16 EC-IC Bypass Evidence
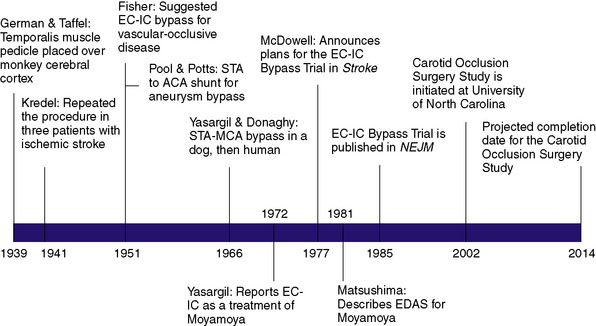
The first description of an operation intended to provide alternative cerebral blood flow (CBF) was given by German and Taffel in 1939, in which a temporalis muscle pedicle was placed over the cerebral cortex in monkeys (Figure 16–1).1 They later subjected both treated and untreated controls to carotid and vertebral occlusion, and reported the monkeys with temporalis pedicles overlying the cortex survived, whereas untreated controls did not. In 1942, Kredel first performed the procedure in three human patients who had suffered ischemic stroke, reporting postoperative clinical improvement in all three patients.2 However, the technique was largely abandoned over the next decade when postoperative angiograms failed to provide evidence of collateral flow through the graft.
C. Miller Fisher shed new light on the concept of re-establishing cerebral perfusion in 1951 when he postulated that extracranial to intracranial (EC-IC) arterial bypass might be performed as a treatment for occlusive vascular disease.3 That same year, Pool and Potts created a superficial temporal artery (STA) to anterior cerebral artery (ACA) shunt during treatment of a giant ACA aneurysm. Although the shunt was successful intraoperatively, postoperative angiogram confirmed the shunt had become occluded.4
The first EC-IC arterial bypass was not described until 1966 when Yasargil and Donaghy successfully performed an STA to middle cerebral artery (MCA) anastomosis in a dog. Shortly thereafter, Yasargil successfully performed the same procedure in a human, providing the neurosurgical community with what was deemed a breakthrough in the treatment of intracranial atherosclerosis. Following Yasargil’s description of the EC-IC arterial bypass, neurosurgeons around the world utilized the procedure with the intent of re-establishing CBF in a variety of cerebrovascular diseases. Surgeons subsequently described bypasses from the occipital and middle meningeal arteries to the MCA, from the occipital artery to the posterior inferior cerebellar artery,5 from the occipital artery to the anterior inferior cerebellar artery (AICA), and from the STA to the superior cerebellar artery.5,6
Over time surgical technique improved, and the most frequently employed bypass procedure became the STA to MCA bypass for the indication of symptomatic internal carotid artery (ICA) or MCA stenosis. However, as was the case with most other surgically treated diseases at the time, there were no extant data to demonstrate that performing the procedure provided patients with any significant short- or long-term benefit. The only early report came in the form of a small retrospective series published by Sundt et al. in 1977, the utility of which was limited by multiple biases. The group reported on a retrospective series of 56 patients who underwent STA-MCA bypass for a wide variety of indications, including TIAs, “orthostatic ischemia,” progressive stroke, and aneurysmal bypass. With regards to graft patency, those who had grafts prior to 1973 had low patency (25%) whereas those operated after 1974 had high patency (95%). The other outcome described was major ischemic stroke or death from the surgery, of which they reported none. The report served more to describe the acceptable safety of the procedure, rather than to examine its efficacy.7
Too often surgical treatment for a particular condition gains enthusiasm and wide use before any clear evidence of effectiveness. This problem has been particularly evident in the field of atherosclerotic vascular disease involving the treatment of its cardiac and cerebral complications. The recent suggestions for extracranial/intracranial arterial anastomosis in the prevention and treatment of stroke have without question a logical basis. This is especially true when atherosclerosis in cerebral vessels lies distal to the surgically accessible portion of the internal carotid artery. Anastomosis of the superficial temporal artery to the middle cerebral artery or one of its branches to improve vertebral circulation in such situations is beginning to be carried out frequently in a number of centers across the United States. In an effort to determine the effectiveness of this therapy 20 major medical centers in the United States and three centers outside the United States have joined together in a collaborative study of this therapy.8
Pending the results of the EC-IC Bypass Study, neurosurgeons published a handful of case reports, series, and commentaries on the appropriate indications, safety, and utility of EC-IC bypass.9–15 In 1983, Chater reported on 400 patients who underwent EC-IC bypass for TIAs and hemodynamic intracranial lesions, with a permanent neurological morbidity rate of approximately 2% and operative mortality of 2.5%, and an ipsilateral postoperative stroke incidence of 0.9% per year after a 55-month follow-up.16 Chater et al. then reported on 105 patients with intracranial ICA stenosis (60% to 98%) and ischemia 1 to 3 months prior to EC-IC bypass. Of these, surgical mortality was 1%, permanent surgical morbidity was 2%, and after 54 months of follow-up, 22 patients had died, of which three were related to stroke; thus the overall late death rate was 4% per year. Although Chater’s reports had begun to examine a more uniform patient population, both of his retrospective series contained no medically treated control group against which the success of surgery could be measured.17
International cooperative study of ec-ic arterial anastomosis
In 1985, nearly 8 years after McDowell’s introduction of the International Cooperative Study of Extracranial-Intracranial Arterial Anastomosis (termed the EC-IC Bypass Trial), the group reported the results of this randomized, prospective trial. Of the 1377 patients enrolled in the trial, 714 (52%) were assigned to medical treatment alone (daily aspirin and aggressive hypertension control) and 663 (48%) were assigned to receive EC-IC bypass in addition to medical therapy. Inclusion and exclusion criteria are presented in Table 16–1.
Table 16–1 Inclusion and Exclusion Criteria for the EC-IC Bypass Trial.
| Inclusion Criteria |
Notably, patients were allowed to enroll in the trial if they had tandem stenosis (two separate stenosis lesions) of the proximal and distal ICA, the proximal lesion being amenable to carotid endarterectomy18 and the distal lesion qualifying for EC-IC bypass. Which surgery was performed first was left to the discretion of the operating surgeons, with the caveat that CEA, if performed first, was to occur at least 30 days prior to randomization in the EC-IC Bypass Trial.
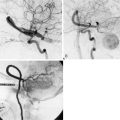
The results of the trial demonstrated that EC-IC bypass provided no significant reduction in major and fatal strokes, ipsilateral strokes, major ipsilateral strokes, or all strokes and deaths combined. Separate analyses in patients with different angiographic lesions did not identify a subgroup with any benefit from surgery. They also reported 30-day surgical mortality and major stroke morbidity rates of 0.6% and 2.5%, respectively. The graft patency rate was reported as 96%, roughly similar to previous series.19
Study Criticism
Furthermore, the bypass study contained no description of patients’ collateral circulation sources into the clinically affected hemisphere. This information was considered critical with the advent of carotid endarterectomy data showing that intraoperative determination of collateral flow by radioactive xenon washout was predictive of subsequent stroke: no infarctions occurred in patients with collateral CBF greater than 40 ml/100 g/min, whereas strokes incidence was 3.4% per year when CBF was less than 25 ml/100 g/min.20 The concept applies to EC-IC bypass in that the ideal candidate would be one with naturally available but insufficient collateral channels, for which the bypass would provide improved blood flow.
Moyamoya Disease: EC-IC Bypass and EDAS
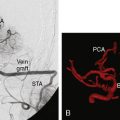
With the advent of angiography, Moyamoya, a disease in children and adults characterized by progressive multiple stenoses of the ICA, MCA, and ACA, was recognized as a physiologic entity distinct from the kind of adult intracranial stenosis heretofore discussed. Moyamoya occlusions result in secondary neovascularization of the lenticulostriate and leptomeningeal circulation, resulting in the characteristic “puff of smoke” appearance of collateral vessels on angiogram. As a consequence of Moyamoya, children typically present with strokes and adults typically present with intracranial hemorrhage related to friable vessels. As bypass procedures emerged, the Moyamoya population was identified as ideal for reconstituting flow for at-risk tissue. Yasargil was the first to report EC-IC bypass for Moyamoya in 1972, and a number of case reports describing STA-MCA bypass followed in the ensuing decades.21–25 The STA-MCA bypass was generally considered the treatment of choice for Moyamoya at the time, though there were a number of patients (children, mostly) whose STA was too small to serve as an adequate pedicle.
In 1981, Matsushima et al. first described the encephaloduroarteriosynangiosis (EDAS), in which the distally intact STA and a strip of galea were mobilized, and simply placed over the cortex and sutured to the dura. The idea of the procedure was to help promote the natural tendency of the disease to develop collateral circulation.26 The procedure was considered simple and safe because the arteries remain intact, requiring no microvascular anastomosis. Matsushima subsequently reported on 50 pediatric and four adult patients, stating that 12-month angiograms showed marked revascularization through the external carotid system.25 Olds and Spetzler also reported early good results with unilateral or bilateral EDAS.27
Although no prospective studies have been performed comparing EDAS to EC-IC bypass or medical treatment, recent case series continue to support its application in the treatment of Moyamoya in both children and adults.28–34 We recently reported long-term outcome in our series of 43 adults treated with EDAS for Moyamoya. Comparing infarct-free survival rates in surgically treated hemispheres and non-surgically treated hemispheres, we found that the 5-year, infarction-free rate was 94% versus 36% in the untreated hemispheres.34
Both indirect and direct revascularization procedures continue to be employed in the treatment of Moyamoya depending on patient characteristics and institutional preference. A large-scale retrospective study was published in Japan in 1997 examining 290 patients with hemorrhagic Moyamoya, including 152 patients who underwent EC-IC bypass and 138 patients who were treated conservatively with medical therapy.35 In the surgical group, 19.1% experienced recurrent hemorrhage, while 28.3% had recurrent hemorrhage in the nonsurgical group. A large prospective trial, the Japan Adult Moyamoya Trial, is now underway in Japan to analyze the efficacy of direct EC-IC bypass for Moyamoya.36
Intracranial Aneurysms, Tumors, and EC-IC Bypass
Aneurysm treatment has been historically linked to carotid occlusion since the first intracranial aneurysm was treated with Hunterian ligation of the internal carotid artery. The publication of the Cooperative Study of Intracranial Aneurysms in 1966 first presented the risk of developing neurological deficits in the event of surgical carotid occlusion: 12% in the treatment of unruptured aneurysms. In both ruptured and unruptured aneurysms, abrupt occlusion of the carotid artery led to neurologic symptoms 59% of the time if the ICA was occluded and 32% if the CCA was occluded.37 With the development of Yasargil’s STA-MCA bypass technique, aneurysm treatment became a clear indication for EC-IC bypass, but its utility quickly decreased as clipping and eventually coiling supplanted parent vessel sacrifice as the major treatment modalities for intracranial aneurysms.
In modern-day neurosurgical practice, EC-IC bypass is an important adjunct when a vessel must be sacrificed due to the presence of a complex aneurysm or skull base tumor. In these cases, practice varies among centers, but often a balloon test occlusion is performed to test for dependency on the vessel to be sacrificed and if neurological symptoms develop, a bypass is performed prior to vessel sacrifice. Given the risks of performing a balloon test occlusion and the possibility that patients might develop ischemic symptoms even if the test is negative, some surgeons prefer to perform an EC-IC bypass in all cases.38 The utility of EC-IC bypass in the treatment of skull base tumors and intracranial aneurysms has been well established and remains an important component of the cerebrovascular neurosurgeon’s armamentarium in the treatment of complex lesions.
Re-Evaluation of EC-IC Bypass for Athero-Occlusive Disease
In the era since the New England Journal of Medicine’s 1985 publication of the EC-IC Bypass Trial results, clinicians have produced scores of critical articles that counter its conclusions or identify study groups in which EC-IC bypass surgery may be effective. Modern criticism elaborates on the responses that followed immediately in the wake of the trial, claiming that although the trial was carried out meticulously, the design itself biased the data against a surgical benefit.39 Continued criticism has been accompanied by more focused clinical trials and a number of prospective clinical studies bolstering support for adapted indications. Finally, the field of neurosurgery has responded by applying new technologies to preoperative hemodynamic evaluation and to the performance of the operation itself.
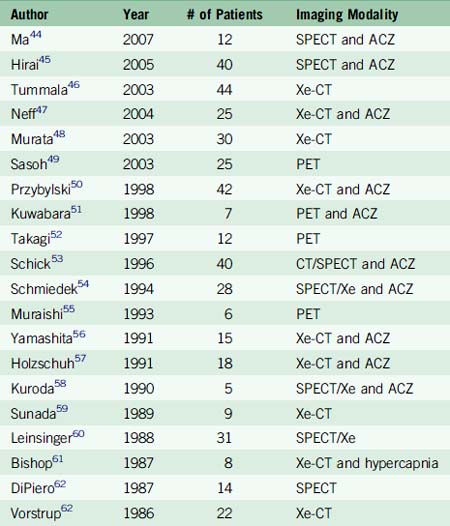
The major criticism of the EC-IC Bypass Trial has been that patient selection biased results toward the null hypothesis by selecting too broad a group of patients, not all for whom bypass would be most effective. In the latter years of the trial and immediately afterward, it became possible to measure cerebral hemodynamic status using physiologic imaging, such as magnetic resonance spectroscopy,40 static xenon inhalation dynamic CT (Xe-CT), or PET in response to acetazolamide (ACZ) or inhaled CO2 to measure CBF and autoregulatory capacity. These major advances permitted surgeons to more precisely select patients who would be likely to benefit from EC-IC bypass and a number of studies have been performed focusing on patients suffering symptomatic hemodynamic failure (Table 16–2).
A recent systematic review of the direct EC-IC bypass literature between 1985 and 2007 identified 22 studies reporting outcomes using the procedure to treat patients with varying stages of cerebral hemodynamic failure.41 A logistic regression model was constructed comparing medically treated and surgically treated patients with intracranial athero-occlusive disease using annual stroke rate as the outcome measure. Patients were stratified by the severity of cerebral hemodynamic compromise evaluated radiographically. Mild stage I hemodynamic failure was defined as decreased vascular reactivity in response to acetazolamide or inhaled CO2. Severe stage I hemodynamic failure was defined as decreased dilatation after inhaled ACZ or CO2, commonly referred to as “steal effect,” which occurs when the arteries are already maximally dilated due to low flow or local ischemia and paradoxically decrease as blood flow is diverted to other dilated vessels. Stage II hemodynamic failure was defined as the phenomenon observed on a PET scan when vessels become maximally dilated and CBF actually declines in response to ACZ or CO2 inhalation. The results demonstrated that surgically treated patients in the selected studies presenting with severe stage I failure significantly benefitted from surgery with an average annual stroke rate of 1.2% versus a stroke rate of 19.3% in the medically treated group. This meta-analysis provides the first evidence of a systematic attempt to compare stratified patient groups to equivalent groups of medically treated patients. These results clearly demonstrate that a modern clinical trial is needed, stratifying patients by the severity of cerebral hemodynamic failure.
A clinical trial, called the Japan EC-IC Bypass Trial (JET), was conducted in Japan to determine whether EC-IC bypass is superior to medical management for ICA and MCA athero-occlusive disease resulting in severe hemodynamic compromise.42 An interim analysis briefly describes the inclusion criteria and results of 206 patients who met the following criteria:
The authors concluded that the surgically treated patients showed a statistically significant decrease in the rate of major stroke, or death, although this data has never been formally presented.43
A second randomized clinical trial, the Carotid Occlusion Surgery Study trial (visit clinicaltrials.gov, NCT00029146), is currently underway with a projected completion date of 2014. This is a multicenter trial with a projected enrollment of 1400 patients. The inclusion and exclusion criteria are listed in Table 16–3. This randomized trial improves on the design of the original EC-IC Bypass Trial in that it precisely examines the cohort of patients for whom this procedures is practically applies, those who have perfusion deficits in the hemisphere ipsilateral to the occlusive lesion. This study also focuses on ICA occlusion following evidence from the original trial and subsequent prospective studies that this procedure may not benefit patients with MCA stenosis or occlusion and may even harm them.19,43
Table 16–3 Inclusion and Exclusion Criteria for the Carotid Occlusion Surgery Study.
| Inclusion Criteria |
1 German W., Taffel M. Surgical Production of Collateral Intracranial Circulation. Proc Soc Ex Biol Med. 1939;42:349-353.
2 Kredel F. Collateral cerebral circulation by muscle graft. Technique of operation with report of 3 cases. South Surg. 1942;10:235-244.
3 Fisher M. Occlusion of the internal carotid artery. AMA Arch Neurol Psychiatry. 1951;65:346-377.
4 Pool J.L., Potts D.G. Aneurysms and Arteriovenous Anomalies of the Brain. New York: Hoeber, 1964.
5 Scamoni C., Dario A., Castelli P., et al. Extracranial-intracranial bypass for giant aneurysms and complex vascular lesions: a clinical series of 10 patients. J Neurosurg Sci. 2008;52:1-9. discussion 9-10
6 Yasargil M.G., editor. Diagnosis and Indications for Operations in Cerebrovascular Occlusive Disease. New York: Academic Press, 1969.
7 Sundt T.M.Jr, Siekert R.G., Piepgras D.G., et al. Bypass surgery for vascular disease of the carotid system. Mayo Clin Proc. 1976;51:677-692.
8 McDowell F.H. The extracranial/intracranial bypass study. Stroke. 1977;8:545.
9 Brambilla G., Paoletti P., Rodriguez y Baena R. Extracranial-intracranial arterial bypass in the treatment of inoperable giant aneurysms of the internal carotid artery. Report of a case. Acta Neurochir (Wien). 1982;60:63-69.
10 Bushe K.A., Bockhorn J. Extracranial-intracranial arterial bypass for giant aneurysms. Acta Neurochir (Wien). 1980;54:107-115.
11 Ditmore Q.M., Watts C., Scharf P. Extracranial-intracranial bypass. a review. Mo Med. 1982;79:272-275.
12 Edwards M.S., Chater N.L., Stanley J.A. Reversal of chronic ocular ischemia by extracranial-intracranial arterial bypass: case report. Neurosurgery. 1980;7:480-483.
13 Olteanu-Nerbe V., Marguth F. Extracranial-intracranial bypass operation in basal tumours. Neurosurg Rev. 1982;5:99-105.
14 Rhodes R.S., Spetzler R.F., Roski R.A. Improved neurologic function after cerebrovascular accident with extracranial-intracranial arterial bypass. Surgery. 1981;90:433-438.
15 Rogers L.A. Selection of patients for extracranial external carotid artery surgery and extracranial-intracranial bypass. Neurosurgery. 1982;11:467-468.
16 Chater N. Neurosurgical extracranial-intracranial bypass for stroke: with 400 cases. Neurol Res. 1983;5:1-9.
17 Weinstein P.R., Rodriguez y Baena R., Chater N.L. Results of extracranial-intracranial arterial bypass for intracranial internal carotid artery stenosis: review of 105 cases. Neurosurgery. 1984;15:787-794.
18 Donadio C., Tramonti G., Garcea G., et al. Azthreonam in the treatment of urinary tract infection: evaluation of efficacy, renal effects and nephrotoxicity. Drugs Exp Clin Res. 1987;13:167-170.
19 Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. The EC/IC Bypass Study Group. N Engl J Med. 1985;313:1191-1200.
20 Whisnant J.P., Sandok B.A., Sundt T.M.Jr. Carotid endarterectomy for unilateral carotid system transient cerebral ischemia. Mayo Clin Proc. 1983;58:171-175.
21 Amine A.R., Moody R.A., Meeks W. Bilateral temporal-middle cerebral artery anastomosis for Moyamoya syndrome. Surg Neurol. 1977;8:3-6.
22 Boone S.C., Sampson D.S. Observations on moyamoya disease: a case treated with superficial temporal–middle cerebral artery anastomosis. Surg Neurol. 1978;9:189-193.
23 Holbach K.H., Wassmann H., Wappenschmidt J. Superficial temporal-middle cerebral artery anastomosis in Moyamoya disease. Acta Neurochir (Wien). 1980;52:27-34.
24 Karasawa J., Kikuchi H., Furuse S., et al. Treatment of moyamoya disease with STA-MCA anastomosis. J Neurosurg. 1978;49:679-688.
25 Matsushima Y., Suzuki R., Yamaguchi T., et al. [Effects of indirect EC/IC bypass operations on adult moyamoya patients]. No Shinkei Geka. 1986;14:1559-1566.
26 Matsushima Y., Fukai N., Tanaka K., et al. A new surgical treatment of moyamoya disease in children: a preliminary report. Surg Neurol. 1981;15:313-320.
27 Olds M.V., Griebel R.W., Hoffman H.J., et al. The surgical treatment of childhood moyamoya disease. J Neurosurg. 1987;66:675-680.
28 Hankinson T.C., Bohman L.E., Heyer G., et al. Surgical treatment of moyamoya syndrome in patients with sickle cell anemia: outcome following encephaloduroarteriosynangiosis. J Neurosurg Pediatr. 2008;1:211-216.
29 Isono M., Ishii K., Kamida T., et al. Long-term outcomes of pediatric moyamoya disease treated by encephalo-duro-arterio-synangiosis. Pediatr Neurosurg. 2002;36:14-21.
30 Kim C.Y., Wang K.C., Kim S.K., et al. Encephaloduroarteriosynangiosis with bifrontal encephalogaleo(periosteal)synangiosis in the pediatric moyamoya disease: the surgical technique and its outcomes. Childs Nerv Syst. 2003;19:316-324.
31 Kim S.K., Wang K.C., Kim I.O., et al. Combined encephaloduroarteriosynangiosis and bifrontal encephalogaleo (periosteal) synangiosis in pediatric moyamoya disease. Neurosurgery. 2008;62:1456-1464.
32 Kim S.K., Wang K.C., Kim I.O., et al. Combined encephaloduroarteriosynangiosis and bifrontal encephalogaleo(periosteal)synangiosis in pediatric moyamoya disease. Neurosurgery. 2002;50:88-96.
33 Matsushima T., Inoue T., Ikezaki K., et al. Multiple combined indirect procedure for the surgical treatment of children with moyamoya disease. A comparison with single indirect anastomosis and direct anastomosis. Neurosurg Focus. 1998;5:e4.
34 Starke R.M., Komotar R.J., Hickman Z.L., et al. Clinical features, surgical treatment, and long-term outcome in adult patients with moyamoya disease. Clinical article. J Neurosurg. 2009;111:936-942.
35 Fujii K., Ikezaki K., Irikura K., et al. The efficacy of bypass surgery for the patients with hemorrhagic moyamoya disease. Clin Neurol Neurosurg. 1997;99(Suppl 2):S194-S195.
36 Miyamoto S. Group JAMT: Study design for a prospective randomized trial of extracranial-intracranial bypass surgery for adults with moyamoya disease and hemorrhagic onset—the Japan Adult Moyamoya Trial Group. Neurol Med Chir (Tokyo). 2004;44:218-219.
37 Nishioka H. Report on the cooperative study of intracranial aneurysms and subarachnoid hemorrhage. Section VII. I. Evaluation of the conservative management of ruptured intracranial aneurysms. J Neurosurg. 1966;25:574-592.
38 Vilela M.D., Newell D.W. Superficial temporal artery to middle cerebral artery bypass: past, present, and future. Neurosurg Focus. 2008;24:E2.
39 Reichman O.H., Origitano T.C., Anderson D.E., et al. Lies, damned lies, and statistics: a neurosurgical perspective on the international randomized trial of extracranial to intracranial arterial bypass surgery. J Stroke Cerebrovasc Dis. 2009;18:389-397.
40 Amin-Hanjani S., Shin J.H., Zhao M., et al. Evaluation of extracranial-intracranial bypass using quantitative magnetic resonance angiography. J Neurosurg. 2007;106:291-298.
41 Garrett M.C., Komotar R.J., Starke R.M., et al. The efficacy of direct extracranial-intracranial bypass in the treatment of symptomatic hemodynamic failure secondary to athero-occlusive disease: a systematic review. Clin Neurol Neurosurg. 2009;111:319-326.
42 Jinnouchi J., Toyoda K., Inoue T., et al. Changes in brain volume 2 years after extracranial-intracranial bypass surgery: A preliminary subanalysis of the Japanese EC-IC trial. Cerebrovasc Dis. 2006;22:177-182.
43 Garrett M.C., Komotar R.J., Merkow M.B., et al. The extracranial-intracranial bypass trial: implications for future investigations. Neurosurg Focus. 2008;24:E4.
44 Ma J., Mehrkens J.H., Holtmannspoetter M., et al. Perfusion MRI before and after acetazolamide administration for assessment of cerebrovascular reserve capacity in patients with symptomatic internal carotid artery (ICA) occlusion: comparison with 99mTc-ECD SPECT. Neuroradiology. 2007;49:317-326.
45 Hirai Y., Fujimoto S., Toyoda K., et al. Superficial temporal artery duplex ultrasonography for improved cerebral hemodynamics after extracranial-intracranial bypass surgery. Cerebrovasc Dis. 2005;20:463-469.
46 Tummala R.P., Chu R.M., Nussbaum E.S. Extracranial-intracranial bypass for symptomatic occlusive cerebrovascular disease not amenable to carotid endarterectomy. Neurosurg Focus. 2003;14:e8.
47 Neff K.W., Horn P., Dinter D., et al. Extracranial-intracranial arterial bypass surgery improves total brain blood supply in selected symptomatic patients with unilateral internal carotid artery occlusion and insufficient collateralization. Neuroradiology. 2004;46:730-737.
48 Murata Y., Katayama Y., Sakatani K., et al. Evaluation of extracranial-intracranial arterial bypass function by using near-infrared spectroscopy. J Neurosurg. 2003;99:304-310.
49 Sasoh M., Ogasawara K., Kuroda K., et al. Effects of EC-IC bypass surgery on cognitive impairment in patients with hemodynamic cerebral ischemia. Surg Neurol. 2003;59:455-460. discussion 460-3
50 Przybylski G.J., Yonas H., Smith H.A. Reduced stroke risk in patients with compromised cerebral blood flow reactivity treated with superficial temporal artery to distal middle cerebral artery bypass surgery. J Stroke Cerebrovasc Dis. 1998;7:302-309.
51 Kuwabara Y., Ichiya Y., Sasaki M., et al. PET evaluation of cerebral hemodynamics in occlusive cerebrovascular disease pre- and postsurgery. J Nucl Med. 1998;39:760-765.
52 Takagi Y., Hashimoto N., Iwama T., et al. Improvement of oxygen metabolic reserve after extracranial-intracranial bypass surgery in patients with severe haemodynamic insufficiency. Acta Neurochir (Wien). 1997;139:52-56. discussion 56-7
53 Schick U., Zimmermann M., Stolke D. Long-term evaluation of EC-IC bypass patency. Acta Neurochir (Wien). 1996;138:938-942. discussion 942-3
54 Schmiedek P., Piepgras A., Leinsinger G., et al. Improvement of cerebrovascular reserve capacity by EC-IC arterial bypass surgery in patients with ICA occlusion and hemodynamic cerebral ischemia. J Neurosurg. 1994;81:236-244.
55 Muraishi K., Kameyama M., Sato K., et al. Cerebral circulatory and metabolic changes following EC/IC bypass surgery in cerebral occlusive diseases. Neurol Res. 1993;15:97-103.
56 Yamashita T., Kashiwagi S., Nakano S., et al. The effect of EC-IC bypass surgery on resting cerebral blood flow and cerebrovascular reserve capacity studied with stable XE-CT and acetazolamide test. Neuroradiology. 1991;33:217-222.
57 Holzschuh M., Brawanski A., Ullrich W., et al. Cerebral blood flow and cerebrovascular reserve 5 years after EC-IC bypass. Neurosurg Rev. 1991;14:275-278.
58 Kuroda S., Takigawa S., Kamiyama H., et al. [Diagnosis of hemodynamic compromise in patients with chronic cerebral ischemia; measurement of cerebral blood volume (CBV) with 99mTc-RBC SPECT]. No Shinkei Geka. 1990;18:259-266.
59 Sunada I. [Measurement of cerebral blood flow by single photon emission computed tomography in cases of internal carotid artery occlusion]. Neurol Med Chir (Tokyo). 1989;29:496-502.
60 Leinsinger G., Schmiedek P., Kreisig T., et al. [133Xe-DSPECT: significance of the cerebrovascular reserve capacity for the diagnosis and therapy of chronic cerebral ischemia]. Nuklearmedizin. 1988;27:127-134.
61 Bishop C.C., Burnand K.G., Brown M., et al. Reduced response of cerebral blood flow to hypercapnia: restoration by extracranial-intracranial bypass. Br J Surg. 1987;74:802-804.
62 Vorstrup S., Brun B., Lassen N.A. Evaluation of the cerebral vasodilatory capacity by the acetazolamide test before EC-IC bypass surgery in patients with occlusion of the internal carotid artery. Stroke. 1986;17:1291-1298.