Compartment Syndrome Evaluation
Open fractures, dislocations, and exposed joints are true orthopedic emergencies that must be managed aggressively to prevent morbidity and mortality. Even when managed appropriately, these injuries may be further complicated by compartment syndrome, a condition of increased pressure within a limited space that results in compromised tissue perfusion and, ultimately, dysfunction of the neural and muscular structures contained within that space.1 The magnitude of the trauma is usually significant, but compartment syndrome may also develop after seemingly minor injuries, prolonged proximal arterial occlusion, or prolonged external pressure in the absence of acute injury or fracture. In addition, compartment syndrome can develop without direct trauma, such as from muscular exertion, from the toxic effects of drugs, and following intramuscular hemorrhage in those with a bleeding diathesis.
Causes of compartment syndrome are categorized into those that decrease compartment volume capacity, those that increase the contents of a compartment, and those that create externally applied pressure (Box 54-1).1 Subtleties in the early signs and symptoms of compartment syndrome or other clinical priorities render some cases simply impossible to recognize and treat early enough to thwart the ultimate disability. About 10% of cases of acute compartment syndrome secondary to fractures will have muscle necrosis requiring débridement at the time of surgery. About 20% of cases of compartment syndrome in non–fracture-associated cases will have muscular necrosis at the time of surgery, thus indicating that even with diligent clinical care, it is not standard to identify all cases before injury to muscles occurs,2 particularly in uncooperative, unconscious, or critically injured patients who are unable to report symptoms. Unfortunately, the vagaries of the clinical scenario that result in failure to recognize the early signs and symptoms of compartment syndrome may have severe and irreversible limb- or life-threatening consequences.
Numerous drugs and toxins have been reported to cause rhabdomyolysis, possibly because of a direct effect or secondary to agitation and exertion, with the theoretical potential for the development of compartment syndrome (Fig. 54-1). This list is exhaustive but includes heroin, various hydrocarbons, cocaine, amphetamines, antidepressants, antipsychotics, salicylates, propoxyphene, nonsteroidal antiinflammatory drugs, succinylcholine, human immunodeficiency virus medications, antimetabolites and cancer drugs, antimalarials, diphenhydramine, baclofen, ecstasy, ethanol, anticoagulants and thrombolytics, strychnine, statins, and phenothiazines.3
Background
Though recognized as a clinical syndrome in the mid-19th century, the pathophysiology of ischemia in extremities was not fully described until more than a century later. Postischemic myoneural dysfunction and its associated contractures were first described in the 1870s by the German surgeon Richard von Volkmann, who recognized the effects of increased pressure causing vascular compromise of the limb.4
Various needles and equipment have been developed to measure compartment pressure (Fig. 54-2).5–11 The wick catheter, originally developed to measure subcutaneous and brain tissue pressure, was modified during the mid-1970s to provide continuous compartment pressure measurements. This catheter is rarely used today because of the fear of catheter breakdown leading to errors in measurement and retained foreign bodies. This prompted development of the slit catheter in 1980.10,11 This catheter has slits at its distal end that prevent the catheter from clogging. The proximal end of the catheter is connected to a transducer and infusion system, which permits continuous monitoring. Both the wick and slit catheters have been shown to offer similar accuracy and reproducibility as long as patency of the catheter is ensured.11–13 A newer approach to predicting the onset of compartment syndrome involves measuring compartment pH as a marker of compromised circulation and decreased tissue perfusion.14 A rise in lactic acid from ischemic tissue lowers the pH of the compartment and has promise as an early predictor of compartment syndrome.
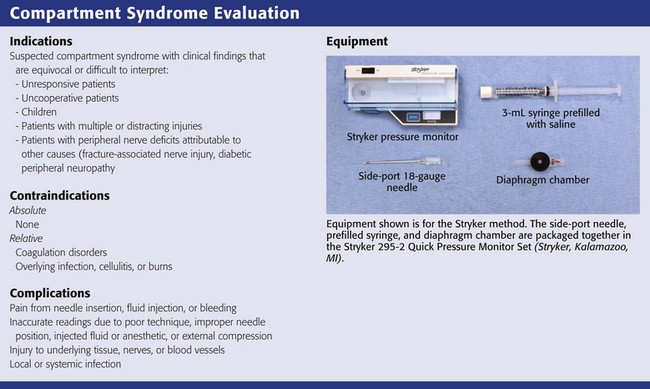
The Stryker Intracompartmental Compartment Pressure Monitoring System (Kalamazoo, MI) has become the most commonly used commercially available device to measure compartment pressure in the emergency department (see Review Box 54-1). This device uses a fluid-filled pressure measurement catheter, a pressure monitor, and a fluid infusion mechanism that maintains catheter patency and ensures accurate measurement. In contrast to earlier devices in which relatively large volumes of fluid were injected into the compartment to measure pressure, the Stryker system uses a minimal amount of saline (<0.3 mL). This helps ensure accurate measurements while reducing the chance of further increases in compartment pressure. The Stryker system also has the ability to record a single measurement or provide continuous compartment pressure recordings when required.
Noninfusion systems such as the transducer-tipped fiberoptic system offer a distinct advantage over conventional fluid-filled systems in that they do not produce hydrostatic pressure artifacts or require the injection of fluid for long-term or continuous measurements. However, the fiberoptic transducer is relatively large, must be attached to a sheath approximately 2.0 mm in diameter, and is likely to cause pain during measurements.15 In recent years, noninvasive, less painful methods of measuring compartment pressure have been studied in patients with both acute and chronic exertional compartment syndrome. Investigations using magnetic resonance imaging (MRI), single-photon emission computed tomography (SPECT), myotonometry, electromyography, ultrasound, near-infrared spectroscopy, and microwave tomography have provided encouraging results in the evaluation of compartment syndrome.16–33 In addition, externally applied devices that measure muscle tissue “hardness” are under investigation as an economic alternative to these modalities, although support of their use has been mixed.34–36
Pathophysiology
Several theories have been proposed to account for the tissue ischemia associated with compartment syndrome, including the “arteriovenous (AV) gradient” theory, which suggests that reduced AV pressure-perfusion gradients prevent adequate blood supply37; the “critical closure” theory, in which blood flow is arrested well before the AV perfusion gradient declines to zero38,39; and the “venous occlusion” theory,40 which states that externally applied pressure, thrombotic events, and reperfusion contribute to the increased compartment pressure and, ultimately, tissue ischemia. Although the exact mechanism has not been agreed on, inherent in each of these theories is a decrease in blood flow to levels below those required to meet the metabolic demands of the tissue. Hence, the final common pathway is cellular hypoxia and muscle necrosis.
Adequate blood flow to tissues is a function of AV gradients across capillary beds. Once blood flow falls below a critical level, delivery of oxygen to these structures is impaired and aerobic cellular metabolism is no longer possible. Anaerobic metabolism then ensues until energy stores become depleted. Muscles become ischemic, and a reduction in venous and lymphatic drainage creates increased pressure within this confined space. It is important to note that ischemia and necrosis of the musculature can occur despite an arterial pressure high enough to produce pulses, so merely assessing distal pulses is insufficient.41
A drop in blood pressure, an increase in compartment pressure, or a combination of the two can reduce AV gradients and lead to insufficient blood flow to tissues. Hypotension can occur in a variety of settings, including hypovolemia, acute blood loss, cardiac disease states (e.g., ischemia), and sepsis. An increase in the contents of a compartment, a decrease in its volume capacity, and external constriction of a compartment can all lead to increases in compartment pressure. Thus, the relationship between intracompartmental pressure and the circulatory status of the extremity is an important factor in the development of compartment syndrome.42 Compartment syndrome may also develop in an extremity in the absence of direct trauma. This is most often due to prolonged ischemia associated with acute arterial occlusion by thrombi or proximal arterial injury.
At rest, normal adult compartment pressure is typically below 10 mm Hg. However, deviations of 2 to 6 mm Hg have been reported.1,8–10,12 Recent data suggest that normal compartment pressures in the lower extremity at rest are higher in children.43 The perfusion pressure of a compartment, also known as the compartment delta pressure (ΔP), is defined as the difference between diastolic blood pressure and intracompartmental pressure.44 A model using legs of normal volunteers has shown that a progression of neuromuscular deficits occurs when intracompartmental pressure rises to within 35 to 40 mm Hg of diastolic blood pressure.45 Above this level, tissue perfusion is interrupted. Studies of neuromuscular tissue ischemia have demonstrated that inflammatory necrosis can occur at pressures between 40 and 60 mm Hg.46,47
Whitesides and colleagues demonstrated that when tissue pressure within a closed compartment rises to within 10 to 30 mm Hg of the patient’s diastolic blood pressure, inadequate perfusion ensues and results in relative ischemia of the involved limb.6 Heppenstall and associates further clarified this relationship by demonstrating that the difference (ΔP) between mean arterial pressure (MAP) and the measured compartment pressure is directly related to blood flow to the tissue.48 They noted that as compartment pressure approaches MAP, ΔP decreases. Once ΔP falls below 30 mm Hg, tissue ischemia becomes more likely. In normal musculature, a ΔP of less than 30 mm Hg results in loss of normal aerobic cellular metabolism.48 In traumatized muscle, a ΔP of less than 40 mm Hg is associated with abnormal cellular function, thus highlighting the importance of maintaining adequate systemic blood pressure in the setting of neuromuscular injury.48
For years, conventional wisdom maintained that immediate reperfusion of traumatized tissue would provide improved motor and neurologic function after injury. In the last decade, research suggests that muscle tissue may remain viable even after prolonged periods of ischemia and that a substantial proportion of the injury is generated on reperfusion.49,50 Tissue acidosis, intracellular and extracellular edema, free radical–mediated injury, loss of adenine nucleotide precursors, and interruption of mitochondrial oxidative rephosphorylation by increased intracellular calcium have been implicated in the development of reperfusion-associated compartment syndrome.49–54 A recent study has identified the potential role of N-acetylcysteine in the attenuation of tissue injury in compartment syndrome.55 Even in the absence of local trauma, ischemia followed by reperfusion has been shown to increase compartment pressure in canine models of hypovolemic shock.56
Evidence also suggests that elevated compartment pressure itself (in addition to causing ischemia) plays a role in the cellular deterioration seen with compartment syndrome.57 In a study by Heppenstall and associates,57 muscle ischemia caused by placement of a tourniquet was compared with an experimentally derived high-pressure compartment syndrome. The authors found no difference in the degree to which phosphocreatine levels fell between groups. However, levels of adenosine triphosphate (ATP) diminished rapidly in the compartment syndrome group in comparison to the tourniquet group. Moreover, phosphocreatine levels, ATP, and tissue pH normalized within 15 minutes of releasing the tourniquet. In the group with compartment syndrome, these levels remained low even after fasciotomy. These results suggest that elevated tissue pressure plays a synergistic role with ischemia in cellular deterioration.
Clinical Features
Any compartment limited by fascial planes is potentially at risk for compartment syndrome. However, because of their propensity for injury and the presence of several low-volume compartments, the lower extremities are most commonly affected. In the leg, the anterior compartment is involved most often,58 whereas the posterior compartment is a site frequently missed. The hands, feet, forearms, upper part of the arms, thighs, abdomen, gluteal musculature, and back are other locations where compartment syndrome is known to occur.1 Increased compartment pressure may be caused by a variety of conditions (see Box 54-1). Risk factors for the development of compartment syndrome include recent trauma to an extremity (including acupuncture, venipuncture, intravenous infusions, or intravenous drug use), bleeding within a compartment, a restrictive cast or splint, a crush or compression injury, a prolonged lithotomy position, placement of a tourniquet during an operative procedure, and circumferential burns.59–63
Long-bone fractures account for about 75% of traumatic compartment syndromes, and the absence of a fracture in a traumatized extremity is a factor in delayed diagnosis. The tibia is most often involved, followed by bones of the forearm. Supracondylar fractures in children are at risk for compartment syndrome. Comminuted fractures increase the risk. Closed fractures are of greatest concern, but an open fracture does not necessarily decompress elevated compartment pressure. Treatment of fractures, by both open and closed reduction, can increase compartment pressure. Compartment pressure may peak immediately after reduction. In addition, some evidence suggests that compartment syndrome may occur in the setting of chronic exertion and muscle overuse.64,65 Although the exact etiology remains elusive, studies have demonstrated elevated lactate and water levels in the tibialis anterior muscle after exercise with a reduction in these levels after fasciotomy.66,67 Increases in muscle mass (related to a rise in blood volume during exertion) and hypertrophy of muscle and fascia with chronic use and exertion have also been reported.68–72
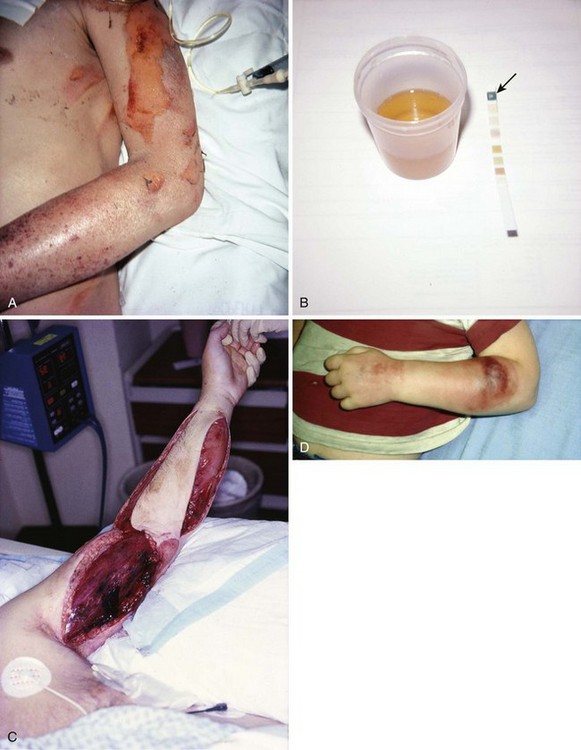
Clinical hallmarks of compartment syndrome include pallor of the extremity, a pulse deficit with respect to the opposite limb, paresis or paralysis of the involved extremity, paresthesias in the distribution of the involved nerves, and pain on passive stretch of the involved musculature (the “5 P’s” of compartment syndrome). Paresthesias are secondary to ischemic nerve dysfunction. These signs and symptoms may be unreliable in pediatric populations.73,74 In addition, though commonly seen, pain and paralysis are late findings. Early, more subtle signs of compartment syndrome include a burning sensation over the involved compartment, nonspecific sensory deficits, or poorly localized deep muscular pain. Pain that seems out of proportion to the apparent injury and clinical examination and pain that intensifies when the musculature is passively stretched are common features.
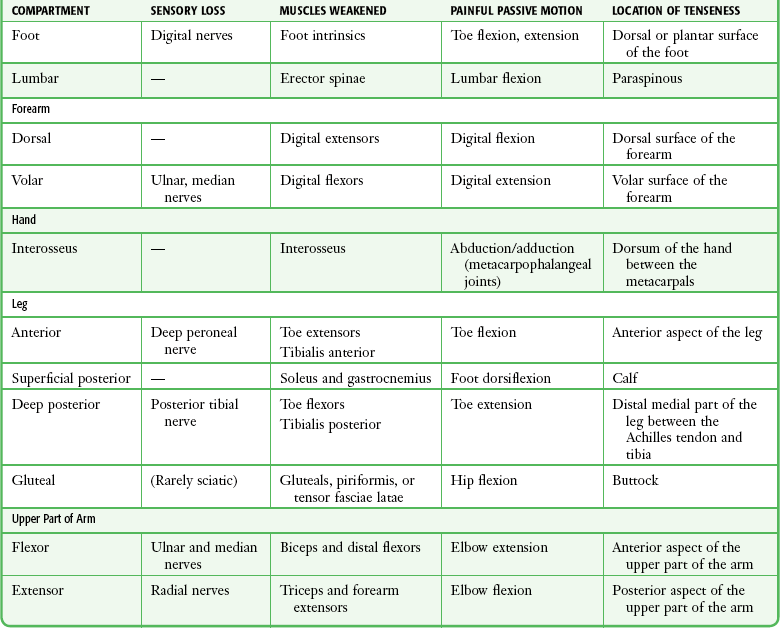
The period between the injury and the onset of symptoms can be as short as 2 hours and as long as 6 days.75 The peak interval appears to be 15 to 30 hours. Frequently, the first symptom described by patients is pain greater than expected given the clinical scenario. Although pain out of proportion to the visible injury may raise the question of drug-seeking behavior, a focused evaluation for the possibility of limb-threatening disorders must precede this diagnosis of exclusion. Physical examination may reveal muscles that are weak and tense with hypoesthesia in the distribution of the involved nerves. Sensory deficits, including loss of two-point discrimination and decreased vibratory sensation, are frequently present.76–78 The presence or absence of a palpable arterial pulse is not an accurate indicator of relative tissue pressure or the risk for compartment syndrome. Pulses may be present in a severely compromised extremity.79 Table 54-1 lists the signs and symptoms of compartment syndrome specific to each compartment.
Diagnosis
The difficulty in diagnosing acute compartment syndrome was highlighted in a report by Vaillancourt and coworkers.80 In a retrospective review of 76 patients who underwent fasciotomy at major university trauma centers or teaching hospitals, the interval from initial patient assessment to diagnosis of compartment syndrome was up to 8 hours. Delay in diagnosis was most common in nontraumatic cases. The interval from the precipitating event to definitive surgery was up to 35 hours, thus reflecting the difficulty in suspecting this diagnosis and instituting definitive therapy in clinical practice. Such statistics describe actual care, which may be less than ideal when compared with theoretical benchmarks.
Notwithstanding the difficulty just described, the diagnosis of compartment syndrome is primarily a clinical one that may be supplemented by direct measurement of compartment pressure. In a study evaluating the utility of clinical findings in making the diagnosis of compartment syndrome, Ulmer noted that the sensitivity and positive predictive value of clinical findings are low whereas the specificity and negative predictive value of these findings are high.81 Nevertheless, the study found that although the sensitivity of an individual clinical finding may be low, the probability of compartment syndrome rises considerably when more than one clinical hallmark is present.81 However, other studies have suggested that the absence of clinical evidence is more useful in excluding compartment syndrome than its presence is in confirming the diagnosis.82–84 All things considered, compartment syndrome remains largely a clinical diagnosis, and a high index of suspicion is paramount.
The differential diagnosis of compartment syndrome is extensive and includes primary vascular, nerve, and muscle injuries that produce similar findings. Acute arterial occlusion, cellulitis, osteomyelitis, neurapraxia, reflex sympathetic dystrophy, synovitis, tenosynovitis, stress fractures, envenomations, necrotizing fasciitis, deep vein thrombosis, and thrombophlebitis are additional diseases that should be considered. Differentiating compartment syndrome from these and other orthopedic disorders requires a detailed history and thorough physical examination, often supplemented by measurement of compartment pressure (Table 54-2).
TABLE 54-2
Clinical Findings in Patients with Compartment Syndrome, Arterial Occlusion, and Neurapraxia
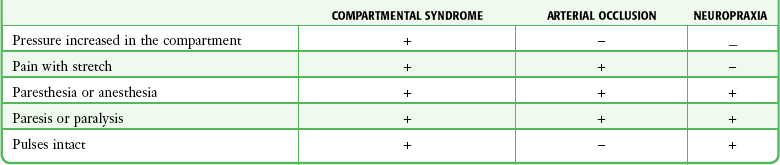
From Mubarak S, Carroll N. Volkman’s contracture in children: etiology and prevention. J Bone Joint Surg Br. 1979;61:290.
Ancillary Studies
In general, laboratory and radiographic studies are not helpful in confirming the diagnosis of compartment syndrome. However, they might be useful in identifying other diagnoses, associated conditions, and complications. Box 54-2 lists useful studies for patients in whom compartment syndrome is suspected.
Invasive Compartment Pressure Monitoring
Indications and Contraindications
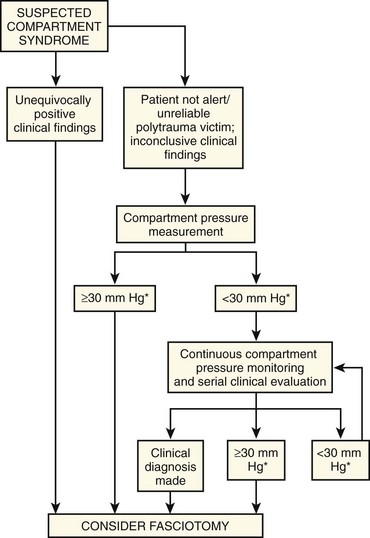
The earliest objective manifestation of acute compartment syndrome is an elevation in the tissue pressure of one or more compartments. However, signs and symptoms do not generally develop until tissue pressure has reached a critical level (see “Pathophysiology”). In some patients the diagnosis of compartment syndrome is clinically obvious, and one can proceed directly to fasciotomy. When the clinical findings are equivocal or difficult to interpret, measurement of tissue pressure may help guide treatment (Fig. 54-3). However, even though pressure measurements may suggest the presence of compartment syndrome, the interpretation of such measurements always requires clinical judgment.
Patient Preparation and Positioning
Explain the procedure to the patient or surrogate. Written informed consent is not a universal standard. Positioning of the patient and extremity for measurement of compartment pressure depends on the extremity and the compartment being studied, coexisting injuries, and the clinical status of the patient. In general, patients should be in the supine position.85–87 The exceptions to supine positioning are discussed in subsequent sections related to the extremity of interest.
For most patients, measurement of compartment pressure is a painful procedure that requires adequate local anesthesia (see Chapter 29), systemic analgesia, or procedural sedation (see Chapter 33). Anesthetize the skin with a small amount of local anesthetic while avoiding the underlying muscle and fascia. Inadvertent injection into the underlying structures may falsely elevate compartment pressure. Patient movement (as a result of inadequate analgesia or improper positioning) may also falsely elevate compartment pressure, particularly if the patient requires limb restraint. To minimize the discomfort of multiple attempts at localizing the correct compartment, some have advocated the use of ultrasound guidance to improve the accuracy of needle insertion.88 However, there is currently no consensus regarding the use of ultrasound guidance in this setting.89–90
Perform compartment pressure measurements with sterile technique, including standard skin preparation and draping at the planned insertion site (see Chapter 34). Avoid placing the needle through areas of overlying burn or infection. If a circumferential cast is present, bivalve the cast or, if necessary, create a window overlying the desired area with a cast saw.
Equipment
Needles commonly used for measurement of compartment pressure include a simple 18-gauge needle, an 18-gauge spinal needle (for deep compartments), and a side-port needle (Stryker Instruments, Kalamazoo, MI). The side-port needle and slit catheter have comparable efficacy when used for measurement of compartment pressure and may be more accurate than simple 18-gauge needles.42 Simple 18-gauge needles are more readily available and more commonly used. The wick catheter and slit catheter methods described previously generally require specialized, cumbersome equipment not often available in most emergency departments and are therefore not described.
In an effort to reduce the pain associated with insertion of larger-gauge needles, Mars and colleagues evaluated the accuracy and reliability of measuring compartment pressure with smaller-gauge needles.91 The authors compared compartment pressures measured with 18- to 25-gauge needles and found that smaller-gauged needles provided results similar to or better than the more traditional 18-gauge needles did. Furthermore, the addition of a side port did not improve accuracy.91 Unfortunately, no additional studies have been done to corroborate these findings. As a result, most clinicians continue to use 18-gauge needles.
Pressure Measurement Systems
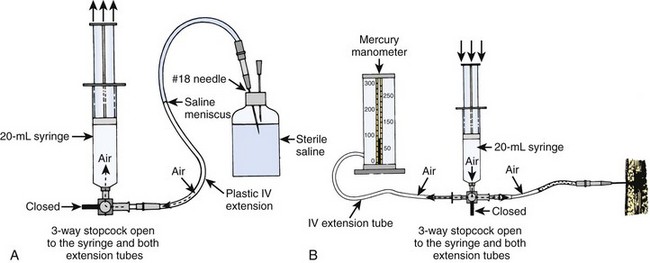
Mercury Manometer System (Fig. 54-4)
1. Position the patient. Prepare and anesthetize the needle insertion site according to the guidelines described earlier.
2. Assemble the syringe, tubing, extension tubing, and needle as shown in Figure 54-4A.
3. Insert the needle into a vented vial of sterile saline. Aspirate a column of saline into the tubing halfway to the stopcock; care should be taken to avoid the formation of bubbles. Close the three-way stopcock to the tube to prevent the loss of saline during insertion of the needle.
4. Insert the needle into the muscle of the compartment being measured (see “Needle Placement Techniques” later in this chapter).
5. Attach the second extension tubing to the monitor and to the third port of the three-way stopcock. Turn the stopcock so that the syringe is open to both extension tubes (see Fig. 54-4B). This closed system has equal pressure in both extension tubes.
6. Increase the pressure in the system gradually by slowly depressing the syringe plunger while simultaneously watching the column of saline. The mercury manometer will rise as pressure in the system increases. When the pressure in the system exceeds that in the tissue, inject saline into the compartment, which causes the saline column to move. Read the manometer at the moment that the saline is noted to move. This reading corresponds to the tissue pressure in millimeters of mercury.
7. To obtain a second reading, completely remove the needle and repeat steps 4 through 6. A third measurement might be necessary to achieve two readings in agreement. Check the needle for tissue plugs and blood clots between readings.
Procedural Caveats: The most common error with this system is depressing the syringe plunger too quickly. Only when saline is injected slowly into the compartment will the mercury column (which has greater inertia) accurately reflect compartment pressure. Another source of error is obstruction of the needle with a plug of tissue (or blood clot) if the plunger of the syringe is pulled back. Finally, aneroid manometers are prone to inaccuracy, are not well calibrated at lower pressure ranges, and should not be substituted for the more accurate mercury manometers for this procedure.
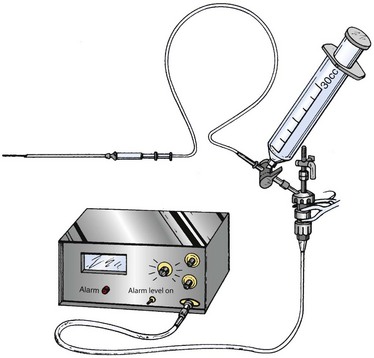
Arterial Line System (Fig. 54-5)
1. Position the patient and prepare and anesthetize the needle insertion site according to the guidelines described earlier.
2. Connect the transducer cable to the pressure monitor.
3. Assemble the stopcocks, transducer, transducer cable, syringe, high-pressure tubing, and needle as shown in Figure 54-5.
4. Fill the syringe with 15 mL of sterile saline and place one of the stopcocks on the syringe. Turn the stopcocks to allow filling of the transducer, high-pressure tubing, and needle. Once filled, close the stopcock to the high-pressure tubing.
5. Open the top stopcock to air and place the transducer at the height of the compartment being measured. Calibrate the transducer to zero and then close the top stopcock.
6. Open the lower stopcock that is attached to the high-pressure tubing.
7. Insert the needle into the desired muscle compartment (see “Needle Placement Techniques” later in this chapter for details). If the needle is in the proper location, applying slight external pressure to the compartment or passively moving the muscles within the compartment should cause a pressure spike on the monitor. Allow the compartment to equilibrate for several seconds after this maneuver, and then measure the mean compartment pressure.
8. To obtain a second reading, completely remove the needle and repeat steps 4 through 7. A third measurement might be necessary to achieve two readings in agreement. Check the needle for tissue plugs and blood clots between readings.
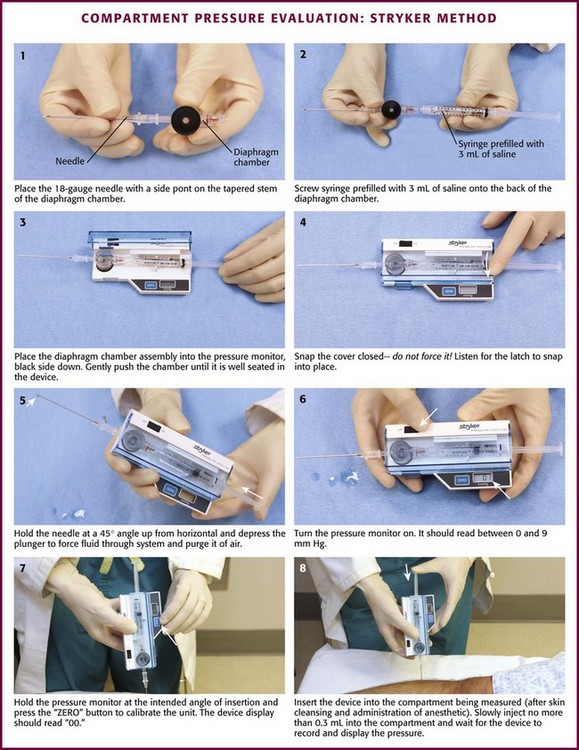
Stryker Intracompartmental Pressure Measurement (Fig. 54-6)
Setup and Procedure (see Fig. 54-6):
1. Position the patient and prepare and anesthetize the needle insertion site according to the guidelines described earlier.
2. Open the Quick Pressure Monitor Set and remove the contents while maintaining sterile conditions.
3. Place the needle firmly on the tapered chamber stem.
4. Remove the cap on the prefilled syringe and screw the syringe onto the remaining chamber stem. Take care to avoid contaminating the fluid pathway.
5. Open the monitor cover and inspect the device for damage or contamination. Place the chamber into the device well (black surface down) and push gently until it seats.
6. Snap the cover closed—DO NOT FORCE IT. The latch must have “snapped” in place.
7. Hold the needle at approximately 45 degrees from the horizontal plane and slowly force fluid through the disposable system to purge it of air. Caution: DO NOT allow saline to roll down the needle into the transducer well.
8. Turn on the unit; it should read between 0 and 9 mm Hg.
9. Hold the device at the intended angle of insertion and press the “ZERO” button to calibrate it. After a few seconds, the display should read “00.” Failure to calibrate the device while in the intended angle of insertion may result in inaccurate readings. Note: The display must read “00” before continuing. If it does not, follow the troubleshooting instructions provided by the manufacturer before proceeding.
10. Now insert the needle into the compartment being measured (see “Needle Placement Techniques” for anatomic details). Slowly inject no more than 0.3 mL of saline into the compartment to equilibrate with the interstitial fluid.
11. Wait for the display to reach equilibrium and record the resulting pressure.
12. For additional measurements, turn the unit off and repeat steps 8 through 11. Recalibrate the unit to zero before each measurement.
13. For continuous monitoring with an indwelling slit catheter, refer to the instructions accompanying the system.
Needle Placement Techniques for Specific Compartments
Accurate pressure measurements depend on careful needle insertion and confirmation of correct placement. Proper needle insertion requires (1) reliable placement in the compartment being measured, (2) avoidance of important neurovascular structures, (3) simplicity and reproducibility, and (4) minimal patient discomfort.92 Most compartments are superficial and easily accessible. Only the deep posterior compartment in the lower part of the leg and the gluteal compartment may require a spinal needle to reach the required depth. Most approaches require that the needle enter the tissue perpendicular to the skin.
Lower Extremity
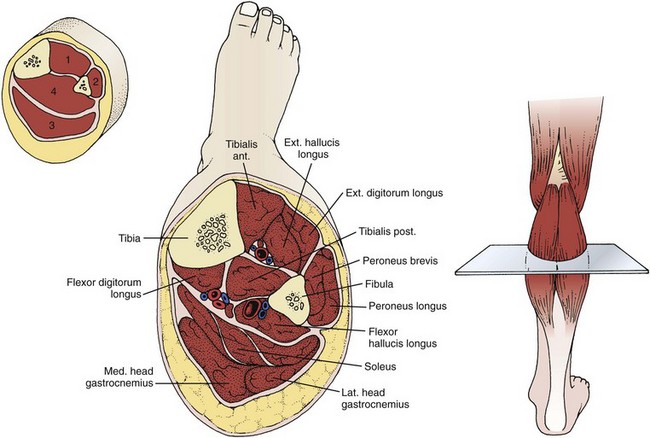
Because of its high vulnerability to injury and limited fascial compliance, the lower part of the leg is predisposed to compartment syndrome. The foreleg traditionally has four compartments: anterior, lateral, deep posterior, and superficial posterior (Fig. 54-7).1 The anterior compartment is the most frequent site of compartment syndrome.45 In some patients the tibialis posterior muscle may occupy its own compartment, separate from the rest of the deep posterior compartment.93–95 Keep this possibility in mind when measuring compartment pressure in this area.
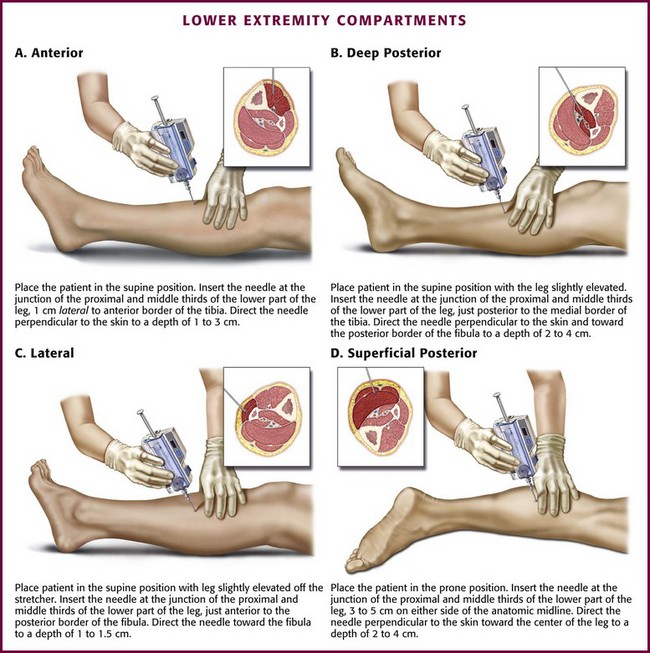
The easiest cross-sectional level for placement of the needle in any foreleg compartment is approximately 3 cm on either side of a transverse line drawn at the junction of the proximal and middle thirds of the lower part of the leg (see Fig 54-7). When measuring compartment pressure in the foreleg, place the patient in the supine position with the leg at the level of the heart. An exception occurs when measuring the superficial posterior compartment. In this case, place the patient in the prone position. Prepare and anesthetize the needle insertion site as described previously in this chapter.
Forearm
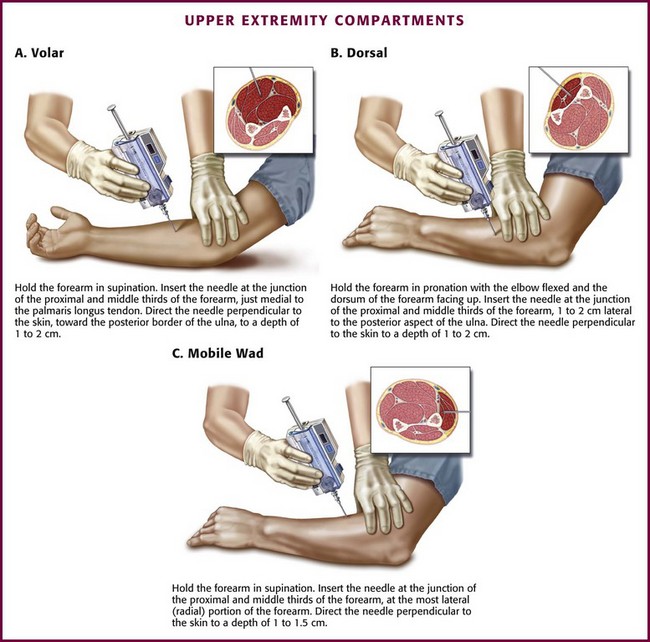
Traditionally, the forearm has been considered a two-compartment limb.1 However, some authors place the extensor carpi radialis brevis, the extensor carpi radialis longus, and the brachioradialis muscles in a third compartment called the “mobile wad.”96–98 The forearm compartments (particularly the volar compartment) are predisposed to compartment syndrome because of their use during vigorous exercise, accessibility for drug use, intravenous infiltration of fluid or medication, and vulnerability to injury and burns.1,99–102
The junction of the proximal and middle thirds of the forearm is the cross-sectional level for insertion of the needle.96 When measuring forearm compartment pressure, place the patient in the supine position with the arm at the level of the heart. Prepare and anesthetize the needle insertion site as previously described.
Gluteal Musculature
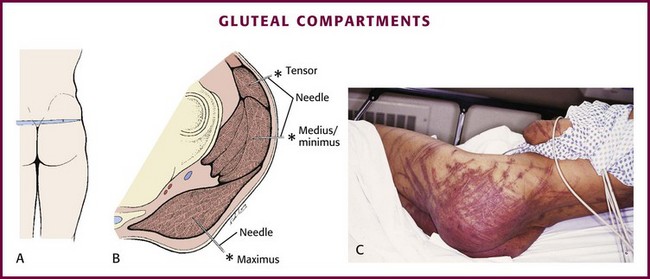
A two-layer fascia encases the muscle bellies of the tensor fasciae latae anteriorly and the gluteus maximus posteriorly. This fascia divides the musculature into three distinct compartments: maximus, tensor, and medius/minimus (Fig. 54-10A). The sciatic nerve is deep to the fascia but lies between the pelvis–external rotator complex and the gluteus maximus, which makes it vulnerable to injury when compartment syndrome occurs here. Gluteal compartment syndrome is very rare and unknown to many clinicians. Most reported cases of gluteal compartment syndrome result from prolonged immobilization and local compression in association with drug or alcohol intoxication.103–107 Prolonged pressure from a toilet seat or significant soft tissue contusions (whipping, paddling) may predispose to this condition. Patients typically have gluteal tenderness that is often attributed to contusion or hematoma. This, combined with the rarity of a compartment syndrome in this area, frequently results in a delayed or missed diagnosis. Rhabdomyolysis should be considered in patients with gluteal compartment syndrome given the large muscle mass involved.
Gluteal Compartments
To measure gluteal compartment pressure, place the patient in the prone position with the gluteal structures at the level of the heart. Prepare and anesthetize the needle insertion site as described previously. Cutaneous landmarks for the three compartments are not consistent from patient to patient. Therefore, in cases of suspected gluteal compartment syndrome, insertion of the needle at the point of maximal tenderness is considered sufficient to provide adequate pressure measurements.103 Insert an 18-gauge spinal needle perpendicular to the skin and direct it toward the point of maximal tenderness to a depth of 4 to 8 cm (see Fig. 54-10B). Confirm proper needle placement by observing a rise in pressure during external compression of the gluteal musculature.
Foot
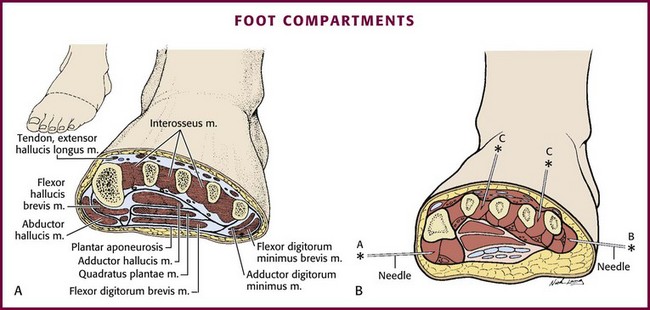
Crush injuries account for the majority of reported cases of compartment syndrome in the foot,108–110 but it may also be seen after vascular injuries, fractures, and other high-energy injuries. Although compartment syndrome of the foot is rare, it is being reported with increasing frequency as clinicians become more aware of this entity. Despite a lack of universal agreement on the number or exact location of the anatomic compartments in the foot,111–115 it is generally accepted that there are at least nine compartments separated into four groups: the central/calcaneal, intrinsic/interosseous, medial, and lateral (Fig. 54–11A).
For measurement of pressure in any of the foot compartments, place the patient in the supine position with the foot at the level of the heart and prepare and anesthetize the needle insertion site as described previously. Note that pedal edema has been shown to increase resting tissue pressure in the foot.116,117
Medial Compartment (see Fig. 54-11B)
The medial compartment contains the abductor hallucis and flexor hallucis brevis muscles. The compartment is bounded medially and inferiorly by an extension of the plantar aponeurosis, laterally by an intramuscular septum, and dorsally by the first metatarsal. To measure pressure in this compartment, insert the needle perpendicular to the skin at the medial aspect of the foot just inferior to the base of the first metatarsal into the abductor hallucis muscle, which is approximately 1 to 1.5 cm deep.118 Confirm proper needle depth by observing a rise in pressure during external compression of the medial compartment of the foot.
Central (Calcaneal) Compartment (see Fig. 54-11B)
The central compartment contains the flexor digitorum brevis, the quadratus plantae, the lumbricals, and the abductor hallucis muscles. Its boundaries are the plantar aponeurosis inferiorly, the osteofascial tarsometatarsal structures dorsally, and the intermuscular septa medially and laterally. To measure pressure in this compartment, insert the needle perpendicular to the skin at the medial aspect of the foot just inferior to the base of the first metatarsal. Advance the needle through the abductor hallucis muscle to a depth of 3 cm.118 Confirm proper needle depth by observing a rise in pressure during external compression of the central compartment of the foot.
Lateral Compartment (see Fig. 54-11B)
The lateral compartment contains the abductor, flexor, and opponens muscles of the fifth toe. The boundaries are the fifth metatarsal dorsally, the plantar aponeurosis inferiorly and laterally, and an intermuscular septum medially.118 To measure pressure in this compartment, insert the needle parallel to the plantar aspect of the foot just inferior to the base of the fifth metatarsal. Advance the needle to a depth of 1 to 1.5 cm. Confirm proper needle depth by observing a rise in pressure during external compression of the lateral compartment of the foot.
Intrinsic (Interosseous) Compartment (see Fig. 54-11B)
The intrinsic compartment contains the seven interossei muscles and is bounded by the metatarsals and the interosseous fascia. Pressure in this compartment is measured in two areas, the second and fourth web spaces. Avoid the first web space to prevent inadvertent puncture or disruption of the dorsalis pedis artery or the deep peroneal nerve.118 Insert the needle perpendicular to the skin at the dorsum of the second and fourth web spaces at the base of the metatarsals, and advance the needle to a depth of 1 cm.118 Confirm proper needle depth by observing a rise in pressure during external compression of the intrinsic compartment adjacent to the needle insertion site.
Interpretation of Compartment Pressure Measurements
Compartment pressures must be interpreted within the context of the clinical picture. Inaccurate measurements are far worse than no measurement at all, and clinical evaluation is more telling than pressure measurements alone. To be most accurate, compartment pressures should be measured in the area of highest pressure and greatest tissue damage.79,119 Inaccurate measurements may result from placement of the needle into tendon or fascia, plugged needles, defective or poorly calibrated devices, injection of fluid into the compartment, and movement during the procedure. Whitesides and colleagues found a 1–mm Hg increase in compartment pressure for every 1 mL of saline infused into the anterior compartment of the lower extremity.6 It is difficult to assess the relevance of this finding, but recognition of the potential for its occurrence is important.
Reports of normal human compartment pressure vary in the literature. In comparing several techniques, Shakespeare and associates found an average normal pressure of 8.5 mm Hg with slightly higher resting pressure in individuals who were physically fit.12 Willy and coworkers found a mean of 15 mm Hg (±8 mm Hg) when using an electronic transducer-tipped probe in healthy volunteers.15 Although the generally accepted range of normal is between 0 and 10 mm Hg, others have noted pressures in normal subjects ranging from 0 to as high as 18 mm Hg.1,8,78,120 Even though compartment pressure measurements have not been studied extensively in pediatric populations, one study reported higher resting compartment pressure in children than in adults.43
When properly performed, each method has acceptable accuracy in the clinical setting. Studies have found standard deviations of between 2 and 6 mm Hg with all the techniques described earlier.1,8–10,12 It is generally agreed that the mercury manometer method of measuring compartment pressure is the least accurate. The arterial line system used with a simple (straight) or side-port needle provides a higher degree of accuracy for simple, episodic readings. The Stryker Intracompartmental Pressure Monitoring System provides consistent, accurate readings for both episodic and continuous pressure monitoring. The development of miniature transducer-tipped devices is ongoing.121 Noninvasive modalities, including MRI, SPECT, myotonometry, electromyography, near-infrared spectroscopy, and ultrasound, continue to be investigated as painless alternatives to needle-driven compartment pressure measurements, and although the early results are promising, they are not currently in widespread use.16–33
Mubarak and coworkers9,122 and Hargens and colleagues suggested that an absolute compartment pressure of 30 mm Hg is the “critical pressure” requiring fasciotomy.123 Others have corroborated these findings.119,124 Despite the fact that this tissue pressure is abnormally high and corresponds to the onset of pain and paresthesias,9 it does not necessarily precipitate compartment syndrome in the absence of other factors and must be interpreted within the context of the clinical scenario. Although compartment syndrome may develop in some patients at this pressure, in others it will not because tolerance to increasing pressure appears to be variable.1,8,12,78
Despite a body of literature addressing measurement of compartment pressure, there continues to be no consensus regarding a specific compartment pressure threshold at which fasciotomy should be performed. Obviously, no measured compartment pressure, by itself, is an indication for fasciotomy, but it may guide consultation and further observation. A single pressure reading can be misleading, and trends in pressure provide more information to the clinician. Some argue that an absolute compartment pressure of 30 mg Hg or greater should be the threshold for fasciotomy.9,122–124 Matsen found that no patients with a pressure lower than 45 mm Hg had symptoms of compartment syndrome1 whereas all patients with a pressure higher than 60 mm Hg had symptoms. Whitesides and colleagues found that fasciotomy was required when intracompartmental pressure approaches 20 mm Hg below diastolic pressure,6 whereas McQueen and associates recommended using a differential pressure (diastolic minus compartment pressure) of less than 30 mm Hg as a criterion for fasciotomy.125 Heppenstall and coworkers concluded that use of either the Whitesides or McQueen criteria would produce similar results and recommended using a ΔP (MAP minus the measured compartment pressure) of 30 mm Hg or less in nontraumatized muscle and a ΔP of 40 mm Hg or less in traumatized muscle as a guide for fasciotomy.48,57
In summary, absolute compartment pressures of 30 mm Hg or higher garner concern for consultation or intervention for compartment syndrome. Based on pathophysiology, it appears quite reasonable to consider the difference between diastolic blood pressure and the measured compartment pressure (ΔP) as a guide for diagnosing compartment syndrome and a strong consideration for fasciotomy. A compartment pressure within 30 mm Hg of the patient’s diastolic blood pressure, essentially a ΔP of less than 30 mm Hg, is generally considered the threshold for consideration of fasciotomy.47,119,126
References
1. Matsen FA, ed. Compartment Syndromes. New York: Grune & Stratton, 1980.
2. Hope, MJ, McQueen, MM. Acute compartment syndrome in the absence of fracture. J Orthop Trauma. 2004;18:220.
3. Coco, T, Klasner, A. Drug-induced rhabdomyolysis. Curr Opin Pediatr. 2004;16:206.
4. von Volkmann, R. Verletzungen und Krankheiten der Bewegungsorgane. Hanbude der Allgemeinen und Speciellen Chirurgie; 1872.
5. Henderson, Y, Oughterson, AW, Greenberg, LA, et al. Muscle tonus, intramuscular pressure, and the venopressure mechanism. Am J Physiol. 1935;114:261.
6. Whitesides, TE, Haney, TC, Morimoto, K, et al. Tissue pressure measurements: a determinant for the need for fasciotomy. Clin Orthop. 1975;113:43.
7. Matsen, FA, Mayo, KA, Sheriden, GW, et al. Monitoring of intramuscular pressure. Surgery. 1976;79:702.
8. Mubarak, SJ, Hargens, AR, Owen, CA, et al. The wick catheter technique for measurement of intramuscular pressure: a new research and clinical tool. J Bone Joint Surg Am. 1976;58:1016.
9. Mubarak, SJ, Owen, CA, Hargens, AR, et al. Acute compartment syndrome: diagnosis and treatment with the aid of a wick catheter. J Bone Joint Surg Am. 1978;60:1091.
10. Rorabeck, CH, Castle, GS, Hardie, R, et al. The slit catheter: a new device for measuring intracompartmental pressure. Surg Forum. 1980;31:513.
11. Rorabeck, CH, Castle, GS, Hardie, R, et al. Compartmental pressure measurements: an experimental investigation using the slit catheter. J Trauma. 1981;21:446.
12. Shakespeare, DT, Henderson, NJ, Clough, G. The slit catheter: a comparison with the wick catheter in the measurement of compartment pressures. Injury. 1981;13:404.
13. Hammerberg, EM, Whitesides, TE, Jr., Seiler, JG, 3rd. The reliability of measurement of tissue pressure in compartment syndrome. J Orthop Trauma. 2012;26:24–31. [discussion 32].
14. Arato, E, Kurthy, M, Sinay, L, et al. Pathology and diagnostic options of lower limb compartment syndrome. Clin Hemorheol Microcirc. 2009;41:1.
15. Willy, C, Gerngross, H, Sterk, J. Measurement of intracompartmental pressure with use of a new electronic transducer-tipped catheter system. J Bone Joint Surg Am. 1999;81:158.
16. Verleisdonk, EJ, van Gils, A, van der Werken, C. The diagnostic value of MRI scans for the diagnosis of chronic exertional compartment syndrome of the lower leg. Skeletal Radiol. 2001;30:321.
17. Rominger, MB, Lukosch, CJ, Bachmann, GF. MR imaging of compartment syndrome of the lower leg: a case control study. Eur Radiol. 2004;14:1432.
18. Trease, L, van Every, B, Bennell, K, et al. A prospective blinded evaluation of exercise thallium-201 SPECT in patients with suspected chronic exertional compartment syndrome of the leg. Eur J Nucl Med. 2001;28:688.
19. Oturai, PC, Lorenzen, T, Norregaard, J, et al. Evaluation of Tc-99m-tetrofosmin single-photon emission computed tomography for detection of chronic exertional compartment syndrome of the leg. Scand J Med Sci Sports. 2006;16:282.
20. Korhonen, RK, Vain, A, Vanninen, E, et al. Can mechanical myotonometry or electromyography be used for the prediction of intramuscular pressure? Physiol Meas. 2005;26:951.
21. Noseworthy, MD, Davis, AD, Elzibak, AH. Advanced MR imaging techniques for skeletal muscle evaluation. Semin Musculoskel Radiol. 2010;14:257.
22. Giannotti, G, Cohn, SM, Brown, M, et al. Utility of near-infrared spectroscopy in the diagnosis of lower extremity compartment syndrome. J Trauma. 2000;48:396.
23. Lynch, JE, Heyman, JS, Hargens, AR. Ultrasonic device for the noninvasive diagnosis of compartment syndrome. Physiol Meas. 2004;25:N1.
24. Gentilello, LM, Sanzone, A, Wang, L, et al. Near-infrared spectroscopy versus compartment pressure for the diagnosis of lower extremity compartmental syndrome using electromyography-determined measurements of neuromuscular function. J Trauma. 2001;51:1.
25. Tobias, JD, Hoernschemeyer, DG. Near-infrared spectroscopy identifies compartment syndrome in an infant. J Pediatr Orthop. 2007;27:311.
26. Cole, AL, Herman, RA, Jr., Heimlich, JB, et al. Ability of near infrared spectroscopy to measure oxygenation in isolated upper extremity muscle compartments. J Hand Surg Am. 2012;37:297.
27. Bariteau, IT, Beutel, BG, Kamal, R, et al. The use of near-infrared spectrometry for the diagnosis of lower-extremity compartment syndrome. Orthopedics. 2011;11:178.
28. Shuler, MS, Reisman, WM, Kinsey, TL, et al. Correlation between muscle oxygenation and compartment pressures in acute compartment syndrome of the leg. J Bone Joint Surg Am. 2010;92:863.
29. Shuler, MS, Reisman, WM, Whitesides, TE, Jr., et al. Near-infrared spectroscopy in lower extremity trauma. J Bone Joint Surg Am. 2009;91:1360.
30. Shuler, MS, Reisman, WM, Cole, AL, et al. Near-infrared spectroscopy in acute compartment syndrome: case report. Injury. 2011;42:1506.
31. Katz, LM, Nauriyal, V, Nagaraj, S, et al. Infrared imaging of trauma patients for detection of acute compartment syndrome of the leg. Crit Care Med. 2008;36:1756.
32. Semenov, S, Kellam, J, Sizov, Y, et al. Microwave tomography of extremities: 1. Dedicated 2D system and physiological signatures. Phys Med Biol. 2011;56:1005.
33. Semenov, S, Kellam, J, Nair, B, et al. Microwave tomography of extremities: 2. Functional fused imaging of flow reduction and simulated compartment syndrome. Phys Med Biol. 2011;56:2019.
34. Dickson, KF, Sullivan, MJ, Steinberg, B, et al. Noninvasive measurement of compartment syndrome. Orthopedics. 2003;26:1215.
35. Steinberg, BD. Evaluation of limb compartments with increased interstitial pressure. An improved noninvasive method for determining quantitative hardness. J Biomech. 2005;38:1629.
36. Joseph, B, Varghese, RA, Mulpuri, K, et al. Measurement of tissue hardness: can this be a method of diagnosing compartment syndrome noninvasively in children? J Pediatr Orthop B. 2006;15:443.
37. Vollmar, B, Westermann, S, Menger, MD. Microvascular response to compartment syndrome–like external pressure elevation: an in vivo fluorescence microscopic study in the hamster striated muscle. J Trauma. 1999;46:91.
38. Whittaker, SRE, Winton, FR. The apparent velocity of blood flowing in the isolated hind limb of the dog and its variation with corpuscular concentration. J Physiol (Lond). 1933;78:339.
39. Burton, AC. On the physical equilibrium of small blood vessels. Am J Physiol. 1951;164:319.
40. Velmahos, GC. Vascular trauma and compartment syndrome. Surg Clin North Am. 2002;82:122.
41. Wheeless’ Textbook of Orthopedics. Available at Wheelessonline.com. [Accessed March 27, 2007].
42. Boody, AR, Wongworawat, MD. Accuracy in the measurement of compartment pressures: a comparison of three commonly used devices. J Bone Joint Surg Am. 2005;87:2415.
43. Staudt, JM, Smeulders, MJ, van der Horst, CM. Normal compartment pressures of the lower leg in children. J Bone Joint Surg Br. 2008;90:215.
44. Menetrey, J, Peter, R. Acute compartment syndrome in the post-traumatic leg. Rev Chir Orthop Reparatice Appar Mot. 1998;84:272.
45. Hargens, AR, Mubarak, SJ. Current concepts in the pathophysiology, evaluation, and diagnosis of compartment syndrome. Hand Clin. 1998;14:371.
46. Sheridan, GW, Matsen, FA. An animal model of the compartmental syndrome. Clin Orthop. 1975;113:36.
47. Matava, MJ, Whitesides, TE Jr, Seiler, JG, 3rd., et al. Determination of the compartment pressure threshold of muscle ischemia in a canine model. J Trauma. 1994;37:50.
48. Heppenstall, RB, Sapega, AA, Scott, R, et al. The compartment syndrome: an experimental and clinical study of muscular energy metabolism using phosphorous nuclear magnetic resonance spectroscopy. Clin Orthop Relat Res. 1988;226:138.
49. Defraigne, JO, Pincemail, J. Local and systemic consequences of severe ischemia and reperfusion of the skeletal muscle. Physiology and prevention. Acta Chir Belg. 1998;98:176.
50. Percival, TJ, White, JM, Ricci, MA. Compartment syndrome in the setting of vascular injury. Perspect Vasc Surg Endovasc Ther. 2011;23:119.
51. Ferrari, RP, Battiston, R, Brunelli, G, et al. The role of allopurinol in preventing oxygen free radical injury to skeletal muscle and endothelial cells after ischemia-reperfusion. J Reconstr Microsurg. 1996;12:447.
52. Tollens, T, Janzine, H, Broos, P. The pathophysiology of acute compartment syndrome. Acta Chir Belg. 1998;98:171.
53. Perler, BA, Tohmeh, AG, Bulkley, GB. Inhibition of the compartment syndrome by the ablation of free radical–mediated reperfusion injury. Surgery. 1990;108:40.
54. Gillani, S, Cao, J, Suzuki, T, et al. The effect of ischemia reperfusion injury on skeletal muscle. Injury. 2012;43:670–675.
55. Kearns, SR, O’Brien, DE, Sheehan, KM, et al. N-Acetylcysteine protects striated muscle in a model of compartment syndrome. Clin Orthop Relat Res. 2010;468:2251.
56. Ablove, RH, Babikian, G, Moy, OJ, et al. Elevation in compartment pressure following hypovolemic shock and fluid resuscitation: a canine model. Orthopedics. 2006;29:443.
57. Heppenstall, RB, Scott, R, Sapega, A, et al. A comparative study of tolerance of skeletal muscle to ischemia. Tourniquet application compared with acute compartment syndrome. J Bone Joint Surg Am. 1986;68:820.
58. Simon RR, Sherman SC, Koenigsknecht SJ, eds. Emergency Orthopedics—The Extremities, 5th ed, New York: McGraw-Hill, 2007.
59. Shah, N, Hing, C, Tucker, K, et al. Infected compartment syndrome after acupuncture. Acupunct Med. 2002;20:105.
60. Chow, CE, Friedell, ML, Freeland, MB, et al. A pitfall of protracted surgery in the lithotomy position: lower extremity compartment syndrome. Am Surg. 2007;73:19.
61. Brinker, A, Doehn, C. Compartment syndrome following surgery in the lithotomy position. Anesthesia. 2007;62:98.
62. Meyer, RS, White, KK, Smith, JM, et al. Intramuscular and blood pressures in legs positioned in the hemilithotomy position: clarification of risk factors for well-leg acute compartment syndrome. J Bone Joint Surg Am. 2002;84:1829.
63. Chase, J, Harford, F, Pinzur, MS, et al. Intraoperative lower extremity compartment pressures in lithotomy positioned patients. Dis Colon Rectum. 2000;43:678.
64. Blackman, PG. A review of chronic exertional compartment syndrome in the lower leg. Med Sci Sports Exerc. 2000;32(suppl):S4.
65. Shah, SN, Miller, BS, Kuhn, JE. Chronic exertional compartment syndrome. Am J Orthop. 2004;33:335.
66. Qvarfordt, P, Christenson, JT, Eklöf, B, et al. Intramuscular pressure, muscle blood flow, and skeletal muscle metabolism in chronic anterior tibial compartment syndrome. Clin Orthop Relat Res. 1983;179:284–290.
67. Wallensten, R, Eriksson, E. Intramuscular pressures in exercise-induced lower leg pain. Int J Sports Med. 1984;5:31.
68. Amendola, A, Rorabeck, CH. Chronic exertional compartment syndrome. In: Welsh RP, Shepard RJ, eds. Current Therapy in Sports Medicine. Toronto: Decker; 1985:250.
69. Detmer, DE, Sharpe, K, Sufit, RL, et al. Chronic compartment syndrome: diagnosis, management, and outcomes. Am J Sports Med. 1985;13:162.
70. Detmer, DE. Chronic shin splints. Sports Med. 1986;3:436.
71. Martens, MA, Backaert, M, Vermaut, G, et al. Chronic leg pain in athletes due to a recurrent compartment syndrome. Am J Sports Med. 1984;12:148.
72. Martens, MA, Moeyersoons, JP. Acute and recurrent effort-related compartment syndrome in sports. Sports Med. 1990;9:62.
73. Bae, DS, Kadiyala, RK, Waters, PM. Acute compartment syndrome in children: contemporary diagnosis, treatment, and outcome. J Pediatr Orthop. 2001;21:680.
74. Prasarn, ML, Ouellette, EA, Livingstone, A, et al. Acute pediatric upper extremity compartment syndrome in the absence of fracture. J Pediatr Orthop. 2009;29:263.
75. Matsen, FA, Clawson, DK. The deep posterior compartmental syndrome of the leg. J Bone Joint Surg Am. 1975;57:34.
76. Phillips, JH, Mackinnon, SE, Beatty, SE, et al. Vibratory sensory testing in acute compartment syndrome: a clinical and experimental study. Plast Reconstr Surg. 1987;5:796.
77. Hutchinson, MR, Ireland, ML. Common compartment syndromes in athletes. Sports Med. 1994;17:200.
78. Gelberman, RH, Garfin, SR, Hergenroeder, PR, et al. Compartment syndromes of the forearm: diagnosis and treatment. Clin Orthop. 1981;161:252.
79. Whitesides, TE, Heckman, MM. Acute compartment syndrome: update on diagnosis and treatment. J Am Acad Orthop Surg. 1996;4:209.
80. Vaillancourt, C, Shrier, I, Falk, M, et al. Quantifying delays in the recognition and management of acute compartment syndromes. Can J Emerg Med. 2001;3:26.
81. Ulmer, T. The clinical diagnosis of compartment syndrome of the lower leg: are clinical findings predictive of the disorder? J Orthop Trauma. 2002;16:572.
82. Shadgan, B, Menon, M, Sanders, D, et al. Current thinking about compartment syndrome of the lower extremity. Can J Surg. 2010;53:329.
83. McQueen, MM, Gatson, P, Court-Brown, MD. Acute compartment syndrome. Who is at risk? J Bone Joint Surg Br. 2000;82:200.
84. White, TO, Howell, GED, Will, EM, et al. Elevated intramuscular compartment pressures do not influence outcome after tibial fracture. J Trauma. 2003;55:1133.
85. Gershuni, DH, Yaru, NC, Hargens, AR, et al. Ankle and knee position as a factor modifying intracompartmental pressure in the human leg. J Bone Joint Surg Am. 1984;66:1415.
86. Al-Hadithy, N, Al-Nammari, S. Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. BET 4. Positioning of compartment pressure monitors in lower limb fractures. Emerg Med J. 2010;27:954.
87. Tsintzas, D, Ghosh, S, Maffulli, N, et al. The effect of ankle position on intracompartmental pressures of the leg [Turkish]. Acta Orthop Traumatol Turc. 2009;43:42.
88. Wiley, JP, Short, WB, Wiseman, DA, et al. Ultrasound catheter placement for deep posterior compartment pressure measurements in chronic compartment syndrome. Am J Sports Med. 1990;18:74.
89. Peck, E, Finnoff, JT, Smith, J, et al. Accuracy of palpation-guided and ultrasound-guided needle tip placement into the deep and superficial posterior leg compartments. Am J Sports Med. 2011;39:1968.
90. Lynch, JE, Lynch, JK, Cole, SL, et al. Noninvasive monitoring of elevated intramuscular pressure in a model compartment syndrome via quantitative fascial motion. J Orthop Res. 2009;27:489.
91. Mars, M, Tufts, MA, Hadley, GP. Toward reducing the trauma of direct intracompartment pressure measurement for children: an in vitro assessment of small-diameter needles. Pediatr Surg Int. 1997;12:172.
92. McDougall, CG, Johnston, GH. A new technique of catheter placement for measurement of forearm compartment pressures. J Trauma. 1991;31:1404.
93. Hislop, M, Tierney, P, Murray, P, et al. Chronic exertional compartment syndrome: the controversial “fifth” compartment of the leg. Am J Sports Med. 2003;31:770.
94. Kwiatkowski, TC, Detmer, DE. Anatomical dissection of the deep posterior compartment and its correlation with clinical reports of chronic compartment syndrome involving the deep posterior compartment. Clin Anat. 1997;10:104.
95. Ruland, RT, April, EW, Meinhard, BP. Tibialis posterior muscle: the fifth compartment? J Orthop Trauma. 1992;6:347.
96. Naidu, SH, Capo, J. Upper extremity compartment syndromes. Techn Orthop. 1997;12:117.
97. Boles, CA, Kannam, S, Cardwell, AB. The forearm: anatomy of muscle compartments and nerves. AJR Am J Roentgenol. 2000;174:151.
98. Ardolino, A, Zeineh, N, O’Connor, D. Experimental study of forearm compartment pressures. J Hand Surg Am. 2010;35:1620.
99. Hwang, RW, de Witte, PB, Ring, D. Compartment syndrome associated with distal radial fracture and ipsilateral elbow injury. J Bone Joint Surg Am. 2009;91:642.
100. Dhawan, V, Borschel, GH, Brown, DL. Acute exertional compartment syndrome of the forearm. J Trauma. 2008;64:1635.
101. Crawford, B, Comstock, S. Acute compartment syndrome of the dorsal forearm following noncontact injury. CJEM. 2010;12:453.
102. Talbot, SG, Rogers, GF. Pediatric compartment syndrome caused by intravenous infiltration. Ann Plast Surg. 2011;67:531.
103. Prynn, WL, Kates, DE, Pollack, CV. Gluteal compartment syndrome. Ann Emerg Med. 1994;24:1180.
104. Iizuka, S, Miura, N, Fukushima, T, et al. Gluteal compartment syndrome due to prolonged immobilization after alcohol intoxication: a case report. Tokai J Exp Clin Med. 2011;35:25.
105. Keene, R, Froelich, JM, Milbrandt, JC, et al. Bilateral gluteal compartment syndrome following robotic-assisted prostatectomy. Orthopedics. 2010;33:852.
106. Kumar, V, Saeed, K, Panagopoulos, A, et al. Gluteal compartment syndrome following joint arthroplasty under epidural anaesthesia: a report of 4 cases. J Orthop Surg (Hong Kong). 2007;15:113.
107. Chew, MH, Xu, GG, Ho, PW, et al. Gluteal compartment syndrome following abdominal aortic aneurysm repair: a case report. Ann Vasc Surg. 2009;23:535.e15–535.e20.
108. Chambers, L, Hame, SL, Levine, B. Acute exertional medial compartment syndrome of the foot after playing basketball. Skeletal Radiol. 2011;40:931.
109. Middleton, S, Clasper, J. Compartment syndrome of the foot—implications for military surgeons. J R Army Med Corps. 2010;156:241.
110. Ojike, NI, Roberts, CS, Giannoudis, PV. Foot compartment syndrome: a systematic review of the literature. Acta Orthop Belg. 2009;75:573.
111. Guyton, GP, Shearman, CM, Salzman, CL. The compartments of the foot revisited. Rethinking the validity of cadaver infusion experiments. J Bone Joint Surg Br. 2001;83:245.
112. Reach, JS, Jr., Amrami, KK, Felmlee, JP, et al. Anatomic compartments of the foot: a 3-tesla magnetic resonance imaging study. Clin Anat. 2007;20:201.
113. Ling, ZX, Kumar, VP. The myofascial compartments of the foot: a cadaver study. J Bone Joint Surg Br. 2008;90:1114.
114. Reach, JS, Jr., Amrami, KK, Felmlee, JP, et al. The compartments of the foot: a 3-tesla magnetic resonance imaging study with clinical correlates for needle pressure testing. Foot Ankle Int. 2007;28:584.
115. Dayton, P, Goldman, FD, Barton, E. Compartment pressure in the foot. Analysis of normal values and measurement technique. J Am Podiatr Med Assoc. 1990;80:521.
116. Lee, BY, Butler, G, Al-Waili, N. Noninvasive assessment of visco-elasticity in the presence of accumulated soft tissue fluid. J Surg Res. 2007;141:289.
117. Myerson, MS. Management of compartment syndromes of the foot. Clin Orthop. 1991;271:239.
118. Myerson, M. Diagnosis and treatment of compartment syndrome of the foot. Orthopedics. 1990;13:711.
119. Saikia, KC, Bhattacharya, TD, Agarwala, V. Anterior compartment pressure measurement in closed fractures of leg. Indian J Orthop. 2008;42:217.
120. Ogunlusi, JD, Oginni, LM, Ikem, IC. Normal leg compartment pressures in adult Nigerians using the Whiteside’s method. Iowa Orthop J. 2005;25:200.
121. Ozerdem, U. Measuring interstitial fluid pressure with fiberoptic pressure transducers. Microvasc Res. 2009;77:226.
122. Mubarak SJ, Hargens AR, Akeson WH, eds. Compartment Syndromes and Volman’s Contracture. Saunders: Philadelphia, 1981:37.
123. Hargens, AR, Schmidt, DA, Evans, KL, et al. Quantitation of skeletal-muscle necrosis in a model compartment syndrome. J Bone Joint Surg Am. 1981;63:631.
124. Shadgan, B, Menon, M, O’Brien, PJ, et al. Diagnostic techniques in acute compartment syndrome of the leg. J Orthop Trauma. 2008;22:581.
125. McQueen, MM, Christie, J, Court-Brown, CM. Acute compartment syndrome in tibial diaphyseal fractures. J Bone Joint Surg Br. 1996;78:95.
126. Gourgiotis, S, Villias, C, Germanous, S, et al. Acute limb compartment syndrome: a review. J Surg Educ. 2007;64:178.