Diabetes Mellitus and Pregnancy
History
Before the discovery of insulin, pregnancy in a woman with DM was little more than a medical curiosity. The few women with DM who survived adolescence were often infertile. Those who conceived frequently underwent therapeutic abortion in view of the alarmingly high rates of both maternal (25%) and perinatal (40% to 50%) mortality present at the time. After therapy with insulin became available, women with diabetes generally reached adulthood with little impairment in fertility. Maternal mortality declined to a rate similar to that of women without DM. A comparable reduction in fetal wastage did not occur until much later. In the 1950s and 1960s, pioneering efforts based on the premise that fetal survival is linked to control of maternal diabetes reduced the rates of fetal loss to 10% to 15%. Further improvements followed the development of technologies for (1) monitoring the integrity of the fetoplacental unit, (2) documenting maternal metabolic control more accurately (i.e., self-monitoring of capillary blood sugar), and (3) sophisticated management of neonatal morbidity. In centers that regularly provide specialized team care to substantial numbers of patients, rates of perinatal loss in diabetic pregnancies (except for those related to major congenital malformations) now approach those of the general obstetric population. Thus attention has increasingly focused on neonatal morbidity and the potential effects of maternal diabetes on the offspring in later life. For a historical perspective, see the comprehensive Technical Review published recently by the American Diabetes Association (ADA).1
In recent years, increasing numbers of women with long duration of type 1 DM are having pregnancies sometimes in the presence of vascular and/or neuropathic complications. In the past decade, the prevalence of preexisting type 2 DM complicating pregnancy has increased throughout the world. Rates of congenital malformations and adverse pregnancy outcome tend to be as high as those in pregnancies complicated by type 1 DM.2
Criteria for the diagnosis of GDM were initially established over 40 years ago3 and with minor modifications remain in widespread use today. These criteria were chosen to identify women at high risk for development of diabetes following pregnancy, not to identify pregnancies at increased risk for adverse perinatal outcomes. Others use criteria for GDM that are the same as those used to classify glucose tolerance in nonpregnant persons.4 Consequently, for about 3 decades, much controversy has existed regarding the medical/obstetrical significance of GDM and the cost effectiveness of its detection and treatment. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study5 has reported strong continuous associations of maternal glucose levels below those diagnostic of diabetes with increased birth weight and increased cord-blood serum C-peptide levels (fetal hyperinsulinemia). Significant associations were also found with the two other primary outcomes (primary cesarean section delivery and clinically defined neonatal hypoglycemia) and with several secondary outcomes (premature delivery [<37 weeks’ gestation], preeclampsia, shoulder dystocia or birth injury, need for intensive neonatal care, or hyperbilirubinemia), but these tended to be weaker. It is anticipated that in the near future, these results and other data will stimulate the adoption of outcome-based criteria for diagnosis and classification of states of hyperglycemia in pregnancy.
Epidemiology
Over the past 2 decades, the age-adjusted prevalence of DM has increased in the general population of women of reproductive age.6 In 1995, based on data from the 1988 National Maternal and Infant Health Survey (NMIHS), Engelau et al.7 reported a 4% prevalence of diabetes complicating pregnancy in the United States. They estimated that GDM accounted for 88% and preexisting DM 12% of cases. Type 2 DM (NIDDM) accounted for two-thirds and type 1 DM (IDDM) one-third of preexisting DM. Studies reported recently provide evidence that the prevalence of both GDM and pregnancy in women with preexisting DM has increased since the NMIHS data were collected.8 For example, the age- and race/ethnicity–adjusted prevalence of preexisting diabetes in pregnancy increased from 0.81% in 1999 to 1.82% in 2005 in the population participating in the Kaiser Permanente Southern California Medical Care Program. Preexisting diabetes represented 10% of deliveries with any form of diabetes complicating pregnancy in 1999. By 2005, it represented 21%. Furthermore, the greatest proportional increase in preexisting DM was found in the youngest cohort, aged 13 to 19 years. Information was not sufficient to distinguish between preexisting type 1 and type 2 DM; however, ethnic/racial distribution and other demographic characteristics suggest that the increase in preexisting DM in pregnancy is primarily in type 2 DM.8 In contrast, the prevalence of GDM, which had been increasing, (see later) did not change during this 6-year interval.
Using a database from the Kaiser Permanente Northern California Medical Care Program that employed the same screening and diagnostic criteria throughout the study period, Ferrara et al.9 found an increase in the incidence of GDM from 1991 to 1997 (from 5.1% to 7.4%) and leveling of the rate from 1997 to 2000. An increase was found in all racial/ethnic groups of the heterogeneous population. In Colorado, Dabelea et al.10 found that the prevalence of GDM doubled between 1994 and 2002 in the population served by Kaiser Permanente, but the absolute prevalences were lower than reported from Northern California. Here also, the same screening and diagnostic criteria were followed throughout the period of observation, but they required higher levels of glucose for the diagnosis of GDM than those used by Ferrara et al.9,11,12 This alone would lead to differences in observed frequency of GDM. In another recent publication based on the National Hospital Discharge Survey (NHDS) of births in the United States between 1989 and 2004, Getahun et al.13 also reported an increase in the prevalence of GDM among all racial and ethnic groups. However, in this report, the largest relative increase was found among blacks. There is also a general impression that GDM prevalence has increased globally14; however, aside from the two reports mentioned above, these impressions are not based on standardized procedures and universal testing of populations.
Pathogenesis
Metabolic Effects of Pregnancy
The metabolic alterations that develop during pregnancy are profound, but they do not occur with equal intensity throughout gestation. Rather, a temporal progression is seen in which increasing insulin resistance and other metabolic changes parallel the growth of the conceptus. In the immediate postpartum period, the profound insulin resistance dissipates rapidly. These metabolic perturbations and their temporal associations suggest that they derive from the conceptus.15 Serial estimates of insulin sensitivity both before and during pregnancy in a relatively small number of women with normal carbohydrate metabolism indicate a slight reduction in insulin sensitivity by 12 to 14 weeks and a further decline by the end of the second trimester.16 During the third trimester insulin sensitivity is 40%-60% lower than in nongravid women.16–19 Catalano and colleagues16 found modest improvement in insulin sensitivity at 12 to 14 weeks in women with GDM when compared with their state of insulin resistance before pregnancy. This modest improvement was followed by progression to severe insulin resistance in late gestation that was equal to or greater than that in subjects with normal glucose tolerance. Women with type 1 DM who are in optimal metabolic control before conception do not have an increase in insulin requirement during the first trimester and may even require some reduction in dosage because of hypoglycemia at the end of the first and beginning of the second trimester (Fig. 30-1).20
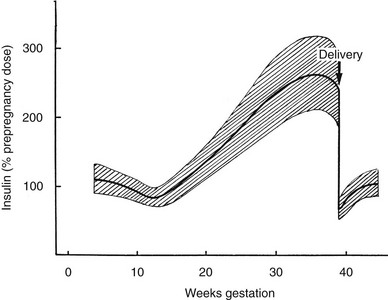
FIGURE 30-1 Schematic representation of changing insulin requirements over the course of pregnancy and after delivery in pregestational diabetes mellitus. (Data from Phelps RL, Metzger BE, Freinkel N: Medical management of diabetes in pregnancy. In Sciarra J (ed): Gynecology and obstetrics, vol 3, Philadelphia, 1988, Harper & Row, pp 1–16.)
In early pregnancy, there is little if any increase in insulin secretion in response to glucose. Conversely, insulin secretion in response to oral or intravenous glucose in the last trimester of pregnancy is approximately 1.5 to 2.5 times greater than that seen in nongravid conditions15 and is accompanied by islet cell hyperplasia. The product of β-cell secretion is primarily insulin and not a disproportionate amount of proinsulin or intermediates, which have substantially less activity than insulin. Insulin does not cross the placenta. Although the human placenta is small in proportion to total maternal mass, it actively degrades insulin and moderately increases insulin clearance in normal pregnancy and GDM.19,21
These changes occur temporally in parallel with increasing size of the placenta and growth of the fetus. However, the specific mediators of increased insulin secretion and insulin resistance are not entirely clear. Table 30-1 lists a number of the many factors potentially implicated in these changes. Numerous studies suggest that progesterone, acting either separately or in concert with estrogens, has direct β-cell cytotropic actions. When the two sex steroids are administered to nonpregnant animals in appropriate molar concentration ratios, effects on plasma insulin and fuel storage in liver and adipose tissue similar to those seen in normal pregnancy are observed without significantly affecting skeletal muscle sensitivity to insulin.22 Higher circulating concentrations of maternal leptin, potentially of placental origin,23 may reflect the change in insulin sensitivity rather than directly contributing to it. During the latter half of pregnancy, circulating levels of human chorionic somatomammotropin (hCS) or placental lactogen, estrogen, and progesterone reach maximal plasma concentrations with increasing placental mass.15 The concentration of pituitary growth hormone decreases,24 but the increasing level of the growth hormone variant (hGH-V) of placental origin may offset the decline.24 Prolactin also increases throughout gestation and may contribute to the insulin resistance. Free cortisol levels increase, but the diurnal variations are maintained25 despite the presence of placental corticotropin and corticotropin-releasing factor. In recent years, several other factors derived from the placenta and/or adipose tissue have been identified as potentially important contributors to insulin resistance in normal pregnancy and GDM. These include increases in tumor necrosis factor α (TNF-α)26 and decreases in adiponectin.27 Several other factors that potentially contribute to insulin resistance in type 2 DM have not been fully evaluated in normal pregnancy or GDM.28
Table 30-1
Factors of Placental Origin That May Influence Maternal Insulin Sensitivity
Estrogens and progesterone
Human chorionic somatomammotropin (hCS) or placental lactogen (HPL)
Prolactin
Placental growth hormone variant (hGH-V)
Corticotropin-releasing factor (CRF) and corticotropin
Leptin
Tumor necrosis factor α (TNF-α)
Adiponectin*
Resistin
Ghrelin
Interleukin 6 (IL-6)
*There is controversy about whether the placenta is or is not a source of adiponectin.
In a recent review, Friedman and colleagues29 concluded that at the molecular level, the insulin resistance of normal pregnancy is multifactorial, involving reduced ability of insulin to phosphorylate the insulin receptor, decreased expression of insulin receptor substrate 1 (IRS-1), and increased levels of a specific kinase. Further changes occur in GDM that inhibit signaling and lead to substantially reduced GLUT4 translocation. The net effect of these combined hormonal and metabolic changes is to oppose insulin action at peripheral (muscle and adipose tissue) and hepatic sites.
Utilization of Maternal Fuels by the Conceptus
The placenta is the conduit through which the conceptus continuously draws maternal fuel for its metabolic and biosynthetic needs, and glucose is the major source of its metabolic energy. In addition, glucose or three-carbon intermediates derived from glucose (lactate) are precursors for glycogen, glycoproteins, and the glyceride-glycerol in triglycerides and phospholipids of the conceptus. Glucose utilization rates as high as 6 mg/kg/min have been estimated in the human fetus at term,30 in contrast to glucose turnover of 2 to 3 mg/kg/min in normal adults. Glucose delivery across the placenta occurs by facilitated diffusion, and maternal glucose usually exceeds fetal glucose concentration by 10 to 20 mg/dL (0.6 to 1.1 mmol/L).
In the third trimester, growth of the human fetus requires the net placental transfer of approximately 54 mmol of nitrogen per day.31 Furthermore, amino acids may be used in the conceptus for oxidative energy. Although quantitative measurements of nitrogen requirement for fetal growth in humans are not available, it is clear that the fetus exerts an unremitting drain on maternal nitrogen reserves.
Although maternal triglyceride represents the largest reserve fuel depot, it can directly support the metabolic needs of the conceptus only to a limited extent. Triglycerides cross the placental barrier poorly, and the net transfer of free fatty acids (FFAs) to the fetus may be limited. Glycerol can cross the placenta readily, but its contribution in nonruminant mammalian species is probably small. Ketones readily cross the placenta, are present in the fetal circulation in concentrations approaching those in maternal blood,32 and the enzymes necessary for ketone oxidation are present in the human fetus. When fetal tissues, including the brain, are incubated in vitro with concentrations of ketones similar to those present during fasting, substantial oxidation of ketones is seen, even in the presence of alternative fuels (i.e., fasting concentrations of glucose, lactate, and amino acids).32 Oxidation of ketones lessens that of the other fuels and may spare them for biosynthetic disposition or other pathways in the fetus.33 However, such diversion to the metabolism of ketones may have adverse consequences. Ketones inhibit pyrimidine and purine synthesis in developing brain cells in the rat fetus33 and at high concentrations disrupt organogenesis in rodent embryos in culture. Controversial epidemiologic evidence suggested that maternal ketonuria during human pregnancy may be associated with reduction in the intelligence quotient (IQ) of the offspring in childhood.34 Rizzo and co-workers35 reported an inverse association between increased plasma FFAs and β-hydroxybutyrate concentrations in the second and third trimesters of pregnancy and intellectual development of offspring at age 2 to 5 years.
Circulating Concentrations of Nutrient Fuels
In Normal Pregnancy: Normal women have a decrease in the concentration of fasting plasma glucose (FPG) during pregnancy. The greatest decline in FPG (10- to 12-hour fast) occurs early in gestation,36 well before the rate of glucose utilization by the fetus is sufficient to increase total maternal glucose turnover. It has been reported that severely obese women do not show a decline of FPG during pregnancy.36 A lower FPG persists during late gestation despite relatively higher postmeal glucose levels. However, reports of diurnal glucose profiles of ambulatory pregnant women obtained by capillary blood glucose monitoring or continuous monitoring of subcutaneous fluid confirm that glycemic excursions vary within a narrow range in normal subjects, even during late gestation.37,38 Basal concentrations of plasma glycerol and FFAs do not change until late gestation, at which time significant elevations occur, and transition to the metabolic profile characteristic of the fasting state is accelerated in association with mounting lipolysis and insulin resistance.39 Progressive increases occur in all major lipid fractions, including triglycerides, cholesterol, and phospholipids.22 Total plasma amino acid concentrations also decline in early pregnancy and persist throughout gestation.40 The reasons for these changes are not clear. The suppressive effects of insulin on plasma amino acids are well known and could account for this finding. However, in later gestation, release of amino acids from skeletal muscle is less restrained by insulin, at least in pregnant rats. This suggests that during this time of insulin resistance, increased fetal removal, as opposed to impaired muscle release, may play a primary role in sustaining maternal hypoaminoacidemia.
In Gestational Diabetes Mellitus: Basal and postprandial levels of glucose, FFAs, triglycerides, and amino acids tend to exceed those of normal pregnant control subjects,41 and the changes tend to persist during dietary intervention, with the extent of the abnormalities paralleling the severity of the GDM.41 Branched-chain amino acids are sensitive to insulin, are often altered in obesity and other insulin-resistant states, and are the most consistently disturbed.41 The propensity to “accelerated starvation” (e.g., a more rapid decline in circulating glucose concentration in association with a greater increase in FFAs and ketones) in women with GDM is similar to that found in women with normal glucose homeostasis.42 Diurnal glucose profiles of ambulatory women with diet-treated GDM obtained by continuous monitoring of subcutaneous fluid show greater glycemic excursions and delay in reaching postprandial peak values than seen in normal subjects.38
In Women With Preexisting Diabetes Mellitus: In pregnant women in whom type 1 DM is well controlled, few disturbances in plasma lipids (FFAs, cholesterol, and triglycerides) have been found, and individual lipoprotein fractions have little change in their lipid content.43 The greatest departures from the norm during pregnancy occur in plasma glucose profiles; plasma amino acid concentrations also may be markedly disturbed. Changes in amino acids and indices of glycemic control (blood glucose self-monitoring records and hemoglobin A1c levels) are poorly correlated, especially in late pregnancy.44 Lipids tend to be altered more extensively in pregnant women with type 2 DM, with higher total plasma triglycerides and an increased triglyceride content of very-low-density lipoproteins.43 The cholesterol content of high-density lipoproteins may be decreased when compared with levels in normal pregnancy or in pregnant women with type 1 DM.43 The relative roles of obesity and diabetes in the development of these lipid aberrations remain to be defined. Studies of amino acid metabolism in type 2 DM in pregnancy have not been reported.
Maternal Metabolism and Pregnancy Outcome
The pioneering hypothesis advanced by Pedersen45 a half century ago stated that maternal hyperglycemia leads to fetal hyperinsulinism, which is responsible for macrosomia and neonatal morbidity. Extensive experimental and clinical evidence indicates that metabolic disturbances in the mother contribute to virtually all the adverse effects of DM on the offspring.15,46,47 The importance of alterations in other metabolic fuels, in addition to glucose, was recognized later.41 Results of the HAPO Study5 indicate that the associations between maternal glycemia, fetal insulin, and parameters of fetal growth extend through the full range from “normal” to those that reflect overt diabetes. Freinkel15 emphasized the temporal relations between a metabolic insult and the adverse outcome expected (“fuel-mediated teratogenesis”) and postulated that the altered intrauterine environment of diabetes can have lifelong as well as perinatal consequences.15,46 The key features of the hypotheses of Pedersen and Freinkel are schematically integrated in Fig. 30-2.
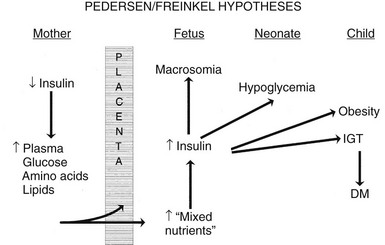
FIGURE 30-2 Effect of maternal fuels on fetal development. The classic hyperglycemia-hyperinsulinemia hypothesis of Pedersen45 has been modified to show the contribution of other insulin-responsive maternal fuels besides glucose. All of these fuels can influence the growth of the fetus and the maturation of its insulin secretion. As indicated here, altered fetal nutrients and enhanced insulin secretion are associated with consequences that extend well beyond the neonatal period. (Data from Silverman BL, Purdy LP, Metzger BE: The intrauterine environment: Implications for the offspring of diabetic mothers, Diabetes Rev 4:21–35, 1996.)
Congenital Malformations and Early Fetal Loss
Increased risks of congenital malformations and spontaneous abortions in diabetic pregnancies are linked to metabolic control at conception.15,47 Good control during the period of organogenesis may reduce the prevalence of these adverse outcomes.47 Risk of spontaneous abortion increases in direct proportion to hemoglobin A1c concentration measured shortly before or after conception.48,49 The specific relation between metabolic control and risk of congenital malformations has been more difficult to define. Greene and associates49 found a prevalence of congenital malformation of about 5% until initial hemoglobin A1c concentrations were in excess of 10 to 12 SD of the mean control value. If put in the context of current analytical methods and reference range for pregnancy,50 this would represent hemoglobin A1c in the range of 9.5 to 10%. Beyond that, the risk of malformations increased steeply. Several groups reported that improving control of DM before conception51 reduces rates of major congenital malformations to those expected in the general obstetric population. In populations in which most pregnancies in women with diabetes are planned, congenital malformations have declined to rates similar to those of the general population.52 However, data from general population–based sources indicate that the overall risk of birth defects remains nearly 10% in pregnancies in women with preexisting diabetes.53,54
In vivo and in vitro animal models suggest that diabetic embryopathy is multifactorial.55 Oxidative stress, increased generation of free radicals, disruption of signaling pathways, including the expression and action of the Pax3 gene, or a combination of these have been implicated.53,54,56 When tight metabolic control is restored, levels of circulating serum factors that may mediate embryopathy decline more slowly than hyperglycemia and hyperketonemia.55 Hypoglycemia also is potentially teratogenic,57 so measurements of blood glucose or hemoglobin A1c may not fully reflect the “toxicity” of the maternal environment for the fetus. This lack of specificity is reflected in the fact that 60% to 70% of offspring of mothers with first-trimester hemoglobin A1c levels indicative of poor metabolic control are normally developed at birth.49 Consequently, neither the precise degree of glycemic control nor the interval over which good control must be maintained to achieve optimal outcome is known.47
Disturbances of Fetal Growth
Development of macrosomia (traditionally defined as birth weight > 4000 g or above the 90th percentile for gestational age) is the quintessential fulfillment of the Pedersen hypothesis and a frequent complication of pregnancies complicated by DM and GDM. Increased adiposity is the primary component of the macrosomia. Infants of diabetic mothers may have up to twice the body-fat content of infants of normal mothers. Increased fat content was reported in infants of mothers with GDM, even with total body weight identical to that of controls,58 and data from the HAPO Study5 showed that the risk of high infant percent body fat increased in association with higher maternal glucose concentration across the entire range of subdiabetic glucose levels. Adiposity tends to be prominent in the shoulder region, enhancing risks for cesarean delivery, shoulder dystocia, and birth trauma.59 Skin-fold measurements may be used to document adiposity at birth and reflect maternal metabolic regulation.60 However, skin-fold measurements are difficult to standardize and are seldom used in routine clinical assessment.60
Asymmetric growth is one hallmark of diabetic fetopathy. In addition to hypertrophy of subcutaneous fat, other organs responsive to insulin (e.g., the heart and liver) may be larger, whereas insulin-insensitive tissues such as the brain are of normal size. Thickness of fetal humeral soft tissue61 or cheek-to-cheek dimensions62 can be used to detect asymmetric growth caused by maternal diabetes. Some investigators use ultrasound-measured abdominal circumference that is greater than the 75th percentile to identify pregnancies at higher risk for macrosomia and target them for intensive therapy with insulin.63
Fetal hyperinsulinemia may develop early in gestation, well before adipose tissue develops.64 Morphometric studies of the pancreas from fetuses of mothers with diabetes demonstrated islet hypertrophy and hyperplasia during the second trimester. We observed a stronger association between fetal islet function near term or at birth and metabolic control in the second trimester (hemoglobin A1c concentration) than in the third trimester.65 Once initiated, β-cell overactivity may promote development of macrosomia, even without sustained elevations in nutrient fuels. Visceromegaly and fat accumulation also resulted from insulin administration to normal fetal monkeys (via implanted insulin pumps), without concurrent infusion of additional nutrients.66 The results of the HAPO Study indicate that the risk of fetal hyperinsulinemia increases in a linear fashion across the range of maternal glucose concentrations below those characteristic of overt diabetes.5 Alterations of multiple nutrient fuels65 may contribute to premature activation of fetal islet function and increases in insulin-like growth factors (IGFs).67
Historically, intrauterine growth restriction (IUGR) was a common finding in offspring of type 1 diabetic mothers. This was thought to be secondary to maternal vascular disease, resulting in uteroplacental insufficiency.45 However, in early pregnancy very poor metabolic control (in the absence of vasculopathy) may retard growth irreparably, even without associated birth defects.1 At the present time, growth restriction is rarely seen with diabetes except in pregnancies complicated by hypertension or nephropathy.
Anthropometric and Metabolic Development in Childhood
Pettitt and colleagues68 found a correlation in Pima Indian mothers between the 2-hour response to oral glucose during pregnancy and the occurrence of obesity in their offspring. Moreover, the risk of obesity was not limited to those whose birth weight was increased.69,70 Greater relative weight for height was reported at age 4 years in the offspring of diabetic mothers whose control was “poor” rather than “good” during the index pregnancy.69 In a prospective long-term follow-up study of offspring of diabetic mothers, our group at Northwestern University found that macrosomia of offspring disappeared by age 1 year. However, by age 8 years, obesity was highly prevalent; nearly half had a weight greater than the 90th percentile.69,71 Recently, Hillier and colleagues reported weight of children (ages 5 to 7 years) from a large multiethnic group cohort whose mothers had glucose challenge tests and/or glucose tolerance tests during pregnancy.72 Risk of obesity in the children increased progressively across the range of subdiabetic maternal glucose values.
In Pima Indians, by age 20 to 24 years, type 2 DM is present in 45.5% of offspring of “diabetic” mothers, 8.6% in offspring of “prediabetic” mothers, and 1.4% of offspring of “nondiabetic” mothers.73 The differences remain after adjustment for diabetes in the father, age at onset of diabetes in either parent, and obesity in the offspring (Fig. 30-3). The authors concluded, “The findings suggest that the intrauterine environment is an important determinant of the development of diabetes and that its effect is in addition to effects of genetic factors.”73 The offspring of diabetic mothers enrolled in the Northwestern University long-term follow-up had a high prevalence of impaired glucose tolerance (IGT),74 particularly during puberty. IGT developed at similar rates in the offspring of mothers with GDM and preexisting DM. Excessive insulin secretion in utero was a strong predictor of both IGT and obesity in childhood, independent of degree of obesity.74 Together, these observations indicate that in offspring of diabetic mothers, nature (genetic factors) and nurture (intrauterine metabolic environment) may interact in predisposing to obesity and type 2 DM.
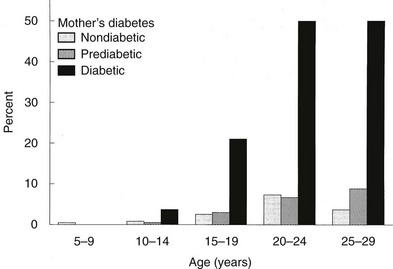
FIGURE 30-3 Age-specific prevalence of non–insulin-dependent diabetes mellitus (plasma glucose > 200 mg/dL 2 hours after oral glucose) in offspring of Pima Indian women without diabetes mellitus (light gray bars), those developing diabetes only subsequent to pregnancy (hatched bars), or those with diabetes during pregnancy (solid bars). (Data from Pettitt DJ, Aleck KA, Baird HR et al: Congenital susceptibility to NIDDM: Role of intrauterine environment, Diabetes 37:622–628, 1988.)
Diagnosis and Classification
We advocate the scheme outlined in Table 30-2 for the classification of carbohydrate intolerance during pregnancy. It incorporates many of the recommendations of an American Diabetes Association expert committee12 and those of the Fourth11 and Fifth75 International Workshop Conferences on GDM.
Table 30-2
Classification of Glucose Metabolism
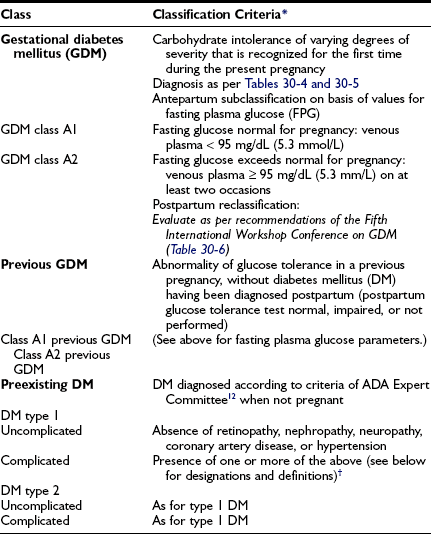
*Classification is based on prevailing practice in the authors’ center and the recommendations of the American Diabetes Association’s Expert Committee and the Fourth and Fifth International Workshop Conferences on Gestational Diabetes.11,12,75
†Designations and definitions of DM types 1 and 2 (designations are appended to primary diagnosis as appropriate [e.g., “diabetes mellitus type 2 uncomplicated”; “diabetes mellitus type 1; BDR, NEPH”]): BDR, Background diabetic retinopathy; CAD, coronary artery disease diagnosed by history, electrocardiogram (ECG), or stress ECG; HTN, hypertension, defined as blood pressure ≥ 140/90 mm Hg consistently; NEPH, diabetic nephropathy, defined as ≥ 0.5 g protein in 24-hr urine collection and/or serum creatinine consistently ≥ 1.2 mg/dL (≥106 mol/L); NEUR, neuropathy, defined as known gastroparesis when not pregnant, orthostatic hypotension, or sensory abnormalities in lower extremities detected at bedside examination; PDR, proliferative diabetic retinopathy.
Gestational Diabetes Mellitus
GDM is subclassified to distinguish between those with FPG values within the normal range for pregnancy (i.e., < 95 mg/dL [5.3 mmol/L]) and those with values exceeding the limits of normal (i.e., ≥ 95 mg/dL [5.3 mmol/L]). Those with higher FPG are at greater risk for progress to a diagnosis of diabetes outside of pregnancy,76 and some have arbitrarily initiated pharmacologic therapy in those with elevated FPG in the diagnostic OGTT.77 Patients are often seen who had GDM in a previous pregnancy but had no postpartum evaluation of glucose metabolism. Others may have had impaired fasting glucose or IGT postpartum, but not DM. From the perspective of proper classification and epidemiology, the diagnosis of GDM should not be assigned to such patients in a subsequent pregnancy. However, for purposes of clinical management, it is appropriate to stratify them on the basis of FPG (see earlier) and to designate them as GDM class A1, previous GDM, or as GDM class A2, previous GDM.
Preexisting Diabetes
We subdivide patients with preexisting DM into those with presumed type 1 or type 2 DM. Historically, the White classification78 was devised to predict pregnancy risk in type 1 DM based on age at onset and duration of diabetes, in combination with microvascular or macrovascular complications. In the present era, fetal loss is uncommon, and the degree of metabolic control throughout pregnancy and the presence or absence of vascular complications, independent of maternal age or duration of DM, are more specific predictors of maternal or fetal morbidity. Therefore, we currently use the White classification only for descriptive purposes. To assist in the estimation of maternal and fetal risks, we designate pregnancies in women with DM as uncomplicated (no known vascular or neuropathic complications) or complicated (one or more complications). Abbreviations for the specific complication(s) are added as a postscript. Hare79 proposed a similar although not identical classification.
Physicians would like to provide pregnant women who have complications of diabetes prospective answers to two questions: Will pregnancy accelerate or worsen preexisting complications? Does the diabetic complication per se contribute to the risk of adverse pregnancy outcome? In many cases, evidence is not sufficient to offer specific advice. These complicated issues have been reviewed in detail in a recent technical report from the American Diabetes Association.43 In the next several paragraphs, we comment on those situations for which the greatest amount of specific information is available.
Retinopathy: Diabetic retinopathy may worsen during gestation. The risk is present primarily in women with active proliferative changes or severe preproliferative retinopathy. Patients with mild background retinopathy or inactive laser-treated proliferative disease rarely experience progression of consequence. An association has been found between worsening retinopathy during pregnancy and the severity of hyperglycemia at enrollment80,81 and the magnitude of improved glycemic control achieved in the first half of gestation.80 This worsening during pregnancy may be analogous to the transient deterioration observed in nonpregnant subjects after the initiation of “tight” control of diabetes.82 Data from the Diabetes Control and Complications Trial82 indicate that pregnancy per se adds independently to the risk of transient progression of retinopathy, and the increased risk of progression may continue during the first postpartum year. Hypertension in pregnancy also is associated with progression of diabetic retinopathy.83 Regardless of the mechanisms involved, women with preexisting retinopathy should be advised of the potential for deterioration and the need for close ophthalmologic follow-up before conception, during pregnancy, and in the postpartum period. Although photocoagulation therapy can be used effectively during gestation, those with active proliferative disease should be advised to postpone pregnancy until photocoagulation treatment has stabilized the retinal condition.
Nephropathy: Diabetic nephropathy (24-hour urine protein ≥ 0.5 g or reduced creatinine clearance) increases risks for both the mother and offspring.43,84 Worsening proteinuria (twofold to threefold increase), hypertension, premature labor, and a need for early induction are common outcomes. The risks of these complications increase with stage of nephropathy (Table 30-3). Most women experience little permanent effect on renal function, despite transient but substantial increases in proteinuria.84,85 Occasionally, patients experience deterioration in renal function that continues in the postpartum period.43 Whether this decline is related to pregnancy or reflects the natural progression of renal impairment is uncertain. The number of subjects with severe diabetic nephropathy is too small to gain definitive information at any single center.
Table 30-3
Stages of the Evolution of Diabetic Nephropathy and Common Effects on Pregnancy
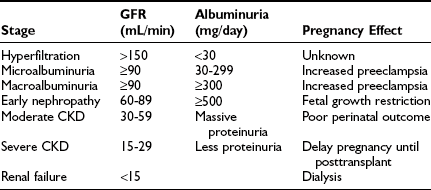
CKD, Chronic kidney disease; GFR, glomerular filtration rate.
Data from Ref. 43, p 375.
Neuropathy: Diabetic neuropathy is commonly found in patients with longstanding diabetes. Little is known about the effect of pregnancy on progression of diabetic neuropathy. However, autonomic neuropathy may contribute to maternal morbidity and adverse pregnancy outcome.43,86,87 Gastroparesis may result in marked glucose lability, inadequate nutrition, and maternal pulmonary aspiration. Bladder dysfunction may increase risk for urinary tract infection and worsening renal function.
Cardiovascular Disease: Both systolic and diastolic blood pressure may increase in pregnancy in type 1 diabetic women.88 In dated studies, myocardial infarction was associated with 50% mortality.89,90 An increased risk for myocardial infarction and congestive heart failure is also found in the postpartum period. The number of subjects with either longstanding type 1 or type 2 DM who experience coronary artery disease during pregnancy is small. At this time, an efficient, cost-effective strategy for detection and treatment of cardiovascular disease before and during pregnancy is not available.43
Diagnosis of Gestational Diabetes Mellitus
The optimal cost-effective strategy for the detection and diagnosis of GDM has been the subject of much controversy for decades. In the United States and a number of other countries, the standard procedure is to do a screening blood glucose (50-gm glucose challenge test [GCT]) followed by a 3-hour OGTT in those with a positive GCT. In some other countries, an OGTT is performed as the only blood glucose test in women with a history of GDM risk factors. It is anticipated that translation of the results of the HAPO Study5 and the increasing prevalence of GDM9,10 (see Epidemiology section) will lead to a less complicated screening and diagnostic algorithm for all pregnant women that will be widely if not globally adopted. Previously diagnosed and undiagnosed type 2 DM have also increased in prevalence8 and are generally asymptomatic. Testing blood glucose concentrations in all women at the first obstetric appointment and serially throughout pregnancy is not cost effective in most populations. However, those with undiagnosed DM or IGT before conception have hyperglycemia in the first trimester and may benefit from early diagnosis and initiation of therapy. Therefore, it is important to have a comprehensive strategy for GDM diagnosis that is tailored to the population served. Participants in the Fourth and Fifth International Workshop Conferences on Gestational Diabetes Mellitus recommended that an assessment of risk for GDM as outlined in Table 30-4 be performed during the first prenatal visit.11,75
Table 30-4
Screening Strategy for Detecting Gestational Diabetes Mellitus11,75
GDM Risk Assessment Should Be Ascertained at the First Prenatal Visit
High risk: Perform blood glucose testing as soon as feasible if one or more present:
If GDM is not diagnosed, blood glucose testing should be repeated at 24–28 weeks or at any time a patient has symptoms or signs suggestive of hyperglycemia.
Average risk: Perform blood glucose testing at 24–28 weeks by using either:
• Two-step procedure: 50-gm glucose challenge test (GCT) followed by a diagnostic oral glucose tolerance test (OGTT) in those meeting threshold value in GCT (see text for details) or
• One-step procedure: Diagnostic OGTT performed on all subjects (see text for details)
Low risk: Blood glucose testing not routinely required if all of these characteristics are present:
We anticipate that strategies for detection and diagnosis of GDM will change when the HAPO Study results are applied to clinical practice, but in the authors’ opinion, universal blood glucose testing should continue to be performed in the interim unless the patient population includes a sizable proportion of women who qualify as low risk. Two points from Table 30-4 deserve emphasis. First, it is critical that women be defined as “low risk” only if they have all of the low-risk characteristics. Second, those who are identified as “high risk” should have their initial blood glucose testing at the first prenatal visit. If normal, they are to be retested at 24 to 28 weeks’ gestation. Women at “average risk” should receive a glucose challenge test (GCT) for the first time at 24 to 28 weeks’ gestation. Those with values of 140 mg/dL (7.8 mmol/L) or more go on to a diagnostic oral glucose tolerance test (OGTT).
It is important that glucose measurements on serum or plasma be made with certified laboratory techniques. Although measurement of capillary blood glucose with portable meters and reagent strips is convenient and rapid, a within-test variability of 10% to 15% markedly reduces both the sensitivity and specificity of this approach.11,75 Measurements of random blood glucose,91 hemoglobin A1c,92 or fructosamine93 also are not sufficiently sensitive for screening purposes.11 Although more palatable, screening with alternative agents such as jelly beans may be less sensitive in detecting GDM.94 Second-trimester amniocentesis for amniotic fluid insulin demonstrates reasonable sensitivity for early diagnosis of GDM95,96 but is invasive and potentially has adverse consequences.
Diagnosis
Patients with an abnormal GCT receive a 3-hour 100-g OGTT, interpreted according to the criteria of O’Sullivan and Mahan3 (extrapolated to venous plasma from whole blood). These criteria for the diagnosis of GDM, initially established over 40 years ago, with minor modifications remain in widespread use today. These criteria were chosen to identify women at high risk for development of diabetes following pregnancy, not to identify pregnancies at increased risk for adverse perinatal outcomes. Others use criteria for GDM that are the same as those used to classify glucose tolerance in nonpregnant persons.4 When the National Diabetes Data Group (NDDG) developed the classification and diagnosis of DM in 1979,97 the AutoAnalyzer colorimetric (ferricyanide-based) analytic method for glucose was the “gold standard.” Currently, glucose assays are primarily enzymatic (glucose oxidase or hexokinase). Carpenter and Coustan98 derived values for interpretation of a 100-g OGTT that more accurately extrapolates the O’Sullivan results to glucose oxidase–based methods. This results in lower plasma glucose values for the diagnosis of GDM than those recommended by the NDDG and about a 50% increase in the number of women with a diagnosis of GDM.9 Several studies found that pregnancy-associated complications occurred in equal frequency in the additional pregnancies defined as GDM with the plasma glucose cut-off values recommended by Carpenter and Coustan and in those cases that also met the higher NDDG criteria.99–101 The Fourth International Workshop Conference on GDM12 and the American Diabetes Association12 endorsed the Carpenter-Coustan translation of the O’Sullivan-Mahan criteria for interpretation of the 100-g OGTT (Table 30-5). The American Diabetes Association also approved criteria for interpretation of a 2-hour 75-g OGTT that are identical to the Carpenter and Coustan levels used in the 100-g OGTT.98 These criteria are loosely based on studies of populations in the United States, Europe, Brazil, and Australia,11,102,103 but they lack validation from perinatal outcomes in a large number of pregnancies.
Table 30-5
Diagnosis of Gestational Diabetes Mellitus: 100 g Oral Glucose Load*

*The test should be performed in the morning after an overnight fast of at least 8 hours but not more than 14 hours and after at least 3 days of unrestricted diet (≥150 gm carbohydrate per day) and physical activity. The subject should remain seated and should not smoke throughout the test.
†Two or more of the values for glucose concentrations must be met or exceeded for a positive diagnosis
A number of investigators have also provided evidence that adverse pregnancy outcomes are common in women with plasma glucose levels during the OGTT that are lower than those currently used in the United States for the diagnosis of GDM. As indicated previously, the HAPO Study results showed continuous associations between perinatal outcome and maternal glycemia across the full subdiabetic range.5 In our opinion, caregivers should continue to apply the diagnostic criteria they presently use until results from the HAPO Study and others lead to new clinical guidelines.
Heterogeneity of Gestational Diabetes Mellitus
Phenotypic Heterogeneity
Substantial heterogeneity has been observed among women with GDM in age, weight, severity of carbohydrate intolerance, and insulin secretion.76 Those who have GDM are older and heavier than their “normal” counterparts yet span the age and weight spectrum of the obstetric population. FPG levels tend to increase in GDM in parallel with the degree of obesity, but lean women with elevated FPG levels are frequently encountered. Fasting plasma immunoreactive insulin (IRI) is greater in obese subjects of both control and GDM groups (except in GDM subjects with elevated FPG).76 Controlling for the effects of age and weight indicates that the short-term (15-minute) and long-term (3-hour integrated) IRI response is attenuated in both obese and lean women with GDM, and the insulinopenia is most pronounced when the fasting plasma glucose level is elevated.76 A small number of gravida with GDM display well-preserved IRI responses to oral glucose.76 Similar heterogeneity exists with respect to the first and second phases of IRI secretion in response to intravenous glucose in GDM.17 Insulin response to mixed meals also is heterogeneous in markedly obese patients with GDM. Severe insulin resistance is characteristic of late normal pregnancy, and several reports indicated that on average, subjects with GDM are even more resistant.17,18,104 In some studies, the issue is confounded by differences in age and weight and potentially by the effects that even mild fasting hyperglycemia may exert on insulin sensitivity.
Genotypic Heterogeneity
Genotypic heterogeneity has been suggested in GDM from findings of some immunologic features that are associated with risk of type 1 DM in comparison to the characteristics of racially matched controls. The prevalence of glutamic acid decarboxylase (GAD) or islet cell antibodies in women with GDM varies with the methods used and populations studied.14,105 Together, the reports suggest a higher prevalence of islet cell antibodies in white women with GDM and elevated FPG at diagnosis. These and other findings suggest that the population with GDM may include some patients with slowly evolving type 1 DM. A retrospective review from Copenhagen concurred and noted that a higher than expected number of women with type 1 DM had the onset during pregnancy.106
Racial and ethnic-group differences in the prevalence of GDM have been observed that are not fully accounted for by differences in maternal age or obesity.107,108 However, specific genetic or environmental factors mediating predisposition to GDM remain to be defined. DM associated with mutations of mitochondrial DNA109,110 may be initially discovered as GDM. Maturity-onset diabetes of the young (MODY), another uncommon and atypical form of type 2 DM, also can be manifested as GDM.111 To date, specific diabetic genetic factors have been demonstrated in only a small fraction of women with GDM.112
Treatment of Diabetes Mellitus Diagnosed Before Pregnancy
Preconception Planning
Education about the special issues related to pregnancy (impact of diabetic complications, metabolic control and adverse pregnancy outcome, family planning, contraception) should begin when young women with diabetes achieve fertility. It is emphasized that 3 to 6 months before intended conception, consultation with the diabetes and pregnancy team should be sought to reassess diabetes status and review the medical and obstetric management both before and during gestation. At the preconception visit, assessment includes determination of vascular complications, measurement of hemoglobin A1c and thyroid-stimulating hormone (TSH), and determination of renal function (often requiring 24-hour creatinine clearance and quantitative urinary protein). Before gestation, prescribed medications which may have adverse fetal effects—such as angiotensin-converting enzyme (ACE) inhibitors, angiotension-receptor blockers (ARBs), thiazolidinediones, and 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors—should be discontinued, substituting other appropriate medications (e.g., antihypertensive agents) where needed. Supplemental intake of folic acid, 0.8 mg daily, is now routinely recommended to reduce risks for neural tube defects in the offspring of women with and without diabetes. Lifestyle factors needing modification such as smoking and alcohol/drug use should be addressed. Pregnancy should not be discouraged on genetic grounds. Long-term follow-up of the offspring of persons with type 1 DM indicated a prevalence of type 1 DM of 1.3% in the offspring of diabetic mothers and 6.1% in the offspring of diabetic fathers.113 Short- and long-term consequences of inadequately controlled maternal diabetes for the offspring should be reviewed. The potential impact of pregnancy on maternal diabetes complications and the time commitment needed to attain optimal control before conception and throughout gestation should be discussed.
As indicated in the Congenital Malformations and Early Fetal Loss discussion, clinical and experimental evidence indicates that the risks of these events in diabetic pregnancies are linked to disturbances in maternal metabolism around the time of conception.15,47 Furthermore, control of maternal diabetes during the period of organogenesis may reduce the prevalence of these adverse outcomes.47 This possibility makes a compelling case for establishing tight control of diabetes before conception.
The preceding considerations have led us to advise prospective mothers to attain stable, near-normal glycemic control before pregnancy and during the period of organogenesis. Striving for complete normalization may lead to frequent hypoglycemia in some women, which we attempt to minimize. The relentless pursuit of completely normal blood glucose levels, particularly in women with hypoglycemia unawareness, may have adverse consequences, as indicated by recent reports of very high prevalence of severe episodes of hypoglycemia in the 3 months prior to pregnancy and during the first trimester of gestation.114 For patients with more than minimal retinopathy who are in poor control, we recommend slow but steady correction of hyperglycemia over a period of several months before conception, in the hope of preventing deterioration in retinal status. Patients are reassured that with careful adherence to medical and obstetric treatment plans, the chances of a favorable outcome are excellent for both the mother and baby. Unfortunately, in the United States, most women with known preexisting DM still fail to begin intensive management of diabetes before their pregnancy,51 despite extensive professional and patient educational efforts for more than a decade.
Diet
Dietary prescriptions are individualized and modified if necessary over the course of gestation. Although they were developed for the general population, we apply the Institute of Medicine of the National Academies of Science guidelines concerning optimal weight gain during gestation115 to patients with DM. It is recommended that weight gain be proportional to the degree of adiposity (based on body mass index [BMI; weight/height2]) in the mother before conception. This may range from as little as a 15-lb (7-kg) gain in the very obese to as much as 40 lb (18 kg) for underweight women. Estimates of the caloric cost of singleton pregnancy have varied widely, for example, from an additional 100 to 150 kcal/day to 300 to 450 kcal/day in the second and third trimesters.116 This underscores the need to individualize dietary prescriptions and make alterations over time as necessary.
It has long been recognized that the increase in blood glucose following ingestion of different foods containing the same amount of carbohydrate is variable. This led to rating the hyperglycemic properties or “glycemic index” of various foods.117 Applying this insight effectively for routine patient care poses difficulties. At least two factors contribute: first, foods with different glycemic indices are often combined in the same meal, with unpredictable effects on blood glucose; and second, the apparent glycemic effects of the same food tend to vary from meal to meal. However, alert patients often discover particular foods or combinations of foods that for them seem to result in an exaggerated glycemic response, and which they then avoid.
Insulin
Short-Acting Insulins: There are four commercially available short-acting insulins that can be used: regular (intact human insulin) and three human insulin analogs that have amino acid sequence substitutions or alterations that lead to more rapid entry into the circulation from subcutaneous injection sites. Regular insulin has been used in pregnancy for several decades, without evidence of harmful effects for mother or offspring. After subcutaneous injection, onset of action begins in 30 to 60 minutes, peak levels are reached in 2 to 3 hours, with duration of 5 to 8 hours. The three modified insulins (lispro, aspart, and glulisine insulin) have almost identical insulin action profiles that more closely approximate secreted insulin profiles following a meal in normal individuals. Onset of action is 5 to 15 minutes after injection, with peak effects at 30 to 90 minutes and duration of 4 to 5 hours. In studies, this “more physiologic” profile has resulted in a reduction in postprandial hyperglycemia and a lesser incidence of hypoglycemia in both pregnant118 and nonpregnant subjects.119 Concern about the safety of insulin analogs centers on the cross-reactivity of insulin and IGF-1 for insulin and IGF-1 receptors. There is evidence that IGF-1 plays a role in embryo implantation and in placental nutrient flow in later gestation. Potentially, altered IGF-1 receptor stimulation by insulin or an insulin analog could adversely affect these processes. Human insulin has little affinity for the IGF-1 receptor compared to IGF-1 itself, and in vitro studies with lispro, aspart, and glulisine insulin indicate IGF-1 receptor affinity of 156%, 81%, and 100%, respectively, compared to native human insulin. Moreover, in vitro estimates of mitogenic potency of the analogs are less than for native insulin (see Ref. 120 for review).
A number of clinical reports of lispro and aspart insulin in pregnancy have been published. They include a large retrospective study (500 pregnancies) of insulin lispro121 and a prospective comparison of aspart insulin and regular insulin in a randomized clinical trial.118 To date, no convincing evidence has arisen that would indicate a lack of safety of these analogs. By comparison, there is as yet little clinical information available concerning glulisine use in pregnancy.
Long-Acting Insulins: Three insulin preparations that can be classified as long-acting are currently available commercially.120 NPH insulin, like regular insulin, has been used in pregnancy for several decades and is considered to have an established safety profile. Its onset of action begins after 1 to 4 hours, with peak effects at 6 to 10 hours and a duration of 11 to 17 hours. Two modified insulins, glargine and detemir, provide more uniform blood levels over approximately 24 hours than NPH and have been referred to as “peakless insulins.” Their use in nonpregnant patients with type 1 DM has been associated with less hypoglycemia, especially overnight, and they allow more flexibility in mealtimes. Glargine has onset of action 1.2 to 1.8 hours after injection, with a duration of effect of 18 to 26 hours. Detemir is similar, except for a somewhat shorter duration of effect. In vitro studies suggest that compared to human insulin, glargine has substantially increased IGF-1 activity and mitogenic potency in certain cell lines. The meaning of these observations in normal cells or intact animals or humans is unknown, but such findings highlight the desirability of randomized clinical trials. Nonetheless, many women who have been using glargine insulin successfully before conception are reluctant to revert to use of NPH or to begin the use of insulin-pump therapy. Thus, use of glargine insulin during pregnancy is increasing. In reports based on a total of 189 patients,122–124 no adverse events that appeared to be related to the use of glargine insulin have been noted. Clinical reports concerning the use of detemir insulin in pregnancy are not currently available.
Our goal is a premeal capillary blood glucose level of 65 to 85 mg/dL (3.6 to 5.3 mmol/L) and 1- and 2-hour postprandial levels lower than 140 mg/dL (7.8 mmol/L) or 120 mg/dL (6.7 mmol/L), respectively, throughout gestation. Measurements of finger-stick capillary blood sugar37 and interstitial fluid glucose using continuous sampling technologies38 in normal pregnant women have shown that average levels are in fact lower than these targets. Women who are able to consistently achieve levels lower than these targets are encouraged to do so. However, for most women with type 1 DM, this is extremely difficult and results in more frequent hypoglycemia. In contrast to findings of adverse effects of hypoglycemia on the embryo in experimental animals and in vitro models,57 episodes of maternal hypoglycemia during the latter two-thirds of pregnancy do not appear to be harmful to the fetus.125 Late in the first trimester (approximately 10 to 14 weeks’ gestation), sensitivity to insulin may increase temporarily. This may be compounded by “morning sickness” and lead to more pronounced blood sugar fluctuations and increased risk for severe hypoglycemia. A greater degree of caution in attempting to normalize blood glucose concentrations is indicated in this time period. After the first trimester, a two- to threefold increase in insulin requirement occurs, reaching a peak in the middle of the third trimester. The greatest rate of change typically occurs between 20 and 30 weeks of gestation (see Fig. 30-1). Dosing requirements are relatively stable in weeks 32 to 38, with only modest changes required from week to week. Some decline in nocturnal insulin dosing is commonly noted in the 1 to 2 weeks before delivery. The challenge of therapy is to modify the insulin dosage in a timely fashion in parallel with these fluctuations in insulin sensitivity while maintaining optimal glycemic control.
Monitoring Control of Diabetes
Self-monitoring of capillary blood glucose is universally employed by women treated with insulin during pregnancy. For those not familiar with this technique (usually those with GDM and often with type 2 DM), a certified diabetes educator gives initial instruction, with follow-up at each clinic visit where venous plasma glucose is obtained with simultaneous patient-measured capillary values. This provides verification of proper functioning of the monitoring equipment. Patients are asked to measure blood from a finger stick rather than from an alternate site (e.g., palm or forearm), because this more accurately reflects simultaneous venous plasma glucose. Blood sugar is measured before each meal, at bedtime, and often at either 1 or 2 hours after meals. Measuring after meals is helpful for determining the appropriate dose of short-acting insulin and may more accurately reflect fuel delivery to the fetus than measuring only premeal values.126 The level of blood glucose before a meal is also of utility in selecting doses of short- or intermediate-acting insulin for the next interval, as well as revealing hypoglycemia in those with hypoglycemia unawareness. When postprandial hyperglycemia persists despite normalization of premeal blood glucose, adjustments in analog insulin, meal size, frequency of feedings, or a combination of these may be of benefit. Monitoring urine glucose is unnecessary.
Hemoglobin A1c
Measurements of hemoglobin A1c are obtained at enrollment and at 4- to 8-week intervals until term. As noted earlier, values in the first or early second trimester provide a general indication of the risk of fetal loss and major congenital malformations47 and help guide decisions about management. Serial assessments provide affirmation for patients that their efforts to achieve better control of diabetes are effective. A disparity between hemoglobin A1c concentrations and blood glucose measurements may signal errors in glucose-monitoring technique or, rarely, falsification of results. Other factors (hemolysis, hemoglobinopathies, variations in analytic technique) can alter the values of hemoglobin A1c appreciably and render such measurements of limited value. In such cases, serial measurements of fructosamine or glycosylated albumin may provide analogous information.
Obstetric Surveillance
Measurements of crown-rump length by ultrasonography in the first trimester provides the most accurate determination of gestational age when dating by menstrual history is in doubt. Viability is assessed by detection of fetal heartbeat, which can be seen in embryos as early as 5 weeks’ gestation.127 Screening to detect aneuploidy, such as Down’s syndrome, and certain single organ defects, such as neural tube defect, is offered to all women. Although different screening protocols are in use, a combination of nuchal translucency thickness at 11 to 13 weeks’ gestation by ultrasound,128,129 with multiple serum markers performed at the same time as the ultrasound and again at 15 to 18 weeks is very sensitive for detecting an increased risk of these defects. If screening is abnormal, diagnostic testing by chorionic villous sampling (CVS) or amniocentesis is offered to rule out aneuploidy, or by amniocentesis to rule out open neural tube defect. Even in the absence of abnormal screening results, a detailed ultrasound examination is offered at 18 to 22 weeks to detect cardiac and other anomalies. Fetal echocardiography, more sensitive than ultrasound in detecting cardiac defects, may be indicated in certain patients.130 Those with normal serum screening but an abnormal nuchal translucency have an increased risk of cardiac defects,128 as do those with poor periconceptional metabolic control. If serious deformities are detected by 22 to 24 weeks, pregnancy termination may be a consideration. If termination of pregnancy is not feasible or desired, detailed advanced information about a birth deformity is of value for planning delivery and for preparing the parents.
In the third trimester, serial ultrasound measurements may be used to assess evolving macrosomia, intrauterine growth retardation, polyhydramnios, and fetal cardiac hypertrophy. Although ultrasound is the best tool available for evaluation of fetal size, predicting macrosomia and planning operative delivery from such estimates is controversial.131
Reassurance of fetal well-being by noninvasive techniques used to detect the risk of stillbirth permits most pregnancies to be carried to term, although there may be indications for early delivery in women with advanced vasculopathy, despite reassuring fetal testing. The biophysical test most commonly used is the nonstress test (NST).132 Two or more accelerations of fetal heart rate in response to spontaneous fetal activity in 20 minutes of continuous fetal heart monitoring is predictive of fetal well-being. If the NST is nonreactive, further evaluation is undertaken with a biophysical profile. Some use the biophysical profile as the primary mode of fetal surveillance. Modeled after the Apgar score, the biophysical profile assesses five parameters (NST plus fetal activity, fetal breathing activity, fetal tone, and the volume of amniotic fluid) determined by fetal ultrasound. Serial fetal testing may be initiated as soon as the fetus is viable in a highly complicated pregnancy but no later than 32 weeks, even in a pregnancy progressing normally.133 Assessments are made once or more each week as dictated by clinical circumstances.
Delivery and Puerperium
During spontaneous or induced labor, the objectives of medical management are to maintain plasma glucose in the physiologic range (70 to 120 mg/dL [3.9 to 6.7 mmol/L]) and to prevent ketosis. Several protocols to achieve these goals have been published.1 The practice of the authors is to administer intravenous glucose as D10W at 5 to 6 g/hour by a constant-infusion pump. Other intravenous fluids are devoid of glucose. Capillary blood sugar is measured every 1 to 4 hours. The glucose infusion may be delayed by 1 to 2 hours when hyperglycemia is present at entry, and insulin administration may be withheld temporarily if the blood glucose level is less than 70 mg/dL (3.9 mmol/L). Patients with type 1 DM may be treated with small doses of short-acting subcutaneous insulin (lispro, aspart, or regular) every 3 to 6 hours as needed. Preferably, a continuous intravenous infusion of regular insulin is given at rates of 0.02 to 0.04 U/hr/kg of body weight (1.4 to 2.8 U/hr in a 70-kg woman), or by CSII in patients using insulin pumps. However, the best determinant of insulin need may be the 24-hour insulin requirement of the patient before delivery, with approximately 30% to 40% of the total dose representing basal requirement. Insulin infusion is initiated at that basal rate, then modified as necessary to achieve a stable blood glucose level within the normal range. Those managed with insulin pumps initially continue the basal insulin dose they are using. This may then be modified according to the capillary blood glucose measurements performed subsequently. Those receiving insulin glargine have “ongoing” basal insulin that will not be dissipated for approximately 24 hours from the time of the last dose and often need no insulin for many hours during labor. Those with type 2 DM often do not require insulin during labor if the rate of glucose infusion does not exceed 5 to 6 g/hr. The relative importance of maternal hyperglycemia during labor and delivery versus that in the weeks before delivery in promoting neonatal hypoglycemia remains controversial. Nonetheless, we feel that it is prudent to maintain normoglycemia throughout the delivery process.
Insulin requirements usually decline dramatically immediately after delivery (often by 50% to 90%) (see Fig. 30-1). After several days, insulin requirements usually return to levels similar to those present before pregnancy. Women who wish to breast feed are maintained at or above their antepartum caloric intake. Limited data indicate that neither glyburide nor glipizide are transported into breast milk or are responsible for hypoglycemia in nursing infants. Moreover, extremely small (considered not clinically significant) amounts of metformin have been detected in breast milk of lactating women using metformin. These agents therefore may be safe for use postpartum in patients with type 2 DM who are nursing infants,75 but until there is more experience, they should be used with caution. Those who do not breast-feed are returned to a diet appropriate for nongravid women (30 to 32 kcal/kg IBW [125 to 135 kJ/kg]). All patients are encouraged to use the diabetes management skills they acquired during gestation.
Treatment of Gestational Diabetes Mellitus
Morbidities
The risks of perinatal loss and neonatal morbidity are increased when GDM is undetected or treated casually.134 In many centers, however, women with GDM receive dietary advice, some form of blood sugar monitoring, and treatment with insulin if hyperglycemia persists.11,12,75 Moreover, pregnancies complicated by GDM are designated “high risk,” which leads to more intensive obstetric supervision, itself a form of intervention.11 With such approaches, little if any increase in perinatal loss occurs in GDM, and the frequency of neonatal morbidity (such as hypoglycemia, hypocalcemia, polycythemia, and hyperbilirubinemia) may decrease toward levels found in the general population.135 Recently, Crowther and colleagues136 reported results of a randomized treatment trial of GDM in which intervention reduced serious perinatal morbidity and the mother’s health-related quality of life. Nonetheless, benefits and cost effectiveness of diagnosis and treatment of GDM have been questioned. For example, the U.S. Preventive Services Task Force recently concluded that “current evidence is insufficient to assess the balance of benefits and harms of screening for gestational diabetes mellitus, either before or after 24 weeks’ gestation”.137
Congenital Malformations
The risk of congenital anomalies is not increased in most pregnancies with GDM.138 However, in GDM with fasting hyperglycemia that is diagnostic of DM, there is an increased risk of birth defects that is proportional to the severity of the hyperglycemia. This has been found in the Latino population of Los Angeles, where the prevalence of both GDM and type 2 DM is high.139 In reality, many of the women with diagnostically elevated FPG probably have had previously undiagnosed DM before pregnancy, which is being identified for the first time as GDM. Thus, identifying pregnancies at risk and achieving better care before conception remain major challenges.
Fetal Hyperinsulinism
Offspring of mothers with GDM or with elevated plasma glucose levels in the nondiabetic range5 are at risk for fetal hyperinsulinemia, increased fetal adiposity, and excess fetal size (macrosomia), which increases the likelihood of birth trauma and operative delivery.11,135 In addition, many studies indicate that the maternal metabolic abnormalities seen in gestational and preexisting diabetes have long-term consequences on weight, pancreatic function, and neurologic development of the offspring (see Anthropometric and Metabolic Development).
Metabolic Surveillance
All patients with GDM are asked to monitor blood or urinary ketones before breakfast and dinner to detect possible deficiencies in dietary carbohydrate. Fasting and 1-hour postprandial plasma glucose measurements are obtained at outpatient visits to monitor for deteriorating glucose tolerance requiring more intensified treatment and to assess the accuracy of glucose self-monitoring, if performed. All insulin-treated patients monitor fasting and premeal capillary blood glucose levels to guide adjustment of insulin doses and include measurements of 1- or 2-hour postprandial glucose concentrations at least twice weekly. Debate exists regarding use of postmeal versus premeal glucose levels to determine insulin doses.140 Participants in the Fourth11 and Fifth75 International Workshop Conferences on GDM concluded that glucose self-monitoring appears to be superior to glucose monitoring performed only at clinic visits for the detection of glucose concentrations that may warrant intensification of therapy beyond standard dietary management, but they stopped short of recommending its use by all women with GDM. We recommend its use in those diet-treated subjects who wish to use the results as an incentive to achieve better adherence to diet and lifestyle recommendations and in patients who require more intensive therapy.
Metabolic Management
The rationale for treatment of GDM has been summarized earlier. Since the risk of perinatal loss is not appreciably increased in the majority with GDM (given the excellent obstetric care presently available), efforts now focus on preventing perinatal morbidity and potential adverse long-term sequelae. Prevention of macrosomia and the morbidity associated with it has received the greatest attention. Restoration of fasting and postmeal glucose values to within normal ranges is the primary goal of treating GDM, with the initial step being lifestyle modification. Although controlled trials have not been performed to identify ideal glycemic targets for the prevention of fetal risk, evidence presented at the Fourth International Workshop Conference on GDM suggests that reducing maternal capillary blood glucose concentrations to 140 mg/dL or less (7.8 mmol/L) at 1 hour, or 120 mg/dL or less (6.7 mmol/L) 2 hours after meals, or both, may reduce the risk of excessive fetal growth.11 The target for fasting and premeal values is commonly less than 95 mg/dL (5.3 mmol/L). Recent studies in normal pregnant women have found blood glucose levels lower than previously expected, with mean glucose concentration 78.3 mg/dL at 38 weeks and mean postprandial glucose values not exceeding 105.2 mg/dL at 1 or 2 hours.37,38 Even in these nondiabetic women, maternal postprandial capillary glucose measurements correlated with fetal size (abdominal circumference).37
Some investigators have provided evidence that it is more cost effective to assess fetal abdominal circumference (AC) by ultrasound in all women with GDM to identify those at low risk for having a large baby (AC < 75th percentile) and concentrate therapeutic efforts on those with much higher risk for delivery of a large baby (AC ≥ 75th percentile).63
Lifestyle Modification
Nutritional Therapy: Medical nutrition therapy (MNT) is referred to as the “cornerstone” of medical or metabolic management of GDM. The objectives of MNT and the approaches used for GDM are the same as already discussed for normal pregnancy and preexisting DM. Adjustments are made to the initial prescription (35 to 38 kcal/kg IBW [145 to 160 kJ/kg]) as needed to maintain weight gain within the range appropriate for the subject’s prepregnancy weight.115 Several “isocaloric” modifications of the standard diet have been investigated. Reduction in carbohydrate content to 30% to 40% can reduce postprandial hyperglycemia141 but is associated with an increased fat or protein content or both. The effects on maternal amino acid, ketone, and lipid levels and on long-term outcomes for the offspring are not known. When the daily dietary intake is ingested as multiple small meals (six or seven), postprandial glycemic peaks are reduced.142 However, fasting levels may not be achieved before the next meal, and mean 24-hour glucose may not differ from the standard approach (three meals plus bedtime snack). Safety, efficacy, and long-term outcomes need further study. Foods with a low glycemic index and fiber-enriched diets have been evaluated for both prevention and treatment of GDM.143,144 Convincing evidence of effectiveness is lacking.
Hypocaloric Diet.: Because caloric restriction in obese nonpregnant subjects with type 2 DM can reduce insulin resistance and correct hyperglycemia, use of a hypocaloric diet in obese women with GDM is appealing. Moderate caloric restriction (25% to 35% below standard diets) results in some correction of hyperglycemia.145–147 Some147 but not all146 groups have noted a reduction in fetal weight in these subjects; however, larger numbers in controlled trials are needed to evaluate immediate and long-term safety and efficacy of this approach. Knopp and associates145 also examined metabolic responses to a more severe (50%) reduction in caloric intake in obese women with GDM. Mean 24-hour glucose, fasting insulin, and triglyceride levels declined substantially, but plasma β-hydroxybutyrate concentrations increased more than twofold, and ketonuria increased significantly. Until more data are available on the effects of such treatment on perinatal and long-term outcomes, caloric restriction of this magnitude should be considered experimental. Monitoring plasma β-hydroxybutyrate or urine ketones would be critical to determine fetal safety of this therapy.
Exercise: Although concern has been expressed about increasing uterine contractility, IUGR, prematurity, fetal bradycardia, and ketonuria in association with exercise, physically active, well-conditioned women have routinely engaged in exercise during pregnancy without apparent adversity. Moreover, cardiovascular fitness training outside of pregnancy is known to increase insulin sensitivity and glucose disposal by recruitment of glucose transporter proteins, thus making exercise an attractive therapeutic possibility in GDM.148 Studies using arm ergometry149 or a recumbent bicycle150 found moderate exercise to be safe and effective in reducing fasting and postprandial blood glucose levels in women with GDM. Others failed to see better glycemic control with the use of moderate exercise.151 Recently, encouraging results were reported (fewer babies with macrosomia) in a prospective (but not randomized) trial that was designed to limit maternal weight gain of obese women with GDM by a combination of diet and exercise.152
Intensified Metabolic Management
When goals for maternal glycemia are not achieved or sustained with the lifestyle modifications outlined earlier, or when signs of excessive fetal growth are demonstrated, it is generally acknowledged that there is need for more intensive metabolic therapy. Operationally, we advise changes in the treatment regimen if more than 20% to 25% of glucose monitoring values are above fasting/premeal or postprandial targets (individually or in combination). Historically, treatment with insulin has been used in such instances, since the use of oral medications was specifically “not recommended.”153 However, on the basis of results from randomized clinical trials, use of the oral medication, glyburide (glybenclamide outside of the United States), is now recognized as being a commonly used alternative to therapy with insulin.75,154 Results of a clinical trial of GDM treatment with metformin have also been published recently.155 Accordingly, the potential use of oral medications will also be considered later.
Insulin
The precise place for insulin therapy in GDM remains difficult to define. It is generally agreed that a woman with overt hyperglycemia diagnostic of DM (FPG ≥ 126 mg/dL [7.0 mmol/L]) should start insulin immediately because the perinatal risks are like those for patients with preexisting diabetes. Approximately 0.5 to 1.4 units of insulin per kilogram of body weight per day is required to maintain fasting/premeal and 1- or 2-hour postprandial values within the target ranges defined earlier. A “mixed/split” insulin regimen (rapid-acting [human regular insulin or analog]/intermediate-acting [NPH) has typically been used for many years, although multiple daily injections may provide greater flexibility in management.156 As noted, during pregnancy, as well as outside of pregnancy, the rapid-acting insulin analogs have an established place in management of preexisting diabetes and are now commonly used in GDM as well. We do not currently use or recommend the use of long-acting analogs in the treatment of GDM.
Oral Antihyperglycemic Agents
Glyburide: On the basis of evidence that transplacental passage of glyburide is very low at term,157 Langer and colleagues conducted a clinical trial comparing glyburide and insulin in patients needing intensified treatment of GDM.158 The primary outcome was glycemic control which was comparable in the insulin and glyburide treatment groups; only 4% of those assigned to glyburide required transfer to insulin treatment. The investigators concluded that “in women with gestational diabetes, glyburide is a clinically effective alternative to insulin therapy”.158 The results have been extensively critiqued, and numerous other reports have followed.154 Subsequent studies have not been randomized trials, nor have they involved numbers of patients that approached the sample size of the initial report, and they have not provided convincing evidence of adverse effects. As a result, the use of glyburide for treatment of GDM appears to be increasing in part because it represents a less expensive, less labor-intensive option. In the summary and recommendations of the Fifth International Workshop Conference on GDM, emphasis was placed on the need to maintain adequate metabolic and obstetric surveillance of all patients to assure that therapeutic objectives are met whether receiving MNT, treatment with insulin, or therapy with oral medications.75 We strongly endorse this position.
Metformin: Although metformin freely crosses the placenta, use of metformin in childbearing women has increased substantially in recent years. It is frequently used to enhance fertility in patients with polycystic ovary syndrome (PCOS). However, there is no compelling evidence that metformin reduces pregnancy loss, and it is currently recommended that metformin be discontinued as soon as pregnancy is confirmed.159
There is also renewed interest in the use of metformin for the treatment of some patients with type 2 DM or GDM. Results of a randomized trial of the use of metformin or insulin for treatment of GDM (MIG Trial) in Australia and New Zealand has been published recently.155 No evidence of adverse effects of metformin on perinatal outcomes was found. However, nearly half of those assigned to the metformin arm required the addition of insulin to achieve glycemic treatment targets. Furthermore, since metformin freely crosses the placenta, conclusive assessment of the safety and benefits of metformin use in pregnancy requires long-term follow-up of the offspring. Thus, we continue to consider metformin use in pregnancy as investigational.
Other Criteria for Initiating Intensified Therapy
Various criteria or algorithms (apart from or in addition to severity of maternal hyperglycemia) have been used to identify pregnancies at highest risk for fetal hyperinsulinemia or increased size or both and to serve as criteria for insulin treatment. Weiss and co-workers95 used elevated amniotic fluid insulin levels (which reflect fetal hyperinsulinemia) to determine the need for insulin therapy and reported good fetal outcomes in uncontrolled trials. Fetal ultrasound to measure shoulder soft tissue61 or abdominal circumference63 has been used to stratify the risk for macrosomia. Those with abdominal circumference less than the 75th percentile were not at increased risk. Those with abdominal circumference at the 75th percentile or higher were considered at risk, and intensive insulin therapy in these patients eliminated that risk. The long-term outcomes associated with the application of these methods must be evaluated further because risk of obesity and glucose intolerance in the offspring is not dependent on the presence of macrosomia at birth.70,71 The hypothesis that a relatively low hemoglobin A1c concentration can identify a subgroup of patients who may be treated by diet therapy alone with no excess risk of fetal complications also warrants further investigation.160
Delivery and Puerperium
Therapeutic goals for the management of gestational diabetes during labor and delivery are the same as those outlined previously for women with preexisting DM. Insulin therapy is rarely required to maintain intrapartum normoglycemia in women with GDM. The vast majority of patients requiring antepartum insulin therapy can discontinue it at delivery. However, measurement of FPG or untimed (random) plasma glucose should be performed prior to hospital discharge to detect the unusual patients with overt diabetes who should be treated75 (Table 30-6).
Table 30-6
Metabolic Assessments After Gestational Diabetes Mellitus*
| Time | Test | Purpose |
| Postdelivery (1–3 days) | Fasting or random plasma glucose | Detect persistent, overt diabetes |
| Early postpartum (around time of the “postpartum visit”) | 75-g 2-hr OGTT | Postpartum classification of glucose metabolism |
| 1 year postpartum | 75-g 2-hr OGTT | Assess glucose metabolism |
| Annually | Fasting plasma glucose | Assess glucose metabolism |
| Triannually | 75-g 2-hr OGTT | Assess glucose metabolism |
| Prepregnancy | 75-g 2-hr OGTT | Classify glucose metabolism |
OGTT, Oral glucose tolerance test.
*Glucose metabolism classified by criteria recommended by the American Diabetes Association12; Data from Ref. 75, p S258.
Postpartum Follow-Up
At the time of diagnosis of GDM, one cannot reliably distinguish between evolving type 1 or type 2 DM and glucose intolerance that will subside in the postpartum period. Thus, reclassification and long-term follow-up after pregnancy are essential. Early reports of O’Sullivan3 and subsequent studies161 established that GDM identifies women at high risk for the later development of DM. Postpartum studies demonstrated that women with previous GDM continue to have insulin resistance and impaired insulin secretion.162 In some of these subjects, abnormalities of glucose tolerance antedate the index pregnancy, but they cannot be identified retrospectively.163
Certain features that can be identified during the index pregnancy or at early postpartum testing can detect those at high risk to progress to DM after pregnancy.161 These include the severity of glucose intolerance at diagnosis, early gestational age at diagnosis, obesity, relative insulinopenia, family history of maternal diabetes, and racial/ethnic origin. Obesity, further weight gain, and impaired insulin secretion are independent predictors of progression to DM in the 5 years following the diagnosis of GDM.
The high incidence of abnormal postpartum glucose tolerance in women with GDM makes a compelling case for early and continuing follow-up. The algorithm for postpartum surveillance that was recommended by the Fifth International Workshop Conference on GDM75 is outlined in Table 30-6. Patient education concerning the risk of development of DM and its complications is paramount. Those with persistent glucose intolerance or DM should receive appropriate counseling and therapy. Those whose initial evaluation is “normal” should be followed as outlined in Table 30-6, because the long-term risk of developing diabetes is also high in this subgroup.
Excellent intervention trials from Finland164 and the United States165,166 were successful in preventing or delaying the onset of DM in high-risk subjects, including women with previous GDM,165,167 using lifestyle intervention or medication (metformin or troglitazone). Features of the metabolic syndrome—hypertension, elevated triglycerides, and low high-density lipoprotein cholesterol (HDL-C)—are frequently found in women with a history of GDM.161 In light of these risks and the potential benefits that may accrue, it is prudent to advise patients to attain and maintain optimal body weight and to exercise regularly. Further studies in this population will be important in defining the pathogenesis of type 2 DM and implementation of strategies to prevent or delay its development in the population at large.
Contraception
The importance of good preconception care should be reinforced in the postpartum period.75 Contraception choices include intrauterine devices, low-dose combination oral contraceptive or transdermal delivery systems, and progestin-only depot preparations. Barrier methods have a failure rate of 5% per year. If possible, avoid the use of high-dose synthetic estrogen/progestin oral contraceptives and progestin-only oral contraceptives, because they may exacerbate insulin resistance.
References
1. Kitzmiller JL, Jovanovic L, Bown F, et al, eds. Managing preexisting diabetes and pregnancy: technical reviews and consensus recommendations for care. American Diabetes Association: Alexandria, VA, 2008:1–827.
2. Cundy, T. Pregnancy loss and neonatal death in women with Type 1 or Type 2 diabetes mellitus. Insulin. 2008;3:167–175.
3. O’Sullivan, JB, Mahan, CM. Criteria for the oral glucose tolerance test in pregnancy. Diabetes. 1964;13:278–285.
4. World Health Organization: WHO Expert Committee on Diabetes Mellitus: Second Report, World Health Organ Tech Rep Ser 646, 1980.
5. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study Cooperative Research Group. Hyperglycemia and Adverse Pregnancy Outcomes. N Engl J Med. 2008;358:1991–2002.
6. Prevalence of diagnosed diabetes per 100 population, females, by age, United States, 1980–2005. < [article online]. Available at http://www.cdc.gov/diabetes/statistics/prev/national/tprevfemage.htm.
7. Engelau, MM, Herman, WH, Smith, PJ, et al. The epidemiology of diabetes and pregnancy in the U.S., 1988. Diabetes Care. 1995;18:1029–1033.
8. Lawrence, JM, Contreras, R, Wansu, C, et al. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999–2005. Diabetes Care. 2008;31:899–904.
9. Ferrara, A, Kahn, HS, Quesenberry, CP, et al. An increase in the incidence of gestational diabetes mellitus: Northern California, 1991–2000. Obstet Gynecol. 2004;103:526–533.
10. Dabelea, D, Snell-Bergeon, JK, Hartsfield, CL, et al. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort. Diabetes Care. 2005;28:579–584.
11. Metzger, BE, Coustan, DR, Organizing Committee. Summary and recommendations of the Fourth International Workshop Conference on Gestational Diabetes Mellitus. Diabetes Care. 1998;21(Suppl 2):B161–B167.
12. American Diabetes Association. Gestational diabetes mellitus: Position Statement. Diabetes Care. 2004;27(Suppl 1):S88–S90.
13. Getahun, D, Nath, C, Ananth, CV, et al. Gestational diabetes in the United States: Temporal trends 1989 through 2004. Am J Obstet Gynecol. 2008;195:525e1–525e6.
14. Metzger, BE, Cho, NH, Brickman, WJ. The rising tide of diabetes mellitus: Implications for women of all ages. In: Reece EA, Coustan DR, Gabbe SG, eds. Diabetes in Women. ed 3. Philadelphia: Lippincott Williams & Wilkins; 2004:9–26.
15. Freinkel, N. The Banting Lecture 1980: of pregnancy and progeny. Diabetes. 1980;29:1023–1035.
16. Catalano, PM, Tyzbir, ED, Wolfe, RR, et al. Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. Am J Physiol. 1993;264:E60–E67.
17. Buchanan, TA, Metzger, BE, Freinkel, N, et al. Insulin sensitivity and β-cell responsiveness to glucose during late pregnancy in lean and moderately obese women with normal glucose tolerance or mild gestational diabetes. Am J Obstet Gynecol. 1990;162:1008–1014.
18. Catalano, PM, Tyzbir, ED, Roman, NM, et al. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am J Obstet Gynecol. 1991;165:1667–1672.
19. Catalano, PM, Huston, L, Amini, SB, et al. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol. 1999;180:903–916.
20. Jovanovic, L, Knopp, RH, Brown, Z, et al. Declining insulin requirement in the first trimester of diabetic pregnancy. Diabetes Care. 2001;24:1130–1136.
21. Catalano, PM, Drago, NM, Amini, SB. Longitudinal changes in pancreatic β-cell function and metabolic clearance rate of insulin in pregnant women with normal and abnormal glucose tolerance. Diabetes Care. 1998;21:403–408.
22. Kalkhoff, RK, Kissebah, AH, Kim, HJ. Carbohydrate and lipid metabolism during normal pregnancy: Relationship to gestational hormone action. Semin Perinatol. 1978;2:291–307.
23. Lepercq, J, Cauzac, M, Lahlou, N, et al. Overexpression of placental leptin in diabetic pregnancy: A critical role for insulin. Diabetes. 1988;47:847–850.
24. Eriksson, L, Frankenne, F, Eden, S, et al. Growth hormone 24-h serum profiles during pregnancy: Lack of pulsatility for the secretion of the placental variant. Br J Obstet Gynaecol. 1989;96:949–953.
25. Nolten, WE, Lindheimer, MD, Rueckert, PA, et al. Diurnal patterns and regulation of cortisol secretion in pregnancy. J Clin Endocrinol Metab. 1980;51:466–472.
26. Kirwan, JP, Hauguel-DeMouzon, S, Challier, JC, et al. TNF-alpha is a predictor of insulin-resistance in human pregnancy. Diabetes. 2002;51:2207–2213.
27. Retnakaran, R, Hanley, AJGN, Raif, N, et al. Adiponectin and beta cell dysfunction in gestational diabetes: pathophysiological implications. Diabetologia. 2005;48:993–1001.
28. Rabe, K, Lehrke, M, Parhofer, KG, et al. Adipokines and insulin resistance. Mol Med. 2008;14:741–751.
29. Barbour, LA, Mccurdy, CE, Teri, L, et al. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care. 2007;30(Suppl 2):S112–S119.
30. Page, EW. Human fetal nutrition and growth. Am J Obstet Gynecol. 1969;104:378–387.
31. Young, M. Placental transfer of glucose and amino acids. In: Camerini-Davalos RA, Cole HS, eds. Early Diabetes in Early Life. New York: Academic Press; 1975:237–242.
32. Shambaugh, GE, III., Mrozak, SC, Freinkel, N. Fetal fuels. I. Utilization of ketones by isolated tissues at various stages of maturation and maternal nutrition during late gestation. Metabolism. 1977;26:623–636.
33. Shambaugh, GE, III., Angulo, MC, Koehler, RR. Fetal fuels. VII. Ketone bodies inhibit the synthesis of purines in fetal rat brain. Am J Physiol. 1984;247:E111–E117.
34. Berendes, HW. Effect of maternal acetonuria on IQ of offspring. In: Camerini-Davalos RA, Cole HS, eds. Early Diabetes in Early Life. New York: Academic Press; 1975:135–140.
35. Rizzo, T, Metzger, BE, Burns, WJ, et al. Correlations between antepartum maternal metabolism and intelligence of offspring. N Engl J Med. 1991;325:911–916.
36. Mills, JL, Jovanovic, L, Knopp, R, et al. Physiological reduction in fasting plasma glucose concentration in the first trimester of normal pregnancy: The Diabetes in Early Pregnancy Study. Metabolism. 1998;47:1140–1144.
37. Paretti, E, Mecacci, F, Papini, M, et al. Third-trimester maternal glucose levels from diurnal profiles in nondiabetic pregnancies. Diabetes Care. 2001;24:1319–1323.
38. Hod, M, Yogev, Y. Goals of metabolic management of gestational diabetes: Is it all about sugar? Diabetes Care. 2007;30(Suppl 2):S180–S187.
39. Metzger, BE, Ravnikar, V, Vilesis, R, et al. Accelerated starvation and the skipped breakfast in late normal pregnancy. Lancet. 1982;1:588–592.
40. Shoengold, DM, DeFiore, RH, Parlett, RC. Free amino acids in plasma throughout pregnancy. Am J Obstet Gynecol. 1978;131:490–499.
41. Metzger, BE, Phelps, RL, Freinkel, N, et al. Effects of gestational diabetes on diurnal profiles of plasma glucose, lipids and individual amino acids. Diabetes Care. 1980;3:402–409.
42. Buchanan, TA, Metzger, BE, Freinkel, N. Accelerated starvation in late pregnancy: A comparison between obese women with and without gestational diabetes mellitus. Am J Obstet Gynecol. 1990;162:1015–1020.
43. Technical Reviews and Consensus Recommendations for Care, Part 2: Management of diabetic/medical complications in pregnancy: Diabetic nephropathy and pregnancy. In: Kitzmiller JL, Jovanovic L, Bown F, et al, eds. Managing Preexisting Diabetes and Pregnancy: Technical Reviews and Consensus Recommendations for Care. Alexandria, VA: American Diabetes Association; 2008:374–399.
44. Kalkhoff, RK, Kandaraki, E, Morrow, PG, et al. Relationship between neonatal birth weight and maternal plasma amino acid profiles in lean and obese nondiabetic women and in type I diabetic women. Metabolism. 1988;37:234–239.
45. Pedersen, J. The Pregnant Diabetic and Her Newborn: Problems and Management. Baltimore: Williams & Wilkins; 1977.
46. Freinkel, N, Metzger, BE. Pregnancy as a tissue culture experience: The critical implications of maternal metabolism for fetal development. In: Elliot K, O’Connor M, eds. Pregnancy Metabolism, Diabetes and the Fetus. CIBA Foundation Symposium No. 63. Amsterdam: Excerpta Medica; 1979:3–23.
47. Metzger, BE, Buchanan, TA. From research to practice: Diabetes and birth defects: Insights from the 1980s, prevention in the 1990s. editors. Diabetes Spectrum. 1990;3:150–184.
48. Mills, JL, Simpson, JL, Driscoll, SG, et al. The NICHHD-Diabetes in Early Pregnancy Study: Incidence of spontaneous abortion among normal women and insulin-dependent diabetic women whose pregnancies were identified within 21 days of conception. N Engl J Med. 1988;319:1617–1623.
49. Greene, MF, Hare, JW, Cloherty, JP, et al. First trimester hemoglobin A1c and risk for major malformations and spontaneous abortion in diabetic pregnancy. Teratology. 1989;39:225–231.
50. Lappin, TR, Savage, GA, Metzger, BE, et alfor the HAPO Study Cooperative Research Group. Normative values of hemoglobin A1c (HbA1c) in non-diabetic pregnancy (24–32 wks gestation): Data from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes. 2006;55(Suppl 1):A217.
51. Ray, JG, O’Brien, TE, Chan, WS. Preconception care and the risk of congenital anomalies in the offspring of women with diabetes mellitus: A meta-analysis. Q J Med. 2001;94:435–444.
52. Herman, WH, Janz, NK, Becker, MP, et al. Diabetes and pregnancy: Preconception care, pregnancy outcomes, resource utilization and costs. J Reprod Med. 1999;44:33–38.
53. Loeken, MR. Current perspectives on the causes of neural tube defects resulting from diabetic pregnancy. Am J Med Genet C Semin Med Genet. 2005;135C:77–87.
54. Correa, A, Gilboa, SM, Besser, LM, et al. Diabetes and birth defects. Am J Obstet Gynecol. 2008;199:237e1–237e9.
55. Buchanan, TA, Denno, KM, Sipes, GF, et al. Diabetic teratogenesis: in vitro evidence for a multifactorial etiology with little contribution from glucose per se. Diabetes. 1994;43:656–660.
56. Eriksson, UJ, Borg, LAH. Diabetes and embryonic malformations: Role of substrate-induced free-oxygen radical production for dysmorphogenesis in cultured rat embryos. Diabetes. 1993;42:411–419.
57. Buchanan, T, Schemmer, JK, Freinkel, N. Embryotoxic effects of brief maternal insulin-hypoglycemia during organogenesis in the rat. J Clin Invest. 1986;78:643–649.
58. Catalano, PM, Thomas, A, Huston-Presley, L, et al. Increased fetal adiposity: A very sensitive marker of abnormal in utero development. Am J Obstet Gynecol. 2003;189:1698–1704.
59. Elliot, JP, Garite, TJ, Freeman, RK. Ultrasonic prediction of fetal macrosomia in diabetic patients. Obstet Gynecol. 1982;60:159–164.
60. Catalano, PM, Thomas, AJ, Huston, LP, et al. Effect of maternal metabolism on fetal growth and body composition. Diabetes Care. 1998;21(Suppl 2):B85–B90.
61. Landon, MB, Sonek, J, Foy, P, et al. Sonographic measurement of fetal humeral soft tissue thickness in pregnancy complicated by GDM. Diabetes. 1991;40(Suppl 2):66–70.
62. Abramowicz, JS, Sherer, DM, Woods, JR, Jr. Ultrasonographic measurement of cheek-to-cheek diameter in fetal growth disturbances. Am J Obstet Gynecol. 1993;162:405–408.
63. Kjos, SL, Schaefer-Graf, UM. Modified therapy for gestational diabetes using high-risk and low-risk fetal abdominal circumference growth to select strict versus relaxed maternal glycemic targets. Diabetes Care. 2007;30(Suppl 2):S200–S205.
64. Reiher, H, Fuhrmann, K, Noack, S, et al. Age-dependent insulin secretion of the endocrine pancreas in vitro from fetuses of diabetic and nondiabetic patients. Diabetes Care. 1983;6:446–451.
65. Metzger, BE. Biphasic effects of maternal metabolism on fetal growth: The quintessential expression of “fuel mediated teratogenesis.”. Diabetes. 1991;40(Suppl 2):99–105.
66. Susa, JB, Neave, C, Sehgal, P, et al. Chronic hyperinsulinemia in the fetal rhesus monkey: Effects of physiologic hyperinsulinemia on fetal growth and composition. Diabetes. 1984;33:656–660.
67. Verhaeghe, J, Van Bree, R, Van Herck, E, et al. C-peptide, insulin-like growth factors I and II and insulin-like growth factor binding protein-1 in umbilical cord serum: correlations with birth weight. Am J Obstet Gynecol. 1993;169:89–97.
68. Pettitt, DJ, Baird, HR, Aleck, KA, et al. Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. N Engl J Med. 1983;308:242–245.
69. Silverman, BL, Purdy, LP, Metzger, BE. The intrauterine environment: Implications for the offspring of diabetic mothers. Diabetes Rev. 1996;4:21–35.
70. Pettitt, DJ, Knowler, WC, Bennett, PH, et al. Obesity in offspring of diabetic Pima Indian women despite normal birth weight. Diabetes Care. 1987;10:76–80.
71. Silverman, BL, Rizzo, T, Green, OC, et al. Long-term prospective evaluation of offspring of diabetic mothers. Diabetes. 1991;40(Suppl 2):121–125.
72. Hillier, TA, Pedula, KL, Schmidt, MM, et al. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30:2287–2292.
73. Pettitt, DJ, Aleck, KA, Baird, HR, et al. Congenital susceptibility for NIDDM: Role of intrauterine environment. Diabetes. 1988;37:622–628.
74. Silverman, BL, Cho, NH, Metzger, BE. Impaired glucose tolerance in adolescent offspring of diabetic mothers: Relationship to fetal hyperinsulinism. Diabetes Care. 1995;18:611–618.
75. Metzger, BE, Buchanan, TA, Coustan, DR, et al. Summary and recommendations of the Fifth International Workshop Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(Suppl 2):S251–S260.
76. Freinkel, N, Metzger, BE. Gestational diabetes: Problems in classification and implications for long-range prognosis. In: Vranic M, Hollenberg CH, Steiner G, eds. Comparison of Type I and Type II Diabetes: Similarities and Dissimilarities in Etiology, Pathogenesis and Complications. New York: Plenum; 1985:47–64.
77. Langer, O, Hod, M. Management of gestational diabetes mellitus. Obstet Gynecol Clin. 23, 1996. [173–159].
78. White, P. Pregnancy complicating diabetes. Am J Med. 1949;7:609–616.
79. Hare, JW. Complicated diabetes complicating pregnancy. Baillieres Clin Obstet Gynaecol. 1991;5:3449–3467.
80. Phelps, RL, Sakol, P, Metzger, BE, et al. Changes in diabetic retinopathy during pregnancy. Correlations with regulation of hyperglycemia. Arch Ophthalmol. 1986;104:1806–1810.
81. Chew, EY, Mills, JL, Metzger, BE, et al. Metabolic control and progression of retinopathy: The diabetes in early pregnancy study. Diabetes Care. 1995;18:631–637.
82. Diabetes Control and Complications Trial Research Group. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. Diabetes Care. 2000;23:1084–1091.
83. Rosenn, B, Miodovnik, M, Kranias, G, et al. Progression of diabetic retinopathy in pregnancy: Association with hypertension in pregnancy. Am J Obstet Gynecol. 1992;166:1214–1218.
84. Reece, EA, Coustan, DR, Hayslett, JP, et al. Diabetic nephropathy: Pregnancy performance and fetomaternal outcome. Am J Obstet Gynecol. 1988;159:56–66.
85. McCance, DR, Traub, AI, Harley, JM, et al. Urinary albumin excretion in diabetic pregnancy. Diabetologia. 1989;32:236–239.
86. Macleod, AF, Smith, SA, Sonksen, PH, et al. The problem of autonomic neuropathy in diabetic pregnancy. Diabet Med. 1990;7:80–82.
87. Airaksinen, KEJ, Anttila, LM, Linnaluoto, MK, et al. Autonomic influence on pregnancy outcome in IDDM. Diabetes Care. 1990;13:756–761.
88. Siddiqi, T, Rosenn, B, Mimouni, F, et al. Hypertension during pregnancy in insulin-dependent diabetic women. Obstet Gynecol. 1991;77:514–519.
89. Hare, JW, White, P. Pregnancy in diabetes complicated by vascular disease. Diabetes. 1977;26:953–955.
90. Reece, EA, Egan, JF, Coustan, DR, et al. Coronary artery disease in diabetic pregnancies. Am J Obstet Gynecol. 1986;154:150–151.
91. Nasrat, AA, Johnstone, FD, Hasan, SAM. Is random plasma glucose an efficient screening test for abnormal glucose tolerance in pregnancy? Br J Obstet Gynaecol. 1988;95:855–860.
92. Cousins, L, Dattel, BJ, Hollingsworth, DR, et al. Glycosylated hemoglobin as a screening test for carbohydrate intolerance in pregnancy. Am J Obstet Gynecol. 1984;150:455–460.
93. Roberts, AB, Baker, JR, Metcalf, P, et al. Fructosamine compared with a glucose load as a screening test for gestational diabetes. Obstet Gynecol. 1990;76:773–775.
94. Lamar, ME, Kuehl, TJ, Cooney, AT, et al. Jelly beans as an alternative to a fifty-gram glucose beverage for gestational diabetes screening. Am J Obstet Gynecol. 1999;181:1154–1157.
95. Weiss, PAM, Hofmann, HM, Winter, RR, et al. Diagnosis and treatment of gestational diabetes according to amniotic fluid insulin levels. Arch Gynecol. 1986;239:81–91.
96. Carpenter, MW, Canick, JA, Star, J, et al. Fetal hyperinsulinism at 14–20 weeks and subsequent gestational diabetes. Obstet Gynecol. 1996;87:89–93.
97. National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039–1057.
98. Carpenter, MW, Coustan, DR. Criteria for screening tests for gestational diabetes mellitus. Am J Obstet Gynecol. 1982;159:768–773.
99. Sermer, M, Naylor, CD, Gare, DJ, et al. Impact of increasing carbohydrate intolerance on maternal-fetal outcomes in 3637 women without gestational diabetes. Am J Obstet Gynecol. 1995;173:146–156.
100. Rust, OA, Bofill, JA, Andrew, ME, et al. Lowering the threshold for the diagnosis of gestational diabetes. Am J Obstet Gynecol. 1996;175:961–965.
101. Deerochanawong, C, Putiyanun, C, Wongsuryrat, M, et al. Comparison of the National Diabetes Data Group and World Health Organization criteria for detecting gestational diabetes mellitus. Diabetologia. 1996;39:1070–1073.
102. Schmidt, MI, Duncan, BB, Reichelt, AJ, et al. Gestational diabetes mellitus diagnosed with a 2-h 75-g OGTT and adverse pregnancy outcomes. Diabetes Care. 2001;24:1151–1155.
103. Weiss, PAM, Haeusler, M, Kainer, F, et al. Toward universal criteria for gestational diabetes: Relationships between seventy-five and one hundred gram glucose loads and between capillary and venous glucose concentrations. Am J Obstet Gynecol. 1998;178:830–835.
104. Buchanan, TA, Catalano, PM. The pathogenesis of GDM: Implications for diabetes after pregnancy. Diabetes Rev. 1995;3:584–601.
105. de Leiva, A, Mauricio, D, Corcoy, R. Diabetes-related autoantibodies and gestational diabetes. Diabetes Care. 2007;30(Suppl 2):S127–S133.
106. Buschard, K, Buch, I, Molsted-Pedersen, L, et al. Increased incidence of true type I diabetes acquired during pregnancy. Br Med J. 1987;294:275–279.
107. Beischer, NA, Oats, JN, Henry, OA, et al. Incidence and severity of gestational diabetes mellitus according to country of birth in women living in Australia. Diabetes. 1991;40(Suppl 2):35–38.
108. Green, JR, Schumacher, LB, Pawson, IG, et al. Influence of maternal body habitus and glucose intolerance on birth weight. Obstet Gynecol. 1991;78:235–240.
109. Maassen, JA, Kadowaki, T. Maternally inherited diabetes and deafness: A new diabetes subtype. Diabetologia. 1996;39:375–382.
110. Gebhart, SSP, Shoffner, JM, Koontz, D, et al. Insulin resistance associated with maternally inherited diabetes and deafness. Metabolism. 1996;45:526–531.
111. Ellard, S, Beards, F, Allen, LI, et al. A high prevalence of glucokinase mutations in gestational diabetic subjects selected by clinical criteria. Diabetologia. 2000;43:250–253.
112. Watanabe, RM, Black, MH, Xiang, AH, et al. Genetics of gestational diabetes mellitus and type 2 diabetes. Diabetes Care. 2007;30(Suppl 2):134–139.
113. Warram, JH, Krolewski, AS, Gottlieb, MS, et al. Differences in risk of insulin-dependent diabetes in offspring of diabetic mothers and diabetic fathers. N Engl J Med. 1984;311:149–152.
114. Evers, IM, Braak, EW, de Valk, HW, et al. Risk indicators predictive for severe hypoglycemia during the first trimester of type 1 diabetic pregnancy. Diabetes Care. 2002;25:554–559.
115. Committee on Nutritional Status During Pregnancy and Lactation, Food and Nutrition Board, Institute of Medicine, National Academy of Sciences. Nutrition During Pregnancy, Part I: Weight Gain. Washington, DC: National Academy Press; 1990. [pp 10–13].
116. Durnin, JVGA. Energy requirements of pregnancy. Diabetes. 1991;40(Suppl 2):152–156.
117. Jenkins, DJ, Wolever, TM, Taylor, RH, et al. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362–366.
118. Mathiesen, ER, Kinsley, B, Amiel, SA, et alfor the Insulin Aspart Pregnancy Study Group. Maternal glycemic control and hypoglycemia in type 1 diabetic subjects: a randomized trial of insulin aspart versus human insulin in 322 pregnant women. Diabetes Care. 2007;30:771–776.
119. Anderson, JH, Jr., Brunelle, RL, Koivisto, VA, et althe Multicenter Insulin Lispro Study Group. Reduction of postprandial hyperglycemia and frequency of hypoglycemia in IDDM patients on insulin-analog treatment. Diabetes. 1997;46:265–270.
120. Technical Reviews and Consensus Recommendations for Care, Part 1: Managing preexisting diabetes in pregnancy. In: Kitzmiller JL, Jovanovic L, Bown F, et al, eds. Managing Preexisting Diabetes and Pregnancy. Alexandria, VA: American Diabetes Association; 2008:1–268.
121. Wyatt, JW, Frias, JL, Hoyme, HE, et alfor the IONS Study Group. Congential anomaly rate in offspring of mothers with type 1 diabetes treated with insulin lispro during pregnancy. Diabet Med. 2005;22:803–807.
122. Pôyhônen-Alho, M, Rônnemaa, T, Saltevo, J, et al. Use of insulin glargine during pregnancy. Acta Obstet Gynecol Scand. 2007;86:1171–1174.
123. Price, N, Bartlett, C, Gillmer, M. Use of insulin glargine during pregnancy: A case-control pilot study. Brit J Obstet Gynaecol. 2007;114:453–457.
124. Gallen, IW, Jaap, A, Roland, M, et al. Survey of glargine use in 115 pregnant women with Type 1 diabetes. Diabet Med. 2008;25:165–169.
125. Roversi, GD, Garginlo, M, Nicolini, U, et al. A new approach to the treatment of diabetic pregnant women. Am J Obstet Gynecol. 1979;135:567–576.
126. Combs, CA, Gunderson, E, Kitzmiller, JL, et al. Relationship of fetal macrosomia to maternal postprandial glucose control during pregnancy. Diabetes Care. 1992;15:1251–1257.
127. American College of Obstetricians and Gynecologists. Ultrasonography in pregnancy. ACOG Practice Bulletin #58. Obstet Gynecol. 2004:1449–1458.
128. Bahado-Singh, RO, Wapner, R, Thom, E, et alfor the First Trimester Maternal Serum Biochemistry and Fetal Nuchal Translucency Screening (BUN) Study Group. Elevated first-trimester nuchal translucency increases the risk of congenital heart defects. Am J Obstet Gynecol. 2005;192:89–95.
129. Yaron, Y, Cherry, M, Kramer, RL, et al. Second-trimester maternal serum marker screening: maternal serum α-fetoprotein, β-human chorionic gonadotropin, estriol, and their various combinations as predictors of pregnancy outcome. Am J Obstet Gynecol. 1999;181:968–974.
130. Friedberg, MK, Silverman, NH. Changing indicators for fetal echocardiography in a University Center population. Prenat Diagn. 2004;24:781–786.
131. Sacks, DA. Etiology, detection, and management of fetal macrosomia in pregnancies complicated by diabetes. Clin Obstet Gynecol. 2007;50:980–989.
132. Devoe, LD, Jones, CR. Nonstress test: evidence-based use in high-risk pregnancy. Clin Obstet Gynecol. 2002;45:986–992.
133. Oats, JN. Obstetrical management of patients with diabetes in pregnancy. Baillieres Clin Obstet Gynaecol. 1991;5:395–411.
134. Pettitt, DJ, Knowler, WC, Baird, HR, et al. Gestational diabetes: Infant and maternal complications of pregnancy in relation to third-trimester glucose tolerance in Pima Indians. Diabetes Care. 1980;3:458–464.
135. Hod, M, Merlob, P, Friedman, S, et al. Gestational diabetes mellitus: A survey of perinatal complications in the 1980s. Diabetes. 1991;40(Suppl 2):74–78.
136. Crowther, CA, Hiller, JE, Moss, JR, et alfor the Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–2486.
137. U.S. Preventive Services Task Force. Screening for gestational diabetes mellitus: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148:759–765.
138. Kalter, H. The non-teratogenicity of gestational diabetes. Paediatr Perinat Epidemiol. 1998;12:456–458.
139. Schaefer, UM, Songster, G, Xiang, A, et al. Congenital malformations in offspring of women with hyperglycemia first detected during pregnancy. Am J Obstet Gynecol. 1997;177:165–171.
140. De Veciana, M, Major, CA, Morgan, MA, et al. Postprandial versus preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N Engl J Med. 1995;333:1237–1241.
141. Peterson, CM, Jovanovic-Peterson, L. Percentage of carbohydrate and glycemic response to breakfast, lunch and dinner in women with gestational diabetes. Diabetes. 1991;40(Suppl 2):172–174.
142. Jovanovic-Peterson, L, Peterson, CM. Dietary manipulation as a primary treatment strategy for pregnancies complicated by diabetes. J Am Coll Nutr. 1990;9:320–325.
143. Reece, EA, Hagay, Z, Gay, KJ, et al. Do fiber-enriched diabetic diets have glucose-lowering effects in pregnancy? Am J Perinatol. 1993;4:272–274.
144. Tieu J, Crowther CA, Middleton P: Dietary advice in pregnancy for preventing gestational diabetes mellitus, Cochrane Database Syst Rev 16:CD006674, 2008.
145. Knopp, RH, Magee, MS, Raisys, V, et al. Metabolic effects of hypocaloric diets in management of gestational diabetes. Diabetes. 1991;40(Suppl 2):165–171.
146. Algert, S, Shragg, P, Hollingsworth, DR. Moderate caloric restriction in obese women with gestational diabetes. Obstet Gynecol. 1985;65:487–491.
147. Dornhorst, A, Nichols, JSD, Probst, F, et al. Caloric restriction for the treatment of gestational diabetes. Diabetes. 1991;40(Suppl 2):161–164.
148. Artal, R. Exercise: The alternative therapeutic intervention for gestational diabetes. Clin Obstet Gynecol. 2003;46:479–487.
149. Jovanovic-Peterson, L, Durak, EP, Peterson, CM. Randomized trial of diet versus diet plus cardiovascular conditioning on glucose levels in gestational diabetes. Am J Obstet Gynecol. 1989;161:415–419.
150. Bung, P, Artal, R, Khodiguian, N, et al. Exercise in gestational diabetes: An optional therapeutic approach? Diabetes. 1991;40(Suppl 2):182–185.
151. Lesser, K, Gruppuso, P, Terry, R, et al. Exercise fails to improve postprandial glycemic excursion in women with gestational diabetes. J Matern Fetal Med. 1996;5:211–217.
152. Artal, R, Catanzaro, RB, Gavard, JA, et al. A lifestyle intervention of weight-gain restriction: Diet and exercise in obese women with gestational diabetes mellitus. Appl Physiol Nutr Metab. 2007;32:596–601.
153. Summary and recommendations of the Second International Workshop Conference on Gestational Diabetes Mellitus. Diabetes. 1985;34(Suppl 2):123–126.
154. Moore, TR. Glyburide for the treatment of gestational diabetes: A critical appraisal. Diabetes Care. 2007;30(Suppl 2):S209–S213.
155. Rowan, JA, Hague, WM, Gao, W, et alfor MIG Trial Investigators. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003–2015.
156. Nachum, Z, Ben-Shlomo, I, Weiner, E, et al. Twice daily versus four times daily insulin dose regimens for diabetes in pregnancy: Randomised controlled trial. Br Med J. 1999;319:1223–1227.
157. Elliott, BD, Schenker, S, Langer, O, et al. Comparative placental transport of oral hypoglycemic agents in humans: A model of human placental drug transfer. Am J Obstet Gynecol. 1994;171:653–660.
158. Langer, O, Conway, DL, Berkus, MD, et al. A comparison of glyburide and insulin in women with gestational diabetes. N Engl J Med. 2000;343:1134–1138.
159. Mathur, R, Alexander, CJ, Yano, J, et al. Use of metformin in polycystic ovary syndrome. Am J Obstet Gynecol. 2008;199:596–609.
160. Agarwal, MM, Hughes, PF, Punnose, J, et al. Gestational diabetes screening of a multiethnic, high risk population using glycated proteins. Diabetes Res Clin Pract. 2001;51:67–73.
161. Kitzmiller, JL, Dang-Kilduff, L, Taslimi, MM. Gestational diabetes after delivery: short-term management and long-term risks. Diabetes Care. 2007;30(Suppl 2):S25–S235.
162. Byrne, MM, Sturis, J, O’Meara, NM, et al. Insulin secretion in insulin-resistant women with a history of gestational diabetes. Metab Clin Exp. 1995;44:1067–1073.
163. Harris, MI. Gestational diabetes may represent discovery of preexisting glucose intolerance. Diabetes Care. 1988;11:402–411.
164. Tuomilehto, J, Lindstrom, J, Eriksson, JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350.
165. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle or metformin. N Engl J Med. 2002;346:393–403.
166. Buchanan, TA, Xiang, AH, Peters, RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes. 2002;51:2796–2803.
167. Ratner, RE, Christophi, CA, Metzger, BE, et alfor the Diabetes Prevention Research Group. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab. 2008;93:4774–4779.