Energy Metabolism and Membrane Physiology of the Erythrocyte
After completion of this chapter, the reader will be able to:
1. List red blood cell (RBC) processes that require energy.
2. Discuss the Embden-Meyerhof anaerobic glycolytic pathway (EMP) in the erythrocyte, with attention to adenosine triphosphate generation and consumption.
3. Name three diversion pathways from the EMP within RBCs and state the function or purpose of each.
4. Describe the role of 2,3-bisphosphoglycerate in erythrocyte metabolism and the sacrifice made to produce it.
5. Name the components related to the hexose-monophosphate pathway that act to detoxify peroxide and the type of chemical process that accomplishes the detoxification.
6. Describe the methemoglobin reductase pathway that diverts from glycolysis.
7. Explain the importance of semipermeability of biologic membranes.
8. Discuss the arrangement of lipids in the RBC membrane.
9. Discuss the function of glycolipids in the RBC membrane.
10. Explain the concept of lipid exchange between the RBC membrane and the plasma, including factors that affect the exchange.
11. Define, locate, and provide examples of transmembranous versus skeletal membrane proteins of RBCs.
12. Discuss the general structure of spectrin and its function and arrangement in the membrane.
13. Discuss how ankyrin, protein 4.1, actin, adducin, tropomodulin, dematin, and band 3 interact with α- and β-spectrin and the lipid bilayer.
14. Describe the functions of transmembranous proteins as they affect RBC function.
15. Cite the relative concentrations of sodium and potassium inside RBCs and name the structure that maintains those concentrations.
16. Name conditions that develop from abnormalities of RBC membrane constituents.
Case Study
Cyanosis is blue skin coloration, most obvious in whites, that occurs when the blood does not deliver enough oxygen to the tissues. It is a common sign of heart or lung disease, in which the blood fails to become oxygenated and/or is distributed improperly throughout the body. In the 1940s, Dr. James Deeny, an Irish physician, was experimenting with the use of vitamin C (ascorbic acid), a potent reducing agent, as a treatment for heart disease.1 To his disappointment, it was ineffective for nearly all patients. However, he discovered two brothers with the distinction of being truly blue men. When he treated them with vitamin C, each turned a healthy pink. Neither man was determined to have either heart or lung disease.
1. What does it mean to say that vitamin C is a reducing agent?
2. What must be happening if vitamin C was able to cure the cyanosis?
3. What is the significance of finding this condition in brothers?
The red blood cell (RBC, erythrocyte) transports oxygen from the lungs to tissues and organs and helps to transport carbon dioxide to the lungs to be exhaled. The mammalian RBC has evolved to do this quite efficiently but has sacrificed greatly for this purpose by losing its nucleus.2,3 The resulting RBC shape facilitates oxygen acquisition and release. However, the cell is left without the ability to synthesize replacement proteins for vital pathways of energy production and membrane integrity, among others. As a result, its life span is limited to about 120 days because these processes inevitably fail. Mechanisms have evolved to maximize oxygen exchange during the life span of the cell and to counter the natural chemical processes that oxidize heme iron, which would leave it nonfunctional for oxygen transport. This chapter examines the energy production pathways of the RBC, discusses mechanisms for maintaining reduced heme iron and facilitating oxygen delivery, and explores the features of the RBC membrane that contribute to effective oxygen delivery for RBCs.
Energy Production—Anaerobic Glycolysis
At the time the RBC ejects its nucleus, the cellular organelles are also packaged and ejected from the cell. Without mitochondria, the RBC is left to rely on glycolysis for energy production. Fortunately, oxygen exchange and oxygen binding to heme iron do not require energy.4 Other RBC metabolic processes do require energy, however, and they are listed in Box 9-1. Loss of energy leads to RBC death. In fact, a hereditary deficiency of nearly every enzyme involved in glycolysis has been identified. A common result of these deficiencies is shortened RBC survival, known as hereditary nonspherocytic hemolytic anemia.
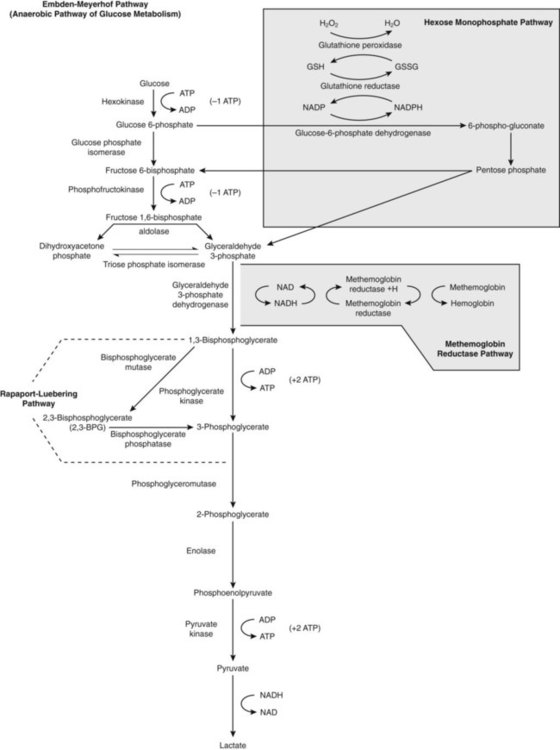
Plasma glucose enters the RBC through a facilitated membrane transport system.5 Anaerobic glycolysis, the Embden-Meyerhof pathway (EMP; Figure 9-1), requires glucose to generate ATP, a high-energy phosphate source that is the greatest reservoir of energy in the RBC. Energy reserves like glycogen are not available in RBCs, so the RBCs rely mostly on external glucose for glycolysis-generated ATP. Via the EMP, glucose is catabolized to pyruvate, consuming two molecules of ATP per molecule of glucose and maximally generating four molecules of ATP per molecule of glucose, for a net gain of two molecules of ATP.
The sequential list of biochemical intermediates involved in glucose catabolism, with corresponding enzymes, is given in Figure 9-1. Tables 9-1 through 9-3 organize this information into three phases for better comprehension.
TABLE 9-1
Glucose Catabolism: First Phase
| Substrates | Enzyme | Products |
| Glucose, ATP | Hexokinase | G6P, ADP |
| G6P | Glucose phosphate isomerase | F6P |
| F6P, ATP | Phosphofructokinase | F-1,6-P; ADP |
| F-1,6-P | Fructodiphosphate adolase | DHAP, G3P |
TABLE 9-2
Glucose Catabolism: Second Phase
| Substrates | Enzyme | Product |
| G3P | Glyceraldehyde-3-phosphate dehydrogenase | 1,3-BPG |
| 1,3-BPG, ADP | Phosphoglycerate kinase | 3-PG, ATP |
| 1,3-BPG | Bisphosphoglyceromutase | 2,3-BPG |
| 2,3-BPG | Bisphosphoglycerate phosphatase | 3-PG |
TABLE 9-3
Glucose Catabolism: Third Phase
| Substrates | Enzyme | Product |
| 3-PG | Monophosphoglyceromutase | 2-PG |
| 2-PG | Phosphopyruvate hydratase (enolase) | PEP |
| PEP, ADP | Pyruvate kinase | Pyruvate, ATP |
The first phase of glucose catabolism involves glucose phosphorylation, isomerization, and diphosphorylation to yield fructose 1,6-bisphosphate (F-1,6-P). F-1,6-P serves as the substrate for aldolase cleavage for the final product of phase 1 glycolysis: glyceraldehyde 3-phosphate (G3P; see Figure 9-1 and Table 9-1). Hexokinase and phosphofructokinase are rate-limiting in steady-state anaerobic glycolysis, even though hexokinase has the lowest activity of all the glycolytic enzymes.
The second phase of glucose catabolism converts G3P to 3-phosphoglycerate (3-PG). The substrates, enzymes, and products for this phase of glycolytic metabolism are summarized in Table 9-2. During the first reaction step, G3P is phosphorylated with a high-energy phosphate and oxidized to 1,3-bisphosphoglycerate (1,3-BPG) through the action of glyceraldehyde-3-phosphate dehydrogenase (G3PD). 1,3-BPG is dephosphorylated by phosphoglycerate kinase, which generates ATP and 3-PG.
The third phase of anaerobic glucose catabolism converts 3-PG to pyruvate with the generation of ATP. Substrates, enzymes, and products are listed in Table 9-3. The product 3-PG is isomerized by phosphoglyceromutase to 2-phosphoglycerate (2-PG). Enolase then converts 2-PG to phosphoenolpyruvate (PEP). Pyruvate kinase (PK) splits off the phosphates, forming ATP and pyruvate. PK activity is allosterically modulated by increased concentrations of F-1,6-P, which enhances the affinity of PK for PEP.5 Thus when the F-1,6-P is plentiful, increased activity of PK favors pyruvate production. Pyruvic acid may diffuse from the erythrocyte or may become a substrate for lactate dehydrogenase with regeneration of the oxidixed form of nicotinamide adenine dinucleotide (NAD+). The ratio of NAD+ to the reduced form (NADH) may modify the activity of this enzyme.
Glycolysis Diversion Pathways (Shunts)
Hexose Monophosphate Pathway
Aerobic or oxidative glycolysis occurs through a diversion of glucose catabolism into the HMP, also known as the pentose phosphate shunt (see Figure 9-1). The HMP detoxifies accumulated peroxide, an agent that oxidizes heme iron, proteins, and lipids, especially those containing thiol groups. Peroxide arises spontaneously from the reduction of oxygen in the cell’s aqueous environment.5 By detoxifying peroxide, the HMP extends the functional life span of the RBC.
During steady-state glycolysis, 5% to 10% of G6P is diverted to the HMP. After oxidative challenge, the activity of the HMP may increase twentyfold to thirtyfold.6 The HMP catabolizes G6P to ribulose 5-phosphate and carbon dioxide by oxidizing G6P at carbon 1. The substrates, enzymes, and products of the HMP are listed in Table 9-4.
TABLE 9-4
Glucose Catabolism: Hexose Monophosphate Pathway
| Substrates | Enzyme | Product |
| G6P | Glucose-6-phosphate dehydrogenase | 6-PG |
| 6-PG |

6-PG, 6-Phosphogluconate; G6P, glucose 6-phosphate; R5P, ribulose 5-phosphate.
G6PD provides the only means of generating NADPH for glutathione reduction, and in its absence erythrocytes are particularly vulnerable to oxidative damage.7 Normal G6PD activity is able to detoxify oxidative compounds and safeguard hemoglobin, sulfhydryl-containing enzymes, and membrane thiols, allowing normally functioning RBCs to carry enormous quantities of oxygen safely. However, G6PD deficiency is the most common human RBC enzyme deficiency worldwide resulting in hereditary nonspherocytic anemia (see Chapter 23).
Methemoglobin Reductase Pathway
Heme iron is constantly exposed to oxygen, an oxidizing agent.8 In addition, the accumulation of peroxide oxidizes heme iron from the ferrous to the ferric state, forming methemoglobin. Although the HMP is able to prevent some hemoglobin oxidation by reducing peroxide, it is not able to reduce methemoglobin once it forms. NADPH is able to do so, but only slowly. The reduction of methemoglobin by NADPH is far more efficient in the presence of methemoglobin reductase, more properly called cytochrome b5 reductase (cytob5r). Using H+ from NADH formed when G3P is converted to 1,3-BPG, soluble cytochrome b5 reductase acts as an intermediate electron carrier, swiftly returning the oxidized iron to its ferrous, oxygen-carrying state. Cytochrome b5 reductase accounts for more than 65% of the methemoglobin-reducing capacity within the RBC.8
Rbc Membrane
RBC Deformability
RBCs are biconcave and average 90 fL in volume.9 Their average surface area is 140 µm2, a 40% excess of surface area compared with a 90-fL sphere. This excess surface-to-volume ratio enables RBCs to stretch undamaged up to 2.5 times their resting diameter as they pass through narrow capillaries and through splenic pores 2 µm in diameter, a property called RBC deformability.10 The RBC plasma membrane, which is 5 µm thick, is 100 times more elastic than a comparable latex membrane, yet has tensile (lateral) strength greater than that of steel. The deformable RBC membrane provides the broad surface area and close tissue contact necessary to support the delivery of oxygen from lungs to body tissue and carbon dioxide from body tissue to lungs.
RBC deformability depends not only on RBC geometry but also on relative cytoplasmic (hemoglobin) viscosity. The normal mean cell hemoglobin concentration (MCHC) ranges from 32% to 36% (see Chapter 14 and inside front cover), and as hemoglobin concentration rises, viscosity rises.11 Hemoglobin concentrations above 36% compromise deformability and shorten the RBC life span, because the more viscous cells cannot accommodate to narrow capillaries or splenic pores. As RBCs age, they lose membrane surface area while retaining hemoglobin. The hemoglobin becomes more and more concentrated, and eventually the RBC, unable to pass through the splenic pores, is destroyed by splenic macrophages. Refer to Chapter 8 for a more complete discussion of RBC senescence theories.
RBC Membrane Lipids
Membrane elasticity (pliancy) is the third property that contributes to deformability. The RBC membrane consists of approximately 8% carbohydrates, 52% proteins, and 40% lipids.12 The lipid portion, equal parts of cholesterol and phospholipids, forms a bilayer universal to all animal cells (see Figure 13-9). Four main phospholipids form an impenetrable fluid barrier as their hydrophilic polar head groups are arrayed upon the membrane’s surfaces, oriented toward both the aqueous plasma and the cytoplasm, respectively.13 Their hydrophobic nonpolar acyl tails arrange themselves to form a central layer dynamically sequestered from the aqueous plasma and cytoplasm. The membrane maintains extreme differences in osmotic pressure, cation concentrations, and gas concentrations between the plasma external to the cell and the internal cytoplasm.14 Phospholipids reseal rapidly when the membrane is torn.
Cholesterol, esterified and largely hydrophobic, resides parallel to the acyl tails of the phospholipids, equally distributed between the outer and inner layers, and evenly dispersed within each layer, approximately one molecule per phospholipid molecule. Cholesterol’s β hydroxyl group, the only hydrophilic portion of the molecule, anchors within the polar head groups, while the rest of the molecule intercalates among and parallel to the acyl tails. Cholesterol confers tensile strength to the lipid bilayer.15
The phospholipids are asymmetrically distributed. Phosphatidylcholine and sphingomyelin predominate in the outer layer; phosphatidylserine (PS) and phosphatidylethanolamine form most of the inner layer. Distribution of the phospholipids is energy dependent, relying on a number of membrane-associated enzymes, called flippases, floppases, and scramblases, for their position.16 When phospholipid distribution is disrupted, as in sickle cell anemia and thalassemia or in RBCs that have reached the end of their 120-day life span, PS, the only negatively charged phospholipid, redistributes (flips) to the outer layer. Splenic macrophages possess receptors that bind PS and destroy senescent RBCs. Refer to Chapter 8 for a complete discussion of RBC senescence theories.
Membrane phospholipids and cholesterol may also redistribute laterally so that the RBC membrane may respond to stresses and deform within 100 milliseconds of being challenged by the presence of a narrow passage, such as when arriving at a capillary. Redistribution becomes limited as the proportion of cholesterol increases. Plasma bile salt concentration also affects cholesterol exchange.17 In liver disease with low bile salt concentration, membrane cholesterol concentration becomes reduced. As a result, the more elastic cell membrane shows a “target cell” appearance when the RBCs are spread on a slide (see Figure 18-1).
Glycolipids (sugar-bearing lipids) make up 5% of the external half of the RBC membrane.18 They associate in clumps or rafts and support carbohydrate side chains that extend into the aqueous plasma to help form the glycocalyx. The glycocalyx is a layer of carbohydrates whose net negative charge prevents microbial attack and protects the RBC from mechanical damage caused by adhesion to neighboring RBCs or to the endothelium. Glycolipids may bear a few copies of carbohydrate-based blood group antigens, for example, antigens of the ABH and the Lewis blood group systems.
RBC Membrane Proteins
Although cholesterol and phospholipids constitute the principal RBC membrane structure, transmembranous (integral) and skeletal (cytoskeletal, peripheral) proteins make up 52% of the membrane structure by mass.19 A proteomic study reveals there are at least 300 RBC membrane proteins, including 105 transmembranous proteins. Of the purported 300 membrane proteins, about 50 have been characterized and named, some with a few hundred copies per cell, others with over a million copies per cell.20
Transmembranous Proteins
The transmembranous proteins serve a number of RBC functions, as listed in Table 9-5.21 Through glycosylation they support surface carbohydrates, which join with glycolipids to make up the protective glycocalyx.22 They serve as transport and adhesion sites and signaling receptors. Any disruption in transport protein function changes the osmotic tension of the cytoplasm, which leads to a rise in viscosity and loss of deformability. Any change affecting adhesion proteins permits RBCs to adhere to each other and to the vessel walls, promoting fragmentation (vesiculation), reducing membrane flexibility, and shortening the RBC life span. Signaling receptors bind plasma ligands and trigger energy activation of submembranous G proteins that then initiate various energy-dependent cellular activities, a process called signal transduction.
TABLE 9-5
Names and Properties of Selected Transmembranous RBC Proteins
| Transmembranous Protein | Band | Molecular Weight (D) | Copies per Cell (×103) | % of Total Protein | Function |
| Aquaporin 1 | Water transporter | ||||
| Band 3 (anion exchanger 1) | 3 | 90,000-102,000 | 1200 | 27% | Anion transporter, supports ABH antigens |
| Ca2+ ATPase | Ca2+ transporter | ||||
| Duffy | 35,000-43,000 | G protein–coupled receptor, supports Duffy antigens | |||
| Glut-1 | 4.5 | 45,000-75,000 | 5% | Glucose transporter, supports ABH antigens | |
| Glycophorin A | PAS-1 | 36,000 | 500-1000 | 85% of glycophorins | Transports negatively charged sialic acid, supports MN determinants |
| Glycophorin B | PAS-4 | 20,000 | 100-300 | 10% of glycophorins | Transports negatively charged sialic acid, supports Ss determinants |
| Glycophorin C | PAS-2 | 14,000-32,000 | 50-100 | 4% of glycophorins | Transport negatively charged sialic acid, supports Gerbich system determinants |
| ICAM-4 | Integrin adhesion | ||||
| K+-Cl– cotransporter | |||||
| Kell | 93,000 | Zn2+-binding endopeptidase, Kell antigens | |||
| Kidd | 46,000-50,000 | Urea transporter | |||
| Na+,K+-ATPase | |||||
| Na+-K+-2Cl– cotransporter | |||||
| Na+-Cl– cotransporter | |||||
| Na+-K+ cotransporter | |||||
| Rh | 30,000-45,500 | D and CcEe antigens | |||
| RhAG | 50,000 | Necessary for expression of D and CcEe antigens; gas transporter, probably of CO2 |
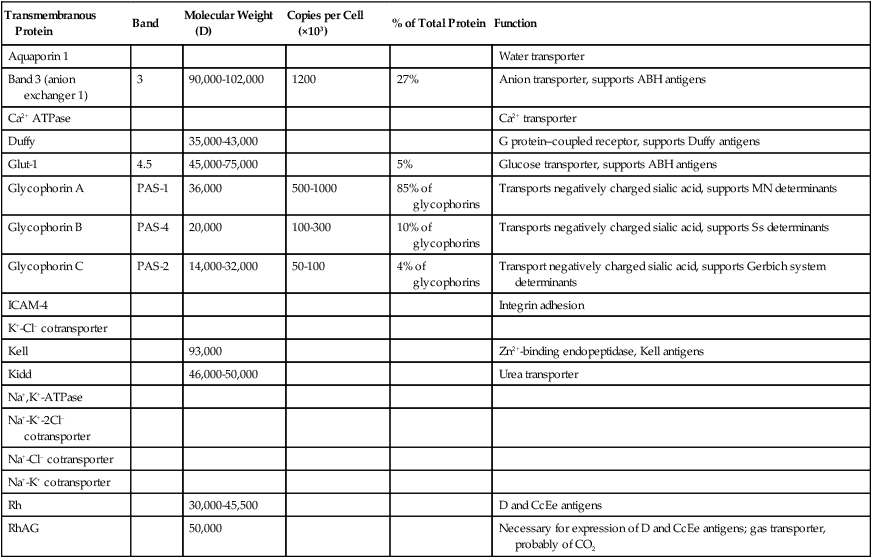
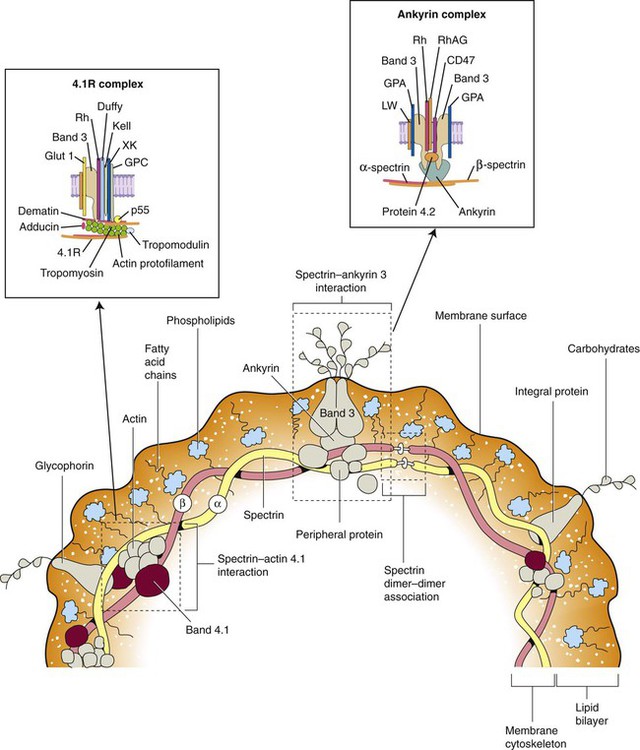
The transmembranous proteins assemble into one of two macromolecular complexes named by their respective skeletal anchorages, ankyrin or protein 4.1 (Figure 9-2). These complexes and their anchorages provide RBC membrane structural integrity, because the membrane relies on the cytoplasmic skeletal proteins positioned immediately within (underneath) the lipid bilayer for its ability to retain (and return to) its biconcave shape despite deformability. The transmembranous proteins provide vertical membrane structure.
Transmembranous proteins support carbohydrate-defined blood group antigens.23 For instance, band 3 (anion transport) and Glut-1 (glucose transport) support the majority of ABH system carbohydrate determinants by virtue of their high copy numbers.24 ABH system determinants are also found on several low copy number transmembranous proteins. Certain transmembranous proteins provide peptide epitopes. For instance, glycophorin A carries the peptide-defined M and N determinants, and glycophorin B carries the Ss determinants, which together comprise the MNSs system.25,26
The Rh system employs two multipass transmembranous lipoproteins and a multipass glycoprotein that each cross the membrane 12 times.27–30 The two lipoproteins present the D and CcEe epitopes, respectively, but expression of the D and CcEe antigens requires the separately inherited glycoprotein RhAG, which localizes near the Rh lipoproteins in the ankyrin complex. Loss of the RhAG glycoprotein prevents expression of both the D and CcEd antigens (Rh-null) and is associated with morphologic RBC abnormalities. Additional blood group antigens localize to the 4.1 complex or specialized proteins.28–30
A few copies of phosphatidylinositol (PI), not mentioned in the RBC Membrane Lipids section, reside in the outer, plasma-side portion of the lipid bilayer. These serve as anchors for two surface proteins, decay-accelerating factor (DAF, or CD55) and membrane inhibitor of reactive lysis (MIRL, or CD59).31,32 These proteins appear to float on the surface of the membrane as they link to PI through a glycan core consisting of multiple sugars. CD55 and CD59 are linked to PI by phosphatidylinositol glycan class A (PIG-A). In paroxysmal nocturnal hemoglobinuria (see Chapter 23), PIG-A acquires a mutation, CD55 and CD59 become deficient, and the cell is susceptible to complement-mediated hemolysis.
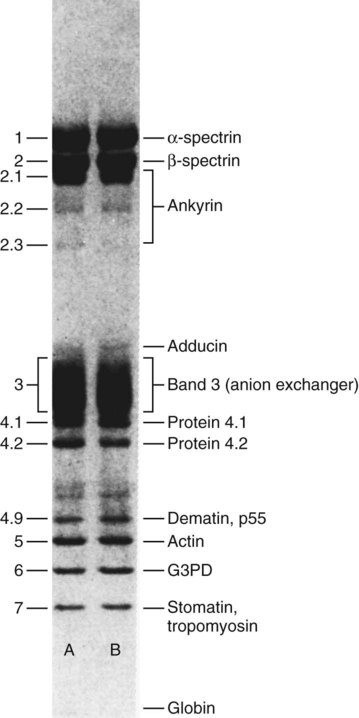
Numerical naming—for instance, band 3, protein 4.1, and protein 4.2—derives from historical (preproteomics) protein identification techniques that distinguished 15 membrane proteins using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), as illustrated in Figure 9-3.33 Bands migrate through the gel, with their velocity a property of their molecular weight and net charge, and are identified using Coomassie blue dye. The glycophorins, with abundant carbohydrate side chains, are stained using periodic acid–Schiff (PAS) dye.
Band 3, protein 4.2, and RhAG, members of the ankyrin complex, link their associated proteins and the bilayer membrane to the skeletal proteins through ankyrin.34–36 Likewise, glycophorin C, Rh, and blood group Duffy link the 4.1 complex through protein 4.1.37 The 4.1 anchorage also includes the more recently defined proteins adducin and dematin, which link with band 3 and Glut-1, respectively.38
Skeletal Proteins
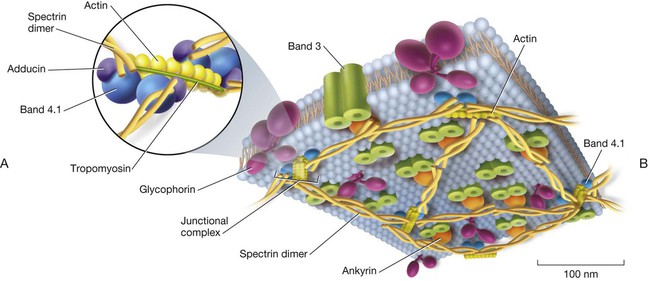
The principal skeletal proteins are the filamentous α-spectrin and β-spectrin (Table 9-6), which assemble to form an antiparallel heterodimer held together with a series of lateral bonds.39 Antiparallel means that the carboxyl (COOH) end of one strand associates with the amino (NH3) end of the other. The spectrins form a hexagonal lattice (Figure 9-4) that is immediately adjacent to the cytoplasmic membrane lipid layer and provides lateral membrane stability.40 Because the skeletal proteins do not penetrate the bilayer, they are also called peripheral proteins.
TABLE 9-6
Names and Properties of Selected Skeletal RBC Proteins
| Skeletal Protein | Band | Molecular Weight (D) | Copies per Cell (×103) | % of Total Protein | Function |
| α-spectrin | 1 | 240,000-280,000 | 240 | 16% | Filamentous antiparallel heterodimer, primary cell skeleton |
| β-spectrin | 2 | 220,000-246,000 | 240 | 14% | |
| Adducin | 2.9 | 80,000-103,000 | 60 | 4% | Caps actin filament |
| Ankyrin | 2.1 | 206,000-210,000 | 120 | 4.5% | Anchors band 3 and protein 4.2 |
| Dematin | 4.9 | 43,000-52,000 | 40 | 1% | Actin bundling protein |
| F-actin | 5 | 42,000-43,000 | 400-500 | 5.5% | Binds β-spectrin |
| G3PD | 6 | 35,000-37,000 | 500 | 3.5% | |
| Protein 4.1 | 4.1 | 66,000-80,000 | 200 | 5% | Anchors 4.1R complex |
| Protein 4.2 (protein kinase) | 4.2 | 72,000-77,000 | 200 | 5% | Anchors ankyrin complex |
| Tropomodulin | 5 | 41,000-43,000 | 30 | — | Caps actin filament |
| Tropomyosin | 7 | 27,000-38,000 | 80 | 1% | Regulates actin polymerization |
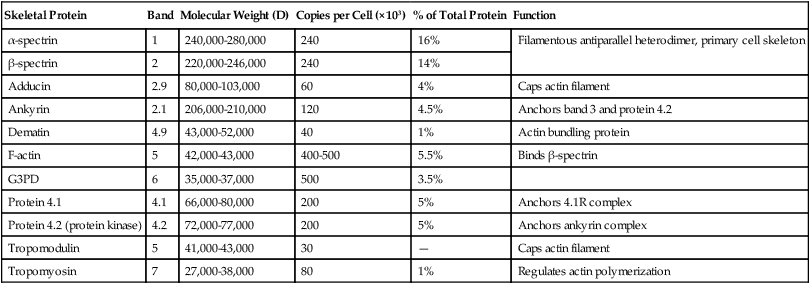
G3PD, Glucose-3-phosphate dehydrogenase glyceraldehyde; RBC, red blood cell.
The secondary structure of both α- and β-spectrin features triple-helical repeats of 106 amino acids each; 20 such repeats make up α-spectrin and 16 make up β-spectrin.41 Essential to the cytoskeleton are the previously mentioned ankyrin, protein 4.1, adducin and dematin, and, in addition, actin, tropomyosin, and tropomodulin (see Figure 9-4).35 A single helix at the amino terminus of α-spectrin consistently binds a pair of helices at the carboxy terminus of the β-spectrin chain, forming a stable triple helix that holds together the ends of the heterodimers.42 Joining these ends are actin and protein 4.1. Actin forms short filaments of 14 to 16 monomers whose length is regulated by tropomyosin. Adducin and tropomodulin cap the ends of actin, and dematin appears to stabilize actin in a manner that is the subject of current investigation.43
Spectrin dimer bonds that appear along the length of the molecules disassociate and reassociate (open and close) during RBC deformation.44 Likewise, the 20 α-spectrin and 16 β-spectrin repeated helices unfold and refold. These flexible interactions plus the spectrin–actin–protein 4.1 junctions are key regulators of membrane elasticity and mechanical stability, and abnormalities in any of these proteins result in deformation-induced membrane fragmentation. For instance, autosomal dominant mutations that affect the integrity of band 3, RhAG, ankyrin, protein 4.1, or spectrin are associated with hereditary spherocytosis (see Chapter 23).45,46 In these cases there are too few vertical anchorages to maintain membrane stability. The lipid membrane peels off in small blebs called vesicles, whereas the cytoplasmic volume remains intact. This generates spherocytes with a reduced membrane-to-cytoplasm ratio. Conversely, hereditary elliptocytosis (ovalocytosis) arises from one of several autosomal dominant mutations affecting spectrin dimer-to-dimer lateral bonds or the spectrin–ankyrin–protein 4.1 junction.47 In hereditary elliptocytosis, the membrane fails to rebound from deformation, and RBCs progressively elongate to form visible elliptocytes, which causes a mild to severe hemolytic anemia.48
Osmotic Balance and Permeability
The RBC membrane is impermeable to Na+, K+, and Ca2+. It is permeable to water and the anions bicarbonate (HCO3–) and chloride (Cl–), which freely exchange between plasma and RBC cytoplasm.49 Aquaporin 1 is a transmembranous protein that forms pores or channels whose surface charges create inward flow of water in response to internal osmotic changes. Glucose is transported without energy expenditure by the transmembranous protein Glut-1.
Adenosine triphosphatase (ATPase)–dependent (energy-dependent) cation pumps (see Table 9-5) regulate the concentrations of Na+ and K+, maintaining intracellular-to-extracellular ratios of 1:12 and 25:1, respectively.50,51 These enzyme-based pump mechanisms, in addition to aquaporin, maintain osmotic balance. Pump mechanism damage permits the influx of Na+, with water following osmotically. The cell swells, becomes spheroid, and eventually ruptures, spilling hemoglobin. This phenomenon is called colloid osmotic hemolysis.
Ca2+ ATPase extrudes calcium, maintaining exceptionally low intracellular levels of 5 to 10 µmol/L. Calmodulin, a cytoplasmic Ca2+-binding protein, controls the function of Ca2+ ATPase.52
Sickle cell disease provides an example of increased cation permeability. When crystallized sickle hemoglobin deforms the cells, internal levels of Na+, K+, and especially Ca2+ rise, which results in hemolysis.53
Summary
• Glucose enters the erythrocyte by facilitated membrane transport.
• Glycolysis in the EMP occurs aerobically and anaerobically.
• The primary reservoir of energy is ATP, which is generated by glycolysis.
• Through anaerobic glycolysis in the EMP, glucose is metabolized to pyruvate, using two molecules of ATP per molecule of glucose and providing four molecules of ATP per molecule of glucose, for a net gain of two molecules of ATP.
• By means of the Rapaport-Luebering pathway (a shunt off the EMP), 2,3-BPG is generated. It is necessary for the facilitation of oxygen delivery to the tissues.
• The methemoglobin reductase pathway (also a bypass from the EMP) is able to convert oxidized heme iron (methemoglobin) to its reduced state, returning the molecule to its oxygen-carrying function.
• Aerobic glycolysis occurs in the HMP, which converts glucose into pentose with the generation of NADPH. NADPH reduces glutathione, which then reduces peroxide to water and oxygen. Proteins, lipids, and heme iron are then protected from oxidation by peroxide.
• The RBC membrane is a typical lipid bilayer with hydrophobic components oriented away from the water-rich plasma and cytoplasm.
• The two lipid layers of the membrane differ in the composition of phospholipids.
• The membrane provides a semipermeable barrier separating the plasma constituents from the cytoplasm, which allows the cell to maintain necessary intracellular differences.
• Membrane cholesterol is restored from the plasma by an enzyme-enhanced process of lipid exchange.
• Abnormalities in membrane lipids lead to abnormal cell shapes, such as the “target cells” that develop with liver disease, which result from the changes in plasma bile salts.
• The membrane proteins include the transmembranous (integral) proteins. These include ion channels and various receptors.
• The proteins that localize to the interior surfaces of the membrane are called skeletal (peripheral) proteins.
• Membrane proteins are extracted using sodium dodecyl sulfate, separated using polyacrylamide gel electrophoresis, and stained with Coomassie blue for visualization. They are numbered from the point of application; lower numbers correlate to high protein molecular weight and lower net charge.
• PAS staining is used to localize electrophoresed proteins with high carbohydrate composition, which are designated as PAS-1 through PAS-4.
• The shape and flexibility of the RBC, which are essential to its function, are related to the cytoskeleton. The cytoskeleton is derived from a group of peripheral proteins on the interior side of the lipid membrane. The major structural proteins are α- and β-spectrin, which are bound together and to the lipid membrane by ankyrin, actin, protein 4.1, adducin, tropomodulin, dematin, and band 3.
• Abnormalities of the peripheral structural proteins such as spectrin and actin lead to abnormal RBC shapes. Hereditary spherocytosis and hereditary elliptocytosis are examples of diseases associated with abnormalities and deficiencies of structural proteins.
• The concentration of potassium inside the RBC is higher than that in the plasma, whereas the sodium concentration is lower. This disequilibrium is maintained by the Na+-K+ pump in the membrane. Failure of the pump leads to an influx of sodium, with water following by osmosis, which results in cell swelling and possible lysis.
Review Questions
1. An RBC process that does not require energy is:
a. Maintenance of intracellular cationic electrochemical gradients
3. Which of the following is true concerning 2,3-BPG?
a. It enhances the release of oxygen from hemoglobin.
b. It provides a source of glucose for the RBC.
c. It is unnecessary for RBC survival.
d. It is the least abundant of all organophosphates in the RBC.
4. The activity of the hexose monophosphate pathway increases the RBC source of:
5. The layer of the erythrocyte membrane that is largely responsible for the shape, structure, and deformability of the cell is the:
6. The glycolipids of the RBC membrane:
7. The lipids of the membrane are arranged:
8. Lipid exchange between the RBC membrane and the plasma occurs:
a. To replace lost lipids in the membrane
b. To provide a mechanism for excretion of lipid-soluble RBC waste products
c. To ensure symmetry between the composition of the interior and exterior lipid layers
9. The numbering system for membrane proteins gives the lowest number to proteins that are:
10. Which of the following is an example of an integral membrane protein?
11. Which of the following statements about spectrin structure is correct?
a. Spectrin forms homodimers that twist into ropelike strands.
b. Spectrin dimers associate into tetramers that are tightly bound to each other throughout their length.
c. Seven spectrin tetramers join at their ends to create a lattice.
d. The spectrin strands are connected to the lipid bilayer by ankyrin.
12. All of the following are functions of band 3 except:
a. Providing a channel for anion movement through the membrane
b. Contributing to membrane flexibility
13. Na+,K+-ATPase maintains a concentration of ions in which:
a. Potassium is in higher concentration inside the RBC than in the plasma, and sodium is in lower concentration in the RBC than in the plasma
b. Sodium and potassium are in higher concentration inside the RBC than in the plasma
c. Sodium is in higher concentration inside the RBC than in the plasma, and potassium is in equilibrium with the plasma
d. Potassium and sodium are in lower concentration inside the RBC than in the plasma