Chapter 54 Central Retinal Vein Occlusion
Central retinal vein occlusion (CRVO) is a retinal vascular condition that may cause significant ocular morbidity. It commonly affects men and women equally and occurs predominantly in persons over the age of 65 years.1–3 In this population there may be associated systemic vascular disease, including hypertension and diabetes.4 Younger individuals who present with a clinical picture of CRVO may have an underlying hypercoagulable or inflammatory etiology.5,6 Population-based studies report the prevalence of CRVO at <0.1 to 0.4%.2,7,8 CRVO is usually a unilateral disease; however, the annual risk of developing any type of retinal vascular occlusion in the fellow eye is approximately 1% per year, and it is estimated that up to 7% of persons with CRVO may develop CRVO in the fellow eye within 5 years of onset in the first eye.1,9 Individuals with CRVO demonstrate a significant decrease in vision-related quality of life with increased healthcare costs and resource use as compared to a reference group without ocular disease.10,11 CRVO may impact a person’s ability to perform activities of daily living, especially in cases of bilateral CRVO or when concurrent ocular disease limits vision in the fellow eye.
Clinical features
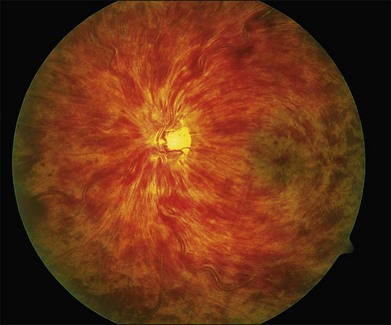
CRVO usually presents with sudden painless loss of vision, but it may also present with a history of gradual visual decline that may correlate with a series of less severe occlusions. The typical clinical constellation in CRVO includes retinal hemorrhages (both superficial flame-shaped and deep blot type) in all four quadrants of the fundus with a dilated, tortuous retinal venous system. The hemorrhages radiate from the optic nerve head, are variable in quantity, and may result in the classic “blood and thunder” appearance (Fig. 54.1). Optic nerve head swelling, cotton-wool spots, splinter hemorrhages, and macular edema are present to varying degrees (Figs 54.2 and 54.3). Breakthrough vitreous hemorrhage may also be observed.
A cilioretinal artery occlusion can occur in association with CRVO. Together, these occlusions have been hypothesized to constitute a distinct clinical entity arising from a sudden increase in the intraluminal capillary pressure due to CRVO, inducing relative occlusion of the cilioretinal artery whose perfusion pressure is lower than the central retinal artery.12,13 Rarely, a central retinal arterial occlusion may also accompany a CRVO.14
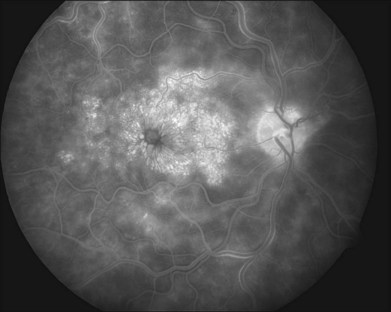
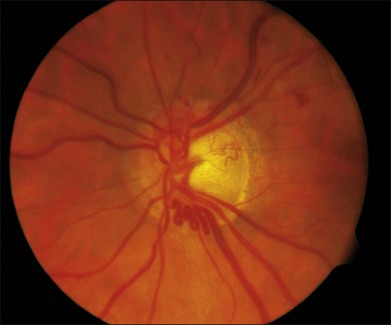
With time, the extent of retinal hemorrhage may decrease or resolve completely with variable degrees of secondary retinal pigment epithelium alterations. The time course for resolution of the hemorrhages varies and is dependent on the amount of hemorrhage produced by the occlusion. Macular edema often chronically persists despite resolution of retinal hemorrhages (Fig. 54.4). An epiretinal membrane may also form. Optociliary shunt vessels can develop on the optic nerve head, a sign of newly formed collateral channels with the choroidal circulation (Fig. 54.5). Neovascularization of the optic disc (NVD) or retinal neovascularization elsewhere (NVE) may develop as a response to secondary retinal ischemia. The vessels that comprise NVD are typically of smaller caliber than optociliary shunt vessels, branch into a vascular network resembling a net, and will leak on fluorescein angiography. Fibrovascular proliferation from NVD or NVE may result in vitreous hemorrhage or traction retinal detachment.
The natural history of CRVO was examined in the Central Vein Occlusion Study (CVOS), a randomized multicenter clinical trial of 728 eyes with CRVO. In this study, visual acuity at the time of presentation was variable but an important prognostic indicator of final visual outcome. Baseline visual acuity was 20/40 or better in 29% of affected eyes, 20/50–20/200 in 43%, and 20/250 or worse in 28%; median baseline acuity was 20/80.3,15 Of those with initial visual acuity of 20/40 or better, the majority maintained this acuity. Individuals with intermediate visual acuity (20/50–20/200) had a variable outcome: 21% improved to better than 20/50, 41% stayed in the intermediate group, and 38% were worse than 20/200. Persons with poor visual acuity at onset (less than 20/200) had only a 20% chance of improvement.9
Anterior-segment findings may include iris and/or angle neovascularization (NVI/NVA). NVI typically begins at the pupillary border but may extend across the iris surface. NVA is detected during undilated gonioscopy as fine branching vessels bridging the scleral spur and may develop without any NVI in 6–12% of eyes with CRVO.3,9,16 The CVOS used an index of any 2 clock-hours of NVI or any NVA as evidence of significant anterior-segment neovascularization, which was found in 16% of eyes with 10–29 disc areas of angiographic nonperfusion and 52% of eyes with 75 disc areas or more of angiographic nonperfusion.9 In the CVOS, worse initial visual acuity correlated with the development of NVI/NVA: 5% in eyes with 20/40 or better, 14.8% in eyes with 20/50–20/200, and 30.8% in eyes with worse than 20/200 acuity.9 Long-standing NVA may lead to secondary angle closure from peripheral anterior synechiae formation. Elevated intraocular pressure associated with NVI/NVA is the hallmark of neovascular glaucoma.
Perfusion status
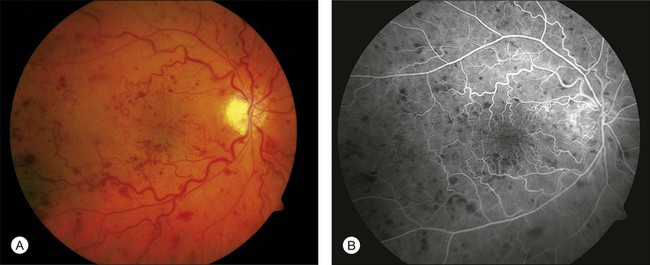
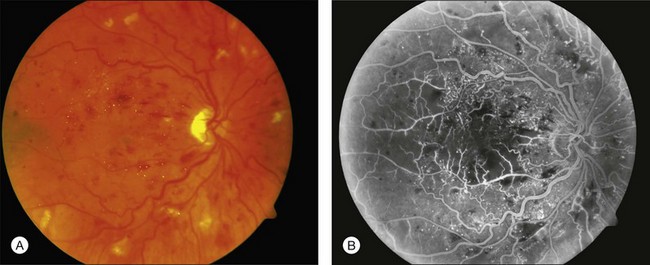
A perfused CRVO (also termed nonischemic, incomplete, or partial) demonstrates less than 10 disc areas of retinal capillary nonperfusion on angiography (Fig. 54.2). These eyes typically have a lesser degree of intraretinal hemorrhage on presentation. Generally, eyes with perfused CRVO have better initial and final visual acuity. A nonperfused CRVO (also termed ischemic, hemorrhagic, or complete) demonstrates 10 or more disc areas of retinal capillary nonperfusion on angiography (Fig. 54.3). Acutely, these eyes demonstrate a greater degree of intraretinal hemorrhage, macular and disc edema, and capillary nonperfusion than in perfused CRVO. A CRVO is categorized as indeterminate when there is sufficient intraretinal hemorrhage to prevent angiographic determination of the perfusion status. Other examination features that may help in determining the perfusion status in the acute phase of a CRVO include baseline visual acuity, presence of an afferent pupillary defect, electroretinography (a negative waveform may be seen), and Goldmann perimetry.5,9,17
The CVOS classification of initial perfusion status of the CRVO was important for determining the natural history of the disease.9 Poor visual acuity and large areas of retinal capillary nonperfusion were significant factors associated with an increased risk of developing NVI/NVA. In eyes initially categorized as perfused, 10% (56/538) developed NVI/NVA compared to 35% (61/176) of eyes initially characterized as nonperfused or indeterminate. At 3 years, there was a 45% chance of developing neovascular glaucoma after onset of ischemic CRVO.1 Overall, 34% of initially perfused eyes converted to nonperfused status after 3 years.9 In the CVOS, 38 eyes (83%) with an indeterminate CRVO at baseline were ultimately determined to be nonperfused. Initial visual acuity was highly correlated with degree of nonperfusion – eyes with nonperfused CRVO were much more likely than those with perfused CRVO to have poor visual acuity at initial presentation and final visit.9,18
Ultrawide-field angiography has enabled mapping of peripheral retinal nonperfusion not easily visualized with a conventional fundus camera. Adjusted protocols for grading extent of nonperfusion are being developed from photographs taken with ultrawide-field angiography, which may prove important in redefining characteristics of perfused versus nonperfused CRVO.19,20
Pathogenesis
The pathophysiology of CRVO is not clearly understood. Histopathologic studies of eyes enucleated for CRVO demonstrated a thrombus occluding the lumen of the central retinal vein at or just proximal to the lamina cribrosa,21 suggesting that the anatomic variations at the level of the lamina cribrosa may be important in the development of a CRVO. Within the retrolaminar portion of the optic nerve, the central retinal artery and vein are aligned parallel to each other in a common tissue sheath. The central retinal artery and vein are naturally compressed as they cross through the rigid sieve-like openings in the lamina cribrosa but typically give off branching collateral vessels just before piercing the lamina. These vessels may be subject to compression from mechanical stretching of the lamina, as with increases in intraocular pressure, which may cause a posterior bowing of the lamina and subsequent impingement on the central retinal vein. Furthermore, local factors may predispose to occlusion of the central retinal vein, including compression by an atherosclerotic central retinal artery or primary occlusion of the central retinal vein from inflammation.
Hemodynamic alterations may produce stagnant flow and subsequent thrombus formation in the central retinal vein, including diminished blood flow, increased blood viscosity, and an altered lumen wall (also known as Virchow’s triad). Experimentally, occlusion of both the retrolaminar central retinal artery and central retinal vein, posterior to the lamina cribrosa and prior to the branching of collateral channels from the main trunk, was required to produce the clinical appearance of a hemorrhagic (ischemic) CRVO.18 This implies that concurrent retinal artery insufficiency or occlusion may play a role in an ischemic CRVO. It is hypothesized that a less hemorrhagic, more likely nonischemic, CRVO may be due to occlusion of the central retinal vein at a site further posterior, allowing normal collateral channels to provide alternative routes of venous drainage.
In the largest histopathologic study of eyes with CRVO, 29 eyes enucleated for acute (within 6 hours) and chronic (up to 10 years) occlusions were reviewed,21 some of which had concurrent neovascular glaucoma. In acute occlusions, a thrombus at the level of the lamina cribrosa was adherent to a portion of the vein wall devoid of an endothelial lining. Subsequently, there was endothelial cell proliferation within the vein and secondary inflammatory cell infiltrates. Recanalization of the thrombus was demonstrated in eyes 1–5 years after the documented occlusion.
Neovascularization of the anterior and posterior segment and severity of macular edema are modulated by growth factors released from the ischemic retina. Green and colleagues demonstrated inner retinal ischemic changes in 25% of eyes enucleated for CRVO.21 In a study of enucleated eyes with CRVO and neovascular glaucoma, intraretinal vascular endothelial growth factor (VEGF) production from areas of ischemic retina was demonstrated.22 Analysis of vitreous fluid from patients with CRVO demonstrated increased levels of VEGF along with other cytokines and growth factors, including interleukin-6 (IL-6), IL-8, interferon-induced protein-10, monocyte chemotactic protein-1, and platelet-derived growth factor-AA.23–25 Intraocular VEGF levels correlate with severity of ocular findings, including neovascularization and vascular permeability,26 prompting the development of anti-VEGF agents for the treatment of CRVO (see below).
Risk factors and associations
Concurrent systemic vascular disease is a risk factor for CRVO (Box 54.1). The Eye Disease Case-Control Study found an increased risk of any type of CRVO in persons with systemic hypertension and diabetes mellitus.4 Similar associations with systemic hypertension were found in other studies.27–31 Diabetes mellitus was more prevalent in individuals with nonperfused CRVO than in matched controls from large population databases.27,28 Hyperlipidemia, arteriosclerosis, and smoking have also been linked to the development of vein occlusions.2,30,32
Box 54.1
Risk factors and associations with central retinal vein occlusion4,5,39
• Systemic vascular diseases: diabetes mellitus, hypertension, carotid insufficiency
• Ocular diseases: open angle glaucoma, ischemic optic neuropathy, pseudotumor cerebri, tilted optic nerve heads, optic nerve head drusen
• Hematologic alterations: hyperviscosity syndromes: dysproteinemias (multiple myeloma), blood dyscrasias (polycythemia vera, lymphoma, leukemia, sickle-cell disease or trait), anemia, elevated plasma homocysteine, factor XII deficiency, antiphospholipid antibody syndrome, activated protein C resistance, protein C deficiency, protein S deficiency
• Inflammatory/autoimmune vasculitis: systemic lupus erythematosus
• Medications: oral contraceptives, diuretics, hepatitis B vaccine
• Infectious vasculitis: HIV, syphilis, herpes zoster, sarcoidosis
Hematologic abnormalities, particularly conditions that predispose to a hypercoagulable state, have been identified in persons with CRVO. Individuals less than 60 years of age may have a greater association with hypercoagulable states and inflammatory conditions compared to older persons with a higher incidence of systemic vascular disease risk factors.5,6 Lahey and colleagues found one abnormal laboratory value suggesting systemic hypercoagulability in 27% of 55 patients younger than 56 years of age.33 Studies have demonstrated an increased incidence of coagulation cascade abnormalities, including protein C and S deficiency, activated protein C resistance, presence of factor V Leiden, presence of antiphospholipid antibodies, hyperhomocysteinemia, antithrombin III deficiency, prothrombin gene mutations, and abnormal fibrinogen levels.34–41 Hyperviscosity from blood dyscrasias, dysproteinemias, and dehydration have also been reported with CRVO.42–45
An increased risk of CRVO is present in eyes with open angle glaucoma.4,46 Other ocular conditions causing deformation or mechanical pressure on the optic nerve head and lamina cribrosa, including ischemic optic neuropathy, tilted optic nerve head, optic nerve head drusen, optic disc traction syndrome, and pseudotumor cerebri,42,47 have also been associated with CRVO. External compression of the globe and optic nerve from thyroid-related ophthalmopathy, mass lesions, or head trauma with orbital fracture may also result in CRVO.5
Clinical evaluation
The ophthalmic examination should be performed on both eyes and include visual acuity, pupillary reaction, and intraocular pressure. Undilated slit-lamp examination is performed to detect NVI or NVA. Undilated gonioscopy is essential to determine the presence of NVA or evidence of angle closure from peripheral anterior synechiae, as NVA may be present without any NVI in up to 12% of eyes.16 Ophthalmoscopic examination will help differentiate a CRVO from intraretinal hemorrhage associated with carotid occlusive disease.48 Adjunctive imaging studies, including optical coherence tomography (OCT) and fluorescein angiography, are helpful in evaluating and following the presence of macular edema and perfusion status.
In general, a systemic workup is not indicated in persons older than 60 years of age with known systemic vascular risk factors for CRVO. Younger patients are more likely to have predisposing conditions resulting in thrombotic disease.6,33 A limited systemic workup may be considered in those with a prior occlusion in the fellow eye, prior systemic thrombotic disease, family history of thrombosis, or other symptoms suggestive of a hematologic or rheumatologic condition. An initial laboratory investigation may include an erythrocyte sedimentation rate, antinuclear antibody, antiphospholipid antibody, and fasting plasma homocysteine levels. An elevated plasma homocysteine level may uncover a correctable etiology of CRVO, which may also influence cardiovascular health.38 Individuals with bilateral, simultaneous CRVO or mixed-type retinal vascular occlusions should have a detailed evaluation for a hypercoagulable condition, as these persons may be at risk for future, nonocular thrombotic events.9
Therapeutic options
Treatment for CRVO is directed at treating the sequelae of CRVO, particularly macular edema and neovascularization. The recent development of intravitreal pharmacotherapy has revolutionized the treatment of CRVO-associated macular edema (Fig. 54.6). While these intravitreal agents can also improve secondary neovascularization, panretinal photocoagulation (PRP) remains the definitive treatment. Alternative experimental therapies have sought to modify the anatomic alterations believed to be responsible for CRVO. Of course, appropriate management of blood pressure and other systemic factors is always of paramount importance.
Treatment of macular edema
Observation
The CVOS group M report studied the effect of grid pattern argon laser photocoagulation to improve visual acuity in 155 eyes with perfused CRVO-associated macular edema and 20/50 acuity or worse.49 Laser treatment involved a grid pattern in the area of leaking capillaries within 2 disc diameters of the foveal center but not within the foveal avascular zone. At 36 months, there was no significant difference in mean visual acuity between treated (20/200) and untreated (20/160) eyes despite reduction of angiographic macular edema. Widespread damage to the perifoveal capillary network has been hypothesized to contribute to the lack of visual recovery. Therefore, the CVOS did not recommend grid laser photocoagulation for CRVO-associated macular edema. In the absence of robust treatment options before the advent of intravitreal pharmacotherapy for retinal diseases,50 standard of care for CRVO-associated macular edema was observation.
Corticosteroid therapy
The exact mechanism of action of corticosteroids in modulating retinal edema is unknown. It is believed that corticosteroids maintain anti-inflammatory effects with modulation of production of cytokines and growth factors, including VEGF. Corticosteroids are also thought to stabilize the blood–retinal barrier with reduction in vascular permeability.51,52 There is little evidence for systemic administration of corticosteroids to treat macular edema from CRVO, unless the vein occlusion is associated with underlying systemic inflammatory disease.53 Intravitreal delivery of corticosteroids provides targeted delivery of the drug to the retinal vessels and macular tissue while limiting potential systemic toxicity.
Following case reports on the use of intravitreal triamcinolone (IVTA) for the treatment of CRVO-associated cystoid macular edema (CME),54–57 the Standard care vs Corticosteroid for Retinal vein occlusion (SCORE) study compared the efficacy and safety of two doses of preservative-free IVTA (1 mg and 4 mg) versus standard of care (i.e., observation per CVOS) for the treatment of CME in 271 eyes with CRVO.58 In this randomized, multicenter clinical trial, eyes were retreated with IVTA every 4 months for 1 year unless one of the following reasons was encountered: (1) significant improvement (central subfield OCT thickness ≤ 225 µm, visual acuity ≥ 20/25, or significant interval improvement with presumed potential for continued improvement without treatment); (2) contraindication due to significant adverse effect (e.g., significant rise in intraocular pressure); or (3) additional treatment considered futile due to no improvement following two consecutive injections.
The SCORE study showed significant improvement in visual acuity with IVTA compared to observation.58 At 1 year, 26% of eyes in the 4-mg group and 27% of eyes in the 1-mg group gained ≥15 letters at 1 year compared to 7% of untreated eyes. Mean change in visual acuity was a loss of only 1.2 letters in both IVTA groups compared to a loss of 12.1 letters in the observation group. A subgroup analysis of pseudophakic eyes revealed a mean gain in visual acuity of two letters in the 1-mg group and a mean loss of visual acuity of one letter in the 4-mg group compared to a mean loss of 14 letters in the observation group. In these pseudophakic eyes, a gain of three or more lines was achieved in 20% of both IVTA groups compared to 6% in the observation group.
Primary ocular adverse events included cataract formation and elevated intraocular pressure.58 Cataract formation was observed in 26% and 33% of eyes in the 1-mg and 4-mg IVTA groups, respectively, compared to 18% in the observation group. Over 2 years, 27% of eyes from the 4-mg group and 3% of eyes in the 1-mg group required cataract surgery, but no eyes from the observation group required cataract surgery. Elevated intraocular pressure was observed in 20% and 35% of eyes in the 1-mg and 4-mg IVTA groups, respectively, compared to 8% in the observation group. At 12 months, there were no cases of endophthalmitis or retinal detachment in any group.
The limited duration of the response to IVTA therapy has prompted the development of sustained-release corticosteroids for the treatment of CRVO-associated CME. In a case series of 14 eyes with chronic refractory CME secondary to CRVO, improvements in visual acuity and macular edema after 1 year were observed in eyes surgically implanted with a sustained-release intravitreal fluocinolone acetonide implant (Retisert).59 In this series, all phakic eyes developed visually significant cataracts, and 92% required medical or surgical intervention for increased intraocular pressure.
In 2009, a sustained-release intravitreal dexamethasone delivery system, Ozurdex, was approved by the Food and Drug Administration (FDA) for the treatment of macular edema secondary to CRVO. A multicentered, international study at 167 sites in 24 countries reported the 6-month outcomes of a 0.35-mg and 0.7-mg Ozurdex dose for CME in eyes with retinal vein occlusion, a group consisting of both branch retinal vein occlusions and CRVO, compared to sham-injected eyes.60 The Ozurdex implant resulted in improved mean visual acuity, increased rate of ≥15 letter gain, and lower rate of ≤15 letter loss. Subgroup analysis was performed on 136 CRVO eyes injected with Ozurdex. The 0.7-mg group demonstrated significant gains of ≥15 letters compared to sham control at 30 days (28% versus 7%) and at 60 days (29% versus 9%), but not at 90 or 180 days. The 0.35-mg group demonstrated significant gains of ≥15 letters compared to sham at 30 days (20% versus 7%), at 60 days (33% versus 9%), and at 90 days (24% versus 10%), but not at 180 days. No significant difference in cataract formation or cataract surgery was identified between the treated and untreated groups. Ocular hypertension occurred in 4% of eyes receiving the drug delivery system, and most were able to be managed with topical medications. There were no significant differences between the sham and treatment groups in vitreous hemorrhage, vitreous floaters, or retinal hemorrhage. Two retinal detachments occurred in the study, one in the sham group and one in the 0.7-mg group. No cases of endophthalmitis were reported.60
Intravitreal anti-VEGF therapy
VEGF plays a key role in the pathophysiology of CRVO and its sequelae. Markedly elevated levels of VEGF have been demonstrated in the vitreous of eyes with ischemic CRVO.23 It has been hypothesized that VEGF may cause capillary endothelial cell proliferation that leads to progressive vascular closure and nonperfusion in CRVO.61 Anti-VEGF therapy may result in enhanced blood flow, lowered intravenous pressure, and the normalization of venous diameter and tortuosity.61 Several intravitreal anti-VEGF treatments have been developed, including ranibizumab (Lucentis), bevacizumab (Avastin), and pegaptanib (Macugen).
The double-masked, multicenter, randomized phase III CRUISE trial prospectively compared monthly intravitreal injections of 0.3 mg or 0.5 mg ranibizumab to sham-injected controls in the treatment of 392 patients with macular edema after CRVO.62 Eyes treated with 0.3 mg and 0.5 mg ranibizumab gained 12.7 and 14.9 letters, respectively, at 6 months compared to a 0.8 letter gain in the sham group. Additionally 46.2% (0.3 mg) and 47.7% (0.5 mg) of eyes treated with intravitreal ranibizumab gained ≥15 letters from baseline compared to only 16.9% in the sham group. The mean change in central foveal thickness was -434 µm (0.3 mg) and -452 µm (0.5 mg) in the treatment groups compared to -167.7 µm in the sham group. These improvements in visual acuity and foveal thickness were statistically significant at 6 months. There were no cases of retinal detachment or endophthalmitis in any of the groups. Systemic adverse events were also rare. There were no reported strokes in any of the groups. One transient ischemic attack occurred in the 0.5-mg group, and one myocardial infarction occurred in each of the groups. Following the 6-month endpoint, the control group was treated with ranibizumab on an as-needed basis, limiting further information on the natural history of CRVO and any long-term comparison in this study between a true control group and the treatment group.15 The results of this study prompted FDA approval of ranibizumab for the treatment of CRVO. However, the long-term effects of ranibizumab and other anti-VEGF agents on macular edema for CRVO are still unknown.
Pegaptanib (Macugen) is currently the only other FDA-approved intravitreal anti-VEGF agent which received approval for the treatment of neovascular age-related macular degeneration. Similar to other anti-VEGF agents, the off-label use of pegaptanib for the treatment of retinal diseases has been investigated. In a phase II double-masked, multicenter, randomized trial, patients with CRVO receiving 0.3 mg or 1 mg pegaptanib every 6 weeks for 24 weeks were prospectively compared to sham-injected controls.63 Patients treated with 0.3 mg and 1 mg pegaptanib had a risk of ≤15 letter loss of 9% and 6%, respectively, which was significantly lower compared to 31% in sham-injected eyes. While there was no significant difference in gain of ≥15 letters among groups, 0.3 mg and 1 mg pegaptanib groups showed 7.1 and 9.9 mean letter improvement, respectively, compared to -3.2 letter loss in sham-injected eyes; only the 1-mg group difference was statistically significant. The 0.3-mg and 1-mg pegaptanib groups also exhibited a significantly greater decrease of -269 µm and -210 µm, respectively, in central retinal thickness compared to -5 µm in the sham group.
Much of our understanding of the role of anti-VEGF agents in the treatment of retinal disease comes from studies with bevacizumab. While bevacizumab is not FDA-approved for intravitreal use, its ophthalmic use quickly grew due to its low cost, reported efficacy, and availability prior to the approval of ranibizumab. Several retrospective and prospective case series have reported decreased retinal thickness and improved visual acuity after intravitreal treatment with bevacizumab in eyes with CRVO-associated macular edema.61,64–67 In a study using a single injection of bevacizumab, the peak increase in visual acuity was reached between 3 and 6 weeks after the injection, followed by a return of macular edema and secondary decrease in visual acuity.66 As a result, in most case series, repeated treatment with bevacizumab was given at 4–8-week intervals to avoid recurrence of CME. Improvements in macular edema and visual acuity in CRVO following intravitreal bevacizumab are greater than would be expected by the natural history alone and have been reported in both ischemic and nonischemic CRVO.67 Additionally, intravitreal bevacizumab has been associated with rapid resolution of anterior-segment neovascularization,68 indicating that neovascular complications of CRVO, including neovascular glaucoma, may respond well to anti-VEGF agents.
The intravitreal use of bevacizumab is off-label. The lack of large, randomized, controlled clinical trials limits the safety profile data on bevacizumab for rare events, and it is difficult to quantify theoretical systemic risks such as stroke and myocardial infarction. In retrospective studies, the side-effect profile of bevacizumab was similar to that of ranibizumab, with an equivalent rate of endophthalmitis of 0.2%.69 In another study, the most common adverse events were conjunctival hyperemia and subconjunctival hemorrhage at the injection site.64 A recent randomized, blinded comparison of bevacizumab and ranibizumab in the treatment of age-related macular degeneration suggests that bevacizumab may be comparable to ranibizumab in efficacy.70 It is unclear if these results are generalizable to the treatment of CRVO or of other retinal disorders.
Given the efficacy of intravitreal pharmacologic agents in the treatment of CRVO, and given their favorable side-effect profile, the use of intravitreal pharmacotherapy has replaced observation as the previous standard of care established by the CVOS for treatment of CRVO-associated macular edema. The specific indications for anti-VEGF agents compared to corticosteroids are currently being investigated along with the efficacy of combined therapy.71 In a small study following 32 eyes with CRVO prospectively randomized to either IVTA or intravitreal bevacizumab treatment every 3 months as needed, improvements in visual acuity and central retinal thickness were equivalent at the 9-month endpoint. Significantly fewer injections were required with IVTA (mean 1.31 injections) than with bevacizumab (mean 2.38 injections, P = 0.004) over 9 months, but IVTA was associated with significantly more adverse events, particularly increases in intraocular pressure and visually significant premacular membranes.72 The long-term role of these agents in the treatment of CRVO, the role for early intervention in improving outcome, and their ability to limit progression to the ischemic variant remain unanswered but are currently being investigated.73,74 Further understanding of duration of effect and frequency of injections required will be important in optimizing dosing of anti-VEGF agents.
Treatment of ocular neovascularization
Laser photocoagulation
The CVOS group N report compared the efficacy of PRP placement at the time of study entry in eyes with nonperfused CRVO that did not have evidence of NVI/NVA (early treatment group, n = 90) with delayed, but prompt, PRP application (no early treatment group, n = 91) only when NVI/NVA was detected.75 NVI/NVA developed in 20% of early treatment and 34% of no early treatment eyes. There was greater resolution of NVI/NVA by 1 month after PRP in 56% of no early treatment eyes compared with 22% of early treatment eyes. The CVOS therefore recommended that PRP be delivered promptly after the development of NVI/NVA but not prophylactically in eyes with nonperfused CRVO. In approximately 90% of cases, the regression of NVI/NVA occurs within 1–2 months of PRP. Persistent neovascularization after PRP should be followed closely, and additional PRP may be applied in attempts to halt its progression. Persons presenting with NVD/NVE without NVI/NVA should be treated with PRP, as performed in eyes with proliferative diabetic retinopathy or branch retinal vein occlusion, to prevent anterior-segment neovascularization. Prophylactic placement of PRP may be considered in eyes with nonperfused CRVO and risk factors for developing NVI/NVA (male gender, short duration of CRVO, extensive retinal nonperfusion, and extensive retinal hemorrhage) or in cases where frequent ophthalmologic follow-up is not possible.
Medical therapy
Topical or systemic antiglaucoma agents may be required to reduce elevated intraocular pressure. Topical corticosteroids can reduce anterior-segment inflammation by stabilizing tight junctions in neovascular tissue, thereby reducing vascular exudation. Cycloplegic agents prevent posterior synechiae formation between the iris and lens. Anti-VEGF agents may result in rapid regression of neovascularization, but these agents should be used as a temporizing adjunctive measure with subsequent placement of PRP for definitive treatment.76 Failure of medical therapy to control intraocular pressure may require surgical intervention (e.g., trabeculectomy or tube placement).
Treatment of systemic medical conditions
Identification and treatment of systemic vascular risk factors, such as systemic hypertension and diabetes mellitus, are of paramount importance in individuals with CRVO. Coordination with the internist is strongly recommended. The role of systemic anticoagulation in CRVO is unclear as there is no evidence that agents, such as aspirin or heparin, can prevent or alter the natural history of CRVO; patients taking warfarin sodium (Coumadin) can still develop CRVO despite maintaining therapeutic levels of anticoagulation.77 Prophylactic use of these medications, however, may help prevent nonocular thrombotic events, especially in individuals with known systemic vascular disease, and may be considered in coordination with the patient’s internist.
Oral pentoxifylline is a potent vasodilator used in systemic vascular diseases to improve perfusion to occluded vessels and enhance the development of collateral circulation. A retrospective series of 11 patients treated with oral pentoxifylline (400 mg three times a day) for an average of 5 months demonstrated a 10% mean reduction in macular thickening by volumetric OCT but did not demonstrate a change in visual acuity or perfusion status.78
The reported increased plasma viscosity in persons with CRVO has prompted interest in systemic hemodilution to increase oxygen supply to the retina. A recent prospective, randomized, controlled clinical trial of selected CRVO patients demonstrated significant visual acuity gains and reduced conversion to nonperfusion.79 Hemodilution is likely not appropriate for patients with anemia, renal insufficiency, or pulmonary insufficiency, which may limit its clinical use.79,80
Alternative treatments
Chorioretinal venous anastomosis
In eyes with perfused CRVO, investigators have bypassed the occluded central retinal vein by creating a chorioretinal anastomosis (CRA) between a nasal branch retinal vein and the choroidal circulation. Successful creation of an anastomosis may allow transretinal retrograde flow of venous blood from the eye and prevent the development of retinal ischemia or the reduction of macular edema. CRAs have been created through a surgical transretinal venipuncture technique81,82 or, more commonly, through argon or neodymium : yttrium aluminum garnet (Nd-YAG) laser delivery directly at a branch retinal vein to rupture the posterior vein wall and Bruch’s membrane.83,84 McAllister and colleagues prospectively randomized 113 patients with nonischemic CRVO to laser-induced CRA or sham treatment.85 Treated eyes demonstrated a significant 8.3 letter mean improvement compared to sham. Successful anastomosis was created in 76.4% of CRVO patients, and subanalysis of this group revealed 11.7 mean letter improvement compared to sham. Neovascularization developed at the site of anastomosis in 18.2% of treated eyes, and 9.1% of treated eyes required vitrectomy because of macular traction or nonclearing vitreous hemorrhage.
Immediate complications from this technique may include intraretinal, subretinal, or vitreous hemorrhage, while long-term complications include nonclearing vitreous hemorrhage, epiretinal avascular proliferation, fibrovascular proliferation, secondary neovascularization (choroidal, retinal, choroidovitreal, anterior segment), and traction retinal detachment.81,86,87 Visual recovery may be limited in spite of successful anastomosis creation due to thrombosis of the treated vein with progressive retinal ischemia and development of macular pigment abnormalities following resolution of chronic macular edema.
Tissue plasminogen activator
Systemic administration of low-dose (50 mg) front-loaded r-tPA has been attempted in two pilot studies with visual acuity improvement in 30–73% of patients.88,89 In a prospective, multicentered randomized trial of 41 patients with CRVO, Hattenbach and colleagues demonstrated significant 1-year improvement of three lines of acuity in 45% of patients undergoing low-dose r-tPA compared to 21% of patients undergoing hemodilution.90 Another study, examining full-dose (≤100 mg) systemic tPA for the treatment of 96 patients with CRVO, reported development of intraocular hemorrhage in 3 patients and a fatal stroke in 1 patient.91 While Hattenbach et al.90 did not observe any serious adverse events in their trial of low dose r-tPA, these complications highlight the importance of approaching systemic administration of r-tPA with caution.
Intravitreal delivery of r-tPA has potential advantages, including decreased risk of systemic complications, directed delivery to the vitreous cavity, and subsequent access to the retinal vessels with low risk of ocular morbidity from the procedure. Of 47 persons in three noncontrolled studies of intravitreal r-tPA for both ischemic and nonischemic CRVO of less than 21 days’ duration, 28–44% had three lines of visual acuity improvement, with 6-month follow-up.92–94 Administration of r-tPA did not significantly alter final perfusion status, especially in pretreatment ischemic eyes. Although there were no significant treatment-related complications, differences in inclusion criteria and dosage of r-tPA used (between 66 and 100 mg) limit generalizations from these studies. Ghazi and colleagues reported using a standard dose of 50 mg of intravitreal r-tPA in 12 eyes with acute CRVO of less than 3 days’ duration.95 In all patients, perfusion status remained unchanged at last follow-up, but marked improvement (20/50 or better) was demonstrated in eyes with perfused CRVO. This report did not include a control arm but suggests that prompt use of intravitreal r-tPA in perfused CRVO may provide visual benefit.
Endovascular delivery of r-tPA involves cannulation of retinal vessels, either through a neuroradiologic or a vitreoretinal approach, with delivery of minute quantities of r-tPA directly to the occluded vessels to release the suspected thrombus.96,97 Weiss and Bynoe reported their technique of pars plana vitrectomy followed by cannulation of a branch vein and infusion of r-tPA towards the optic nerve head.98 In their report, 50% of 28 eyes with CRVO of greater than 1 month’s duration and worse than 20/400 preoperative acuity recovered more than three lines of acuity by a mean follow-up of 12 months. There was a trend towards increased perfusion by fluorescein angiography attributed in part to the resolution of intraretinal hemorrhages after the procedure. This study did not include a control arm. Complications included vitreous hemorrhage in seven eyes and treated retinal detachment in one eye. In another prospective study of 13 patients undergoing endovascular r-tPA delivery, visual recovery did not correspond with successful thrombolysis, and complications including retinal detachment, phthisis, neovascular glaucoma, and cataract were considered unacceptably high.99
Surgical treatments
Vitrectomy
Pars plana vitrectomy may be useful to address complications of CRVO and even to attempt to alter the natural course of the disease. Eyes with nonclearing vitreous hemorrhage from secondary retinal neovascularization may benefit from surgical evacuation. At the time of vitrectomy, clearing of the hemorrhage can be combined with removal of epiretinal membranes and removal of fibrovascular proliferations, if present, and the placement of complete endolaser PRP.100 Although this technique may prevent or aid in regression of anterior-segment neovascularization, visual outcomes may be limited due to the extent of underlying retinal nonperfusion.101 In eyes with extensive anterior-segment neovascularization and neovascular glaucoma, pars plana vitrectomy and endolaser PRP may be combined with pars plana placement of a glaucoma drainage device to avoid anterior-chamber hemorrhage at the time of tube placement.
The potential role for pars plana vitrectomy with peeling of the internal limiting membrane has also been investigated for treatment of CME secondary to CRVO. Small studies have demonstrated an improvement in CME accompanied by improvement in visual acuity.102–104 In contrast, one study showed no significant improvement in visual acuity despite improvement of central foveal thickness.105
Radial optic neurotomy
Opremcak and colleagues first reported combining pars plana vitrectomy with radial optic neurotomy (RON) involving transvitreal incision of the nasal scleral ring to release pressure on the central retinal vein at the level of the scleral outlet. In a nonrandomized study of 117 consecutive eyes undergoing RON, Opremcak et al. reported anatomic resolution of CME in 95% and visual improvement in 71% of eyes.106 Interpretation of these impressive results must consider the nonrandomized nature of the study and the absence of a control group. While subsequent reports on RON have also demonstrated visual improvement,107–109 no study has replicated the 71% improvement in Opremcak’s study, and some studies have reported that visual improvement following RON is comparable to natural history.109 In contrast, other studies have not demonstrated improvement in visual acuity110 or in central retinal hemodynamics,111,112 questioning the role for RON in CRVO treatment. Importantly, RON has been associated with signficant risks, including postoperative visual field defects, laceration of central retinal vessels, globe perforation, choroidal neovascularization, and retinal detachment.107,109,110,113 Evidence of the efficacy of RON in management of CRVO is limited by the absence of randomized prospective trials but currently does not clearly demonstrate a beneficial role. With the availability of effective intravitreal pharmacologic agents, the use of RON for patients with CRVO has largely been abandoned.
Follow-up
Prior to the availability of intravitreal pharmacotherapy for the treatment of CRVO-associated macular edema, follow-up for eyes with CRVO to detect neovascular complications was typically guided by visual acuity at initial presentation. Eyes with initial acuity of 20/40 or better were generally examined every 1–2 months for 6 months, then annually if stable. Eyes with initial acuity worse than 20/200 were seen monthly for the initial 6 months, then bimonthly for the next 6 months, as these eyes have a greater degree of nonperfusion and a higher risk of developing NVI/NVA. Eyes with acuity between 20/50 and 20/200 have an intermediate risk of developing NVI/NVA and were also typically examined monthly for the first 6 months. Eyes that experienced a drop in visual acuity below the 20/200 level during follow-up were re-evaluated with assessment of perfusion status and presence of neovascularization, and monthly follow-up for an additional 6 months was recommended for these eyes.9 With the development of intravitreal pharmacologic agents, this follow-up paradigm has changed. Follow-up intervals for patients undergoing treatment with intravitreal pharmacotherapy should currently be based on clinical response to treatment.
Conclusion
CRVO is a sight-threatening disease with significant ocular morbidity, including macular edema and ocular neovascularization. Before the recent advent of intravitreal pharmacotherapy in the management of CRVO, standard of care was guided by results from the CVOS, which recommended observation of macular edema and retinal ischemia with management of neovascular sequelae using PRP. In the absence of robust treatment options for CRVO,50 other approaches, including the administration of r-tPA, creation of CRA, and various surgical interventions, had been reported with variable success and often unacceptable adverse effects. More recently, intravitreal corticosteroids and particularly anti-VEGF agents have demonstrated impressive improvements in macular edema, visual acuity, and even neovascular complications with a favorable side-effect profile. The use of ranibizumab (Lucentis) and a sustained-release dexamethasone implant (Ozurdex) have been FDA-approved for the treatment of CRVO. Intravitreal pharmacotherapy has now replaced observation as the standard of care for the management of macular edema associated with CRVO.
1 Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrence and demographic characteristics. Am J Ophthalmol. 1994;117:429–441.
2 Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains Eye Study. Arch Ophthalmol. 1996;114:1243–1247.
3 The Central Vein Occlusion Study. Baseline and early natural history report. Arch Ophthalmol. 1993;111:1087–1095.
4 The Eye Disease Case-Control Study Group. Risk factors for central retinal vein occlusion. Arch Ophthalmol. 1996;114:545–554.
5 Gutman FA. Evaluation of a patient with central retinal vein occlusion. Ophthalmology. 1983;90:481–483.
6 Fong AC, Schatz H. Central retinal vein occlusion in young adults. Surv Ophthalmol. 1993;37:393–417.
7 Klein R, Klein BE, Moss SE, et al. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98:133–141. discussion 41–3
8 Rogers S, McIntosh RL, Cheung N, et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117:313–319. e1
9 The Central Vein Occlusion Study Group. Natural history and clinical management of central retinal vein occlusion. Arch Ophthalmol. 1997;115:486–491.
10 Deramo VA, Cox TA, Syed AB, et al. Vision-related quality of life in people with central retinal vein occlusion using the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2003;121:1297–1302.
11 Fekrat S, Shea AM, Hammill BG, et al. Resource use and costs of branch and central retinal vein occlusion in the elderly. Curr Med Res Opin. 2010;26:223–230.
12 Schatz H, Fong AC, McDonald HR, et al. Cilioretinal artery occlusion in young adults with central retinal vein occlusion. Ophthalmology. 1991;98:594–601.
13 Hayreh SS, Fraterrigo L, Jonas J. Central retinal vein occlusion associated with cilioretinal artery occlusion. Retina. 2008;28:581–594.
14 Brown GC, Duker JS, Lehman R, et al. Combined central retinal artery-central vein obstruction. Int Ophthalmol. 1993;17:9–17.
15 Decroos FC, Fekrat S. The natural history of retinal vein occlusion: what do we really know? Am J Ophthalmol. 2011;151:739–741. e2
16 Browning DJ, Scott AQ, Peterson CB, et al. The risk of missing angle neovascularization by omitting screening gonioscopy in acute central retinal vein occlusion. Ophthalmology. 1998;105:776–784.
17 Hayreh SS, Klugman MR, Beri M, et al. Differentiation of ischemic from non-ischemic central retinal vein occlusion during the early acute phase. Graefes Arch Clin Exp Ophthalmol. 1990;228:201–217.
18 Hayreh SS, Podhajsky PA, Zimmerman MB. Natural history of visual outcome in central retinal vein occlusion. Ophthalmology. 2011;118:119–133. e1–2
19 Tsui I, Kaines A, Havunjian MA, et al. Ischemic index and neovascularization in central retinal vein occlusion. Retina. 2011;31:105–110.
20 Spaide RF. Peripheral areas of nonperfusion in treated central retinal vein occlusion as imaged by wide-field fluorescein angiography. Retina. 2011;31:829–837.
21 Green WR, Chan CC, Hutchins GM, et al. Central retinal vein occlusion: a prospective histopathologic study of 29 eyes in 28 cases. Retina. 1981;1:27–55.
22 Pe’er J, Folberg R, Itin A, et al. Vascular endothelial growth factor upregulation in human central retinal vein occlusion. Ophthalmology. 1998;105:412–416.
23 Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487.
24 Funk M, Kriechbaum K, Prager F, et al. Intraocular concentrations of growth factors and cytokines in retinal vein occlusion and the effect of therapy with bevacizumab. Invest Ophthalmol Vis Sci. 2009;50:1025–1032.
25 Noma H, Funatsu H, Mimura T, et al. Vitreous levels of interleukin-6 and vascular endothelial growth factor in macular edema with central retinal vein occlusion. Ophthalmology. 2009;116:87–93.
26 Boyd SR, Zachary I, Chakravarthy U, et al. Correlation of increased vascular endothelial growth factor with neovascularization and permeability in ischemic central vein occlusion. Arch Ophthalmol. 2002;120:1644–1650.
27 Elman MJ, Bhatt AK, Quinlan PM, et al. The risk for systemic vascular diseases and mortality in patients with central retinal vein occlusion. Ophthalmology. 1990;97:1543–1548.
28 Hayreh SS, Zimmerman B, McCarthy MJ, et al. Systemic diseases associated with various types of retinal vein occlusion. Am J Ophthalmol. 2001;131:61–77.
29 Koizumi H, Ferrara DC, Brue C, et al. Central retinal vein occlusion case-control study. Am J Ophthalmol. 2007;144:858–863.
30 Cheung N, Klein R, Wang JJ, et al. Traditional and novel cardiovascular risk factors for retinal vein occlusion: the multiethnic study of atherosclerosis. Invest Ophthalmol Vis Sci. 2008;49:4297–4302.
31 Di Capua M, Coppola A, Albisinni R, et al. Cardiovascular risk factors and outcome in patients with retinal vein occlusion. J Thromb Thrombolysis. 2010;30:16–22.
32 O’Mahoney PR, Wong DT, Ray JG. Retinal vein occlusion and traditional risk factors for atherosclerosis. Arch Ophthalmol. 2008;126:692–699.
33 Lahey JM, Tunc M, Kearney J, et al. Laboratory evaluation of hypercoagulable states in patients with central retinal vein occlusion who are less than 56 years of age. Ophthalmology. 2002;109:126–131.
34 Williamson TH, Rumley A, Lowe GD. Blood viscosity, coagulation, and activated protein C resistance in central retinal vein occlusion: a population controlled study. Br J Ophthalmol. 1996;80:203–208.
35 Gottlieb JL, Blice JP, Mestichelli B, et al. Activated protein C resistance, factor V Leiden, and central retinal vein occlusion in young adults. Arch Ophthalmol. 1998;116:577–579.
36 Hayreh SS, Zimmerman MB, Podhajsky P. Hematologic abnormalities associated with various types of retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2002;240:180–196.
37 Hvarfner C, Hillarp A, Larsson J. Influence of factor V Leiden on the development of neovascularisation secondary to central retinal vein occlusion. Br J Ophthalmol. 2003;87:305–306.
38 Cahill MT, Stinnett SS, Fekrat S. Meta-analysis of plasma homocysteine, serum folate, serum vitamin B(12), and thermolabile MTHFR genotype as risk factors for retinal vascular occlusive disease. Am J Ophthalmol. 2003;136:1136–1150.
39 Yap YC, Barampouti F. Central retinal vein occlusion secondary to protein S deficiency. Ann Ophthalmol (Skokie). 2007;39:343–344.
40 Rehak M, Rehak J, Muller M, et al. The prevalence of activated protein C (APC) resistance and factor V Leiden is significantly higher in patients with retinal vein occlusion without general risk factors. Case–control study and meta-analysis. Thromb Haemost. 2008;99:925–929.
41 Incorvaia C, Parmeggiani F, Costagliola C, et al. The heterozygous 20210 G/A genotype prevalence in patients affected by central and branch retinal vein occlusion: a pilot study. Graefes Arch Clin Exp Ophthalmol. 2001;239:251–256.
42 Ciardella AP, Clarkson JG, Guyer DR, et al. Central retinal vein occlusion: a primer and review. In: Guyer DR, Yannuzzi LA, Chang S, et al. Retina – vitreous – macula. New York: W.B. Saunders, 1999.
43 Francis PJ, Stanford MR, Graham EM. Dehydration is a risk factor for central retinal vein occlusion in young patients. Acta Ophthalmol Scand. 2003;81:415–416.
44 Alexander P, Flanagan D, Rege K, et al. Bilateral simultaneous central retinal vein occlusion secondary to hyperviscosity in Waldenstrom’s macroglobulinaemia. Eye (Lond). 2008;22:1089–1092.
45 Al-Abdulla NA, Thompson JT, LaBorwit SE. Simultaneous bilateral central retinal vein occlusion associated with anticardiolipin antibodies in leukemia. Am J Ophthalmol. 2001;132:266–268.
46 Dev S, Herndon L, Shields MB. Retinal vein occlusion after trabeculectomy with mitomycin C. Am J Ophthalmol. 1996;122:574–575.
47 Rumelt S, Karatas M, Pikkel J, et al. Optic disc traction syndrome associated with central retinal vein occlusion. Arch Ophthalmol. 2003;121:1093–1097.
48 Kearns TP. Differential diagnosis of central retinal vein obstruction. Ophthalmology. 1983;90:475–480.
49 The Central Vein Occlusion Study Group M report. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. Ophthalmology. 1995;102:1425–1433.
50 Hayreh SS. Management of central retinal vein occlusion. Ophthalmologica. 2003;217:167–188.
51 Nauck M, Karakiulakis G, Perruchoud AP, et al. Corticosteroids inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. Eur J Pharmacol. 1998;341:309–315.
52 Felinski EA, Antonetti DA. Glucocorticoid regulation of endothelial cell tight junction gene expression: novel treatments for diabetic retinopathy. Curr Eye Res. 2005;30:949–957.
53 Shaikh S, Blumenkranz MS. Transient improvement in visual acuity and macular edema in central retinal vein occlusion accompanied by inflammatory features after pulse steroid and anti-inflammatory therapy. Retina. 2001;21:176–178.
54 Jonas JB, Kreissig I, Degenring RF. Intravitreal triamcinolone acetonide as treatment of macular edema in central retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2002;240:782–783.
55 Ip MS, Kumar KS. Intravitreous triamcinolone acetonide as treatment for macular edema from central retinal vein occlusion. Arch Ophthalmol. 2002;120:1217–1219.
56 Greenberg PB, Martidis A, Rogers AH, et al. Intravitreal triamcinolone acetonide for macular oedema due to central retinal vein occlusion. Br J Ophthalmol. 2002;86:247–248.
57 Park CH, Jaffe GJ, Fekrat S. Intravitreal triamcinolone acetonide in eyes with cystoid macular edema associated with central retinal vein occlusion. Am J Ophthalmol. 2003;136:419–425.
58 Ip MS, Scott IU, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol. 2009;127:1101–1114.
59 Ramchandran RS, Fekrat S, Stinnett SS, et al. Fluocinolone acetonide sustained drug delivery device for chronic central retinal vein occlusion: 12-month results. Am J Ophthalmol. 2008;146:285–291.
60 Haller JA, Bandello F, Belfort R, Jr., et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117:1134–1146. e3
61 Ferrara DC, Koizumi H, Spaide RF. Early bevacizumab treatment of central retinal vein occlusion. Am J Ophthalmol. 2007;144:864–871.
62 Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary endpoint results of a phase III study. Ophthalmology. 2010;117:1124–1133. e1
63 Wroblewski JJ, Wells JA, 3rd., Adamis AP, et al. Pegaptanib sodium for macular edema secondary to central retinal vein occlusion. Arch Ophthalmol. 2009;127:374–380.
64 Costa RA, Jorge R, Calucci D, et al. Intravitreal bevacizumab (avastin) for central and hemicentral retinal vein occlusions: IBeVO study. Retina. 2007;27:141–149.
65 Pai SA, Shetty R, Vijayan PB, et al. Clinical, anatomic, and electrophysiologic evaluation following intravitreal bevacizumab for macular edema in retinal vein occlusion. Am J Ophthalmol. 2007;143:601–606.
66 Stahl A, Agostini H, Hansen LL, et al. Bevacizumab in retinal vein occlusion-results of a prospective case series. Graefes Arch Clin Exp Ophthalmol. 2007;245:1429–1436.
67 Kriechbaum K, Michels S, Prager F, et al. Intravitreal Avastin for macular oedema secondary to retinal vein occlusion: a prospective study. Br J Ophthalmol. 2008;92:518–522.
68 Ehlers JP, Spirn MJ, Lam A, et al. Combination intravitreal bevacizumab/panretinal photocoagulation versus panretinal photocoagulation alone in the treatment of neovascular glaucoma. Retina. 2008;28:696–702.
69 Fintak DR, Shah GK, Blinder KJ, et al. Incidence of endophthalmitis related to intravitreal injection of bevacizumab and ranibizumab. Retina. 2008;28:1395–1399.
70 Martin DF, Maguire MG, Ying GS, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–1908.
71 Ehlers JP, Fekrat S. Differential effects of triamcinolone and bevacizumab in central retinal vein occlusion. Can J Ophthalmol. 2011;46:88–89.
72 Ding X, Li J, Hu X, et al. Prospective study of intravitreal triamcinolone acetonide versus bevacizumab for macular edema secondary to central retinal vein occlusion. Retina. 2011;31:838–845.
73 Chang LK, Spaide RF, Klancnik JM, et al. Longer-term outcomes of a prospective study of intravitreal ranibizumab as a treatment for decreased visual acuity secondary to central retinal vein occlusion. Retina. 2011;31:821–828.
74 DeCroos FC, Ehlers JP, Stinnett S, et al. Intravitreal bevacizumab for macular edema due to central retinal vein occlusion: perfused vs. ischemic and early vs. late treatment. Curr Eye Res. 2011;36:1164–1170.
75 The Central Vein Occlusion Study Group N report. A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. Ophthalmology. 1995;102:1434–1444.
76 Iliev ME, Domig D, Wolf-Schnurrbursch U, et al. Intravitreal bevacizumab (Avastin) in the treatment of neovascular glaucoma. Am J Ophthalmol. 2006;142:1054–1056.
77 Mruthyunjaya P, Wirostko WJ, Chandrashekhar R, et al. Central retinal vein occlusion in patients treated with long-term warfarin sodium (Coumadin) for anticoagulation. Retina. 2006;26:285–291.
78 Park CH, Scott AW, Fekrat S. Effect of oral pentoxifylline on cystoid macular edema associated with central retinal vein occlusion. Retina. 2007;27:1020–1025.
79 Glacet-Bernard A, Atassi M, Fardeau C, et al. Hemodilution therapy using automated erythrocytapheresis in central retinal vein occlusion: results of a multicenter randomized controlled study. Graefes Arch Clin Exp Ophthalmol. 2011;249:505–512.
80 Chen HC, Wiek J, Gupta A, et al. Effect of isovolaemic haemodilution on visual outcome in branch retinal vein occlusion. Br J Ophthalmol. 1998;82:162–167.
81 Fekrat S, de Juan E, Jr. Chorioretinal venous anastomosis for central retinal vein occlusion: transvitreal venipuncture. Ophthalmic Surg Lasers. 1999;30:52–55.
82 Peyman GA, Kishore K, Conway MD. Surgical chorioretinal venous anastomosis for ischemic central retinal vein occlusion. Ophthalmic Surg Lasers. 1999;30:605–614.
83 McAllister IL, Constable IJ. Laser-induced chorioretinal venous anastomosis for treatment of nonischemic central retinal vein occlusion. Arch Ophthalmol. 1995;113:456–462.
84 Fekrat S, Goldberg MF, Finkelstein D. Laser-induced chorioretinal venous anastomosis for nonischemic central or branch retinal vein occlusion. Arch Ophthalmol. 1998;116:43–52.
85 McAllister IL, Gillies ME, Smithies LA, et al. The Central Retinal Vein Bypass Study: a trial of laser-induced chorioretinal venous anastomosis for central retinal vein occlusion. Ophthalmology. 2010;117:954–965.
86 McAllister IL, Douglas JP, Constable IJ, et al. Laser-induced chorioretinal venous anastomosis for nonischemic central retinal vein occlusion: evaluation of the complications and their risk factors. Am J Ophthalmol. 1998;126:219–229.
87 Bavbek T, Yenice O, Toygar O. Problems with attempted chorioretinal venous anastomosis by laser for nonischemic CRVO and BRVO. Ophthalmologica. 2005;219:267–271.
88 Hattenbach LO, Steinkamp G, Scharrer I, et al. Fibrinolytic therapy with low-dose recombinant tissue plasminogen activator in retinal vein occlusion. Ophthalmologica. 1998;212:394–398.
89 Hattenbach LO, Wellermann G, Steinkamp GW, et al. Visual outcome after treatment with low-dose recombinant tissue plasminogen activator or hemodilution in ischemic central retinal vein occlusion. Ophthalmologica. 1999;213:360–366.
90 Hattenbach LO, Friedrich Arndt C, Lerche R, et al. Retinal vein occlusion and low-dose fibrinolytic therapy (R.O.L.F.): a prospective, randomized, controlled multicenter study of low-dose recombinant tissue plasminogen activator versus hemodilution in retinal vein occlusion. Retina. 2009;29:932–940.
91 Elman MJ. Thrombolytic therapy for central retinal vein occlusion: results of a pilot study. Trans Am Ophthalmol Soc. 1996;94:471–504.
92 Lahey JM, Fong DS, Kearney J. Intravitreal tissue plasminogen activator for acute central retinal vein occlusion. Ophthalmic Surg Lasers. 1999;30:427–434.
93 Glacet-Bernard A, Kuhn D, Vine AK, et al. Treatment of recent onset central retinal vein occlusion with intravitreal tissue plasminogen activator: a pilot study. Br J Ophthalmol. 2000;84:609–613.
94 Elman MJ, Raden RZ, Carrigan A. Intravitreal injection of tissue plasminogen activator for central retinal vein occlusion. Trans Am Ophthalmol Soc. 2001;99:219–221. discussion 22–3
95 Ghazi NG, Noureddine B, Haddad RS, et al. Intravitreal tissue plasminogen activator in the management of central retinal vein occlusion. Retina. 2003;23:780–784.
96 Paques M, Vallee JN, Herbreteau D, et al. Superselective ophthalmic artery fibrinolytic therapy for the treatment of central retinal vein occlusion. Br J Ophthalmol. 2000;84:1387–1391.
97 Weiss JN. Treatment of central retinal vein occlusion by injection of tissue plasminogen activator into a retinal vein. Am J Ophthalmol. 1998;126:142–144.
98 Weiss JN, Bynoe LA. Injection of tissue plasminogen activator into a branch retinal vein in eyes with central retinal vein occlusion. Ophthalmology. 2001;108:2249–2257.
99 Feltgen N, Junker B, Agostini H, et al. Retinal endovascular lysis in ischemic central retinal vein occlusion: one-year results of a pilot study. Ophthalmology. 2007;114:716–723.
100 Lam HD, Blumenkranz MS. Treatment of central retinal vein occlusion by vitrectomy with lysis of vitreopapillary and epipapillary adhesions, subretinal peripapillary tissue plasminogen activator injection, and photocoagulation. Am J Ophthalmol. 2002;134:609–611.
101 Yeshaya A, Treister G. Pars plana vitrectomy for vitreous hemorrhage and retinal vein occlusion. Ann Ophthalmol. 1983;15:615–617.
102 Liang XL, Chen HY, Huang YS, et al. Pars plana vitrectomy and internal limiting membrane peeling for macular oedema secondary to retinal vein occlusion: a pilot study. Ann Acad Med Singapore. 2007;36:293–297.
103 Raszewska-Steglinska M, Gozdek P, Cisiecki S, et al. Pars plana vitrectomy with ILM peeling for macular edema secondary to retinal vein occlusion. Eur J Ophthalmol. 2009;19:1055–1062.
104 Park DH, Kim IT. Long-term effects of vitrectomy and internal limiting membrane peeling for macular edema secondary to central retinal vein occlusion and hemiretinal vein occlusion. Retina. 2010;30:117–124.
105 DeCroos FC, Shuler RK, Jr., Stinnett S, et al. Pars plana vitrectomy, internal limiting membrane peeling, and panretinal endophotocoagulation for macular edema secondary to central retinal vein occlusion. Am J Ophthalmol. 2009;147:627–633. e1
106 Opremcak EM, Rehmar AJ, Ridenour CD, et al. optic neurotomy for central retinal vein occlusion: 117 consecutive cases. Retina. 2006;26:297–305.
107 Weizer JS, Stinnett SS, Fekrat S. Radial optic neurotomy as treatment for central retinal vein occlusion. Am J Ophthalmol. 2003;136:814–819.
108 Hasselbach HC, Ruefer F, Feltgen N, et al. Treatment of central retinal vein occlusion by radial optic neurotomy in 107 cases. Graefes Arch Clin Exp Ophthalmol. 2007;245:1145–1156.
109 Arevalo JF, Garcia RA, Wu L, et al. Radial optic neurotomy for central retinal vein occlusion: results of the Pan-American Collaborative Retina Study Group (PACORES). Retina. 2008;28:1044–1052.
110 Martinez-Jardon CS, Meza-de Regil A, Dalma-Weiszhausz J, et al. Radial optic neurotomy for ischaemic central vein occlusion. Br J Ophthalmol. 2005;89:558–561.
111 Crama N, Gualino V, Restori M, et al. Central retinal vessel blood flow after surgical treatment for central retinal vein occlusion. Retina. 2010;30:1692–1697.
112 Skevas C, Wagenfeld L, Feucht M, et al. Radial optic neurotomy in central retinal vein occlusion does not influence ocular hemodynamics. Ophthalmologica. 2011;225:41–46.
113 Opremcak EM, Bruce RA, Lomeo MD, et al. Radial optic neurotomy for central retinal vein occlusion: a retrospective pilot study of 11 consecutive cases. Retina. 2001;21:408–415.