Decontamination of the Poisoned Patient
In 2009, the National Poison Data System of the American Association of Poison Control Centers reported 2,479,355 human toxic exposures and 1452 resultant fatalities.1 Of these total exposures, 24.1% were managed in a health care facility. Massive exposure to some toxic agents (e.g., calcium channel blockers, tricyclic and other antidepressants, antipsychotics, β-blockers, colchicine, chloroquine, cyanide, Amanita phalloides mushrooms, paraquat) will probably result in severe morbidity or fatality regardless of even the most sophisticated and timely medical interventions. With general supportive care and the use of a few specific antidotes, however, the mortality rate in unselected overdose patients is less than 1% if the patient arrives at the hospital in time for the clinician to intervene.
Key management of poisoned patients seen in health care facilities initially focuses on confirming the diagnosis of possible exposure to a toxin, providing standard cardiovascular and respiratory supportive care, and using a small cadre of specific antidotes. In rare and yet undefined selected instances, prevention of further absorption of toxin by various decontamination procedures may theoretically ameliorate the morbidity or reduce mortality. Although a better final outcome after gastric decontamination may seem intuitively reasonable, there is no definitive evidence from prospective clinical trials that the use of various decontamination techniques positively alters the morbidity or mortality of a poisoned patient.2
Before the availability of objective or experimental evidence addressing gastric-emptying procedures, most clinicians instituted previously performed unproven decontamination procedures in the emergency department (ED) as a reflex response for the majority of patients suspected of drug overdose, often without much forethought and certainly without confirming data. Mounting evidence relegates any benefit from any form of gastric decontamination to selected cases and specific individual scenarios. In fact, because of the lack of demonstrable benefit and the mounting evidence for potential harm, syrup of ipecac, once a mainstay in the management of poisonings, is no longer recommended; the American Heart Association and American Red Cross International Consensus on First Aid Science state that syrup of ipecac should not be used in first aid treatment of acute poisonings,3 and parents have been instructed by the American Academy of Pediatrics to remove it from the home.4 Parents and health care providers appear to be heeding this recommendation since less than 0.01% of poisoned patients received ipecac in 2009.1
Gastric Decontamination
Background
The use of gastric lavage in poisoned patients has decreased significantly since the 1990s, and in the United States it is currently reported to be used in less than 1% of overdose case.1,5,6 Numerous animal and human volunteer studies have been conducted to examine the effectiveness of gastric lavage in removing toxins from the stomach, especially with respect to other gastrointestinal decontamination methods.7–18 The reported efficacy of gastric lavage in removing markers from the stomach varies significantly in these studies. The difference in these study results is due in part to the variability of the methods used (different fluid-instilled markers, animal models, positioning, amount of lavage, and lavage tube size) and the time that elapsed from instillation of the marker in the stomach until gastric lavage was performed. Even within individual studies, the range of effectiveness of gastric lavage in removing the marker varied considerably. For example, Tandberg and coworkers performed gastric lavage 10 minutes after ingestion of the marker and reported that its effectiveness in removing the marker varied from 18.9% to 67.7%.13
Many of these studies do not replicate the typical clinical scenario encountered in emergency medicine.2,19 The efficiency of gastric lavage in removing a marker significantly decreases with increasing time after ingestion. This is due to the fact that as time increases after ingestion, there is more time for the marker to be absorbed and pass out of the stomach. For example, Shrestha and colleagues reported that more than 70% of the marker used in their study passed out of the stomach by 60 minutes.20 It is rare that gastric lavage can be performed within the first hour after toxic ingestion. Not only does it take time for these patients to arrive at the ED, but it also takes time for evaluation, stabilization, and performance of gastric lavage. Watson and associates reported that the mean time required by experienced emergency medicine nurses to perform lavage was 1.3 hours.16 Gastric lavage may also propel the marker from the stomach into the small intestine, thereby decreasing the effectiveness of removing the toxin from the stomach and actually enhancing the rate of absorption.21
Three major studies have examined whether gastric lavage positively influences the outcome of poisoned patients.22–24 In a study performed by Kulig and coworkers, there was no difference in outcome in patients who received gastric lavage followed by charcoal versus charcoal alone when these interventions were performed more than 1 hour after ingestion.22 In patients who were treated within 1 hour of ingestion, gastric lavage followed by charcoal provided a small but statistically significant advantage over activated charcoal alone. Merigian and colleagues demonstrated that in symptomatic patients, the rate of intensive care admission and need for intubation was significantly higher in patients who received gastric lavage followed by charcoal than in those who received charcoal alone.23 This increased admission and intubation rate was directly attributed to the aspiration of gastric contents as a result of gastric lavage. Pond and associates replicated the Kulig and colleagues’ study.24 They found no difference in outcome between patients who received gastric lavage followed by charcoal and those receiving charcoal alone, regardless of the time of performance of gastric lavage. They concluded that “gastric emptying procedures can be omitted from the treatment regimen for adults after acute overdose, including those who present within 1 hour of overdose and those that manifest severe toxicity.”
Indications
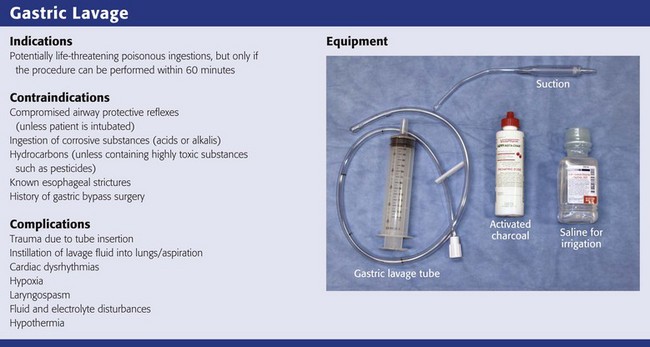
Based on the literature available, gastric lavage should not be routinely used in the management of poisoned patients (Fig. 42-1).25 There is no universally accepted standard of care that can be applied to the use of gastric lavage in unselected poisoned patients in the ED. Under certain circumstances, however, there may be a theoretical benefit from gastric emptying, and the local poison center should be contacted to assist in making a decision whether gastric lavage may be of benefit. Whether specific subsets of overdose patients may benefit from gastric lavage has not been clearly defined.
Contraindications
Though generally safe, gastric lavage is not an innocuous procedure. Performance of gastric lavage is contraindicated in any person who demonstrates compromised airway protective reflexes, unless that person is intubated.26 Many clinicians opt for lavage in a seriously ill patient who is intubated because airway protection is already accomplished. Tracheal intubation, however, does not ensure a totally protected airway. Paralyzing plus intubating a patient merely to initiate gastric lavage is generally eschewed.
Gastric lavage is contraindicated in persons who have ingested corrosive substances (acids or alkalis) or hydrocarbons (unless they contain highly toxic substances such as pesticides) or have known esophageal strictures or a history of gastric bypass surgery.27 Caution should be exercised in performing gastric lavage in combative patients and in those who have medical conditions such as bleeding diatheses that could be compromised by performing this procedure.
Equipment and Preparation
If the decision is made to perform gastric lavage, careful attention to the details of the procedure results in increased safety for the patient and more effective removal of the ingested poison. Before lavage, the patient should have intravenous access secured and continuous cardiac monitoring and pulse oximetry initiated (Fig. 42-2, step 1). A large, rigid suction catheter should be available immediately.
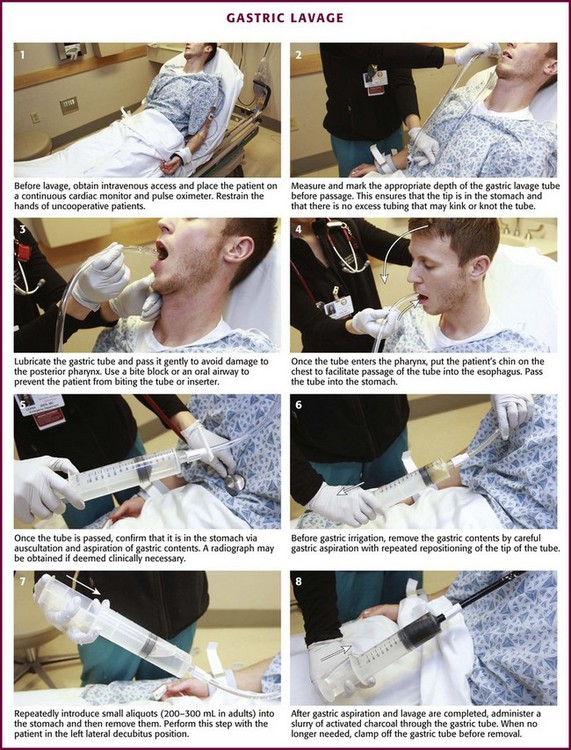
Figure 42-2 Gastric lavage.
The position of the patient during gastric lavage is important. Place all patients in the left lateral decubitus, Trendelenburg position (≈20-degree tilt on the table) (Fig. 42-3). This position diminishes the passage of gastric contents into the duodenum during lavage and decreases the risk for pulmonary aspiration of gastric contents should vomiting or retching occur. Restrain the hands of an uncooperative patient to prevent removal of the gastric or endotracheal tube. Intubated patients on a ventilator may be lavaged in the supine position because of logistic reasons (Fig. 42-4). Under no circumstances should a nonintubated patient undergo lavage in the restrained supine position. Such positioning invites aspiration and diminishes the patient’s natural protective maneuvers, such as coughing and sitting up.
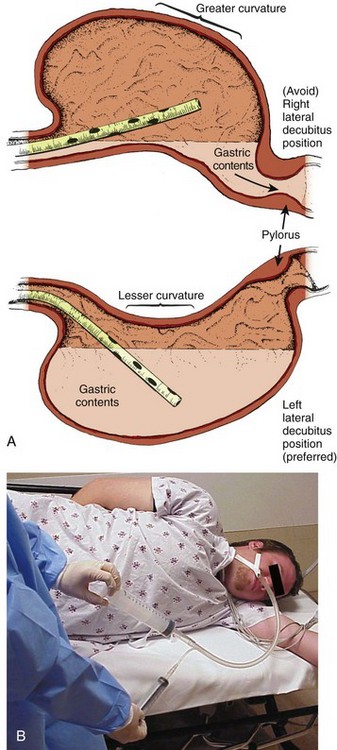
Figure 42-3 A, Effect of patient positioning on lavage. B, The left lateral decubitus position is preferred.
Most clinicians prefer the oral route for gastric lavage, but in selected circumstances a standard large-bore nasogastric (NG) tube (Salem sump pump) may be used. Large-diameter gastric hoses with extra holes cut near the tip have traditionally been recommended for gastric lavage. There are no convincing data on humans to refute or support this recommendation, and one study of a small number of dogs failed to show any difference in efficacy with lavage through a 32-Fr tube versus a 16-Fr lavage tube.28 It is generally held that large-diameter NG or orogastric tubes (>1 cm) are more likely to retrieve particulate matter successfully, but the tube size is such that whole pills are unlikely to pass (Fig. 42-5). Smaller, more flexible tubes may kink and are significantly more difficult to pass. An NG tube may be passed through the mouth or nose, but orogastric hoses should not be passed through the nose. Because most pills disintegrate in the stomach in a few minutes, significant amounts of particulate matter may be retrieved with a large-bore NG tube such as an 18-Fr Salem sump tube. NG tubes are considerably easier to pass and less traumatic for the patient. NG tubes are preferred for liquid ingestions and in children (Fig. 42-6).
In most cases, a 36- to 40-Fr or a 30-English gauge tube (external diameter, 12 to 13.3 mm) should be used in adults and a 24- to 28-Fr gauge (diameter, 7.8 to 9.3 mm) tube in children.25 Before passage, estimate the length of tube required to enter the stomach by approximating the distance from the corner of the mouth to the midepigastrium. Premeasurement avoids the curling and kinking of excess hose in the stomach (see Fig. 42-2, step 2). Passage of an excessive length of hose may cause gastric distention, bruising, and perforation, whereas passage of an insufficient length of hose may result in lavage of the esophagus and increased risk for emesis and aspiration. Commercial lavage systems are available and often use either a gravity fill-and-empty system with a Y connector or a closed irrigation syringe system. Alternatively, an irrigation syringe can be used for intermittent input and withdrawal of lavage fluid.
Technique
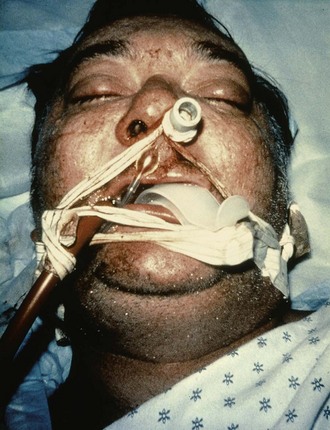
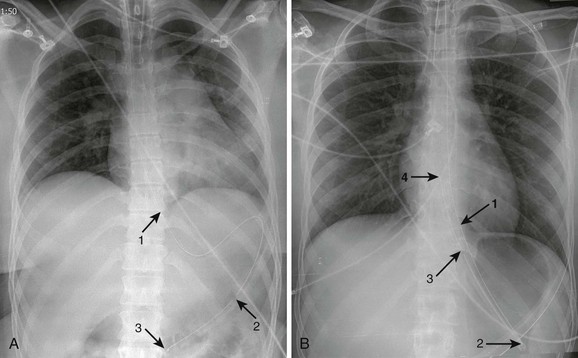
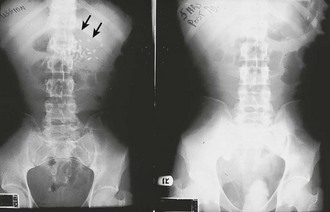
Lubricate the gastric tube and pass it gently to avoid damage to the posterior pharynx (see Fig. 42-2, step 3). Use of a bite block or an oral airway may prevent the patient from chewing on the orogastric tube and biting the fingers of the inserter. If the patient is obtunded or paralyzed, extend the jaw to facilitate passage. Never use force to pass the tube. Once the pharynx has been entered, put the patient’s chin on the chest to facilitate passage of the tube into the esophagus (see Fig. 42-2, step 4). Cough, stridor, or cyanosis indicates that the tube has entered the trachea; withdraw the tube immediately and reattempt passage. Once the tube is passed, confirm that it is in the stomach. Intragastric placement is usually evident on clinical grounds by the spontaneous egress of gastric contents but may be confirmed by auscultation of the stomach during injection of air with a 50-mL syringe followed by successful aspiration of gastric contents (see Fig. 42-2, step 5). In an intubated or obtunded patient or a young child, confirm tube position radiographically before lavaging, although this is not routinely performed (Fig. 42-7A). A misplaced tube may irrigate the esophagus with a tube that has doubled back on itself during passage (see Fig. 42-7B). The most serious complication, other than esophageal perforation, is inadvertent passage of the tube into the lungs. Tracheal passage of a lavage tube should be readily obvious in an awake patient before lavage, and obtunded patients are intubated, thereby obviating this problem. If an awake patient begins to vomit during lavage, immediately remove the tube to allow the patient to protect the airway.
Before gastric irrigation, remove the gastric contents by careful gastric aspiration with repeated repositioning of the tip of the tube (see Fig. 42-2, step 6). With the Y-connector closed system, perform lavage by clamping the drainage arm of the Y adapter and infusing aliquots of fluid into the stomach from a reservoir. Clamp the reservoir arm of the Y, and then open the drainage arm to permit drainage of the stomach contents by gravity. Repeat this procedure. Some resistance is produced by the Y connector and tubing. Apply suction intermittently to the drainage tubing to enhance emptying of the stomach.
Lavage can be performed adequately with tap water in adults. Because electrolyte disturbances have occurred in children who underwent lavage with tap water, prewarmed (45°C) normal saline is generally recommended for children.29–31 Warmed lavage fluid increases the solubility of most substances, delays gastric emptying, and should theoretically increase the effectiveness of the procedure.32,33 Repeatedly introduce small aliquots of lavage solution (200 to 300 mL in adults and 10 mL/kg body weight in children up to a maximum of 300 mL) into the stomach and then remove them (see Fig. 42-2, step 7). Larger amounts of fluid create the potential for an increased risk of washing the gastric contents into the duodenum or lungs. Much smaller amounts are not clinically practical because of the dead space in the tubing (≈50 mL in a 36-Fr hose) and the increased time required. The amount of fluid that is returned should approximate the amount introduced. Manual agitation of the patient’s stomach by gently “kneading” it with a hand placed on the abdomen may increase recovery.32 Continue lavage until the fluid becomes clear.
After gastric aspiration and lavage have been completed, administer a slurry of activated charcoal through the gastric tube (see Fig. 42-2, step 8). When no longer needed, clamp off the gastric tube during removal to avoid “dribbling” fluid into the airway. With the increasing use of repetitive doses of activated charcoal, an NG tube may be left in place, or passed, after the lavage procedure is completed. A patient who remains obtunded may receive additional doses via a standard NG tube. Because the large gastric tube is irritating and may predispose the patient to gagging, drooling, or aspiration, it should be removed. Alert patients should take subsequent doses of charcoal orally as necessary.
Complications
A correctly performed procedure in the appropriate environment is generally safe, but numerous complications have been associated with gastric lavage.26 The complications can be divided into those caused by mechanical trauma and those resulting from the lavage fluid.
Depending on the route selected for insertion of the tube, damage to the nasal mucosa, turbinates, pharynx, esophagus, and stomach has been reported.34–37 After insertion of the tube, it is imperative to confirm correct placement. Scalzo and associates found radiographically that 7 of 14 children had improper tube placement (too high or too low) despite positive gastric auscultation in all cases, although the clinical effects of such misplacement was not evaluated. Radiographic confirmation of tube placement should be considered in young children and intubated patients (see Fig. 42-7).38 Instillation of lavage fluid and charcoal into the lungs through tubes inadvertently misplaced within the airways has been reported.39
During lavage, changes in cardiorespiratory function have been noted. Thompson and coworkers reported that during lavage 36% of patients had atrial or ventricular ectopy, 4.8% had transient ST elevation, and 29% had a fall in oxygen tension to 60 mm Hg or lower.40 Laryngospasm may also occur during gastric lavage.25
The lavage fluid itself is a potential source of complications. The large amount of fluid administered during lavage has been reported to cause fluid and electrolyte disturbances. These disturbances have been seen with the use of both hypertonic and hypotonic lavage fluid in the pediatric population.29–31 Hypothermia is a possible complication if the lavage fluid is not prewarmed.
Pulmonary aspiration of gastric contents or lavage fluid is the primary potential risk during gastric lavage, especially in patients with compromised airway protective reflexes.41–43 Merigian and colleagues reported a 10% incidence of aspiration pneumonia in patients who underwent gastric lavage.23 This risk is reduced by using small aliquots of lavage fluid, adequately positioning the patient, and intubating patients with compromised airway protective reflexes.
If the lavage tube cannot be removed easily, do not force it. Kinking or knotting of the tube can occur, but occasionally a tube may become stuck because of lower esophageal spasm. If fluoroscopy or radiography demonstrates no deformation of the lavage tube, 1 to 2 mg of intravenous glucagon can be infused in an attempt to relieve lower esophageal spasm.44 Surgical removal may be necessary if the gastric tube is deformed by kinking or knotting.
Activated Charcoal
Activated charcoal is a carbon product that is subjected to heat and oxidized to increase its surface area (Fig. 42-8). It has the capacity to adsorb substances onto the porous surface of the charcoal. The use of activated charcoal for poisoning has been recognized for almost 2 centuries. To demonstrate charcoal’s effectiveness, in 1930 French pharmacist Touery ingested several times the lethal dose of strychnine mixed with 15 g of activated charcoal. He performed this act in front of a class of colleagues and exhibited no ill effects.
Activated charcoal acts both by adsorbing a wide range of toxins present in the gastrointestinal tract and by enhancing elimination of toxin if systemic absorption has already occurred. It enhances elimination by creating a concentration gradient between the contents of the bowel and the circulation, but it also has the potential of interrupting enterohepatic circulation if the particular toxin is secreted in bile and enters the gastrointestinal tract before reabsorption.45 Oral activated charcoal is given as a single dose or in multiple doses. The adsorptive capacity of charcoal depends on the inherent properties of the toxin and the local milieu, such as pH. Adsorption begins within minutes of contact with a toxin but may not reach equilibrium for 20 to 30 minutes. Desorption of toxins from charcoal occurs over time, although this has little clinical significance for most patients and can be overcome by administering additional charcoal.
Indications
For years, administration of a single oral dose of activated charcoal for essentially all overdoses has been routine. However, with the emergence of new guidelines in overdose management,6 use of charcoal has declined to less than 5% of all potential toxin exposures (Fig. 42-9).1 Clearly, charcoal binds many toxins in the gut, thereby decreasing some systemic absorption. Despite a lack of scientific data demonstrating a decrease in morbidity and mortality and firm evidence to support its widespread use, charcoal is a reasonable intervention for most poisoned patients encountered in the ED if it can be administered easily and safely. The exact indications are not established, and no universally accepted standard of care has been promulgated.46 A single dose of activated charcoal is indicated if the clinician estimates that a clinically significant fraction of the ingested substance remains in the gastrointestinal tract, the toxin is adsorbed by charcoal, further absorption may result in clinical deterioration, and it can be administered safely. This will usually be a clinical decision because adequate historical data may often be lacking. It may also be administered by multiple dosing if the clinician anticipates that the charcoal will result in increased clearance of an already absorbed drug. It is most effective within the first 60 minutes after an oral overdose and decreases in effectiveness over time. Charcoal is generally considered to provide superior gut decontamination over gastric lavage. There is no definitive evidence, however, that administration of activated charcoal improves outcome.
Contraindications
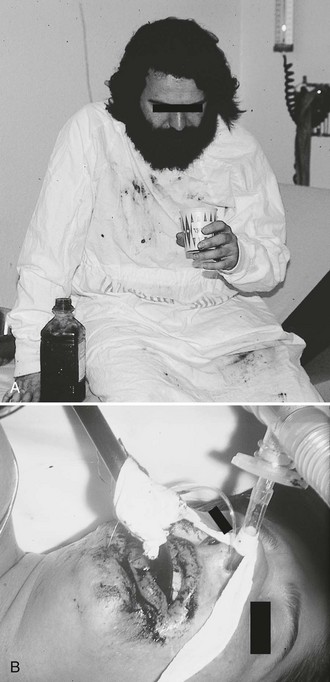
Administration of charcoal is contraindicated in any person who demonstrates compromised airway protective reflexes unless already intubated.46 It is absolutely contraindicated in persons who have ingested corrosive substances (acids or alkalis). Not only does charcoal provide no benefit in a corrosive ingestion, but its administration could also precipitate vomiting, obscure endoscopic visualization, and lead to complications if a perforation developed and charcoal entered the mediastinum, peritoneum, or pleural space. Charcoal should be avoided with ingestion of a pure aliphatic petroleum distillate. Hydrocarbons are not well adsorbed by activated charcoal, and its administration could lead to further risk for aspiration. Many hydrocarbons are potential systemic toxins (e.g., carbon tetrachloride and benzene) or are mixed with other potentially significant toxins such as pesticides. In these cases data are lacking, but charcoal administration can be considered. Caution should be exercised in using charcoal in patients with medical conditions that could be further compromised by charcoal ingestion, such as gastrointestinal perforation or bleeding. Charcoal is not indicated for isolated ingestion of ethanol or metals (e.g., iron, lithium) because these substances are not adsorbed. If the airway is not secure, charcoal should be given with caution to minimally symptomatic patients who have ingested a toxin that may suddenly induce seizures. Because it is often impossible to determine the exact nature of an ingestion, a liberal use policy is advocated for potentially mixed overdoses.
Charcoal administration by paramedics and other emergency response personnel should be performed with caution.47 There is insufficient evidence to recommend for or against administration of activated charcoal in the prehospital setting.3 The same indications and contraindications apply as for patients who are in the hospital. The motion of the ambulance during transport may make the patient more prone to emesis. Either spilling of charcoal or vomiting of charcoal may result in significant contamination of the transport vehicle and subsequently place that vehicle out of commission until it can be cleaned.
Technique
There is no universally accurate dose for charcoal. A 10 : 1 ratio (charcoal to toxin) is recommended if the amount of ingestion is known. Dosing of charcoal should be considered in light of the specific ingestion, but the following empirical doses of single-dose activated charcoal (standard aqueous products such as Liqui-Char) are recommended46:
Because in many charcoal formulations the contents settle with time, shake the preparation vigorously before administering it to the patient. Follow this by rinsing the container with a small amount of tap water before administering it to the patient to allow ingestion of the full dose.48 Aqueous activated charcoal has a gritty texture that most patients find unpleasant, but attempts have been made to improve its taste and texture. Mixing activated charcoal with chocolate milk, chocolate- or cherry-flavored syrup, or ice cream may increase palatability, but mixing with these additives has been suggested, though not proved, to cause a decrease in the adsorptive capacity of activated charcoal.49 Rangan and colleagues reported no decrease in adsorption after mixing superactivated charcoal with a noncaffeinated cola.50 Scharman and associates demonstrated that a regular, sugared cola was favored by children over a diet cola, but only 20% of the time were they able to cajole even nonpoisoned children younger than 3 years to drink a therapeutic amount of flavored charcoal.51
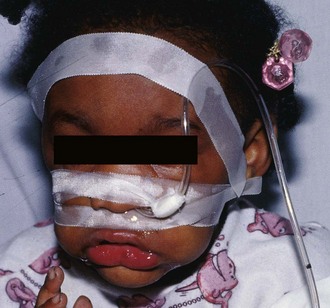
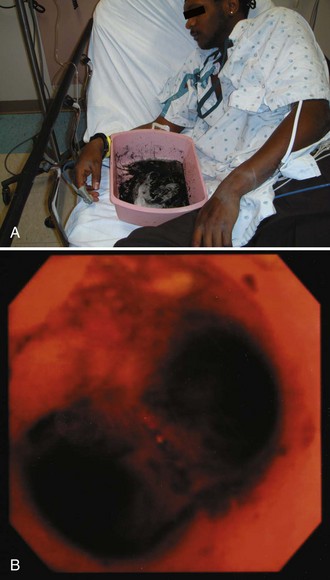
Give activated charcoal orally if the patient is awake and cooperative and by NG tube if the patient is unconscious. If an NG tube is inserted, it is imperative that correct placement be verified. Confirm correct tube placement radiographically before administering charcoal, especially in obtunded or intubated patients (see Fig. 42-7). Instillation of charcoal into the lungs has been reported after inadvertent misplacement within the airways, and massive aspiration can be fatal (Fig. 42-10).39,52 Intubation is protective, but it is not uncommon to see some charcoal in the airway even if the patient has been intubated.
A clinical conundrum exists when a patient refuses to drink charcoal and an NG tube must be passed without consent if charcoal is deemed advisable. The common tactic of passing an NG tube in an awake but uncooperative patient merely to administer charcoal is controversial, and no standards exist. Such a scenario is more likely to result in trauma from placement of the tube, a misplaced tube, or subsequent emesis from the rapid administration of charcoal. Given the unproven efficacy of charcoal, the authors advise against routine insertion of an NG tube simply to administer charcoal in an awake and minimally symptomatic patient. Such a decision is, however, a clinical one that must be made by the health care provider and be based on the entire clinical milieu (Fig. 42-11).
Complications
Administration of activated charcoal is not without risks and complications. Published reports have demonstrated adverse effects associated with activated charcoal therapy, including childhood deaths.53 The most common complications of charcoal administration include constipation, diarrhea, and vomiting.54,55 Bowel perforation has been described in a patient with diverticular disease.56 Pulmonary aspiration of activated charcoal is a dreaded complication that can result in pneumonitis, obstruction of the respiratory tree, bronchiolitis obliterans,36,57–59 acute lung injury, and barotrauma.53 Risk factors for serious aspiration are large amounts of charcoal instilled over a short period, multiple-dose charcoal in the setting of ileus, charcoal administration in a patient who becomes obtunded, charcoal that is inappropriately diluted, or forced administration of charcoal via an NG tube, especially in a restrained supine patient. These complications can be prevented by prudent dosing of charcoal and associated cathartic therapy, as well as by monitoring the patient’s fluid and electrolyte status and clinical condition and performing abdominal examinations. Trivial aspiration of charcoal is common and usually innocuous even if the patient is intubated. Studies show a 4% to 39% incidence of aspiration pneumonia in intubated patients who received activated charcoal while intubated.60 It has been shown that even in patients with a protected airway and a cuffed endotracheal tube, vomiting can lead to pulmonary aspiration of the charcoal.53 This can result in a significant increase in lung microvascular permeability and lead to lung edema and pulmonary compromise.61
Multiple Doses of Activated Charcoal
The use of multiple-dose activated charcoal (MDAC) may be indicated in selected cases (Fig. 42-12).62 Its use has been advocated for two purposes: first, to prevent continued absorption of a drug that may still be present in the gastrointestinal tract and, second, to increase serum clearance of a drug that has already been absorbed (Box 42-1).
MDAC prevents continued absorption by either binding a drug that may be present throughout the gastrointestinal tract or binding a drug that exists as extended-release or enteric-coated preparations. MDAC enhances elimination of a drug by interrupting enterobiliary recirculation or augmenting enterocapillary exsorption.54 By interrupting enterobiliary recirculation, charcoal binds to an active drug that is secreted by the biliary system, thus subsequently preventing reabsorption. By augmentation of enterocapillary exsorption, charcoal produces sink conditions that drive diffusion of the drug from capillaries into the entraluminal space, where it is subsequently eliminated. This process is called intestinal dialysis.63 Drug characteristics associated with enhanced systemic clearance via MDAC include a low intrinsic clearance, a prolonged distributive phase, low protein binding, and a small volume of distribution.64
MDAC has been shown to increase total-body clearance of multiple drugs, including carbamazepine,65 dapsone,66 phenobarbital,67 quinine,68 and theophylline.62 Despite the reported increase in drug clearance associated with the use of MDAC, improved clinical outcomes have not been definitively demonstrated. For example, Pond and coworkers described 10 comatose patients following phenobarbital overdose who were randomized to receive either single-dose activated charcoal or MDAC.69 Despite the fact that the MDAC group had a significantly shorter phenobarbital serum half-life, no difference was found between the groups with regard to the duration of intubation or hospitalization. In a rural, developing world setting, routine use of MDAC was not found to alter mortality rates.70
Contraindications
MDAC is contraindicated in patients with evidence of bowel obstruction. Ileus is a relative contraindication.71 Many ill patients in whom ileus develops may be candidates for MDAC if the airway is protected. Administration of MDAC is contraindicated in any patient who does not have an intact or protected airway. MDAC should be avoided in patients with repetitive emesis, especially when associated with decreased mental status or a decreased gag reflex. Concurrent use of cathartics with MDAC remains unproved and is not recommended.72 MDAC with cathartics should not be administered to young children because of the propensity for laxatives to cause fluid and electrolyte imbalance. For example, MDAC with sorbitol has been associated with hypernatremia and dehydration,73,74 and MDAC with magnesium cathartics has been associated with hypermagnesemia, neuromuscular weakness, and coma.75,76
Technique
Give 1 g/kg (≤100 g) for the first dose of charcoal. If a cathartic is used, administer it only with the first dose of charcoal to decrease the potential risk for cathartic-induced electrolyte abnormalities, especially in children.73–76 Follow the initial dose of charcoal with 0.5 g/kg (≤50 g) every 4 hours. Stop giving MDAC if repeated examination reveals an absence of bowel sounds or a distended abdomen. In this case, consider placing an NG tube and put it on low intermittent suction. Patients receiving MDAC may be at increased risk for emesis because of the larger total dose of activated charcoal received. The use of antiemetics may help decrease the incidence of vomiting associated with MDAC.77,78 Charcoal therapy should be continued until clinical improvement is evident and plasma drug levels have fallen to acceptable levels.
Complications
The complications encountered with single-dose activated charcoal are also encountered with MDAC. In addition, there have been reports of gastrointestinal obstruction and perforation with MDAC therapy, especially in conjunction with the ingestion of drugs that have anticholinergic properties.79–83
Cathartics
The use of cathartics is intended to decrease the absorption of substances by accelerating expulsion of the poison from the gastrointestinal tract. Cathartics are often used in conjunction with activated charcoal because of charcoal’s side effect of constipation. The mechanism of action of cathartics is such that theoretically, it would minimize the possibility of desorption of drug bound to activated charcoal. There is little evidence that a single dose of aqueous activated charcoal is significantly constipating; however, cathartics are often given for this potential problem. The majority of data suggest negligible clinical benefit from the use of cathartics.84,85
Indications
Routine administration of a cathartic in combination with activated charcoal is not endorsed by the American Academy of Clinical Toxicology or the European Association of Poison Centres and Clinical Toxicologists.86 Administration of a cathartic alone has no role in the management of a poisoned patient.
Technique
There are two types of osmotic cathartics: saccharide cathartics (sorbitol) and saline cathartics (magnesium citrate, magnesium sulfate, and sodium sulfate). The optimal dose of sorbitol or magnesium citrate remains to be determined. The recommended dose of sorbitol is approximately 1 to 2 g/kg of body weight or 1 to 2 mL/kg of 70% sorbitol in adults and 4.3 mL/kg of 35% sorbitol in children (single administration only).86 Many charcoal formulations come premixed with sorbitol, but the sorbitol content varies considerably. The recommended dose of magnesium citrate is 250 mL of a 10% solution in an adult and 4 mL/kg body weight of a 10% solution in a child. Multiple doses of cathartics should be avoided.
Complications
Administration of sorbitol has been associated with vomiting, abdominal cramps, nausea, diaphoresis, and transient hypotension.87–89 Because the sorbitol content varies between different charcoal-sorbitol combination products, pay attention to the sorbitol content in each brand to avoid excessive sorbitol administration. Be aware that multiple doses of sorbitol have been associated with volume depletion.73 Multiple doses of magnesium-containing cathartics have been linked to severe hypermagnesemia.75,76 Children are particularly susceptible to the adverse effects of cathartics, and therefore use caution or totally avoid using cathartics in children.
Whole Bowel Irrigation
WBI involves the enteral administration of an osmotically balanced polyethylene glycol electrolyte solution (PEG-ES) in a sufficient amount and rate to physically flush ingested substances through the gastrointestinal tract, thereby purging the toxin before absorption can occur.14 PEG-ES (CoLyte, GoLYTELY) is isosmotic, is not systemically absorbed, and will not cause electrolyte or fluid shifts. The data available suggest that the large volumes of this solution needed to mechanically propel pills, drug packets (such as in body packers or stuffers), or other substances through the gastrointestinal tract are safe, including use in pregnant women and young children.90–92
Clinical data on the efficacy of WBI remain limited. Ly and colleagues found that the effect of WBI on reduction of acetaminophen concentration versus time was not statistically significant.93 However, WBI did have a mechanical effect on radiopaque markers in the gastrointestinal tract, with 8 of 10 subjects’ markers congregating in the right hemicolon after WBI. WBI was shown to mobilize lead BB pellets in a child to the large bowel, where less absorption occurs and the foreign bodies could be removed by colonoscopic intervention.94 In addition, PEG-ES may play a role in the pharmacologic conversion of some toxins. For example, it has been shown that the relatively high pH of PEG-ES increases the rate of spontaneous conversion of cocaine to its inactive metabolite benzoylecgonine.95
Indications
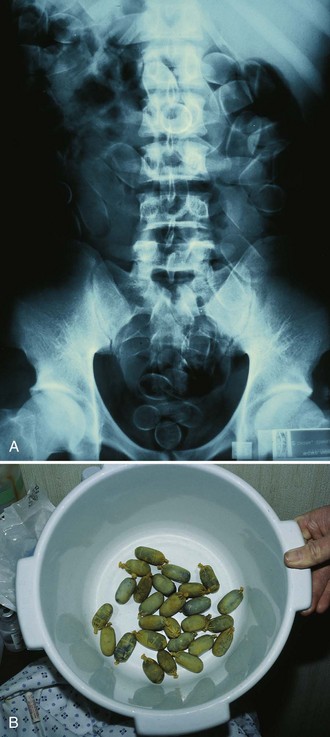
WBI may be considered for the ingestion of exceedingly large quantities of potentially toxic substances, toxins that are poorly adsorbed to activated charcoal (e.g., iron, lithium), and delayed-release formulations; late arrival at the ED after ingestion of a toxin; the presence of pharmacobezoars; and body stuffers or packers (Fig. 42-13).95–97 WBI remains a theoretical option for these ingestions and is often performed on body packers who have ingested many times the lethal amount of heroin or cocaine (Fig. 42-14). No definitive evidence exists that WBI improves the outcome of poisoned patients.98 Though not a proven procedure, WBI is often suggested by toxicologists, and its use in selected cases is intuitively reasonable and supported by the authors. The most common indication for WBI in the ED is for the treatment of toxic sustained-release medications (such as iron, calcium channel blockers, β-blockers, theophylline, and lithium) and iron tablets (Fig. 42-15).98
Contraindications
WBI is contraindicated in patients with gastrointestinal obstruction, perforation, ileus, and ingestion of a corrosive agent. It should also be avoided in patients with hemodynamic instability or an unprotected airway.98 WBI should likewise be avoided with patients who have repetitive emesis, especially when associated with decreased mental status or a decreased gag reflex. WBI should be used cautiously in debilitated patients.
Technique
PEG-ES is marketed in a powder form. Add tap water to make a total volume of 4 L. The recommended rate of administration is as follows98:
Cooperative patients with intact airway protective reflexes may drink the solution. The large volume and taste often limit even the most motivated patient’s ability to comply. If the patient is unable or unwilling to drink this solution, administer it through a small-bore NG tube after placement is confirmed. Even cooperative patients have difficulty drinking adequate fluid for effective WBI. Because it is common for WBI to be delayed while the patient and medical personnel attempt to administer the large volumes of oral WBI solution required to be effective, it is suggested that NG instillation be instituted early in the ED course (Fig. 42-16). Unconscious patients with protected airways may receive WBI via an NG tube. In one study, patients vomited shortly after beginning WBI infusion at a rate of 1.5 to 2 L/hr. Antiemetics such as ondansetron, as well as gradually advancing the infusion rate over a 60-minute period, can help ease this side effect. Prewarming the irrigant to a temperature of approximately 37°C avoids the potential complication of hypothermia. To collect the waste products, ask an awake patient to sit on a commode. In an obtunded patient, insert a rectal tube to collect the waste. Many toxicologists recommend adding two to three bottles of activated charcoal to each liter of WBI solution. The benefit is unproved, but there is little theoretical downside to this technique, and it is supported by the authors. The binding capacity of charcoal is decreased when combined with PEG-ES, but the clinical consequences of this observation are unknown and probably minimal. Empirically, metoclopramide (10 to 20 mg intravenously) may be coadministered to decrease nausea and facilitate gastrointestinal passage.
The end point of WBI is the arrival of clear rectal effluent or resolution of the toxic effect (or both).98 There are rare case reports of late purging of drug packets, plant parts, and tablets after the arrival of clear effluent.96,99 Radiographic studies may also be beneficial to determine the end point in body packers or in patients who have ingested radiopaque medications.
Complications
Few complications from WBI therapy, especially pertaining to acute poisonings, have been reported. Nausea, vomiting, abdominal cramps, bloating, and aspiration have been described.71,100 Nausea and vomiting may make administration of WBI difficult. Antiemetics and a 15- to 30-minute break followed by a slower rate may allow readministration. As discussed with the other methods of decontamination, attention should be directed to the airway and the potential for aspiration. Administration of a large amount of chilled or room-temperature WBI fluid to pediatric patients could potentially cause hypothermia. Consider warmed fluids in these patients. If activated charcoal is administered concurrently with WBI, desorption of toxin from charcoal might occur.101–103
Dermal Decontamination
Numerous hazardous material (HAZMAT) incidents occur each year in the United States. In the first 6 months of 2009, 3458 HAZMAT events occurred in 13 states as reported to the Hazardous Substances Emergency Events Surveillance System.104 HAZMAT events frequently result in injuries, and ED treatment of contaminated HAZMAT patients is not a rare event. Many of these patients, including those involved in past terrorist events, transport themselves to the ED. For example, in the Tokyo sarin gas attack, 93% of 498 patients reporting to St. Luke’s Hospital arrived by means other than ambulance.105 The risk for injury to medical personnel incurred while treating contaminated patients is significant. After the Tokyo attack, 13 of 15 clinicians (87%) reported symptoms while treating patients in the ED, and 23% of involved hospital staff complained of acute poisoning symptoms.106 Burgess and associates reported that 13% of Washington state emergency care facilities had evacuated their ED or another part of the hospital because of contamination during a 5-year period.107 Ghilarducci and coworkers surveyed level 1 trauma centers in the United States and reported that only 6% had the necessary equipment required for safe decontamination.108 Less than 36% of emergency medicine staff had received appropriate training in handling contaminated patients, and 5.6% had experienced injuries to their staff from contact with contaminated patients during a 1-year period. It is imperative that EDs have plans in place to handle patients who have been exposed to potential toxins, provide adequate decontamination facilities, and ensure the safety of the treating medical staff.109
Technique
There are a number of key components in the management of HAZMAT incidents and the care of contaminated patients seen in the ED.110 These components should include early recognition of a HAZMAT event, rapid activation of a plan to manage contaminated patients, initiation of primary triage, appropriate patient registration, patient decontamination, secondary triage, and final treatment.
First, the ED must be able to recognize that an event has occurred before contaminated patients gain entrance into the health care facility (Fig. 42-17, step 1). Communication with the local fire, police, and paramedic systems provides early detection of such events and allows preparation before patients arrive. Security should be arranged to prevent contaminated patients from entering the hospital, and “lockdown” of the facility should be considered.
Second, the ED should have the authority to activate a plan expeditiously to prepare the decontamination facility and allow appropriate preselected personnel to don personal protective equipment (PPE) (see Fig. 42-17, step 2). If necessary, the hospital disaster plan should be activated quickly at the discretion of the ED clinician who is in contact with scene operations and incoming patients. Specific data to determine the appropriate level of PPE to maintain protection of hospital workers remain limited. The minimum PPE for hospital-based decontamination (level C) consists of a splash-proof, chemical-resistant suit with tape, double-layer protective gloves, and a powered air-purifying respirator per the National Institute of Occupational Safety and Health. Higher levels of protection, such as a level A self-contained breathing apparatus (SCBA), fully encapsulated chemical-resistant suit, or level B SCBA chemical-resistant suit, are recommended with unknown chemical and biologic exposures and for entering hot zones, but these levels of protection are not readily available in EDs.111,112 Fortunately, most chemical exposures are known. For those that occur in the workplace, Material Safety Data Sheets can be obtained and either the local poison center or the Agency for Toxic Substances and Disease Registry (ATSDR) can be contacted for advice on what level of protection is appropriate.
Third, appropriate primary triage should take place (see Fig. 42-17, step 3). Contaminated patients should not enter the ED until proper decontamination has occurred to ensure that the hospital staff will not be subjected to secondary contamination. Appropriate triage should then take place, with experienced personnel performing an initial brief assessment of each patient. The triage and decontamination areas should be organized into several “zones” to prevent further contamination. The “hot” zone is the location with the highest level of contaminant or where the incident occurred. In most cases of hospital-based decontamination, there is no hot zone because patients have been removed from the initial chemical insult. On average, patients arrive at the ED 20 minutes after the event and have had significant off-gassing by this time; however, the majority of patients will have transported themselves and will not have received any prehospital decontamination by emergency medical services/HAZMAT personnel. Basic lifesaving treatments, airway and hemorrhage control, antidote administration (e.g., for cyanide or nerve agents), and decontamination occur in the “warm” zone. The “cold” zone is safe from contaminant.112
Fourth, a brief sign-in process in the warm zone should capture the patient’s name and date of birth, with full registration to occur after decontamination. Contaminated clothing and valuables should be placed in an impervious bag to avoid potential off-gassing.113,114
Fifth, decontamination should be performed (see Fig. 42-17, step 4). The hospital ED should have preexisting HAZMAT incident protocols that designate the decontamination area and the triage and decontamination team. Ideally, a hospital should have a permanent decontamination facility capable of handling a small number of chemically exposed patients and, in addition, a large portable unit for mass casualties. The decontamination area should meet several qualifications: (1) it should be secured to prevent spread to other areas of the hospital, (2) the ventilation system should be separate from the rest of the hospital or it should be shut off to prevent airborne spread of contaminants, and (3) provisions must be made to collect the rinse water from contaminated patients to prevent contamination of the facility and water supply. At most facilities the best place to begin initial treatment and evaluation is outdoors. Portable decontamination facilities are available, but their cost may be prohibitive for many institutions. A practical alternative is to have a warm shower nozzle, soap, and a wading pool available outside the entrance to the ED. A tent or screen can provide privacy.
For the ED to care for contaminated patients, protocols should be in place and be regularly rehearsed by the facility. Train staff in the procedures and protocols, establish communication between community agencies and hospitals, regularly inspect equipment, and rehearse setups. Obtain template protocols from both the peer-reviewed medical literature and the government literature if needed.115,116 For example, guidelines for managing HAZMAT incidents are available from ATSDR. In addition, prompted by the 2001 terrorist attacks, the U.S. Department of Veterans Affairs and several policy experts have developed a “comprehensive hospital-wide emergency mass casualty decontamination program.” This program has been applied at most Veterans Affairs medical centers and has been demonstrated to be a cost-effective protocol suitable for implementation at other U.S. hospitals.117
References
1. Bronstein, AC, Spyker, DA, Cantilena, LR, Jr., et al. 2009 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 27th Annual Report. Clin Toxicol (Phila). 2010;48:979–1178.
2. Heard, K. Gastrointestinal decontamination. Med Clin North Am. 2005;89:1067–1078.
3. Markenson, D, Ferguson, JD, Chameides, L, et al. Part 13: first aid: 2010 American Heart Association and American Red Cross International Consensus on First Aid Science With Treatment Recommendations. Circulation. 2010;122(16 Suppl 2):S582–S605.
4. Poison treatment in the home. American Academy of Pediatrics Committee on Injury, Violence, and Poison Prevention. Pediatrics. 2003;112:1182–1185.
5. Larkin, GL, Claassen, C. Trends in emergency department use of gastric lavage for poisoning events in the United States, 1993-2003. Clin Toxicol (Phila). 2007;45:164–168.
6. Chyka, PA, Winbery, SL. Quality improvement process in the adherence to gastric decontamination guidelines for poison exposures as recommended by a poison control center. Qual Manag Health Care. 2006;15:263–267.
7. Arnold, FJ, Jr., Hodges, JB, Jr., Barta, RA, Jr. Evaluation of the efficacy of lavage and induced emesis in treatment of salicylate poisoning. Pediatrics. 1959;23:286–301.
8. Auerbach, PS, Osterloh, J, Braun, O, et al. Efficacy of gastric emptying: gastric lavage versus emesis induced with ipecac. Ann Emerg Med. 1986;15:692–698.
9. Boxer, L, Anderson, FP, Rowe, DS. Comparison of ipecac-induced emesis and lavage in the treatment of acute salicylate ingestion. J Pediatr. 1969;74:800.
10. Burton, BT, Bayer, MJ, Barron, L, et al. Comparison of activated charcoal and gastric lavage in the prevention of aspirin absorption. J Emerg Med. 1984;1:411–416.
11. Comstock, EG, Boisaubin, EV, Comstock, BS, et al. Assessment of the efficacy of activated charcoal following gastric lavage in acute drug emergencies. J Toxicol Clin Toxicol. 1982;19:149–165.
12. Corby, DG, Lisciandro, RC, Lehman, RH, et al. The efficiency of methods used to evacuate the stomach after acute ingestions. Pediatrics. 1967;40:871–874.
13. Tandberg, D, Diven, BG, McLeod, JW. Ipecac-induced emesis versus gastric lavage: a controlled study in normal adults. Am J Emerg Med. 1986;4:205–209.
14. Tenenbein, M. Whole bowel irrigation as a gastrointestinal decontamination procedure after acute poisoning. Med Toxicol Adverse Drug Exp. 1988;3(2):77–84.
15. Underhill, TJ, Greene, MK, Dove, AF. A comparison of the efficacy of gastric lavage, ipecacuanha and activated charcoal in the emergency management of paracetamol overdose. Arch Emerg Med. 1990;7:148–154.
16. Watson, W, Leighton, J, Guy, J. Recovery of cyclic antidepressants with gastric lavage. J Emerg Med. 1989;7:373.
17. Young, WF, Jr., Bivins, HG. Evaluation of gastric emptying using radionuclides: gastric lavage versus ipecac-induced emesis. Ann Emerg Med. 1993;22:1423–1427.
18. Li, Y, Tse, ML, Gawarammana, I, et al. Systematic review of controlled clinical trials of gastric lavage in acute organophosphorus pesticide poisoning. Clin Toxicol (Phila). 2009;47:179–192.
19. Manoguerra, AS. Gastrointestinal decontamination after poisoning. Where is the science? Crit Care Clin. 1997;13:709–725.
20. Shrestha, M, George, J, Chiu, MJ, et al. A comparison of three gastric lavage methods using the radionuclide gastric emptying study. J Emerg Med. 1996;14:413–418.
21. Saetta, JP, March, S, Gaunt, ME, et al. Gastric emptying procedures in the self-poisoned patient: are we forcing gastric content beyond the pylorus? J R Soc Med. 1991;84:274–276.
22. Kulig, K, Bar-Or, D, Cantrill, SV, et al. Management of acutely poisoned patients without gastric emptying. Ann Emerg Med. 1985;14:562–567.
23. Merigian, KS, Woodard, M, Hedges, JR, et al. Prospective evaluation of gastric emptying in the self-poisoned patient. Am J Emerg Med. 1990;8:479–483.
24. Pond, SM, Lewis-Driver, DJ, Williams, GM, et al. Gastric emptying in acute overdose: a prospective randomised controlled trial. Med J Aust. 1995;163:345–349.
25. Vale, JA. Position statement: gastric lavage. American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. J Toxicol Clin Toxicol. 1997;35:711–719.
26. Eddleston, M, Haggalla, S, Reginald, K, et al. The hazards of gastric lavage for intentional self-poisoning in a resource poor location. Clin Toxicol (Phila). 2007;45:136–143.
27. Dunning, K, Plymyer, MR. Charcoal peritonitis causing chronic pelvic pain: a unique complication following bariatric surgery. Obes Surg. 2006;16:1238–1242.
28. Fane, LR, Combs, HF, Decker, WJ. Physical parameters in gastric lavage. Clin Toxicol. 1971;4:389–395.
29. Bachrach, L, Correa, A, Levin, R, et al. Iron poisoning: complications of hypertonic phosphate lavage therapy. J Pediatr. 1979;94:147–149.
30. Carter, RF, Fotheringham, FJ. Fatal salt poisoning due to gastric lavage with hypertonic saline. Med J Aust. 1971;1:539–541.
31. Peterson, CD. Electrolyte depletion following emergency stomach evacuation. Am J Hosp Pharm. 1979;36:1366–1369.
32. McDougal, CB, Maclean, MA. Modifications in the technique of gastric lavage. Ann Emerg Med. 1981;10:514–517.
33. Ritschel, WA, Erni, W. The influence of temperature of ingested fluid on stomach emptying time. Int J Clin Pharmacol Biopharm. 1977;15:172–175.
34. Askenasi, R, Abramowicz, M, Jeanmart, J, et al. Esophageal perforation: an unusual complication of gastric lavage. Ann Emerg Med. 1984;13:146.
35. Coutselinis, A, Poulos, L, Boukis, D. A lethal complication to gastric lavage leading to malpractice suit: a case report. Forensic Sci Int. 1979;13:81–86.
36. Mariani, PJ, Pook, N. Gastrointestinal tract perforation with charcoal peritoneum complicating orogastric intubation and lavage. Ann Emerg Med. 1993;22:606–609.
37. Wald, P, Stern, J, Weiner, B, et al. Esophageal tear following forceful removal of an impacted oral-gastric lavage tube. Ann Emerg Med. 1986;15:80–82.
38. Scalzo, AJ, Tominack, RL, Thompson, MW. Malposition of pediatric gastric lavage tubes demonstrated radiographically. J Emerg Med. 1992;10:581–586.
39. Justiniani, FR, Hippalgaonkar, R, Martinez, LO. Charcoal-containing empyema complicating treatment for overdose. Chest. 1985;87:404–405.
40. Thompson, AM, Robins, JB, Prescott, LF. Changes in cardiorespiratory function during gastric lavage for drug overdose. Hum Toxicol. 1987;6:215–218.
41. Chan, TY, Critchley, JA. Pulmonary aspiration following Dettol poisoning: the scope for prevention. Hum Exp Toxicol. 1996;15:843–846.
42. Hack, JB, Gilliland, MG, Meggs, WJ. Images in emergency medicine. Activated charcoal aspiration. Ann Emerg Med. 2006;48:522. [531].
43. Matthew, H, Mackintosh, TF, Thompsett, SL, et al. Gastric aspiration and lavage in acute poisoning. British Medical Journal. 1966;1:1333.
44. Thoma, ME, Glauser, JM. Use of glucagon for removal of an orogastric lavage tube. Am J Emerg Med. 1995;13:219–222.
45. Park, GD, Spector, R, Goldberg, MJ, et al. Effect of the surface area of activated charcoal on theophylline clearance. J Clin Pharmacol. 1984;24:289–292.
46. Chyka, PA, Seger, D. Position statement: single-dose activated charcoal. American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. J Toxicol Clin Toxicol. 1997;35:721–741.
47. Alaspaa, AO, Kuisma, MJ, Hoppu, K, et al. Out-of-hospital administration of activated charcoal by emergency medical services. Ann Emerg Med. 2005;45:207–222.
48. Krenzelok, EP, Lush, RM. Container residue after the administration of aqueous activated charcoal products. Am J Emerg Med. 1991;9:144–146.
49. Levy, G, Soda, DM, Lampman, TA. Inhibition by ice cream of the antidotal efficacy of activated charcoal. Am J Hosp Pharm. 1975;32:289–291.
50. Rangan, C, Nordt, SP, Hamilton, R, et al. Treatment of acetaminophen ingestion with a superactivated charcoal–cola mixture. Ann Emerg Med. 2001;37:55–58.
51. Scharman, EJ, Cloonan, HA, Durback-Morris, LF. Home administration of charcoal: can mothers administer a therapeutic dose? J Emerg Med. 2001;21:357–361.
52. Menzies, DG, Busuttil, A, Prescott, LF. Fatal pulmonary aspiration of oral activated charcoal. BMJ. 1988;297:459–460.
53. Donoso, A, Linares, M, Leon, J, et al. Activated charcoal laryngitis in an intubated patient. Pediatr Emerg Care. 2003;19:420–421.
54. Neuvonen, PJ, Olkkola, KT. Oral activated charcoal in the treatment of intoxications. Role of single and repeated doses. Med Toxicol Adverse Drug Exp. 1988;3:33–58.
55. Osterhoudt, KC, Alpern, ER, Durbin, D, et al. Activated charcoal administration in a pediatric emergency department. Pediatr Emerg Care. 2004;20:493–498.
56. Green, JP, McCauley, W. Bowel perforation after single-dose activated charcoal. CJEM. 2006;8:358–360.
57. Benson, B, VanAntwerp, M, Hergott, T. A fatality resulting from multiple dose activated charcoal therapy [abstract]. Vet Hum Toxicol. 1989;31:335.
58. Geller, RJ. Death complicating gastrointestinal decontamination—time to rethink “routine therapy” [abstract]? Vet Hum Toxicol. 1993;35:335.
59. Pollack, MM, Dunbar, BS, Holbrook, PR, et al. Aspiration of activated charcoal and gastric contents. Ann Emerg Med. 1981;10:528–529.
60. Moll, J, Kerns, W, Tomaszewski, C, et al. Incidence of aspiration pneumonia in intubated patients receiving activated charcoal. J Emerg Med. 1999;17:279–283.
61. Arnold, TC, Willis, BH, Xiao, F, et al. Aspiration of activated charcoal elicits an increase in lung microvascular permeability. J Toxicol Clin Toxicol. 1999;37:9–16.
62. Chyka, PA, Holley, JE, Mandrell, TD, et al. Correlation of drug pharmacokinetics and effectiveness of multiple-dose activated charcoal therapy. Ann Emerg Med. 1995;25:356–362.
63. Levy, G. Gastrointestinal clearance of drugs with activated charcoal. N Engl J Med. 1982;307:676–678.
64. Chyka, P. Multi-dose activated charcoal and enhancement of systemic drug clearance: summary of studies in animals and human volunteers. Clin Toxicol. 1995;33:399–405.
65. Montoya-Cabrera, MA, Sauceda-Garcia, JM, Escalante-Galindo, P, et al. Carbamazepine poisoning in adolescent suicide attempters. Effectiveness of multiple-dose activated charcoal in enhancing carbamazepine elimination. Arch Med Res. 1996;27:485–489.
66. Neuvonen, PJ, Elonen, E, Haapanen, EJ. Acute dapsone intoxication: clinical findings and effect of oral charcoal and haemodialysis on dapsone elimination. Acta Med Scand. 1983;214:215–220.
67. Veerman, M, Espejo, MG, Christopher, MA, et al. Use of activated charcoal to reduce elevated serum phenobarbital concentration in a neonate. J Toxicol Clin Toxicol. 1991;29:53–58.
68. Prescott, LF, Hamilton, AR, Heyworth, R. Treatment of quinine overdosage with repeated oral charcoal. Br J Clin Pharmacol. 1989;27:95–97.
69. Pond, SM, Olson, KR, Osterloh, JD, et al. Randomized study of the treatment of phenobarbital overdose with repeated doses of activated charcoal. JAMA. 1984;251:3104–3108.
70. Eddleston, M, Juszczak, E, Buckley, NA, et al. Multiple-dose activated charcoal in acute self-poisoning: a randomised controlled trial. Lancet. 2008;371:579–587.
71. Cumpston, KL, Aks, SE, Sigg, T, et al. Whole bowel irrigation and the hemodynamically unstable calcium channel blocker overdose: primum non nocere. J Emerg Med. 2010;38:171–174.
72. Position statement and practice guidelines on the use of multi-dose activated charcoal in the treatment of acute poisoning. American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. J Toxicol Clin Toxicol. 1999;37:731–751.
73. Allerton, J, Strom, J. Hypernatremia due to repeated doses of charcoal and sorbitol. Am J Kidney Dis. 1991;17:581.
74. McCord, M. Toxicity of sorbitol-charcoal suspension. J Pediatr. 1987;110:307.
75. Jones, J, Heiselman, D, Dougherty, J. Cathartic-induced magnesium toxicity during overdose management. Ann Emerg Med. 1986;15:1214.
76. Smilkstein, M, Steedle, D, Kulig, K, et al. Magnesium levels after magnesium containing cathartics. J Toxicol Clin Toxicol. 1988;26:51.
77. Brown, SG, Prentice, DA. Ondansetron in the treatment of theophylline overdose. Med J Aust. 1992;156:512.
78. Roberts, JR, Carney, S, Boyle, SM, et al. Ondansetron quells drug-resistant emesis in theophylline poisoning. Am J Emerg Med. 1993;11:609–610.
79. Atkinson, SW, Young, Y, Trotter, GA. Treatment with activated charcoal complicated by gastrointestinal obstruction requiring surgery. BMJ. 1992;305:563.
80. Gomez, HF, Brent, JA, Munoz, DC, et al. Charcoal stercolith with intestinal perforation in a patient treated for amitriptyline ingestion. J Emerg Med. 1994;12:57–60.
81. Goulbourne, KB, Cisek, JE. Small-bowel obstruction secondary to activated charcoal and adhesions. Ann Emerg Med. 1994;24:108–110.
82. Ray, MJ, Radin, DR, Condie, JD, et al. Charcoal bezoar. Small-bowel obstruction secondary to amitriptyline overdose therapy. Dig Dis Sci. 1988;33:106–107.
83. Watson, WA, Cremer, KF, Chapman, JA. Gastrointestinal obstruction associated with multiple-dose activated charcoal. J Emerg Med. 1986;4:401–407.
84. al-Shareef, AH, Buss, DC, Allen, EM, et al. The effects of charcoal and sorbitol (alone and in combination) on plasma theophylline concentrations after a sustained-release formulation. Hum Exp Toxicol. 1990;9:179–182.
85. Minton, NA, Henry, JA. Prevention of drug absorption in simulated theophylline overdose. J Toxicol Clin Toxicol. 1995;33:43–49.
86. Barceloux, D, McGuigan, M, Hartigan-Go, K. Position statement: cathartics. American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. J Toxicol Clin Toxicol. 1997;35:743–752.
87. Goldberg, MJ, Spector, R, Park, GD, et al. The effect of sorbitol and activated charcoal on serum theophylline concentrations after slow-release theophylline. Clin Pharmacol Ther. 1987;41:108–111.
88. Keller, RE, Schwab, RA, Krenzelok, EP. Contribution of sorbitol combined with activated charcoal in prevention of salicylate absorption. Ann Emerg Med. 1990;19:654–656.
89. Minocha, A, Herold, DA, Bruns, DE, et al. Effect of activated charcoal in 70% sorbitol in healthy individuals. J Toxicol Clin Toxicol. 1984;22:529–536.
90. Beckley, I, Ansari, NA, Khwaja, HA, et al. Clinical management of cocaine body packers: the Hillingdon experience. Can J Surg. 2009;52:417–421.
91. Tenenbein, M. Whole bowel irrigation for toxic ingestions. J Toxicol Clin Toxicol. 1985;23:177–184.
92. Van Ameyde, KJ, Tenenbein, M. Whole bowel irrigation during pregnancy. Am J Obstet Gynecol. 1989;160:646–764.
93. Ly, BT, Schneir, AB, Clark, RF. Effect of whole bowel irrigation on the pharmacokinetics of an acetaminophen formulation and progression of radiopaque markers through the gastrointestinal tract. Ann Emerg Med. 2004;43:189–195.
94. Clifton, JC, Sigg, T, Burda, AM, et al. Acute pediatric lead poisoning: combined whole bowel irrigation, succimer therapy, and endoscopic removal of ingested lead pellets. Pediatr Emerg Care. 2002;18:200–202.
95. Farmer, JW, Chan, SB. Whole body irrigation for contraband bodypackers. J Clin Gastroenterol. 2003;37:147–150.
96. Hoffman, RS, Smilkstein, MJ, Goldfrank, LR. Whole bowel irrigation and the cocaine body-packer: a new approach to a common problem. Am J Emerg Med. 1990;8:523–527.
97. Kirshenbaum, LA, Mathews, SC, Sitar, DS, et al. Whole-bowel irrigation versus activated charcoal in sorbitol for the ingestion of modified-release pharmaceuticals. Clin Pharmacol Ther. 1989;46:264–271.
98. Tenenbein, M, Cohen, S, Sitar, DS. Whole bowel irrigation as a decontamination procedure after acute drug overdose. Arch Intern Med. 1987;147:905–907.
99. Scharman, EJ, Lembersky, R, Krenzelok, EP. Efficiency of whole bowel irrigation with and without metoclopramide pretreatment. Am J Emerg Med. 1994;12:302–305.
100. Ernstoff, JJ, Howard, DA, Marshall, JB, et al. A randomized blinded clinical trial of a rapid colonic lavage solution (GoLYTELY) compared with standard preparation for colonoscopy and barium enema. Gastroenterology. 1983;84:1512–1516.
101. Hoffman, RS, Chiang, WK, Howland, MA, et al. Theophylline desorption from activated charcoal caused by whole bowel irrigation solution. J Toxicol Clin Toxicol. 1991;29:191–201.
102. Kirshenbaum, LA, Sitar, DS, Tenenbein, M. Interaction between whole-bowel irrigation solution and activated charcoal: implications for the treatment of toxic ingestions. Ann Emerg Med. 1990;19:1129–1132.
103. Makosiej, FJ, Hoffman, RS, Howland, MA, et al. An in vitro evaluation of cocaine hydrochloride adsorption by activated charcoal and desorption upon addition of polyethylene glycol electrolyte lavage solution. J Toxicol Clin Toxicol. 1993;31:381–395.
104. Hazardous Substances Emergency Events Surveillance (HSEES) system. Available at http://www.atsdr.cdc.gov/HS/HSEES/. [Accessed November 28, 2011].
105. Okumura, T, Suzuki, K, Fukuda, A, et al. The Tokyo subway sarin attack: disaster management, part 2: hospital response. Acad Emerg Med. 1998;5:618–624.
106. Nozaki, H, Hori, S, Shinozawa, Y, et al. Secondary exposure of medical staff to sarin vapor in the emergency room. Intensive Care Med. 1995;21:1032–1035.
107. Burgess, JL, Blackmon, GM, Brodkin, CA, et al. Hospital preparedness for hazardous materials incidents and treatment of contaminated patients. West J Med. 1997;167:387–391.
108. Ghilarducci, DP, Pirrallo, RG, Hegmann, KT. Hazardous materials readiness of United States level 1 trauma centers. J Occup Environ Med. 2000;42:683–692.
109. George, G, Ramsay, K, Rochester, M, et al. Facilities for chemical decontamination in accident and emergency departments in the United Kingdom. Emerg Med J. 2002;19:453–457.
110. Macintyre, AG, Christopher, GW, Eitzen, E, Jr., et al. Weapons of mass destruction events with contaminated casualties: effective planning for health care facilities. JAMA. 2000;283:242–249.
111. Georgopoulos, PG, Fedele, P, Shade, P, et al. Hospital response to chemical terrorism: personal protective equipment, training, and operations planning. Am J Ind Med. 2004;46:432–445.
112. Houston, M, Hendrickson, RG. Decontamination. Crit Care Clin. 2005;21:653–672. [v].
113. Huff, JS. Lessons learned from hazardous materials incidents. Emerg Care Q. 1991;7(3):17–22.
114. Schultz, M, Cisek, J, Wabeke, R. Simulated exposure of hospital emergency personnel to solvent vapors and respirable dust during decontamination of chemically exposed patients. Ann Emerg Med. 1995;26:324–329.
115. Burgess, JL, Kirk, M, Borron, SW, et al. Emergency department hazardous materials protocol for contaminated patients. Ann Emerg Med. 1999;34:205–212.
116. Koplan, JP, Falk, H, DeRosa, CT. Managing Hazardous Materials Incidents in Hospital Emergency Departments: A Planning Guide for the Management of Contaminated Patients. Washington, D.C.: U.S. Department of Health and Human Services, Public Health Service Agency for Toxic Substances and Disease Registry; 2000.
117. Brown, M, Beatty, J, O’Keefe, S, et al. Planning for hospital emergency mass-casualty decontamination by the US Department of Veterans Affairs. Disaster Manag Response. 2004;2(3):75–80.