Cytokines and Biologic Modifiers
Learning Objectives
• Differentiate between a cytokine and an interleukin (IL)
• List the cells that produce IL-1
• Identify the three biologic functions of IL-1
• Design a treatment regimen for CAPS
• List the cells that produce IL-2
• Compare and contrast IL-2 receptors on activated and naïve T cells
• Compare the roles of IL-2 in autocrine and paracrine signaling
• Recognize the biologic role of aldesleukin
• Identify the biologic role(s) of IL-11 and the clinical usefulness of oprelvekin
• Compare and contrast type I and type II interferons (IFNs) and their biologic functions
• List the cellular sources of type I and type II IFNs
• Describe the role of IFNs in viral infections
• List the commercially available natural and synthetic IFNs
• Define tumor necrosis factor (TNF)
• Compare the functions of TNF at low and high concentrations
• Discuss the role of TNF in the pathophysiology of rheumatoid arthritis (RA)
• Identify the two monoclonal antibodies used in the treatment of RA
• Recognize the biologic target of etanercept
• Differentiate between tumor-specific antigen (TSA) and tumor-associated antigen (TAA)
• Compare and contrast the structure of heat shock proteins (HSPs) from normal and malignant cells
• Compare and contrast normal and malignant carbohydrate tumor antigens
• Identify the two antigens present on germ-line tumor cells
• Discuss the advantages and disadvantages of whole-cell tumor vaccines
• Discuss the advantages and disadvantages of dendritic cell vaccines
• Identify the advantages and disadvantages of viral vector vaccines
• Explain the relationship between B cell receptors (BCRs), B cell malignancies, and idiotype vaccines
Key Terms
Colony-stimulating factor (CSF)
Cryopyrin-associated periodic syndrome (CAPS)
Cytokines
Dendritic cell cancer vaccine
Idiotypic cancer vaccine
Interferon (IFN)
Interleukin 1 (L-1)
Interleukin 2 (L-2)
Interleukin 11 (L-11)
Tumor-associated antigen (TAA)
Tumor necrosis factor (TNF)
Tumor-specific antigen (TSA)
Whole-cell cancer vaccine
Introduction
Biologic response modifiers (BRMs) are natural or synthetic substances that stimulate the immune system or restore normal numbers of immunocompetent cells in peripheral blood. BRMs include cytokines, interleukins (ILs), interferons (IFNs), and colony-stimulating factors (CSFs). In the broadest sense, human cancer vaccines are considered BRMs, since they modify the immune response to tumors.
Cytokines and Interleukins
Cytokines are a family of small-molecular-weight, soluble proteins that activate or inhibit the function of cells. Within the cytokine family are ILs, IFNs, and other factors that stimulate the differentiation of stem cells in bone marrow. ILs and IFNs facilitate cross-talk between immunocompetent cells, which results in an alteration of biologic function. Bone marrow–stimulating factors induce the differentiation of macrophages and granulocytes and their release into peripheral blood.
Interleukin 1
IL-1 is synthesized by macrophages, monocytes, dendritic cells, and keratinocytes. It comprises two different polypeptides (IL-1α and IL-1β), each having a molecular weight of 17 kilodaltons (kDa). The effects of IL-1 are wide ranging and concentration dependent. At low concentrations, IL-1 upregulates leukocyte adhesion factors, which facilitates the tethering of immunocompetent cells before their transmigration into tissue. As part of an innate immune response, IL-1 acts as an endogenous pyrogen, which raises the body temperature above 100.4o F (see Chapter 2). The temperature increase prevents microbial growth and accelerates lymphocyte division and the onset of an adaptive immune response.
Interleukin 1 and Disease
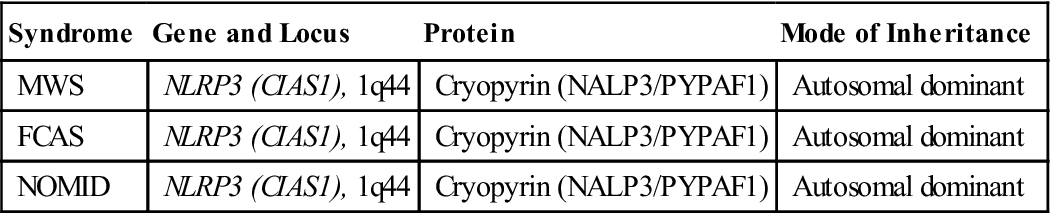
Cryopyrin-associated periodic syndrome (CAPS) comprises a group of inherited diseases that are characterized by the overproduction of IL-1β. CAPS is characterized by short, intense inflammatory reactions with rashes, fever or chills, redness of eyes, joint pain, and adolescent deafness. Included in the CAPS family are:
Individuals with CAPS have an autosomal dominant mutation in the NLRP3 gene that prevents the synthesis of cryopyrin (Table 23-1). Under normal conditions, cryopyrin interacts with apoptosis-associated Speck-like protein and caspase 1 to increase the synthesis of IL-1β (see Table 23-1).
Table 23-1
Gene Abnormalities in Cryopyrin-Associated Periodic Syndrome (CAPS)
< ?comst?>
| Syndrome | Gene and Locus | Protein | Mode of Inheritance |
| MWS | NLRP3 (CIAS1), 1q44 | Cryopyrin (NALP3/PYPAF1) | Autosomal dominant |
| FCAS | NLRP3 (CIAS1), 1q44 | Cryopyrin (NALP3/PYPAF1) | Autosomal dominant |
| NOMID | NLRP3 (CIAS1), 1q44 | Cryopyrin (NALP3/PYPAF1) | Autosomal dominant |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Modified from Hereditary periodic fever syndromes. Available at www.emedicine.medscape.com/article/952254-overview. Accessed 11/01/10.
IL-1 as a Biologic Response Modifier
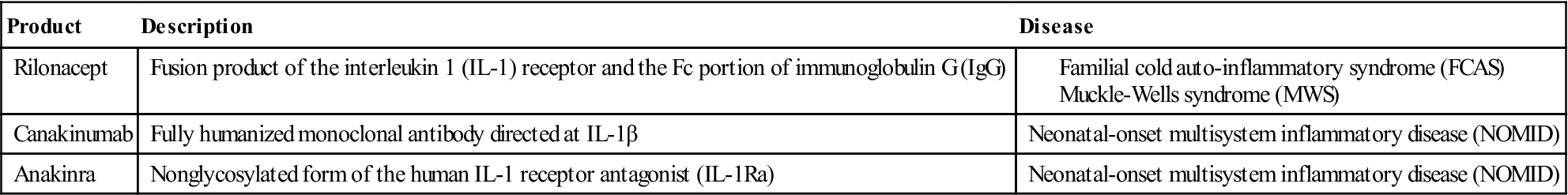
Fusion proteins, receptor antagonists, and monoclonal antibodies are used to neutralize the physiologic effects of soluble IL-1 or block the interactions with the receptor. Rilonacept is a fusion protein that contains the IL-1 receptor coupled with the Fc portion of immunoglobulin G (IgG). It binds and neutralizes soluble IL-1 before it can interact with its receptor. Canakinumab is a humanized monoclonal antibody directed at IL-1β. It blocks the effects of IL-1β and has no cross-reactivity with IL-1α or the IL-1 receptor. Anakinra is an IL-1 receptor antagonist that downregulates or blocks intracellular signaling. Many of these BRMs are used to treat patients with CAPS (Table 23-2).
Table 23-2
Treatment for Cryopyrin-Associated Periodic Syndrome (CAPS)
< ?comst?>
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Interleukin 2
Interleukin 2 (or T cell growth factor) is a glycosylated protein synthesized by activated T cells. Synthesis is transient, with peak activity occurring 4 hours after T cell activation. Autocrine IL-2 stimulation of activated T cells is necessary for the activation and proliferation of CD8+ and CD4+ cells (see Chapter 7). By increasing the synthesis of perforin and granzymes, IL-2 transforms natural killer (NK) cells into lymphokine-activated killer (LAK) cells.
IL-2 as a Biologic Response Modifier
Administration of IL-2 can augment or inhibit immune function. Blocking the interaction between IL-2 and the high-affinity IL-2 receptor prevents autocrine stimulation of T cells and the initiation of an immune response. Two commercially available monoclonal antibodies block autocrine stimulation. Daclizumab is a humanized IgG1, and basiliximab is a chimeric (murine–human) antibody. Both antibodies are directed at the α-chain of the human high-affinity IL-2 receptor. They are administered in concert with cyclosporine and corticosteroid therapy to prevent the rejection of allografts.
Recombinant IL-2 is also used to stimulate the immune system. Aldesleukin is a recombinant human IL-2 produced in Escherichia coli. It is indicated for the treatment of metastatic renal cell carcinoma in adults.
Aldesleukin has serious side effects. In some patients, it induces capillary leak syndrome (CLS), which is characterized by leakage of fluid into tissue and the loss of vascular tone. The rapid drop in blood pressure results in reduced organ perfusion, hypotension, and death.
Interleukin 11
IL-11 is one of several uncharacterized proteins that stimulate the proliferation and differentiation of platelet progenitors.
IL-11 as a Biologic Response Modifier
Oprelvekin is a recombinant IL-11 produced in E. coli. It is indicated for the prevention of severe thrombocytopenia following immunosuppressive therapy in patients with nonmyeloid malignancies.
Interferons
IFNs, a group of proteins usually produced in response to viral infections, are divided into two broad groups: type I and type II. The two subclasses of type I interferons are known as IFN-α and IFN-β. Type I IFN-α is produced by lymphocytes. Type I IFN-β is produced by fibroblasts. IFN-γ is also produced by lymphocytes and is the only known type II IFN. IFN-γ has a unique role in immune responses. It directs immune responses by recruiting Th1 cells to the inflammatory site and by downregulating the activity of Th2 cells. At the site of the inflammatory reaction, IFN-γ upregulates the expression of vascular adhesion factors that tether immunocompetent cells to the vascular endothelium before diapedesis.
Interferons and Viral Infections
Containment of viral infections is the primary biologic role of type I IFNs. Infected cells secrete IFNs, which warn adjacent cells of the infection risk. Uninfected, IFN-stimulated cells produce a unique 2’5’ oligoadenylate synthetase that activates several nucleases and kinases. Nucleases cleave viral ribonucleic acid (RNA) preventing transcription and translation of viral proteins and the synthesis of new viral RNA. Kinases also inhibit the synthesis of viral proteins by downregulating protein elongation factors (ELF-2).
Synthetic and Natural Interferons and Biologic Modifiers
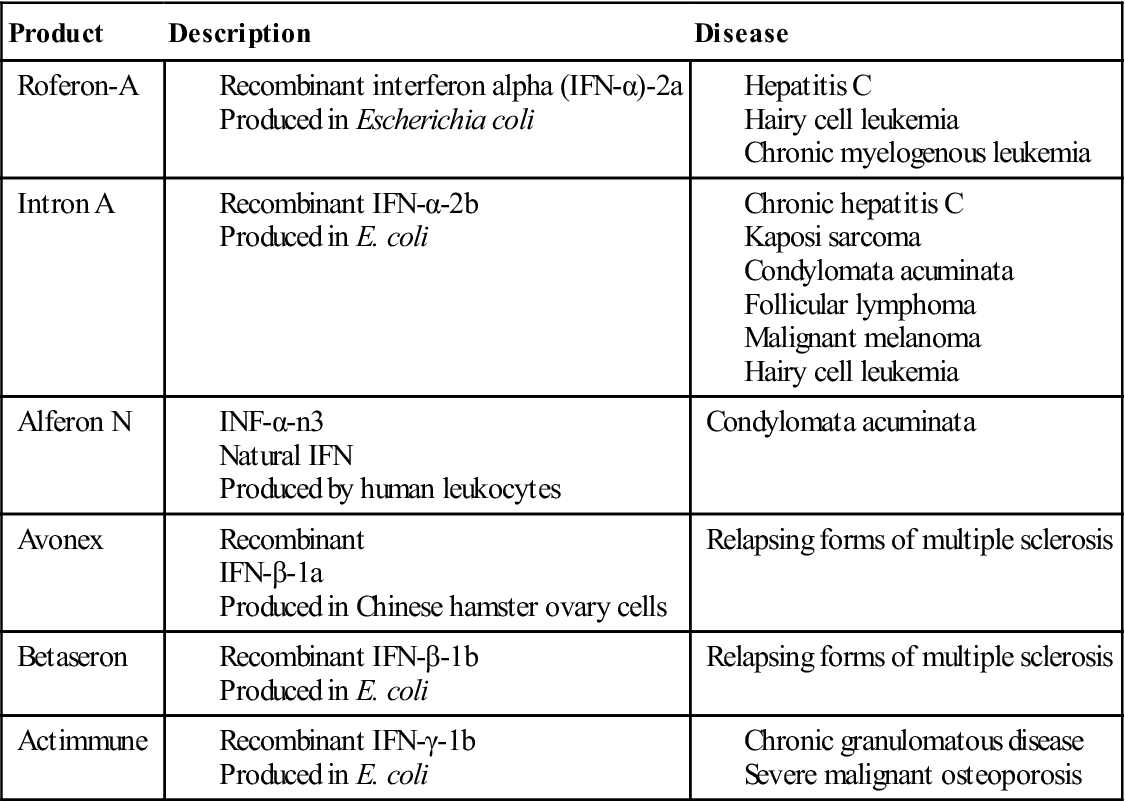
A number of synthetic and natural IFN-α, IFN-β, and IFN-γ are used in immunotherapy for viral infections, melanoma, and various lymphocyte malignancies. A list of commercially available interferons is provided in Table 23-3.
Table 23-3
Commercially Available Natural and Synthetic Interferons
< ?comst?>
| Product | Description | Disease |
| Roferon-A | ||
| Intron A | ||
| Alferon N | Condylomata acuminata | |
| Avonex | Relapsing forms of multiple sclerosis | |
| Betaseron | Relapsing forms of multiple sclerosis | |
| Actimmune |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Tumor Necrosis Factor
Tumor necrosis factor (TNF) is a 17-kDal protein produced by monocytes, macrophages, and T cells after exposure to bacterial endotoxins. The name is derived from in vitro studies, which showed that nonphysiologic TNF levels killed tumor cells. At low physiologic concentrations, TNF activates macrophages, which release endogenous pyrogens and proinflammatory prostaglandins that increase phagocytosis, chemotaxis of neutrophils, and the expression of vascular adhesion factors.
At high concentrations, TNF causes tissue injury, intravascular coagulation, shock, and circulatory collapse. TNF perpetuates coagulation by downregulating activated protein C (aPC) expressed on endothelial cells. aPC acts as an anti-coagulant by degrading clotting factors Va and VIIIa. In the absence of aPC, excessive coagulation causes disseminated intravascular coagulopathy (DIC). Microthrombi localize in the small capillaries of the hands and feet, blocking blood flow and creating gangrenous lesions.
TNF contributes to vascular collapse by activating nitric oxide (NO) synthetase, which catalyzes the formation of NO. NO decreases myocardial contractibility and relaxes smooth muscle in the vasculature, which depresses blood pressure and tissue perfusion. The clinical endpoints are hypovolemic shock and death.
Tumor Necrosis Factor and Disease
TNF plays a major role in the pathogenesis of rheumatoid arthritis (RA) (see Chapter 16). Synovial macrophages secrete high concentrations of TNF and IL-2, which interact with receptors on fibroblasts. Bone matrix degrading proteases, prostaglandin E2 (PGE2), IL-6, and IL-8 are secreted by activated fibroblasts. The ILs stimulate polymorphonuclear leukocytes (PMNs) to produce additional bone-degrading proteases and B cells to produce rheumatoid factor (RF).
Tumor Necrosis Factor as a Biologic Response Modifier
In addition to standard anti-inflammatory agents, three biologic response modifiers are often used to treat RA, psoriatic and juvenile arthritis, Crohn’s disease, ulcerative colitis, and ankylosing spondylitis. Biologic response modifiers (BRMs) neutralize the activity of TNF by preventing interactions with transmembrane receptors. Infliximab and adalimumab are monoclonal antibodies that bind to soluble TNF with high affinity (10-11M). Etanercept is a fusion protein that consists of the TNF receptor linked to the Fc portion of human IgG1.
Colony-STimulating Factors
Colony-stimulating factors (CSFs) are a diverse group of glycoproteins that induce differentiation of white blood cells in bone marrow. The CSF family includes:
• Multiple-colony-stimulating factor, or interleukin 3 (IL-3)
• Macrophage–colony-stimulating factor (M-CSF)
Multiple-Colony-Stimulating Factors or Interleukin 3
IL-3 stimulates bone marrow stem cells to differentiate into myeloid progenitor cells. It also stimulates the proliferation of mature granulocytes, monocytes, and dendritic cells. IL-3 is secreted by lymphocytes, epithelial cells, and astrocytes. The role of IL-3 in disease is unclear.
Macrophage–Colony-Stimulating Factor
M-CSF is a nonglycosylated protein that induces the mobilization and differentiation of macrophage precursors. When used in conjunction with G-CSF, it also mobilizes endothelial cell precursors and revascularizes ischemic tissue.
Granulocyte-Macrophage–Colony-Stimulating Factor
GM-CSF is secreted by a wide variety of lymphoid and nonlymphoid cells. Stimulation of stem cells induces maturation and differentiation of granulocytes (e.g., polymorphonuclear leukocytes [PMNs], eosinophils, and basophils) and monocytes.
Granulocyte–Colony-Stimulating Factor
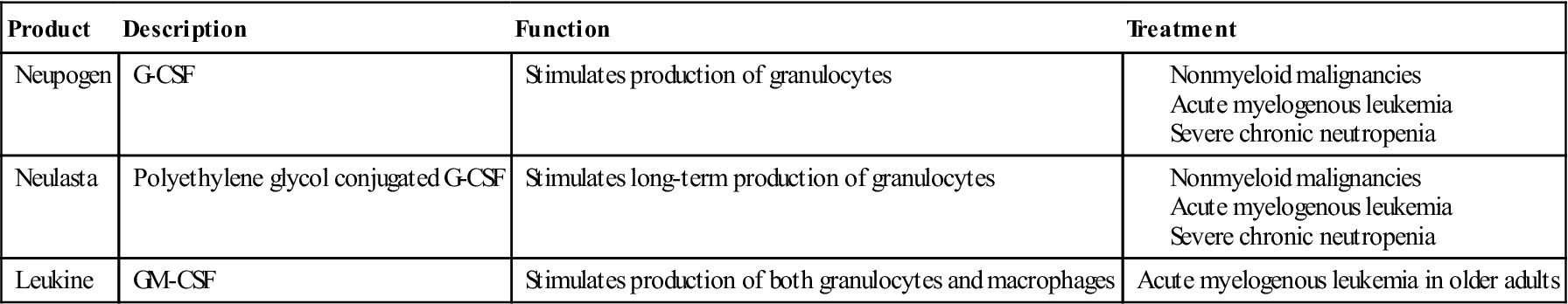
G-CSF stimulates the production of PMNs, which are necessary for the ingestion and intracellular destruction of bacteria. It is often used to increase neutrophil numbers and decrease infections in patients with nonmyeloid malignancies. It is also indicated in patients with acute myeloid leukemia, bone marrow transplants, and severe, chronic neutropenia.
Colony-Stimulating Factors as Biologic Response Modifiers
A number of commercially available CSFs are indicated for the treatment of diseases ranging from malignancies, viral infections, and autoimmune diseases. A list of commercially available CSFs is provided in Table 23-4.
Table 23-4
Commercially Available Colony-Stimulating Factors
< ?comst?>
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Tumor Antigens
Two different tumor antigens are used in the preparation of cancer vaccines. Tumor-specific transplantation antigens (TSTAs) are unique to tumor cells and are not expressed on normal cells. Tumor-associated transplantation antigens (TATAs) are expressed on both normal and cancer cells.
Tumor-Specific Transplantation Antigens
Tyrosinase
Tyrosinase catalyzes the oxidation of tyrosine to produce melanin and is considered a specific marker for melanoma. In mutant melanoma cells, a misfolded tyrosinase variant is not subject to normal controls or proteolysis. Therefore, increased production of melanin characteristic of melanoma occurs.
MAGE-A3
MAGE-A3 is a large-molecular-weight protein expressed on melanoma, breast, and glial tumors and non–small-cell lung cancers (NSCLC). Because MAGE-A3 preferentially stimulates cytotoxic (CD8+) T cells, it is an excellent candidate for inclusion in a vaccine. However, CD8 responses are elicited when antigens are presented by human leukocyte antigen (HLA)-A1, -A2, -A24, -B37, and -B44. An experimental MAGE-A3 vaccine has been shown to reduce the re-emergence of NSCLC after conventional surgery.
Carcinoembryonic Antigen
Carcinoembryonic antigen (CEA) is an 180-kDal polypeptide produced during fetal development and functions as a cellular adhesion factor during organ formation. Individuals with colorectal cancers have elevated levels of CEA in the blood. CEA is immunogenic and causes a vigorous T cell response.
Alpha Fetal Protein
Alpha fetal protein (AFP) is the major protein produced by the liver during fetal development. Levels decrease rapidly after birth, reaching steady state levels at 8 to 12 months. AFP is elevated in the serum of individuals with hepatocellular carcinoma, germ-cell tumors, and metastatic cancers.
Heat Shock Proteins
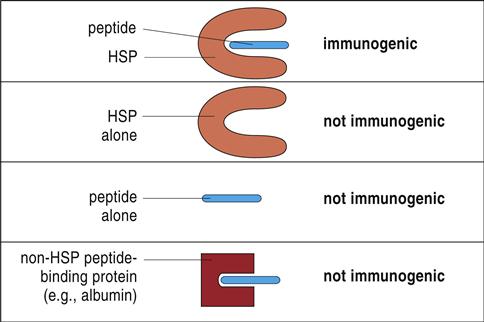
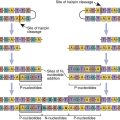
Most tumor antigens belong to a family of heat shock proteins (HSPs) present in the cytosol, endoplasmic reticulum (ER), and mitochondria. HSPs are produced in response to environmental stress (e.g., heat or cold) or cellular stress and act as molecular chaperones that assist in the proper folding of proteins or mark proteins for degradation. On the basis of molecular size, HSPs are subdivided into HSP90 (gp96), HSP70 (HSP/c70), calreticulin, and HSP170 (grp170). Tumor-specific proteins are bound in the binding cleft of the HSPs from tumor cells. Only the peptide–HSP complex is immunogenic. Neither the HSP nor the tumor protein alone stimulates the immune system (Figure 23-1).
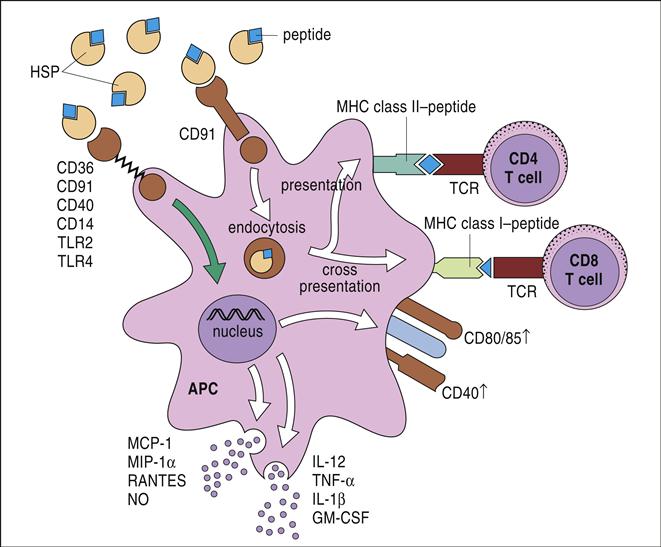
The HSP tumor protein complex binds to macrophages and dendritic cells (DCs) using a CD91 receptor. Once internalized, the peptide is processed in both the endogenous and exogenous pathways and presented to lymphocytes using class I and class II molecules (Figure 23-2). HSPs are currently being tested for efficacy in the treatment of patients with recurrent or progressive glioma.
Melanoma Antigen Recognized by T Cells
Melanoma antigen recognized by T cells (MART-1) is a 180-amino-acid protein antigen found on cells in the skin, retina, and melanocytes. This marker is overexpressed in melanoma cells and stimulates a vigorous T cell response.
NY-ESO-1
NY-ESO-1 is a 180-amino-acid protein, which is expressed in many different tumors. It is unique in that it stimulates both CD4 and CD8 cells. However, the CD4 response is genetically restricted to antigens presented by DRB1 and DRB4.
Mucins
Mucins (MUCs) are a family of large-molecular-weight glycoproteins found on epithelial cells. MUC-1 is expressed in almost all solid tumors and some leukemia, lymphoma, multiple myeloma, and breast cancer cells. Over 80% of tumor cells express an underglycosylated, truncated MUC-1 that exposes a neoantigen.
Sialyl Tn Antigen
Some mucins have increased sialylation of terminal structures and are known as sialyl Tn (sTn) antigens. The mucin core contributes to tumor cell aggregation and protects metastatic tumor cells from degradation in blood. Tn antigens are overexpressed in gastric, colorectal, ovarian, breast, and pancreatic carcinomas.
Cancer Vaccines
With the exception of the viral vaccines used to prevent cervical and liver cancers, most vaccines are designed to stop cancer tumor growth or eliminate cancer cells remaining after chemotherapy. Vaccines administered to the host can consist of tumor-specific proteins or tumor-associated proteins or carbohydrates, modified autologous or allogeneic tumor cells, antigen-pulsed dendritic cells, idiotypic antigens, viral vectors, or DNA.
Whole-Cell Vaccines
Whole-cell vaccines are prepared using irradiated autologous or allogeneic tumor cells. Autologous vaccines are prepared from a patient’s tumor cells and reinjected into the same patient. These vaccines have limited usefulness because tumor cells rarely express co-stimulatory molecules (e.g., B7-1 or B7-2) necessary to stimulate the immune system. Allogeneic vaccines are prepared from cells grown in the laboratory. Often, these vaccines contain a mixture of cells obtained from several different individuals. To increase immunogenicity, whole-cell vaccines are genetically modified to express co-stimulatory molecules or combined with an adjuvant. Adjuvants create an insoluble depot of antigens, which are slowly released over time and continually stimulate the immune system.
Idiotypic Antigen Vaccines
Idiotypic vaccines are a therapeutic target for treatment of B cell malignancies. Since the malignant cells are derived from a single B cell clone, each B cell in the malignant population has a B cell receptor (a monomeric IgM) with a homogeneous idiotope. Isolation and purification of idiotypic molecules form the basis for the vaccine. Idiotypic vaccines have several limitations. First, the B cell receptor idiotype differs with each patient, and each vaccine must be patient specific. Second, the idiotypic molecules are haptens that must be coupled with an immunogenic protein such as keyhole limpet hemocyanin (KLH) to stimulate the immune system. Idiotypic vaccines produce strong anti-tumor effects and improved clinical outcomes in patients with follicular lymphoma.
Dendritic Cell Vaccines
To prepare the vaccine, intact tumor cells or tumor-specific antigens are reacted with mature dendritic cells isolated from peripheral blood. Tumor cells are ingested and processed and presented in context with class I and class II molecules. In vitro, dendritic cells are often stimulated with GM-CSF, IL-4, and interferon gamma (IFN-γ) that induce dendritic cell maturation. Antigen-pulsed dendritic cells are re-injected into the host to stimulate an immune response. Antigen-pulsed dendritic cell vaccines are used in the treatment of glioblastoma, glioma, ductal cell carcinoma, and metastatic breast cancer.
At the present time, only one vaccine is licensed by the U.S. Food and Drug Administration (FDA) for cancer treatment. Sipuleucel-T is a vaccine for treatment of asymptomatic, hormone-independent prostate cancer. To prepare the vaccine, peripheral blood dendritic cells and monocytes are incubated with a fusion protein containing prostate-specific antigen (PSA) and GM-CSF. The prostate antigen stimulates the immune system, and the GM-CSF augments dendritic cell development and longevity. When infused into patients, the antigen-pulsed dendritic cells stimulate CD8 cells, which kill the tumor cells that express PSAs.
Viral Vector Vaccines
Viral vector vaccines are the leading edge of vaccine development. To prepare the vaccine, tumor antigen genes (transgenes) are inserted into a gene-depleted adenovirus or modified vaccinia Ankara virus that can infect, but not replicate in, human cells.
Continued transcription and translation of transgene DNA results in the production of tumor antigens. Modified adenovirus-containing CEA and GM-CSF genes are being evaluated for the treatment of CEA-expressing tumors.
DNA Vaccines
In the preparation of DNA vaccines, tumor antigen genes are inserted into a small circular, extrachromosomal DNA fragment called a plasmid. When injected into the patient, the plasmid begins the transcription and translation of tumor antigens and cytokines.
A plasmid vaccine is currently in clinical trials for the treatment of melanoma. The SCIB1 plasmid vaccine uses a novel mechanism to generate an anti-tumor response, which causes inhibition of tumor growth and regression of both primary and metastatic melanoma cells. The plasmid genes code for a human antibody molecule that expresses a tyrosinase and two helper cell epitopes. The antibody is taken up by dendritic cells and presented to T cells. Activated cells attack any melanoma cells expressing the tyrosinase antigen.
A major limitation of nucleic acid vaccines is that only a small fraction of the injected plasmids reach the nucleus. The application of an electrical pulse to the cell membrane (electroporation) increases membrane permeability, the number of plasmids entering the cell, and the strength of the immune response.
Summary