Diagnosis and Treatment Of Thyroid Disease During Pregnancy
Background and Historical Perspective
Thyroid diseases occur commonly in women of reproductive age and are well-described complications of reproductive dysfunction, pregnancy, and the puerperium.1 Historically, in Egyptian and Roman times, an enlarging thyroid gland was viewed as a positive sign of pregnancy in younger women,2 but it remains controversial whether significant goiter is an acceptable physiologic accompaniment of pregnancy. Regardless, increasing numbers of women are referred to physicians for clinical evaluation of thyroid illness during pregnancy. The spectrum of thyroid disease in pregnancy is similar to that in the normal female population (Table 25-1), although the prevalence of thyroid disease is likely higher in this subpopulation because many cases of thyrotoxicosis can be attributed to the physiologic, thyroid-stimulating effects of human chorionic gonadotropin (hCG) or related molecules.3 Furthermore, an estimated 1 in 20 women experience postpartum thyroiditis (PPT).4 The clinical manifestations of thyroid disease overlap those of normal pregnancy, and results of traditional tests of thyroid and metabolic status may be abnormal because of pregnancy itself.5,6 Fetal considerations influence thyroid diagnostic protocols and therapeutic options for women of reproductive age or those currently pregnant.7,61 Fortunately, improved assays for thyroid-stimulating hormone (TSH)7,8,117 have recently permitted better assessment of thyroid status in pregnancy. The availability of effective therapy, in conjunction with close clinical follow-up and monitoring, generally ensures a safe pregnancy for the mother and can reduce the fetal morbidity and mortality caused by spontaneous abortion, intrauterine growth retardation, stillbirth, and neonatal death.7
Table 25-1
Thyroid Disorders in Pregnancy
Hyperthyroidism
Subacute (painful) thyroiditis
Lymphocytic (painless) thyroiditis
Gestational trophoblastic neoplasia
Pregnancy itself may be viewed as a clinically euthyroid state amid the complex changes in endocrine and cardiovascular physiology that characterize gestation.9,10 Pregnancy can have a favorable effect on the course of maternal autoimmune thyroid disorders, although the tendency is for exacerbation postpartum.11,74 This favorable effect is due to generalized suppression of humoral and cell-mediated immunity during gestation, which is itself an example of a successful allograft bearing a complement of maternal antigens.12,13 The loss of immune suppression with delivery often results in a rebound during the postpartum period.
Thyroid Disease and Reproductive Health
Thyroid disease has been implicated in several reproductive disorders, including menstrual abnormalities, infertility, hyperprolactinemia, and pregnancy wastage.1 Whether reproductive status has an effect on the risk of thyroid disease for women is not clear.
Hypothyroidism
Effects on Menstruation, Fertility, and Maternal Health
Overt hypothyroidism may be accompanied by oligomenorrhea and anovulation and is sometimes associated with elevated concentrations of prolactin (PRL), galactorrhea, or an enlarged sella turcica.14 Increased production of thyrotropin-releasing hormone (TRH) is often responsible for elevations in thyroid-stimulating hormone (TSH) and PRL. Another proposed mechanism is a defect in hypothalamic dopamine turnover, which would also explain the observation of increased luteinizing hormone levels.15–17 Ovulatory defects, increased PRL, and galactorrhea are thought to be reversible with thyroxine replacement therapy in most cases. Mild hypothyroidism may be associated with menorrhagia.18 Anovulatory cycles and luteal phase dysfunction contribute to infertility and may accompany mild or subclinical hypothyroidism.19,20 Subclinical hypothyroidism may also be associated with slight elevations in serum PRL.21 Studies in infertile women with mild hypothyroidism have also found increased PRL responses to metoclopramide challenge.19,22 Contrary to earlier reports,19 treatment of latent hyperprolactinemia in hypothyroidism with dopamine agonists is not effective in improving pregnancy rates.22 At present, normalization of thyroid function remains the recommended therapeutic intervention. An ovarian hyperstimulation syndrome (multiple giant follicular cysts) with normal PRL and gonadotropin levels has been reported in a patient with primary hypothyroidism23; thyroxine therapy resulted in cyst involution. Specific to maternal health, a study investigating the effects of subclinical and overt hypothyroidism on maternal health documented a two- to threefold increase in gestational hypertension (eclampsia, preeclampsia, and pregnancy-induced hypertension) in affected women compared with euthyroid controls.24
Effects on Miscarriage and Pregnancy
In some patients, recurrent abortions have been attributed to the presence of hypothyroidism.25 Up to a fourfold increase in fetal death has been reported with overt hypothyroidism compared with control women.26 Lower serum total thyroxine (T4) levels may be due to a fall in thyroxine-binding globulin (TBG) associated with declining estrogen levels in a nonviable pregnancy rather than to the presence of hypothyroidism.7 Adequate thyroxine replacement for women with mild or overt hypothyroidism in early pregnancy results in term deliveries in more than 90%. However, failure to achieve a normal serum TSH level during pregnancy has been reported to be associated with term deliveries in only 20% of women.27 These and other studies suggest that thyroxine replacement therapy increases the chance of a successful pregnancy outcome even when known thyroid dysfunction is present. Finally, several studies have associated an increased risk of pregnancy complications with maternal hypothyroidism, even if delivery of a live infant occurs. Casey and colleagues investigated a cohort of 17,298 pregnant women, detecting a 2.3% overall prevalence of subclinical or overt hypothyroidism. Placental abruption was approximately three times more likely in women with subclinical hypothyroidism compared with controls. Preterm birth (delivery before 34 weeks’ gestation) was almost twice as likely in the women with subclinical hypothyroidism. Although not all parameters differed between groups, these findings suggest a subtle but important adverse effect on maternal and fetal health resulting from even mild thyroid dysfunction.28
Recent studies have reported a doubling of the spontaneous miscarriage rate early in gestation among women who have serum antithyroid antibodies (either anti–thyroid peroxidase [TPO] or antithyroglobulin) detected in the first trimester.29–32 Most of these antibody-positive women who miscarry have normal thyroid function. Furthermore, the presence of antithyroid antibodies in the first trimester is not correlated with that of anticardiolipin antibodies, which are known to be associated with pregnancy loss. The mechanism linking thyroid autoimmunity and miscarriage is not known. It may be a marker for more generalized activation of the immune system or for subtle changes in maternal/fetal thyroid metabolism. In addition, among women undergoing in vitro fertilization, although the presence of antithyroid antibodies does not alter pregnancy rate, the miscarriage rate is significantly higher, as reported in a study by Poppe and colleagues.33 Separately, a report investigating more than 1500 Pakistani women also reported a threefold higher rate of preterm delivery in women with known antithyroid antibodies compared with antibody-negative women.34 The authors speculate that the high levels of preterm delivery in antibody-positive women may contribute to a high rate of low birth rate nationally. A recent meta-analysis on this subject confirms a consistent pattern of higher rates of preterm delivery in women who have serum antithyroid antibodies.35
Most recently, a randomized, prospective study has suggested that levothyroxine replacement in women with serum thyroid antibodies can reduce the risk of both miscarriage and premature deliveries.36 The authors prospectively treated a cohort of 57 TPO antibody–positive women with a low dose of levothyroxine beginning in the first trimester of pregnancy. Adverse events were compared with a cohort of 58 antibody-positive women who were not treated, as well as with 869 antibody-negative women. Results confirmed that TPO antibody–positive women who received levothyroxine therapy were about four times less likely to miscarry, and overall risk approximated that of the antibody-negative cohort. Similar reductions in preterm delivery were also documented. Confirmation of these data is awaited; however, the prospective, randomized nature of this trial suggests that adoption of such an intervention is reasonable at this time. In extrapolating from these data, it can be seen that most thyroid antibody–positive pregnant women (if euthyroid or subclinically hypothyroid) are reasonable candidates for 25 to 50 mcg of levothyroxine administration daily. Such therapy may also be reasonable for thyroid antibody–positive women with repeated miscarriages, although the benefit is less clear for those with persistent infertility.
Premenstrual Syndrome
It had been suggested that mild hypothyroidism, defined by isolated elevation of serum TSH levels or even an exaggerated TSH response to TRH, is associated with premenstrual syndrome (PMS) in a significant proportion of cases.37,38 This finding was not confirmed in a prospective study of patients with PMS and age-matched controls.39 There now would seem to be little basis for associating PMS with thyroid dysfunction or for recommending thyroxine replacement therapy in this condition.
Thyrotoxicosis
Mild to moderate thyrotoxicosis does not necessarily affect fertility.7 Such thyrotoxic women remain ovulatory and have a normal chance of becoming pregnant. Severe thyrotoxicosis, however, may be accompanied by oligoamenorrhea or amenorrhea.1 The exact mechanism is not known. Hyperthyroidism is a hyperestrogenic state, in part caused by increased conversion of weak androgens to estrogen.40 Gonadotropin levels may be elevated with loss of the midcycle luteinizing hormone surge41 yet may remain responsive to exogenous gonadotropin-releasing hormone (GnRH).42 Nutritional, weight, and psychological changes in thyrotoxicosis may also contribute to menstrual dysfunction.43 Recent data suggest that only severe thyrotoxicosis is likely to be associated with an increased risk of spontaneous abortion.44 Studies of this complex subject are often confounded, as women with thyrotoxicosis in early pregnancy are usually already treated, and adequate control data for untreated thyrotoxicosis during gestation are lacking. The appearance of biochemical hyperthyroidism can be mimicked by the physiologic effects of maternal hCG upon the thyroid. Differentiation between the physiologic effects of hCG and pathologic hyperthyroid illness can be difficult. Adequate treatment of thyrotoxicosis should restore fertility and menstruation and reduce early pregnancy wastage.
Reproductive Status and Thyroid Disease Risk
Epidemiologic studies of thyroid disease, including autoimmune thyroid disease, nodular thyroid disease, and thyroid carcinoma, indicate a high prevalence among women, typically those in their late-reproductive or postreproductive years.45,46 This high prevalence may suggest possible influences of sex hormones on the development of thyroid disease. Experimentally, autoimmune thyroiditis in rats and chickens is modulated by exposure to estrogens and androgens, with androgens having a protective effect,47 Estrogen exposure leads to a reduction in suppressor/cytotoxic T cells that may permit an increase in autoantibody synthesis.48 A case-control study of 89 patients with autoimmune thyroiditis (Hashimoto’s disease) found no association of thyroiditis with parity.49 However, a longer reproductive span (early menarche and/or late menopause) was associated with a twofold to threefold increased relative risk of euthyroid or hypothyroid Hashimoto’s disease. A prospective study in pregnancy from an area of marginally low iodine intake reported that a greater number of pregnancies and increased parity were associated with an increased prevalence of nodular thyroid disease and goiter in women with thyroid autoimmunity, or in women with a past history of thyroid disease, compared with controls.50 These changes were independent of maternal age, biochemical thyroid status, or evidence of thyroid autoimmunity. Iodide levels in the population may have played a role.
Maternal and Fetal Thyroid Physiology in Pregnancy
Basal Metabolic Rate
The basal metabolic rate increases from 15% to 20% between 4 and 8 months’ gestation.5 Most of this increase is due to oxygen consumption by the fetoplacental unit; the balance is accounted for by changes in cardiovascular physiology that accompany pregnancy. Difficulty distinguishing the true basal metabolic rate, which could be a useful indicator of thyroid function status, from total metabolism in the setting of pregnancy mitigates against its use for diagnosis or measurement of therapeutic response to treatment.
Maternal Iodine Economy
Glomerular filtration rates increase by 50% in pregnancy, resulting in a sustained increase in iodide clearance.5 Reduced tubular reabsorption of iodide by the kidneys may also contribute to increased renal clearance.51 Plasma inorganic iodide levels may fall as a result. Similar changes in renal iodide clearance have been observed in women treated postpartum with diethylstilbestrol.51 Additionally, iodide readily crosses the placenta with a reported fetal-maternal gradient of 5:1, suggesting an active transport process.52 Iodide accumulates in the fetal thyroid primarily after 90 days’ gestation. Lactation is another source of iodide loss in the mother.53 This iodide loss during pregnancy has implications for maternal and fetal thyroid hormone economy in view of the major problems still encountered with endemic iodine deficiency disorders on a global basis.54 In many geographic areas outside North America, iodide intake is marginal, that is, average intake is less than 100 µg/day.50,51,55 Goiter is unlikely to develop unless plasma inorganic iodide levels are less than 0.08 µg/dL.56 Levels are considerably higher in North America, with no differences in iodide balance reported in pregnant versus nonpregnant women.57 Although such studies are now contraindicated, previous measurements of thyroid radioiodine uptake have shown increases in pregnancy that depend on changes in plasma inorganic iodide and thyroid-stimulating activity.5,58,59 These studies used 123I. In some cases, 131I treatment was inadvertently given to thyrotoxic pregnant women.
Therefore, to compensate for this increased iodine loss during pregnancy, increased daily intake of iodine is required during pregnancy. Initial recommendations favored daily iodine intake of at least 150 µg/day.60 More recently, expert consensus favors maternal daily iodine intake of 250 mcg/day or more.61 The World Health Organization has also adopted this recommendation. This supplementation should continue throughout pregnancy, as well as during lactation. The most recent National Health and Nutrition Examination Survey in the United States reported a substantial increase in the number of women with a low urinary iodine excretion (<50 µm per gram of creatinine) over the last 20 years (from <1% to 5%).62 In this survey, very low urine iodine concentrations were documented in nearly 7% of pregnant women and in nearly 15% of women of child-bearing age. A recent survey conducted by one of the authors found that about 50% of prescription prenatal vitamins may not contain iodine, although recent campaigns have lobbied drug makers to address this shortcoming. Together, the above data support a recommendation that aggressive assessment of iodine nutritional status should be performed on every woman of child-bearing age (or actively pregnant). Physicians should have a low threshold for recommending iodine supplementation as needed to reach the goal of 250 mcg/day.
Serum Thyroid Hormones and Protein Binding
Circulating TBG concentrations double in pregnancy as a result of estrogen stimulation of hepatic production63 and reduction in clearance of TBG secondary to sialylation.64 Transthyretin (prealbumin) and albumin levels are reduced. As a result, total serum thyroxine (T4), triiodothyronine (T3), and reverse T3 levels are frankly elevated in pregnancy because of increased hormone-TBG binding65–67; binding to transthyretin and albumin is paradoxically reduced. The increase in total T4 during gestation is predictable, with a suggested adjustment of the nonpregnant reference range by a factor of 1.5.68 Indirect estimates of free thyroid hormone status using the resin T3 uptake test may be reduced as the result of increased TBG, and the free thyroxine (FT4) index calculated from the resin T3 uptake test and total serum T4 generally remains within normal limits. However, this technique does not yield a particularly accurate estimate of free hormone status when TBG concentrations are greatly increased.69 Women with congenital TBG deficiency show little TBG rise or change in serum T4 in pregnancy.70 Hypothyroid patients receiving low-dose replacement therapy fail to increase protein-bound iodine (T4) after estrogen therapy even though T4-binding capacity, or TBG, is increased. This finding indicates that an increase in T4 production is required during normal pregnancy, along with increased binding capacity.
It is now well recognized that many hypothyroid women require an increase of 25% to 40% in thyroxine dosage during pregnancy.71,83 A prospective study of 19 women demonstrated that thyroid hormone demand increases early in the first half of pregnancy, climbs through 20 weeks’ gestation, and plateaus thereafter. If mothers do not have adequate endogenous thyroid function, the increased hormone demand of pregnancy will induce a hypothyroid state in most individuals unless their levothyroxine dose is increased. Separate retrospective analysis supports this conclusion. Serum thyrotropin has long been assumed to be the major stimulus for the necessary increase in thyroxine production from the thyroid. However, recent data suggest the importance of hCG in this physiologic process as well.72 Serum thyroglobulin levels also increase during pregnancy and in some studies returned to normal by 6 weeks’ postpartum.50,73 In euthyroid women with no known thyroid dysfunction, early studies of free thyroid hormone concentrations during pregnancy suggest that they remain within normal limits.66,74 Results from longitudinal studies reveal significant fluctuations in free thyroid hormone levels throughout pregnancy, although these concentrations also generally remain within normal reference limits.8,73–75 FT4 and FT3 levels may be slightly increased in the first trimester at between 6 and 12 weeks and may fall progressively throughout gestation, often to levels below the nonpregnant assay-specific reference ranges; TBG saturation is reduced (Figs. 25-1, 25-2). This pattern is consistent regardless of the FT4 assay method used (dialysis, ultrafiltration, gel filtration and adsorption, or free hormone immunoassay).8,76,79 Thus, reductions in free thyroid hormone levels in late pregnancy seem to be a real phenomenon that cannot be accounted for by changes in serum albumin, nonesterified fatty acids, or TBG. The physiologic relevance of these observations is unclear, especially for patients with no evidence of thyroid pathology.
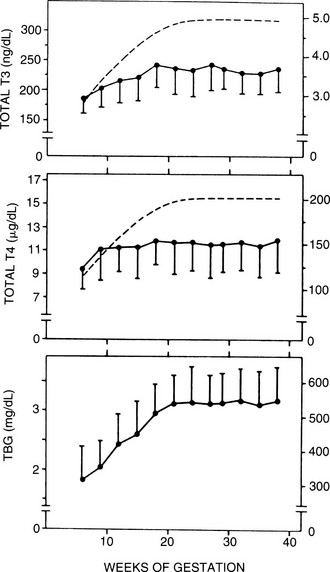
FIGURE 25-1 Serum thyroxine (T4), triiodothyronine (T3), and thyroxine-binding globulin (TBG) as a function of gestational age. Each point gives the mean value (±1 standard deviation [SD]) of determinations performed at the initial evaluation, pooled for 3 weeks, between 5 and 28 weeks (n = 510), and again for samples obtained between 28 and 39 weeks (n = 355). The latter samples include both late initial evaluations and the second series of determinations at 30 to 33 weeks. Each point represents an average of 72 individual determinations. The dashed lines illustrate the theoretical curves of T3 and T4 concentrations required to yield the average molar ratios of T4/TBG and T3/TBG that correspond to nonpregnant reference subjects (0.37 for T4/TBG and 0.0089 for T3/TBG with a molecular weight of 57 kDa for TBG). (Data from Glinoer D, De Nayer P, Bourdoux P, et al: Regulation of maternal thyroid during pregnancy, J Clin Endocrinol Metab 71:276. ©1990 by The Endocrine Society.)
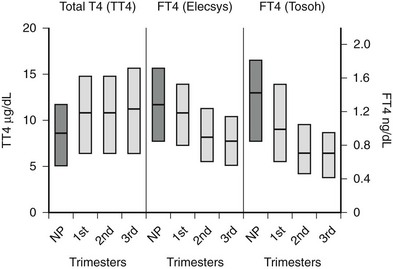
FIGURE 25-2 Serum total thyroxine (TT4) and free thyroxine (FT4) levels by trimester. Interquartile ranges are shown by the shaded boxes, with the median value indicated by the line. Serum TT4 levels rise to approximately 1.5 times the normal nonpregnant reference range. Although serum FT4 ranges were method dependent, as shown by differences in measurement by the Elecsys system, Roche Diagnostic and Tosoh, Tosoh Corporation methods, both methods show a consistent decrease in FT4 as pregnancy progresses. (NP, Nonpregnant [n = 62]; 1st, first trimester [n = 105]; 2nd, second trimester [n = 39]; 3rd, third trimester [n = 64].)
(Data courtesy of Carole Spencer, PhD.) (From Chan GW, Mandel SJ: Therapy insight: management of Graves’ disease during pregnancy, Nat Clin Pract Endocrinol Metab 3:470. © 2007.)
In pregnant women, the T3/T4 ratio is increased in the third trimester. Increased binding of T4 and T3 to monocyte nuclear receptors has also been reported in human pregnancy.77 Unfortunately, except for the equilibrium dialysis FT4 assay, none of the other commercial assays has reported trimester-specific and method-specific FT4 reference ranges during pregnancy. The commercially available automated FT4 assays that use two-step or labeled antibody methods are protein sensitive and therefore are affected by pregnancy-induced changes in serum albumin or TBG.78,79 Consequently, no universal absolute FT4 value can be used to define a low serum FT4 level across methods. It has been suggested that the normal range for the serum total T4 level during pregnancy is 1.5 times the nonpregnant reference range.68 Until validated pregnancy reference ranges are available for serum FT4 assays, the serum total T4 level (adjusted for protein binding) may be more reliable for use during pregnancy.
Because serum free thyroid hormone measurements are difficult to assess during pregnancy, serum TSH measurements remain the best assessment of a pregnant woman’s thyroid status. However, population-specific normal ranges for serum TSH are derived primarily from healthy, nonpregnant individuals. Recently, some have advocated “trimester-specific” reference ranges for TSH.80,81,117 These data derive from analysis of serum TSH in healthy euthyroid women who then are assessed during pregnancy (Fig. 25-3). Serum TSH values in the first trimester range much lower than would be expected in a nonpregnant individual, generally between 0.03 mIU/L and 2.5 mIU/L. In the second and third trimesters, greater variance is seen, although the lower limit of “normal” remains below what would be expected for nonpregnant individuals.80 Debate on how these data should be translated into clinical practice is ongoing. Regardless, these data suggest that, compared with nonpregnant reference ranges, mildly suppressed TSH in a pregnant woman should be viewed as safe, and perhaps physiologically normal.
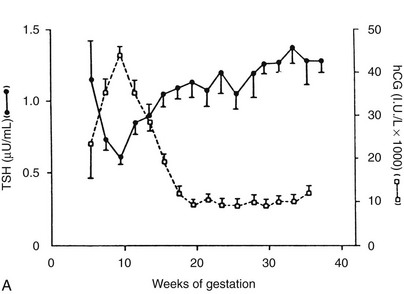
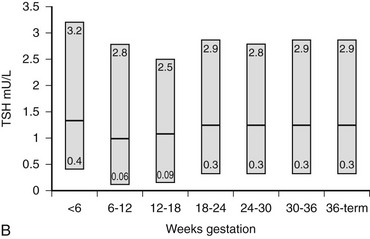
FIGURE 25-3 Serum thyroid-stimulating hormone (TSH) and human chorionic gonadotropin (hCG) as a function of gestational age. A, Serum hCG was determined at the initial evaluation and TSH at the initial evaluation and during late gestation. The symbols give the mean value (±standard error [SE]) for samples pooled for 2 weeks’ gestation. Each point corresponds to an average of 33 determinations for hCG and 49 for TSH.
(From Glinoer D, De Nayer P, Bourdoux P, et al: Regulation of maternal thyroid during pregnancy, J Clin Endocrinol Metab 71:276. © 1990 by The Endocrine Society.)
B, Gestational age–specific serum TSH concentrations in women without thyroid autoimmunity. The shaded areas represent the 2.5th to the 97.5th percentile values, with the median value indicated by the line. (Data graphed from Stricker R, Echenard M, Eberhart R, et al: Evaluation of maternal thyroid function during pregnancy: the importance of using gestational age-specific reference intervals, Eur J Endocrinol 157:509, 2007.)
Thyroid Stimulation and Regulation
The histologic picture of the thyroid gland during pregnancy is one of active stimulation. Columnar epithelium can be seen lining hyperplastic follicles.82 The increase in maternal T4 production that occurs during normal gestation is most evident from clinical observations of thyroxine-replaced hypothyroid women who require a 25% to 40% dosage increase to maintain normal serum TSH levels in pregnancy.71,83 Furthermore, findings of relative hypothyroxinemia and slightly increased serum TSH levels during pregnancy in women from areas of borderline iodine sufficiency (<100 mcg/day) support the view that pregnancy constitutes a stress for the maternal thyroid by stimulating thyroid hormone production.84
Several factors are known to tax gravid thyroid economy, and each may have relative importance at a different time in gestation. In early pregnancy, the serum concentration of TBG increases rapidly and more thyroid hormone may be needed to saturate binding sites. Glomerular filtration rate increases, resulting in increased iodide clearance. Later, with placental growth, metabolism of T4 to its inactive metabolite reverse T3 is increased by the high levels of placental type III deiodinase.85 In addition, transplacental passage of maternal T4 may occur.86 Finally, alterations in the volume of distribution of thyroid hormone may occur because of both gravid physiology and the fetal/placental unit.
Human Chorionic Gonadotropin
Serum hCG has thyromimetic effects and is responsible for the hyperthyroidism associated with trophoblastic disease.87–94 In normal pregnancy, hCG is a physiologic regulator of thyroid function early in gestation.73,74,95–100 Clinically, hCG levels peak in pregnancy at 50 to 100 times 100,000-200,000 IU/L at between 9 and 14 weeks; this peak correlates with reduced TSH levels in the first trimester73,98 (see Fig. 25-3). Levels decline thereafter and are undetectable by a few weeks postpartum. An overall increase in thyromimetic activity in the sera of women during early pregnancy may be due to hCG, as determined by immunoadsorption studies using hCG monoclonal antibodies.99,100
Ekins and colleagues have effectively argued that an alternative control system such as hCG may regulate maternal thyroid activity in early pregnancy, when the most important changes in TBG and T4 secretion occur, to ensure an adequate supply of thyroid hormones to the placenta and embryo (Fig. 25-4).98,101,102 Experimentally, in vitro studies show that hCG binds to the TSH receptor (TSHR), as assessed by radioreceptor assays using porcine and human thyroid membranes incubated with 125I-TSH,103–106 stimulates adenylate cyclase activity and cyclic adenosine monophosphate (cAMP) generation, and enhances T3 secretion in human and porcine thyroid slices.107 More recently, hCG has been shown to stimulate growth, iodide uptake, and cAMP generation in the rat thyroid cell line FRTL5.99,100,108–110 Species differences111,112 and microheterogeneity of hCG molecules through pregnancy and in gestational trophoblastic diseases may account for the variable thyrotropic activities reported.98–100,106–108,113–115 However, with reported TSH bioactivity of up to 0.7 µU/U hCG,106,108 the hCG levels obtained in early pregnancy could produce a noticeable thyrotropic effect, and it has been reported that up to 9% of pregnant women may have an isolated subnormal serum TSH level in the first trimester.116 A recent study reported greater susceptibility to hCG-associated suppression of serum TSH in pregnant women whose serum TSH levels were in the lowest 25th percentile in early pregnancy.72 Overall, maternal serum TSH concentrations decrease in the first half of pregnancy compared with the nonpregnant state and remain lower until term (see Fig. 25-3B).117 Consequently, in healthy pregnant women at between 6 and 18 weeks’ gestation, the lower limit of the 95% confidence interval for serum TSH levels is between 0.03 and 0.09 mIU/L, which then rises to 0.3 mIU/L as pregnancy progresses.80,117
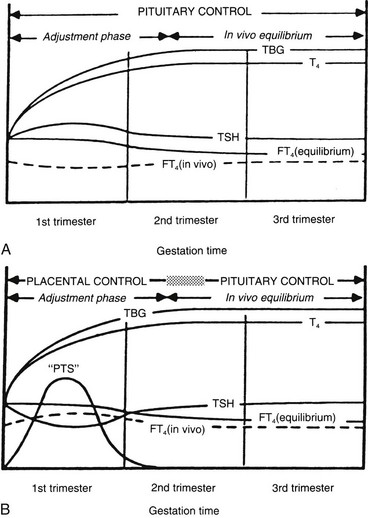
FIGURE 25-4 A, Conventional model of maternal thyroid gland control throughout pregnancy if based on the traditional hypothalamic-pituitary feedback mechanism. B, Hypothetical model of maternal thyroid gland control throughout pregnancy if a putative “placental thyroid stimulator” (PTS), possibly human chorionic gonadotropin, assumes regulatory control over maternal thyroid secretion. FT4, Free T4; T4, thyroxine; TBG, thyroxine-binding globulin; TSH, thyroid-stimulating hormone. (From Ballabio M, Poshyachinda M, Ekins RP: Pregnancy-induced changes in thyroid function: role of human chorionic gonadotropin as a putative regulator of maternal thyroid, J Clin Endocrinol Metab 73:824. © 1991 by The Endocrine Society.)
Fetal Thyroid Function
The fetal hypothalamic-pituitary-thyroid axis develops autonomously and has been extensively studied in the human, sheep, and rat.52,118–120 A number of agents and maternal factors may affect fetal thyroid function, depending on whether they cross the placenta (Table 25-2).
Table 25-2
Placental Transfer and Fetal Thyroid Function
| Without Difficulty | Some Transfer | Little or No Transfer |
| Iodides | T4 | TSH |
| Thionamides | T3 | |
| Thyroid autoantibodies | ||
| TRH |
Modified from Burrow GN: Thyroid diseases in pregnancy. In Burrow GN, Oppenheimer JH, Volp JR (eds): Thyroid function and disease, Philadelphia, 1989, WB Saunders, p 292.
Placental Transfer
The placenta is impermeable to TSH but permeable to TRH, although endogenous maternal levels are probably too low to influence fetal thyroid function.121 Pituitary and serum TSH in the fetus may be under the control of pancreatic TRH before the maturation of hypothalamic TRH after 20 weeks’ gestation.122–126 Injection of TRH in the mother is accompanied by increased cord serum TSH, T4, and T3 levels, thus indicating that endogenous TSH stimulates the fetal thyroid.127
Before the onset of human fetal thyroid gland function at 10 to 12 weeks’ gestation,128–132 any requirement for thyroid hormone would be met by the maternal supply. The presence of human fetal tissue thyroid hormones and receptors before 12 to 18 weeks, when fetal serum T4 production increases, is consistent with an early requirement for thyroid hormones from the mother.133 The placenta has generally been viewed as a substantial barrier to thyroid hormone transfer, in part because of preferential 5′-monodeiodination of T4 to reverse T3.119,121 Studies in rats have provided good evidence for transfer of maternal thyroid hormones to the fetus in early and late pregnancy, which may be important for early brain development and later brain growth and neuronal differentiation.134–139 In the rat, maternal T4 is the principal source of intracellular T3 in the early developing brain.139 The local intracellular generation of T3 from T4 of maternal origin protects the fetal brain from T3 deficiency in cases of fetal thyroid failure, because cerebral 5′ type 2 deiodinase activity increases markedly in response to a minor decrease in T4.140 In humans and sheep, maternal thyroid hormones have more limited access to the fetal circulation.119 Recent human studies have confirmed the presence of T4 and T3 in coelomic and amniotic fluid in the first trimester. Although the concentrations of total T4 and T3 are 100-fold lower than those in maternal serum, because of the lack of binding protein in these fetal compartment fluids, the concentration of FT4 is biologically relevant.141
The apparent normality of sporadic congenitally hypothyroid infants at birth indicates the role of maternal thyroid hormones. The devastating effects of maternal and fetal/neonatal thyroid hormone deficiency in endemic cretinism in humans underscore the overall importance of thyroid hormones to the fetus.54,142,143 Fortunately, this problem does not seem to occur in areas where iodine intake is just marginal,55 but concern remains with respect to any effect of maternal hypothyroxinemia on early fetal brain development and its effects on progeny.101,102,133,144–146 Early studies suggested limited transfer of T4 and T3 across the placenta in humans in the later part of pregnancy and at term,147–153 with T3 crossing more readily than T4. Vulsma and colleagues have convincingly demonstrated maternal-to-fetal T4 transfer in neonates born with a complete organification defect. These infants have subnormal fetal T4 concentrations when compared with normal newborns, but their levels are approximately 40% of the maternal concentration.86 Because of their absolute inability to produce thyroid hormone, this T4 must be maternal in origin.
Iodide is actively transported to the fetus.121 The fetal and neonatal thyroid is susceptible to iodine-induced hypothyroidism and goiter with excessive exposure.119,154,155 This complication can occur after intravenous, oral, mucosal, or topical exposure and absorption in the mother,156 after amniography,157 and as a result of postnatal topical absorption,158 as well as through breast milk.159 A number of pregnant women have been treated with amiodarone, an antiarrhythmic drug containing 75 mg of iodine per 200-mg dose that partially crosses the placenta and increases maternal and fetal iodide levels.160 Although thyroid function may remain normal, case reports have described fetal or neonatal goiter, hypothyroidism, or hyperthyroxinemia in association with maternal amiodarone therapy.161–163
Diagnosis of Fetal Thyroid Disorders In Utero
The possibility of thyroid disease in the fetus is usually considered because of maternal thyroid disease. Fetal hyperthyroidism is generally encountered in the setting of maternal active or previously ablated Graves’ disease via the transplacental passage of maternal TSH receptor–stimulating antibodies. Fetal hypothyroidism is associated with fetal thyroid maldevelopment, iodine deficiency disorders, thyroid autoimmunity, and excessive maternal antithyroid drug therapy.164–166 In fetal hyperthyroidism, ultrasound usually demonstrates a fetal goiter with increased vascularity, and fetal bone maturation is accelerated.167 In hypothyroidism, a fetal goiter may be visible on ultrasound,167,168 or the radiographic appearance of distal femoral or proximal tibial epiphyses may be delayed.169,170 The latter has limited clinical application. Although the fetus incurs serious risk, measurement of T4 and TSH in serum collected by percutaneous umbilical cord sampling (cordocentesis)120 is currently the most reliable means of diagnosing hypothyroidism167,171 or hyperthyroidism in utero.167,172 This technique has advantages over measurement of thyroid hormones or TSH in amniotic fluid, which has not been shown to reliably predict fetal thyroid status.173,174 Fortunately, the diagnosis of fetal thyroid dysfunction can usually be inferred from the clinical scenario presented by the maternal thyroid disease status (see “Fetal and Neonatal Thyrotoxicosis”). The fetus can absorb thyroid hormones injected into amniotic fluid, and such therapy has been used successfully in the treatment of hypothyroidism and goiter in utero.171,175
Goiter and Pregnancy
Goiter has historically been associated with pregnancy, but its incidence and prevalence vary with the geographic area and iodine status of the general population. Up to 70% of pregnant women in Scotland and Ireland were considered on clinical grounds (visible and palpable thyroid gland) to have a goiter versus 38% of nonpregnant controls.176 No cumulative influence of successive pregnancies was observed, inasmuch as goiters were seen in 39% of nulliparous women and 35% of nonpregnant parous women. These studies were conducted in areas of relatively low iodine intake. A comparative study in Iceland, an area of iodine sufficiency, showed a lower basal prevalence of goiter (20%) and no increase in the incidence of goiter in pregnancy.177 Similar results have been reported in studies from North America,178 which has led some authors to suggest that goiter in pregnancy is a myth.179 Most goiters during pregnancy in North America are related to autoimmune thyroid disease, colloid nodular disease, or thyroiditis.
Ultrasonography has added a quantitative perspective to the assessment of goiter in pregnancy. In Denmark, an area of marginal iodine intake, a 30% increase in thyroid volume has been documented at between 18 and 36 weeks’ gestation.180 Volume returned to baseline postpartum, and no evidence of thyroid dysfunction or thyroid autoimmunity was apparent. Only 25% of the women actually had a goiter on clinical grounds. Serum thyroglobulin levels were also increased during pregnancy.181 Only a 13% increase in thyroid volume was reported in a North American study.182 The largest longitudinal and cross-sectional study of thyroid volume in pregnancy involved more than 600 women from Belgium, another area of marginal iodine intake.73 Seventy percent of the women had a 20% or greater increase in thyroid volume during pregnancy, although only 9% had a significant goiter as defined by thyroid volume in excess of 23 mL. Thyroid volume showed positive correlations with higher serum thyroglobulin levels and an increased serum T3/T4 ratio. No correlation was seen with urine iodide excretion, and a negative correlation with serum TSH levels was noted. The latter may have been due to the influence of hCG during pregnancy. The same authors from Belgium prospectively studied preexisting mild thyroid abnormalities through pregnancy and noted a significant goitrogenic effect as well as an increase in the incidence and prevalence of thyroid nodules.50 Many of these nodules were subclinical and were detected only on thyroid ultrasound. Serum thyroglobulin levels were disproportionately increased in women with goiters and nodules when compared with controls and pregnant women with autoimmune thyroid disease or a history of previous thyroid abnormalities. The authors further suggested that previous pregnancies were a significant risk factor for goiter and thyroid nodules. This same risk was also suggested in a study from the Netherlands.183 It should be noted that an increase in thyroid volume during pregnancy does not necessarily denote increased mitotic activity because increased colloid volume, cell hypertrophy, inflammation, or increased thyroid blood flow could account for some of the enlargement.
No evidence of adverse effects on fetal development or neonatal thyroid function has been seen in these studies from areas of marginal iodine uptake.50,55,73 Nor does there appear to be an increase in risk of neonatal thyroid dysfunction in goitrous, iodine-sufficient areas.184 This finding contrasts with results from areas with endemic iodine deficiency.54,185 Maternal smoking has been shown to be a risk factor for neonatal thyroid enlargement, as determined ultrasonographically.186 Neonatal thyroid volume correlated with cord serum thyroglobulin and thiocyanate levels, but no evidence of neonatal thyroid dysfunction was found.
Pathology of Thyrotoxicosis in Pregnancy
All forms of thyroid disease are more common in women than in men, and thyrotoxicosis is not a rare event during pregnancy. It occurs in about 2 of every 1000 pregnancies. Autoimmune thyrotoxicosis, or Graves’ disease, the most common cause of thyrotoxicosis in pregnant women, accounts for about 90% of cases. Toxic adenomas or nodular goiters are much less common in this age group. Other causes of thyrotoxicosis in pregnancy include gestational trophoblastic neoplasia93 and hyperemesis gravidarum (see Table 25-1).
Gestational Thyrotoxicosis
A spectrum of hCG-induced hyperthyroidism occurs during pregnancy, and this entity has been referred to recently as “gestational thyrotoxicosis.”187–189 This disorder differs from Graves’ disease in several ways: (1) nonautoimmune origin, with negative antithyroid and anti–TSH receptor antibodies (TRAbs); (2) absence of large goiter; and (3) resolution in almost all patients after 20 weeks.187
Findings range from an isolated subnormal serum TSH concentration (approximately 9% of pregnancies116) to elevations of free thyroid hormone levels in the clinical setting of hyperemesis gravidarum. Systematic screening of 1900 consecutive pregnant women in Belgium demonstrated low TSH and elevated FT4 in 2.4%, half of whom had weight loss, lack of weight gain, or unexplained tachycardia.189
Hyperemesis gravidarum, or pernicious nausea and vomiting in pregnancy, is usually associated with weight loss and fluid and electrolyte disturbances. Its manifestation and diagnosis can be complicated because other causes of severe nausea and vomiting in pregnancy must be excluded. Suppressed TSH levels may occur in 60% of women with hyperemesis gravidarum, along with elevated FT4 levels in almost 50%.188–192 Serum hCG concentrations correlate with FT4 levels and inversely with TSH determinations. The magnitude of the deviation from normal values increases with the severity of nausea and vomiting.116,193 Only 12% of such women have an elevated free T3 index, which may help to differentiate this entity from Graves’ disease.116 Furthermore, thyroid-stimulating activity, as measured by adenylate cyclase activity per international unit of hCG, is reported to be greater in women with hyperemesis gravidarum than in those with occasional or no vomiting.187
Similar thyroid hormone changes and emetic symptoms may be present in multiple gestations, which are associated with higher peak and more sustained hCG levels.194 In addition, a recent case report further supports the concept of hCG-induced thyrotoxicosis. A woman with recurrent gestational thyrotoxicosis and her mother with the same obstetric history were found to have a missense mutation in the extracellular domain of the TSH receptor. When this receptor was studied in transfected COS-7 cells, it caused a twofold to threefold increase in cAMP generation when exposed to hCG as compared with wild-type receptor.192 This genetic mutation induced hyperresponsiveness to hCG as well as thyrotoxicosis.
Gestational thyrotoxicosis is transient and usually resolves within 10 weeks of diagnosis.195 Treatment with antithyroid drugs is not recommended61 but may be needed if there is concomitant Graves’ disease. The clinician may consider antithyroid drug therapy for patients with hyperemesis who remain symptomatic after 20 weeks’ gestation and continue to have elevated thyroid hormone concentrations and suppressed TSH levels, or who have evidence of significant clinical thyrotoxicosis.
Diagnosis
Despite the difficulty associated with interpretation of thyroid function tests because of the elevated TBG concentration during pregnancy, the diagnosis of hyperthyroidism in pregnant women depends on laboratory testing. A sensitive TSH determination with a value less than 0.01 mU/L196,197 and an elevated serum FT4 (or total T4 concentration above pregnancy reference values) is generally diagnostic. This illustrates the need for the manufacturers of commercial FT4 assays to report trimester-specific and method-specific reference ranges during pregnancy. As noted, during the first trimester, the serum TSH may be below the nonpregnant reference range in response to an increase in serum hCG concentration.73 The delay in TRAb measurement renders it generally impractical for routine diagnostic use in this clinical scenario. If the diagnosis is not clearcut, one can usually wait 3 to 4 weeks and then can repeat the thyroid function tests because most pregnant women tolerate mild to moderate thyrotoxicosis without difficulty.224
Treatment
Once the diagnosis of thyrotoxicosis has been established in a pregnant woman, therapy should be instituted. Treatment of a pregnant woman with thyrotoxicosis is limited to antithyroid drug therapy or surgery because radioactive iodine is absolutely contraindicated.198–200 After the 10th to the 12th week of gestation, once the fetal thyroid has the ability to concentrate iodine, congenital hypothyroidism may be induced by 131I treatment. In one study, in which 182 fetuses were inadvertently exposed to 131I therapy during the first trimester, pregnancy resulted in two (1.1%) spontaneous abortions, two (1.1%) intrauterine deaths, six (3.3%) hypothyroid children, and four (2.2%) mentally retarded children. If 131I treatment is inadvertently administered to a woman in early pregnancy, the effects on the thyroid could be blocked by iodide administration, but the optimal dosing of iodide has not been studied.
Thionamide Therapy
The thionamides propylthiouracil, methimazole, and carbimazole have all been prescribed for the treatment of thyrotoxicosis during pregnancy. Carbimazole, which is metabolized to methimazole, is used mainly in Europe. All these agents cross the placenta and are also secreted in breast milk.201 The serum half-lives of propylthiouracil and methimazole are 1 hour and 5 hours, respectively.202–206 These two antithyroid drugs have been used interchangeably. Thionamides block the synthesis but not the release of thyroid hormone. Propylthiouracil does have the potential additional advantage of partially blocking the conversion of T4 to T3. With propylthiouracil or methimazole, the typical patient will note some improvement after 1 or 2 weeks and may approach euthyroidism after 6 to 8 weeks of treatment, with no difference in the median time to lowering the FT4 index to appropriate pregnant levels.207
If minor drug reactions occur, the thionamides may be interchanged, but cross-sensitivity is seen in about half of patients.208 The most common reactions include fever, nausea, skin rash, pruritus, and metallic taste.209 Transient leukopenia, not an uncommon reaction to thionamide therapy, occurs in about 12% of adults.209,210 This association may be complicated because mild leukopenia is not uncommon in untreated Graves’ disease.202 This mild leukopenia is not a sign of agranulocytosis, which occurs in about 0.5% of patients, usually within 12 weeks of initiation of therapy, and may be an autoimmune phenomenon.211–214 Hepatitis and vasculitis have also been reported as rare side effects of thionamide therapy, specifically with propylthiouracil215–217; these complications have not been reported to affect the fetus, although they have occurred in the pregnant mother.
Additionally, the possibility has been raised that methimazole is associated with the development of aplasia cutis of the scalp in the treated mother’s offspring.218–220 Some endocrinologists nonetheless recommend propylthiouracil as initial therapy during pregnancy because no cases of aplasia cutis have been reported in babies born to propylthiouracil-treated mothers.61,220 In addition, perhaps of greater concern than aplasia cutis are recent descriptions of a methimazole embryopathy, which may include findings of choanal atresia, tracheal-esophageal fistulas, and hypoplastic nipples.126 A recent case-control study raised the possibility that maternal hyperthyroidism itself, rather than methimazole treatment, might be the causal factor for this embryopathy, specifically choanal atresisa.221 When the significance of all these reports is considered, it must be emphasized that no case reports of aplasia cutis or other congenital anomalies have been associated with propylthiouracil exposure. This generally remains the preferred drug therapy of maternal hyperthyroidism, in pregnancy. However, some have recently advocated propylthiouracil use only during the first trimester. Thereafter, methimazole may be substituted for propylthiouracil and continued until delivery.
The goal of antithyroid drug therapy is to gain control of the maternal thyrotoxicosis to ensure favorable gestational outcomes and to minimize the impact on the fetus.61,222 Studies show a strong correlation between maternal and neonatal levels of free T4, indicating that maternal thyroid status is the most clinically practical index of fetal thyroid status.199,223 To optimize neonatal thyroid function and minimize the incidence of transient newborn hypothyroidism, maternal serum FT4 should be maintained at or slightly higher than (<10%) the nonpregnant reference range.61,223 An alternative approach would be to keep the total T4 concentration in the high normal range for pregnancy (1.5 times the nonpregnant reference range).61,68 When detectable, serum TSH concentrations at or just below the trimester-specific 95% confidence interval are acceptable.
Therefore, the therapeutic target for maternal thyroid hormone levels is actually very mild hyperthyroidism as compared with true “normal” levels for gestational physiology. Fortunately, subclinical and mild hyperthyroidism during pregnancy is not associated with adverse maternal gestational outcomes.224
Propylthiouracil (PTU) usually is started at doses sufficient to control the hyperthyroidism; the dose may be increased after 4 weeks if necessary to gain control of the thyrotoxicosis. Some women may require high doses (up to 450 mg/day of PTU) for this purpose and must be carefully monitored. Doses of this magnitude may cause fetal hypothyroidism and may justify a change in therapy to surgery. The need for larger doses may correlate with low serum concentrations of PTU225 and could be caused by documented individual variability in serum propylthiouracil levels after an oral dose226 or poor compliance with the medication. Methimazole should be used if PTU is not available, the side effect profile of PTU is deemed unacceptable, or if there is difficulty with adherence to a multidose, multi-pill PTU regimen. Free or total T4 and sensitive TSH measurements should be performed monthly during pregnancy and the dose of thionamide decreased to maintain the therapeutic targets for FT4, total T4, and TSH indicated above. If a requirement for higher doses of PTU (>450 mg/day) or methimazole (>30 mg/day) continues and effects on the fetus are a concern, the clinician should consider thyroid surgery.
β-Adrenergic Blockers
If it is necessary to give drugs to a pregnant woman, they should be the least toxic agents possible. For this reason, the use of β-adrenergic blocking drugs has been advocated for the treatment of pregnant women with hyperthyroidism.227 β-Blockers have been used in large numbers of pregnant women to treat hypertension without apparent significant side effects.228 However, intrauterine growth retardation with a small placenta, impaired response to anoxic stress, postnatal bradycardia, and hypoglycemia have been reported in the offspring of mothers receiving these agents, indicating the need for caution in their use.229 β-Blockers are particularly useful for rapid control of the β-adrenergic manifestations of thyrotoxicosis such as tremor and tachycardia. Propranolol, 20 to 40 mg three or four times a day, or atenolol, 50 to 100 mg/day, is usually adequate to control the maternal heart rate at 80 to 90 beats per minute. Esmolol, an ultra–short-acting cardioselective β-blocker given intravenously, controlled the heart rate in a pregnant woman with hyperthyroidism who required emergency surgery and was unresponsive to large doses of propranolol.230 Current practice is to control hyperthyroidism with antithyroid drugs and to add β-blockers only in exceptional cases.
Surgery
In pregnant thyrotoxic women with poor compliance or for whom maternal or fetal toxicity from antithyroid drug therapy is a concern, subtotal thyroidectomy may be advised.61 Thyrotoxicosis needs to be controlled medically before subtotal thyroidectomy can be performed. This treatment includes antithyroid drugs, β-blockers, and, rarely, possible short-term use of oral iodides.231 A useful clinical parameter of control is a resting heart rate of 80 to 90 beats per minute. Because of concern about spontaneous abortion, surgery is often delayed until after the first trimester. The small but real anesthetic and surgical risk is probably greater than the risks associated with thionamide therapy.232,233
Iodide
Iodide has not been recommended in the routine treatment of hyperthyroidism during pregnancy because of its association with neonatal goiter and hypothyroidism when given in conjunction with thionamides. One study in which gravidas with mild Grave’s disease (GD) were treated with low-dose iodine alone (6 to 40 mg daily) showed that 6% of neonates had elevated serum TSH levels, but none had a goiter.231 With such little evidence, iodide should not be considered as primary therapy but may be used short term for control of thyrotoxicosis prior to thyroidectomy, or in the management of thyroid storm.
Pregnancy Outcome
Severe maternal thyrotoxicosis significantly increases morbidity in both the fetus and the mother. The prevalence of low birth weight offspring is higher, with a trend toward increased neonatal mortality.26,234 Whether fetal wastage is increased in established pregnancy is not clear. In one study of 57 thyrotoxic pregnancies, the fetal wastage rate was 8.4%, which compares favorably with the estimated overall fetal wastage rate of 17% in normal women, including those undergoing spontaneous abortion.44 Very early spontaneous abortions could easily be missed in thyrotoxic women. A recent study reported a higher miscarriage rate in mothers affected by resistance to thyroid hormone. The inference is that these miscarriages may have predominantly involved genetically unaffected fetuses who therefore were exposed to high maternal thyroid hormone levels with resultant fetal thyrotoxicosis that proved toxic. This study provides valuable insight about the effects of in utero thyrotoxicosis without accompanying maternal gestational hyperthyroid physiology.235 A higher incidence of minor congenital malformations has been suggested to occur in the children of thyrotoxic women who were untreated during the first trimester of pregnancy,236 but this finding has not been confirmed by others.207 Only one study has reported Down syndrome to occur more frequently in children born to women with hyperthyroidism.237 Preterm delivery, perinatal mortality, and maternal congestive heart failure were markedly increased in untreated and inadequately treated thyrotoxic patients in a retrospective study of 60 pregnant women with hyperthyroidism admitted to an inner-city hospital over a 12-year period.238 Women with newly diagnosed thyrotoxicosis during pregnancy had a higher incidence of morbidity and mortality than did women in whom the condition was diagnosed and treated before conception. Socioeconomic conditions might have played a role in the severity of hyperthyroidism or poor outcomes, but treatment of thyrotoxicosis is indicated nonetheless. These patients are at risk for congestive heart failure because the hyperdynamic state of thyrotoxicosis is superimposed on the increased cardiac output of normal pregnancy.239 Management of diseases such as diabetes mellitus is also complicated in a pregnant woman with thyrotoxicosis, causing erratic glycemic control and a need for increased insulin.240 Fortunately, these adverse outcomes are observed only with untreated overt or poorly controlled hyperthyroidism, not with subclinical hyperthyroidism.224 In addition, successful treatment of overt hyperthyroidism with antithyroid drugs by the third trimester reduces the risk of low birth weight newborns to that of a control euthyroid populaton.241
Fetal and Neonatal Thyrotoxicosis
Fetal hyperthyroidism complicates pregnancy in 1% of women with active or treated Graves’ disease, including those who have become hypothyroid after radioactive iodine therapy. These women have thyroid-stimulating immunoglobulins that, similar to other immunoglobulins such as IgG, cross the placenta.242 In high enough concentrations, they may cause fetal or neonatal thyrotoxicosis. Maternal IgG antibodies, particularly subclasses 1 and 3, and thyroid autoantibodies are able to cross the placenta after 20 weeks’ gestation242,243 by micropinocytosis after IgG binding to Fc receptors on the syncytiotrophoblast. Maternal levels are indicative of the degree of fetal exposure and the potential for fetal thyroid stimulation.244
Measurement of maternal TSH receptor antibody (TRAb) levels may provide prognostic information about the development of fetal Graves’ disease.245–247 At present, TSH receptor antibodies can be measured by two techniques: an immunoassay (TSH binding inhibitory immunoglobulin [TBII]) or a functional assay of biological stimulation (thyroid-stimulating immunoglobulin [TSI]).119,248 These antibodies may exhibit stimulating or blocking activity, resulting in fetal/neonatal hyperthyroidism or hypothyroidism.166,245,249,250 Alternating neonatal hyperthyroidism and hypothyroidism have been described in infants born to the same mother.251
TSH receptor antibodies should be measured by the end of the second trimester in mothers with current Graves’ disease, a history of Graves’ disease and treatment with 131I or thyroidectomy, or birth of a previous neonate with Graves’ disease.61 Women who have a negative TRAb and do not require antithyroid drug therapy have a very low risk of fetal or neonatal thyroid dysfunction.
In a woman with active or previously ablated Graves’ disease, the diagnosis of fetal hyperthyroidism should be considered if persistent fetal tachycardia (>180 beats per minute) without beat-to-beat variation and advanced bone maturation are present, sometimes with fetal growth restriction.167 The diagnosis is strengthened by the ultrasound documentation of a goiter with diffusely increased vascularity.167 Diagnosis can be confirmed by cordocentesis if considered necessary after discussion of the potential risks, but this usually is not needed.252 The pathology of fetal thyrotoxicosis includes goiter, visceromegaly, adenopathy, and pulmonary hypertension.253 If fetal thyrotoxicosis occurs in the setting of active maternal Graves’ disease, usually the mother is overtly hyperthyroid. Appropriate maternal antithyroid therapy will treat both the mother and the fetus because, as in the mother, fetal thyroid hormone synthesis represents the balance between the transplacental passage of inhibitory maternal antithyroid drug and stimulating maternal TRAb concentrations.
Levothyroxine replacement in women with a history of Graves’ disease and prior 131I ablation or thyroidectomy antedating pregnancy may still produce TRAb, causing fetal thyrotoxicosis. In this scenario, maternal therapy with propylthiouracil, 150 mg/day, may decrease the fetal heart rate to the normal range (140 to 160 beats per minute) within 2 weeks.244,254 Maternal thyroid hormone levels should be monitored regularly, with thyroxine supplementation given if hypothyroxinemia occurs.
TSH receptor antibody levels usually decline progressively toward term,13,251,255 and the PTU drug dose can be lowered to 50 to 75 mg/day or even discontinued with titration to the fetal heart rate and growth. In women with persistent elevations of TSH receptor antibodies at term (more than threefold elevated above reference values),167,256,257 neonatal hyperthyroidism may occur, requiring continued antithyroid drug therapy after birth. In addition, the presence of cord blood TSH receptor antibodies is highly predictive of the development of neonatal hyperthyroidism.256–259 If the mother has been treated with antithyroid drugs during pregnancy, the manifestations of neonatal thyrotoxicosis may be delayed until 5 to 10 days of life, when the pharmacologic effect of the transplacentally acquired antithyroid drug has cleared.258 The neonate may become irritable and have feeding problems. Other manifestations include proptosis, goiter, and failure to thrive. In severe cases, congestive heart failure, jaundice, and thrombocytopenia may occur and can cause significant mortality.260 Usually, the disease runs a self-limited course over a period of several months.119 Temporary treatment with iodides, β-blockers, and antithyroid drugs is indicated. As maternal antibody levels decrease over the first 3 months of life, treatment generally can be discontinued.259
In addition, it has been reported that infants with central hypothyroidism have been born to mothers with uncontrolled Graves’ disease. The hypothesis is that these infants are exposed to high levels of thyroid hormone in utero, which results in impaired development of the fetal hypothalamic-pituitary-thyroid axis and eventual central hypothyroidism.258,261 The incidence of such central congenital hypothyroidism is estimated to be 1 in 35,000 live births.262
Lactation
Until the last decade, lactation was strongly discouraged in women receiving thionamide therapy. The doctrine against lactation in women receiving antithyroid drug therapy originated in a 1948 report, which stated that the concentration of thiouracil measured 2 hours after ingestion was three times higher in breast milk than in serum.263 Over the last 20 years, several studies have examined the extent of secretion of propylthiouracil and methimazole in breast milk and have evaluated the thyroid function of infants nursed by mothers taking these drugs. Propylthiouracil is more tightly protein bound than methimazole; therefore, the mean milk-to-serum concentration ratio is lower for propylthiouracil, at approximately 0.67,264 than for methimazole, for which the ratio is 1.0.265,266 Therefore, the mean total amount of methimazole excreted in breast milk is greater than that of propylthiouracil (0.14% versus 0.025%264,265).
Several studies have prospectively evaluated whether maternal antithyroid therapy affects the thyroid function of breastfed infants. Thyroid function remained unaffected in 171 infants whose mothers received daily doses of 50 to 300 mg of propylthiouracil, 5 to 20 mg of methimazole, or 5 to 15 mg of carbimazole for periods ranging from 3 weeks to 8 months.264,267–269 In fact, serum TSH and T4 levels remained normal even in six women in whom elevated serum TSH levels (19 mU/L and 120 mU/L) had developed while they were receiving propylthiouracil.269 Therefore, although the number of infants monitored is small, breastfeeding with continued antithyroid drugs may be contemplated as long as the doses are less than 300 mg daily for propylthiouracil and 20 mg daily for methimazole. It is important to remember that these antithyroid drugs have other nonthyroidal effects such as agranulocytosis; no data are available on the possible occurrence of such effects in infants nursed by mothers taking these drugs.
Pathology of Hypothyroidism in Pregnancy
Incidence and Etiology
Hypothyroidism is encountered more often in pregnancy than is hyperthyroidism. The most recent epidemiologic data have been obtained through the analysis of banked serum obtained at approximately 16 weeks’ gestation in healthy pregnant women. In these women (without prior thyroid disease), two independent studies documented a 2% to 3% incidence of an elevated serum TSH concentration in midpregnancy.26,28 In industrialized societies, the main causes of maternal hypothyroidism in pregnancy are related to thyroid autoimmunity: Hashimoto’s thyroiditis and postablative hypothyroidism in Graves’ disease (see Table 25-1, Fig. 25-5). A third cause that has been noted increasingly is primary hypothyroidism due to surgical thyroidectomy. In most women, the hypothyroidism has already been diagnosed and treated before pregnancy. Worldwide, the main cause of maternal hypothyroidism is iodine deficiency, which has been estimated to affect more than one billion individuals. Population screening programs have documented that a substantial proportion of women of child-bearing age may also have mild TSH elevations. Furthermore, even those hypothyroid women treated to a goal TSH within the normal range, often are found to have abnormal hormone levels.270,271 The former point is notable in that the average maternal age at pregnancy has been increasing in the United States over the past three decades. In summary, it is estimated that between 4% and 7% of women of child-bearing age in the United States are hypothyroid and unaware of it, or are at risk for hypothyroidism during pregnancy if their levothyroxine dose is not adjusted upon conception. This impressive number draws attention to the importance of this medical problem.
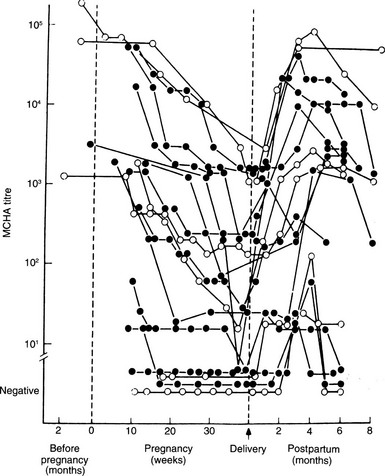
FIGURE 25-5 Sequential changes in serum antithyroid antibodies (MCHA) during pregnancy and after delivery in patients with Graves’ disease (black dots) and autoimmune thyroiditis (open dots). (From Amino N, Kuro R, Tanizawa O, et al: Changes in serum antithyroid antibodies during and after pregnancy in autoimmune thyroid diseases, Clin Exp Immunol 31:30, 1978.)
Evidence of thyroid autoimmunity is seen in other autoimmune endocrine disorders such as insulin-dependent (type 1) diabetes mellitus,272 including that occurring during pregnancy.273 Up to 40% of diabetic women are thyroid antibody positive, and up to 10% are mildly hypothyroid with elevated TSH levels. Hypothyroidism does not appear to progress in diabetic pregnancy unless proteinuria develops, in which case overt hypothyroidism may ensue.274 Hypothyroidism may result from increased urinary loss of thyroid hormones as occurs with proteinuria, combined with the preexisting impaired thyroid reserve. Thyroxine therapy in this situation is appropriate but may result in an increase in insulin requirements.
Diagnosis and Screening
Clinical assessment of thyroid status during pregnancy is important, but it can be imprecise. Normal pregnancy symptoms such as lethargy and weight gain may be suggestive of hypothyroidism. Paresthesias resulting from median nerve compression (carpal tunnel syndrome) are seen in both hypothyroidism and pregnancy. Delayed relaxation of deep tendon reflexes (pseudomyotonia) is a good clinical sign of severe hypothyroidism, if present, but is rarely seen in mild thyroid failure. If present, signs of myxedema such as decreased body temperature, periorbital edema, swelling, thick tongue, and hoarse voice should be apparent in pregnancy but may be confused with the features of preeclampsia.275 Goiter may be present, but its absence does not detract from a diagnosis of hypothyroidism. The most sensitive indicator of primary hypothyroidism in pregnancy is an elevated serum TSH level.276 Serum TSH values during pregnancy are lower than those seen in healthy nonpregnant individuals.80,81 Presently, serum TSH values greater than 4.5 to 5 mIU/L should be considered abnormal, warranting further evaluation and possible intervention. Some authors argue that TSH values greater than 3 to 3.5 mIU/L are abnormal and suggest the diagnosis of thyroid dysfunction. While further research findings are awaited, however, no data currently demonstrate the clinical superiority of using trimester-specific data. In contrast, TSH values between 3.0 and 5.0 mIU/L in pregnant women should indicate that repeat laboratory studies are required because the risk of overt hypothyroidism may be increased. When treating patients with levothyroxine for a known thyroid disorder, it is generally accepted that the therapeutic target is a TSH within the normal range. The presence of TPO antibodies indicates a probable autoimmune origin. Hypothalamic or pituitary hypothyroidism is rarely encountered; its presence is suggested by low FT4 or total T4 for gestational age with normal or slightly elevated TSH.
Currently, no recommendations have been put forth regarding universal screening of women for thyroid dysfunction before or during pregnancy.61 This type of screening program has both political and medical implications, which remain under active discussion by numerous public health and professional societies. Extensive published data have provided insight into the harmful effects of maternal hypothyroidism on the cognitive and neurologic development of offspring. Most often cited is an investigation by Haddow and colleagues, in which a case-control study demonstrated a decrement in intelligence quotient (IQ) among children born to untreated hypothyroid mothers.277 In this study, 62 children born to untreated hypothyroid mothers (average age, approximately 8 years) were studied prospectively and compared with 124 children born to euthyroid mothers. IQ scores averaged seven points lower in children of hypothyroid mothers. Furthermore, nearly three times as many children had IQ scores lower than 85, in comparison with those born to euthyroid women. Although the lack of randomization has been acknowledged, these data provide the strongest evidence yet suggesting that maternal hypothyroidism is harmful to neurocognitive development and should be prevented. However, many argue that recommendations for universal screening of a population should be implemented only if data also confirm the benefit of a particular treatment intervention (such as levothyroxine replacement). No investigation to date has yet fulfilled this criterion in a prospective, randomized fashion, although several studies are ongoing. In the absence of recommendations for universal screening, it is presently believed that women deemed at high risk for development of hypothyroidism during pregnancy should undergo measurement of serum TSH before pregnancy or during early gestation. This would include women with a history of autoimmune thyroid disease or positive antithyroid antibodies, women with symptoms that are suggestive of hypothyroidism, women with other autoimmune disorders (such as type 1 diabetes mellitus) or a family history of autoimmune thyroid disease, women with a goiter on clinical examination, and women who have undergone previous thyroid surgery or who have a history of head or neck irradiation (including 131I ablation).61 Additionally, women 35 years of age and older are considered at higher risk for undiagnosed hypothyroidism, warranting evaluation. If a “case-finding” approach is followed, data suggest that nearly 70% of hypothyroid women can be identified in a general population.278 However, these data also demonstrate that 30% of pregnant women may have elevated TSH during gestation and may avoid detection, even if clinical profiling is instituted.
Separate from consideration of universal screening, clinicians must identify and monitor all thyroxine-replaced hypothyroid women during pregnancy and must adjust levothyroxine early in gestation (Fig. 25-6).61 A study of 20 pregnancies in 19 women prospectively assessed thyroid function every 2 to 4 weeks throughout gestation and adjusted levothyroxine doses to maintain normal TSH values.71 Results demonstrated an increased demand for levothyroxine during pregnancy, primarily in the first 16 to 20 weeks. At 20 weeks’ gestation, the increased requirement for levothyroxine persisted, although it had plateaued. To prevent abnormal elevations in maternal serum TSH, this study strongly suggests that pregnant women receiving levothyroxine therapy be required to undergo initial biochemical evaluation as early as 6 weeks’ gestation. Women who become pregnant via assisted reproductive techniques appear to require the earliest adjustment in thyroid hormone doses, although further validation of this finding is required. These findings have important implications for populations. Because nearly 2% to 4% of women of child-bearing age currently require levothyroxine to maintain normal thyroid function, physicians must actively counsel such women to immediately notify a health care professional upon suspicion of a possible pregnancy. Confirmatory testing should then be performed, in conjunction with evaluation of thyroid status. If biochemical testing is delayed until the time of the first obstetric visit, serum TSH values may already be elevated, thus exposing the developing fetus to a period of maternal hypothyroxinemia. Some have advocated that hypothyroid women treated with levothyroxine should be instructed to proactively increase their levothyroxine dosage (by two extra tablets per week) once pregnancy is suspected, while they await further medical testing. This recommendation has not been universally adopted.
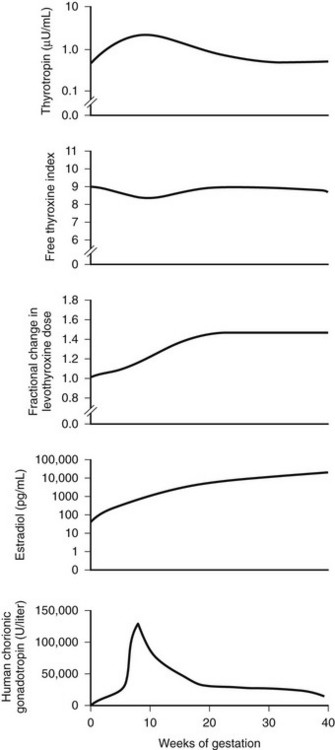
FIGURE 25-6 Changes in maternal hormone concentrations and the levothyroxine dose during gestation. The graphs depict the best-fit curves for serum thyrotropin (range, 0.5 to 5.0 µU per milliliter), the free thyroxine index (range, 5 to 11), the fractional increase in the dose of levothyroxine, the maternal estradiol concentration (range, 10 to 80 pg per milliliter), and the concentration of human chorionic gonadotropin (range, less than 5 U per liter) throughout pregnancy in the 14 women who required an increase in the levothyroxine dose during a full-term pregnancy. To convert the values for estradiol to picomoles per liter, multiply by 3.67. (Data from Alexander EK, Marqusee E, Lawrence J, et al: Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism, N Engl J Med 351:241, 2004.)
Treatment
If hypothyroidism is diagnosed during pregnancy, thyroxine therapy should be initiated. When no endogenous thyroid function remains, full thyroxine replacement can be accomplished with a dosage of approximately 2 mcg/kg/day, a dose slightly higher than the replacement dose for a nonpregnant woman.61 This usually is well tolerated in otherwise healthy individuals. However, in women with mild hypothyroidism who retain limited endogenous thyroid function, a lesser dose of thyroxine (1 mcg/kg/day) is often initiated and successfully achieves an adequate treatment response. Regardless of initial dose, serum TSH determinations should be repeated at 1 month and the levothyroxine dose adjusted accordingly, with the goal of maintaining TSH within normal limits for pregnancy, which translates practically to a serum TSH level ≤2.5 to 3.0 mIU/L. Women in whom subclinical hypothyroidism is diagnosed may be treated with lower thyroxine dosages. Although many advocate that subclinical hypothyroidism need not be treated in the general population, consensus indicates that even mild hypothyroidism during pregnancy can be harmful, and therefore levothyroxine therapy should be initiated.279
Women for whom levothyroxine is initiated or adjusted during pregnancy should be advised by their clinician to have serum TSH concentration tested every 4 weeks during the first half of pregnancy, with dosage adjusted as needed to maintain a normal TSH level. Even if the initial serum TSH concentration is normal, it should be retested at 4-week intervals because as many as 30% of patients may later require a dose change, and data confirm a dynamic physiology of increasing thyroxine requirements over 20 weeks’ gestation, as was noted previously. After delivery, the thyroxine dosage should be lowered to the preconception level, with serum TSH testing conducted at 6 weeks’ postpartum. Patients should be instructed to separate thyroxine ingestion by 4 hours from ingestion of prenatal vitamins containing iron and iron supplements, calcium supplements, and soy milk, all of which can interfere with thyroxine absorption.280,281
Pregnancy Outcome
Although hypothyroidism has long been known to negatively affect pregnancy, numerous reports over the past 100 years have documented hypothyroid women carrying their pregnancies to term.282–284 Early studies reported up to a 20% incidence of perinatal mortality and congenital malformations associated with maternal hypothyroxinemia25 (i.e., untreated or inadequately treated hypothyroidism), with up to 60% of surviving children having evidence of impaired mental or physical development. An increase in congenital malformations was seen even in the infants of women considered to have adequate thyroxine replacement therapy, although details from these early reports are difficult to fully evaluate because the biochemical assessment of thyroid hormone status in these studies was imprecise. In more recent studies, patients were well characterized with respect to degree of hypothyroidism and the adequacy of thyroxine replacement therapy.284 Still, untreated or inadequately treated overtly hypothyroid women experienced up to a 40% incidence of anemia, preeclampsia, placental abruption, and postpartum hemorrhage; 30% of the neonates were small for gestational age; and a 10% incidence of perinatal mortality and congenital anomalies was noted. Women with untreated subclinical hypothyroidism (elevated serum TSH only) had approximately one-third the incidence of these problems, and in both groups it appeared that maternal and fetal outcomes were improved by adequate thyroxine therapy. Other studies confirm a pattern of increased pregnancy complications and maternal morbidity.28
A separate study focusing on gestational hypertension reported a 15% incidence in women with subclinical hypothyroidism and a 22% incidence in those with overt disease versus 7.6% in the general population. Preeclampsia occurred in 66% of women who were inadequately treated and remained hypothyroid, resulting in premature delivery.24 Two investigations have suggested that fetal distress and fetal death may occur more frequently in hypothyroid women.26,285 Wasserstrum and coworkers found that the likelihood of fetal distress, defined as an abnormal heart rate during labor, was significantly higher in women with overt hypothyroidism (56%) detected during gestation, especially if the serum TSH level remained elevated at term, than it was in women with only mild TSH elevations (3%).285 In a screening study from Maine in the United States, an elevated serum TSH greater than 6 mIU/L at 15 to 18 weeks’ gestation was associated with a higher rate of fetal death (3.8%) than occurred in euthyroid women (0.9%).26 Many studies reporting gestational complications of hypothyroidism have consisted of populations in which, on average, the initial antenatal visit occurred at between 16 and 20 weeks’ gestation—less than optimal prenatal care. These women often had other medical problems that could be associated with adverse fetal outcomes. However, the consistency of findings across several studies supports a contributing causal role of hypothyroidism.
Separate from maternal complications related to hypothyroidism, substantial interest has shifted toward assessing possible neurocognitive decline among the offspring of hypothyroid women. As was noted previously, a case-control study reported a 7-point IQ deficit in children born to untreated, hypothyroid mothers compared with euthyroid controls.277 Beyond cognitive deficits, significantly worse attention, behavior, and motor skills were demonstrated in these children. Detailed anatomic brain imaging of offspring born to hypothyroid mothers has revealed that anatomic abnormalities are also detected in the visual, hippocampal, and other important areas of the brain.24 Together, these data strongly suggest that maternal and fetal complications are increased during pregnancy in untreated, hypothyroid women. Even if the pregnancy remains uncomplicated, the data support long-term adverse effects on the fetus’ cognitive abilities and development if maternal thyroid status remains undertreated.
An article by Abalovich confirms the importance of adequate thyroxine replacement during the remainder of the gestational period, once maternal hypothyroidism has been detected. Abalovich and colleagues correlated the pregnancy outcomes of 51 hypothyroid women (diagnosed in the first trimester) with the adequacy of subsequent thyroxine therapy.27 Regardless of whether the initial diagnosis was overt or subclinical hypothyroidism, those with normalization of serum TSH levels had term pregnancies (93%), except for two preterm deliveries (7%). However, only 21% of those with inadequate thyroxine therapy had term deliveries; 67% had spontaneous abortions and 12% had preterm deliveries. In this as in other studies,24,284 appropriate thyroxine therapy may ameliorate some of the adverse outcomes even if thyroid hormone deficiency is detected early during pregnancy. These data support the current belief that any degree of maternal hypothyroidism should be actively treated with levothyroxine replacement, with the goal of normalizing serum TSH. Presently, no available data support an adverse effect on the pregnancy or the fetus if mild hypothyroidism of short duration is effectively treated. However, further research is required.
Neonatal Hypothyroidism
The incidence of congenital hypothyroidism is 1 per 3000 to 4000 live births, with most cases involving primary hypothyroidism.119 Any delay in thyroxine treatment beyond 4 to 6 weeks of life reduces the chance of normal development and future intellectual performance of the offspring.286,287 Because clinical diagnosis is imprecise, the solution has been to institute a neonatal screening program to measure heel-prick blood samples drawn between 3 and 5 days’ postpartum for TSH alone or thyroxine. As needed, subsequent TSH measurements are performed on samples with the lowest decile T4 values.83,275 Most cases of congenital hypothyroidism are considered sporadic and likely to be permanent. A proportion of cases may be due to maternal autoimmunization or may be associated with maternal TSH receptor antibodies that inhibit thyroid growth or function288,289 or with cytotoxic antibodies.290 Antibodies to thyroglobulin or TPO probably are not causal in hypothyroidism. Maternal thyroid autoimmunity may play a role in transient neonatal hypothyroidism that results from transplacental passage of TSH receptor antibodies that block thyroid function or growth, or both, in utero or postpartum.291 This transient hypothyroidism accounts for up to 10% of cases of congenital hypothyroidism. Familial forms are also recognized.245 The main risk seems to involve a maternal history of primary myxedema rather than goitrous Hashimoto’s disease.165 Endemic cretinism has also been associated with the finding of maternal thyroid growth-blocking antibodies,292 but this association has yet to be confirmed.
Postpartum Thyroid Disease
Thyroid dysfunction is a well-recognized complication of the puerperium, and it follows that close surveillance of mothers with thyroid disease or a history of thyroid disease should be continued after delivery. Although Graves’ thyrotoxicosis and Hashimoto’s thyroiditis tend to improve (or remit) in later pregnancy, a relapse often occurs postpartum11,293 and may be transient or protracted. The reason for relapse at this time is most likely the rebound in immune surveillance that occurs postpartum.12 TPO antibodies often are increased postpartum in Hashimoto’s disease and Graves’ disease, but correlation of TSHR antibody status and the onset or relapse of thyrotoxicosis has been relatively weak.294 Rarely, postpartum thyroid dysfunction is associated with hypothalamic-pituitary disease as part of pituitary failure in Sheehan’s syndrome or lymphocytic hypophysitis.295–297 Transient isolated thyrotropin deficiency in the postpartum period has been described.297 Most often, thyroid dysfunction in the puerperium is part of the spectrum of postpartum thyroiditis (PPT), which differs significantly from the aforementioned disorders in terms of its pathogenesis, treatment, and outcome. Otherwise, diagnosis and treatment of thyroid disease in the postpartum period proceed as outlined earlier and are subject to the usual precautions relevant to pregnancy or breastfeeding.
Postpartum Thyroiditis
PPT is now well recognized as a distinct variant of silent (painless) thyroiditis298–300 associated with thyroid autoimmunity and transient thyroid dysfunction (hypothyroidism and/or hyperthyroidism). Since the first modern descriptions of PPT more than 25 years ago, much has been learned about the pathophysiology, clinical course, treatment, and outcome of this disorder. The incidence of PPT averages 5%,4 with a range as low as 1% to 2%, to as high as 20%. The prevalence of PPT appears to be higher in those with separate autoimmune disorders. For example, patients with type 1 diabetes mellitus appear to have a prevalence of PPT of 25% to 30%.301,302
Thyrotoxic Phase
Typically, 70% of women experience a transient period of thyrotoxicosis with onset between 6 weeks’ and 3 months’ postpartum and lasting 1 to 2 months before spontaneously resolving. A goiter develops in 50% of cases. Thyrotoxic symptoms usually are milder than in Graves’ disease and may be overlooked or attributed to the adjustment to motherhood. Fatigue and palpitations may be prominent. Hypertension is seen occasionally.303 Specific tests to detect biochemical thyrotoxicosis are the same as those generally used in pregnancy (Table 25-3). A caveat is that some patients in whom PPT develops have antibodies to T4 and T3, which may lead to spuriously high or low results for total or free thyroid hormone levels, depending on the immunoassay method used.304 TPO antibodies usually are present in women with PPT. Higher TPO antibody titers are associated with more severe disease and a greater risk of subsequent hypothyroidism. In one study, nearly 40% of patients who had TPO antibody positivity developed postpartum thyroid dysfunction, in contrast to <1% of patients who were TPO antibody negative. In this series, nearly 90% of patients who developed postpartum thyroid dysfunction were TPO antibody positive.305 Overall, the risk of developing postpartum thyroid dysfunction for mothers who are TPO antibody negative appears to be 100-fold lower than in TPO antibody–positive women.306
Table 25-3
Diagnostic Measures in Postpartum Thyroiditis
| Investigation | Results |
| Thyroid function tests | TSH suppressed, FT4 or FT3 elevated in thyrotoxicosis; TSH increased, FT4 normal or low in hypothyroidism |
| Thyroid autoantibodies | Positive result indicates autoimmune thyroid disorder; higher titers associated with increased risk of hypothyroidism |
| Isotope uptake and scan (thyrotoxicosis only) | Low or absent uptake and scan in PPT; increased uptake and scan in Graves’ disease |
Upon evaluating a patient who is thyrotoxic postpartum, it is important to distinguish between thyrotoxicosis caused by PPT and that caused by Graves’ disease. Radioactive iodine uptake in the thyroid readily differentiates the two, with elevation seen in Graves’ disease and reduction or absence noted in thyrotoxic PPT. However, this test is contraindicated in actively lactating mothers. If such testing is required, mothers should interrupt breastfeeding, while continuing to express and discard milk, for 1 to 3 days after receiving tracer doses of technetium 99m or 123I.307 Thereafter, lactation can be resumed. Rarely other causes of reduced radioactive iodine uptake include iodine-induced thyrotoxicosis and iatrogenic thyrotoxicosis secondary to thyroid hormone administration.
When symptoms of PPT are more severe, the differential diagnosis includes postpartum psychotic depression (postpartum psychosis). Typically, this condition occurs earlier than PPT, 1 to 2 weeks after delivery, and is less common, often reported in 1 per 1000 deliveries.308 Although hyperthyroxinemia and impaired TSH responses to TRH may be seen in patients with acute psychiatric disorders, thyrotoxicosis has not been confirmed in women with postpartum psychosis. Thyroid antibodies are typically negative in those with postpartum psychosis.
Hypothyroid Phase
Most commonly, with or without symptoms or documentation of a preceding episode of thyrotoxicosis, primary hypothyroidism occurs 3 to 6 months’ postpartum. Symptoms of lethargy, cold intolerance, and impaired memory and concentration are typically mild. Fatigue can be indistinguishable from that related to poor sleep patterns commonly experienced by mothers in the postpartum state. An increase in depressive symptoms and depression scores has been reported in patients with PPT hypothyroidism as compared with euthyroid postpartum controls.309 Up to 20% of patients were considered mildly depressed on the basis of symptom scores, although the results did not achieve statistical significance. Moreover, depressed mood related to the course of hypothyroidism and to positive thyroid antibodies was seen more often in women with postpartum depression.309 It has not yet been proven that postpartum hypothyroidism is a major cause of postpartum depression, however. The largest trial investigating this question enrolled nearly 750 women who were 6 months’ postpartum.310 Biochemical testing for thyroid dysfunction and TPO antibody status was complemented by a self-administered questionnaire for depression. Both the patient and the physician scoring the questionnaire were blinded regarding test data. Although nearly 12% of patients were found to have postpartum thyroid dysfunction, this trial failed to show any relation between thyroid status and the incidence of depression. The prevalence rate of depression was 9.4% among all women, as tested at 6 months’ postpartum. However, hypothyroidism should be considered in patients with postpartum depression because treatment with thyroid hormone may result in clinical improvement when identified.
Although spontaneous recovery is usual by 10 to 12 months’ postpartum, 15% to 20% of women remain permanently hypothyroid. The development of permanent hypothyroidism is correlated with antibody titer and severity of the hypothyroid phase of PPT.311,312 A high prevalence of organification defects and susceptibility to iodine-induced hypothyroidism is observed after PPT.313 The risk of recurrence after subsequent pregnancies is up to 70%.314 Even with full biochemical recovery, women with a history of postpartum thyroid dysfunction have a markedly increased risk of developing permanent primary hypothyroidism in the years thereafter. For this reason, it is recommended that these women receive annual testing of serum TSH, even if asymptomatic.61
Predisposing Factors
The major risk factor for the development of PPT is, of course, the postpartum state. The condition may also occur after miscarriage or abortion300,315 and has been described after pregnancy losses as early as 5 to 6 weeks’ gestation.316 Women with a personal or family history of autoimmune thyroid disease are also at increased risk, specifically those with Hashimoto’s thyroiditis. The presence of TPO antibodies in the first trimester is associated with a 30% to 35% incidence of PPT,317 whereas PPT may develop in two thirds of women who remain TPO antibody positive 2 days’ postpartum. White and Asian women, as opposed to blacks, may be at increased risk of PPT, as are cigarette smokers.318 Maternal age, parity, presence of a goiter, breastfeeding, and infant birth weight have not been associated with an increased risk of PPT.318 It is controversial whether the sex of the infant has any association with PPT.315,318 On the other hand, a lower ponderal index272 and a reduced early neonatal growth rate have been reported in association with maternal thyroid-antibody positivity in pregnancy and the subsequent development of PPT. Maternal thyroid function was normal in the women during these pregnancies. Iodide exposure may also be a risk factor for the development of PPT,319,320 although a similar incidence of PPT is seen in geographic areas with high315 and low iodine intake.
Genetic, Humoral, and Cellular Mechanisms
Immune injury to the thyroid gland in PPT is mediated by humoral and cellular mechanisms. A rebound in the immune response is thought to exacerbate autoimmune thyroid disease postpartum.12,321 A decline in serum cortisol levels at this time may also be important.322 The risk of development of PPT has been associated with HLA haplotypes B8, DR3, DR4, DR5, DRW3, DRW8, and DRW9.311,323–326 The relative risk is increased twofold to fivefold. The risk of PPT is reduced in association with the HLA-DR2 haplotype.311 Variability in HLA haplotypes associated with PPT risk may be due to geographic and population differences. Also, as proposed by DeGroot and Quintans, it is the interaction of genetics along with immune dysfunction and environmental factors that contributes to the clinical expression of autoimmune thyroid disorders.327 TPO antibodies reflect disease activity in PPT. Total IgG concentrations are elevated in women with PPT.328 Thyroid antibodies are predominantly associated with IgG subclasses 1 and 4.328,329 A nonspecific increase in anti-DNA antibodies is seen in postpartum relapses of thyroid autoimmune disorders, including the thyrotoxic phase of PPT.330
Thyroid cytolytic activity resulting from T cell or killer cell attack320,327 may be important in the release of thyroid hormones in PPT, as opposed to thyroid antibodies, which serve as markers of the disease process.329 A significant increase in circulation of large peripheral granular lymphocytes with killer cell and cytotoxic activity has been reported in women with PPT as opposed to euthyroid controls or patients with Graves’ disease.331 Others have found no differences in natural killer cell activity between postpartum euthyroid controls and patients with PPT,332 although both groups showed a relative increase when compared with the reduced killer cell activity noted immediately postpartum. Similarly, these and other investigators showed no change in antibody-dependent cell-mediated cytotoxicity in PPT.331,332 Analysis of circulating lymphocyte subsets showed an increase in activated T cells with helper-inducer activity in PPT,333 opposite the findings in Graves’ thyrotoxicosis. Stagnaro-Green and colleagues prospectively studied T cell phenotypes during pregnancy and postpartum and found that women in whom PPT developed had higher ratios of CD4+ to CD8+ T cells throughout gestation than did unaffected women, and therefore exhibited a lesser degree of immunosuppression.317 Another study reported no change in peripheral blood lymphocytes in PPT but observed an increase in intrathyroidal activated B lymphocytes and T cells with helper-inducer activity.334 Overall, the cellular mechanisms involved in the pathogenesis of PPT, although undoubtedly important, remain only partially defined.
Treatment and Prevention
A recent investigation by Negro and colleagues suggested that selenium supplementation during pregnancy may reduce the risk of PPT in women who are TPO antibody positive.335 In a prospective, randomized fashion, 2143 euthyroid pregnant women were screened for TPO antibody. Approximately 8% were found to have TPO antibody positivity. Women in this cohort were randomized to selenomethionine (200 µg/day) or placebo. A separate group of TPO antibody–negative women without intervention served as a dual control. TPO antibody–positive women who received selenium supplementation had a significant reduction in the prevalence of PPT (49%) compared with those receiving placebo (29%). These data are notable, although most clinicians have not incorporated selenium supplementation into clinical practice, in part because of lack of confirmatory data. Safety data regarding administration of this vitamin supplement throughout gestation are also limited. Notably, selenium administration has been associated recently with an increased risk of diabetes mellitus.
Thyroid Nodules and Carcinoma During Pregnancy
Ultrasonographic data suggest that thyroid nodules appear more frequently in multiparous women.50,183,336 However, these studies have been conducted in areas with lower iodine intake than is found in the United States. Thyroid radioisotope scanning is contraindicated in the workup of a patient with a thyroid nodule during pregnancy, and although ultrasound provides excellent definition of anatomy, it is relatively nonspecific in terms of thyroid histology or pathology. If a nodule is clinically palpable, the diagnosis can be made most specifically by fine-needle aspiration (FNA) biopsy.
For this reason, FNA (with ultrasound guidance) should be considered the recommended diagnostic intervention for any pregnant women with a thyroid nodule larger than 1.0 to 1.5 cm in maximal diameter.337,338 Generally, FNA is a safe procedure that is easily tolerated, with few contraindications. Because the primary risk is hematoma formation, the major relative contraindication is the concomitant use of anticoagulants such as heparin. Local anesthesia in the form of subcutaneous lidocaine is generally accepted.
Cancer per se is relatively uncommon in pregnancy. It is generally believed that pregnancy has little effect on the development or progression of thyroid carcinoma, and that thyroid carcinoma has little effect on pregnancy outcome.337,339 Although some authors have reported an increased prevalence of neoplasia in thyroid nodules initially discovered during pregnancy, these reports fail to demonstrate a causative association between pregnancy and increased cancer risk. Of a series of 39 patients who underwent FNA biopsy during pregnancy, more than 60% had benign cytologic results and histology confirmed neoplasia in only 20%, half of whom had benign adenomas and the others, papillary thyroid cancer.339 These data are similar to expected distributions in nonpregnant cohorts. Although theoretically important, the roles of estrogens, thyroid stimulators, and other growth factors, as well as of immunosuppression, in the development or progression of thyroid neoplasia during pregnancy remain uncertain. In addition, several authors have reported that the prognosis of patients with papillary and follicular cancers diagnosed during pregnancy does not differ from that of nonpregnant women with respect to disease stage, recurrence, or mortality.340,341
Although surgery during the second trimester is often considered for nodules that are suspicious for or diagnostic of thyroid cancer, some authors recommend delay until the postpartum period.342 This is generally considered a reasonable approach, although patients must be evaluated on a case-by-case basis. For example, one might consider surgery to remove a cancerous nodule during pregnancy if the thyroid cancer was diagnosed early in gestation and if the nodule is enlarging by midpregnancy. Conversely, a small tumor identified late in pregnancy would likely warrant consideration of treatment following delivery. Very often, the anxiety caused by the cytologic diagnosis of possible cancer drives the patient’s desire for operation in the near term. Among pregnant women in whom thyroid cancer is diagnosed, long-term outcomes do not differ between those undergoing surgery during pregnancy and those operated on postpartum.340,343
Surgery remains the initial therapy for thyroid cancer. As was previously noted, radioisotope administration (such as 131I) is contraindicated in any patient with thyroid cancer who is currently pregnant or is actively breastfeeding. Although no studies have systematically investigated the appropriate timing of future pregnancy after high-dose radioactive iodine therapy is received for ablation of thyroid tissue, most endocrinologists recommend waiting 6 months or longer before attempting conception.344 For patients with a known history of thyroid cancer (and on suppressive levothyroxine therapy) who become pregnant, it is prudent to maintain close clinical follow-up and ensure adequate thyroid hormone replacement (and possible TSH suppression) throughout gestation (Fig. 25-7). No standard recommendation has been put forth with regard to continued TSH suppression throughout gestation in patients with cancer. For low-risk patients, many physicians target a TSH value of approximately 0.5 mIU/L; high-risk patients often remain on levothyroxine doses that maintain prepregnancy levels of TSH suppression. No data presently support benefit or harm associated with either approach. Mild TSH suppression during pregnancy is considered safe.224
FIGURE 25-7 Gestational week of the initial increase in the levothyroxine dose. A shows the week at which the levothyroxine dose was first increased in 11 women with primary hypothyroidism; in these women, the dose was increased when the thyrotropin concentration was greater than 5.0 µU per milliliter. B shows the week at which the levothyroxine dose was first increased in women with a history of thyroid cancer; in these women, the dose was increased when the thyrotropin concentration was greater than 0.5 µU per milliliter. (Data from Alexander EK, Marqusee E, Lawrence J, et al: Timing and magnitude of increase in levothyroxine requirements during pregnancy in women with hyperthyroidism, N Engl J Med 351:241, 2004.)
References
1. Thomas, R, Reid, RL. Thyroid disease and reproductive dysfunction: a review. Obstet Gynecol. 1987;70:789.
2. Medvei, VC. A history of endocrinology. Boston: MTP; 1982. [p 58].
3. Lazarus, JH, Othman, S. Thyroid disease in relation to pregnancy. Clin Endocrinol. 1991;34:91.
4. Gerstein, HC. How common is postpartum thyroiditis? A methodological overview of the literature. Arch Intern Med. 1990;150:1397.
5. Abdoul-Khair, SA, Crooks, J, Turnbull, AC, et al. The physiologic changes in thyroid function during pregnancy. Clin Sci. 1964;27:195.
6. Wong, TK, Pekary, AE, Hoo, GS, et al. Comparison of methods for measuring free thyroxin in nonthyroidal illness. Clin Chem. 1992;38:720.
7. Burrow, GN. Thyroid diseases in pregnancy. In: Burrow GN, Oppenheimer JH, Volp JR, eds. Thyroid function and disease. Philadelphia: WB Saunders; 1989:292.
8. Wiersinga, WM, Vet, T, Berghout, A, et al. Serum free thyroxine during pregnancy: a meta-analysis. In: Beckers C, Reinwein D, eds. The thyroid and pregnancy. Stuttgart, Germany: Shattauer; 1991:79.
9. Burrow, GN. Pituitary and adrenal disorders. In: Burrow GN, Ferris TF, eds. Medical complications during pregnancy. ed 3. Philadelphia: WB Saunders; 1988:254.
10. Burrow, GN, Fisher, DA, Larsen, PR. Maternal and fetal thyroid function. N Engl J Med. 1994;331:1072.
11. Salvi, M, How, J. Pregnancy and autoimmune thyroid disease. Endocrinol Metab Clin North Am. 1987;16:431.
12. Lewis, JE, Coulam, CB, Moore, SB. Immunologic mechanisms in the maternal-fetal relationship. Mayo Clin Proc. 1986;61:655.
13. Amino, N, Izumi, Y, Hidaka, Y, et al. No increase of blocking type anti-thyrotropin receptor antibodies during pregnancy in patients with Graves’ disease. J Clin Endocrinol Metab. 2003;88:5871.
14. Goldsmith, R, Sturgis, S, Lerman, J, et al. Menstrual pattern in thyroid disease. J Clin Endocrinol Metab. 1952;12:846.
15. Feek, CM, Sawers, JSA, Brown, NS, et al. Influence of thyroid status on dopaminergic inhibition of thyrotropin and prolactin secretion. J Clin Endocrinol Metab. 1980;51:585.
16. Kramer, M, Kauschansky, A, Genel, M. Adolescent secondary amenorrhea: association with hypothalamic hypothyroidism. Pediatrics. 1979;94:300.
17. Scanlon, MF, Chan, V, Heath, M, et al. Dopaminergic control of thyrotropin α-subunit, thyrotropin, β-subunit, and prolactin in euthyroidism and hypothyroidism: dissociated responses to dopamine receptor blockade with metoclopramide in hypothyroid subjects. J Clin Endocrinol Metab. 1981;53:360.
18. Willansky, DL, Greisman, B. Early hypothyroidism in patients with menorrhagia. Am J Obstet Gynecol. 1989;160:673.
19. Bohnet, HG, Fieldler, K, Leidenberger, FA. Subclinical hypothyroidism and infertility. Lancet. 1981;2:1278.
20. Gerhard, I, Becker, T, Eggert-Kruse, W, et al. Thyroid and ovarian function in infertile women. Hum Reprod. 1991;6:338.
21. Seki, K, Kato, K. Increased thyroid-stimulating hormone response to thyrotropin-releasing hormone in hyperprolactinemic women. J Clin Endocrinol Metab. 1985;61:1138.
22. Gerhard, I, Eggert-Kruse, W, Merzoug, K, et al. Thyrotropin-releasing hormone (TRH) and metoclopramide testing in infertile women. Gynecol Endocrinol. 1991;5:15.
23. Rotmensch, S, Scommegna, A. Spontaneous ovarian hyperstimulation syndrome associated with hypothyroidism. Am J Obstet Gynecol. 1989;160:1220.
24. Rovet, J, Simic, N. The role of transient hypothyroxinemia of prematurity in development of visual abilities. Semin Perinatol. 2008;32:431.
25. Greenman, GW, Gabrielson, MA, Howard-Flanders, I, et al. Thyroid dysfunction in pregnancy: fetal loss and follow-up of evaluation of surviving infants. N Engl J Med. 1962;267:426.
26. Allan, WC, Haddow, JE, Palomaki, GE, et al. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J Med Screen. 2000;7:127.
27. Abalovich, M, Gutierrez, S, Alcaraz, G, et al. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2002;12:63.
28. Casey, B, Dashe, JS, Wells, CE, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;105:239.
29. Glinoer, D, Fernandez-Soto, D, Bourdoux, P, et al. Pregnancy in patients with mild thyroid abnormalities: maternal and neonatal repercussions. J Clin Endocrinol Metab. 1991;73:421.
30. Iijima, T, Tada, H, Hidaka, Y, et al. Effects of autoantibodies on the course of pregnancy and fetal growth. Obstet Gynecol. 1997;90:364.
31. Bagis, T, Gokcel, A, Saygili, ES. Autoimmune thyroid disease in pregnancy and the postpartum period: relationship to spontaneous abortion. Thyroid. 2001;11:1049.
32. Prummel, MF, Wiersinga, WM. Thyroid autoimmunity and miscarriage. Eur J Endocrinol. 2004;150:751.
33. Poppe, K, Glinoer, D, Tournaye, H, et al. Assisted reproduction and thyroid autoimmunity: an unfortunate combination? J Clin Endocrinol Metab. 2003;88:4149.
34. Ghafoor, F, Mansoor, M, Malik, T, et al. Role of thyroid peroxidase antibodies in the outcome of pregnancy. J Coll Physicians Surg Pak. 2006;16:468.
35. Stagnaro-Greene, A. Maternal thyroid disease and preterm delivery. J Clin Endocrinol Metab. 2009;94:21.
36. Negro, R, Formoso, G, Mangieri, T, et al. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. J Clin Endocrinol Metab. 2006;91:2587.
37. Brayshaw, ND, Brayshaw, DD. Thyroid hypofunction in premenstrual syndrome. N Engl J Med. 1986;315:1486.
38. Roy-Byrne, PP, Rubinow, DR, Hoban, MC, et al. TSH and prolactin response to TRH in patients with premenstrual syndrome. Am J Psychiatry. 1987;144:480.
39. Casper, RF, Patel-Christopher, A, Powell, AM. Thyrotropin and prolactin responses to thyrotropin releasing hormone in premenstrual syndrome. J Clin Endocrinol Metab. 1989;68:608.
40. Southern, AL, Olivio, J, Gorelon, GG, et al. The conversion of androgens to estrogen in hyperthyroidism. J Clin Endocrinol Metab. 1974;38:207.
41. Akande, E, Hockaday, T. Plasma luteinizing hormone levels in women with thyrotoxicosis. J Endocrinol. 1972;53:173.
42. Tanaka, T, Tamai, H, Kuma, K, et al. Gonadotropin response to luteinizing hormone releasing hormone in hyperthyroid patients with menstrual disturbances. Metabolism. 1981;30:323.
43. Roger, J. Menstruation and systemic disease. N Engl J Med. 1958;259:676.
44. Anselmo, J, Cao, D, Karrison, T, et al. Fetal loss associated with excess thyroid hormone exposure. JAMA. 2004;292:691.
45. Amino, N. Autoimmunity and hypothyroidism. In: Lazarus JH, Hall R, eds. Hypothyroidism and goiter. London: WB Saunders; 1988:591.
46. Rojeski, MT, Gharib, H. Nodular thyroid disease: evaluation and management. N Engl J Med. 1985;313:428.
47. Ansar Ahmed, S, Young, PR, Penhale, WJ. Beneficial effect of testosterone in the treatment of chronic autoimmune thyroiditis in rats. J Immunol. 1986;136:143.
48. Talal, N, Ansar Ahmed, S. Immunomodulation by hormones: an area of growing importance. J Rheumatol. 1987;14:191.
49. Phillips, DIW, Lazarus, JH, Butland, BK. The influence of pregnancy and reproductive span on the occurrence of autoimmune thyroiditis. Clin Endocrinol. 1990;32:301.
50. Glinoer, D, Fernandez Soto, M, Bourdoux, P, et al. Pregnancy in patients with mild thyroid abnormalities: maternal and neonatal repercussions. J Clin Endocrinol Metab. 1991;73:421.
51. Beckers, C. Iodine economy in and around pregnancy. In: Beckers C, Reinwein D, eds. The thyroid and pregnancy. Stuttgart, Germany: Schattauer; 1991:25.
52. Roti, E. Regulation of thyroid-stimulating hormone (TSH) secretion in the fetus and neonate. J Endocrinol Invest. 1988;11:145.
53. Gushurst, CA, Mueller, JA, Green, JA, et al. Breast milk iodide: reassessment in 1980. Pediatrics. 1984;73:354.
54. Hetzel, BS. Iodine deficiency disorders and their eradication. Lancet. 1983;2:1136.
55. Delange, F, Burgi, H. Iodine deficiency disorders in Europe. Bull World Health Organ. 1989;67:317.
56. Alexander, WD, Koutras, DA, Crooks, J, et al. Quantitative studies of iodine metabolism in thyroid disease. Q J Med. 1966;31:281.
57. Dworkin, HJ, Jacquez, JA, Beirewaltes, WH. Relationship of iodine ingestion to iodine excretion in pregnancy. J Clin Endocrinol Metab. 1966;26:1329.
58. Halnan, KE. Radioiodine uptake of the human thyroid in pregnancy. Clin Sci. 1958;17:281.
59. Pochin, EE. The iodine uptake of the human thyroid throughout the menstrual cycle and in pregnancy. Clin Sci. 1952;11:441.
60. Delange, F. Optimal iodine nutrition during pregnancy, lactation and the neonatal period. Int J Endocrinol Metab. 2004;2:1.
61. Abalovich, M, Nobuyuki, A, Barbour, LA, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2007;92:S1.
62. Hollowell, JG, Staehling, NW, Hannon, WH, et al. Iodine nutrition in the United States. Trends and public health implications: iodine excretion data from National Health and Nutrition Examination Surveys I and III. J Clin Endocrinol Metab. 1998;83:3401.
63. Glinoer, D, Gershengorn, MC, Dubois, A, et al. Stimulation of thyroxine-binding globulin synthesis by isolated rhesus monkey hepatocytes after in vivo 8-estradiol administration. Endocrinology. 1977;100:807.
64. Ain, KB, Mori, Y, Refetoff, S. Reduced clearance of thyroxine binding globulin (TBG) with increased sialylation: a mechanism for estrogen-induced elevation of serum TBG concentration. J Clin Endocrinol Metab. 1987;65:686.
65. Oppenheimer, JH. Role of plasma proteins in the binding, distribution and metabolism of thyroid hormones. N Engl J Med. 1968;278:1153.
66. Osathanondh, R, Tulchinsky, D, Chopra, IJ. Total and free thyroxine and triiodothyronine in normal and complicated pregnancy. J Clin Endocrinol Metab. 1976;42:98.
67. Whitworth, AS, Midgley, JEM, Wilkins, TA. A comparison of free T4 and the ratio of total T4 to T4-binding globulin in serum through pregnancy. Clin Endocrinol. 1982;17:307.
68. Demers, LM, Spencer, CA. NACB guidelines: laboratory medicine practice guidelines: laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13:11.
69. Wilke, TJ. A challenge of several concepts of free thyroxine index for assessing thyroid status in patients with altered thyroid-binding protein capacity. Clin Chem. 1983;29:56.
70. Premachandra, BN, Gossain, VV, Perlstein, IB. Effect of pregnancy on thyroxine binding globulin (TBG) in partial TBG deficiency. Am J Med Sci. 1977;274:189.
71. Alexander, EK, Marqusee, E, Lawrence, J, et al. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N Engl J Med. 2004;351:241.
72. Haddow, JE, McClain, MR, Lambert-Messerlain, G, et al. Variability in thyroid-stimulating hormone suppression by human chorionic gonadotropin during early pregnancy. J Clin Endocrinol Metab. 2008;93:3341.
73. Glinoer, D, De Nayer, P, Bourdoux, P, et al. Regulation of maternal thyroid during pregnancy. J Clin Endocrinol Metab. 1990;71:276.
74. Yamamoto, T, Amino, N, Tanizawa, O, et al. Longitudinal study of serum thyroid hormones, chorionic gonadotropin and thyrotropin during and after normal pregnancy. Clin Endocrinol. 1979;10:459.
75. Pachiarotti, A, Martino, E, Bartalena, L, et al. Serum thyrotropin by ultrasensitive immunoradiometric assay and free thyroid hormones in pregnancy. J Endocrinol Invest. 1986;9:185.
76. Gow, SM, Kellett, HA, Seth, J, et al. Limitations of new thyroid function tests in pregnancy. Clin Chim Acta. 1985;152:325.
77. Kvetny, J, Poulsen, HK. Nuclear thyroxine and 3,5,3′-triiodothyronine receptors in human mononuclear blood cells during pregnancy. Acta Endocrinol. 1984;105:19.
78. Calvo, R, Obregon, MJ, Riuz de Ona, C, et al. Thyroid hormone economy in pregnant rats near term: a physiological animal model of non-thyroidal illness? Endocrinology. 1990;126:10.
79. Sapin, R, d’Herbomez, M. Free thyroxine measured by equilibrium dialysis and nine immunoassays in sera with various serum thyroxine-binding capacities. Clin Chem. 2003;49:1531.
80. Soldin, OP, Tractenberg, RE, Hollowell, JG, et al. Trimester-specific changes in maternal thyroid hormone, TSH, and thyroglobulin concentrations during gestation: trends and associations across trimesters in iodine sufficiency. Thyroid. 2004;14:1084.
81. Dashe, JS, Casey, BM, Wells, CE, et al. Thyroid-stimulating hormone in singleton and twin pregnancy: importance of gestational age-specific reference ranges. Obstet Gynecol. 2005;106:753.
82. Stoffer, RP, Koeneke, IA, Chesky, VE, et al. The thyroid in pregnancy. Am J Obstet Gynecol. 1957;74:300.
83. Mandel, SJ, Larsen, PR, Seely, EW, et al. Increased need for thyroxine during pregnancy in women with primary hypothyroidism. N Engl J Med. 1990;323:91.
84. Glinoer, D, Delange, F, Laboureur, I, et al. Maternal and neonatal thyroid function at birth in an area of marginally low iodine intake. J Clin Endocrinol Metab. 1992;75:800.
85. Burrow, GN, Fisher, DA, Larsen, PR. Maternal and fetal thyroid function. N Engl J Med. 1994;331:1072.
86. Vulsma, T, Gous, MH, De Vijlder, JJM. Maternal-fetal transfer of thyroxine in congenital hypothyroidism due to a total organification defect or thyroid agenesis. N Engl J Med. 1989;321:13.
87. Rajatanavin, R, Chailurkit, L, Srisupandit, S, et al. Trophoblastic hyperthyroidism: clinical and biochemical features in five cases. Am J Med. 1988;85:237.
88. Kenimer, JC, Hershman, JM, Higgins, HP. The thyrotropin in hydatidiform moles in human chorionic gonadotropin. J Clin Endocrinol Metab. 1975;40:482.
89. Valdalem, JL, Pirens, G, Hennen, G, et al. Thyroliberin and gonadoliberin tests during pregnancy and the puerperium. Acta Endocrinol. 1977;86:695.
90. Ylikorkala, O, Kivinen, S, Reinila, M. Serial prolactin and thyrotropin responses to thyrotropin-releasing hormone throughout normal human pregnancy. J Clin Endocrinol Metab. 1979;48:288.
91. Burrow, GN, Polackwich, R, Donabedian, R. The hypothalamic-pituitary-thyroid axis in normal pregnancy. In: Fisher DA, Burrow GN, eds. Perinatal thyroid physiology and disease. New York: Raven; 1975:1.
92. Chan, BY, Swaminanthan, R. Serum thyrotropin concentration measured by sensitive assays in normal pregnancy. Br J Obstet Gynaecol. 1988;95:1332.
93. Desai, RK, Norman, RJ, Jialal, I, et al. Spectrum of thyroid function abnormalities in gestational trophoblastic neoplasia. Clin Endocrinol. 1988;29:583.
94. Cave, WT, Jr., Dunn, JT. Choriocarcinoma with hyperthyroidism: probable identity of the thyrotropin with human chorionic gonadotropin. Ann Intern Med. 1976;85:60.
95. Guillaume, J, Schussler, GC, Goldman, J, et al. Components of the total serum thyroxine during pregnancy: high free thyroxine and blunted thyrothropin (TSH) response to TSH-releasing hormone in the first trimester. J Clin Endocrinol Metab. 1985;60:678.
96. Harada, A, Hershman, JM, Reed, AW, et al. Comparison of thyroid stimulators and thyroid hormone concentrations in the sera of pregnant women. J Clin Endocrinol Metab. 1979;48:793.
97. Pekonen, F, Alfthan, H, Stenman, UH, et al. Human chorionic gonadotropin (HCG) and thyroid function in early human pregnancy: circadian variation and evidence for intrinsic thyrotropic activity of HCG. J Clin Endocrinol Metab. 1988;66:853.
98. Ballabio, M, Poshyachinda, M, Ekins, RP. Pregnancy-induced changes in thyroid function: role of human chorionic gonadotropin as a putative regulator of maternal thyroid. J Clin Endocrinol Metab. 1991;73:824.
99. Yoshikawa, N, Nishikawa, M, Horimoto, M, et al. Thyroid stimulating activity in sera of normal pregnant women. J Clin Endocrinol Metab. 1989;69:891.
100. Kinmura, M, Amino, N, Tamaki, H, et al. Physiologic thyroid activation in normal pregnancy is induced by circulating HCG. Obstet Gynecol. 1990;75:775.
101. Ekins, R. Roles of serum thyroxine binding proteins and maternal thyroid hormones in fetal development. Lancet. 1985;1:1129.
102. Ekins, R, Sinha, A, Ballabio, M, et al. Role of maternal carrier proteins in the supply of thyroid hormones to the feto-placental unit: evidence of a feto-placental requirement for thyroxine. In: Delange F, Fisher DA, Glinoer D, eds. Research in congenital hypothyroidism. New York: Plenum; 1989:45.
103. Carayon, P, Lefort, G, Nisula, B. Interaction of human chorionic gonadotropin and human luteinizing hormone with human thyroid membranes. Endocrinology. 1980;106:1907.
104. Davies, TF, Taliadouros, GS, Catt, KJ, et al. Assessment of urinary thyrotropin-competing activity in choriocarcinoma and thyroid disease: further evidence for human chorionic gonadotropin interacting at the thyroid cell membrane. J Clin Endocrinol Metab. 1979;49:353.
105. Silverberg, J, O’Donnel, J, Sugenaya, A, et al. Effect of human chorionic gonadotropin on human thyroid tissue in vitro. J Clin Endocrinol Metab. 1978;46:420.
106. Carayon, P, Amir, S, Nisula, B, et al. Effect of carboxypeptidase digestion of the human chorionic gonadotropin molecule on its thyrotropic activity. Endocrinology. 1981;108:1891.
107. Mann, K, Schneider, N, Hoermann, R. Thyrotropic activity of acidic isoelectric variants of human chorionic gonadotropin from trophoblastic tumors. Endocrinology. 1986;118:1558.
108. Hershman, JM, Lee, HY, Sugawara, M, et al. Human chorionic gonadotropin stimulates iodide uptake, adenylate cyclase and deoxyribonucleic acid synthesis in cultured rat thyroid cells. Endocrinology. 1988;67:74.
109. Davies, TF, Platzer, M. HCG-induced TSH receptor activation and growth acceleration in FRTL-5 cells. Endocrinology. 1986;118:2149.
110. Ballabio, M, Sinha, AK, Ekins, RP. Thyrotropic activity of crude HCG in FRTL-5 rat thyroid cells. Acta Endocrinol. 1987;116:479.
111. Amir, SM, Eudo, K, Osathanoudh, R, et al. Divergent responses by human and mouse thyroids to human chorionic gonadotropin in vitro. Mol Cell Endocrinol. 1985;39:31.
112. Pekary, AE, Azukizawa, M, Hershman, JM. Thyroidal responses to human chorionic gonadotropin in the chicken and rat. Horm Res. 1983;17:36.
113. Uchimura, H, Nagataki, S, Ito, K, et al. Inhibition of the thyroid adenylate cyclase response to thyroid-stimulating immunoglobulin G by crude and asialo-human choriogonadotropin. J Clin Endocrinol Metab. 1982;55:347.
114. Amir, S, Shimohigahsi, Y, Carayon, P, et al. Role of carbohydrate moiety of the human chorionic gonadotropin molecule in its thyrotropic activity. Arch Biochem Biophys. 1984;229:170.
115. Fein, HG, Rosen, SW, Weintraub, BD. Increased glycosylation of serum human chorionic gonadotropin and subunits from eutopic and ectopic sources: comparison with placental and urinary forms. J Clin Endocrinol Metab. 1980;50:1111.
116. Goodwin, TM, Montoro, M, Mestman, JH, et al. The role of chorionic gonadotropin in transient hyperthyroidism of hyperemesis gravidarum. J Clin Endocrinol Metab. 1992;75:1333.
117. Stricker, R, Echenard, M, Eberhart, R, et al. Evaluation of maternal thyroid function during pregnancy: the importance of using gestational age-specific reference intervals. Eur J Endocrinol. 2007;157:509.
118. Fisher, DA, Dussault, JH, Sack, J, et al. Ontogenesis of hypothalamic-pituitary-thyroid function in man, sheep and rat. Recent Prog Horm Res. 1977;33:59.
119. Fisher, DA, Polk, DH. Development of the thyroid. Baillieres Clin Endocrinol Metab. 1989;3:627.
120. Thorpe-Beeston, JG, Nicolaides, KH, Felton, CV, et al. Maturation of the secretion of thyroid hormone and thyroid-stimulating hormone in the fetus. N Engl J Med. 1991;324:532.
121. Roti, E, Gnudi, A, Braverman, LE. The placental transport, synthesis and metabolism of hormones and drugs which affect thyroid function. Endocr Rev. 1983;4:131.
122. Greenberg, AH, Czernichow, P, Reba, RC, et al. Observations on the maturation of thyroid function in early fetal life. J Clin Invest. 1970;49:1790.
123. Koivusalo, F. Evidence of thyrotropin releasing hormone activity in autopsy pancreata from newborns. J Clin Endocrinol Metab. 1981;5:734.
124. Leduque, P, Aratan-Spire, S, Czernichow, P, et al. Ontogenesis of thyrotropin releasing hormone in human fetal pancreas. J Clin Invest. 1986;78:1028.
125. Polk, DH, Reviczky, AL, Lam, RW, et al. Thyrotropin releasing hormone: effect of thyroid status on tissue concentrations in fetal sheep. Clin Res. 1988;36:203.
126. DiGianantonio, E, Schaefer, C, Mastroiacova, PP, et al. Adverse effects of prenatal methimazole exposure. Teratology. 2001;64:262.
127. Roti, E, Gundi, A, Braverman, LE, et al. Human cord blood concentrations of thyrotropin, thyroglobulin and iodothyronines after maternal administration of thyrotropin-releasing hormone. J Clin Endocrinol Metab. 1981;53:813.
128. Evans, TC, Kretschmar, RM, Hodges, RE, et al. Radioiodine uptake studies of the human fetal thyroid. J Nucl Med. 1967;8:157.
129. Johnson, JR. Fetal thyroid dose from intake of radioiodine by the mother. Health Physics. 1955;43:573.
130. Hodges, RE, Evans, TC, Bradbury, JT, et al. The accumulation of radioactive iodine by human fetal thyroid. J Clin Endocrinol Metab. 1955;15:661.
131. Chapman, EM, Corner, GW, Robinson, D, et al. The collection of radioactive iodine by human fetal thyroid. J Clin Endocrinol. 1948;8:717.
132. Fisher, DA, Dussault, J. Development of the mammalian thyroid gland. In: Greer MA, Solomon DH, eds. Handbook of physiology: endocrinology. III. Baltimore: Williams & Wilkins; 1974:21.
133. Bernal, J, Pekonen, F. Ontogenesis of the nuclear 3,5,3′-triiodothyronine receptor in the human fetal brain. Endocrinology. 1984;114:677.
134. Calvo, R, Obregon, MJ, Ruiz de Ona, C, et al. Congenital hypothyroidism as studied in rats: crucial role of maternal thyroxine but not 3,5,3′-triiodothyronine in the protection of the fetal brain. J Clin Invest. 1990;86:889.
135. Morreale de Escobar, G, Obregon, MJ, Escobar del Rey, F. Maternal-fetal thyroid hormone relationships and the fetal brain. Acta Med Austriaca. 1988;15:66.
136. Morreale de Escobar, G, Obregon, MJ, Ruiz de Ona, C, et al. Comparison of maternal to fetal transfer of 3,5,3′-triiodothyronine versus thyroxine in rats. Acta Endocrinol. 1989;120:20.
137. Ruiz de Ona, C, Obregon, MJ, Escobar del Rey, F, et al. Developmental changes in rat brain 5′-deiodinase and thyroid hormones in the fetal period. Pediatr Res. 1988;24:588.
138. Vaccari, A. Teratogenic mechanisms of dysthyroidism in the central nervous system. Prog Brain Res. 1988;73:71.
139. Morreale de Escobar, G, Calvo, R, Obregon, MJ, et al. Contribution of maternal thyroxine to fetal thyroxine pools in normal rats near term. Endocrinology. 1990;126:2765.
140. Calvo, R, Obregon, MJ, Ruiz de Oña, C, et al. Congenital hypothyroidism, as studied in rats: crucial role of maternal thyroxine but not of 3,5,3′-triiodothyronine in the protection of the fetal brain. J Clin Invest. 1990;86:889.
141. Calvo, RM, Jauniaux, E, Gulbis, B, et al. Fetal tissues are exposed to biologically relevant free thyroxine concentrations during early phases of development. J Clin Endocrinol Metab. 2002;87:1768.
142. Hetzel, BS. Progress in the prevention and control of iodine-deficiency disorders. Lancet. 1987;2:266.
143. Hetzel, BS, Mano, MT. A review of experimental studies of iodine deficiency in fetal development. J Nutr. 1989;119:145.
144. Ferreiro, B, Bernal, J, Goodyear, G, et al. Estimation of nuclear thyroid hormone receptor saturation in human fetal brain and lung during early gestation. Endocrinology. 1988;67:853.
145. Man, EB, Jones, WS, Holden, RH, et al. Thyroid function in human pregnancy. VIII. Retardation of progeny aged 7 years: relationships to maternal age and maternal thyroid function. Am J Obstet Gynecol. 1971;111:905.
146. Man, EB, Brown, JF, Serunian, SA. Maternal hypothyroxinemia: psychoneurological deficits of progeny. Ann Clin Lab Sci. 1991;21:227.
147. Raiti, S, Holsman, GB, Scott, RL, et al. Evidence for placental transfer of triiodthyronine in human beings. N Engl J Med. 1967;277:456.
148. Dussault, J, Row, VV, Lickrish, G, et al. Studies of serum triiodothyronine concentration in maternal and cord blood. J Clin Endocrinol Metab. 1969;29:595.
149. Fisher, DA, Lehman, H, Lackey, C. Placental transport of thyroxine. J Clin Endocrinol Metab. 1964;24:393.
150. Grumbach, MM, Werner, SC. Transfer of thyroid hormones across the human placenta at term. J Clin Endocrinol Metab. 1956;16:1392.
151. Carr, EA, Bierwaltes, WH, Raman, G, et al. The effect of maternal thyroid function on fetal thyroid function and development. J Clin Endocrinol Metab. 1959;19:1.
152. Kearns, JE, Hutson, W. Tagged isomers and analogs of thyroxine: Their transmission across the human placenta and other studies. J Nucl Med. 1963;4:453.
153. Myant, NB. Passage of thyroxine and triiodothyronine from mother to fetus in pregnant women. Clin Sci. 1958;17:75.
154. Penel, C, Rognoni, JB, Pastiani, P. Thyroid autoregulation. Am J Physiol. 1987;16:E165.
155. Theodoropoulos, T, Braverman, LE, Vagenakis, AG. Iodine-induced hypothyroidism. Science. 1979;205:502.
156. Mahillon, I, Peers, W, Bourdoux, P, et al. Effects of vaginal douching with povidone-iodine during early pregnancy on the iodine supply to mother and fetus. Biol Neonate. 1989;56:210.
157. Stubbe, P, Heidemann, P, Schurnbrand, P, et al. Transient congenital hypothyroidism after amniofetography. Eur J Pediatr. 1980;135:97.
158. Smerdley, P, Boyages, SC, Wu, D, et al. Topical iodine-containing antiseptics and neonatal hypothyroidism in very low birth weight infants. Lancet. 1989;2:661.
159. Danziger, Y, Pertzelan, A, Mimouni, M. Transient congenital hypothyroidism after topical iodine in pregnancy and lactation. Arch Dis Child. 1987;62:295.
160. Rey, E, Bachrach, LK, Burrow, GN. Effects of amiodarone during pregnancy. Can Med Assoc J. 1987;136:959.
161. DeWolf, D, DeSchepper, J, Verhaaren, H, et al. Congenital hypothyroid goiter and amiodarone. Acta Paediatr Scand. 1988;77:616.
162. Tubman, R, Jenkins, J, Lim, J. Neonatal hyperthyroxinemia associated with maternal amiodarone therapy. Ir J Med Sci. 1988;157:243.
163. Widehorn, J, Bhandari, AK, Bughi, S, et al. Fetal and neonatal adverse effects profile of amiodarone treatment during pregnancy. Am Heart J. 1991;122:1162.
164. Dussault, JH, Rousseau, F. Immunologically mediated hypothyroidism. Endocrinol Metab Clin North Am. 1987;16:417.
165. Tamaki, H, Amino, N, Aozasa, M, et al. Effective method for prediction of transient neonatal hypothyroidism in infants born to mothers with chronic thyroiditis. Am J Perinatol. 1989;6:296.
166. Matsuura, N, Konishi, J. Transient hypothyroidism in infants born to mothers with chronic thyroiditis: a nationwide study of 23 cases. Endocrinol Jpn. 1990;37:369.
167. Luton, D, Le Gac, I, Vuillard, E, et al. Management of Graves’ disease during pregnancy: the key role of fetal thyroid gland monitoring. J Clin Endocrinol Metab. 2005;90:6093.
168. Perelman, AH, Johnston, RL, Clemons, RD, et al. Intrauterine diagnosis and treatment of fetal goitrous hypothyroidism. J Clin Endocrinol Metab. 1990;71:618.
169. Glorieux, J, Desjardins, M, Letarte, J, et al. Useful parameters to predict eventual mental outcome of hypothyroid children. Pediatr Res. 1988;24:6.
170. Virtanen, M, Perheentupa, J. Bone age at birth: method and effect of hypothyroidism. Acta Paediatr Scand. 1989;78:412.
171. Davidson, KM, Richards, DS, Schatz, DA, et al. Successful in utero treatment of fetal goiter and hypothyroidism. N Engl J Med. 1991;324:543.
172. Wenstrom, KD, Weiner, CP, Williamson, RA, et al. Prenatal diagnosis of fetal hyperthyroidism using funipuncture. Obstet Gynecol. 1990;76:513.
173. Yoshida, K, Sakurada, T, Takahashi, T, et al. Measurement of TSH in human amniotic fluid. Clin Endocrinol. 1986;25:313.
174. Hollingsworth, DR, Alexander, NM. Amniotic fluid concentrations of iodothyronines and thyrotropin do not reliably predict fetal thyroid status in pregnancies complicated by maternal thyroid disorders or anencephaly. J Clin Endocrinol Metab. 1983;57:349.
175. Lightner, ES, Fisher, DA, Giles, H, et al. Intra-amniotic injections of thyroxine to a human fetus. Am J Obstet Gynecol. 1977;127:487.
176. Drury, MI. Hyperthyroidism in pregnancy. J R Soc Med. 1986;79:317.
177. Crooks, J, Tulloch, MI, Turnbull, AC, et al. Comparative incidence of goiter in pregnancy in Iceland and Scotland. Lancet. 1964;2:625.
178. Long, TJ, Felice, ME, Hollingsworth, DR. Goiter in pregnant teenagers. Am J Obstet Gynecol. 1985;152:670.
179. Levy, RP, Newman, DM, Rejali, LS, et al. The myth of goiter in pregnancy. Am J Obstet Gynecol. 1980;137:701.
180. Rasmussen, NG, Hornnes, PJ, Hegedus, L. Ultrasonographically determined thyroid size in pregnancy and postpartum: the goitrogenic effect of pregnancy. Am J Obstet Gynecol. 1989;160:1216.
181. Pedersen, KM, Borlum, KG, Knudson, PR, et al. Urinary iodine excretion is low and serum thyroglobulin high in pregnant women in parts of Denmark. Acta Obstet Gynecol Scand. 1988;67:413.
182. Nelson, M, Wickus, GG, Caplan, RH, et al. Thyroid gland size in pregnancy. J Reprod Med. 1987;32:888.
183. Struve, C, Ohlen, S. The influence of previous pregnancies on the prevalence of goitre and thyroid nodules in women without clinical evidence of thyroid disease. Dtsch Med Wochenschr. 1990;115:1050.
184. Gaitan, E, Cooksey, RC, Meydrech, EF, et al. Thyroid function in neonates from goitrous and non-goitrous iodine-sufficient areas. J Clin Endocrinol Metab. 1989;69:359.
185. Liu, JL, Zhuang, ZJ, Cao, XM. Changes in thyroid, cerebral cortex and bones of therapeutically aborted fetuses from endemic goiter region supplied with iodized salt for 5 years. Chin Med J. 1988;101:133.
186. Chanoine, JP, Toppet, V, Bourdoux, P, et al. Smoking during pregnancy: a significant cause of neonatal thyroid enlargement. Br J Obstet Gynaecol. 1991;98:65.
187. Kimura, M, Amino, N, Tamaki, H, et al. Gestational thyrotoxicosis and hyperemesis gravidarum: possible role of HCG with higher stimulating activity. Clin Endocrinol. 1993;38:345.
188. Goodwin, TM, Hershman, JM. Hyperthyroidism due to inappropriate production of human chorionic gonadotropin. Clin Obstet Gynecol. 1997;40:32.
189. Glinoer, D, Merck, AG, European Thyroid Symposium. Thyroid hyperfunction during pregnancy. Thyroid. 1998;8:859.
190. Chin, RKH, Lao, TTH. Thyroxine concentration and outcome of hyperemetic pregnancies. Br J Obstet Gynaecol. 1988;95:507.
191. Lao, TT, Chin, RKH, Panesar, NS, et al. Observations on thyroid hormones in hyperemesis gravidarum. Asia Oceania J Obstet Gynaecol. 1988;14:449.
192. Rodien, P, Bremont, C, Sanson, ML, et al. Familial gestational hyperthyroidism caused by a mutant thyrotropin receptor hypersensitive to human chorionic gonadotropin. N Engl J Med. 1998;339:1823.
193. Mori, M, Amino, N, Tamaki, H, et al. Morning sickness and thyroid function in normal pregnancy. Obstet Gynecol. 1988;72:355.
194. Grun, JP, Meuris, S, De Nayer, P, et al. The thyrotrophic role of human chorionic gonadotrophin (HCG) in the early stages of twin (versus single) pregnancies. Clin Endocrinol. 1997;46:719.
195. Goodwin, TM, Montoro, M, Mestman, JH. Transient hyperthyroidism and hyperemesis gravidarum: Clinical aspects. Am J Obstet Gynecol. 1992;167:648.
196. de los Santos, ET, Mazzaferri, EL. Sensitive thyroid-stimulating hormone assays: clinical applications and limitations. Compr Ther. 1988;14:26.
197. Bassett, F, Cresswell, J, Eastman, CJ, et al. Diagnostic value of thyrotropin concentrations in serum as measured by a sensitive immunoradiometric assay. Clin Chem. 1986;32:461.
198. Burrow, GN. The management of thyrotoxicosis in pregnancy. N Engl J Med. 1985;313:562.
199. Momotani, N, Noh, J, Oyanagi, H, et al. Antithyroid drug therapy for Graves’ disease during pregnancy, optimal regimen for fetal thyroid status. N Engl J Med. 1986;315:24.
200. Pekonen, F, Lamberg, B-A. Thyrotoxicosis during pregnancy. Ann Chir Gynaecol. 1978;67:165.
201. Mutjaba, Q, Burrow, GN. Treatment of hyperthyroidism in pregnancy with propylthiouracil and methimazole. Obstet Gynecol. 1975;46:282.
202. Cooper, DS, Saxe, VC, Meskell, M, et al. Acute effects of propylthiouracil (PTU) on thyroidal iodide organification and peripheral iodothyronine deiodination: correlation with serum PTU levels measured by radioimmunoassay. J Clin Endocrinol Metab. 1982;54:101.
203. Cooper, DS, Bode, HH, Nath, B, et al. Methimazole pharmacology in man: studies using a newly developed radioimmunoassay for methimazole. J Clin Endocrinol Metab. 1984;58:473.
204. Sitar, DS, Abu-Bakare, A, Gardiner, RJ. Propylthiouracil disposition in pregnancy and postpartum women. Pharmacology. 1982;25:57.
205. Skellern, GG, Knight, BI, Otter, M, et al. The pharmacokinetics of methimazole in pregnant patients after oral administration of carbimazole. Br J Clin Pharmacol. 1980;9:145.
206. Marchant, B, Brownlie, BEW, Hart, DM, et al. The placental transfer of propylthiouracil, methimazole and carbimazole. J Clin Endocrinol Metab. 1977;45:1187.
207. Wing, DA, Millar, LK, Koonings, PP, et al. A comparison of propylthiouracil versus methimazole in the treatment of hyperthyroidism in pregnancy. Am J Obstet Gynecol. 1994;170:90.
208. Amrhein, JA, Kenny, FM, Ross, D. Granulocytopenia, lupus-like syndrome, and other complications of propylthiouracil therapy. J Pediatr. 1970;76:54.
209. Jackson, IMD. Management of thyrotoxicosis. J Maine Med Assoc. 1975;66:224.
210. Wing, ES, Jr., Asper, SP, Jr. Observations on the use of propylthiouracil in hyperthyroidism with special reference to long-term treatment. Bull Johns Hopkins Hosp. 1952;90:152.
211. Bilezikian, SB, Lalei, U, Tsan, M-F, et al. Immunological reactions involving leukocytes. III. Agranulocytosis induced by antithyroid drugs. Johns Hopkins Med J. 1976;138:124.
212. Wall, JR, Fang, SL, Kuroki, T, et al. In vitro immunoactivity to propylthiouracil, methimazole, and carbimazole in patients with Graves’ disease: a possible cause of antithyroid drug–induced agranulocytosis. J Clin Endocrinol Metab. 1984;58:868.
213. Weitzman, SA, Stossel, TP, Desmond, M. Drug-induced immunological neutropenia. Lancet. 1978;1:1068.
214. Tajiri, J, Noguchi, S, Murakami, T, et al. Antithyroid drug–induced agranulocytosis. Arch Intern Med. 1990;150:621.
215. Romaldini, JH, Bromberg, N, Werner, RS, et al. Comparison of effects of high and low dosage regimens of antithyroid drugs in the management of Graves’ hyperthyroidism. J Clin Endocrinol Metab. 1983;57:563.
216. Safani, MM, Tatro, DS, Rudd, P. Fatal propylthiouracil-induced hepatitis. Arch Intern Med. 1982;142:838.
217. Vasily, DB, Tyler, WB. Propylthiouracil-induced cutaneous vasculitis: case presentation and review of the literature. JAMA. 1980;23:458.
218. Milham, S. Scalp defects in infants of mothers treated for hyperthyroidism with methimazole or carbimazole during pregnancy. Teratology. 1985;32:231.
219. Van Dijke, CP, Heydendael, RJ, De Kleine, MJ. Methimazole, carbimazole and congenital skin defects. Ann Intern Med. 1987;106:60.
220. Mandel, SJ, Brent, GA, Larsen, PR. Review of antithyroid drug use during pregnancy and report of a case of aplasia cutis. Thyroid. 1994;4:129.
221. Barbero, P, Valdez, R, Rodriguez, H, et al. Choanal atresia associated with maternal hyperthyroidism treated with methimazole: a case-control study. Am J Med Genet A. 2008;146A:2390.
222. Chan, GW, Mandel, SJ. Therapy insight: management of Graves’ disease during pregnancy. Nat Clin Pract Endocrinol Metab. 2007;3:470.
223. Momotani N, Iwama S, Noh JY, et al: Anti–thyroid drug therapy for Graves’ disease during pregnancy: mildest thyrotoxic maternal free thyroxine concentrations to avoid fetal hypothyroidism. In 77th Annual Meeting of the American Thyroid Association, Phoenix, AZ, 2006.
224. Casey, BM, Dashe, JS, Wells, CE, et al. Subclinical hyperthyroidism and pregnancy outcomes. Obstet Gynecol. 2006;107(2 Pt 1):337.
225. Sato, K, Mimura, H, Kato, S, et al. Serum propylthiouracil concentration in patients with Graves’ disease with various clinical courses. Acta Endocrinol. 1983;104:189.
226. Gardner, DF, Cruikshank, DP, Hays, PM, et al. Pharmacology of propylthiouracil (PTU) in pregnant hyperthyroid women: correlation of maternal PTU concentrations with cord serum thyroid function tests. J Clin Endocrinol Metab. 1986;62:217.
227. Bullock, JL, Harris, RL, Young, R. Treatment of thyrotoxicosis during pregnancy with propranolol. Am J Obstet Gynecol. 1975;121:242.
228. Rubin, PC. Current concepts: beta-blockers in pregnancy. N Engl J Med. 1981;305:1323.
229. Pruyn, SC, Phelan, JP, Buchanan, GC. Long-term propranolol therapy in pregnancy: maternal and fetal outcome. Am J Obstet Gynecol. 1979;135:485.
230. Isley, WL, Dahl, S, Gibbs, H. Use of esmolol in managing a thyrotoxic patient needing emergency surgery. Am J Med. 1990;89:122.
231. Momotani, N, Hisaoka, T, Noh, J, et al. Effects of iodine on thyroid status of fetus versus mother in treatment of Graves’ disease complicated by pregnancy. J Clin Endocrinol Metab. 1992;75:738.
232. Brodsky, JF, Cohen, EN, Brown, BW, Jr., et al. Surgery during pregnancy and fetal outcome. Am J Obstet Gynecol. 1980;138:1165.
233. Weingold, AB. Surgical diseases in pregnancy. Clin Obstet Gynecol. 1983;26:793.
234. Mitsuda, N, Tamaki, H, Amino, N, et al. Risk factors for developmental disorders in infants born to women with Graves’ disease. Obstet Gynecol. 1992;80:359.
235. Anselmo, J, Cao, D, Karrison, T, et al. Fetal loss associated with excess thyroid hormone exposure. JAMA. 2004;292:691.
236. Momotani, N, Ito, K, Hamada, N, et al. Maternal hyperthyroidism and congenital malformation in the offspring. Clin Endocrinol. 1984;20:695.
237. Dinani, S, Carpenter, S. Down’s syndrome and thyroid disorder. J Ment Defic Res. 1990;34:187.
238. Davis, LE, Lucas, MJ, Hankins, GDV, et al. Thyrotoxicosis complicating pregnancy. Am J Obstet Gynecol. 1989;160:63.
239. Easterling, TR, Schmucker, BC, Carlson, KL, et al. Maternal hemodynamics in pregnancies complicated by hyperthyroidism. Obstet Gynecol. 1991;78:348.
240. Bruner, JP, Landon, MB, Gabbe, SG. Diabetes mellitus and Graves’ disease complicated by maternal allergies to antithyroid drugs. Obstet Gynecol. 1988;72:443.
241. Phoojaroenchanachai, M, Sriussadaporn, S, Peerapatdit, T, et al. Effect of maternal hyperthyroidism during late pregnancy on the risk of neonatal low birth weight. Clin Endocrinol (Oxf). 2001;54:365.
242. Pitcher-Willmott, RW, Hindocha, P, Wood, CBS. The placental transfer of IgG subclasses in human pregnancy. Clin Exp Immunol. 1980;41:308.
243. Ewin, DL, McGregor, AM. Pregnancy and autoimmune thyroid disease. Trends Endocrinol Metab. 1990;1:296.
244. Fisher, DA. Fetal thyroid function: diagnosis and management of fetal thyroid disorders. Clin Obstet Gynecol. 1997;40:16.
245. Matsuura, N, Yamada, Y, Nohara, Y, et al. Familial neonatal transient hypothyroidism due to maternal TSH-binding inhibitor immunoglobulins. N Engl J Med. 1980;303:738.
246. Mitsuda, N, Tamaki, H, Amino, N, et al. Risk factors for developmental disorders in infants born to women with Graves’ disease. Obstet Gynecol. 1992;80:359.
247. Tamajki, H, Amino, N, Aozasa, M, et al. Universal predictive criteria for neonatal overt thyrotoxicosis requiring treatment. Am J Perinatol. 1988;5:152.
248. McKenzie, JM, Zakarija, M. The clinical use of thyrotropin receptor antibody measurements. J Clin Endocrinol Metab. 1989;69:1093.
249. Clavel, S, Madec, AM, Bornet, H, et al. Anti–TSH-receptor antibodies in pregnant patients with autoimmune thyroid disorder. Br J Obstet Gynaecol. 1990;97:1003.
250. Iseki, M, Shimizu, YM, Oikawa, T, et al. Sequential serum measurements of thyrotropin binding inhibition immunoglobulin G in transient familial neonatal hypothyroidism. J Clin Endocrinol Metab. 1983;57:384.
251. Zakarija, M, McKenzie, JM, Hoffman, WH. Prediction and therapy of intrauterine and late onset neonatal hyperthyroidism. J Clin Endocrinol Metab. 1986;62:368.
252. Bruinse, HW, Vermeulen-Meiners, C, Wit, JM. Fetal therapy for thyrotoxicosis in nonthyrotoxic pregnant women. Fetal Ther. 1988;3:152.
253. Page, DV, Brady, K, Mitchell, J, et al. The pathology of intrauterine thyrotoxicosis: two case reports. Obstet Gynecol. 1988;72:479.
254. Wallace, C, Couch, R, Ginsberg, J. Fetal thyrotoxicosis: a case report and recommendations for prediction, diagnosis, and treatment. Thyroid. 1995;5:125.
255. Mortimer, RH, Tyack, SA, Galligan, JP, et al. Graves’ disease in pregnancy: TSH receptor binding inhibiting immunoglobulins and maternal and neonatal thyroid function. Clin Endocrinol. 1990;32:141.
256. Tamaki, H, Amino, N, Takeoka, K, et al. Prediction of later development of thyrotoxicosis or central hypothyroidism from the cord serum TSH level in neonates born to mothers with Graves’ disease. J Pediatr. 1989;115:318.
257. Zakarija, M, McKenzie, JM. Pregnancy-associated changes in the thyroid-stimulating antibody of Graves’ disease and the relationship to neonatal hyperthyroidism. J Clin Endocrinol Metab. 1983;57:1036.
258. Tamaki, H, Amino, N, Takeoka, K, et al. Prediction of later development of thyrotoxicosis of central hypothyroidism from the cord serum thyroid-stimulating hormone level in neonates born to mothers with Graves’ disease. J Pediatr. 1989;115:318.
259. Skuza, KA, Sills, IN, Stene, M, et al. Prediction of neonatal hyperthyroidism in infants born to mothers with Graves’ disease. J Pediatr. 1996;128:264.
260. Delange, F. Effect of maternal thyroid function during pregnancy on fetal development. In: Beckers C, Reinwein D, eds. The thyroid and pregnancy. Stuttgart, Germany: Schattauer; 1991:7.
261. Mandel, S, Hanna, C, LaFranchi, S. Thyroid function of infants born to mother with Graves’ disease. J Pediatr. 1990;117:169.
262. Kempers, MJ, van Tijn, DA, van Trotsenburg, JA, et al. Central congenital hypothyroidism due to gestational hyperthyroidism: detection where prevention failed. J Clin Endocrinol Metab. 2003;88:5851.
263. Williams, RH, Kay, GA, Jandorf, BJ. Thiouracil, its absorption, distribution, and excretion. J Clin Invest. 1943;23:613.
264. Kampmann, JP, Hansen, JM, Johansen, K, et al. Propylthiouracil in human milk: revision of a dogma. Lancet. 1980;1:736.
265. Johansen, K, Andersen, AN, Kampmann, JP, et al. Excretion of methimazole in human milk. Eur J Clin Pharmacol. 1982;23:39.
266. Cooper, DS, Bode, HH, Nath, B, et al. Methimazole pharmacology in man: studies using a newly developed radioimmunoassay for methimazole. J Clin Endocrinol Metab. 1984;58:473.
267. Lamberg, BA, Ikonen, E, Österlund, K, et al. Antithyroid treatment of maternal hyperthyroidism during lactation. Clin Endocrinol. 1984;21:81.
268. Momotani, N, Yamashita, R, Yoshimoto, M, et al. Recovery from foetal hypothyroidism: evidence for the safety of breast-feeding while taking propylthiouracil. Clin Endocrinol. 1989;31:591.
269. Azizi, F, Khoshniat, M, Bahrainian, M, et al. Thyroid function and intellectual development of infants nursed by mothers taking methimazole. J Clin Endocrinol Metab. 2000;85:3233.
270. Canaris, GJ, Manowitz, NR, Mayor, G, et al. The Colorado Thyroid Disease Prevalence Study. Arch Intern Med. 2000;160:526.
271. Hollowell, JG, Staehling, NW, Flanders, WD, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489.
272. Gray, RS, Dorsey, DQ, Seth, J, et al. Prevalence of subclinical thyroid failure in insulin dependent diabetes. J Clin Endocrinol Metab. 1980;50:1034.
273. Bech, K, Hoier-Madsen, M, Feldt-Rasmussen, U, et al. Thyroid function and autoimmune manifestations in insulin-dependent diabetes mellitus during and after pregnancy. Acta Endocrinol. 1991;124:534.
274. Jovanovic-Peterson, L, Peterson, CM. De novo clinical hypothyroidism in pregnancies complicated by type I diabetes, subclinical hypothyroidism and proteinuria. Am J Obstet Gynecol. 1989;104:909.
275. Patel, S, Robinson, S, Bidgood, RJ, et al. A pre-eclampsia-like syndrome associated with hypothyroidism during pregnancy. Q J Med. 1991;79:435.
276. Klein, RZ, Haddow, JE, Faix, JD, et al. Prevalence of thyroid deficiency in pregnant women. Clin Endocrinol. 1995;35:41.
277. Haddow, JE, Palomaki, GE, Allan, WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549.
278. Vaidya, B, Anthony, S, Bilous, M, et al. Detection of thyroid dysfunction in early pregnancy: universal screening or targeted high-risk case finding? J Clin Endocrinol Metab. 2007;92:203.
279. Surks, MI, Ortiz, E, Daniels, GH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291:228.
280. Singh, N, Singh, PN, Heshman, JM. Effect of calcium carbonate on the absorption of levothyroxine. JAMA. 2000;283:2822.
281. Bell, DS, Ovalle, F. Use of soy protein supplement and resultant need for increased dose of levothyroxine. Endocr Pract. 2001;7:193.
282. Tamaki, H, Amino, N, Takeoka, K, et al. Thyroxine requirement during pregnancy for replacement therapy of hypothyroidism. Obstet Gynecol. 1990;76:230.
283. Balen, AH, Kurtz, AB. Successful outcome of pregnancy with severe hypothyroidism: case report and literature review. Br J Obstet Gynaecol. 1990;97:536.
284. Montoro, M, Collea, JV, Frasier, SD, et al. Successful outcome of pregnancy in hypothyroid women. Ann Intern Med. 1986;94:31.
285. Wasserstrum, N, Anania, CA. Perinatal consequences of maternal hypothyroidism in early pregnancy and inadequate replacement. Clin Endocrinol. 1995;42:353.
286. New England Congenital Hypothyroidism collaborative. Neonatal hypothyroidism screening: status of patients at six years of age. J Pediatr. 1985;107:915.
287. New England Congenital Hypothyroidism Collaborative. Elementary school performance of children with congenital hypothyroidism. J Pediatr. 1990;116:27.
288. Van der Gaag, RD, Drexhage, HA, Dussault, JH. Role of maternal immunoglobulin blocking TSH-induced growth in sporadic forms of congenital hypothyroidism. Lancet. 1985;1:246.
289. Brown, RS, Keating, P, Mitchell, E. Maternal thyroid-blocking immunoglobulins in congenital hypothyroidism. J Clin Endocrinol Metab. 1990;70:1341.
290. Bogner, U, Graters, AH, Sigle, B, et al. Cytotoxic antibodies in congenital hypothyroidism. J Clin Endocrinol Metab. 1989;68:671.
291. Takasu, N, Mori, T, Koizumi, Y, et al. Transient neonatal hypothyroidism due to maternal immunoglobulins that inhibit thyrotropin-binding and post-receptor processes. J Clin Endocrinol Metab. 1984;59:142.
292. Boyages, SC, Halpern, JP, Maberly, GF, et al. Endemic cretinism: possible role for thyroid autoimmunity. Lancet. 1989;2:529.
293. Jansson, R, Dahlberg, PA, Winsa, B, et al. The postpartum period constitutes an important risk for the development of clinical Graves’ disease in young women. Acta Endocrinol. 1987;116:321.
294. Tamaki, H, Amino, N, Aozasa, M, et al. Serial changes in thyroid-stimulating antibody and thyrotropin binding inhibitor immunoglobulin at the time of postpartum occurrence of thyrotoxicosis in Graves’ disease. J Clin Endocrinol Metab. 1988;65:324.
295. Asa, SL, Bilbao, JM, Kovacs, K, et al. Lymphocytic hypophysitis of pregnancy resulting in hypopituitarism: a distinct clinicopathologic entity. Ann Intern Med. 1981;96:166.
296. Kumar, S. Isolated thyroid stimulating hormone deficiency following childbirth. Proc R Soc Med. 1966;59:1281.
297. Merenich, JA, McDermott, MT, Kidd, GS. Transient isolated thyrotropin deficiency in the postpartum period. Am J Med. 1989;86:361.
298. Papetrou, PD, Jackson, IMD. Thyrotoxicosis due to silent thyroiditis. Lancet. 1975;1:361.
299. Muller, AF, Drexhage, HA, Berghout, A. Postpartum thyroiditis and autoimmune thyroiditis in women of childbearing age: recent insights and consequences for antenatal and postnatal care. Endocr Rev. 2001;22:605.
300. Stagnaro-Green, A. Clinical review 152: postpartum thyroiditis. J Clin Endocrinol Metab. 2002;87:4042.
301. Alvarez-Marfany, M, Roman, SH, Drexler, AJ, et al. Long-term prospective study of postpartum thyroid dysfunction in women with insulin dependent diabetes mellitus. J Clin Endocrinol Metab. 1994;79:10.
302. Gerstein, HC. Incidence of postpartum thyroid dysfunction in patients with type 1 diabetes mellitus. Ann Intern Med. 1993;118:419.
303. White, WB, Andreoli, JW. Severe, accelerated postpartum hypertension associated with hyperthyroxinemia. Br J Obstet Gynaecol. 1986;93:1297.
304. Rhys, J, Othman, S, Parkes, AB, et al. Interference in thyroid function tests in postpartum thyroiditis. Clin Chem. 1991;37:1397.
305. Amino, N, Nori, H, Iwatani, Y, et al. High prevalence of transient post-partum thyrotoxicosis and hypothyroidism. N Engl J Med. 1982;306:849.
306. Amino, N, Tada, H, Hidaka, Y. Postpartum autoimmune thyroid syndrome: a model of aggravation of autoimmune disease. Thyroid. 1999;9:705.
307. Dydek, GJ, Blue, PW. Human breast milk excretion of iodine-131 following diagnostic and therapeutic administration to a lactating patient with Graves’ disease. J Nucl Med. 1988;29:407.
308. Robinson, GE, Stewart, DE. Postpartum psychiatric disorders. Can Med Assoc J. 1986;134:31.
309. Pop, VJM, de Rooy, HAM, Vadar, HL, et al. Postpartum thyroid dysfunction and depression in an unselected population. N Engl J Med. 1991;324:1815.
310. Kent, GN, Stuckey, BG, Allen, JR, et al. Postpartum thyroid dysfunction: clinical assessment and relationship to psychiatric affective morbidity. Clin Endocrinol (Oxf). 1999;51:429.
311. Tachi, J, Amino, N, Tamaki, H, et al. Long-term follow-up and HLA association in patients with postpartum hypothyroidism. J Clin Endocrinol Metab. 1988;66:480.
312. Othman, S, Phillips, DIW, Parkes, AB, et al. A long-term follow-up of postpartum thyroiditis. Clin Endocrinol. 1990;32:559.
313. Roti, E, Minelli, R, Gardini, E, et al. Impaired intrathyroidal organification and iodine-induced hypothyroidism in euthyroid women with a previous episode of postpartum thyroiditis. J Clin Endocrinol Metab. 1991;73:958.
314. Lazarus, JH. Postpartum thyroiditis. Thyroid Int. 1996;5:3.
315. Amino, N, Mori, H, Iwatani, Y, et al. High prevalence of transient post-partum thyrotoxicosis and hypothyroidism. N Engl J Med. 1982;306:849.
316. Marqusee, E, Hill, JA, Mandel, SJ. Thyroiditis after pregnancy loss. J Clin Endocrinol Metab. 1997;82:2455.
317. Stagnaro-Green, A, Roman, SH, Cobin, RH, et al. A prospective study of lymphocyte-initiated immunosuppression in normal pregnancy: evidence of a T-cell etiology for postpartum thyroid dysfunction. J Clin Endocrinol Metab. 1992;74:645.
318. Fung, HYM, Kologlu, M, Collison, K, et al. Postpartum thyroid dysfunction in Mid Glamorgan. Br Med J. 1988;296:241.
319. Kampe, O, Jansson, R, Karlsson, FA. Effects of l-thyroxine and iodide on the development of autoimmune postpartum thyroiditis. J Clin Endocrinol Metab. 1990;70:1014.
320. Bech, K. Importance of cytolytic activity and dietary iodine in the pathogenesis of postpartum thyroiditis. Allergy. 1988;43:161.
321. Sridama, V, Pacini, F, Yan, SL, et al. Decreased levels of helper T cells: a possible cause of immunodeficiency in pregnancy. N Engl J Med. 1982;307:352.
322. Takasu, N, Komiya, I, Nagasawa, Y, et al. Exacerbation of autoimmune thyroid dysfunction after unilateral adrenalectomy in patients with Cushing’s syndrome due to an adrenocortical adenoma. N Engl J Med. 1990;322:1708.
323. Vargas, MT, Briones-Urbina, R, Gladman, D, et al. Antithyroid microsomal autoantibodies and HLA-DR5 are associated with postpartum thyroid dysfunction: evidence supporting an autoimmune pathogenesis. J Clin Endocrinol Metab. 1988;67:327.
324. Kologlu, M, Fung, H, Darke, C, et al. Postpartum thyroid dysfunction and HLA status. Eur J Clin Invest. 1990;20:56.
325. Jansson, R, Safwenberg, J, Dahlberg, PA. Influence of the HLA-DR4 antigen and iodine status on the development of autoimmune postpartum thyroiditis. J Clin Endocrinol Metab. 1985;60:168.
326. Pryds, O, Lervang, HH, Ostergaard-Kristensen, HP, et al. HLA-DR factors associated with postpartum hypothyroidism: an early manifestation of Hashimoto’s thyroiditis? Tissue Antigens. 1987;30:34.
327. DeGroot, LJ, Quintans, J. The causes of autoimmune thyroid disease. Endocr Rev. 1989;10:537.
328. Weetman, AP, Fung, HYM, Richards, CJ, et al. IgG subclass distribution and relative functional affinity of thyroid microsomal antibodies in postpartum thyroiditis. Eur J Clin Invest. 1990;20:133.
329. Briones-Urbina, R, Parkes, AB, Bogner, U, et al. Increase in antimicrosomal antibody–related IgG1 and IgG4 and titers of antithyroid peroxidase antibodies but not antibody dependent cell-mediated cytotoxicity in post-partum thyroiditis with transient hyperthyroidism. J Endocrinol Invest. 1990;13:879.
330. Tachi, J, Amino, N, Iwatani, Y, et al. Increase in antideoxyribonucleic acid antibody titer in postpartum aggravation of autoimmune thyroid disease. J Clin Endocrinol Metab. 1988;67:1049.
331. Iwatani, Y, Amino, N, Tamaki, H, et al. Increase in peripheral large granular lymphocytes in postpartum autoimmune thyroiditis. Endocrinol Jpn. 1988;35:447.
332. Hayslip, CC, Baker, JR, Wartofsky, L, et al. Natural killer cell activity and serum autoantibodies in women with postpartum thyroiditis. J Clin Endocrinol Metab. 1988;66:1089.
333. Chan, JYC, Walfish, PG. Activated (Ia+) T-lymphocytes and their subsets in autoimmune thyroid diseases: analysis by dual laser flow microfluorocytometry. J Clin Endocrinol Metab. 1986;62:403.
334. Jansson, R, Totterman, TH, Sallstrom, J, et al. Intrathyroidal and circulating lymphocyte subsets in different stages of autoimmune postpartum thyroiditis. J Clin Endocrinol Metab. 1984;58:942.
335. Negro, R, Greco, G, Mangieri, T, et al. The influence of selenium supplementation on postpartum thyroid status in pregnant women with thyroid peroxidase autoantibodies. J Clin Endocrinol Metab. 2007;92:1263.
336. Struve, CW, Haupt, S, Ohlen, S. Influence of frequency of previous pregnancies on the prevalence of thyroid nodules in women without clinical evidence of thyroid disease. Thyroid. 1993;3:7.
337. Cooper, DS, Doherty, GM, Haugen, B, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109.
338. Pacini, F, Schlumberger, M, Dralle, H, et al. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154:787.
339. Tan, GH, Gharib, H, Goellner, JR, et al. Management of thyroid nodules in pregnancy. Arch Intern Med. 1996;156:2317.
340. Moosa, M, Mazzaferri, EL. Outcome of differentiated thyroid cancer diagnosed on pregnant woman. J Clin Endocrinol Metab. 1997;82:2862.
341. McTiernan, AM, Weiss, NS, Daling, JR. Incidence of thyroid cancer in women in relation to reproductive and hormonal factors. Am J Epidemiol. 1984;120:423.
342. Rosen, IB, Walfish, PG, Nikore, V. Pregnancy and surgical thyroid disease. Surgery. 1985;98:1135.
343. Herzon, FS, Morris, DM, Segal, MN, et al. Coexistent thyroid cancer and pregnancy. Arch Otolaryngol Head Neck Surg. 1994;120:1191.
344. Balan, KK, Critchley, M. Outcome of pregnancy following treatment of well-differentiated thyroid cancer with 131iodine. Br J Obstet Gynaecol. 1992;99:1021.







