38 Discography
Part A Theoretical Aspects
There are four important aspects to the concept of discography that should be implicitly understood (Table 38-1). First, although there are substantial clinicopathologic correlates in the lumbar spine, it does not define a specific pathologic process that might have rendered the disc painful. It is, in particular, a procedural test that attempts to identify a cohort of otherwise labeled nonspecific spinal pain (NSSP) patients with a particular type of discogenic pain (DP), called internal disc disruption (IDD). Second, in IDD there is no breach of the outer annulus fibrosus (AF); thus, when the disc is pressurized for the subjective part of the discogram, it is possible to reproduce the patient’s pain in the case of provocation discography (PD) and reduce pain in the case of AD, as the injected material is confined to the disc. Third, discography cannot identify patients with putative DP in whom there is a breach of the external border of the AF, as consequent leakage of injected material is prone to both false-negative results (the pressure is insufficient to propagate intradiscal nociception or local anesthetic concentrations are insufficient to block nociception) and false-positive results (due to anesthetization subsequent to epidural spread of the local anesthetic). Fourth, there is no definitively proven treatment that has arisen for patients with IDD.
| Discography is a quasi-subjective test that attempts to define the disc as a source of pain: it does not establish the pathophysiology of the pain. |
| In internal disc disruption (IDD) there is, by definition, disruption of the normal internal disc architecture, often with radial or circumferential tears in the annulus fibrosis. |
| Discography cannot identify patients with putative discogenic pain in whom there is a breach of the external border of the AF. |
| There is no proven treatment that has arisen for patients with pain with IDD. |
History and Development of Discography
After 1934, when Mixter and Barr published on disc prolapse and neural compression,1 most back pain was thought to arise from disc prolapse, yet it became apparent that neural compression was found only in a relatively small number of patients with spinal pain. Furthermore, disc prolapse has been shown to be common in the asymptomatic population (for example, a prevalence of 37% in the thoracic spine).2
Discography, pioneered from 1947 by investigators such as Inman and Saunders,3 Falconer and colleagues4 and Wiberg,5 arose before the disc was considered or proven to be innervated and at the time when the cause of back pain and radicular pain was considered to be disc prolapse. The first clue to the possibility of intrinsic DP occurred when back pain was produced by probing the posterior AF during lumbar disc surgery performed under local anesthesia.6 Despite the prevailing view that the disc was aneural, various authors reported that there was a relationship between disc morphology and reproduction of back pain; and, more particularly, that morphologically normal discs were rarely painful, in contrast to degenerative discs with posterior bulges and discs with epidural leaks on discography which were virtually always painful.7,8
Researchers then attempted to determine if abnormal discs occurred in patients without pain. Massie and Stevens, who reported their findings on discography in 52 normal subjects and 570 patients, found that abnormal discs were associated with age and far more common in patients with pain than in asymptomatic controls, and that in asymptomatic subjects, abnormal discs were rarely painful on PD.9 After this PD fell into disrepute when Holt reported a false-positive rate of 37% in a group of asymptomatic prisoners,10 but a later in-depth study refuted this report on methodologic grounds.11 Subsequent to this there was a period of debate about the role of discography, leading in 1988 to a position statement on discography by NASS that emphasized the pain-response as the most important part of the procedure,12 thereby restating the fact that abnormal morphology is of minimal relevance when the diagnostic methodology seeks to establish if the disc is the source of an individual’s pain.
Then, using a more refined technique, Walsh and colleagues studied a selection of normal volunteers and patients with low back pain.13 In this study, the discographer was blinded in all respects, and the patient responses were filmed and assessed by two external observers. This study revealed that normal discs in asymptomatic volunteers do not hurt, and only abnormal discs in patients are painful. At this time, therefore, PD performed in this manner was considered a highly specific procedure for symptomatic (painful) lumbar discs.
The next development was the finding that axial view CT discography revealed radial dispersal of contrast medium in distinct patterns of radial and circumferential fissures in the AF.14,15 From this arose the Dallas discogram scale,16 later modified by Aprill and Bogduk,17 where Grade 0 describes contrast medium contained wholly within a regular NP, grades 1 to 3 describe extension of contrast medium along radial fissures into the inner third, middle third, and outer third of the AF, respectively, grade 4, in which grade 3 fissures extend circumferentially around the AF by at least 30 degrees of arc, and grade 5 in which the fissures breach the outer lamellae of the AF. The morphologic patterns of disc disruption and the innervation of these different areas lead to a consideration that there was a greater chance of the more peripheral fissures being painful. By this time it was established that the inner third of the AF is never innervated, the outer third is regularly innervated, and the middle third may or may not be innervated.
Subsequently, Vanharanta and coworkers corroborated this clinicopathologic model by determining that on PD grade 0 and grade 1 disruptions are rarely painful, in contrast to grade 3 disruptions, which are associated with exact or similar pain reproduction in 75% of cases.18 Moneta reported that only grade 3 and 4 fissures reproduced pain that closely approximated the patient’s reporting of pain, and that annular fissures were not a feature of disc aging or degeneration.19
Since then, a number of researches, particularly Carragee, have studied PD in depth. The first criticism leveled on PD is that it is nonspecific and fails because it produces excessive false-positive results.20–25 It should also be noted that use of pain provocation is questionable in other pain diagnosis areas, such as in the detection of zygapophysial joint pain.26
Studies that question the validity of PD include:
Discography as Criterion Standard Test
In medicine, a primary aim is to make a diagnosis based on solid criteria that definitively establish if a specific disease or condition exists. The criteria that underpin a reliable diagnosis are measured by diagnostic tests. Examples of the best criterion standards include pathologic specimens to diagnose cancer and infection, imaging for bone disease and fracture and angiography to detect vascular disease. Certain standards have been iterated for rating the tests used to confirm a diagnosis.27–29
Some criterion standard tests are not routinely used because they are expensive, inaccessible, invasive, and risky.30 As a consequence, other diagnostic tests are used as surrogate criterion standard tests.30 Although surrogate tests might be cheaper, more available, less invasive, and safer; they might also be less accurate.
Robust diagnosis is not possible in many areas of pain medicine, and especially in the diagnosis of NSSP, because of the lack of a good criterion standard test. In putative DP even tissue biopsy is unhelpful in determining whether or not the disc is a source of pain. MRI may have a role in the detection of DP, but overall it has not been accepted as valid because it is not generally predictive of pain. Thus, in NSSP there is no criterion standard based on pathology, and no simple imaging test acting as an acceptable surrogate. Discography has been advocated as the best test, but it comes with costs of limited availability, some risks, limited reliability, and less than ideal validity.
The diagnostic dilemma encountered where there is no reasonable criterion standard is overcome by using a classification system that is based not on pathology, but on a manifesting symptom. Notable examples of such well recognized classification systems predicated on symptoms rather than on robust criterion-based tests include the classification system for psychiatric disorders, called the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV),32 and the classification system for headache, called the International Classification of Headache Disorders (ICHD).33 DSM-IV is a categoric classification system for psychiatric diagnosis in which the categories are prototypes, and a patient with a close approximation to the prototype is said to have that disorder. The ICHD classifies all headache disorders into major groups and each group is then subdivided one, two, or three times into headache types, subtypes, and subforms. Migraine is a diagnosis in this system. There is no test that confirms the diagnosis. It is a symptom-based diagnosis which has subtypes of migraine such as “migraine with aura,” further subgrouped into subforms such as “typical aura with migraine headache.” The ICHD uses all kinds of available evidence (clinical description, longitudinal studies of cohorts of patients, epidemiologic studies, treatment results, genetics) to establish such a diagnostic entity.
A diagnosis is required not only for individual patient management, but also so that diagnosis based management can be evaluated for all populations. Discography reveals the likely source of pain for some patients presenting with otherwise classified NSSP. It also provides a mechanism for the pain, namely IDD. The validity of the diagnosis IDD, and hence the assessment of treatment related to IDD, is predicated primarily on the validity of discography (Table 38-2).
Table 38-2 Diagnostic Criteria for Discogenic Pain Secondary to Internal Disc Disruption (IDD)∗
| Patient’s pain is reproduced by stimulation of the affected disc. |
| This pain must have an intensity of at least 7 on a 10-point scale. |
| This pain must be reproduced at a low to intermediate pressure of stimulation: 15-30 psi above opening pressure (1-3 kg/cm2) |
| Stimulation of adjacent disc(s) must not reproduce pain. |
| Postdiscography CT must demonstrate a grade 3 or 4 fissure. |
∗ From International Spine Intervention Society (ISIS, 2004).31 All categories must be satisfied. These criteria are being modified.
Disc Morphology
Anatomy
The lumbar discs consist of a central NP surrounded by the AF which consists of concentric laminae of collagen fibers thickest anteriorly and laterally.34 In contrast, cervical discs consist of a crescent-shaped anterior interosseous ligament with thick anterior collagen fibers that taper laterally toward the uncinate processes; they are essentially deficient posterolaterally where there is only a thin layer of paramedian, vertically orientated fibers.35 The anterior longitudinal ligament covers the front of the cervical disc, and the posterior longitudinal ligament reinforces the deficient posterior AF with longitudinal and alar fibers.35
Cervical discs have a different chemical morphology to thoracic and lumbar discs. In particular, they contain a higher collagen content in the NP and higher glycosaminoglycans content in the AF, both of which allow for the greater demands of bending and twisting movements in the cervical spine compared to other spinal regions.36
Because discs are innervated, they have the capacity to be sources of pain.37 The normal disc is avascular and aneural except for the outer third of the AF.38,39 Lumbar discs are innervated by the lumbar sinuvertebral nerves, and branches of the lumbar ventral rami and grey rami comunicantes.40–42 There are five types of nerve terminations found in the lumbar disc: these have various morphologies and include simple and complex free nerve endings that concentrate particularly in the lateral disc, with a smaller amount posteriorly and the least amount anteriorly.43 The posterolateral portion of lumbar discs are innervated by the lumbar ventral rami that arise just lateral to the intervertebral foramen and by a branch of the grey rami communicans just before it connects with the ventral ramus.40 The sinuvertebral nerves also innervate the posterior longitudinal ligament, and the grey rami communicantes also innervate the lateral disc and the anterior longitudinal ligament.40,41 Additionally, the innervation by the grey rami communicantes is not a direct sympathetic innervation;40 it has been postulated that somatic and afferent fibers from lumbar structures use the grey rami as transmission pathways only.41
Cervical discs are innervated laterally by branches of the vertebral nerve and more generally by branches of the cervical sinuvertebral nerves; these nerves penetrate as deeply as the outer third of the AF.38,44 The end-plate adjacent to the NP is also innervated.45
In discogram positive discs, histologic studies have shown that there is there is a more extensive and deeper innervation of the disc, with free nerve endings expressing substance P reaching into the outer part of the NP,37,46,47 as well as the adjacent end plate and vertebral body.48 The ingrowth of nerves in the discogram positive disc is within a region of vascularized and innervated granulation tissue that accompanies fissures extending from the AF to the outer part of the NP.47 These nerves contain increased substance P (SP), neurofilament (NF), and vasointestinal peptide (VIP) immunoreac-tive nerve fibers, and are consistent with the concept of DP.47
Disc Degeneration
Disc degeneration and similar morphologically descriptive terms including degenerative disc disease and zygapophysial joint spondylitis, are imaging-related labels that should not be used as diagnostic labels for a person presenting with NSSP. These terms describe mid- to late-stage degenerative change seen on imaging techniques. Although there may be a mild link between some imaging changes and NSSP,49 there is at present no reliable or valid method to detect this relationship in any individual case.50,51
The IVDs are mainly composed of extracellular matrix molecules and have only about 1% by volume cell content, with the content of cells decreasing from the periphery inward, reflecting the relative avascularity of the disc.52 These cells are of vital importance for disc homeostasis because they regulate the synthesis and metabolism of the extracellular matrix.
DD is represented at a molecular level by a relative rise in the quantity of senescent cell population on the background of an overall fall in the total number of cells within the disc.53 The percentage of senescent cells within a disc is also inversely related to the ability of other cells to proliferate, and the relative quantity of senescent and proliferating cells is independent of age.53 DD ends with structural failure, represented macroscopically by thickened vertebral end plates, increased cracks and fissures in the matrix, and delamination and tears in the AF.54 The radiologic endpoint of DD is disc space narrowing and osteophyte formation.54
Cellular function within the disc is mediated by at least five major factors: genetics, nutrition (diffusion of nutrients and oxygen across the disc matrix), cell function regulation via IL-1, TNF-α and TNF-β, age and senescence, and mechanical loading.55 The contribution to DD by genetic factors is highly significant; it may be as high as 80% in the cervical spine,56 and general heritability for DD ranges from 34% to 61% in different regions of the spine.57 In the lumbar spine, the genetic contribution is between 29% and 54%, with environmental influences of about equal importance.58 For example, smoking has a moderate influence on the prevalence of DD,59 presumably due to its effects on disc nutrition. This emphasizes that DD is not primarily or significantly due to aging60 or to mechanically induced “wear and tear” processes.57,61
Studies on symptomatic and asymptomatic populations with various imaging modalities emphasize that DD does not imply NSSP. In the cervical spine, radiologic DD is present in 13% of men and 5% of women during the third decade, in 85% to 90% of the population by the sixth decade, and nearly 100% by the age of 70.62 It occurs most commonly at C5-6, C6-7, and C4-5, respectively.63,64 In people aged 60 to 65 years without neck pain, about 95% of men and 70% of women have at least one degenerative change on their cervical spine plain x-rays.63 Although plain x-ray changes, including vertebral end-plate changes, disc space narrowing, spondylolisthesis, spondylolysis, sacral lumbarization, wedge vertebra, a sagittal diameter of less than 12 mm, and abnormal lumbar lordotic angle,49,65,66 have some predictive value for low back pain, the relationship is mild at best,67 and their detection is largely not helpful in the management of NSLBP because changes occur frequently in the asymptomatic population. As a consequence, plain x-rays should not be ordered unless there is suspicion of a red-flag condition.68–71
The relevance of computed tomography (CT) scanning is similar to plain x-rays; it is an excellent test for some red-flag conditions, demonstrates DD well, but it is not helpful in the detection of DP. CT is better than MRI in detecting ZJ spondylitis,72 but this is of no particular clinical relevance. F-PET/CT (fluoride positron emission tomography with addition of CT) may have a role to play in that it is more likely to be positive in symptomatic patients, and in that it might identify the source of pain.73
Plain x-rays combined with MRI provide the best method to detect DD.74 Plain x-rays are perhaps better than MRI at detecting early stages of DD; MRI detects later stages of DD.74 However, such information is only relevant to research because the detection of DD does not help in the diagnosis of NSSP. Studies of asymptomatic subjects have shown degenerative rates of more than 30% in the general population.75
Discography and Pain
Discography can theoretically cause pain from the disc itself by two fundamental mechanisms, annular distention and end-plate deflection. Annular distention during discography can initiate nociception because the peripheral AF is innervated. In vitro study has shown that in radiographically nondegenerated discs, discography causes measurable end-plate deflection ranging from 0.12 to 0.69 mm centrally and from 0.06 to 0.35 mm at locations between the center and the periphery of the end plate.76 Hsu investigated 692 discs in patients with back pain; he showed that during PD end-plate disruption is uncommon (2% of all discograms) but that when it occurs even with lightly induced pressure, the prevalence of moderate or severe fully concordant pain is very high (in this series, 13/14 or 93%); in contrast, 42.3% of the remaining discs without end-plate disruption had moderate-to-severe fully concordant pain on PD, and 57.7% had either mild or zero pain.77
Pain reproduction during the disc stimulation component of PD is considered integral to its validity. All discs, if provoked enough, will produce pain. However, a study on asymptomatic subjects revealed that even if grade 3 annular tears are present, pain induced on PD is likely to be mild—even at high manometric pressures.78 From this study, pressures below which pain does not occur or it occurs at low intensity have been identified for asymptomatic subjects. The assumption is that this effect should be mirrored in studies of symptomatic patients, so that manometric pressure readings can be titrated against pain scores to set parameters above which the procedure can be called positive. For example, at a pressure of 50 psi any pain score above 6/10 is not seen in asymptomatic volunteers: at a pressure of 40 psi, the cut-off score is 5/10. Put another way, there is close to zero chance of an asymptomatic subject having any pain at 20 psi or less, and of having pain greater than 6/10 up to a pressure of 70 psi.79 When the criterion for a positive PD pain response is 6/10 or more at 50 psi, the false-positive rates in asymptomatic subjects falls to 10%.79
Derby and colleagues then found that there was a correlation between postdiscography CT scan levels of annular disruption and the pain score; using VAS of 6/10 or more at less or equal to 50 psi above opening pressure, they found that these parameters revealed negligible positive results at annular disruption below grade 3, and 44% positive results with grade 3 or greater annular disruption, with severe pain being proportional to the pressure in grade 4 and 5 discs.80
Subsequent to this study, Carragee and coworkers found the false-positive rate of low-pressure painful disc stimulation injections in subjects without chronic NSLBP to be about 25%.20 However, there were some drawbacks in this retrospective study including that some of these subjects had undergone previous back surgery. The conclusion from this study is that although the false-positive rate of PD may be as high as 25% in an imperfectly selected asymptomatic population, this figure is likely to be less in subjects who do not have a chronic pain state or who have not had previous spinal surgery.
In an effort to decrease the rate of false-positive findings, the methodology of discography is undergoing modification. The development currently is in AD, in which various local anesthetic agents are injected into the disc, and FAD, in which a balloon-tipped catheter is placed into the disc so that provocation maneuvers can be undertaken in the recovery room.81,82
Clinical Application
The procession into this type of management should be understood by patient and clinician to be somewhat experimental. Patients should be alerted to the fact that the discography is a controversial test and that the management of IDD is contentious.79 Some basic issues that need to be understood by the patient at this stage include an understanding of referred pain and of the relative uselessness of special tests in the diagnosis of these referred pain problems.
Diagnosis is Rarely Tissue or Pathology Specific
The problem for the clinician is what to recommend to a patient with chronic disabling NSLBP. Discography is useful only from an interventional perspective if it rules in or rules out some form of treatment; options are confined to intradiscal procedures and spinal surgery. But these treatments have not been shown to be effective for the general population of patients with DP established on PD. Conversely, rehabilitation and pain management programs also have not had significant impact on the management of NSSP,83–86 although exercise programs combined with fear-avoidance application have proven merit in the prevention of chronicity,87–91 and pain management programs are often recommended as being more effective than traditional medical conservative treatment for chronic pain in general.92 In any case, the decision to move into interventional management for any patient typically occurs after such attempts have failed.
Predicting long-term outcome is probably impossible in individual cases. Trends exist: but the course of back pain is not as optimistic as has been conveyed.93 Although about 76% of patients with a history of recent (within 6 months) low back pain recover or have low levels of pain, 14% have persistent, high disability with moderate-to-severe limitation of function.94
Will the patient with severe disabling back pain ever get better? Many do not; however, some do. Smith performed a retrospective study of 25 NSLBP patients with positive PD in whom surgery was considered to be indicated yet was not undertaken.95 At a mean follow-up time of 5 years, 68% had improved, 8% were the same, and 24% had worsened. Of those who had become worse, 67% had psychiatric disease. As an aside, in this study 80% of those receiving workers’ compensation improved.
If the patient does go to surgery consequent upon PD, what will be the long-term result? Knox and Chapman performed a retrospective study on 22 patients who had anterior lumbar interbody fusion for discogram-concordant lower back pain and found that all double level fusions did poorly and in single level fusions, 35% had good, 18% fair, and 47% poor results, with previous surgery and workers’ compensation doing the worst.96 Parker prospectively studied 23 patients treated by a single surgeon with posterolateral fusion.97 All underwent preoperative discography and were monitored for an average of 4 years postoperatively, at which time 39% had a good-to-excellent result, 13% a fair result, and 48% a poor result, with 90% of those on compensation having a poor result. Wetzel performed a retrospective review of 48 patients with low back pain who had discogram/CT, then lumbar arthrodesis, with a success rate (good outcome) of 46%.98 Carragee performed total discectomy and fusion on patients with positive discography and achieved highly effective outcome in 27% of patients, and minimally acceptable or better outcome in 43%.99
Is discography useful to detect pain possibly derived from discs adjacent to another disc that is to be fused? Willems found that PD adjacent to an intended to be fused segment (determined by temporary external transpedicular fixation) in patients with persistent back pain was not helpful and that patient outcomes after lumbar fusion were the same whether or not the adjacent disc was normal or degenerated.100
Discography has also been used as a pretest for intradiscal procedures including intradiscal electrothermal therapy (IDET) and nucleoplasty. Results from these procedures suggest that there may be cohorts within the IDD subtype that do well.101,102
Can Other Tests Replace Discography?
How Useful is MRI in the Assessment of NSSP and IDD?
MRI changes occur in the asymptomatic population.51,103,104 In the lumbar spine, Boden and colleagues studied subjects with no past history of LBP and sciatica; in those younger than age 60, 20% had a herniated disc; in the 60 years and older group, 36% had a herniated disc and 21% had spinal stenosis. Disc degeneration or bulging at one or more levels occurred in 35% of subjects between 20 and 39 years, and in all but one of the subjects aged 60 to 80 years.103 MRI findings are common in asymptomatic individuals and the extent of changes is not predictive of the development or duration of future back pain.104
In the evolution of PD, attempts have been made to ascertain whether or not MRI can be used to predict PD outcome because PD is not without morbidity.105,106 Although MRI is somewhat predictive of a positive PD test when a high intensity zone (HIZ) or Modic type I or II changes are found, it is somewhat insensitive.
The HIZ, defined on sagittal MRI as a very bright signal (equal to or brighter than CSF on T2 weighted scans) contained within the posterior AF,17 has been shown to have appreciable but variable correlation with positive PD in patients presenting with NSLBP.79 The initial study showed that an HIZ increases the odds that PD will be positive in that patient at that level by a factor of 6.5.17 Studies on the HIZ since that time have all shown that HIZ is highly specific (range 0.74 to 0.93)107–111 but variable sensitivity, from as low as 0.09,108 to as high as 0.78.110 Thus, it is uncommon for an HIZ to occur in a disc that is not painful to PD, and the calculated likelihood ratios (ranging from 1.3 to 6.5 and averaging 4.1) indicate that an HIZ increases the odds that a PD will be positive by at least 50%.79 Additionally, although HIZs are present in both asymptomatic and symptomatic subjects, they are found significantly more in symptomatic (prevalence 60% ± 15%) than asymptomatic subjects (24% ± 11%).112
Modic changes have also been studied in relationship to NSLBP and PD.113,114 The overall rate of vertebral end-plate signal changes (VEPSC) is about 43% in patients with NSLBP and 6% in those without.115 The most common association of VEPSC and NSLBP is extensive Modic type I changes at L5-S1.116
Overall, VEPSCs are relatively insensitive, but quite specific, for the diagnosis of IDD established by discography, with sensitivity of 23.2%, specificity 96.8%, PPV 91.3%, and NPV 46.5% in one study.25 When Modic changes are studied according to type, the type I end plate has the highest PPV for positive PD at 81% (±7%).114
It is possible for PD to be positive in the presence of a normal MRI. Zucherman reported on 18 symptomatic patients who had positive PD an average of 2.5 months after a normal MRI; positive meant production of concordant pain and abnormal disc morphology.117 This study was performed more than 20 years ago and whether or not this applies with current MRI technology is unknown.
Can Clinical Tests Predict IDD?
The prevalence of IDD in a population of NSLBP patients thought to have sufficient pain and disability to undergo PD is about 35%.118,119 The prevalence of IDD in a group of patients with positive clinical indicators increases to 52% to 69%.118 The centralization phenomenon, or pain centralization, which is the retreat of referred pain toward the spinal midline during specified clinical examination, a history of persistent pain between acute episodes, a significant loss of extension and a subjective report of so-named “vulnerability in the neutral zone” individually and in combination increase the likelihood of an eventual diagnosis of IDD in a group of highly disabled and psychosocially distressed patients with otherwise diagnosed NSLBP.118,120 Additionally, if vibration applied over individual spinous processes is considered a positive test, then the odds of a positive PD increases significantly.121,122
Technical Performance of Discography
Optimal lumbar discography is based on consensus guidelines published by International Spine Intervention Society (ISIS). However, optimal technique is uncommon; in a survey of physicians performing discography, the adherence to patient safety guidelines were as follows: preoperative antibiotics (83.81%), intradiscal antibiotics (84.97%), postprocedure antibiotics (9.82%), double-needle technique (64.16%). Additionally, the adherence to technical guidelines were as follows: optional use of CT scan (64.78%), pain assessment sheet (66.47%), entering on the side opposite symptoms (48.55%), manometry for opening pressure (65.31%), manometry of pain reproduction pressure (72.25%), injecting a control disc first (78.61%), injecting discs adjacent to the painful disc (56.64%).119 It is also evident that there is considerable variation from one specialty to another in discography performance.119
Interpretation of Discography
The International Spine Intervention Society (ISIS) describes positive PD as follows:49 (1) reproduction of the patient’s pain by stimulation of the affected disc; (2) such that the evoked pain has an intensity of at least 7 on a 10-point scale; and (3) pain is reproduced at a pressure of stimulation of <15 psi; (4) provided that stimulation of adjacent discs does not reproduce pain; and (5) postdiscography CT demonstrates a grade 3 or 4 fissure.
Provocation/Analgesia Assessment
Disc Manometry
Disc manometry is an estimate of the hydrodynamic competence of a disc. It is formally measured with manometric pressure systems.13,123,124 In IDD, NP pressure decreases irregularly, and AF stresses rise. The depressurization of the NP reflects the degradation of the nuclear matrix, which can no longer retain water efficiently, to sustain axial loading. This results in extra load having to be borne by the posterior AF. Both of these findings correlate with a positive provocation component.125
Disc Morphology
Postdiscography CT scan performed within 3 hours of the procedure provides more information about disc morphology and is said to improve diagnostic accuracy, at least for disc prolapse.126 CT-discography importantly provides a better ability to subtype PD according to the Dallas system.16 Where CT is not available, caudad gantry views can show the discs close to AP.
Cervical and Thoracic Discography
It is not possible to determine if a cervical disc is a major source of pain using MRI or other imaging techniques, particularly as MRI findings do not correlate well even with advanced histologic DD.127,128 However, the cervical disc is likely to be a source of pain. If cervical DP is diagnosed, the ability of such knowledge to improve outcome in the management of neck pain is questionable.129–136 Cervical discography has hardly been studied,137 but has advocates. A review of cervical provocation discography, which includes disc stimulation and morphologic evaluation, found that there were few studies of good standard, and concluded the cervical discography is a useful tool for evaluating chronic cervical pain, without disc herniation or radiculitis, with the strength of evidence for diagnostic accuracy being at Level II-2.138 However, because the morphology of the cervical disc is substantially different from the lumbar disc, no comparative conclusions can be made from the studies on lumbar discography. The cervical AF does not consist of concentric laminae of collagen fibers as in lumbar discs; it is a crescentic mass of collagen that is thick anteriorly and tapers laterally toward the uncinate processes, and is deficient posterolaterally.114 There are no established postdiscography CT scan findings such as is seen in the Dallas description of these in the lumbar spine. There are no clinicopathologic correlations that underpin cervical, or thoracic, discography.
The technique of cervical provocation discography was first published in 1957,139 having been pioneered by Cloward,140 and subsequently developed.141–144 In the lumbar spine, abnormal morphology and precise pain reproduction are two of the more important components that contribute to a positive discogram.145 This is not the case in the cervical spine because fissuring of cervical discs is not reflective of trauma but of normal anatomy and aging,146–149 and, hence, extravasation of contrast from the disc in cervical discography cannot be considered to be indicative of an abnormal disc.149–152 The essential component of cervical discography is, therefore, not morphologic, but it is in what happens during the injection or “stimulation” phase because stimulation of cervical discs in asymptomatic volunteers is painless or only minimally painful,148 meaning that cervical disc stimulation has at least some negative predictive value: if the disc does not hurt when stimulated, it is unlikely to be a source of pain. Additionally, to diminish the possibility of false positives, subsequent opinion and study has discerned that adjacent structures should be similarly stimulated to ensure that pain is not apparent in multiple sites.
If cervical discography is used, it should be understood that it is in the very experimental stage of evolution. If performed, the recommended protocol is to perform multilevel procedures that stimulate pain: positive disc stimulation occurs when: (1) provocation of the target disc reproduces the patient’s (concordant) pain, and (2) adjacent site stimulation does not reproduce pain.153
It is evident from the use of cervical facet joint blocks and disc stimulation that more than one source of pain can be detected at any one cervical intervertebral segment.154 As a consequence, when disc stimulation is used, a positive test requires: (1) reproduction of the patient’s concordant pain at a magnitude of at least 7 on a 10-point VAS, (2) exclusion of cervical facet joint pain at any segmental level where disc stimulation is positive because the false-positive rate without exclusion is 67%,154 and (3) not finding reproduction of the patient’s pain in adjacent structures.
Thoracic discography is even less studied. In a study on 10 asymptomatic subjects and 10 patients with chronic thoracic pain undergoing PD, it was found that: (1) even in asymptomatic people, thoracic discography could be intensely painful, especially when prominent Schmorl nodes were present, (2) MRI changes did not always correlate with disc morphology on discography, and (3) in the symptomatic group, 50% of studied discs were concordantly painful.155
Complications of Discography
Discitis
Discitis is a nasty complication. A review of cases revealed the following:156
There are no randomized or controlled trials on the efficacy of prophylactic antibiotics.158 Based on animal experiments,106 the incidence of postdiscography discitis is lowered with the use of cefazolin given intravenously or in the intradiscal suspension injection. In high concentrations, contrast medium can itself inhibit bacterial growth at least to some degree, and it does not reduce the inhibitory effect of antibiotics.159 A follow-up of 127 consecutive patients having lumbar discography with contrast containing cefazolin 1 mg/mL revealed no cases of discitis.121 Willems and colleagues had no cases of discitis when they performed two-needle technique discography without preemptive antibiotics on 200 patients and followed them all for a minimum period of 3 months.160 The reported overall incidence of discitis without prophylactic antibiotics is in the region of 0.25% of all patients or 0.094% of all discograms.160
It is of course important to know where the injectate is going. Initial injection of antibiotic containing material should not be made if the needle tip site is unknown. For example, intrathecal administration of cefazolin can be fatal.161
Can Discography Cause Harm?
Experimental animal studies have revealed that disc puncture can induce accelerated disc degeneration. Carragee followed two matched cohorts of 75 each without serious LBP “illness.”105 Each subject was assessed with MRI and one group underwent L3-4, L4-5, and L5-S1 discography examination in 1997. Between 7 and 10 years after enrollment, each subject underwent a further MRI: 50 discography subjects and 52 control subjects were reassessed. The results were summarized as follows: (1) in all graded or measured parameters, discs that had been exposed to puncture and injection had greater progression of degenerative findings compared to control (noninjected) discs: progression of disc degeneration, 54 discs (35%) in the discography group compared to 21 (14%) in the control group; 55 new disc herniations in the discography group compared to 22 in the control group (2) new disc herniations were disproportionately found on the side of the AF puncture, (3) the quantitative measures of disc height and disc signal also showed significantly greater loss of disc height and signal intensity in the discography disc compared to the control disc.
Additionally, discography can produce long-term pain in subjects with abnormal psychological profiles. A 12-month follow up of subjects without LBP revealed that no subject with normal psychometric testing had persistent pain after discography, but pain of those with abnormal psychometric test results reported significant new low back pain.162
1. Mixter W.J., Barr J.S. Ruptures of the intervertebral disc with involvement of the spinal canal. N Engl J Med. 1934;211:210-215.
2. Wood K.B., Blair J.M., Aepple D.M., et al. The natural history of asymptomatic thoracic disc herniations. Spine. 1997;22:525-529.
3. Inman V.T., Saunders J.B. Anatomicophysiological aspects of injuries to the intervertebral disc. J Bone Joint Surg AM. 1947;29:461-465.
4. Falconer M.A., McGeorge M., Begg A.C. Observations on the cause and mechanism of symptom-production in sciatica and low-back pain. J Neurol Neurosurg Psychiat. 1948;11:13-26.
5. Wiberg G. Back pain in relation to the nerve supply of the intervertebral disc. Acta Orthop Scand. 1949;19:211-221.
6. Hirsch C., Ingelmark B.E., Miller M. The anatomical basis for low back pain. Studies on the presence of sensory nerve endings in ligamentous, capsular and intervertebral disc structures in the human lumbar spine. Acta Orthop Scand. 1963;33:1-17.
7. Feinberg S.B. The place of diskography in radiology as based on 2,320 cases. Am J Roentgenol Radium Ther Nucl Med. 1964;92:1275-1281.
8. Friedman J., Goldner M.Z. Discography in evaluation of lumbar disk lesions. Radiology. 1955;65:653-662.
9. Massie W.K., Stevens D.B. A critical evaluation of discography. J Bone Joint Surg Am. 1967;49A:1243-1244.
10. Holt E.P.Jr. The question of lumbar discography. J Bone Joint Surg Am. 1968;50:720-725.
11. Simmons J.W., Aprill C.N., Dwyer A.P., Brodsky A.E. A reassessment of Holt’s data on: “the question of lumbar discography”. Clin Orthop Relat Res. 1988;237:120-124.
12. Executive Committee of the North American Spine Society. Position statement on discography. Spine. 1988;13:1343.
13. Walsh T.R., Weinstein J.N., Spratt K.F., et al. Lumbar discography in normal subjects. A controlled, prospective study. J Bone Joint Surg Am. 1990;72:1081-1088.
14. McCutcheon M.E., Thompson W.C.3rd. CT scanning of lumbar discography: A useful diagnostic adjunct. Spine. 1986;11:257-259.
15. Videman T., Malmivaara A., Mooney V. The value of the axial view in assessing discograms. An experimental study with cadavers. Spine. 1987;12:299-304.
16. Sachs B.L., Vanharanta H., Spivey M.A., et al. Dallas discogram description. A new classification of CT/discography in low-back disorders. Spine. 1987;12:287-294.
17. Aprill C., Bogduk N. High-intensity zone: A diagnostic sign of painful lumbar disc on magnetic resonance imaging. Br J Radiol. 1992;65:361-369.
18. Vanharanta H., Sachs B.L., Spivey M.A., et al. The relationship of pain provocation to lumbar disc deterioration as seen by CT/discography. Spine. 1987;12:295-298.
19. Moneta G.B., Videman T., Kaivanto K., et al. Reported pain during lumbar discography as a function of anular ruptures and disc degeneration. A re-analysis of 833 discograms. Spine. 1994;19:1968-1974.
20. Carragee E.J., Alamin T.F., Carragee J.M. Low-pressure positive Discography in subjects asymptomatic of significant low back pain illness. Spine. 2006;31:505-509.
21. Carragee E.J., Alamin T.F., Miller J., Grafe M. Provocative discography in volunteer subjects with mild persistent low back pain. Spine J. 2002;2:25-34.
22. Carragee E.J., Alamin T.F., Miller J.L., et al. Discographic, MRI and psychosocial determinants of low back pain disability and remission: A prospective study in subjects with benign persistent back pain. Spine J. 2005;5:24-35.
23. Carragee E.J., Chen Y., Tanner C.M., et al. Provocative discography in patients after limited lumbar discectomy: A controlled, randomized study of pain response in symptomatic and asymptomatic subjects. Spine. 2000;25:3065-3071.
24. Carragee E.J., Tanner C.M., Khurana S., et al. The rates of false-positive lumbar discography in select patients without low back symptoms. Spine. 2000;25:1373-1380.
25. Carragee E.J., Tanner C.M., Yang B., et al. False-positive findings on lumbar discography. Reliability of subjective concordance assessment during provocative disc injection. Spine. 1999;24:2542-2547.
26. Schwarzer A.C., Derby R., Aprill C.N., et al. The value of the provocation response in lumbar zygapophyseal joint injections. Clin J Pain. 1994;10:309-313.
27. Gluud C., Gluud L.L. Evidence-based diagnostics. BMJ. 2005;330:724-726.
28. Reid M.C., Lachs M.S., Feinstein A.R. Use of methodological standards in diagnostic test research. Getting better but still not good. JAMA. 1995;274:645-651.
29. Sackett D.L., Haynes R.B. The architecture of diagnostic research. BMJ. 2002;324:539-541.
30. Elavunkal J, Sinert R. Screening and Diagnostic Tests. [emedicine]. 2009. http://emedicine.medscape.com/article/773832-overview.
31. International Spine Intervention Society. Lumbar disc stimulation. In Bogduk N (ed): Practice Guidelines for Spinal Diagnostic and Treatment Procedures. San Francisco, 2004:20-46.
32. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.—Text Revision (DSMIV-TR). American Psychiatric Association; 2000.
33. International Headache Society. IHS Classification ICHD-II. International Headache Society. 2002.
34. Bogduk N., Twomey L.T. The inter-body joints and the intervertebral discs. Clinical Anatomy of the Lumbar Spine. 1987:11-24. Edinburgh
35. Mercer S., Bogduk N. The ligaments and annulus fibrosus of human adult cervical intervertebral discs. Spine. 1999;24:619-626.
36. Scott J.E., Bosworth T.R., Cribb A.M., et al. The chemical morphology of age-related changes in human intervertebral disc glycosaminoglycans from cervical, thoracic and lumbar nucleus pulposus and annulus fibrosus. J Anat. 1994;184(Pt 1):73-82.
37. Coppes M.H., Marani E., Thomeer R.T., Groen G.J. Innervation of “painful” lumbar discs. Spine. 1997;22:2342-2349.
38. Bogduk N., Windsor M., Inglis A. The innervation of the cervical intervertebral discs. Spine. 1988;13:2-8.
39. Mendel T., Wink C.S., Zimny M.L. Neural elements in human cervical intervertebral discs. Spine. 1992;17:132-135.
40. Nerves of the lumbar spine. In: Bogduk N., Twomey L.T., editors. Clinical Anatomy of the Lumbar Spine. Edinburgh: Churchill Livingstone; 1987:92-102.
41. Bogduk N., Tynan W., Wilson A.S. The nerve supply to the human lumbar intervertebral discs. J Anat. 1981;132:39-56.
42. Taylor J.R., Twomey L.T. Innervation of lumbar intervertebral discs. Med J Aust. 1979;2:701-702.
43. Malinsky J. The ontogenetic development of nerve terminations in the intervertebral discs of man. (Histology of intervertebral discs, 11th communication). Acta Anat. 1959;38:96-113.
44. Jackson H.C., Winkelmann R.K., Bickel W.H. Nerve endings in the human lumbar spinal column and related structures. J Bone Joint Surg Am. 1966;48:1272-1281.
45. Fagan A., Moore R., Vernon Roberts .B., et al. ISSLS prize winner: The innervation of the intervertebral disc: a quantitative analysis. Spine. 2003;28:2570-2576.
46. Freemont A.J., Peacock T.E., Goupille P., et al. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178-181.
47. Peng B., Wu W., Hou S., et al. The pathogenesis of discogenic low back pain. J Bone Joint Surg Br. 2005;87:62-67.
48. Brown M.F., Hukkanen M.V., McCarthy I.D., et al. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Joint Surg Br. 1997;79:147-153.
49. Inaoka M., Yamazaki Y., Hosono N., et al. Radiographic analysis of lumbar spine for low-back pain in the general population. Arch Orthop Trauma Surg. 2000;120:380-385.
50. Binder A.I. Cervical spondylosis and neck pain. BMJ. 2007;334:527-531.
51. Boden S.D., McCowin P.R., Davis D.O., et al. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:1178-1184.
52. Urban J.P., Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120-130.
53. Gruber H.E., Ingram J.A., Davis D.E., et al. Increased cell senescence is associated with decreased cell proliferation in vivo in the degenerating human annulus. Spine J. 2009;9:210-215.
54. Hadjipavlou A.G., Tzermiadianos M.N., Bogduk N., Zindrick M.R. The pathophysiology of disc degeneration: A critical review. J Bone Joint Surg Br. 2008;90:1261-1270.
55. Freemont A.J. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford). 2009;48:5-10.
56. Sambrook P.N., Macgregor A.J., Spector T.D. Genetic influences on cervical and lumbar disc degeneration: A magnetic resonance imaging study in twins. Arthritis Rheum. 1999;42:366-372.
57. Kalichman L., Hunter D.J. The genetics of intervertebral disc degeneration. Familial predisposition and heritability estimation. Joint Bone Spine. 2008;75:383-387.
58. Battie M.C., Videman T., Levalahti E., et al. Genetic and environmental effects on disc degeneration by phenotype and spinal level: A multivariate twin study. Spine. 2008;33:2801-2808.
59. Battie M.C., Videman T., Gill K., et al. 1991 Volvo Award in clinical sciences. Smoking and lumbar intervertebral disc degeneration: An MRI study of identical twins. Spine. 1991;16:1015-1021.
60. Kettler A., Werner K., Wilke H.J. Morphological changes of cervical facet joints in elderly individuals. Eur Spine J. 2007;16:987-992.
61. Battie M.C., Videman T., Kaprio J., et al. The Twin Spine Study: Contributions to a changing view of disc degeneration. Spine J. 2009;9:47-59.
62. Irvine D.H., Foster J.B., Newell D.J., Klukuin B.N. Prevalence of cervical spondylosis in a general practice. Lancet. 1965;1:1089-1092.
63. Gore D.R., Sepic S.B., Gardner G.M. Roentgenographic findings of the cervical spine in asymptomatic people. Spine. 1986;11:521-524.
64. Rowe L.J. Diagnostic imaging of mechanical and degenerative syndromes of the cervical spine. In: Giles L.G.F., Singer K.P., editors. Clinical Anatomy of Cervical Spine Pain. Newton, Mass: Butterworth-Heinemann; 1998:89-111.
65. Steinberg E.L., Luger E., Arbel R., et al. A comparative roentgenographic analysis of the lumbar spine in male army recruits with and without lower back pain. Clin Radiol. 2003;58:985-989.
66. Torgerson W.R., Dotter W.E. Comparative roentgenographic study of the asymptomatic and symptomatic lumbar spine. J Bone Joint Surg Am. 1976;58:850-853.
67. van Tulder M.W., Assendelft W.J., Koes B.W., et al. Spinal radiographic findings and nonspecific low back pain. A systematic review of observational studies. Spine. 1997;22:427-434.
68. Chou R., Fu R., Carrino J.A., Deyo R.A. Imaging strategies for low-back pain: systematic review and meta-analysis. Lancet. 2009;373:463-472.
69. Deyo R.A., Diehl A.K. Lumbar spine films in primary care: current use and effects of selective ordering criteria. J Gen Intern Med. 1986;1:20-25.
70. Deyo R.A., Diehl A.K., Rosenthal M. Reducing roentgenography use. Can patient expectations be altered? Arch Intern Med. 1987;147:141-145.
71. Heller C.A., Stanley P., Lewis-Jones B., Heller R.F., et al. Value of x ray examinations of the cervical spine. Br Med J (Clin Res Ed). 1983;287:1276-1278.
72. Fujiwara A., Tamai K., Yamato M., et al. The relationship between facet joint osteoarthritis and disc degeneration of the lumbar spine: An MRI study. Eur Spine J. 1999;8:396-401.
73. Gamie S., El-Maghraby T. The role of PET/CT in evaluation of facet and disc abnormalities in patients with low back pain using (18)F-Fluoride. Nucl Med Rev Cent East Eur. 2008;11:17-21.
74. Benneker L.M., Heini P.F., Anderson S.E., et al. Correlation of radiographic and MRI parameters to morphological and biochemical assessment of intervertebral disc degeneration. Eur Spine J. 2005;14:27-35.
75. Simmons J.W., Emery S.F., McMillin J.N., et al. Awake discography. A comparison study with magnetic resonance imaging. Spine. 1991;16:S216-S221.
76. Heggeness M.H., Doherty B.J. Discography causes end plate deflection. Spine. 1993;18:1050-1053.
77. Hsu K.Y., Zucherman J.F., Derby R., et al. Painful lumbar end-plate disruptions: A significant discographic finding. Spine. 1988;13:76-78.
78. Derby R., Lee S.H., Kim B.J., et al. Pressure-controlled lumbar discography in volunteers without low back symptoms. Pain Med. 2005;6:213-221.
79. Bogduk N. Why I pursue discogenic pain. Annual Scientific Meeting of the German Pain Society; 2010. 10-20-2005
80. Derby R., Kim B.J., Chen Y., et al. The relation between annular disruption on computed tomography scan and pressure-controlled diskography. Arch Phys Med Rehabil. 2005;86:1534-1538.
81. Bartynski W.S., Rothfus W.E. Pain improvement after intradiskal lidocaine administration in provocation lumbar diskography: Association with diskographic contrast leakage. AJNR Am J Neuroradiol. 2007;28:1259-1265.
82. Derby R., Baker R., Wolfer L., et al. Analgesic discography: can analgesic testing identify a painful disc? Spine Line. 2008. November-December
83. Linton S.J., Gotestam K.G. A controlled study of the effects of applied relaxation and applied relaxation plus operant procedures in the regulation of chronic pain. Br J Clin Psychol. 1984;23(Pt 4):291-299.
84. Moseley G.L., Nicholas M.K., Hodges P.W. A randomized controlled trial of intensive neurophysiology education in chronic low back pain. Clin J Pain. 2004;20:324-330.
85. Stewart M.J., Maher C.G., Refshauge K.M., et al. Randomized controlled trial of exercise for chronic whiplash-associated disorders. Pain. 2007;128:59-68.
86. van Tulder M.W., Ostelo R., Vlaeyen J.W., et al. Behavioral treatment for chronic low back pain: A systematic review within the framework of the Cochrane Back Review Group. Spine. 2000;25:2688-2699.
87. Indahl A., Haldorsen E.H., Holm S., et al. Five-year follow-up study of a controlled clinical trial using light mobilization and an informative approach to low back pain. Spine. 1998;23:2625-2630.
88. Indahl A., Velund L., Reikeraas O. Good prognosis for low back pain when left untampered. A randomized clinical trial. Spine. 1995;20:473-477.
89. McGuirk B., Bogduk N. Evidence-based care for low back pain in workers eligible for compensation. Occup Med (Lond). 2007;57:36-42.
90. McGuirk B., King W., Govind J., et al. Safety, efficacy, and cost effectiveness of evidence-based guidelines for the management of acute low back pain in primary care. Spine. 2001;26:2615-2622.
91. Von Korff M., Balderson B.H., Saunders K., et al. A trial of an activating intervention for chronic back pain in primary care and physical therapy settings. Pain. 2005;113:323-330.
92. Gatchel R.J., Okifuji A. Evidence-based scientific data documenting the treatment and cost-effectiveness of comprehensive pain programs for chronic nonmalignant pain. J Pain. 2006;7:779-793.
93. Coste J., Delecoeuillerie G., Cohen de Lara .A., et al. Clinical course and prognostic factors in acute low back pain: an inception cohort study in primary care practice. BMJ. 1994;308:577-580.
94. Von Korff M., Deyo R.A., Cherkin D., Barlow W. Back pain in primary care. Outcomes at 1 year. Spine. 1993;18:855-862.
95. Smith S.E., Darden B.V., Rhyne A.L., Wook K.E. Outcome of unoperated discogram-positive low back pain. Spine. 1995;20:1997-2000.
96. Knox B.D., Chapman T.M. Anterior lumbar interbody fusion for discogram concordant pain. J Spinal Disord. 1993;6:242-244.
97. Parker L.M., Murrell S.E., Boden S.D., et al. The outcome of posterolateral fusion in highly selected patients with discogenic low back pain. Spine. 1996;21:1909-1916.
98. Wetzel F.T., LaRocca S.H., Lowery G.L., et al. The treatment of lumbar spinal pain syndromes diagnosed by discography. Lumbar arthrodesis. Spine. 1994;19:792-800.
99. Carragee E.J., Lincoln T., Parmar V.S., Alamin T. A gold standard evaluation of the “discogenic pain” diagnosis as determined by provocative discography. Spine. 2006;31:2115-2123.
100. Willems P.C., Elmans L., Anderson P.G., et al. Provocative discography and lumbar fusion: Is preoperative assessment of adjacent discs useful? Spine. 2007;32:1094-1099.
101. Bogduk N., Karasek M. Two-year follow-up of a controlled trial of intradiscal electrothermal anuloplasty for chronic low back pain resulting from internal disc disruption. Spine J. 2002;2:343-350.
102. Pauza K.J., Howell S., Dreyfuss P., et al. A randomized, placebo-controlled trial of intradiscal electrothermal therapy for the treatment of discogenic low back pain. Spine J. 2004;4:27-35.
103. Boden S.D., Davis D.O., Dina T.S., et al. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403-408.
104. Borenstein D.G., O’Mara J.W.Jr., Boden S.D., et al. The value of magnetic resonance imaging of the lumbar spine to predict low-back pain in asymptomatic subjects: A seven-year follow-up study. J Bone Joint Surg Am. 83-A, 2001. 1306–1311
105. Carragee E.J., Don A.S., Hurwitz E.L., et al. 2009 ISSLS Prize Winner: Does discography cause accelerated progression of degeneration changes in the lumbar disc: A ten-year matched cohort study. Spine. 2009;34:2338-2345.
106. Osti O.L., Fraser R.D., Vernon-Roberts B. Discitis after discography. The role of prophylactic antibiotics. J Bone Joint Surg Br. 1990;72:271-274.
107. Ito M., Incorvaia K.M., Fredrickson B.E., et al. Predictive signs of discogenic lumbar pain on magnetic resonance imaging with discography correlation. Spine. 1998;23:1252-1260.
108. Ricketson R., Simmons J.W., Hauser B.O. The prolapsed intervertebral disc. The high-intensity zone with discography correlation. Spine. 1996;21:2758-2762.
109. Saifuddin A., Braithwaite I., White J., et al. The value of lumbar spine magnetic resonance imaging in the demonstration of anular tears. Spine. 1998;23:453-457.
110. Schellhas K.P., Pollei S.R., Gundry C.R., et al. Lumbar disc high-intensity zone. Correlation of magnetic resonance imaging and discography. Spine. 1996;21:79-86.
111. Smith B.M., Hurwitz E.L., Solsberg D., et al. Interobserver reliability of detecting lumbar intervertebral disc high-intensity zone on magnetic resonance imaging and association of high-intensity zone with pain and anular disruption. Spine. 1998;23:2074-2080.
112. Carragee E.J., Paragioudakis S.J., Khurana S. Lumbar high-intensity zone and discography in subjects without low back problems. Spine. 2000;25:2987-2992.
113. Braithwaite I., White J., Saifuddin A., et al. Vertebral end-plate (Modic) changes on lumbar spine MRI: Correlation with pain reproduction at lumbar discography. Eur Spine J. 1998;7:363-368.
114. Thompson K.J., Dagher A.P., Eckel T.S., et al. Modic changes on MR images as studied with provocative diskography: clinical relevance—a retrospective study of 2457 disks. Radiology. 2009;250:849-855.
115. Jensen T.S., Karppinen J., Sorensen J.S., et al. Vertebral endplate signal changes (Modic change): A systematic literature review of prevalence and association with non-specific low back pain. Eur Spine J. 2008;17:1407-1422.
116. Kuisma M., Karppinen J., Niinimaki J., et al. Modic changes in endplates of lumbar vertebral bodies: Prevalence and association with low back and sciatic pain among middle-aged male workers. Spine. 2007;32:1116-1122.
117. Zucherman J., Derby R., Hsu K., et al. Normal magnetic resonance imaging with abnormal discography. Spine. 1988;13:1355-1359.
118. Laslett M., Aprill C.N., McDonald B., et al. Clinical predictors of lumbar provocation discography: A study of clinical predictors of lumbar provocation discography. Eur Spine J. 2006;15:1473-1484.
119. Schwarzer A.C., Aprill C.N., Derby R., et al. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine. 1995;20:1878-1883.
120. Laslett M., Oberg B., Aprill C.N., et al. Centralization as a predictor of provocation discography results in chronic low back pain, and the influence of disability and distress on diagnostic power. Spine J. 2005;5:370-380.
121. Vanharanta H., Ohnmeiss D.D., Aprill C.N. Vibration pain provocation can improve the specificity of MRI in the diagnosis of symptomatic lumbar disc rupture. Clin J Pain. 1998;14:239-247.
122. Yrjama M., Tervonen O., Kurunlahti M., et al. Bony vibration stimulation test combined with magnetic resonance imaging. Can discography be replaced? Spine. 1997;22:808-813.
123. Quinnell R.C., Stockdale H.R., Harmon B. Pressure standardized lumbar discography. Br J Radiol. 1980;53:1031-1036.
124. Quinnell R.C., Stockdale H.R., Willis D.S. Observations of pressures within normal discs in the lumbar spine. Spine. 1983;8:166-169.
125. McNally D.S., Shackleford I.M., Goodship A.E., Mulholland R.C. In vivo stress measurement can predict pain on discography. Spine. 1996;21:2580-2587.
126. Min K., Leu H.J., Perrenoud A. Discography with manometry and discographic CT: Their value in patient selection for percutaneous lumbar nucleotomy. Bull Hosp Jt Dis. 1996;54:153-157.
127. Christe A., Laubli R., Guzman R., et al. Degeneration of the cervical disc: histology compared with radiography and magnetic resonance imaging. Neuroradiology. 2005;47:721-729.
128. Viikari-Juntura E., Raininko R., Videman T., Porkka L. Evaluation of cervical disc degeneration with ultralow field MRI and discography. An experimental study on cadavers. Spine. 1989;14:616-619.
129. Buenaventura R.M., Shah R.V., Patel V., et al. Systematic review of discography as a diagnostic test for spinal pain: An update. Pain Physician. 2007;10:147-164.
130. Connor P.M., Darden B.V.2nd. Cervical discography complications and clinical efficacy. Spine. 1993;18:2035-2038.
131. Grubb S.A., Kelly C.K. Cervical discography: Clinical implications from 12 years of experience. Spine. 2000;25:1382-1389.
132. Guyer R.D., Ohnmeiss D.D., Mason S.L., et al. Complications of cervical discography: findings in a large series. J Spinal Disord. 1997;10:95-101.
133. Simmons E.H., Bhalla S.K. Anterior cervical discectomy and fusion. A clinical and biomechanical study with eight-year follow-up. J Bone Joint Surg Br. 1969;51:225-237.
134. Singh V. The role of cervical discography in interventional pain management. Pain Physician. 2004;7:249-255.
135. Whitecloud T.S.III, Seago R.A. Cervical discogenic syndrome. Results of operative intervention in patients with positive discography. Spine. 1987;12:313-316.
136. Zeidman S.M., Thompson K., Ducker T.B. Complications of cervical discography: Analysis of 4400 diagnostic disc injections. Neurosurgery. 1995;37:414-417.
137. Menkowitz M., Stieber J.R., Wenokor C., et al. Intradiscal pressure monitoring in the cervical spine. Pain Physician. 2005;8:163-166.
138. Manchikanti L., Dunbar E.E., Wargo B.W., et al. Systematic review of cervical discography as a diagnostic test for chronic spinal pain. Pain Physician. 2009;12:305-321.
139. Smith G.W., Nichols P.Jr. The technic of cervical discography. Radiology. 1957;68:718-720.
140. Cloward R.B. Cervical discography. Acta Radiol Diagn (Stockh). 1963;1:675-688.
141. Cloward R.B. Cervical diskography; technique, indications and use in diagnosis of ruptured cervical disks. Am J Roentgenol Radium Ther Nucl Med. 1958;79:563-574.
142. Cloward R.B. Cervical diskography. A contribution to the etiology and mechanism of neck, shoulder and arm pain. Ann Surg. 1959;150:1052-1064.
143. Cloward R.B. The clinical significance of the sinu-vertebral nerve of the cervical spine in relation to the cervical disk syndrome. J Neurol Neurosurg. Psychiatry. 1960;23:321-326.
144. Smith G.W. The normal cervical diskogram; with clinical observations. Am J Roentgenol Radium Ther Nucl Med. 1959;81:1006-1010.
145. Wolfer L.R., Derby R., Lee J.E., Lee S.H. Systematic review of lumbar provocation discography in asymptomatic subjects with a meta-analysis of false-positive rates. Pain Physician. 2008;11:513-538.
146. Oda J., Tanaka H., Tsuzuki N. Intervertebral disc changes with aging of human cervical vertebra. From the neonate to the eighties. Spine. 1988;13:1205-1211.
147. Parfenchuck T.A., Janssen M.E. A correlation of cervical magnetic resonance imaging and discography/computed tomographic discograms. Spine. 1994;19:2819-2825.
148. Schellhas K.P., Smith M.D., Gundry C.R., et al. Cervical discogenic pain. Prospective correlation of magnetic resonance imaging and discography in asymptomatic subjects and pain sufferers. Spine. 1996;21:300-311.
149. Sneider S.E., Winslow O.P.Jr., Pryor T.H. Cervical diskography: Is it relevant? JAMA. 1963;185:163-165.
150. Klafta L.A.Jr., Collis J.S.Jr. The diagnostic inaccuracy of the pain response in cervical discography. Cleve Clin Q. 1969;36:35-39.
151. Meyer R.R. Cervical diskography. A help or hindrance in evaluating neck, shoulder, arm pain? Am J Roentgenol Radium Ther Nucl Med. 1963;90:1208-1215.
152. Stuck R.M. Cervical discography. Am J Roentgenol Radium Ther Nucl Med. 1961;86:975-982.
153. Bogduk N., Merskey H. Classification of Chronic Pain. Descriptions of Chronic Pain Syndromes and Definition of Pain Terms, 2nd ed. Seattle: IASP Press. 1994.
154. Bogduk N., Aprill C. On the nature of neck pain, discography and cervical zygapophysial joint blocks. Pain. 1993;54:213-217.
155. Wood K.B., Schellhas K.P., Garvey T.A., Aeppli D. Thoracic discography in healthy individuals. A controlled prospective study of magnetic resonance imaging and discography in asymptomatic and symptomatic individuals. Spine. 1999;24:1548-1555.
156. Guyer R.D., Collier R., Stith W.J., et al. Discitis after discography. Spine. 1988;13:1352-1354.
157. Carrino J.A., Swathwood T.C., Morrison W.B., et al. Prospective evaluation of contrast-enhanced MR imaging after uncomplicated lumbar discography. Skeletal Radiol. 2007;36:293-299.
158. Sharma S.K., Jones J.O., Zeballos P.P., et al. The prevention of discitis during discography. Spine J. 2009;9:936-943.
159. Langer R.D., Usmani A., van Gorkom K.N., et al. In vitro assessment of the antibiotic efficacy of contrast media and antibiotics and their combinations at various dilutions. Br J Radiol. 2010;83:394-400.
160. Willems P.C., Jacobs W., Duinkerke E.S., De Kleuver M. Lumbar discography: Should we use prophylactic antibiotics? A study of 435 consecutive discograms and a systematic review of the literature. J Spinal Disord Tech. 2004;17:243-247.
161. Boswell M.V., Wolfe J.R. Intrathecal cefazolin-induced seizures following attempted discography. Pain Physician. 2004;7:103-106.
162. Carragee E.J., Chen Y., Tanner C.M., et al. Can discography cause long-term back symptoms in previously asymptomatic subjects? Spine. 2000;25:1803-1808.
Part B Intervertebral Disc Access and Stimulation: Lumbar, Thoracic, and Cervical
Low back pain commonly seen with referral into the hip, buttock and thigh, is a common malady afflicting ~15% of the adult population in any given year, and 80% will suffer from this symptom during their lifetime. It is well known that disc pain accounts for 39% of chronic low back pain complaints,2 with zygapophysial3 and sacroiliac4 pain running a poor second and third. This makes pain from the lumbar disc the most commonly found and objectively determined source of low back pain. If a pain specialist is not trained to provide discography, or elects not to do so, this physician is unable to provide a diagnosis for approximately 40% of his patients suffering from this very prevalent complaint. The prevalence of discogenic thoracic pain is unknown, while the cervical spine, disc pain accounts for only16%, with zygapophysial pain approximately 65%.5
Although in the past it was argued that the intervertebral disc was devoid of innervation, presently, there is no doubt that the intervertebral discs are innervated6,7 and can therefore be the source of pain .Painful diseases of the disc associated with imaging pathology are well known, for example, discitis. However, it is also quite evident that asymptomatic patients are often are noted to have significant pathology on imaging studies. Pathology does not equal pain. MRI and CT imaging provide wonderfully detailed pictures of the spine, but they are merely images and provide only clues as to the pain generator. Stimulation of the intervertebral disc is the sole method of determining is the discs are painful under a mechanical load.
Indications
Prior to the advent of modern imaging modalities, discography was proposed by Lindblum to diagnose protrusions and other changes in the outer disc morphology.8 With the current availability of CT and MRI, discography is no longer indicated for diagnosis of radicular pain, sciatica, and elucidation of the external disc morphology.
Discography is frequently used to validate a surgical procedure. In that surgical success rates for low back pain, as determined by 5-year pain relief and functionality studies are not stellar, every patient with a positive discogram should not be considered a surgical candidate. Surgery for discogenic pain is a consequence of societal norms, unrealistic patient expectations and insistence, economic variables, medical legal concerns, medical referral patterns, and the proclivity of some surgeons towards highly invasive, extraordinarily well- reimbursed procedures.9 At the present time treatments for discogenic pain are limited, but new technologies including minimally invasive procedures and injectable therapies are being given a high priority and hopefully will see fruition in the coming years. However, lack of a validated, effective treatment does not negate the responsibility of the interventional pain physician to provide the patient with an accurate diagnosis.
Discography must not be performed for capricious, unjustifiable reasons. For idiopathic low back pain, a well thought out algorithm must be utilized. The well respected International Spine Intervention Society (ISIS) has published such an algorithm based on the best evidence available.10 Discography should be considered the primary interventional diagnostic test when significant, chronic low back pain is present, the intervertebral discs are not pristine, and a diagnosis required. In addition, with regard to the societal cost of medical care, discography is a fiscally sound course of action in that a positive discogram essentially exits the patient from the interventional pain algorithm and prevents other questionable procedures, such as the ubiquitous, grossly over utilized interlaminar epidural steroid injection, from being foisted on a susceptible population.
Contraindications
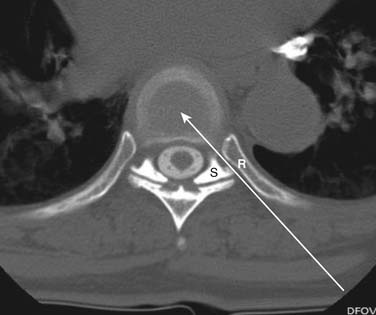
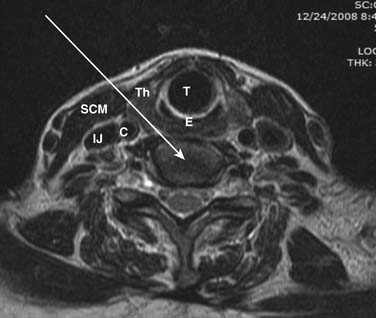
In regard to cervical discography, further contraindications exist. A case of iatrogenic quadriplegia has been reported2 owing to iatrogenic disc herniation and severe cord compression resulting from a disc injection at a level with little reserve space secondary to spinal stenosis. An anterior-posterior (AP) spinal canal diameter of less than 10 mm is an absolute contraindication, whereas an AP diameter of less than 11 mm constitutes a relative contraindication, to the performance of cervical discography at the specific or adjacent levels. To ascertain whether cervical discography is safe, the physician injectionist must personally interpret a recent MRI or CT scan to ensure that the cervical canal is ample.
Complications
Prior to the routine use of prophylactic antibiotics, discitis was the most feared, although nonlife threatening, complication to disc access. However, with precautions as will be detailed subsequently, this problem has become exceedingly rare. A comprehensive discussion of complications attributable to discography, mild to severe, is beyond the scope of this chapter, and the reader is directed to several excellent reviews and papers on the subject.12–19
Equipment and Training
Radiation safety precautions as discussed in Chapter 5 should be followed.
In regard to radiographic guidance, although the use of computed tomography (CT), guidance has been advocated by a minority of practitioners,20 this technique should be avoided. Using CT allows one to accurately delineate the skin entry point, however the advancement of the needle toward the target is accomplished crudely, without imaging assistance. This adds to the physical discomfort of the patient, the time required to complete the procedure, and the chance for needle misadventures. CT necessitates the injection of contrast for verification of needle placement without the benefit of active, real time imaging. Therefore, during the injection phase of the procedure (i.e., disc stimulation) the emerging contrast pattern cannot be monitored for disruption of the annulus, end-plate ruptures, and increase in height of the disc. In addition, vascular injections will, in all likelihood, escape detection owing to the rapid “washout” of contrast secondary to blood flow within the vein or artery. Marked increase in total radiation exposure to the patient is inherent in CT and should be of concern to all involved. There is no literary evidence indicating any benefit to the use of CT for discography. Specialty group guidelines published by the International Spine Intervention Society (ISIS)21 and the Physiatric Association of Spine, Sports, and Occupational Rehabilitation (PASSOR),22 specifically do not mention CT for use with disc access.
Miscellaneous Supplies
Monitoring devices, such as pulse oximetry, noninvasive blood pressure, and ECG, must at the least be immediately available, and their use is required if any sedation is contemplated. An ongoing conversation with the patient is always an appropriate and accurate monitor of the patient’s comfort, level of consciousness, cardiac, and respiratory status pre-, peri-, and postprocedure. Whether the procedure is performed in an office, ambulatory surgery center, or hospital setting, emergency equipment, pharmaceuticals, oxygen, airway supplies, suction, and other resuscitation provisions must be immediately available and checked regularly to ensure proper functioning. In addition, trained personnel must be available in case of an emergency.
Medications
In that the very name discography indicates an imaging study of the intervertebral disc, contrast is required to detail the internal disc architecture. Only nonionic, water-soluble contrast media should be used. Iohexol (Omnipaque) or iopamidol (Isovue) in concentrations of 180 to 240 mg/mL are safe with allergic reactions occurring in the 1:500,000 range. Contrast of the 300 mg/mL concentration, may be useful in cervical discography where the small volumes used may require a higher concentration for good visualization with fluoroscopy. Today, the addition of antibiotics to the contrast medium should be considered routine. The excellent paper by Klessig and colleagues23 details this practice. Gentamicin and cefazolin in a concentration of 1 mg/mL of contrast, or clindamycin 7.5 mg/mL exceeds the minimum inhibitory concentration of Staphylococcus aureus, Staphylococcus epidermidis, and Escherichia coli, the three most commonly implicated organisms in discitis events.
Sedation
One problem facing discographers is that patients on chronic opioid therapy have developed some degree of altered pain response, including hyperalgesia and allodynia because of their long and often questionable narcotic treatment. Having these patients withdrawn entirely from their medications is often not an option. In this patient subset, provocation discography can be performed using their present state of medication load as a baseline pain level. Although quantification of their pain response might be difficult, determination of pain response to specific disc levels is often easily accomplished. When presenting these results to the referring physician or surgeon, the outcome must be understood in the context of the patient response to mild-to-moderate pain, such as creation of the local anesthetic skin wheal with a 25-gauge needle.
Techniques
Prior to beginning a discographic study, a decision as to what levels need to be included must be made. This selection is based on the physical examination, imaging studies, and patient symptomatology. Accurate pain referral maps are available for the cervical24–26 and thoracic27,28 regions and, although based on induced zygapophysial joint pain, appear to be accurate for intervertebral disc-mediated pain as well. In that negative control levels rostral and caudal to any substantiated painful disc provide an internal negative control validation, the suspected intervertebral disc and the two adjoining discs should be included. Although extraordinary exceptions may exist, disc stimulation of more than four levels is rarely indicated.
Criteria for Discogenic Pain
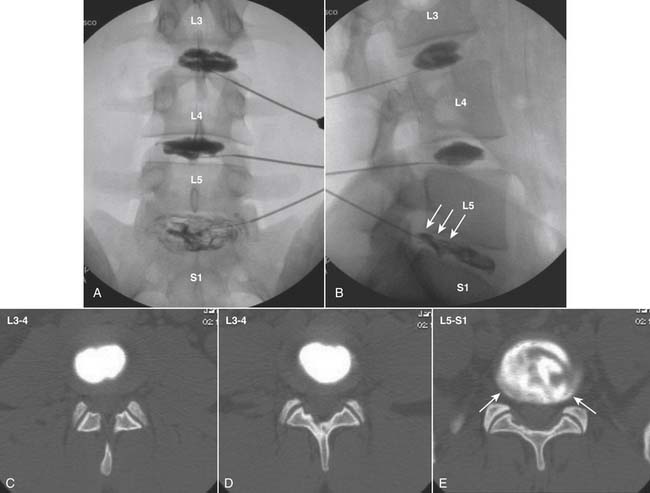
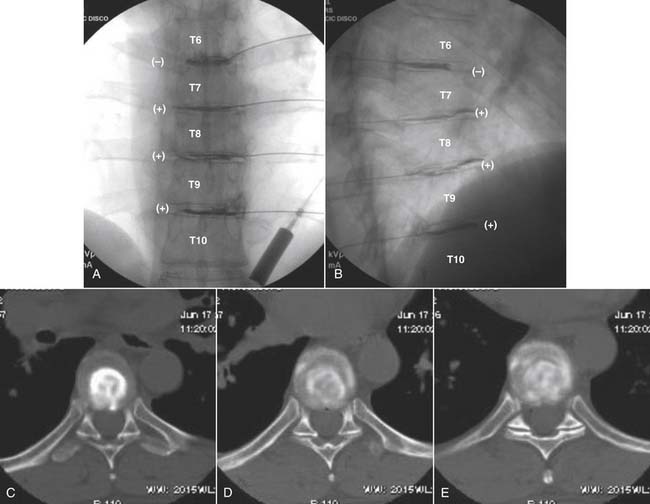
The stricter the criteria for establishing a diagnosis, the fewer false-positive results will occur. In the case of a diagnosis of discogenic pain, surgery of a highly invasive nature with the possibly of a lifelong disability may be based on the results; therefore strict criteria are mandated. For the lumbar spine: (1) the pain produced from the disc simulation must be concordant, exactly reproducing the patients ongoing index pain; (2) the pain on stimulation must be significant at a VAS of ≥7 on a scale of 10; (3) an internal anatomic control must be found where stimulation of an adjacent level is nonpainful or minimally painful29,30; (4) pain is noted at <50 psi above opening pressure; (5) except on rare occasions, pain occurs with a volume of contrast <3.5 mL; (6) internal disc disruption is noted preferably on a postprocedure CT scan, with a radial fissure to the outer third of the annulus (i.e., a grade 3 or higher lesion).31,32
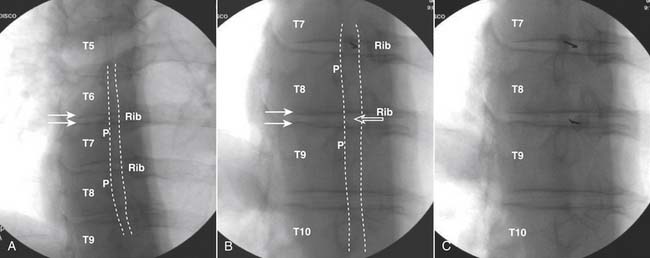
Although there is little information regarding criteria for a positive thoracic discogram,33 using the preceding lumbar standards would be prudent at this time.
The cervical intervertebral disc does not have a well defined nucleus which is bounded by an annulus.34 Fissures within the “core,” (i.e., internal disc disruptions), are prevalent in nearly all individuals older than 30 years of age. Pain can be elicited at nearly all levels. Therefore, the criteria for a positive level involve strict concordance of pain at a VAS of ≥7/10, and a negative internal anatomic control level. Multiple positive levels are frequently present.35
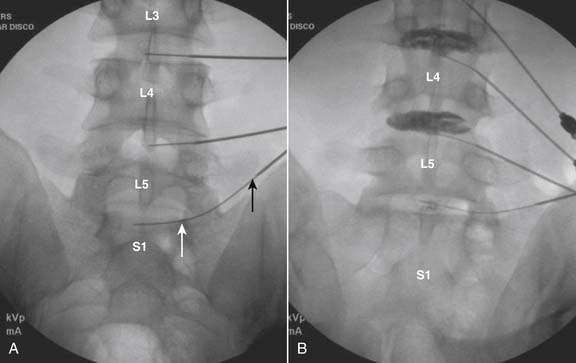
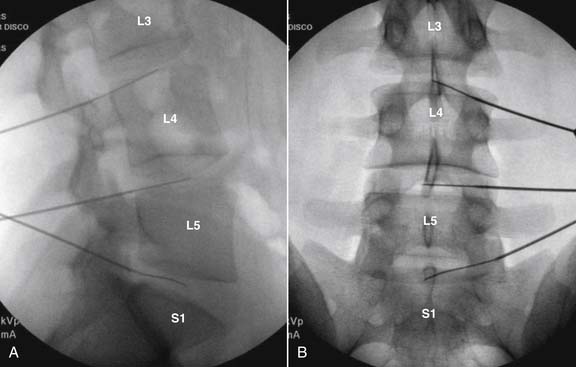
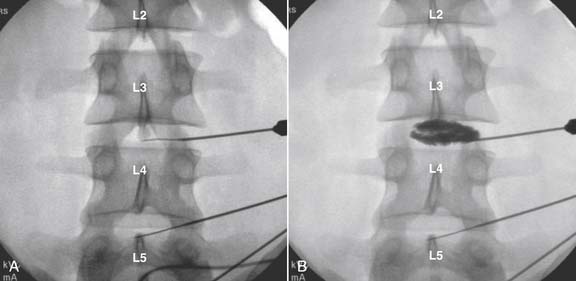
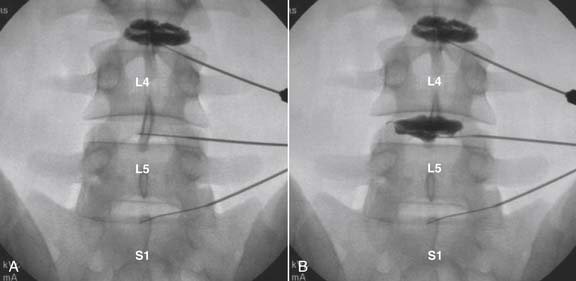
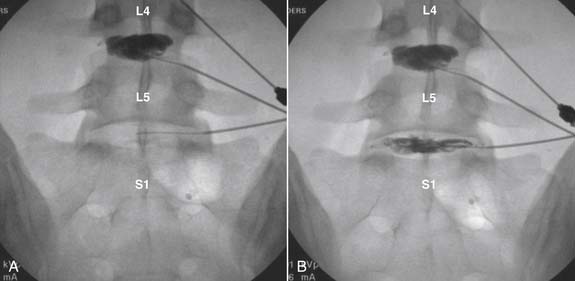
Lumbar
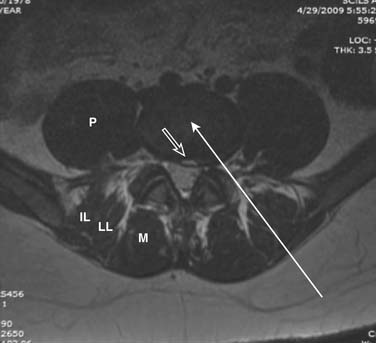
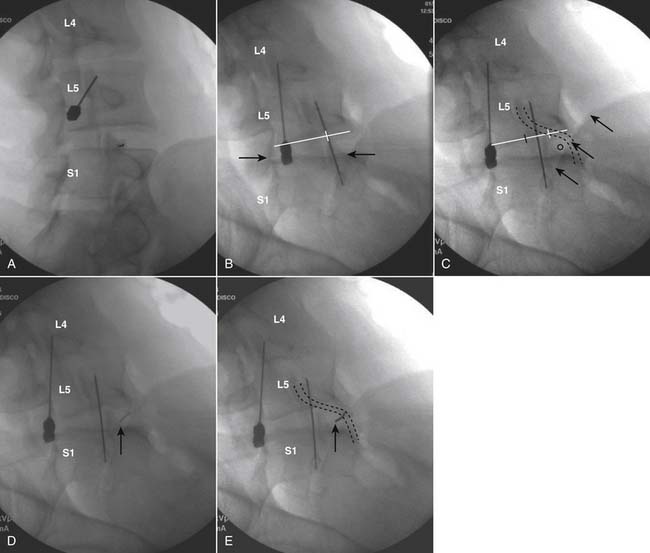
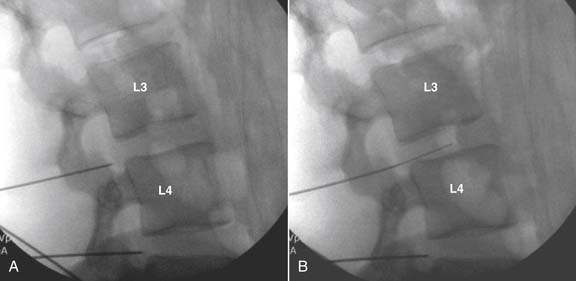
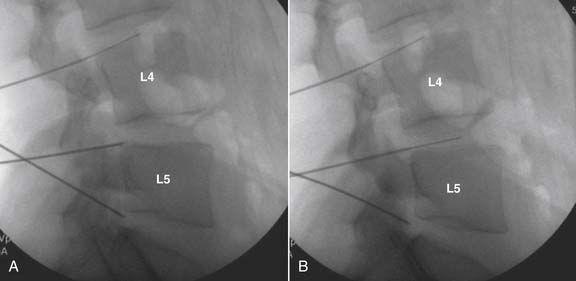
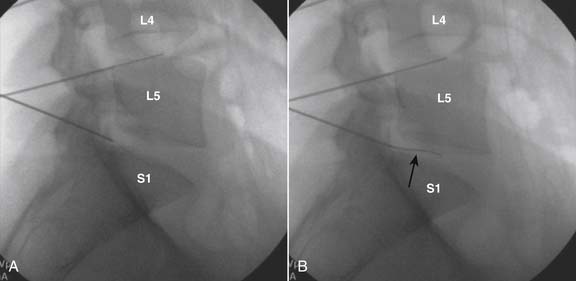
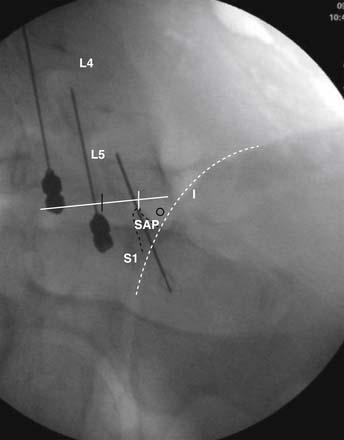
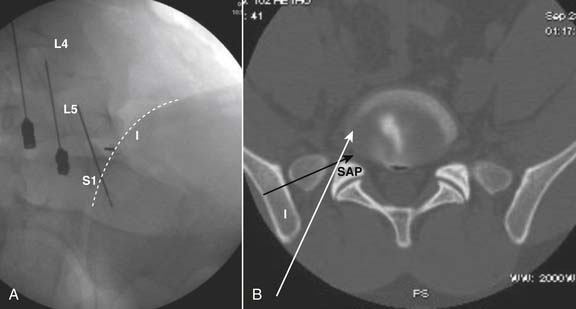
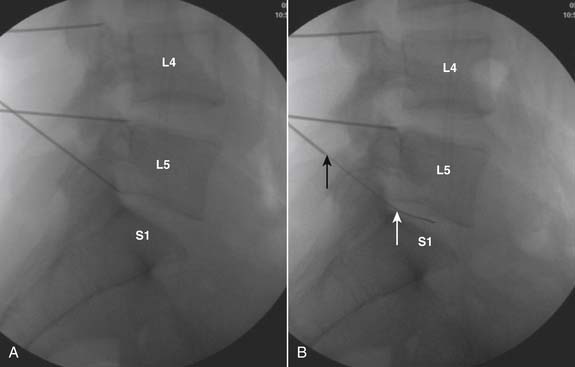
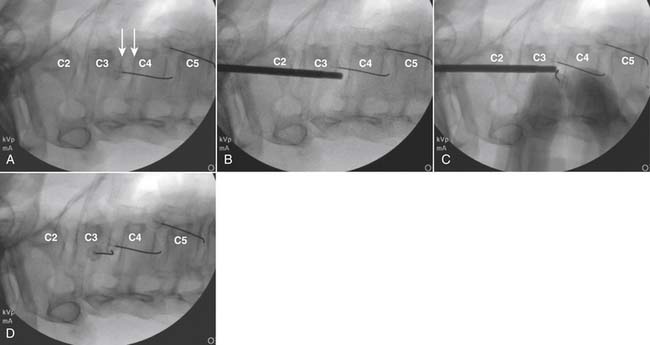
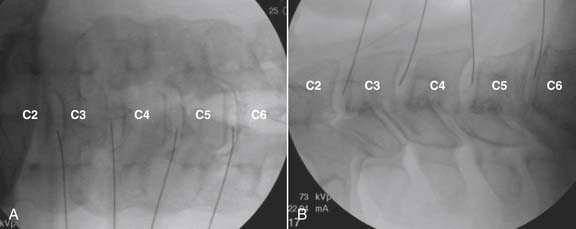
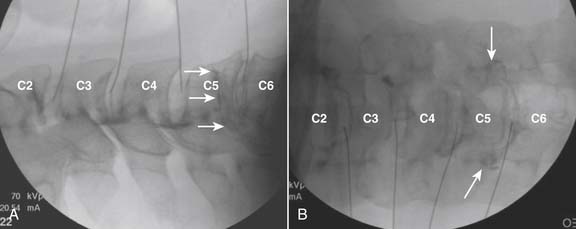
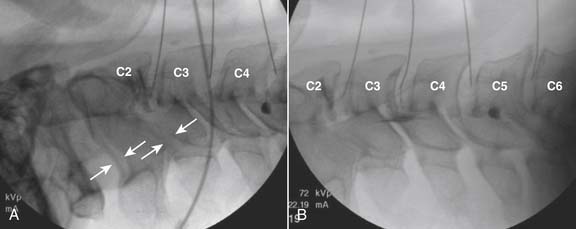
The lateral or extrapedicular approach to the lumbar intervertebral disc provides safe access with minimal morbidity (see Fig. 38-1). Historically, lumbar disc access was performed using a posterior, interpedicular, transdural approach. Because this technique requires puncture of the ventral and dorsal dura, it is used infrequently today except in those rare, very specific instances in which postsurgical changes or anatomic considerations necessitate.
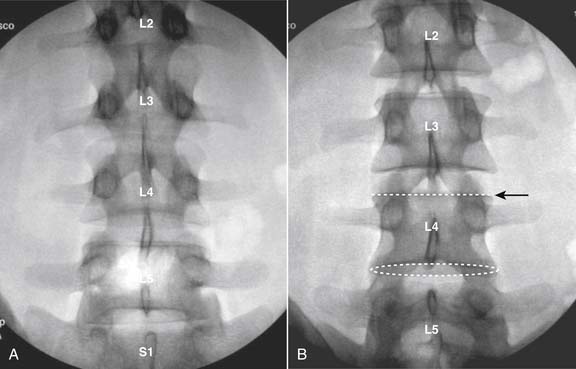
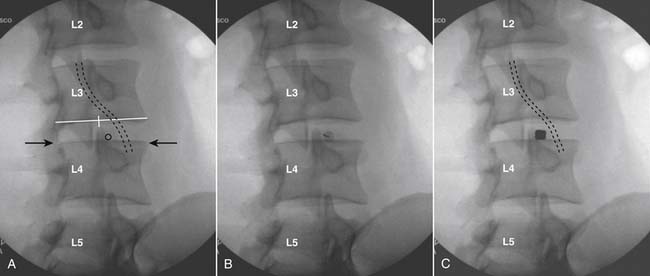
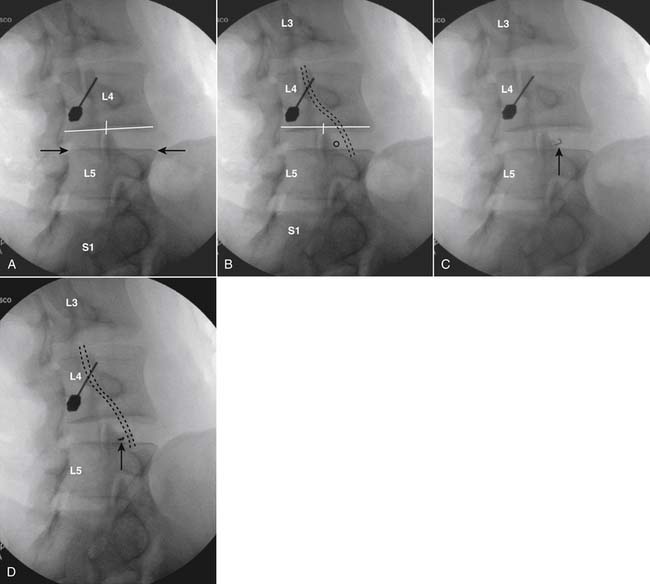
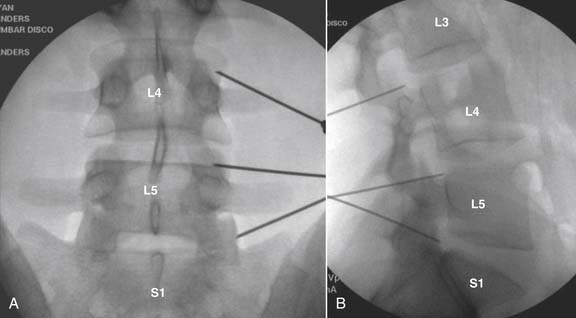
The lumbar spine is examined to ensure no segmental anomalies are present (see Fig. 38-2, A). Non–rib-bearing vertebral bodies are counted to ensure sacralized L5, or nonsacralized S1 vertebral bodies are identified. These important variants must be described on the procedure note.
The targeted intervertebral discs are identified in AP view, and the C-arm is maneuvered in a cephalocaudal tilt motion so that the superior subchondral end plate of the vertebral body caudal to the chosen disc is parallel to the x-ray beam. This end plate will be seen as a straight line rather than oval when in optimal position (see Fig. 38-2, B).
After “squaring” the subchondral end plate, the C-arm is rotated until the superior articular process (SAP) of the level below appears to transect or lie under the midpoint of the inferior end plate of the level above (see Fig. 38-3, A). This imaging manipulation allows placement of the needle using “tunnel vision,” (i.e., needle insertion parallel to the beam when the skin puncture site is aligned with the target endpoint). The needle will travel medial and caudal to the segmental ventral ramus, which courses medial to lateral, and dorsal to ventral through the foramen. Using this technique, the final needle tip position will be in the center of the nucleus when seen in an AP and lateral orientation.
When the oblique view is perfected, the skin is marked overlying the target, just lateral to the SAP, and over the caudal aspect of the targeted disc. A skin wheal is made and a 25-gauge, 3.5 inch needle is advanced toward the lateral aspect of the SAP (see Fig. 38-3, B).
Proponents of both single and dual needle lumbar discography techniques can be found. Prior to the use of prophylactic antibiotics, Fraser and colleagues36 reported an incidence of discitis of 2.7% using a single-needle technique with nonstylet needles versus 0.7% when an introducer needle was employed. However, using a single stylet needle technique, Professor Charles Aprill noted just one case of discitis in more than 2000 patients (<0.05% per patient). The author has accessed well over 5000 intervertebral discs without a single case of discitis (<0.02%) using a technique which will be described, calling for a skin puncture with a 15-gauge needle, 18-gauge introducer needle, 22-gauge disc puncture needle, and the use of both IV and intradiscal prophylactic antibiotics. The International Spine Intervention Society (ISIS)37 and the North American Spine Society (NASS)38 recommend the two-needle approach.
Following localization of the skin and underlying structures, as noted earlier, a puncture is made with a 15- or 16-gauge, 1.5-inch needle. This prevents any needle that may come in contact with the disc from actually being passed through the skin. An introducer needle, 18 or 22 gauge and 3.5 or 5 inches long, is then advanced through the anesthetic track toward the lateral aspect of the SAP and toward the disc entry site (see Fig. 38-3, C).
Advancement is halted when resistance is felt. This procedure is repeated until introducer needles have been placed at all levels to be tested see Figs. 38-4 and 38-5. A lateral view is then used to check needle depth (see Fig. 38-6).
Using active lateral fluoroscopy, the disc puncture needles, 22 or 25 gauge, 6 to 10 inches in length, are now individually inserted slowly through the introducer needle, and will be seen to traverse the foramen and felt to contact the intervertebral disc(see Figs. 38-7 to 38-9). To facilitate needle maneuverability and control, a slight bend on the disc puncture needle tip, opposite the bevel, is advocated. The L5-S1 intervertebral disc access is occasionally complicated by a “high” ilium preventing direct access to the center of the nucleus pulposus (see Fig. 38-10). This normal anatomic variant is much more common in men than in women. As illustrated in Figures 38-11 to 38-14, a curved needle technique can be used to provide adequate needle placement within the intervertebral disc. This variant requires the use of a dual-needle technique.
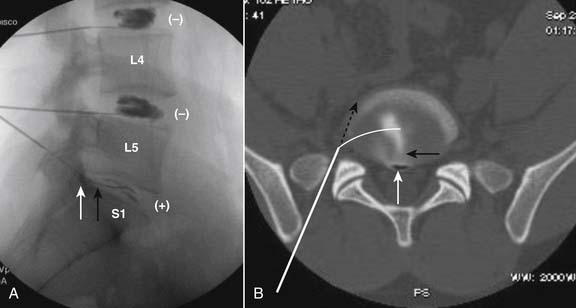
Figure 38-14 A, Lateral view. Results of stimulation (±) noted. L5-S1 positive concordant pain at 0.5 and 0.75 mL. Pressure 2 ± 5 psi above opening. B, Postprocedure axial CT image, bone window of L5-S1 disc from Fig. 38-14, A. Access to the nucleus pulposis requires a curved disc puncture needle (depicted by the curved white line) be advanced through a larger bore introducer needle (straight white line). Broken black line represents a straight needle track coursing medial to the ilium and lateral to the SAP entering only the far right lateral aspect of the annulus.
Using active lateral fluoroscopy and the bent needle tip to maneuver within the substance of the disc, the needle is positioned into the center of each disc nucleus. AP and lateral images of needle placement are saved for documentation of acceptable needle placement prior to injection of contrast (see Fig. 38-15).
When excellent needle position is noted within the disc nucleus, disc stimulation can commence. As indicated previously, injection is made using a manometer, with contrast containing antibiotic. The disc thought most likely to be positive from MRI pathology, should be stimulated last. The patient is blinded as to level and onset of stimulation. To ensure accurate visualization of the internal disc architecture during injection, the end plates of the contiguous vertebral bodies should be parallel to the beam. Injection into the disc is made slowly, in small aliquots of approximately 0.5 mL. Active fluoroscopy is used with many injectionists preferring a lateral view of the disc during active injection. The author prefers to use an AP view during injection in that it appears to provide a more accurate indication of the disc anatomy. Others suggest that a lateral view provides additional information about posterior annular disruptions. During injection, contrast should be noted within the nucleus (i.e., center of the disc) in AP and lateral views.
Following disc stimulation, AP and lateral images are saved for the permanent medical record (see Figs. 38-16 to 38-19).
In intervertebral discs shown to be positive, analgesic discography has been advocated by some.39,40 This entails injection of local anesthetic into the disc shown to provoke pain on stimulation. Significant decrease in pain is assumed to confirm the results of the provocation test. A recent multicenter study41 evaluating so called functional analgesic discography (FAD) using balloon tipped catheters inserted into the intervertebral discs shows only 39% of patients with positive provocation disc stimulation evidencing ≥75% relief with local anesthetic.
Following the disc provocation phase of the procedure, the needles are removed, the back is cleaned of the dried preparation solution, sterile self-adhesive dressings are applied, and analgesia is provided as necessary.
Postprocedure
Following the procedure, the patient is taken to recovery. Results of the procedure are discussed realizing that the medications used for sedation may render the patient amnestic. If positive concordant pain was noted at any level, a CT scan to document grade of annular disruption can be considered (see Fig. 38-19).
As with all medical procedures, a true and accurate post procedure report must be generated by the discographer. This record must include all pertinent information and be of such detail that a reviewing physician can appreciate what actually occurred during the procedure itself. If a postprocedure CT was ordered, interpretation of this by the discographer is required. Correlation between the provocation discography and the CT images is essential and has validity only if performed by that physician involved with actually stimulating the intervertebral discs and evaluating the patient during the procedure. Interpretation by a noninvolved radiologist, separated by time and distance, is at best meaningless and inappropriate. See example in Appendix A.
Thoracic Discography
Literature pertaining to thoracic discography is limited to technical considerations.42,43 There is no evidence currently available on the validity and utility of this procedure. However, the thoracic intervertebral discs are in many ways analogous to the related lumbar structures; and negative stimulation is noted in seemingly nonpathologic discs and provocation of concordant pain in chronic thoracic pain patients can be frequently elicited—indications that thoracic discography appears to be an appropriate presumptive diagnostic procedure. Realization of the tentative nature of this procedure must be taken into account in regard to any clinical applications.
As one progresses from lumbar, to thoracic, and finally to cervical interventional spine procedures, target structure size is reduced, tolerances become narrower, and the consequences of unintentional, errant needle placement become more profound. The injectionist contemplating thoracic procedures must assure himself of his expertise in interpreting the radiographic anatomy that will be encountered. Specifically, the dorsal region, the ribs, lungs, heart and other anatomic structures introduce a level of complexity which is unique to this area. Fluoroscopic images rarely provide the conspicuity as in the lumbar and cervical spine. The proximity of the pleura creates the real possibility of iatrogenic pneumothorax. Disc access in the thoracic spine involves needle introduction under conditions of close tolerances traversing a narrow corridor between rib head and superior articular process (see Fig. 38-20). Thoracic discography is technically demanding and should only be attempted by physicians whose skills have been well honed by extensive experience with fluoroscopically guided spinal procedures.
Procedure
The patient is positioned prone on a radiolucent procedure table. Monitors are attached, IV antibiotic is provided, and mild sedation as above is initiated. Depending on the chosen procedure levels, the corresponding thoracic region is prepared and draped. In that most of the twelve thoracic levels are difficult to differentiate from each other, meticulous care must be taken to identify the targeted intervertebral discs. Counting down from T1, and then up from T12, should provided an identical answer. At each level to be studied, the end plates surrounding the disc are aligned so as to be parallel to the x-ray beam by using cephalocaudal tilt of the image intensifier (see Fig. 38-21). The end plates will evidence a linear rather than an oval appearance. As with the lumbar procedure, an oblique view is then used contralateral to the dominant pain (see Fig. 38-22, A).
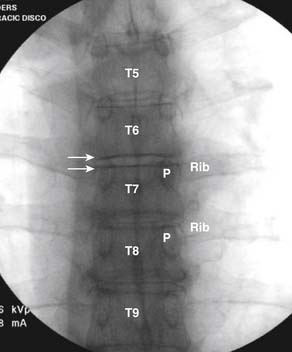
Figure 38-21 Scout anteroposterior (AP) midthoracic spine. T6-7 disc end plates parallel to x-ray beam, arrows. Pedicle (P).
As the C-arm is rotated, the spinous process will appear to move toward the contralateral side, followed by the pedicle and rib head of the infrasegmental level. A hyperlucent rectangle, or “box,” will become distinct over the target disc. When the pedicle is seen to be positioned approximately 30% to 40% across the vertebral body further rotation should cease. The hyperlucent “magic box” will appear, and is easily identified over the disc with its sides defined rostrally by the inferior end plate of the vertebral body above; caudally by the superior end plate of the vertebral body below; laterally by the medial rib head line (i.e., a line oriented rostral-caudal connecting the medial aspect of the rib heads; and medially by the medial pedicular line which approximates the superior articular process and lamina which is at times quite indistinct (see Fig. 38-22, B).
The skin is marked over the hyperlucent “box” and a local anesthetic skin wheal is made. A 25-gauge, 3.5-inch needle can now be advanced through the wheal toward the target and a local anesthetic track can be injected during withdrawal. A puncture can be made with an 18-gauge needle so that the disc puncture needle need not pierce and potentially carry skin and detritus into the disc. Depending on level and body habitus, a 22-gauge, 5-inch, or 25-gauge, 3.5-inch disc puncture needle, with slight bend at the tip opposite the bevel, is introduced and using the “tunnel vision,” down the beam approach, guided toward the target “box” (see Fig. 38-22, C).
The needle is incrementally advanced using intermittent fluoroscopy to make corrections. It is important to stay medial to the medial rib head line, the costovertebral joint, because the lung lies just lateral. Os is often contacted during needle advancement because the needle is transiting a space between rib head and superior articular process (see Fig. 38-20). If contact is made, withdrawing the needle tip from os 1 to 2 mm, rotating the needle and reinserting slowly, the bent needle tip will allow one passage through this narrow space with minimal difficulty. Needle advancement is stopped when the intervertebral disc annulus is contacted as can be appreciated by its unique tactile feeling. The other levels are now accessed to the point of disc contact. When all needles are positioned in proximity to the discs (see Fig. 38-23), the C-arm is positioned for a lateral view, and the needles will be seen as aligned parallel to the respective vertebral end plates in the infraneural, caudal position within their foramen. The needles are then advanced slowly using active lateral fluoroscopy, using rotation to provide directional control secondary to the bent needle tips. The disc is felt and seen to be entered, and the needles are inserted sequentially into the center of the intervertebral discs.
Needle position within the nucleus pulposus is then verified, and documentary AP and lateral images are saved (see Fig. 38-24).
Injection of contrast with antibiotic is then made into each disc using active lateral imaging. The disc felt most likely to be positive by MRI pathology should be injected last. The patient is blinded as to level and onset of stimulation. As discussed previously, either a manometer is used, or 3 mL syringes with a small-bore, low-volume extension tubing. Tubing length must be limited in that perceived pressure increases in proportion to length. The injected volume into a thoracic disc will range from 0.5 to 2.5 mL depending on level, the more rostral discs resembling a cervical disc and accepting little volume. During injection, if a manometer is used, the same objective data as collected for lumbar discography is recorded. If one elects to use 3 mL syringes, approximate pressure generated, and characteristic of the endpoint (soft or firm) are recorded in lieu of opening and final pressure as determined with a manometer. AP and lateral images postcontrast are then saved (see Fig. 38-25, A and B). A true and exact, detailed procedure record of the entire procedure must be produced by the discographer as a historical documentation of the study. Postprocedure care is identical to that for lumbar discography. CT imaging postthoracic discography although never validated as having diagnostic utility, might be obtained if beneficial information is expected (see Fig. 38-25, C-E).
Cervical
Cervical spinal injections of any variety are “demanding, technically intensive, and unforgiving procedures”43 fraught with severe consequences at the slightest inadvertent misstep. Cervical discography should not be attempted by physicians of any primary specialty without extensive experience in fluoroscopically guided spine injection techniques and significant hands on instruction by an expert practitioner.
Procedure
After positioning, preprocedure fluoroscopic screening ensures adequate visualization of all planned levels in AP, lateral, and oblique views. Beards prevent adequate sterile preparation of the skin and must be removed before the procedure, preferably the day before. The cervical region is prepared and draped in a sterile manner. A wide preparation is advisable and should include the entire anterior and lateral neck, extend down to the clavicles, and include the mandibular area. Both shoulders are incorporated so that the discographer can push the shoulders down manually if adequate visualization so demands. As with all spinal procedures, “An ounce of prevention is worth a pound of cure”. 45
Excellent discogenic pain referral maps for the cervical region are available,46 and will help guide the discographer; however, multilevel discogenic pain is common47 and a minimum of four levels should be studied to prevent omitting a painful disc and providing unreliable information. If pain is noted as referred into the occiput, C2-3 must be studied, whereas if scapular pain is present C6-7 is included.
Unlike the thoracic and lumbar procedures, in cases of cervical pain, the zygapophysial joint must be evaluated prior to proceeding with provocation discography. An extraordinarily high false-positive rate (68%) has been noted by Bogduk and Aprill,48 when cervical discography was not preceded by cervical medial branch anesthetic injections to block the segmental zygapophysial joints.
In that the esophagus is prominent on the left, needle insertion is always from the right independent as to side of dominant pain. The skin entry will be approximately along the medial border of the sternocleidomastoid muscle (see Fig. 38-26) but will vary slightly with the individual level. At the C6-7 level the apex of the lung, the thyroid, and common carotid arteries require a slight medial approach. The thyroid cartilage is present at C5-6 and will be readily evident if contacted on insertion, whereas a somewhat more lateral needle insertion is used at C2-3 to prevent entry and passage through a possibly distended hypopharynx.
With the patient positioned as above, the fluoroscope is obliqued toward the right into the so-called “foraminal view” where the foramen are seen at their widest extent in both the cephalocaudal and ventral-dorsal dimensions. This is the same view advocated for cervical transforaminal and cervicothoracic (stellate) ganglion injections. The objective disc is identified by counting down from the readily identifiable C2-3 level, and the C-arm is manipulated so that end plates of the contiguous vertebral bodies are seen to be parallel to the x-ray beam. It must be emphasized that the course of the vertebral artery runs in a cephalocaudal orientation over the uncinate line (see Fig. 38-27).
A target point is selected over the disc approximately one third the distance between the uncinate process and the apparent ventral disc margin (see Fig. 38-28, B). The skin entry site is marked, and will be seen to be medial to the sternocleidomastoid and carotid artery, which can be easily palpated. If desired, a small skin wheal is made with local anesthetic. Using a blunt sterile instrument, pressure is then applied over the insertion site to decrease the distance between the skin and disc and also to distract vulnerable soft tissue structures away from the needle track. The patient is asked to refrain from vocalization, swallowing, or coughing, and the needle is then quickly advanced over the tip of the blunt instrument, and using active fluoroscopy is maneuvered toward the disc in one fluid movement (see Fig. 38-28, C). Resistance to further insertion is noted as the needle enters the disc, and needle advancement is halted when purchase in the disc is accomplished. Total time elapsed between needle skin puncture and disc entry is often less than 2 seconds. During needle insertion, the bend on the needle tip allows precise directional control by rotation. Some radiation of the dominant hand is inevitable; however, using pulsed 8/second, and low-dose fluoroscopic modes, it is minimal and an acceptable risk.
When needles are positioned in the periphery of all levels to be studied (see Fig. 38-28, D) the fluoroscope is rotated into a lateral view. Using active lateral fluoroscopy the needles are advanced into the approximate center of each disc and the position is verified in AP view. Care must be taken to ensure that the needle is not advanced through the disc, which could involve entry into the spinal canal and probable cord penetration. AP and lateral views are then saved to document-safe needle position (see Fig. 38-29). At C6-7, if body habitus prevents adequate lateral visualization, AP, ipsilateral oblique, and contralateral oblique images can be substituted on the rare occasion.
Active lateral fluoroscopy is the used during disc stimulation (i.e., injection of contrast). The patient is blinded as to level and onset of stimulation. Pressure of injection is slowly increased until the intrinsic internal pressure within the disc is overcome and a small amount of contrast is seen within the disc. At this point, injection is halted. Occasionally, some momentary pain will be felt by the patient at opening pressure. This is usually transient and is probably a response to enlargement of a fissure. If high pressure is evident without intradiscal contrast noted, the needle opening may be lodged in dense discal material. Slight withdrawal of the needle and repositioning within the disc will often rectify this problem. Prior to reinjection, safe needle position must be verified. When opening pressure has been noted, slow injection of contrast can proceed. Often firm resistance or pain will be noted with injection of as little as 0.2 mL. Injection into any nonpathologic cervical intervertebral disc will be limited to less than 0.5 mL and high pressure noted.49 Cervical intervertebral discs that appear to hold more than 0.5 mL will invariably be noted to have pathology on imaging. In normal discs, the endplates will be seen to separate owing to an increase in disc height during injection. AP and lateral images for the permanent medical record must be archived (see Fig. 38-31).
Endpoints of stimulation as per the ISIS guidelines cited previously include: concordant pain reproduced at a VAS of ≥7;
significant nonconcordant pain; neurologic symptoms experienced by the patient; contrast escaping from the disc; displacement of the vertebral body end plates; or firm resistance to injection.
Analgesic discography although first described in the cervical region as referenced earlier,26 has not been validated as providing accurate information on which to base clinical decisions. Bogduk and Aprill34 were able to provide analgesia in only 7 of 34 (21%) of discs evidencing pain on stimulation, which only comes close to the level of placebo effect. Further study is needed.
Summary
No doubt procedural nuances will be instituted in the future to further refine our use and interpretation of disc stimulation, and discography will continue to provide a diagnosis for and guidance in the treatment of our patients.
A Example of Procedure Note for Lumbar Discography
Mr. Pain tolerated the procedure well, was taken to recovery, and then for a post-procedure CT scan. He will follow up with his primary care physician in the near future. A consultation with Dr. Surgeon might be considered to discuss possible surgical treatment options. Mr. Pain knows to follow up with this physician if any problems were to develop.
Interpretations of CT Post-Discography
1. Heschel A.J. The Patient as a Person. San Francisco. American Medical Association Annual Meeting 1964. In: The Insecurity of Freedom: Essays on Existence. New York: Schochen; 1966:24-38.
2. Schwarzer A.C., Aprill C.N., Derby R., et al. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine. 1995;20:1878-1882.
3. Schwarzer A.C., Aprill C.N., Derby R., et al. Clinical features of patients with pain stemming from the lumbar zygapophysial joints. Is the lumbar facet syndrome a clinical entity. Spine. 1994;19:1132-1137.
4. Schwarzer A.C., Aprill C.N., Bogduk N. The sacroiliac joint in chronic low back pain. Spine. 1995;20:31-37.
5. Yin W., Bogduk N. The nature of neck pain in a private pain clinic in the United States. Spine. 2006;9:196-203.
6. Bogduk N., Tynan W., Wilson A.S. The nerve supply to the lumbar intervertebral disc. J Anat. 1981;132:39-56.
7. Bogduk N. The innervations of the lumbar spine. Spine. 1983;8:286-293.
8. Lindblom K. Diagnostic puncture of the intervertebral discs in sciatica. Acta Orthop Scand. 1948;17:231.
9. Deyo R.A., Mirza S.K., Martin B.I., et al. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303:1259-1265.
10. Algorthim for Investigation of Low Back Pain. In: Bogduk N., editor. Practice Guidelines for Spinal Diagnostic and Treatment Procedures. San Francisco: International Spine Intervention Society; 2005:87-94.
11. Laun A., Lorenz R., Agnoli A.L. Complications of cervical discography. J Neurosurg Sci. 1981;25:17-20.
12. Aprill C.A. Diagnostic Disc Injection. I. Cervical Disc Injection. In: Frymoyer J.W., editor. The Adult Spine: Principles and Practice. Philadelphia: Lippincott-Raven; 1997:523-538.
13. Aprill C.A. Diagnostic Disc Injection. II. Lumbar Disc Injection. In: Frymoyer J.W., editor. The Adult Spine: Principles and Practice. Philadelphia: Lippincott-Raven; 1997:539-562.
14. Landers M.H. Diskography. In: Waldman S.W., editor. Pain Management. Philadelphia: Saunders-Elsevier; 2007:118-144.
15. Fraser R.D., Osti O.L., Vernon-Roberts B. Iatrogenic discitis: The role of intravenous antibiotics in prevention and treatment. An experimental study. Spine. 1989;14:1025.
16. Osti O., Fraser R.D., Vernon-Roberts B. Discitis after discography. The role of prophylactic antibiotics. J Bone Joint Surg Br. 1990;72:271.
17. Connor P.M., Darden B.V.2nd. Cervical discography complications and clinical efficacy. Spine. 1993;18:2035.
18. Zeidman S.M., Thompson K., Ducker T.B. Complications of cervical discography: Analysis of 4400 diagnostic disc injections. Neurosurgery. 1995;37:414.
19. Boswell M.V., Wolfe J.R. Intrathecal cefazolin-induced seizures following attempted discography. Pain Physician. 2004;7:103.
20. Fenton D.S., Czervionke L.F. Discography. In: Fenton D.S., Czervionke L.F., editors. Image-Guided Spine Intervention. Philadelphia: Saunders; 2003:227-255.
21. Bogduk N., editor. Practice Guidelines: Spinal diagnostic & treatment procedures. San Francisco: International Spine Intervention Society, 2004.
22. Pauza K.J. PASSOR Educational Guidelines for the performance of spinal injection procedures. PASSOR Board of Governors and AAPMR Board of Governors; 2001.
23. Klessig H.T., Showsh S.A., Sekorski A. The use of intradiscal antibiotics for discography: An in vitro study of gentamycin, cefazolin, and clindamycin. Spine. 2003;28:1735-1738.
24. Bogduk N., Marsland A. The cervical zygapophysial joints as a source of neck pain. Spine. 1988;13:610-617.
25. Dwyer A., Aprill C., Bogduk N. Cervical zygapophysial joint pain patterns. 1: A study in normal volunteers. Spine. 1990;15:453-457.
26. Fukui S., Ohseto K., Shiotani M., et al. Referred pain distribution of the cervical zygapophysial joints and cervical dorsal rami. Pain. 1996;68:79-83.
27. Fukui S., Ohseto K., Shiotani M. Patterns of pain induced by distending the thoracic zygapophysial joints. Reg Anesth. 1997;22:332-336.
28. Dreyfuss P., Tibiletti C., Dreyer S.J. Thoracic zygapophysial joint pain patterns. A study in normal volunteers. Spine. 1994;19:807-811.
29. Lumbar disc stimulation (Provocation Discography). In: Bogduk N., editor. Practice Guidelines: Spinal diagnostic & treatment procedures. San Francisco: International Spine Intervention Society; 2004:20-46.
30. Mersky H., Bogduk N., editors. Classification of Chronic Pain. Descriptions of chronic pain syndromes and definition of pain terms. 2nd ed. Seattle: IASP Press. 1994:180-181.
31. Moneta G.B., Videman T., Kaivanto K., et al. Reported pain during lumbar discography as a function of annular ruptures and disc degeneration. A re-analysis of 833 discograms. Spine. 1994;17:1968-1974.
32. Sachs B.L., Vanharanta H., Spivey M.A., et al. Dallas discogram description: A new classification of CT/discography in low back disorders. Spine. 1987;12:287-294.
33. Bogduk N., editor. Thoracic provocation discography Practice Guidelines: In Spinal diagnostic & treatment procedures. San Francisco: International Spine Intervention Society. 2004:287-313.
34. Mercer S., Bogduk N. The ligaments and annulus fibrosus of human adult cervical intervertebral discs. Spine. 1999;24:619.
35. Cervical disc stimulation (provocation discography). In: Bogduk N., editor. Practice Guidelines: In Spinal diagnostic & treatment procedures. San Francisco: International Spine Intervention Society; 2004:95-111.
36. Fraser R.D., Osti O.L., Vernon-Roberts B. Discitis after discography. J Bone Joint Surg Br. 1987;69:26-35.
37. Lumbar disc stimulation (Provocation Discography). In: Bogduk N., editor. Practice Guidelines: Spinal diagnostic & treatment procedures. San Francisco: International Spine Intervention Society; 2004:20-46.
38. Guyer R.D., Ohnmeiss D.D. Lumbar discography: position statement from the North American Spine Society Diagnostic and Therapeutic Committee. Spine. 1995;20:2048-2059.
39. Roth D.A. Cervical analgesic discography. A new test for the definitive diagnosis of painful-disk syndrome. JAMA. 1976;235:1713.
40. Ohtori S., Kinoshita T., Nakamura S., et al. Surgical results for discogenic low back pain randomized study using discography vs. discoblock. Spineweek 5/29/2008. 2008:59.
41. DePalma M.J., Lee J.E., Peterson L., et al. Are outer annular fissures stimulated during diskcography the source of diskogenic low-back pain? An analysis of analgesic diskography data. Pain Medicine. 2009;10:488-494.
42. Schellhas K.P., Pollei S.R., Dorwart R.H. Thoracic discography: A safe and reliable technique. Spine. 1994;19:2103-2109.
43. Thoracic provocation discography Practice Guidelines. In: Bogduk N., editor. Spinal diagnostic & treatment procedures. San Francisco: International Spine Intervention Society; 2004:287-313.
44. Cervical disc stimulation (provocation discography). In: Bogduk N., editor. Practice Guidelines: In Spinal diagnostic & treatment procedures. San Francisco: International Spine Intervention Society; 2004:95-111.
45. Franklin B. Poor Richard’s Almanack: Philadelphia. 1733.
46. Schellhas K.P., Smith M.D., Gundry C.R., Pollei S.R. Cervical discogenic pain. Prospective correlation of magnetic resonance imaging and discography in asymptomatic subjects and pain sufferers. Spine. 1996;21:300-312.
47. Grubb S.A., Kelly C.K. Cervical discography: Clinical implications from 12 years of experience. Spine. 2000;25:1382-1389.
48. Bogduk N., Aprill C. On the nature of neck pain, discography and cervical zygapophysial joint blocks. Pain. 1993;54:213-217.
49. Kambin P., Abda S., Kurpicki F. Intradiskal pressure and volume recording: Evaluation of normal and abnormal cervical disks. Clin Orthop Relat Res. 1980;146:144.