46 Electrical Stimulation
Historical Perspective
Long before the development of modern electricity, “natural electricity” was used for its therapeutic properties of inducing analgesia. In the earliest written description of an electric fish, Aristotle remarked, “The torpedo is known to cause a numbness even in human beings.”1 Perhaps the earliest record of local electrical analgesia was when Scribonius Largusm wrote in circa CE 46 that he advocated piscine “electrotherapy” for the relief of pain.1 Centuries later, in the mid 1740s, the application of an electrical apparatus was first used in medicine by Kratzenstein.1 In 1858, Francis received a patent for an electrical device that he claimed relieved dental pain; he boasted that he had performed 164 successful tooth extractions and that most had resulted in no pain.1
Electrical Stimulation in Modern Medicine
As a physical modality, electrical stimulation has been prescribed to strengthen muscles, promote healing, decrease urinary incontinence, and increase circulation, as well as for pain management.2
Electrical stimulation is a nonpharmacologic and, in most cases, a noninvasive pain-management method that has been promoted for its analgesic properties. It has demonstrated efficacy for a variety of pain conditions including chronic low back pain,3–4 dysmenorrhea,5 hemiplegic shoulder pain,6 and arthritic pain.40
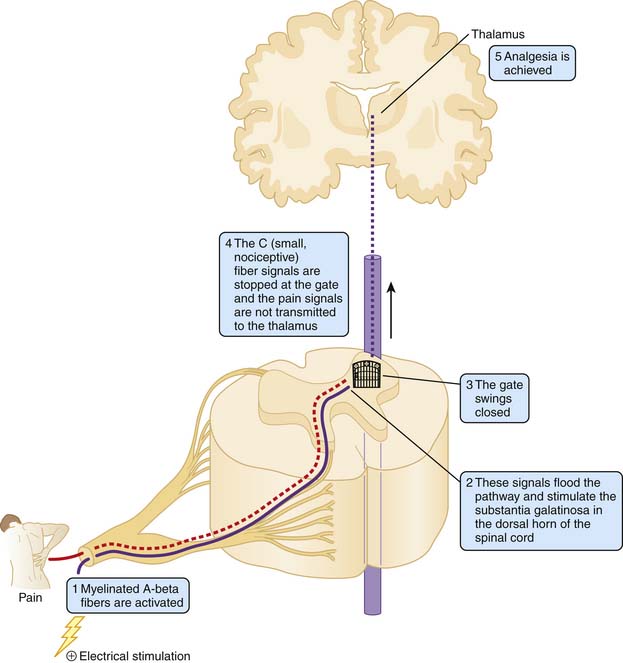
In all three types of electrical stimulation that will be explored in this chapter, an electrical stimulation device is turned on and electrical current is sent through electrodes that are applied to the body causing a tingling sensation and/or muscle contractions in the underlying skin and muscle. This electrical signal disrupts the regular pain signals that are being sent from the affected area to its surrounding nerves. By interrupting this signal pathway, the recipient perceives less pain (see Fig. 46-2).
Neuromuscular Electrical Stimulation (NMES), sometimes known as Electrical Muscle Stimulation (EMS), is often referred to as functional electrical stimulation (FES) when it is used to activate paralyzed muscles in a precise sequence and magnitude so as to directly accomplish functional tasks.6,8 Beyond its neurorehabilitation potential, NMES has shown efficacy in pain management. NMES uses high-intensity electrical stimulation to elicit intermittent contraction and relaxation of proximal muscle fibers and is widely prescribed following surgery and trauma.
Developed in the early 1950s, interferential current (IFC) therapy produces two alternating currents of slightly differing medium frequency using wave interference to create a resultant amplitude of therapeutic stimulation.9 Interferential current therapy is mainly used to relieve pain that is felt in deep tissues. Depending on the configuration of the electrodes applied to the skin, the effects can be localized or adjusted to be more general. Unlike other methods of low-frequency electrical stimulation, IFC encounters low electrical resistance and therefore can penetrate deeply without causing unnecessary discomfort.
Transcutaneous Electrical Nerve Stimulation
TENS is a method of pain relief in which a device transmits low-voltage electrical impulses through electrodes on the skin to an area of the body that is in pain.
Proposed Mechanisms of Pain Control
Research indicates that TENS can be a noninvasive, safe method for managing and reducing both acute and chronic pain. Although a uniform mechanism has not been established, there are a number of theories to explain the modulation of pain associated with TENS. Two main theories on pain transmission are the gate theory and the endorphin theory.10 The gate theory pioneered by Melzack and Wall has been attributed to motivating the development of electrotherapeutic equipment, and the endorphin theory has led to a decrease on the reliance of pain medication for treatment of postoperative pain. An additional theory includes the acupuncture theory, which hypothesizes that management of pain is related to energy lines and its associated acupuncture points.
Gate Theory
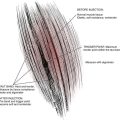
The gate theory asserts that stimulation of large, highly myelinated (afferent A-beta fibers) block the transmission of pain signals by relatively small, nociceptive fibers with little or no myelination (A-delta and C fibers) at the level of the spinal cord. Small, unmyelinated C fibers are responsible for chronic, throbbing pain, whereas the thicker A-delta fibers with slight myelination make them more conducive to transmitting faster more intense pain information. It is postulated that electrical stimulation decreases the perception of pain by increasing the activation of A-beta fibers and therefore flooding the pain signal pathway and ‘closing the gate’ of transmission in the spinal cord.11,12 These target cells are located in the substantia gelatinosa (Rexed’s laminae I, II, and IV) of the dorsal horn (Fig. 46-1).11,13
Endorphin Theory
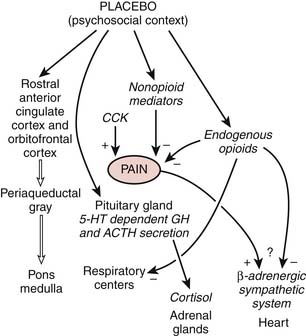
Also known as the opiate-mediated theory, the endorphin theory is based on the discovery of the presence of natural opiates in the body (Fig. 46-2).14,15 Acting as the body’s own natural pain relievers, they are produced in the spinal cord and pituitary gland as enkephalins and beta-endorphins, respectively. These endogenous opiates are effective at decreasing the perception of pain and, in turn, mimicking the action of narcotic drugs. Basic science studies show that high and low-frequency TENS produce their effects by activation of opioid receptors in the central nervous system. High-frequency TENS and low-frequency TENS activate delta-opioid and mu-opioid receptors, respectively, both in the spinal cord and supraspinally (in the medulla). Studies suggest that TENS also stimulates the body’s production of endogenous opiates that interact with specific receptor cites in the central and peripheral nervous systems, thereby blocking the perception of pain.
Cheng and Pomerantz demonstrated that pain relief produced at 4 Hz of stimulation (low frequency) was blocked by the opiate antagonist, naloxone, whereas pain relief induced at 200 Hz was not blocked by naloxone. When administered with a strong, subnoxious intensity at an adequate frequency, TENS has been demonstrated to decrease reliance on pain medication for postoperative pain.16–22
Acupuncture Theory
A lesser-known theory that has been presented as a possible explanation for the ability of TENS to be effective in pain management is the acupuncture theory. Some believe that the use of TENS opens and stimulates common acupuncture points along the same meridians or energy lines used in traditional acupuncture. Acupuncture points can lie on, be adjacent to, or be distant from the site of pain. When applied to these points, TENS is believed to modify the flow of energy or chi resulting in a decrease in pain.23,24 Some theorists propose that hyperirritable spots in the skeletal muscle that are associated with palpable nodules in taut bands of muscle fibers, known as trigger points, can be stimulated by TENS to successfully decrease pain. It is believed that trigger points cause tissue ischemia and that the application of TENS causes vasodilation to occur, which modifies the ischemic area, thereby decreasing pain.25–28
Practical Application
Clinical Use and Application
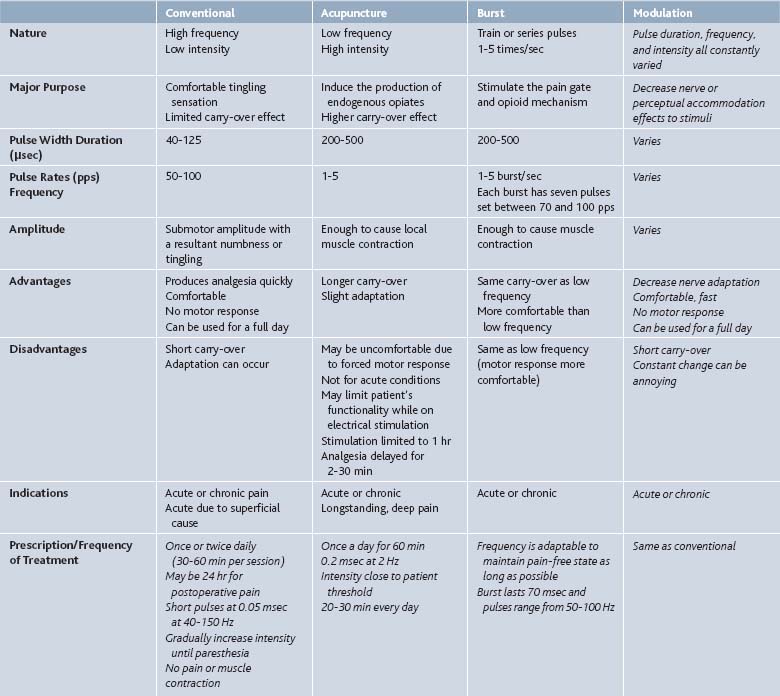
There are four main types of stimulation (Table 46-1) namely: conventional mode TENS, acupuncture mode TENS, burst or pulse mode TENS, and modulation mode TENS.
Conventional Mode TENS
This mode has high-frequency (40 to 150 Hz) and low intensity (10 to 30 mA) stimulation, with amplitude being adjusted to induce minimal sensory discomfort. Studies have shown it is the most effective type of stimulation. This mode is most useful for neuropathic pain. Patients customarily apply the electrodes and have them in place all day, turning it on for approximately 30-minute intervals throughout the day. This mode activates A-beta and A-alpha nerves that cause interneurons from the substantia gelatinosa to inhibit the transmission of pain by T cells in lamina V neurons of the spinal cord (Table 46-2).
| External use only |
| Keep from children |
| Skin irritation at electrode placement sites |
| Avoid water content |
Acupuncture Mode TENS
Characterized as low-frequency (1-10 Hz) and high-intensity (close to the tolerance limit of the patient) stimulation, Acupuncture mode TENS is useful for musculoskeletal pain. It is thought that this mode stimulates C-fibers causing counterirritation. This method is often considered for patients who do not respond to conventional TENS. Acupuncture mode stimulates the hypothalamus which, in turn, causes the release of endogenous opiates, specifically beta-endorphin, evidenced by the fact that the TENS effect can be reversed by naloxone (see Table 46-2).
Burst or Pulse Mode TENS
In this mode, the unit delivers high-frequency stimulation bursts interrupted by low-frequency intervals. The recurrent burst discharge at 1 to 2 or 2 to 3 Hz and the frequency of the impulses with each burst is at about 100 Hz. There is a delayed onset of analgesia with this type of TENS, and no particular advantage has been established for pulse method over the conventional method. Like the acupuncture mode, burst mode causes the body to release endogenous opiates (see Table 46-2).
Modulated Mode TENS
Pulse duration, frequency, and intensity all are constantly varied in an attempt to avoid neurohabituation in this particular mode. Because of the constantly changing stimuli, perceptual adaptation (which can occur under the use of constant unchanging stimuli) is decreased. Modulation mode works by the same mechanism as the conventional mode, which is closely related to the gate theory (see Table 46-2).
Neuromuscular Electrical Stimulation
Neuromuscular electrical stimulation uses low-frequency, low-amplitude electrical current to targeted muscle groups to activate motoneurons, resulting in involuntary muscle contraction. NMES uses high-intensity electrical stimulation to elicit intermittent contraction and relaxation of proximal muscle fibers.29 Clinical NMES systems stimulate either the nerve directly or the motor point of the nerve proximal to the neuromuscular junction. Most clinical NMES systems fall into two broad categories: surface (transcutaneous) and implanted (usually percutaneous) systems.6 NMES systems are either voltage or current regulated. Despite variable motor response, voltage-regulated stimulation is more common with transcutaneous NMES systems because, as resistance increases (due to electrode-to-skin interface changes), the current decreases.29
Proposed Mechanisms of Pain Control
Like TENS, NMES is associated with gate theory and endorphin theory as possible explanations for the mechanism of analgesia owing to stimulation. It is believed that because NMES provokes muscle contractions in the applied area, it acts as a counterirritant stimulus, reduces muscle spasms, and increases muscle strength and endurance.3 It is believed that NMES reduces pain through muscle toning, and prevents disuse atrophy and muscle degeneration that is frequently associated with chronic myofascial pain.29
Practical Application
Electrode Placement for NMES
The most commonly used NMES electrode is the transcutaneous electrode that is applied to the skin and stimulates directly over the peripheral nerve or motor point of the targeted muscle groups. Two electrodes, which are placed in a monopolar or bipolar configuration, are required to produce an electrical current flow.6
However, trans-NMES for the treatment of some pain conditions, such as hemiplegic shoulder pain, has not been widely accepted due to stimulation-induced pain (because it can activate the subcutaneous pain fibers), poor muscle selectivity, and difficulty in daily application of electrodes.30 When used to treat shoulder subluxation, Yu and colleagues found that percutaneous-NMES was less painful than transcutaneous-NMES31 because tissues with C-fiber nociceptors that respond to strong mechanical, thermal, and chemical stimuli lie between the electrode and the muscle motor point making stimulation of these nociceptors difficult to avoid.
Therefore, some practitioners and patients choose the minimally invasive percutaneous intramuscular electrodes that can reduce the risk of tissue injury. But with percutaneous-NMES systems, there is a risk of displacement or breakage associated with the anchoring of the external lead and electrode-related infection and granuloma formation secondary to retained electrode fragments. All percutaneous electrodes are anchored in place for the duration of the treatment. The electrodes then connect to lead wires that exit the skin and connect to the stimulator; this system eliminates skin resistance, cutaneous pain issues, allows greater muscle selectivity, and allows use of lower stimulation currents.6
Interferential Current Stimulation
Interferential stimulation is primarily effective in allowing deeper tissue penetration extending over a larger tissue volume than TENS is capable of stimulating.39 This technique differs from TENS in that it uses two alternating currents of slightly different medium frequency.
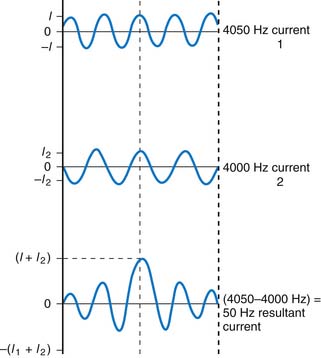
IFC applies these principals by using waves of a slightly different frequency. As one wave peak catches up to the other, constructive interference occurs, causing an increase in resultant amplitude; conversely, this resultant amplitude decreases as the waves drift out of phase and interfere in a destructive manner (Fig. 46-3). The rate or ”beat frequency” at which the resultant amplitude rises and falls depends on the difference between the two frequencies being used. This resultant amplitude’s frequency or beat frequency is actually the therapeutic stimulation.9
Proposed Mechanisms of Pain Control
All of the electrical stimulation methods described in this chapter are believed to be effective owing to the gate and endorphin theories of pain. But because of the different current flowing between each pair of electrodes in IFC, it is speculated to have an even more complex mechanism. Although the current flowing between each pair of electrodes is insufficient to stimulate nerve and muscle directly, when amplitude is modulated by interference, IFC creates a deeper signal.
Wedensky Inhibition
Repetitive stimulation at any frequency of at least 4000 Hz up to its maximum will cause action potentials to flow in the axon at the same rate. As the rate of stimulation increases above this value, successive stimuli fall within the relative and eventually the absolute refractory period of the preceding action potential. Therefore, a larger than normal flow of current is needed to stimulate a refractory neuronal membrane and thus the sensitivity of the nerve decreases. Prolonged stimulation at a supramaximal frequency will cause Wednesky inhibition of type C nociceptive fibers, eventually causing the axon to cease conducting. Accommodation of the neuron is responsible for this effect, caused by an increased threshold for pain and synaptic fatigue.9
Practical Application
Electrode Placement
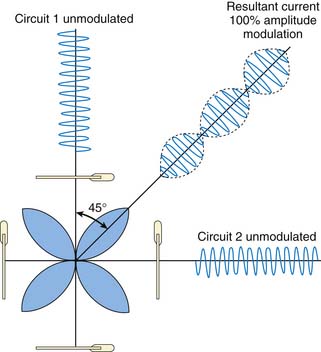
Most units use four electrodes to be placed at four corners around the area to be stimulated, with pairs using the same frequency at opposite corners. The stimulated area will then be a four-leaf clover-shaped area with the clover leaves at a 45-degree angle to the resultant intersection of the opposing frequencies (Fig. 46-4).
Clinical Use and Application
Like TENS and NMES, IFC uses two pairs of electrodes but one pair of the electrodes has a fixed frequency of 4000 Hz, whereas the other has a variable frequency between 4000 Hz and 4250 Hz. The variable electrode is then amplified to a therapeutically useful intensity. Some units operate at a base frequency of 2 kHz, which some practitioners believe can stimulate muscles more effectively.9
Safety Precautions for Electrical Stimulation
There are many safety concerns when considering the application of electricity directly to the human body. TENS, NMES, and IFC are contraindicated for use over the anterior cervical region, carotid sinuses, heart, transthoracic area, insensate skin, and the abdomen of a pregnant woman. Electrodes should never be placed on or near the eyes, in the mouth, transcerebrally, on the front of the neck. Electrical stimulation should not be used by people with an artificial demand-type cardiac pacemaker owing to risk of interference and failure of the device (Table 46-3).9 It is not to be used on anyone with an implanted defibrillator, or any other implanted electrical device.32 Stimulation should not be done during electrocardiogram testing or while operating diathermy devices. Electronic stimulation should not be used over swollen, infected, or inflamed areas or skin eruptions (e.g., phlebitis, thrombophlebitis, varicose veins). Patients may report discomfort or skin irritation if the intensity is too high; skin irritation can be resolved if electrode positions are shifted or if a different conducting gel is used (see Table 46-2).
Table 46-3 Common Indications and Contraindications for Use of TENS
| Common Indications | Common Contraindications |
|---|---|
| Diabetic neuropathic pain | Stimulation over the carotid sinuses |
| Posttraumatic pain | Cardiac pacemakers (controversial) |
| Postsurgical pain | Pregnancy (although risk from distal treatment appears to be negligible) |
| Peripheral nerve injury | Inability to report effects or discomfort |
| Chronic musculoskeletal pain | Atrophic skin |
| Phantom limb pain | Allergies to electrodes or gels |
| Sympathetically mediated pain (reflex sympathetic dystrophy, causalgia) |
Evidence of Efficacy
TENS
A 2008 systematic review conducted by Poitras and Brosseau in the relief of nonspecific or rheumatic chronic lower back pain (CLBP) identified six randomized controlled trials on the use of TENS. Number and length of treatment sessions varied among studies, as did the TENS parameters. Two studies recorded a statistically significant reduction in pain intensity assessed immediately after high-frequency TENS. When pain perception was assessed after the TENS protocol was complete results were mixed. After contacting the authors, they concluded that contradictory postintervention results could be explained by the differences in assessment periods. They concluded that those researchers that assessed immediately after the last session were capturing immediate results rather than short-term ones. In the negative results, delays in outcome measurement (TENS being used at home and evaluations conducted in the clinic) were the probably cause. This review also found that high-frequency TENS was more effective than low-frequency TENS.3
Dowswell and his team found that overall there was little difference in pain ratings between TENS and control groups when assessing the effect of TENS during labor. Although they did find that women receiving TENS to acupuncture points were less likely to report severe pain. Where TENS was used as an adjunct to epidural analgesia there was no evidence that it reduced pain.33
In a 2009 article in the Clinical Journal of Pain, DeSantana and colleagues used both high-frequency (100 Hz) and low-frequency (4 Hz) TENS, at strong, but comfortable sensory intensity. They were applied for 20 minutes through four electrodes placed around the surgical incision immediately after laparoscopic tubal ligation surgery (LS). They found that high- and low-frequency TENS significantly decreased postoperative pain intensity when compared with before administration of TENS. They proposed that TENS in combination with standard pharmacologic analgesic treatment was efficacious for postoperative pain relief after LS.34 But systematic reviews conducted by Robb and colleagues and Khadilkar’s team found insufficient evidence to support the use of TENS to treat cancer pain or lower back pain, respectively.35
NMES
A comprehensive review of the clinical uses of NMES in 2007 found that seven of eight studies that evaluated radiographic inferior glenohumeral subluxation reported improvements. Six of seven trials found NMES had a significant effect on subluxation. But only two reported sustained improvements beyond the end of treatment.6
Glaser and colleagues found that when comparing standardized exercise therapy in conjunction with functional electrical muscle stimulation to a standardized exercise therapy alone that 4 months after the end of the electrical stimulation (during the exercise alone intervention period) the group that initially had electrical stimulation for the first 2 months of the intervention had statistically significantly improved lumbar spine function over the entire 6 months. They suggested that electrical muscle stimulation can be an effective adjunctive treatment modality for nonacute low back pain.36
Gaines when studying the effect of NMES on arthritis knee pain, found an immediate reduction in pain 15 minutes after the NMES treatment sessions but it lacked long-term improvement in pain. Renzenbrink found that when using a percutaneous NMES, there was a significant reduction in body pain and pain intensity up to 24 weeks after intervention. But other measures were not statistically significant after 24 weeks.8
IFC
In a study that induced heat pain on healthy subjects to assess the tolerance created by the use of TENS and IFC found that both increased the heat pain threshold to a similar extent during stimulation but the poststimulation effect lasted longer in IFC than TENS.37
Tugay and her team found that both TENS and IFC were effective in the treatment of dysmenorrhea and were free from the potentially adverse effects of analgesics. They performed a 10-minute protocol for IFC and a 20-minute protocol for TENS. When patients were asked to assess their pain 8 hours and 8 to 24 hours after the protocol, their pain levels were below the baseline assessed immediately after the protocol. Although 8- to 24-hour measurements were increased significantly from the 8-hour assessment.5
In 2006, Walker reported that IFC, when used to treat psoriatic arthritis (PsA), improved SF-36 assessed body pain but not other SF-36 subscales. Thus, IFC had analgesic effects in PsA.37 Almeida reported in Pain that a combined ultrasound and interferential current therapy on pain and sleep in fibromyalgia led to subjective improvement of pain in terms of number and intensity of painful areas, and objective improvement with a decrease in tender point count and increased tenderness threshold. 39
Conclusion
Because of its wide variability, conflicting research remains to haunt this sector of medicine. Proponents of this method of pain analgesia believe that conflicting research about the extent of it efficacy in pain management is lacks consensus when it comes to the basic issues of its prescription, such as electrode placement, treatment length, and the best waveform and stimulation parameters. Clinical trials suggest that adequate dosing, particularly intensity, is critical to obtaining pain relief with electrical stimulation. Electrical stimulation is growing as a modality for pain management but continued research is needed to ensure that this technology is maximally utilized. And with this in mind, continued advancements in technology, a better understanding of optimal stimulation parameters, and a better understanding of physiologic changes induced by this technology are required.
1. Kane K., Taub A. A history of local electrical analgesia. Pain. 1975;1:125-138.
2. Cuccurullo S.J. Electrotherapy. Physical Medicine and Rehabilitation Board Review. New York: Demos Medical; 2004. 562–567
3. Poitras S., Brosseau L. Evidence-informed management of chronic low back pain with transcutaneous electrical nerve stimulation, interferential current, electrical muscle stimulation, ultrasound and thermotherapy. Spine J. 2008;8:226-233.
4. Khadilkar A., Odebiyi D.O., Brosseau L., Wells G.A. Transcutaneous electrical nerve stimulation (TENS) versus placebo for chronic low-back pain. Cochrane database of systematic reviews. 2, 2009.
5. Tugay N., Akbayrak T., Demirtürk F., et al. Effectiveness of transcutaneous electrical nerve stimulation and interferential current in primary dysmenorrhea. Pain Med. 2007;8(4):295-300.
6. Sheffler L.R., Chae J. Neuromuscular electrical stimulation in neurorehabilitation. Muscle Nerve. 2007;35:562-590.
7. Gaines J.M., Metter E.J., Talbot L.A. The effect of neuromuscular electrical stimulation on arthritis knee pain in older adults with osteoarthritis of the knee. Appl Nurs Res. 2004;17(30):201-206.
8. Mysiw W.J., Jackson R.D. Electrical Stimulation. In: Braddom R.L., editor. Physical Medicine and Rehabilitation. 3rd ed. Philadelphia: WB Saunders; 2006:495.
9. Goats G.C. Interferential current therapy. Br J Sports Med. 1990;24(2):87-92.
10. Braccinano A. Transcutaneous Electrical Stimulation. Physical Agent Modalities Theory & Application for the Occupational Therapist, 2nd ed. Thorofare, NJ: Slack Inc. 2008. 219-240
11. Melzack R., Wall P.D. Pain mechanisms: a new theory. Science. 1965;150:971-977.
12. Melzack R., Wall P. The gate control theory of pain. In: Soulairac A., Cahn J., Carpentier J., editors. Pain: Proceedings of the International Symposium on Pain. London: Academic Press, 1968.
13. Jessel T., Kelly D. Pain and analgesia. In: Kandel E., Schwartz J., Jessel T., editors. Priniciples of Neural Science. 3rd ed. New York: Elsevier, 1991.
14. Bonica J. The Management of Pain. Malvern, Pa: Lea & Febiger; 1990.
15. Kandel E., Schwartz J., Jessel T. Principles of neural science. New York: Elsevier; 1991.
16. Allais G., De Lorenzo C., Quirico P.E., et al. Non-pharmacological approaches to chronic headaches: transcutaneous electrical nerve stimulation, laser therapy and acupuncture in transformed migraine treatment. Neurological Sci. 2003;24(2 suppl):S138-S142.
17. Benedetti F. Placebo and endogenous mechanisms of analgesia. Handbook of Experimental Pharmacology. 2007;177:393-413.
18. Bjordal J.M., Johnson M.I., Ljunggreen A.E. Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption. A meta-analysis with assessment of optimal treatment parameters for postoperative pain. Eur J Pain. 2003;7:181-188.
19. Goffaux P., Redmond W.J., Rainville P., Marchand S. Descending analgesia—when the spine echoes what the brain expects. Pain. 2007;130(1-2):137-143.
20. McGaraughty S., Honore P., Wismer C.T., et al. Endogenous opioid mechanisms partially mediate P2X3/P2X2/3-related antinociception in rat models of inflammatory and chemogenic pain but not neuropathic pain. Br J Pharmacol. 2005;146:180-188.
21. Washington L.L., Gibson S.J., Helme R.D. Age-related differences in the endogenous analgesic response to repeated cold water immersion in human volunteers. Pain. 2000;89:89-96.
22. Zhang R.X., Lao L., Wang L., et al. Involvement of opioid receptors in electroacupuncture-produced anti-hyperalgesia in rats with peripheral inflammation. Brain Res. 2004;1020:12-17.
23. Barlas P., Ting S.L., Chesterton L.S., et al. Effects of intensity of electroacupuncture upon experimental pain in healthy human volunteers: a randomized, double-blind, placebo-controlled study. Pain. 2006;122:81-89.
24. Kawakita K., Shinbara H., Imai K., et al. How do acupuncture and moxibustion act? Focusing on the progress in Japanese acupuncture research. J Pharmacol Sci. 2006;100:443-459.
25. Fernánadez-de-Las-Peñas C., Alonso-Blanco C., Cuadrado M.L., Pareja J.A. Myofascial trigger points in the suboccipital muscles in episodic tension-type headache. Man Ther. 2006;11:225-230.
26. Fernándaz-de-Las-Peñas C., Cuadrado M.L., Pareja J.A. Myofascial trigger points, neck mobility and forward head posture in unilateral migraine. Cephalalgia: IntJ Headache. 2006;26:1061-1070.
27. Gam A.N., Warming S., Larsen L.H., et al. Treatment of myofascial trigger-points with ultrasound combined with massage and exercise—a randomised controlled trial. Pain. 1998;77:73-79.
28. Ge H.Y., Fernández-de-las-Peñas C., Arendt-Nielsen L. Sympathetic facilitation of hyperanalgesia evoked from myofascial tender and trigger points in patients with unilateral shoulder pain. Clin Neurophysiol. 2006;117:1545-1550.
29. Moore S.R., Shurman J. Combined neuromuscular electrical stimulation and transcutaneous electrical nerve stimulation for treatment of chronic back pain: a double-blind, repeated measures comparison. Arch Phys Med Rehabil. 1997;78:55-60.
30. Renzenbrink G.J., IJzerman M.J. Percutaneous neuromuscular electrical stimulation (P-NMES) for treating shoulder pain in chronic hemiplegia. Effects on shoulder pain and quality of life. Clin Rehabil. 2004;18:359-365.
31. Yu D.T., Chae J., Walker M.E., et al. Comparing stimulation-induced pain during percutaneous (intramuscular) and transcutaneous neuromuscular electrical stimulation for treating shoulder subluxation in hemiplegia. Arch Phys Med Rehabil. 2001;82:756-760.
32. O’Young B.J., Young M.A., Stiens S.A. Physical Medicine & Rehabilitation Secrets, 3rd ed. Amsterdam: The Netherlands, Mosby; 2007.
33. Dowswell T., Bedwell C., Lavender T., Neilson J.P. Transcutaneous electrical nerve stimulation (TENS) for pain relief in labour. Cochrane Database Sys Rev. 2, 2009.
34. DeSantana J.M., Sluka K.A., Lauretti G.R. High and low frequency TENS reduce postoperative pain intensity after laparoscopic tubal ligation: a randomized controlled trial. Clin J Pain. 2009;25(1):12-19.
35. Robb K., Oxberry S.G., Bennett M.I., et al. A cochrane systematic review of transcutaneous electrical nerve stimulation for cancer pain. J Pain Symptom Manage. 2009;37(4):746-753.
36. Glaser J.A., Baltz M.A., Nietert P.J., Bensen C.V. Electrical muscle stimulation as an adjunct to exercise therapy in the treatment of nonacute low back pain: a randomized trial. J Pain. 2001;2(5):295-300.
37. Cheing G.L., Hui-Chan C.W. Analgesic effects of transcutaneous electrical nerve stimulation and interferential currents on heat pain in healthy subjects. J Rehabil Med. 2003;35:15-19.
38. Reference deleted in proofs
39. Almeida T.F., Roizenblatt S., Benedito-Silva A.A., Tufik S. The effect of combined therapy (ultrasound and interferential current) on pain and sleep in fibromyalgia. Pain. 2003;104:665-672.
40. Kaplan R. Physical Medicine and Rehabilitation Review: Pearls of Wisdom, 2nd ed. London: McGraw-Hill/Appleton & Lange; 2005.