Chapter 75C Esophageal varices
Operative devascularization and splenectomy
Treatment of Esophageal Varices
With the development of advanced vasoactive agents (see Chapter 75B), endoscopic therapies (Chapter 75B), and transjugular intrahepatic portosystemic shunting (TIPS; see Chapter 76E), the need for surgical treatment for esophagogastric varices has decreased (Cello et al, 1984). Most patients with esophagogastric varices have cirrhosis and portal hypertension; for patients in advanced stages of liver failure, liver transplantation (see Chapter 97A, Chapter 97B, Chapter 97C, Chapter 97D, Chapter 97E ) is the optimal treatment, although shunting (see Chapter 78) or nonshunting operations can be considered for treatment of bleeding complications. Rikkers (1998) reported that portosystemic shunts and esophagogastric devascularization have an important role in selected patients with bleeding related to portal hypertension.
Compared with the risk of postoperative hepatic encephalopathy in surgical shunts and TIPS, nonshunting procedures maintain postoperative portal perfusion and long-term hepatic and systemic hemodynamics in patients with cirrhosis, which results in a low incidence of postoperative encephalopathy (Lin et al, 1993; Vons et al, 1996). The applications of shunting operations are limited in patients with extensive thrombosis of the mesenteric venous system, but nonshunting operations do not have such limitations. In addition, nonshunting operations do not alter vascular anatomy and complicate future liver transplant surgery, although they can cause significant upper abdominal adhesions, which are associated with increased bleeding at the time of transplantation.
In the era of multimodalities for bleeding esophagogastric varices, the role of nonshunting operations is redefined as 1) an emergency procedure when medical treatment, endoscopic ligation or sclerotherapy, and TIPS have failed; 2) a part of combined therapies to achieve better outcomes in selected patients (Burroughs et al, 1989); and 3) a long-term bridge to liver transplantation, particularly in patients with splanchnic thrombosis or in the absence of a facility capable of TIPS (Selzner et al, 2001).
In general, nonshunting operations include the devascularization procedure, splenectomy, and esophagus transection. The devascularization procedure has been described as obliteration of varices or disconnection of the esophagogastric veins from the hypertensive portal tributaries. These approaches, known as the Hassab (Hassab, 1998) and Sugiura procedures (Sugiura & Futagawa, 1973), and other modified procedures are discussed.
Effects of Devascularization and Splenectomy for Esophageal Varices
The principle of devascularization procedures for variceal bleeding is based on anatomic features. The coronary vein and gastric veins are anastomosed with tributaries of the superior vena cava by collateral channels in the submucosa of the esophagus, between the two muscular layers, and in the periesophageal region. In portal hypertension, the increased venous pressure can produce varices throughout the length of the esophagus and down into the upper stomach. In the region of the esophageal hiatus, periesophageal vein dilation does not usually occur, and the most blood flow goes through the esophageal submucosal varices. Hence, most bleeding occurs in the distal segment of the esophagus, in the last 5 cm, usually at the esophagogastric junction (Spence, 1984).
Spence and colleagues (1983) applied a computerized image analysis system to study the venous anatomy of the esophagus; serial sections have shown that the large vessels in the lamina propria communicate directly with dilated intraepithelial blood channels via the epithelial papillae. These intraepithelial channels seen histologically represent the cherry-red spots viewed endoscopically (Spence & Terblanche, 1987). An ideal technique of control for bleeding varices would be permanent obliteration of the varices in the lower periesophageal vessels and intraepithelial dilated vessels.
Portal hypertension, however, is not decreased even with the ideal devascularization procedures. Some methods to reduce the portal hypertension include medication with β2-blockers, octreotide, and vasopressin (Dell’Era et al, 2008) and surgical treatment. Liver transplantation is the most effective treatment to reduce portal hypertension, but splenectomy may also have benefit in this regard. Zhang and colleagues (2009) found a decreased portal venous pressure gradient, decreased portal venous flow, and increased hepatic artery flow after splenectomy with periesophagogastric devascularization, which resulted in short-term improvement in hepatic functional reserve. De Cleva and colleagues (2007) compared esophagogastric devascularization plus splenectomy with distal splenorenal shunt in hemodynamic evaluation and found similar improved hyperdynamic circulation in schistosomal portal hypertension.
History of Esophageal Transection, Devascularization, and Splenectomy
Crile (1950) proposed transthoracic ligation of esophageal varices, and subsequently many modifications of his technique have been devised. Boerema (1949) and Vosschulte (1957) introduced the simple button technique for transabdominal esophageal transection, but unfortunately, this method was a major source of stricture formation (Johnston & Kelly, 1976). Walker (1964) used a vertical incision for the muscle layers of the esophagus and a transverse division of the mucosa and submucosa to reduce the risk of posttransection leakage. About the same time, Tanner (1961) proposed a subcardiac portoazygos disconnection with gastric transection. Hassab (1967) found that in patients with bleeding esophageal varices secondary to schistosomiasis, an adequate gastroesophageal devascularization procedure via the abdomen was possible. This technique, known as the Hassab procedure, included splenectomy but not esophageal or gastric transection, and Hassab (1998) reported a remarkably low rate of rebleeding with this method.
Japanese surgeons devised an extensive transthoracic paraesophageal devascularization and esophageal transection combined with an abdominal component consisting of splenectomy, devascularization of the upper stomach, vagotomy, and pyloroplasty (Sugiura & Futagawa, 1973). Thereafter, some Japanese surgeons modified this Sugiura procedure with a sole abdominal approach and achieved similar survival (Inokuchi, 1985).
In 1974, Vankemmel published the first report of the use of a circular stapling gun for esophageal transection. This technique removes approximately 1 cm of full-thickness esophageal wall, provides a safe suture line, and reduces the risk of stricture formation. Jin and Rikkers (1996) reported the American experience with a modified Sugiura procedure without esophagus transection as an effective method for treating variceal hemorrhage. Johnson and colleagues (2006) proposed that the same results could be achieved with a transabdominal modified devascularization procedure without esophageal stapler transection. Nakamura and colleagues (1996) simultaneously used the Hassab procedure and endoscopic injection sclerotherapy for esophageal varices. Hashizume and colleagues (1998) first applied the laparoscopic technique to perform the Hassab procedure and splenectomy successfully in 10 patients with cirrhosis and varices. Jiang and colleagues (2009) compared open and laparoscopic splenectomy with azygoportal devascularization and concluded that the laparoscopic procedure was a safe and effective method in carefully selected patients.
Hassab Procedure
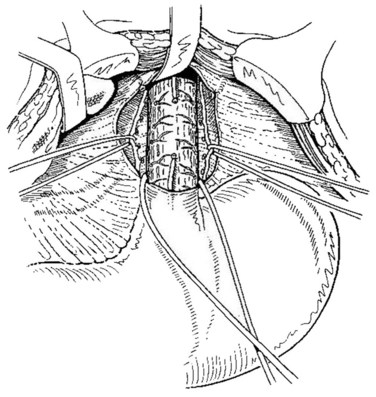
Hassab (1967) proposed a method of gastroesophageal decongestion and splenectomy (GEDS) for management of bleeding varices. In this approach, the patient is in a supine position; a 30-degree, head-up tilt improves the exposure of the operative field. A midline epigastric incision is generally used. Occasionally, an extended left subcostal incision or L-shaped incision of the left upper abdomen is used for extreme splenomegaly. The left lobe of the liver is retracted. In case of left lobe hypertrophy in cirrhotic patients, division of the left lobe ligaments may be useful. Splenectomy was initially performed, and the splenic artery could be suture ligated from the upper part of the pancreatic body if pancreatic tail oozing persisted. Next, the gastrohepatic ligament is divided in the area where it is thin and usually transparent, exposing the right crus of the esophageal hiatus. The incision is carried out superiorly over the anterior aspect of the esophagus, dividing the reflection of the parietal peritoneum at the junction between the esophagus and the diaphragm. The incision is pursued down the left side of the esophagus until the left crus of the esophageal hiatus is identified. The abdominal esophagus is isolated by circumferential dissection and looped with a tape (Fig. 75C.1).
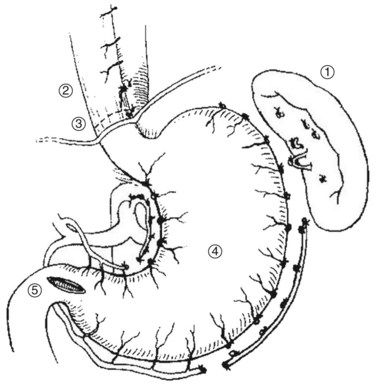
The Hassab procedure consists of splenectomy, perihiatal devascularization of the lower esophagus, ligation of the left gastric vessels, devascularization of the proximal half of the stomach, and separation of the stomach from its bed through the abdominal approach (Fig. 75C.2). The points of this technique are 1) perihiatal devascularization of the lower 3 to 4 inches of the esophagus with ligation of any vessels or vagus nerves ascending through the hiatus and diaphragm; 2) ligation of the left gastric artery branches to the stomach, taking care to avoid injury to any accessory left hepatic artery; 3) devascularization of the proximal half of the stomach, taking care to reperitonealize the greater curvature of the stomach to avoid rebleeding; and 4) aggressive pulmonary toilet, which is necessary to avoid basal collapse and chest infection. As an additional step, suction drainage of the splenic bed was suggested by Hassab (1998) to avoid subphrenic infection, although this is probably unnecessary.
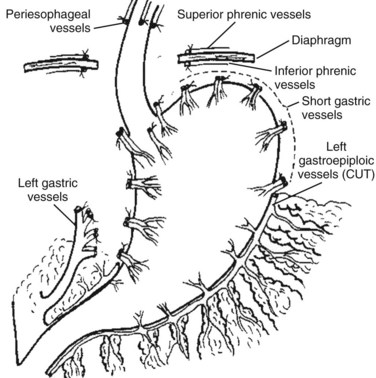
FIGURE 75C.2 Diagrammatic representation of the Hassab procedure.
(From Hassab MA, 1967: Gastro-esophageal decongestion and splenectomy in the treatment of esophageal varices in bilharzial cirrhosis: further studies with a report on 355 operations. Surgery 61:170.)
Hassab (1998) suggested that combined sclerotherapy or endoscopic ligation could reduce rebleeding if there were missed obliterated esophageal perforators or if devascularization at operation was incomplete. Nakamura and colleagues (1992) applied endoscopic ultrasonography and reported that GEDS is effective for the extramural connections, but for the intramural connections, combination with sclerotherapy is necessary. Hassab (1998) reported that esophageal transection is unnecessary and instead disconnected the perigastroesophageal portoazygos connections; this is contrary to Sugiura and Futagawa (1973), who preserved them and interrupted gastroesophageal continuity with stapled transection and reanastomosis. Zhang (1991) compared treatment of variceal bleeding treated with GEDS or GEDS plus esophageal transection and found that the simple procedure without transection was effective in maintaining good quality of life with no encephalopathy. He reported maintenance of liver function and an upper gastrointestinal (GI) bleeding rate of 3.1% within 2 years. The addition of esophageal transection did not change the bleeding rate and added more serious complications early after operation.
Sugiura and Modified Sugiura Procedures
The Sugiura procedure is a nonshunting technique aimed at the eradication of esophageal varices. The operation consists of an extensive paraesophagogastric devascularization with esophageal transection and splenectomy through successive thoracic and abdominal incisions. The results obtained by Sugiura and Futagawa (1973, 1984) in Japan were impressive and led some authors to evaluate the efficacy of the operation in Western countries. In the West, the Sugiura procedure was believed to be technically complex and time consuming and was largely ignored or abandoned. Interest was renewed, however, when simplified procedures using an exclusive abdominal approach and stapled transection were described (Peracchia et al, 1980). The original procedure proposed by Sugiura and Futagawa (1973) and the modified procedure described by Ginsberg and colleagues (1982) and Umeyama and colleagues (1983), which is the procedure used routinely use today (Liao et al, 2009), are described.
Sugiura Procedure
The original Sugiura procedure (Fig. 75C.3) is performed in two steps; a thoracic approach is taken first, followed by an abdominal procedure 4 to 6 weeks later (Sugiura & Futagawa, 1973). The thoracic procedure is carried out through a left lateral thoracotomy using the sixth or seventh intercostal space. The mediastinal pleura over the esophagus is opened longitudinally. The lower part of the thoracic esophagus is surrounded by dilated (adventitial) veins that form a plexus. These adventitial veins run parallel to the esophageal wall and communicate with the submucosal veins (variceal channels) via large perforating veins. All these perforating veins must be completely and systematically ligated and divided to devascularize the esophagus; at the same time, great care must be taken not to damage the adventitial veins, which represent a spontaneous portacaval shunt. Usually, 30 to 50 perforating veins must be ligated over a 12- to 18-cm length of the thoracic esophagus. At the level of the hiatus, periesophageal channels are bulky and esophageal varices predominate. Thorough devascularization of this region is paramount because it prepares the area for esophageal transection. After completion of the devascularization, esophageal transection is performed at the level of the diaphragm. During this step, esophageal varices are electively occluded with sutures. A nasogastric tube is left in the stomach and the thorax is closed with appropriate drainage.
The abdominal procedure is subsequently performed through an upper midline or left subcostal laparotomy, and splenectomy is performed. The abdominal esophagus and the cardia are devascularized, as are the greater curvature and the posterior aspect of the stomach. The posterior vagus nerve is divided as in a selective vagotomy (Sugiura & Futagawa, 1973). Devascularization of the lesser curvature of the stomach follows, and the cardioesophageal branches of the left gastric vessels are ligated and divided. Devascularization is performed over more than 7 cm of gastric wall from the cardia (Sugiura & Futagawa, 1973), and the esophagus and the cardia are completely mobilized and freed from the adjacent anatomic structures. Devascularization is facilitated by the division of the anterior vagus nerve; because of this, pyloroplasty is performed, and the abdominal incision is closed. Both key steps of the abdominal procedure may be performed during the same operative session by using synchronous incisions. If such an option is elected, the abdominal procedure is carried out through a left subcostal incision.
Modified Sugiura Procedure
To improve the exposure of the esophagus, the diaphragmatic orifice of the esophagus is opened. The pericardium is freed from the upper aspect of the diaphragm, which is divided sagittally. Esophageal devascularization is performed close to the esophageal wall by dividing only the vessels running transversely (perforating veins) and coursing to perforate the esophageal wall. Devascularization is pursued over a distance of 10 to 12 cm; this step of the operation is facilitated by using clips. In patients who have had previous sclerotherapy with periesophageal fibrosis, completely safe devascularization may be technically impossible; but at the very least, devascularization should be done within the limits of safety to avoid damaging the esophagus. The degree of difficulty during this step of the procedure most likely is linked to the type of sclerosant used and the number of sclerotherapy sessions carried out before surgery (Alam et al, 1996; Chaudhary & Aranya, 1991).
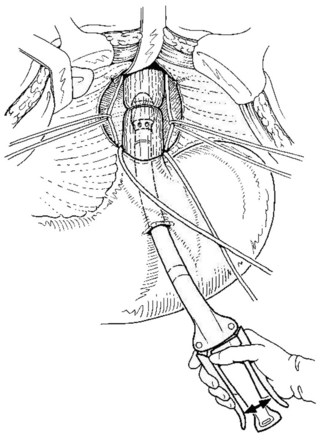
Esophageal transection is performed with a mechanical stapler introduced through a short gastrotomy (Fig 75C.4). In cases of previous sclerotherapy, thickness of the lower end of the esophagus and inflammatory changes preclude the use of a large stapler. A 29-mm circular stapler is optimal for this procedure; however, a smaller stapler (25 mm) may be required, especially in patients treated by previous sclerotherapy (Mariette et al, 1994).
The Sugiura operation includes five procedures, and only devascularization of the gastroesophagus remains in many versions of the modified Sugiura operation. Ginsberg and coworkers (1982) proposed that esophagogastric devascularization be performed without dividing the main vagus trunks; only a proximal gastric vagotomy is done, thereby avoiding a pyloroplasty. Hidalgo Huerta and colleagues (1983) proposed using a Nissen fundoplication to allow wrapping of the transection line, which may prevent reflux possibly caused by extensive dissection of the cardia. Johnson and colleagues (2006) reported that devascularization without esophageal transection has similar safety and outcome as devascularization with esophageal transection to control variceal bleeding. In a prospective, controlled, randomized trial that included 55 patients, Orozco and colleagues (1998) reported that splenectomy may be unnecessary in the Sugiura procedure for control of variceal bleeding; they showed that the no-splenectomy group had fewer blood transfusions and less postoperative portal vein thrombosis, and no difference was reported in rebleeding and encephalopathy rates compared with the splenectomy group.
Laparoscopic Devascularization
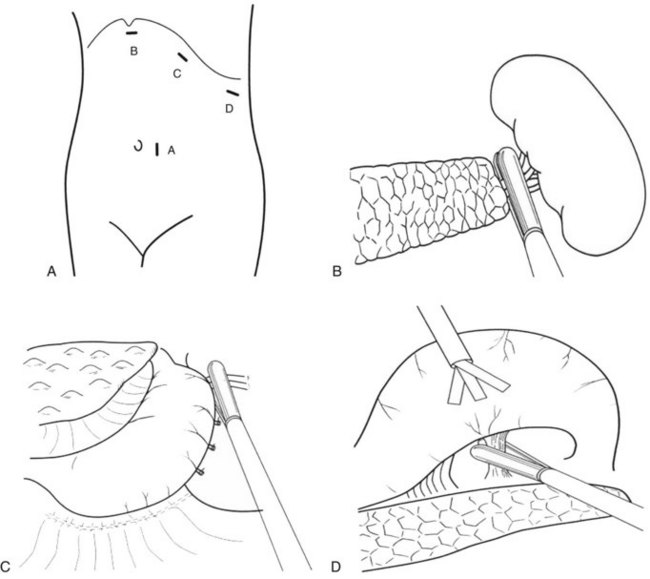
With the development of laparoscopic instrumentation and advanced surgical techniques, the gastroesophageal devascularization procedure can now be performed laparoscopically. The patient is placed in a right semidecubitus position; the trocar port sites are shown in Fig. 75C.5A. The laparoscope is introduced through the cannula close to the umbilicus, and three other 12-mm trocars are inserted into the left subcostal region. The splenocolic ligament is divided with the Harmonic Scalpel (Ethicon, Cincinnati, OH), and the spleen is mobilized from splenophrenic and splenorenal ligaments by both blunt dissection and coagulation. Exposure of the splenic hilum is completed by a division of the gastrosplenic ligament, and the short gastric vessels are divided with a Harmonic Scalpel or autosuture device (Linear Cutter; Ethicon); the autosuture device can also be useful for resecting the spleen at the splenic hilum (Fig. 75C.5B). A large, nylon surgical sack (Endo Catch II; U.S. Surgical, Norwalk, CT) is introduced into the abdominal cavity, and the spleen is maneuvered into the sack, where it is grasped by forceps and crushed with a Kelly clamp and removed.
Devascularization of the short gastric vessels is completed along the greater curvature of the upper stomach after splenectomy. First, the posterior wall of the fundus is dissected and devascularized with the autosuture device (Fig. 75C.5C). Next, the lesser omentum is opened with the Harmonic Scalpel, and the esophageal branches of the left gastric vessels are cut along the lower esophagus. The lesser curvature of the stomach is then dissected until the left gastric artery and vein are exposed, while the greater curvature is lifted up with a retractor (Fig. 75C.5D). Finally, the upper stomach is freed from its bed, and devascularization completed.
Kitano and associates (1994) first reported seven cirrhotic patients with bleeding esophagogastric varices treated by laparoscopic devascularization of the lower esophagus and upper stomach. Hashizume and colleagues (1998) successfully applied the laparoscopic Hassab procedure for sclerotherapy-resistant esophagogastric varices combined with hypersplenism. In subsequent years, other authors (Danis et al, 2004; Jiang et al, 2009; Wang et al, 2008; Yamamoto et al, 2006) reported their experience with laparoscopic devascularization with splenectomy and concluded that this is a feasible, relatively safe, and minimally invasive surgical procedure in selected patients.
Results
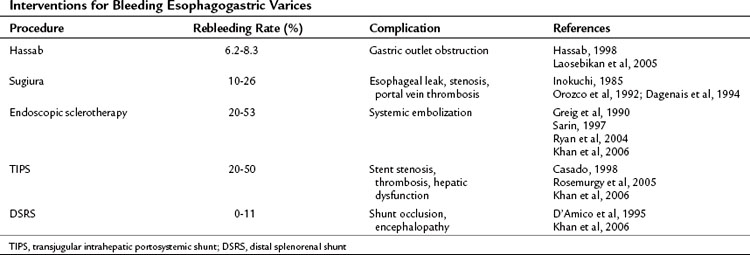
A number of treatment options exist for bleeding esophagogastric varices, including surgical shunting, nonshunt operations, and endoscopy with sclerotherapy or variceal ligation (Table 75C.1). The two kinds of devascularization of gastroesophageal varices are the Hassab and Sugiura procedures, and the reported rebleeding rates, respectively, are 6.2% to 8.3% and 10% to 26%. A concerning complication of the Hassab operation is gastric outlet obstruction as a result of vagus trunk transection without pyloroplasty. The specific complications of the Sugiura operation are esophageal leak and stenosis from the esophageal transection procedure. Compared with the rebleeding rate of 20% to 50% in endoscopic sclerotheapy, devascularization operations seem to be better for rebleeding control. The distal splenorenal shunt has a superior rebleeding control rate, but shunt occlusion and encephalopathy have limited its clinical application in some centers. Although the devascularization operation is complex, it can achieve rebleeding control.
Operative Mortality
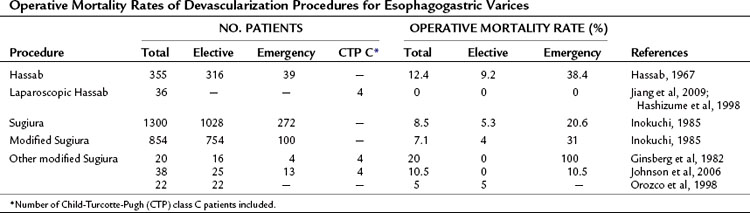
The overall operative mortality rate of the Hassab procedure in Egypt (Hassab, 1967) is 12.4%, and the overall operative mortality rate is 8.5% for the Sugiura operation in Japan (Inokuchi, 1985). The rates compare favorably with the operative mortality rates of various other abdominal operations performed in cirrhotic patients (Franzetta et al, 2003; Sirinek et al, 1987). When the devascularization operation is used as an emergency procedure, the operative mortality rate increases dramatically to 38.4% in the Hassab and 20.6% in the Sugiura procedure (Table 75C.2). The overall and emergency operative mortality rates with the modified Sugiura operation from Japanese reports (Inokuchi, 1985) are 7.1% and 31%, respectively. A laparoscopic Hassab operation was recently developed, but experience is still limited and it has only been performed in highly select patients. Other variations of the Sugiura procedure have been proposed outside Japan; however, given the limited case experience, it is not possible to gauge surgical mortality rates accurately with the modified procedures.
Operative Morbidity
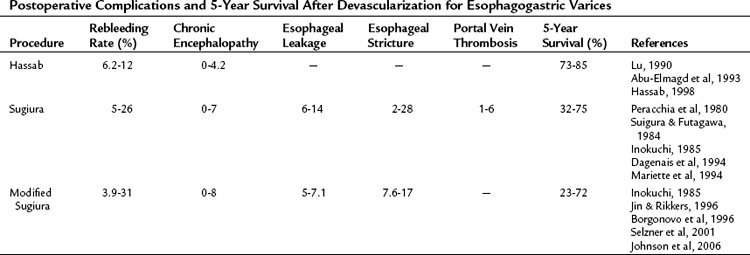
The rates of complications and 5-year survival in various devascularization operations are different (Table 75C.3). The correlative factors should be related to the severity of the liver cirrhosis (e.g., Child-Turcotte-Pugh class C), etiology of the liver disease (schistosomal or cirrhotic portal hypertension), and the surgical methods used (with or without esophageal transection). The rebleeding rates are 6.2% to 12%, 5% to 26%, and 3.9% to 31% with the Hassab, Sugiura, and modified Sugiura procedures, respectively. The range of the rebleeding rates may be attributable to the complete or incomplete devascularization procedure (Hassab, 1998). Most devascularization procedures do not result in deterioration of liver function, and chronic encephalopathy is generally not exacerbated. Complications of esophageal leakage and stenosis are related to esophageal transection. The rate of esophageal leakage and stenosis with the Sugiura operation are 6% to 14% and 2% to 28%, respectively. The incidence rates for the modified Sugiura operation are similar, and the risk of portal vein thrombosis after the Sugiura procedure has been emphasized by Sugiura and Futagawa (1984). Portal vein thrombosis may be linked to thrombocythemia or to a decrease in portal blood flow after splenectomy (Takenaka et al, 1990) and affects 1% to 6% of patients. The 5-year survival rate with the Hassab operation is 73% to 85%, seemingly better than other devascularization procedures. However, these improved results may be attributable to the frequent use of schistosomal portal hypertension with preserved liver function. The 5-year survival rate of the Sugiura and modified Sugiura operations is approximately 70% and dramatically decreases to about 30% in the emergency setting.
Role of Splenectomy and Devascularization in Liver Transplantation
Shunt and nonshunt operations cannot improve liver function in cirrhotic patients in the long term; this can only be accomplished with liver transplantation. Some shunt operations impair liver function and result in worsening of hepatic encephalopathy (Henderson et al, 2006). Nonshunt operations, such as the Hassab and Sugiura procedures, seem to preserve liver function and increase hepatic artery flow in the short term (Zhang et al, 2009). Technique and experience accumulation has improved the success rate of endoscopic variceal ligation for variceal bleeding; however, endoscopic variceal ligation and devascularization with splenectomy can occlude collateral veins both inside and outside the esophageal wall (Liu et al, 2006). Laparoscopic devascularization with splenectomy combined with endoscopic variceal ligation may be an ideal procedure for consideration as a bridge to liver transplantation in cirrhotic patients with variceal bleeding. This approach not only controls variceal bleeding, but also may maintain liver function and may be associated with decreased surgical adhesions when compared with open devascularization procedures.
Abu-Elmagd KM, et al. Ten years of experience with patients with chronic active liver disease variceal bleeding: ablative versus selective decompressive therapy. Surgery. 1993;114:868-881.
Alam MK, et al. Effect of previous sclerotherapy on the outcome of gastro-esophageal devascularization and esophageal transection in bleeding esophageal varices. Br J Surg. 1996;83:1702-1705.
Boerema I. Surgical therapy of bleeding varices of esophagus during hepatic cirrhosis and Banti’s disease. Ned Tijdschr Geneeskd. 1949;93:4174-4182.
Borgonovo G, et al. Comparison of a modified Sugiura procedure with portal systemic shunt for prevention of recurrent variceal bleeding in cirrhosis. Surgery. 1996;119:214-221.
Burroughs AK, et al. A comparison of sclerotherapy with staple transection of the esophagus for the emergency control of bleeding from esophageal varices. N Engl J Med. 1989;321:857-862.
Casado M. Clinical events after transjugular intrahepatic portosystemic shunt: correlation with hemodynamic findings. Gastroenterology. 1998;114:1296-1303.
Cello JP, et al. Endoscopic sclerotherapy versus portacaval shunt in patients with severe cirrhosis and variceal hemorrhage. N Engl J Med. 1984;311:1589-1594.
Chaudhary A, Aranya RC. Devascularization following endoscopic sclerotherapy of esophageal varices: dangers and difficulties. Br J Surg. 1991;78:1249-1251.
Crile G. Transesophageal ligation of bleeding esophageal varices. Arch Surg. 1950;61:654-660.
D’Amico G, et al. The treatment of portal hypertension: a meta-analytic review. Hepatology. 1995;22:332-354. 1995
Dagenais M, et al. Experience with radical esophagogastric devascularization procedures (Sugiura) for variceal bleeding outside Japan. World J Surg. 1994;18:222-228.
Danis J, et al. Novel technique of laparoscopic azygoportal disconnection for treatment of esophageal varicosis: preliminary experience with five patients. Surg Endosc. 2004;18:702-705.
de Cleva R, et al. Pre- and postoperative systemic hemodynamic evaluation in patients subjected to esophagogastric devascularization plus splenectomy and distal splenorenal shunt: a comparative study in schistomomal portal hypertension. World J Gastroenterol. 2007;13:5471-5475.
Dell’Era A, et al. Acute variceal bleeding: pharmacological treatment and primary/secondary prophylaxis. Best Pract Res Clin Gastroenterol. 2008;22:279-294.
Franzetta M, et al. Prognostic factors of cirrhotic patients in extra-hepatic surgery. Minerva Chir. 2003;58:541-544.
Ginsberg RJ, et al. A modified Sugiura procedure. Ann Thorac Surg. 1982;34:258-264.
Greig JD, et al. Management of gastric variceal haemorrhage. Br J Surg. 1990;77:297-299.
Hashizume M, et al. Laparoscopic gastric devascularization and splenectomy for sclerotherapy-resistant esophagogastric varices with hypersplenism. J Am Coll Surg. 1998;187:263-270.
Hassab MA. Gastro-esophageal decongestion and splenectomy in the treatment of esophageal varices in bilharzial cirrhosis: further studies with a report on 355 operations. Surgery. 1967;61:169-176.
Hassab MA. Gastro-esophageal decongestion and splenectomy GEDS (Hassab), in the management of bleeding varices. Int Surg. 1998;83:38-41.
Henderson JM, et al. Distal splenorenal shunt versus transjugular intrahepatic portal systematic shunt for variceal bleeding: a randomized trial. Gastroenterology. 2006;130:1643-1651.
Hidalgo Huerta M, Cabrero Gómez F, Salomón Giordani P. Gastroesophageal devascularization, splenectomy, circular suture of the esophagus and fundoplication in the treatment of esophageal varices (results in 18 cases). Rev Esp Enferm Apar Dig. 1983;63:395-400.
Inokuchi K. Present status of surgical treatment of esophageal varices in Japan: a nationwide survey of 3,588 patients. World J Surg. 1985;9:171-180.
Jiang XZ, et al. Laparoscopic and open splenectomy and azygoportal disconnection for portal hypertension. World J Gastroenterol. 2009;15:3421-3425.
Jin G, Rikkers LF. Transabdominal esophagogastric devascularization as treatment for variceal hemorrhage. Surgery. 1996;20:641-647.
Johnson M, et al. Transabdominal modified devascularization procedure with or without esophageal stapler transection—an operation adequate for effective control of a variceal bleed: is esophageal stapler transection necessary? World J Surg. 2006;30:1507-1518.
Johnston GW, Kelly JM. Early experience with the Boerema button for bleeding oesophageal varices. Br J Surg. 1976;63:117-121.
Khan S, et al. Portosystemic shunts versus endoscopic therapy for variceal rebleeding in patients with cirrhosis. Cochrane Database Syst Rev. 4, 2006. CD000553
Kitano S, et al. Laparoscopy-assisted devascularization of the lower esophagus and upper stomach in the management of gastric varices. Endoscopy. 1994;26:470-473.
Laosebikan AO, et al. Schistosomal portal hypertension. J Am Coll Surg. 2005;200:795-806.
Liao GS, et al. Transection of the esophagus is optional in the modified Sugiura procedure. Hepatogastroenterology. 2009;56:133-138.
Lin PW, et al. Effects of splenectomy, devascularization and esophageal transection on portal venous pressure and portal perfusion in cirrhotic patients with bleeding esophageal varices. J Formos Med Assoc. 1993;92:871-875.
Liu B, et al. Evaluation of the effects of combined endoscopic variceal ligation and splenectomy with pericardial devascularization on esophageal varices. World J Gastroenterol. 2006;12:6889-6892.
Lu XS. Long-term results of the Hassab operation in portal hypertension: 10 year’s follow-up. Zhonghua Wai Ke Za Zhi. 1990;28:143-146.
Mariette D, et al. The Sugiura procedure: a prospective experience. Surgery. 1994;115:282-289.
Nakamura H, et al. Selection of the treatment for esophagogastric varices: analysis of collateral structures by endoscopic ultrasonography. Surg Endoscop. 1992;6:228-234.
Nakamura H, et al. Hassab operation with intraoperative endoscopic injection sclerotherapy (“Hassab-EIS”) for esophagogastric varices: with an autopsied case after excessive gastric vascular damage. Hepatogastroenterology. 1996;43:980-986.
Orozco H, et al. Elective treatment of bleeding varices with the Sugiura operation over 10 years. Am J Surg. 1992;163:585-589.
Orozco H, et al. Is splenectomy necessary in devascularization procedures for treatment of bleeding portal hypertension? Arch Surg. 1998;133:36-38.
Peracchia A, et al. A new technique for the treatment of esophageal bleeding in portal hypertension. Int Surg. 1980;65:401-404.
Rikkers LF. The changing spectrum of treatment for variceal bleeding. Ann Surg. 1998;228:536-546.
Rosemurgy AS, et al. H-graft portocaval shunts versus TIPS, ten-year follow-up of a randomized trial with comparison to predicted survivals. Ann Surg. 2005;241:238-246.
Ryan BM, et al. A pathophysiologic, gastroenterologic, and radiologic approach to the management of gastric varices. Gastroenterology. 2004;126:1175-1189.
Sarin SK. Long-term follow-up of gastric variceal sclerotherapy: an eleven-year experience. Gastroinest Endosc. 1997;46:8-14.
Selzner M, et al. Current indication of a modified Sugiura procedure in the management of variceal bleeding. J Am Coll Surg. 2001;193:166-173.
Sirinek KR, et al. Improving survival in patients with cirrhosis undergoing major abdominal operations. Arch Surg. 1987;122:271-273.
Spence RAJ. The venous anatomy of the lower esophagus in normal subjects and in patients with varices: an image analysis study. Br J Surg. 1984;71:739-744.
Spence RAJ, Terblanche J. Venous anatomy of the lower esophagus: a new perspective on varices. Br J Surg. 1987;74:659-660.
Spence RAJ, et al. Oesphageal mucosal changes in patients with varices. Gut. 1983;24:1024-1029.
Sugiura M, Futagawa S. A new technique for treating esophageal varices. J Thorac Cardiovasc Surg. 1973;66:677-685.
Sugiura M, Futagawa S. Esophageal transection with para-esophagogastric devascularization (the Sugiura procedure) in the treatment of esophageal varices. World J Surg. 1984;8:673-682.
Takenaka H, et al. Hemodynamic study after devascularization procedure in patients with esophageal varices. Surgery. 1990;107:55-62.
Tanner NC. Direct operations in the treatment of complications of portal hypertension. J Int Coll Surg. 1961;36:308-314.
Umeyama K, et al. Transabdominal esophageal transection for esophageal varices: experience in 101 patients. Br J Surg. 1983;70:419-422.
Vankemmel M. Resection-anastomose de l’oescophage sus-cardial pour rupture de varices oesophagiennes. Nauvelle Presse Medicale. 1974;5:1123.
Vons C, et al. Long-term hemodynamic effects of portocaval shunt and Sugiura procedure in patients with cirrhosis. HPB Surg. 1996;9:209-213.
Vosschulte K. Place de la section par ligature de l’oesophage dans le traitement de l’hypertension portale. Lyon Chir. 1957;53:519-525.
Walker RM. Esophageal transection for bleeding varices. Surg Gynecol Obstet. 1964;118:323-329.
Wang YD, et al. Laparoscopic splenectomy and azygoportal disconnection for bleeding varices with hypersplenism. J Laparoendosc Adv Surg Tech A. 2008;18:37-41.
Yamamoto J, et al. Hand-assisted laparoscopic splenectomy and devascularization of the upper stomach in the management of gastric varices. World J Surg. 2006;30:1520-1525.
Zhang DC. Hassab’s procedure with or without power esophageal transection in the treatment of portal hypertension: a prosective controlled study. Zhonghua Wai Ke Za Zhi. 1991;19:561-563.
Zhang Y, et al. The changes of hepatic hemodynamics and functional hepatic reserve after splenectomy with periesophagogastric devascularization. Hepatogastroenterology. 2009;56:835-839.