Chapter 19 Diagnostic angiography in hepatobiliary and pancreatic disease
Indications
Imaging Overview
In the 1980s and 1990s, selective angiography was commonly used as a diagnostic and staging technique. Indications would include ascertaining the organ of origin of an abdominal mass, assessing for vascular invasion by pancreatic or cholangiocarcinoma, and preoperative planning prior to hepatic resection. CT (see Chapter 16) and MRI (see Chapter 17) have completely replaced catheter angiography for these indications. In a prior edition of this book published in 2000, it was suggested that catheter angiography was a dying specialty and that angiographers should accept their fate with grace. However, a new age has dawned, and the volume of selective visceral angiography has increased considerably. The majority of these arteriograms are performed for the purpose of a diagnostic assessment prior to performing catheter-based interventions including radioembolizations (see Chapter 84A), chemoembolizations (see Chapter 83), chemoperfusions (see Chapters 86 and 89), and embolizations of bleeding arteries (see Chapter 25). In addition, endovascular therapies have replaced open surgical arterial interventions in patients with primary vascular pathology using angioplasties, stents, and the placement of stent grafts.
Arteries
Arteriographic Anatomy
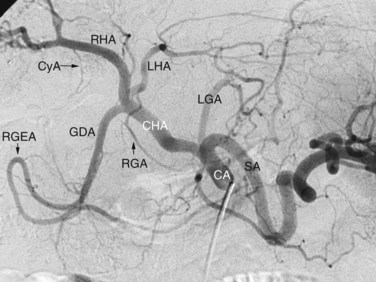
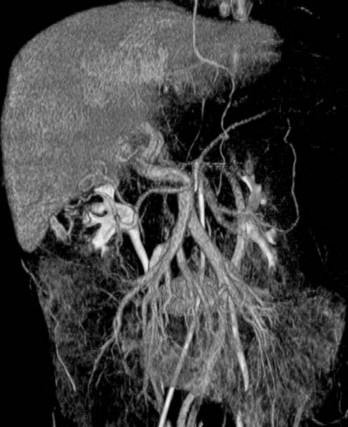
Arterial anatomy has been discussed elsewhere (see Chapter 1A, Chapter 1B ) and will only be briefly reviewed here. The most frequently encountered anatomy (Fig. 19.1) is the left gastric artery, splenic artery (SA), and common hepatic artery (HA) taking origin from the celiac axis. The common HA divides into the gastroduodenal artery (GDA) and proper HA, with the latter dividing into the right and left hepatic arteries. The right gastric artery most often originates from the base of the left HA, and the cystic artery most often originates from the right HA, but considerable variations in the origins of these arteries exist. Moreover, accessory duodenal arteries, either representing a supraduodenal or a retroduodenal artery, are frequently encountered; this is critical to recognize when planning chemoembolization and radioembolization.
The normal arterial supply to the liver is shown in Figure 19.2 (see the Extrahepatic Vasculature section in Chapter 1B), which shows the commonly recognized variations of the left hepatice artery (LHA), taking origin from the left gastric artery, and the right hepatic artery (RHA), taking origin from the superior mesenteric artery (SMA). It is important to recognize that either a part or the entirety of the right and left hepatic arteries may have these variant origins. When the entire vessel has a variant origin, it is termed replaced. If the entire trunk does not take a variant origin, the vessel is termed accessory. For example, if the right lobe is supplied by a right HA originating from the common HA, as well as a right HA taking origin from the SMA, the latter would be termed an accessory right HA. If the entire right lobe was supplied by an artery taking origin from the SMA, it would be called a replaced right HA.
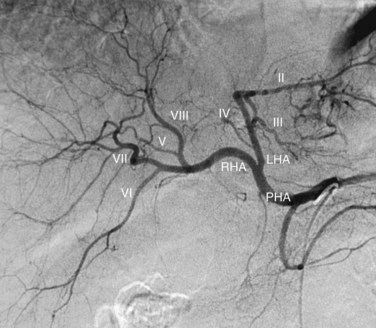
The RHA conventionally divides into an anterior (ventral) and posterior (dorsal) branch. The anterior branch usually is more vertically oriented and supplies segments V and VIII. The posterior branch is usually more horizontally oriented and supplies segments VI and VII. More than one projection is usually required to ascertain with certainty which is the anterior branch and which is the posterior branch. In the right anterior oblique projection, the anterior branch moves medially and the posterior branch moves laterally when compared with the posteroanterior (PA) projection. The entire segmental arterial supply to the liver should be accounted for prior to hepatic arterial therapy, major hepatic resection, partial hepatectomy, or living-donor liver transplantation (LDLT, see Chapter 98B).
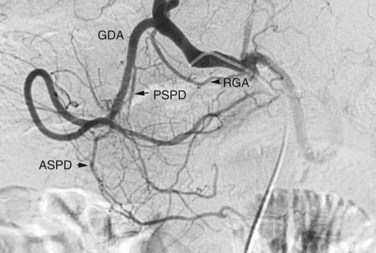
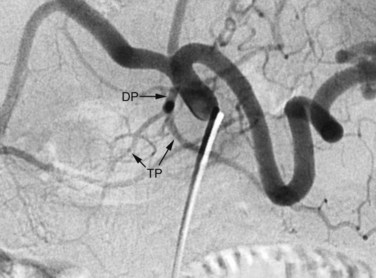
The arterial supply to the pancreas is somewhat variable. The most consistent supply is to the pancreatic head, afforded by an arcade formed by the inferior pancreaticoduodenal (IPD) branch of the SMA to the anterior superior and posterior superior pancreaticoduodenal branches of the GDA (Fig. 19.3). The transverse pancreatic artery runs along the middle portion of the long axis of the pancreas and may take origin from the arterial arcade in the head of the pancreas, directly from the GDA, or as a branch of the dorsal pancreatic artery, which variably originates from the common HA or SA (Fig. 19.4). A number of small branches from the SA supply the pancreatic body and tail, but the number and location of these arteries vary and must be identified in each individual patient when clinically relevant.
Preoperative Angiography: Historical Perspective
Prior to the advent of multidetector computed tomography angiography (MDCTA), visceral angiography was performed to assess the resectability of pancreatic and biliary tract tumors by demonstrating the presence or absence of vascular invasion. The sensitivity of catheter angiography for this purpose was low, and MDCTA has replaced catheter angiography for this indication. Using angiography for diagnosis of a hepatic mass, such as differentiating a cavernous hemangioma from a hepatocellular carcinoma, is no longer performed, as no improvement in diagnostic accuracy can be achieved over conventional cross-sectional imaging techniques (see Chapters 16 and 17).
Arteriography in Conjunction with Arterial Interventions
Accurate delineation of arterial anatomy has increased in importance remarkably as radioembolization (see Chapter 84A) and chemoembolization (see Chapter 83) have gained widespread application. The inadvertent administration of radiation or chemotherapeutic agents into arteries supplying the stomach or duodenum can lead to significant adverse outcomes. When performing procedures designed to necrose tumor, it is imperative that normal structures be spared. To accomplish this, variant anatomy must be recognized. The importance of accessory branches to the duodenum, including the supraduodenal and retroduodenal arteries, is now well known. Communications from intrahepatic branches to the lower esophagus, stomach, and diaphragm are of equal importance (Miyayama et al, 2009; Liu et al, 2005).
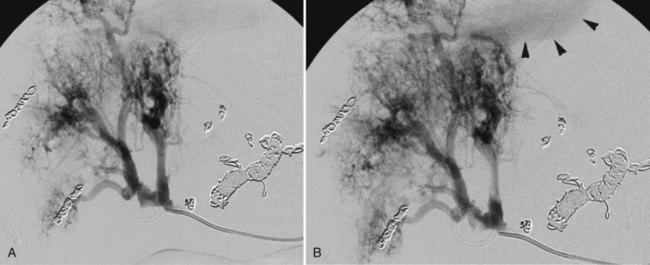
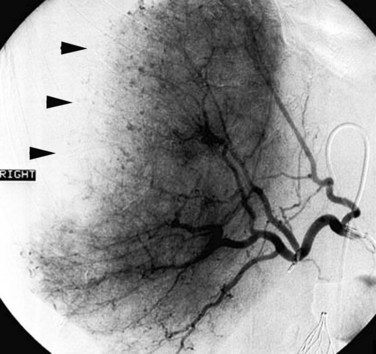
With current angiographic technology, it is possible to obtain a volumetric digital image during the arterial injection of contrast media and to display the images in a computerized tomographic format (i.e., C-arm CT) (Wallace et al, 2008). Using this technique it is possible to identify extrahepatic perfusion from aberrant hepatic arterial branches that may be unrecognized on the basis of the angiogram alone (Fig. 19.5).
Hemorrhage
Splenic Bleeding
The most common cause of bleeding from the spleen is blunt trauma, and nonoperative management is currently the standard of practice. SA embolization has been established as a method to increase the success rate of nonoperative management of traumatic splenic injuries (Dent et al, 2004). A comparative study between two cohorts consisting of 625 patients over a 15-year period revealed an improved success rate of nonoperative management from 77% to 96% with the advent of splenic embolization (Rajani et al, 2006). The indications for splenic arteriography and splenic arterial embolization are based upon CT findings and include active contrast extravasation, splenic vascular injuries, and significant hemoperitoneum. Moreover, the American Association for the Surgery of Trauma recommends angiography for grade III, IV, and V splenic injuries (Raikhlin et al, 2008).
Two techniques are used to perform splenic embolization: the first is occlusion of the proximal splenic artery with coils or Amplatzer plugs (AGA Medical, Plymouth, MN), and the second is selective small intrasplenic arterial embolization with a gelatin sponge or coils. Collaterals through the short gastric arteries and the gastroepiploic arcade usually maintain splenic viability following occlusion of the proximal splenic artery. Intrasplenic embolization generally results in a variable degree of splenic infarction, depending upon the size of the artery occluded, as intrasplenic arteries have no significant collateral routes. Both techniques are associated with a risk of complications. Minor complications including fever, pleural effusion, and partial splenic infarction have been reported in up to 62% of patients. Major complications including total splenic infarction, splenic atrophy, and postprocedure bleeding have been observed in 28.5% of patients (Wu et al, 2008).
Hepatic Bleeding
Hemorrhage from the liver may be encountered from blunt or penetrating trauma (see Chapter 102) but is most commonly due to an iatrogenic injury related to a biopsy (see Chapter 20) or percutaneous transhepatic biliary drainage (PTBD; see Chapters 18 and 28). Arteriographic findings indicating a source of hemorrhage include extravasation, pseudoaneurysm formation, and/or arteriovenous fistula (see Chapter 104). Superselective catheterization using coaxial microcatheters to deposit coil-spring emboli both distal and proximal to the area of injury is the preferred technique for embolization when a discrete bleeding site can be identified. In contrast to the spleen, a rich collateral network exists in the hepatic arterial bed, making proximal occlusion of the offending artery ineffective in many cases. For more diffuse injuries with multiple bleeding sites secondary to blunt trauma, using particulate embolization with a gelatin sponge may be a useful adjunct.
As with blunt splenic injuries, nonoperative management has become the preferred method of management in hemodynamically stable patients, with a success rate exceeding 90% (Christmas et al, 2005). In the setting of blunt trauma, hepatic arteriography may also be indicated following surgical exploration and packing of a hepatic parenchymal injury with evidence of continued bleeding or hemodynamic instability. Even patients who present with hemodynamic instability who can be successfully resuscitated can be treated effectively with transcatheter embolization (Misselbeck et al, 2009). The yield of arteriography in identifying an arterial injury amenable to embolization is higher when the CT scan suggests a vascular injury.
Pancreas
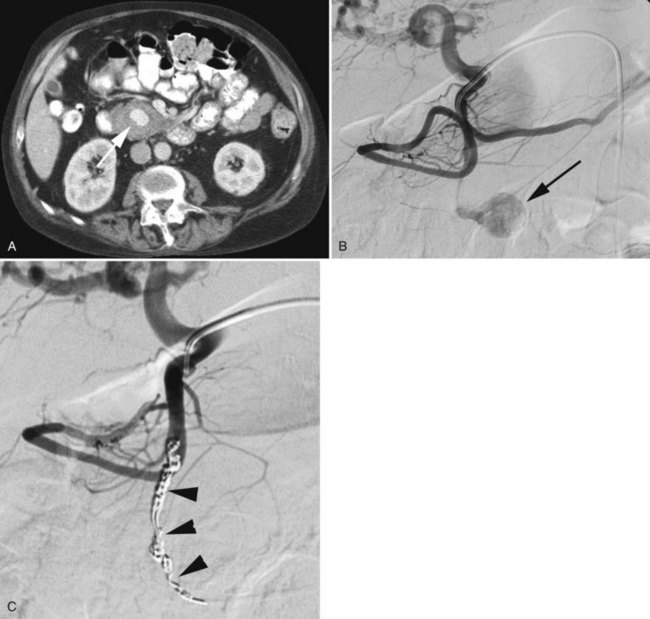
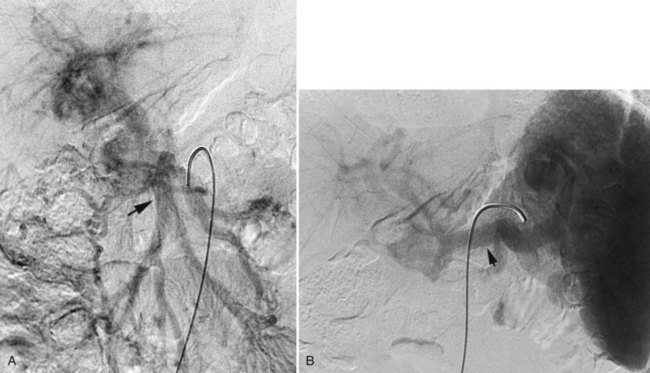
Bleeding from the pancreas is not common following trauma (see Chapter 103). Bleeding is more commonly encountered in patients with pancreatitis (see Chapter 54) or pancreatic surgery (see Chapter 62A, Chapter 62B ). Hemorrhage can occur in the retroperitoneum, or it may extend from the retroperitoneum into the peritoneal cavity or into the gastrointestinal tract via communication with the pancreatic duct (hemosuccus pancreaticus). Hemorrhage complicating acute or chronic pancreatitis is usually arterial in origin. Although this complication is encountered in only 2% to 5% of cases, it may be life threatening. Proteolytic enzymes coupled with intense inflammation can erode small arterial branches and create foci of extravasation or create small pseudoaneurysms. Pseudocysts may erode small arterial branches, leading to hemorrhage within the pseudocyst, or it may erode into larger arterial branches and create a large pseudoaneurysm (Fig. 19.6). These pseudoaneurysms are most frequently identified in the splenic artery or its branches (60% to 65%), followed by the GDA (20% to 25%), pancreaticoduodenal arteries (10% to 15%), HA (5% to 10%), and left gastric artery (2% to 5%) (Kirby et al, 2008).
MDCT has been reported to have 90% sensitivity in detecting pseudoaneurysms in patients with acute pancreatitis, however, tiny aneurysms in the intrapancreatic arteries may not be visible (Hyare et al, 2006). When a patient has clinical evidence of significant hemorrhage, or a CT scan reveals a pseudoaneurysm, arteriography followed by embolization is indicated. When the bleeding site is identified angiographically, it can be controlled with embolotherapy in up to 88% of patients (Mansueto et al, 2007).
In the patient who has bleeding following pancreatic surgery, angiography and embolization may also be useful. Significant hemorrhage was noted in 5.7% of 1669 consecutive patients following partial or total pancreatectomy (Yekebas et al, 2007). Bleeding may manifest clinically as gastrointestinal hemorrhage, retroperitoneal or intraperitoneal bleeding, or bleeding through percutaneous or surgically placed drains. When a pancreaticojejunal anastomosis has been created, disruption of the anastomosis may lead to false localization of the bleeding site. Specifically, an extraluminal bleeding site may drain into the bowel through the dehisced anastomosis, or bleeding from the bowel at the anastomosis may extend outside the lumen to cause an intraperitoneal hematoma or hemorrhage through a drain.
Angiography can be extremely useful in the diagnosis and treatment of these patients. In 25 of 43 patients undergoing angiography to diagnose and treat postpancreatectomy hemorrhage, a bleeding site was located and embolized with an 80% success rate in controlling the hemorrhage (Yekebas et al, 2007). The relatively low rate of positive angiograms may be explained by the high incidence of venous bleeding encountered in the postpancreatectomy patient. Many of these patients develop regional portal hypertension as a result of splenic or superior mesenteric venous compression or occlusion secondary either to the underlying pathology or a surgical complication. Venous extravasation is rarely identified by arteriography but may be suggested by CT. If the underlying etiology is compression of the splenic vein (SV), percutaneous transhepatic stenting of the SV may be potentially useful.
Primary Arterial Disorders
Visceral Artery Aneurysms
Aneurysms of the celiac, splenic, and hepatic arteries, and less commonly the SMA, have clinical relevance (see Chapter 104). True aneurysms are usually atherosclerotic or developmental in origin and differ from those encountered in pancreatic inflammatory disease, which are typically pseudoaneurysms. Splenic artery aneurysms in women of childbearing age are of particular concern because of their propensity to rupture during childbirth. This clinical circumstance is associated with a 75% mortality rate (Grotemeyer et al, 2009).
Aneurysms of the pancreaticoduodenal arcade are also associated with stenosis of the celiac axis, most commonly secondary to median arcuate ligament compression. In this situation, the increased flow and altered rheologic dynamics through the inferior pancreaticoduodenal arcade likely lead to aneurysm formation (Sugiyama et al, 2007).
Traditionally, visceral artery aneurysms have been managed with a variety of surgical options. More recently, endovascular therapy using stent grafts has been successful (Rossi et al, 2008). For aneurysms located in nonessential vascular beds—such as the gastroduodenal, pancreaticoduodenal, and proximal splenic arteries—deployment of coil spring emboli in the artery proximal and distal to the aneurysm origin has been successful. Using this technique, a 72% success rate in managing 22 patients with visceral artery aneurysms was recently reported (Ikeda et al, 2008).
Segmental Arterial Mediolysis
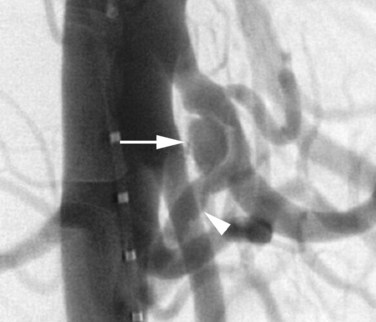
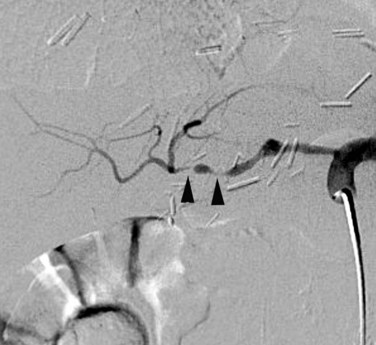
A rare vascular disorder, segmental arterial mediolysis (SAM), may also require catheter angiography for definitive diagnosis. This disorder is characterized pathologically by noninflammatory destruction of smooth muscle cytoplasm, leading to arterial dissection and aneurysm formation. Multifocal lesions with skip areas involving the superior mesenteric, hepatic, renal, and middle colic arteries in patients in their fourth to sixth decades are typical (Fig. 19.7). Clinically these lesions are prone to both occlusion by dissection as well as rupture, leading to catastrophic abdominal hemorrhage. Treatment with coil embolization within noncritical arterial beds has been reported (Davran et al, 2009).
Hereditary Hemorrhagic Telangiectasia
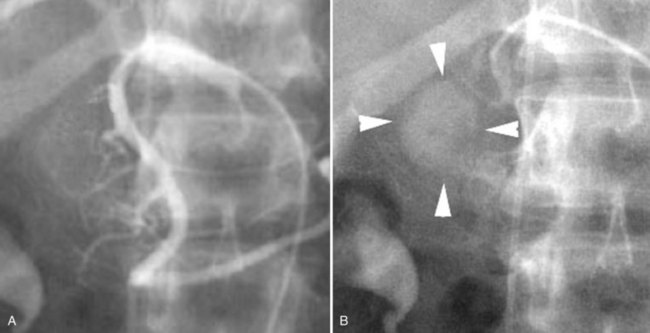
An additional condition in which diagnostic arteriography can be a useful adjunct to cross-sectional imaging is hereditary hemorrhagic telangiectasia (HHT). This disorder, also known as Osler-Weber-Rendu syndrome, is hereditary; it is transmitted as an autosomal dominant trait, characterized by telangiectasias of the skin and mucous membranes. HHT is also associated with arteriovenous malformations in the pulmonary and hepatic circulation that potentially pose a threat to life. The diagnosis can usually be established on the basis of the family history coupled with abnormalities visible on cross-sectional imaging. However, because of the variability in severity of clinical manifestations, individuals may be unaware that the condition exists in their family. The hepatic arterial malformations that shunt blood into the hepatic venous system may be initially noted on a cross-sectional imaging study, but the findings may be nonspecific. Catheter angiography can be diagnostic by depicting the malformations shunting hepatic arterial blood into the hepatic venous system (Fig. 19.8). Although this disorder has been treated with transcatheter techniques, embolotherapy has currently fallen out of vogue because of the risk of precipitating hepatic failure. OLT is curative if the patient develops high-output cardiac failure.
Peliosis Hepatis
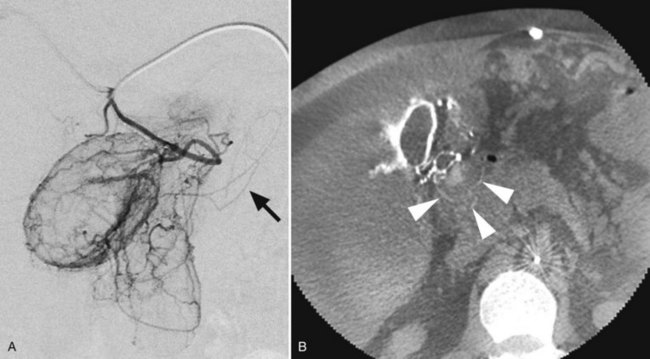
Peliosis is an abnormality of the reticuloendothelia system that is most commonly encountered in the liver (peliosis hepatis). Pathologically it is characterized by blood-filled cystic spaces that range in size from a few millimeters to almost a centimeter. This abnormality has been associated with HIV infection as well as with the use of certain drugs including immunosuppressives, antimetabolites, and oral contraceptives. Although usually benign, it has been associated with spontaneous massive hemorrhage (Choi et al, 2009) and therefore may be encountered angiographically during the investigation of hepatic bleeding. Angiographically (Fig. 19.9) the lesions are easily visible as a disorganized collection of amorphous channels not dissimilar to those observed in HHT or hemangioma; however, the lack of shunting to the hepatic venous system distinguishes it from HHT, and the absence of sharp definition with persistence into the late venous phase distinguishes it from hemangioma.
Arterial Anastomotic Stenoses
Hepatic arterial anastomotic stenoses are observed in up to 12% of patients following OLT (Bechstein et al, 1987; see Chapter 98A, Chapter 98B, Chapter 98C ). Although these stenoses are usually detected by surveillance duplex ultrasound and confirmed with MDCTA, catheter angiography is often performed to improve morphologic delineation and assess the degree of stenosis in preparation for endovascular treatment. Stenosis of the HA anastomosis usually leads to allograft dysfunction, and stenoses of the HA may also be more diffuse (Fig. 19.10). Severe stenosis may lead to hepatic artery thrombosis (HAT). The HA supplies the bile duct, and the bile duct has been rendered devoid of collateral arterial supply during donor hepatectomy. Arterial insufficiency typically leads to biliary strictures and to potentially diffuse biliary ductal infarction. HA thrombosis may lead to irretrievable allograft damage and may necessitate retransplantation. When a hepatic arterial stenosis is identified prior to advanced biliary injury, endovascular therapy with either balloon angioplasty and/or stent placement is warranted.
Localization of Functioning Pancreatic Neuroendocrine Tumors
Insulinomas
Insulinomas are the most common tumors originating from the islets of Langerhans (see Chapter 61). Because these tumors are usually 2 cm in diameter or smaller, they may not be detected by noninvasive imaging techniques. In a series of 75 surgically proven insulinomas, the sensitivities of CT and MRI were 28% and 35%, respectively (Guettier et al, 2009). This series incorporated cross-sectional imaging dating back to the late 1980s that likely reduced the overall sensitivity. When cross-sectional imaging techniques were examined after 1994, the sensitivity of CT and MRI improved to 80% and 70%, respectively, owing to improved imaging technology (Nikfarjam et al, 2008). Moreover, endoscopic ultrasound (EUS; see Chapter 14) has been used to localize insulinomas with a reported sensitivity ranging from 82% to 94%, making this technique a valuable adjunct in the management of these patients (McLean, 2004). EUS requires considerable expertise, is not available at all centers, and has reduced sensitivity for lesions in the tail of the pancreas, ranging from 50% to 60% (McLean, 2004).
Calcium stimulation arteriography was developed and described in 1991 (Doppman et al, 1991). Although unnecessary when imaging can confidently detect the offending pancreatic lesion, it is an extremely useful adjunct when the location of the lesion cannot be defined with confidence (Fig. 19.11). When 1 mL of 10% calcium gluconate is injected into an artery supplying the pancreas, tumor cells degranulate and release insulin into the portal venous circulation. Sequential blood samples obtained from the hepatic vein will demonstrate a significant rise in the concentration of insulin when the portion of the pancreas containing the tumor is injected. By sequentially injecting the arterial supplies to different regions of the pancreas, it is possible to ascertain the location of the tumor. The GDA supplies the head of the pancreas, the SMA supplies the head and uncinate process, and the SA supplies the body and tail. The origin of small branches supplying the pancreas from the SA and common HA are important to note in determining the approximate areas of pancreatic arterial supply. In the aforementioned article by Guettier and colleagues (2009), calcium stimulation arteriography was the most sensitive technique for localizing surgically proven insulinomas with an accuracy of 84%, a false-negative rate of 11%, and a false-positive rate of 4%.
Gastrinomas
The diagnosis and location of a gastrinoma can be established with a combination of somatostatin receptor scintigraphy and EUS in approximately 90% of patients. When an occult gastrinoma is encountered, angiography has been used for localization. The principles are identical to the localization of insulinomas; however, secretin has been used in addition to calcium gluconate as the stimulating agent. Sensitivities of arterial stimulation venous sampling (ASVS) in the detection of gastrinomas have ranged from 70% to 100% (Cohen et al, 1997; Turner et al, 2002).
Glucagonoma
Glucagonomas may also occur sporadically, or they may be associated with MEN type 1. These tumors are usually larger than gastrinomas or insulinomas at presentation, therefore localization can generally be achieved with noninvasive imaging or EUS. A single case of successfully using ASVS with calcium gluconate as the stimulating agent to localize an occult glucagonoma has been reported (Okauchi et al, 2009).
Veins
Venographic Technique
The most common technique to visualize the splanchnic vein is transarterial portography, in which selective splenic and superior mesenteric arteriograms are performed with delayed imaging into the venous phase, depicting the splenic and superior mesenteric veins, respectively (Fig. 19.12). When detailed visualization of the venous anatomy is required, a higher dose of contrast media can be used for the arterial injection, increasing the clarity of the venous opacification.
When an extremely detailed evaluation is required, a combination of transarterial portography during MDCT can be performed (Fig. 19.13). The increased contrast sensitivity of the MDCT coupled with multiplanar and three-dimensional reconstruction allows visualization of the veins that cannot be achieved by any other single technique. This is particularly useful in the presence of portal vein (PV) occlusion, when a complex venous reconstruction or bypass is being considered.
Direct venography of the splanchnic veins can be achieved by three routes: transjugular, transhepatic, and transsplenic. In the transjugular approach, a catheter is placed into the jugular vein and manipulated into a hepatic vein. Obtaining pressure measurements through this catheter in both the free and wedged position will yield the corrected sinusoidal pressure in patients being assessed for portal hypertension. A biopsy of the hepatic parenchyma (transjugular liver biopsy) may be performed when desirable. Injection of carbon dioxide through a catheter wedged in a hepatic vein or through a balloon catheter will often yield an image of the PV. If direct portography is warranted, one of several specially designed needles can be inserted to puncture the PV through the intervening hepatic parenchyma and through catheters advanced into the portal, splenic, or superior mesenteric venous system. This procedure is performed almost exclusively in patients undergoing a transjugular intrahepatic portosystemic shunt (TIPS), but it is also used to manage selected patients with portal and superior mesenteric vein (SMV) thrombosis (Liu et al, 2009; see Chapter 76E).
The portal venous system may also be accessed by a percutaneous transhepatic approach (Semiz-Oysu et al, 2007). After sterile preparation of the right upper quadrant, an intrahepatic portal venous radicle is punctured under real-time ultrasonographic guidance, permitting a vascular access sheath to be placed using a Seldinger technique. Standard guidewire and catheter manipulations are then used through the sheath for selectively catheterizing the SV and the SMV or its branches. Once the diagnostic images have been taken or venous interventions have been performed, the catheter is removed. The transhepatic tract is then occluded by a variety of techniques including insertion of Gelfoam pledgets, deployment of coils, or injection of fibrin glue. The transhepatic venographic procedure is usually performed as part of a direct pancreatic venous sampling, lobar portal venous embolization to stimulate contralateral hypertrophy prior to major hepatic resection, assessment and management of an anastomotic portal venous stenosis following OLT, pharmacomechanical lysis of a splanchnic vein thrombosis, or rarely to control bleeding from a splanchnic vein.
Percutaneous catheterization of the splanchnic veins is also possible from a transsplenic approach using a technique identical to that described for transhepatic catheterization (Tuite et al, 2007). This procedure is usually done in conjunction with a percutaneous procedure to assess a PV stenosis or occlusion following OLT, but it may also be used in conjunction with the assessment and treatment of a splenorenal venous bypass, when access cannot be achieved from the systemic venous circulation.
Visceral Venographic Anatomy
The SV and SMV join to form the main PV (see Chapter 1B). The inferior mesenteric vein (IMV) usually enters the SV adjacent to the confluence, but it may also enter the SMV either at or just caudal to the confluence. The coronary vein most often drains into the cephalic aspect of the main PV just beyond the confluence of the SMV and SV. The number and location of veins draining the pancreas is variable. Multiple small, unnamed veins drain directly into the SV. Typically the anterior-superior pancreaticoduodenal vein drains directly into the PV, and the posterior-superior pancreaticoduodenal vein drains into the SMV. The inferior pancreaticoduodenal veins drain into the SMV at the caudal margin of the pancreas, and the PV courses obliquely cephalad from near the midline toward the liver, where it divides to supply the right and left lobes. This division, as well as the division into segmental branches, is variable and must be delineated when clinically relevant for each individual patient.
Preoperative Planning for Portal Decompression
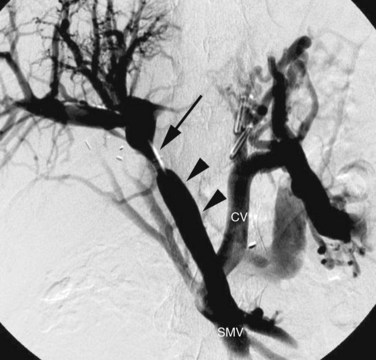
An alternative procedure to portosystemic shunting in selected patients with symptomatic portal hypertension secondary to extrahepatic portal venous obstruction is the Rex shunt (Vanderlan et al, 2009). This procedure involves a venous bypass from the superior mesenteric vein to the umbilical segment of the left PV, the latter being accessed through the fossa of Rex (Fig. 19.14). When successful, this type of bypass avoids the complication of encephalopathy, as all of the splanchnic blood drains through the liver. Careful preoperative imaging is needed prior to this procedure to confirm the patency and demonstrate the size of the umbilical segment of the left PV. Arterial portography coupled with MDCT is ideal for this indication. When insufficient, direct transhepatic portography may be necessary.
Bechstein WO, et al. Surgical complications in 200 consecutive liver transplants. Transplant Proc. 1987;19(5):3830-3831.
Choi SK, et al. Spontaneous liver rupture in a patient with peliosis hepatis: a case report. World J Gastroenterol. 2009;15(43):5493-5497.
Christmas AW, et al. Selective management of blunt hepatic injuries including non-operative management is a safe and effective strategy. Surgery. 2005;138:606-610.
Cohen MS, et al. Prospective study of provocative angiograms to localize functional islet cell tumors of the pancreas. Surgery. 1997;122(6):1091-1100.
Davran RC, et al. Radiological findings and endovascular management of three cases with segmental arterial mediolysis. Cardiovasc Intervent Radiol. 2009;33(3):601-606.
Dent DA, et al. Blunt splenic injuries: high nonoperative management rate can be achieved with selective embolization. J Trauma. 2004;56:1063-1067.
Doppman JL, et al. Insulinomas: localization with selective intraarterial injection of calcium. Radiology. 1991;178(1):237-241.
Grotemeyer DD, et al. Visceral artery aneurysms: follow-up of 23 patients with 31 aneurysms after surgical or interventional therapy. Langenbuchs Arch Surg. 2009;394:1093-1100.
Guettier JM, et al. Localization of insulinomas to regions of the pancreas by intraarterial calcium stimulation: the NIH experience. J Clin Endocrinol Metab. 2009;94(4):1074-1080.
Hyare H, et al. Multi-section CT angiography compared with digital subtraction angiography in diagnosing major arterial hemorrhage in inflammatory pancreatic disease. Eur J Radiol. 2006;59(2):295-300.
Ikeda O, et al. Nonoperative management of unruptured visceral artery aneurysms: treatment by transcatheter coil embolization. J Vasc Surg. 2008;47(6):1212-1219.
Kirby JV, et al. Vascular complications of pancreatitis: imaging and intervention. Cardiovasc Intervent Radiol. 2008;31:957-970.
Liu DS, et al. Angiographic considerations in patients undergoing liver-directed therapy. J Vasc Interv Radiol. 2005;16:911-935.
Liu FW, et al. Interventional treatment for symptomatic acute–subacute portal and superior mesenteric thrombosis. World J Gastroenterol. 2009;15:5028-5034.
Mansueto G, et al. Endovascular treatment of arterial bleeding in patients with pancreatitis. Pancreatology. 2007;7(4):360-369.
McLean A. Endoscopic ultrasound in the detection of pancreatic islet cell tumors. Cancer Imaging. 2004;4:84-91.
Misselbeck TS, et al. Hepatic angioembolization in trauma patients: indications and complications. J Trauma. 2009;67(4):769-773.
Miyayama SY, et al. Anastomosis between the hepatic artery and the extrahepatic collateral or between extrahepatic collaterals: observation on angiography. J Med Imaging Radiat Oncol. 2009;53:271-282.
Nikfarjam MW, et al. Improved contemporary surgical management of insulinomas: a 25-year experience at the Massachusetts General Hospital. Ann Surg. 2008;247:165-172.
Okauchi Y, et al. Glucagonoma diagnosed by arterial stimulation and venous sampling (ASVS). Intern Med. 2009;48(12):1025-1030.
Raikhlin AB, et al. Imaging and transcatheter arterial embolization for traumatic splenic injuries: review of the literature. Can J Surg. 2008;51:464-472.
Rajani RR, et al. Improved outcome of adult blunt splenic injury: a cohort analysis. Surgery. 2006;140(4):625-631. discussion 631-632
Rossi M, et al. Endovascular exclusion of visceral artery aneurysms with stent-grafts: technique and long-term follow-up. Cardiovasc Intervent Radiol. 2008;31(1):36-42.
Semiz-Oysu A, et al. Interventional radiological management of prehepatic obstruction of the splanchnic venous system. Cardiovasc Intervent Radiol. 2007;30(4):688-695.
Sugiyama KT, et al. Analysis of five cases of splanchnic artery aneurysm associated with celiac artery stenosis due to compression by the median arcuate ligament. Clin Radiol. 2007;62:688-693.
Tuite DJ, et al. Percutaneous transsplenic access in the management of bleeding varices from chronic portal vein thrombosis. J Vasc Interv Radiol. 2007;18(12):1571-1575.
Turner JJ, et al. Localization of gastrinomas by selective intra-arterial calcium injection. Clin Endocrinol (Oxf). 2002;57(6):821-825.
Vanderlan WB, et al. Adult portal hypertension secondary to posttraumatic extrahepatic portal vein thrombosis treated with Rex shunt. J Trauma. 2009;66(1):260-263.
Wallace MK, et al. Three-dimensional C-arm cone-beam CT: applications in the interventional suite, for the Technology Assessment Committee of the Society of Interventional Radiology. J Vasc Interv Radiol. 2008;19:799-813.
Wu SC, et al. Complications associated with embolization in the treatment of blunt splenic injury. World J Surg. 2008;32(3):476-482.
Yekebas EF, et al. Postpancreatectomy hemorrhage: diagnosis and treatment—an analysis in 1669 consecutive pancreatic resections. Ann Surg. 2007;246(2):269-280.