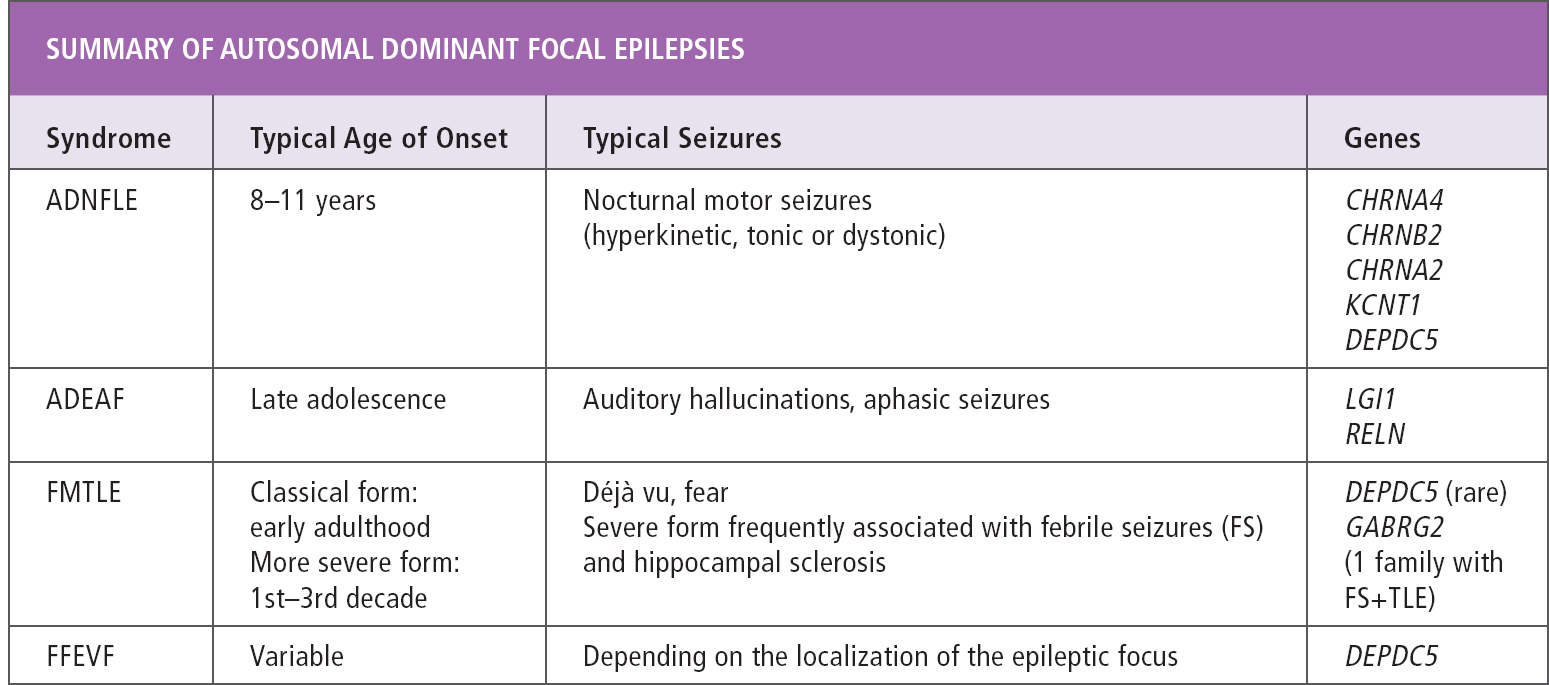
The familial focal epilepsies with onset in childhood or adolescence are classified according to the focus of seizure onset in different members of the same family. Seizure onset can be uniform in all affected individuals in a family, as in autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE), autosomal dominant epilepsy with auditory features (ADEAF), and familial mesial temporal lobe epilepsy (FMTLE). Alternatively, different types of focal epilepsy can co-occur in the same family, as in familial focal epilepsy with variable foci (FFEVF). A diagnosis of any of these syndromes can only be made in the presence of a family history of focal epilepsy, as individual affected family members do not differ clinically from patients with sporadic focal epilepsies. During the past few years, a genetic cause has been identified for many of these syndromes, and a thorough familial history taking should be part of every workup of patients with focal epilepsy (Table 16.1).
AUTOSOMAL DOMINANT NOCTURNAL FRONTAL LOBE EPILEPSY
Clinical Manifestations
Autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE) was the first familial focal epilepsy syndrome described, and the first epilepsy syndrome for which a genetic cause was identified (1,2). Within families, the epilepsy follows autosomal dominant inheritance with a clinical penetrance of about 70%, and the severity of the epilepsy varies greatly among the different affected members of a family (1,3,4). The core seizure phenotype consists of clusters of motor seizures arising from sleep, with a mean age at seizure onset between 8 and 11.5 years (1,3,5). Patients have a mean of eight seizures per night that often cluster over 1 or 2 hours. A nonspecific aura can precede the motor seizure, such as a somatosensory aura with tingling, for example, in the back or in the head. A sensation of being out of breath often accompanies the seizures (6). The ictal motor component includes hyperkinetic (frantic movements of bipedal activity, pelvic thrashing), tonic, or dystonic (sudden, abruptly changing dystonic puppet-like postures) elements. Seizures are of short duration, usually lasting less than 1 minute (1). Awareness is most often retained throughout the seizure. Because of the sometimes bizarre seizure semiology, a misdiagnosis of parasomnias, night terrors, or hysteria is frequent before the diagnosis of epilepsy is finally made (7).
Sleep deprivation (30% of the patients) and stress (30%) are possible provocative factors. Diurnal seizures may be observed in the most severe cases, usually during periods of poor seizure control. Rare secondarily generalized seizures are reported in nearly half of the patients, occurring at the onset of epilepsy or during the course of the disease.
Neurological clinical examination is normal. However, a high incidence of behavioral disorders, such as irritability, aggressiveness, and impulsive behavior, has been reported during adolescence (3), and personality disorders and/or depression can be present during adulthood. Neuropsychological disturbances may occur, but are most often subtle, with features of frontal and sometimes even temporal lobe dysfunction (8). A few reported families present with a more severe phenotype: psychosis and other psychiatric disorders were reported in several individuals from a Norwegian family (9) and in two other families with a severe ADNFLE phenotype; status epilepticus was present in 24% of the patients; behavioral and psychiatric disorders were reported in half of the patients; and intellectual disability was seen in 25% (10). In nearly half of the subjects from four families with ADNFLE, mild intellectual disability was present, showing that cognitive dysfunction is an integral part of the phenotype of ADNFLE (8). As discussed in this chapter, the severity of the epilepsy and associated neuropsychiatric features seem to be at least partly related to the underlying gene mutations.
TABLE 16.1
EEG and Neuroimaging
Interictal EEG is normal, or may show nonspecific focal epileptiform abnormalities in frontotemporal anterior regions, often only visible during sleep recording (1,3,5). Scalp ictal EEG is noncontributive in about half of the patients (4), and movement artifacts often obscure interpretation. Seizures occur during non-rapid eye movement (non-REM) sleep, mainly from stage 2, and start after a transition from sleep to an arousal (6). By using ictal video-EEG recordings and functional neuroimaging, Hayman et al showed different regions of onset within the frontal lobe in different family members, concordant with the differences in ictal symptomatology in individuals from the same family (11).
Magnetic resonance imaging (MRI) studies were classically considered normal in all patients; however, recent reports have described focal cortical dysplasias in families with DEPDC5 mutations (see following text) (12,13). A hypometabolism was observed in a few regions, including the right anterior orbitofrontal cortex, in a fluorodeoxyglucose (FDG)-positron emission tomography (PET) study of several ADNFLE patients (14).
Treatment and Evolution
The seizure semiology in a given individual is very stereotyped throughout life, except for some cases with seizure onset in early childhood, where seizures may evolve from tonic attacks to classical NFLE seizures including dystonic or hyperkinetic components (4). In many patients, seizures persist for years, but then tend to disappear with age, particularly around the fourth or fifth decade, without relapse after cessation of drug therapy (3). Carbamazepine is the most effective antiepileptic medication in this syndrome. It leads to a complete suppression of seizures in about 70% of patients. Interestingly, low dosages of carbamazepine (around 600 mg/day in adults) are usually sufficient, which may indicate pathophysiological mechanisms that are different from those of other epilepsies (15). However, drug resistance to carbamazepine and other antiepileptic drugs is observed in about 30% of patients.
Molecular Genetics
Mutations in genes coding for two different subunits of the neuronal acetylcholine nicotinic receptors (nAChRs), namely CHRNA4 and CHRNB2, have been identified in 10% to 15% of the ADNFLE families (4,16–18). A mutation in CHRNA2 was identified in two Italian families with ADNFLE (19,20). The nAChRs are ligand-gated ion channel receptors. Being the first epilepsy genes to be identified, they contributed to the concept of epilepsy being a channelopathy. Nearly all reported nAChR mutations are missense mutations. A study analyzing the clinical features of 19 ADNFLE families with a total of 150 patients showed that some nicotinic receptor mutations, like the Ser284Leu mutation in CHRNA4, appear to be associated with an increased risk for major psychiatric or neuropsychological symptoms such as mental retardation, schizophrenia-like symptoms, or marked specific cognitive deficits, but the risk is low for most other mutations (21).
More recently, in a small percentage of ADNFLE families, mutations have been identified in KCNT1, which encodes a Na+-gated K+ channel (22), and in DEPDC5, a gene encoding DEP (Disheveled, Egl-10 and Pleckstrin) domain containing protein 5 (23,24).
Mutations in KCNT1 were identified in four families with severe forms of ADNFLE. They had an earlier mean age at onset of 6 years, compared to 10 years in the classical form of ADNFLE, and had more frequent intellectual disability, behavioral, or psychiatric problems (22). Remarkably, in the same journal issue, mutations in KCNT1 were described in patients with the seemingly unrelated and much more severe syndrome epilepsy of infancy with migrating focal seizures (EIMFS), previously called malignant migrating partial seizures of infancy (25). Since then, KCNT1 mutations seem to have given rise to a whole spectrum of focal or multifocal epilepsies with different degrees of intellectual impairment (26). Although functional studies support some degree of genotype–phenotype correlation, a few mutations leading to both ADNFLE and EIMFS have been described (26,27). There is preliminary evidence for a positive effect of the antiarrhythmic drug quinidine, a potassium channel blocker, in epilepsies due to KCNT1 mutations (28,29).
Recent studies have further reported DEPDC5 mutations in different familial focal epilepsy syndromes, in particular, familial focal epilepsy with variable foci (FFEVF), but several smaller families with ADNFLE or familial temporal lobe epilepsy (FTLE) have been described too (13,23,24,30). It is possible that the families with ADNFLE or FTLE, in whom DEPDC5 mutations were found, were too small to identify the variable foci within the family, and the subsequent finding of additional affected members might allow us to correct the classification of the epilepsy syndrome. In general, families with ADNFLE and DEPCD5 mutations have a higher rate of drug resistance compared to the classical ADNFLE (24). DEPDC5 is an upstream negative regulator of mammalian target of rapamycin mTOR complex 1 (mTORC1) (12,31). Other proteins of the same mTORC1 system are involved in tuberous sclerosis (32). Interestingly, MRI abnormalities, in particular focal cortical dysplasias, were described in some patients with familial focal epilepsy and DEPDC5 mutations, with a preferential location in the frontal lobe or in the insula (12,13). Some of these drug-resistant patients benefited from surgical treatment, and had a good long-term outcome (13).
Finally, a few mutations have also been identified in the corticotropin-releasing hormone (CRH) gene (33,34). However, functional studies have shown contradictory results, consisting of both increased and diminished levels of CRH expression, and more evidence is needed to securely implicate the gene in the etiology of ADNFLE.
Pathophysiology
ADNFLE Caused by nAChR Mutations
The nAChRs are ligand-gated ion channels permeable for cations (Na+, K+, Ca2+). The endogenous ligand is acetylcholine (ACh), whereas nicotine is an exogenous ligand. The nAChRs are pentameric receptors, and they occur in different functional subunit combinations. The most widely expressed nAChR in the human brain is the α4β2 nAChR. Most of the α4β2 nAChRs are presynaptic and have a neuromodulatory role: They enhance the release of the neurotransmitter present in the neurons on which they are located (GABA, glutamate, dopamine, norepinephrine, serotonin, or ACh). Other nAChRs are postsynaptic and mediate fast excitatory synaptic transmission. The nAChRs are involved in brain development and plasticity and participate in many different brain functions, including modulation of sensory inputs, sleep, mood, attention, reward, and learning (35). As for their role in ADNFLE, electrophysiological studies of ADNFLE mutations tend to show a gain of function of the mutant receptor (36), and mouse models point toward alterations in the control of GABAergic transmission in the prefrontal cortex. The precise mechanisms through which mutations in a ubiquitously expressed gene lead to a frontal lobe nocturnal epilepsy are, however, still little understood (16).
ADNFLE Caused by KCNT1 Mutations
The KCNT1 or slack channel, is a sodium-gated potassium channel; its presence in the central nervous system is ubiquitous, but its location in the cortex is most prominent in the frontal lobe, according to a study performed in rodents (37). In vitro functional studies showed an increased current of the mutant KCNT1 channels (27). This gain of function was generally greater for mutations identified in EIMFS than in ADNFLE. Many mutations are located in the large cytoplasmic C-terminal domain of KCNT1, which interacts with a large network of proteins, among which is the FMRP protein. Loss of FMRP is the cause of Fragile X mental retardation, and the disturbance of the interaction between KCNT1 and FMRP might possibly play a role in the frequent occurrence of cognitive impairment in KCNT1-related pathology (38).
DEPDC5-Related ADNFLE
See paragraph on FFEVF.
FAMILIAL TEMPORAL LOBE EPILEPSIES: ADEAF AND FMTLE
Temporal lobe epilepsy is the most common focal epilepsy type in adolescents and adults. Many patients have underlying congenital or acquired brain lesions, and the entity is, therefore, often considered a typical lesional epilepsy. Nevertheless, through the years several familial cases have been described, and a genetic cause has been identified for a substantial subset of them. Based on seizure semiology, two types of familial temporal lobe epilepsies can be distinguished: a lateral form with typically auditory or dysphasic features (autosomal dominant epilepsy with auditory features, ADEAF) and a mesial form with prominent psychic and vegetative symptomatology (familial mesial temporal lobe epilepsy, FMTLE). Whereas the mesial form of familial TLE encompasses a heterogeneous group of epilepsies, familial lateral temporal lobe epilepsy is clinically and genetically a much more homogeneous entity.
AUTOSOMAL DOMINANT EPILEPSY WITH AUDITORY FEATURES (ADEAF)
Clinical Manifestations
Autosomal dominant epilepsy with auditory features (ADEAF, previously called familial lateral temporal lobe epilepsy, or autosomal dominant partial epilepsy with auditory features, or ADPEAF) is a familial focal epilepsy syndrome characterized by seizures with auditory symptoms and/or receptive aphasia. The diagnosis of epilepsy might be overlooked in some family members due to the sometimes subtle symptomatology. Careful clinical history taking is therefore crucial.
Seizure onset is mostly in adolescence, but onset at any age between 4 years and 50 years has been described (39,40). Affected individuals have focal seizures with or without disturbance of consciousness. Most patients also have secondarily generalized tonic–clonic seizures, but these are rare, and often nocturnal (41). Auras most frequently consist of simple auditory symptoms as humming, buzzing, or ringing. More complex symptoms, such as distortion of sounds, volume changes, or auditory hallucinations, are also seen. A subset of patients has focal seizures consisting of ictal receptive aphasia, during which they lose the ability to understand spoken language despite conserved consciousness (40,42,43). Reflex seizures due to sudden noise, like a ringing telephone, have been described in several families (41). The characteristic seizure semiology of the syndrome is thought to reflect seizure onset in the lateral temporal lobe; hence also the name “autosomal dominant lateral temporal lobe epilepsy (ADLTLE),” which can be found throughout the literature. Although the presence of auditory or aphasic seizures is mandatory for the diagnosis of this syndrome, other seizure types with sensory (eg, visual, olfactory, cephalic) auras and motor or autonomic symptoms can co-occur (42,44,45). They are nevertheless much rarer.
Seizures are usually infrequent and well controlled with any antiepileptic drug used for focal seizures. Rare families, including family members with therapy-resistant seizures, have been described, though, and two family members of a large Norwegian family with predominantly aphasic seizures died suddenly during sleep (40,46). Affected individuals do not show other abnormalities besides seizures, and neurological exam is normal.
EEG, Neuroimaging, and Other Investigations
Interictal EEG is most often normal; however, focal epileptic discharges in the temporal lobe are present in one-third of patients (40,42,47). Ictal EEGs have been described in a few patients, and showed ictal onset in the mid-temporal, anterior temporal, or fronto-temporal region (40,46,47). Standard MRI of the brain is normal. One study showed aspecific malformations of the temporal lobe, such as volumetric enlargement, but this has not been confirmed in further studies (48,49). However, several functional studies showed evidence for subtle abnormalities of the lateral temporal lobe network and of language processing: on functional MRI with an auditory description decision task, individuals with ADEAF had significantly less activation than controls, and magnetoencephalography with auditory stimuli showed a significantly delayed peak 2 auditory evoked field latency in affected individuals (49). Furthermore, in the Norwegian family with predominant aphasic seizures, a strong asymmetry of long-latency auditory evoked potentials with reduced left N1-P2 amplitudes was shown (50), and abnormal phonologic processing was demonstrated in a Sardinian family by means of a fused dichotic listening task (51).
Molecular Genetics
Following the description of three families with linkage to chromosome 10q22, two groups independently described mutations in the gene LGI1 (leucin rich, glioma inactivated 1) as a cause of ADEAF (45,52). LGI1 is a secreted protein, and it was the first non-ion channel gene linked to epilepsy. Subsequent studies have shown LGI1 mutations to be present in 33% to 50% of familial cases, and 2% of sporadic cases with lateral temporal lobe epilepsy (41,42,53). Penetrance is reduced, and is thought to be around 67% (54). There seems to be no major phenotypic differences between families with or without mutations, although families with mutations tend to have more family members with auditory symptoms and fewer with autonomic symptoms (42). Truncating mutations, rare whole gene deletions, and missense mutations have all been described, suggesting a loss of function mechanism for LGI1-related epilepsy. In line with this mechanism, most examined missense mutations have been shown to lead to a loss of secretion of the protein, with accumulation in the endoplasmatic reticulum and Golgi apparatus before their premature degradation (55–57).
Interestingly, LGI1 is also a target for autoantibodies in a form of acquired autoimmune limbic encephalitis, an adult-onset neurological disorder including cognitive impairment and epilepsy, with typically faciobrachial dystonic seizures (58).
For years, LGI1 was the only known gene linked to ADEAF. More recently, however, heterozygous missense mutations in the gene RELN (reelin), encoding another secreted protein, have been described in 7 of 40 (17.5%) Italian ADEAF families (59). Many clinically unaffected mutation carriers were present, suggesting a relatively low penetrance rate of 60%. Reelin is also secreted by the liver, and serum levels of RELN were shown to be reduced in patients with RELN mutations. Recessive loss-of-function mutations in this gene were already known to be a cause of lissencephaly with cerebellar hypoplasia (LCH), a severe disorder with cerebral migration defects, intellectual disability, and seizures (60). Epilepsy has not been reported in the heterozygous mutation carriers in the few LCH families described to date, but this can be compatible with the significantly reduced penetrance seen in ADEAF families.
Pathophysiology
ADEAF Due to LGI1 Mutations
LGI1 is a primarily neuron-expressed protein, consisting of a N terminal signal peptide defining it as a secreted protein, a leucine-rich repeat domain, and another C-terminal repeat region called the epitempin repeat (61,62). The protein derives its name from the fact that it was initially found to be downregulated in high-grade gliomas, and tumor expression studies suggest a role for LGI1 in reducing tumor invasion (63). Families with LGI1 mutations, however, do not have an excess of gliomas or other brain tumors. Little was known about the function of the protein at the time mutations in families with ADEAF were identified, but cellular and animal model studies have greatly contributed to our understanding of the function of the protein since then. First of all, copurification studies have identified the main binding partners of LGI1 as the presynaptic voltage-gated potassium channel Kv.1.1, the presynaptic transmembrane proteins ADAM11 and ADAM23, and the postsynaptic protein ADAM22 (64–66). Most likely, all together they form a trans-synaptic multiprotein complex (67). The nature of LGI1’s binding partners supports a role of LGI1 in regulation of synaptic transmission. The Kv1.1 channel is predominantly localized to the axons and nerve terminals, and controls the neuronal firing rate and neurotransmission through its hyperpolarizing action. LGI1 has been shown to slow the Kvβ1 subunit-mediated inactivation of the Kv1.1 subunit (64). More extensively studied is LGI1’s interaction with ADAM22, a postsynaptic protein that itself binds to the postsynaptic scaffolding protein PSD-95. Through its interaction with this complex, LGI1 may increase the number of AMPA receptors, thus increasing glutamatergic neurotransmission (65). Besides its role in synaptic transmission, LGI1 also seems to be involved in neurodevelopment, as it was shown to stimulate axonal outgrowth and to play a role in maturation and pruning of glutamatergic neurons (68–70).
Several rodent models have further confirmed the role of LGI1 haploinsufficiency in the pathogenesis of epilepsy. Homozygous knock-out animals have early-onset frequent spontaneous seizures and die after 2 to 3 weeks. Heterozygous knock-out animals, which more closely recapitulate the human genotype, have a normal life span and do not have spontaneous seizures, but show an increased susceptibility to sound-induced and PTZ-induced seizures (65,71–73). A conditional knock-out mouse model underlined the key role of glutamatergic neurotransmission in LGI1-related epilepsy, by showing that deletion of LGI1 in cortical glutamatergic neurons alone is sufficient to reproduce the epileptic phenotype, while deletion in GABAergic interneurons did not change the seizure threshold (74). In summary, while it is clear that LGI1 loss of function leads to seizures, the precise molecular processes through which this occurs remain unclear. More specifically, its differential role during different stages of life and its predilection for lateral temporal lobe pathology remain topics for further study.
ADEAF Due to RELN Mutations
Reelin is a very large (3460 amino acids) secreted glycoprotein, mainly expressed in the brain and liver. The protein gets its name from the identification of homozygous deletions of its encoding gene in the reeler mouse, which has tremor, ataxia, and neuronal migration defects (75). Prenatally, reelin is secreted by the Cajal Retzius cells in the marginal zone of the developmental cortex and by granule cells in the cerebellum. In the postnatal brain it is mainly expressed in GABAergic interneurons (76). Reelin is known to have an important role in the correct cortical lamination during embryogenesis and in postnatal modulation of synaptic plasticity (77,78). It is thought to act by binding to apolipoprotein E receptor 2 (ApoE2) and very low-density lipoprotein receptor (VLDLR), which, in turn, leads to phosphorylation of the intracellular adaptor protein disabled homolog 1 (DAB1) (79). Interestingly, after the recent discovery of mutations in RELN in families with ADEAF, colocalization of reelin and LGI1 was shown in neurons of the hippocampus and temporal lobe cortex of immature and mature rats (59). These findings suggest a functional interplay between both proteins, and the understanding of this common pathway might help to elucidate the molecular biology underlying ADEAF.
FAMILIAL MESIAL TEMPORAL LOBE EPILEPSY (FMTLE)
Clinical Manifestations and Investigations
Whereas mesial temporal lobe epilepsies are generally thought of as lesional, and thus acquired, epilepsies, twin studies and family studies have made it clear that a genetic etiology exists in a subset of patients (80). Two subgroups of FMTLE have been described. A first subgroup is considered a relatively benign syndrome, consisting of infrequent, typically well-controlled mesial temporal lobe seizures with onset in adolescence or early adulthood (81–84). MRI of the brain is normal and the incidence of FS in these families is not different from the general population (2.5%) (85). Seizures have prominent psychic and autonomic ictal symptomatology, especially déjà vu, fear, dream-like states, and nausea. Secondarily generalized seizures can occur, but are rare. Interictal EEG is most often normal. There is no impact of seizures on learning or memory. Symptoms in some family members might be so mild that the differential diagnosis between epileptic auras (such as déjà vu) and normal experiental phenomena can be difficult.
A clinically more severe and more heterogeneous subgroup of FMTLE has subsequently been described, with an earlier age of onset (first to third decade, mean 10 years), a higher frequency of hippocampal sclerosis (HS) (30%–50% of family members) and history of FS, and frequent drug resistance (48,86). Intra- and interfamilial variability is high, and family members with hippocampal sclerosis without seizures have been identified, suggesting that the genetic background rather than seizure severity plays a role in the genesis of hippocampal sclerosis in these families. Interictal EEGs often show temporal discharges. A study on postoperative outcome in refractory patients with FMTLE and hippocampal sclerosis showed a favorable outcome, comparable to what is seen in nonfamilial cases, provided that hippocampal abnormalities were clearly unilateral or asymmetric (48).
Finally, it should be noted that a few families have been described with both FS and temporal lobe seizures, but without hippocampal abnormalities (87,88). These families are now considered to be part of the GEFS+ spectrum, a syndrome initially termed generalized epilepsy with febrile seizures plus, but later renamed genetic epilepsy with febrile seizures plus.
Molecular Genetics
Linkage analysis in large FMTLE families identified several candidate loci, including a locus on chromosome 4q13.2-21.3 in a four-generation family with pure benign FMTLE without HS or antecedent FS (89), on chromosome 5q31.3-32 for presumed temporal lobe epilepsy without HS (90), on chromosome 12q22-q23.3 and chromosome 3q25-q26 for FMTLE with antecedent FS (88,91), and on 18p11.31 in FMTLE with hippocampal abnormalities (92). However, responsible genes have not been identified in any of these larger families so far. One exception is a large family with TLE + FS without hippocampal sclerosis, in which a truncating mutation in GABRG2 was found, a gene usually causing FS + generalized epilepsy (57). In clinical practice, the majority of families are generally small, consisting of a handful of affected family members only. Some of these families (2% of an Italian cohort) might have a mutation in the gene DEPDC5 (see FFEVF) (23,93). A large study of 20 families with FMTLE concluded that most smaller families have a complex (either polygenic or multifactorial) inheritance, though similar to what is seen in idiopathic generalized epilepsy (94).
FAMILIAL FOCAL EPILEPSY WITH VARIABLE FOCI (FFEVF)
Clinical Manifestations
Familial focal epilepsy with variable foci (FFEVF) refers to an inherited epilepsy syndrome with seizures arising from different cortical regions among different family members, but with each individual in the family having a single focal seizure type (95). FFEVF is therefore a diagnosis at the family level, not at the level of the single individual. Consequently, the diagnosis of FFEVF can remain uncertain in small pedigrees with too few affected individuals to establish the existence of variable foci.
In the FFEVF spectrum, age of seizure onset ranges from infancy to adult, and may vary considerably within families, as do seizure frequency and drug response. In general, seizures are nevertheless well controlled. Seizure semiology is heterogeneous and varies according to the location of the epileptic focus. Frontal and temporal lobe seizures are the most frequent seizure type, but centroparietal and occipital seizures are also seen. Especially in smaller families, it is sometimes difficult to make the differential diagnosis of FFEVF rather than one of the other focal epileptic syndromes, in particular ADNFLE (3,96–98). In FFEVF, frontal lobe seizures occur predominantly during wakefulness and are less frequent, clusters are rare, and there is a higher prevalence of secondarily generalized seizures and interictal abnormalities. Temporal lobe seizures are typically of mesial origin. Whereas development is generally normal, a small subset of affected individuals has been reported to have intellectual disability, autistic features, and psychiatric disorders or behavioral problems (95,97,99).
EEG, Neuroimaging, and Other Investigations
Interictal EEG shows focal abnormalities that are constant over time in each affected family member, but vary among family members. Conventional structural brain MRI studies are usually unremarkable, but some family members with structural malformations, including focal cortical dysplasia, have been reported (12,13).
Molecular Genetics
FFEVF is transmitted following an autosomal dominant inheritance pattern with a low penetrance (50% to 70%). Despite a great phenotypic heterogeneity, several families of French-Canadian, Dutch, Spanish, and Australian origin presented genetic homogeneity and mapped to chromosome 22q12 (96–100). In 2013, the 22q12 gene was discovered, when mutations were identified in DEPDC5 (Dishevelled, Egl-10 and Pleckstrin (DEP) domain-containing protein 5) in seven of eight large families with FFEVF (30). In a cohort of 19 European families, DEPDC5 mutations were found in 37% (6 out of 16) of the families with focal epilepsies, including 3 FFEVF families, 1 ADNFLE family and 2 TLE families (23). Overall, DEPDC5 mutations were found in 5% (4 of 79) of familial and sporadic focal epilepsy cases in a French-Canadian cohort (101), in 13% (4 of 30) of a series of small European ADNFLE families (24), 12% (10 of 82) of small families with focal epilepsies (30), and some rare patients with Rolandic epilepsy (3 of 207) and unclassified focal childhood epilepsy (3 of 82) (102).
The phenotype spectrum of DEPDC5 mutations was further extended with the identification of germline mutations in patients with focal cortical dysplasia (FCD I and IIa), one patient with focal band heterotopia, and one case of hemimegalencephaly (12,13,103,104). Interestingly, most of these patients were part of larger FFEVF families, in whom many affected family members had a normal MRI. In a French FFEVF family with a germline DEPDC5 mutation, one affected family member with FCD also carried an additional somatic DEPDC5 mutation in the resected brain tissue after epilepsy surgery, suggesting that focal cortical dysplasia is caused by second-hit mutations (13). Thus, DEPDC5 mutations are associated with diverse focal epileptic phenotypes, ranging from apparently nonlesional focal epilepsies to malformation-associated focal epileptic syndromes.
With nearly 40 mutations reported, DEPDC5 is to date the most common genetic cause across the whole spectrum of focal epilepsy syndromes. Penetrance of mutations is reduced, and is as low as 60% in some families. Half of DEPDC5 mutations are nonsense; 20% of them are missense, located throughout the gene, with the remainder being frameshift or splice mutations. Several nonsense mutations were proven to be targeted by nonsense-mediated mRNA decay (NMD), leading to transcript degradation (23,24). Loss of function is therefore the most likely general mechanism underlying DEPDC5-related epilepsy.
Pathophysiology
DEPDC5 encodes a highly conserved protein of 1,603 amino acids with no homology to ion channels or membrane receptor proteins. In vitro, DEPDC5 was shown to be part of the GATOR1 complex (together with the proteins NPRL2 and NPRL3), which inhibits the mTORC1 pathway (complex 1 of mTOR) in mammalian cells (31) and in yeast (105). The mTORC1 signaling pathway regulates many major cellular processes, including cell proliferation, growth, survival, and protein synthesis. The role of DEPDC5 in the mTORC1 signaling cascade is particularly interesting because mutations in other inhibitors of mTORC1 (TSC1, TSC2, PTEN, STRADA) are known to increase mTORC1 activity and lead to malformations of cortical development and intractable seizures in tuberosis sclerosis, hemimegalencephaly, Cowden syndrome, and Pretzel syndrome (32). Additionally, enhanced constitutive mTORC1 activation is seen in balloon cells found in FCD type IIb (106). A recent study performed in vitro functional assessment of several disease-causing DEPDC5 mutations and found that only three of seven variants identified in familial focal epilepsy syndromes clearly disrupted the DEPDC5-dependent inhibition of mTORC1 (107). In summary, the mechanisms underlying DEPDC5-related epilepsy remain to be elucidated, and further functional studies are needed to demonstrate that seizures due to loss of DEPDC5 are indeed caused by mTORC1 upregulation. In addition, the role of rapamycin, an mTOR inhibitor, in the treatment of these milder epilepsy syndromes requires further investigation.
REFERENCES
1. Scheffer IE, Bhatia KP, Lopes-Cendes I, et al. Autosomal dominant nocturnal frontal lobe epilepsy: a distinctive clinical disorder. Brain. 1995;118(Pt 1):61–73.
2. Steinlein OK, Mulley JC, Propping P, et al. A missense mutation in the neuronal nicotinic acetylcholine receptor alpha 4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet. 1995;11(2):201–203.
3. Picard F, Baulac S, Kahane P, et al. Dominant partial epilepsies: a clinical, electrophysiological and genetic study of 19 European families. Brain. 2000;123(Pt 6):1247–1262.
4. Picard F, Scheffer IE. Genetically determined focal epilepsies. In: Bureau M, Genton P, Dravet C, eds. Epileptic Syndromes in Infancy, Childhood and Adolescence. 5th ed. Vol 12. Paris: John Libbey Eurotext; 2012:600.
5. Oldani A, Zucconi M, Asselta R, et al. Autosomal dominant nocturnal frontal lobe epilepsy: a video-polysomnographic and genetic appraisal of 40 patients and delineation of the epileptic syndrome. Brain. 1998;121(Pt 2):205–223.
6. Picard F, Brodtkorb E. Familial frontal lobe epilepsies. In: Engel J, Pedley TA, eds. Epilepsy: A Comprehensive Textbook, Vol. 3. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:2495–2502.
7. Scheffer IE, Bhatia KP, Lopes-Cendes I, et al. Autosomal dominant frontal epilepsy misdiagnosed as sleep disorder. Lancet. 1994;343(8896):515–517.
8. Picard F, Pegna AJ, Arntsberg V, et al. Neuropsychological disturbances in frontal lobe epilepsy due to mutated nicotinic receptors. Epilepsy Behav. 2009;14(2):354–359.
9. Magnusson A, Stordal E, Brodtkorb E, Steinlein O. Schizophrenia, psychotic illness and other psychiatric symptoms in families with autosomal dominant nocturnal frontal lobe epilepsy caused by different mutations. Psychiatr Genet. 2003;13(2):91–95.
10. Derry CP, Heron SE, Phillips F, et al. Severe autosomal dominant nocturnal frontal lobe epilepsy associated with psychiatric disorders and intellectual disability. Epilepsia. 2008;49(12):2125–2129.
11. Hayman M, Scheffer IE, Chinvarun Y, Berlangieri SU, Berkovic SF. Autosomal dominant nocturnal frontal lobe epilepsy: demonstration of focal frontal onset and intrafamilial variation. Neurology. 1997;49(4):969–975.
12. Scheffer IE, Heron SE, Regan BM, et al. Mutations in mammalian target of rapamycin regulator DEPDC5 cause focal epilepsy with brain malformations. Ann Neurol. 2014;75(5):782–787.
13. Baulac S, Ishida S, Marsan E, et al. Familial focal epilepsy with focal cortical dysplasia due to DEPDC5 mutations. Ann Neurol. 2015;77(4):675–683.
14. Picard F, Bruel D, Servent D, et al. Alteration of the in vivo nicotinic receptor density in ADNFLE patients: a PET study. Brain. 2006;129(Pt 8):2047–2060.
15. Picard F, Bertrand S, Steinlein OK, Bertrand D. Mutated nicotinic receptors responsible for autosomal dominant nocturnal frontal lobe epilepsy are more sensitive to carbamazepine. Epilepsia. 1999;40(9):1198–1209.
16. Becchetti A, Aracri P, Meneghini S, et al. The role of nicotinic acetylcholine receptors in autosomal dominant nocturnal frontal lobe epilepsy. Front Physiol. 2015;6:22.
17. di Corcia G, Blasetti A, De Simone M, et al. Recent advances on autosomal dominant nocturnal frontal lobe epilepsy: “understanding the nicotinic acetylcholine receptor (nAChR).” Eur J Paediatr Neurol. 2005;9(2):59–66.
18. Ferini-Strambi L, Sansoni V, Combi R. Nocturnal frontal lobe epilepsy and the acetylcholine receptor. Neurologist. 2012;18(6):343–349.
19. Conti V, Aracri P, Chiti L, et al. Nocturnal frontal lobe epilepsy with paroxysmal arousals due to CHRNA2 loss of function. Neurology. 2015;84(15):1520–1528.
20. Aridon P, Marini C, Di Resta C, et al. Increased sensitivity of the neuronal nicotinic receptor alpha 2 subunit causes familial epilepsy with nocturnal wandering and ictal fear. Am J Hum Genet. 2006;79(2):342–350.
21. Steinlein OK, Hoda JC, Bertrand S, Bertrand D. Mutations in familial nocturnal frontal lobe epilepsy might be associated with distinct neurological phenotypes. Seizure. 2012;21(2):118–123.
22. Heron SE, Smith KR, Bahlo M, et al. Missense mutations in the sodium-gated potassium channel gene KCNT1 cause severe autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet. 2012;44(11):1188–1190.
23. Ishida S, Picard F, Rudolf G, et al. Mutations of DEPDC5 cause autosomal dominant focal epilepsies. Nat Genet. 2013;45(5):552–555.
24. Picard F, Makrythanasis P, Navarro V, et al. DEPDC5 mutations in families presenting as autosomal dominant nocturnal frontal lobe epilepsy. Neurology. 2014;82(23):2101–2106.
25. Barcia G, Fleming MR, Deligniere A, et al. De novo gain-of-function KCNT1 channel mutations cause malignant migrating partial seizures of infancy. Nat Genet. 2012;44(11):1255–1259.
26. Møller RS, Heron SE, Larsen LH, et al. Mutations in KCNT1 cause a spectrum of focal epilepsies. Epilepsia. 2015;56(9):e114–e120.
27. Kim GE, Kronengold J, Barcia G, et al. Human slack potassium channel mutations increase positive cooperativity between individual channels. Cell Rep. 2014;9(5):1661–1672.
28. Bearden D, Strong A, Ehnot J, et al. Targeted treatment of migrating partial seizures of infancy with quinidine. Ann Neurol. 2014;76(3):457–461.
29. Milligan CJ, Li M, Gazina EV, et al. KCNT1 gain of function in 2 epilepsy phenotypes is reversed by quinidine. Ann Neurol. 2014;75(4):581–590.
30. Dibbens LM, de Vries B, Donatello S, et al. Mutations in DEPDC5 cause familial focal epilepsy with variable foci. Nat Genet. 2013;45(5):546–551.
31. Bar-Peled L, Chantranupong L, Cherniack AD, et al. A tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science. 2013;340(6136):1100–1106.
32. Crino PB. A pathogenic signaling pathway in developmental brain malformations. Trends Mol Med. 2011;17(12):734–742.
33. Combi R, Dalprà L, Ferini-Strambi L, Tenchini ML. Frontal lobe epilepsy and mutations of the corticotropin-releasing hormone gene. Ann Neurol. 2005;58(6):899–904.
34. Sansoni V, Forcella M, Mozzi A, et al. Functional characterization of a CRH missense mutation identified in an ADNFLE family. PLoS One. 2013;8(4):e61306.
35. Smythies J. Section I. The cholinergic system. Int Rev Neurobiol. 2005;64:1–122.
36. Hoda JC, Gu W, Friedli M, et al. Human nocturnal frontal lobe epilepsy: pharmocogenomic profiles of pathogenic nicotinic acetylcholine receptor beta-subunit mutations outside the ion channel pore. Mol Pharmacol. 2008;74(2):379–391.
37. Bhattacharjee A, Gan L, Kaczmarek LK. Localization of the slack potassium channel in the rat central nervous system. J Comp Neurol. 2002;454(3):241–254.
38. Kim GE, Kaczmarek LK. Emerging role of the KCNT1 slack channel in intellectual disability. Front Cell Neurosci. 2014;8:209.
39. Winawer MR, Ottman R, Hauser WA, Pedley TA. Autosomal dominant partial epilepsy with auditory features: defining the phenotype. Neurology. 2000;54(11):2173–2176.
40. Brodtkorb E, Gu W, Nakken KO, et al. Familial temporal lobe epilepsy with aphasic seizures and linkage to chromosome 10q22-q24. Epilepsia. 2002;43(3):228–235.
41. Michelucci R, Poza JJ, Sofia V, et al. Autosomal dominant lateral temporal epilepsy: clinical spectrum, new epitempin mutations, and genetic heterogeneity in seven European families. Epilepsia. 2003;44(10):1289–1297.
42. Ottman R, Winawer MR, Kalachikov S, et al. LGI1 mutations in autosomal dominant partial epilepsy with auditory features. Neurology. 2004;62(7):1120–1126.
43. Kanemoto K, Kawasaki J. Familial aphasic episodes: another variant of partial epilepsy with simple inheritance? Epilepsia. 2000;41(8):1036–1038.
44. Sadleir LG, Agher D, Chabrol E, et al. Seizure semiology in autosomal dominant epilepsy with auditory features, due to novel LGI1 mutations. Epilepsy Res. 2013;107(3):311–317.
45. Kalachikov S, Evgrafov O, Ross B, et al. Mutations in LGI1 cause autosomal-dominant partial epilepsy with auditory features. Nat Genet. 2002;335–413.
46. Di Bonaventura C, Carni M, Diani E, et al. Drug resistant ADLTE and recurrent partial status epilepticus with dysphasic features in a family with a novel LGI1mutation: electroclinical, genetic, and EEG/fMRI findings. Epilepsia. 2009;50(11):2481–2486.
47. Winawer MR, Martinelli Boneschi F, Barker-Cummings C, et al. Four new families with autosomal dominant partial epilepsy with auditory features: clinical description and linkage to chromosome 10q24. Epilepsia. 2002;43(1):60–67.
48. Kobayashi E, D’Agostino MD, Lopes-Cendes I, et al. Hippocampal atrophy and T2-weighted signal changes in familial mesial temporal lobe epilepsy. Neurology. 2003;60(3):405–409.
49. Ottman R, Rosenberger L, Bagic A, et al. Altered language processing in autosomal dominant partial epilepsy with auditory features. Neurology. 2008;71(24):1973–1980.
50. Brodtkorb E, Steinlein OK, Sand T. Asymmetry of long-latency auditory evoked potentials in LGI1-related autosomal dominant lateral temporal lobe epilepsy. Epilepsia. 2005;46(10):1692–1694.
51. Pisano T, Marini C, Brovedani P, et al. Abnormal phonologic processing in familial lateral temporal lobe epilepsy due to a new LGI1 mutation. Epilepsia. 2005;46(1):118–123.
52. Morante-Redolat JM, Gorostidi-Pagola A, Piquer-Sirerol S, et al. Mutations in the LGI1/epitempin gene on 10q24 cause autosomal dominant lateral temporal epilepsy. Hum Mol Genet. 2002;11(9):1119–1128.
53. Bisulli F, Tinuper P, Scudellaro E, et al. A de novo LGI1 mutation in sporadic partial epilepsy with auditory features. Ann Neurol. 2004;56(3):455–456.
54. Rosanoff MJ, Ottman R. Penetrance of LGI1 mutations in autosomal dominant partial epilepsy with auditory features. Neurology. 2008;71(8):567–571.
55. Chabrol E, Popescu C, Gourfinkel-An I, et al. Two novel epilepsy-linked mutations leading to a loss of function of LGI1. Arch Neurol. 2007;64(2):217–222.
56. Yokoi N, Fukata Y, Kase D, et al. Chemical corrector treatment ameliorates increased seizure susceptibility in a mouse model of familial epilepsy. Nat Med. 2015;21(1):19–26.
57. Boillot M, Baulac S. Genetic models of focal epilepsies. J Neurosci Methods. 2016;260:132–143.
58. Irani SR, Stagg CJ, Schott JM, et al. Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain. 2013;136(Pt 10):3151–3162.
59. Dazzo E, Fanciulli M, Serioli E, et al. Heterozygous reelin mutations cause autosomal-dominant lateral temporal epilepsy. Am J Hum Genet. 2015;96(6):992–1000.
60. Hong SE, Shugart YY, Huang DT, et al. Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nature Genet. 2000;26(1):93–96.
61. Gu W, Wevers A, Schröder H, et al. The LGI1 gene involved in lateral temporal lobe epilepsy belongs to a new subfamily of leucine-rich repeat proteins. FEBS Letters. 2002;519(1–3):71–76.
62. Scheel H, Tomiuk S, Hofmann K. A common protein interaction domain links two recently identified epilepsy genes. Hum Mol Genet. 2002;11(15):1757–1762.
63. Chernova OB, Somerville RP, Cowell JK. A novel gene, LGI1 from 10q24 is rearranged and downregulated in malignant brain tumors. Oncogene. 1998;17(22):2873–2881.
64. Schulte U, Thumfart JO, Klöcker N, et al. The epilepsy-linked Lgi1 protein assembles into presynaptic Kv1 channels and inhibits inactivation by Kvbeta1. Neuron. 2006;49(5):697–706.
65. Fukata Y, Adesnik H, Iwanaga T, et al. Epilepsy-related ligand/receptor complex LGI1 and ADAM22 regulate synaptic transmission. Science. 2006;313(5794):1792–1795.
66. Sagane K, Ishihama Y, Sugimoto H. LGI1 and LGI4 bind to ADAM22, ADAM23 and ADAM11. Int J Biol Sci. 2008;4(6):387–396.
67. Fukata Y, Lovero KL, Iwanaga T, et al. Disruption of LGI1-linked synaptic complex causes abnormal synaptic transmission and epilepsy. Proc Natl Acad Sci USA. 2010;107(8):3799–3804.
68. Zhou YD, Lee S, Jin Z, et al. Arrested maturation of excitatory synapses in autosomal dominant lateral temporal lobe epilepsy. Nat Med. 2009;15(10):1208–1214.
69. Zhou YD, Zhang D, Ozkaynak E, et al. Epilepsy gene LGI1 regulates postnatal developmental remodeling of retinogeniculate synapses. J Neurosci. 2012;32(3):903–910.
70. Owuor K, Harel NY, Englot DJ, et al. LGI1-associated epilepsy through altered ADAM23-dependent neuronal morphology. Mol Cell Neurosci. 2009;42(4):448–457.
71. Chabrol E, Navarro V, Provenzano G, et al. Electroclinical characterization of epileptic seizures in leucine-rich, glioma-inactivated 1-deficient mice. Brain. 2010;133(9):2749–2762.
72. Yu YE, Wen L, Silva J, et al. Lgi1 null mutant mice exhibit myoclonic seizures and CA1 neuronal hyperexcitability. Hum Mol Genet. 2010;19(9):1702–1711.
73. Baulac S, Ishida S, Mashimo T, et al. A rat model for LGI1-related epilepsies. Hum Mol Genet. 2012;21(16):3546–3557.
74. Boillot M, Huneau C, Marsan E, et al. Glutamatergic neuron-targeted loss of LGI1 epilepsy gene results in seizures. Brain. 2014;137(Pt 11):2984–2996.
75. D’Arcangelo G, Miao GG, Chen SC, et al. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374(6524):719–723.
76. Pesold C, Impagnatiello F, Pisu MG, et al. Reelin is preferentially expressed in neurons synthesizing gamma-aminobutyric acid in cortex and hippocampus of adult rats. Proc Natl Acad Sci USA. 1998;95(6):3221–3226.
77. Rice DS, Curran T. Role of the reelin signaling pathway in central nervous system development. Ann Rev Neurosci. 2001;24:1005–1039.
78. Dulabon L, Olson EC, Taglienti MG, et al. Reelin binds alpha3beta1 integrin and inhibits neuronal migration. Neuron. 2000;27(1):33–44.
79. Hiesberger T, Trommsdorff M, Howell BW, et al. Direct binding of reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 1999;24(2):481–489.
80. Berkovic SF, McIntosh A, Howell RA, et al. Familial temporal lobe epilepsy: a common disorder identified in twins. Ann Neurol. 1996;40(2):227–235.
81. Aguglia U, Gambardella A, Le Piane E, et al. Mild non-lesional temporal lobe epilepsy. A common, unrecognized disorder with onset in adulthood. Can J Neurol Sci. 1998; 25(4):282–286.
82. Regesta G, Tanganelli P. Temporal lobe epilepsy of adult age of possible idiopathic nature. Seizure. 2002;11(2):131–135.
83. Striano P, Gambardella A, Coppola A, et al. Familial mesial temporal lobe epilepsy (FMTLE): a clinical and genetic study of 15 Italian families. J Neurol. 2008;255(1):16–23.
84. Gambardella A, Messina D, Le Piane E, et al. Familial temporal lobe epilepsy autosomal dominant inheritance in a large pedigree from southern Italy. Epilepsy Res. 2000;38(2–3):127–132.
85. Verity CM, Butler NR, Golding J. Febrile convulsions in a national cohort followed up from birth: I—Prevalence and recurrence in the first five years of life. Br Med J (Clin Res. Ed). 1985;290(6478):1307–1310.
86. Cendes F, Lopes-Cendes I, Andermann E, Andermann F. Familial temporal lobe epilepsy: a clinically heterogeneous syndrome. Neurology. 1998;50(2):554–557.
87. Baulac S, Picard F, Herman A, et al. Evidence for digenic inheritance in a family with both febrile convulsions and temporal lobe epilepsy implicating chromosomes 18qter and 1q25-q31. Ann Neurol. 2001;49(6):786–792.
88. Claes L, Audenaert D, Deprez L, et al. Novel locus on chromosome 12q22-q23.3 responsible for familial temporal lobe epilepsy associated with febrile seizures. J Med Genet. 2004;41(9):710–714.
89. Hedera P, Blair MA, Andermann E, et al. Familial mesial temporal lobe epilepsy maps to chromosome 4q13.2-q21.3. Neurology. 2007;68(24):2107–2112.
90. Angelicheva D, Tournev I, Guergueltcheva V, et al. Partial epilepsy syndrome in a Gypsy family linked to 5q31.3-q32. Epilepsia. 2009;50(7):1679–1688.
91. Chahine L, Abou-Khalil B, Siren A, et al. A new locus for familial temporal lobe epilepsy on chromosome 3q. Epilepsy Res. 2013;106(3):338–344.
92. Maurer-Morelli CV, Secolin R, Morita ME, et al. A locus identified on chromosome18P11.31 is associated with hippocampal abnormalities in a family with mesial temporal lobe epilepsy. Front Neurol. 2012;3:124.
93. Striano P, Serioli E, Santulli L, et al. DEPDC5 mutations are not a frequent cause of familial temporal lobe epilepsy. Epilepsia. 2015;56(10):e168–e171.
94. Crompton DE, Scheffer IE, Taylor I, et al. Familial mesial temporal lobe epilepsy: a benign epilepsy syndrome showing complex inheritance. Brain. 2010;133(11):3221–3231.
95. Scheffer IE, Phillips HA, O’Brien CE, et al. Familial partial epilepsy with variable foci: a new partial epilepsy syndrome with suggestion of linkage to chromosome 2. Ann Neurol. 1998;44(6):890–899.
96. Morales-Corraliza J, Gómez-Garre P, Sanz R, et al. Familial partial epilepsy with variable foci: a new family with suggestion of linkage to chromosome 22q12. Epilepsia. 2010;51(9):1910–1914.
97. Callenbach PM, van den Maagdenberg AM, Hottenga JJ, et al. Familial partial epilepsy with variable foci in a Dutch family: clinical characteristics and confirmation of linkage to chromosome 22q. Epilepsia. 2003;44(10):1298–1305.
98. Berkovic SF, Serratosa JM, Phillips HA, et al. Familial partial epilepsy with variable foci: clinical features and linkage to chromosome 22q12. Epilepsia. 2004;45(9):1054–1060.
99. Klein KM, O’Brien TJ, Praveen K, et al. Familial focal epilepsy with variable foci mapped to chromosome 22q12: expansion of the phenotypic spectrum. Epilepsia. 2012;53(8):e151–e155.
100. Xiong L, Labuda M, Li DS, et al. Mapping of a gene determining familial partial epilepsy with variable foci to chromosome 22q11-q12. Am J Hum Genet. 1999;65(6):1698–1710.
101. Martin C, Meloche C, Rioux M-F, et al. A recurrent mutation in DEPDC5 predisposes to focal epilepsies in the French-Canadian population. Clin Genet. 2014;75(5):788–792.
102. Lal D, Reinthaler EM, Schubert J, et al. DEPDC5 mutations in genetic focal epilepsies of childhood. Ann Neurol. 2014;75(5):788–792.
103. D’Gama AM, Geng Y, Couto JA, et al. Mammalian target of rapamycin pathway mutations cause hemimegalencephaly and focal cortical dysplasia. Ann Neurol. 2015;77(4):720–725.
104. Scerri T, Riseley JR, Gillies G, et al. Familial cortical dysplasia type IIA caused by a germline mutation in DEPDC5. Ann Clin Transl Neurol. 2015;2(5):575–580.
105. Panchaud N, Péli-Gulli MP, De Virgilio C. Amino acid deprivation inhibits TORC1 through a GTPase-activating protein complex for the Rag family GTPase Gtr1. Sci Signal. 2013;6(277):ra42.
106. Lim KC, Crino PB. Focal malformations of cortical development: new vistas for molecular pathogenesis. Neuroscience. 2013;252:262–276.
107. van Kranenburg M, Hoogeveen-Westerveld M, Nellist M. Preliminary functional assessment and classification of DEPDC5 variants associated with focal epilepsy. Hum Mutat. 2015;36(2):200–209.