Cytotoxic T Cells
Learning Objectives
• Identify the role of dendritic cells in cytotoxic T cell activation
• Identify the two signals necessary to activate cytotoxic T cells
• Identify the molecules that stabilize the interaction between cytotoxic T cells and target cells
• Identify the three different mechanisms that cytotoxic T cells use to kill target cells
• Identify the role of perforin in the lysis of target cells
• Identify the role of granulysin in the lysis of target cells
• Understand the mechanism of perforin-granzyme–induced cell death
• Identify the cellular location of CD95L (FasL) and CD95R (FasR)
• Understand the mechanism of FasL-FasR–induced cell death
• Compare and contrast the biologic functions of tumor necrosis factor alpha (TNF-α) and TNF-β
• Compare and contrast the immunologic functions of CD8 populations
• Identify the characteristics of a tumor infiltrating lymphocyte (TIL)
• Identify two factors that may inhibit the function of TILs
• Speculate on the role of CD4 cytotoxic cells in microbial and tumor immunity
• Identify the four different mechanisms that tumor cells use to evade cytotoxic T cells
• Identify the two mechanisms that influenza viruses use to evade cytotoxic T cells
• Identify the mechanisms used by latent viruses to evade the immune system
• Recognize the immunologic defect in familial erythrophagocytic lymphohistiocytosis type 2 (FHLH2)
• Identify the therapeutic agents used to treat FHLH2
• Identify the immunologic defect in autoimmune lymphoproliferative syndrome type 2 (APLS2)
• Recognize the therapeutic agents used to treat APLS2
• Identify the immunologic defect in Papillon–Lefèvre syndrome (PLS)
Key Terms
Antigen drift
Antigen shift
Cathepsin C
Cytotoxic T cells
Fas-associated protein with death domains (FADD)
Granulysin
Granzyme
Fas ligand (FasL)
Fas receptor (FasR)
Perforin
Serglycin
Tumor necrosis factor receptor–associated protein with death domains (TRADD)
Trail protein
Tumor necrosis factor-β
Tumor infiltrating lymphocyte (TIL)
Introduction
Evolutionary pressures resulted in the development of efficient cell-based methods to kill infected or abnormal cells. CD8, CD4, and natural killer (NK) cells (see Chapter 21) are the major effector cells involved in this cell-mediated response. Cytotoxic CD8 and CD4 cells are antigen specific and genetically restricted. In contrast, NK cells can kill a wide variety of target cells without the need for antigen presentation on class I proteins. Lysis of target cells follows cell-to-cell contact between effector and target cells. Other cells such as monocytes and eosinophils also have the ability to kill cells. However, killing by these cells is facilitated by the secretion of cytolytic cytokines or proteins and does not require cell-to-cell contact or antigen presentation by class I or II molecules.
CD8 Cytotoxic Cells
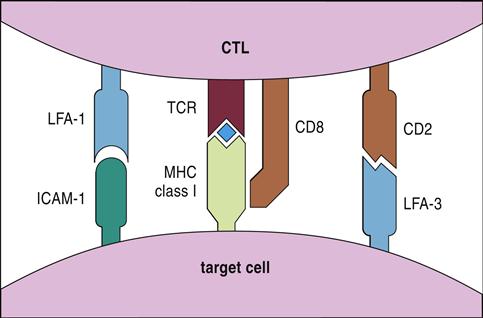
Cytotoxic T cells are activated by dendritic cells that express antigen-loaded class I molecules. Dendritic cells ingest intact cells (cross-priming) or free antigens. After processing, antigens are presented in the context of class I or class II molecules. Three signals are required for T cell activation and proliferation. The initial signal is provided by the interaction between the CD8 T cell receptor (TCR) and antigen-loaded class I molecules on dendritic cells (Figure 20-1). The second signal is provided by CD28 on T cells interacting with B7-1 on antigen-presenting cells. Proliferation of activated cytotoxic T cells requires the production of interleukin 12 (IL-12) by dendritic cells and autocrine production of IL-2.
CD8 Subpopulations
Two subpopulations of CD8 cells can be differentiated by patterns of cytokine production. For example, CD8Tc1 cells, which provide defense against tumors and viral infections, secrete IL-2, interferon gamma (IFN-γ), and TNF-β. Conversely, CD8Tc2 cells secrete IL-4, IL-5, and IL-10. Within the CD8Tc2 population are two other populations known as Tc2a and Tc2b. The biologic function of CD8Tc2a cells is ill defined. They are strongly cytotoxic and may play a role in neurologic and autoimmune diseases. CD8Tc2b cells are only weakly cytotoxic but secrete proteins that prevent intracellular viral replication. In organs that are critical to survival (e.g., brain), CD8Tc2b cells resolve viral infections without destroying the organs.
CD4 Cytotoxic Cells
One of the basic paradigms of immunology is that CD4 cells are involved in inflammatory reactions or antibody production and that CD8 cells are cytotoxic cells. This paradigm has been challenged by recent findings of cytotoxic CD4 cells in peripheral blood. Normally, this population represents less than 2% of the total CD4 population but can expand rapidly during some infections. Although the total numbers of CD4 cells is low in patients with acquired immuno deficiency syndrome (AIDS), 50% of circulating CD4 cells phenotype as cytotoxic CD4 memory cells. These cells have granules containing perforin and granzymes and toxicity is restricted to class II protein–expressing cells. Like CD8 cytotoxic cells, CD4 lysis of infected cells occurs via the perforin, FasL–FasR, and TNF pathways.
The physiologic role of CD4 cytotoxic cells in immunity is not fully delineated. It is tempting to speculate, however, that these cells are involved in the containment of viral infections of B cells and macrophages (Epstein-Barr virus [EBV]–infected B cells or human immunodeficiency virus [HIV]–infected CD4 cells).
Lysis of Target Cells
Target cells can be lysed in three ways: (1) One mechanism involves cytoplasmic granules containing perforin and granzymes. (2) A second mechanism involves the interaction between a Fas ligand (FasL) molecule expressed on cytotoxic T cells and a Fas receptor (FasR) molecule expressed on most target cells. (3) The third mechanism involves ligation of TNF-β receptors (TNFR) expressed on target cells.
Perforin–Granzyme Cytolysis
In perforin–granzyme–target cell lysis, cytotoxic T cells bind antigen-loaded class I antigens on the target cell. The initial reaction is stabilized by CD8, CD2, and leukocyte function–associated antigen 1 (LFA-1) (see Figure 20-1), which creates a small immunologic synapse between the two cells.
Following synapse formation, microtubules within the T cell align themselves so that unidirectional movement of intracellular granules occurs toward the synapse.
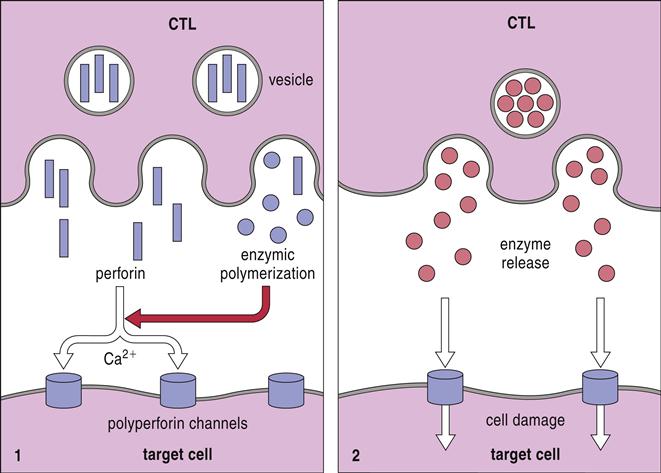
Granules containing granulysin, perforin, granzymes, and cathepsin C move along the microtubules and fuse with the target cell plasma membrane (Figure 20-2).
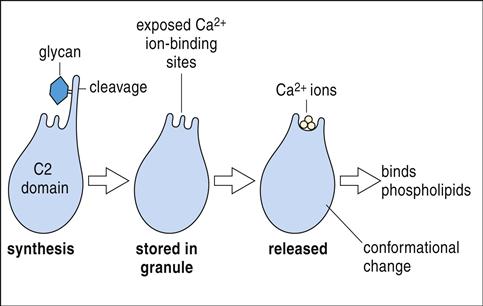
Perforin may contribute to cytolysis by two different mechanisms: (1) In one mechanism, granulysin alters membrane permeability and facilitates the insertion of perforin into target cell membranes. Perforin forms homopolymeric pores, through which granzymes are injected into the target cell. (2) In an alternative method, a protein known as serglycin assembles a complex of granulysin, perforin, and granzymes in the small gap between effector and target cells. The complex is ingested by target cells by using receptor-mediated endocytosis and is placed into a cytoplasmic endosome. Granulysin and perforin create pores in the endosomal membrane and release several granzymes into the cytoplasm. A summary of the perforin–granzyme pathway is provided in Figure 20-3.
Granzymes
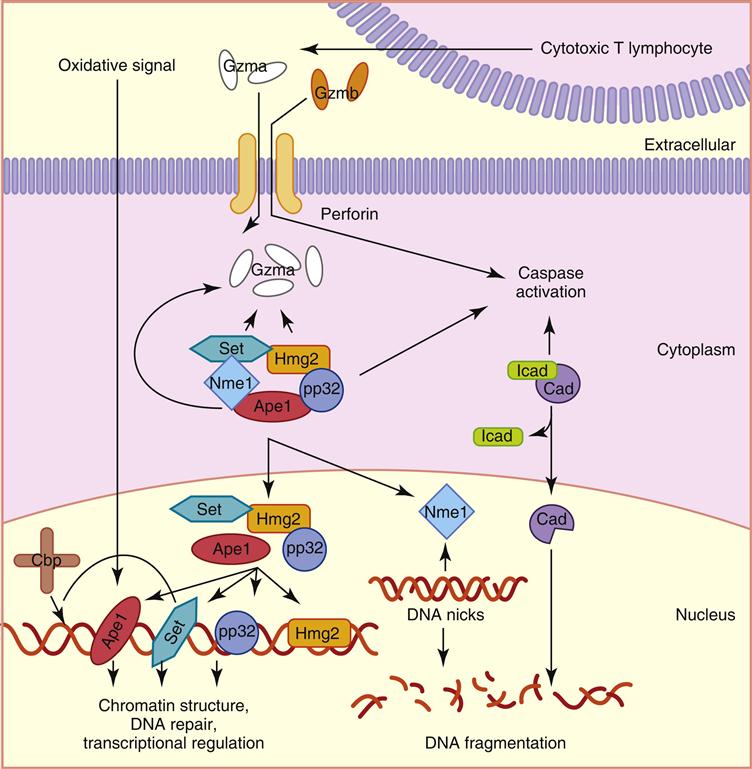
Of the five different granzymes that cause apoptosis in target cells, the most biologically important are granzymes A and B. They are synthesized as inactive precursors and activated when a small piece of protein is removed by cathepsin C. Granzyme A has several different effects on target cells. It enters the mitochondria and disrupts the electron transport chain which produces singlet oxygen that is toxic to target cells. In addition, granzyme A initiates target cell apoptosis by activating a protein called SET, which relieves the inhibition of a second protein called NME-1, an enzyme that destroys deoxyribonucleic acid (DNA) (Figure 20-4). Granzyme B has a more limited biologic function and causes apoptosis by activating a caspase-dependent cascade, which cleaves procaspases to activate caspases 10, 3, and 7.
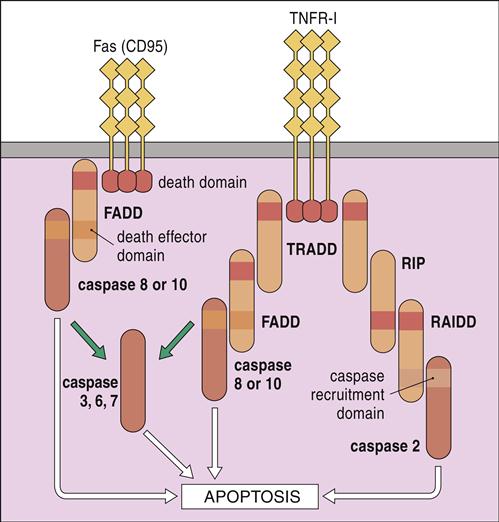
FasL–FasR Cytolysis
FasL, or CD95L, is expressed on the surface of activated CD8 cells. Ligation of a homotrimeric FasR (CD95) on target cells activates death domains, which bind to an adaptor protein called Fas-associated protein with death domains (FADD). In turn, FADD activate caspases 8 and 10. When high calcium concentrations are present, additional caspases (3, 6, and 7) activate endonucleases, which fragment DNA (Figure 20-5).
Tumor Necrosis Factor Beta–Tumor Necrosis Factor Receptor 1 Cytolysis
TNF-β belongs to the TNF superfamily that also includes TNF-α. TNF-β, or lymphotoxin, is produced by activated T and B cells. It has many of the proinflammatory properties of TNF-α, but it also is involved in biologic processes that include cell proliferation and apoptosis (see Figure 20-5). Ligation of TNF-β and the TNF receptor 1 (TNFR-1) on the target cell activates one of two apoptotic pathways: (1) In one pathway, receptor trimerization creates a binding site for the TNFR-associated proteins with death domains (TRADD). TRADD complexes with proteins known as RIP and RAIDD and activates caspase 2 of the apoptotic pathway. In the TNF-related apoptosis-inducing ligand (TRAIL) pathway, TRADD complexes with FADD and activates caspase 8 and 10 that are critical to the induction of apoptosis.
Tumor-Infiltrating Lymphocytes
Tumor-infiltrating lymphocytes (TILs) are often found in melanomas and solid tumors of the kidney, colon, and lung. TILs comprise CD8 cells and a small population of cytotoxic CD4 cells and are the result of antigen-driven recruitment and proliferation of oligoclonal T cells within the tumor. The presence of inflammatory cells and CD8 TILs in tumors is considered evidence of a vigorous anti-tumor response and is associated with a favorable clinical prognosis in some forms of cancer.
Evasion of T Killer Cells by Tumor Cells
Tumor cells have well-developed active mechanisms to evade lymphocyte killer cells. Downregulation of class I molecule expression occurs in every solid tumor, but the frequency varies with tumor types. Other tumors lack B7 co-stimulatory molecules (CD80) necessary to activate T cells. High levels of FasL (CD95L) are also expressed by NK lymphomas, large granular lymphocytic leukemia cells, melanomas, hepatocellular carcinomas, and astrocytomas. Activated T cells express both Fas and FasL. Engagement of FasL on the tumor cell and FasR on the T cell initiates the apoptotic sequence, which results in the death of the T cell and downregulation of the immune response. Some tumors also produce transforming growth factor beta (TGF-β), which inhibits IL-2–dependent proliferation of T and B cells.
Evasion of the Immune Response by Viruses
Viruses have well-developed strategies to evade the immune system and promote their replication. Influenza viruses express hemagglutinins and neuraminidase, which are recognized by the immune system. Over time, mutations result in slight changes in the protein structure and formation of new antigenic epitopes. This phenomenon is known as antigenic drift. Yearly influenza epidemics are a reflection of antigenic drift. During yearly epidemics, the immune system creates memory cells that recognize hemagglutinin and neuraminidase antigens. Because of selection and environmental pressures, influenza antigens drift over the next 12 months. In the next influenza season, the new epitopes that are distinct from previous virus strains are not recognized by the influenza memory cells. By the time the immune response is mounted (7–10 days), the virus has been transmitted to another susceptible individual.
A more problematic change in hemagglutinins is antigenic shift, which results from genetic recombination of animal and human influenza viruses. For example, the great influenza pandemic of 1918 occurred when swine and human influenza strains exchanged genetic material to create major changes in hemagglutinins and neuraminidase. Unlike normal influenza strains, which only infect the lung, the recombinant influenza infected many organs in the body. The vigorous Tc1 killer cell response caused the destruction of many organs. The pandemic strain killed 10 to 38 million people within 18 months.
Viruses use a number of different mechanisms that prevent the expression of antigen-loaded class I molecules on the cell surface. For example, proteins produced by herpes virus inhibit the function of transporter-associated antigen processing (TAP), preventing the transfer of antigenic fragments from the cytosol to the endoplasmic reticulum (ER). Adenoviruses, which infect the respiratory tract, eyes, and intestine, produce a number of different proteins that prevent the transfer of the class I molecules from the ER to the Golgi apparatus.
Immunodeficiency and T Killer Cells
Familial Erythrophagocytic Lymphohistiocytosis Type 2
Familial erythrophagocytic lymphohistiocytosis type 2 (FHLH2) is a potentially fatal disease caused by the nonmalignant proliferation of macrophages and T lymphocytes. The term denotes the fact that activated, proliferating tissue macrophages (histiocytes) ingest red blood cells, white blood cells, and platelets. Unless the patient is rescued by bone marrow transplantation, the disease is invariably fatal.
The major immunologic defect is impaired perforin and granzyme secretion from CD8 cells and NK cells. The defect is compounded by the uncontrolled release of proinflammatory (e.g., IL-1, IL-6, and TNF-α) cytokines. A positive feedback loop amplifies cytokine production, resulting in a cytokine storm that causes significant tissue damage in the liver, spleen, central nervous system, and bone marrow.
Treatment for Familial Erythrophagocytic Lymphohistiocytosis Type 2
Several forms of FHLH2 exist. The low-risk, non–life-threatening FHLH2 is usually treated with cyclosporine A, steroids, and pooled intravenous immunoglobulin (IVIG). As stated in previous chapters, IVIG downregulates the production of proinflammatory cytokines and blocks the Fc receptors on macrophages. Severe and aggressive FHLH2 caused by EBV infections requires etoposide and dexamethasone therapy for 8 weeks. Etoposide is a topoisomerase II inhibitor that arrests cell division in the S or G2 phases of cell division. Intrathecal administration of methotrexate also effectively controls neurologic involvement. Long-term remission greater than 2 years is possible with combined therapy of anti-thymocyte globulin (ATG), steroids followed by cyclosporine A, and intrathecal methotrexate.
Autoimmune Lymphoproliferative Syndrome Type 2
Autoimmune lymphoproliferative syndrome type 2 (APLS2), or Canale-Smith syndrome, is characterized aberrant lymphocyte proliferation, which results in splenomegaly and lymphadenopathy in the neck. Individuals with ALPS2 have germ-line mutations in the FasR, which prevent intracellular signaling and apoptosis. The defective FasR allows the accumulation of activated lymphocytes, which produce antibodies directed at red blood cells (autoimmune hemolytic anemia) and neutrophils (autoimmune neutropenia).
Treatment of Autoimmune Lymphoproliferative Syndrome Type 2
Prednisone therapy is the first-line therapy for autoimmune lymphoproliferative syndrome type 2 (APLS2). When combined with IVIG, prednisone is effective in controlling acute clinical episodes. Patients with refractory disease can be treated with rituximab, which is directed at CD20 on B cells.
Papillon–Lefèvre Syndrome
Papillon–Lefèvre syndrome (PFS) is a rare autosomal recessive disorder characterized by severe destruction of bone, which affects both primary or permanent teeth, and hyperkeratosis (dry scaly patches) on the palms and soles of the feet. Germ-line mutation results in loss of function in dipeptidyl peptidase I (DPPI) or cathepsin C. Without cathepsin C, granzymes cannot be activated, and infected cells or tumor cells cannot be killed.
Treatment for Papillon–Lefèvre Syndrome
PLS can be treated with acitretin, which interacts with β-retinoid and γ-retinoid receptors in the skin and prevents cell growth and keratinization. Acitretin poses a slight risk for birth defects, so women should not take the drug if they are pregnant or plan to become pregnant within 3 years. Periodontal disease is difficult to control with antibiotics alone and may require extraction of teeth.
Summary