbilical cord blood IL-6 levels were reduced in the corticosteroid group, although the differences were not significant. Fetal condition was excellent in both groups.
The mechanism by which epidural analgesia might induce inflammation in a subgroup of women remains unclear. In nonrandomized investigations, differences in patient factors among women selecting epidural analgesia may indicate confounding risk factors for inflammation. Even in randomized trials, differences in obstetric management could also confound the association, because it is not possible to blind the treatment group in trials of epidural analgesia.163 For example, epidural analgesia is associated with greater use of oxytocin in randomized trials, and it is conceivable that women randomly assigned to receive epidural analgesia may also receive earlier artificial rupture of membranes, more frequent cervical examinations, and greater use of internal monitoring in order to titrate oxytocin administration.52,152 Conversely, a direct action of epidural local anesthetic in stimulating inflammation has been proposed, with the effect mediated through activation of the vanilloid receptor TRPV1.181
Clinical Impact
Epidural analgesia–associated maternal hyperthermia has become a subject of significant controversy. As discussed earlier, maternal fever attributed to clinical chorioamnionitis and other infection is associated with adverse neonatal condition as well as more severe neurologic injuries, including seizures, encephalopathy, and cerebral palsy. However, the effect of epidural analgesia–associated fever has not been specifically elucidated. In randomized trials comparing epidural and nonepidural analgesia, neonatal condition as measured by umbilical arterial acidosis or 5-minute Apgar score is consistently better in the epidural analgesia groups and neonatal intensive care unit admission does not differ between groups.164 In a large retrospective analysis, epidural analgesia with fever was associated with adverse neonatal outcomes, but in the absence of fever epidural analgesia was not.24 To date, no evidence has linked noninfectious epidural analgesia–associated fever to severe neonatal adverse outcomes.
It is possible that the mother and neonate may be placed at risk indirectly as a result of the interventions triggered by the occurrence of maternal fever.41,150 For example, antibiotic administration is more likely in the setting of maternal fever. Mayer et al.41 retrospectively analyzed the records of 300 low-risk nulliparous women who received systemic opioids, epidural analgesia, or both and identified an increased incidence of both fever (≥ 37.8° C) and antibiotic administration among the two groups who received epidural analgesia. Nonetheless, only 5 of 10 patients with culture- or pathology-proven chorioamnionitis developed fever and none had fever as the only presenting sign or symptom. The authors suggested that obstetricians seek additional evidence of infection before treating all maternal fever with antibiotics.
In another retrospective study, Lieberman et al.150 reanalyzed the records of 1657 low-risk nulliparous women originally enrolled in a trial of active management of labor.182 They, too, found a higher incidence of maternal temperature (> 38° C) in parturients who received epidural analgesia compared with those who did not (15% versus 1%, respectively). Neonates in the epidural group had a higher incidence of sepsis evaluation (34% versus 10%, respectively) and antibiotic treatment (15% versus 4%, respectively) compared with neonates in the nonepidural group. The incidence of actual neonatal sepsis was very low in both groups (0.3% and 0.2%, respectively). As in all studies in which women self-select their analgesia, the women receiving epidural analgesia were already at risk for intrapartum fever. For example, they had larger infants, longer labors, and a twofold increase in the rate of induction of labor.150 Moreover, the active labor management protocol mandated frequent cervical examinations and early amniotomy, which may have increased the risk for fever.183 Of interest, two thirds of the sepsis evaluations occurred in infants of mothers who did not have intrapartum fever.150,183
To further elucidate the relationship between epidural analgesia and neonatal sepsis evaluation, the same group reanalyzed the subcohort of women182 admitted in spontaneous labor, in whom intrapartum temperature remained below 38° C throughout labor.184 Epidural analgesia was associated with both major (rupture of membranes > 24 hours, FHR > 160 beats/min) and minor criteria (maternal temperature > 37.5° C, rupture of membranes 12 to 24 hours) for sepsis evaluation. It is likely that many of the features of labor that led to sepsis evaluations also predisposed women to choose epidural analgesia.
Subsequent work by other investigators has suggested the role of maternal temperature elevation as the key feature related to neonatal sepsis evaluations. Philip et al.152 demonstrated that women randomized to receive epidural analgesia had a higher incidence of fever (≥ 38° C) than those randomized to receive intravenous analgesia (15% versus 4%, respectively). Neonatal sepsis evaluations were also much more common in women with fever than in those without (96% versus 13%, respectively). However, within both the febrile and the afebrile cohorts, the incidence of neonatal sepsis evaluation was independent of analgesic type. The authors concluded152:
… our results indicate that in the absence of maternal fever, epidural analgesia during labor has no bearing on the need for such neonatal management and therefore should not be considered a predictor per se for neonatal sepsis evaluations. We attribute this finding to the minimization of ascertainment bias as a result of randomization of analgesia.
Other investigators have highlighted the importance of neonatal practice style in determining the rate of sepsis evaluations. Yancey et al.147 studied the rates of maternal fever and neonatal sepsis evaluation during a period in which epidural analgesia use rapidly increased from 1% to 83% after the introduction of a round-the-clock, on-demand epidural analgesia service. The incidence of fever greater than 37.5° C increased threefold, and fever greater than 38° C increased 18-fold after the introduction of epidural analgesia, although numerous indices of the patients’ admission status and intrapartum obstetric management did not change. The incidence of screening neonatal blood counts and blood cultures increased modestly (RR, 1.5 and 1.7, respectively), but there was no change in antibiotic treatment. The authors contrasted their results to those of Lieberman150 and attributed the difference to neonatal practice patterns that did not require antibiotic therapy solely on the basis of maternal fever or antibiotic exposure.147
Kaul et al.157 also emphasized neonatologists’ practice style. They analyzed the records of 1177 nulliparous women and their neonates. Women with epidural analgesia had an increased risk for fever compared with women who received parenteral opioid analgesia (7% versus 0%, respectively), but multivariate logistic regression failed to demonstrate a significant association between epidural analgesia or maternal fever and neonatal sepsis evaluation. In contrast to Lieberman et al.,150 the authors attributed the lack of effect of epidural analgesia to more stringent guidelines for neonatal sepsis evaluation that required at least one neonatal sign or symptom of infection regardless of the maternal condition.157
The ability of epidural analgesia to stimulate maternal fever is likely a real phenomenon and not purely an artifact of higher-risk patients selecting epidural analgesia more frequently. Because of the growing evidence that maternal inflammation and infection, manifesting as fever, can be detrimental to the fetal brain, anesthesiologists cannot dismiss this unique physiologic effect as a mere curiosity. Further study to elucidate the link between epidural analgesia, pregnancy and labor, and fever is crucial to developing effective and safe strategies to minimize this link. In addition, attention must be paid to the indirect effects of maternal fever on the clinical decision-making of obstetricians and neonatologists to minimize unnecessary maternal and fetal interventions. In the meantime, if maternal pyrexia occurs, good clinical practice dictates that efforts be made to lower maternal temperature and identify and treat a presumed maternal infection.
Neuraxial Anesthesia in the Febrile or Infected Patient
Clinicians have long suspected an association between the performance of dural puncture during a period of bacteremia and the subsequent development of meningitis. Some clinicians have feared that diagnostic lumbar puncture may cause meningitis rather than aid in its diagnosis. They reasoned that lumbar puncture may disrupt the rich venous plexus surrounding the spinal cord and allow the direct introduction of infected blood into the CNS by the spinal needle. Alternatively, others have speculated that disruption of the dural barrier may permit hematogenous spread of infection into the CNS without direct vessel trauma. Similar concerns apply to the performance of neuraxial anesthesia and the development of spinal-epidural abscess (see Chapter 32). Administration of continuous epidural analgesia often results in blood vessel trauma, and it includes the introduction of a foreign body. Theoretically, this technique could produce a nidus for subsequent infection.
Laboratory Studies
Carp and Bailey185 performed a study to assess the risk for meningitis after the performance of dural puncture in bacteremic rodents. In this study, rats were made bacteremic by producing a flank abscess using E. coli bacteria. The bacteremia was similar in magnitude to that which occurs during the early phase of sepsis in humans. Cisternal dural puncture was performed after the onset of bacteremia. After 24 hours, the cisterna magna was drained surgically, and the cerebrospinal fluid (CSF) was cultured for evidence of meningitis. Of the 40 animals that underwent dural puncture during E. coli bacteremia, 12 developed meningitis (Table 37-2). None of the 40 bacteremic animals not subjected to dural puncture developed meningitis. Furthermore, dural puncture did not result in infection in the 30 animals without bacteremia. Importantly, none of the 30 bacteremic animals given a dose of gentamicin 15 minutes before dural puncture developed meningitis.
TABLE 37-2
The Association between Bacteremia and the Recovery of Escherichia coli from Cerebrospinal Fluid after Dural Puncture in Rats

* Data expressed as mean ± SD (range in parentheses).
† Gentamicin administered before dural puncture.
‡ Data expressed as the number of animals in which E. coli cultured from spinal fluid per total number of animals in that group.
§ P < .05 compared with other groups.
‖ Not significantly different from that in the bacteremic groups undergoing cisternal puncture.
CFU, colony-forming unit(s); CSF, cerebrospinal fluid; n, number of rats in each group.
Modified from Carp H, Bailey S. The association between meningitis and dural puncture in bacteremic rats. Anesthesiology 1992; 76:739-42.
This study is consistent with earlier laboratory studies that observed the development of meningitis after the performance of dural puncture in bacteremic laboratory animals.186,187 Although animal models of disease permit careful control of experimental conditions, these studies do not duplicate clinical conditions. Thus, there are limitations in the application of the rat study185 to clinical practice. First, the level of bacteremia produced in the rats exceeded the transient, low-grade bacteremia that often occurs clinically. Also, these animals most likely had hemodynamic and metabolic changes characteristic of early sepsis. Second, although E. coli is a common cause of bacteremia in surgical and obstetric patients, it is an uncommon cause of meningitis. Third, the relative size of the dural tear produced by the 26-gauge needle used in this study is greater in rats than humans. Fourth, the cisternal site of dural puncture is not used clinically. Fifth, spinal and epidural anesthesia involves the injection of local anesthetics, and these drugs appear to be bacteriostatic.188 Finally, the investigators knew the identity of the organism (E. coli) and also knew that it was susceptible to gentamicin.
In summary, this study suggests that high-grade bacteremia may increase the risk for meningitis after dural puncture. However, antibiotic therapy before dural puncture appears to reduce if not eliminate this risk.
Clinical Studies
At least six retrospective clinical studies have evaluated diagnostic lumbar puncture and the risk for meningitis.189–194 (These studies did not evaluate the risk of neuraxial anesthesia or analgesia.) These reports provided conflicting conclusions regarding the risk for meningitis after the performance of dural puncture in bacteremic patients. Two studies suggested an association between dural puncture and meningitis.189,192 However, both studies had serious methodologic flaws. One study was performed during an epidemic of meningitis.189 Although the authors observed a high rate of meningitis after lumbar puncture, they did not evaluate a comparable control group who did not undergo lumbar puncture. Teele et al.192 reported an association between lumbar puncture and meningitis only in bacteremic children younger than 1 year of age. However, they acknowledged the possibility that clinical judgment might have prompted their pediatricians to perform diagnostic lumbar puncture in children with incipient meningitis before the CSF provided diagnostic evidence of infection.
The remaining four studies clearly did not support an association between dural puncture and meningitis.190,191,193,194 Shapiro et al.194 concluded:
The development of bacterial meningitis in children with occult bacteremia is strongly associated with the species of bacteria that causes the infection, but not with a lumbar puncture.…Children with high-density bacteremia may appear to be more severely ill than children who have bacteremia with lower concentrations of bacteria, and therefore may be more likely to undergo a lumbar puncture.
In an editorial, Chestnut195 stated, “Physicians often perform diagnostic lumbar puncture in patients with fever and/or bacteremia of unknown origin. If dural puncture during bacteremia results in meningitis, one would expect that unequivocal clinical data should exist.” However, no epidemiologic study has clearly established a causal relationship between the performance of dural puncture during bacteremia and the subsequent development of meningitis or epidural abscess. Part of the uncertainty regarding the risk of dural puncture results from awareness that processes other than meningeal integrity may help protect against the occurrence of CNS infection. For example, as many as 35% of epidural catheters used postoperatively are colonized by bacteria,196 but epidural abscess is a very rare complication. Some anesthesiologists have cited anecdotal reports of meningitis after spinal anesthesia during presumed, but not documented, bacteremia as evidence that dural puncture may cause meningitis.197–200 In one of these reports, the physicians used reusable equipment and the source of infection was traced to inadequately sterilized supplies.197 The use of sterile, disposable equipment and strict attention to aseptic technique have largely eliminated these factors as a source of infection.
Some evidence points to external contamination as the source of meningitis after spinal anesthesia (see Chapter 32).201–204 Rubin203 reported six cases of meningitis after spinal anesthesia over a 5-year period; all procedures were performed by the same anesthesiologist and the infections were all caused by the same organism. Videira et al.205 reported three cases of meningitis after 38,128 spinal anesthetics (1 : 12,709). In two cases, streptococci presumed to be skin or nasopharyngeal flora were cultured. The authors concluded that lapses in sterile technique may have been responsible for the meningitis. Poor attention to asepsis was also apparently responsible for three cases of meningitis reported in a 3-year period in a single hospital.206 In one case, the offending organism was cultured from the nose of the anesthesiologist performing the procedure. A 2012 outbreak of fungal meningitis in patients receiving otherwise uncomplicated epidural steroid injections was traced to contaminated methylprednisolone.207
Several reports have described the occurrence of meningitis after CSE analgesia in obstetric patients.208–210 None of these patients was febrile during the CSE procedure. Furthermore, in the cases with positive CSF cultures, the authors concluded that contamination by skin flora was the most likely mechanism of infection. In general, evidence does not support a greater risk for meningitis with the CSE technique than with other neuraxial procedures, but rather a reporting bias involving complications of a relatively new technique.211
Large epidemiologic studies have found a very low incidence of CNS infection after the administration of neuraxial anesthesia. Dripps and Vandam212 prospectively studied 8460 patients who received 10,098 spinal anesthetics between 1948 and 1951. Similarly, Phillips et al.213 reported the administration of spinal anesthesia to 10,440 patients between 1964 and 1966. A large number of the patients in both studies underwent obstetric or urologic procedures. Undoubtedly some patients had bacteremia during or after the performance of spinal anesthesia. However, neither study reported a single case of CNS infection.212,213
Some evidence supports a lower frequency of infectious complications in obstetric patients than in general surgical patients. A 10-year review in Sweden of 1,260,000 spinal anesthetics and 450,000 epidural procedures (including 200,000 labor analgesia procedures) found 29 cases of meningitis and 13 epidural abscesses. Among the obstetric patients, there were no episodes of meningitis and one abscess.214 Similarly, four reviews that included a total of more than 500,000 obstetric patients who received epidural anesthesia reported no cases of meningitis and two cases of epidural infection.215–218 A 2009 national audit conducted in the United Kingdom gathered data on all central neuraxial procedures over a 1-year period (707,425 total cases and 320,425 obstetric cases).219 Three cases of meningitis and 15 epidural abscesses were reported, but no meningitis and only one abscess was observed in the obstetric population.
Undoubtedly some parturients are bacteremic during the administration of epidural or spinal anesthesia, given the frequency with which parturients develop fever and infection during labor. For example, Blanco et al.50 found a 1% incidence of bacteremia in a random sample of patients on the labor ward. Other studies have noted an incidence of bacteremia ranging from 2.3% to 12% in parturients with chorioamnionitis.47,49,51 Unfortunately, there are no good predictive factors for identifying the subgroup of febrile patients with chorioamnionitis who are bacteremic at the time of anesthesia. The severity of fever does not reliably predict the likelihood of bacteremia in these patients.220 For example, Blanco et al.50 reported that 86 (49%) of 176 patients with documented bacteremia had a temperature below 38.8° C. Furthermore, Bader et al.220 reported no significant difference in the mean temperatures of bacteremic and nonbacteremic patients with chorioamnionitis. Similarly, in a study of 146 women with chorioamnionitis, Goodman et al.51 found no differences in temperature, leukocytosis, or maternal symptoms between patients with positive and negative blood cultures.
Bader et al.220 retrospectively observed no cases of CNS infection after the administration of epidural or spinal anesthesia for labor and/or cesarean delivery in 279 patients with chorioamnionitis. Only 43 of these 279 women received antibiotic therapy before the administration of neuraxial anesthesia. At least three women had positive blood cultures consistent with bacteremia, and none of these three women received antibiotics before the administration of anesthesia. Similarly, Goodman et al.51 found no cases of meningitis or epidural abscess among 531 patients with chorioamnionitis (proven by culture or pathologic examination) who received epidural (n = 517) or spinal (n = 14) anesthesia. Eleven of 45 patients with fever before initiation of the neuraxial procedure, and 174 of 229 patients with preexisting leukocytosis, received no antibiotics before instrumentation of the epidural or subarachnoid space.
Together, these clinical studies suggest that meningitis and epidural abscess are rare complications of epidural or spinal anesthesia. Furthermore, bacteremia itself does not appear to increase the risk for CNS infection after the administration of neuraxial anesthesia. However, published studies of neuraxial anesthesia in patients with chorioamnionitis were small and retrospective. Given the infrequent occurrence of CNS infection among noninfected patients undergoing neuraxial anesthesia, none of these studies was sufficiently large to exclude the possibility that chorioamnionitis increases the risk for meningitis or epidural abscess. Moreover, the retrospective study design introduces the possibility of selection bias; anesthesia providers may have avoided neuraxial anesthesia in the sickest patients with chorioamnionitis.
Recommendations
In our judgment, the anesthesiologist may safely administer spinal or epidural anesthesia to healthy patients at risk for bacteremia. The anesthesiologist need not avoid administration of neuraxial anesthesia in patients at risk for transient, low-grade bacteremia after the administration of anesthesia. Moreover, appropriate antibiotic therapy may lessen the risk for meningitis or epidural abscess in patients with established infection. In our practice, we administer spinal or epidural anesthesia to patients with evidence of systemic infection, provided that appropriate antibiotic therapy has begun. Thus, it often is appropriate for the anesthesia provider to request the initiation of antibiotic therapy before administration of analgesia/anesthesia. Finally, although the choice of anesthesia must be individualized, it seems prudent to avoid spinal or epidural anesthesia in untreated patients with overt clinical signs of sepsis.
Chestnut reviewed this subject and concluded195:
We do not give regional [neuraxial] anesthesia in the absence of other relevant information. Rather, we provide care for febrile patients who require anesthesia for labor, delivery, or emergency surgery. When one considers the risks of infection with regional anesthesia, one should ask: What are the alternatives? What are the consequences of withholding regional anesthesia in a febrile patient? For example, what is the greater risk in a febrile parturient: meningitis or epidural abscess after spinal or epidural anesthesia, or failed intubation and aspiration during general anesthesia?
Finally, both physicians and patients should recognize that most cases of meningitis and epidural abscess occur spontaneously. Eng and Seligman191 concluded, “Even if an appropriate temporal sequence…is documented…one cannot differentiate spontaneous meningitis from lumbar puncture–induced meningitis in the individual patient.”
Genital Herpes Infection
Herpes simplex virus type 2 (HSV-2) causes locally recurring disease that is characterized by asymptomatic periods interrupted by episodes of viral reactivation from sites in the sensory ganglia.221 Genital herpes infection typically presents as painful vesicular or papular lesions on the skin or mucous membranes of the genital tract, including the labia, vulva, perineum, cervix, and urethra.222 Primary maternal HSV-2 infection is associated with transient viremia,222 and up to 2% of pregnant women will be primarily infected during gestation.221 Epidemiologic evidence suggests that HSV-1, formerly associated only with perioral lesions (herpes labialis), is now the predominant cause of new genital herpes infections in some populations.223 Patients with primary infection may have systemic symptoms, including fever, headache, and lymphadenopathy, although asymptomatic primary infection is common. Hepatitis, aseptic meningitis, encephalitis, and cauda equina syndrome are uncommon complications of primary genital herpes infection. During recurrent (i.e., secondary) infection, maternal antibodies prevent the recurrence of viremia. Thus systemic symptoms are less severe—or do not occur at all—during episodes of recurrent infection. However, recurrent infection may result in severe symptoms localized to the site of the lesions on the external genitalia. Prodromal symptoms, including vulvar pain or burning, often precede development of recurrent lesions. Unfortunately, asymptomatic shedding of the virus also may occur in the genital tract.221,222
Interaction with Pregnancy
During the first 20 weeks of pregnancy, primary genital herpes infection may be associated with an increased risk for pregnancy loss,224 although contemporary cohort studies have disputed the risk for fetal death.225 However, the major obstetric concern is the potential for transmission of the virus to the infant at the time of birth. The infant may become infected in one of two ways.222 First, infection can occur as the fetus comes in direct contact with the virus during vaginal delivery. Second, intrauterine infection can occur by ascent of the organism after rupture of membranes. Neonatal HSV infection is a life-threatening infection with the potential for permanent CNS sequelae.221,222,224,226 The severity of the neonatal infection may be reduced by the early initiation of antiviral therapy. Meta-analysis of seven randomized trials of antepartum antiviral therapy with acyclovir or valacyclovir demonstrated a reduction in the presence of active genital lesions at delivery (RR, 0.28) and requirement for cesarean delivery (RR, 0.30).227 However, these trials could not demonstrate a reduction in neonatal herpes infection (there were no cases in any trial), and failure of suppressive therapy to prevent such transmission has been reported.226
Retrospective studies suggest that the risk for neonatal HSV infection associated with a primary maternal infection is much greater than that associated with recurrent maternal infection or asymptomatic shedding of the virus.224,228–230 The risk for transmission in the setting of active recurrent herpes has been estimated at 3% and in infected women without genital lesions at delivery at 2 per 10,000.221 Most likely there is a greater risk that the infant will be exposed to the virus during episodes of primary maternal infection; in recurrent herpes there is evidence that passive transfer of antibodies to HSV from the mother to the fetus is protective.
Obstetric Management
A large epidemiologic study of 58,362 women provided direct evidence that cesarean delivery dramatically reduces the overall risk for HSV transmission to the neonate when HSV cultures of the cervix and external genitalia taken at the time of labor were positive (OR, 0.14).228 Other risk factors included maternal HSV seronegativity, positive cervical culture, use of invasive obstetric monitoring, and HSV-1 (compared with HSV-2) infection.228
The ACOG has reviewed the obstetric management of parturients with HSV infections and has concluded the following221:
Cesarean delivery is indicated in women with active genital lesions or prodromal symptoms, such as vulvar pain or burning at delivery, because these symptoms may indicate an impending outbreak. The incidence of neonatal disease is low when there is recurrent maternal disease, but cesarean delivery is recommended because of the potentially serious nature of the disease.…In patients with active HSV infection and ruptured membranes at or near term, a cesarean delivery should be performed as soon as the necessary personnel and equipment can be readied. There is no evidence that there is a duration of rupture of membranes beyond which the fetus does not benefit from cesarean delivery.…In pregnancies remote from term, especially in women with recurrent disease, there is increasing support for continuing the pregnancy to gain benefit from time and use of corticosteroids. There is no consensus on the gestational age at which the risks of prematurity outweigh the risks of HSV.
Of interest, the mothers of most infants infected with HSV have no history of HSV infection and no obvious lesions at the time of delivery.221,230 Previously, it was thought that antenatal viral cultures could predict asymptomatic viral shedding at the time of delivery and reduce the incidence of neonatal infection. Unfortunately, there is little correlation between antepartum HSV culture results and viral shedding at the time of delivery, even in women with a history of previous HSV infection.230 Thus, the ACOG concluded that there was no evidence for cost-effectiveness of various antenatal screening protocols.221
Anesthetic Management
There are at least five published retrospective studies of the use of neuraxial anesthesia in patients with genital HSV infection. These studies reported no serious neurologic sequelae related to the use of neuraxial anesthesia.231–235 However, most of the patients in these studies had recurrent (secondary) infection. Two studies232,233 were limited to patients with recurrent infection, and a third report231 did not indicate whether the patients had primary or recurrent infection. Bader et al.234 reported outcomes for 169 women with genital herpes infection who underwent cesarean delivery. Five of the 169 women in this study had primary infections, and three of these women received spinal anesthesia. Of the three women, one had transient, postoperative weakness of the left leg. The authors234 stated, “None of the cases of primary infection had associated systemic symptoms; it is therefore possible that some of these cases were actually misdiagnosed recurrent infections. The safety of regional anesthesia in patients with primary HSV infection remains unclear.”
Viremia may accompany primary episodes of genital herpes infection. However, viremia rarely complicates recurrent episodes of genital herpes infection. It is unlikely that a spinal or epidural needle could introduce virus into the CNS in patients with recurrent genital herpes infection. Thus, a consensus exists that it is safe to administer spinal or epidural anesthesia to women with recurrent genital herpes infection and no systemic symptoms. There are insufficient data to allow a definitive recommendation regarding the safety of neuraxial anesthesia in patients with primary infection who may be viremic. If the anesthesia provider is confronted with a patient with primary infection, the theoretical risk for CNS infection should be weighed against the risks of alternative methods of analgesia and anesthesia.
Finally, several studies have suggested that spinal or epidural administration of morphine, commonly administered for postcesarean delivery analgesia, increases the incidence of recurrence of oral HSV infection. This phenomenon was confirmed in randomized prospective trials for both epidural236 and intrathecal237 morphine. The cause is unknown, but some investigators have speculated that pruritus and scratching play a role in reactivation of oral lesions. Boyle238 concluded that facial pruritus is a marker of the migration of morphine to the trigeminal nucleus but not the cause of HSV recrudescence. He suggested that immunologic modulation by the opioid within this ganglion is the primary cause of the viral reactivation. Substantial evidence now supports this mechanism.239 A case of HSV-1 meningitis after unintentional dural puncture and passage of an intrathecal catheter has been reported.240 The patient underwent cesarean delivery with spinal bupivacaine, fentanyl, and morphine; the authors suggested reactivation of HSV-1 due to morphine and the presence of the intrathecal catheter may have contributed to the infection.240 To our knowledge, there are no reports suggesting that epidural or intrathecal administration of opioids increases the risk for recurrent genital herpes infection or neonatal herpes infection.
References
1. Wunderlich C. Das Verhalten der Eigenwärme in Krankenheiten. Ott Wigard: Leipzig, Germany; 1868.
2. Mackowiak PA, Wasserman SS, Levine MM. A critical appraisal of 98.6 degrees F, the upper limit of the normal body temperature, and other legacies of Carl Reinhold August Wunderlich. JAMA. 1992;268:1578–1580.
3. Nakamura K. Central circuitries for body temperature regulation and fever. Am J Physiol Regul Integr Comp Physio. 2011;301:R1207–R1228.
4. Dinarello CA. Infection, fever, and exogenous and endogenous pyrogens: some concepts have changed. J Endotoxin Res. 2004;10:201–222.
5. Novy MJ, Liggins GC. Role of prostaglandins, prostacyclin, and thromboxanes in the physiologic control of the uterus and in parturition. Semin Perinatol. 1980;4:45–66.
6. Walker DW, Wood C. Temperature relationship of the mother and fetus during labor. Am J Obstet Gynecol. 1970;107:83–87.
7. Laburn HP, Faurie A, Mitchell D. The fetus and fever. J Therm Biol. 2003;28:107–116.
8. Macaulay JH, Bond K, Steer PJ. Epidural analgesia in labor and fetal hyperthermia. Obstet Gynecol. 1992;80:665–669.
9. Duff P, Gibbs RS, Sweet RL. Maternal and fetal infections. Creasy RK, Resnik R, Iams JD, et al. Maternal-Fetal Medicine: Principles and Practice. 6th edition. Philadelphia: Saunders-Elsevier; 2009:739–796.
10. Anderson B, Gaitanis M, Sessler DI. Nonviral infectious diseases in pregnancy. Powrie RO, Greene MF, Camann W. de Swiet’s Medical Disorders in Pregnancy. 5th edition. Wiley-Blackwell: West Sussex; 2010:404–430.
11. Malaeb S, Dammann O. Fetal inflammatory response and brain injury in the preterm newborn. J Child Neurol. 2009;24:1119–1126.
12. Snyder-Keller A, Stark PF. Prenatal inflammatory effects on nigrostriatal development in organotypic cultures. Brain Res. 2008;1233:160–167.
13. Morishima HO, Glaser B, Niemann WH, James LS. Increased uterine activity and fetal deterioration during maternal hyperthermia. Am J Obstet Gynecol. 1975;121:531–538.
14. Cefalo RC, Hellegers AE. The effects of maternal hyperthermia on maternal and fetal cardiovascular and respiratory function. Am J Obstet Gynecol. 1978;131:687–694.
15. Harris WH, Pittman QJ, Veale WL, et al. Cardiovascular effects of fever in the ewe and fetal lamb. Am J Obstet Gynecol. 1977;128:262–265.
16. Milunsky A, Ulcickas M, Rothman KJ, et al. Maternal heat exposure and neural tube defects. JAMA. 1992;268:882–885.
17. Li DK, Janevic T, Odouli R, Liu L. Hot tub use during pregnancy and the risk of miscarriage. Am J Epidemiol. 2003;158:931–937.
18. Duong HT, Shahrukh Hashmi S, Ramadhani T, et al. Maternal use of hot tub and major structural birth defects. Birth Defects Res A Clin Mol Teratol. 2011;91:836–841.
20. Lieberman E, Lang J, Richardson DK, et al. Intrapartum maternal fever and neonatal outcome. Pediatrics. 2000;105:8–13.
21. Lieberman E, Eichenwald E, Mathur G, et al. Intrapartum fever and unexplained seizures in term infants. Pediatrics. 2000;106:983–988.
22. Perlman JM. Maternal fever and neonatal depression: preliminary observations. Clin Pediatr. 1999;38:287–291.
23. Shalak LF, Perlman JM, Jackson GL, Laptook AR. Depression at birth in term infants exposed to maternal chorioamnionitis: does neonatal fever play a role? J Perinatol. 2005;25:447–452.
24. Greenwell EA, Wyshak G, Ringer SA, et al. Intrapartum temperature elevation, epidural use, and adverse outcome in term infants. Pediatrics. 2012;129:e447–e454.
25. Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. JAMA. 1997;278:207–211.
26. Wu YW, Colford JM Jr. Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA. 2000;284:1417–1424.
27. Wu YW, Escobar GJ, Grether JK, et al. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA. 2003;290:2677–2684.
28. Eastman NJ, Deleon M. The etiology of cerebral palsy. Am J Obstet Gynecol. 1955;69:950–961.
29. Yoon BH, Romero R, Park JS, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182:675–681.
30. Impey L, Greenwood C, MacQuillan K, et al. Fever in labour and neonatal encephalopathy: a prospective cohort study. BJOG. 2001;108:594–597.
31. Dammann O, Drescher J, Veelken N. Maternal fever at birth and non-verbal intelligence at age 9 years in preterm infants. Dev Med Child Neurol. 2003;45:148–151.
32. Zerbo O, Iosif AM, Walker C, et al. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. J Autism Dev Disord. 2013;43:25–33.
33. Previc FH. Prenatal influences on brain dopamine and their relevance to the rising incidence of autism. Med Hypotheses. 2007;68:46–60.
34. Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr Bull. 2009;35:959–972.
35. Rodts-Palenik S, Wyatt-Ashmead J, Pang Y, et al. Maternal infection-induced white matter injury is reduced by treatment with interleukin-10. Am J Obstet Gynecol. 2004;191:1387–1392.
36. Impey LW, Greenwood CE, Black RS, et al. The relationship between intrapartum maternal fever and neonatal acidosis as risk factors for neonatal encephalopathy. Am J Obstet Gynecol. 2008;198:49. e1–6.
37. Shipp TD, Zelop C, Cohen A, et al. Post-cesarean delivery fever and uterine rupture in a subsequent trial of labor. Obstet Gynecol. 2003;101:136–139.
38. Benson MD, Haney E, Dinsmoor M, Beaumont JL. Shaking rigors in parturients. J Reprod Med. 2008;53:685–690.
39. Gleeson NC, Nolan KM, Ford MR. Temperature, labour, and epidural analgesia. Lancet. 1989;2:861–862.
40. Panzer O, Ghazanfari N, Sessler DI, et al. Shivering and shivering-like tremor during labor with and without epidural analgesia. Anesthesiology. 1999;90:1609–1616.
41. Mayer DC, Chescheir NC, Spielman FJ. Increased intrapartum antibiotic administration associated with epidural analgesia in labor. Am J Perinatol. 1997;14:83–86.
42. Goetzl L, Cohen A, Frigoletto F Jr, et al. Maternal epidural analgesia and rates of maternal antibiotic treatment in a low-risk nulliparous population. J Perinatol. 2003;23:457–461.
43. Lieberman E, Cohen A, Lang J, et al. Maternal intrapartum temperature elevation as a risk factor for cesarean delivery and assisted vaginal delivery. Am J Public Health. 1999;89:506–510.
44. Newton ER. Chorioamnionitis and intraamniotic infection. Clin Obstet Gynecol. 1993;36:795–808.
45. Cohen-Cline HN, Kahn TR, Hutter CM. A population-based study of the risk of repeat clinical chorioamnionitis in Washington State, 1989-2008. Am J Obstet Gynecol. 2012;207:473. e1–7.
46. Seaward PG, Hannah ME, Myhr TL, et al. International Multicentre Term Prelabor Rupture of Membranes Study: evaluation of predictors of clinical chorioamnionitis and postpartum fever in patients with prelabor rupture of membranes at term. Am J Obstet Gynecol. 1997;177:1024–1029.
47. Gibbs RS, Castillo MS, Rodgers PJ. Management of acute chorioamnionitis. Am J Obstet Gynecol. 1980;136:709–713.
48. Duff P, Sanders R, Gibbs RS. The course of labor in term patients with chorioamnionitis. Am J Obstet Gynecol. 1983;147:391–395.
49. Yoder PR, Gibbs RS, Blanco JD, et al. A prospective, controlled study of maternal and perinatal outcome after intra-amniotic infection at term. Am J Obstet Gynecol. 1983;145:695–701.
50. Blanco JD, Gibbs RS, Castaneda YS. Bacteremia in obstetrics: clinical course. Obstet Gynecol. 1981;58:621–625.
51. Goodman EJ, DeHorta E, Taguiam JM. Safety of spinal and epidural anesthesia in parturients with chorioamnionitis. Reg Anesth. 1996;21:436–441.
52. Riley LE, Celi AC, Onderdonk AB, et al. Association of epidural-related fever and noninfectious inflammation in term labor. Obstet Gynecol. 2011;117:588–595.
53. Roberts DJ, Celi AC, Riley LE, et al. Acute histologic chorioamnionitis at term: nearly always noninfectious. PLoS One. 2012;7:e31819.
54. Sperling RS, Newton E, Gibbs RS. Intraamniotic infection in low-birth-weight infants. J Infect Dis. 1988;157:113–117.
55. Satin AJ, Maberry MC, Leveno KJ, et al. Chorioamnionitis: a harbinger of dystocia. Obstet Gynecol. 1992;79:913–915.
56. Qureshi F, Jacques SM, Bendon RW, et al. Candida funisitis: a clinicopathologic study of 32 cases. Pediatr Dev Pathol. 1998;1:118–124.
57. Gerber S, Vial Y, Hohlfeld P, Witkin SS. Detection of Ureaplasma urealyticum in second-trimester amniotic fluid by polymerase chain reaction correlates with subsequent preterm labor and delivery. J Infect Dis. 2003;187:518–521.
58. Romero R, Espinoza J, Goncalves LF, et al. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39.
59. Nath CA, Ananth CV, Smulian JC, et al. Histologic evidence of inflammation and risk of placental abruption. Am J Obstet Gynecol. 2007;197:319 e1–6.
60. Tran TS, Jamulitrat S, Chongsuvivatwong V, Geater A. Risk factors for postcesarean surgical site infection. Obstet Gynecol. 2000;95:367–371.
61. Rouse DJ, Leindecker S, Landon M, et al. The MFMU Cesarean Registry: uterine atony after primary cesarean delivery. Am J Obstet Gynecol. 2005;193:1056–1060.
62. Mark SP, Croughan-Minihane MS, Kilpatrick SJ. Chorioamnionitis and uterine function. Obstet Gynecol. 2000;95:909–912.
63. Hernandez JS, Nuangchamnong N, Ziadie M, et al. Placental and uterine pathology in women undergoing peripartum hysterectomy. Obstet Gynecol. 2012;119:1137–1142.
64. Rouse DJ, Landon M, Leveno KJ, et al. The Maternal-Fetal Medicine Units cesarean registry: chorioamnionitis at term and its duration-relationship to outcomes. Am J Obstet Gynecol. 2004;191:211–216.
65. Gaudet LM, Smith GN. Cerebral palsy and chorioamnionitis: the inflammatory cytokine link. Obstet Gynecol Surv. 2001;56:433–436.
66. YW Wu. Systematic review of chorioamnionitis and cerebral palsy. Ment Retard Dev Disabil Res Rev. 2002;8:25–29.
67. Shatrov JG, Birch SC, Lam LT, et al. Chorioamnionitis and cerebral palsy: a meta-analysis. Obstet Gynecol. 2010;116:387–392.
68. Lee J, Croen LA, Backstrand KH, et al. Maternal and infant characteristics associated with perinatal arterial stroke in the infant. JAMA. 2005;293:723–729.
69. Resch B, Vollaard E, Maurer U, et al. Risk factors and determinants of neurodevelopmental outcome in cystic periventricular leucomalacia. Eur J Pediatr. 2000;159:663–670.
70. Shimoya K, Taniguchi T, Matsuzaki N, et al. Chorioamnionitis decreased incidence of respiratory distress syndrome by elevating fetal interleukin-6 serum concentration. Hum Reprod. 2000;15:2234–2240.
71. Van Marter LJ, Dammann O, Allred EN, et al. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J Pediatr. 2002;140:171–176.
73. Mwanyumba F, Gaillard P, Inion I, et al. Placental inflammation and perinatal transmission of HIV-1. J Acquir Immune Defic Syndr. 2002;29:262–269.
74. American College of Obstetricians and Gynecologists. Management of intrapartum fetal heart rate tracings. [ACOG Practice Bulletin No. 116. Washington, DC, November 2010] Obstet Gynecol. 2010;116:1232–1240.
75. Mead PB. When to treat intra-amniotic infection. Obstet Gynecol. 1988;72:935–936.
76. Gibbs RS, Dinsmoor MJ, Newton ER, Ramamurthy RS. A randomized trial of intrapartum versus immediate postpartum treatment of women with intra-amniotic infection. Obstet Gynecol. 1988;72:823–828.
77. Gilstrap LC 3rd, Leveno KJ, Cox SM, et al. Intrapartum treatment of acute chorioamnionitis: impact on neonatal sepsis. Am J Obstet Gynecol. 1988;159:579–583.
78. Fiadjoe P, Kannan K, Rane A. Maternal urological problems in pregnancy. Eur J Obstet Gynecol Reprod Bio. 2010;152:13–17.
79. Millar LK, Cox SM. Urinary tract infections complicating pregnancy. Infect Dis Clin North Am. 1997;11:13–26.
80. Harris RE, Gilstrap LC 3rd. Cystitis during pregnancy: a distinct clinical entity. Obstet Gynecol. 1981;57:578–580.
81. Gilstrap LC 3rd, Ramin SM. Urinary tract infections during pregnancy. Obstet Gynecol Clin North Am. 2001;28:581–591.
82. Angel JL, O’Brien WF, Finan MA, et al. Acute pyelonephritis in pregnancy: a prospective study of oral versus intravenous antibiotic therapy. Obstet Gynecol. 1990;76:28–32.
83. Hill JB, Sheffield JS, McIntire DD, Wendel GD Jr. Acute pyelonephritis in pregnancy. Obstet Gynecol. 2005;105:18–23.
84. Wing DA. Pyelonephritis in pregnancy: treatment options for optimal outcomes. Drugs. 2001;61:2087–2096.
85. Wing DA, Hendershott CM, Debuque L, Millar LK. Outpatient treatment of acute pyelonephritis in pregnancy after 24 weeks. Obstet Gynecol. 1999;94:683–688.
86. Kaul AK, Khan S, Martens MG, et al. Experimental gestational pyelonephritis induces preterm births and low birth weights in C3H/HeJ mice. Infect Immun. 1999;67:5958–5966.
87. Jolley JA, Kim S, Wing DA. Acute pyelonephritis and associated complications during pregnancy in 2006 in US hospitals. J Matern Fetal Neonatal Med. 2012;25:2494–2498.
88. Gilstrap LC 3rd, Cunningham FG, Whalley PJ. Acute pyelonephritis in pregnancy: an anterospective study. Obstet Gynecol. 1981;57:409–413.
89. Cunningham FG, Lucas MJ, Hankins GD. Pulmonary injury complicating antepartum pyelonephritis. Am J Obstet Gynecol. 1987;156:797–807.
90. Towers CV, Kaminskas CM, Garite TJ, et al. Pulmonary injury associated with antepartum pyelonephritis: can patients at risk be identified? Am J Obstet Gynecol. 1991;164:974–978.
91. Twickler DM, Lucas MJ, Bowe L, et al. Ultrasonographic evaluation of central and end-organ hemodynamics in antepartum pyelonephritis. Am J Obstet Gynecol. 1994;170:814–818.
92. U.S. Preventive Services Task Force. Screening for asymptomatic bacteriuria in adults: reaffirmation recommendation statement. Am Fam Physician. 2010;81:505.
93. Smaill F, Vazquez JC. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev. 2007;(2).
94. Guinto VT, De Guia B, Festin MR, Dowswell T. Different antibiotic regimens for treating asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev. 2010;(9).
95. Widmer M, Gulmezoglu AM, Mignini L, Roganti A. Duration of treatment for asymptomatic bacteriuria during pregnancy. Cochrane Database Syst Rev. 2011;(12).
96. Korst LM, Reyes C, Fridman M, et al. Gestational pyelonephritis as an indicator of the quality of ambulatory maternal health care services. Obstet Gynecol. 2006;107:632–640.
97. Lim WS, Macfarlane JT, Colthorpe CL. Treatment of community-acquired lower respiratory tract infections during pregnancy. Am J Respir Med. 2003;2:221–233.
98. Toppozada H, Michaels L, Toppozada M, et al. The human respiratory nasal mucosa in pregnancy: an electron microscopic and histochemical study. J Laryngol Otol. 1982;96:613–626.
99. Brito V, Niederman MS. Pneumonia complicating pregnancy. Clin Chest Med. 2011;32:121–132.
100. Benedetti TJ, Valle R, Ledger WJ. Antepartum pneumonia in pregnancy. Am J Obstet Gynecol. 1982;144:413–417.
101. Leontic EA. Respiratory disease in pregnancy. Med Clin North Am. 1977;61:111–128.
102. Landsberger EJ, Hager WD, Grossman JH 3rd. Successful management of varicella pneumonia complicating pregnancy: a report of three cases. J Reprod Med. 1986;31:311–314.
103. Ahmad H, Mehta NJ, Manikal VM, et al. Pneumocystis carinii pneumonia in pregnancy. Chest. 2001;120:666–671.
104. Fiore AE, Shay DK, Broder K, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58:1–52.
105. Haberg SE, Trogstad L, Gunnes N, et al. Risk of fetal death after pandemic influenza virus infection or vaccination. N Engl J Med. 2013;368:333–340.
106. Steinhoff MC, Omer SB, Roy E, et al. Influenza immunization in pregnancy—antibody responses in mothers and infants. N Engl J Med. 2010;362:1644–1646.
107. Siston AM, Rasmussen SA, Honein MA, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303:1517–1525.
108. Hansen C, Desai S, Bredfeldt C, et al. A large, population-based study of 2009 pandemic Influenza A virus subtype H1N1 infection diagnosis during pregnancy and outcomes for mothers and neonates. J Infect Dis. 2012;206:1260–1268.
109. Kankuri E, Kurki T, Carlson P, Hiilesmaa V. Incidence, treatment and outcome of peripartum sepsis. Acta Obstet Gynecol Scand. 2003;82:730–735.
110. Locksmith GJ, Duff P. Assessment of the value of routine blood cultures in the evaluation and treatment of patients with chorioamnionitis. Infect Dis Obstet Gynecol. 1994;2:111–114.
111. Smaill FM, Gyte GM. Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database Syst Rev. 2010;(1).
112. Hadiati DR, Hakimi M, Nurdiati DS. Skin preparation for preventing infection following caesarean section. Cochrane Database Syst Rev. 2012;(9).
113. Haas DM, Morgan Al Darei S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Syst Rev. 2010;(3).
114. Chongsomchai C, Lumbiganon P, Laopaiboon M. Prophylactic antibiotics for manual removal of retained placenta in vaginal birth. Cochrane Database Syst Rev. 2006;(2).
115. Liabsuetrakul T, Choobun T, Peeyananjarassri K, Islam M. Antibiotic prophylaxis for operative vaginal delivery. Cochrane Database Syst Rev. 2004;(3).
116. van Schalkwyk J, Van Eyk N, Society of Obstetricians and Gynaecologists of Canada Infectious Diseases Committee. Antibiotic prophylaxis in obstetric procedures. J Obstet Gynaecol Can. 2010;32:878–892.
117. Martin SR, Foley MR. Intensive care in obstetrics: an evidence-based review. Am J Obstet Gynecol. 2006;195:673–689.
118. Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655.
119. Lappen JR, Keene M, Lore M, et al. Existing models fail to predict sepsis in an obstetric population with intrauterine infection. Am J Obstet Gynecol. 2010;203:573 e1–5.
120. Nduka OO, Parrillo JE. The pathophysiology of septic shock. Crit Care Clin. 2009;25:677–702.
121. Cantwell R, Clutton-Brock T, Cooper G, et al. Saving Mothers’ Lives: reviewing maternal deaths to make motherhood safer: 2006-2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011;118(Suppl 1):1–203.
123. Galvagno SM Jr, Camann W. Sepsis and acute renal failure in pregnancy. Anesth Analg. 2009;108:572–575.
124. Ho CS, Bhatnagar J, Cohen AL, et al. Undiagnosed cases of fatal Clostridium-associated toxic shock in Californian women of childbearing age. Am J Obstet Gynecol. 2009;201:459 e1–7.
125. Fischer M, Bhatnagar J, Guarner J, et al. Fatal toxic shock syndrome associated with Clostridium sordellii after medical abortion. N Engl J Med. 2005;353:2352–2360.
126. Ayadi S, Carbillon L, Varlet C, et al. Fatal sepsis due to Escherichia coli after second-trimester amniocentesis. Fetal Diagn Ther. 1998;13:98–99.
127. Rode ME, Morgan MA, Ruchelli E, Forouzan I. Candida chorioamnionitis after serial therapeutic amniocenteses: a possible association. J Perinatol. 2000;20:335–337.
128. Winer N, David A, Leconte P, et al. Amniocentesis and amnioinfusion during pregnancy: report of four complicated cases. Eur J Obstet Gyn Reprod Biol. 2001;100:108–111.
129. Hamanishi J, Itoh H, Sagawa N, et al. A case of successful management of maternal septic shock with multiple organ failure following amniocentesis at midgestation. J Obstet Gynaecol Res. 2002;28:258–261.
130. Slabbert DR, Kruger TF, Siebert TI, Stead P. Endotoxic shock after gamete intrafallopian transfer. Fertil Steril. 2005;83:1041.
131. Kramer HM, Schutte JM, Zwart JJ, et al. Maternal mortality and severe morbidity from sepsis in the Netherlands. Acta Obstet Gynecol Scand. 2009;88:647–653.
132. Pazos M, Sperling RS, Moran TM, Kraus TA. The influence of pregnancy on systemic immunity. Immunol Res. 2012;54:254–261.
133. Beller FK, Schmidt EH, Holzgreve W, Hauss J. Septicemia during pregnancy: a study in different species of experimental animals. Am J Obstet Gynecol. 1985;151:967–975.
134. Ledger WJ, Norman M, Gee C, Lewis W. Bacteremia on an obstetric-gynecologic service. Am J Obstet Gynecol. 1975;121:205–212.
135. Lee W, Clark SL, Cotton DB, et al. Septic shock during pregnancy. Am J Obstet Gynecol. 1988;159:410–416.
136. Mabie WC, Barton JR, Sibai B. Septic shock in pregnancy. Obstet Gynecol. 1997;90:553–561.
137. American College of Obstetricians and Gynecologists. Septic shock. ACOG Technical Bulletin No. 204. [Washington, DC, April 1995] Int J Gynaecol Obstet. 1995;50:71–79.
138. Lucas DN, Robinson PN, Nel MR. Sepsis in obstetrics and the role of the anaesthetist. Int J Obstet Anesth. 2012;21:56–67.
139. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637.
140. Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377.
141. Jones AE, Focht A, Horton JM, Kline JA. Prospective external validation of the clinical effectiveness of an emergency department-based early goal-directed therapy protocol for severe sepsis and septic shock. Chest. 2007;132:425–432.
142. Mesotten D, Van den Berghe G. Glycemic targets and approaches to management of the patient with critical illness. Curr Diab Rep. 2012;12:101–107.
143. Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–871.
144. Matsukawa T, Sessler DI, Christensen R, et al. Heat flow and distribution during epidural anesthesia. Anesthesiology. 1995;83:961–967.
145. Fusi L, Steer PJ, Maresh MJ, Beard RW. Maternal pyrexia associated with the use of epidural analgesia in labour. Lancet. 1989;1:1250–1252.
146. Camann WR, Hortvet LA, Hughes N, et al. Maternal temperature regulation during extradural analgesia for labour. Br J Anaesth. 1991;67:565–568.
147. Yancey MK, Zhang J, Schwarz J, et al. Labor epidural analgesia and intrapartum maternal hyperthermia. Obstet Gynecol. 2001;98:763–770.
148. Vinson DC, Thomas R, Kiser T. Association between epidural analgesia during labor and fever. J Fam Pract. 1993;36:617–622.
149. Ploeckinger B, Ulm MR, Chalubinski K, Gruber W. Epidural anaesthesia in labour: influence on surgical delivery rates, intrapartum fever and blood loss. Gynecol Obstet Invest. 1995;39:24–27.
150. Lieberman E, Lang JM, Frigoletto F Jr, et al. Epidural analgesia, intrapartum fever, and neonatal sepsis evaluation. Pediatrics. 1997;99:415–419.
151. Herbst A, Wolner-Hanssen P, Ingemarsson I. Risk factors for fever in labor. Obstet Gynecol. 1995;86:790–794.
152. Philip J, Alexander JM, Sharma SK, et al. Epidural analgesia during labor and maternal fever. Anesthesiology. 1999;90:1271–1275.
153. Agakidis C, Agakidou E, Philip Thomas S, et al. Labor epidural analgesia is independent risk factor for neonatal pyrexia. J Matern Fetal Neonatal Med. 2011;24:1128–1132.
154. Dashe JS, Rogers BB, McIntire DD, Leveno KJ. Epidural analgesia and intrapartum fever: placental findings. Obstet Gynecol. 1999;93:341–344.
155. de Orange FA, Passini R Jr, Amorim MM, et al. Combined spinal and epidural anaesthesia and maternal intrapartum temperature during vaginal delivery: a randomized clinical trial. Br J Anaesth. 2011;107:762–768.
156. Halpern SH, Muir H, Breen TW, et al. A multicenter randomized controlled trial comparing patient-controlled epidural with intravenous analgesia for pain relief in labor. Anesth Analg. 2004;99:1532–1538.
157. Kaul B, Vallejo M, Ramanathan S, Mandell G. Epidural labor analgesia and neonatal sepsis evaluation rate: a quality improvement study. Anesth Analg. 2001;93:986–990.
158. Nafisi S. Effects of epidural lidocaine analgesia on labor and delivery: a randomized, prospective, controlled trial. BMC Anesthesiol. 2006;6:15.
159. Lucas MJ, Sharma SK, McIntire DD, et al. A randomized trial of labor analgesia in women with pregnancy-induced hypertension. Am J Obstet Gynecol. 2001;185:970–975.
160. Ramin SM, Gambling DR, Lucas MJ, et al. Randomized trial of epidural versus intravenous analgesia during labor. Obstet Gynecol. 1995;86:783–789.
161. Sharma SK, Alexander JM, Messick G, et al. Cesarean delivery: a randomized trial of epidural analgesia versus intravenous meperidine analgesia during labor in nulliparous women. Anesthesiology. 2002;96:546–551.
162. Sharma SK, Sidawi JE, Ramin SM, et al. Cesarean delivery: a randomized trial of epidural versus patient-controlled meperidine analgesia during labor. Anesthesiology. 1997;87:487–494.
163. Segal S. Labor epidural analgesia and maternal fever. Anesth Analg. 2010;111:1467–1475.
164. Anim-Somuah M, Smyth RM, Jones L. Epidural versus non-epidural or no analgesia in labour. Cochrane Database Syst Rev. 2011;(12).
165. Goetzl L, Rivers J, Zighelboim I, et al. Intrapartum epidural analgesia and maternal temperature regulation. Obstet Gynecol. 2007;109:687–690.
166. Gelfand T, Palanisamy A, Tsen LC, Segal S. Warming in parturients with epidurals is an averaging artifact. Anesthesiology. 2007;106:A5.
167. Frolich MA, Esame A, Zhang K, et al. What factors affect intrapartum maternal temperature? A prospective cohort study: maternal intrapartum temperature. Anesthesiology. 2012;117:302–308.
168. Goodlin RC, Chapin JW. Determinants of maternal temperature during labor. Am J Obstet Gynecol. 1982;143:97–103.
169. Glosten B, Savage M, Rooke GA, Brengelmann GL. Epidural anesthesia and the thermoregulatory responses to hyperthermia—preliminary observations in volunteer subjects. Acta Anaesthesiol Scand. 1998;42:442–446.
170. Hagerdal M, Morgan CW, Sumner AE, Gutsche BB. Minute ventilation and oxygen consumption during labor with epidural analgesia. Anesthesiology. 1983;59:425–427.
171. Negishi C, Lenhardt R, Ozaki M, et al. Opioids inhibit febrile responses in humans, whereas epidural analgesia does not: an explanation for hyperthermia during epidural analgesia. Anesthesiology. 2001;94:218–222.
173. Evron S, Ezri T, Protianov M, et al. The effects of remifentanil or acetaminophen with epidural ropivacaine on body temperature during labor. J Anesth. 2008;22:105–111.
174. Soper DE, Mayhall CG, Dalton HP. Risk factors for intraamniotic infection: a prospective epidemiologic study. Am J Obstet Gynecol. 1989;161:562–566.
175. Dolak JA, Brown RE. Epidural analgesia and neonatal fever. Pediatrics. 1998;101:492.
176. Cahill AG, Duffy CR, Odibo AO, et al. Number of cervical examinations and risk of intrapartum maternal fever. Obstet Gynecol. 2012;119:1096–1101.
177. Vallejo MC, Kaul B, Adler LJ, et al. Chorioamnionitis, not epidural analgesia, is associated with maternal fever during labour. Can J Anaesth. 2001;48:1122–1126.
178. Goetzl L, Evans T, Rivers J, et al. Elevated maternal and fetal serum interleukin-6 levels are associated with epidural fever. Am J Obstet Gynecol. 2002;187:834–838.
179. Goetzl L, Zighelboim I, Badell M, et al. Maternal corticosteroids to prevent intrauterine exposure to hyperthermia and inflammation: a randomized, double-blind, placebo-controlled trial. Am J Obstet Gynecol. 2006;195:1031–1037.
180. Wang LZ, Hu XX, Liu X, et al. Influence of epidural dexamethasone on maternal temperature and serum cytokine concentration after labor epidural analgesia. Int J Gynaecol Obstet. 2011;113:40–43.
181. Kozlov I. Why labor epidural causes fever and why lidocaine burns on injection? Role of TRPV-1 receptor in hyperthermia: possible explanation of mechanism of hyperthermia during labor epidural and burning sensation on injection of local anesthetics. Open J Anesth. 2012;2:134–137.
182. Frigoletto FD Jr, Lieberman E, Lang JM, et al. A clinical trial of active management of labor. N Engl J Med. 1995;333:745–750.
183. Tarshis J, Camann WR, Datta S. Epidural analgesia and neonatal fever. Pediatrics. 1998;101:490–491.
184. Goetzl L, Cohen A, Frigoletto F Jr, et al. Maternal epidural use and neonatal sepsis evaluation in afebrile mothers. Pediatrics. 2001;108:1099–1102.
185. Carp H, Bailey S. The association between meningitis and dural puncture in bacteremic rats. Anesthesiology. 1992;76:739–742.
186. Weed LH, Wegeforth P, Ayer JB, Felton LD. The production of meningitis by release of cerebrospinal fluid during an experimental septicemia: preliminary note. JAMA. 1919;72:190–193.
187. Petersdorf RG, Swarner DR, Garcia M. Studies on the pathogenesis of meningitis. II. Development of meningitis during pneumococcal bacteremia. J Clin Invest. 1962;41:320–327.
188. James FM, George RH, Naiem H, White GJ. Bacteriologic aspects of epidural analgesia. Anesth Analg. 1976;55:187–190.
189. Wegefroth P, Lastham JR. Lumbar puncture as a factor in the causation of meningitis. Am J Med Sci. 1919;158:183–185.
190. Pray L. Lumbar puncture as a factor in the pathogenesis of meningitis. Am J Dis Child. 1941;295:62–68.
191. Eng RH, Seligman SJ. Lumbar puncture-induced meningitis. JAMA. 1981;245:1456–1459.
192. Teele DW, Dashefsky B, Rakusan T, Klein JO. Meningitis after lumbar puncture in children with bacteremia. N Engl J Med. 1981;305:1079–1081.
193. Smith KM, Deddish RB, Ogata ES. Meningitis associated with serial lumbar punctures and post-hemorrhagic hydrocephalus. J Pediatr. 1986;109:1057–1060.
194. Shapiro ED, Aaron NH, Wald ER, Chiponis D. Risk factors for development of bacterial meningitis among children with occult bacteremia. J Pediatr. 1986;109:15–19.
195. Chestnut DH. Spinal anesthesia in the febrile patient. Anesthesiology. 1992;76:667–669.
196. Kost-Byerly S, Tobin JR, Greenberg RS, et al. Bacterial colonization and infection rate of continuous epidural catheters in children. Anesth Analg. 1998;86:712–716.
197. Barrie H. Meningitis following spinal anesthesia. Lancet. 1941;237:242–243.
198. Lee JJ, Parry H. Bacterial meningitis following spinal anaesthesia for caesarean section. Br J Anaesth. 1991;66:383–386.
199. Kilpatrick ME, Girgis NI. Meningitis—a complication of spinal anesthesia. Anesth Analg. 1983;62:513–515.
200. Roberts SP, Petts HV. Meningitis after obstetric spinal anaesthesia. Anaesthesia. 1990;45:376–377.
201. Cohen S, Hunter CW, Sakr A, Hijazi RH. Meningitis following intrathecal catheter placement after accidental dural puncture. Int J Obstet Anesth. 2006;15:171.
202. Reynolds F. Infection as a complication of neuraxial blockade. Int J Obstet Anesth. 2005;14:183–188.
203. Rubin L, Sprecher H, Kabaha A, et al. Meningitis following spinal anesthesia: 6 cases in 5 years. Infect Control Hosp Epidemiol. 2007;28:1187–1190.
204. Baer ET. Post-dural puncture bacterial meningitis. Anesthesiology. 2006;105:381–393.
205. Videira RL, Ruiz-Neto PP, Brandao Neto M. Post spinal meningitis and asepsis. Acta Anaesthesiol Scand. 2002;46:639–646.
206. Trautmann M, Lepper PM, Schmitz FJ. Three cases of bacterial meningitis after spinal and epidural anesthesia. Eur J Clin Microbiol Infect Dis. 2002;21:43–45.
207. Kainer MA, Reagan DR, Nguyen DB, et al. Fungal infections associated with contaminated methylprednisolone in Tennessee. N Engl J Med. 2012;367:2194–2203.
208. Harding SA, Collis RE, Morgan BM. Meningitis after combined spinal-extradural anaesthesia in obstetrics. Br J Anaesth. 1994;73:545–547.
209. Cascio M, Heath G. Meningitis following a combined spinal-epidural technique in a labouring term parturient. Can J Anaesth. 1996;43:399–402.
210. Bouhemad B, Dounas M, Mercier FJ, Benhamou D. Bacterial meningitis following combined spinal-epidural analgesia for labour. Anaesthesia. 1998;53:292–295.
211. Rawal N, Holmstrom B, Crowhurst JA, Van Zundert A. The combined spinal-epidural technique. Anesthesiol Clin North America. 2000;18:267–295.
212. Dripps RD, Vandam LD. Long-term follow-up of patients who received 10,098 spinal anesthetics. JAMA. 1954;156:1486–1491.
213. Phillips OC, Ebner H, Nelson AT, Black MH. Neurologic complications following spinal anesthesia with lidocaine: a prospective review of 10,440 cases. Anesthesiology. 1969;30:284–289.
214. Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990-1999. Anesthesiology. 2004;101:950–959.
215. Hellmann K. Epidural anaesthesia in obstetrics: a second look at 26,127 cases. Can Anaesth Soc J. 1965;12:398–404.
216. Crawford JS. Some maternal complications of epidural analgesia for labour. Anaesthesia. 1985;40:1219–1225.
217. Scott DB, Hibbard BM. Serious non-fatal complications associated with extradural block in obstetric practice. Br J Anaesth. 1990;64:537–541.
218. Paech MJ, Godkin R, Webster GS. Complications of obstetric epidural analgesia and anaesthesia: a prospective analysis of 10,995 cases. Int J Obstet Anesth. 1998;7:5–11.
219. Cook TM, Counsell D, Wildsmith JA. Major complications of central neuraxial block: report on the Third National Audit Project of the Royal College of Anaesthetists. Br J Anaesth. 2009;102:179–190.
220. Bader AM, Gilbertson L, Kirz L, Datta S. Regional anesthesia in women with chorioamnionitis. Reg Anesth. 1992;17:84–86.
221. American College of Obstetricians and Gynecologists. Management of herpes in pregnancy. [ACOG Practice Bulletin No. 82. Washington, DC, June 2007 (Reaffirmed 2009)] Obstet Gynecol. 2007;109:1489–1498.
222. Corey L, Adams HG, Brown ZA, Holmes KK. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med. 1983;98:958–972.
223. Roberts CM, Pfister JR, Spear SJ. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex Transm Dis. 2003;30:797–800.
224. Brown ZA, Vontver LA, Benedetti J, et al. Effects on infants of a first episode of genital herpes during pregnancy. N Engl J Med. 1987;317:1246–1251.
225. Eskild A, Jeansson S, Stray-Pedersen B, Jenum PA. Herpes simplex virus type-2 infection in pregnancy: no risk of fetal death: results from a nested case-control study within 35,940 women. BJOG. 2002;109:1030–1035.
226. Pinninti SG, Angara R, Feja KN, et al. Neonatal herpes disease following maternal antenatal antiviral suppressive therapy: a multicenter case series. J Pediatr. 2012;161 [134-8 e1–3] .
228. Brown ZA, Wald A, Morrow RA, et al. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289:203–209.
229. Prober CG, Sullender WM, Yasukawa LL, et al. Low risk of herpes simplex virus infections in neonates exposed to the virus at the time of vaginal delivery to mothers with recurrent genital herpes simplex virus infections. N Engl J Med. 1987;316:240–244.
230. Arvin AM, Hensleigh PA, Prober CG, et al. Failure of antepartum maternal cultures to predict the infant’s risk of exposure to herpes simplex virus at delivery. N Engl J Med. 1986;315:796–800.
231. Ravindran RS, Gupta CD, Stoops CA. Epidural analgesia in the presence of herpes simplex virus (type 2) infection. Anesth Analg. 1982;61:714–715.
232. Ramanathan S, Sheth R, Turndorf H. Anesthesia for cesarean section in patients with genital herpes infections: a retrospective study. Anesthesiology. 1986;64:807–809.
233. Crosby ET, Halpern SH, Rolbin SH. Epidural anaesthesia for caesarean section in patients with active recurrent genital herpes simplex infections: a retrospective review. Can J Anaesth. 1989;36:701–704.
234. Bader AM, Camann WR, Datta S. Anesthesia for cesarean delivery in patients with herpes simplex virus type-2 infections. Reg Anesth. 1990;15:261–263.
235. Birnbach DJ, Bourlier RA, Choi R, Thys DM. Anaesthetic management of caesarean section in a patient with active recurrent genital herpes and AIDS-related dementia. Br J Anaesth. 1995;75:639–641.
236. Boyle RK. Herpes simplex labialis after epidural or parenteral morphine: a randomized prospective trial in an Australian obstetric population. Anaesth Intensive Care. 1995;23:433–437.
237. Davies PW, Vallejo MC, Shannon KT, et al. Oral herpes simplex reactivation after intrathecal morphine: a prospective randomized trial in an obstetric population. Anesth Analg. 2005;100:1472–1476.
238. Boyle RK. A review of anatomical and immunological links between epidural morphine and herpes simplex labialis in obstetric patients. Anaesth Intensive Care. 1995;23:425–432.
239. Bauchat JR. Focused review: neuraxial morphine and oral herpes reactivation in the obstetric population. Anesth Analg. 2010;111:1238–1241.
240. Hoesni S, Bhinder R, Tan T, et al. Herpes simplex meningitis after accidental dural puncture during epidural analgesia for labour. Int J Obstet Anesth. 2010;19:466–467.