Factors That Influence the Immune Response
Learning Objectives
• Compare and contrast the determinant selection and hole in the T cell repertoire models
• Recognize how the physical form of the antigen influences the immune response
• Compare the effects of low-dose and high-dose antigens on T and B cells
• Compare and contrast the three different CD4 regulator cells
• Identify the role of FOXp3 in the downregulation of an immune response
• Identify the disease caused by mutations in FOXp3
• Compare and contrast the characteristics of antigen-stimulated CD4 regulator cells
• Recognize the role of Qa-1 in immune regulation by CD8 cells
• Explain activation-induced cell death (AICD)
• Identify the defects or alterations in AICD in individuals with systemic lupus erythematosus (SLE)
• Differentiate between an idiotope and an idiotype
• Explain idiotype network theory
• Recognize the two mechanisms by which anti-idiotype antibodies can downregulate an immune response
Key Terms
Anergy
FOXp3
Idiotope
Idiotype
NTreg cell
Qa-1
Regulator cells
Treg1 cell
TH3 cell
Introduction
The generation of an immune response is determined by genetic factors related to the nature of the antigen, dose, and route of administration. Once a response is mounted, it must be carefully controlled to ensure that it is not directed at normal host tissue. A response must also be easily downregulated when no more antigen is present to drive the immune response.
The immune system has multiple control mechanisms available to damper an immune response after the antigen has been reduced or eliminated. A network of CD4 and CD8 regulatory cells (Tregs) suppresses the function of the lymphocytes involved in autoimmune diseases, allergies, and transplant rejection but without disrupting the function of lymphocytes involved in normal adaptive immune responses. Responses are dampered by two different mechanisms: (1) Some responses are terminated by the killing of CD4 effector cells by a mechanism called activation-induced cell death (AICD). (2) Other responses may be terminated by a perforin and granzymes, anti-inflammatory cytokines, or an anti-idiotypic network.
Genetics and the Immune Response
Human Leukocyte Antigen Glycoproteins and the Immune Response
Some individuals are genetically restricted and are unable to respond to specific antigens. Failure to respond to a specific antigen can be explained by the determinant selection and hole in the T cell repertoire models. The determinant selection model proposes that class II molecules differ in their ability to bind processed antigens. In essence, individuals lack human leukocyte antigen (HLA) molecules that present a specific antigen to T cells. Conversely, the hole in the T cell repertoire model proposes that class II antigen presentation is normal but that some individuals lack a lymphocyte with a T cell receptor (TCR) that recognizes the specific antigen. In both cases, T cell activation fails to occur.
Physical and Biologic Factors Influencing the Immune Response
Form of Antigen and Route of Administration
The physical form of the antigen is important in the generation of an immune response. Because insoluble antigens are easily processed by macrophages, large aggregates favor the generation of an immune response. In contrast, small, soluble antigens cannot be processed by antigen-presenting cells and generate a tolerogenic effect.
The route of administration also determines whether the host mounts an immune response or becomes tolerogenic. Intramuscular and subcutaneous administration of antigens usually elicits an immune response by favoring antigen uptake and presentation by Langerhans cells. In contrast, inhalation, oral, or intravenous administration often fails to generate an immune response.
Maturity of the Antigen-Presenting Cell
Dendritic cells (DCs) are the major antigen-presenting cells in the body. They originate in bone marrow and are released into peripheral blood before migration to lymph nodes. In tissues and lymph nodes, DCs are present as “immature,” partially differentiated cells. These cells can capture and present the antigen in the context of class II molecules but lack the co-stimulatory molecules B7-1 and B7-2, which normally engage CD28 receptors on T cells (see Chapter 6). T cells interacting with immature DCs become anergic. Anergic T cells cannot proliferate or respond to antigen stimulation. In contrast, mature DCs in tissue express class I or class II molecules as well as co-stimulatory molecules. They are efficient in presenting antigens to T cells within the lymph node.
Antigen Concentration
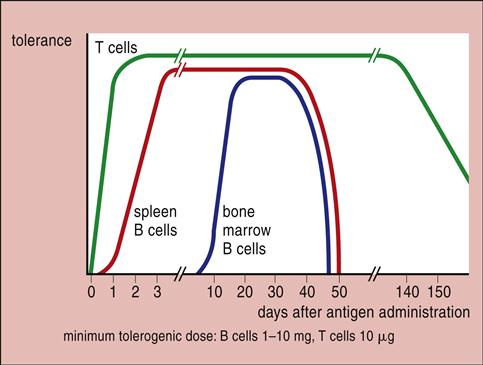
When the antigen is administered over a wide concentration range, only intermediate concentrations induce an immune response. High doses paralyze the immune system by inactivating B cells. Low doses or subimmunogenic doses administered over an extended period inactivate T cells (Figure 22-1).
Passively Administered Immunoglobulin
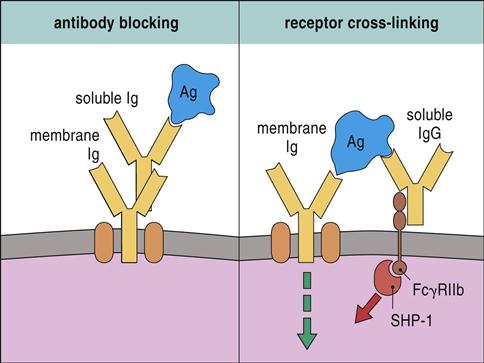
High doses of passively administered immunoglobulin can suppress the immune response. Pooled immunoglobulin contains antibodies to common bacterial and viral pathogens. Interaction between antibodies in pooled serum and microbial antigens creates immune complexes that block B cell receptor (BCR) activation. Pooled immunoglobulin also suppresses an immune response by engaging the FcγRIIB receptors expressed by monocytes, macrophages, and lymphocytes. FcγRIIB has a cytoplasmic immuno-receptor tyrosine-based inhibitory motif (ITIM), which associates with a tyrosine phosphatase (SHP-1) and downregulates intracellular signaling (Figure 22-2).
Regulator Cells
The concept of suppressor cells was introduced by Gershon and Kondo in 1971. They demonstrated that the transfer of immunocompetent cells from an immunized mouse to a naïve mouse downregulated an immune response to the same antigen. Because of an inability to identify suppressor cells in lymphoid organs, the concept of regulator cells was largely discarded. The advent of flow cytometry and cell sorting made it possible to identify and isolate CD4 and CD8 regulator cell populations. Regulator cells play a role in downregulating auto-reactive lymphocytes, reducing inflammation, mediating tolerance to superantigens, and maintaining self-tolerance.
CD4 Regulator Cells
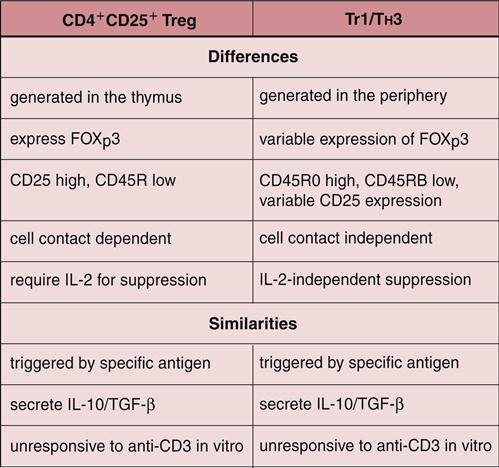
A population of CD3+, CD4+, and CD25+ cells in peripheral blood suppresses autoimmune reactions and response to infectious disease. These cells have been designated T regulator (Treg) cells. Three subpopulations of CD4 Tregs have been identified and are known as natural Tregs, Treg1, and TH3.
Natural CD4 Tregs
Natural Tregs (NTregs) are educated in the thymus during the negative selection process (see Chapter 1). In the thymus, naïve cells bind to macrophages and dendritic cells, expressing high levels of HLA self-peptides. Thymocytes binding to the HLA molecules with intermediate affinity are considered NTregs that exit the thymus and circulate in peripheral blood. NTregs require antigen presentation by class II molecules, the presence of co-stimulatory molecules, stimulation of the high-affinity interleukin 2 (IL-2) receptor by exogenous IL-2, and cell-to-cell contact.
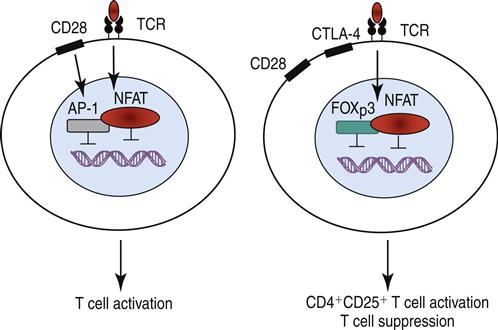
Stimulation of the high-affinity IL-2 receptor activates the JAK1–JAK3 pathway, which phosphorylates STAT3 and STAT5. The STATs, which are translocated to the nucleus, upregulate the synthesis of intracellular forehead helix or winged transcription factor (FOXp3). Binding of FOXp3 to NFAT (nuclear factor of activated T cells) alters the translocation within the nucleus and upregulates the expression of CD25 (the α-chain of the high-affinity IL-2 receptor), CTLA-4, and a glucocorticoid-induced tumor necrosis receptor (GITR). The mechanism is shown in Figure 22-3.
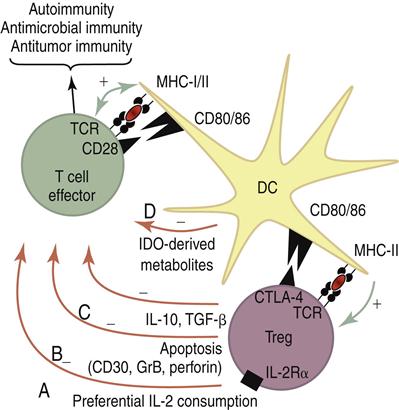
NTregs downregulate an immune response by two different mechanisms: (1) Using CTLA-4 ligation with B7 molecules on dendritic cells, NTregs downregulate dendritic cells and damper the activation of T cells. Activated NTregs also produce IL-10 and transforming growth factor beta (TGF-β). Both cytokines have immuno-regulatory functions. A summary of the possible interactions between NTregs and conventional T cells is shown in Figure 22-4.
FOXp3 Deficiency
Mutations in the FOXp3 gene occur in the forkhead domain that is critical to NFAT binding. Impaired FOXp3 function results in an immune polyendocrinopathy, enteropathy, and X-linked disorder (IPEX). Symptoms include cachexia and growth retardation. Patients present with severe watery diarrhea, type I insulin-dependent diabetes, thyroid disorders, or eczema. Without treatment, affected children usually die within the first 2 years of life. Severe enteric infections associated with catheters or diarrhea contribute to mortality.
Treatment for X-Linked Disorder
Little information on the treatment of IPEX is available. Immunosuppressive therapy and bone marrow transplantation have shown variable results. Immunosuppressive therapy is difficult because of toxicity and infectious complications.
Treg1 Cells
A small population of circulating CD4+, CD25–, and IL-10+ cells have regulatory activity and have been designated T regulatory cells type 1 (Treg1). These cells have variable expression of FOXp3, require antigen stimulation, and do not need IL-2 stimulation or cell-cell contact for activation. Treg1 cells lyse effector cells using the classical perforin-granzyme lytic pathway.
TH3 Regulator Cells
Another population of regulator cells (TH3) is found in the intestinal mucosa and is important in establishing oral tolerance or in downregulating the immune response to ingested antigens. The similarities and differences between Treg cells are shown in Figure 22-5.
CD8 Regulator Cells
CD8 regulator cells kill CD4 cells by a unique mechanism. Activated CD4 cells express Qa-1, a nonclassic HLA glycoprotein that presents a small hydrophobic peptide (AMAPRTLLL) derived from the leader sequences of other HLA class I molecules. Engagement of the Qa-1 and CD8 TCR activates a population of CD8 suppressor cells, which differentiate into CD8 Tc1 cells with high levels of FOXp3. Using the perforin–granzymes lytic pathway, activated CD8 suppressor cells kill any antigen-driven, activated CD4 cells expressing the Qa-1 self-peptide.
Mucosal CD8 cells may play a role in the downregulation of an allergic response. Initial exposure to allergen-naïve T cells default to a Th2 cytokine pattern and production of immunoglobulin E (IgE). Repeated exposure to TGF-β produced by antigen-driven CD4 regulator cells generates a population of CD8 regulatory cells. These cells react with Qa-1 markers on activated CD4Th2 cells and downregulate IgE synthesis.
Activation-Induced Cell Death
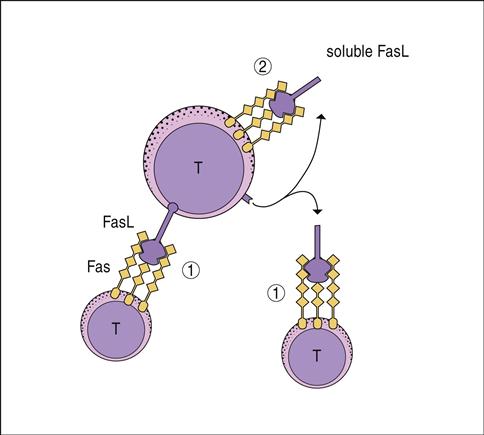
Antigen-driven expansion of T cells and B cells can be inhibited by a process called activation-induced cell death (AICD). Cell death is mediated by FasL–FasR engagement. Fas ligand (FasL) is a homotrimeric membrane molecule on the regulator cells that bind three Fas molecules on target cells. Clustering of Fas receptor (FasR) molecules brings intracellular death domains and adaptor proteins close to create a death-inducing signal complex (DISC). Engagement of FasL and FasR activates the DISC complex and triggers downstream endonucleases that degrade deoxyribonucleic acid (DNA).
CD4 regulator cells express FasL, and activated T cells express both FasR and FasL. FasL–FasR interactions between the regulatory and effector cells is often fatal to the activated T cell. Reduction in activated T cells downregulates the immune response. However, termination of an immune response also requires “suicide” by any remaining activated T cells. Autologous killing is achieved by the cleavage of the membrane-bound FasL by a zinc-dependent metalloprotease called matrilysin. Soluble FasL binds to FasR on the same cell in an autocrine manner to kill the T cell and terminate the response (Figure 22-6).
FasL–FasR Engagement and Disease
Defective FasL–FasR engagement plays a role in select autoimmune diseases. In animal models of systemic lupus erythematosus (SLE), auto-reactive cells persist in the host because of defective FasL–FasR interactions. FasR-induced apoptosis may also play a role in the termination of viral diseases. Human immunodeficiency virus (HIV)–infected CD4 cells express high numbers of FasR. It has been proposed that the destruction of HIV-infected CD4 cells may be the result of FasL–FasR interactions. In the case of HIV infections, the mechanism may serve as an alternative to classic cytotoxic T cell killing mediated by perforin and granzymes.
Idiotype Network Theory
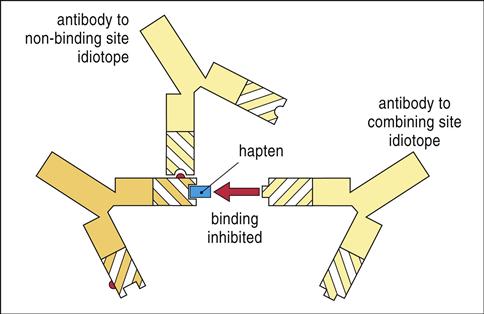
The idiotype network theory was proposed by Jerne in 1974 as a means for explaining immune modulation. The amino acid sequences in the complementarity-determining regions (CDRs) of antibodies that make contact with the antigen are a mirror image of the antigen that stimulated its production. Each CDR amino acid sequence is called an idiotope. Although most idiotopes are associated with the antigen-binding region of the antibody, they can be associated with other sites on the antibody. Collectively, clusters of idiotopes in and around the CDR are called the antibody idiotype.
Idiotopes are considered foreign to the host, and antibodies directed at the idiotype can be generated. Mechanistically, the initial antibody idiotope (Ab1) is the three-dimensional mirror image of the antigen that elicited its production. To interact with Ab1, the anti-idiotope antibody (Ab2) would have a CDR that is consistent with the three-dimensional structure of the antigen that stimulated the production of Ab1. Since Ab2 has the structure of a specific antigenic epitope, it can react with the TCR or BCR of the lymphocyte clone that is specific for the antigen and block the engagement of the TCR and antigen-loaded class I or II glycoproteins (Figure 22-7) on dendritic cells.
The immune response to idiotypes located outside the CDRs facilitates the formation of large immune complexes. Interactions between the immune complexes and FcγRIIB activate a cytoplasmic ITIM that downregulates auto-reactive B cells.