Chapter 339 Embryology, Anatomy, and Physiology
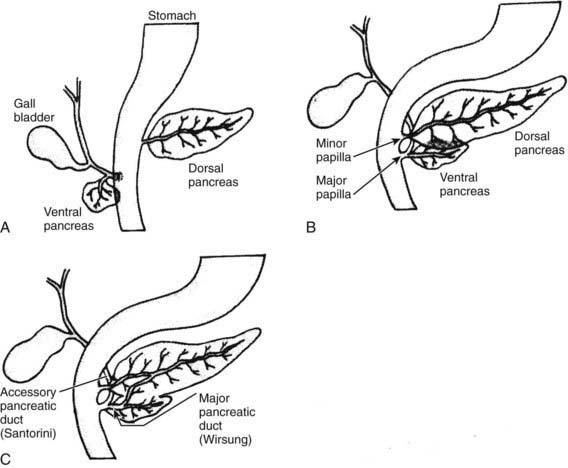
The human pancreas develops from the ventral and dorsal domains of the primitive duodenal endoderm beginning at about the 5th wk of gestation (see ![]() Fig. 339-1 on the Nelson Textbook of Pediatrics website at www.expertconsult.com). The larger dorsal anlage, which develops into the tail, body, and part of the head of the pancreas, grows directly from the duodenum. The smaller ventral anlage develops as 1 or 2 buds from the primitive liver and eventually forms the major portion of the head of the pancreas. At about the 17th wk of gestation, the dorsal and ventral anlagen fuse as the buds develop and the gut rotates. The ventral duct forms the proximal portion of the major pancreatic duct of Wirsung, which opens into the ampulla of Vater. The dorsal duct forms the distal portion of the duct of Wirsung and the accessory duct of Santorini, which empties independently in ∼5% of people. Variations in fusion might account for pancreatic developmental anomalies. Pancreatic agenesis has been associated with a base pair deletion in the ipf1 HOX gene, PDX1, and possibly in the PTF1A and FS123TER genes. Other recessive genes involved in pancreatic organogenesis include the IHH, SHH or sonic hedgehog gene, SMAD2, and transforming growth factor (TGF)-1β genes.
Fig. 339-1 on the Nelson Textbook of Pediatrics website at www.expertconsult.com). The larger dorsal anlage, which develops into the tail, body, and part of the head of the pancreas, grows directly from the duodenum. The smaller ventral anlage develops as 1 or 2 buds from the primitive liver and eventually forms the major portion of the head of the pancreas. At about the 17th wk of gestation, the dorsal and ventral anlagen fuse as the buds develop and the gut rotates. The ventral duct forms the proximal portion of the major pancreatic duct of Wirsung, which opens into the ampulla of Vater. The dorsal duct forms the distal portion of the duct of Wirsung and the accessory duct of Santorini, which empties independently in ∼5% of people. Variations in fusion might account for pancreatic developmental anomalies. Pancreatic agenesis has been associated with a base pair deletion in the ipf1 HOX gene, PDX1, and possibly in the PTF1A and FS123TER genes. Other recessive genes involved in pancreatic organogenesis include the IHH, SHH or sonic hedgehog gene, SMAD2, and transforming growth factor (TGF)-1β genes.
The pancreas lies transversely in the upper abdomen between the duodenum and the spleen in the retroperitoneum (Fig. 339-2). The head, which rests on the vena cava and renal vein, is adherent to the C loop of the duodenum and surrounds the distal common bile duct. The tail of the pancreas reaches to the left splenic hilum and passes above the left kidney. The lesser sac separates the tail of the pancreas from the stomach.
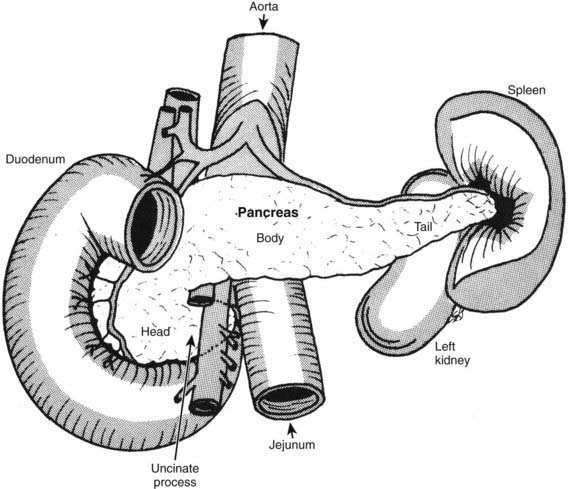
Figure 339-2 Anterior view of the pancreas: relationship to neighboring structures.
From Werlin SL: The exocrine pancreas. In: Kelly VC, editor: Practice of pediatrics, vol 3, Hagerstown, MD, 1980, Harper and Row.)
339.1 Anatomic Abnormalities
Complete or partial pancreatic agenesis is a rare condition. Complete agenesis is associated with severe neonatal diabetes and usually death at an early age (Chapter 583).
Cano D, Hebrok M, Zenker M. Pancreatic development and disease. Gastroenterology. 2007;132:745-762.
Howard ER. Congenital abnormalities. In: Howard ER, Stringer MD, Colombani PM, editors. Surgery of the liver and bile ducts in children. ed 2. London: Arnold; 2002:493-502.
Klein SD, Affronti JP. Pancreas divisum, an evidence-based review: part I, pathophysiology. Gastrointest Endosc. 2004;60:9-25.
Klein SD, Affronti JP. Pancreas divisum, an evidence-based review: part II, patient selection and treatment. Gastrointest Endosc. 2004;60:585-589.