CHAPTER 54 Continuous Electroencephalography in Neurological-Neurosurgical Intensive Care
Applications and Value
Electroencephalography (EEG) provides a noninvasive means of assessing brain function dynamically. Recent advances in computer technology, networking, and data storage have made continuous EEG (cEEG) monitoring at the bedside increasingly practical, and it is now a commonly used modality for diagnosing seizures and monitoring response to treatment in many neuro-ICUs. Methods for analyzing and compressing the vast amounts of data generated by cEEG are now available and allow neurophysiologists to more efficiently review recordings from many patients monitored simultaneously and provide frequent, timely information for guiding treatment. In this chapter, we review current indications and potential uses for cEEG in the critically ill (summarized in Table 54-1) and identify future areas for research.
TABLE 54-1 Indications for Continuous Electroencephalographic Monitoring
|
1 Detection of nonconvulsive seizures and characterization of spells in patients with altered mental status and
|
Adapted with permission from Friedman D, Claassen J, Hirsch LJ. Continuous EEG monitoring in the intensive care unit. Anesth Analg. 2009;109:506.
Detection of Nonconvulsive Seizures and Status Epilepticus
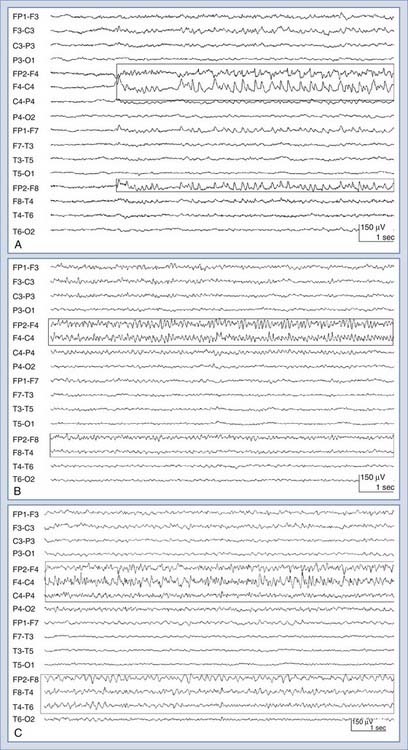
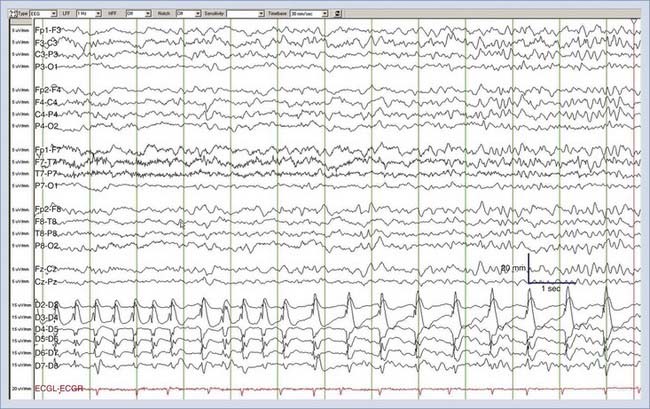
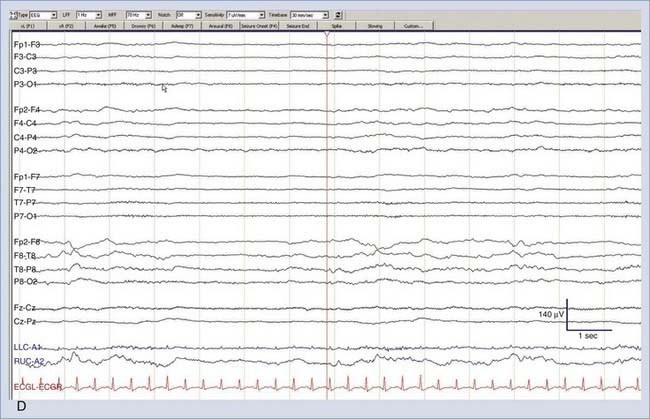
Nonconvulsive seizures (NCSzs) are electrographic seizures with little or no overt clinical manifestations (Fig. 54-1). The EEG criteria for definite NCSzs are outlined in Table 54-2. They are an increasingly commonly recognized entity in neuro-ICUs, where 8% to 34% of comatose patients may have NCSzs, depending on the clinical setting.1–11 Major studies investigating NCSzs in various patient populations are summarized in Table 54-3. Nonconvulsive status epilepticus (NCSE) occurs when NCSzs are prolonged; a commonly used definition is continuous or nearly continuous electrographic seizures of at least 30 minutes’ duration.12 Although some forms of NCSzs may occur in ambulatory patients (typically manifested as confusion and easily treated), our main focus in this chapter is on NCSzs in neuro-ICU patients, for whom the most common manifestation is a depressed level of consciousness.12 Most patients with NCSzs have purely electrographic seizures,8 but other subtle signs that have been associated with NCSzs include face and limb myoclonus, nystagmus, eye deviation, pupillary abnormalities (including hippus), and autonomic instability.12–15 However, none of these clinical findings are highly specific for NCSzs, and cEEG is usually necessary to confirm or refute the diagnosis of NCSzs. A recent study showed that findings from cEEG monitoring in 287 patients led to a change in AED prescribing in 52% of all studies, with initiation of AED therapy in 14%, modification of AED therapy in 33%, and discontinuation of AED therapy in 5% of all studies. The detection of electrographic seizures led to a change in AED therapy in 28% of all studies.15a
TABLE 54-2 Criteria* for Definite Nonconvulsive Seizure
* Satisfying these criteria is adequate for confirming nonconvulsive seizure activity. However, failing to meet these criteria does not rule out nonconvulsive seizure activity; clinical judgment and correlation are required in this situation.
Adapted from Chong DJ, Hirsch LJ: Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophysiol. 2005;22:79, as modified from Young GB, Jordan KG, Doig GS. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: an investigation of variables associated with mortality. Neurology. 1996;47:83.
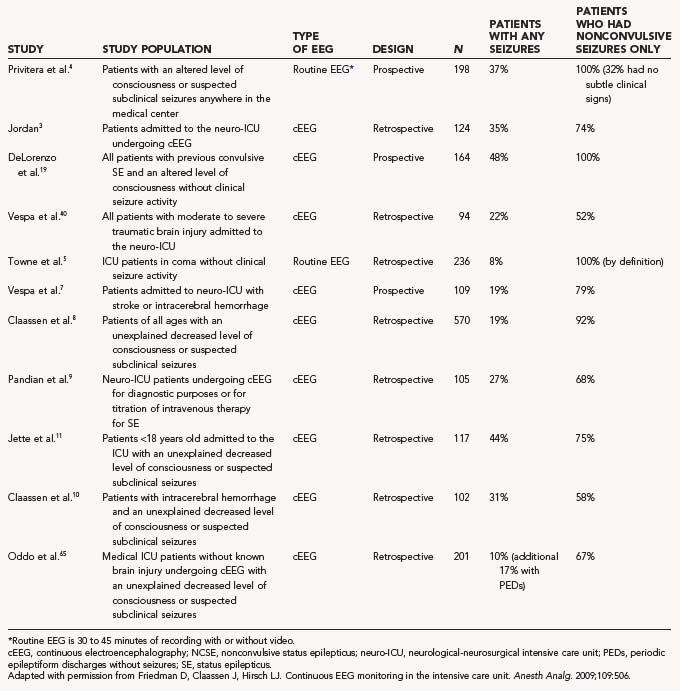
TABLE 54-3 Studies Using Continuous Electroencephalographic Monitoring in Critically Ill Patients for Detection of Nonconvulsive Seizures
The causes of NCSzs and NCSE in neuro-ICU patients may vary by age and patient group but in general are similar to the causes of convulsive seizures in these patients, including structural lesions, infections, metabolic derangements, toxins, drug withdrawal, and epilepsy, all of which are common diagnoses in critically ill patients.16 However, nonconvulsive or subtle seizures are much more common than clinically overt seizures in critically ill patients (see Table 54-3). In this section we review the incidence of NCSzs/NCSE in neuro-ICU patients by diagnostic and demographic category.
Convulsive Status Epilepticus
In many patients with convulsive status epilepticus (SE), electrographic seizures can persist even when convulsive seizures have stopped.17,18 In a prospective study, DeLorenzo and colleagues found that 48% of the patients monitored with cEEG for 24 hours after convulsive SE had stopped experienced NCSzs and 14% had NCSE.19 In most of these patients, coma was the only clinical manifestation. Patients with NCSE after convulsive SE had a twofold greater mortality than did patients whose seizures ended when convulsive activity stopped in this study, as well as in the Veterans Affairs Cooperative Study.18 Therefore, cEEG should be performed on any patient who does not quickly regain consciousness after a convulsive seizure to detect ongoing seizure activity. This includes patients who are sedated or paralyzed (or both) during the treatment of SE in whom the level of consciousness cannot be assessed adequately.
Subarachnoid Hemorrhage
Aneurysmal SAH has long been known to be associated with seizures. Studies have reported 4% to 9% rates of convulsive seizures after the initial bleeding,20–23 often in the setting of a focal clot.21,23,24 However, several more recent studies using cEEG suggest that these seizure rates underestimate the incidence of electrographic seizures after SAH, especially in comatose patients. In the Columbia series of 570 patients who underwent cEEG for altered mental status or suspicion of seizures, 19% of 108 SAH patients had seizures.8 Most of these seizures were NCSzs, and 70% of the patients with seizures had NCSE. In a study of 11 patients with SAH and NCSE, the following clinical factors were associated with NCSE: advanced age, female sex, need for ventriculostomy, poor-grade SAH (Hunt-Hess grade III, IV, or V), thick cisternal blood clots, and structural lesions (ICH and stroke).25 Because both convulsive seizures and NCSzs are associated with a poor outcome on multivariate analysis in patients with a diagnosis of SAH,20,26 it may follow that seizures occurring after SAH are likely to worsen the brain injury (see the later section “Impact of Nonconvulsive Seizures in the Critically Ill”). However, the currently available data are insufficient to confirm a causal relationship.
Intracerebral Hemorrhage
ICH is associated with a 3% to 19% rate of in-hospital convulsive seizures.10,24,27–30 In two recent studies using cEEG, 18% to 21% of patients with ICH were shown to have NCSzs.7,10 cEEG may also predict outcome after ICH. Vespa and coworkers found that NCSzs were associated with an increased midline shift and a trend toward worse outcomes after controlling for hemorrhage size.7 In a study of patients with ICH by Claassen and associates, NCSzs were associated with expansion of hemorrhage volume and a trend toward worse outcomes as well.10 In addition, periodic epileptiform discharges (PEDs) were an independent predictor of poor outcome.
Ischemic Stroke
Population- and hospital-based studies have reported rates of acute clinical seizures after ischemic stroke ranging from 2% to 9%.24,27,29,31–33 Again, more recent studies using cEEG have shown that this may be an underestimate. In the Columbia series, 11% of 56 patients with ischemic stroke undergoing cEEG had seizures; these seizures were purely nonconvulsive in 5 of these 6 patients.8 In a recent study using cEEG, 6% of 46 patients with ischemic stroke demonstrated nonconvulsive seizure activity.7 In the work of Jordan, who used cEEG in 57 consecutive patients admitted to the ICU with cerebral ischemia, 26% of the patients had EEG-defined NCSzs during the period of monitoring.3 Several studies have shown that acute clinical seizures are associated with increased mortality in patients with ischemic stroke.24,34,35 The relationship between NCSzs and outcome after stroke is currently unknown. However, in a rodent model of acute stroke, NCSzs were associated with increased infarct volume and a threefold increase in mortality.36
Traumatic Brain Injury
The incidence of convulsive seizures within the first week after TBI is 4% to 14%37–39 and increases to 15% in patients with severe TBI.37,39 Data regarding the incidence of NCSzs after TBI are relatively scant, but rates from 18% to 28% have been reported.1,6,8 In 96 consecutive patients with moderate or severe TBI undergoing cEEG, Vespa found that 22% had seizures; more than half of the patients with seizures had NCSzs only, and many of these patients had therapeutic phenytoin serum levels.40 In the Columbia series, 18% of the 51 patients with TBI monitored with cEEG had seizures, all of them had NCSzs, and 8% had NCSE.8 The exact relationship between seizures and outcome is unclear, but some studies have shown that early posttraumatic seizures are an independent risk factor for poor outcome in adults41 and children with severe TBI.42 Whether NCSzs have a similar impact has not been studied properly.
Postoperative Patients
Neurosurgical procedures, especially those involving supratentorial lesions, are associated with a 4% to 17% risk for postoperative clinical seizures.43–46 Patients with a presurgical history of epilepsy have a risk for postoperative seizures that has been reported to be as high as 34%.46 Little is known about the rate of NCSzs in these patients. In the Columbia cEEG series, 3 of 13 patients monitored after neurosurgery (excluding patients with SAH) had seizures, all NCSzs, and 1 had NCSE.8 Postoperative seizures can occur in any postoperative setting in which there is an acute neurological injury, a high risk for metabolic derangement, or neurotoxicity for any reason.
One particularly high-risk non-neurosurgical group is transplant patients. Seizures are common after pancreas, liver, lung, heart, kidney, and bone marrow transplantation47–57 and often occur in the immediate postoperative period. Patients undergoing cardiac surgery are at risk for the development of acute neurological complications, including stroke or hypoxia,58 that may predispose them to seizures in the perioperative or postoperative period.59 The incidence of NCSzs and NCSE in these patients has not been studied adequately.
Hypoxic-Ischemic Injury
In a series of comatose patients with NCSE, 42% of the patients had hypoxic/anoxic injury.5 Rates of seizures have been reported to be as high as 35% after cardiac arrest.60,61 Twenty percent of the patients with hypoxic-ischemic injury monitored in the Columbia series had seizures, most of which were NCSzs.8 Aside from being a potential contributor to decreased mental status in these patients, the presence of seizures after cardiac arrest may have important prognostic implications (see the later section “Prognostication”).62 In addition, as hypothermia becomes more widely implemented for neuroprotection after cardiac arrest, cEEG may become an important tool for identifying NCSzs, especially during rewarming.63
Toxic-Metabolic Encephalopathy
Critically ill patients are susceptible to many toxic, metabolic, and electrolytic imbalances that may cause both changes in mental status and seizures. Such conditions include but are not limited to hypoglycemia and hyperglycemia, hyponatremia, hypocalcemia, drug intoxication or withdrawal, uremia, liver dysfunction, hypertensive encephalopathy, and sepsis.16 In the Columbia series, 21% of the patients monitored with cEEG who had toxic-metabolic encephalopathy as their primary neurological diagnosis experienced NCSzs.8 In other series, 5% to 10% of patients with acute NCSzs had metabolic derangements as the probable cause of their seizures.5,64 In a recent study of 201 medical ICU patients without known brain injury who underwent cEEG, 22% had PEDs or seizures; sepsis and acute renal failure were significantly associated with electrographic seizures.65
Impact of Nonconvulsive Seizures in the Critically Ill
As evidenced by the preceding discussion, NCSzs are clearly common in critically ill patients, and this prompts the question of whether NCSzs require rapid identification and treatment and whether this would have an impact on patient outcome. There is previous evidence that delay in diagnosis and the duration of NCSE are each independent predictors of a worse outcome, including higher mortality,6,64 although mortality in patients with NCSE may be most influenced by the underlying cause.66 In addition, although NCSE may be associated with a poor prognosis in the critically ill elderly,67 aggressive treatment of NCSzs and NCSE itself may be associated with worse outcomes in this population.68 Definitive proof that NCSzs worsen outcomes is lacking, however; to date, there has not been a single prospective controlled trial to determine whether treating NCSzs or NCSE improves neurological outcomes. Therefore, much of the justification for identifying and treating NCSzs in the critically ill comes from human and animal data demonstrating that seizures can lead to neuronal injury.
There is a large body of evidence that prolonged seizures, including NCSzs, can lead to neuronal damage in several animal models. Meldrum and colleagues found that paralyzed and artificially ventilated baboons exhibited hippocampal cell loss after treatment with a convulsant.69 Despite careful control of factors such as oxygenation, temperature, and metabolic status, cell death occurred after 60 minutes of continuous electrographic seizures. Electrical- and chemoconvulsant-induced SE in rodents leads to cell loss, free radical production, inflammation, gliosis, and synaptic reorganization.70 Pathologic changes can be seen in the absence of overt convulsions and can have profound long-term effects such as impaired performance on cognitive tasks71 and the development of epilepsy.72 There is also some evidence from animal models that even single or multiple brief seizures may lead to cell death and cognitive impairment.73,74 SE in humans has likewise been associated with hippocampal cell loss in postmortem studies75 and evidence of cell injury in hospitalized patients as demonstrated by elevated levels of serum neuron-specific enolase76,77 (NSE). Although the sequelae of NCSzs and NCSE are not as well understood, evidence suggests that they can lead to neuronal damage in humans. In a study of NSE levels after seizures, DeGiorgio and associates showed that NSE levels were especially high after NCSzs and seizures of partial onset and that elevations were seen even in absence of acute brain injury.77
In addition to direct pathologic effects, seizures can place increased metabolic, excitotoxic, and oxidative stress on at-risk brain and lead to irreversible injury that may worsen the extent of the primary neurological insult. Microdialysis studies in patients with TBI have demonstrated that extracellular glutamate increases to excitotoxic levels after NCSzs.78 Significant elevations in the ratio of lactate to pyruvate (suggesting neuronal stress and potential death), glycerol (suggesting membrane breakdown),79 and intracranial pressure (ICP) are also seen.80 As mentioned earlier, NCSzs in patients with ICH were associated with increased mass effect on serial imaging7 and expansion of hematoma size.10 NCSzs have been associated with increased infarct volumes after occlusion of the middle cerebral artery in rats,36 treatment of which was shown to result in reduced infarct volumes.81 In addition, even brief seizures can lead to hemodynamic changes, such as increased cerebral blood flow (CBF), which may lead to transient and potentially injurious elevations in ICP, even in the absence of tonic-clonic activity.82,83 Hippocampal atrophy can be seen on long-term follow-up MRI ipsilateral to NCSz.84
Periodic Patterns on Continuous Electroencephalography—What Do They Mean?
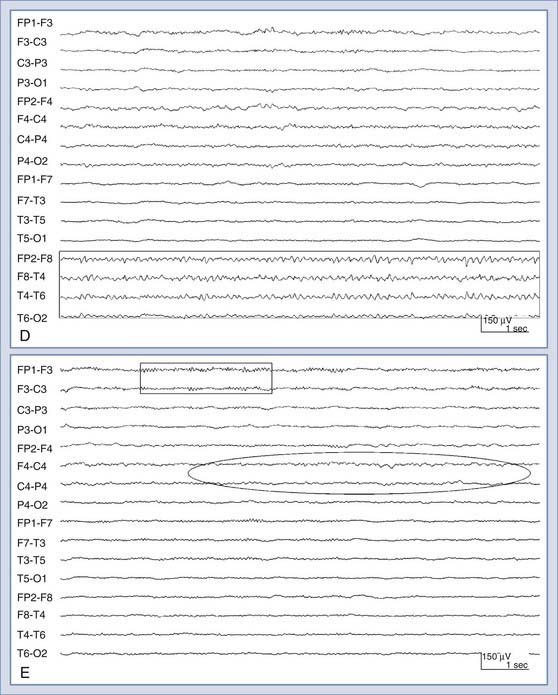
There are several periodic patterns commonly seen in critically ill patients in which the relationship to seizures is unknown.85 Although certain periodic discharges may be more closely related to systemic metabolic abnormalities, such as triphasic waves in patients with hepatic encephalopathy, others reflect injured tissue at high risk for seizures, such as periodic lateralized epileptiform discharges (PLEDs, also known as lateralized periodic discharges86). See Figure 54-2 for an example of PLEDs evolving into a focal seizure. Furthermore, periodic patterns are sometimes definitively ictal, such as when they are associated with time-locked contralateral clonic jerking. Periodic discharges are typically thought to be interictal or on an interictal-ictal continuum.85 However, the evidence that PLEDs are occasionally ictal include the following: positron emission tomography in patients with frequent PLEDs demonstrates increased regional glucose metabolism similar to that seen with focal seizures,87 single-photon emission computed tomography in patients with PLEDs demonstrates increased regional cerebral perfusion that normalizes when the PLEDs resolve,88,89 and there are reports of PLEDs causing reversible confusion that resolves with antiepileptic drugs. In one series, seven patients older than 60 years experienced recurrent confusional episodes associated with PLEDs on EEG with interdischarge intervals as long as 4 seconds.90 The clinical deficits resolved with a slowing of the EEG discharges, whether spontaneously or prompted by intravenous benzodiazepines. Carbamazepine appeared to be effective in preventing recurrences.
A common practice used to distinguish ictal from nonictal EEG patterns in the critically ill is to see whether the periodic pattern is abolished by a trial of short-acting benzodiazepines. However, almost all periodic discharges, including the periodic triphasic waves seen in patients with metabolic encephalopathy, are attenuated by benzodiazepines.91 Thus, unless clinical improvement accompanies the EEG change, the test is not helpful diagnostically. Unfortunately, improvement can take substantial time even if the activity represents NCSE and is aborted with benzodiazepines. It is important to recognize that lack of immediate clinical improvement does not exclude NCSE—it simply does not help determine its presence or absence. Our protocol for attempting to prove the presence of NCSE is shown in Table 54-4. In a recent study of 91 ICU patients with SE diagnosed on EEG, 68 nonanoxic patients were treated with new or additional antiepileptic drugs after the diagnostic EEG. Thirty-eight (56%) had some improvement in level of consciousness that seemed to be due to treatment with the antiepileptic drugs. This improvement was never immediate but was often evident on the same day. The response was similar across medical or surgical ICUs and causes, regardless of whether patients had earlier clinical seizures. Improvement was significantly more likely in stuporous or confused patients (81%) than in comatose patients, but half (48%) of the comatose patients still improved. Patients with evidence of focal seizure onset improved more often (68%) than did those with generalized (50%) or myoclonic seizures (25%) alone, but this did not reach statistical significance. Two thirds of these nonanoxic patients died. Although there is some evidence that the presence of periodic discharges is an independent risk factor for a worse prognosis in patients with ICH and SAH, it is unclear whether the presence of these discharges should change management. This is currently an area of active clinical research.
TABLE 54-4 Benzodiazepine Trial for the Diagnosis of Nonconvulsive Status Epilepticus
ECG, electrocardiography; EEG, electroencephalography.
Adapted from Jirsch J, Hirsch LJ. Nonconvulsive seizures: developing a rational approach to the diagnosis and management in the critically ill population. Clin Neurophysiol. 2007;118:1660.
Another common pattern in encephalopathic ICU patients is ictal or quasi-ictal–appearing activity triggered by stimulation or arousal. The evoked activity is typically on the interictal-ictal continuum, and we have termed it stimulus-induced rhythmic, periodic, or ictal discharges (SIRPIDs).92 There is usually no clinical correlate, as with most ICU seizures, but a small proportion of patients will have focal motor seizures consistently elicited by alerting stimuli.93 This phenomenon of seizures or highly epileptiform patterns induced by alerting is most likely a result of hyperexcitable cortex activated by the usual arousal pathways that involve the upper brainstem, thalamus, and widespread thalamocortical projections. The treatment and prognostic implications of SIRPIDs are currently unknown, but the relationship between ictal discharges and arousal raises the possibility that limiting unnecessary stimulation in patients with SIRPIDs may be beneficial.
Continuous Electroencephalographic Monitoring with Depth Electrodes
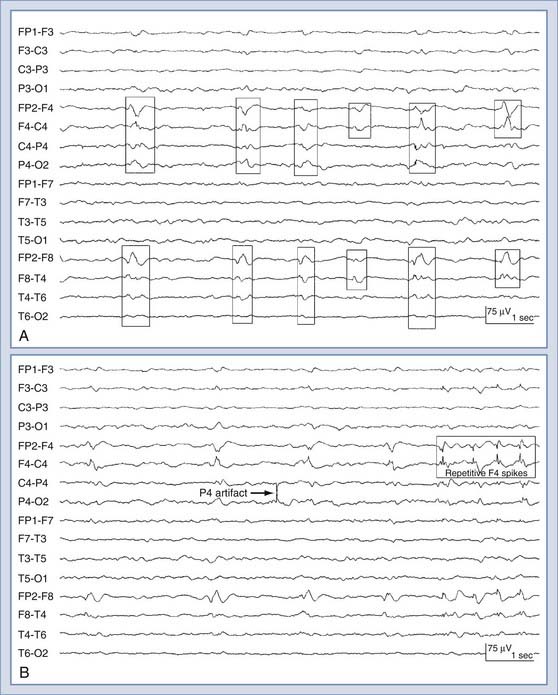
Despite all the aforementioned potential applications, the use of conventional scalp electrode–derived EEG for continuous monitoring has significant limitations in the ICU setting. Interpretation of scalp EEG can be hampered by a poor signal-to-noise ratio, suboptimal long-term contact with the scalp, artifact from the numerous electrical devices used in ICU care, and other patient-related factors (such as movement artifact from standard clinical care). These confounding factors (most notably artifact) have largely precluded the development of practical bedside scalp EEG–driven alarm systems that could provide real-time information regarding ongoing or potentially reversible neurological injury. Early experience with the use of depth electrodes suggests that many of the limitations of EEG recordings derived from scalp electrodes can be overcome. We recently described our experience with recordings obtained from a small, intracortical eight-contact depth electrode (referred to as intracortical EEG) in a cohort of 16 ICU patients with acute brain injury requiring other invasive brain monitoring.94 Depth electrode–specific EEG abnormalities were identified in 12 of 16 patients (75%), including electrographic seizures (n = 10) and PEDs without seizures (n = 2) (Fig. 54-3). The majority of seizures seen with the depth electrode were not seen with the scalp electrode: 6 of the 10 patients with electrographic seizures never had a scalp EEG recording correlate with the seizures seen intracranially; 2 showed an intermittent scalp correlate that could be considered potentially ictal, and 2 had intermittent delta rhythm only, without clear evolution and without a pattern that would traditionally be considered ictal. Significant nonepileptiform EEG changes were observed with the depth electrode only (not with the scalp electrode) in 2 patients who suffered secondary neurological complications during the monitoring period. In 1 patient with SAH and underlying vasospasm, widespread cerebral infarction develop after sepsis-associated hypoxia/hypotension, and in the other, hemorrhagic conversion of a large middle cerebral artery infarction developed. In both patients, dramatic changes in depth EEG tracings (marked attenuation or suppression-burst patterns) appeared before other devices detected any changes. These changes were not evident in simultaneous scalp EEG recordings (because of prominent muscle artifact on the scalp EEG or diminished signal amplitude). These depth EEG changes preceded the detection of concerning changes from other implanted neuromonitoring devices (by 2 to 6 hours) or changes in clinical examination (by >8 hours). These preliminary results indicate that the use of intracranial EEG can improve detection of abnormal brain electrical activity and provide early warning of secondary neurological complications. Intracortical EEG may thus facilitate the development of EEG-based alarm systems and ultimately improve outcomes in patients with neurological injury.
Multimodality Monitoring
Invasive multimodality brain monitoring is increasingly being used to monitor comatose patients with severe brain injury.95 Many different devices are available to measure and track either upstream effectors or downstream indicators of neuronal health, including neuronal activity, brain metabolism, brain tissue oxygenation, and perfusion.96 Surface and depth EEG monitoring may become an integral part of multimodality monitoring, but few studies have investigated this potential to date.78,80,94 Most investigators agree that focusing on a single parameter will not allow one to interpret changes adequately but that taking into account information from multiple devices may be helpful in detecting and defining clinical changes with reasonable sensitivity and specificity.
Cerebral Microdialysis
Cerebral microdialysis monitors are increasingly being used as part of routine care in some neuro-ICUs. Many cerebral metabolites can be measured, but those that are now most commonly evaluated in the brain include glucose, lactate, pyruvate (energy metabolites), glutamate (an excitatory and potentially toxic amino acid known to be increased in patients with cerebral ischemia and seizures), and glycerol (a marker of cell membrane damage).97–104 The principal microdialysis marker for neuronal stress is the lactate-pyruvate ratio (LPR). An LPR higher than 40 (normal, <30) is commonly seen in patients with permanent neuronal injury. Observing trends in individuals is probably more useful than interpreting absolute values.105 Electrographic posttraumatic seizures have been shown to result in sustained elevations of ICP and LPR on microdialysis. Extracellular glucose is also reduced after TBI and may be related to a poor outcome.106–108
Detection of Ischemia
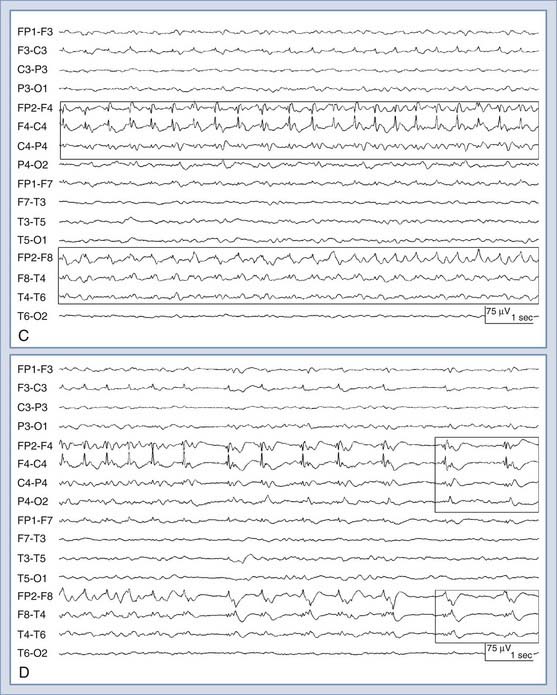
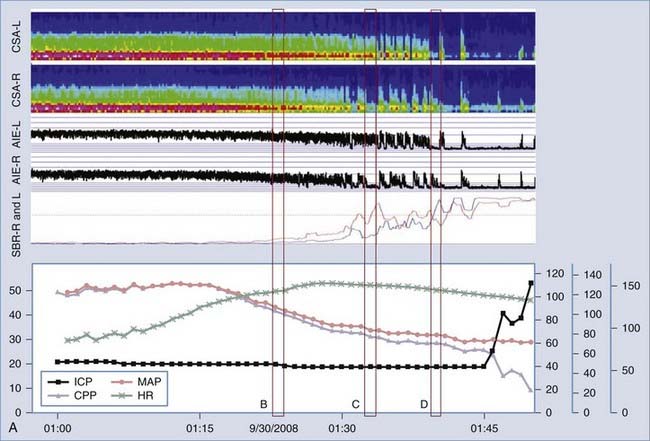
It is well-known that EEG changes occur within seconds of a reduction in CBF, and this is the basis for intraoperative EEG monitoring of patients undergoing surgeries with a high risk for cerebral ischemia, such as carotid clamping during endarterectomy.109–111 As CBF falls below 25 to 30 mL/100 g per minute, there is a progressive loss of higher frequencies and prominent slowing of background EEG activity. All EEG frequencies are suppressed when CBF falls below 8 to 10 mL/100 g per minute, a value low enough to cause irreversible cell death.112,113 Therefore, cEEG can detect a window where intervention can potentially prevent permanent brain injury. This is becoming important inasmuch as thrombolytic and endovascular therapies have been shown to be effective in treating acute stroke and vasospasm, especially if the treatment is provided very early.114,115 Recent advances in computing have allowed real-time application of quantitative EEG (qEEG) algorithms for using time-frequency data to measure changes in the background EEG rhythms and to set alarms leading to more detailed assessment for possible ischemia. See Figure 54-4 for an example.
In monitoring for cerebral ischemia, applications of qEEG include the detection of delayed cerebral ischemia (DCI) secondary to vasospasm after SAH and the detection of acute-onset cerebral ischemia. Vasospasm is common after aneurysmal SAH and causes symptomatic DCI in up to 36% of patients, which is associated with a 1.5- to 3-fold increase in mortality. Vasospasm typically occurs 3 to 12 days after the initial SAH and is diagnosed by a combination of serial clinical examinations, transcranial Doppler (TCD), and possibly CO2 reactivity and invasive tissue oxygen monitoring, all typically with angiographic confirmation as needed.116 TCD can detect abnormal cerebrovascular velocities only at one point in time (or rarely continuously for a short period), is typically performed every 24 hours, and has just moderate sensitivity and specificity for detecting symptomatic vasospasm.117–119 The clinical examination of a comatose patient is also limited. Therefore, cEEG-based tools are well suited to detect DCI secondary to vasospasm in poor-grade SAH patients before it is apparent by current screening techniques. Several studies have demonstrated the feasibility of using qEEG for the early detection of DCI. An early report by Labar and colleagues using only two recording channels found that a reduction in total spectral power correlated with DCI and preceded clinical changes in 4 of 11 patients.120 In a study of 32 patients with primarily good-grade SAH, Vespa and coworkers found that a reduction in the variability of relative alpha frequency (6 to 14 Hz, expressed as a percentage of total power between 1 and 20 Hz) was 100% sensitive and 50% specific for vasospasm as detected by TCD or angiography.121 In the majority of patients, qEEG changes preceded the diagnosis of vasospasm by more than 2 days. In a study of 34 patients with poor-grade SAH (Hunt-Hess grade IV and V) monitored from postoperative days 2 to 14, Claassen and colleagues found that a reduction in the poststimulation ratio of alpha to delta frequency power of greater than 10% relative to baseline in six consecutive epochs of cEEG was 100% sensitive and 76% specific for DCI.122 A reduction of greater than 50% in a single epoch was 89% sensitive and 84% specific. The authors examined cEEG epochs after routine stimulation of the ICU patient by caregivers and family to minimize the variability in EEG findings caused by levels of sedation or sleep-wake cycles.
Patients with acute ischemic stroke can also deteriorate after their initial event because of extension of the original infarct, a new infarct,123 or reocclusion of a recanalized vessel124 and thus may also benefit from cEEG for detection of new-onset ischemia. In a pilot study,125 the brain symmetry index (BSI), a qEEG algorithm based on the difference in the mean spectral power for 1 to 25 Hz between the left and right hemispheres, was highly correlated with National Institutes of Health (NIH) stroke scale scores in patients with anterior circulation infarcts. The BSI was also found to correlate with changes on NIH stroke scale immediately after thrombolysis126 and with outcome after stroke.127,128 Other changes in the cEEG background pattern, such as regional attenuation without delta frequency (RAWOD), are useful to identify patients with massive acute ischemic strokes and can identify them earlier than computed tomography or magnetic resonance imaging can; these patients may benefit from early surveillance and intervention for cerebral edema, and the appearance of RAWOD on cEEG may assist in prognostication.129
Monitoring the Efficacy of Therapy
cEEG is often used to evaluate the response to interventions aimed at reducing neuronal activity to slow brain metabolism. It is commonly used to guide the treatment of refractory SE with intravenous infusions of anesthetic agents such as midazolam, propofol, or pentobarbital. cEEG may be helpful in adjusting infusion rates to maintain sufficient suppression, in preventing seizures while minimizing adverse drug effects, and in guiding withdrawal of anesthetics in these patients. Pharmacologic coma has been a tool for the treatment of refractory elevated ICP in head trauma; although it can help reduce ICP, it is also associated with a high risk for systemic complications such as hypotension, hepatic dysfunction, and renal failure.130 cEEG is used to achieve the therapeutic goal of a suppression-burst pattern with the lowest dose of barbiturates possible in an attempt to limit adverse effects.131 Finally, cEEG can provide information about the level of sedation in critically ill patients, especially in the setting of neuromuscular blockade. qEEG-based tools such as the bispectral index,132 patient state index,133 and Narcotrend134 have been in use intraoperatively and postoperatively for more than a decade to monitor the depth of sedation in neurologically normal patients. Unlike cEEG, these devices cannot detect seizures or ischemia reliably, and their performance has not been tested adequately in brain-injured patients.
Prognostication
cEEG monitoring can provide prognostic information after brain injury that can guide treatment decisions for clinicians and family members alike. Prolonged monitoring can provide a continuous assessment of EEG reactivity, sleep architecture, seizures, and epileptiform discharges, all of which have been found to be factors in determining the prognosis in comatose patients.135 Burst-suppression patterns after hypoxic-ischemic injury are almost always associated with a poor outcome and a lack of neurological recovery.71 EEG patterns that are not affected by stimulation, such as alpha coma, are associated with an 88% likelihood of a persistent vegetative state or death after anoxia.72 qEEG tools, such as amplitude-integrated EEG, can also be used to predict outcome after cardiac arrest, mostly by identifying very low-amplitude tracings or burst-suppression patterns.136 It has also been shown that convulsive SE and NCSE after cardiac arrest are associated with less than a 25% chance of a good neurological outcome.60,137 However, therapeutic hypothermia was not used in these studies investigating neurological outcomes in postanoxic patients. Therapeutic hypothermia has been shown in two recent, randomized controlled trials to improve neurological outcome in patients after out-of-hospital cardiac arrest secondary to ventricular fibrillation and without subsequent circulatory shock138,139 and has therefore been recommended in this setting.140 More recently, there is evidence suggesting that this benefit extends to patients in shock and is particularly notable when the duration of cardiac arrest is short (<30 minutes).60,137,141 Thus, one cannot necessarily extrapolate findings from the older studies that did not use hypothermia to prognosticate after cardiac arrest. In fact, there are already case reports of patients with postanoxic coma who had refractory SE, myoclonic status, or generalized PEDs on EEG yet had excellent outcomes. Thus, the prognosis can no longer be presumed to be uniformly poor in these groups if therapeutic hypothermia is used; prognostic studies need to be repeated in the current era to evaluate this.62,63,136,142
EEG can provide useful prognostic information in other clinical settings. After convulsive SE, Jaitly and colleagues found that normalization of the EEG background was associated with an excellent outcome whereas patterns such as burst suppression and continued electrographic seizures were associated with mortality rates greater than 50%.143 Many of the survivors with these patterns remained dependent. Periodic discharges also increased the risk for a poor outcome, but to a lesser degree. In a study of 116 patients with poor-grade SAH, absence of sleep architecture and the presence of PLEDs were independent risk factors for a poor outcome. All patients with absent EEG reactivity, generalized PEDs, bilaterally independent PLEDs, or NCSE had poor outcomes.144 The percent alpha variability in the first 3 days after TBI has been shown to correlate with Glasgow Outcome Scale scores after 6 months, with a sensitivity of 87% and negative predictive value of 82%.145,146
Future Directions
Finally, research is needed to determine whether conventional scalp EEG recording methods are sufficient for all patients. Recent studies with subdural electrodes in patients with severe TBI found episodes of cortical spreading depression and slow and prolonged peri-injury depolarizations lasting minutes near injured brain.147 Similar types of cortical depolarizations have been observed in animal models of stroke, where they are associated with infarct enlargement because of N-methyl-D-aspartate receptor–mediated injury.148 Recently, these events have also been demonstrated in patients with large middle cerebral artery strokes149 and in patients with SAH,150 in whom they have been associated with DCI. As described earlier, preliminary data suggest that depth electrodes inserted near injured cortex in severely brain-injured patients are able to detect seizures and changes in background activity not readily apparent with scalp electrodes.94 Whether targeting these events for therapy can improve patient outcomes needs to be determined before more widespread application of these invasive recording techniques.
Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557.
Chong DJ, Hirsch LJ. Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophysiol. 2005;22:79.
Claassen J, Jette N, Chum F, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology. 2007;69:1356.
Claassen J, Mayer SA, Kowalski RG, et al. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743.
DeGiorgio CM, Heck CN, Rabinowicz AL, et al. Serum neuron-specific enolase in the major subtypes of status epilepticus. Neurology. 1999;52:746.
DeLorenzo RJ, Waterhouse EJ, Towne AR, et al. Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia. 1998;39:833.
Dohmen C, Sakowitz OW, Fabricius M, et al. Spreading depolarizations occur in human ischemic stroke with high incidence. Ann Neurol. 2008;63:720.
Fabricius M, Fuhr S, Bhatia R, et al. Cortical spreading depression and peri-infarct depolarization in acutely injured human cerebral cortex. Brain. 2006;129:778.
Fountain NB, Waldman WA. Effects of benzodiazepines on triphasic waves: implications for nonconvulsive status epilepticus. J Clin Neurophysiol. 2001;18:345.
Hirsch LJ, Claassen J, Mayer SA, et al. Stimulus-induced rhythmic, periodic, or ictal discharges (SIRPIDs): a common EEG phenomenon in the critically ill. Epilepsia. 2004;45:109.
Hirsch LJ, Pang T, Claassen J, et al. Focal motor seizures induced by alerting stimuli in critically ill patients. Epilepsia. 2008;49:968.
Husain AM, Horn GJ, Jacobson MP. Non-convulsive status epilepticus: usefulness of clinical features in selecting patients for urgent EEG. J Neurol Neurosurg Psychiatry. 2003;74:189.
Jirsch J, Hirsch LJ. Nonconvulsive seizures: developing a rational approach to the diagnosis and management in the critically ill population. Clin Neurophysiol. 2007;118:1660.
Jordan KG. Emergency EEG and continuous EEG monitoring in acute ischemic stroke. J Clin Neurophysiol. 2004;21:341.
Oddo M, Carrera E, Claassen J, et al. Continuous electroencephalography in the medical intensive care unit. Crit Care Med. 2009;37:2051.
Oddo M, Schaller MD, Feihl F, et al. From evidence to clinical practice: effective implementation of therapeutic hypothermia to improve patient outcome after cardiac arrest. Crit Care Med. 2006;34:1865.
Towne AR, Waterhouse EJ, Boggs JG, et al. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology. 2000;54:340.
Vespa P. Continuous EEG monitoring for the detection of seizures in traumatic brain injury, infarction, and intracerebral hemorrhage: “to detect and protect. ”. J Clin Neurophysiol. 2005;22;:99.
Vespa PM, Miller C, McArthur D, et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007;35:2830.
Vespa PM, Nuwer MR, Juhasz C, et al. Early detection of vasospasm after acute subarachnoid hemorrhage using continuous EEG ICU monitoring. Electroencephalogr Clin Neurophysiol. 1997;103:607.
Vespa PM, Nuwer MR, Nenov V, et al. Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J Neurosurg. 1999;91:750.
Vespa PM, O’Phelan K, Shah M, et al. Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology. 2003;60:1441.
Vespa P, Prins M, Ronne-Engstrom E, et al. Increase in extracellular glutamate caused by reduced cerebral perfusion pressure and seizures after human traumatic brain injury: a microdialysis study. J Neurosurg. 1998;89:971.
Waziri A, Arif H, Oddo M, et al. Early experience with a cortical depth electrode for ICU neurophysiological monitoring in patients with acute brain injury. Epilepsia. 2007;48(suppl 6):208.
Young GB, Jordan KG, Doig GS. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: an investigation of variables associated with mortality. Neurology. 1996;47:83.
1 Jordan K. Nonconvulsive status epilepticus in acute brain injury. J Clin Neurophysiol. 1999;16:332.
2 Narayanan JT, Murthy JM. Nonconvulsive status epilepticus in a neurological intensive care unit: profile in a developing country. Epilepsia. 2007;48:900.
3 Jordan KG. Neurophysiologic monitoring in the neuroscience intensive care unit. Neurol Clin. 1995;13:579.
4 Privitera M, Hoffman M, Moore JL, et al. EEG detection of nontonic-clonic status epilepticus in patients with altered consciousness. Epilepsy Res. 1994;18:155.
5 Towne AR, Waterhouse EJ, Boggs JG, et al. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology. 2000;54:340.
6 Vespa PM, Nuwer MR, Nenov V, et al. Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J Neurosurg. 1999;91:750.
7 Vespa PM, O’Phelan K, Shah M, et al. Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology. 2003;60:1441.
8 Claassen J, Mayer SA, Kowalski RG, et al. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743.
9 Pandian JD, Cascino GD, So EL, et al. Digital video-electroencephalographic monitoring in the neurological-neurosurgical intensive care unit: clinical features and outcome. Arch Neurol. 2004;61:1090.
10 Claassen J, Jette N, Chum F, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology. 2007;69:1356.
11 Jette N, Claassen J, Emerson RG, et al. Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol. 2006;63:1750.
12 Jirsch J, Hirsch LJ. Nonconvulsive seizures: developing a rational approach to the diagnosis and management in the critically ill population. Clin Neurophysiol. 2007;118:1660.
13 Husain AM, Horn GJ, Jacobson MP. Non-convulsive status epilepticus: usefulness of clinical features in selecting patients for urgent EEG. J Neurol Neurosurg Psychiatry. 2003;74:189.
14 Kaplan PW. Behavioral manifestations of nonconvulsive status epilepticus. Epilepsy Behav. 2002;3:122.
15 Lowenstein DH, Aminoff MJ. Clinical and EEG features of status epilepticus in comatose patients. Neurology. 1992;42:100.
15a Kilbride RD, Costello DJ, Chiappa KH. How seizure detection by continuous electroencephalographic monitoring affects the prescribing of antiepileptic medications. Arch Neurol. 2009;66:723.
16 Abou Khaled KJ, Hirsch LJ. Advances in the management of seizures and status epilepticus in critically ill patients. Crit Care Clin. 2006;22:637.
17 Bauer LA. Interference of oral phenytoin absorption by continuous nasogastric feedings. Neurology. 1982;32:570.
18 Treiman DM, Meyers PD, Walton NY, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med. 1998;339:792.
19 DeLorenzo RJ, Waterhouse EJ, Towne AR, et al. Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia. 1998;39:833.
20 Butzkueven H, Evans AH, Pitman A, et al. Onset seizures independently predict poor outcome after subarachnoid hemorrhage. Neurology. 2000;55:1315.
21 Hasan D, Schonck RS, Avezaat CJ, et al. Epileptic seizures after subarachnoid hemorrhage. Ann Neurol. 1993;33:286.
22 Rhoney DH, Tipps LB, Murry KR, et al. Anticonvulsant prophylaxis and timing of seizures after aneurysmal subarachnoid hemorrhage. Neurology. 2000;55:258.
23 Sundaram MB, Chow F. Seizures associated with spontaneous subarachnoid hemorrhage. Can J Neurol Sci. 1986;13:229.
24 Bladin CF, Alexandrov AV, Bellavance A, et al. Seizures after stroke: a prospective multicenter study. Arch Neurol. 2000;57:1617.
25 Little AS, Kerrigan JF, McDougall CG, et al. Nonconvulsive status epilepticus in patients suffering spontaneous subarachnoid hemorrhage. J Neurosurg. 2007;106:805.
26 Dennis LJ, Claassen J, Hirsch LJ, et al. Nonconvulsive status epilepticus after subarachnoid hemorrhage. Neurosurgery. 2002;51:1136.
27 Arboix A, Garcia-Eroles L, Massons JB, et al. Predictive factors of early seizures after acute cerebrovascular disease. Stroke. 1997;28:1590.
28 Faught E, Peters D, Bartolucci A, et al. Seizures after primary intracerebral hemorrhage. Neurology. 1989;39:1089.
29 Szaflarski JP, Rackley AY, Kleindorfer DO, et al. Incidence of seizures in the acute phase of stroke: a population-based study. Epilepsia. 2008;49:974.
30 Weisberg LA, Shamsnia M, Elliott D. Seizures caused by nontraumatic parenchymal brain hemorrhages. Neurology. 1991;41:1197.
31 Camilo O, Goldstein LB. Seizures and epilepsy after ischemic stroke. Stroke. 2004;35:1769.
32 Reith J, Jorgensen HS, Nakayama H, et al. Seizures in acute stroke: predictors and prognostic significance. The Copenhagen Stroke Study. Stroke. 1997;28:1585.
33 Shinton RA, Gill JS, Melnick SC, et al. The frequency, characteristics and prognosis of epileptic seizures at the onset of stroke. J Neurol Neurosurg Psychiatry. 1988;51:273.
34 Arboix A, Comes E, Massons J, et al. Relevance of early seizures for in-hospital mortality in acute cerebrovascular disease. Neurology. 1996;47:1429.
35 Vernino S, Brown RDJr, Sejvar JJ, et al. Cause-specific mortality after first cerebral infarction: a population-based study. Stroke. 2003;34:1828.
36 Hartings JA, Williams AJ, Tortella FC. Occurrence of nonconvulsive seizures, periodic epileptiform discharges, and intermittent rhythmic delta activity in rat focal ischemia. Exp Neurol. 2003;179:139.
37 Annegers JF, Grabow JD, Groover RV, et al. Seizures after head trauma: a population study. Neurology. 1980;30:683.
38 Lee ST, Lui TN, Wong CW, et al. Early seizures after moderate closed head injury. Acta Neurochir (Wien). 1995;137:151.
39 Temkin NR, Dikmen SS, Wilensky AJ, et al. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990;323:497.
40 Vespa P. Continuous EEG monitoring for the detection of seizures in traumatic brain injury, infarction, and intracerebral hemorrhage: “to detect and protect”. J Clin Neurophysiol. 2005;22:99.
41 Wang HC, Chang WN, Chang HW, et al. Factors predictive of outcome in posttraumatic seizures. J Trauma. 2008;64:883.
42 Chiaretti A, Piastra M, Pulitano S, et al. Prognostic factors and outcome of children with severe head injury: an 8-year experience. Childs Nerv Syst. 2002;18:129.
43 Baker CJ, Prestigiacomo CJ, Solomon RA. Short-term perioperative anticonvulsant prophylaxis for the surgical treatment of low-risk patients with intracranial aneurysms. Neurosurgery. 1995;37:863.
44 Foy PM, Copeland GP, Shaw MD. The incidence of postoperative seizures. Acta Neurochir (Wien). 1981;55:253.
45 Kvam DA, Loftus CM, Copeland B, et al. Seizures during the immediate postoperative period. Neurosurgery. 1983;12:14.
46 Matthew E, Sherwin AL, Welner SA, et al. Seizures following intracranial surgery: incidence in the first post-operative week. Can J Neurol Sci. 1980;7:285.
47 Adams DH, Ponsford S, Gunson B, et al. Neurological complications following liver transplantation. Lancet. 1987;1:949.
48 Kiok MC. Neurologic complications of pancreas transplants. Neurol Clin. 1988;6:367.
49 Martinez AJ, Estol C, Faris AA. Neurologic complications of liver transplantation. Neurol Clin. 1988;6:327.
50 McEnery PT, Nathan J, Bates SR, et al. Convulsions in children undergoing renal transplantation. J Pediatr. 1989;115:532.
51 Perez-Miralles F, Sanchez-Manso JC, Almenar-Bonet L, et al. Incidence of and risk factors for neurologic complications after heart transplantation. Transplant Proc. 2005;37:4067.
52 Snider S, Bashir R, Bierman P. Neurologic complications after high-dose chemotherapy and autologous bone marrow transplantation for Hodgkin’s disease. Neurology. 1994;44:681.
53 Vaughn BV, Ali II, Olivier KN, et al. Seizures in lung transplant recipients. Epilepsia. 1996;37:1175.
54 Veroux P, Veroux M, Puliatti C, et al. Tacrolimus-induced neurotoxicity in kidney transplant recipients. Transplant Proc. 2002;34:3188.
55 Wijdicks EF, Plevak DJ, Wiesner RH, et al. Causes and outcome of seizures in liver transplant recipients. Neurology. 1996;47:1523.
56 Wijdicks EF, Wiesner RH, Krom RA. Neurotoxicity in liver transplant recipients with cyclosporine immunosuppression. Neurology. 1995;45:1962.
57 Wong M, Mallory GBJr, Goldstein J, et al. Neurologic complications of pediatric lung transplantation. Neurology. 1999;53:1542.
58 Llinas R, Barbut D, Caplan LR. Neurologic complications of cardiac surgery. Prog Cardiovasc Dis. 2000;43:101.
59 Cheung AT, Weiss SJ, Kent G, et al. Intraoperative seizures in cardiac surgical patients undergoing deep hypothermic circulatory arrest monitored with EEG. Anesthesiology. 2001;94:1143.
60 Krumholz A, Stern BJ, Weiss HD. Outcome from coma after cardiopulmonary resuscitation: relation to seizures and myoclonus. Neurology. 1988;38:401.
61 Wright WL, Geocadin RG. Postresuscitative intensive care: neuroprotective strategies after cardiac arrest. Semin Neurol. 2006;26:396.
62 Rossetti AO, Logroscino G, Liaudet L, et al. Status epilepticus: an independent outcome predictor after cerebral anoxia. Neurology. 2007;69:255.
63 Hovland A, Nielsen EW, Kluver J, et al. EEG should be performed during induced hypothermia. Resuscitation. 2006;68:143.
64 Young GB, Jordan KG, Doig GS. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: an investigation of variables associated with mortality. Neurology. 1996;47:83.
65 Oddo M, Carrera E, Claassen J, et al. Continuous electroencephalography in the medical intensive care unit. Crit Care Med. 2009;37:2051.
66 Shneker BF, Fountain NB. Assessment of acute morbidity and mortality in nonconvulsive status epilepticus. Neurology. 2003;61:1066.
67 Bottaro FJ, Martinez OA, Pardal MM, et al. Nonconvulsive status epilepticus in the elderly: a case-control study. Epilepsia. 2007;48:966.
68 Litt B, Wityk RJ, Hertz SH, et al. Nonconvulsive status epilepticus in the critically ill elderly. Epilepsia. 1998;39:1194.
69 Meldrum BS, Vigouroux RA, Brierley JB. Systemic factors and epileptic brain damage. Prolonged seizures in paralyzed, artificially ventilated baboons. Arch Neurol. 1973;29:82.
70 Holmes GL. Seizure-induced neuronal injury: animal data. Neurology. 2002;59:S3.
71 Young GB. The EEG in coma. J Clin Neurophysiol. 2000;17:473.
72 Kaplan PW, Genoud D, Ho TW, et al. Etiology, neurologic correlations, and prognosis in alpha coma. Clin Neurophysiol. 1999;110:205.
73 Cavazos JE, Das I, Sutula TP. Neuronal loss induced in limbic pathways by kindling: evidence for induction of hippocampal sclerosis by repeated brief seizures. J Neurosci. 1994;14:3106.
74 Kotloski R, Lynch M, Lauersdorf S, et al. Repeated brief seizures induce progressive hippocampal neuron loss and memory deficits. Prog Brain Res. 2002;135:95.
75 DeGiorgio CM, Tomiyasu U, Gott PS, et al. Hippocampal pyramidal cell loss in human status epilepticus. Epilepsia. 1992;33:23.
76 DeGiorgio CM, Gott PS, Rabinowicz AL, et al. Neuron-specific enolase, a marker of acute neuronal injury, is increased in complex partial status epilepticus. Epilepsia. 1996;37:606.
77 DeGiorgio CM, Heck CN, Rabinowicz AL, et al. Serum neuron-specific enolase in the major subtypes of status epilepticus. Neurology. 1999;52:746.
78 Vespa P, Prins M, Ronne-Engstrom E, et al. Increase in extracellular glutamate caused by reduced cerebral perfusion pressure and seizures after human traumatic brain injury: a microdialysis study. J Neurosurg. 1998;89:971.
79 Vespa P, Martin NA, Nenov V, et al. Delayed increase in extracellular glycerol with post-traumatic electrographic epileptic activity: support for the theory that seizures induce secondary injury. Acta Neurochir Suppl. 2002;81:355.
80 Vespa PM, Miller C, McArthur D, et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007;35:2830.
81 Williams AJ, Tortella FC, Lu XM, et al. Antiepileptic drug treatment of nonconvulsive seizures induced by experimental focal brain ischemia. J Pharmacol Exp Ther. 2004;311:220.
82 Gabor AJ, Brooks AG, Scobey RP, et al. Intracranial pressure during epileptic seizures. Electroencephalogr Clin Neurophysiol. 1984;57:497.
83 Marienne JP, Robert G, Bagnat E. Post-traumatic acute rise of ICP related to subclinical epileptic seizures. Acta Neurochir Suppl. 1979;28:89.
84 Vespa P, McArthur DL, Xu Y, et al. Nonconvulsive seizures after traumatic brain injury are associated with hippocampal atrophy. Neurology. 2010;75:792-798.
85 Chong DJ, Hirsch LJ. Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophysiol. 2005;22:79.
86 Hirsch LJ, Brenner RP, Drislane FW, et al. The ACNS subcommittee on research terminology for continuous EEG monitoring: proposed standardized terminology for rhythmic and periodic EEG patterns encountered in critically ill patients. J Clin Neurophysiol. 2005;22:128.
87 Handforth A, Cheng JT, Mandelkern MA, et al. Markedly increased mesiotemporal lobe metabolism in a case with PLEDs: further evidence that PLEDs are a manifestation of partial status epilepticus. Epilepsia. 1994;35:876.
88 Assal F, Papazyan JP, Slosman DO, et al. SPECT in periodic lateralized epileptiform discharges (PLEDs): a form of partial status epilepticus? Seizure. 2001;10:260.
89 Bozkurt MF, Saygi S, Erbas B. SPECT in a patient with postictal PLEDs: is hyperperfusion evidence of electrical seizure? Clin Electroencephalogr. 2002;33:171.
90 Terzano MG, Parrino L, Mazzucchi A, et al. Confusional states with periodic lateralized epileptiform discharges (PLEDs): a peculiar epileptic syndrome in the elderly. Epilepsia. 1986;27:446.
91 Fountain NB, Waldman WA. Effects of benzodiazepines on triphasic waves: implications for nonconvulsive status epilepticus. J Clin Neurophysiol. 2001;18:345.
92 Hirsch LJ, Claassen J, Mayer SA, et al. Stimulus-induced rhythmic, periodic, or ictal discharges (SIRPIDs): a common EEG phenomenon in the critically ill. Epilepsia. 2004;45:109.
93 Hirsch LJ, Pang T, Claassen J, et al. Focal motor seizures induced by alerting stimuli in critically ill patients. Epilepsia. 2008;49:968.
94 Waziri A, Arif H, Oddo M, et al. Early experience with a cortical depth electrode for ICU neurophysiological monitoring in patients with acute brain injury. Epilepsia. 2007;48(suppl 6):208.
95 Sahuquillo J. Does multimodality monitoring make a difference in neurocritical care? Eur J Anaesthesiol Suppl. 2008;42:83-86.
96 Wartenberg KE, Schmidt JM, Mayer SA. Multimodality monitoring in neurocritical care. Crit Care Clin. 2007;23:507.
97 Persson L, Valtysson J, Enblad P, et al. Neurochemical moinitoring using intracerebral microdialysis in patients with subarachnoid hemorrhage. J Neurosurg. 1996;84:606-616.
98 Goodman JC, Valadka AB, Gopinath SP, Uzura M, et al. Extracellular lactate and glucose alterations in the brain after head injury measured by microdialysis. Crit Care Med. 1999;27:1965-1973.
99 Cesarini KG, Enblad P, Ronne-Engström E, et al. Early cerebral hyperglycolysis after subarachnoid haemorrhage correlates with favourable outcome. Acta Neurochir (Wien). 2002;144:1121-1131.
100 Kett-White R, Hutchinson PJ, al-Rawi PG, et al. Extracellular lactate/pyruvate and glutamate changes in patients during pere-operative episodes of cerebral ischaemia. Acta Neurochir Suppl. 2002;81:363-365.
101 Sarrafzadeh A, Haux D, Küchler I, et al. Poor-grade aneurysmal subarachnoid hemorrhage: relationship of cerebral metabolism to outcome. J Neurosurg. 2004;100:400-406.
102 Staub F, Graf R, Gabel P, et al. Multiple interstitial substances measured by microdialysis in patients with subarachnoid hemorrhage. Neurosurgery. 2000;47:1106-1115.
103 Helbok R, Madineni RC, Schmidt MJ, et al. Intracerebral monitoring of silent infarcts after subarachnoid hemorrhage. Neurocrit Care. 2010 Dec 2. [Epub ahead of print]
104 Helbok R, Kurtz P, Schmidt JM, et al. Effect of mannitol on brain metabolism and tissue oxygenation in severe haemorrhagic stroke. J Neurol Neurosurg Psychiatry. 2010 Sep 30. [Epub ahead of print]
105 De Georgia MA, Deogaonkar A. Multimodal monitoring in the neurological intensive care unit. Neurologist. 2005;11:45.
106 Vespa PM. Multimodality monitoring and telemonitoring in neurocritical care: from microdialysis to robotic telepresence. Curr Opin Crit Care. 2005;11:133.
107 Vespa PM, McArthur D, O’Phelan K, et al. Persistently low extracellular glucose correlates with poor outcome 6 months after human traumatic brain injury despite a lack of increased lactate: a microdialysis study. J Cereb Blood Flow Metab. 2003;23:865.
108 Vespa PM, Miller C, McArthur D, et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007;35:2830.
109 Arnold M, Sturzenegger M, Schaffler L, et al. Continuous intraoperative monitoring of middle cerebral artery blood flow velocities and electroencephalography during carotid endarterectomy. A comparison of the two methods to detect cerebral ischemia. Stroke. 1997;28:1345.
110 Sharbrough FW, Messick JMJr, Sundt TMJr. Correlation of continuous electroencephalograms with cerebral blood flow measurements during carotid endarterectomy. Stroke. 1973;4:674.
111 Zampella E, Morawetz RB, McDowell HA, et al. The importance of cerebral ischemia during carotid endarterectomy. Neurosurgery. 1991;29:727.
112 Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia—the ischemic penumbra. Stroke. 1981;12:723.
113 Jordan KG. Emergency EEG and continuous EEG monitoring in acute ischemic stroke. J Clin Neurophysiol. 2004;21:341.
114 Dorsch NW. Therapeutic approaches to vasospasm in subarachnoid hemorrhage. Curr Opin Crit Care. 2002;8:128.
115 Wardlaw JM, Zoppo G, Yamaguchi T, et al. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2003;3:CD000213.
116 Komotar RJ, Zacharia BE, Valhora R, et al. Advances in vasospasm treatment and prevention. J Neurol Sci. 2007;261:134.
117 Grosset DG, Straiton J, McDonald I, et al. Use of transcranial Doppler sonography to predict development of a delayed ischemic deficit after subarachnoid hemorrhage. J Neurosurg. 1993;78:183.
118 Lysakowski C, Walder B, Costanza MC, et al. Transcranial Doppler versus angiography in patients with vasospasm due to a ruptured cerebral aneurysm: a systematic review. Stroke. 2001;32:2292.
119 Naval NS, Thomas CE, Urrutia VC. Relative changes in flow velocities in vasospasm after subarachnoid hemorrhage: a transcranial Doppler study. Neurocrit Care. 2005;2:133.
120 Labar DR, Fisch BJ, Pedley TA, et al. Quantitative EEG monitoring for patients with subarachnoid hemorrhage. Electroencephalogr Clin Neurophysiol. 1991;78:325.
121 Vespa PM, Nuwer MR, Juhasz C, et al. Early detection of vasospasm after acute subarachnoid hemorrhage using continuous EEG ICU monitoring. Electroencephalogr Clin Neurophysiol. 1997;103:607.
122 Claassen J, Hirsch LJ, Kreiter KT, et al. Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clin Neurophysiol. 2004;115:2699.
123 Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology. 2004;62:569.
124 Grotta JC, Welch KM, Fagan SC, et al. Clinical deterioration following improvement in the NINDS rt-PA Stroke Trial. Stroke. 2001;32:661.
125 van Putten MJ, Tavy DL. Continuous quantitative EEG monitoring in hemispheric stroke patients using the brain symmetry index. Stroke. 2004;35:2489.
126 de Vos CC, van Maarseveen SM, Brouwers PJ, et al. Continuous EEG monitoring during thrombolysis in acute hemispheric stroke patients using the brain symmetry index. J Clin Neurophysiol. 2008;25:77.
127 Rose SE, Janke AL, Griffin M, et al. Improved prediction of final infarct volume using bolus delay-corrected perfusion-weighted MRI: implications for the ischemic penumbra. Stroke. 2004;35:2466.
128 Finnigan SP, Walsh M, Rose SE, et al. Quantitative EEG indices of sub-acute ischaemic stroke correlate with clinical outcomes. Clin Neurophysiol. 2007;118:2525.
129 Schneider AL, Jordan KG. Regional attenuation without delta (RAWOD): a distinctive EEG pattern that can aid in the diagnosis and management of severe acute ischemic stroke. Am J Electroneurodiagnostic Technol. 2005;45:102.
130 Schalen W, Sonesson B, Messeter K, et al. Clinical outcome and cognitive impairment in patients with severe head injuries treated with barbiturate coma. Acta Neurochir (Wien). 1992;117:153.
131 Winer JW, Rosenwasser RH, Jimenez F. Electroencephalographic activity and serum and cerebrospinal fluid pentobarbital levels in determining the therapeutic end point during barbiturate coma. Neurosurgery. 1991;29:739.
132 Simmons LE, Riker RR, Prato BS, et al. Assessing sedation during intensive care unit mechanical ventilation with the Bispectral Index and the Sedation-Agitation Scale. Crit Care Med. 1999;27:1499.
133 Prichep LS, Gugino LD, John ER, et al. The Patient State Index as an indicator of the level of hypnosis under general anaesthesia. Br J Anaesth. 2004;92:393.
134 Bauerle K, Greim CA, Schroth M, et al. Prediction of depth of sedation and anaesthesia by the Narcotrend EEG monitor. Br J Anaesth. 2004;92:841.
135 Koenig MA, Kaplan PW, Thakor NV. Clinical neurophysiologic monitoring and brain injury from cardiac arrest. Neurol Clin. 2006;24:89.
136 Rundgren M, Rosen I, Friberg H. Amplitude-integrated EEG (aEEG) predicts outcome after cardiac arrest and induced hypothermia. Intensive Care Med. 2006;32:836.
137 Simon RP, Aminoff MJ. Electrographic status epilepticus in fatal anoxic coma. Ann Neurol. 1986;20:351.
138 Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549.
139 Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557.
140 Nolan JP, Morley PT, Vanden Hoek TL, et al. Therapeutic hypothermia after cardiac arrest: an advisory statement by the Advanced Life Support Task Force of the International Liaison Committee on Resuscitation. Circulation. 2003;108:118.
141 Oddo M, Schaller MD, Feihl F, et al. From evidence to clinical practice: effective implementation of therapeutic hypothermia to improve patient outcome after cardiac arrest. Crit Care Med. 2006;34:1865.
142 Bleck TP. Prognostication and management of patients who are comatose after cardiac arrest. Neurology. 2006;67:556.
143 Jaitly R, Sgro JA, Towne AR, et al. Prognostic value of EEG monitoring after status epilepticus: a prospective adult study. J Clin Neurophysiol. 1997;14:326.
144 Claassen J, Hirsch LJ, Frontera JA, et al. Prognostic significance of continuous EEG monitoring in patients with poor-grade subarachnoid hemorrhage. Neurocrit Care. 2006;4:103.
145 Hebb MO, McArthur DL, Alger J, et al. Impaired percent alpha variability on continuous electroencephalography is associated with thalamic injury and predicts poor long-term outcome after human traumatic brain injury. J Neurotrauma. 2007;24:579.
146 Vespa PM, Boscardin WJ, Hovda DA, et al. Early and persistent impaired percent alpha variability on continuous electroencephalography monitoring as predictive of poor outcome after traumatic brain injury. J Neurosurg. 2002;97:84.
147 Fabricius M, Fuhr S, Bhatia R, et al. Cortical spreading depression and peri-infarct depolarization in acutely injured human cerebral cortex. Brain. 2006;129:778.
148 Hartings JA, Rolli ML, Lu XC, et al. Delayed secondary phase of peri-infarct depolarizations after focal cerebral ischemia: relation to infarct growth and neuroprotection. J Neurosci. 2003;23:11602.
149 Dohmen C, Sakowitz OW, Fabricius M, et al. Spreading depolarizations occur in human ischemic stroke with high incidence. Ann Neurol. 2008;63:720.
150 Dreier JP, Woitzik J, Fabricius M, et al. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129:3224.