Discontinuing Ventilatory Support
After reading this chapter you will be able to:
 Discuss the relationship between ventilatory demand and ventilatory capacity as well as their relationship with ventilator discontinuance.
Discuss the relationship between ventilatory demand and ventilatory capacity as well as their relationship with ventilator discontinuance.
 List the factors associated with ventilator dependence.
List the factors associated with ventilator dependence.
 Explain how to evaluate a patient before attempting ventilator discontinuation or weaning.
Explain how to evaluate a patient before attempting ventilator discontinuation or weaning.
 List acceptable values for specific weaning indices used to predict a patient’s readiness for discontinuation of ventilatory support.
List acceptable values for specific weaning indices used to predict a patient’s readiness for discontinuation of ventilatory support.
 Describe factors that should be optimized before an attempt is made at ventilator discontinuation or weaning.
Describe factors that should be optimized before an attempt is made at ventilator discontinuation or weaning.
 Describe techniques used in ventilator weaning, including daily spontaneous breathing trials, synchronized intermittent mandatory ventilation, pressure support ventilation, and other newer methods.
Describe techniques used in ventilator weaning, including daily spontaneous breathing trials, synchronized intermittent mandatory ventilation, pressure support ventilation, and other newer methods.
 Contrast the advantages and disadvantages associated with various weaning methods and techniques.
Contrast the advantages and disadvantages associated with various weaning methods and techniques.
 Describe how to assess a patient for extubation.
Describe how to assess a patient for extubation.
 List the primary reasons why patients fail a ventilator discontinuance trial.
List the primary reasons why patients fail a ventilator discontinuance trial.
 Explain why some patients cannot be successfully weaned from ventilatory support.
Explain why some patients cannot be successfully weaned from ventilatory support.
The purpose of mechanical ventilation is to support the patient until the disease state or condition that caused the need for support is alleviated or resolved.1–3 Ventilatory support can sustain life, but it cannot cure disease. Further, many complications and hazards are associated with mechanical ventilation. Consequently, ventilatory support should be withdrawn as soon as the patient is able to adequately resume spontaneous breathing.1–4 All patients who are mechanically ventilated should be evaluated on a daily basis for their ability to wean from ventilatory support.5,6
After the problem or condition that caused the need for mechanical ventilation is resolved, most patients can be quickly and easily removed from ventilatory support. For example, for most patients who need mechanical ventilation as a result of drug overdose or severe asthma, for those who are recovering from postoperative anesthesia, and for those who have received ventilation for 72 hours or less, one may simply discontinue ventilation when the precipitating condition has resolved.1,5 However, some patients require mechanical ventilation for longer periods. The term ventilator dependent is usually reserved for patients who need ventilatory support for lengthy periods (i.e., 2 weeks or more) or who have not responded to attempts at ventilator discontinuation.3 For these patients, a more formal ventilator discontinuation process is required.3
Ventilator discontinuation should be carefully timed.4 Premature removal from the ventilator may severely stress the cardiopulmonary system and delay the patient’s recovery.4 Premature discontinuation also exposes the patient to the hazards of reintubation. However, delays in discontinuing ventilation expose the patient to an increased risk of complications, including nosocomial pneumonia, myocardial infarction, and death.6
There are three basic methods for discontinuing ventilatory support:
1. Spontaneous breathing trials (SBTs) alternating with mechanical ventilation
Other techniques that may facilitate ventilator discontinuation include the use of volume-support ventilation (VSV), adaptive support ventilation (ASV); automatic tube compensation (ATC); proportional assist ventilation (PAV), which is also known as proportional pressure support (PPS); and continuous positive airway pressure (CPAP).4,7–9 However, little data exist that support the use of any of these techniques except CPAP during the ventilator discontinuation process.
Techniques for predicting when patients are ready for ventilator discontinuation and weaning have been studied extensively.6 Many weaning indices have been used, and a number of different weaning methods have been advocated. Despite this, there are no universally applicable rules for predicting success. Of all of the methods studied, SBTs and PSV have been shown to be the most effective methods for ventilator discontinuation and weaning. Evidence-based reviews recommend the use of at least daily SBTs.3 Protocols for ventilator discontinuation administered by an interdisciplinary team of respiratory therapists, nurses, and physicians can be highly effective, and the use of ventilator discontinuation protocols has also been recommended.3,6,10–13 Regardless of the method used, success is unlikely unless the precipitating problems that caused the ventilator dependency have been resolved.1,3,7,14 After these problems are resolved, an organized plan or protocol should be followed, and variations should be based on each patient’s response.3,6,10
Some patients cannot be successfully removed from mechanical ventilatory support. This group of ventilator-dependent patients poses clinical, economic, and ethical concerns.15
Many clinicians use the term weaning as a general term to refer to the process of discontinuing ventilatory support, regardless of the time frame or method involved.7,8,16 The term has also been used to refer to reductions in fractional inspired oxygen concentration (FiO2), PEEP, and CPAP.8 Alternatively, the term ventilator discontinuation has been used to refer to the process of disconnecting a patient from mechanical ventilatory support. For the purposes of this chapter, the term weaning is defined as a gradual reduction in the level of ventilatory support,1,7–9 whereas discontinuing ventilatory support refers to the overall process of removing the patient from the ventilator, regardless of the method used.3 In general, patients who are being considered for removal from ventilatory support fall into one of four categories:
1. Those for whom removal is quick and routine, which is normally the vast majority of ventilated patients
2. Those who need a more systematic approach to discontinuing ventilatory support, which is normally about 15% to 20% of ventilated patients
3. Those who require days to weeks to wean from ventilatory support, which is usually less than 5% of ventilated patients
4. Those ventilator-dependent or “unweanable” patients, who compose less than 1% of patients who require ventilatory support17
Reasons for Ventilator Dependence
Patients may require mechanical ventilation because of apnea, acute or impending ventilatory failure, or severe oxygenation problems that necessitate high levels of PEEP or CPAP. Regardless of the reason for initiating mechanical ventilation, patients remain dependent on the ventilator because of respiratory, cardiovascular, neurologic, or psychologic factors.3
Ventilatory Workload or Demand
Patients who need mechanical ventilation often have a ventilatory workload or demand that exceeds their ventilatory capacity. This is the most common cause of ventilator dependence.1,2 The term ventilatory workload refers to the amount of work that the respiratory muscles are asked to perform to provide an appropriate level of ventilation. A patient’s total ventilatory workload is primarily determined by the following: (1) the level of ventilation needed; (2) the compliance of the lungs and thorax; (3) the resistance to gas flow through the airways, and (4) any imposed work of breathing (WOBi) due to mechanical factors.1,7
Mechanical factors that can increase the work of breathing include artificial airways (i.e., endotracheal and tracheotomy tubes), partial obstruction of the airway, ventilator circuits, demand flow systems, auto-PEEP, and inappropriate ventilator flow and sensitivity settings. Factors that may increase ventilatory workload are summarized in Box 47-1.
Ventilatory Capacity
Ventilatory capacity is determined by CNS drive, ventilatory muscle strength, and ventilatory muscle endurance.18 Most patients who are being withdrawn from ventilatory support have a normal or an increased drive to breathe. Patients with neuromuscular disorders and those who are receiving sedatives, narcotics, or neuromuscular blocking agents may have a reduced drive to breathe or impaired neuromuscular transmission. Patients with metabolic alkalosis, hypothyroidism, and sleep deprivation also may have reduced ventilatory drive. Box 47-2 summarizes the factors that may reduce ventilatory drive.
Muscle strength is influenced by age, sex, muscle bulk, and overall health. Malnutrition, starvation, and electrolyte imbalances (especially involving calcium, magnesium, potassium, and phosphate) can lead to ventilatory muscle weakness.18,19 Critical illness myopathy, critical illness polyneuropathy, and the prolonged use of neuromuscular blocking agents are major causes of the development of ventilatory muscle weakness in the intensive care unit (ICU).20 Controlled ventilation for prolonged periods can result in ventilatory muscle discoordination and atrophy.18 Ventilatory muscle endurance is a function of energy supply versus demand. Energy supply is related to nutrition, perfusion, and cell use, whereas demand is related to the amount of work performed and is a function of minute ventilation, compliance, and resistance. Figure 47-1 summarizes the relationship between ventilatory demands and capabilities.
Global Criteria for Discontinuing Ventilatory Support
Success with the discontinuation of ventilatory support is related to the patient’s condition in four main areas1,2,7:
Simply put, when ventilatory workload or demand exceeds ventilatory capacity, successful ventilator discontinuation is unlikely.1,2 Excessive ventilatory workload may lead to ventilatory muscle fatigue. When the ventilatory muscles fatigue, they must be rested for at least 24 hours to recover.3,4,19 Ventilatory workload increases with decreased compliance, increased airway resistance, or an increased level of ventilation. Ventilatory capacity can be reduced by ventilatory muscle fatigue and by a loss of muscle strength and endurance.
Other factors that may contribute to ventilator dependence include inadequate arterial oxygenation, poor tissue oxygen delivery, myocardial ischemia, arrhythmias, low cardiac output, and cardiovascular instability.7,16 Neurologic problems that may contribute to ventilator dependence include decreased central drive to breathe and impaired peripheral nerve transmission.3 Psychologic issues that may contribute to ventilatory dependence include the fear of removal of the life support system, anxiety, stress, depression, and sleep deprivation.3 Box 47-3 summarizes the major factors that contribute to ventilator dependence.
Patient Evaluation
An important factor to consider as part of this assessment is the length of time that the patient has been receiving mechanical ventilation. In general, those who receive support for 72 hours or less often can be removed quickly from the ventilator.1,5,21 Those who need a longer period of support may need a more structured approach. Current guidelines recommend that patients who need mechanical ventilation for more than 48 to 72 hours be carefully assessed to determine all of the possible causes of ventilator dependence.3 These include the respiratory, cardiovascular, neurologic, and psychologic causes of ventilator dependence that are listed in Box 47-3. This recommendation is especially important for the care of patients who have had unsuccessful attempts at the discontinuation of ventilation.3 Factors associated with readiness for the discontinuation of ventilatory support are summarized in Box 47-4.
The Most Important Criterion
The single most important criterion to consider when evaluating a patient for ventilator discontinuation or weaning is whether there has been significant alleviation or reversal of the disease state or condition that necessitated use of the ventilator in the first place.3,7,14 The clinician should determine whether the patient’s condition is improving, whether the initial reason for providing ventilatory support is improved or resolved, and whether the patient’s clinical condition is stable. The following specific questions for patient evaluation have been suggested3:
1. Is there evidence of improvement or reversal of the disease state or condition that caused the need for mechanical ventilation?
2. Is the patient’s oxygenation status adequate? Specific criteria may include the following: partial pressure of oxygen in the arteries PaO2 of 60 mm Hg or more, FiO2 of less than 0.40 to 0.50, PEEP of less than 5 to 8 cm H2O; PaO2/FiO2 of 150 to 200 or more; and pH of 7.25 or more.
3. Is the patient medically and hemodynamically stable? Specific criteria may include the absence of acute myocardial ischemia or marked hypotension. Patients should have adequate blood pressure without vasopressor therapy or with only low-dose vasopressor therapy (i.e., less than 5 µg/kg/min of dopamine or dobutamine).
4. Can the patient breathe spontaneously? The patient must be able to breathe spontaneously and have a sufficient drive to breathe if ventilator discontinuation is being considered.
If the patient’s condition is improving, if the alleviation or reversal of the precipitating disease state or condition has occurred, if the patient is capable of spontaneous breathing, and if the oxygenation status and hemodynamic values are stable, then a formal evaluation for ventilator discontinuation should be performed.3
Weaning Indices
Mechanical ventilation is hazardous, and unnecessary delays in ventilator discontinuation increase the associated complication rate.3,4 Unfortunately, premature ventilator discontinuation may also cause serious problems, including difficulty with reestablishing the artificial airway and serious compromise of the patient’s clinical status.3,4 Clinical judgment has been found to be a poor guide to determining whether a patient is ready for ventilator discontinuation, and more specific indicators have been sought.4 Ideally, specific indicators or weaning indices would clearly show whether a patient is ready to have the ventilator removed and would help to avoid inappropriate ventilator discontinuation. Unfortunately, none of the current weaning indices are capable of predicting readiness for ventilator discontinuance with a high level of accuracy.
Traditional discontinuation indices include the PaO2/FiO2 ratio, the alveolar-to-arterial partial pressure of oxygen difference [P(A–a)O2], the maximum inspiratory pressure (MIP), the vital capacity (VC), the spontaneous minute ventilation (VEsp), and the maximum voluntary ventilation (MVV).5,21 Newer indices include the rapid, shallow breathing index (f/Vt), airway occlusion pressure (P0.1), and measures of WOB.6–8 Although all of these values can be useful, there are enormous discrepancies in the literature regarding their accuracy with regard to the prediction of “weanability.”3,6,7 With respect to the more traditional discontinuation indices, vital capacity and MIP can be highly variable, whereas minute ventilation, respiratory rate (f), and f/Vt tend to be more reliable.22 However, these measures may not correlate well with discontinuation success among all patients and especially among those receiving long-term ventilatory support, the elderly, and those with major pulmonary abnormalities.5,18,23
A comprehensive evidence-based review identified a possible role for 66 specific measurements as predictors of weaning success.6 Of these, eight values were found to be the most useful for the prediction of successful ventilator discontinuation.3,6 Useful predictive measures included spontaneous respiratory rate, spontaneous tidal volume, f/Vt, minute ventilation, MIP, P0.1, P0.1/MIP, and a combined index called the CROP score that included compliance, rate, oxygenation, and MIP.3,6 Unfortunately, these measures all have limitations and relatively high false-positive predictions in specific settings.
It is doubtful that a single index will be found that can be used for consistent discrimination between discontinuation success and failure.7 Moreover, none of these traditional indicators alone has proved useful for the prediction of improvements in patient outcome or in the selection of a particular discontinuation method.7,10 The likely explanation for this failure to identify any consistently powerful discontinuation predictor is that patients’ conditions vary greatly and, for research purposes, clinicians already fully consider information from predictors when choosing patients for trials of the reduction or discontinuation of ventilatory support.10,23
Notwithstanding these limitations, the measurement of discontinuation indices in the difficult-to-wean patient may provide guidance with regard to the reasons that patients fail discontinuation trials. Many find it useful to trend these data on a daily basis for those patients who require lengthy weaning times.15 Specific values for respiratory indices that are used to predict the successful discontinuation of ventilatory support are found in Table 47-1.
TABLE 47-1
Indices That Are Used to Predict the Success of Weaning and Ventilator Discontinuation
| Measurement | Criterion |
| Oxygenation | |
| FiO2 | ≤0.40 to 0.50 |
| PEEP (cm H2O) | ≤5 to 8 |
| PaO2 (mm Hg) | ≥60 |
| SaO2 (%) | ≥90 |
| SvO2 (%) | ≥60 |
| PaO2/PAO2 ratio | ≥0.35 |
| PaO2/FiO2 ratio | >150 to 200 |
| P(A-a)O2 (mm Hg) | <350 |
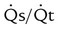 (% shunt) (% shunt) |
<15% to 20% |
No lactic acidosis, adequate 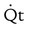 , blood pressure , blood pressure |
|
| Ventilation | |
| PaCO2 (mm Hg) | <50 |
| pH | ≥7.35 |
| Ventilatory Mechanics | |
| Respiratory rate (f) (breaths/min) | 12 to 30 |
| Tidal volume (VT) (ml/kg) | >5 |
| Vital capacity (VC) (ml/kg) | >10 to 15 |
| Static compliance (ml/cm H2O) | >25 |
| f/VT | <105 |
| Respiratory Muscle Strength | |
| Maximum inspiratory force (MIF) (cm H2O) | <−20 to −30 |
| Ventilatory Drive (Demand) | |
Minute ventilation (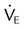 ) for ) for |
|
| Normal PCO2 (L/min) | <10 |
| VDS/VT | <0.55 to 0.60 |
| P0.1 (cm H2O) | <6 |
| P0.1/MIP | <0.30 |
| Work of Breathing | |
| Spontaneous work of breathing | <1.6 kg·m/min (<0.14 kg·m/L) |
| Pressure–time index | <0.15 to 0.18 |
| Ventilatory Reserve | |
| Maximum voluntary ventilation (MVV) (L/min) | >20; more than twice the 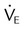 |
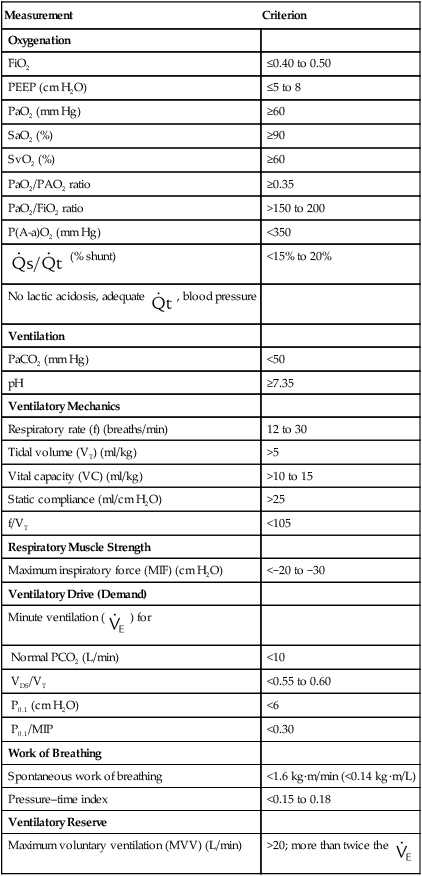
Data from MacIntyre NR, Cook DJ, Ely EW, et al: Evidence-based guidelines for weaning and discontinuing ventilator support: a collective task force facilitated by the American College of Chest Physicians, the American Association for Respiratory Care, and the American College of Critical Care Medicine. Chest 120:375S–395S, 2001; AHRQ publication no. 01-E010, Rockville, MD, 2000, Agency for Healthcare Research and Quality; American College of Chest Physicians: Chest 104:1833, 1993; Burns SM et al: Am J Crit Care 4:4, 1995; Sharar S: Resp Care 40:239, 1995; Bassili HR, Deitel M: JPEN J Parenter Enteral Nutr 5:161, 1981.
Ventilation
Increased thoracic cage movement during spontaneous breathing and asynchronous chest-wall-to-diaphragm movement are related to an increased workload that may lead to ventilatory muscle fatigue and failure.19 Tachypnea (i.e., more than 30 to 35 breaths/min) is a sensitive marker of respiratory distress, but it can prolong intubation if it is used as an exclusive criterion.25 Irregular spontaneous breathing or periods of apnea indicate that the patient is at risk for weaning failure. Asynchronous and rapid, shallow breathing patterns—although not definitive—suggest respiratory decompensation.19
The evaluation of patients for the presence of palpable scalene muscle use during inspiration, an irregular ventilatory pattern, palpable abdominal muscle tensing during expiration, and the inability to alter the ventilatory pattern on command can be helpful for the assessment of the potential for prolonged spontaneous ventilation. Patients with none of these signs have a 90% chance of success.26 Patients with one or two of these signs usually need continued support. The presence of three or more of these signs can mean that the patient’s condition is unstable and that the patient has a poor prognosis for ventilator removal.
P0.1 is the inspiratory pressure that is measured 100 milliseconds after airway occlusion.21 The P0.1 is effort independent, and it correlates well with central respiratory drive.19 Ventilator-dependent patients with COPD who have a P0.1 of more than 6 cm H2O tend to be difficult to wean.21
The f/Vt is the ratio of spontaneous breathing frequency (breaths/min) to tidal volume (liters), and it has been found to be a good predictor of discontinuation success for the care of many patients who need mechanical ventilation.4,6,23 The f/Vt has less predictive power for the care of patients who need ventilatory support for longer than 8 days, and it may be less useful for predicting discontinuation success among elderly patients.8,23,27 Adjusting the threshold value for f/Vt to 130 or less measured at 3 hours was very effective for predicting discontinuation success among patients 70 years old and older. Despite these limitations, an f/Vt of less than 105 can be an accurate and early predictor of weaning outcome, and an f/Vt of 80 is associated with an almost 95% probability of successful discontinuation.4,23 The ratio must be calculated during 1 minute of unsupported spontaneous breathing, and the addition of pressure support significantly reduces the predictive value of the ratio.4
The P0.1/MIP ratio has been found to be a good early predictor of discontinuation success,3,19 and it may be more useful than the MIP by itself. The f/Vt also has been found to be a better predictor of discontinuation success than the MIP alone.2,3 However, even with f/Vt of less than 105, as many as 20% of patients have false-positive results (i.e., they cannot be discontinued from ventilation despite a favorable index) as a result of unpredictable factors such as congestive heart failure, aspiration, or the development of a new pulmonary lesion.28 However, some patients can be successfully discontinued from ventilatory support despite poor f/Vt values.29
WOB would seem to be an excellent way to gauge spontaneous ventilatory workload. Successful weaning has been found to be less likely among patients with spontaneous WOB levels of more than 1.6 kg/m/min (16 J/min) or 0.14 kg/m/L (1.4 J/L).5 However, WOB may not be predictive of weaning success for specific patients.7 This may be because WOB does not take into account ventilatory muscle capacity or fatigue. Consequently, WOB may be less accurate than other conventional discontinuation indices, and it is very difficult to measure at the bedside.30
< ?xml:namespace prefix = "mml" />
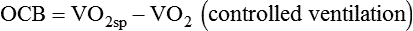
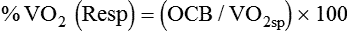
Both OCB and %VO2 have been correlated with the number of days required to wean patients.19 Patients with an OCB of 15% or less of the total VO2 may be more likely to achieve discontinuation success. In one study, OCB was a better predictor of discontinuation success than was f/Vt.31
Pressure–time product (i.e., the area under the inspiratory pressure–time curve) and pressure–time index (PTI) may be the best measures of ventilatory workload for the care of patients who are receiving mechanical support.1 The PTI can be calculated as follows:
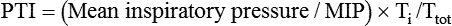
where MIP is maximum inspiratory pressure, Ti is the inspiratory time in seconds, and Ttot is the total respiratory cycle. The Ttot can be calculated by dividing 60 by the respiratory rate (f) (i.e., 60/f). A PTI of more than 0.15 to 0.18 has been associated with diaphragmatic fatigue,32 and a PTI of more than 0.15 cannot be sustained indefinitely.1 There is currently no well accepted and reliable way to measure ventilatory muscle fatigue for patients who are receiving mechanical ventilation.5
Oxygenation
Poor oxygenation status is associated with weaning failure.3,7 Arterial blood gas (ABG) analysis, pulse oximetry, and continuous mixed venous oximetry have been used to monitor and assess the oxygenation status of patients before and during a discontinuation trial. In general, a PaO2 of more than 60 mm Hg (or of more than 55 mm Hg for patients with COPD with carbon dioxide retention) with an FiO2 of less than 0.40 to 0.50 and a PEEP of 5 to 8 cm H2O or less should be adequate for ventilator discontinuation.3,7 The PaO2/FiO2 ratio should be 150 to 200 mm Hg or more.3 With these values, a normal hemoglobin level, a normal oxygen saturation (SaO2), and adequate cardiac output and tissue perfusion are assumed. Specific indices used to assess oxygenation status are found in Table 47-1.
Metabolic Factors
Metabolic factors primarily affect discontinuation in those patients who require long-term ventilatory support. Although nutritional factors are important for all patients, they are unlikely to affect discontinuation in those who only require short-term ventilatory support. Nutrition should be adequate to maintain respiratory muscle mass and contractile force.16 Feeding should be adjusted according to individual patient needs; most patients need 1.5 to 2.0 times their resting energy expenditure.33 In addition, protein intake should be between 1 and 1.5 g/kg per day. Excessive carbohydrate feeding can increase carbon dioxide production and may precipitate acute hypercapnic respiratory failure.16 Parenteral nutrition solutions that contain amino acid formulations (e.g., arginine/lysine) can cause metabolic acidosis and thus increase ventilatory demand.33 Metabolic rate can increase as a result of fever or sepsis. Increased WOB, shivering, seizures, and agitation can also increase oxygen demand and should be evaluated.
Renal Function and Electrolytes
Adequate renal function is required to maintain acid–base homeostasis, electrolyte concentrations, and fluid balance.34 The patient ideally should have an adequate urine output (i.e., more than 1000 ml/day), and there should be no inappropriate weight gain or edema.
Renal insufficiency can lead to metabolic acidosis, which increases respiratory drive. Electrolyte disorders can impair ventilatory muscle function.3,16 Key electrolytes should be normal (i.e., magnesium, 1.8 to 3 mEq/L; phosphate, 2.5 to 4.8 mEq/L; potassium, 3.5 to 5 mEq/L); see Chapter 16 for details. Fluid overload can lead to congestive heart failure and pulmonary edema, which may impair pulmonary gas exchange.
Cardiovascular Function
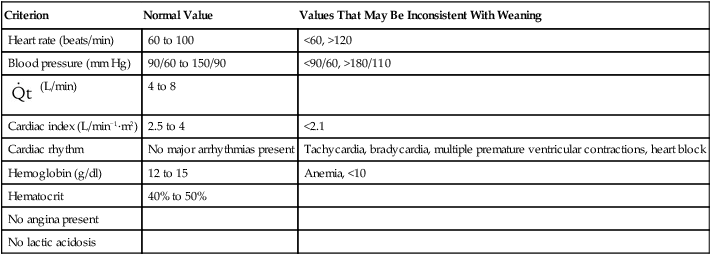
Adequate cardiovascular function is needed to provide sufficient oxygen delivery to the tissues. Cardiac rate and rhythm and blood pressure should be evaluated.3,16 Tachycardia (i.e., a heart rate of more than 100 to 120 beats/min) and bradycardia (i.e., a heart rate of less than 60 to 70 beats/min) should be controlled. The presence of arrhythmias, hypotension (i.e., a blood pressure of less than 90/60 mm Hg), and severe hypertension (i.e., a blood pressure of more than 180/110 mm Hg) should be evaluated carefully before the discontinuation of ventilatory support is considered.3,19
Cardiac output and index measurements as well as central line and pulmonary arterial pressures (i.e., central venous pressure, pulmonary arterial pressure, and pulmonary capillary wedge pressure) may be helpful for the evaluation of cardiovascular function. Left ventricular dysfunction, myocardial ischemia, and cardiovascular instability are associated with decreased discontinuation success.3,21 Table 47-2 provides criteria for confirming cardiovascular stability. However, very few patients without primary cardiovascular dysfunction have pulmonary artery catheters in place. The assessment of cardiac output is usually by noninvasive means, and only arterial and central venous pressures are generally available.
TABLE 47-2
Criteria for Confirming Cardiovascular Stability
| Criterion | Normal Value | Values That May Be Inconsistent With Weaning |
| Heart rate (beats/min) | 60 to 100 | <60, >120 |
| Blood pressure (mm Hg) | 90/60 to 150/90 | <90/60, >180/110 |
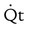 (L/min) (L/min) |
4 to 8 | |
| Cardiac index (L/min−1·m2) | 2.5 to 4 | <2.1 |
| Cardiac rhythm | No major arrhythmias present | Tachycardia, bradycardia, multiple premature ventricular contractions, heart block |
| Hemoglobin (g/dl) | 12 to 15 | Anemia, <10 |
| Hematocrit | 40% to 50% | |
| No angina present | ||
| No lactic acidosis |
Psychologic Factors and Central Nervous System Assessment
Adequate CNS function is needed to ensure stable ventilatory drive, adequate secretion clearance (i.e., coughing and deep breathing), and the protection of the airway (i.e., gag reflex and swallow). In addition, the level of consciousness, dyspnea, anxiety, depression, and motivation can affect discontinuation success.3,5
The patient ideally is awake and alert, free of seizures, and able to follow instructions.3,34 Patients should have an intact central drive to breathe and peripheral nerve function. Brainstem strokes, electrolyte disturbances, sedation, neuromuscular blocking agents, and narcotic drugs can impair the central neurologic control of ventilation.3 Mental status is a good predictor of discontinuation success, and patients who are not alert are at risk for upper airway obstruction, aspiration, and secretion retention. Obtunded patients should, at a minimum, have an adequate gag reflex and cough. Decreased levels of consciousness are associated with aspiration after extubation. The level of consciousness is affected by the use of narcotic, sedative, and analgesic drugs. Drugs with CNS depressant effects should be discontinued, if possible, before the withdrawal of ventilatory support and extubation.16 Protocols to reduce sedation and the daily cessation of sedative drugs may reduce weaning time12,35,36 (see the section on Spontaneous Awaking Trials). The use of neuromuscular blocking agents to allow for controlled ventilation should be avoided, because they may prolong mechanical ventilation.37
Psychologic factors may be among the most important nonrespiratory contributing factors that lead to ventilator dependence.3 Fear, anxiety, and stress should be minimized, and frequent communication among the staff, the patient, and the patient’s family can be helpful.3 Box 47-5 summarizes nonrespiratory factors that affect discontinuation success.
Integrated Indices
Many factors are associated with discontinuation success. In an analysis of 217 patients, discontinuation success could be accurately predicted in about 75% of the patients when multiple predictors were combined.24 For all patients, regardless of diagnosis, days of mechanical ventilation (DMV), f/Vt; MIP, P0.1, maximum expiratory pressure, and VC—when used in combination—were the best predictors.24 For patients with acute respiratory failure, the best predictors were DMV, P0.1/MIP, MIP, f/Vt, and age (76.1% accurate); for patients with COPD, the best predictors were DMV, f/Vt, P0.1, P0.1/MIP, MIP, and age (93.9% accurate); and for patients with neuromuscular disease, MIP, maximum expiratory pressure, f/Vt, and P0.1 were the best predictors (73.9% accurate).24 Integrated indices improve prediction by combining several measures of ability to breathe without ventilatory support. Current examples of integrated indexes include the CROP score, the Adverse Factor/Ventilator Score, the weaning index, and the Burns Weaning Assessment Program.7,19 The CROP score combines measures of ventilatory load, respiratory muscle strength, and gas exchange.7,23
The Adverse Factor/Ventilator Score combines ratings of 15 adverse factors, including hemodynamic values, infection, nutrition, and neurologic/psychiatric state, with ratings of six ventilator factors, including FiO2, compliance, minute ventilation, and rate.19 The weaning index combines measures of ventilatory strength, endurance, and efficiency of gas exchange.19 A weaning index of less than 4 suggests successful discontinuation from mechanical ventilation.18,32 The Burns Weaning Assessment Program is a 26-item assessment that combines 12 general and 14 respiratory factors into a single score.19 Although integrated indices appear promising, no single index has emerged as superior for use in diverse patient populations. Despite the success of these integrated indices in very specific settings, the best approach to determining if a patient can be successfully discontinued from ventilatory support is the patient’s performance on spontaneous breathing trials. All patients should be assessed daily, and their ability to breathe spontaneously should be the primarily variable to determine if the ventilator can be discontinued.
Evaluation of the Airway
It is important for the clinician to separate the decision to discontinue ventilatory support from the decision to extubate.29 The clinician must also be aware that most weaning indices do not evaluate airway patency or protection (see the section on Extubation). The inability to protect or maintain the natural airway is a clear contraindication to extubation. Some patients who can be successfully removed from a ventilator should not be extubated.29 Tracheotomy may be considered for the care of patients who need artificial airways for an extended period.
Tracheotomy may improve patient comfort, allow for more effective suctioning, decrease airway resistance, enhance mobility, and allow the patient to eat and speak.3 Some patients also need high levels of sedation to tolerate the endotracheal tube.3 Consequently, tracheotomy should be considered for the care of patients who are likely to benefit and for those who need prolonged mechanical ventilatory support.3 See Chapter 33 for details about airway management and extubation.
Other patients with good airway patency and protection may be unable to maintain spontaneous breathing for prolonged periods without assistance.29 These patients may be candidates for noninvasive ventilation (NIV) after extubation; see Chapter 46 for details.18,38
Preparing the Patient
Optimizing the Patient’s Medical Condition
Before the removal of ventilatory support is attempted, the patient’s oxygenation status, ventilation, cardiovascular status, and overall medical condition should be optimized. Disease-imposed ventilatory load is minimized with the management of infection, bronchospasm, and airway edema.1 Bronchodilator therapy should be maximized and the appropriate use of anti-inflammatory agents considered.1,29 Techniques to facilitate secretion clearance (e.g., suctioning, adequate humidification, bronchial hygiene techniques), good nutrition, and optimal positioning should be used.29 The patient should be rested and not fatigued or sleep deprived, which can be common in the ICU. The patient should be allowed to sleep at night while maintained on a level of ventilatory support that ensures ventilatory muscle rest.16,39
The patient’s ventilatory workload should be minimized with PSV.29 Flow-trigger or flow-by ventilation or ATC may also be helpful for minimizing imposed ventilatory work. Intrinsic PEEP during mechanical ventilation may increase trigger work, and small amounts of PEEP or CPAP can help to overcome this problem.1,2 Box 47-6 lists factors that should be optimized before ventilatory support is discontinued.
Patients’ Psychologic and Communication Needs
As many as 47% of patients who spend more than 5 days in an ICU may experience psychologic disturbances.38 The cause may be the stress of critical illness, the interruption of nighttime sleep, or the use of sedatives, tranquilizers, hypnotics, narcotics, and other drugs that affect the CNS.39 The patient’s environment should be optimized, anxiety and depression should be evaluated, and communication should be maximized.3,19,21,39
Environmental considerations that may improve the patient’s sense of well-being and outlook include reducing extraneous noise and providing clocks, calendars, pictures, a radio, a television, and, if possible, a room with windows.38 Daily mobility should be considered, including getting the patient up in a chair or placing the patient in the hall in a chair.39 Patients whose condition has been stable can sometimes even be helped to walk with the use of an E cylinder for oxygen, a manual resuscitator bag, and two or more health care workers for support.
Anxiety and agitation should be minimized, and communication and encouragement may be helpful.39 The patient should be told what is planned and be given some control over the situation. Patients should be asked to participate and help in the weaning process. Depression or a lack of motivation may increase weaning time.5,39 If depression is present, assessment and treatment by an appropriate health care provider should be considered.39
Methods
There are three basic methods of discontinuing ventilatory support4:
1. Spontaneous breathing trials (via the mechanical ventilator or with a T-piece) alternating with mechanical ventilatory support
Box 47-7 summarizes evidence-based criteria and related conclusions regarding methods for ventilator discontinuation.
Rapid Ventilator Discontinuation
When the precipitating disease state or condition that necessitates support has been significantly alleviated or reversed, most patients can be rapidly removed from mechanical ventilation.16,21 These patients typically have acceptable blood gas levels with mechanical ventilation, adequate myocardial function, and no underlying cardiovascular, pulmonary, neurologic, or neuromuscular disorders.21
After a careful evaluation, patients in stable condition who have been treated with a ventilator for less than 72 hours and who have a good spontaneous respiratory rate and minute ventilation may undergo an SBT on the ventilator or with a T-piece for 30 to 120 minutes.3,7 If the patient does well, the endotracheal tube can then be removed if there is no reason to maintain an artificial airway.
Today, most SBTs are performed with the patient attached to a ventilator that is set on zero PSV and zero CPAP. This allows for a stable FiO2 and ongoing monitoring. Others have recommended treating the patient with a low level of PSV (i.e., 5 to 7 cm H2O) to overcome the resistance caused by the ventilator circuit, the demand flow system, and the artificial airway.2 Others may prefer simply using CPAP (i.e., 5 to 7 cm H2O).2,3 These methods have the advantage of allowing the clinician to maintain ventilator alarm settings that can provide a margin of safety in the event of apnea or severe hypoventilation. Low levels of CPAP may be useful for maintaining lung volumes and overcoming intrinsic PEEP.2 Some experts recommend PSV in combination with PEEP or CPAP.2 However, the most widely accepted guidelines3 recommend a simple spontaneous breathing trial for the first SBT using the ventilator at zero CPAP and zero PEEP or a T-piece.
Patients Who Need Progressive Weaning of Ventilatory Support
Patients who have been receiving mechanical ventilation for more than 72 hours and those with marginal oxygenation, ventilatory, cardiovascular, or medical statuses may need a more prolonged period for ventilator discontinuation.19,21 The most common methods for accomplishing this are SBTs interspersed with continued ventilatory support, SIMV, and PSV.4
Spontaneous Breathing Trials
The oldest discontinuation method is an SBT via either the ventilator or T-piece, which allows for spontaneous breathing several times per day interspersed with periods of mechanical ventilatory support. For gradual weaning, initial SBTs may last only 5 to 10 minutes.4,7 Full ventilatory support is resumed for a 1- to 4-hour rest period in the assist-control or pressure-support mode. The time off of the ventilator is gradually increased until the patient is able to stay off of the ventilator for an extended period. SBTs can progress rapidly to ventilator removal.
When weaning is very difficult, the process can last days or even weeks. When a T-piece is used instead of the ventilator, the patient must be more carefully monitored, because none of the ventilator alarms are active during the SBT. If distress occurs, mechanical ventilation is resumed. In no case should the patient be overstressed during the SBT, because exhaustion can delay weaning.2,8 An example of an SBT is presented in Box 47-8.
In one major multicenter study, one or more daily SBTs resulted in three times faster extubation than with SIMV and two times faster than with PSV40 (Figure 47-2). Results of another study favored PSV over T-tube trials or SIMV47 (Figure 47-3). In a third study, SBTs were more effective than SIMV for weaning quadriplegic patients.41 Advantages of SBTs include the early and frequent testing of the patient’s ability to breathe spontaneously without support.2,3 The use of SBTs several times per day has become unpopular, because it requires a great deal of time on the part of ICU staff, and a single daily SBT seems to be just as effective for most patients.3,4,10 PSV and CPAP are sometimes used during the SBT, and patients who tolerate spontaneous breathing with a low level of PSV (i.e., 7 cm H2O or less) should be able to tolerate extubation.42 Current recommendations suggest a single daily trial of spontaneous breathing may be preferable to other methods of weaning.3 Results of randomized, controlled studies have shown SBTs to be faster than SIMV and at least as effective as PSV.3,4,10 Furthermore, performing an SBT one time per day is as effective as performing SBTs several times per day, and 30-minute trials are as effective as 120-minute trials.4
A collective task force representing the American College of Chest Physicians, the American Association for Respiratory Care, and the American College of Critical Care Medicine published evidence-based guidelines for weaning and discontinuing ventilatory support.3 These guidelines were based in part on a comprehensive review of the evidence collected under the direction of the U.S. Agency for Health Care Policy and Research.6
The task force made 12 specific recommendations (Box 47-9). These include the regular use of formal and carefully monitored SBTs to guide clinical decision making regarding the discontinuation of ventilatory support.3 Specifically, the task force recommended that, for the care of patients who need mechanical ventilation for more than 24 hours, a search for all possible causes of ventilatory dependence should be conducted and an attempt should be made to reverse or optimize each condition. Spontaneously breathing patients with evidence of reversal of the underlying condition that necessitated the ventilatory support, adequate oxygenation, and hemodynamic stability should be assessed with an SBT. Table 47-3 lists criteria to be used when determining whether patients who are receiving high levels of ventilatory support can be considered for SBTs.
TABLE 47-3
| Criteria | Description |
| Objective measurements | Adequate oxygenation (e.g., PO2 of ≥60 mm Hg with an FiO2 of ≤0.40 to 0.50; PEEP of ≤5 to 8 cm H2O; PO2/FiO2 ratio of ≥150 to 200) |
| Stable cardiovascular system (e.g., heart rate ≤140 beats/min; stable blood pressure; no [or minimal] pressors) | |
| Afebrile (i.e., temperature about 37° C) | |
| No significant respiratory acidosis | |
| Adequate hemoglobin (e.g., ≥8 to 10 g/dl) | |
| Adequate mentation (e.g., arousable, Glasgow coma score of ≥13, no continuous sedative infusions) | |
| Stable metabolic status (e.g., acceptable electrolyte levels) | |
| Subjective clinical assessments | Resolution of acute phase of disease; physician believes discontinuation is possible; adequate cough |
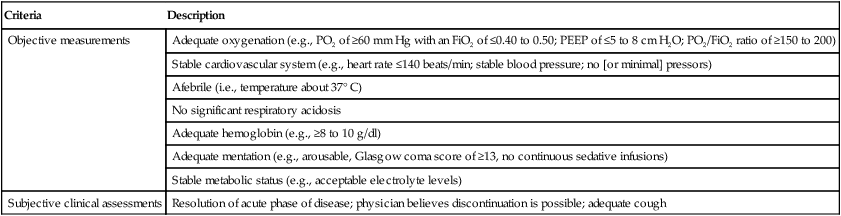
Modified from MacIntyre NR, Cook DJ, Ely EW, et al: Evidence-based guidelines for weaning and discontinuing ventilator support: a collective task force facilitated by the American College of Chest Physicians, the American Association for Respiratory Care, and the American College of Critical Care Medicine. Chest 120:375S–395S, 2001.
A daily screen that includes the assessment of oxygenation, PEEP, and the use of vasopressors and sedatives should be conducted. A PaO2/FiO2 ratio of more than 150 to 200, a PEEP value of less than 5 to 8 cm H2O, and a lack of a continuous infusion of vasopressors or sedatives (i.e., less than 5 mg/kg/min of dopamine) were good predictors of successful extubation and survival.41
The formal SBT may be performed in combination with low levels of PSV (i.e., 5 to 7 cm H2O), CPAP (i.e., 5 cm H2O or less), or automatic tube compensation applied to just offset the imposed WOB caused by the endotracheal tube.3 During the SBT, the patient is carefully observed for breathing comfort, anxiety, agitation, dyspnea, or diaphoresis. Respiratory rate, heart rate, blood pressure, and SpO2 are monitored. The SBT is terminated if signs of distress are observed, including the following:
• Agitation, anxiety, diaphoresis, or a change in mental status
• A sustained respiratory rate of more than 35 breaths/min
• A sustained SpO2 of less than 90%
• A more than 20% increase or decrease in heart rate or a heart rate of more than 120 to 140 beats/min
• A systolic blood pressure of more than 180 mm Hg or of less than 90 mm Hg
Patients with unsuccessful results of an SBT are returned to full ventilatory support for 24 hours to allow the ventilatory muscles to recover. During this period, the causes of failure are identified and corrected, if possible, and the patient is then reevaluated. If the criteria listed in Table 47-3 continue to be met, SBTs are repeated every 24 hours.3 Patients who tolerate the formal SBT for 30 to 120 minutes remain off of the ventilator, and extubation is considered. Box 47-10 is a sample protocol for an SBT for the discontinuation of ventilatory support.
Continuous Positive Airway Pressure
CPAP is used during an SBT in many facilities. CPAP has the advantages of maintaining the lung volume during the weaning phase and thus of improving the patient’s oxygenation status. Minimal levels of CPAP may be useful for reducing WOB and compensating for auto-PEEP, particularly in patients with obstructive lung disease.2 CPAP is usually provided through the use of the CPAP mode that is available on most mechanical ventilators. By using the ventilator in the CPAP mode, the clinician can take advantage of the alarm systems that are available. This may provide an improved margin of safety as compared with the use of a T-piece. However, in one study, CPAP was no more effective as compared with T-piece trials for the weaning of patients who were recovering from coronary artery bypass graft surgery.43
Synchronized Intermittent Mandatory Ventilation
SIMV has been advanced since the early 1970s as a method that can speed weaning and ventilator discontinuation.8 SIMV can be used to provide full or partial ventilatory support. Weaning from SIMV involves the gradual reduction of the machine rate on the basis of the results of ABG analysis and patient assessment.7 Early claims that SIMV allowed for faster weaning times8 have not been substantiated by subsequent studies.44 However, SIMV is a commonly used mode of ventilation throughout the United States.45,46
Patients who are receiving ventilation in the SIMV mode uncouple their breathing efforts from the support provided by the machine.2 They continue to make spontaneous breathing efforts during the delivery of a “mandatory breath.” Evidence also suggests that, once the machine cycling rate is reduced to approximately 50% of the full ventilatory support value, the patient breathes approximately as hard per cycle as when ventilatory support is completely withdrawn.2 SIMV provides a higher-level of WOB than control46 or assist-control47 ventilation, although the increase in WOB is not always evident.46 This additional work can be overcome with the use of pressure support (i.e., 5 to 10 cm H2O). However, the addition of pressure support further complicates the weaning process. For initial ventilator setup, the SIMV rate and the tidal volume usually are set at values that are equivalent to those used during volume control-continuous manditory ventilation (VC-CMV) or pressure control-continuous mandatory ventilation (PC-CMV). When the patient’s condition has been stabilized, one of two approaches can be used. Some clinicians prefer to continue at these settings until the patient’s precipitating disease state or condition has improved considerably.2 At that point, the rate is reduced in a stepwise manner until complete spontaneous breathing can be achieved.2 Other clinicians prefer to immediately reduce the level of mechanical ventilation, thereby forcing the patient to perform additional WOB.1,2 From the beginning, attempts are made to reduce the SIMV rate, and the patient is challenged to provide a portion of the required ventilation.2 The clinician adjusts the ventilator to make up the difference between what the patient is able to do and the total ventilation needed.2 The rationale for this partial ventilatory support approach is that only the minimal level of mechanical support should be provided. The level of partial ventilatory support is titrated up or down the entire time that the patient is ventilated on the basis of changes in the patient’s condition.
Unfortunately, research indicates that SIMV prolongs ventilatory support and that it is the least effective method of weaning from ventilatory support as compared with either SBT or PSV.6,10,40,49
Pressure Support Ventilation
PSV is a mode of ventilatory support that allows the patient to have significant control over the process of ventilatory support. The only gas-delivery variable directly controlled by the ventilator is peak airway pressure; see Chapters 42 and 44 for details.
For initial ventilator setup in the PSV mode, the beginning pressure level can be adjusted to deliver an appropriate tidal volume, which is usually approximately 6 to 10 ml/kg of the ideal body weight based on the patient’s condition and the desired tidal volume. PSV is then gradually reduced to a minimal value that only compensates for the WOB imposed by the artificial airway.48–50 Generally this is about 5 to 8 cm H2O. After this reduction is accomplished, extubation can be performed directly from the low level pressure support, or an SBT may be conducted for 30 to 120 minutes.2,3
PSV allows the clinician to manipulate the level of patient work, but the benefit of this to weaning is questionable.1,2 In general, patients who can spontaneously breathe comfortably at 5 to 8 cm H2O of PSV can be extubated without problems.4 However, if upper airway edema is present, WOB after extubation may be about the same as that caused by the endotracheal tube.4 In these cases, low levels of pressure support may give a false impression about the patient’s ability to tolerate extubation4 (Box 47-11).
Synchronized Intermittent Mandatory Ventilation With Pressure Support Ventilation
Although it is clear that the addition of PSV during SIMV can reduce or eliminate imposed work caused by mechanical factors, it has not been shown how this affects weaning. In one study, SIMV with PSV increased tidal volume and reduced respiratory rate but did not significantly reduce weaning time or success as compared with SIMV alone.51 On the basis of all of the weaning trials, it can be concluded that this approach to weaning can only increase the length of ventilatory support.38,49 Current guidelines recommend the use of SBT to rapidly wean patients from ventilatory support.3
Spontaneous Awaking Trials
Concern regarding the use of sedation in critically ill patients has increased markedly during the last few years. This concern has focused on the issue of delirium.52 The indiscriminate use of sedatives in the ICU is considered inappropriate patient management. In general, sedatives should always be considered last when trying to handle an agitated patient. Something has caused the patient’s agitation, and it should be addressed and corrected, if possible, before sedation is administered. In addition, it is becoming clearer that the use of excessive sedation lengthens the time of mechanical ventilation. A recent clinical trial compared an SBT alone with SBT with a spontaneous awaking trial (SAT). The SAT required that all of the sedation that a patient was receiving be stopped at midnight and the patient reassessed. If the patient did continue to require sedation, it was restarted at half the dose that the patient previously received. The use of the SAT along with the daily SBT resulted in the faster weaning of patients from ventilatory support as well as a decrease in mortality.53 The authors argued that the decrease in morality was a result of patients being exposed for a shorter time to the complications of mechanical ventilation. Throughout the course of mechanical ventilation, sedation should be kept to the bare minimum necessary; patient sedation should be periodically stopped and the patient carefully assessed before additional sedation is given.53
Newer Techniques for Facilitating Ventilator Discontinuance
Mandatory Minute Volume Ventilation
Mandatory minute volume ventilation (MMV) was described by Hewlett, Plott, and Terry and introduced in 1977 in Great Britain.54 With this mode of ventilation, the total minute volume is set, and the patient may elect to inspire all of the minute volume, part of the minute volume, or none of the minute volume spontaneously. The ventilator would automatically provide a clinician set level of minute volume. It was originally assumed that, as the patient’s status improved, he or she would assume a greater and greater percentage of the set minute volume, eventually inspiring it all spontaneously. However, this did not occur; patients generally settled into a ventilator pattern where the overall WOB was shared between the patient and the ventilator, and the patient never assumed a greater percentage of the work.55 There is no data to support the use of MMV over SBT or PSV to wean patients from ventilatory support.
Adaptive Support Ventilation
ASV is a newer mode of ventilation that maintains a minimum minute ventilation with an optimal breathing pattern (tidal volume and rate) and that is based on the work of Otis;56,57 see Chapters 42 and 44 for details. ASV automatically adjusts inspiratory pressure and ventilator breath rate to achieve the target minute volume set. As the patient’s status improves, the target minute volume is reduced, and the level of pressure required each breath diminishes. When a minimal level is reached, the patient is considered weaned from ventilatory support and ready for extubation.
Preliminary studies of ASV for the care of patients who are recovering from cardiac surgery show a reduction in the duration of mechanical ventilation as compared with an SIMV weaning protocol.57,58 The ASV weaning protocol used in the study incorporated two 50% reductions in minute ventilation. Each reduction was followed by an assessment of patient tolerance according to criteria that were similar to those suggested for SBTs. Additional data about ASV indicates that it works very well for patients who are under controlled ventilation,59 but additional data from a large heterogeneous group of patients will be necessary to determine if AVS truly improves the speed of ventilator discontinuation.
Computer-Based Weaning
Current versions of MMV and ASV are examples of computer-controlled mechanical ventilation. Several more complex systems have been developed, including Ventilation Manager, VQ-attending, ESTER, Continuous Respiratory Evaluator (CORE), KUSIVAR, and WEAN-PRO.19 The desire to develop computer-based weaning protocols is based on two factors. First, weaning is a time-consuming and labor-intensive process. If computer control can expedite or simplify this process, considerable time and money can be saved. Second, because most weaning decisions are based on objective data, computer-based weaning protocols are relatively easy to develop. In one study in which physician-directed weaning was compared with computer-based weaning, the computer-based system resulted in the less-frequent assessment of ABGs and shorter weaning times.19 Another study showed that a handheld computer weaning protocol for use by respiratory therapists was more effective than a paper-based weaning protocol.60
The most successful computerized application for weaning is the Smart Care approach, which is based on data from Laurent Brochard’s group.61 The system adjusts pressure support on the basis of the patient’s tidal volume, respiratory rate, and end-tidal carbon dioxide level. When the pressure support has been decreased to a predefined level, the ventilator automatically begins an SBT. If the patient fails the SBT, which is determined by changes in the patient’s respiratory rate, tidal volume, and end-tidal carbon dioxide level, then the ventilator automatically reassumes ventilatory support. If the patient passes the SBT, the ventilator also returns to baseline ventilatory support but notifies the clinician that the patient is ready for ventilator discontinuation. In a randomized comparison with clinician-applied SBT, this system weaned patients faster and decreased the total number of days that patients were maintained in the ICU as well as the need for postextubation NIV. One criticism of this study was that the clinician-applied SBTs were not always performed properly; however, this is clinical reality, and if an automated system can wean patients faster, it will have a place in the care of critically ill patients.
Automatic Tube Compensation
ATC is an option on newer mechanical ventilators that compensates for the flow-dependent pressure decrease across the endotracheal tube during both inspiration and expiration; see Chapters 42 and 44 for details. ATC reduces WOB and may improve patient comfort.62 Because it compensates for the imposed WOB caused by the artificial airway, ATC has been referred to as electronic extubation. Patients who are able to breathe adequately with the addition of ATC at low peak airway pressure (i.e., less than 8 cm H2O) should tolerate extubation. ATC is similar to pressure support in that an inspiratory pressure is used to compensate for imposed WOB; however, ATC varies the pressure, depending on the size of the endotracheal tube and the patient’s inspiratory flow rate. Alternatively, PSV delivers a preset inspiratory pressure that may overcompensate or undercompensate imposed WOB at any given point in time. However, no data is available to indicate that ATC weans patients faster than SBTs or PSV.
Volume Support
A major problem with VC is the excessive reduction of airway pressure in the presence of high ventilatory demand, thereby increasing patient effort.63 With most ventilators offering this mode, no lower limit to ventilating airway pressure is set, and inspiratory airway pressure can decrease all the way to the PEEP level. Volume support may offer a sort of automatic weaning from pressure support. As the patient’s spontaneous tidal volume improves, the level of pressure support is automatically reduced. Clinical trials indicate no benefit to the use of VC to wean patients.64
Noninvasive Ventilation
1. Transitioning patients with COPD who failed SBTs from invasive ventilation to spontaneous breathing65–67
2. Supporting patients who passed SBTs but who were considered to be at high risk for failing the extubation68–70
3. Supporting patients who developed hypoxemic respiratory failure after extubation71,72
The literature strongly supports the first two indications but does not support the third application. Refer to Chapter 45 for details regarding the application of NIV during weaning and the use of NIV in general.
Respiratory-Therapist–Driven Protocols
A number of randomized, controlled clinical trials have demonstrated that patients are weaned faster with the use of protocols than with individual physician orders.73–76 In all of these studies, the therapists, nurses, and physicians who normally manage patients in the unit developed the protocol. In addition, in the individual physician order group, the physicians who wrote the protocol also wrote the individual weaning orders. In all of these trials, patients who were randomized to the protocol group weaned faster and with less complications then the patients randomized to the individual physician group.73–76
What these results do not mean is that therapists wean patients better than physicians. However, what they do mean is that, when an evidence-based approach to patient management is developed and followed precisely, care is generally better than with individual physician orders. The reason for this is that protocols empower the clinician at the bedside to advance care when the patient meets specific criteria without waiting for the physician to come and write an order. In many community hospitals, the wait for the physician could be an hour or a whole shift or more. Thus, with protocols, care processes rapidly on the agreed-upon course. In addition, the individual bias of the caregiver does not affect the manner in which care is provided. Thus, the approach does not change from day to day depending on which physician is at the bedside. An example of a respiratory-therapist–directed ventilatory discontinuance protocol is presented in Box 47-12.
Selecting An Approach
Current evidence suggests that, for progressive weaning from mechanical support, it may be best to avoid SIMV.3,6,10 It has also been suggested, regardless of the mode of weaning used, that protocols administered by respiratory therapists and other health care workers be developed.3,6,10 These protocols should be designed to begin testing for the opportunity to reduce support very soon after intubation and to reduce the level of ventilatory support at every opportunity.6,73 The approach to weaning that seems to wean patients most rapidly is the SBT. This should be the approach that is used to identify readiness for ventilator discontinuance in the vast majority of patients.6 In addition, NIV should be considered as part of the total ventilator discontinuance process.74
One area that requires additional research is the role of ventilatory muscle conditioning in patients who require long-term ventilatory support. Endurance conditioning of the ventilatory muscles can be achieved by the continuous repetition of low levels of ventilatory work. Strength conditioning can be achieved by maximal ventilatory effort for short periods. In theory, PSV would allow for improving ventilatory muscle endurance, whereas intermittent SBTs would favor the development of muscle strength. Inspiratory resistive training to improve ventilatory muscle strength has been tried as an adjunct to weaning for the care of patients undergoing long-term ventilation.19 Unfortunately, the role of ventilatory muscle rest and load in the care of difficult-to-wean patients has not been established.19
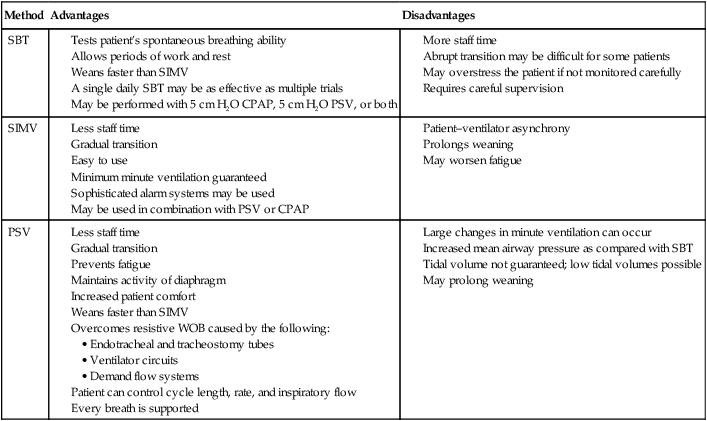
In summary, a single daily SBT that lasts from 30 minutes to 2 hours has been recommended as the primary approach to weaning.3,4 If the trial is successful, extubation is considered. If the trial is unsuccessful, at least 24 hours of ventilatory support is provided before another trial is attempted.3 There are advantages and disadvantages to each of the methods used to conduct ventilator weaning. Table 47-4 compares SBT, SIMV, and PSV as weaning techniques. The best approach may be the one with which a given clinician is most familiar.8 It should be based on knowledge of the patient’s condition, a sound rationale, and good clinical experience.2,8 The method chosen should include careful patient assessment, and the patient’s condition should be optimized before weaning. For the vast majority of patients, a protocolized approach to ventilator discontinuance is most efficient.
TABLE 47-4
Monitoring the Patient During Weaning
Ventilatory Status
Respiratory rate and pattern are easy to monitor and may be the most reliable indicators of patient progress during weaning.1,5 Weaning may proceed as quickly as the patient’s respiratory rate and subjective tolerance allow.1 However, in no case should patients be pushed beyond their physiologic limits; to do so may result in diaphragmatic dysfunction and further delay the weaning process.2,4,6 If the patient is allowed to fatigue during a weaning trial, it will require at least 24 hours for the muscles to recover.77,78 Dyspnea should be monitored during weaning and may be quantified with a visual analog scale or a modified Borg scale79 (Table 47-5). The onset or worsening of discomfort, respiratory distress, fatigue, sweating, signs of increased WOB (e.g., accessory muscle use, abdominal paradox), deterioration in vital signs, or changes in mental status (e.g., agitation, anxiety, somnolence, coma) may be signs of intolerance of a weaning trial.3
TABLE 47-5
Modified Borg Scale for Dyspnea
| Grade | Degree of Dyspnea |
| 0 | None |
| 0.5 | Very, very slight (just noticeable) |
| 1 | Very slight |
| 2 | Slight |
| 3 | Moderate |
| 4 | Somewhat severe |
| 5 | Severe |
| 6 | Very severe |
| 7 | |
| 8 | |
| 9 | Very, very severe (almost maximal) |
| 10 | Maximal |
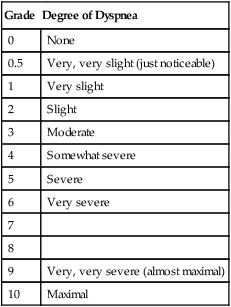
Modified from Mahler DA: Dyspnea, Mt. Kisco, NY, 1990, Futura.
The single best index of ventilation remains the measurement of PaCO2. However, the assessment of a patient’s tolerance of an SBT should be based on clinical presentation rather than PaCO2. A patient with a PaCO2 of 40 mm Hg but a clinical presentation defined by a rapid, shallow breathing pattern with abdominal paradox, tachycardia, and hypertension has not passed an SBT. The results of capnography should not be used to guide the weaning of patients with pulmonary parenchymal disease.80 End-tidal PCO2 values can be highly misleading as an estimate of effective ventilation in sick patients.80 Gastric pH has been used as a predictor of patient status, and gastrointestinal acidosis may be an early sign of weaning failure.81
Oxygenation
Continuous pulse oximetric (SpO2) monitoring can provide a sensitive indicator of oxygenation status during weaning.5 Arterial blood gas analysis for PaO2 and SaO2 and the calculation of the oxygen content of the arterial blood (CaO2) should be performed if doubt exists when assessing a patient’s oxygenation status during weaning.
Cardiovascular Status
Pulse, blood pressure, and cardiac rhythm should be monitored, and arrhythmias should be assessed to determine whether weaning should be continued. Tachycardia, bradycardia, and abnormalities in blood pressure should be promptly evaluated and the patient returned to ventilatory support or a higher level of support, if indicated. Silent myocardial ischemia may occur frequently in some postoperative patients during weaning.82 Table 47-6 summarizes changes that may occur during the withdrawal of ventilatory support.
TABLE 47-6
Changes During the Withdrawal of Ventilatory Support
| Expected Change | Deleterious Change |
| Respiratory | |
| Respiratory rate minimally increased | Respiratory rate of ≥35 breaths/min |
Stable 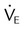 |
Large increase or decrease in 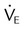 |
| SpO2 ≥ 90% | Decrease in SpO2 to ≤90% |
| 5 to 10 mm Hg swing in PaO2 | PaO2 of <60 mm Hg |
| 5 to 10 mm Hg swing in PaCO2 | An increase of >10 mm Hg in PaCO2 |
| pH of >7.30 and <7.50 | pH of <7.30 |
| Minimal use of accessory muscles | Increased use of accessory muscles |
| No paradoxical breathing | Paradoxical breathing Diaphoresis Dyspnea |
| Cardiovascular | |
| Heart rate increased by 15 to 20 beats/min | Persistent tachycardia of ≥120 to 140 beats/min |
| Blood pressure increased 10 to 15 mm Hg | Hypotension (blood pressure of <90/60 mm Hg) Hypertension (systolic blood pressure of >180 mm Hg) |
| Increased cardiac index | Decreased cardiac index |
| Increased stroke volume | Decreased stroke volume Angina New arrhythmias Increased pulmonary capillary wedge pressure |
| Other | |
| Mental status good (i.e., awake, alert, responsive) | Anxiety, agitation, somnolence, coma |
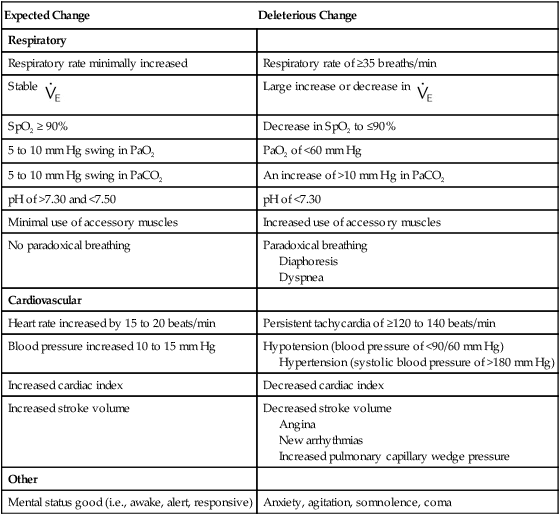
Extubation
Artificial Airways and Weaning
The effect on a patent of a properly sized artificial airway on WOB is controversial. In a study with 14 successfully extubated patients, at the end of a 2-hour SBT, there was no difference in WOB before and after extubation.83 However, many endotracheal tubes are partially obstructed and as a result impose a significant increase in WOB. Removing the endotracheal tube may markedly improve the patients’ clinical status.
There is little difference in the airway resistance of healthy adults with low minute ventilation (i.e., 8 L/min) breathing through endotracheal tubes with an internal diameter (ID) of 7, 8, or 9 mm.84 Decreases in ID and increases in minute ventilation increase WOB. Adults have a critical increase in workload when the tube has an ID of less than 7 mm.84 It is thought that the added work due to the presence of an artificial airway may contribute to ventilator dependency among patients with borderline pulmonary function or ventilatory muscle weakness.84,85
Endotracheal tubes themselves can cause reflex bronchoconstriction. Consequently, some experts believe that the routine use of bronchodilators is justified in the care of all intubated patients. Dried secretions in the endotracheal tube can cause dramatic increases in airway resistance, especially among infants and children.84,85 Taking care to provide adequate humidification can help to avoid this problem.
Tracheotomy may substantially reduce the WOB of patients who need mechanical ventilatory support.74 The short length of tracheotomy tubes results in an overall decrease in resistance as compared with the resistance of an endotracheal tube, even though the curvature of the tracheotomy tube is greater.3 It appears that the performance of a tracheotomy improves airway resistance and reduces the load of the ventilatory muscles. The clinical benefit of this improvement in terms of weaning has not been established.3
Weaning and extubation should be separate decisions.29 Weaning indices are not predictive of the adequacy of airway patency or the need for the protection of the airway.29 The reintubation rate among patients with prolonged postoperative ventilation as a result of respiratory failure can range from 5% to 20%.29
Patients who have been successfully extubated generally have the following characteristics: (1) the resolution of the disease state or condition; (2) hemodynamic stability; (3) the absence of sepsis; (4) adequate oxygenation status with a decreased FiO2 and decreased PEEP or CPAP; and (5) adequate ventilatory status and PaCO2.29 The decision to extubate should be based on the assessment of upper airway patency and protection.29 No one indicator is 100% sensitive and specific with regard to the prediction of successful extubation.29 Practical guidelines for extubation are presented in Box 47-13. Regardless of the weaning technique used, an SBT is recommended before extubation to ensure that the patient can sustain spontaneous unsupported ventilation.2
Some patients may be successfully extubated even if extubation criteria are not met.29 If the patient is at risk, trained personnel who are able to perform reintubation must be immediately available before extubation is attempted. At a minimum, those who perform the extubation must be prepared to provide an airway and ventilatory support in the event that problems develop immediately after extubation. Extubation should be postponed when myocardial ischemia is present, when the patient has upper gastrointestinal hemorrhage, or when a procedure that necessitates reintubation is impending. If difficult reintubation is anticipated, trained personnel should be immediately available.29
Many patients report hoarseness and sore throat after extubation, and patients should be advised that these symptoms may occur. Other common problems after extubation include airway obstruction, increased risk of aspiration, and difficulty with secretion clearance.29 Patients with neurologic or neuromuscular disorders and those with excessive secretions are at increased risk after extubation.28 The compression of the airway as a result of a traumatic or postoperative hematoma of the neck, infectious masses or abscesses, and malignant tumors or compression after major head or neck surgery can lead to upper airway obstruction after extubation.29 The cuff leak test may detect airway obstruction before extubation; however, results of studies addressing this are controversial.86,87 An air leak of less than 11% to 12% or 110 to 130 ml has been shown to be predictive of stridor.86,87 Box 47-14 describes the cuff leak test.
After extubation, glottic edema can result in partial airway obstruction, which can cause mild to severe stridor.29 Postextubation stridor occurs in 2% to 16% of patients in the ICU and should be viewed with concern.88 Severe edema after extubation can lead to complete airway obstruction.29 Children, patients with epiglottitis or angioedema (i.e., dermal, subcutaneous, or submucosal edema of the face or larynx), and patients who have sustained smoke inhalation are at greater risk.29 Postextubation edema occurs in as many as 47% of children with trauma injuries or burns.29 See Chapter 33 for detailed information about airway management and postextubation care.
Ventilator Discontinuance Failure
As many as 25% of patients who have been removed from ventilatory support experience respiratory distress that is severe enough to necessitate the reinstitution of mechanical ventilation.4 In patients who are unlikely to be successfully weaned, rapid, shallow breathing begins almost immediately after the ventilator is disconnected.4 As spontaneous breathing continues, respiratory mechanics worsen in these patients for reasons that are not clearly understood.4 Approximately half of patients who have poor results after the discontinuation of ventilation experience marked hypercapnia as a result of rapid, shallow breathing.4 An unsuccessful SBT also causes considerable cardiovascular stress.4 Myocardial ischemia may occur frequently among ventilator-dependent patients, and it has been associated with weaning failure.89 Critical illness polyneuropathy has been cited as a frequent cause of neuromuscular weaning failure among critically ill patients.19,90 Unsuspected neuromuscular disease may be an important factor in ventilator dependency.19
Inability to wean can sometimes be attributed to psychologic dependence, poor oxygenation status, or cardiovascular instability (i.e., congestive heart failure or ischemia).2 However, the most common cause of the inability to wean is an imbalance between ventilatory capability and ventilatory demand.2 Inability to wean is usually caused by a concurrent pathologic process that necessitates treatment.2,3 Common causes of weaning failure are summarized in Box 47-13.
Prolonged Mechanical Ventilation
Prolonged mechanical ventilation (PMV) may be required for 3% to 7% of patients who are receiving mechanical ventilation.3 Patients who require a lengthy course of ventilatory support after the acute phase of their disease has resolved may be suffering from some of the items listed in Box 47-14. Any patient who is repeatedly failing SBTs should undergo a complete review of all systems to determine if something had been overlooked that may add to the patient’s workload, thereby preventing them from weaning. In many patients, their pulmonary mechanics have not been optimized and thus they are unable to assume the workload required of spontaneous breathing. In others, underlying cardiac disease may not have been properly treated. Always a concern is the patient’s acid–base status. If a patient had baseline compensated respiratory acidosis, then he or she will not be able to wean if the compensation has been eliminated. Every time these patients are trialed on an SBT, their carbon dioxide levels will rise, and they will develop respiratory failure. If this is the problem, the baseline acid–base status must be slowly reestablished if these patients are to wean. In other patients, sedation, opiates, nutritional status, electrolytes, and other underlying issues many be the concern. The more carefully all systems are reviewed, the greater the likelihood that the problem will be identified and the patient weaned from ventilatory support.
Chronically Ventilator-Dependent Patients
Chronically ventilator-dependent patients (i.e., less than 1% of those requiring ventilatory support) present ethical, economic, and practical problems.17,91 From an economic point of view, the long-term care of ventilator-dependent patients in an ICU is prohibitively expensive. Often these patients are transferred to subacute or long-term care facilities, where they can be cared for in an environment that is less intrusive and more like home.91 For cases in which the family has adequate resources, the patient may be cared for in the home. Regional weaning centers have been developed and have reported success with weaning most patients who are undergoing long-term mechanical ventilation.3,16,91 Current guidelines suggest that, unless there is clear evidence of an irreversible cause of ventilator dependency (e.g., a high spinal cord injury, amyotrophic lateral sclerosis), patients should not be considered permanently ventilator dependent until 3 months of weaning attempts have failed.3 If the patient is unweanable, the goal should be to restore the patient to the highest level of independent function possible.15 For example, portable wheelchair-mounted ventilators have been effective for providing a surprising level of mobility and independence to persons who are quadriplegic. Table 47-7 describes the management of common problems among difficult-to-wean patients. Box 47-15 lists the goals of weaning of patients who are receiving long-term ventilation.
a. Apply pressure support or ATC.
b. Change the size of the small endotracheal tube.
c. Cut the length of endotracheal tube if it is 2 in (5 cm) past the mouth.
d. Deflate the cuff if all breathing is spontaneous and the risk of aspiration is minimal.
1. Provide exercise therapy to increase muscle function, prevent contracture, and maintain joint integrity (i.e., passive-to-active range of motion and sitting to walking).
2. Increase strength during activities of daily living.
3. Secure a physiotherapy consultation.
4. Consider the use of an exercise bicycle.
5. Encourage wheelchair rides or walks with a portable ventilator.
1. Secure early psychiatric consultation.
3. Demonstrate staff accountability and honesty.
4. Provide a communication method.
5. Decrease environmental stress.
7. Provide mental stimulation.
9. Provide rewards for reaching short-term goals.
11. Allow other patients to visit.
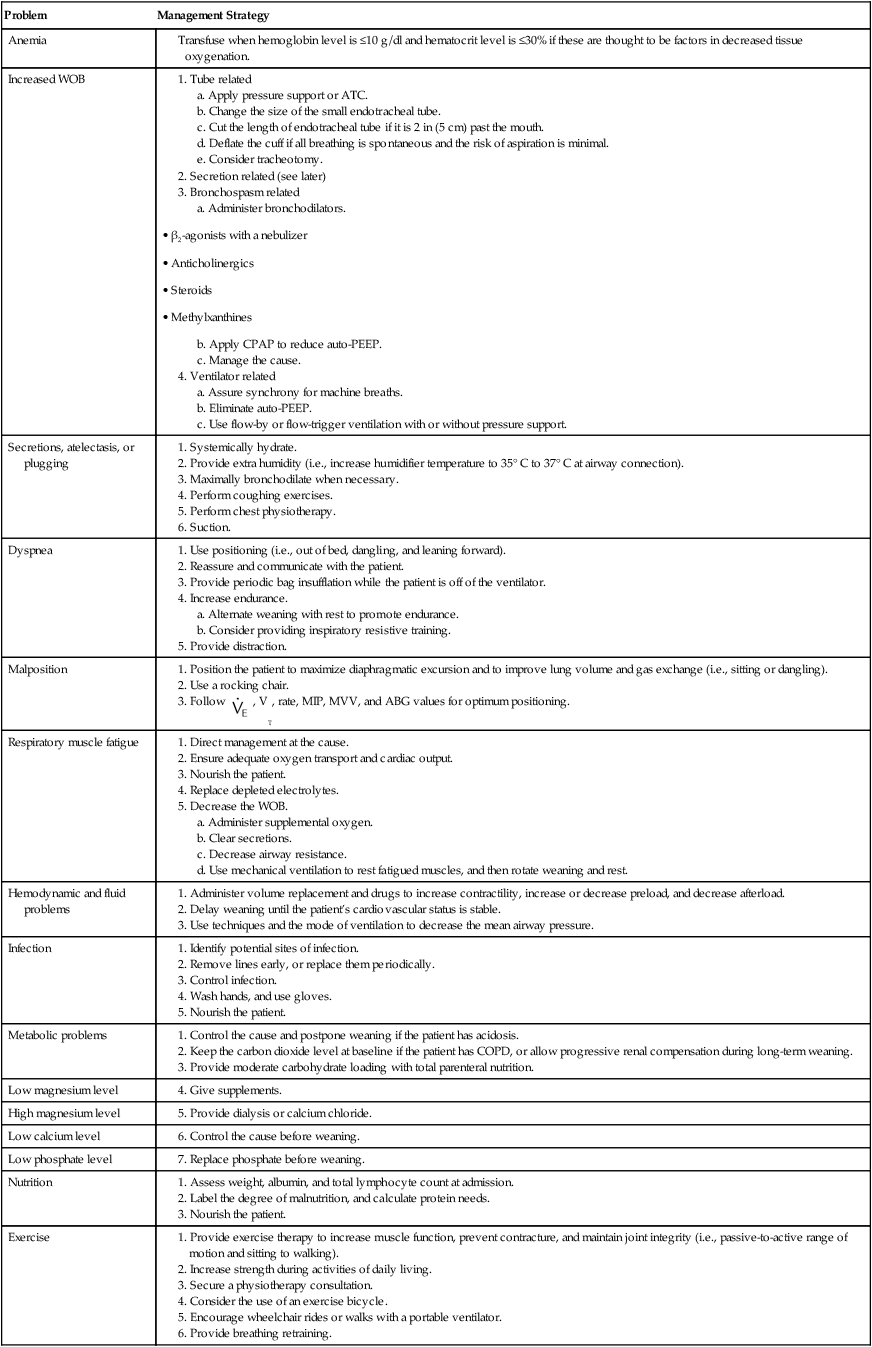
Modified from Norton LC, Neureuter A: Weaning the long-term ventilator-dependent patient: common problems and management. Crit Care Nurse 9:42–52, 1989.
Terminal Weaning
The term terminal weaning has been used to refer to the discontinuation of mechanical ventilatory support in the face of a catastrophic and irreversible illness.92 The decision to proceed with disconnecting the ventilator when such an act is likely to cause the death of a patient is fraught with ethical, emotional, and practical problems. The decision should be made by the family in consultation with the patient’s physician and in accordance with established ethical and legal guidelines.92 Determinants of the decision to withdraw ventilation include the patient’s desire to not continue with life support, the predictions of a low chance of survival in the ICU (i.e., less than 10%), the likelihood that future cognitive function would be severely impaired, and the continuous need for inotropes or vasopressors to maintain blood pressure.93 After the decision has been made, the process is generally one of ventilator disconnection rather than weaning. The method of terminal weaning should be as humane and as comfortable as possible and should not be done in a way that further burdens the family.92

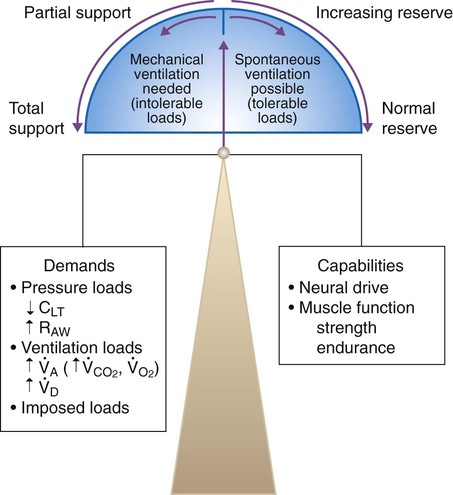
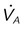 , minute alveolar ventilation;
, minute alveolar ventilation; 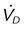 , minute dead space ventilation.
, minute dead space ventilation. 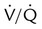
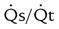



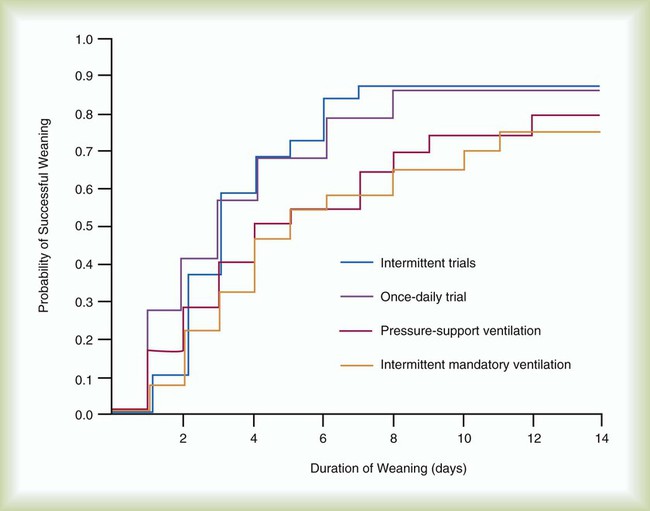
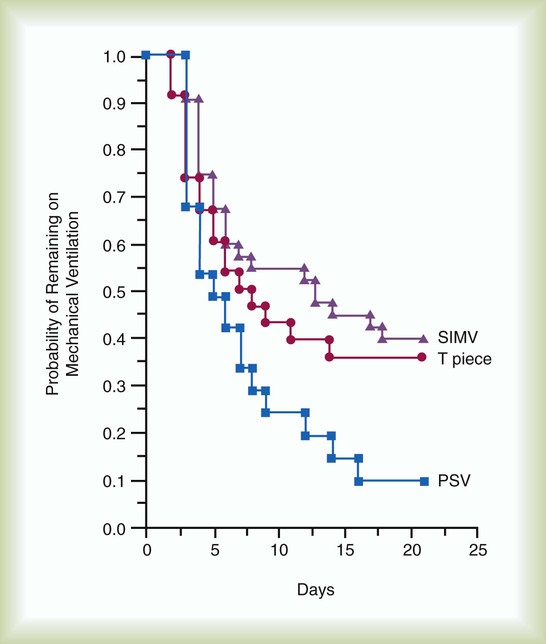
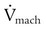 ) = 60 L/min (1 L/s)
) = 60 L/min (1 L/s)
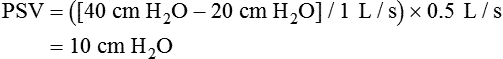
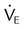 , VT, rate, MIP, MVV, and ABG values for optimum positioning.
, VT, rate, MIP, MVV, and ABG values for optimum positioning.



