39 Discogenic Pain, Internal Disc Disruption, and Radicular Pain
This chapter concerns the diagnosis of spinal pain; diagnosis being the cornerstone of a doctor/patient relationship. Various spinal interventions will be discussed in following chapters. Each intervention requires particular knowledge and technical ability. Unfortunately, knowledge of the indications for these procedures and more importantly, their technical performance, have often fallen short of the mark—even in scientific publications.1,2 This has lead to a persistent skepticism about their efficacy. This fact is most evident in the development of radiofrequency neurotomy (RFN) treatments, and in subsequent meta-analyses and systematic reviews that gave equal credence to results, whether or not the technique was accurate.3
Social and Economic Dimension of Spinal Pain
Low back pain (LBP) is a major health problem particularly in industrialized countries,4 affecting approximately 60% to 80% of the adult population at some stage5–7 and about 6% of people each day.6 LBP affects up to 80% of the working population during their lifetime and is the second most common reason for physician visits,8 and for work disability.9 Although LBP is typically self-limiting,10 it is still associated with substantial health care costs and absenteeism from work.4,11,12 Neck pain is also extremely common with a lifetime prevalence of 70%, a 1-year prevalence of 40%, and a point prevalence of 10% to 20%.13 Generalized musculoskeletal pain is also common: in one study of kitchen-hand workers it was present in 87%, and often present in multiple sites. In this study, neck pain was present in 71%, LBP in 50%, and forearm/hand pain in 49%;14 73% had pain in at least two sites, 36% in four or more, and 10% in six to seven sites.14 In another study of 4006 workers from industrial and service companies, only 7.7% were free of regional pain of any description.15
In Australia, back problems are the most frequently seen musculoskeletal condition in general practice and the seventh most common reason for seeking care.16 In Australia, a 2001 survey found the point prevalence of LBP to be 26%, the 12-month prevalence 68%, and lifetime prevalence 79%.7 Only about 50% of the adult population experience low-intensity pain and low disability from it and another 11% experience high-intensity-pain but still low disability in a 6-month period.7 However, about 11% of the population experience high-disability LBP,7,17 and it is to this group that most resources are presumably directed.
LBP is a costly problem. In early 2000, the cost of headache, LBP, arthritis and other muscle and joint pain to U.S. employers was more than $60 billion per year (these costs may have been underestimated because lost productivity among workers affected by a coworker’s diminished productivity was not taken into account).18 The majority of these costs (77%) related to reduced performance rather than work absence (workers who experienced lost productive time from a pain condition lost a mean of 4.6 hours/week). Workers who reported arthritis or LBP had mean lost productive times of 5.2 hours/week. It was established that these pains were directly associated with a 13% loss in productive time. Headache was the most common (5.4%) pain condition resulting in lost productive time. It was followed by LBP (3.2%), arthritis pain (2.0%), and other musculoskeletal pain (2.0%).
Furthermore, the total health care expenditure only for LBP in the United States is even more alarming. When studied in 1998, total health care expenditures incurred by individuals with LBP in the United States reached $90.7 billion and total incremental expenditures attributable to LBP among these persons were approximately $26.3 billion. On average, individuals with LBP incurred health care expenditures at 60% higher than people without LBP.19 The lead researcher, Xuemei Luo, put these figures into the perspective of the U.S. economy by noting: “The total $90 billion spent in 1998 represented 1% of the U.S. Gross Domestic Product, and the $26 billion in direct back pain costs accounted for 2.5% of all health care expenditures for that year.”
The largest proportion of direct medical costs for LBP is spent on physical therapy (17%) and inpatient services (17%), followed by pharmacy (13%) and primary care (13%).20 However, indirect costs, especially resulting from lost work productivity, outweigh other costs substantially.20
Nonspecific Spinal Pain
Careful history and examination may at least be helpful in determining that the pain comes from the spine and whether or not it is caused by a red-flag condition. If clinical examination produces pain, it is likely that the pain derives from the spine. As a corollary, the absence of any painful restriction of movement on clinical examination should alert the clinician to the possibility of distant referred pain and such a negative finding is, in itself, a “red-flag sign”.21 Radiologic techniques are not helpful in resolving whether or not regional spinal pain originates from a spinal structure at all; imaging can exclude only red-flag or exotic causes of pain.
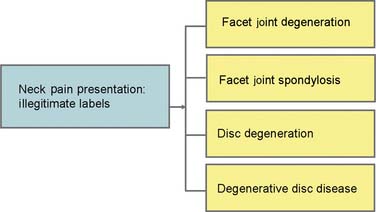
Numerous studies on all imaging modalities used in the detection of morphologic changes in symptomatic and asymptomatic populations have failed to find significant differences that can be considered useful in any individual presentation of NSSP.22–46 Astoundingly, this well developed scientific fact has still not penetrated into standard health care practice; consequently, certainly the public and regrettably, some heath practitioners, remain misinformed about the relevance of technology as it applies to NSSP. The perceived contribution of the disc to spinal pain has been skewed by inappropriate use of labeling in the field of radiology.47 The label degenerative disc disease (DDD) pervades imaging reports and the scientific literature, yet there is no reasonable evidence to confirm DDD as being of any particular relevance to any single manifestation of NSSP. DDD is not a legitimate label for a patient with NSSP.
NSSP concentrates along the spine; early studies on pain referral patterns have shown that all of the innervated back structures can produce local pain with or without more distant referred pain.48–54 When the pain is concentrated in the lumbar spine, it can be called nonspecific low back pain (NSLBP); in the cervical region, it is called nonspecific neck pain (NSNP). These terms are synonymous and equally as useful as terms such as idiopathic back (or neck) pain and low back (or neck) pain of unknown origin.
Referred Pain
Referred pain can theoretically derive from any locally innervated spinal structure. The mechanism for referred pain is convergence.55 Somatic referred pain is pain evoked by the stimulation of the peripheral endings of nociceptive afferent fibers and is perceived in an ambiguous site due to the phenomenon of convergence when these afferents converge on second-order or third-order neurons in the central nervous system that happen also to receive afferents from the region to which the pain is referred.56 Under those conditions, and in the absence of additional sensory input to clarify the situation, the brain is unable to identify the source of the pain accurately, and attributes it erroneously to the entire area subtended by the common neurons.57,58 Ambiguity as to the source of information arises, either or both, because the painful structure is not densely innervated, and the central pathways along which the information is relayed are not highly organized somatotopically.59 NSSP is, therefore, different to radicular pain. Hence, lumbar ZJ pain,60 lumbar disc pain,61–63 and sacroiliac joint pain64 are described very differently to lumbar radicular pain.
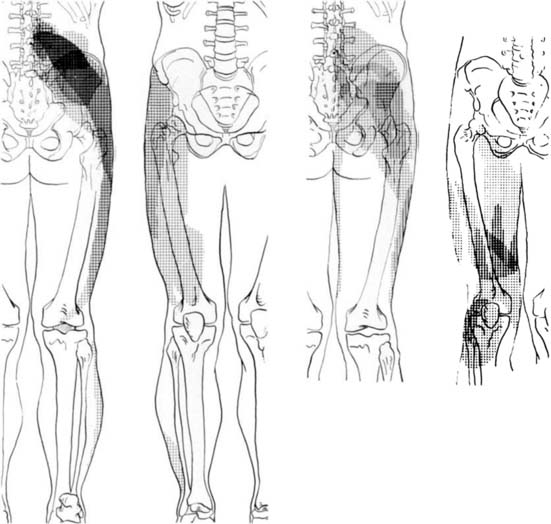
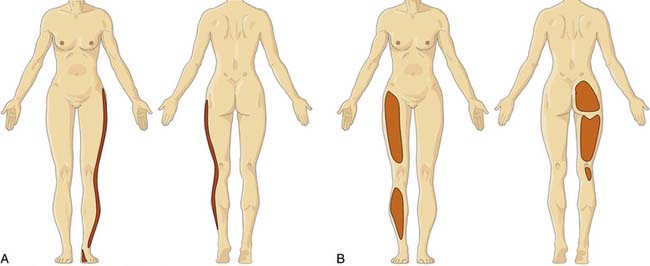
Information about referred pain from deep somatic structures has arisen from numerous important studies that identified the nature and spread of referred pain.48–54 Experiments on deep somatic referred pain by Kellgren52 in 1939 and Feinstein44 in 1954 showed that pain from deep somatic structures can be felt not only locally but also in distant areas; pain from deep lumbar structures refers into the legs as far as the feet; similarly pain from deep cervical structures refers into the arms and hands (Fig. 39-1). Later Hirsch and colleagues placed needles into various lumbar structures in people with LBP and reported that the disc was the most sensitive area for LBP.54 In this experiment, needles were placed into one or both of the lower two discs and into the ZJs, followed by the ligamentum flavum and the posterior ligamentous structures. When the disc was injected with 0.3 mL of saline a deep aching occurred across the low back. When the ZJ was injected, also with 0.3 mL saline, the ache spread also into the buttocks and lateral hips. Additionally, deep somatic pain felt three dimensional; it was described typically as deep and aching, but other terms used included gripping, boring, crampy, and lumpy.48 Subsequently the pain from lumbar PD, in which intradiscal pressures probably rise above that used by Hirsch and colleagues, has been shown to be experienced as central low back pain that can also spread diffusely into the legs,65 and certainly below the knees.66 From these studies, various referral pain maps have been created, demonstrating that segmental referral patterns overlap substantially, and structures at one segmental level have similar pain referrals to other structures at the same level.67 Thus, although the site of pain may be a clue to a particular spinal segment, it is not a pointer to the specific anatomic origin of pain.
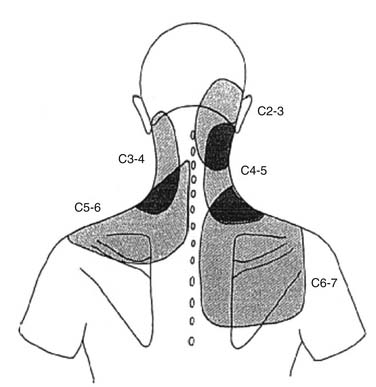
Similar patterns exist in the cervical spine. Cervical ZJ pain, as ascertained by anesthetic blocks rather than provocation, extends beyond the local region to the ipsilateral occiput, shoulder and/or periscapular region (Fig. 39-2).67 Cervical disc pain, as ascertained by PD, can spread into the thoracic spine and the arms including the forearms, hands, and fingers.68–70 Cervical PD at any level produces local neck pain that is unilateral as often as it is bilateral, with referral patterns as follows: C2-3 suboccipital and facial; C3-4 suboccipital, trapezius, anterior neck, face, shoulder, interscapular, and upper limb; C4-5 shoulder, interscapular, trapezius, extremity, face, chest and suboccipital; C5-6 trapezius, interscapular, suboccipital, anterior neck, chest and face; C6-7 interscapular, trapezius, shoulder, extremity and suboccipital; and C7-T1 interscapular (Fig. 39-3).71 Thus, cervical referred pain includes headache as well as arm, chest, shoulder, and thoracic pain.
Radicular Pain
NSSP and radicular pain can extend to the limbs; it is therefore important to ascertain which type of pain is more likely because each must be considered differently from clinical and pathophysiologic perspectives (Fig. 39-4).72 Radicular pain is not the same as referred pain.74 Radicular pain is a particular type of neurogenic pain caused by direct injury to a sensory nerve root or dorsal root ganglion of a spinal nerve.74,75 Radicular pain is sometimes accompanied by objective signs of deficit or loss of neurologic function in a segmental distribution as a result of conduction block and it can coexist with spinal or somatic referred pain.74,75
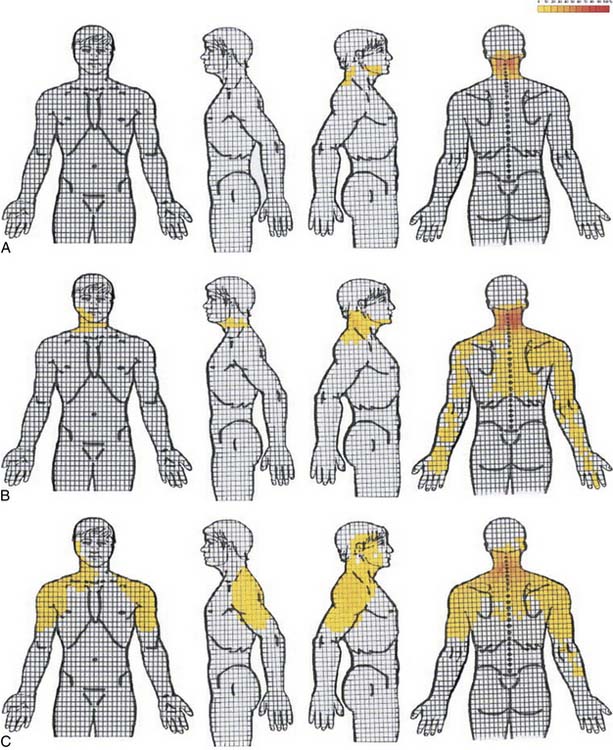
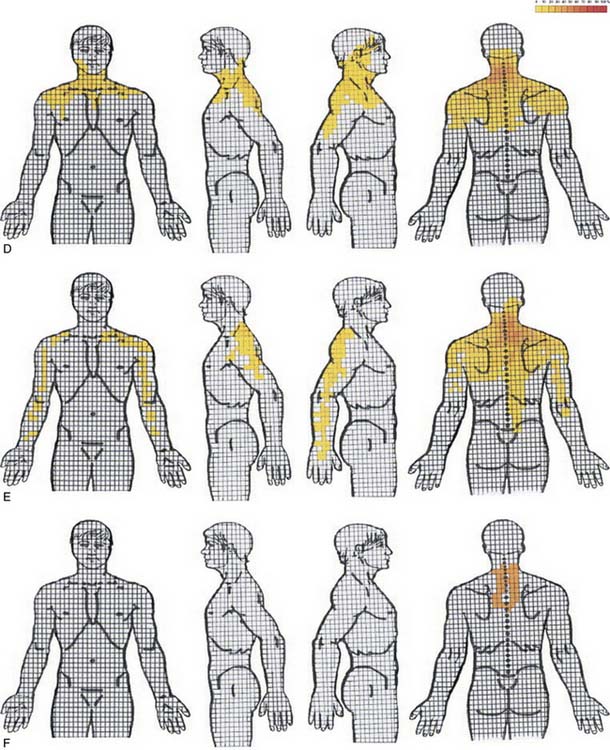
Cervical radicular pain tends to be deep, severe, aching pain, and as such is different from lumbar radicular pain.74 Cervical radicular pain is experienced predominantly in the upper limb and shoulder girdle but when it occurs in the limb its distribution does not correspond to dermatomal maps of sensory deficit due to cervical radiculopathy.73 Cervical radicular pain from the C6, C7, and C8 nerve roots is felt in the arm with pain extending into the forearm and hand (Fig. 39-5).75 However, limb pain can also occur in referred pain, and because cervical radicular pain is uncommon, with an annual prevalence rate of 0.083% in one large population study,76 deep aching arm pain is much more likely to be somatic referred pain than is cervical radicular pain.
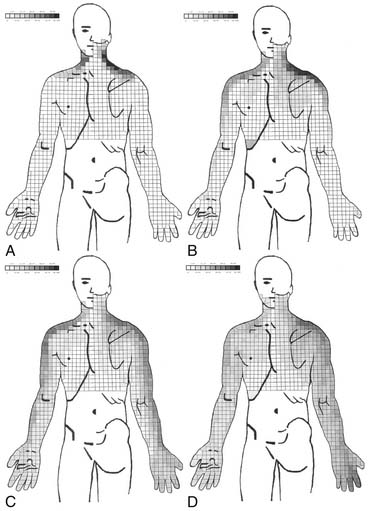
Figure 39-5 Percent occurrence of symptom provocation per bit for C4 to C7 nerve roots. A, C4; B, C5; C, C6; D, C7.73 Note that the figures depict anterior and posterior views.
In contrast, lumbar radicular pain is classically an intense narrow band of lancinating, sometimes burning pain that refers down the limb, and often to the foot.77 The leg pain is typically more prominent than any LBP (indeed, LBP frequently is absent), and the leg pain tends to concentrate distally. Coughing, sneezing, straining at the toilet, and lifting will all classically exacerbate radicular pain, but such aggravating features are not specific to radicular pain. Pain is not limited to the dermatome and it may also be experienced in deep tissues innervated by the nerve.78
Concepts: Discogenic Pain, Internal Disc
As at least the outer one third of the annulus fibrosus (AF) is innervated,79–81 the intervertebral disc is considered to be a possible source of pain that is otherwise labeled NSSP. There are five types of nerve terminations found in the lumbar disc: these have various morphologies and include simple and complex free nerve endings that concentrate particularly in the lateral disc, with a smaller amount posteriorly and the least amount anteriorly.79 Discogenic pain (DP) is pain that is considered to arise intrinsically from the disc. DP is frequently bandied about and assumed as a diagnosis, but the plain fact is that it is a diagnosis that cannot be substantiated. With the use of PD, the entity IDD has been defined according to specific diagnostic criteria.82 IDD is considered to be a subtype of DP.
Is There a Relationship Between Disc Degeneration and Discogenic Pain?
Cellular function within the disc is mediated by at least five major factors: genetics, nutrition (diffusion of nutrients and oxygen across the disc matrix), cell function regulation (via IL-1, TNF-α, and TNF-β), age and senescence, and mechanical loading.83 The contribution to DD by genetic factors is highly significant; it may be as high as 80% in the cervical spine,84 and general heritability for DD ranges from 29% to 80% in different regions of the spine.84–86 In the lumbar spine, the genetic contribution is between 29% and 54%, with environmental influences of about equal importance.87 For example, smoking has a moderate influence on the prevalence of DD,87 presumably due to its effects on disc nutrition. This emphasizes that DD is not primarily or significantly caused by aging88 or by mechanically induced “wear and tear” processes.85,89
Studies with various imaging modalities on symptomatic and asymptomatic populations further emphasize that DD does not imply NSSP. In the cervical spine, radiologic DD is present in 13% of men and 5% of women during the third decade, in 85% to 90% of the population by the sixth decade, and nearly 100% by the age of 70 years.90 It occurs most commonly at C5-6, C6-7, and C4-5, respectively.42,91 In people aged 60 to 65 years without neck pain, about 95% of men and 70% of women have at least one degenerative change on their cervical spine plain radiographs.42 Lumbar degeneration, defined as a grade of ≥2 by the Kellgren-Lawrence scale; is present in Japanese females in 9.7% of the ≤ 39 age group, in 28.6% of those 40 to 49 years, in 41.7% of those 50 to 59 years, in 55.4% of those 60 to 69 years, in 75.1% of those 70 to 79 years, and 78.2% of those ≥ 80 years; and in Japanese males in 14.3% of the ≤ 39 age group, in 45.5% of those 40 to 49 years, in 72.9% of those 50 to 59 years, in 74.6% of those 60 to 69 years, in 85.3% of those 70 to 79 years, and 90.1% of those ≥ 80 years.92 Although plain radiographic changes, including vertebral end-plate changes, disc space narrowing, spondylolisthesis, spondylolysis, sacral lumbarization, wedge vertebra, a sagittal diameter of less than 12 mm and abnormal lumbar lordotic angle,44,93,94 have some predictive value for LBP, the relationship is mild at best,95 and their detection is largely not helpful in the management of NSLBP because such changes occur frequently in the asymptomatic population.96 Although disc space narrowing at 2 or more levels from L1-2 to L4-5 shows the strongest radiologic relationship with LBP,97 similar comments apply. As a consequence, plain radiographs should not be ordered unless there is suspicion of a red-flag condition.98–101
The relevance of CT scanning is similar to plain radiographs; it is an excellent test for some red-flag conditions, demonstrates DD well, but it is not helpful in the detection of DP or ZJ pain. CT is better than MRI in detecting ZJ spondylitis,102 but this is of no particular clinical relevance. A newer technology, F-PET/CT (fluoride positron emission tomography with addition of CT) is more likely to be positive in symptomatic patients,103 but only time will tell if it has satisfactory validity in detecting a truly painful structure.
Diagnosis: Discogenic Pain and Internal Disc Disruption
The Diagnosis of Discogenic Pain Using Specific Nerve Blocks
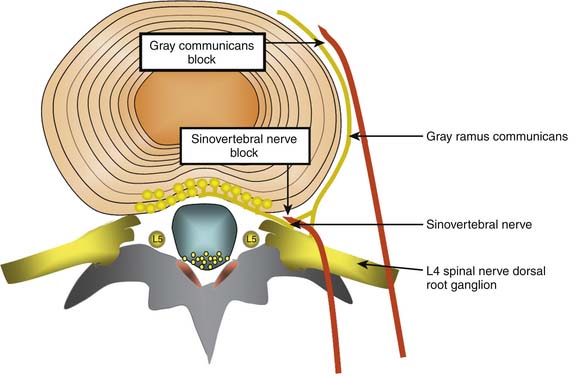
Lumbar discs are innervated by the lumbar sinuvertebral nerves, and branches of the lumbar ventral rami and gray rami communicantes (Fig. 39-6).104–106 The posterior and posterolateral portion of each lumbar disc is innervated by a branch of the ventral ramus arising just lateral to the intervertebral foramen and by a branch of the gray ramus communicans just before it connects with the ventral ramus.106 The sinuvertebral nerves also innervate the posterior longitudinal ligament; the gray rami communicantes also innervate the lateral disc and the anterior longitudinal ligament,104,106 and in rats the DRG innervates other lumbar structures, such as, lamina, spinous process, back muscle fascia, and skin.107 The innervation by the gray rami communicantes is not a direct sympathetic innervation;106 it has been postulated that somatic and afferent fibers from lumbar structures use the gray rami as transmission pathways only.104,108,109 The rami communicantes branch from the spinal nerves just after they enter the intervertebral foramina, and then run anteriorly along the inferior third aspect of the vertebral body where they connect to the sympathetic trunk before branching to the lateral and anterolateral aspects of the discs above and below.104,110,111
Various studies have been used to support or refute the utility of blocking the innervation of the disc.112–115 Although the nerve supply of the disc is nonspecific, if a local anesthetic block aimed at these nerves totally eradicated pain under controlled conditions, it might be possible to devise a treatment directed at the nerve similar to that performed for medial branch RF neurotomy. If this was the case, the interpretation would be that the pain was mediated by this particular nerve, not that the pain was necessarily discogenic.
The Diagnosis of Discogenic Pain or IDD Using Anesthetic Discography
The disc itself can be blocked by intradiscal injection. Analgesic discography (AD) and functional analgesic discography are refinements of the technique of PD aimed at increasing the utility of discography and, in particular, its specificity.116 However, the current definition of IDD does not include any statement about AD. There is no role for AD in discs with breaches through the outer lamellae (Dallas grade V) because pain reduction with leakage of any anesthetic agent into the epidural space could be a false-positive finding. This is the primary reason why there is at present no method for the diagnosis of DP in general; it is probable that discs with breaches in the outer lamellae can be a source of pain, but there is no valid method to make this connection. The problem with AD is that the anesthetic may not reach the painful radial tear owing to the dilution effect of contrast. If AD is to be used, it probably should be done directly via injection into the radial fissure.
The Diagnosis of IDD with Discography
Although lumbar DP cannot be diagnosed using imaging, there is a significant relationship between disc morphology as determined by discography, and clinical pain as determined by the subjective provocation phase of PD. As a consequence, a particular cohort of otherwise labeled NSLBP patients can be defined as having IDD,117 a specific subtype of DP.
Studies and reports on intradiscal therapies for putative DP have largely been drawn from patients diagnosed with IDD on the basis of PD. Discussion about the role and interpretation of PD can be found in Chapter 38. PD is used to confirm or deny the diagnosis of IDD, allowing for (1) enhanced ability to make a decision on interventional treatment—be it intradiscal therapy or spinal surgery; and (2) cessation of the search for other pain sources. The specificity of PD and, hence, the robustness of the diagnosis IDD has been questioned because of its propensity for false-positive findings.118–123 This can be minimized by careful patient selection; the risk of false positive findings is substantially diminished by selecting subjects with normal psychometric profiles who do not have pain in other regions.119,120 It would be folly to perform PD on a patient with fibromyalgia! The criteria for IDD are specifically applied to the lower lumbar spine (Table 39-1). There is insufficient research on other areas of the spine to consider that intradiscal therapies have any traction in the cervical and thoracic spine for such regional NSSP presentations. In the cervical spine the disc cannot be pressurized because fissures are present in normal adult discs.124 Nevertheless, intradiscal therapies have been trialed in other regions.
| Chronic, disabling NSLBP |
| Failure to respond to noninvasive treatments |
| No red-flag conditions |
| No evidence of radicular or neuropathic pain |
| No psychological barriers |
| No greater than 25% loss of disc height |
| Criteria for IDD by provocation discography satisfied |
IDD, internal disc disruption; NSLBP, nonspecific low back pain.
This last point is likely to be highly significant. The histology of discs that have been diagnosed with IDD using PD is different for discs in patients without LBP that have been assessed by PD as negative but degenerate on MRI and for cadaver discs that are macroscopically normal; the major difference is that in IDD there is a chronic inflammatory reaction with variable blood vessel infiltration.129 More specifically, the AF loses its normal lamellar structure, is disorganized and disrupted, and the fibers are cross-fused; the NP is markedly fibrosed, inflamed, and infiltrated with blood vessels.129 Additionally, immunohistochemical staining shows strong connective tissue growth factor (CTGF) expression in IDD discs, weak expression in asymptomatic DDs, and no expression in adjacent control discs in patients with IDD.129
CTGF is the downstream effector mediated by transforming growth factor-β1 (TGF-β1).130 CTGF is “closely associated with the regulation of cell proliferation and differentiation and the fibrosis of tissues and organs, and can induce the in vivo expression of the gene involved with fibroblast extracellular matrix composition.”130 CTGF has a number of functions, one of which is the creation of some of the components of fibrosis by the production and accumulation of extracellular matrix.131 Healing of disc tissue is different to most other tissues because it is relatively avascular and takes place either from peripheral structures such as the outer AF and posterior longitudinal ligament,131 or via the end plate.132,133 Healing after injury to the AF or end plate is promoted and accompanied by vascular ingrowth, which stimulates vascular inflammatory reactions and the production of growth factors including TGF-β1 and CTGF.133 Aberrations of growth factor contribution to healing after injury to the AF or end plate are postulated to be a significant cause of DP.
The validity of the diagnosis IDD is predicated first on the methodology of the PD, and second on patient selection for the procedure. The International Spine Interventional Society mandates the protocol as follows:82 (1) reproduction of the patient’s pain by stimulation of the affected disc; (2) such that the evoked pain has an intensity of at least 7 on a 10-point scale; and (3) pain is reproduced at a pressure of stimulation of < 15 psi; (4) provided that stimulation of adjacent discs does not reproduce pain; and (5) post-discography CT demonstrates a grade III or IV fissure. That is, the disc is morphologically abnormal internally, but it is intact peripherally. A diagnosis of IDD can be made, therefore, on a disc that is normal on CT scan, and, dependent on the sensitivity of MRI, on a normal MRI.134 Patients with concordant pain at 15 to 50 psi might be labeled as having indeterminate pain, and those without pain until above 50 psi as definitely negative. Presumably, alterations in the pressure parameters used to diagnose IDD alter the robustness of PD to truly detect DP; however, this is a somewhat ethereal statement because there is no standardized criterion against which the concept of IDD can be tested.
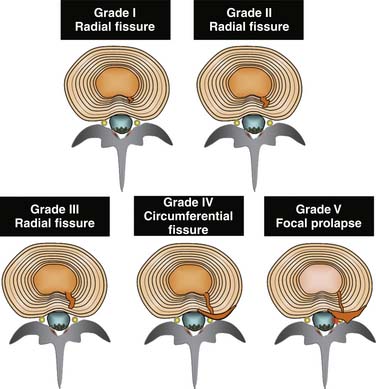
The features of IDD are characterized morphologically by: (1) degradation of the matrix of the NP, and (2) radial fissures, with or without a circumferential extension, that penetrate the AF without breaching the outer lamella. Fissures are graded on their extent, depending on whether they reach the inner, middle, or outer third of the AF,135 or if they extend circumferentially.136 The more extensive fissures that do not breach the outer lamellae of the AF correlate strongly with clinical pain and are associated with abnormal stress profilometry throughout the disc, including reduction and irregularity of NP stress, and increase in AF stress.125 Additionally, altered NP pressure can arise experimentally from end-plate fatigue failure,126 which has, in turn, been demonstrated to occur with loads that are consistent with moderately heavy work activities.
Although it is likely that intrinsic DP can occur in discs that are disrupted to the point where the outer lamellae of the AF are breached (a grade 5 annular tear), it is not possible to determine that such a disc is the source of pain with PD (Fig. 39-7). On morphologic grounds alone, a discogram finding of a grade 5 disc disruption negates the diagnosis of IDD. On pain provocation grounds it is not possible to state that a disc with grade 5 change is the source of pain because of the high chance of invalid responses. If the outer lamellae are breached during discography, the provocation phase might not only produce a false-positive result because contrast and pressure is exerted into the epidural space, but also a false-negative response because the disc may not be able to be pressurized to the degree required by protocol or to a sufficient degree to reproduce the index pain.
How Useful Is MRI as a Predictor of IDD?
As discussed earlier, MRI changes occur in the asymptomatic population,38,137, 138 and apart from the finding of a high intensity zone (HIZ) or Modic type 1 and 2 changes in the lumbar spine, the extent of changes are not predictive of the development or duration of future back pain.138 Attempts have been made to determine whether or not MRI can be used to predict PD outcome because PD is not without morbidity.139–141 Although MRI is somewhat predictive of a positive PD test when an HIZ or Modic type I or II changes are found, it is somewhat insensitive.
The HIZ, defined on sagittal MRI as a very bright signal (equal to or brighter than CSF on T2-weighted scans) contained within the posterior AF,136 has been shown to have appreciable but variable correlation with positive PD in patients presenting with NSLBP.127 The initial study showed that an HIZ increases the odds that PD will be positive in that patient at that level by a factor of 6.5.136 Studies on the HIZ since that time have all shown that HIZ is highly specific (range 0.74 to 0.93)142–146 but that it has variable sensitivity, from as low as 0.09,146 to as high as 0.78.144 Thus, it is uncommon for an HIZ to occur in a disc that is not painful to PD, and the calculated likelihood ratios (ranging from 1.3 to 6.5 and averaging 4.1) indicate that an HIZ increases the odds that a PD will be positive by at least 50%.127 Additionally, although HIZs are present in both asymptomatic and symptomatic subjects, they are found significantly more in symptomatic (prevalence 60% ± 15%) than asymptomatic subjects (24% ± 11%).147
Modic changes have also been studied in relationship to NSLBP and PD.148,149 The overall rate of vertebral end-plate signal changes (VEPSC) is about 43% in patients with NSLBP and 6% in those without.150 The most common association of VEPSC and NSLBP is extensive Modic type 1 changes at L5-S1.151 Overall, VEPSCs are relatively insensitive, but quite specific, for the diagnosis of IDD established by discography, with sensitivity of 23.2%, specificity 96.8%, PPV 91.3%, and NPV 46.5% in one study.148 When Modic changes are studied according to type, the type 1 end plate has the highest PPV for positive PD at 81% (±7%).149
One of the problems of proton MRI is that the traditional T1 and T2 images measure the end stages of proteoglycan breakdown, by which stage water depletion is more severe. The earlier, more subtle, changes are not able to be detected with these sequences. As proteoglycans are highly negatively charged they attract cations, particularly Na+. Sodium MRI measures Na+ levels in the disc, thereby giving a direct measurement of proteoglycan levels. Depletion of Na+ is, therefore, a direct measure of degree of disc degeneration.152 Sodium MRI does not have the resolution of proton MRI, but it can now be studied as a potential surrogate for PD.
Can Clinical Tests Predict IDD?
The prevalence of IDD in a population of NSLBP patients considered to be having sufficient pain and disability to undergo PD is about 35% to 40%.61,153 The prevalence of IDD in a group of patients with positive clinical indicators increases to 52% to 69%.153 The centralization phenomenon, or pain centralization, which is the retreat of referred pain toward the spinal midline during specified clinical examination, a history of persistent pain between acute episodes, a significant loss of extension, and a subjective report of so-named “vulnerability in the neutral zone” individually and in combination increase the likelihood of an eventual diagnosis of IDD in a group of highly disabled and psychosocially distressed patients with otherwise diagnosed NSLBP.153,154 Additionally, if vibration applied over individual spinous processes is considered a positive test, then the odds of a positive PD increases significantly.155,156 These clinical tests and protocols will need further validity and reliability studies before they can be considered to be adequate surrogates for PD.
The Diagnosis of Radicular Pain Due to a Contained Disc Prolapse
The nature of radicular pain has been described. Apart from the description of pain, various clinical tests such as the straight leg raise test are used as aids to the diagnostic process, but their clinical utility is questionable,157 and radicular pain lends itself to imaging investigation. In the cervical spine, the neck compression, the axial manual traction test, and the shoulder abduction test are highly specific but poorly sensitive for the diagnosis of root compression.158 CT scan, MRI, and CT myelography can all be used in different clinical settings. If the morphologic findings match the clinical picture, then the cause of radicular pain can be assumed. The most common cause is some degree of disc prolapse. However, it should be noted that disc prolapse is also commonly seen in the asymptomatic population.34,36,41,159
Which Patients Should Be Considered for Intradiscal Therapies?
Internal Disc Disruption and Discogenic Pain
The patient with putative IDD or DP being considered for an intradiscal therapy should have had considerable NSLBP for at least 6 months. Each patient should have exhausted the options provided in less invasive care, and the pattern of pain should be such that it might reasonably be expected that the condition will not recover spontaneously. By the time a patient is being considered for PD, there is a reasonable chance that the pain and disability will be similar at 2 years, and perhaps improved by 5 years. The natural history of IDD over a 5-year period has been studied in a group of patients who elected not to have the subsequently offered posterolateral fusion surgery.160 In this study, 25 patients underwent PD after experiencing incapacitating NSLBP for a period of at least 6 months. The patients (n = 36) were selected from all patients who underwent PD (n = 432) over a 3-year period. Eligibility included single level PD positive (morphology and pain response), normal plain radiographs, and a minimum of 3-year follow-up. Of these, 25 patients were able to be studied, with average age at time of discography being 43 years, and at a mean follow-up time of 4.9 years, 68% had improved, 8% had stayed the same, and 24% were worse; 80% of those receiving worker’s compensation had improved. No patient had recovered fully. The natural history of IDD in a group of 36 patients undergoing placebo intradiscal injection over a 2-year period has also been documented by Peng and colleagues in their study on methylene blue.161 In this study, patients also had IDD using similar selection criteria but they elected to have the injection treatment. At 2 years the placebo group, with an average age of 42, had not undergone much change in either disability or pain levels: there was a weak statistically insignificant trend to improvement of pain.
The ideal patient has focal pain, with or without a component of referred pain, little or no other pain in other areas of the body, minimal disc space narrowing at the level to be treated, and a fairly normal psychosocial profile (Fig. 39-8). These are the ideal prerequisites in any case for a patient to be assessed with high quality PD that complies with recognized standards. If the PD is positive, the diagnosis of IDD (and thus DP) is then made (Figs. 39-8 through 39-10).
Figure 39-8 Ideal prerequisites if provocation discography (PD) is to be used as a test before an intradiscal procedure.
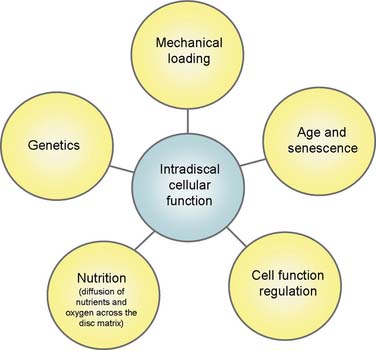
Figure 39-9 Cellular function within the disc is mediated by at least five major factors.
(From Freemont AJ: The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology [Oxford] 2009;48:5-10.)
The diagnosis of IDD does not imply that specific interventional treatment including surgery to the disc will produce good results.162,163 The cohort or cohorts of patients who might respond best to treatments arising from a diagnosis of IDD are still being defined.
Radicular Pain due to Contained Disc Prolapse
The patient with radicular pain due to a contained disc prolapse considered appropriate for an intradiscal therapy should have considerable pain that is not showing signs of recovery despite the passage of time and perhaps one or more epidural injections. This can be a hard situation to call. Also, if such a therapy is suggested, the clinician should be able to justify using an intradiscal treatment over minimally invasive treatments including minimally invasive spinal surgery.
Surgery for radicular pain caused by a disc protrusion has been considered successful for many years since the discovery of the disc prolapse and its effects on the neural system.164 It is instructive to consider that the natural history for radicular pain due to disc prolapse is reasonably good in any case,165–168 and surgery has proved popular not necessarily because of its better long-term outcome,169 but because it provides the quickest route to recovery.165–169 Surgery may be superior to conservative care within 12 months but by 4 years there is no difference in outcome.170 Large disc protrusions or extrusions treated conservatively for the presentation of radicular pain do resolve when followed with imaging techniques: smaller protrusions tend not to resolve.171
Which Patients Should Not Be Considered for Intradiscal Therapies?
Purely on face validity grounds, the more that the presenting pain is localized into one region, the more likely that it is derived from a local structure. As a corollary, chronic widespread pain, which is present in about 4.5% of the population,172 could derive from multiple regions or from an underlying pain dysfunction, such as central sensitization. There are schools of thought postulating that most, if not all, chronic NSSP is caused by central nervous system processing dysfunction independent of any initiating musculoskeletal event.
Fibromyalgia (FM), the most common known cause of chronic, widespread pain,173 is an example of a presentation that could mimic DP. It is considered to be present in between 0.5% and 7% of the population at any given time.174–176 It is not uncommon for a patient, who presents to a spinal clinic with a pain diagram demonstrating only focal LBP, to reveal under closer questioning a profile consistent with FM. Additionally, there are others who present somewhere short of the FM diagnostic requirements.177 They might get labeled with an entity such as diffuse myofascial pain. The essence is that they do not have relatively focal regional pain like that described earlier for focal deep somatic pain referral, and they might have some of the accompanying symptoms of FM, such as sleep disturbance, unrefreshed sleep, and general autonomic symptoms.
Local neuropathic pain should also alert the clinician to be wary. Neuropathic pain can be challenging to diagnose because diagnostic criteria are still developing, and neuropathic pain can manifest simultaneously with somatic referred or other pain.178 The diagnosis of neuropathic pain is based primarily on history and physical examination.179 Although neuropathic pain can have deep aching and shooting characteristics, more typical of lumbar radicular pain,75 it also attracts other descriptors such as burning, crushing, punishing, and cruel,179 and it can be associated with abnormal sensations such as formication. Because it is often unrecognized by clinicians,180 a range of neuropathic questionnaires, such as StEP,181 PainDETECT,182 DN4,183,184 LANSS185 and NPS186 have been developed and reviewed.187 The prevalence of neuropathic pain in the general population is about 7% of which 70% have pain in the moderate to severe range.188 Additionally, although some of these questionnaires should be considered a screening tool only and population surveys should be treated with some scepticism,189 one survey report using PainDETECT found that the prevalence of predominantly neuropathic pain in a population of people with LBP was 37%.182 In an orthopedic setting of patients with predominantly LBP, those with a positive PainDETECT questionnaire had a median VAS of 5.0, and some had features typically representative of radicular pain and/or radiculopathy, including typical radicular pain (40%), positive Laségue sign (18%), or absent reflex (17%).190 More than two neuropathic pain characteristics were present in 34% of these patients. Although neuropathic pain may represent radicular pain, it is fair to say that when a person presents with LBP that has neuropathic descriptors rather than deep somatic pain descriptors, clinicians should consider that the pain is more complex than being simply derived from a local somatic structure such as a disc. Pain centralization may be a significant factor.
The patient with significant psychological features in association with pain is difficult to assess from a pain origin perspective. Although it has been reported that alleviation of pain from a surgical procedure can return abnormal psychological parameters to normal,191 the false-positive rate of interventional diagnostic tests such as discography is an example of the difficulty in assessing such a presentation.120
Why Is There Such A Problem with NSSP?
Specific traditional treatments have not proved effective in controlling the epidemic of back pain. Because about 70% of the acute back pain population recovers rapidly, therapies that are no better than placebo have been ascribed healing properties they do not deserve. The challenges for those who manage back pain are to provide a management strategy that actually alters the long-term outcome and to identify, if possible, and as early as possible, the patients who are less likely to recover.
To this end, a number of evidence-based guidelines for the clinical management of acute back pain in primary care have been developed.192–199 In general, the guidelines subscribe to the concept that exercise is beneficial, fear of movement is harmful, that consequent pain should not be understood to be further injury, and that chronicity is contributed to by avoidance of these concepts. More specifically, they (1) identify potentially serious causes of acute low back pain (< 5%); (2) promote effective self-management of symptoms through the provision of timely and appropriate advice; and (3) maximize functional status and minimize disability.
A multidimensional approach is required to translate these concepts into reality for the public and the medical profession.200,201 One way to promulgate the guideline concepts is with mass-media campaigns, which have been shown to be effective in delivering health information to medical and allied health practitioners and the general public,202,203 although the effective penetration relates more to public perception changes than alterations in the attitudes or behaviors of health professionals.204 A mass media campaign that outlined some evidence-based facts about back pain was implemented205 and was found to be useful.206,207 The campaign issued sharply focused, unambiguous advice directed toward staying active and exercising, not resting for prolonged periods, and remaining at work.208–211
Such programs do not necessarily translate into huge savings.210 Guidelines in general do not lead to improvement of clinical outcomes, primarily because they are not followed by practitioners.210,212–218 Reasons for this include poor penetration of clinical guidelines into graduate and postgraduate medical education, lack of awareness, inability to understand the ramifications of referred pain, lack of commitment to change, incomplete understanding of the natural history of LBP, disagreement with evidence-based recommendations, concern for litigation, a belief that guidelines cannot be adequately explained to patients and particularly lack of uniformity in management by the various health professionals.212,218–222 It is also not easy to remedy lack of compliance to guidelines.210,220,223
It is apparent that the proper use of clinical guidelines in the management of LBP produces better outcomes.224–226 Guidelines tend to portray LBP as a benign, self-limiting disease, as single attacks tend to recover.227 Consequently it is not surprising that some primary care practitioners adopt a blasé attitude to an acute presentation of LBP, despite evidence clearly demonstrating that early diagnosis and suitable advice underpins the long-term outcome for many patients.224
The end result of lack of compliance to guidelines is prolonged unnecessary disability and increased usage of the health-care system. For example, a survey on the usage of the health care system over a 12-month period in a longitudinal prospective cohort study following presentation with LBP revealed alarming data.227 Of the 1342 patients, 57% were seeking additional specialist care, 46% had some form of imaging, 49% underwent physiotherapy, and 31% received massage. Because specialist care resulted in more imaging and intervention, it was considered that a significant contribution to the problem was the lack of a well-functioning primary care gate-keeping force.227
So, one problem is the dissemination and utilization of evidence-based clinical guidelines to those practitioners in the front line of LBP management. Promoting adherence to LBP guidelines requires more than enhancing knowledge about its evidence-based management.212 Public education and an interdisciplinary consensus are important requirements for successful guideline implementation into daily practice.212 Guideline recommendations need to be adapted to the infrastructure of the health care system in general.212 Most guidelines refer to the management of LBP in general. However, in respect to work-related acute LBP, specific guidelines do exist.228–230 In the first instance, we need to know if these guidelines are of sufficient quality on which to base the management of LBP.
Preventative Measures
In Indahl’s study,225–226 in which workers with LBP were put into one of two groups and followed up for 5 years, 19% of the patients in the intervention group, compared with 34% in the control group, were still on sick leave after 5 years. The successfully treated group was managed with an approach that included clinical examination combined with information for patients about the nature of the problem provided in a manner designed to reduce fear and give them reason to resume light activity. It might be reasonably assumed that Indahl was able to successfully rehabilitate the 15% of people who had a reversible primary psychosocial reason for nonrecovery of function, and that the other 19% who remained disabled had significant underlying pathologic processes or some other unknown factor responsible for this disability.
Evidence-based care has been further studied. A seminal study on WorkCover patients which offered these workers a choice between Indahl’s evidence-based care and usual care (e.g., from the family general practitioner) in four district hospitals in Newcastle, Australia, also showed the benefit of early decisive management of LBP.224 In this study, the 65% who accepted evidence-based care were compared to the other group. The specialist in this clinic practiced according to Australian Evidence-Based Guidelines for Management of Acute LBP.198 These guidelines are similar to other published guidelines except that they do not use treatment by manual therapy, a treatment that seems to lack significant positive evidence.231 The emphasis is on explanation, reassurance, encouragement to remain at work, simple analgesics, avoidance of passive therapies, and worksite visit/intervention if indicated. Imaging was performed only if there was a clinical red-flag indicator. Instructions on how to implement these guidelines have been discussed in detail.232
This study followed 253 patients. Of these, 62 elected usual care (private general practitioner, etc.), 191 (75%) consulted the staff specialist (of these, 27 [14%] elected to change to usual care after the initial consultation), and 164 (65%) remained under the care of the staff specialist. The outcomes are summarized in Table 39-2.A group of 84 patients who saw the staff specialist were identified as having credible back pain but alleged disability dissonant with the history of injury. Psychosocial factors in this subgroup included job dissatisfaction or dislike of the supervisor. Of these 84 patients, 13 transferred to usual care but 71 remained under evidence-based care. Although they required a longer initial consultation and a greater number of consultations, their outcomes were favorable and did not statistically differ from other patients who pursued evidence-based care. Additionally, of the entire group, 32% accepted evidence-based care readily, 24% came to the consultation with preconceived beliefs about back pain management, and 44% expressed job dissatisfaction and psychosocial difficulties in the workplace. When evidence-based care was explained, most of the group with preconceived beliefs accepted evidence-based care. Patients with psychosocial difficulties required more consultations and care, but ultimately had similar outcomes as the rest.
Table 39-2 Outcomes of Workers Presenting with Acute Back Pain
| Evidence-Based Care (%) | Usual Care (%) | |
|---|---|---|
| Immediately back to normal duties | 63 | 0 |
| Modified duties | 37 | 92 |
| Time off work | One person | 35 |
| Percentage fully recovered | 98 | 84 |
| Recurrence | 6 | 27 |
From McGuirk B, Bogduk N: Evidence-based care for low back pain in workers eligible for compensation. Occup Med [Lond.] 2007;57:36-42.
1. Shealy C.N. Percutaneous radiofrequency denervation of spinal facets. Treatment for chronic back pain and sciatica. J Neurosurg. 1975;43:448-451.
2. van Wijk R.M., Geurts J.W., Wynne H.J., et al. Radiofrequency denervation of lumbar facet joints in the treatment of chronic low back pain: a randomized, double-blind, sham lesion-controlled trial. Clin J Pain. 2005;21:335-344.
3. Bogduk N., Dreyfuss P., Govind J. A narrative review of lumbar medial branch neurotomy for the treatment of back pain. Pain Med. 2009;10:1035-1045.
4. Engers A., Jellema P., Wensing M., et al. Individual patient education for low back pain. Cochrane Database Syst Rev. 2008. Art. No: CD004057 DOI: 10.1002/14651858.CD004057.pub3
5. Deyo R.A., Rainville J., Kent D.L. What can the history and physical examination tell us about low back pain? JAMA. 1992;268:760-765.
6. Kinkade S. Evaluation and treatment of acute low back pain. Am Fam Physician. 2007;75:1181-1188.
7. Walker B.F., Muller R., Grant W.D. Low back pain in Australian adults: prevalence and associated disability. J Manipulative Physiol Ther. 2004;27:238-244.
8. Linton S. A review of psychological risk factors in back and neck pain. In: Nachemson A.L., Jonsson E., editors. Neck and Back Pain: The Scientific Evidence of Causes, Diagnosis, And Treatment. Philadelphia: Lippincott Williams & Wilkins, 2000.
9. Waddell G., Newton M., Henderson I., et al. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. 1993;52:157-168.
10. Waddell G. 1987 Volvo award in clinical sciences. A new clinical model for the treatment of low-back pain. Spine. 1987;12:632-644.
11. Maniadakis N., Gray A. The economic burden of back pain in the UK. Pain. 2000;84:95-103.
12. van Tulder M.W., Koes B.W., Bouter L.M. A cost-of-illness study of back pain in The Netherlands. Pain. 1995;62:233-240.
13. Fejer R., Kyvik K.O., Hartvigsen J. The prevalence of neck pain in the world population: a systematic critical review of the literature. Eur Spine J. 2006;15:834-848.
14. Haukka E., Leino-Arjas P., Solovieva S., et al. Co-occurrence of musculoskeletal pain among female kitchen workers. Int Arch Occup Environ Health. 2006;80:141-148.
15. Andersen J.H., Haahr J.P., Frost P. Risk factors for more severe regional musculoskeletal symptoms: a two-year prospective study of a general working population. Arthritis Rheum. 2007;56:1355-1364.
16. Australian Institute of Health and Welfare. Australia’s health 2000: The seventh biennial health report of the Australian Institute of Health and Welfare. Canberra: AIHW; 2000.
17. Von Korff M., Deyo R.A., Cherkin D., Barlow W. Back pain in primary care. Outcomes at 1 year. Spine. 1993;18:855-862.
18. Stewart W.F., Ricci J.A., Chee E., et al. Lost productive time and cost due to common pain conditions in the U.S. workforce. JAMA. 2003;290:2443-2454.
19. Luo X., Pietrobon R., Sun S.X., et al. Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine. 2004;29:79-86.
20. Dagenais S., Caro J., Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8:8-20.
21. Bogduk N., Bolton P., Jull G., et al. Acute Neck Pain. In: Brooks P., March L., Bogduk N., et al, editors. Evidence-based Management of Acute Musculoskeletal Pain. Brisbane, Australia: Australian Academic Press; 2003:83-118.
22. Castinel B.H., Adam P., Milburn P.D., et al. Epidemiology of cervical spine abnormalities in asymptomatic adult professional rugby union players using static and dynamic MRI protocols: 2002 to 2006. Br J Sports Med. 2010;44:194-199.
23. Alyas F., Turner M., Connell D. MRI findings in the lumbar spines of asymptomatic, adolescent, elite tennis players. Br J Sports Med. 2007;41:836-841.
24. Belfi L.M., Ortiz A.O., Katz D.S. Computed tomography evaluation of spondylolysis and spondylolisthesis in asymptomatic patients. Spine. 2006;31:E907-E910.
25. Pfirrmann C.W., Metzdorf A., Elfering A., et al. Effect of aging and degeneration on disc volume and shape: A quantitative study in asymptomatic volunteers. J Orthop Res. 2006;24:1086-1094.
26. Jarvik J.G., Hollingworth W., Heagerty P.J., et al. Three-year incidence of low back pain in an initially asymptomatic cohort: clinical and imaging risk factors. Spine. 2005;30:1541-1549.
27. Carragee E.J., Barcohana B., Alamin T., van den Haak E. Prospective controlled study of the development of lower back pain in previously asymptomatic subjects undergoing experimental discography. Spine. 2004;29:1112-1117.
28. Chung C.B., Vande Berg B.C., Tavernier T., et al. End plate marrow changes in the asymptomatic lumbosacral spine: frequency, distribution and correlation with age and degenerative changes. Skeletal Radiol. 2004;33:399-404.
29. Fukuta S., Masaki K., Korai F. Prevalence of abnormal findings in magnetic resonance images of asymptomatic knees. J Orthop Sci. 2002;7:287-291.
30. Siivola S.M., Levoska S., Tervonen O., et al. MRI changes of cervical spine in asymptomatic and symptomatic young adults. Eur Spine J. 2002;11:358-363.
31. Danielson B., Willen J. Axially loaded magnetic resonance image of the lumbar spine in asymptomatic individuals. Spine. 2001;26:2601-2606.
32. Gore D.R. Roentgenographic findings in the cervical spine in asymptomatic persons: a ten-year follow-up. Spine. 2001;26:2463-2466.
33. Borenstein D.G., O’Mara J.W.Jr., Boden S.D., et al. The value of magnetic resonance imaging of the lumbar spine to predict low-back pain in asymptomatic subjects: a seven-year follow-up study. J Bone Joint Surg Am. 2001:1306-1311. 83-A
34. Boos N., Semmer N., Elfering A., et al. Natural history of individuals with asymptomatic disc abnormalities in magnetic resonance imaging: predictors of low back pain-related medical consultation and work incapacity. Spine. 2000;25:1484-1492.
35. Weishaupt D., Zanetti M., Hodler J., Boos N. MR imaging of the lumbar spine: prevalence of intervertebral disk extrusion and sequestration, nerve root compression, end plate abnormalities, and osteoarthritis of the facet joints in asymptomatic volunteers. Radiology. 1998;209:661-666.
36. Wood K.B., Blair J.M., Aepple D.M., et al. The natural history of asymptomatic thoracic disc herniations. Spine. 1997;22:525-530.
37. Hald H.J., Danz B., Schwab R., et al. [Radiographically demonstrable spinal changes in asymptomatic young men]. Rofo. 1995;163:4-8.
38. Boden S.D., McCowin P.R., Davis D.O., et al. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:1178-1184.
39. Boden S.D., Davis D.O., Dina T.S., et al. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403-408.
40. Weinreb J.C., Wolbarsht L.B., Cohen J.M., et al. Prevalence of lumbosacral intervertebral disk abnormalities on MR images in pregnant and asymptomatic nonpregnant women. Radiology. 1989;170:125-128.
41. Teresi L.M., Lufkin R.B., Reicher M.A., et al. Asymptomatic degenerative disk disease and spondylosis of the cervical spine: MR imaging. Radiology. 1987;164:83-88.
42. Gore D.R., Sepic S.B., Gardner G.M. Roentgenographic findings of the cervical spine in asymptomatic people. Spine. 1986;11:521-524.
43. Wiesel S.W., Tsourmas N., Feffer H.L., et al. A study of computer-assisted tomography. I. The incidence of positive CAT scans in an asymptomatic group of patients. Spine. 1984;9:549-551.
44. Torgerson W.R., Dotter W.E. Comparative roentgenographic study of the asymptomatic and symptomatic lumbar spine. J Bone Joint Surg Am. 1976;58:850-853.
45. Hitselberger W.E., Witten R.M. Abnormal myelograms in asymptomatic patients. J Neurosurg. 1968;28:204-206.
46. Elias F. Roentgen findings in the asymptomatic cervical spine. N Y State J Med. 1958;58:3300-3303.
47. Roland M. van Tulder M. Should radiologists change the way they report plain radiography of the spine? Lancet. 1998;352:229-230.
48. Feinstein B., Langton J.N., Jameson R.M., Schiller F. Experiments on pain referred from deep somatic tissues. J Bone Joint Surg Am. 1954;36-A:981-987.
49. Hackett G.S. Referred pain and sciatica in diagnosis of low back disability. J Am Med Assoc. 1957;163:183-185.
50. Hackett G.S. Referred pain from low back ligament disability. AMA Arch Surg. 1956;73:878-883.
51. Kellgren J.H. Observations on referred pain arising from muscle. Clin Sci. 1938;3:175-190.
52. Kellgren J.H. On the distribution of pain arising from deep somatic structures with charts of segmental pain areas. Clin Sci. 1939;4:35-46.
53. Hockaday J.M., Whitty C.W. Patterns of referred pain in the normal subject. Brain. 1967;90:481-496.
54. Hirsch C., Ingelmark B.E., Miller M. The anatomical basis for low back pain. Studies on the presence of sensory nerve endings in ligamentous, capsular and intervertebral disc structures in the human lumbar spine. Acta Orthop Scand. 1963;33:1-17.
55. Borowczyk J. Visceral pain and nociception. In: Schmidt R.F., Willis W., editors. Encyclopedia of Pain. Berlin, Heidelberg, New York: Springer; 2007:2617-2619.
56. Bogduk N. Diagnosing lumbar zygapophysial joint pain. Pain Med. 2005;6:139-142.
57. Bogduk N. Clinical Anatomy of the Lumbar Spine and Sacrum, 3rd ed. Edinburgh: Churchill Livingstone; 1987. 187–213
58. Bogduk N., McGrath D., Vivian D. Neurogenic pain. Australasian Faculty of Musculoskeletal Medicine. 2005. Unpublished Work
59. Roselt D. Somatic referred pain. In: Schmidt R.F., Willis W., editors. Encyclopedia of Pain. Berlin, Heidelberg, New York: Springer; 2007:p2192-p2196.
60. Schwarzer A.C., Aprill C.N., Derby R., et al. Clinical features of patients with pain stemming from the lumbar zygapophysial joints. Is the lumbar facet syndrome a clinical entity? Spine. 1994;19:1132-1137.
61. Schwarzer A.C., Aprill C.N., Derby R., et al. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine. 1995;20:1878-1883.
62. Wiley J.J., Macnab I., Wortzman G. Lumbar discography and its clinical applications. Can J Surg. 1968;11:280-289.
63. Venner R.M., Crock H.V. Clinical studies of isolated disc resorption in the lumbar spine. J Bone Joint Surg Br. 1981;63B:491-494.
64. Dreyfuss P., Dreyer S.J., Cole A., Mayo K. Sacroiliac joint pain. J Am Acad Orthop Surg. 2004;12:255-265.
65. Kim H.G., Shin D.A., Kim H.I., et al. Clinical and radiological findings of discogenic low back pain confirmed by automated pressure-controlled discography. J Korean Neurosurg Soc. 2009;46:333-339.
66. O’Neill C.W., Kurgansky M.E., Derby R., et al. Disc stimulation and patterns of referred pain. Spine. 2002;27:2776-2781.
67. Dwyer A., Aprill C., Bogduk N. Cervical zygapophyseal joint pain patterns. I: A study in normal volunteers. Spine. 1990;15:453-457.
68. Schellhas K.P., Garvey T.A., Johnson B.A., et al. Cervical diskography: analysis of provoked responses at C2-C3, C3-C4, and C4-C5. AJNR Am J Neuroradiol. 2000;21:269-275.
69. Schellhas K.P., Smith M.D., Gundry C.R., Pollei S.R. Cervical discogenic pain. Prospective correlation of magnetic resonance imaging and discography in asymptomatic subjects and pain sufferers. Spine. 1996;21:300-311.
70. Grubb S.A., Kelly C.K. Cervical discography: Clinical implications from 12 years of experience. Spine. 2000;25:1382-1389.
71. Slipman C.W., Plastaras C., Patel R., et al. Provocative cervical discography symptom mapping. Spine J. 2005;5:381-388.
72. Bogduk N., McGuirk B. Management of Acute and Chronic Neck Pain. Amsterdam: Elsevier; 2006.
73. Slipman C.W., Plastaras C.T., Palmitier R.A., et al. Symptom provocation of fluoroscopically guided cervical nerve root stimulation. Are dynatomal maps identical to dermatomal maps? Spine. 1998;23:2235-2242.
74. Govind J. Lumbar radicular pain. Aust Fam Physician. 2004;33:409-412.
75. Bogduk N. The anatomy and pathophysiology of neck pain. Phys Med Rehabil Clin N Am. 2003;14:455-472.
76. Radhakrishnan K., Litchy W.J., O’Fallon W.M., et al. Epidemiology of cervical radiculopathy. A population-based study from Rochester, Minnesota, 1976 through 1990. Brain. 1994;117:325-335.
77. Smyth M.J., Wright V. Sciatica and the intervertebral disc; an experimental study. J Bone Joint Surg Am. 1958;40A:1401-1418.
78. Chao S.C., Lee H.T., Kao T.H., et al. Percutaneous pulsed radiofrequency in the treatment of cervical and lumbar radicular pain. Surg Neurol. 2008;70:59-65.
79. Malinsky J. The ontogenetic development of nerve terminations in the intervertebral discs of man. (Histology of intervertebral discs, 11th communication). Acta Anat. 1959;38:96-113.
80. Bogduk N., Windsor M., Inglis A. The innervation of the cervical intervertebral discs. Spine. 1988;13:2-8.
81. Mendel T., Wink C.S., Zimny M.L. Neural elements in human cervical intervertebral discs. Spine. 1992;17:132-135.
82. International Spinal Intervention Society. Lumbar disc stimulation. N. Bogduk. San Francisco: Practice Guidelines for Spinal Diagnostic and Treatment Procedures. 2004:20-46.
83. Freemont A.J. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology. 2009;48:5-10.
84. Sambrook P.N., Macgregor A.J., Spector T.D. Genetic influences on cervical and lumbar disc degeneration: A magnetic resonance imaging study in twins. Arthritis Rheum. 1999;42:366-372.
85. Kalichman L., Hunter D.J. The genetics of intervertebral disc degeneration. Familial predisposition and heritability estimation. Joint Bone Spine. 2008;75:383-387.
86. Battie M.C., Videman T., Levalahti E., et al. Genetic and environmental effects on disc degeneration by phenotype and spinal level: a multivariate twin study. Spine. 2008;33:2801-2808.
87. Battie M.C., Videman T., Gill K., et al. 1991 Volvo Award in clinical sciences. Smoking and lumbar intervertebral disc degeneration: an MRI study of identical twins. Spine. 1991;16:1015-1021.
88. Kettler A., Werner K., Wilke H.J. Morphological changes of cervical facet joints in elderly individuals. Eur Spine J. 2007;16:987-992.
89. Battie M.C., Videman T., Kaprio J., et al. The Twin Spine Study: contributions to a changing view of disc degeneration. Spine J. 2009;9:47-59.
90. Irvine D.H., Foster J.B., Newell D.J., et al. Prevalence of cervical spondylosis in a general practice. Lancet. 1965;1:1089-1092.
91. Rowe L.J. Diagnostic imaging of mechanical and degenerative syndromes of the cervical spine. In: Giles L.G.F., Singer K.P., editors. Clinical anatomy of cervical spine pain. Newton, Mass: Butterworth-Heinemann; 1998:89-111.
92. Yoshimura N., Muraki S., Oka H., et al. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: The research on osteoarthritis/osteoporosis against disability study. J Bone Miner Metab. 2009;27:620-628.
93. Inaoka M., Yamazaki Y., Hosono N., et al. Radiographic analysis of lumbar spine for low-back pain in the general population. Arch OrthopTrauma Surg. 2000;120:380-385.
94. Steinberg E.L., Luger E., Arbel R., et al. A comparative roentgenographic analysis of the lumbar spine in male army recruits with and without lower back pain. Clin Radiol. 2003;58:985-989.
95. van Tulder M.W., Assendelft W.J., Koes B.W., Bouter L.M. Spinal radiographic findings and nonspecific low back pain. A systematic review of observational studies. Spine. 1997;22:427-434.
96. Muraki S., Oka H., Akune T., et al. Prevalence of radiographic lumbar spondylosis and its association with low back pain in elderly subjects of population-based cohorts: The ROAD study. Ann Rheum Dis. 2009;68:1401-1406.
97. de Schepper E.I., Damen J., van Meurs J.B., et al. The association between lumbar disc degeneration and low back pain: the influence of age, gender, and individual radiographic features. Spine. 2010;35:531-536.
98. Heller C.A., Stanley P., Lewis-Jones B., et al. Value of x ray examinations of the cervical spine. Br Med J (Clin Res Ed). 1983;287:1276-1278.
99. Deyo R.A., Diehl A.K. Lumbar spine films in primary care: current use and effects of selective ordering criteria. J Gen Intern Med. 1986;1:20-25.
100. Deyo R.A., Diehl A.K., Rosenthal M. Reducing roentgenography use. Can patient expectations be altered? Arch Intern Med. 1987;147:141-145.
101. Chou R., Fu R., Carrino J.A., Deyo R.A. Imaging strategies for low-back pain: systematic review and meta-analysis. Lancet. 2009;373:463-472.
102. Fujiwara A., Tamai K., Yamato M., et al. The relationship between facet joint osteoarthritis and disc degeneration of the lumbar spine: an MRI study. Eur Spine J. 1999;8:396-401.
103. Gamie S., El-Maghraby T. The role of PET/CT in evaluation of Facet and Disc abnormalities in patients with low back pain using (18)F-Fluoride. Nucl Med Rev Cent East Eur. 2008;11:17-21.
104. Bogduk N., Tynan W., Wilson A.S. The nerve supply to the human lumbar intervertebral discs. J Anat. 1981;132:39-56.
105. Taylor J.R., Twomey L.T. Innervation of lumbar intervertebral discs. Med J Aust. 1979;2:701-702.
106. Bogduk N., Twomey L.T. Nerves of the lumbar spine. In: Clinical Anatomy of the Lumbar Spine. Edinburgh: Churchill Livingstone; 1987:92-102.
107. Takahashi Y., Chiba T., Kurokawa M., et al. Stereoscopic structure of sensory nerve fibers in the lumbar spine and related tissues. Spine. 2003;28:871-880.
108. Nakamura S., Takahashi K., Takahashi Y., et al. Origin of nerves supplying the posterior portion of lumbar intervertebral discs in rats. Spine. 1996;21:917-924.
109. Chen J., Hou S., Peng B., et al. Effect of the L2 ramus communicans on the nociceptive pathway in lumbar intervertebral discs in rats. Eur J Pain. 2008;12:798-803.
110. Bogduk N. The innervation of the lumbar spine. Spine. 1983;8:286-293.
111. Suseki K., Takahashi Y., Takahashi K., et al. Sensory nerve fibres from lumbar intervertebral discs pass through rami communicantes. A possible pathway for discogenic low back pain. J Bone Joint Surg Br. 1998;80:737-742.
112. Murata Y., Kato Y., Miyamoto K., et al. Clinical study of low back pain and radicular pain pathways by using l2 spinal nerve root infiltration: a randomized, controlled, clinical trial. Spine. 2009;34:2008-2013.
113. Nakamura S.I., Takahashi K., Takahashi Y., et al. The afferent pathways of discogenic low-back pain. Evaluation of L2 spinal nerve infiltration. J Bone Joint Surg Br. 1996;78:606-612.
114. Simopoulos T.T., Malik A.B., Sial K.A., et al. Radiofrequency lesioning of the L2 ramus communicans in managing discogenic low back pain. Pain Physician. 2005;8:61-65.
115. Ohtori S., Yamashita M., Inoue G., et al. L2 spinal nerve-block effects on acute low back pain from osteoporotic vertebral fracture. J Pain. 2009;10:870-875.
116. Derby R., Baker R., Wolfer L., et al. Analgesic discography: Can analgesic testing identify a painful disc? Spine Line. November-December, 2008.
117. Crock H.V. Internal disc disruption. A challenge to disc prolapse fifty years on. Spine. 1986;11:650-653.
118. Carragee E.J., Tanner C.M., Yang B., et al. False-positive findings on lumbar discography. Reliability of subjective concordance assessment during provocative disc injection. Spine. 1999;24:2542-2547.
119. Carragee E.J., Chen Y., Tanner C.M., et al. Provocative discography in patients after limited lumbar discectomy: A controlled, randomized study of pain response in symptomatic and asymptomatic subjects. Spine. 2000;25:3065-3071.
120. Carragee E.J., Tanner C.M., Khurana S., et al. The rates of false-positive lumbar discography in select patients without low back symptoms. Spine. 2000;25:1373-1381.
121. Carragee E.J., Alamin T.F., Miller J., et al. Provocative discography in volunteer subjects with mild persistent low back pain. Spine J. 2002;2:25-34.
122. Carragee E.J., Alamin T.F., Carragee J.M. Low-pressure positive discography in subjects asymptomatic of significant low back pain illness. Spine. 2006;31:505-509.
123. Carragee E.J., Alamin T.F., Miller J.L., et al. Discographic, MRI and psychosocial determinants of low back pain disability and remission: a prospective study in subjects with benign persistent back pain. Spine J. 2005;5:24-35.
124. Mercer S., Bogduk N. The ligaments and annulus fibrosus of human adult cervical intervertebral discs. Spine. 1999;24:619-628.
125. Adams M.A., McNally D.S., Wagstaff J., et al. Abnormal stress concentrations in lumbar intervertebral discs following damage to the vertebral bodies: a cause of disc failure? Eur Spine J. 1993;1:214-221.
126. Liu Y.K., Njus G., Buckwalter J., et al. Fatigue response of lumbar intervertebral joints under axial cyclic loading. Spine. 1983;8:857-865.
127. Bogduk N: Why I pursue discogenic pain. In: Lindgren KA, ed. LBP. Controversies in Clinical Practice and Research. 8th Physiatric Summer School. Rehabilitation ORTON, Helsinki, 2008:49-71.
128. Holm S., Holm A.K., Ekstrom L., et al. Experimental disc degeneration due to endplate injury. J Spinal DisordTech. 2004;17:64-71.
129. Peng B., Chen J., Kuang Z., et al. Expression and role of connective tissue growth factor in painful disc fibrosis and degeneration. Spine. 2009;34:E178-E182.
130. Rachfal A.W., Brigstock D.R. Connective tissue growth factor (CTGF/CCN2) in hepatic fibrosis. Hepatol Res. 2003;26:1-9.
131. Chen Y., Blom I.E., Sa S., et al. CTGF expression in mesangial cells: involvement of SMADs, MAP kinase, and PKC. Kidney Int. 2002;62:1149-1159.
132. Crock H.V., Goldwasser M. Anatomic studies of the circulation in the region of the vertebral end-plate in adult Greyhound dogs. Spine. 1984;9:702-706.
133. Lotz J.C., Ulrich J.A. Innervation, inflammation, and hypermobility may characterize pathologic disc degeneration: review of animal model data. J Bone Joint Surg Am. 2006;88(suppl 2):76-82.
134. Peng B.G., Pang X.D., Li D.M., et al. [A special type of discogenic low back pain: normal signal intensity of magnetic resonance imaging with abnormal discography]. Zhonghua Yi.Xue Za Zhi. 2009;89:2894-2897.
135. Sachs B.L., Vanharanta H., Spivey M.A., et al. Dallas discogram description. A new classification of CT/discography in low-back disorders. Spine. 1987;12:287-294.
136. Aprill C., Bogduk N. High-intensity zone: A diagnostic sign of painful lumbar disc on magnetic resonance imaging. Br J Radiol. 1992;65:361-369.
137. Boden S.D., Davis D.O., Dina T.S., et al. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403-408.
138. Borenstein D.G., O’Mara J.W.Jr., Boden S.D., et al. The value of magnetic resonance imaging of the lumbar spine to predict low-back pain in asymptomatic subjects: A seven-year follow-up study. J Bone Joint Surg Am. 2001:1306-1311. 83-A
139. Osti O.L., Fraser R.D., Vernon-Roberts B. Discitis after discography. The role of prophylactic antibiotics. J Bone Joint Surg Br. 1990;72:271-274.
140. Carragee E.J., Don A.S., Hurwitz E.L., et al. Does discography cause accelerated progression of degeneration changes in the lumbar disc: a ten-year matched cohort study. Spine. 2009;34:2338-2345.
141. Chen Z.Y., Ma L., Li T. Imaging of low back pain: Comparative role of high intensity zone in diagnosing the discogenic low back pain with evidence-based radiology. Chin Med J.. 2009;122:3062-3065.
142. Ito M., Incorvaia K.M., Fredrickson B.E., et al. Predictive signs of discogenic lumbar pain on magnetic resonance imaging with discography correlation. Spine. 1998;23:1252-1260.
143. Saifuddin A., Braithwaite I., White J., et al. The value of lumbar spine magnetic resonance imaging in the demonstration of anular tears. Spine. 1998;23:453-457.
144. Schellhas K.P., Pollei S.R., Gundry C.R., et al. Lumbar disc high-intensity zone. Correlation of magnetic resonance imaging and discography. Spine. 1996;21:79-86.
145. Smith B.M., Hurwitz E.L., Solsberg D., et al. Interobserver reliability of detecting lumbar intervertebral disc high-intensity zone on magnetic resonance imaging and association of high-intensity zone with pain and anular disruption. Spine. 1998;23:2074-2080.
146. Ricketson R., Simmons J.W., Hauser B.O. The prolapsed intervertebral disc. The high-intensity zone with discography correlation. Spine. 1996;21:2758-2762.
147. Carragee E.J., Paragioudakis S.J., Khurana S. 2000 Volvo Award winner in clinical studies: Lumbar high-intensity zone and discography in subjects without low back problems. Spine. 2000;25:2987-2992.
148. Braithwaite I., White J., Saifuddin A., et al. Vertebral end-plate (Modic) changes on lumbar spine MRI: correlation with pain reproduction at lumbar discography. Eur Spine J. 1998;7:363-368.
149. Thompson K.J., Dagher A.P., Eckel T.S., et al. Modic changes on MR images as studied with provocative diskography: clinical relevance—a retrospective study of 2457 disks. Radiology. 2009;250:849-855.
150. Jensen T.S., Karppinen J., Sorensen J.S., et al. Vertebral endplate signal changes (Modic change): a systematic literature review of prevalence and association with non-specific low back pain. Eur Spine J. 2008;17:1407-1422.
151. Kuisma M., Karppinen J., Niinimaki J., et al. Modic changes in endplates of lumbar vertebral bodies: prevalence and association with low back and sciatic pain among middle-aged male workers. Spine. 2007;32:1116-1122.
152. Wang C., McArdle E., Fenty M., et al. Validation of sodium magnetic resonance imaging of intervertebral disc. Spine. 2010;35:505-510.
153. Laslett M., Aprill C.N., McDonald B., et al. Clinical predictors of lumbar provocation discography: a study of clinical predictors of lumbar provocation discography. Eur Spine J. 2006;15:1473-1484.
154. Laslett M., Oberg B., Aprill C.N., McDonald B. Centralization as a predictor of provocation discography results in chronic low back pain, and the influence of disability and distress on diagnostic power. Spine J. 2005;5:370-380.
155. Vanharanta H., Ohnmeiss D.D., Aprill C.N. Vibration pain provocation can improve the specificity of MRI in the diagnosis of symptomatic lumbar disc rupture. Clin J Pain. 1998;14:239-247.
156. Yrjama M., Tervonen O., Kurunlahti M., Vanharanta H. Bony vibration stimulation test combined with magnetic resonance imaging. Can discography be replaced? Spine. 1997;22:808-813.
157. van der Windt D.A., Simons E., RiphagenII, et al. Physical examination for lumbar radiculopathy due to disc herniation in patients with low-back pain. Cochrane Database Syst Rev. 2010:CD007431.
158. Viikari-Juntura E., Porras M., Laasonen E.M. Validity of clinical tests in the diagnosis of root compression in cervical disc disease. Spine. 1989;14:253-257.
159. Gorman W.F., Hodak J.A. Herniated intervertebral disc without pain. J Okla State Med Assoc. 1997;90:185-190.
160. Rhyne A.L., Smith S.E., Wood K.E., et al. Outcome of unoperated discogram-positive low back pain. Spine. 1995;20:1997-2001.
161. Peng B., Pang X., Wu Y., et al. A randomized placebo-controlled trial of intradiscal methylene blue injection for the treatment of chronic discogenic low back pain. Pain. 2010;149:124-129.
162. Carragee E.J., Lincoln T., Parmar V.S., et al. A gold standard evaluation of the “discogenic pain” diagnosis as determined by provocative discography. Spine. 2006;31:2115-2123.
163. Pauza K.J., Howell S., Dreyfuss P., et al. A randomized, placebo-controlled trial of intradiscal electrothermal therapy for the treatment of discogenic low back pain. Spine J. 2004;4:27-35.
164. Mixter W.J., Barr J.S. Ruptures of the intervertebral disc with involvement of the spinal canal. N Engl J Med. 1934;211:210-215.
165. Persson L.C., Carlsson C.A., Carlsson J.Y. Long-lasting cervical radicular pain managed with surgery, physiotherapy, or a cervical collar. A prospective, randomized study. Spine. 1997;22:751-758.
166. Takada E., Takahashi M., Shimada K. Natural history of lumbar disc hernia with radicular leg pain: Spontaneous MRI changes of the herniated mass and correlation with clinical outcome. J Orthop Surg. 2001;9:1-7.
167. Bush K., Cowan N., Katz D.E., Gishen P. The natural history of sciatica associated with disc pathology. A prospective study with clinical and independent radiologic follow-up. Spine. 1992;17:1205-1212.
168. Saal J.A., Saal J.S. Nonoperative treatment of herniated lumbar intervertebral disc with radiculopathy. An outcome study. Spine. 1989;14:431-437.
169. Weber H. Lumbar disc herniation. A controlled, prospective study with ten years of observation. Spine. 1983;8:131-140.
170. Weber H. The natural history of disc herniation and the influence of intervention. Spine. 1994;19:2234-2238.
171. Dullerud R., Nakstad P.H. CT changes after conservative treatment for lumbar disk herniation. Acta Radiol. 1994;35:415-419.
172. Coster L., Kendall S., Gerdle B., et al. Chronic widespread musculoskeletal pain—a comparison of those who meet criteria for fibromyalgia and those who do not. Eur J Pain. 2008;12:600-610.
173. Rao S.G., Gendreau J.F., Kranzler J.D. Understanding the fibromyalgia syndrome. Psychopharmacol Bull. 2007;40:24-67.
174. Pamuk G.E., Pamuk O.N., Set T., et al. An increased prevalence of fibromyalgia in iron deficiency anemia and thalassemia minor and associated factors. Clin Rheumatol. 2008;27:1103-1108.
175. Akbik H., Butler S.F., Budman S.H., et al. Validation and clinical application of the Screener and Opioid Assessment for Patients with Pain (SOAPP). J Pain SymptomManage. 2006;32:287-293.
176. Katz D.L., Greene L., Ali A., Faridi Z. The pain of fibromyalgia syndrome is due to muscle hypoperfusion induced by regional vasomotor dysregulation. Med Hypotheses. 2007;69:517-525.
177. Wolfe F., Smythe H.A., Yunus M.B., et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160-172.
178. Dworkin R.H., O’Connor A.B., Backonja M., et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237-251.
179. Gilron I., Watson C.P., Cahill C.M., et al. Neuropathic pain: a practical guide for the clinician. CMAJ. 2006;175:265-275.
180. EU Neuropathic Pain Patient Flow Survey. 2004. Pfizer.
181. Scholz J., Mannion R.J., Hord D.E., et al. A novel tool for the assessment of pain: validation in low back pain. PLoS Med. 2009;6:e1000047.
182. Freynhagen R., Baron R., Gockel U., Tolle T.R. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22:1911-1920.
183. Bouhassira D., Attal N., Alchaar H., et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114:29-36.
184. Bouhassira D., Attal N., Fermanian J., et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain. 2004;108:248-257.
185. Kaki A.M., El-Yaski A.Z., Youseif E. Identifying neuropathic pain among patients with chronic low-back pain: use of the Leeds Assessment of Neuropathic Symptoms and Signs pain scale. Reg Anesth Pain Med. 2005;30:422-428.
186. Galer B.S., Jensen M.P. Development and preliminary validation of a pain measure specific to neuropathic pain: The Neuropathic Pain Scale. Neurology. 48, 1997. 332-338
187. Cruccu G., Truini A. Tools for assessing neuropathic pain. PLoS Med. 6, 2009. e1000045
188. Bouhassira D., Lanteri-Minet M., Attal N., et al. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136:380-387.
189. Weingarten T.N., Watson J.C., Hooten W.M., et al. Validation of the S-LANSS in the community setting. Pain. 2007;132:189-194.
190. Freynhagen R., Baron R., Tolle T., et al. Screening of neuropathic pain components in patients with chronic back pain associated with nerve root compression: a prospective observational pilot study (MIPORT). Curr Med Res Opin. 2006;22:529-537.
191. Wallis B.J., Lord S.M., Bogduk N. Resolution of psychological distress of whiplash patients following treatment by radiofrequency neurotomy: A randomised, double-blind, placebo-controlled trial. Pain. 1997;73:15-22.
192. Waddell G. Acute Low Back Pain in Adults: Assessment and Treatment. Rockville, Md: Agency for Health Care Policy and Research, US Department of Health and Human Services; 1987.
193. American Academy of Orthopaedic Surgeons. Evidence based recommendations for patients with acute activity intolerance due to low back symptoms. Orthop Update. 1995;5:632.
194. Accident Rehabilitation and Compensation Insurance Corporation. Core services, Ministry of Health. Clinical Practice Guidelines. Acute Low Back Problems in Adults: Assessment and Treatment. Wellington, New Zealand: National Advisory Committee on Core health and Disability Services, Accident Rehabilitation and Compensation Insurance Corporation; 1995.
195. Dutch College of General Practitioners (NHG). Practice Guideline Low Back Pain. Utrecht, The Netherlands, 1996. Nederlands Huisartsen Genootschap.
196. Royal College of General Practitioners CSoPOAoGBBCANBPA. Clinical Guidelines for the Management of Acute Low Back Pain. Royal College of General Practitioners. London, UK, 1996.
197. Koes B.W., van Tulder M.W., Ostelo R., et al. Clinical guidelines for the management of low back pain in primary care: a international comparison. Spine. 2001;26:2504-2513.
198. Australian Acute Musculoskeletal Pain Guidelines Group, March L, Trevena L, et al: Evidence-Based Management of Acute Musculoskeletal Pain: Acute Low Back Pain. Brisbane, Australia, Australian Academic Press, 2003.
199. Evidence-based Management of Acute Musculoskeletal Pain. 2003. NHMRC (Australian Government).
200. Grimshaw J.M., Shirran L., Thomas R., et al. Changing provider behavior: an overview of systematic reviews of interventions. Med Care. 2001;39:I12-I45.
201. Gross D.P., Ferrari R., Russell A.S., et al. A population-based survey of back pain beliefs in Canada. Spine. 2006;31:2142-2145.
202. Grilli R., Freemantle N., Minozzi S., et al. Mass media interventions: Effects on health services utilisation. Cochrane Database Syst Rev. 2000. CD000389
203. Byles J.E., Sanson-Fisher R.W., Redman S., et al. Effectiveness of three community based strategies to promote screening for cervical cancer. J Med Screen. 1994;1:150-158.
204. Werner E.L., Gross D.P., Lie S.A., et al. Healthcare provider back pain beliefs unaffected by a media campaign. Scand J Prim Health Care. 2008;26:50-56.
205. Buchbinder R., Jolley D., Wyatt M. 2001 Volvo Award Winner in Clinical Studies: Effects of a media campaign on back pain beliefs and its potential influence on management of low back pain in general practice. Spine. 2001;26:2535-2542.
206. Buchbinder R., Jolley D., Wyatt M. Population based intervention to change back pain beliefs and disability: three part evaluation. BMJ. 2001;322:1516-1520.
207. Buchbinder R., Gross D.P., Werner E.L., et al. Understanding the characteristics of effective mass media campaigns for back pain and methodological challenges in evaluating their effects. Spine. 2008;33:74-80.
208. Burton A.K., Waddell G., Tillotson K.M., Summerton N. Information and advice to patients with back pain can have a positive effect. A randomized controlled trial of a novel educational booklet in primary care. Spine. 1999;24:2484-2491.
209. Roland M., Waddell G., Moffat J., et al. The Back Book. London: Stationery Office; 1996.
210. Webster B.S., Courtney T.K., Huang Y.H., et al. Physicians’ initial management of acute low back pain versus evidence-based guidelines. Influence of sciatica. J Gen Intern Med. 2005;20:1132-1135.
211. Victorian WorkCover Authority. Guidelines for the management of employees with compensable low back pain. Melbourne, Victorian Government Publisher, Australia, 1996.
212. Chenot J.F., Scherer M., Becker A., et al. Acceptance and perceived barriers of implementing a guideline for managing low back in general practice. Implement Sci. 2008;3:7.
213. Little P., Cantrell T., Roberts L., et al. Why do GPs perform investigations?: The medical and social agendas in arranging back X-rays. Fam Pract. 1998;15:264-265.
214. Little P., Smith L., Cantrell T., et al. General practitioners’ management of acute back pain: A survey of reported practice compared with clinical guidelines. BMJ. 1996;312:485-488.
215. Deyo R.A., Phillips W.R. Low back pain. A primary care challenge. Spine. 1996;21:2826-2832.
216. Chew-Graham C., May C. Chronic low back pain in general practice: the challenge of the consultation. Fam Pract. 1999;16:46-49.
217. Deyo R.A. Acute low back pain: a new paradigm for management. BMJ. 1996;313:1343-1344.
218. Fullen B.M., Maher T., Bury G., et al. Adherence of Irish general practitioners to European guidelines for acute low back pain: a prospective pilot study. Eur J Pain. 2007;11:614-623.
219. Bishop P.B., Wing P.C. Compliance with clinical practice guidelines in family physicians managing worker’s compensation board patients with acute lower back pain. Spine J. 2003;3:442-450.
220. Bishop P.B., Wing P.C. Knowledge transfer in family physicians managing patients with acute low back pain: a prospective randomized control trial. Spine J. 2006;6:282-288.
221. Cabana M.D., Rand C.S., Powe N.R., et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458-1465.
222. Gonzalez-Urzelai V., Palacio-Elua L., Lopez-de-Munain J. Routine primary care management of acute low back pain: adherence to clinical guidelines. Eur Spine J. 2003;12:589-594.
223. McKenzie J.E., French S.D., O’Connor D.A., et al. IMPLEmenting a clinical practice guideline for acute low back pain evidence-based manageMENT in general practice (IMPLEMENT): cluster randomised controlled trial study protocol. Implement Sci. 2008;3:11.
224. McGuirk B., Bogduk N. Evidence-based care for low back pain in workers eligible for compensation. Occup Med. 2007;57:36-42.
225. Indahl A., Haldorsen E.H., Holm S., et al. Five-year follow-up study of a controlled clinical trial using light mobilization and an informative approach to low back pain. Spine. 1998;23:2625-2630.
226. Indahl A., Velund L., Reikeraas O. Good prognosis for low back pain when left untampered. A randomized clinical trial. Spine. 20, 1995. 473-477
227. Chenot J.F., Leonhardt C., Keller S., et al. The impact of specialist care for low back pain on health service utilization in primary care patients: a prospective cohort study. Eur J Pain. 2008;12:275-283.
228. Waddell G., Burton A.K. Occupational health guidelines for the management of low back pain at work: evidence review. Occup Med. 2001;51:124-135.
229. Staal J.B., Hlobil H., van Tulder M.W., et al. Occupational health guidelines for the management of low back pain: an international comparison. Occup Environ Med. 2003;60:618-626.
230. Faculty of Occupational Medicine. Occupational Health Guidelines for the Management of Low Back Pain at Work-Principal Recommendations. Carter JT. London, UK: Birrell LN; 2000.
231. Hancock M.J., Maher C.G., Latimer J., et al. Assessment of diclofenac or spinal manipulative therapy, or both, in addition to recommended first-line treatment for acute low back pain: a randomised controlled trial. Lancet. 2007;370:1638-1643.
232. Bogduk N., McGuirk B. History. In: Bogduk N., McGuirk B., editors. Medical Management of Acute and Chronic Low Back Pain. An Evidence-Based Approach. Amsterdam, The Netherlands: Elsevier; 2002:27-40.