Disorders of the Respiratory Pump
Neuromuscular Disease Affecting the Muscles of Respiration
Specific Diseases
The major neuromuscular diseases that can affect the muscles of respiration are listed in Table 19-1; several are discussed here.
Table 19-1
DISORDERS OF THE RESPIRATORY PUMP
| Neuromuscular Diseases | Chest Wall Diseases |
| Guillain-Barré syndrome | Kyphoscoliosis |
| Myasthenia gravis | Obesity |
| Poliomyelitis | Ankylosing spondylitis |
| Postpolio syndrome | |
| Amyotrophic lateral sclerosis | |
| Cervical or thoracic spinal cord injury | |
| Polymyositis | |
| Muscular dystrophy |
Pathophysiology and Clinical Consequences
Impairment of inspiratory muscle strength may render patients unable to maintain sufficient minute ventilation for adequate CO2 elimination. In addition, patients often alter their pattern of breathing, taking shallower and more frequent breaths. Although this pattern of breathing may require less muscular effort and be more comfortable, it is also less efficient because a greater proportion of each breath is wasted on ventilating the anatomic dead space (see Chapter 1). Therefore, even if total minute ventilation is maintained, alveolar ventilation (and thus CO2 elimination) is impaired by the altered pattern of breathing.
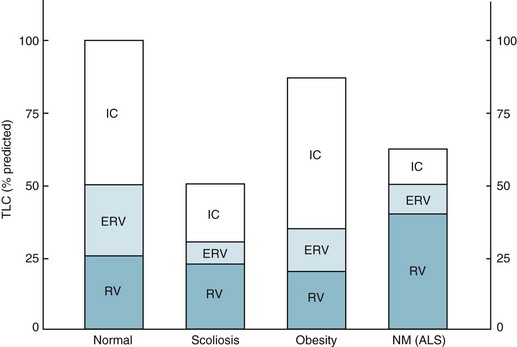
With severe neuromuscular disease, pulmonary function tests show a restrictive pattern of impairment. Although muscle weakness is the primary cause of restriction, compliance of the lung and chest wall may be secondarily affected, further contributing to the restrictive pattern. The decrease in pulmonary compliance presumably is due to microatelectasis (i.e., multiple areas of alveolar collapse) resulting from the shallow tidal volumes. At the same time, stiffening of various components of the chest wall (e.g., tendons, ligaments, and joints) over time is thought to be responsible for decreased distensibility of the chest wall. Functional residual capacity (FRC) is normal or decreased, depending on how much respiratory system compliance is altered. Total lung capacity is decreased primarily as a result of inspiratory muscle weakness, but changes in respiratory system compliance may contribute as well. Residual volume (RV) frequently is increased as a result of expiratory muscle weakness (Fig. 19-1). The degree of muscle weakness can be quantified by measuring the maximal inspiratory and expiratory pressures the patient is able to generate with maximal inspiratory and expiratory efforts against a closed mouthpiece. Both maximal inspiratory pressure (MIP) and maximal expiratory pressure may be significantly depressed.
Diaphragmatic Disease
Diaphragmatic Fatigue
Inefficient diaphragmatic contraction is another factor that may contribute to diaphragmatic fatigue, especially in patients with obstructive lung disease. When the diaphragm is flattened and its fibers are shortened as a result of hyperinflated lungs, the force or pressure developed during contraction is less for any given level of diaphragmatic excitation (see Chapter 17). Therefore, a higher degree of stimulation is necessary to generate comparable pressure by the diaphragm, and increased energy consumption results.
Unilateral Diaphragmatic Paralysis
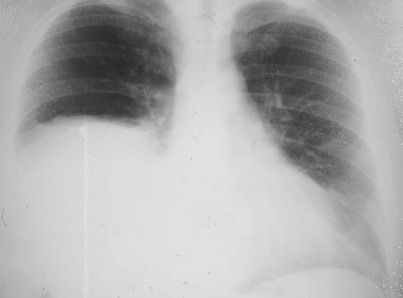
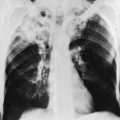
The possibility of unilateral diaphragmatic paralysis is usually first suggested by a characteristic appearance on the chest radiograph (Fig. 19-2). The affected hemidiaphragm is elevated above its usual position in the absence of any associated lobar atelectasis or other reason for volume loss on the affected side. Standard chest radiographs taken during a full inspiration (to total lung capacity) reveal that the normal hemidiaphragm descends during inspiration, whereas the paralyzed hemidiaphragm cannot. Patients may or may not be symptomatic with dyspnea as a result of the paralyzed hemidiaphragm, often depending on the presence or absence of additional underlying lung disease.
Bilateral Diaphragmatic Paralysis
Paralysis of both diaphragms has much more serious clinical implications than unilateral paralysis, because the patient must depend on the accessory muscles of inspiration to maintain minute ventilation. The causes of bilateral diaphragmatic paralysis are the neuromuscular diseases listed in Table 19-1, with bilateral diaphragmatic paralysis being the most severe consequence of respiratory involvement by these disorders.
Diseases Affecting the Chest Wall
With certain diseases of the chest wall, difficulty in expanding the chest may impede normal inspiration (see Table 19-1). This section focuses on two specific disorders that pose the greatest clinical problems: kyphoscoliosis and obesity.
Kyphoscoliosis
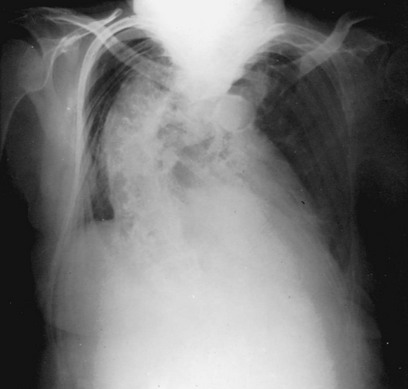
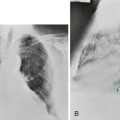
Kyphoscoliosis is an abnormal curvature of the spine in both the anterior (kyphosis) and lateral (scoliosis) directions (Fig. 19-3). This deformity causes the rib cage to become stiffer and more difficult to expand (i.e., chest wall compliance is decreased). Respiratory difficulties are common in patients with significant kyphoscoliosis. In particularly severe cases, chronic respiratory failure is the consequence. Although some cases of kyphoscoliosis actually are secondary to neuromuscular disease such as poliomyelitis, the majority of severe cases associated with respiratory impairment are idiopathic.
Pulmonary function tests in patients with kyphoscoliosis are notable for a restrictive pattern of impairment with a decrease in total lung capacity. Vital capacity is significantly decreased, whereas RV tends to be relatively preserved. FRC, determined by the outward recoil of the chest wall balanced by the inward recoil of the lung, is decreased because the poorly compliant chest wall has a diminished propensity to recoil outward (see Fig. 19-1).
Surgical therapy aimed at improving or correcting the spinal deformity may be useful in children or adolescents but rarely is effective in adults. Supportive therapy that may be beneficial includes a variety of measures that provide ventilatory assistance to the patient. Treatments with an intermittent positive pressure breathing machine augment tidal volume by delivering positive pressure to the patient during inspiration. The increase in tidal volume improves microatelectasis and lung compliance, affording the patient several hours with decreased work of breathing after each treatment. At night, ventilatory assistance with either inspiratory positive pressure delivered via a mask or through a tracheostomy tube or negative pressure around the chest wall allows the respiratory muscles to rest. Nocturnal ventilatory support may provide sufficient rest for the inspiratory muscles to diminish daytime respiratory muscle fatigue. These types of ventilatory support are discussed further in Chapter 29.
Obesity
Another factor that contributes to the overall clinical picture in many massively obese patients is upper airway obstruction during sleep (i.e., the obstructive form of sleep apnea syndrome). Soft tissue deposition in the neck and tissues surrounding the upper airway presumably predisposes the person to episodes of complete upper airway obstruction during sleep (see Chapter 18). In a large percentage of cases, somnolence that occurs in patients who supposedly have obesity-hypoventilation syndrome is related to the presence of obstructive sleep apnea.
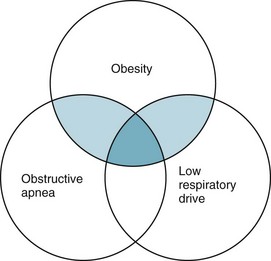
Although obesity, depressed respiratory drive, respiratory muscle weakness, and sleep apnea syndrome contribute to respiratory dysfunction, exactly how they interact in individual patients is often difficult to assess. Because sleep apnea syndrome and depressed respiratory drive also occur in patients who are not obese, it is reasonable to view some of the contributing pathophysiologic factors in terms of a Venn diagram (Fig. 19-4). Probably the most marked symptoms and respiratory dysfunction are seen in patients who are represented at the intersection of the three circles.
Pulmonary function tests frequently demonstrate a restrictive pattern of dysfunction, with a decrease in total lung capacity. The diaphragm is pushed up in massively obese patients, reducing FRC, which in these patients is much closer to RV; thus, spirometric examination shows the expiratory reserve volume is greatly reduced. This pattern of functional impairment is shown in Figure 19-1.
Weight loss is crucial in the treatment of obese patients with respiratory dysfunction. If weight loss is successfully achieved by either diet or bariatric surgery, respiratory problems may resolve in some patients. Unfortunately, attempts at significant and sustained weight loss are often unsuccessful, and some patients who successfully lose weight do not manifest respiratory benefits. Thus in most patients, other modes of therapy must be instituted. Nocturnal positive pressure ventilation is generally helpful in improving daytime sleepiness, particularly if the patient has concomitant obstructive sleep apnea syndrome (see Chapter 18). In some patients who hypoventilate, respiratory stimulants, especially progesterone (a centrally acting respiratory stimulant), have been used with limited success.
Neuromuscular Disease Affecting the Muscles of Respiration
Ambrosino, N, Carpenè, N, Gherardi, M. Chronic respiratory care for neuromuscular diseases in adults. Eur Respir J. 2009;34:444–451.
Bach, JR. Management of post-polio respiratory sequelae. Ann N Y Acad Sci. 1995;753:96–102.
Benditt, JO. Management of pulmonary complications in neuromuscular disease. Phys Med Rehabil Clin North Am. 1998;9:167–185.
Bittner, EA, Martyn, JA, George, E, et al. Measurement of muscle strength in the intensive care unit. Crit Care Med. 2009;37:S321–S330.
Derenne, J-P, Macklem, PT, Roussos, C. The respiratory muscles: mechanics, control, and pathophysiology. Am Rev Respir Dis. 1978;118:581–601.
Dhand, UK. Clinical approach to the weak patient in the intensive care unit. Respir Care. 2006;51:1024–1040.
Kiernan, MC, Vucic, S, Cheah, BC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377:942–955.
Lacomis, D. Myasthenic crisis. Neurocrit Care. 2005;3:189–194.
Mansel, JK, Norman, JR. Respiratory complications and management of spinal cord injuries. Chest. 1990;97:1446–1452.
Mehta, S. Neuromuscular disease causing acute respiratory failure. Respir Care. 2006;51:1016–1021.
Orlikowski, D, Prigent, H, Sharshar, T, et al. Respiratory dysfunction in Guillain-Barré syndrome. Neurocrit Care. 2004;1:415–422.
Piepers, S, van den Berg, JP, Kalmijn, S, et al. Effect of non-invasive ventilation on survival, quality of life, respiratory function and cognition: a review of the literature. Amyotroph Lateral Scler. 2006;7:195–200.
Roussos, C, Macklem, PT. The respiratory muscles. N Engl J Med. 1982;307:786–797.
Smith, PEM, Calverley, PM, Edwards, RH, et al. Practical problems in the respiratory care of patients with muscular dystrophy. N Engl J Med. 1987;316:1197–1205.
Seneviratne, J, Mandrekar, J, Wijdicks, EF, et al. Non-invasive ventilation in myasthenia crisis. Arch Neurol. 2008;65:54–58.
Sunderrajan, EV, Davenport, J. The Guillain-Barré syndrome: pulmonary-neurologic correlations. Medicine. 1985;64:333–341.
Thorsteinsson, G. Management of postpolio syndrome. Mayo Clin Proc. 1997;72:627–638.
Aldrich, TK. Respiratory muscle fatigue. Clin Chest Med. 1988;9:225–236.
Aubier, M, De Troyer, A, Sampson, M, et al. Aminophylline improves diaphragmatic contractility. N Engl J Med. 1981;305:249–252.
Celli, B. The diaphragm and respiratory muscles. Chest Surg Clin North Am. 1998;8:207–224.
Groth, SS, Andrade, RS. Diaphragm plication for eventration or paralysis: a review of the literature. Ann Thorac Surg. 2010;89:S2146–S2150.
Mador, MJ. Respiratory muscle fatigue and breathing patterns. Chest. 1991;100:1430–1435.
Maish, MS. The diaphragm. Surg Clin North Am. 2010;90:955–968.
McCool, FD, Tzelepis, GE. Dysfunction of the diaphragm. N Engl J Med. 2012;366:932–942.
Ottenheijm, CA, Heunks, LM, Dekhuijzen, PN. Diaphragm muscle fiber dysfunction in chronic obstructive pulmonary disease: toward a pathophysiological concept. Am J Respir Crit Care Med. 2007;175:1233–1240.
Pacia, EB, Aldrich, TK. Assessment of diaphragm function. Chest Surg Clin North Am. 1998;8:225–236.
Roussos, C, Macklem, PT. The respiratory muscles. N Engl J Med. 1982;307:786–797.
Tripp, HF, Bolton, JW. Phrenic nerve injury following cardiac surgery: a review. J Card Surg. 1998;13:218–223.
Diseases Affecting the Chest Wall
Baydur, A, Milic-Emili, J. Respiratory mechanics in kyphoscoliosis. Monaldi Arch Chest Dis. 1993;48:69–79.
Berger, KI, Ayappa, I, Chatr-Amontri, B, et al. Obesity hypoventilation syndrome as a spectrum of respiratory disturbances during sleep. Chest. 2001;120:1231–1238.
Bergofsky, EH. Respiratory failure in disorders of the thoracic cage. Am Rev Respir Dis. 1979;119:643–669.
Chen, Y, Rennie, D, Cormier, YF, et al. Waist circumference is associated with pulmonary function in normal-weight, overweight, and obese subjects. Am J Clin Nutr. 2007;85:35–39.
Davis, G, Patel, JA, Gagne, DJ. Pulmonary considerations in obesity and the bariatric surgical patient. Med Clin North Am. 2007;91:433–442.
Kafer, ER. Respiratory and cardiovascular functions in scoliosis. Bull Eur Physiopathol Respir. 1977;13:299–321.
Koenig, SM. Pulmonary complications of obesity. Am J Med Sci. 2001;321:249–279.
Luce, JM. Respiratory complications of obesity. Chest. 1980;78:626–631.
McMaster, MJ, Glasby, MA, Singh, H, et al. Lung function in congenital kyphosis and kyphoscoliosis. J Spinal Disord Tech. 2007;20:203–208.
Mokhlesi, B, Tulaimat, A. Recent advances in obesity hypoventilation syndrome. Chest. 2007;132:1322–1336.
Piper, AJ, Grunstein, RR. Obesity hypoventilation syndrome: mechanisms and management. Am J Respir Crit Care Med. 2011;183:292–298.
Romero-Corral, A, Caples, SM, Lopez-Jimenez, F, et al. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010;137:711–719.
Sutton, FD, Jr., Zwillich, CW, Creagh, CE, et al. Progesterone for outpatient treatment of pickwickian syndrome. Ann Intern Med. 1975;83:476–479.