INTRODUCTION AND DEFINITION
GEFS+ is an acronym for generalized epilepsy febrile seizures plus. It was defined by Scheffer et al in 1997 and was based upon a very careful and extensive examination of large families with multiple affected individuals (1). Family members had febrile seizures alone (FS); febrile seizures plus implies febrile seizures beyond the typical age limit of 6 years or a history of febrile seizures along with afebrile generalized seizures, or afebrile seizures. Early descriptions of the afebrile seizures in these individuals highlighted generalized seizures including absence, convulsions, myoclonus, and atonic seizures. Later descriptions included a richer variety of seizures and epilepsy syndromes. Perhaps the most startling revelation was the fact that some families contained children with severe epilepsies, including myoclonic–astatic epilepsy as described by the Doose syndrome, Dravet syndrome, temporal lobe epilepsy, and infantile spasms. It was quickly recognized that this was a very important contribution to our understanding of the genetic contributions to epilepsy. Some characteristics appeared to be inherited in an autosomal dominant fashion, but the extremely broad clinical spectrum of affected individuals in some families strongly suggested polygenic contributions. These genetic influences may involve complex interactions between susceptibility genes, modifier genes, and the environment.
EPIDEMIOLOGY
The discovery of GEFS+ postdates many of the classic and extensive epidemiologic studies of childhood epilepsy. The original patients described were from large families, which are undoubtedly quite rare. GEFS+ more commonly presents in small families, or perhaps occurs with spontaneous mutations such that the genetic contributions may not be appreciated by the physicians caring for the patients, unless the individual presents with a severe phenotype, such as Dravet syndrome, and undergoes SCN1A testing. These facts, coupled with the large and diverse clinical spectrum of the disorder, make it difficult to establish the precise incidence and prevalence. It is nevertheless clear that families with GEFS+ have been confirmed in many different regions of the world with diverse racial backgrounds and that the phenomenon is not isolated to a restricted gene pool. There is no clear gender preponderance.
CLINICAL MANIFESTATIONS
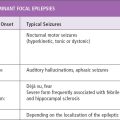
The clinical manifestation of GEFS+ are diverse (1,2). The most common phenotype is typical febrile seizures, defined as convulsions in the setting of fever (above 38°C) in children between the ages of 3 months and 6 years. The second most common phenotype is febrile seizures plus, which indicates that febrile seizures continued beyond 6 years of age or that there were afebrile convulsions in addition to the febrile seizures. The least common phenotype is isolated afebrile convulsions in childhood. Generalized seizure types seen include absence, atonic, and myoclonic seizures. Focal seizures arising from the temporal or frontal regions have also been reported (3). Differentiation from the typical child with febrile seizures is suggested by the presence of other individuals in the family with various forms of epilepsy.
Two of the most severe phenotypes reported in families with GEFS+ include myoclonic–astatic epilepsy as described by Doose et al, and Dravet syndrome. About one-third of children with myoclonic-astatic epilepsy have a history of febrile seizures. Boys are more commonly affected. Myoclonic–astatic seizures are the most common manifestation, but myoclonic, atonic, absence and generalized clonic–tonic–clonic seizures are also observed. Tonic seizures may be present, particularly during the night, and nonconvulsive status epilepticus is also seen. Generalized spike–wave discharges are common, as are rhythmic bursts of bi-parietal theta activity. Many patients will go into remission with several years of onset, but the outcome is variable and some patients will be left with substantial cognitive disabilities (4).
Children with Dravet syndrome typically present in the first year of life with febrile hemiclonic seizures or generalized convulsions (5). Children then develop a variety of afebrile seizures, including absence, atonic, myoclonic, or focal seizures. The EEG may initially show focal spikes in the posterior derivations, but later, generalized spike–wave discharges develop in most individuals, and a photoparoxysmal response may been seen in some.
EEG FEATURES
EEG backgrounds are usually normal. Interictal features appear to vary according to the clinical phenotype. Those individuals with febrile seizures alone often have normal EEGs (1). Those with febrile seizures and generalized afebrile seizures may have generalized spike–wave discharges. Patients with the myoclonic-atonic epilepsy (MAE) or Dravet phenotype have the corresponding EEG features. Patients with focal seizures may have focal spikes or focal slowing (3).
PATHOPHYSIOLOGY
The pathophysiology of GEFS+ has been well studied. Mutations involving three different genes have been shown to be relevant, but these genes alone cannot explain the large phenotypic variation seen in the families of affected individuals. It is likely that other genes yet to be discovered, the environment, or both play additional roles in determining the phenotype. It has been speculated that a fuller knowledge of these interactions may illuminate important pathophysiologic mechanisms in other forms of epilepsy.
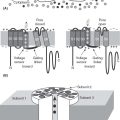
The genes involved in GEFS+ encode the GABAA receptor and the neuronal sodium channel. The gene encoding the alpha 1 subunit of the sodium channel, SCN1A, is the most frequently mutated in GEFS+. This subunit has four domains that create the pore of the channel, a voltage sensor, and an anchor to the axonal membrane. Missense mutations are found at many different loci and may cause gain of function, loss of function, or no discernable change in the channel properties (6–8). Mutations of the beta subunit, SCN1B, have also been found, but these are less common (9). The mutations affect an extracellular portion of the protein that is important in altering properties of the channel. Finally, mutations of the gene that encodes the GABAA receptor are reported in GEFS+. These mutations involve the gamma 2 subunit, GABRG2, and the delta subunit, GABRD (10,11). These mutations are the most rare in GEFS+, but may be of particular importance because of their association with idiopathic generalized epilepsies. Most recently, mutations in STX1B have been found in individuals with the GEFS+ phenotype (12).
DIAGNOSTIC EVALUATION AND DIFFERENTIAL DIAGNOSIS
Patients with febrile seizures are common, and as many as 15% to 20% of all patients with epilepsy have had febrile seizures. The main features suggesting GEFS+ are a positive family history, the persistence of seizures beyond 6 years of age, and the coexistence of other afebrile seizures. From our standpoint, a positive family history is the most relevant and distinguishing clinical feature. The evolution to severe forms of epilepsy is likely to be phenotypically consistent with Dravet syndrome or myoclonic–astatic epilepsy. Genetic testing for SCN1A gene mutations (targeted at identification of individuals with the severe or Dravet phenotype) is now commercially available in the United States.
TREATMENT
The large amount of emerging information regarding the pathogenesis of the seizures in GEFS+ should ultimately direct the development of rational therapies for patients with GEFS+. So far, though, such recommendations are lacking (13). Patients at risk for prolonged or repeated febrile seizures may be prescribed rectal diazepam to be used as needed under these circumstances. There are no formal guidelines for determining when, if ever, one should initiate prophylactic treatment in children with repeated febrile seizures, particularly those whose febrile seizures persist beyond the expected cutoff age of 6 years. Treatment of patients with repeated afebrile seizures would depend upon the seizure type. A recent review covers the excitement and challenges associated with drug development for these types of channelopathies (14). Treatment of Dravet syndrome and myoclonic–astatic epilepsy is described elsewhere in the text.
COURSE AND PROGNOSIS
The course is generally favorable. The majority of patients do not have refractory epilepsies and their epilepsy, if it develops, usually remits spontaneously, often by mid-childhood (2). Patients with a clinical phenotype consistent with Dravet syndrome have a severe prognosis and those with myoclonic–astatic epilepsy must be considered to have a guarded prognosis.
REFERENCES
1. Scheffer IE, Berkovic SF. Generalized epilepsy with febrile seizures plus: a genetic disorder with heterogeneous clinical phenotypes. Brain. 1997;120(Pt 3):479–490.
2. Singh R, Scheffer IE, Crossland K, Berkovic SF. Generalized epilepsy with febrile seizures plus: a common childhood-onset genetic epilepsy syndrome. Ann Neurol. 1999;45(1):75–81.
3. Abou-Khalil B, Ge Q, Desai R, et al. Partial and generalized epilepsy with febrile seizures plus and a novel SCN1A mutation. Neurology. 2001;57(12):2265–2272.
4. Guerrini R, Aicardi J. Epileptic encephalopathies with myoclonic seizures in infants and children (severe myoclonic epilepsy and myoclonic-astatic epilepsy). J Clin Neurophysiol. 2003;20(6):449–461.
5. Dravet C, Bureau M, Oguni H, et al. Severe myoclonic epilepsy in infancy: Dravet syndrome. Adv Neurol. 2005;95:71–102.
6. Mulley JC, Scheffer IE, Petrou S, et al. SCN1A mutations and epilepsy. Hum Mutat. 2005;25(6):535–542.
7. Lossin C, Rhodes TH, Desai RR, et al. Epilepsy-associated dysfunction in the voltage-gated neuronal sodium channel SCN1A. J Neurosci. 2003;23(36):11289–11295.
8. Spampanato J, Aradi I, Soltesz I, Goldin AL. Increased neuronal firing in computer simulations of sodium channel mutations that cause generalized epilepsy with febrile seizures plus. J Neurophysiol. 2004;91(5):2040–2050.
9. Audenaert D, Claes L, Ceulemans B, et al. A deletion in SCN1B is associated with febrile seizures and early-onset absence epilepsy. Neurology. 2003;61(6):854–856.
10. Baulac S, Huberfeld G, Gourfinkel-An I, et al. First genetic evidence of GABA(A) receptor dysfunction in epilepsy: a mutation in the gamma2-subunit gene. Nat Genet. 2001;28(1):46–48.
11. Dibbens LM, Feng HJ, Richards MC, et al. GABRD encoding a protein for extra- or peri-synaptic GABAA receptors is a susceptibility locus for generalized epilepsies. Hum Mol Genet. 2004;13(13):1315–1319.
12. Schubert J, Siekierska A, Langlois M, et al. Mutations in STX1B, encoding a presynaptic protein, cause fever-associated epilepsy syndromes. Nat Genet. 2014;46(12):1327–1332.
13. Burgess DL. Neonatal epilepsy syndromes and GEFS+: mechanistic considerations. Epilepsia. 2005;46(Suppl 10):51–58.
14. Catterall WA. Sodium channels, inherited epilepsy, and antiepileptic drugs. Ann Rev Pharmacol Toxicol. 2014;54:317–338.