Developmental Disorders
Causes, Mechanisms, and Patterns
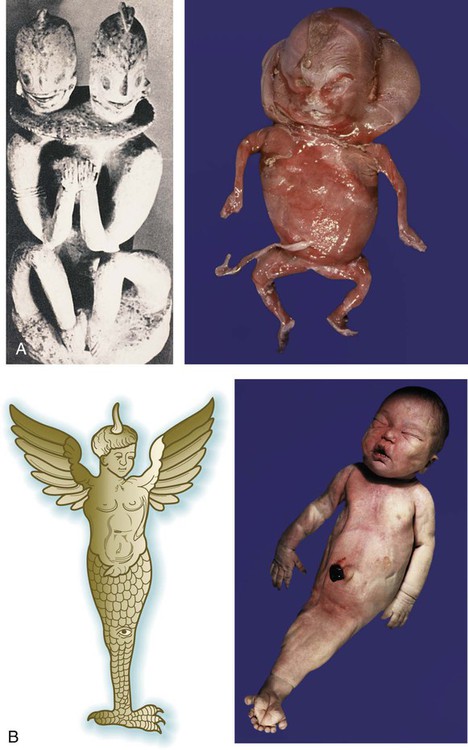
Early representations of some malformed infants are remarkable in their anatomical accuracy, and it is often possible to diagnose specific conditions or syndromes from the ancient art (Fig. 8.1A). By the Middle Ages, however, representations of malformations were much more imaginative, with hybrids of humans and other animals often represented (Fig. 8.1B).
Thalidomide is a very effective sedative that was widely used in West Germany, Australia, and other countries during the late 1950s. Soon, physicians began to see infants born with extremely rare birth defects. One example is phocomelia (which means “seal limb”), a condition in which the hands and feet seem to arise almost directly from the shoulder and hip (Fig. 8.2). Another is amelia, in which a limb is entirely missing. Thalidomide was identified as the certain cause after some careful epidemiological detective work involving the collection of individual case reports and sorting of the drugs taken by mothers during the early period of their pregnancies. Thalidomide, which is an inhibitor of tumor necrosis factor-α, is still a drug of choice in the treatment of leprosy and multiple myeloma. With the intense investigations that followed the thalidomide disaster, modern teratology came of age. Despite much effort, however, the causes of most congenital malformations are still unknown.
General Principles
Birth defects present themselves in a variety of forms and associations, ranging from simple abnormalities of a single structure to often grotesque deformities that may affect an entire body region. Some of the common classes of malformations are listed in Table 8.1.
Table 8.1
| Abnormalities of Individual Structures | |
| Malformation | A structural defect of part of or an entire organ or larger part of a body region that is caused by an abnormal process intrinsic to its development (e.g., coloboma) (see p. 285) |
| Disruption | A defect in an organ or body part caused by process that interferes with an originally normal developmental process (e.g., thalidomide-induced phocomelia) (see p. 149) |
| Deformation | A structural abnormality caused by mechanical forces (e.g., amniotic band constriction) (see Fig. 8.16) |
| Dysplasia | An abnormality of a tissue due to an abnormal intrinsic developmental process (e.g., ectodermal dysplasia) (see p. 150) |
| Defects Involving More Than One Structure | |
| Sequence | A pattern of multiple malformations stemming from a disturbance of a prior developmental process or mechanical factor (e.g., Potter sequence) (see p. 384) |
| Syndrome | A group of malformations of different structures due to a single primary cause, but acting through multiple developmental pathways (e.g., trisomy 13 syndrome) (see Fig. 8.10) |
| Association | A group of anomalies seen in more than one individual that cannot yet be attributed to a definitive cause |

Based on Spranger J and others: J Pediatr 100:160-165, 1982.
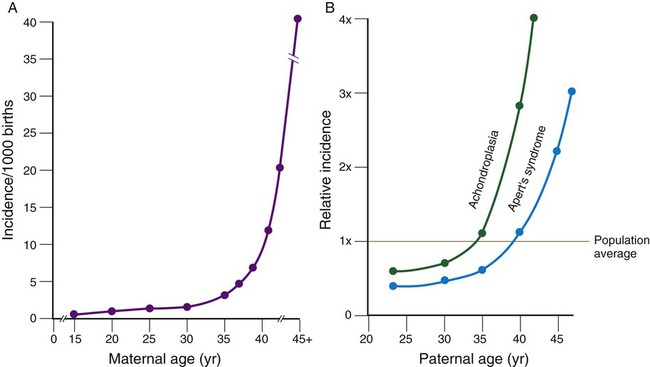
Well-known correlations exist between parental age and the incidence of certain malformations. A classic correlation is the increased incidence of Down syndrome (Fig. 8.3; see Fig. 8.9) in children born to women older than 35 years of age. Other conditions are related to paternal age (see Fig. 8.3).
Some types of anomalies have a higher incidence among infants born at certain seasons of the year. Anencephaly (Fig. 8.4) occurs more frequently in January. Recognizing that the primary factors leading to anencephaly occur during the first month of embryonic life, researchers must seek the potential environmental causes that are more prevalent in April. Anencephaly has been shown to be highly correlated with maternal folic acid deficiency. The high incidence of this anomaly in pregnancies beginning in the early spring may relate to nutritional deficiencies of mothers during the late winter months. Folic acid supplementation in the diet of women of childbearing age significantly reduces the incidence of neural tube defects, such as anencephaly.
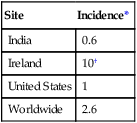
The relationship between the country of residence and an increased incidence of specific malformations can be related to various factors, including racial tendencies, local environmental factors, and even governmental policies. A classic example of the last is the incidence of severely malformed infants as a result of exposure to thalidomide. These cases were concentrated in West Germany and Australia because the drug was commonly sold in these locations. Because thalidomide was not approved by the Food and Drug Administration, the United States was spared from this epidemic of birth defects. Another classic example of the influence of country as a factor in the incidence of malformations is seen in neural tube defects (Table 8.2). The reason neural tube defects (especially anencephaly) were historically so common in Ireland has been the topic of much speculation. In light of the recognition of the importance of folic acid in the prevention of neural tube defects, it is possible that the high incidence of anencephaly in Ireland resulted from poor nutrition in pregnant women during the winter. A greater than threefold decrease in the incidence of neural tube defects in Ireland from 1980 to 1994 may be related to both better nutrition and folic acid supplementation by a certain percentage of pregnant women.
Periods of Susceptibility to Abnormal Development
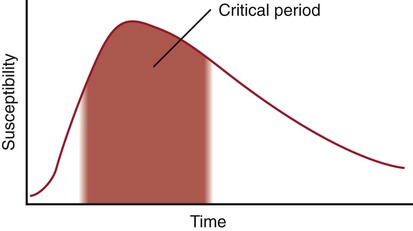
Typically, a developing organ has a curve of susceptibility to teratogenic influences similar to that illustrated in Figure 8.5. Before the critical period, exposure to a known teratogen has little influence on development. During the first days of the critical period, the susceptibility, measured as incidence or severity of malformation, increases sharply and then declines over a much longer period.
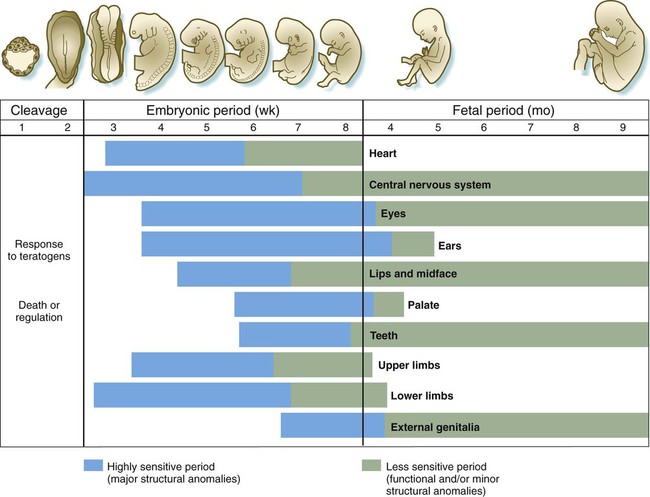
Different organs have different periods of susceptibility during embryogenesis (Fig. 8.6). Organs that form the earliest (e.g., heart) tend to be sensitive to the effects of teratogens earlier than organs that form later (e.g., external genitalia). Some very complex organs, especially the brain and major sense organs, show prolonged periods of high susceptibility to disruption of normal development.
Not all teratogenic influences act in the same developmental periods (Table 8.3). Some influences cause anomalies if the embryo is exposed to them early in development, but they are innocuous at later periods of pregnancy. Others affect only later developmental periods. A good example of the former is thalidomide, which has a very narrow and well-defined danger zone during the embryonic period (4 to 6 weeks). In contrast, tetracycline, which stains bony structures and teeth, exerts its effects after hard skeletal structures in the fetus have formed.
Table 8.3
Developmental Times at Which Various Human Teratogens Exert Their Effects
| Teratogens | Critical Periods (Gestational Days) | Common Malformations |
| Rubella virus | 0-60 | Cataract or heart malformations |
| 0-120+ | Deafness | |
| Thalidomide | 21-40 | Reduction defects of limbs |
| Androgenic steroids | Earlier than 90 | Clitoral hypertrophy and labial fusion |
| Later than 90 | Clitoral hypertrophy only | |
| Warfarin (Coumadin) anticoagulants | Earlier than 100 | Nasal hypoplasia |
| Later than 100 | Possible mental retardation | |
| Radioiodine therapy | Later than 65-70 | Fetal thyroid deficiency |
| Tetracycline | Later than 120 | Staining of dental enamel in primary teeth |
| Later than 250 | Staining of crowns of permanent teeth |
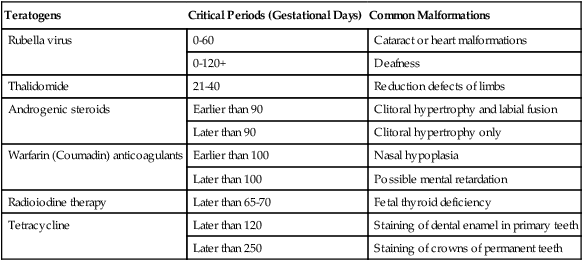
Adapted from Persaud TVN, Chudley AE, Skalko RG, eds: Basic concepts in teratology, New York, 1985, Liss.
Causes of Malformations
Despite considerable research since the 1960s, the cause of at least 50% of human congenital malformations remains unknown (Fig. 8.7). Roughly 18% of malformations can be attributed to genetic causes (chromosomal defects or mutations based on mendelian genetics), and 7% of malformations are caused by environmental factors, such as physical or chemical teratogens. Of all malformations, 25% are multifactorial, for example, caused by environmental factors acting on genetic susceptibility.
Genetic Factors
Abnormal Chromosome Numbers
Monosomy and Trisomy
Monosomy (the lack of one member of a chromosome pair) and trisomy (a triplet instead of the normal chromosome pair) are typically the result of nondisjunction during meiosis (see Fig. 1.7). When this happens, one gamete shows monosomy, and the other shows trisomy of the same chromosome.
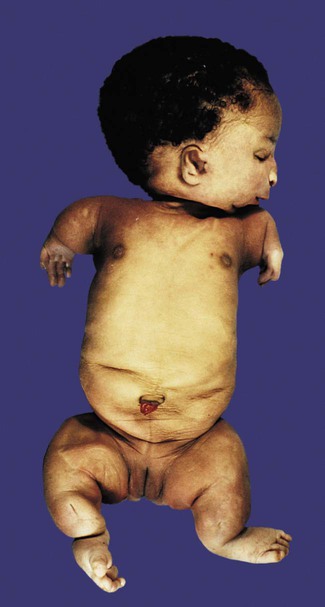
In most cases, embryos with monosomy of the autosomes or sex chromosomes are not viable. Some individuals with monosomy of the sex chromosomes (45XO genotype) can survive, however (Fig. 8.8). Such individuals, who are said to have Turner’s syndrome, exhibit a female phenotype, but the gonads are sterile.
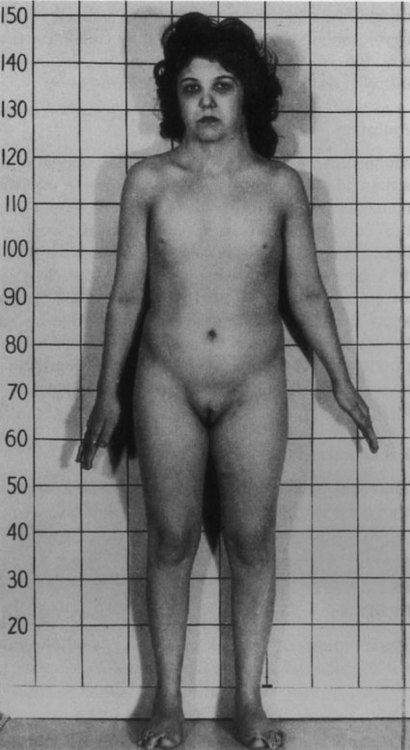
Note the short stature, webbed neck, and infantile sexual characteristics. (From Connor J, Ferguson-Smith M: Essential medical genetics, ed 2, Oxford, 1987, Blackwell Scientific.)
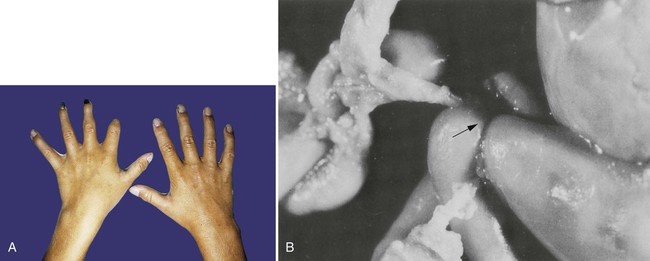
Three autosomal trisomies produce infants with characteristic associations of anomalies. The best known is trisomy 21, also called Down syndrome. Individuals with Down syndrome are typically mentally retarded and have a characteristic broad face with a flat nasal bridge, wide-set eyes, and prominent epicanthic folds. The hands are also broad, and the palmar surface is marked by a characteristic transverse simian crease (Fig. 8.9). Heart defects, especially atrial and ventricular septal defects, are common, with an incidence approaching 50%. Duodenal atresia and other intestinal anomalies are also seen in patients with Down syndrome. Individuals with Down syndrome are prone to the early appearance of Alzheimer’s disease and typically have a shortened life span.
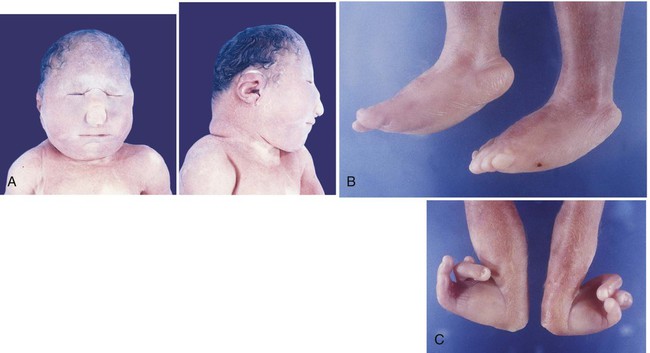
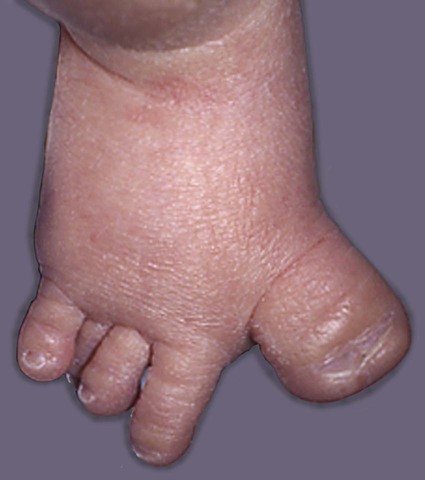
Trisomies of chromosomes 13 and 18 result in severely malformed fetuses, many of which do not survive to birth. Infants with trisomy 13 and trisomy 18 show severe mental retardation and other defects of the central nervous system. Cleft lip and cleft palate are common. Polydactyly is often seen in trisomy 13, and infants with both syndromes exhibit other anomalies of the extremities, such as “rocker bottom feet,” meaning a rounding under and protrusion of the heels (Fig. 8.10). Most infants born with trisomy 13 or trisomy 18 die within the first 1 or 2 months after birth.
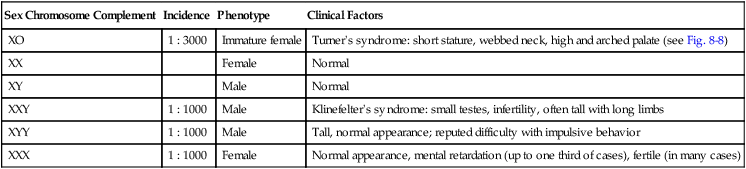
Abnormal numbers of the sex chromosomes are relatively common and can be detected by examination of the sex chromatin (X chromosome) or the fluorescence reactions of the Y chromosomes. Table 8.4 summarizes some of the various types of deletions and duplications of the sex chromosomes.
Table 8.4
Variations in Numbers of Sex Chromosomes
| Sex Chromosome Complement | Incidence | Phenotype | Clinical Factors |
| XO | 1 : 3000 | Immature female | Turner’s syndrome: short stature, webbed neck, high and arched palate (see Fig. 8-8) |
| XX | Female | Normal | |
| XY | Male | Normal | |
| XXY | 1 : 1000 | Male | Klinefelter’s syndrome: small testes, infertility, often tall with long limbs |
| XYY | 1 : 1000 | Male | Tall, normal appearance; reputed difficulty with impulsive behavior |
| XXX | 1 : 1000 | Female | Normal appearance, mental retardation (up to one third of cases), fertile (in many cases) |
Abnormal Chromosome Structure
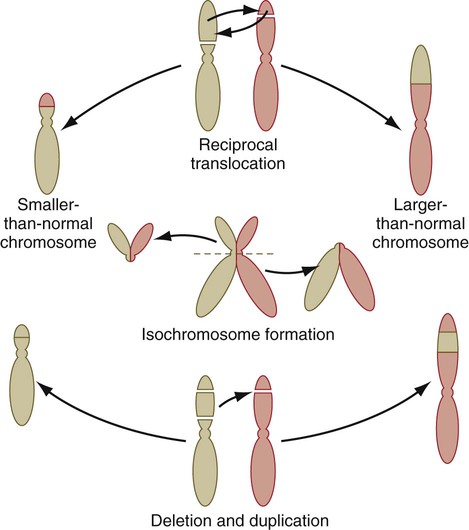
Other types of structural abnormalities of chromosomes are generated during meiosis and, if present in the germ cells, can be inherited. Common types of errors in chromosome structure are reciprocal translocations, isochromosome formation, and deletions and duplications (Fig. 8.11). One well-defined congenital malformation resulting from a deletion in the short arm of chromosome 5 is the cri du chat syndrome. Infants with this syndrome are severely mentally retarded, have microcephaly, and make a cry that sounds like the mewing of a cat.
Genetic Mutations
Many genetic mutations are expressed as morphological abnormalities. These mutations can be of dominant or recessive genes of either the autosomes or the sex chromosomes. For some of these conditions (e.g., hemophilia, Lesch-Nyhan syndrome, muscular dystrophy, cystic fibrosis), the molecular or biochemical lesion has been identified, but the manner in which these defects are translated into abnormal development is unclear. Many of these conditions are discussed extensively in textbooks of human genetics, and only representative examples are listed here (Table 8.5).
Table 8.5
Genetic Mutations Leading to Abnormal Development
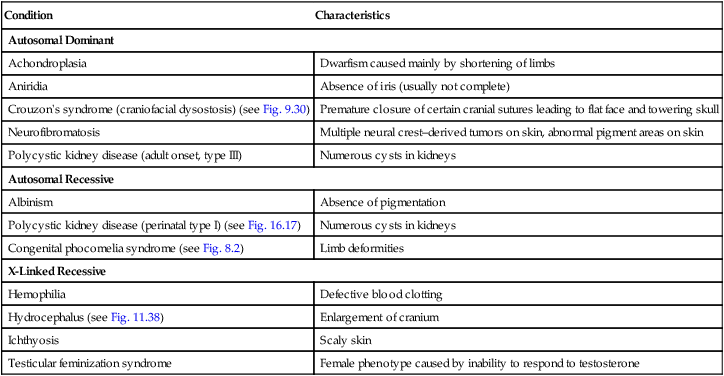
| Condition | Characteristics |
| Autosomal Dominant | |
| Achondroplasia | Dwarfism caused mainly by shortening of limbs |
| Aniridia | Absence of iris (usually not complete) |
| Crouzon’s syndrome (craniofacial dysostosis) (see Fig. 9.30) | Premature closure of certain cranial sutures leading to flat face and towering skull |
| Neurofibromatosis | Multiple neural crest–derived tumors on skin, abnormal pigment areas on skin |
| Polycystic kidney disease (adult onset, type III) | Numerous cysts in kidneys |
| Autosomal Recessive | |
| Albinism | Absence of pigmentation |
| Polycystic kidney disease (perinatal type I) (see Fig. 16.17) | Numerous cysts in kidneys |
| Congenital phocomelia syndrome (see Fig. 8.2) | Limb deformities |
| X-Linked Recessive | |
| Hemophilia | Defective blood clotting |
| Hydrocephalus (see Fig. 11.38) | Enlargement of cranium |
| Ichthyosis | Scaly skin |
| Testicular feminization syndrome | Female phenotype caused by inability to respond to testosterone |
Environmental Factors
Maternal Infections
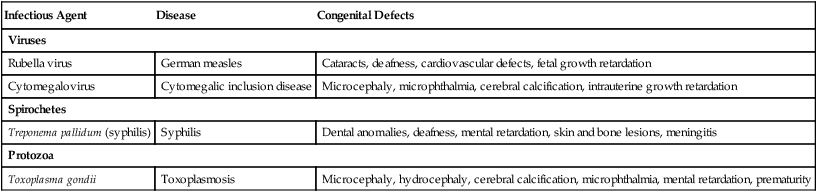
Most infectious diseases that cause birth defects are viral, with toxoplasmosis (caused by the protozoan Toxoplasma gondii) and syphilis (caused by the spirochete Treponema pallidum) being notable exceptions. (A summary of the infectious diseases known to cause birth defects in humans is given in Table 8.6.)
Table 8.6
Infectious Diseases That Can Cause Birth Defects
| Infectious Agent | Disease | Congenital Defects |
| Viruses | ||
| Rubella virus | German measles | Cataracts, deafness, cardiovascular defects, fetal growth retardation |
| Cytomegalovirus | Cytomegalic inclusion disease | Microcephaly, microphthalmia, cerebral calcification, intrauterine growth retardation |
| Spirochetes | ||
| Treponema pallidum (syphilis) | Syphilis | Dental anomalies, deafness, mental retardation, skin and bone lesions, meningitis |
| Protozoa | ||
| Toxoplasma gondii | Toxoplasmosis | Microcephaly, hydrocephaly, cerebral calcification, microphthalmia, mental retardation, prematurity |
Chemical Teratogens
Many substances are known to be teratogenic in animals or are associated with birth defects in humans, but convincing evidence that links the substance directly to congenital malformations in humans exists for only a relatively small number (Table 8.7). Testing drugs for teratogenicity is difficult because what can cause a high incidence of severe defects in animal fetuses (e.g., cortisone and cleft palate in mice) may not cause malformations in other species of animals or in humans. Conversely, the classic teratogen thalidomide is highly teratogenic in humans, rabbits, and some primates, but not in commonly used laboratory rodents.
Table 8.7
| Agent | Effects |
| Alcohol | Growth and mental retardation, microcephaly, various malformations of face and trunk |
| Androgens | Masculinization of females, accelerated genital development in males |
| Anticoagulants (warfarin, dicumarol) | Skeletal abnormalities; broad hands with short fingers; nasal hypoplasia; anomalies of eye, neck, central nervous system |
| Antithyroid drugs (e.g., propylthiouracil, iodide) | Fetal goiter, hypothyroidism |
| Chemotherapeutic agents (methotrexate, aminopterin) | Variety of major anomalies throughout body |
| Diethylstilbestrol | Cervical and uterine abnormalities |
| Lithium | Heart anomalies |
| Organic mercury | Mental retardation, cerebral atrophy, spasticity, blindness |
| Phenytoin (Dilantin) | Mental retardation, poor growth, microcephaly, dysmorphic face, hypoplasia of digits and nails |
| Isotretinoin (Accutane) | Craniofacial defects, cleft palate, ear and eye deformities, nervous system defects |
| Streptomycin | Hearing loss, auditory nerve damage |
| Tetracycline | Hypoplasia and staining of tooth enamel, staining of bones |
| Thalidomide | Limb defects, ear defects, cardiovascular anomalies |
| Trimethadione and paramethadione | Cleft lip and palate, microcephaly, eye defects, cardiac defects, mental retardation |
| Valproic acid | Neural tube defects |
Androgenic Hormones
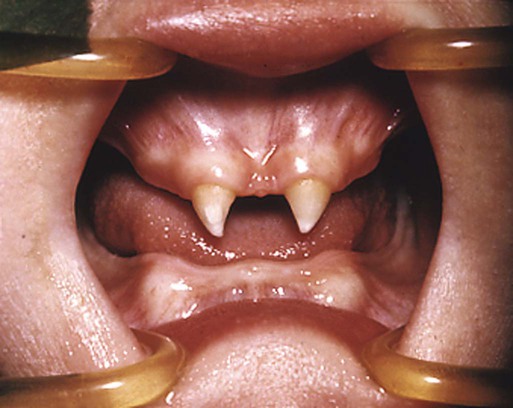
The administration of androgenic hormones to pregnant women either to treat tumors or to prevent threatened abortion resulted in the birth of hundreds of female infants with various degrees of masculinization of the external genitalia. The anomalies consisted of clitoral hypertrophy and often varying amounts of fusion of the genital folds to form a scrotumlike structure (Fig. 8.12).
Anticonvulsants
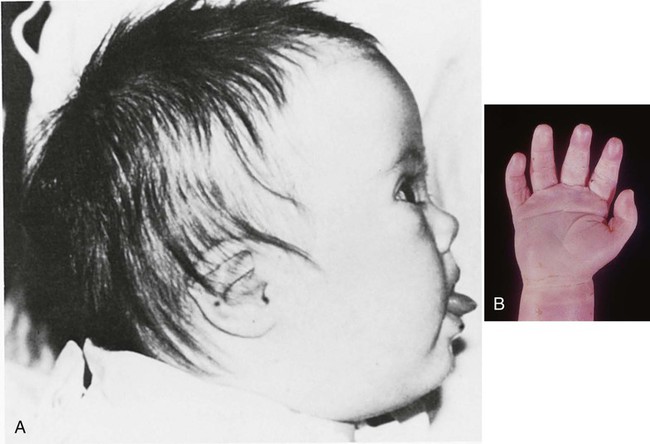
Several commonly used anticonvulsants are known or strongly suspected to be teratogenic. Phenytoin (previously known as diphenylhydantoin) produces a “fetal hydantoin syndrome” of anomalies, including growth anomalies, craniofacial defects, nail and digital hypoplasia, and mental retardation in up to one third of embryos exposed to this drug during pregnancy (Fig. 8.13). Trimethadione also produces a syndrome of anomalies involving low-set ears, cleft lip and palate, and skeletal and cardiac anomalies.
Alcohol
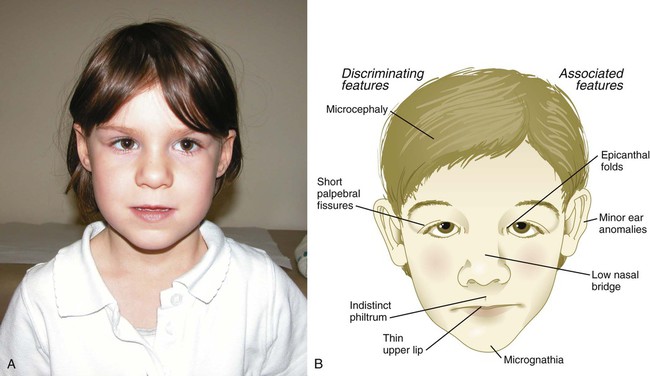
Accumulated evidence now leaves little doubt that maternal consumption of alcohol during pregnancy can lead to a well-defined constellation of developmental abnormalities that includes poor postnatal growth rate, microcephaly, mental retardation, heart defects, and hypoplasia of facial structures (Fig. 8.14). This constellation of abnormalities is now popularly known as fetal alcohol syndrome, and estimates suggest that some form of fetal alcohol syndrome may affect as many as 1% to 5% of all live births. Ingestion of 3 oz of alcohol in a day during the first 4 weeks of pregnancy can lead to extremely severe malformations of the holoprosencephaly type (see p. 309).
Exposure to alcohol later in pregnancy is less likely to cause major anatomical defects in the fetus, but because of the complex course of physiological maturation in the brain throughout pregnancy, more subtle behavioral defects can result. Nevertheless, there are often striking differences from normal in the size and shape of the corpus callosum, the main connecting link between the right and left sides of the brain, and in the cerebellum, which may be hypoplastic. Much of the abnormal development of the face and forebrain can be attributed to the death of cells in the anterior neural ridge (see Fig. 6.4B), which serves as a signaling center in the early embryo. Although the intelligence quotient (IQ) of an individual with fetal alcohol syndrome may be normal, such individuals may show deficits in recognition of the consequences of actions or in planning into the future.
Retinoic Acid (Vitamin A)
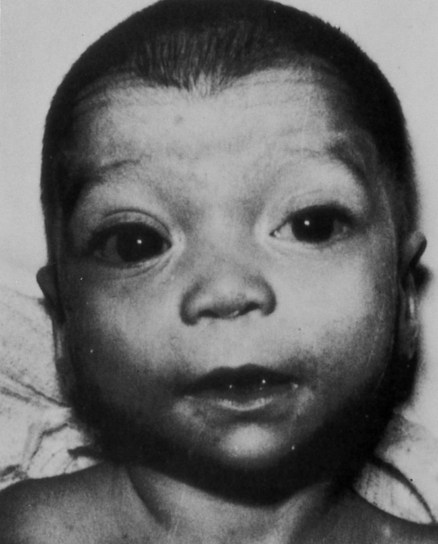
Derivatives of retinoic acid are used in the treatment of acne, but investigators have established that retinoic acid acts as a potent teratogen when it is taken orally. Retinoic acid can produce a wide spectrum of defects, most of which are related to derivatives of the cranial neural crest (see p. 259). These involve a variety of facial structures, the outflow tract of the heart, and the thymus (Fig. 8.15).
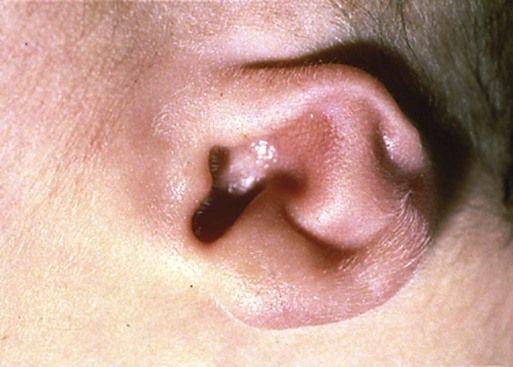
Among multiple facial anomalies, this infant had a highly deformed ear. (From the Robert J. Gorlin collection, Division of Oral and Maxillofacial Pathology, University of Minnesota Dental School, courtesy of Dr. Ioannis Koutlas.)
Through a complex sequence of cytoplasmic binding proteins and nuclear receptors (see Fig. 4.18), retinoic acid affects Hox genes, especially genes expressed in the cranial and pharyngeal region (see Fig. 11.12), with resulting alterations of the anterior rhombomeres and the neural crest cells derived from them. As discussed later, neural crest cells emanating from rhombomeres are instrumental in patterning many structures of the face and neck and contribute to the developing heart and thymus, hence the pattern of retinoic acid–induced defects previously outlined. In view of the increasing recognition of the important role of retinoic acid or its metabolites in pattern formation during early development, extreme caution is recommended when vitamin A is used in doses greater than those needed for basic nutritional requirements.
Mechanical Factors
Amniotic bands constricting digits or extremities of the fetus have been implicated as causes of intrauterine amputations (Fig. 8.16). These bands form as the result of tears to the extraembryonic membranes during pregnancy. Chorionic villus sampling results in a low percentage of transverse limb defects, but the mechanism underlying the defective limb development is not well understood.
Developmental Disturbances Resulting in Malformations
Duplications and Reversal of Asymmetry
The classic example of duplication is identical twinning. Under normal circumstances, both members of the twin pair are completely normal, but rarely the duplication is incomplete, and conjoined twins result (see Figs. 3.15 and 3.16). Twins can be conjoined at almost any site and to any degree. With modern surgical techniques, it is now possible to separate members of some conjoined pairs. A type of conjoined twinning is the condition of parasitic twinning, in which one member of the pair is relatively normal, but the other is represented by a much smaller body, often consisting of just the torso and limbs, attached to an area such as the mouth or lower abdomen of the host twin (see Fig. 3.17). In numerous conjoined twins, one member of the pair has reversed asymmetry in relation to the other (see Fig. 3.16).
In rare instances (approximately 1 in 10,000 births), an otherwise normal individual is found to have a partial or complete reversal of the asymmetry of the internal organs, a condition called situs inversus (see Fig. 5.15). Molecular research on early embryonic stages (see Fig. 5.13) has begun to provide a mechanistic explanation for this condition.
Absence of Normal Cell Death
Genetically or epigenetically (environmental influences imposed on the genetic background) controlled cell death is an important mechanism in sculpting many regions of the body. The absence of normal interdigital cell death has been implicated in syndactyly (webbed digits) (see Fig. 10.23A) and abnormal persistence of the tail (see Fig. 9.25A for normal tail). The latter phenomenon has sometimes been considered an example of atavism (the persistence of phylogenetically primitive structures).
Failure of Tube Formation
The formation of a tube from an epithelial sheet is a fundamental developmental mechanism. A classic case of failure of tube formation is seen in the spina bifida anomalies, which are based on the incomplete fusion of the neural tube (see Fig. 11.42). (Some of the possible mechanisms involved in normal formation of the neural tube are discussed in Chapter 11.)
Disturbances in Tissue Resorption
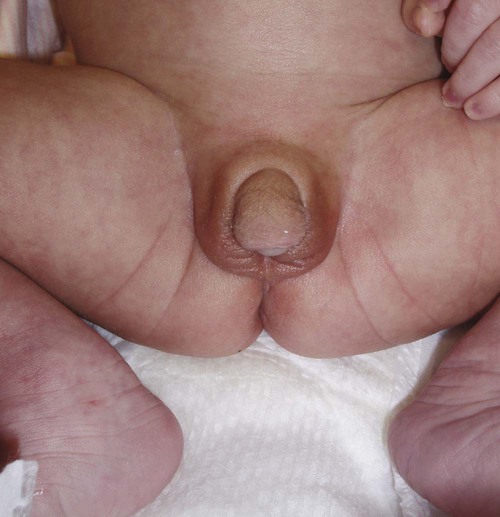
Some structures present in the early embryo must be resorbed for subsequent development to proceed normally. Examples are the membranes that cover the future oral and anal openings. These membranes are composed of opposing sheets of ectoderm and endoderm, but if mesodermal cells become interposed between the two and this tissue becomes vascularized, breakdown typically fails to occur. Anal atresia is a common anomaly of this type (see Fig. 15.19).
Failure of Migration
Migration is an important developmental phenomenon that occurs at the level of cells or entire organs. The neural crest is a classic example of massive migrations at the cellular level, and disturbances in migration can cause abnormalities in any of the structures for which the neural crest is a precursor (e.g., thymus, outflow tracts of the heart, adrenal medulla). At the organ level, the kidneys undertake a prominent migration into the abdominal cavity from their origin in the pelvic region, and the testes migrate from the abdominal cavity into the scrotum. Pelvic kidneys (see Fig. 16.15) and undescended testes (cryptorchidism) are relatively common.
Developmental Arrest
Early in the history of teratology, some malformations were recognized as the persistence of structures in a state that was normal at an earlier stage of development. Many of the patterns of cleft lip and cleft palate (see Figs. 14.16 and 14.17) are examples of developmental arrest, although it is incorrect to assume that development has been totally arrested since the sixth to eighth weeks of embryogenesis. Another example of the persistence of an earlier stage in development is a thyroglossal duct (see Fig. 14.45), in which persisting epithelial cells mark the path of the thyroid gland as it migrates from the base of the tongue to its normal position.
Destruction of Formed Structures
Many teratogenic diseases or chemicals produce malformations by the destruction of structures already present. If the structure is in the early primordial stage, any tissues to which the primordium would normally give rise are missing or malformed. Interference with the blood supply of a structure can cause unusual patterns of malformations. In the genesis of phocomelia (see Fig. 8.2), damage to proximal blood vessels could destroy the primordia of the proximal limb segments, but the cells of the distal limb bud that give rise to the hands or feet could be spared if the distal microvasculature of the limb bud remained intact.
Hypoplasia and Hyperplasia
The normal formation of most organs and complex structures requires a precise amount and distribution of cellular proliferation. If cellular proliferation in a forming organ is abnormal, the structure can become too small (hypoplastic) (see Fig. 16.12B) or too large (hyperplastic). Even minor growth disturbances can cause severe problems in complex regions such as the face. Occasionally, gigantism of a structure such as a digit (Fig. 8.17) or whole limb occurs. The mechanism underlying this excessive growth remains obscure.
Receptor Defects
Some congenital malformations can be attributed to defects in specific receptor molecules. One of the earliest recognized is the testicular feminization syndrome, in which the lack of testosterone receptors results in the development of a typical female phenotype in a genetic male (see Fig. 9.13A).
Defective Fields
Proper morphogenesis of many regions of the body is under the control of poorly understood morphogenetic fields. These regions of the body are under the control of an overall developmental blueprint. Disturbances in the boundaries or overall controls of fields can sometimes give rise to massive anomalies. One example is the fusion of lower limb fields, which is probably associated with a larger defect in the field controlling the development of the caudal region of the body. This mermaidlike anomaly is called sirenomelia (see Fig. 8.1B), and it is an extreme example of what is called the caudal regression syndrome, resulting from abnormal T gene function (see p. 82).
Effects Secondary to Other Developmental Disturbances
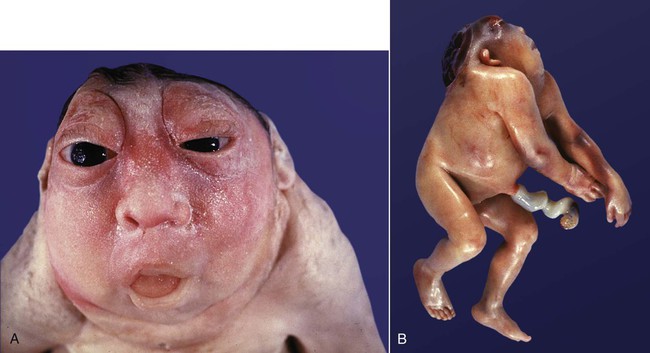
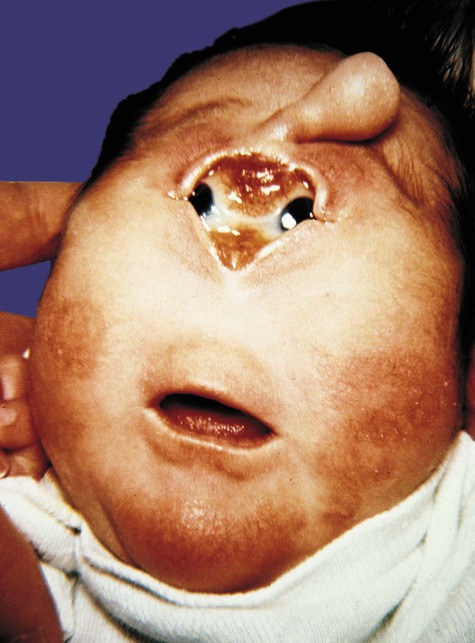
The single or widely separated tubular probosces that appear in certain major facial anomalies, such as cyclopia (Fig. 8.18), are very difficult to explain unless it is understood that one of several primary defects, whether too much or too little tissue of the midface, prevented the two nasal primordia from joining in the midline. In the case of cyclopia, the primary defect is usually a deficiency of forebrain tissue that results from deficient sonic hedgehog signaling (see Clinical Correlation 14.1), and the facial defects are secondary to that.
Germ Layer Defects
An understanding of normal development can explain the basis for a seemingly diverse set of anomalies (Clinical Correlation 8.1). Ectodermal dysplasias, which are based on abnormalities in the ectodermal germ layer, can include malformations as diverse as thin hair, poorly formed teeth, short stature, dry and scaly skin, and hypoplastic nails (Fig. 8.19). Other syndromes with diverse phenotypic abnormalities are related to defects of the neural crest (see Chapter 12).
Summary
 Developmental disorders have been recognized for centuries, but a direct connection between environmental teratogens and human birth defects was not shown until 1941.
Developmental disorders have been recognized for centuries, but a direct connection between environmental teratogens and human birth defects was not shown until 1941.
 Abnormal development is often the result of environmental influences imposed on genetic susceptibility. The factors involved in abnormal development include age, race, country, nutrition, and time of year. The study of abnormal development is teratology, and an agent that causes abnormal development is a teratogen.
Abnormal development is often the result of environmental influences imposed on genetic susceptibility. The factors involved in abnormal development include age, race, country, nutrition, and time of year. The study of abnormal development is teratology, and an agent that causes abnormal development is a teratogen.
 Genetic factors cause a significant number of birth defects. Abnormal chromosome numbers are associated with prenatal death and syndromes of abnormal structures. Common causes of abnormalities are monosomies and trisomies, which are often the result of nondisjunction during meiosis. Other malformations are based on abnormalities of chromosome structure. Certain malformations are based on genetic mutations.
Genetic factors cause a significant number of birth defects. Abnormal chromosome numbers are associated with prenatal death and syndromes of abnormal structures. Common causes of abnormalities are monosomies and trisomies, which are often the result of nondisjunction during meiosis. Other malformations are based on abnormalities of chromosome structure. Certain malformations are based on genetic mutations.
 Environmental factors leading to defective development include maternal infections, chemical teratogens, physical factors such as ionizing radiation, maternal factors, and mechanical factors.
Environmental factors leading to defective development include maternal infections, chemical teratogens, physical factors such as ionizing radiation, maternal factors, and mechanical factors.
 A variety of disturbed developmental mechanisms may be involved in the production of a given congenital malformation, including duplications, faulty inductive tissue interactions, absence of normal cell death, failure of tube formation, disturbances in tissue resorption, failure of migration, developmental arrest, destruction of an already formed structure, failure to fuse or merge, hypoplasia or hyperplasia, receptor defects, defective fields, effects secondary to other developmental disturbances, and germ cell layer defects.
A variety of disturbed developmental mechanisms may be involved in the production of a given congenital malformation, including duplications, faulty inductive tissue interactions, absence of normal cell death, failure of tube formation, disturbances in tissue resorption, failure of migration, developmental arrest, destruction of an already formed structure, failure to fuse or merge, hypoplasia or hyperplasia, receptor defects, defective fields, effects secondary to other developmental disturbances, and germ cell layer defects.
 With technological developments, it is now possible to diagnose increasing numbers of birth defects in utero. Diagnostic techniques include karyotyping and sex chromosome analysis on cells obtained from amniotic fluid, biochemical analysis of amniotic fluid, biochemical and molecular analysis of cells obtained from amniotic fluid or chorionic villus sampling, and imaging techniques, especially ultrasonography. There have been a few attempts to correct malformations by surgery in utero.
With technological developments, it is now possible to diagnose increasing numbers of birth defects in utero. Diagnostic techniques include karyotyping and sex chromosome analysis on cells obtained from amniotic fluid, biochemical analysis of amniotic fluid, biochemical and molecular analysis of cells obtained from amniotic fluid or chorionic villus sampling, and imaging techniques, especially ultrasonography. There have been a few attempts to correct malformations by surgery in utero.