Chapter 139 Epidemiology of Posterior Uveal Melanoma
Introduction
The underlying causes of uveal melanoma are not clearly established. Because we do not have a means to prevent this disease, epidemiologic research is key to determine associated factors and to better understand the mechanisms of disease development. This chapter will focus on posterior uveal melanoma (choroidal and ciliary body melanoma) and will not include a discussion of iris melanoma, which can be reviewed elsewhere.1
Posterior uveal melanoma is an uncommon disease with an incidence of 5–6 cases per 1 million population per year. It is usually diagnosed in the sixth decade of life, and its incidence rises steeply with age. It is the most common primary intraocular malignancy, and the leading primary intraocular disease which can be fatal in adults. Although posterior uveal tract melanoma is the most common noncutaneous form of melanoma, the incidence rate is one eighth that of cutaneous melanoma in the USA.2
Incidence
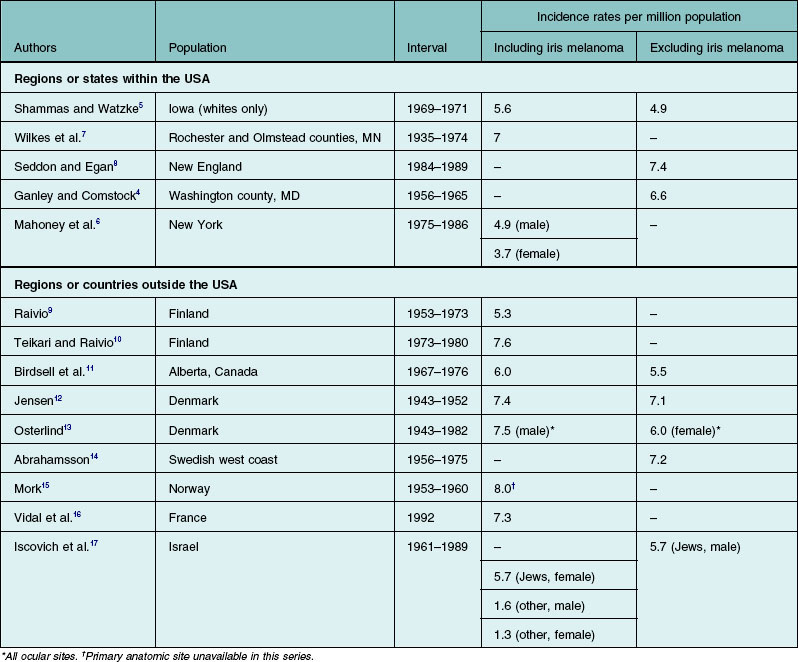
According to the most recent report of data obtained from the Surveillance, Epidemiology, and End Results (SEER) program database in the USA (1973–2008), the mean age-adjusted incidence estimate for ocular melanoma in the USA is 5.1 cases per 1 million population.3 Similar estimates for uveal melanomas of the choroid and ciliary body4,5 or the choroid, ciliary body, and iris5–7 have been reported for individual states or regions within the USA. Other surveys of primarily white populations have found incidence rates similar to those of the USA (Table 139.1).8–17 The incidence rate in black Africans is unknown but thought to be extremely low.18–21 In the USA, the rate of the disease in African Americans is less than one-eighth of the rate for whites.2 Although small fluctuations in several populations have been observed,22,23 the incidence and mortality rates of uveal melanoma have been shown to be fairly stable over the past several decades.24,25 It is important to note that comparative incidence rates across populations must be interpreted with caution – differences in case definition, methods of case ascertainment, and methods of age-adjustment could affect the relative incidence rates.
Host factors
Age and sex
Uveal melanoma is rare in children.26–28 In most series, the median age at diagnosis is 55 years.9,12 In Jensen’s series,12 rates of disease decreased in males after age 69 years. An evaluation of uveal melanoma cases reported to the Finnish Cancer Registry between 1953 and 1982,9,10 found that rates of disease in females leveled off beginning in the mid-60s, but in males of the same age, rates continued to increase. Data from Norway show rates dropping in both sexes after age 70 years.15 This is in contrast to the majority of adult cancers in which incidence increases exponentially with age.
In many large surveys of uveal melanoma patients, there is a slight predominance of males.2,6,12,16,29 Higher rates in males have been reported in many studies that presented gender-specific rates of ocular melanoma. Higher rates in males have also been found in studies that used all eye cancers in persons aged 15 years or older as a surrogate for ocular melanomas.22,23,25 It has been shown in white populations that over 90% of these eye cancers are ocular melanomas22 with the majority involving the uveal tract.3 In New England, however, the overall rate during a 6-year period was similar in males and females.8 Age-specific incidence rates were 2.3 per million for persons aged 15–44 years, 15 per million for persons aged 45–64 years, and 25.3 per million for persons aged 65 years and older.
Race and ancestral origin
Uveal melanoma is rare in nonwhite races. Data from the Third National Cancer Survey indicate that in the USA, whites have more than eight times the risk of developing the disease than blacks.3 The New England incidence survey data for a 6-year period suggest that the rate among whites is 9.4 times that of African Americans.8 A recent analysis of population-based registry data in the Surveillance Epidemiology and End Results (SEER) program30 found a relative risk of ocular melanoma for white males compared with African American males of 7.4; the risk for white women in comparison with African American women was 53. Surveys of eye disease in African populations reveal the same low risk in black Africans.18–21 The risk of uveal melanoma is also low among Chinese, Japanese, and Thais.31–33 In the USA, the disease is rare among Americans of Asian extraction.3 A series of three posterior uveal melanomas in Vietnamese Asians with clinical and cytogenetic characteristics was reported recently by McCannel et al.34 The small number of cases reported among Native Americans and Hispanics in the USA suggests that this diagnosis is also rare in these groups. Two cases of ocular melanoma among south-western Native Americans were found in a review of the New Mexico tumor and melanoma registries,35 and one report described four Native Americans with choroidal melanoma evaluated for the Collaborative Ocular Melanoma Study.36 A series of 20 choroidal melanoma patients of Hispanic origin was described by Hudson et al.37
Among whites with uveal melanoma, ancestral origin from more northern latitudes was the strongest risk factor found in a large case–control study.38 Northern European ancestry was associated with more than a sixfold increased risk, and British ancestry was associated with more than a twofold increased risk, as compared with southern European and Mediterranean heritage. The roles of ancestry and race were examined in an evaluation of the incidence of uveal melanoma using data from the Israeli Cancer Registry.17 Jews immigrating to Israel from Europe or North America and Israeli-born Jews had a threefold to fourfold increased incidence compared with Jews from Asia or Africa; non-Jews had the lowest risk.
Cancer genetics
A number of clusters of uveal melanoma occurring among blood relatives have been reported. Familial clusters of uveal melanoma cases have been identified in several large series of patients. Among 1600 patients with uveal melanoma treated by proton beam irradiation over a 10-year period, only 11 families were found to have more than one verified case of the disease.39 In a series of 4500 cases diagnosed between 1976 and 1993, 27 families having at least two blood relatives with an uveal melanoma diagnosis were identified.40 Although the overall incidence among familial cases in that study was small, it was significantly higher than the expected incidence of sporadic cases. Therefore, it was presumed that the familial clustering was associated with inherited genetic or common environmental factors. Nevertheless, most cases are sporadic with no known family history of the disease.
Although family history of uveal melanoma is rare, some cases may have a heritable component. Mutations in G-α proteins, which are believed to be responsible for tumor development have been recently identified in uveal melanoma and may be present in at least 84% of tumors. Among the most common of these somatic G-α protein mutations are GNAQ41 and GNA11,42 which are mutually exclusive. Cutaneous melanoma is now recognized as an inherited disease in as many as 10% of all cases.43 Recent evidence suggests that family members of persons with cutaneous melanoma who have large numbers of dysplastic nevi have several hundred times the risk of developing cutaneous melanoma compared with the general population.43 Reports of cutaneous melanoma and uveal melanoma occurring as double primary malignancies, some in the presence of dysplastic nevi,44–46 and melanomas of both sites occurring among family members,43,46–49 have led to speculation that cutaneous and uveal melanoma may have a common heritable variant. Persons with cutaneous melanoma have been found to be more likely to possess iris nevi50 or have a larger number of iris nevi51 compared with controls. These studies reported similar, although not statistically significant, patterns for choroidal nevi. A potential bias is that the examiners were not masked with respect to the diagnosis of skin melanoma, and nevi may have been more likely to be identified in patients with skin melanoma. There have been no reports of a higher frequency of ocular melanoma among persons with cutaneous melanoma.
The occurrence of bilateral tumors has been suggested as indicative of genetic predisposition to cancer.52 However, few cases of bilateral primary uveal melanoma have been reported,46,52,53 and most reported cases did not exhibit other characteristics associated with genetically inherited cancer predisposition, such as early age of onset or familial clustering of uveal melanoma.52
Although heredity may not play a role in most cases of uveal melanoma, cytogenetic analyses of uveal melanoma tissue have revealed that alterations in chromosomes 3 and 8 may be associated with increased metastasis-related mortality.54–56 Jay and McCartney57 documented an unusual family with eight presumed cases of malignant ocular melanoma spanning four generations; mutations in the p53 tumor-suppressor gene were detected in the two tumors for which preserved material was available. Application of molecular genetic research to uveal melanoma may lead to new insights into the pathogenesis and prognosis of this malignancy.
Ocular and cutaneous nevi and melanocytosis
Nevi on the skin have been shown to increase the risk of cutaneous melanoma.58,59 Similarly, the majority of uveal melanomas are thought by some to arise from pre-existing choroidal nevi.60 However, the available literature suggests that the risk of choroidal and ciliary body melanomas associated with nevi of the uveal tract is low. Ganley and Comstock4 estimated that 3% of the population over the age of 30 years have choroidal nevi posterior to the equator of the eye. Because nevi may also occur anterior to the equator,61 the prevalence of choroidal nevi may be as much as twice that reported. Each year, only 1 in 5000 persons with such nevi develop a melanoma (assuming all melanomas arise from pre-existing nevi).4 Because of the low risks associated with these conditions, current guidelines and recommendations for management and follow-up may not be cost-effective.
Other melanocytic conditions that have been linked to uveal melanoma include ocular (melanosis oculi) and oculodermal (nevus of Ota) melanocytosis. These are typically congenital, unilateral conditions involving hyperpigmentation of the episclera and uveal tract in ocular melanocytosis and of the periorbital skin in oculodermal melanocytosis. Both conditions are more common in females, and the highest prevalence has been reported in Asians.62 Gonder et al.63 found that the prevalence of both forms of melanocytosis was higher in persons with uveal melanoma compared with a clinic population. Singh et al.64 estimated the lifetime prevalence of uveal melanoma in white patients with ocular or oculodermal melanocytosis to be 2.6 cases per 1000 population, as compared with an estimated prevalence of uveal melanoma of 7.5 cases per 10 000 in the general population.
Case–control studies suggest that presence of cutaneous nevi may be a risk factor for uveal melanoma.38,65,66 A significant trend for an increase in the risk of uveal melanoma with more cutaneous nevi was found by Seddon et al.38 Dysplastic nevus syndrome (DNS)41 and atypical mole syndrome65,67 also have been associated with uveal melanoma. In one study, persons with dysplastic nevi were more likely to possess conjunctival, iris, and choroidal nevi.68 Results of a case–control study comparing the presence of DNS in Hungarian patients with uveal melanoma or with cutaneous melanoma and in volunteer controls indicated that DNS was significantly more common in both groups of melanoma patients than in the control group (the odds ratio for uveal melanoma as compared with controls was 4.36).69 Van Hees et al.67 reported a dose–response relationship between number of atypical nevi and uveal melanoma. After adjustment for age and sex, the presence of one or two atypical nevi was associated with nearly a threefold increased risk, and the presence of three or more atypical nevi with a fivefold increased risk of melanoma in comparison with absence of atypical nevi. Similarly, Bataille et al.65 found a trend of increasing odds ratios for greater numbers of atypical nevi. Although DNS and ocular nevi have been linked to risk of uveal melanoma, DNS was not found to be associated with the prevalence of iris or choroidal nevi in a case–control study from Sweden,70 nor was a higher-than-expected rate of dysplastic nevi demonstrated in a group of patients with ocular melanoma evaluated by Taylor et al.71 The association between DNS and uveal melanoma remains controversial.
Hormones and reproductive factors
Hormonal influences are suspected to be a factor in cutaneous melanoma based on reports of an increased risk for women in their childbearing years72,73 and the seemingly adverse influence of pregnancy on prognosis.74–76 Pregnancy may also pose an added risk for uveal melanoma, although reports of presentation77–81 and tumor growth79 during pregnancy are rare. Increases in mortality resulting from tumors of the eye73 and in the incidence of ocular melanomas2 during the childbearing years have been reported. On the other hand, the hormonal environment had no appreciable influence on risk of metastases in younger women with uveal melanoma in one series.82 Potential mechanisms are speculative and include a hormonal effect from estrogens or melanocyte-stimulating hormone. However, one study showed an absence of estrogen receptors in melanoma and surrounding choroidal tissue.79
Epidemiologic studies comparing uveal melanoma cases to controls without melanoma have evaluated hormonal and reproductive factors.83,84 Findings suggest a weak or no association and are not consistent between the two reports. For example, one found an increased risk83 and the other a decreased risk84 for ever having been pregnant. Similarly, increased risk83 and no change in risk84 for use of postmenopausal estrogens were reported. Further studies are needed to evaluate these relationships.
Eye and skin color
Some studies suggest that persons with light irides are at increased risk of developing uveal melanoma.33,66,85 In one of these studies,86 persons with blue or gray eyes were found to have three times the risk of disease (unadjusted for other host factors) compared with persons with brown eyes. In a larger study of over 400 cases that combined melanoma of the iris with other uveal melanomas, the risk for blue-eyed persons was 1.7 times that of persons with brown eyes. Hair and skin color were not found to be independent risk factors after adjusting for eye color.85 Similarly, Holly et al.66 found a twofold risk for lighter eye color. However, in a case–control study with sibling and population-based controls, Seddon et al. found that skin color was significantly associated with uveal melanoma after adjusting for ancestral origin, with a relative risk of 3.8 comparing light to darker skin color among whites with uveal melanoma, but lighter eye color was only weakly related and was not significant in multivariate analyses.38
The iris is the only part of the uveal tract positioned in front of the lens, an effective ultraviolet filter. One study found a higher prevalence of blue and gray eyes among patients with iris melanoma compared with controls with ciliary body and choroidal melanoma.87 Kliman et al.88 also found that light irides were more common in persons with melanocytic lesions of the iris but suggested that such lesions may simply be more noticeable in lighter irides. However, the well-documented tendency for iris melanomas to occur in the inferior sector of the eye,9,12,89–91 where exposure to sunlight is presumably greatest, supports the view that the origin of these iris tumors may be environmentally related.
History of nonocular malignancy
To examine whether persons with a previous diagnosis of other cancers are at increased risk for uveal melanoma, a few epidemiologic studies have compared patients with melanoma with controls regarding their past medical history of other malignancies.92–94 In a comparison of a series of uveal melanoma cases with population data from the Connecticut Tumor Registry, prevalence of previous malignancy was found to be significantly higher in women with uveal melanoma but not in men.94 A recent investigation using SEER data identified an elevated risk of ocular melanoma among women with a history of invasive ovarian cancer, suggesting a possible common hormonal etiology.95 In general, the results of these studies do not support a consistent association between prior malignancies and subsequent diagnosis of uveal melanoma. A weak and nonsignificant increased relative risk of melanoma associated with history of skin cancers was noted in several studies.92–94,96 This suggests that cutaneous malignancies and uveal melanoma may share some common risk factors. Further studies are needed to evaluate this potential association.
Environmental factors
Sunlight exposure
Sunlight exposure has been examined as a potential environmental risk factor for a number of ocular diseases, including age-related macular degeneration97,98 and senile cataract,99,100 as well as melanoma of the uveal tract.37,38,66,85,86,89 Incident cases of choroidal melanoma in a consecutive series of patients demonstrated a strong predilection for initial presentation at the perifoveal area. Initiation rates decreased with distance from the macula; this gradient decrease correlated with decreasing illuminance of light on the retina from macula to periphery, suggesting a possible role of sun exposure in uveal melanoma.101 However, a meta-analysis by Shah et al. revealed that chronic ultraviolet exposure both in birth latitude and occupational sunlight exposure was not a significant risk factor for uveal melanoma development.102 Earlier and more frequent presentation of perifoveal tumors is likely due to visual symptoms related to the location of these tumors. Intermittent ultraviolet exposure in the form of welding was found to have a possible association with uveal melanoma,66 described in the meta-analysis by Shah et al.102 See section below on “Geography”.
Diet and smoking
There are currently no data in the literature regarding the influence of diet on the incidence of uveal melanoma. A study was conducted looking at dietary intake in enhancing the recurrence-free interval, but both numbers of subjects and follow-up time were small.103 A population-based case–control study in Germany found that smoking and alcohol consumption were not associated with an increased risk for uveal melanoma.104 In a large prospective study, the risk of metastases the first 3 years after radiation was not altered by smoking.105
Geography
Uveal melanoma may differ from cutaneous melanoma in that there does not appear to be a strong latitudinal gradient with regard to the incidence of ocular disease.2,9,22,23 In one study of patients in Veterans Administration hospitals, higher rates of uveal melanoma were reported in southern hospitals106 but this may have reflected a tendency for elderly veterans to retire to the southern United States. In a Canadian study, altitude, not latitude, was positively correlated with the incidence of uveal melanoma.11 The association of exposure to solar radiation early in life with risk of ocular melanoma was evaluated using state of birth among cases identified from SEER data107; birth in a southern state or in a state with higher levels of solar radiation was not found to be related to the risk of ocular melanoma.
There are several possible explanations for the lack of a clear latitudinal gradient in uveal melanoma rates, assuming that sunlight is an etiologic factor in the disease. First, higher rates of uveal melanoma might not be seen in southern latitudes because the greater intensity of overhead sunlight in the south may be offset by the greater reflection of ultraviolet radiation as a result of snow cover in the north.108 Second, the quality of correlational studies depends on both uniform case ascertainment and risk for disease throughout the populations studied. Geographic patterns could be obscured by variations in the completeness of case finding, a problem in several of the studies described above. Regional differences in the racial or ethnic mixes of the populations with different susceptibilities to melanoma may also obscure differences in rates for this rare tumor. Northern latitudinal ancestry is a risk factor among whites,38 whereas sunlight exposure is greatest at lower latitudes.
A problem with the possible link between sunlight exposure and uveal melanoma lies in establishing whether ultraviolet radiation actually reaches the uveal tract through the effective filters of the cornea and lens. In both animal models and studies of enucleated eyes,109 it has been shown that virtually no ultraviolet (UV)-A or UV-B radiation is transmitted through both the lens and cornea in adults. The juvenile lens, on the other hand, may transmit small amounts of ultraviolet radiation.110 Others have argued, however, that tissues directly overlying uveal tract structures, including the retina, would provide the necessary protection if significant amounts of ultraviolet radiation penetrated the lens.111 The net effect then would be the total blockage of ultraviolet radiation from direct contact with the choroid and ciliary body at all ages.
In addition to a direct effect, sunlight may also act indirectly by causing a systemic alteration in immunologic function112 or by production of a “solar” circulating factor.113 Such compromise of the immunologic system by ultraviolet radiation would make ocular parameters less important than other bodily exposures.
A few studies have compared sunlight exposure histories of patients with uveal melanoma with those of controls.38,66,85,86,114,115 All but one86 of these studies suggest a low to moderate adverse effect of certain UV exposures on the risk of uveal melanoma, but results are not consistent. These exposures include residence in the southern States, use of sunlamps, and history of intense sun exposure38; occupational exposure to UV light114; tendency to sunburn, and welding burn or sunburn66; and gardening and lack of eye protection when outdoors.85 In the study that did not find an association between sun sensitivity or exposures and uveal melanoma,86 the sample size was small, and hence the power to detect weak or moderate association was low.
Occupational and chemical exposures
A number of rare cancers are caused by chemical or radiation exposures occurring in the workplace,116 and an occupational exposure has also been sought in the etiology of uveal melanoma. Jensen12 found approximately the same occupational distribution among patients with uveal melanoma as was found in the general population of Denmark. Swerdlow23 reported that patients with eye cancer in England and Wales were more likely to be nonmanual than manual laborers, and a higher risk was found among electrical workers in particular. Gallagher et al.86 did not find an elevated risk for electrical workers or any other specific occupation in western Canada but noted an excess of cases of uveal melanoma among government workers; a managerial job classification. Four patients with uveal melanoma and none of the controls reported having worked as a welder in one of the case–control studies discussed above.
A population-based case–control study evaluated occupational exposures117 using two systems of coding occupations and industries. Results suggested an increased risk of uveal melanoma for the group of agricultural, fishery, and forestry occupations and industries, as well as for those exposed to certain chemicals, but these associations were weak and not statistically significant. This exploratory study suggested possible areas for future research that could test these and other specific hypotheses. In another case–control study,114 associations between ocular melanoma and exposure to welding and asbestos were identified; specific occupations at high risk included chemical-related, maritime, and health-related occupations.
Several reports of occupational or community clusters of uveal melanoma cases have led to speculation regarding a common etiologic occupational or chemical exposure. One report described a cluster of cases that occurred among employees of a chemical plant in West Virginia.118 Four cases were diagnosed between 1972 and 1978, and a fifth case had been diagnosed in 1952. In a small Pennsylvania community, three choroidal melanomas were diagnosed over a 2.5-year period.119 A cluster of four cases in a manufacturing plant in Louisiana was also recently reported.120 However, on investigation, no specific causative agent could be identified in any of these clusters.
Ocular melanomas and other ocular tumors have been produced in laboratory animals after administration of methylcholanthrene121 N-2-fluorenylacetamide and ethionine122 radium123 and nickel subsulfide.124 In the cluster of cases identified in the Pennsylvania community,119 although no common exposure was identified among the patients with choroidal melanoma, laboratory mice given community water developed anterior lens capsule abnormalities that were not seen in control mice. The basis for this association is not clear.
Mobile phone use
Over the past decade there has been public concern regarding microwave energy in mobile or wireless phone use and the development of cancer. Using a questionnaire, a case–control study of over 400 uveal melanoma case patients were matched to controls to identify mobile phone use, and logistic regression analyses revealed that regular mobile phone use was not associated with risk of developing uveal melanoma.125
Other environmental exposures
A number of other environmental exposures may contribute to the etiology of uveal melanoma. Albert et al.50 have summarized the available evidence for a viral cause of the disease. Melanoma-like tumors have been induced in laboratory animals after injection of viral-transformed uveal tissue.50 In addition, virus and virus-like particles have been demonstrated in human uveal melanomas.126 However, the meaning of these findings is unclear, since virus particles are often constituents of normal tissues. Trauma has been cited as a possible cause of some cutaneous melanomas127,128 and there are a few reports of uveal melanomas129,130 occurring at the site of a previous injury.
1 Henderson E, Margo CE. Iris melanoma. Arch Pathol Lab Med. 2008;132:268–272.
2 Scotto J, Fraumeni JF, Lee JAH. Melanomas of the eye and other noncutaneous sites: epidemiologic aspects. J Natl Cancer Inst. 1976;56:489–491.
3 Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment and survival. Ophthalmology. 2011;118:1881–1885.
4 Ganley JP, Comstock GW. Benign nevi and malignant melanomas of the choroid. Am J Ophthalmol. 1973;76:19–25.
5 Shammas HF, Watzke RC. Bilateral choroidal melanomas: case report and incidence. Arch Ophthalmol. 1977;95:617–623.
6 Mahoney MC, Burnett WS, Majerovics A, et al. The epidemiology of ophthalmic malignancies in New York State. Ophthalmology. 1990;97:1143–1147.
7 Wilkes SR, Robertson DM, Kurland LT, et al. Incidence of uveal malignant melanoma in the resident population of Rochester and Olmsted County, Minnesota. Ophthalmology. 1979;117:516–520.
8 Seddon JS, Egan KM. Application of epidemiologic methods to ophthalmology: uveal melanoma. In: Albert DJ, ed. The principles and practice of ophthalmology. Philadelphia: WB Saunders; 1993:1245–1266.
9 Raivio I. Uveal melanoma in Finland: an epidemiological, clinical, histological, and prognostic study. Acta Ophthalmol Suppl (Copenh). 1977;133:1–64.
10 Teikari JM, Raivio I. Incidence of choroidal malignant melanoma in Finland in the years 1973–1980. Acta Ophthalmol. 1985;63:661–665.
11 Birdsell JM, Gunther BK, Boyd TA, et al. Ocular melanoma: a population-based study. Can J Ophthalmol. 1980;15:9–12.
12 Jensen OA. Malignant melanomas of the uvea in Denmark 1943–1952: a clinical, histopathologic, and prognostic study. Acta Ophthalmol Suppl (Copenh). 1963;75:17–78.
13 Osterlind A. Trends in incidence of ocular malignant melanoma in Denmark 1943–1982. Int J Cancer. 1987;40:161–164.
14 Abrahamsson M. Malignant melanoma of the choroid and the ciliary body 1956–1975. Acta Ophthalmol. 1983;61:600–610.
15 Mork T. Malignant neoplasms of the eye in Norway: incidence, treatment and prognosis. Acta Ophthalmol. 1961;39:824–831.
16 Vidal JL, Bacin F, Albuisson E, et al. Melanome 92. Etude epidemiologique des melanomes uveaux en France. J Fr Ophtalmol. 1995;18:520–528.
17 Iscovich J, Ackerman C, Andreev H, et al. An epidemiologic study of posterior uveal melanoma in Israel. Int J Cancer. 1995;61:291–295.
18 Klauss V, Chana HS. Ocular tumors in Africa. Soc Sci Med. 1983;17:1743–1750.
19 Malik MOA, El Sheikh EH. Tumors of the eye and adnexa in the Sudan. Cancer. 1979;44:293–303.
20 Miller B, Abrahams C, Cole GC, et al. Ocular malignant melanoma in South African blacks. Br J Ophthalmol. 1981;65:720–722.
21 Templeton AC. Tumors of the eye and adnexa in Africans of Uganda. Cancer. 1967;20:1689–1698.
22 Hakulinen T, Teppop L, Saxen E. Cancer of the eye, a review of trends and differentials. World Health Stat Q. 1978;31:143–158.
23 Swerdlow AJ. Epidemiology of eye cancer in adults in England and Wales. Am J Epidemiol. 1983;118:294–300.
24 Jensen OA, Prause JU. Malignant melanomas of the human uvea in Denmark: incidence and a 25-year follow-up of cases diagnosed between 1943 and 1952. In: Lommatzsch PB, ed. Intraocular tumors. Berlin: Springer-Verlag, 1983.
25 Strickland D, Lee JAH. Melanomas of eye: stability of rates. Am J Epidemiol. 1981;113:700–702.
26 Apt L. Uveal melanomas in children and adolescents. Int Ophthalmol Clin. 1962;2:403–410.
27 Shields CL, Shields JA, Milte J, et al. Uveal melanomas in teenagers and children: a report 40 cases. Ophthalmology. 1991;98:1662–1666.
28 Stanford DG, Hart R, Thompson JF. Ocular melanoma in childhood. Aust NZ J Surg. 1993;63:729–731.
29 Shammas HF, Blodi FC. Prognostic factors in choroidal and ciliary body melanomas. Arch Ophthalmol. 1977;95:63–69.
30 Neugut AI, Kizelnik-Freilich S, Ackerman C. Black–white differences in risk for cutaneous, ocular, and visceral melanomas. Am J Public Health. 1994;84:1828–1829.
31 Kaneko A. Incidence of malignant melanoma of the eye in Japan. Rinsho Ganka. 1979;33:941–947.
32 Kuo PK, Puliafito CA, Wang KM, et al. Uveal melanoma in China. Int Ophthalmol Clin. 1982;22:57–71.
33 Shuangshoti S, Panyathanya R. Retinoblastoma and uveal melanoma: a study of 206 cases. J Med Assoc Thailand. 1973;56:331–336.
34 McCannel TA, Wu MY, Burgess BL. Clinical and cytogenetic characteristics of choroidal melanoma in Vietnamese Asians. Mol Vis. 2011;17:231–236.
35 Black WC, Wiggins C. Melanoma among southwestern American Indians. Cancer. 1985;55:2899–2902.
36 Wells CG, Bradford RH, Fish GE, et al. Choroidal melanomas in American Indians: COMS Group, Collaborative Ocular Melanoma Study. Arch Ophthalmol. 1996;114:1017–1018.
37 Hudson HL, Valluri S, Rao NA. Choroidal melanomas in Hispanic patients. Am J Ophthalmol. 1994;118:57–62.
38 Seddon JS, Gragoudas ES, Glynn RJ, et al. Host factors, UV radiation, and risk of uveal melanoma: a case-control study. Arch Ophthalmol. 1990;108:1274–1280.
39 Young LH, Egan KM, Walsh SM, et al. Familial uveal melanoma. Am J Ophthalmol. 1994;117:516–520.
40 Singh AD, Wang MX, Donoso LA, et al. Familial uveal melanoma. III. Is the occurrence of familial uveal melanoma coincidental? Arch Ophthalmol. 1996;114:1101–1104.
41 Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations in GNAQ in uveal melanoma and blue nevi. Nature. 2009;457:599–602.
42 Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363:2191–2199.
43 Greene MH, Sanders RJ, Chu FC, et al. The familial occurrence of cutaneous melanoma, intraocular melanoma, and the dysplastic nevus syndrome. Am J Ophthalmol. 1983;96:238–245.
44 Abramson DH, Rodriguez-Sains RS, et al. B-K mole syndrome: cutaneous and ocular malignant melanoma. Arch Ophthalmol. 1980;98:1397–1399.
45 Bellet RE, Shields JA, Soll DB, et al. Primary choroidal and cutaneous melanomas occurring in a patient with the B-K mole syndrome phenotype. Am J Ophthalmol. 1980;89:567–570.
46 Oosterhuis JA, Went LN, Lynch HT. Primary choroidal and cutaneous melanomas, bilateral choroidal melanomas, and familial occurrence of melanomas. Br J Ophthalmol. 1982;66:230–233.
47 Albert DM, Chang MA, Lamping K, et al. The dysplastic nevus syndrome’s pedigree with primary malignant melanomas of the choroid and skin. Ophthalmology. 1985;92:1728–1734.
48 Paton D, Thomas LB. Simultaneous occurrence of primary malignant melanomas of the eye and the skin. Arch Ophthalmol. 1959;62:645–652.
49 Turkington RW. Familial factor in malignant melanoma. JAMA. 1965;192:72–82.
50 Albert DM, Searl SS, Forget B, et al. Uveal findings in patients with cutaneous melanoma. Am J Ophthalmol. 1983;95:474–479.
51 Nordlund JJ, Kirkwood J, Forget BM, et al. Demographic study of clinically atypical (dysplastic) nevi in patients with melanoma and comparison subjects. Cancer Res. 1985;45:1855–1861.
52 Singh AD, Shields CL, Shields JA, et al. Bilateral primary uveal melanoma. Bad luck or bad genes? Ophthalmology. 1996;103:256–262.
53 Ascaso FJ, Cascante JM, Castillo JM, et al. Simultaneous bilateral primary choroidal melanoma. Eur J Ophthalmol. 1996;6:87–89.
54 Prescher G, Bornfeld N, Hirche H, et al. Prognostic implications of monosomy 3 in uveal melanoma. Lancet. 1996;347:1222–1225.
55 Scholes AG, Damato BE, Nunn J, et al. Monosomy 3 in uveal melanoma: correlation with clinical and histologic predictors of survival. Invest Ophthalmol Vis Sci. 2003;44:1008–1011.
56 Sisley K, Rennie IG, Parsons MA, et al. Abnormalities of chromosomes 3 and 8 in posterior uveal melanoma correlate with prognosis. Genes Chromosomes Cancer. 1997;19:22–28.
57 Jay M, McCartney AC. Familial malignant melanoma of the uvea and p53: a Victorian detective story. Surv Ophthalmol. 1993;37:457–462.
58 Elwood JM, Williamson C, Stapleton PJ. Malignant melanoma in relation to moles, pigmentation and exposure to fluorescent and other lighting sources. Br J Cancer. 1986;53:65–74.
59 Holman CDJ, Mulroney CD, Armstrong BK. Epidemiology of preinvasive and invasive malignant melanoma in Western Australia. Int J Cancer. 1980;25:317–323.
60 Yanoff M, Zimmerman LE. Histogenesis of malignant melanomas of the uvea. II. Relationship of uveal nevi to malignant melanomas. Cancer. 1967;20:493–507.
61 Hale PN, Allen RA, Straatsma BR. Benign melanomas (nevi) of the choroid and ciliary body. Arch Ophthalmol. 1965;74:532–538.
62 Gonder JR, Shields JA, Shakin JL, et al. Bilateral ocular melanocytosis with malignant melanoma of the choroid. Br J Ophthalmol. 1981;65:843–845.
63 Gonder JR, Shields JA, Albert DM, et al. Uveal malignant melanoma associated with ocular and oculodermal melanocytosis. Ophthalmology. 1982;89:953–960.
64 Singh AD, De Potter P, Fijal BA, et al. Lifetime prevalence of uveal melanoma in white patients with oculo(dermal) melanocytosis. Ophthalmology. 1998;105:195–198.
65 Bataille V, Sasieni P, Cuzick J, et al. Risk of ocular melanoma in relation to cutaneous and iris naevi. Int J Cancer. 1995;60:622–626.
66 Holly EA, Aston DA, Char DH, et al. Uveal melanoma in relation to ultraviolet light exposure and host factors. Cancer Res. 1990;50:5773–5777.
67 van Hees CL, de Boer A, Jager MJ, et al. Are atypical nevi a risk factor for uveal melanoma? A case-control study. J Invest Dermatol. 1994;103:202–205.
68 Rodriguez-Sains RS. Ocular findings in patients with dysplastic nevus syndrome. Ophthalmology. 1986;93:661–665.
69 Hammer H, Olah J, Toth-Molnar E. Dysplastic nevi are a risk factor for uveal melanoma. Eur J Ophthalmol. 1996;6:472–474.
70 Seregard S, Trampe E, Mansson-Brahme E, et al. Prevalence of primary acquired melanosis and nevi of the conjunctiva and uvea in the dysplastic nevus syndrome: a case-control study. Ophthalmology. 1995;102:1524–1529.
71 Taylor MR, Guerry DI, Bondi EE, et al. Lack of association between intraocular melanoma and cutaneous dysplastic envi. Am J Ophthalmol. 1984;98:478–482.
72 Lee JAH, Storer BE. Excess of malignant melanomas in women in the British Isles. Lancet. 1980;2:1337–1339.
73 Lee JAH, Storer BE. Malignant melanoma female/male death ratios. Lancet. 1981;1:1419.
74 Pack GT, Scharnagel IM. The prognosis for malignant melanoma in the pregnant woman. Cancer. 1951;4:324–334.
75 Reintgen DS, McCarty KS, Vollmer R, et al. Malignant melanoma and pregnancy. Cancer. 1985;55:1340–1344.
76 Shiu MH, Schottenfeld D, Maclean B, et al. Adverse effects of pregnancy on melanoma: a reappraisal. Cancer. 1976;37:181–187.
77 Borner R, Goder G. Melanoblastoma der Uvea und Schwangerschaft. Klin Monatsbl Augenheilkd. 1966;149:684–693.
78 Frenkel M, Klein HZ. Malignant melanoma of the choroid in pregnancy. Am J Ophthalmol. 1966;62:910–913.
79 Seddon JS, MacLaughlin DT, Albert DM, et al. Uveal melanomas presenting during pregnancy and the investigation of estrogen receptors in melanomas. Br J Ophthalmol. 1982;66:695–704.
80 Shields CL, Shields JA, Eagle RC, et al. Uveal melanoma and pregnancy: a report of 16 cases. Ophthalmology. 1991;98:1667–1673.
81 Siegel R, Ainslie WH. Malignant ocular melanoma during pregnancy. JAMA. 1963;185:542–543.
82 Egan KM, Walsh SM, Seddon JM, et al. An evaluation of the influence of reproductive factors on the risk of metastases from uveal melanoma. Ophthalmology. 1993;100:1160–1166.
83 Hartge P, Tucker MA, Sheilds JA, et al. Case-control study of female hormones and eye melanoma. Cancer Res. 1989;49:4622–4625.
84 Holly EA, Aston DA, Ahn DK, et al. Uveal melanoma, hormonal and reproductive factors in women. Cancer Res. 1991;51:1370–1372.
85 Tucker MA, Shields JA, Hartge P, et al. Sunlight exposure as risk factor for intraocular malignant melanoma. N Engl J Med. 1985;313:789–792.
86 Gallagher RP, Elwood JM, Rootman J, et al. Risk factors for ocular melanoma: Western Canada Melanoma Study. J Natl Cancer Inst. 1985;74:775–778.
87 Rootman J, Gallagher RP. Color as a risk factor in iris melanoma. Am J Ophthalmol. 1984;98:558–561.
88 Kliman GH, Augsburger JJ, Shields JA. Association between iris color and iris melanocytic lesions. Am J Ophthalmol. 1985;100:547–548.
89 Horn EP, Hartge P, Shields JA, et al. Sunlight and risk of uveal melanoma. J Natl Cancer Inst. 1994;86:1476–1478.
90 Jakobiec FA, Silbert G. Are most iris “melanomas” really nevi? A clinicopathologic study of 189 lesions. Arch Ophthalmol (Copenh). 1981;99:2117–2132.
91 Rones B, Zimmerman LE. The prognosis of primary tumors of the iris treated by iridectomy. Arch Ophthalmol. 1958;60:193–205.
92 Holly EA, Aston DA, Ahn DK, et al. No excess prior cancer in patients with uveal melanoma. Ophthalmology. 1991;98:608–611.
93 Lischko AM, Seddon JM, Gragoudas ES, et al. Evaluation of prior primary malignancy as a determinant of uveal melanoma: a case-control study. Ophthalmology. 1989;96:1716–1721.
94 Turner BJ, Siatkowski RM, Augsburger JJ, et al. Other cancers in uveal melanoma patients and their families. Am J Ophthalmol. 1989;107:601–608.
95 Travis LB, Curtis RE, Boice JD, et al. Second malignant neoplasms among long-term survivors of ovarian cancer. Cancer Res. 1996;56:1564–1570.
96 Richtig E, Langmann G, Mullner K, et al. Ocular melanoma: epidemiology, clinical presentation and relationship with dysplastic nevi. Ophthalmologica. 2004;218:111–114.
97 Blumenkranz MS, Russell SR, Robey MG, et al. Risk factors in age-related maculopathy complicated by choroidal neovascularization. Ophthalmology. 1986;93:552–558.
98 Hyman LG, Lilienfeld AM, Ferris FL, et al. Senile macular degeneration: a case-control study. Am J Epidemiol. 1983;118:213–227.
99 Brilliant LB, Grasset NC, Pokhrel RP, et al. Associations among cataract prevalence, sunlight hours, and altitude in the Himalayas. Am J Epidemiol. 1983;118:250–264.
100 Hiller R, Giacometti L, Yuen K. Sunlight and cataract: an epidemiologic investigation. Am J Epidemiol. 1977;105:450–459.
101 Li W, Judge H, Gragoudas ES, et al. Patterns of tumor initiation in choroidal melanoma. Cancer Res. 2000;60:3757–3760.
102 Shah CP, Weis E, Lajous M, et al. Intermittent and chronic ultraviolet light exposure and uveal melanoma: a meta-analysis. Ophthalmology. 2005;112:1599–1607.
103 Tallberg T, Uusitalo R, Sarna S, et al. Improvement of the recurrence-free interval using biological adjuvant therapy in uveal melanoma. Anticancer Res. 2000;20:1969–1975.
104 Stang A, Ahrens W, Anastassiou G, et al. Phenotypical characteristics, lifestyle, social class and uveal melanoma. Ophthalmic Epidemiol. 2003;10:293–302.
105 Egan KM, Gragoudas ES, Seddon JM, et al. Smoking and the risk of early metastases from uveal melanoma. Ophthalmology. 1992;99:537–541.
106 Keller AZ. Histology, survivorship, and related factors in the epidemiology of eye cancers. Am J Epidemiol. 1973;97:386–393.
107 Schwartz SM, Weiss NS. Place of birth and incidence of ocular melanoma in the United States. Int J Cancer. 1988;41:174–177.
108 Sliney DH. Physical factors in cataractogenesis: ambient ultraviolet radiation and temperature. Invest Ophthalmol Vis Sci. 1986;27:781–790.
109 Zigman S. Effects of near ultraviolet radiation on the lens and retina. Doc Ophthalmol. 1983;55:375–391.
110 Boettner EA, Wolter JR. Transmission of the ocular media. Invest Ophthalmol Vis Sci. 1962;1:776–783.
111 Lerman S. Sunlight and intraocular melanoma. N Engl J Med. 1986;314:712–713.
112 Hersey P, Haran G, Hasic E, et al. Alteration of T cell subsets and induction of suppressor T cell activity in normal subjects after exposure to sunlight. J Immunol. 1983;31:171–174.
113 Lee JAH, Merrill JM. Sunlight and the etiology of malignant melanoma: a synthesis. Med J Aust. 1970;2:846–851.
114 Holly EA, Aston DA, Ahn DK, et al. Intraocular melanoma linked to occupations and chemical exposures. Epidemiology. 1996;7:55–61.
115 Vajdic CM, Kricker A, Giblin M, et al. Sun exposure predicts risk of ocular melanoma in Australia. Int J Cancer. 2002;101:175–182.
116 Althouse R, Huff J, Tomatis L, et al. An evaluation of chemicals and industrial processes associated with cancer in humans based on human and animal data: IARC monographs, volumes 1 to 20. Cancer Res. 1980;40:1–12.
117 Ajani US, Seddon JM, Chung-Cheng H, et al. Occupation and risk of uveal melanoma. Cancer. 1992;70:2891–2900.
118 Albert DM, Puliafito CA, Fulton AB, et al. Increased incidence of choroidal malignant melanoma occurring in a single population of chemical workers. Am J Ophthalmol. 1980;89:323–337.
119 Louria DB, Coumbis RJ, Lavenhar MA, et al. An apparent small cluster of choroidal melanoma cases. Am J Ophthalmol. 1982;94:172–180.
120 Ganley JP, Fontenot K. Epidemiologic study of time and space clustering of 4 cases of choroidal malignant melanoma. Arch Ophthalmol. 1997;115:537–541.
121 Patz A, Wulff LB, Rogers SW. Experimental production of ocular tumors. Am J Ophthalmol. 1959;48:98–117.
122 Benson WR. Intraocular tumor after ethionine and N-2-fluorenyl-acetamide. Arch Pathol. 1962;73:404–406.
123 Taylor GN, Dougherty TF, Mays CW, et al. Radium-induced eye melanomas in dogs. Radiat Res. 1972;51:361–373.
124 Albert DM, Gonder JR, Paple J, et al. Induction of ocular neoplasms in Fischer rats by intraocular injection of nickel subsulfide. Invest Ophthalmol Vis Sci. 1982;22:768–782.
125 Stang A, Schmidt-Pokrzywniak A, Lash TL, et al. Mobile phone use and risk of uveal melanoma: results of the risk factors for uveal melanoma case-control study. J Natl Cancer Inst. 2009;101:120–123.
126 Albert DM. The association of viruses with uveal melanoma. Trans Am Ophthalmol Soc. 1979;77:367–421.
127 Gellin GA, Epstein WL. Malignant melanoma from thermal burn scar. Arch Dermatol. 1975;111:1214–1215.
128 Kirsch N. Malignant melanoma developing in a tattoo. Arch Dermatol. 1969;99:596–598.
129 El Baba F, Blumenkranz MS. Malignant melanoma at the site of penetrating ocular trauma. Arch Ophthalmol. 1986;104:405–409.
130 Vicary D. Malignant melanoma at the site of penetrating ocular trauma. Arch Ophthalmol. 1986;104:1130.