Chapter 9 Diagnostic Ophthalmic Ultrasound
![]() For additional online content visit http://www.expertconsult.com
For additional online content visit http://www.expertconsult.com
Ultrasound – past and present
In 1880, the Curie brothers first demonstrated that a difference in electric potential could be created by mechanically pressing opposing surfaces of a tourmaline crystal.1–7 This phenomenon is called the piezoelectric effect. This effect is the basis for ultrasound technology and was first applied in underwater sonar systems during World War II.8 During that same era, the medical community also adopted the use of ultrasound technology. Scientists realized the diagnostic potential of this technology when they were able to use acoustic wavelengths to study the consistency of a material without damaging the material itself.
In 1949, Ludwig used ultrasound to detect gallstones in patients. The first publication on the use of ophthalmologic ultrasound appeared in the medical literature in 1956.9 By the mid-1970s, ophthalmologists were using ultrasound to determine axial length in a clinical setting. These measurements facilitated calculations of intraocular lens power which led to a revolution in cataract surgery.10 Further innovations came when Baum and Greenwood introduced their two-dimensional B-mode image to ophthalmology.11 Soon afterwards, Bronson et al.12 developed a hand-held contact transducer for this type of image acquisition which led to the rapid dissemination of ultrasound devices within ophthalmology clinics. The B-mode images could be used to delineate accurately retinal detachments, vitreous membranes, and choroidal tumors. In the early 1990s, new technology made it possible to image the anterior segment of the eye with devices that captured images at higher frequencies of 35–50 MHz. This improved image resolution four- to fivefold and is still the gold standard for analysis of certain anterior-segment disease such as ciliary body effusions, infiltrates, and tumors.
Examination techniques
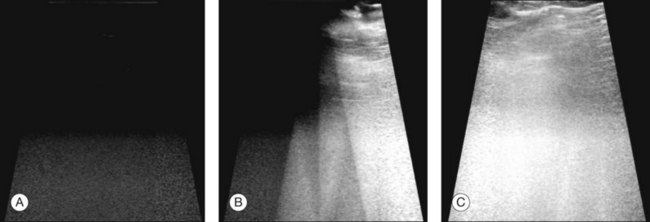
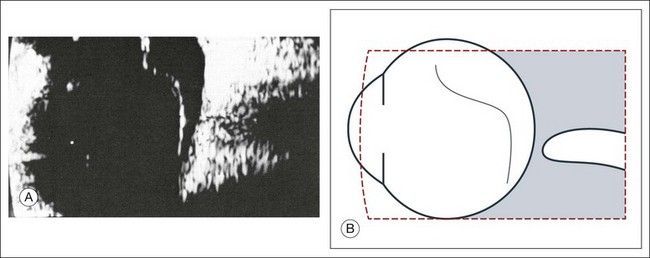
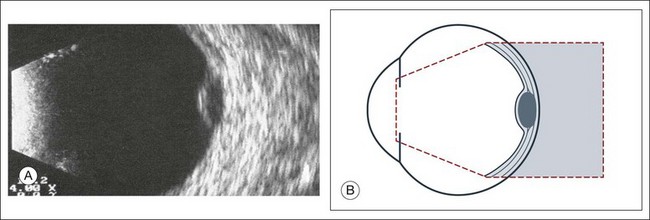
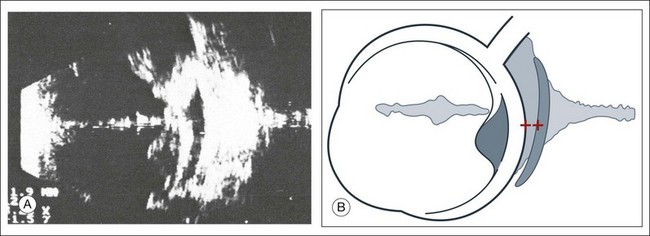
The ultrasound examination is performed with the patient in a reclined position. The frequency of the ultrasound cannot pass through air; therefore, a coupling medium is needed to transmit the sound waves from the transducer to the ocular tissues. A common coupling agent is methylcellulose (Fig. 9.1). The coupling agent is applied to the tip of the transducer probe, which is then placed on the patient’s anesthetized cornea.
B-mode technique
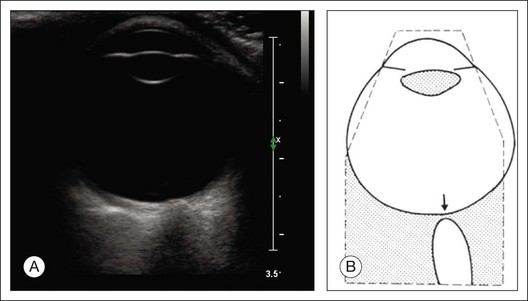
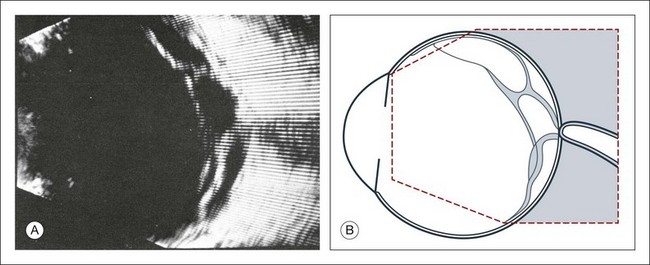
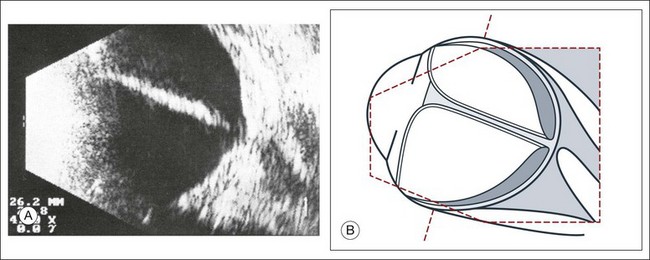
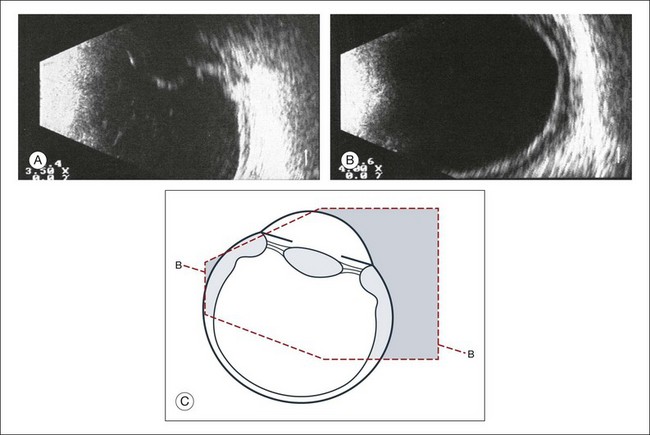
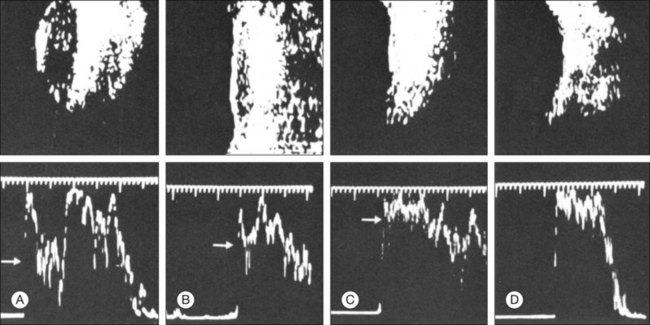
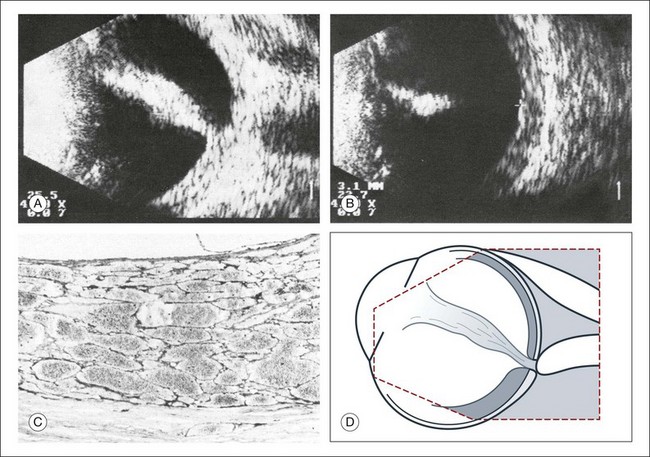
The reflected sound waves are recorded by the device and can be viewed as a two-dimensional image on the screen (Fig. 9.2). The ocular structures can be examined individually. The cornea is characterized ultrasonographically by two separate acoustic interfaces. The anterior chamber appears planoconvex in cross-section. The iris diaphragm cannot be satisfactorily imaged because of the limited lateral resolution power of the normal B-mode. A clear lens is acoustically empty and appears as an ellipsoid structure in axial sections. Similarly, normal vitreous does not give an acoustic signal; however, the presence of a detached posterior vitreous membrane presents an interface that can be imaged by increasing the amplification of the echo signal. The sclera is the most strongly reflecting structure on ocular ultrasonography.
High-frequency ultrasound technique
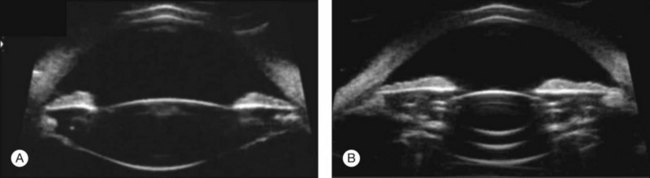
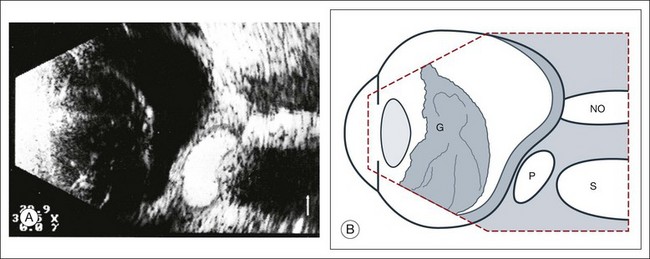
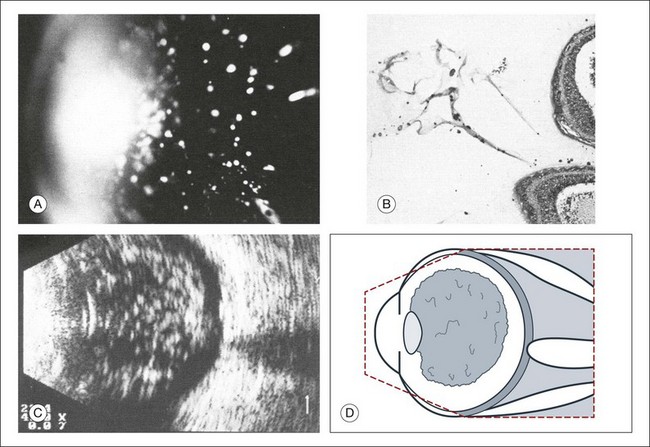
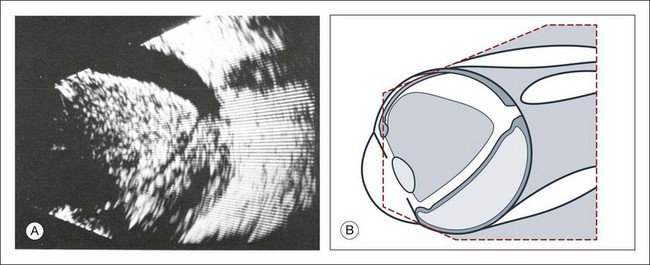
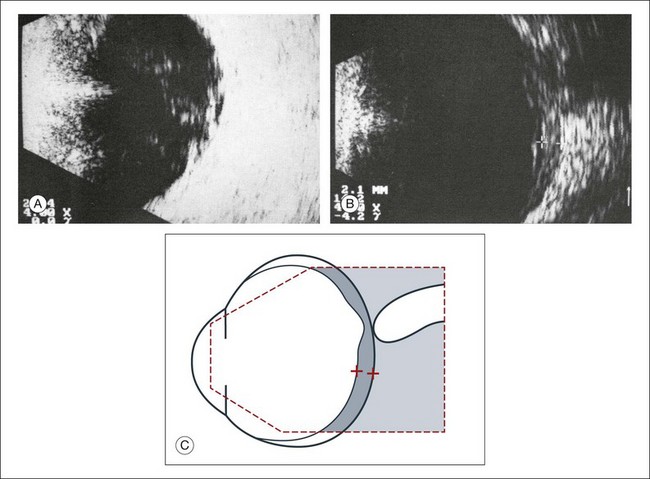
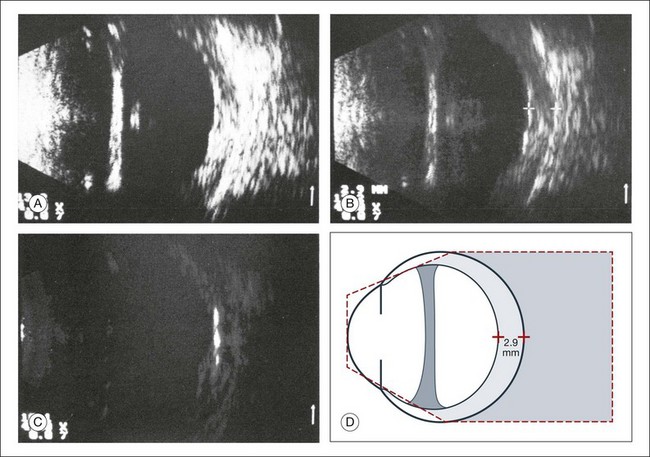
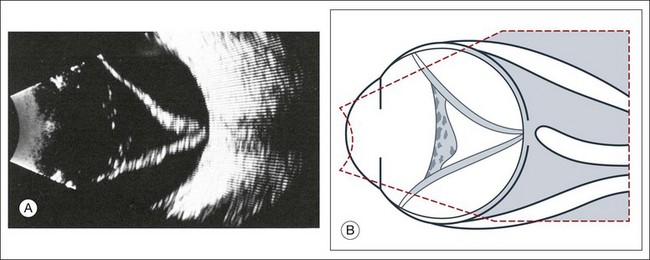
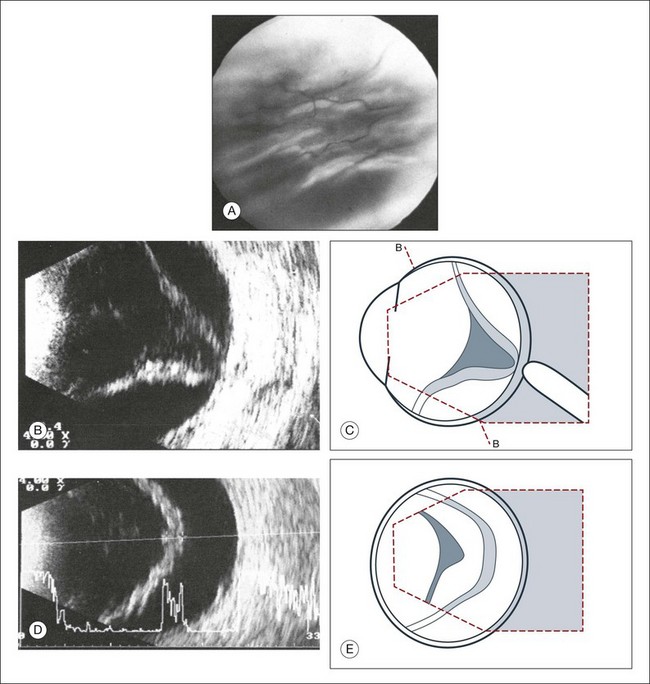
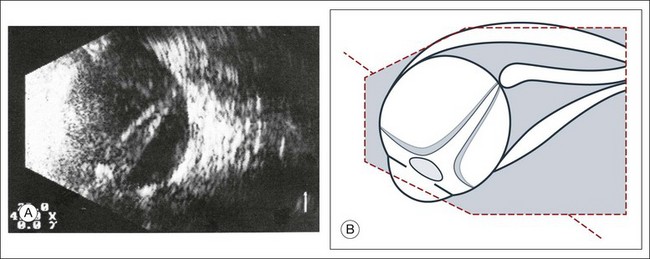
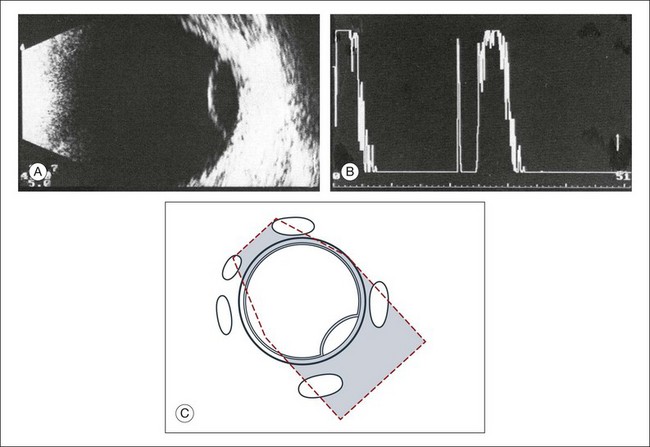
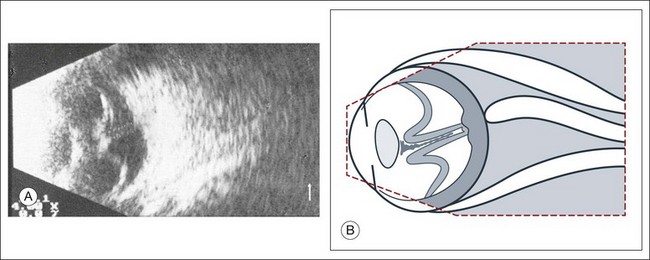
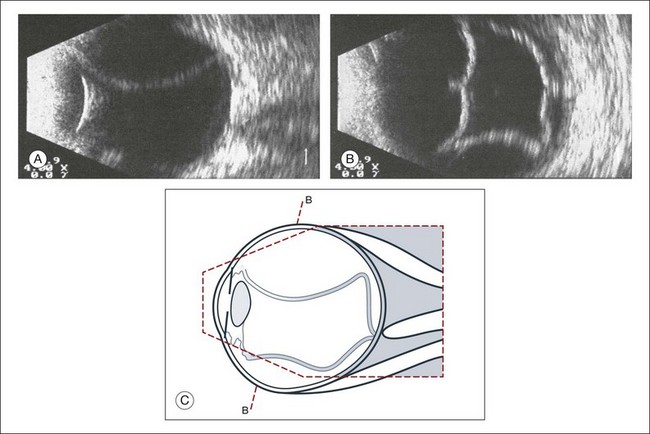
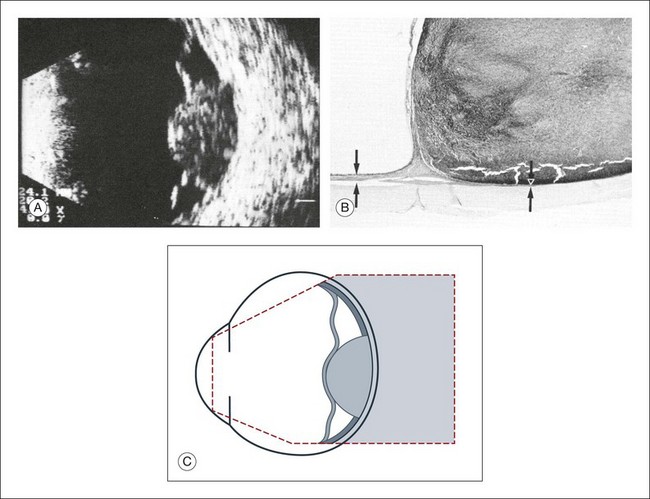
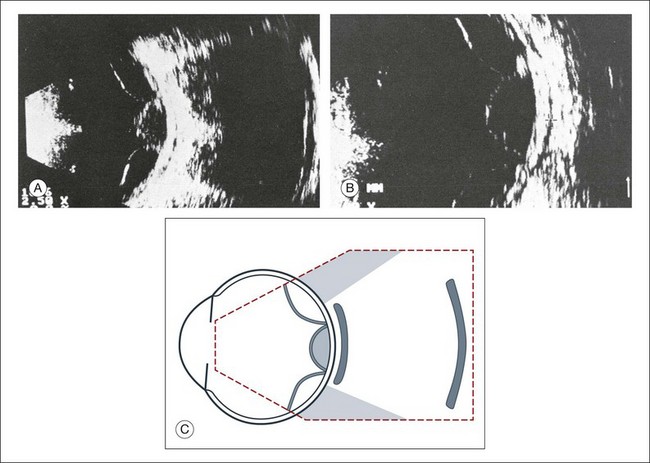
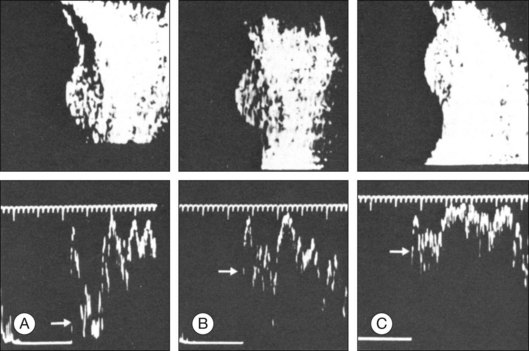
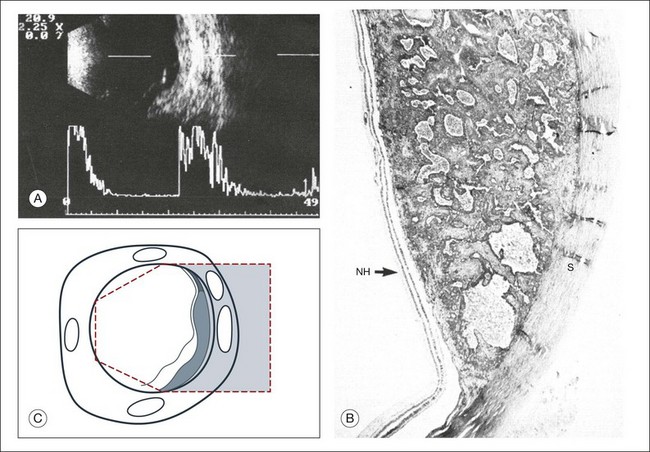
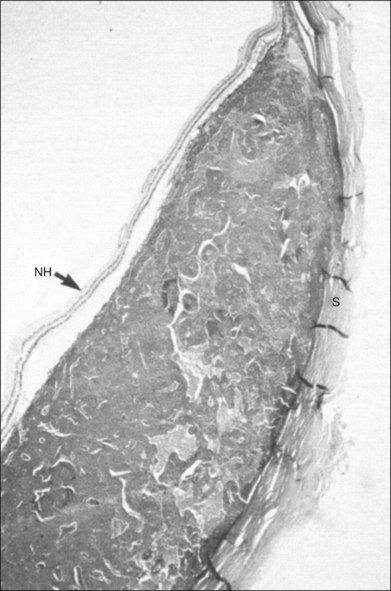
High-frequency echograms can be used for ultrasound biomicroscopy (UBM). The shorter wavelengths provide better resolution of the anterior structures of the eye, including the cornea, lens, aqueous (Fig. 9.3), and ciliary body (Fig. 9.4).13 High-frequency probes range from 50 to 100 MHz.14–16 The 50-MHz probe provides the best balance between depth and resolution for UBM technique. One limitation of this technique is that the shorter wavelengths, from the higher frequency, have poor depth of penetration. UBM cannot visualize structures deeper than 4 mm from the surface.
UBM requires immersion of the transducer in a medium to transmit the higher-frequency wavelengths. Saline or methylcellulose can be used as the coupling agent and is held in place over the eye with the use of a custom cup during the examination. UBM is performed through open eyelids in order to obtain a good reflection signal. Images produced by UBM have a resolution of 30–40 µm, which is similar to that seen with a low-power microscope.17
Doppler ultrasound
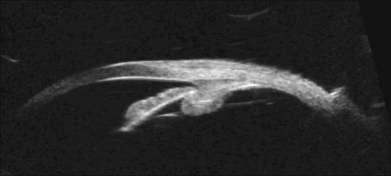
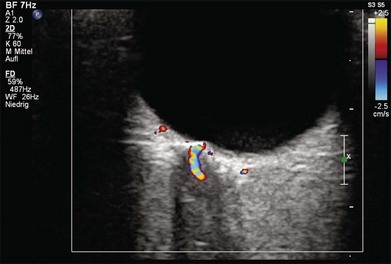
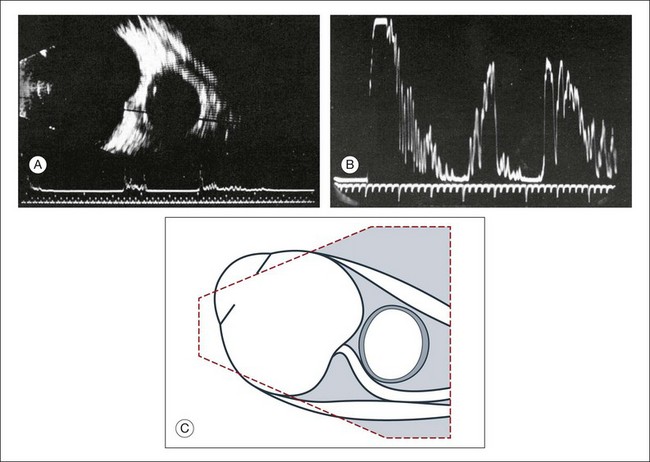
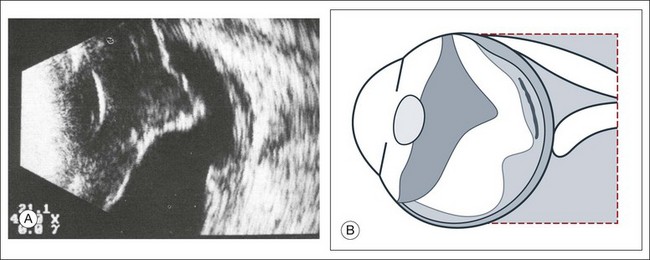
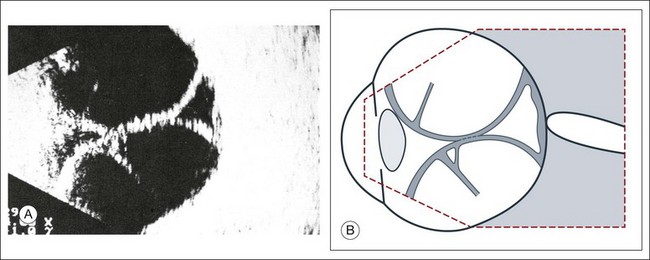
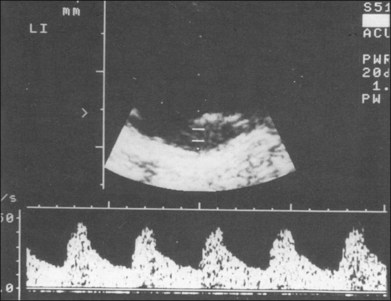
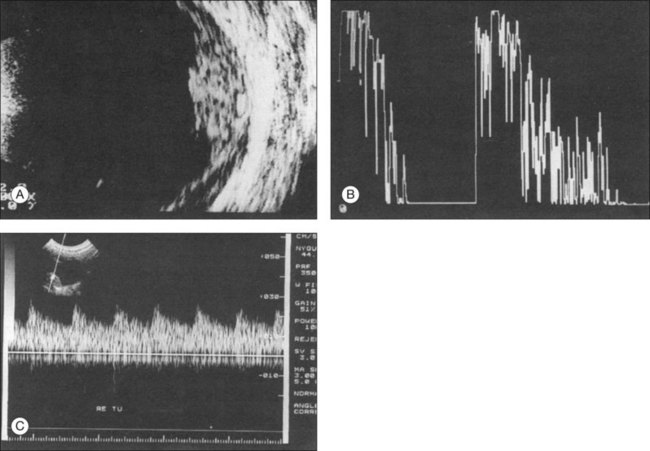
Doppler images are obtained by using frequency shifts from acoustic reflections to measure movements within a tissue and flow conditions within vessels. These frequency shifts can be observed in tissue volumes of less than 10 mm. False color can be added to the images based on ultrasound frequency to distinguish between higher and lower flow states, which aids in the interpretation of the final result (Fig. 9.5).
Ultrasound biometry
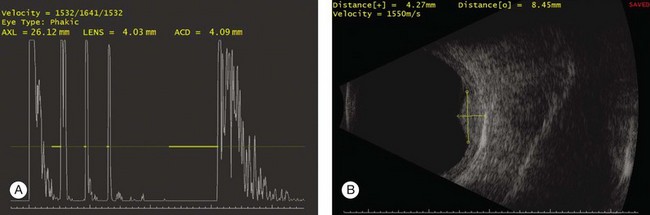
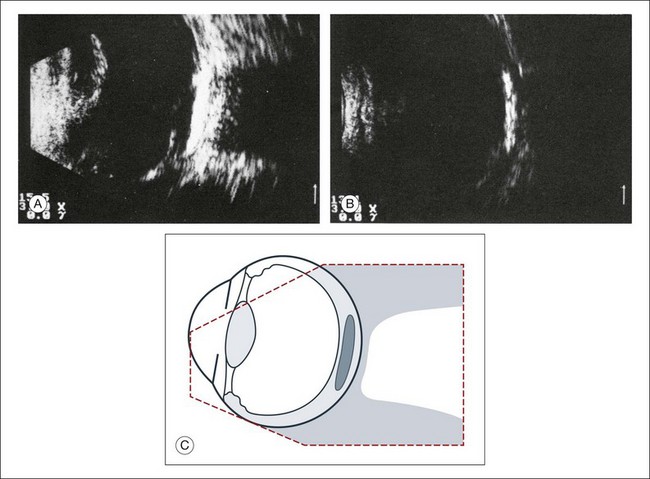
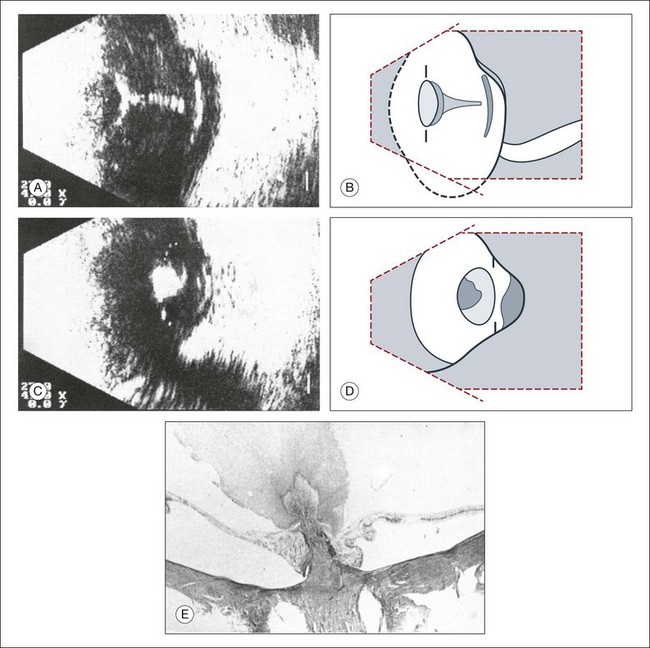
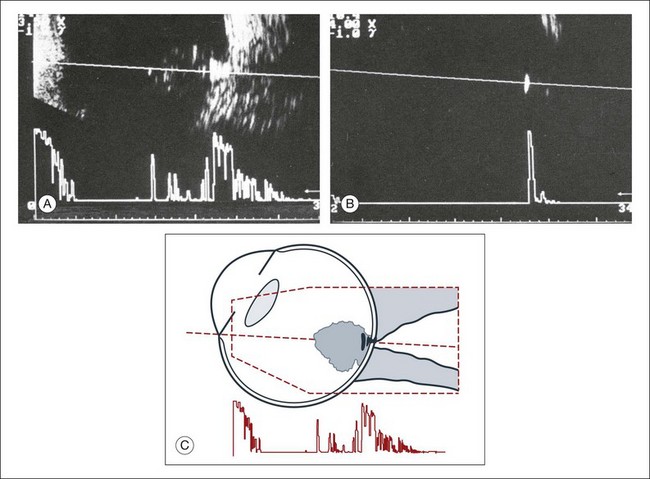
Basic physics formulae can be used to calculate the speed of sound as it passes through various ocular tissues. This number can then be used to calculate distance measurements within the eye (Fig. 9.6). In order to obtain accurate measurements, the specific speed of sound of the different intraocular media, such as the lens, aqueous, and vitreous, must be known.18 These formulae provide precise measurements that can be used to measure intraocular tumors or to deduce the axial length of the globe for intraocular lens power calculations.
Three-dimensional reconstructions
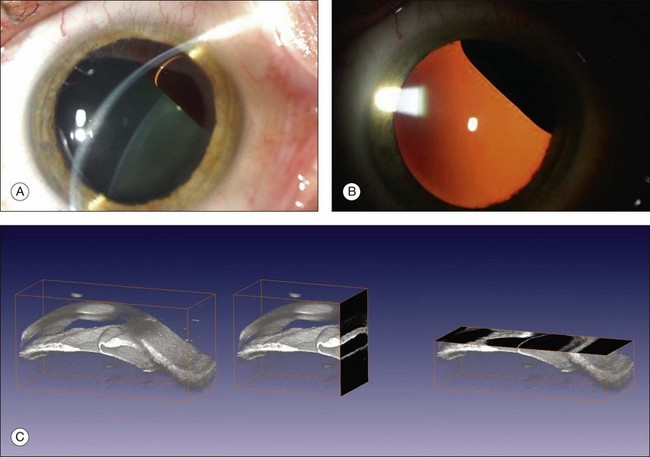
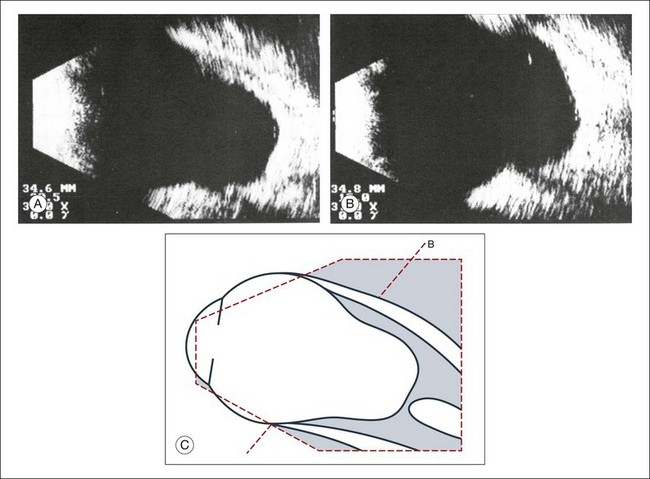
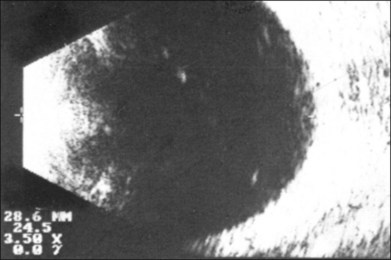
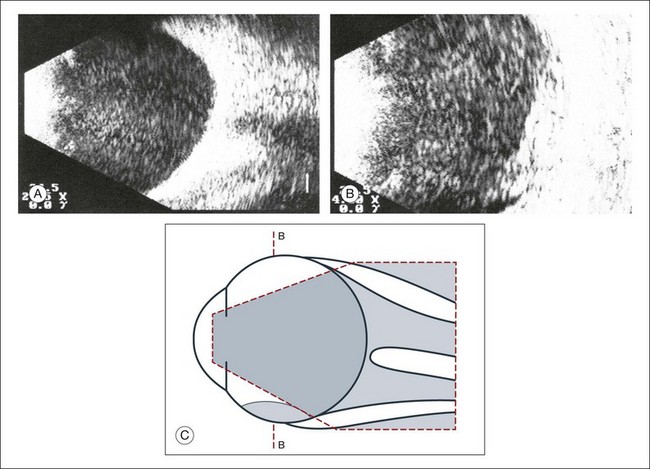
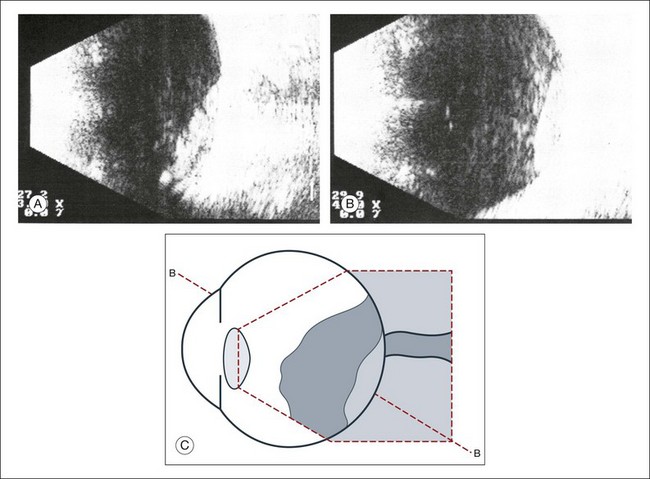
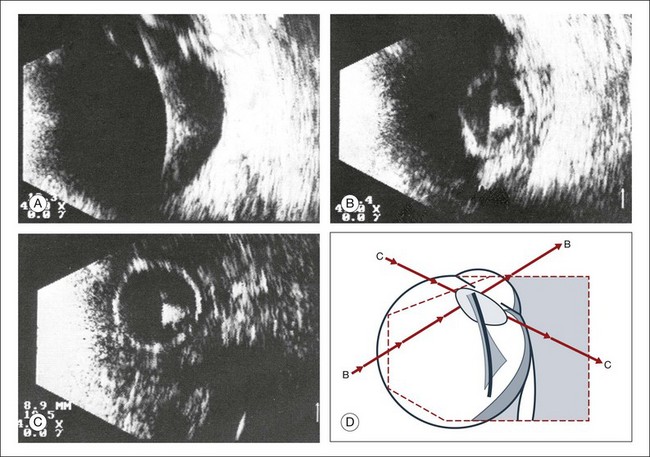
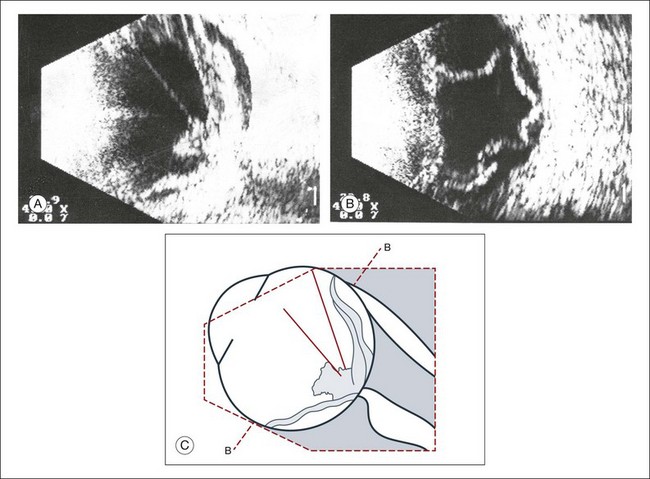
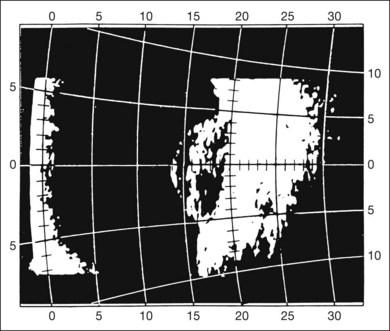
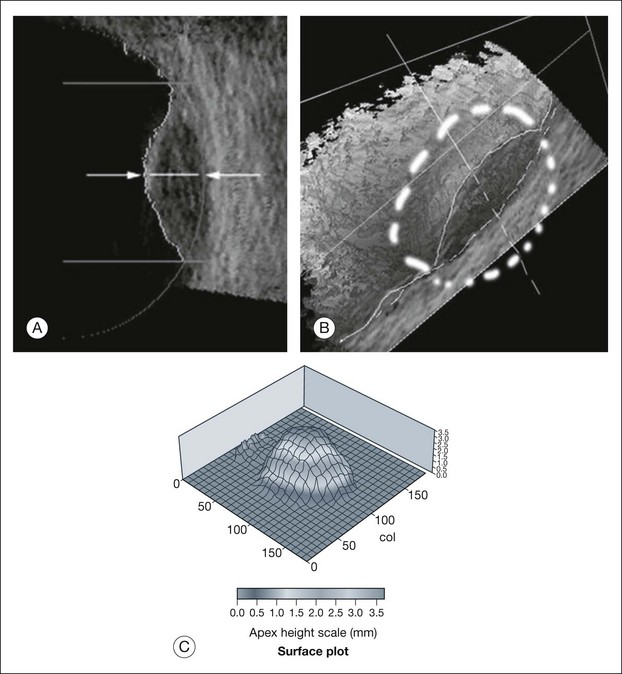
Real-time three-dimensional (3D) and four-dimensional (4D) images are currently used in some medical specialties, including gynecology, obstetrics, and cardiology, but their use in ophthalmology is limited. 3D ultrasonic images can be produced from a series of scan planes.19–22 Silverman et al.23 characterized the ciliary bodies in rabbits and human subjects using 3D high-resolution ultrasound. In the authors’ laboratory a simple extension of the Ultrasound Biomicroscope Model 840 (Humphrey Instruments, Carl Zeiss Group) and VuMax UBM 35/50 (Sonomed) into a user-friendly 3D ultrasonic imaging system was developed (Figs 9.7 and 9.8).24–28
Ultrasound in intraocular pathology
Changes in the shape of the globe
Staphyloma
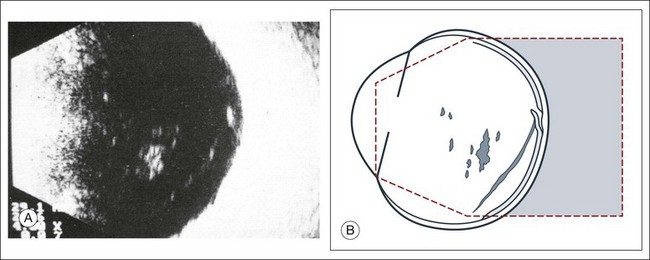
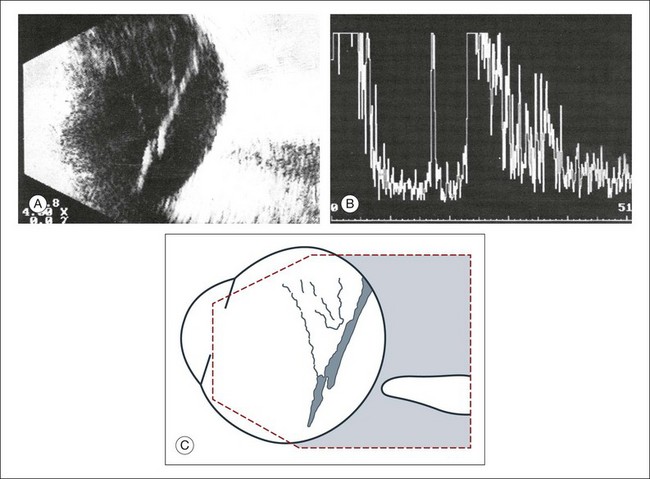
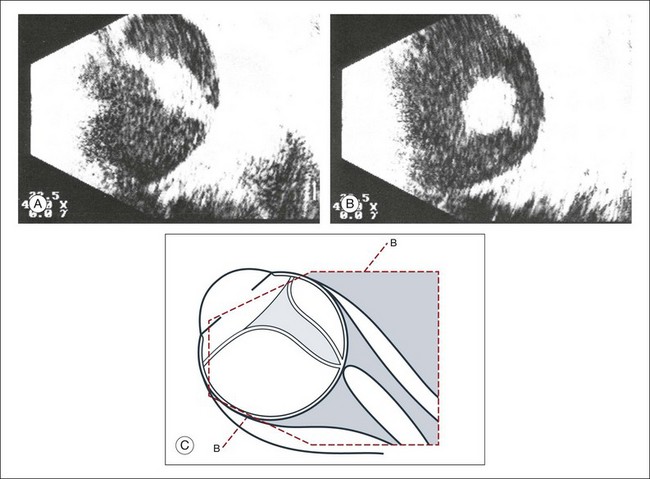
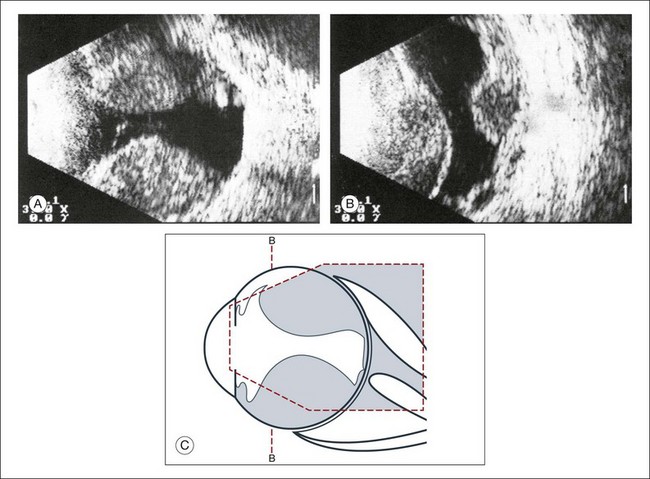
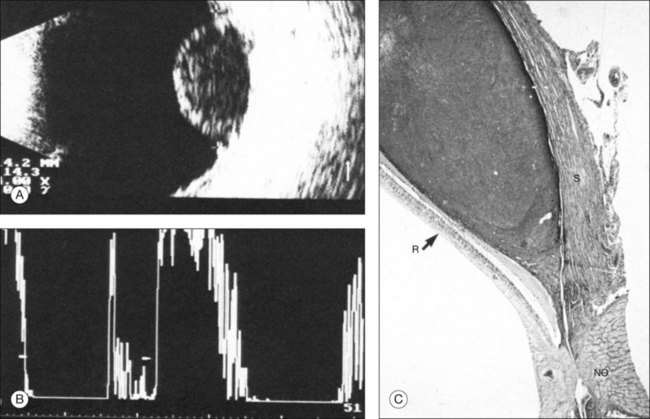
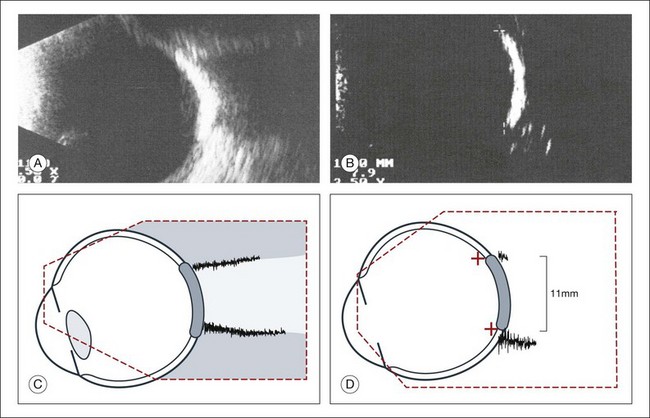
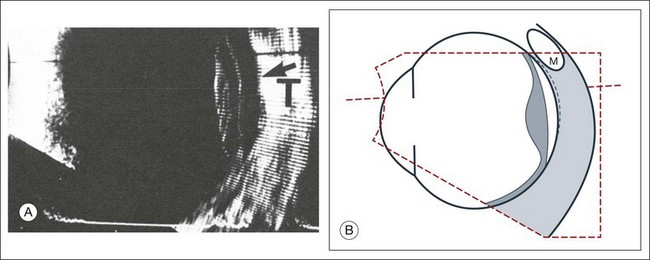
A staphyloma is an abnormal ectasia of the globe that involves uveal tissue. The ectasia typically has a smaller radius of curvature then the normal sclera of the globe. It can be identified on ultrasound by taking axial cross-sectional scans with the transducer probe (Fig. 9.9).
Scleral buckle
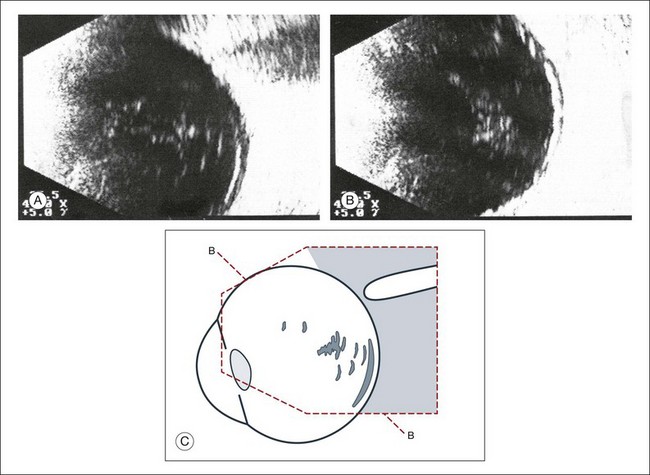
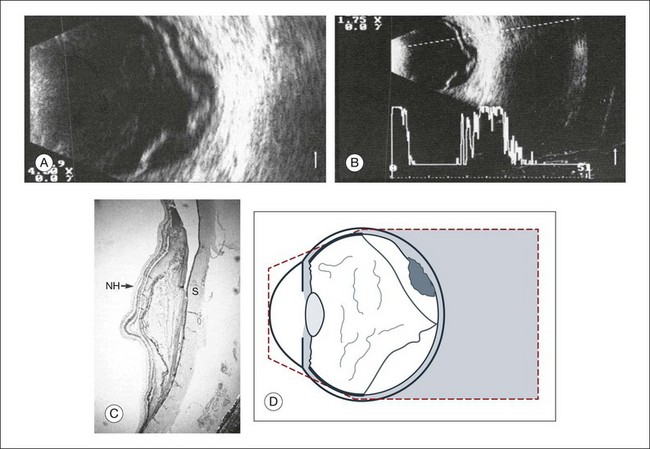
A scleral buckle can create a posterior scleral deformity that looks similar to a staphyloma. It can be distinguished from a true staphyloma by a careful history or identification of the encircling band around the anterior sclera. Also, if silicone oil was used for repair, the higher index of refraction within the silicone oil can alter the reflectance of the ultrasound wavelengths, which might provide a false impression of globe deformation (Fig. 9.10).
Microphthalmos
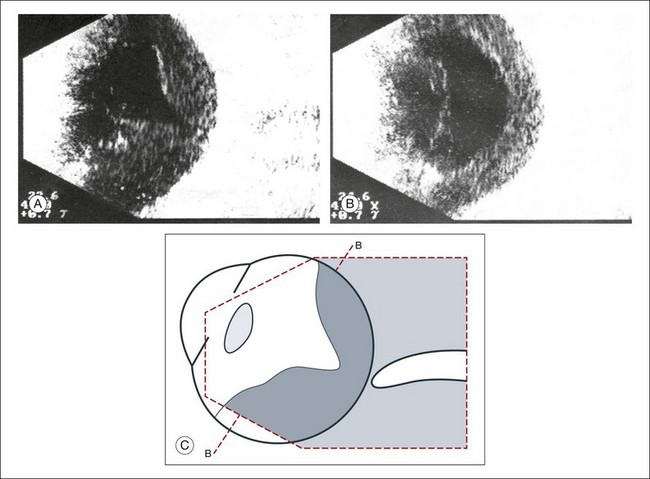
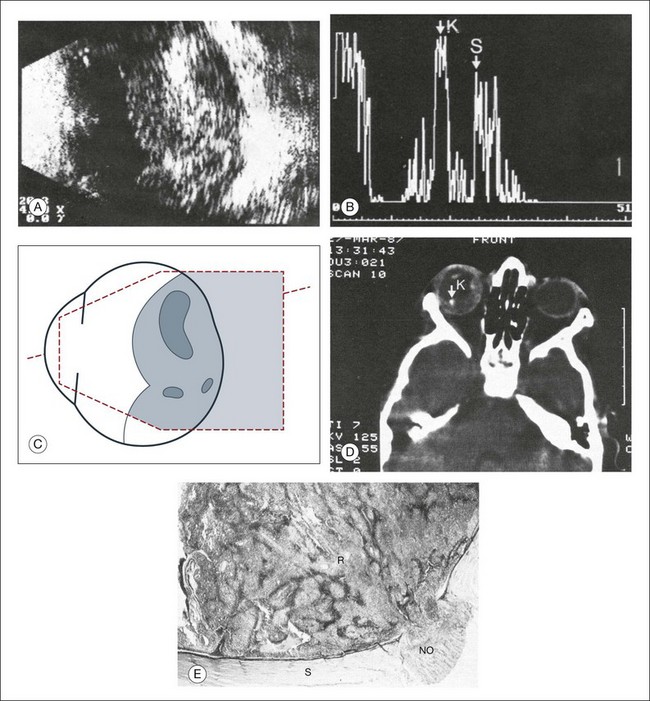
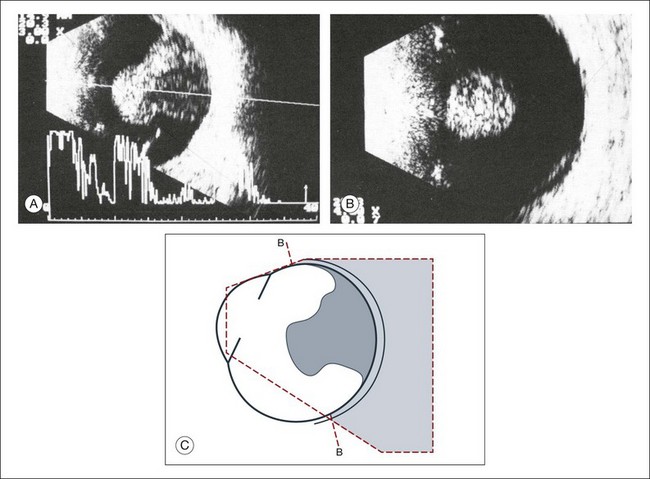
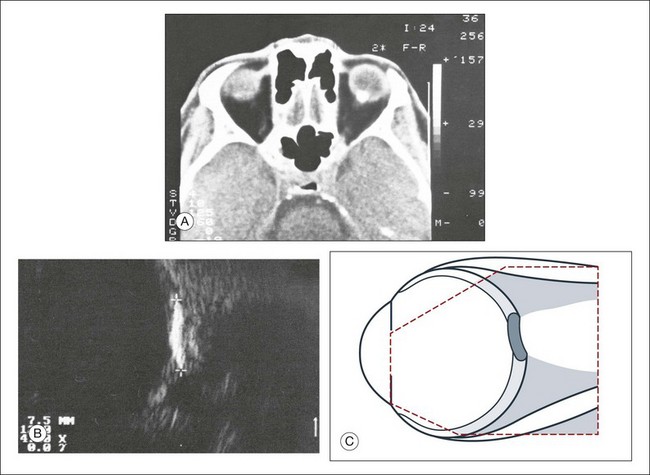
Congenital microphthalmos is an abnormally small eye that can be associated with other ocular abnormalities. The main finding in microphthalmos is axial shortening. This can be identified with A-scan measurements. The B-scan mode can be used to obtain radial and transverse scans to identify abnormalities in the vitreous and posterior segment of the eye, which can also be associated features of microphthalmos. These features include the presence of a coloboma of the retina or optic nerve head, orbital cysts (Fig. 9.11), or persistent hyperplastic vitreous.
Phthisis
Phthisis is defined as severe atrophy of the globe associated with hypotony. Phthisis is characterized ultrasonographically by a thickened outer scleral wall. Occasionally, calcification or ossification may be observed (Fig. 9.12). This may be due to degenerative processes and from metaplasia of the retinal pigment epithelium (RPE). In advanced cases of phthisis, the sclera and choroid can represent up to 70% of the total volume of the globe. The degree of thickening of the globe in chronic hypotony can be an indication of impending phthisis, but the precise thickness threshold for phthisis formation is unknown.
Vitreous
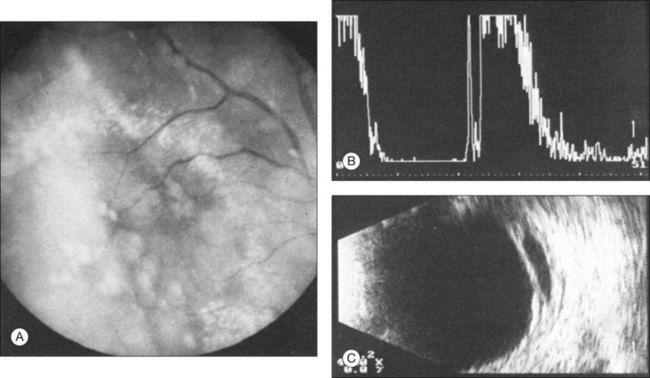
Echographic examination can provide information on vitreous structure which is particularly useful when visualization of the posterior pole is poor due to anterior media opacities. Ultrasonographic findings allow the examiner to differentiate dot-, strand-, and membrane-like reflections (Fig. 9.13). Table 9.1 summarizes the most frequent conditions associated with pathologic changes in the vitreous.
Table 9.1 Clinical conditions with ultrasonographically demonstrable vitreous changes
Vitreous degeneration
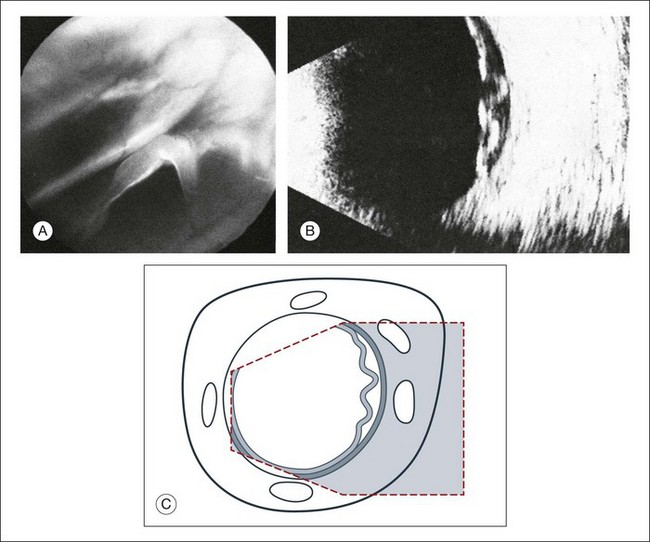
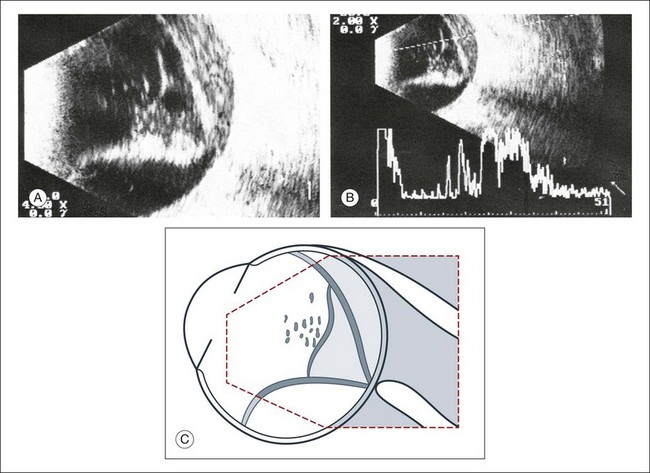
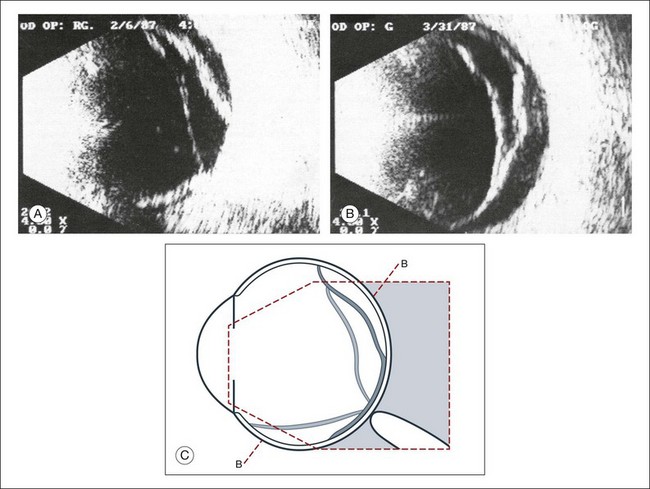
Vitreous syneresis can appear as dot-like reflections which can be more pronounced in myopia or senile vitreous. During a symptomatic posterior vitreous detachment, the B-mode echo may demonstrate various stages of vitreous syneresis and may reveal the remaining adhesions of the hyaloid membrane to the retinal surface (Fig. 9.14).
Asteroid hyalosis
The calcium-containing lipids of asteroid hyalosis are suspended in the vitreous framework and act as distinctive sound reflectors (Fig. 9.15). They can demonstrate the dynamics of vitreous movements.
Persistent and hyperplastic primary vitreous
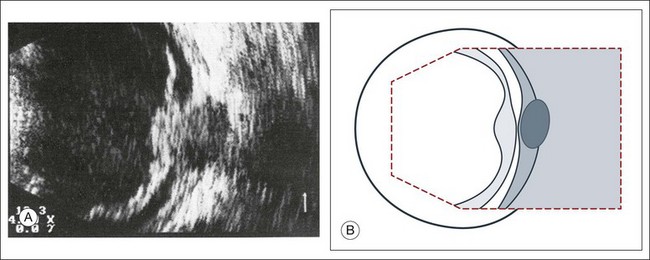
The primary vitreous contains the tunica vasculosa lentis, which is part of the fetal vasculature system. During development, the tunica vasculosa lentis emanates from the optic nerve head and supplies the posterior lens. This structure should involute prior to birth. Failure of the primary vitreous to regress fully is termed persistent hyperplastic primary vitreous. As mentioned earlier, this can be associated with microphthalmos and cataract formation in the newborn. The condition persistent hyperplastic primary vitreous can be ultrasonographically characterized by two features. The first is a strand of membrane that extends between the posterior surface of the lens and the area of the optic nerve head. The second is the reduced axial length of the globe from microphthalmos on ultrasound biometry (Fig. 9.16). If the anomaly is only mild, the lens may be clear at birth but may become cataractous when the posterior lens capsule ruptures.
Vitreous hemorrhages
An acute vitreous hemorrhage is an important indication for ultrasonography. Acute hemorrhages can fill the vitreous cavity with small opacities from the particles of the red blood cells. These opacities usually accumulate after a few hours in the lower circumference of the vitreous base (Fig. 9.17).
If a detachment of the posterior hyaloid membrane precedes a vitreous hemorrhage, the erythrocytes frequently precipitate on to a vitreous strand (Fig. 9.18). This strand may be responsible for the development of a retinal tear, and its traction can be demonstrated directly in acoustic sectioning (Fig. 9.19). A circumscribed thickening of the ocular wall in cross-section may indicate the presence of a retinal operculum (Fig. 9.20). This area should be localized echographically and then carefully scrutinized with ophthalmoscopy if possible.
In larger hemorrhages, the blood can also disseminate into multiple pre-existing vitreous compartments. In the early phase of this process, the erythrocytes will collect in the retrovitreal space (Fig. 9.21). The retrovitreal space may completely clear after a few days or weeks due to its high fluid exchange rate; however, blood on the vitreous framework absorbs much more slowly (Fig. 9.22).
Vitreous hemorrhage from neovascularization
Hemorrhages that develop from proliferative changes in patients with diabetic retinopathy and retinal neovascularization will always be accompanied by pathologic changes in the vitreous. Vitreous membranes tent rectilinearly between the adhesions to the retina. The normal aftermovements that should occur in the vitreous after eye movements are extinguished in the presence of peripheral neovascular tufts. The vitreous tufts create adhesions that encircle the posterior pole. This is an ominous sign, which is indicative of early retinal tractional detachment from these circular adhesions (Fig. 9.23). Choroidal neovascularization from age-related macular degeneration will have hemorrhage in multiple layers of the eye (Fig. 9.24).
Terson syndrome
Terson’s sign is a multilayered, intraocular hemorrhage at the posterior pole that typically occurs after blunt trauma to the head. This is usually accompanied by a subarachnoid hemorrhage. If the posterior hyaloid membrane is still attached, the preretinal bleeding will slowly diffuse into the formed vitreous (Fig. 9.25). This can damage the underlying retina and may be an indication for an early vitrectomy.
Intraocular infections
Ocular infection that extends toward the anterior segment or results in a hypopyon formation will have changes within the anterior vitreous space that are demonstrable on ultrasound. A thickening of the retina or choroid can be seen if the inflammation penetrates to the outer layers of the globe (Fig. 9.26). After only a few hours, these changes may involve the entire vitreous body (Fig. 9.27). If panophthalmitis follows a perforating injury, ultrasound evaluation can detect a local reaction at the entrance point of the infection (Figs 9.28 and 9.29).
Vitreous inflammation
Inflammatory and hemorrhagic vitreous changes cannot be differentiated on the basis of ultrasonographic findings alone. Both conditions may cause densification of pre-existing vitreous structures with subsequent shrinkage of the vitreous; tractional detachment of the retina can occur, especially if there are postinflammatory adhesions between the vitreous and retina. In chronic uveitis, an early and complete posterior vitreous detachment can occur and cause the formed vitreous to shrink and form a frontal membrane that extends across the vitreous base (Fig. 9.30). If this membrane adheres to the ciliary body, it may detach the ciliary body and produce subsequent ocular hypotony.29
Intraocular foreign bodies
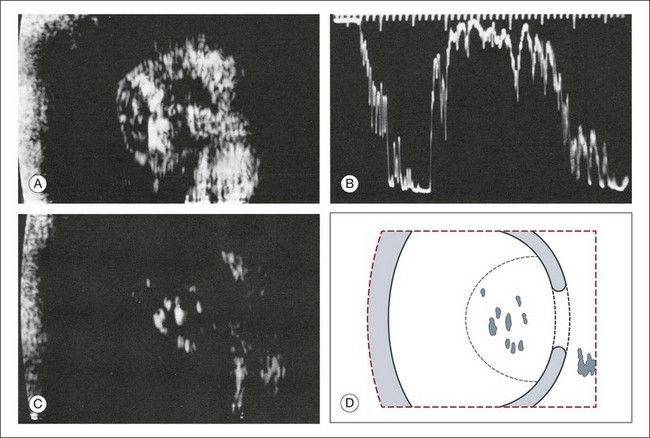
Intraocular foreign bodies induce a change in echo reflectivity which is based on the composition of the material (Figs 9.31–9.33). The change in the reflectivity on the image should be a helpful clue in the localization of the foreign body within the globe; however, this is not always the case since the foreign bodies can also create signal artifact on echograms that can make identifying their exact location difficult. For example, large metallic foreign bodies have significant artifacts from strong reflected signals that can distort their true location. In addition, foreign bodies from trauma can be associated with air bubbles within the vitreous that can mask the presence of the nearby foreign body within the acoustic shadow (Fig. 9.34).
Retina
Acute retinal detachment
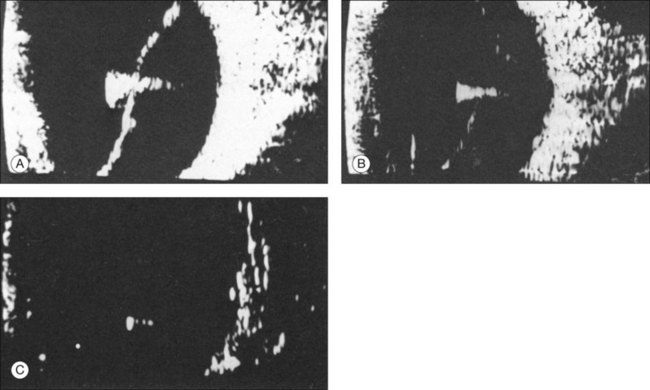
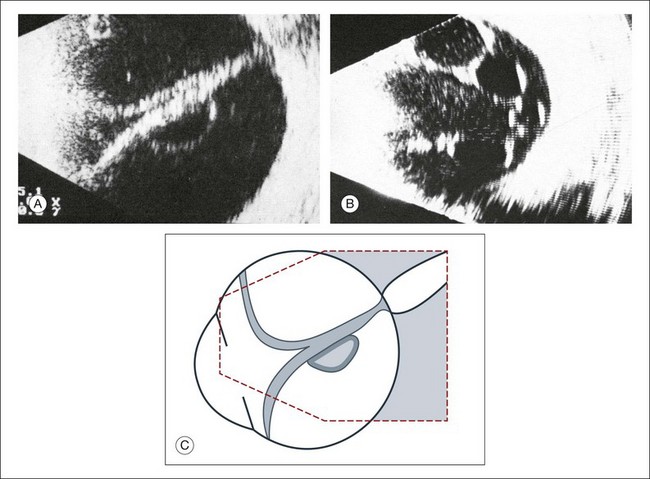
In a retinal detachment, the neurosensory retina separates from the RPE layer. This development allows fluid to collect in the potential space between these two layers. The detached neurosensory retina appears as a membrane in the vitreous space on ultrasound. Partial retinal detachments may still maintain connections to the optic nerve or ora serrata since these areas have the strongest connections to the retina. Identification of these connections on ultrasound can distinguish a partial retinal detachment from a vitreous or choroidal detachment, which would have different anatomical connections (Fig. 9.35). A complete retinal detachment can form a funnel shape due to the retina folding in the center of the globe.
Complicated retinal detachments with severe pathology can make it difficult to identify all the structures on ultrasound (Fig. 9.36). For example, in severe trauma cases that are associated with proliferative vitreoretinopathy or in advanced diabetic disease associated with proliferative retinopathy, the membranes formed within the vitreous can appear similar to a true retinal detachment. The following questions can guide the ultrasound examination of the retina in order to differentiate these common causes of vitreous membranes:
• What is the spatial extent of the membrane? At which point is there contact with the ocular wall?
• What is the shape of a cross-section of the membrane, especially in the optic nerve head area?
• How great is the difference of the spike from the membrane in question to the scleral standard or to the echo from a standard reflector?
These questions should be clarified in the following way:
1. What is the spatial extent of the membrane? At which point is there contact with the ocular wall? A recent rhegmatogenous retinal detachment can be characterized in cross-section by a membranous structure of high reflectivity that converges in an acute angle toward the ocular wall. If the imaged acoustic section is centered on the optic nerve head, then the border of the detached retina will be captured as it connects to the nerve head (Fig. 9.37). If the membrane passes over the optic nerve head instead of connecting to the optic nerve in the echo image, it is not a retinal detachment (Fig. 9.38). This feature can help identify retinal membranes form vitreous membranes.
2. What is the shape of a cross-section of the membrane, especially at the optic nerve head? In order to appreciate the shape of a retinal detachment, the performance of sonographic examinations in various planes is indicated. First, in a sagittal section, a total detachment looks like an isosceles triangle which is open toward the anterior segment (the sides of which may be unevenly tented: Fig. 9.35). Next, frontal-plane sections should be examined with the disc centered in the image. These sectional plans are obtained at a right angle to the sagittal. The frontal planes can be examined best with the transducer probe placed in the temporal part of the lid fissure when the globe is maximally adducted. In these images, the conical shape of the detachment will appear oval to nearly circular in the various sections (Fig. 9.39).
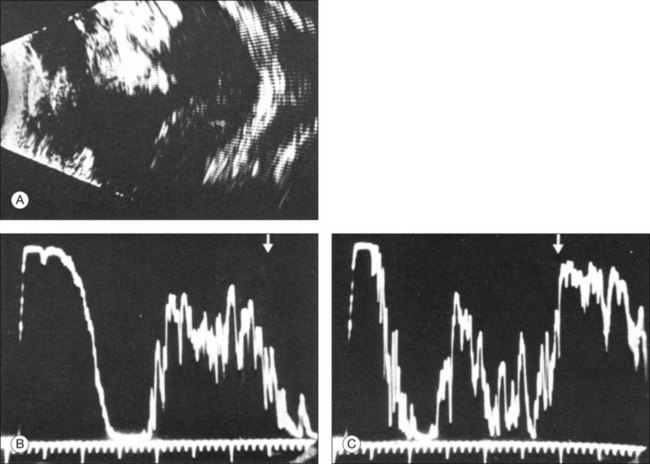
3. Which aftermovements occur? Dynamic ultrasound can be obtained with patient participation. The quality of tissue movement at the end of the ocular saccade, or the aftermovement of the tissue, can be used to distinguish vitreous tissue from retina tissue. An acute rhegmatogenous retinal detachment shows aftermovements of short duration that extend with a whiplash effect from the area where the retina is still attached, which is usually the optic nerve head. The amplitudes of these aftermovements are smaller and less extensive than those seen in the sinusoidal movements of a vitreous hemorrhage or in asteroid hyalosis.30
4. How great is the difference of the spike from the membrane in question to the scleral standard or to the echo from a standard reflector? Quantitative ultrasonography can detect a difference in the echo of the retina compared to that of the sclera, extending from 8 to 15 db.31 Unfortunately, these measurements provide only guidelines. Well-developed connective tissue membranes may show reflection properties quite similar to those of a detached retina. In complicated cases it may be difficult to correlate an isolated A-spike to the multiple membrane structures as they appear on B-mode.
Chronic retinal detachment
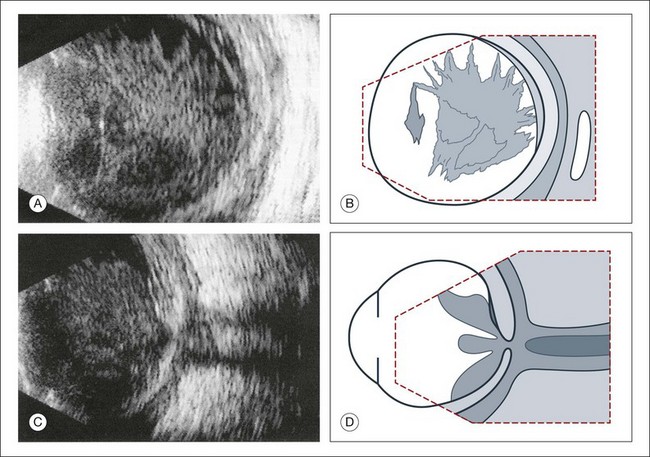
A few weeks after a retinal detachment develops, changes in the proliferation of Müller cells and astrocytes will lead to alterations in the mechanical properties of the detached retina. The massive periretinal proliferation of these cells creates a decrease in the aftermovement amplitude of the retina (Fig. 9.40). The large excursions are replaced by a high-frequency flicker and the retina may appear thickened. After a long-standing retinal detachment, cyst-like cavities within the detached retina can be seen on ultrasound (Fig. 9.41). A long-standing detachment can also result in a funnel-shaped retinal detachment. The first step in this process is the proliferation of the epiretinal connective tissue that causes the vitreous to contract. This stage can be identified on ultrasound by the presence of new acoustic interfaces, which are seen as increased vitreous signals that surround the detached retina (Fig. 9.42). The vitreous shrinks, which creates a narrowing of the retinal cavity, which typically begins to narrow anterior to the optic nerve head and continues to the posterior lens. Frontal “cyclitic membranes” can often be seen early, extending from the vitreous base (Fig. 9.43). Next, the peripheral retina is pulled closer to this membrane until the retinal detachment does not show any recognizable cavity of the original funnel. In frontal sections the shrunken retina appears as a strand, only a few millimeters in diameter (Fig. 9.44). Occasionally, a few retinal folds can be observed.
In addition to the above findings, the consistency of the subretinal space can change with increased duration of the detachment. The protein content will increase and may precipitate out of the subretinal fluid. This can be seen as free-floating opacities on ultrasound (Fig. 9.45). If these opacities appear as static particles and not free-floating, then intraocular tumor has to be excluded. Under these circumstances, ultrasonography provides a vital role in obtaining a diagnosis by distinguishing the freely moving protein precipitation in long-standing detachments from the fixed hyperechoic particles of malignant tumors.
Retinoschisis
Retinoschisis is a splitting within the neurosensory layer of the retina. This condition frequently occurs in the inferotemporal quadrant. A cross-section echogram can display a membranous structure in the far periphery that has a convex border facing the vitreous (Fig. 9.46). In this situation, clinical correlation is important in order to distinguish the retinoschisis from a retinal detachment since these two entities can appear identical on static ultrasound. However, use of dynamic ultrasound and evaluation of the aftermovements can show that the aftermovements in retinoschisis are less conspicuous than in a retinal detachment. This is because in retinoschisis the retina is attached and there are no vitreous adhesions.
Coats disease
Coats disease is an exudative retinopathy most commonly seen unilaterally. It mainly affects males in the first decade of life. A clinical diagnosis can be made if aneurysmal malformations of retinal vessels and yellowish subretinal plaques are associated with an exudative detachment. Coats disease should be differentiated from retinoblastoma, which can have a similar clinical appearance in this patient group. Ultrasound can differentiate these conditions with careful evaluation of the subretinal space. Retinoblastoma typically shows calcifications with high reflectivity. The exudative detachment that is seen in Coats disease has different echo quality due to the subretinal cholesterol deposits (Fig. 9.47). In Coats disease, the crystals floating in the subretinal space are seen as floating opacities similar to the appearance of crystals in synchysis scintillans. They appear in an A-mode echogram as flickering spikes.32 On B-mode images these high-frequency motions of the echo spikes produce a blurred pattern in the subretinal space.
Retinoblastoma
Retinoblastoma is a life-threatening tumor that can present in children as isolated leukocoria or with a constellation of other ocular findings. Some findings can mimic benign ocular conditions. Since treatment of retinoblastoma can include ocular enucleation, making the appropriate diagnosis is critical. Ophthalmoscopy should be performed but sometimes is limited if there is cataract, hypopyon, vitreous seeding, or opacity. Ultrasound plays a critical role in these patients, especially since certain features on echography can be pathognomonic for retinoblastoma. The presence of calcium deposits in retinoblastoma lesions produces a high sound reflection that creates an acoustic shadow on more distant structures such as the sclera (Fig. 9.48). The calcium deposits can be selectively demonstrated on the image by decreasing the amplification in the cross-section echogram until they are the only remaining tissue structure visible on the screen (Fig. 9.49). In some retinoblastomas the calcium appears only in few areas, but any calcification is indicative of tumor burden and is an important feature for the diagnosis. In some cases, ultrasound can also define extraocular tumor extension in retinoblastoma and is of great prognostic and therapeutic importance. Additional imaging to supplement ultrasonography is important in retinoblastoma. Computed tomography can show calcification and magnetic resonance imaging can show tumor extension outside the orbit, optic nerve invasion, or pinealoma (Fig. 9.50).
Retinopathy of prematurity
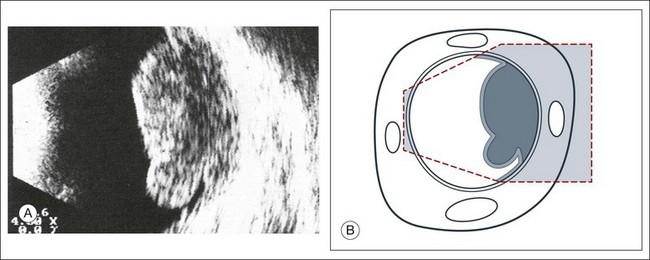
Early stages of retinopathy of prematurity can be diagnosed by indirect ophthalmoscopy. However, later stages with partial or complete tractional retinal detachments from extensive fibrovascular tissue require ultrasonography for diagnosis and surgical planning. Machemer and Aalberg33 emphasized the importance of information obtained by cross-section ultrasonography in these stages, such as the diameter of the retinal funnel in the retrolental space (Fig. 9.51).
Optic nerve
Coloboma of the ocular fundus
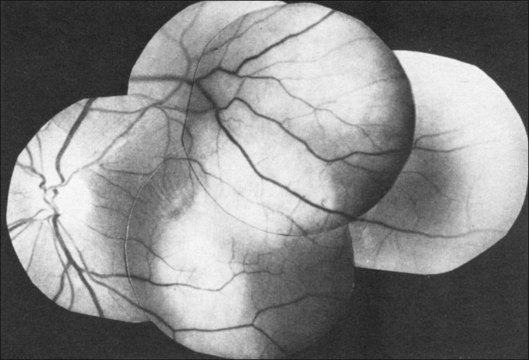
Colobomas can occur in the iris, lens, retina, and optic nerve. Some are spontaneous and some occur in association with systemic syndromes. Colobomas are also in the differential diagnosis of retinoblastoma.34 Colobomas can be seen clearly by ultrasonography even if the fundus cannot be visualized optically (Fig. 9.52).
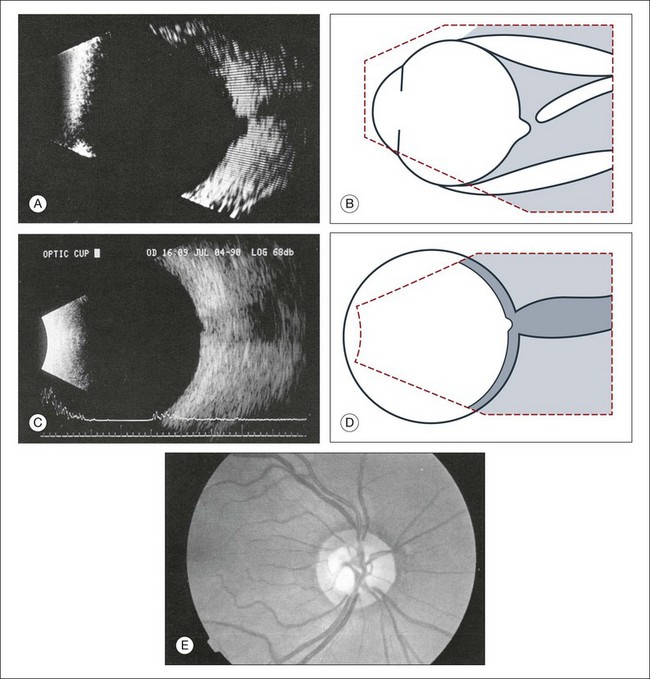
Assessment of optic nerve cupping
Evaluation of optic nerve cupping is important for treatment of glaucomatous changes. This is difficult with anterior media opacity. Recently, with improvement of the lateral resolution of B-scan images, optic nerve head cupping can be reliably measured and reproduced on ultrasound images.35
Choroid
Changes in the ocular layers due to hypotony
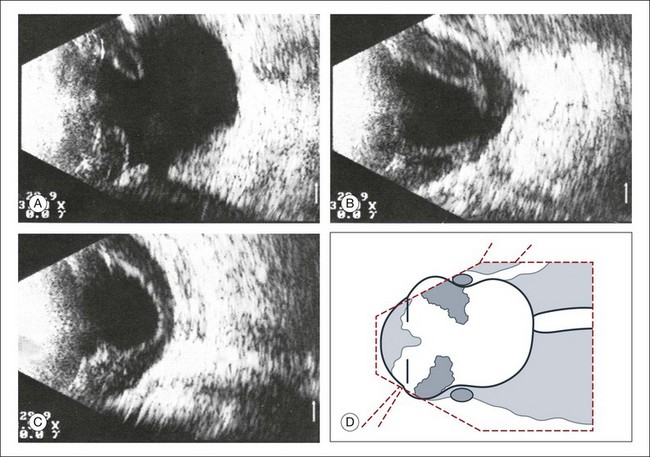
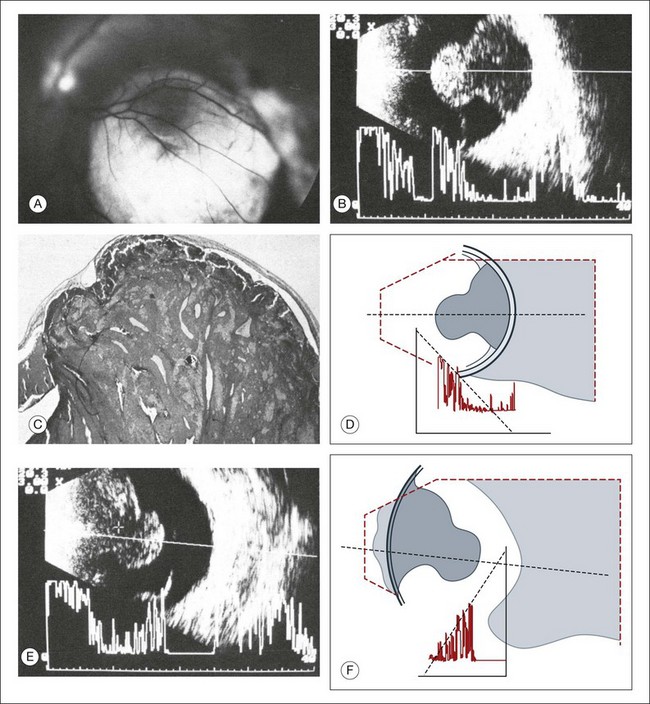
In acute hypotony, exudation into the suprachoroidal space creates an increase in the choroidal vascular pressure. This can lead to a choroidal detachment which will then exacerbate the globe hypotony (Fig. 9.53). This situation is ultrasonographically characterized by a convex border on a cross-section image that denotes the location of the choroidal detachment. This convex shape is formed between the pars plana and location of the vortex veins, both of which have strong choroidal attachments. Knowledge of the choroidal anatomic structure and its attachments can aid in ultrasound diagnosis. In addition to the pars plana and vortex veins, the choroid is firmly attached to the optic nerve. The choroid inserts at the optic nerve at a blunt angle, as compared to the retina, which has a steeper insertion. This feature can be used to help differentiate choroidal and retinal detachments of the posterior pole on ultrasound images. In addition, a choroidal detachment would begin at the ciliary body and not the ora serrate, as in a retinal detachment (Fig. 9.54). Occasionally, the detached ciliary body can compress the lens. In severe cases, choroidal detachments may meet in the center of the globe. This feature has been termed “kissing choroidals” and needs to be surgically corrected (Fig. 9.55). In typical cases of serous choroidal detachments the subchoroidal space is acoustically silent. In contrast, an expulsive choroidal hemorrhage can be identified by the hyperechoic signal on ultrasound that the blood clots create within the detachment (Fig. 9.56).
Choroidal neovascularization
Choroidal neovascularization is a complication seen in many eye diseases, most commonly in macular degeneration. The histological findings include a vascularized collection of connective tissue that extends from the choriocapillaris through defects in Bruch’s membrane and then into the subretinal pigment epithelial or subretinal space. This heterogeneous structure produces mixed ultrasound features, including the strong acoustic reflections from the fibrovascular membranes and dense connective tissue septa and the acoustically silent areas of exudate and subretinal fluid (Figs 9.24 and 9.57). Peripheral choroidal neovascularization membranes can appear similar to choroidal melanomas; therefore, familiarity with the ultrasonographic features of these lesions is important, especially if vitreous hemorrhage prevents direct visualization.
Choroidal melanoma
Evaluation of choroidal melanomas includes a comprehensive examination with ophthalmoscopy combined with ultrasound imaging. In choroidal melanomas, the signals produced within the tumor are complicated by overlapping and dampening echo processes. Connective tissue septa and vessels vary in their prominence and interfere with the signals from the tumor tissue. Furthermore, the resolution of the image is limited because the densely packed tumor cells are separated by a considerably shorter distance than the ultrasound wavelength. A thorough understanding of the principles of ultrasonography and a strong knowledge base of the tissue complexity in metastatic processes combined with an extensive case reference aids in the image interpretation of choroidal melanomas. The interpretation of echograms in A- and B-mode is based on reports on this topic which were published by Oksala,36 Baum,37 Buschmann and Trier,38 Till and Ossoinig,32 Trier,39 Coleman et al.,40 and Silverman et al.,41 which include data from the original scans of ocular tissues. The next section will review the principles used to image choroidal tumors and the features of a choroidal melanoma on B-mode and A-mode imaging.
The characteristics of a choroidal melanoma on B-mode echography
In B-mode echograms, a melanoma appears as a biconvex lesion. The internal structure is relatively homogeneous so produces markedly fewer signals than the tumor surface or the sclera (Fig. 9.58). If the tumor has broken through Bruch’s membrane, then a mushroom-shaped lesion, or a collar button, can be demonstrated in cross-section and serves as a pathognomonic sign (Fig. 9.59). If present, this perforation does not necessarily occur at the peak of the tumor; careful ultrasound examination of the entire lesion is needed (Fig. 9.60). In some sections, the collar button of a tumor may even simulate a tumor mass lying free in the vitreous (Fig. 9.59). In addition, the collar button portion of the lesion has unique ultrasound features due to the presence of the mass above Bruch’s membrane in some places and below Bruch’s membrane in other places. Previously this feature was attributed to sound attenuation, which is the continuous decay of spike altitudes in the A-mode and was called the angle kappa. However, the change in signal is most likely due to more than just an attenuation phenomenon. The features of the collar button can be explained on the basis of echographic–histopathologic correlations (Fig. 9.61). Usually, a tumor lying immediately beneath the sensory retina reflects ultrasound more than the portion of the tumor beneath Bruch’s membrane. In a collar button, the part of the tumor growing in front of Bruch’s membrane will be drained less well, leading to a dilatation of the vessels in this part of the tumor.31,42 This creates new acoustic interfaces with higher signal intensity, resulting in brighter dots in B-mode and higher spikes in A-mode. In the area of the tumor base, a “choroidal excavation” can be seen (Figs 9.62 and 9.63). This excavation in the acoustic section develops at the edge of the tumor where melanoma tissue intersects the adjacent strongly reflecting intact choroid.37,43
The characteristics of a choroidal melanoma on A-mode ultrasonography
The ultrasonographic internal structure of a tumor is quantitatively best described in the A-mode echogram. An unfocused A-mode transducer can obtain a summation signal from the large tissue area,31 which typically demonstrates spikes of relatively uniform amplitudes within the large biconvex tumor if the lesion has not extensively broken through Bruch’s membrane (Fig. 9.58). This corresponds to the uniform histological structure of a melanoma (Fig. 9.58). Exceptions occur when features of hemorrhage or necrosis within the tumor develop.44,45
Most melanomas consist of solid tissue; therefore, no aftermovements occur as might be expected with a large subretinal or choroidal hemorrhage. In addition, flickering spike complexes may be seen on the A-mode echogram within the tumor itself. Various authors interpret them differently. It is assumed that they are due to blood circulating in large tumor vessels. Although the features of the ultrasound combined with a thorough clinical examination can establish the diagnosis of choroidal melanoma with enough certainty to recommend treatment, a preoperative correlation between the sonographic pattern and the histopathological findings is currently still in debate.40,41
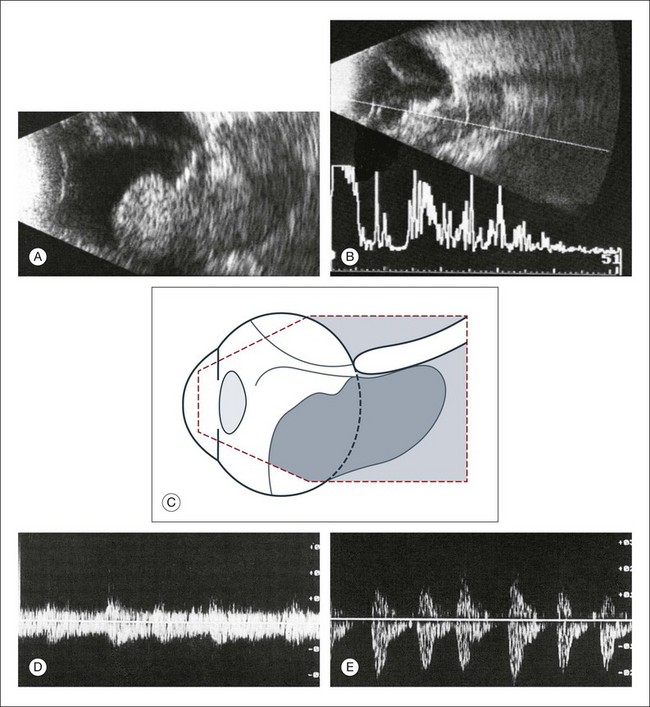
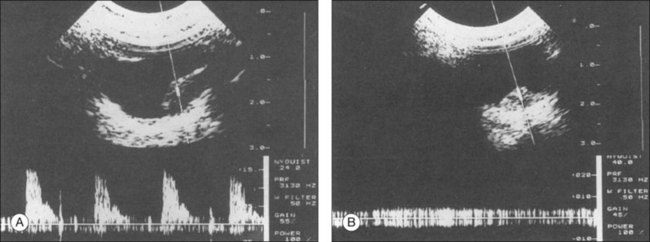
B-mode Doppler devices can obtain signals from the interior of the tumor by using a high-resolution power and a frequency between 7 and 20 MHz. This method is more sensitive than the visual evaluation of a time amplitude echogram46 (Fig. 9.64). In some patients it was possible to determine the blood flow within the tumor using Doppler color-coding technology. In a series of 50 eyes with melanoma, Doppler shifts could be detected in all patients except one. The tumor size varied between 80 and 1500 mm3. Doppler imaging has also been used to study an eye with secondary glaucoma and significantly increased intraocular pressure. In this patient the tumor perforated the sclera and invaded the muscle cone (Fig. 9.65). An intraocular pressure of 45 mmHg compressed the intraocular tumor, which influenced the maximal flow velocities measured by Doppler sonography (Fig. 9.65).
Determining the volume of a choroidal melanoma by ultrasonography
Ultrasonography produces spikes in the recorded signal when interfaces with different media are encountered. In a normal eye, the first acoustic interface after the signal passes through the vitreous is the retina. In the case of a choroidal tumor the apex is adjacent to the retina spike and the base is anterior to the scleral spike. Identifying these positions, however, can be challenging in some settings. For example, if the retina is attached to the tumor surface, no separate discrimination of the two interfaces is possible (Fig. 9.58). On the other hand, if the accompanying retinal detachment is also present over the peak of the tumor, then the retina will be imaged as a separate membranous structure lying in front of the lesion (Fig. 9.66). Then the transition between tumor and scleral must be identified. In most cases, the scleral spike is still the strongest signal even in the presence of a choroidal tumor. If the tumor breaks through the scleral barrier, the continuity of this strong signal is interrupted at the site of perforation. An exact measurement of the volume of the tumor is important when measuring the lesion height, planning a treatment, or analyzing the effect of therapy.
Ultrasonography is capable of providing precise measurements of tumor size. Ultrasound devices have measurement tools that can be applied in the window of the image. The examiner can use this feature to measure the height of the largest and smallest diameter of the tumor. In biconvex tumors, volume data and tumor can be calculated from these linear measurements or it can be evaluated using special equipment designed for 3D evaluation (Fig. 9.67).
The role of ultrasonography for planning the treatment of choroidal melanomas
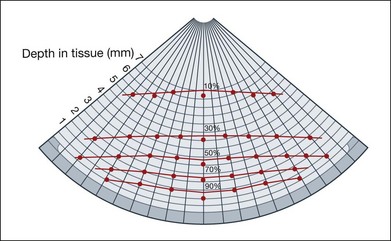
After a choroidal melanoma is diagnosed by careful clinical examination and ultrasound interpretation, the next step for the physician is to formulate a treatment plan. Radiation therapy is successfully used for the treatment of medium or small ocular melanomas. Scleral contact radiotherapy using ruthenium-l06, cobalt-90, or iodine-125 applicators shows a relatively steep decay of radiation dosage. At a distance of 8 mm from the radiation surface, only 10% of the energy is still available. In order to achieve complete tumor necrosis in this area, an application of 10 times the duration of radiation is needed. Figure 9.68 shows the isodose curves for ruthenium-106 applicators. The aim of any radiation therapy is to destroy the tumor tissue and to spare the adjacent normal ocular tissues as much as possible. The radiation pellets are placed on custom scleral plaques that are designed to cover the full extent of the tumor. It is therefore important to have an exact measurement of the size of the tumor and have precise placement of the scleral plaque over the tumor (Figs 9.69 and 9.70). Ultrasonography provides the most accurate measurements for this purpose. Dynamic imaging using the ultrasound B-mode can also provide information on adjacent pathology such as overlying retinal detachments, which may overestimate the total height of the tumor if not properly identified.
Serial examination with ultrasonography posttreatment can be used to monitor the effect of the radiation plaque by measuring the tumor elevation, tumor base, and reflectivity of the internal tumor structure as well as by identifying the presence of tumor vessels. Figure 9.71 illustrates the successful treatment of a choroidal melanoma that was documented echographically. In this case, after 4 months there was a marked increase in reflectivity in the internal tumor structures. Within 10 months the volume was reduced from 380 mm3 to 0 mm3.
In some cases of successfully treated tumors, the increased reflectivity within the tumor is the only ultrasonographic parameter that changes after radiotherapy (Fig. 9.72). Increased reflectivity may demonstrate tumor necrosis; however, this may not always be the case, as some tumors remain viable even with the increase in reflectivity noted on ultrasound.
Determining blood flow by B-mode Doppler sonography is an additional parameter that could be used to monitor the treatment response in highly vascularized choroidal tumors, such as choroidal melanomas. Doppler can detect a decrease in blood flow in the necrotic mass that is left after radiation therapy. The necrotic tissue from treated tumors can be absorbed by the adjacent blood vessels, but radiation may damage these vessels and prevent the clearing of these products, which explains the residual mass on ultrasound seen after treatment with radiation: this mass is called tumefaction. This may explain the increased reflectivity of treated tumors. Figure 9.73 shows an elevated tumor before radiation.
Metastatic choroidal tumors
Choroidal metastasis is a known complication of several cancers, including all types of carcinomas and sarcomas. The most frequent primary tumors are breast (40%) and lungs (29%). One study examined 230 eyes from deceased patients with known systemic carcinoma and showed that 12% of the eyes had choroidal metastasis on pathological specimens.47 Many of these tumors remained undetected at the time of death. The detection of such lesions can be a poor prognostic factor. The average survival time after treatment for choroidal metastasis is 7.4 months.48 Therefore, many of these patients are not followed for extended periods of time by their ophthalmologist.
Typically carcinoma metastasis presents as a large, highly reflective thickening of the ocular outer layers. The internal acoustic properties can resemble a disciform macular degeneration or a choroidal hemangioma. A metastatic adenocarcinoma typically presents with high reflectivity from the strong acoustic interfaces from its adenoid-like histological structure (Fig. 9.74).
In contrast, there are several atypical presentations of choroidal metastasis that have also been reported.49,50 Pathological examination of an eye that was enucleated because of suspicion for choroidal melanoma from ultrasound examination revealed a choroidal metastasis from small-cell bronchial carcinoma (Fig. 9.75).49
Choroidal hemangioma
Choroidal hemangioma may be an isolated lesion or may be associated with Sturge–Weber syndrome. Isolated hemangiomas, described as circumscribed choroidal hemangiomas, usually occur at the posterior pole. The lesions are typically diffuse, slightly elevated, and may have indistinct margins. These features make ophthalmic interpretation of the lesion difficult and often they can be missed. However, associated features of these circumscribed lesions can include retinal detachment and secondary changes of the RPE, which makes their presence more conspicuous on exam. Ultrasonographically, choroidal hemangiomas appear as a strongly reflecting, nearly concentric widening of the outer layers of the eye (Fig. 9.76). In spite of the abundant vascularization, ultrasound images of choroidal hemangiomas do not show the circulating blood as in choroidal melanomas. Instead, the echogram of a choroidal hemangioma is similar to that of metastatic adenocarcinoma or disciform macular degeneration, which may be indicative of a slower circulation more consistent with laminar flow in the cavities of dilated blood vessels shown by Doppler ultrasonography (Fig. 9.77). In long-standing cases the epichoroidal layer may ossify and produce sound shadows. In this stage, a hemangioma may appear identical to an osteoma or a metastatic calcification of the choroid.51,52
Choroidal osteoma – metastatic calcifications
Choroidal osteoma is a rare condition which is characterized ultrasonographically by a localized area of high reflectivity at the outer wall (Fig. 9.78). Several conditions can mimic the appearance of a choroidal osteoma. As mentioned above, a chronic circumscribed hemangioma can ossify and resemble a choroidal osteoma.53–55 In addition, several choroidal lesions can contain calcium, including ocular metastasases of the choroid and the sclera51 or metabolic disorders of calcium metabolism that create a localized calcium deposition within the choroid known as an osseous choristoma (Fig. 9.79).54
Choroidal tuberculoma
Choroidal tuberculomas are another extremely rare choroidal lesion (Fig. 9.80).56 Tuberculomas can also appear similar to choroid melanoma (Figs 9.81). Images that are suggestive of choroidal tuberculomas exemplify the importance of placing the ultrasound image in context with the rest of examination of the patient in order to make the proper diagnosis. Tuberculomas are found in patients with disseminated tuberculosis and a complete examination can identify other tuberculomas to confirm the diagnosis. Laboratory confirmation of mycobacterium by blood or sputum sampling is also helpful. In addition, these lesions should respond to antituberculous treatment.
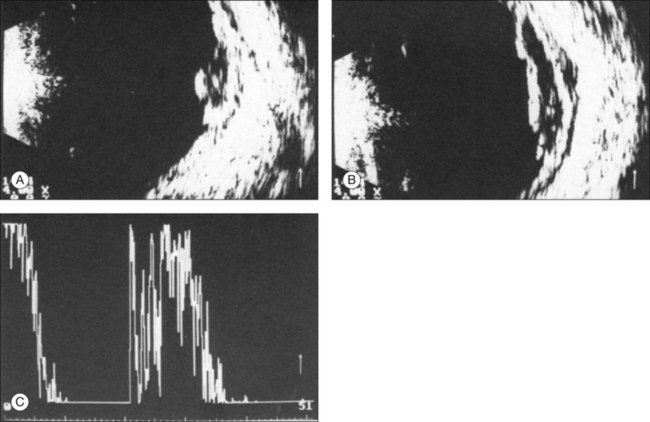
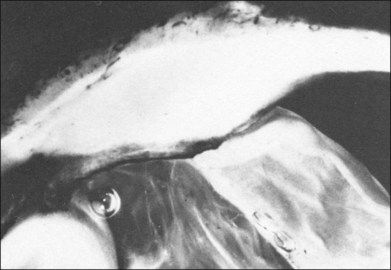
Fig. 9.81 (A,B) Echogram of the fundus lesion shown in Fig. 9.80. (C) The lesion is characterized by low acoustic reflectivity. Tenon’s space can be well demonstrated. The reflectivity corresponds to that of a choroidal melanoma. The infiltration of Tenon’s space points toward inflammatory etiology.
The uveal effusion syndrome
Patients with uveal effusion syndrome can have partial or circular choroidal detachment combined with an exudative retinal detachment (Fig. 9.82). Ultrasonography is very important in this condition. It can reveal fluid in the suprachoroidal space or differentiate choroidal hemorrhage or tumor from the serous fluid seen on imaging from choroidal effusion syndrome.49,57
Sclera
Posterior scleritis
Ultrasonography is the diagnostic method of choice for posterior scleritis.52,58,59 The clinical picture is characterized by an acute loss of vision associated with folds at the posterior pole. The normal high reflectivity of the sclera will be decreased by changes in the tissue from inflammatory swelling. The result is a low reflective signal from a thickened choroid, which is suggestive of posterior scleritis. Choroid and vitreous may appear normal. Occasionally, there is a slight exudation into the subretinal space with accompanying disc edema. In 50% of the patients, fluid accumulates in the Tenon’s space.60 This signal can be similar to a diffuse choroidal melanoma. There is one case report of an eye that was enucleated because of suspicion of an ocular melanoma based on the finding of brawny scleral thickening (Figs 9.83 and 9.84). This case highlights the importance of proper ultrasound interpretation in the clinical context of each patient.61
Ultrasound imaging used to differentiate ocular disease
Ultrasonography is invaluable in the diagnosis of certain ocular and orbital conditions. Proper imaging with ultrasound can narrow the differential diagnosis in certain conditions and guide further workup and management. These conditions include choroidal folds (Tables 9.2–9.4 and see Fig. 9.85), leukocoria (Table 9.5 and see Fig. 9.86), and vitreous hemorrhage (Table 9.6 and see Fig. 9.87). First, choroidal folds can be from orbital masses, ocular inflammation, disc edema in an atypical presentation, or from idiopathic causes.49 Next, leukocoria is a condition of a white pupil that blocks ophthalmic examination, typically diagnosed in infancy. This finding can be from retinoblastoma. Differentiating this disease from other benign causes of leukocoria is critical for providing appropriate treatment of the patient. Finally, vitreous hemorrhage may manifest from a variety of changes in the posterior globe; the urgency of treatment is dependent on the diagnosis of a retinal detachment. All of these conditions require ultrasound technology to establish the proper diagnosis. The tables listed above provide information on the differential diagnosis of each condition and ultrasound features that will help guide image interpretation.
| Diagnosis | Number of patients |
|---|---|
| Graves orbitopathy | 11 |
| Sinusitis | 5 |
| Mucocele | 2 |
| Hemangioma | 6 |
| Orbital pseudotumor | 5 |
| Various orbital tumors | 8 |
| Unexplained | 6 |
| Total | 43 |
Table 9.3 Ocular causes of choroidal folds
| Diagnosis | Number of patients |
|---|---|
| Hyperopic eye | 17 |
| Macular degeneration | 12 |
| Ocular hypotony | 12 |
| Posterior scleritis | 9 |
| Buckling operation | 9 |
| Trauma | 6 |
| Intraocular tumor | 3 |
| Miscellaneous | 10 |
| Total | 78 |
Table 9.4 Choroidal folds: ultrasonographic findings helpful for differential diagnosis
| Diagnosis | Ultrasonographic findings |
|---|---|
| Myositis Graves orbitopathy1 | Thickened extraocular muscles |
| Periorbital space-occupying lesions2 | Change in the relief of the orbital wall, sound propagation into perinasal sinuses |
| Orbital neoplasm3 | Directly evident (it may be difficult to demonstrate a small cavernous hemangioma because of its high acoustic reflectivity) |
| Inflammatory orbital pseudotumor4 | Widening of normal orbital structures, low acoustic reflectivity, Tenon’s space may be demonstrated |
| Disc edema5 | Widened dural diameter of the optic nerve |
| Axial hyperopia6 | Axial length below 22 mm, ocular walls concentrically thickened |
| Ocular hypotony7 | Ocular walls concentrically thickened |
| Macular degeneration | Thickening of the ocular walls in the area of the macula, high acoustic reflectivity |
| Scleritis8 | Circumscribed widening of the ocular walls Tenon’s space apparent |
For companion schematic drawing, see Fig. 9.85.
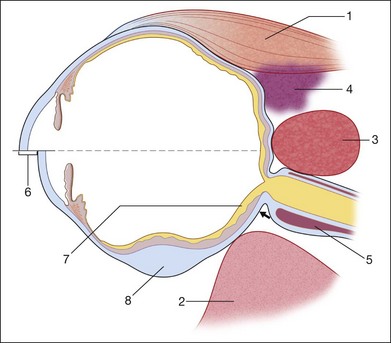
Fig. 9.85 Choroidal folds: ultrasonographic contribution to the differential diagnosis (for detail, see Table 9.4).
Table 9.5 Leukocoria: ultrasonographic findings helpful for differential diagnosis
| Diagnosis | Ultrasonographic findings |
|---|---|
| Normal axial length for the patient’s age | |
| Retinoblastoma1 | Widening of the ocular walls, extremely high acoustic reflectivity, shadowing effect, atypical findings possible |
| Congenital cataract2 | Increased reflectivity from the posterior lens surface, vitreous space empty, ocular walls normal |
| Shortened axial length | |
| Retinopathy of prematurity3 | In stages IV and V, beginning or complete traction detachment (normal findings in stages I–III) |
| Persistent hyperplastic primary vitreous (PHPV)4 | Dense strand of tissue between optic nerve head and posterior lens pole; formes frustes may occur (posterior or anterior PHPV) |
| Retinal anomalies | Membranes in the vitreous, atypical detachment, which in part appears solid (no typical echogram) |
| Fundus coloboma5 | Directly demonstrable protrusion of ocular wall, sometimes with orbital cyst (microphthalmos with cyst) |
| Coats disease6 | Floating crystals in the vitreous and subretinal space (fast-flickering spikes on A-mode) |
For companion schematic drawing, see Fig. 9.86.
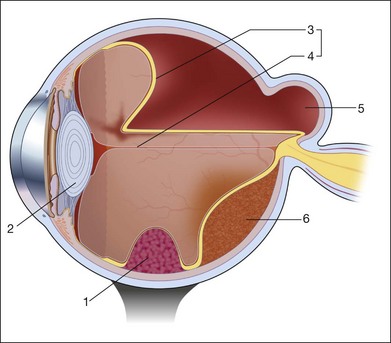
Fig. 9.86 Leukocoria: echographic contribution to the differential diagnosis (for detail, see Table 9.5).
Table 9.6 Vitreous hemorrhage: ultrasonographic findings helpful for establishing the etiology
| Diagnosis | Ultrasonographic findings |
|---|---|
| Symptomatic posterior vitreous detachment1 | Thickened detached posterior hyaloid membrane, occasionally early retinal detachment |
| Recently formed retinal break with torn vessel2 | Blood-covered vitreous strands converge toward the retinal break; occasionally a high-floating operculum may be detected |
| Proliferative retinopathy3 | Strands or membranes extending from the optic nerve head or the posterior pole, high acoustic reflectivity |
| Terson syndrome (vitreous hemorrhage after subchoroidal bleeding4 | Vitreous opacities in front of the optic nerve head or behind the detached vitreous |
| Disciform macular degeneration5 | Widening of the ocular walls in the macular area, high acoustic reflectivity, vitreous strands extending from the macula |
| Choroidal melanoma6 | Biconvex thickening of the ocular wall, low acoustic reflectivity, sometimes mushroom-shaped; accompanying retinal detachment distant from the tumor |
For companion schematic drawing, see Fig. 9.87.
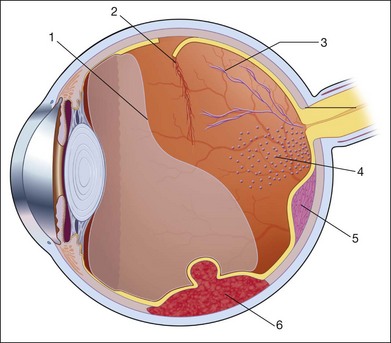
Fig. 9.87 Vitreous hemorrhage: echographic contribution to determine the pathogenesis (for detail, see Table 9.6).
Furthermore, ultrasonography can aid in the preoperative planning and patient counselling for surgery. Table 9.7 lists some of the possible ultrasonographic information and subsequent conclusions that can be obtained for operative planning (and see Fig. 9.88).
Table 9.7 Examination before a vitrectomy: ultrasonographic findings helpful for planning the operation
| Questions on the ultrasonographic examination | Consequences for planning the operation |
|---|---|
| Is the choroidal detachment including the pars plana?1 | Avoiding this area when inserting the ports |
| Is the posttraumatic vitreous hemorrhage associated with a detached retina? | If retina is detached, strong indication for vitrectomy |
| Is an intraocular foreign body demonstrable? If so, where is it in regard to ocular outer wall?2 | Choice of surgical approach, magnet extraction possible? |
| Estimating the prognosis | |
| Is there a choroidal detachment caused by blood?3 | Extremely poor prognosis that may not benefit from surgical intervention |
| Is there a free-floating retinal detachment?4 | Reattachment probable, can be achieved without tamponade from the inside |
| Is there a rigid retinal detachment? Is there a thickened retina?5 | Removal of preretinal membranes necessary |
| Are there vitreous strands with traction effect demonstrable?6 | Consider intraoperatively cutting the strands |
| Is there retinal detachment that is displaced anteriorly to be near the lens?7 | Special care indicated when entering the vitreous space |
| Are there thickened ocular walls in the macula that may indicate a disciform lesion from macular degeneration?8 | Poor prognosis for central vision |
| Are there indications of a secondary, e.g., solid retinal detachment?9 | Additional diagnostic examinations; enucleation may be necessary |
| Is there attached retina with minimal remaining vision? | Reconsider indication for operation, prognosis for vision extremely poor |
For companion schematic drawing, see Fig. 9.88.
Future developments
Many medical fields are using advances in technology to improve ultrasound images. These improvements involve the use of new piezoelectric materials and broadband transducers.62 Current and future developments can be focused on the following trends:
1 Mundt G, Hughes W. Ultrasonics in ocular diagnosis. Am J Ophthalmol. 1956;41:488.
2 Baum G, Greenwood J. The application of ultrasonic locating techniques to ophthalmology, part 1. Am J Ophthalmol. 1958;46:319.
3 Oksala A, Lehtinen A. Diagnostic of detachment of the retina by means of ultrasound. Acta Ophthalmol. 1957;35:461–467.
4 Oksala A, Lehtinen A. Diagnostic value of ultrasonics in ophthalmology. Ophthalmologica. 1957;134:387–395.
5 Oksala A, Lehtinen A. Diagnostic of rupture of the sclera by means of ultrasound. Acta Ophthalmol. 1958;36:37–42.
6 Oksala A, Lehtinen A. Measurement of the velocity of sound in some parts of the eye. Acta Ophthalmol. 1958;36:633–639.
7 Ossoinig K. Standardized echography: basic principles, clinical applications and results. Int Ophthalmol Clin. 1979;19:127.
8 Buschmann W, Haigis W. Influence of equipment parameters on results in ophthalmic ultrasonography. Doc Ophthalmol Proc Ser. 1981;29:487.
9 Ossoinig K, Till P. 10 years’ study on clinical echography in orbital disease. Bibl Ophthalmol. 1975;83:200.
10 Gernet H. Ultrasonic biometry of the eye. Klin Monatsbl Augenheilkd. 1967;151:853–871.
11 Baum G, Greenwood J. Ultrasound in ophthalmology. Am J Ophthalmol. 1960;49:249–261.
12 Bronson N, Fisher Y, Pickering N, et al. Ophthalmic contact B-scan ultrasonography. Westport, CT: Intercontinental; 1976.
13 Pavlin C, Foster F. Ultrasound biomicroscopy of the eye. New York: Springer; 1995.
14 Sherar M, Starkowski B, Taylor W, et al. A 100 Mhz B-scan ultrasound backscatter microscope. Ultrasound Imaging. 1989;11:95–105.
15 Pavlin C, Harasiwicz K, Sherar M, et al. Clinical use of ultrasound biomicroscopy. Ophthalmology. 1991;98:287–295.
16 Sherar M, Foster F. The design and fabrication of high frequency transducer. Ultrasound Imaging. 1989;11:75–94.
17 Pavlin C, Harasiwicz K, Foster F. Ultrasound biomicroscopy of anterior segment structures in normal and glaucomatous eyes. Am J Ophthalmol. 1992;113:381–389.
18 Atta HR. New applications in ultrasound technology. Br J Ophthalmol. 1999;83:1246–1249.
19 Iezzi R, Rosen R, Tello C, et al. Personal computer-based 3-dimensional ultrasound biomicroscopy of the anterior segment. Arch Ophthalmol. 1996;114:520–524.
20 Cusumano A, Coleman D, Silverman R, et al. Three dimensional ultrasound imaging – clinical applications. Ophthalmology. 1998;105:300–306.
21 Coleman D, Silverman R, Daly S. Advances in ophthalmic ultrasound. Radiol Clin North Am. 1998;36:1073–1082.
22 Reinstein D, Raevsky T, Coleman D. Improved system for ultrasonic imaging and biometry. J Ultrasound Med. 1997;16:117–124.
23 Silverman R, Lizzi F, Ursea B, et al. High-resolution ultrasonic imaging and characterization of the ciliary body. Invest Ophthalmol Vis Sci. 2001;42:885–894.
24 Stachs O, Martin H, Behrend D, et al. Three-dimensional ultrasound biomicroscopy, environmental and conventional scanning electron microscopy investigations of the human zonula ciliaris for numerical modelling of accommodation. Graefes Arch Clin Exp Ophthalmol. 2006;244:836–844.
25 Stachs O, Martin H, Kirchhoff A, et al. Monitoring accommodative ciliary muscle function using three-dimensional ultrasound. Graefes Arch Clin Exp Ophthalmol. 2002;240:906–912.
26 Kirchhoff A, Stachs O, Guthoff R. Three-dimensional ultrasound findings of the posterior iris region. Graefes Arch Clin Exp Ophthalmol. 2001;239:968–971.
27 Stachs O, Schneider H, Stave J, et al. Potentially accommodating intraocular lenses – an in vitro and in vivo study using three-dimensional high-frequency ultrasound. J Refract Surg. 2005;21:37–45.
28 Schneider H, Stachs O, Göbel K, et al. Changes of the accommodative amplitude and the anterior chamber depth after implantation of an accommodative intraocular lens. Graefes Arch Clin Exp Ophthalmol. 2006;244:322–329.
29 Jaffe NS. Complications of acute posterior vitreous detachment. Arch Ophthalmol. 1968;79:568–571.
30 McLeod D. (chair) Round table discussion on vitreous pathology. Doc Ophthalmol Proc. 1981;Ser:547.
31 Ossoinig KC. Advances in diagnostic ultrasound. Acta 14th Int Congr Ophthalmol. 1983:89.
32 Till P, Ossoinig KC. 10 years’ study on clinical echography in intraocular disease. Bibl Ophthalmol. 1975;83:49.
33 Machemer R, Aalberg TM. Glaskörperchirurgie, Vitrektomie. Indikationen und Technik. Berne: Huber Verlag, 1981.
34 Shapiro DR, Stone RD. Ultrasonic characteristics of retinopathy of prematurity presenting with leucokoria. Arch Ophthalmol. 1885;103:1690.
35 Darnley-Fisch DD, Frazier-Byrne S, Hughes JR, et al. Contact B-scan echography in the assessment of optic nerve cupping. Am J Ophthalmol. 1990;109:55.
36 Oksala A. Echogram in melanoma of the choroid. Br J Ophthalmol. 1959;43:408.
37 Baum G. Use of ultrasonography in the differential diagnosis of ocular tumors. In: Boniuk M, ed. Ocular and adnexal tumors. St Louis: Mosby; 1964:308.
38 Buschmann W, Trier H. Ophthalmologische Ultraschalldiagnostik, mit Atlas, Standardisierung und Einordnung in den ophthalmologischen Untersuchungsbefund. Berlin: Springer; 1989.
39 Trier H. Gewebsdifferenzierung mit Ultraschall. Bibl Ophthalmol. 1977;86:92.
40 Coleman DJ, Silverman R, Rondeau MJ, et al. Explaining the current role of high frequency ultrasound in ophthalmic diagnosis (ophthalmic ultrasound). Expert Rev Ophthalmol. 2006;1:63–76.
41 Silverman R, Kong F, Chen Y, et al. High-resolution photoacoustic imaging of ocular tissues. Ultrasound Med Biol. 2010;36:733–742.
42 Guthoff R. Ultraschall in der ophthalmologischen Diagnostik. In: Naumann GOH, Hollwich F, Gloor B. Ein Leitfaden für die Praxis. Stuttgart: Enke-Verlag, 1988.
43 Wolter J. Parallel horizontal choroidal folds secondary to an orbital tumor. Am J Ophthalmol. 1974;77:669.
44 Coleman D, Lizzi S, Jack R. Ultrasonography of the eye and orbit. Philadelphia: Lea and Febiger; 1977.
45 Bujara K, von Domarus D, Guthoff R. Necrotic malignant melanoma of the choroid with unusual clinical, echographical and histological case study. Ophthalmologica. 1980;180:222–227.
46 Guthoff R, Berger R, Helmke K, et al. Doppersonographische Befunde bei intraokulären Tumoren Forstschr Ophthalmol. 1989;86:239.
47 Bloch RS, Gartner S. The incidence of ocular metastatic carcinoma. Arch Ophthalmol. 1971;85:673.
48 Ferry AP, Font RI. Carcinoma metastatic to the eye and the orbit: a clinical pathological study of 227 cases. Arch Ophthalmol. 1974;92:276.
49 Verbeek A. Echographic findings in 36 patients with choroidal folds. Doc Ophthalmol Proc Ser. 1981:29.
50 Manschot WA. The relation between histopathological and ultrasonographyin intraocular tumors. Doc Ophthalmol Proc Ser. 1981;29:71.
51 Shields CL, Shields JA, Augsburger JJ, editors. Update on choroidal osteomas. Proceedings of the 2nd international meeting on the diagnosis and treatment of intraocular tumors; Lyons; 1987.
52 Wende S, Aulich A, Nover A, et al. Computer tomography of orbital lesions: a cooperative study of 210 cases. Neuroradiology. 1978;13:123.
53 Gass JDM, Guerry RK, Jack RI, et al. Choroidal osteoma. Arch Ophthalmol. 1978;96:428.
54 Gass JDM. New observations concerning choroidal osteomas. Int Ophthalmol. 71, 1979.
55 Wing GI, Schepenz CL, Trempe CL, et al. Serous choroidal detachment and the thickened choroidal sign detected by ultrasonography. Am J Ophthalmol. 1982;94:499.
56 Blodi FC. Ein Tuberkulom der Aderhaut, ein melanom vortäuschend. Klin Monatsbl Augenheilkd. 1977;170:845.
57 Wirold J, Orlowski-Szczypinski J. Ultrasonography of the subretinal space in rhegmatogeneous retinal detachment. Z Mod Probl Ophthalmol. 1976;18:40.
58 Cappaert W, Purnell E, Frank K. Use of B-scan ultrasound in the diagnosis of benign choroidal folds. Am J Ophthalmol. 1977;84:375.
59 Singh G, Guthoff RF, Foster S. Observations on long-term follow-up of posterior scleritis. Am J Ophthalmol. 1986;101:570.
60 Guthoff RF. Die differentialdiagnostische Bedeutung des Tenon’schen Raumes. Fortschr Ophthalmol. 1974;81:388.
61 Feldon S, Sigelman J, Albert D, et al. Clinical manifestations of brawny scleritis. Am J Ophthalmol. 1978;85:781.
62 Claudon M, Tranquart F, Evans DH, et al. Advances in ultrasound. Eur Radiol. 2002;12:7–18.